Abstract
BACKGROUND
Ethanol has diverse effects on nervous system development, which includes development and survival of GABAergic neurons in a sonic hedgehog (Shh) and fibroblast growth factor (Fgf)-dependent mechanism. Cannabinoids also function as inhibitors of Shh signaling, raising the possibility that ethanol and cannabinoids may interact to broadly disrupt neuronal function during brain development.
METHODS
Zebrafish embryos were exposed to a range of ethanol and/or cannabinoid receptor 1 (CB1R) agonist concentrations at specific developmental stages, in the absence or presence of morpholino oligonucleotides that disrupt shh expression. In situ hybridization was employed to analyze glutamic acid decarboxylase (gad1) gene expression as a marker of GABAergic neuron differentiation, and zebrafish behavior was analyzed using the novel tank diving test as a measure of risk taking behavior.
RESULTS
Combined acute subthreshold ethanol and CB1R agonist exposure results in a marked reduction in gad1 mRNA expression in zebrafish forebrain. Consistent with the ethanol and cannabinoid effects on Shh signaling, fgf8 mRNA overexpression rescues the ethanol- and cannabinoid-induced decrease in gad1 gene expression and also prevents the changes in behavior induced by ethanol and cannabinoids.
CONCLUSIONS
These studies provide evidence that forebrain GABAergic neuron development, and zebrafish risk-taking behavior, are sensitive to both ethanol and cannabinoid exposure in a Shh- and Fgf-dependent mechanism, and provide additional evidence that a signaling pathway involving Shh and Fgf crosstalk is a critical target of ethanol and cannabinoids in FASD.
Keywords: Cannabinoid, Shh, Fgf, gad1, FASD
INTRODUCTION
The effects of alcohol exposure on central nervous system (CNS) development are well documented, with the incidence of fetal alcohol spectrum disorders (FASD) in humans being estimated at 2–5% of live births (May et al., 2014). The most severe form of the spectrum, fetal alcohol syndrome (FAS), results in a range of CNS perturbations that include ocular (Dangata and Kaufman 1997; Stromland and Pinazo-Duran 1994), forebrain (Clarren et al., 1978; Mattson and Riley 1996) and hindbrain developmental dysmorphologies (Cragg and Phillips 1985; Goodlett et al., 1990). It is well established that the developmental stage of alcohol exposure, as well as the dosage of alcohol exposure, impacts the CNS regions that are affected, with exposure during gastrulation and neurulation having marked effects on ocular and forebrain development (Sulik et al., 1981, 1984; Maier et al., 1997; Parnell et al., 2009; Godin et al., 2010).
A wealth of studies provide strong evidence for ethanol exposure disrupting key signaling pathways, in particular growth factors such as the Fgfs and morphogens such as Shh. In mouse forebrain both Fgf2 and Fgf8 expression levels are altered following prenatal ethanol exposure in mice (Aoto et al., 2008; Rubert et al., 2006). In zebrafish Shh has emerged as a critical target of embryonic ethanol exposure, as shh mRNA overexpression rescues most of the morphological and behavioral phenotypes induced by ethanol (Ahlgren et al., 2002; Li et al., 2007; Aoto et al., 2008; Loucks and Ahlgren 2009; Zhang et al., 2011; Zhang et al., 2013; Burton et al., 2017; Boa-Amponsem et al., 2019). Likewise, ethanol exposure in Shh pathway mutant mice exacerbates the effects of ethanol, indicating a key role for Shh signaling in ethanol teratogenicity (Hong and Krauss, 2012; Kietzman et al., 2014; Fish et al., 2017; Kahn et al., 2017).
GABAergic neuron differentiation in both rodents and zebrafish requires Shh and Fgf signaling, providing a possible mechanism for ethanol-mediated perturbation of GABAergic neuron differentiation. Ventral forebrain GABAergic neuron specification in mice occurs via crosstalk between Fgf and Shh signaling pathways (Yung et al., 2002; Gulasci and Lillien, 2003), and similarly this specification of GABAergic neurons in zebrafish forebrain involves coordinated signaling between Shh and Fgf19 (Miyake et al., 2005). Both Fgf3 and Fgf8 gene expression in zebrafish forebrain are also controlled by Shh signaling (Miyake et al., 2005), suggesting that ethanol-mediated reductions in forebrain Fgf8 gene expression (Aoto et al., 2008) may be due to disruption of Shh function by ethanol. Our studies in zebrafish support this argument, with ethanol, in combination with agrin or shh morpholinos (MOs), disrupting forebrain and hindbrain GABAergic neuron development based on gad1, parv7 and atonal1a gene expression, with these treatments also producing marked reductions in gli1 and fgf19 mRNA expression (Zhang et al, 2013). The effects of ethanol and MO on GABAergic neuron development was shown to be rescued by overexpression of shh, fgf8 or fgf19 mRNAs (Zhang et al., 2013).
Like ethanol, cannabinoid exposure during CNS development can induce developmental phenotypes that include FASD-like defects, which are dependent on activation of CB1Rs (Subbanna et al., 2013; Boa-Amponsem et al., 2019; Fish et al, 2019). As combined alcohol and marijuana use is becoming more prevalent with the legalization of cannabis, studies on the effects of these two drugs on CNS development are becoming more important. Marijuana is now reported as the most extensively used illicit drug during pregnancy, with recent surveys indicating 2.8% of pregnant women report using marijuana during the first trimester of pregnancy (Sobrian 2016). In addition, 20% of pregnant women under 24 years of age have tested positive for cannabis during pregnancy (Young-Wolff et al., 2017). Relevant to this use of ethanol and cannabis during pregnancy, combined CB1R agonist and ethanol exposure enhances the susceptibility of the neonatal brain to neurodegeneration, as observed with exposure to high doses of ethanol alone (Hansen et al., 2008). In both zebrafish and mice, previous studies indicate that treatment with the non-selective CB1R and CB2R agonist, CP55940, produces morphological abnormalities that are remarkably similar to high ethanol doses (Fish et al., 2019; Gilbert et al., 2016). Insight into possible mechanisms by which endocannabinoids (eCBs) and alcohol may interact to induce FASD phenotypes are becoming better understood, with eCBs acting as inhibitors of the Shh pathway (Khaliullina et al., 2015; Fish et al., 2019). In addition, using zebrafish our recent studies show that eCBs and alcohol interact to alter zebrafish behavior, with shh mRNA overexpression preventing the behavioral alterations induced by combined ethanol/CB1R agonist exposure (Boa-Amponsem et al., 2019). The activation of CB1R signaling is therefore a possible mechanism by which ethanol alters CNS development and may contribute to the pathophysiology of FASD. We therefore hypothesized that combined ethanol and cannabinoid exposure during early zebrafish embryogenesis will disrupt GABAergic neuron differentiation in an Fgf-dependent mechanism, in light previous studies documenting the role of Shh and Fgf crosstalk in GABAergic neuron differentiation (Miyake et al, 2005; Zhang et al, 2013). The results from the present study indicate that zebrafish forebrain GABAergic neuron development is impaired by subthreshold eCBs combined with subthreshold ethanol, with both drugs altering Shh and Fgf signaling. Our behavioral analysis of juvenile zebrafish shows that fgf8 mRNA overexpression also can prevent the behavioral changes induced by ethanol and/or eCB exposure. These studies support a mechanism where critical signaling pathways involving Shh and Fgf are sensitive to ethanol and eCBs, resulting in the occurrence of morphological and behavioral phenotypes observed in FASD.
MATERIALS AND METHODS
Animals
Zebrafish of the AB strain (Danio rerio) were obtained from the Zebrafish International Resource Center and were bred and maintained as previously described (Zhang et al, 2013; Boa-Amponsem et al, 2019). Fish were group housed with <20 fish per 3L tank on a 14:10 hour (light: dark) cycle 7 days per week. Approximately 20–30 fish per ethanol exposure condition were reared for behavioral testing. Behavioral testing was conducted during the light cycle between the hours of 11:00 AM and 5:00 PM as previously described (Boa-Amponsem et al, 2019). All fish were fed twice daily with brine shrimp (Brine Shrimp Direct, Ogden, Utah) in the morning and dry flake fish food (TetraMin®, Tropical Flakes, Melle, Germany) in the evening; experimental fish were not fed in the morning prior to testing and evening feeding was always withheld until after completion of behavioral testing. All protocols pertaining to the breeding and care of zebrafish were conducted according North Carolina Central University IACUC policy.
Ethanol treatment of zebrafish embryos
Zebrafish embryos in fish water, containing a 1:500 dilution of 0.1% methylene blue (to prevent fungal infection) and 0.003% 1-phenyl-2-thiourea (PTU, to inhibit pigmentation), were exposed to 0.5%−1.0% ethanol from 8–10 hpf or 24–27 hpf as previously described (Zhang et al, 2013; Boa-Amponsem et al, 2019). Following ethanol exposure embryos were washed once with fresh fish water, and then transferred to fresh fish water for the remainder of the experimental time-course. All treatments were conducted with embryos with an intact chorion.
Cannabinoid Treatment of Zebrafish Embryos
Zebrafish embryos were exposed to the CB1R-selective agonist 1mg/L Arachidonoyl 2’-Chloroethylamide (ACEA) or 3mg/L ACEA (Cayman Chemical, Ann Arbor, MI), or ethanol and ACEA, from late gastrulation to the beginning of neurulation (8–10 hpf) or during late neurulation (24–27 hpf) as previously described (Boa-Amponsem et al, 2019). ACEA is a potent and selective CB1R agonist with similar efficacy to the eCBs. Following treatments, fish were washed with fresh fish water and then maintained until morphological or behavioral analyses as previously described (Boa-Amponsem et al, 2019).
Antisense morpholino injection
The properties and characterization of the antisense morpholinos (MOs) to shh (Gene Tools, Philomath, OR) used in the current studies have been previously described (Zhang et al., 2013). MOs (shh or Gene Tools control MO) were solubilized in water (0.1–1.0 mM) and injected into one- to two-cell stage embryos. The control MO at the same concentration and volume produces no detectable zebrafish abnormalities (Kim et al., 2007).
For subthreshold shh MO experiments, embryos were injected with 0.3 pmol Shh MO, and embryos were then exposed to 0.5% ethanol from 8–10 hpf or 24–27 hpf. For fgf8 mRNA rescue of Shh MO/ACEA/ethanol exposure, embryos were co-injected with 0.3 pmol Shh MO and 0.1 pg fgf8 mRNA. Fgf8 was synthesized according to previously published methods (Zhang et al., 2013). PCR primers for fgf8 were CGGAATTCATGAGACTCATACCTTCACGG and GCTCTAGACTAACGCTCTCCTGAGTAGC. The PCR product was subcloned into pCS2 vector and confirmed by sequencing. Capped mRNA was synthesized with Ambion cap mRNA kit.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was carried out as previously described (Kim et al., 2007; Liu et al., 2008). Digoxygenin-labeled riboprobe to gad1 was transcribed as previously described (Zhang et al., 2013). PCR primers for gad1 were GACTGATGGCGTCTTCTGC and CACCAGGATCTGCTCCAGG. All embryos (control and treatments) were developed for riboprobe staining for identical times, and analyzed to score embryos for altered or normal gad1 staining. A second laboratory member was blinded to the treatments and their scoring of gad1 levels was the same as the unblinded laboratory member.
Real-time PCR analysis of gad1 expression
Embryos treated with ethanol or ACEA as described above were collected, pooled (n=15) into tubes, and mRNA was extracted with RNAzol (Molecular Research Center, INC). 1 μg mRNA was used for cDNA synthesis using iScript reverse transcriptase (Bio-Rad) with a mix of oligo(dT) and random hexamers as primers. 50 ng cDNA was used for real time PCR amplification, which was performed with TaqMan® Gene Expression Master Mix (Life Technology) and TaqMan Gene Expression Assay ID (Dr03080474_g1) (Life Technology) for zebrafish gad1 expression or TaqMan Gene Expression Assay ID (Dr03432610_m1) (Life technology) for endogenous control zebrafish ß-actin expression. The PCR reaction plate was run on an Applied Biosystems real-time quantitative PCR instrument in triplicates using the default PCR thermal cycling conditions. Quantitation of gad1 was normalized to the internal ß-actin. Relative gad1 mRNA levels from each treatment group were compared against control, untreated gad1 mRNA levels.
Eye phenotype Scoring
Eye size was measured at 48 hpf using embryos not exposed to PTU (normal pigmentation), as previously described (Zhang et al., 2011; 2014). This involved measuring the longest axis along the eye, with a diameter of less than 240 μm as designated as the “small eye” phenotype, since untreated embryos were always observed to have an eye diameter of at least 250 μm. Both eyes were assessed for changes in response to drug and/or MO treatments.
Behavioral Testing
Fish were assessed for behavioral testing during the late fry juvenile stage, at approximately 2 months of age. The novel tank diving assay (Levin et al. 2007; Levin 2011) was utilized to assess alteration of risk-taking/anxiety-like behavior by alcohol or cannabinoids, as previously described by our laboratory (Burton et al, 2017; Boa-Amponsem et al, 2019). Zebrafish were individually tested as described previously (Boa-Amponsem et al, 2019). Behavior was tracked in real-time using an Everfocus camcorder (Duarte, CA) and EthoVision tracking software (Noldus Information and Technology, Wageningen, The Netherlands). During the behavioral assay the mean distance from the tank floor was quantified, with a total water depth of 10 cm in the test tank. Testing duration was 5 minutes and commenced immediately following placement of a fish in the novel test tank. Video was captured using an Everfocus camcorder (Duarte, CA) that was positioned approximately 88 cm from the testing tanks. A minimum of 12 fish were tested for each treatment group.
Statistics
All ANOVA statistical analysis was performed using SPSS Statistics 25 (International Business Machines Corp, Armonk, New York) as previously described (Boa-Amponsem et al, 2019). Ethanol, CB1R agonist, shh MO, and vehicle (control) were used as the between factors, with mean distance to tank floor and total distance traveled per minute of the assay period as the repeated measure. To control for sphericity, the Greenhouse-Geisser adjustment was applied to all behavioral data. Tukey’s post hoc test was used to determine specific differences in the Mean between treatment groups. Morphological data were analyzed in GraphPad Prism using one-way ANOVA, and Tukey post-hoc test was used to compare the mean of each treatment with the mean of every other treatment.
RESULTS
Effects of shh, ethanol and cannabinoids on zebrafish development are rescued by fgf8 mRNA overexpression
A major focus of the developmental consequences of ethanol exposure during zebrafish embryogenesis has been on eye development, with considerable understanding of the effects of ethanol on eye development resulting from zebrafish studies. A common phenotype observed in zebrafish following ethanol exposure during embryogenesis is microphthalmia (Reimers et al., 2004; Dlugos and Rabin, 2007; Kashyap et al., 2007; Loucks et al., 2007; Ali et al., 2011; Zhang et al., 2011). To assess dysmorphologies resulting from alcohol or CB1R agonist treatment, zebrafish embryos were acutely exposed to ethanol in the presence or absence of shh MO or the CB1R agonist ACEA, from either 8–10 hpf (late gastrulation/early neurulation), or 24–27 hpf (late neurulation) (Figure 1). Treated embryos were then assessed for microphthalmia at 48 hpf. While exposure to 1% ethanol alone, or 0.5% ethanol in combination with 0.3 pm Shh MO, did not induce any dysmorphogenesis as assessed by eye diameter (Figure 2), combined 1% ethanol and 0.3pm Shh MO treatment at 8–10 hpf induced microphthalmia at 48 hpf (Figure 2) for both 8–10 hpf (13/43=30% embryos) and 24–27 hpf exposures (17/53= 32% embryos).
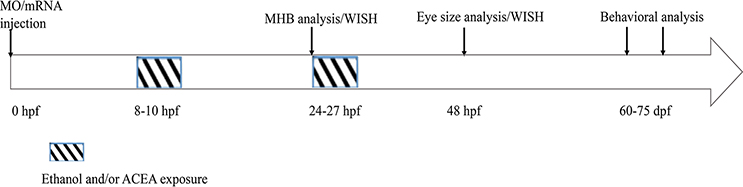
Figure 1:
Timeline for MO/ mRNA microinjection and ethanol and/or cannabinoid treatments are indicated, as well as times of morphological analysis of embryos (MHB and eye size) and behavioral testing.
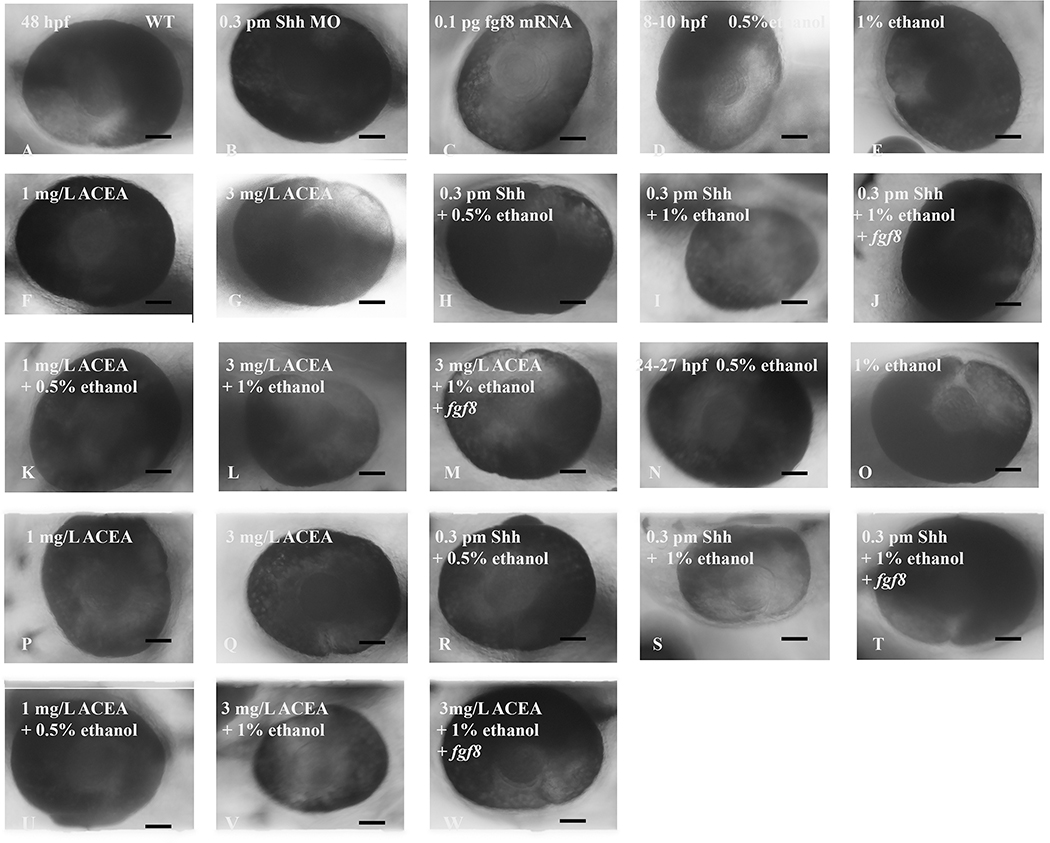
Figure 2:
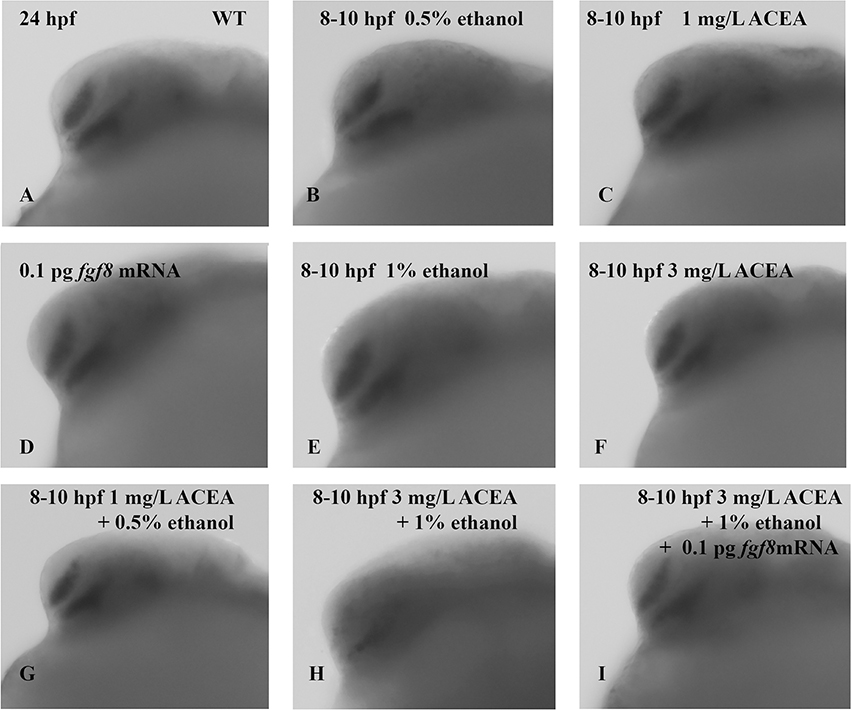
Fgf8 mRNA rescued small eye phenotype induced by combined acute exposure to shh MO and ethanol. Embryos were pre-injected with shh MO alone or with fgf8 mRNA and exposed to ethanol from 8–10 hpf and 24–27 hpf. Eye diameter was measured in 48 hpf embryos as described in Methods and representative photomicrographs are shown. A, wild-type embryos; B, embryos injected with shh MO alone at one- to two-cell stage; C, embryos injected with fgf8 mRNA alone at one- to two-cell stage; D, embryos exposed to 0.5% ethanol alone from 8–10 hpf; E, embryos exposed to 1% ethanol alone from 8–10 hpf; F, embryos exposed to 1 mg/L ACEA alone from 8–10 hpf; G, embryos exposed to 3 mg/L ACEA alone from 8–10 hpf; H, embryos injected with shh MO at one- to two-cell stage and exposed to 0.5% ethanol from 8–10 hpf; I, embryos injected with shh MO at one- to two-cell stage and exposed to 1% ethanol from 8–10 hpf; J, embryos co-injected with shh MO and fgf8 mRNA at one- to two-cell stage and exposed to 1% ethanol from 8–10 hpf; K, embryos exposed to 1 mg/L ACEA and 0.5% ethanol from 8–10 hpf; L, embryos exposed to 3 mg/L ACEA and 1% ethanol from 8–10 hpf; M, embryos exposed to both 3 mg/L ACEA and 1% ethanol from 8–10 hpf, and injected with fgf8 mRNA at the one- to two-cell stage; N, embryos exposed to 0.5% ethanol alone from 24–27 hpf; O, embryos exposed to 1% ethanol alone from 24–27 hpf; P, embryos exposed to 1 mg/L ACEA alone from 24–27 hpf; Q, embryos exposed to 3 mg/L ACEA alone from 24–27 hpf; R, embryos injected with shh MO at one- to two-cell stage and exposed to 0.5% ethanol from 24–27 hpf; S, embryos injected with shh MO at one- to two-cell stage and exposed to 1% ethanol from 24–27 hpf; T, embryos co-injected with shh MO and fgf8 mRNA at one- to two-cell stage and exposed to 1% ethanol from 24–27 hpf; U, embryos exposed to 1 mg/L ACEA and 0.5% ethanol from 24–27 hpf; V, embryos exposed to 3 mg/L ACEA and 1% ethanol from 24–27 hpf; W, embryos exposed to both 3 mg/L ACEA and 1% ethanol from 24–27 hpf, and injected with fgf8 mRNA at the one- to two-cell stage. Microphthalmia was observed only in embryos exposed to ethanol after shh MO injection (I and S) or exposed to both 3 mg/L ACEA and 1% ethanol (L and S). The small eye phenotype was rescued by fgf8 mRNA overexpression (J, M, T and W). The calibration bar is 50 μm. N≥ 30/group.
Embryos were also exposed to the CB1-R specific agonist ACEA, to assess effects on eye development. The doses of ACEA employed in these studies were derived from previous studies where a dose-dependent examination of ACEA on zebrafish eye development was determined (Boa-Amponsem et al, 2019). Exposure to the lower dose of ACEA alone (1 mg/L) or in combination with 0.5% ethanol did not produce microphthalmia. However, while a higher concentration of ACEA (3 mg/L) did not induce microphthalmia (0/21 at 8–10 hpf and 0/27 at 24–27 hpf; Figure 2), in combination with 1% ethanol this ACEA dose produced the small eye phenotype for both 8–10 hpf (10/31, 32%) and 24–27 hpf (14/38, 37%) (Figure 2). Figures 3 and 4 summarize the average eye diameter for each of the treatments, and indicates a significant decrease in eye diameter as a result of co-exposure to ethanol and ACEA. This effect of ACEA on eye development is similar to the effects observed using THC or cannabidiol (CBD) in mouse and a non-selective agonist for CB1R and CB2R (Fish et al, 2019).
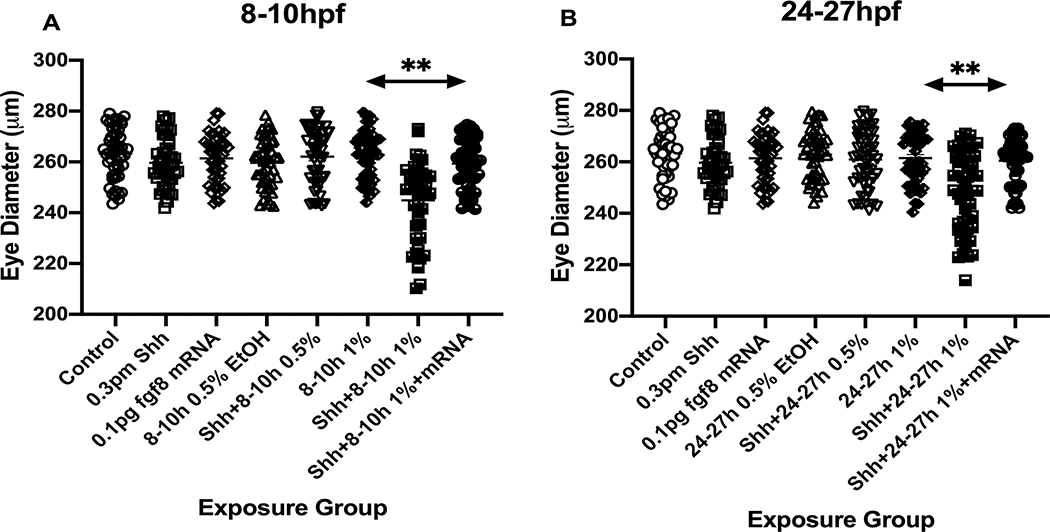
Figure 3:
Effects of ethanol exposure and shh MO treatments on eye diameter. The Mean eye diameter for each treatment group was quantified, with only combined ethanol and shh MO treatment resulting in a significant difference in eye diameter from other treatments, and fgf8 mRNA overexpression rescuing the effects of ethanol and shh MO treatments on eye diameter. A, 8–10 hpf ethanol exposure; B, 24–27 hpf ethanol exposure. ** significantly different from Control Mean, P< 0.001.
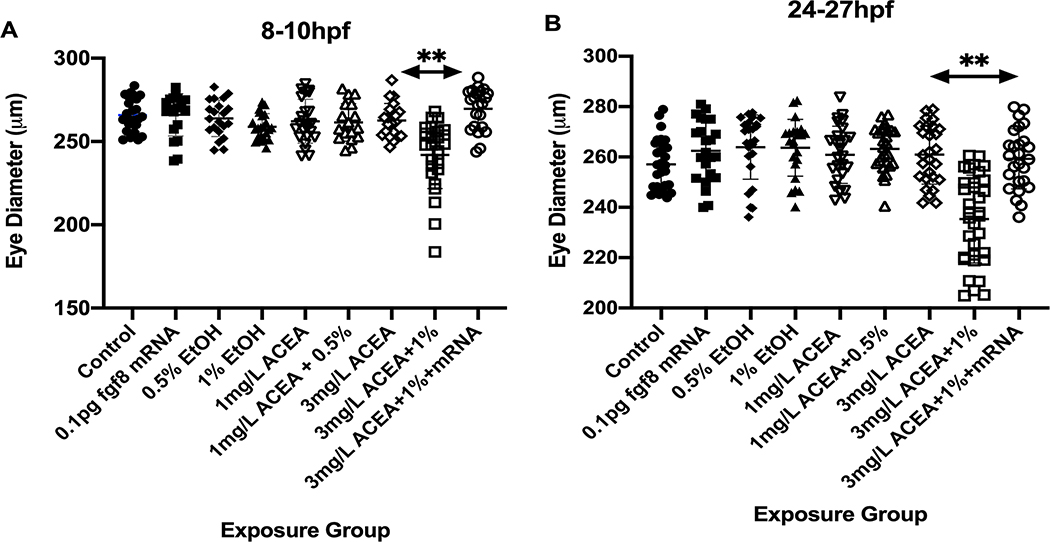
Figure 4:
Effects of ethanol and ACEA exposure on eye diameter. The Mean eye diameter for each treatment group was quantified, with only combined 3 mg/L ACEA + 1% ethanol treatment resulting in a significant difference in eye diameter from other treatments, and fgf8 mRNA overexpression rescuing the effects of combined ethanol and ACEA treatment on eye diameter. A, 8–10 hpf ethanol exposure; B, 24–27 hpf ethanol exposure. ** significantly different from Control Mean, P< 0.001.
To confirm the role of Fgf signaling and Fgf-Shh crosstalk in ethanol-induced dysmorphologies, we examined whether fgf8 mRNA injection of one- to two-cell embryos would rescue the observed eye malformation phenotypes. Fgf8 mRNA overexpression rescued microphthalmia, as only 4/57 (7%) of embryos injected with 0.3 pm shh MO and exposed to 1% ethanol from 8–10 hpf, or 3/60 (5%) of embryos exposed from 24–27 hpf, exhibited decreased eye size following fgf8 mRNA rescue (Figures 2 and 3). Fgf8 mRNA overexpression also rescued the effects of ACEA on eye development, as 0/22 (0%) of embryos co-exposed to 1% ethanol and 3 mg/L ACEA from 8–10 hpf, and 0/26 (0%) of embryos exposed from 24–27 hpf, exhibited the small eye phenotype (Figures 2 and 4). These treatments therefore effectively rescued the dysmorphology produced by combined ethanol combined with either ACEA or shh MO treatment.
Zebrafish forebrain GABAergic neuron development is sensitive to ethanol
Forebrain GABAergic neuron development in zebrafish has been shown to require Fgf signaling acting downstream of Shh signaling (Miyake et al., 2005). Zhang et al. (2013) showed that GABAergic neuron development was disrupted by ethanol treatment based on gad1, parv7 and atonal1a mRNA expression, that these treatments down-regulated Shh signaling and Shh-Fgf crosstalk based on decreased gli1 and fgf19 expression, and that the gad1 expression could be rescued by overexpression of shh or fgf8/fgf19 mRNAs. Numerous studies have provided clear evidence that during development Shh signaling is a major target of ethanol, and recent studies suggest crosstalk between the Shh and eCB signaling pathways is also sensitive to ethanol exposure (Boa-Amponsem et al., 2019; Fish et al., 2019). We therefore predicted that co-exposure with ethanol and the CB1R specific agonist ACEA would impair GABAergic neuron development via a shared Shh and Fgf signaling mechanism, using gad1 gene expression as a proxy of GABAergic neuron differentiation. Gad1 mRNA expression in 24 hpf forebrain is observed following 8–10 hpf exposure to 1% ethanol or 3 mg/L ACEA alone, but is reduced following a combined exposure to 1% ethanol and 3 mg/L ACEA (Figure 5). Consistent with the previously demonstrated role of Fgf signaling in zebrafish forebrain GABAergic neuron development, fgf8 mRNA overexpression rescued the effects of combined ethanol and ACEA on gad1 expression (Figure 5). The number of embryos displaying reduced gad1 mRNA expression was quantified, with these data showing: control 0/62 (0%); 0.5% ethanol 0/32 (0%); 1 mg/L ACEA 0/34 (0%); 1% ethanol 2/60 (3%); 3mg/l ACEA 2/64 (3%); 0.1pg fgf8 mRNA 1/58 (2%); 1 mg/L ACEA + 0.5% ethanol 0/32 (0%); 3mg/L ACEA + 1% EtOH 31/59 (53%); 3mg/L ACEA + 1% EtOH + fgf8 mRNA 5/56 (9%).
Figure 5:
Gad1 gene expression is reduced in 24 hpf embryos following acute 1% ethanol and 3 mg/L ACEA co-exposure from 8–10 hpf and is rescued by fgf8 mRNA. Embryos were analyzed at 24 hpf for gad1 gene expression by whole-mount in situ hybridization. A, wild-type embryos; B, 0.5% ethanol alone; C, 1 mg/L ACEA alone; D, embryos injected with fgf8 mRNA alone at one- to two-cell stage; E, embryos exposed to 1% ethanol alone; F, embryos exposed to 3mg/L ACEA alone; G, embryos co-exposed to 0.5% ethanol and 3 mg/L ACEA; H, embryos co-exposed to 3 mg/L ACEA and 1% ethanol; I, embryos injected with fgf8 mRNA at one- to two-cell stage and co-exposed to 3 mg/L ACEA and 1% ethanol. Note: Decreased gad1 gene expression was only observed with combined 1% ethanol and 3 mg/L ACEA treatment, and fgf8 mRNA overexpression was able to reverse this reduced gene expression (F). N≥ 30/group.
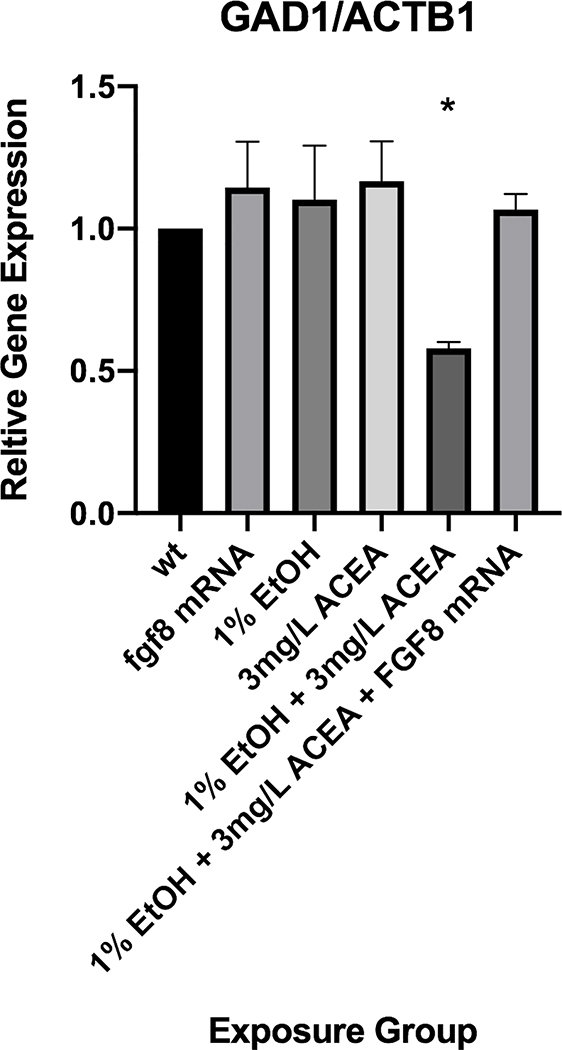
To verify the visual, qualitative assessment of the effects of ethanol and ACEA on gad1 expression using WISH, we quantified gad1 mRNA levels using qRT-PCR (Figure 6). These data corroborate the WISH studies, showing that gad1 mRNA levels were only significantly decreased, compared to control and control treatments, with exposure to 3 mg/L ACEA + 1% ethanol (Figure 6). In addition, fgf8 mRNA injection rescued the decreased gad1 mRNA expression in the presence of 3 mg/L ACEA + 1% ethanol (Figure 6).
Figure 6:
Quantitation of gad1 mRNA expression by quantitative RT-PCR. Gad1 mRNA levels were quantified by qRT-PCR and normalized to an internal ß-actin mRNA control. * significantly different from control expression, P< 0.05.
Fgf8 mRNA is capable of reversing the effects of embryonic exposure to shh MO or CB1R agonist and ethanol on zebrafish risk-taking behavior
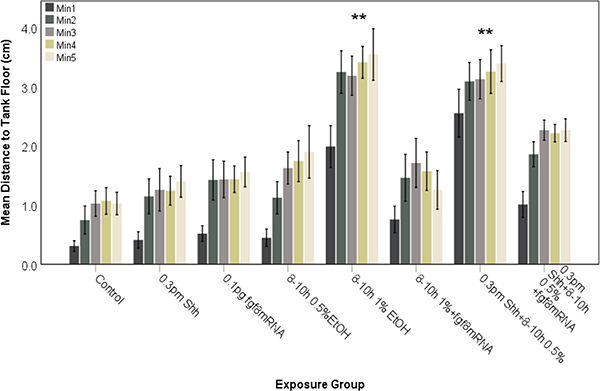
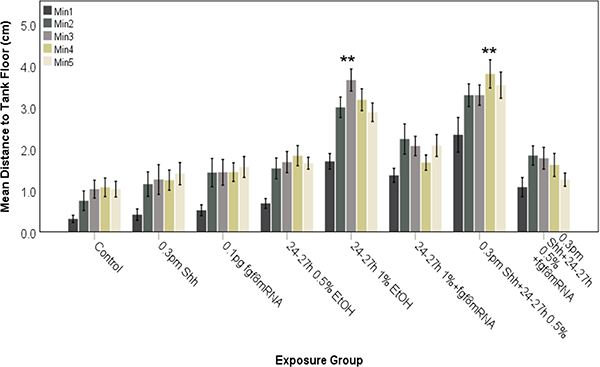
Our previous studies have analyzed novel tank diving behavior of juvenile zebrafish and found that exposure to 1% ethanol alone is capable of inducing behavioral alterations (Bailey et al., 2015; Burton et al., 2017; Boa-Amponsem et al., 2019), which could be reversed with prior injection of shh mRNA (Burton et al., 2017). Since crosstalk between Fgf and Shh signaling is well established in forebrain GABAergic neuron specification (Miyake et al, 2005; Zhang et al, 2013), and is disrupted by combined ethanol and ACEA exposure (Figures 5 and 6), we wanted to establish whether behavioral alteration by ethanol and/or ACEA could also be rescued by fgf8 mRNA overexpression. Figures 7 and 8 examine the effects of ethanol or ethanol combined with shh MO on risk-taking behavior in zebrafish, as measured using the novel tank dive test. In these experiments 1% ethanol alone, or 0.5% ethanol combined with shh MO, alters behavior following either 8–10 hpf exposure (Fig. 7) or 24–27 hpf exposure (Fig. 8). Control fish remained near the bottom of the test tank during the first minute and for the most part did not swim into the upper quadrant of the tanks (Figures 7 and 8). In contrast, fish exposed to 1% ethanol alone or 0.5% ethanol and shh MO explored the entire tank while swimming, and entered the upper quadrant even during the first minute of the testing (Figures 7 and 8). In order to ascertain the role of Fgf signaling in ethanol-mediated effects on altered novel tank diving behavior, zebrafish embryos were injected with fgf8 mRNA to prevent the altered behavior produced by ethanol treatment (Figures 6 and 7). With ethanol exposure, fgf8 mRNA overexpression prevented the altered novel tank diving behavior, with control and fgf8 mRNA injected fish showing swimming behavior that more closely resembled control fish (Figures 7 and 8). The fgf8 injected fish behavior was significantly different when compared to 1% ethanol treatment alone or 0.5% ethanol combined with 0.3 pm shh MO injection for both 8–10 hpf (Figure 7; F (3.007, 249.596)= 33.467, P<0.0001) and 24–27 hpf (Figure 8; F (3.431, 168.750)= 36.932, P<0.0001) treated embryos.
Figure 7:
Behavioral analysis of zebrafish embryos injected with shh MO and exposed to ethanol during late gastrulation to early neurulation (8–10 hpf). Embryos were assessed for novel tank diving response at 60–75 days. As controls, embryos were either untreated (Control), exposed to ethanol alone or injected with shh MO or fgf8 mRNA alone. Altered tank diving response, indicative of increased risk-taking behavior, is only observed for fish exposed to both shh MO and ethanol. Overexpression of fgf8 mRNA was able to attenuate the risk-taking behavior. Each bar represents the mean distance from the tank floor for one minute (min 1–5). Error bars represent SEM. (**significantly different from control (post-hoc) across all time points analyzed, P<0.001). N≥ 15 per group.
Figure 8:
Behavioral analysis of zebrafish embryos injected with shh MO and exposed to ethanol during late neurulation (24–27 hpf). Embryos were assessed for novel tank diving response at 60–75 days. As controls, embryos were either untreated (Control), injected with shh MO or fgf8 mRNA alone. Altered tank diving response is only observed for fish exposed to both shh MO and ethanol. Overexpression of fgf8 mRNA was able to attenuate the risk-taking behavior. Each bar represents the mean distance from the tank floor for one minute (min 1–5). Error bars represent SEM. (**significantly different from control (post-hoc) across all time points analyzed, P<0.001). N≥ 15 per group.
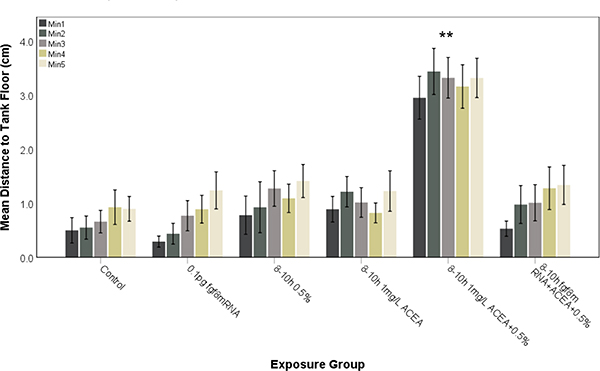
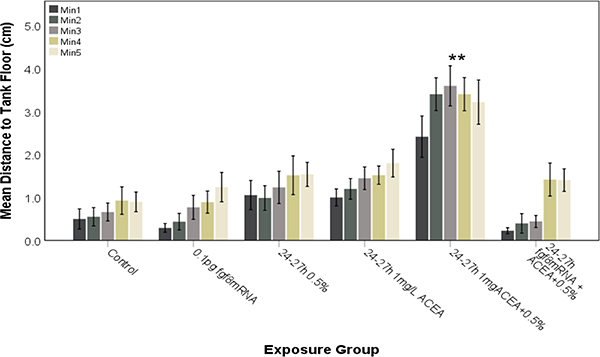
The behavioral studies were extended to examine the effects of 0.5 % ethanol combined with 1 mg/L ACEA on novel tank diving behavior (Figures 9 and 10), with 0.5 % ethanol or 1 mg/L ACEA alone having no effect on swimming behavior, but ethanol combined with 1 mg/L ACEA altering behavior following either 8–10 hpf exposure (Fig. 9) or 24–27 hpf exposure (Fig. 10). Again, with combined ethanol and ACEA exposure, the treated fish explored the entire tank from the beginning of the testing period, indicating a marked increase in risk-taking behavior (Figures 9 and 10). In order to confirm the role of Shh and Fgf signaling in cannabinoid-mediated effects on novel tank diving behavior, zebrafish embryos were injected with fgf8 mRNA to prevent the altered behavioral effects (Figures 9 and 10). Fgf8 mRNA overexpression attenuated the altered novel tank diving behavior of ethanol and ACEA co-exposure, with control and fgf8 mRNA injected fish exhibiting behaviors that were significantly different when compared to 0.5% ethanol combined with 1 mg/L ACEA for 8–10 hpf (Figure 9; F (3.384, 152.282)= 7.422, P<0.0001) and 24–27 hpf (Figure 10; F (3.196, 163.001)= 13.403, P<0.0001) exposed embryos.
Figure 9:
Subthreshold ethanol and ACEA co-exposure during late gastrulation to early neurulation (8–10 hpf) alters risk-taking behavior in zebrafish embryos and is rescued by fgf8 mRNA overexpression. Embryos were exposed to ethanol and/or ACEA from 8–10 hpf and then assessed for novel tank diving response at 60–75 days. As controls, embryos were either untreated (Control) or exposed to fgf8 mRNA alone. Altered tank diving response, indicative of increased risk-taking behavior, is only observed for fish exposed to both ethanol and ACEA. Pre-injection of fgf8 mRNA was able to attenuate the risk-taking behavior. Each bar represents the mean distance from the tank floor for one minute (min 1–5). Error bars represent SEM. (**significantly different from control (post-hoc) across all time points analyzed, P<0.001). N≥ 15 per group.
Figure 10:
Subthreshold ethanol and ACEA co-exposure during late neurulation (24–27 hpf) alters risk-taking behavior in zebrafish embryos and is rescued by fgf8 mRNA overexpression. Embryos were exposed to ethanol and/or ACEA from 24–27 hpf and then assessed for novel tank diving response at 60–75 days. As controls, embryos were either untreated (Control) or exposed to fgf8 mRNA alone. Altered tank diving response, indicative of increased risk-taking behavior, is only observed for fish exposed to both ethanol and ACEA. Pre-injection of fgf8 mRNA was able to attenuate the risk-taking behavior. Each bar represents the mean distance from the tank floor for one minute (min 1–5). Error bars represent SEM. (**significantly different from control (post-hoc) across all time points analyzed, P<0.001). N≥ 15 per group.
DISCUSSION
A wealth of experimental evidence demonstrates that prenatal exposure to alcohol (Sulik et al., 1981; Watson et al., 2008; Kietzman et al., 2014) or cannabinoids (Gilbert et al., 2016; Gunn et al., 2016) causes developmental deformities, although the mechanisms underlying their effects remain unclear (Gunn et al., 2016). However, recent studies have begun to provide some insight, as eCBs have been shown to act as inhibitors of Smoothened and Shh signaling (Khaliullina et al., 2015), and that ethanol and eCBs both decrease Shh signaling to produce FASD phenotypes (Boa-Amponsem et al, 2019; Fish et al, 2019). Our laboratory has shown recently that acute or chronic exposure of zebrafish embryos to the CB1R agonist, ACEA, alone or with ethanol, induces FASD-like phenotypes that are prevented with shh mRNA overexpression or CB1R antagonist pre-treatment (Boa-Amponsem et al., 2019). Likewise, Burton et al. (2017) also found that dysmorphologies and altered behavior caused by ethanol alone or with Shh MO injection could be reversed by shh mRNA overexpression. These previous findings provided the rationale to extend these studies to better understand the contribution of Shh, in combination with crosstalk with Fgf signaling pathways, on ethanol and eCB interactions that result in FASD-like phenotypes.
Previous studies in our laboratory have shown that exposure of zebrafish embryos to high concentrations of ethanol (1.5% or 2%) resulted in a marked reduction in gad1 mRNA expression, suggesting perturbation of GABAergic neuron differentiation (Zhang et al, 2013). We extended these studies to ascertain whether ethanol and cannabinoid treatment, which produces downstream effects on Shh and/or Fgf signaling, could disrupt gad1 mRNA expression in zebrafish forebrain. These present studies indicate that ethanol and cannabinoid treatment diminishes forebrain gad1 mRNA expression in 24 hpf zebrafish embryos. These results indicate that like recent studies demonstrating disruption of ocular development as a result of ethanol and eCBs perturbing Shh signaling (Boa-Amponsem et al, 2019; Fish et al, 2019), forebrain GABAergic neuron differentiation that is regulated by Shh and Fgf signaling is also sensitive to ethanol and eCB exposure. These studies therefore begin to provide insight into possible molecular basis for the effects of ethanol on forebrain GABAergic neuron development, with ethanol and cannabinoids disrupting a signaling pathway involving Fgf8/Fgf19 and Shh.
A major focus of the current study was whether the effects of combined exposure to ethanol and ACEA on zebrafish behavior and GABAergic neuron development would be reversed with fgf8 mRNA overexpression, since Shh and Fgf signaling pathways are known to exhibit crosstalk (Miyaka et al, 2005; Zhang et al, 2013). A major conclusion from these studies is the confirmation of a role for Fgf signaling in the Shh-dependent mechanisms that are perturbed by ethanol and eCBs. eCBs are known to interfere with GABAergic neurotransmission in multiple brain regions, such as the prefrontal cortex, the amygdala, and the hippocampus (Naderi et al., 2008). Inhibition of GABAergic neuronal activity may result in disinhibition of other neurotransmitter pathways in brain regions affected by ethanol, such as the nucleus accumbens and prefrontal cortex (Naderi et al., 2008). Consistent with previous studies in mouse and zebrafish, which suggested a dependence of forebrain GABAergic neuron specification on Fgf and Shh signaling (Yung et al., 2002; Gulasci and Lillien, 2003; Miyake et al., 2005; Zhang et al., 2013), we show that overexpression of fgf8 mRNA is capable of rescuing the disrupted gad1 expression phenotype that results from combined ethanol and ACEA exposure. This study is the first demonstration that ethanol and cannabinoid co-exposure alters both Shh and Fgf signaling in forebrain, leading to diminished gad1 expression that in the present study was utilized as a proxy of forebrain GABAergic neurons. Our previous studies showed that several markers of GABAergic neuron differentiation were affected by ethanol exposure in a Shh- and Fgf-dependent manner (Zhang et al, 2013), suggesting that combined ethanol and ACEA may also be disrupting this normal differentiation as assessed by gad1 expression. Our results therefore are in agreement with the Shh and Fgf crosstalk that regulates forebrain GABAergic neuron development, since fgf8 mRNA overexpression could rescue both ethanol and shh MO exposure as well as ethanol and ACEA exposure. In zebrafish, disruption of Shh function results in perturbed differentiation of GABAergic neurons, and also disrupts normal fgf3 and fgf8 gene expression (Miyake et al., 2005). Our data, along with these previous results, supports the hypothesis that the reduced fgf8 gene expression observed following prenatal ethanol exposure (Aoto et al., 2008) may be due to disrupted Shh signaling. Interestingly, Shh and Fgf8 signaling are also required for specification of some dopaminergic neurons during CNS development in zebrafish, as well as chicken and mouse (Hynes et al., 1995a, 1995b; Ye et al., 1998; Holzschuh et al., 2003). These studies suggest that the development of multiple classes of neurons could be a target of prenatal ethanol and/or cannabinoid exposure due to disruption of Shh and/or Fgf signaling.
One major focus of the present study was to utilize the novel tank diving assay in elucidating the role of Fgf signaling in the behavioral abnormalities caused by combination of ethanol and eCBs. In addition, the use of shh MO combined with ethanol allowed confirmation of Shh and Fgf crosstalk as a result of rescue of altered behavior by fgf8 mRNA. As previously demonstrated, juvenile zebrafish exposed as embryos to acute doses of 1% ethanol displayed an altered novel tank dive behavior, spending significantly more time swimming near the top of the tank than did the controls. This is despite the fish not showing any morphological defects as embryos (Burton et al., 2017; Boa-Amponsem et al., 2019). Similarly, embryos exposed to acute subthreshold doses of ethanol (0.5%) and 0.3 pm shh MO or 1 mg/L ACEA, which display no morphological abnormalities, exhibited altered juvenile zebrafish behavior. Attenuation of this altered zebrafish risk-taking behavior by pre-injection of fgf8 mRNA further supports our hypothesis that there is a shared signaling pathway between Shh/Fgfs and eCBs that regulates both forebrain neuron differentiation as well as zebrafish behavior.
In summary, the present studies have demonstrated that ethanol- and cannabinoid-mediated disruption of forebrain GABAergic neuron development is via signaling pathways involving Shh and Fgfs. These data may suggest a role for Shh/Fgf signaling, and eCBs in development of the neural circuitry involved in the zebrafish novel tank diving behavior, since this behavior is altered following manipulation of these pathways, and can be reversed with fgf8 mRNA overexpression. While the altered tank-diving behavior might be explained by the combined ethanol and ACEA treatment affecting visual function, the doses employed in this study did not induce a small eye phenotype. In addition, previous studies using this behavioral assay and ethanol exposures that induced a small eye phenotype did not alter a visual based spatial discrimination task, indicting that visual function is unaffected by the embryonic ethanol treatments (Bailey et al, 2015). The protective effects of fgf8 mRNA on ethanol and shh MO or CB teratogenicity provides further support for a role for Shh and Fgf signaling in FASD. Importantly, these studies indicate that zebrafish forebrain GABAergic neuron development, and zebrafish risk-taking behavior, share a common signaling pathway that is sensitive to ethanol and cannabinoid exposure in a Shh- and Fgf-dependent mechanism. Moreover, due to the substantial crosstalk that occurs between Shh and Fgf signaling pathways in multiple parts of the CNS as well as multiple organs that include limb and heart development, it is clear that combined ethanol and cannabis exposure during pregnancy may have a greater impact on development than has been previously appreciated.
ACKNOWLEDGEMENTS
The authors thank Shanta Mackinnon for zebrafish care and husbandry. The authors also thank Pryiash Hafiz for assistance in behavioral analyses.
Grant information: This work was supported by NIH grants U54 AA019765 and R21 AA025400.
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- Ahlgren SC, Thakur V, Bronner-Fraser M (2002) Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci USA 99: 10476–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Champagne DL, Alia A, Richardson MK (2011) Large-scale analysis of acute ethanol exposure in zebrafish development: a critical time window and resilience. PLoS One 6: e20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Higashiyama D, Shiota K, Motoyama J (2008) Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res (Part A) 82: 224–231. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Oliveri AN, Zhang C, Frazier JM, Mackinnon S, Cole GJ, Levin ED (2015) Long-term behavioral impairment following acute embryonic ethanol exposure in zebrafish. Neurotoxicology and Teratology 48: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boa-Amponsem O, Zhang C, Mukhopadhyay S, Ardrey A, Cole GJ (2019) Ethanol and cannabinoids interact to alter behavior in a zebrafish fetal alcohol spectrum disorder model. Birth Defects Research 1–14. [DOI] [PubMed] [Google Scholar]
- Burton D, Zhang C, Boa-Amponsem O, Mackinnon S, Cole GJ (2017) Long-term behavioral changes as a result of acute ethanol exposure in zebrafish: evidence for a role for sonic hedgehog but not retinoic acid signaling. Neurotoxicol. Teratol 61, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC Jr, Sumi SM, Streissguth AP, Smith DW (1978) Brain malformations related to prenatal exposure to ethanol. J Pediatrics 92: 64–67. [DOI] [PubMed] [Google Scholar]
- Cragg BG, Phillips SC (1985) Natural loss of Purkinje cells during development and increased loss with alcohol. Brain Res 325: 151–160. [DOI] [PubMed] [Google Scholar]
- Dangata YY, Kaufman MH (1997) Morphometric analysis of the postnatal mouse optic nerve following prenatal exposure to alcohol. J Anat 191: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA (2007) Ocular deficits associated with alcohol exposure during zebrafish development. J. Comp. Neurol 502: 497–506. [DOI] [PubMed] [Google Scholar]
- Fish EW, Holloway HT, Rumple A, Baker LK, Wieczorek LA, Moy SS, Paniagua B, Parnell SE (2016) Acute alcohol exposure during neurulation: Behavioral and brain structural consequences in adolescent C57BL/6J mice. Behavioral Brain Research 311: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Sulik KK, Williams KP, Parnell SE (2017) Genetic vulnerabilities to prenatal alcohol exposure: limb defects in sonic hedgehog and GLI2 heterozygous mice. Birth defects Res 109: 860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Zhang C, Boschen KE, Boa-Amponsem O, Mendoza-Romero HN, Tarpley M, Chdid L, Mukhopadhyay S, Cole GJ, Williams KP, Parnell SE (2019) Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction. Sci Rep 9: 16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Sulik KK, Fish EW, Baker LK, Dehart DB, Parnell SE (2016) Dose-dependent teratogenicity of the synthetic cannabinoid CP- 55,940 in mice. Neurotoxicology and Teratology 58: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin EA, O’Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK (2010) Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res 34: 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR (1990) A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol 7: 107–114. [DOI] [PubMed] [Google Scholar]
- Gulasci A, Lillien L (2003) Sonic hedgehog and bone morphogenetic protein regulate interneuron development from dorsal telencephalic progenitors in vitro. J Neurosci 23: 9862–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JK, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, Ehiri JE (2016) Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 6: e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Krutz B, Sifringer M, Stefovska V, Bittigau P, Pragst F, Marsicano G, Lutz B, Ikonomidou C (2008) Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Annals of Neurology 64: 42–52. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Hauptmann G, Driever W (2003) Genetic analysis of the roles of Hh, FGF8, and Nodal signaling during catecholaminergic system development in the zebrafish brain. J. Neurosci 23: 5507–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Krauss RS (2012) Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disorders in mice. PLoS Genetics 8: e1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A (1995a) Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 15: 35–44. [DOI] [PubMed] [Google Scholar]
- Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A (1995b) Control of neuronal diversity by the floor plate: contact mediated induction of midbrain dopaminergic neurons. Cell 80: 95–101. [DOI] [PubMed] [Google Scholar]
- Kahn BM, Corman TS, Lovelace K, Hong M, Krauss RS and Epstein DJ (2017) Prenatal ethanol exposure in mice phenocpoies Cdon mutation by impeding Shh function in the etiology of optic nerve hypoplasia. Dis Model Mech 10: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap B, Frederickson LC, Stenkamp DL (2007) Mechanisms for persistent microphthalmia following ethanol exposure during retinal neurogenesis in zebrafish embryos. Visual Neuroscience 24: 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzman HW, Everson JL, Sulik KK, and Lipinski RJ (2014) The teratogenic effects of prenatal ethanol exposure are exacerbated by sonic hedgehog or GLI2 haploinsufficiency in the mouse. PLos One 9:e89448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliullina H, Bilgin M, Sampaio JL, Shevchenko A, Eaton S (2015) Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc Natl Acad Sci U S A 112: 3415–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Liu IH, Song Y, Lee JA, Halfter W, Balice-Gordon RJ, Linney E, Cole GJ (2007) Agrin is required for posterior development and axon pathway formation in embryonic zebrafish. Glycobiology 17: 231–247. [DOI] [PubMed] [Google Scholar]
- Levin ED (2011) Zebrafish assessment of cognitive improvement and anxiolysis: filling the gap between in vitro and rodent models for drug development. Reviews in the Neurosciences 22: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90: 54–58. [DOI] [PubMed] [Google Scholar]
- Li YX, Yang HT, Zdanowicz M, Sicklick JK, Qi Y, Camp TJ, Diehl AM (2007) Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest 87: 231–240. [DOI] [PubMed] [Google Scholar]
- Liu IH, Zhang C, Kim MJ, Cole GJ (2008) Retina development in zebrafish requires the heparan sulfate proteoglycan agrin. Dev. Neurobiol 68: 877–898. [DOI] [PubMed] [Google Scholar]
- Loucks EJ, Schwend T, Ahlgren SC (2007) Molecular changes associated with teratogen-induced cyclopia. Birth Defects Res. (Part a) 79: 642–651. [DOI] [PubMed] [Google Scholar]
- Loucks EJ, Ahlgren SC (2009) Deciphering the role of Shh signaling in axial defects produced by ethanol exposure. Birth Defects Res (Part A) 85: 556–567. [DOI] [PubMed] [Google Scholar]
- Maier SE, Chen WJ, Miller JA, West JR (1997) Fetal alcohol exposure and temporal vulnerability regional differences in alcohol-induced microencephaly as a function of the timing of binge-like alcohol exposure during rat brain development. Alcohol Clin Exp Res 21: 1418–1428. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR (1999) Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res 23: 726–734. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP (1996) Brain anomalies in fetal alcohol syndrome, in Abel EL, editor, Fetal Alcohol Syndrome: From Mechanism to Prevention, Boca Raton: CRC Press; p 50–68. [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Nakayama Y, Konishi M, Itoh N (2005) Fgf19 regulated by Hh signaling is required for zebrafish forebrain development. Dev Biol 288: 259–275. [DOI] [PubMed] [Google Scholar]
- Naderi N, Haghparast A, Saber-Tehrani A, Rezaii N, Alizadeh A-M, Khani A, Motamedi F (2008) Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacology, Biochemistry and Behavior 89: 64–75. [DOI] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK (2009) Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcohol Clin Exp Res 33: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers MJ, Flockton AR, Tanguay RL (2004) Ethanol- and acetaldehyde- mediated developmental toxicity in zebrafish. Neurotoxicol Teratol 26:769–781. [DOI] [PubMed] [Google Scholar]
- Rubert G, Minana R, Pascual M, Guerri C (2006) Ethanol exposure during embryogenesis decreases the radial glial progenitor pool and affects the generation of neurons and astrocytes. J Neurosci Res 84: 483–496. [DOI] [PubMed] [Google Scholar]
- Sobrian SK (2016) Developmental cannabinoid exposure: New perspectives on outcomes and mechanisms. Neurotoxicology and Teratology 58: 1–4. [DOI] [PubMed] [Google Scholar]
- Stromland K, Pinazo-Duran MD (1994) Optic nerve hypoplasia: comparative effects in children and rats exposed to alcohol during pregnancy. Teratology 50: 100–111. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS (2013) Anandamide CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J Neurosci 33: 6350–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA (1981) Fetal alcohol syndrome: embryogenesis in a mouse model. Science 214: 36–938. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Lauder JM, Dehart DB (1984) Brain malformations in prenatal mice following acute maternal ethanol administration. Int J Dev Neurosci 2 :203–214. [DOI] [PubMed] [Google Scholar]
- Watson S, Chambers D, Hobbs C, Doherty P, Graham A (2008) The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Molecular and Cellular Neurosciences 38: 89–97. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubinstein JL, Hynes MA, Rosenthal A (1998) FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93: 755–766. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker L-Y, Alexeeff S, Armstrong MA, Conway A, Weisner C, Goler N (2017) Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009–2016. JAMA 318:2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF (2002) Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci USA 99: 16273–16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Turton QM, MacKinnon S, Sulik KK, Cole GJ (2011) Agrin function associated with ocular development is a target of ethanol exposure in embryonic zebrafish. Birth Defects Res (Part A) 91: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ojiaku P, Cole GJ (2013) Forebrain and hindbrain development in zebrafish is sensitive to ethanol exposure involving agrin, Fgf and Sonic hedgehog function. Birth Defects Res (Part A) 97: 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Frazier JM, Chen H, Liu Y, Lee JA, Cole GJ (2014) Molecular and morphological changes in zebrafish following transient ethanol exposure during defined developmental stages. Neurotoxicology and Teratology 44: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]