Abstract
Study Objectives:
Determine relationship between cannabis use with 1) expectations of cannabis being a sleep aid, 2) subjective sleep outcomes, and 3) the influence of age on these relationships.
Methods:
In 152 moderate cannabis users with a wide age range (67% female, mean age = 31.45, SD = 12.96, age range = 21–70; mean days of cannabis use in prior two weeks = 5.54, SD = 5.25) we examined the influence of cannabis use history and behaviors on expectations of cannabis being a sleep aid and sleep outcomes via the Pittsburgh Sleep Quality Index (PSQI). Moderation analysis examined the role of age in the relationship between cannabis use and sleep outcomes.
Results:
Endorsing current cannabis use and more days of cannabis use were associated with increased expectations that cannabis use improves sleep (all β > 0.03, p < 0.04). Frequency of recent use and reported average THC or CBD concentration were largely not associated with sleep outcomes. However, endorsing current cannabis use was associated with worse subjective sleep quality (β = 1.34, p = 0.02) and increased frequency of consuming edibles was associated with worse subjective sleep efficiency (β = 0.03, p = 0.04), lower sleep duration (β = 0.03, p = 0.01), and higher global PSQI scores (worse overall sleep) (β = 0.10, p = 0.01). Additionally, age had a moderating influence on the relationship between increased self-reported concentration of CBD and both better sleep duration and sleep quality (both p < 0.03). While the main effects of cannabis use on sleep outcomes did not survive multiple comparisons correction test (all p adj > 0.34), the adjusted p values for the main effects of cannabis behaviors/history on expectations of cannabis as a sleep aid (p adj = 0.07–0.09) and the main effects of CBD concentration on sleep duration (p adj = 0.08), as well as the interaction terms of CBD and age for that model (p adj = 0.07), were trending.
Conclusion:
Cannabis users have increased expectations of cannabis being a sleep aid, but few associations existed between cannabis use and sleep outcomes. The two exceptions were endorsing any cannabis use and frequency of edible use. Additionally, age may be an important moderator of the potential positive influence CBD concentration can have on sleep.
Keywords: cannabis, THC, CBD, sleep, age
1. Introduction
Sleep is an important process necessary for daily functioning. Over a third of adults do not get the recommended 7 hours of sleep per night1 and sleeping disorders such as insomnia have symptoms and diagnosis prevalence rates as high as 30% and 5–10% respectively2. Sleep disturbances have been linked with a wide array of maladaptive outcomes including cognitive performance deficits, mental health consequences such as depression and anxiety, increases in medical conditions (such as obesity, diabetes, and hypertension) and increased mortality3–7. Cannabis is frequently associated with expectations of improved sleep8–10 and improvements in sleep are often cited as a primary motive for using cannabis11,12, but existing research is largely inconsistent with the notion that cannabis aids sleep.
While there is evidence of acute cannabis use improving sleep, most research points to chronic use being associated with sleep deficits. Tetrahydrocannabinol (THC) and Cannabidiol (CBD) are two of the most prominent cannabinoids in cannabis, with THC being the main psychoactive component and CBD (non-psychoactive) being associated with anxiolytic and sedating effects13. There is some evidence that specific cannabinoids have a positive influence on sleep, with high-dose CBD and acute low-dose THC having potential therapeutic effects on sleep14. Likewise, acute effects of pre-sleep administered cannabis (often studied in isolated or synthetic form) include shorter sleep onset latency, longer sleep duration, improved sleep maintenance and greater subjective sleep satisfaction15–18.
Though acute use might be associated with sleep enhancements, low dose CBD and high dose THC is associated with negative sleep outcomes and consecutive days of using cannabis as a sleep aid may promote development of tolerance to any positive outcomes with a habituation effect that includes negative sleep outcomes14. More frequent use of cannabis is associated with a large array of sleep deficits including sleep quality problems19–24, sleep disturbances21, prolonged latency to sleep onset25, lower sleep duration25–28, and insomnia19,21,29–31. Interestingly, a recent study found that daily cannabis users demonstrated more insomnia symptoms and worse sleep quality than both non-daily users and non-users, and that non-daily users and non-users had similar sleep scores alluding to the idea that intermittent users might not experience the negative effects of cannabis on sleep that daily chronic users report21.
With evidence that frequent cannabis use can have harmful effects on sleep, the public’s perception of cannabis as a sleep aid has potential negative consequences for both sleep health and general well-being. Positive expectancies about the relationship between cannabis and sleep are correlated with increased frequency of cannabis use as well as health/mental health problems11,12,32 and positive expectations of cannabis in general are thought to lead to a higher likelyhood of cannabis related problems33,34. Expectations of cannabis as a sleep aid might lead to increased long-term cannabis use which could progress into negative effects on sleep as well as additional cannabis related issues such as substance use disorders and sleep problems related to cannabis withdrawals35.
One component often neglected in cannabis studies is the lack of assessment of alternative routes of administration36. Recent legalization in the United States has been linked to increased use of alternate methods of cannabis administration, particularly cannabis edibles37, and these different routes of administration can affect the onset and duration of the effects of cannabis38. For example, when smoking cannabis, the effects can take minutes to begin and can last from two to four hours39,40, but with edibles the onset is delayed 30 to 60 minutes and the effects can last up to six hours39. Additionally, information on these different routes of administration as well as the influence of different concentrations of THC and CBD on health outcomes (such as sleep) remain scarce41. Current studies fail to asses cannabis use in great detail and it is important to analyze the effect of alternative methods of administration of cannabis (such as edibles) as well as THC/CBD concentration and their associations of sleep outcomes.
Another component that has yet to be extensively studied in the relationship of cannabis and sleep outcomes is the potential influence of age. There is evidence that the endocannabinoid system may modulate sleep disorders and circadian rhythm uniquely42,43 as adults age throughout adulthood. There is also evidence of age related differences in pharmacokinetics and pharmacodynamics that can influence the biobehavioral effects of substances such as cannabis as adults mature44–46. While exact biological studies are lacking that are focused on comparing differing age groups and cannabis metabolism, It has been theorized that various biological changes that are associated with increased age such as decreases in hepatic blood flow, slower metabolisms, increase adipose tissue, decrease in total body water, and decreases in lean body mass47,48 can not only increase the distribution rate and volume of lipophilic drugs like THC and CBD but also decrease the elimination of them, potentially increasing the side effects of cannabis49–51. It has been speculated that differences between younger and older age groups in cognitive impairments, psychotomimetic symptoms, and subjective feelings of being high in response to cannabis might be based on a linear developmental change process as one ages, with younger age groups having faster basal metabolisms that allow cannabis and its by-products to be metabolized more quickly in comparison to older adults52. Furthermore, there is evidence of age related difference in cannabis use behaviors, such as evidence of daily/almost daily cannabis use prevalence increasing 150% for those ages 26 and older compared to 49% for those age 18–2553, prevalence of cannabis use in the past 12 months increasing for certain older adult age groups (ages 25–44, 45–64, 65 and older) but not in young adults ages 18–2454, and evidence of cannabis users (age 31–50) being more likely to use cannabis to help ease insomnia than younger adult users55. With evidence of potential unique age-related biological effects of cannabis as well as changes in use patterns as individuals age throughout adulthood, it is important to consider the role of age in the relationship of cannabis and sleep.
Prior studies focused on the relationship between cannabis and sleep outcomes have failed to analyze the associations of novel cannabis administration methods (such as edibles) and THC/CBD concentration nor have they examined the influence of age. In the current study we tested the associations cannabis use history and behaviors (including frequency of typical modes of administration and reported THC and CBD concentration) and various sleep outcomes, including higher expectations of cannabis as a sleep aid and self-reported sleep outcomes and function metrics in a sample that consisted of a wide age range (age range = 21–70). We hypothesize that increased cannabis frequency and behaviors would be associated with increased sleep deficit outcomes. Given the role of aging in both sleep and cannabis use, we were particularly interested in age as a moderator of the relationship among cannabis metrics and sleep; thus, we tested an age x cannabis interaction measure to evaluate this further. An interaction between age and cannabis use would indicate that the association of cannabis use and sleep outcomes differs by age (a moderating effect). We would expect, based on evidence of changes in both the modulation of the endocannabinoid system and metabolism rates of substances changing as people age, that the effect of cannabis on sleep may be intensified for older individuals.
2. Methods
2.1. Participants
This study includes baseline data from a community sample recruited for an ongoing longitudinal study of cannabis use and health. Participants were 152 individuals (67% female, mean age = 31.45, SD = 12.96, age range = 21–70) who were recruited for a longitudinal study of cannabis use and anxiety symptoms and experienced at least mild anxiety, as measures by the Generalized Anxiety Scale (GAD-7). Worth mentioning, is the wide age range used in the study which consisted of a high percentage of adults that could be classified into age groups older than young adults (64% (n = 97) are age 21–29, 15% (n = 23) are age 30–39, 14% (n = 21) are age 40–64, and 5% (n = 8) age 65 and up). Subjects were recruited from the Denver area from social media postings and mailed flyers advertising research on anxiety and cannabis to those who 1) use cannabis and want to start using cannabis for anxiety. Participants were screened for the study by an experienced research assistant over the phone with an approved screening script or through an approved and confidential REDCap online survey.
Criteria to be eligible for the study included: 1) Provided informed consent; 2) Age: 21–70; 3) Anxiety: reported ≥5 on GAD-7; 4) Other drug use: No anti-viral medications or psychotropic drug use (ADHD medications allowed), or other drug use for 72 hours and negative toxicology test and blood alcohol content breathalyzer (Intoximeter, Inc., St. Louis, MO); 5) Diagnoses/Treatment: No immune-related diseases or in treatment for psychotic disorder, bipolar disorder, or major depression disorder with suicidal ideation or a history with these disorders, substance use disorder (or actively seeking treatment); 6) No intention to become pregnant and negative pregnancy test (if applicable); Used marijuana at least once and has a desire to use marijuana to cope with anxiety. We had data from 4 subjects who met all of the criteria for the study but did not use cannabis at the time of data collection. Our sample had a race/ethnicity background as follows; White (83%), other (7%), Asian (4%), African American (3%), American Indian or Alaska Native (1%), Hawaiian or Pacific Islanders (1%) and Not Provided (1%) with 11% of the sample identifying as Hispanic or Latino.
Once participants were deemed eligible and scheduled, they arrived at our University Lab, completed the informed consent process, and completed a series of questionnaires in-person. Additional psychological and cognitive tasks related to the longitudinal aims of the study were completed during this 2-hour appointment; data collection for these longitudinal outcomes is ongoing and have not been analyzed. All research protocols were reviewed and approved, and all procedures followed guidelines by the University of Colorado’s Investigational Review Board. Documented informed consent was obtained from all study participants. One subject responded as transgender and their gender score was coded as NA in the analysis.
2.2. Measures
2.2.1. Cannabis Sleep Expectancies
Subjects were asked “Which of the following benefits do you expect to get from cannabis? (please select the level of change you expect)” with one category of responses being “Improved sleep”. Possible responses included “Very improved” (28%), “Somewhat improved” (54%), “Not very improved” (10%), “No improvement at all” (8%), and “Not applicable (Non-user group)”, with responses reverse coded from 3–0 (mean = 2.01 SD = 0.84) and NA (n = 3).
2.2.2. Detailed Assessment of Cannabis Use Behaviors and History
Endorsing any current cannabis use was assessed via a yes (n =129) no (n = 21) question asking, “Do you use cannabis?” Prior regular use was assessed via a yes (n =102) no (n = 30) question “Were you ever a regular cannabis user? (regular use is defined as at least once per week)”. Both of these measures were coded as yes = 1 and no = 0. Frequency of edible use/consumption was assessed via a question “On average, how often do you consume cannabis orally or consume edibles? This question refers to anything that you consume orally like capsules, food, or drink (e.g. baked goods, candies, drinks, hemp oil, cannabis oil, Rick Simpson oil, tinctures, etc.)” with responses including “I never use edibles” (23%), “Less than once a month” (23%), “One day a month” (9%), “Two days a month” (15%), “Three days a month” (10%), “One day a week” (5%), “Two days a week” (7%), “ Three days a week” (2%), “Four days a week” (3%) “ Five days a week” (0%), “Six days a week” (2%), and “ Daily” (1%) (with responses being coded as 0, 0.5, 1, 2, 3, 4, 8, 12, 16, 20, 24 and 30 respectively; mean = 13.22, SD = 12.89). Frequency of smoking flower cannabis was assessed via “How often do you vaporize or smoke cannabis?”. Responses to this question included “I never smoke/vaporize cannabis” (23%), “Less than once a month” (10%), “One day a month” (3%), “Two days a month” (1%), “Three days a month” (5%), “One day a week” (3%), “Two days a week” (6%), “ Three days a week” (7%), “Four days a week” (3%), “ Five days a week” (5%), “Six days a week” (3%), and “ Daily” (31%) (with responses being coded as 0, 0.5, 1, 2, 3, 4, 8, 12, 16, 20, 24 and 30 respectively; mean = 3.07 SD = 5.52).
Recent cannabis use measures were gathered from the Time Line Follow Back (TLFB) measure, modified for online and detailed cannabis specific assessment over the past two weeks (O-TLFB)57. Questions included total number of days that cannabis flower was used during the past two weeks (Mean = 3.87, SD = 4.92, Range = 0–14), total number of days that cannabis edibles were used in the past two weeks (Mean = 0.89, SD = 2.01, Range = 0–14), and total number of days any (i.e., flower, edible, concentrate, topical, other) cannabis use was reported in the past two weeks (Mean = 5.54, SD = 5.25, Range = 0–14). TLFB cannabis questions are conceptualized as cannabis history provided that they are a detailed account of the days cannabis was used in the past two weeks, whereas the cannabis frequency measures imply more general cannabis use behavior over time.
Reported average THC and CBD concentration of cannabis was measured by two questions asking “How much THC and/or CBD is in the cannabis that you use most often? If you are not sure, please take your best guess” with responses including “0”, “Less than 5%”, “5–10%”, “10–15%”, “15–20%”, “20–25%”, “25–30%”, “Greater than 30%” (with responses being coded as 0–7; mean reported THC = 4.24, SD = 1.63; mean reported CBD = 2.57, SD = 1.85). Similar self-reports amongst cannabis users who are aware of the THC/CBD potency of the cannabis they use show consistency across different time points and methods57.
2.2.3. Pittsburgh Sleep Quality Index
Subjects reported on their past two week sleep behavior via the Pittsburgh Sleep Quality Index (PSQI)58 (a modification of the standard PSQI which typically looks at past month sleep outcomes). The PSQI is a 19-item self-report measure that assesses individual sleep habits, sleep quality, and sleep outcomes. The first four questions ask participants to report on their bedtime, waketime, time to fall asleep, and sleep duration. The remaining questions asses sleep efficiency, sleep disturbances, use of sleep medications, and dysfunction while awake. These questions are scored and summed to make a global PSQI score (α = 0.70; Mean = 7.75, SD = 3.12, Range = 1–18) with higher scores reflecting worse sleep. The questions can also be scored to make subscales of sleep efficiency, sleep latency, sleep quality, sleep disturbances, sleep medication, and daytime dysfunction.
2.2.4. Sleep-related Covariates
Our analyses controlled for established correlates of sleep outcomes including gender59, age60, current alcohol use61, and depression/anxiety/stress symptoms62,63. Alcohol use was assessed using the Alcohol Use Disorders Identification Test (AUDIT)64, which is a 10-item screening tool used to asses alcohol use and problems (α = 0.82; Mean = 6.13 SD = 4.02, Range = 0–23). We used the 21-tem self-report version of the Depression Anxiety Stress Scales (DASS)65 (α = 0.88; Mean = 41.75 SD = 19.79, Range = 4–114) as a composite measure of current depression, anxiety and stress symptomology.
Using a univariate outlier approach, we detected several extreme values for the DASS and AUDIT measures that were larger than 1.5 times the inter quartile range for each measure (the difference between 75th and 25th quartiles). We conducted a sensitivity analysis with and without these values and they did not influence the significance of our results. Given that these values are still answers within the realm of possibility and that they appear to not influence our findings, we did not remove or edit these values from our data. Additionally, we conducted residual diagnostics for all regression models (accounting for all variables) via inspection of externally studentized residual plots, using observations with externally studentized residual larges than 3 in absolute values as an indicator of outliers66. While a majority of the models were free of outliers, this inspection led to the identification of two separate data points that were potential outliers across multiple models involving the PSQI sleep disturbance variable. After examining the relevant data points in each model and determining that each value was within the realm of possibility, and conducting a sensitivity analysis with and without these data points and concluding that none of these data points influenced the significance of our results, we did not alter these data points and included them in our analyses.
2.3. Statistical Analyses
We calculated descriptive statistics, linear regressions, and ordinal logistic regression via R version 3.4.467. Linear regression is largely robust to minor distributional issues when the sample is of an adequate size68 and the skewness and kurtosis measures for our PSQI subscales of sleep were within reasonable thresholds69 thus appropriate for linear regression. Furthermore, a majority of our PSQI sleep subscales had responses that were either appropriately distributed or were summed from multiple questions to form the scale (meaning that the computed subscale actually has considerably more points than the items of which it is comprised, thus it approximates a continuous variable), making many of these subscales appropriate for linear regression (such as sleep efficiency, sleep latency, sleep disturbances, and daytime dysfunction). Contrastingly, due to an inadequate distribution across the four responses for three of the PSQI subscales (sleep duration, sleep quality, and sleep medication), we conducted ordinal logistic regression for all models containing those specific variables. Our output coefficients for ordinal logistic regression are log-odds values and for consistency sake, we report the coefficients similarly to linear models. We reported the R squared for each linear regression model as well as a logistic analog to R squared for logistic regression models70,71 which indicates model deviance accounted for by the predictors. We conducted a series of regression models in order to determine both the main effects of cannabis on subjective sleep outcomes as well as to analyze a potential moderating effect of age on the relationship between cannabis behaviors and subjective sleep outcomes via an interaction term between age and cannabis (with all appropriate variables mean centered)72,73. A main effect of cannabis that lacked a significant interaction effect would imply an association of cannabis use and sleep outcomes controlling for all covariates. A main effect of cannabis and a significant interaction term would imply that differences in age possibly influence the association of cannabis use and sleep outcomes. We implemented Benjmamini and Hochberg’s74 correction for multiple testing using the p.adjust function from the stats package found in R. This method provides adjusted p-values to a group of estimated p-values. We generated a series of adjusted p-values for both the main effects and interaction effects for models involving cannabis behaviors/history on expectations of cannabis being a sleep aid, cannabis behaviors/history on PSQI sleep outcomes, and reported average THC and CBD on PSQI sleep outcomes. We report these adjusted p values after all significant or trending associations in our results.
3. Results
3.1. Expectations of cannabis as a sleep aid
Table 1 includes descriptive statistics for the TLFB cannabis, sleep, and covariate measures used in the study. Table 2 provides individual regression outputs involving cannabis behaviors/history and expectations of cannabis being a sleep aid controlling for gender, age, current depression/anxiety/stress, and alcohol use. Endorsing currently using cannabis, number of days that cannabis flower was used in the past two weeks, and number of days any cannabis was used in the past two weeks were all associated with higher expectations of cannabis as a sleep aid (all β > 0.03, p < 0.04; all p adj = 0.07 – 0.09) with no significant interaction effects between age and any of our cannabis measures in these models (all p > 0.16; all p adj > 0.21)
Table 1.
Descriptive statistics for the TLFB cannabis, sleep, and additional covariate measures used in the study.
| Mean (SD) sample characteristics | |||
|---|---|---|---|
| Full sample (n=151) |
Female (n=102) |
Male (n=48) |
|
| Age (years) | 31.45 (12.96) | 31.00 (12.48) | 32.54 (14.12) |
| Depression Anxiety Stress Scales (DASS) | 41.75 (19.79) | 42.61 (19.76) | 40.17 (20.11) |
| The Alcohol Use Disorders Identification Test (AUDIT) | 6.13 (4.02) | 6.00 (3.69) | 6.31 (4.68) |
| Days that cannabis flower was used in past two weeks (TLFB) | 3.87 (4.92) | 3.17 (4.65) | 5.12 (5.11) |
| Days that cannabis edibles were used in past two weeks (TLFB) | 0.89 (2.01) | 1.19 (2.31) | 0.27 (0.94) |
| Days any cannabis was used in past two weeks (TLFB) | 5.54 (5.25) | 4.83 (4.98) | 6.85 (5.19) |
| Sleep efficiency (PSQI) | 2.38 (0.90) | 2.33 (0.94) | 2.49 (0.83) |
| Sleep latency (PSQI) | 1.80 (0.75) | 1.77 (0.74) | 1.83 (0.75) |
| Sleep quality (PSQI) | 1.27 (0.59) | 1.22 (0.58) | 1.35 (0.63) |
| Sleep duration (PSQI) | 2.45 (0.76) | 2.51 (0.72) | 2.34 (0.81) |
| Sleep disturbances (PSQI) | 1.53 (0.59) | 1.54 (0.61) | 1.50 (0.58) |
| Sleep medication (PSQI) | 0.62 (1.04) | 0.72 (1.07) | 0.42 (0.96) |
| Daytime dysfunction (PSQI) | 1.44 (0.74) | 1.44 (0.76) | 1.46 (0.68) |
| Global overall PSQI score | 7.75 (3.12) | 7.77 (3.27) | 7.71 (2.83) |
Possible PSQI subscales scores range from 0–3 and global overall PSQI scores range from 0–21. Lower scores indicate less sleep difficulty and higher scores indicate severe difficulty and worse sleep.
Table 2.
Individual variable effects of regression model results for expectations of cannabis being a sleep aid by cannabis behaviors/history controlling for gender, AUDIT, DASS, age, and the interaction of age and cannabis behavior.
| Do you use cannabis? | Ever been a regular user? | Days of cannabis flower use in past two weeks (TLFB) | Days that cannabis edibles were used in the past two weeks (TLFB) | Days any cannabis was used in the past two weeks (TLFB) | Current frequency of vaporizing and smoking | Current frequency of consuming edibles | |
|---|---|---|---|---|---|---|---|
| Cannabis Expectations | 0.52* | 0.22 | 0.03* | 0.01 | 0.03* | 0.01 | 0.01 |
| Age | −0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Age X Cannabis behavior/history | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.02 |
| Gender | 0.04 | 0.02 | 0.00 | 0.08 | 0.00 | −0.03 | 0.08 |
| DASS | 0.00 | 0.05 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 |
| AUDIT | −0.02 | 0.02 | 0.00 | −0.02 | −0.01 | −0.01 | −0.02 |
| R2 | 0.03 | 0.00 | 0.18 | −0.01 | 0.03 | 0.01 | 0.00 |
Note. Unadjusted p values,
p < 0.05, R2 is the adjusted R squared value for each regression analyses.
3.2. Cannabis Behavior/History and PSQI
Table 3 provides individual regression outputs involving cannabis behaviors/history and global PSQI score controlling for gender, age, current depression/anxiety/stress, and alcohol use. Increased frequency of consuming edibles was associated with higher global PSQI scores (worse sleep) (β = 0.10, p = 0.01; p adj = 0.34) but no other cannabis use variables showed significant associations (all p > 0.05). We did not find a significant interaction effects between age and cannabis in this model (β = 0.00, p = 0.88; p adj = 0.99). Both endorsing currently using cannabis (β =1.36, p = 0.05; p adj = 0.42) and increased number of days that cannabis edibles were used in the past two weeks (β =0.23, p = 0.06; p adj = 0.51) were marginally associated with higher global PSQI scores (worse sleep). Neither of these models had significant interaction effects between age and cannabis (both p > 0.17; both p adj > 0.88). None of the cannabis frequency/history measures were associated with any of the PSQI subscales (sleep efficiency, sleep latency, sleep quality, sleep duration, sleep disturbances, sleep medication, and daytime dysfunction) with the exception of endorsing currently using cannabis which was associated with worse subjective sleep quality (β = 1.34, p = 0.02; p adj = 0.34) and increased frequency of using edibles which was associated with worse subjective sleep efficiency (β = 0.03, p = 0.04; p adj = 0.42) and lower sleep duration (β = 0.03, p = 0.01; p adj = 0.34). These models did not have significant interaction terms (all p > 0.41; all p adj > 0.59). We found trending associations between increased frequency of smoking cannabis and worse sleep efficiency (β = 0.01, p = 0.05; p adj = 0.42). This model did not have a significant interaction term (β = 0.01, p = 0.57; p adj = 0.99).
Table 3.
Individual variable effects of regression model results for global PSQI score by cannabis behavior/history controlling for gender, AUDIT, DASS, age, and the interaction of age and cannabis behavior
| Do you use cannabis? | Ever been a regular user? | Days of cannabis flower use in past two weeks (TLFB) | Days that cannabis edibles were used in the past two weeks (TLFB) | Days any cannabis was used in the past two weeks (TLFB) | Current frequency of vaporizing and smoking | Current frequency of consuming edibles | |
|---|---|---|---|---|---|---|---|
| PSQI | 1.36# | 0.04 | −0.01 | 0.23# | 0.00 | 0.03 | 0.10* |
| Age | 0.06 | 0.00 | 0.04* | 0.05* | 0.04* | 0.04# | 0.04# |
| Age X Cannabis behavior/history | −0.02 | 0.05 | 0.00 | 0.02 | 0.00 | 0.01 | 0.00 |
| Gender | −0.06 | 0.09 | 0.19 | 0.31 | 0.17 | −0.09 | 0.23 |
| DASS | 0.06* | 0.06* | 0.06* | 0.06* | 0.07* | 0.06* | 0.06* |
| AUDIT | 0.04 | 0.07 | 0.08 | 0.07 | 0.08 | 0.07 | 0.07 |
| R2 | 0.15 | 0.14 | 0.17 | 0.19 | 0.16 | 0.14 | 0.16 |
Note. Unadjusted p values,
p = 0.05–0.06;
p < 0.05, R2 is the adjusted R squared value for each regression analyses.
3.3. Association of Reported Average Cannabinoid (THC/CBD) Concentration of Cannabis with Sleep Outcomes
Table 4 displays the frequencies of the reported average THC and CBD concentration of cannabis. Tables 5 and 6 includes individual regression outputs for models focused on the associations of reported average THC and CBD concentration and all sleep outcomes controlling for gender, age, current depression/anxiety/stress, and alcohol use. Reported average THC concentration was not significantly associated with global PSQI nor with any of the PSQI subscales (all p > 0.05), although increased reported average THC concentration was trending in its association with increased sleep disturbances (β =0.06, p = 0.09; p adj = 0.81). The interaction effect was not significant for this model (β =0.00, p = 0.52; p adj = 0.65). Increased reported average CBD concentration, however, was significantly associated with better sleep efficiency (β = −0.11, p = 0.01; p adj = 0.08) and sleep duration (β = −0.25, p = 0.03; p adj = 0.21) and the interaction terms for both models were significant (both β < − 0.01, p < 0.03; p adj = 0.07 – 0.08) suggesting a potential moderating influence of age on these relationships. For ease of presentation, Figure 1 depicts the pattern of this age x reported average CBD concentration interaction on both sleep duration and sleep efficiency with age divided into two age groups separated by the mean age (m = 31.45), demonstrating both trending and significant associations of the older age group on these sleep outcomes in comparison to non-significant relationships in the younger age group.
Table 4.
Number of people who endorse the percentage of reported average THC and CBD concentration of cannabis.
| 0% | < 5% | 5–10% | 10–15% | 15–20% | 20–25% | 25–30% | >30% | |
|---|---|---|---|---|---|---|---|---|
| THC | 1 | 4 | 18 | 16 | 27 | 36 | 12 | 13 |
| CBD | 14 | 27 | 27 | 25 | 12 | 9 | 5 | 6 |
Table 5.
Individual variable effects of regression model results for all PSQI measures by reported average THC concentration controlling for gender, AUDIT, DASS, age, and the interaction of age and THC concentration.
| Sleep efficiency | Sleep quality | Sleep latency | Sleep duration | Sleep disturbances | Sleep medication | Daytime dysfunction | Global PSQI | |
|---|---|---|---|---|---|---|---|---|
| THC | 0.07 | −0.12 | −0.02 | 0.05 | 0.06# | −0.07 | 0.00 | 0.05 |
| Age | 0.01* | 0.03# | 0.00 | 0.02 | 0.01# | 0.01 | 0.00 | 0.04 |
| Age X Reported average THC concentration | 0.00 | −0.01 | 0.00 | 0.01 | 0.00 | −0.01 | 0.00 | 0.00 |
| Gender | −0.31# | 0.56 | 0.05 | 0.32 | −0.03 | -1.11* | 0.05 | −0.33 |
| DASS | 0.01** | 0.04** | 0.01** | 0.03** | 0.01** | 0.03 | 0.01** | 0.07** |
| AUDIT | −0.03 | 0.09 | 0.02 | −0.03 | 0.03* | 0.01 | 0.02 | 0.05 |
| R2 | 0.12 | 0.41+ | 0.12 | 0.44+ | 0.08 | 0.44+ | 0.08 | 0.17 |
Note. Unadjusted p values,
p = 0.05–0.09;
p < 0.05;
p < 0.01, R2 is the adjusted R squared value for each regression analyses.
A logistic analog to R squared for logistic regression which indicates model deviance accounted for by the predictors.
Table 6.
Individual variable effects of regression model results for all PSQI measures by reported average CBD concentration controlling for gender, AUDIT, DASS, age, and the interaction of age and CBD concentration.
| Sleep efficiency | Sleep quality | Sleep latency | Sleep duration | Sleep disturbances | Sleep medication | Daytime dysfunction | Global PSQI | |
|---|---|---|---|---|---|---|---|---|
| CBD | −0.11* | −0.09 | −0.04 | −0.25* | 0.02 | 0.13 | 0.04 | −0.12 |
| Age | 0.01# | 0.04* | 0.00 | 0.01 | 0.01 | 0.02 | 0.00 | 0.04 |
| Age X Reported average CBD concentration | −0.01** | 0.00 | 0.00 | −0.02* | 0.00 | −0.02 | 0.00 | −0.02 |
| Gender | −0.29# | 0.41 | 0.00 | 0.35 | 0.02 | −1.09* | 0.06 | −0.30 |
| DASS | 0.01** | 0.04** | 0.01** | 0.03** | 0.01** | 0.01 | 0.01** | 0.07** |
| AUDIT | −0.03# | 0.10* | 0.02 | −0.04 | 0.03# | 0.05 | 0.02 | 0.05 |
| R2 | 0.19 | 0.41+ | 0.14 | 0.44+ | 0.06 | 0.45+ | 0.09 | 0.19 |
Note. Unadjusted p values,
p = 0.06–0.08;
p < 0.05;
p < 0.01, R2 is the adjusted R squared value for each regression analyses.
A logistic analog to R squared for logistic regression which indicates model deviance accounted for by the predictors.
Figure 1. Difference in slope of reported average cannabidiol (CBD) concentration and PSQI sleep duration (Panel A) and sleep efficiency (Panel B) based on differing age groups.
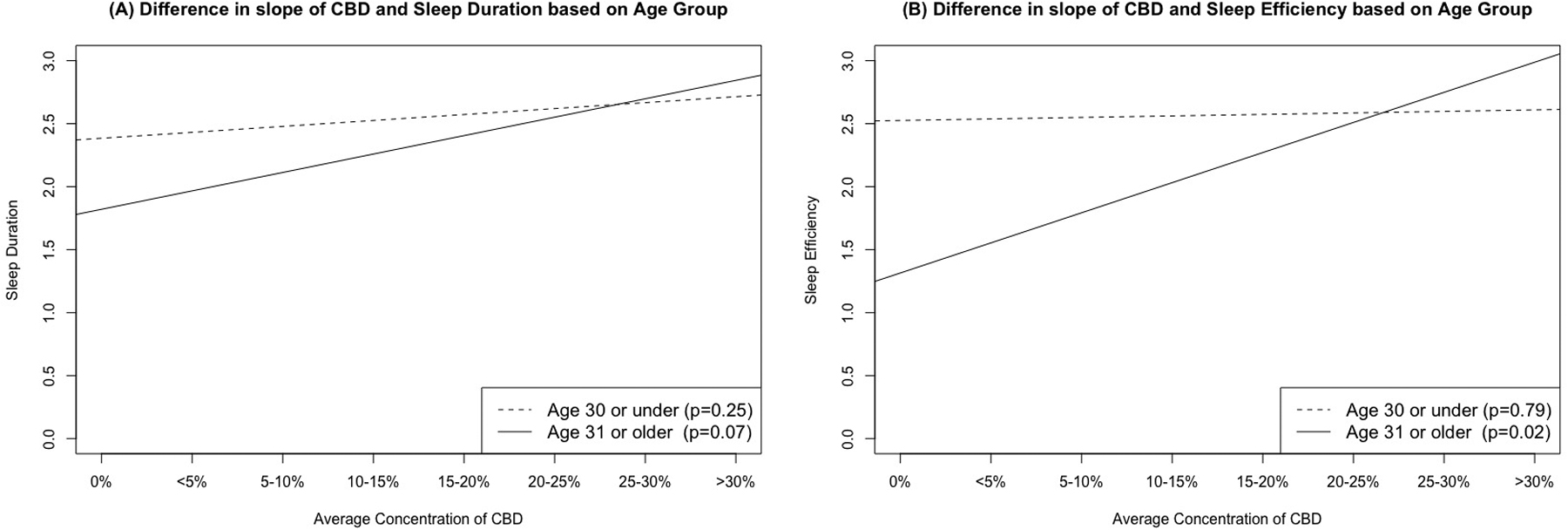
The figure shows trends lines that depict the age x CBD interactions in predicting PSQI sleep duration and sleep efficiency, dashed lines represent age 31 or under (both p > 0.25), solid lines represent age 31 or older (both p < .07). These findings demonstrate both trending and significant associations of the older age group on these sleep outcomes in comparison to non-significant relationships in the younger age group.
None of the main effects of cannabis use on sleep outcomes survived multiple comparison correction tests via adjusted p values (all p adj > 0.34). Our main effects of currently using cannabis, days of cannabis used in the past two weeks, days any cannabis was used in the past two weeks on expectations of cannabis as a sleep aid were trending (all p adj = 0.07– 0.09) and the main effect of reported average CBD concentration on sleep duration was trending (p adj = 0.08) as was the interaction terms of CBD and age for this model (p adj = 0.07) after adjusted p value correction tests.
Discussion
In a community sample of adult moderate cannabis users (ranging from lighter to heavier use) with a wide age range, we found that cannabis use in general and increased number of days of cannabis use was associated with increased expectations of cannabis being a sleep aid. We found that endorsing currently using cannabis was associated with worse subjective sleep quality and that higher frequency of the use of edibles in particular was associated with worse subjective sleep efficiency, lower sleep duration, and higher global PSQI scores (worse sleep). We also found that higher reported average CBD concentration among current users was associated with better sleep efficiency and sleep duration scores and that this effect was significantly moderated by age, such that older participants demonstrated a larger effect. None of these significant main effects nor the significant interaction terms survived multiple correction tests but several of these were trending in significance in their adjusted p values.
Our results are consistent with research that has found that using cannabis is often associated with expectations of improved sleep8–10,75–78 and that expectations of cannabis being a sleep aid is associated with increased cannabis use quantity and frequency11,12. Additionally, prior studies support our result of current cannabis use being associated with poor subject sleep quality19,20,22,23,79, but we failed to find a prior study focused on the specific effects of cannabis edibles in particular on sleep. While prior studies have used oral administered CBD and THC (often in isolated or synthetic forms not typically used outside the laboratory) to look at sleep, this is the first observational study to look specifically at the associations of cannabis edibles and sleep. Importantly, our measures of cannabis use included administration details not typically assessed in prior studies (e.g. specifics of amount and form of cannabis used), which allows us to report a novel association among frequency of cannabis edibles and negative sleep outcomes. Replication of this finding is important as cannabis is becoming increasingly available and administered through a variety of methods and increased potencies which may have varying impacts on sleep outcomes.
Although we didn’t find any significant associations between reported THC concentration and sleep outcomes, our analysis showing that higher reported average CBD concentration was associated with sleep improvements aligns with prior research. Research isolating the effects of CBD has shown that medium to high dose CBD has a sedating effect80–83 and is associated with increased total sleep time, decreased frequency of night arousals, and lower overall global PSQI scores84 implying higher CBD concentration is associated with sleep improvements. However, some research has found CBD has no effect on sleep85 and further investigation is needed to understand its influence alone and in combination with varying ratios of THC.
Our results of age moderating the relationship between reported average CBD concentration and sleep are novel and it has been speculated that there are age differences in cannabis effects. As mentioned, the endocannabinoid system may regulate sleep disorders and circadian rhythm distinctively in older individuals42,43 and it is theorized that cannabis may be metabolized more slowly as adults age46,49–52 resulting in unique effects of cannabis use compared to younger adult individuals. In other words, the moderation effects found in the study could be explained by an increase in age being associated with differences in pharmacokinetics and pharmacodynamics that can influence biobehavioral effects of substances44,45. While no prior study has collaborated this theory using biological methods, we would speculate that increases in age might lead to biological alterations that would influence metabolism, potentially increasing the volume/distribution of CBD in a user’s system and increasing the positive effects of CBD on sleep factors. Studies focused on larger samples of middle aged and older adults as well as studies using biological markers to analyze the potential changes and factors in THC/CBD metabolism should be conducted.
There are several noteworthy limitations of this study. First are the potential limitations regarding the sample. Many of the published studies that show increased cannabis use frequency having a negative influence on sleep are of a much larger sample size and are typically from population-based samples, while our study was a cannabis using community sample. Our sample was mostly women and consisted of individuals who met criteria for mild anxiety disorders. Additionally, our sample was of a largely homogenous racial/ethnic breakdown (predominantly identifying as white) and this limits the generalizability of these findings to other racial/ethnic groups. Future studies should strive for an equal balance of men and women, a sample with a more diverse racial background, and a sample that is potentially free from anxiety disorders. Furthermore, our sample was from a state (Colorado) where cannabis is recreationally legal and individuals in this study might have more positive views of cannabis than those in states with more restrictive cannabis laws. Second, are the limitations regarding some of our cannabis measures. We relied on self-report measures which limited our knowledge of the exact concentration of CBD/THC, thus our responses could have potential recall bias and response error. Our measure of cannabis edibles included all forms (capsules, food, and drink) and future studies should differentiate modes of administration at an even more fine-grained level. Additionally, our cannabis expectations question was general in nature and did not allow for nuanced interpretation (in terms of specific sleep outcomes) nor for potential responses of cannabis exacerbating sleep problems and future studies should include more finite measures of expectations of improved sleep. Third, are the limitations regarding our sleep measures. The sleep measures used in the current study (PSQI) were broad and general in nature and using more nuanced and detailed measurements such as daily sleep diaries9 could provide more precise findings. Furthermore, subjective sleep measures have demonstrated reliability and consistency problems when compared to objective measures of sleep behaviors (such as actigraphy)86–88. Future studies should include both subjective and objective sleep measures in order to provide a comprehensive analysis that allows these separate measures to complement one another.
Moreover, this is a cross-sectional design and we are hesitant to draw causal conclusions from our results, provided it is possible that those with worse sleep problems are preferentially selecting edibles or higher CBD concentrations, perhaps because they think a longer duration of effect might have more benefits for sleep maintenance or that CBD might aid their sleep issues. Provided there is evidence of a bidirectional relationship between these traits, such that early cannabis use predicts later sleep problems31,89,90 and early generalized sleep problems predict later cannabis use31,91–94 we cannot make definitive causal claims. Lastly, our main effects of cannabis use on sleep outcomes did not survive multiple correction tests although our main effects of cannabis behaviors/history on expectations of cannabis as a sleep aid were trending as were both the main effect of reported average CBD concentration on sleep duration and the interaction terms of CBD and age for this model. Given both the exploratory nature of this study as well as the novel aspects and relevance to the emerging field of cannabis research, we believe the results of this study have merit and importance for future research designs and implementation.
5. Conclusions
In summary, we found evidence of cannabis users having increased expectations of cannabis as a sleep aid but found limited support of cannabis use being associated with sleep outcomes, with the exception of current cannabis use endorsement being associated with worse subjective sleep quality and increased frequency of edible use being associated with worse subjective sleep efficiency, lower sleep duration, and worse global PSQI scores. Additionally, we found novel evidence of age moderating a positive association between reported average strength of CBD concentration and both better sleep duration and efficiency. Future research should focus on novel cannabis administration methods as well as the therapeutic effects of CBD on sleep across the age spectrum.
Highlights.
Cannabis use associated with increased expectation that cannabis improves sleep
Limited support that cannabis is associated with sleep outcomes (PSQI)
Currently using cannabis predicts decreased subjective sleep quality
Increased frequency of consuming edibles predicted worse subjective sleep efficiency, lower sleep duration, and higher global PSQI scores.
Age likely moderates the effect of reported concentration of CBD on better sleep duration and efficiency
Acknowledgments:
The authors wish to thank participants and the research assistants for their time and contribution to the data for this manuscript.
Funding: Supported by grants National Institutes of Health (R01DA044131 to LCB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: None
References
- 1.Sheehan CM, Frochen SE, Walsemann KM, Ailshire JA. Are U.S. adults reporting less sleep?: Findings from sleep duration trends in the National Health Interview Survey, 2004–2017. Sleep. 2019. doi: 10.1093/sleep/zsy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth T Insomnia: Definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007. [PMC free article] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004. doi: 10.1016/j.jpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005. doi: 10.1210/jc.2005-0035 [DOI] [PubMed] [Google Scholar]
- 6.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010. doi: 10.1093/sleep/33.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010. doi: 10.1016/j.socscimed.2010.05.041 [DOI] [PubMed] [Google Scholar]
- 8.Pedersen W, Sandberg S. The medicalisation of revolt: A sociological analysis of medical cannabis users. Sociol Heal Illn. 2013. doi: 10.1111/j.1467-9566.2012.01476.x [DOI] [PubMed] [Google Scholar]
- 9.Goodhines PA, Gellis LA, Kim J, Fucito LM, Park A. Self-Medication for Sleep in College Students: Concurrent and Prospective Associations With Sleep and Alcohol Behavior. Behav Sleep Med. 2019. doi: 10.1080/15402002.2017.1357119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachhuber M, Arnsten JH, Wurm G. Use of Cannabis to Relieve Pain and Promote Sleep by Customers at an Adult Use Dispensary. J Psychoactive Drugs. 2019. doi: 10.1080/02791072.2019.1626953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blevins CE, Banes KE, Stephens RS, Walker DD, Roffman RA. Change in motives among frequent cannabis-using adolescents: Predicting treatment outcomes. Drug Alcohol Depend. 2016. doi: 10.1016/j.drugalcdep.2016.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CM, Neighbors C, Hendershot CS, Grossbard JR. Development and preliminary validation of a comprehensive Marijuana motives questionnaire. J Stud Alcohol Drugs. 2009. doi: 10.15288/jsad.2009.70.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015. doi: 10.1016/j.schres.2015.01.033 [DOI] [PubMed] [Google Scholar]
- 14.Babson KA, Sottile J, Morabito D. Cannabis, Cannabinoids, and Sleep: a Review of the Literature. Curr Psychiatry Rep. 2017. doi: 10.1007/s11920-017-0775-9 [DOI] [PubMed] [Google Scholar]
- 15.Bedi G, Foltin RW, Gunderson EW, et al. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: A controlled laboratory study. Psychopharmacology (Berl). 2010. doi: 10.1007/s00213-010-1995-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cousens K, DiMascio A. (−)δ9 THC as an hypnotic - An experimental study of three dose levels. Psychopharmacologia. 1973. doi: 10.1007/BF00437513 [DOI] [PubMed] [Google Scholar]
- 17.Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Med Rev. 2008. doi: 10.1016/j.smrv.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 18.Tassinari CA, Ambrosetto G, Peraita-Adrado MR, Gastaut H. The neuropsychiatric syndrome of Delta(9)-tetrahydrocannabinol and cannabis intoxication in naive subjects - A clinical and polygraphic study during wakefulness and sleep. In: Marihuana and Medicine. ; 1999. [Google Scholar]
- 19.Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001. doi: 10.1016/S0376-8716(00)00222-2 [DOI] [PubMed] [Google Scholar]
- 20.Klonoff H, Clark C. Drug patterns in the chronic marijuana user. Subst Use Misuse. 1976. doi: 10.3109/10826087109045531 [DOI] [PubMed] [Google Scholar]
- 21.Conroy DA, Kurth ME, Strong DR, Brower KJ, Stein MD. Marijuana use patterns and sleep among community-based young adults. J Addict Dis. 2016. doi: 10.1080/10550887.2015.1132986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakier N, Wild LG. Associations among sleep problems, learning difficulties and substance use in adolescence. J Adolesc. 2011. doi: 10.1016/j.adolescence.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 23.Ogeil RP, Phillips JG, Rajaratnam SMW, Broadbear JH. Risky drug use and effects on sleep quality and daytime sleepiness. Hum Psychopharmacol. 2015. doi: 10.1002/hup.2483 [DOI] [PubMed] [Google Scholar]
- 24.Ogeil RP, Cheetham A, Mooney A, et al. Early adolescent drinking and cannabis use predicts later sleep-quality problems. Psychol Addict Behav. 2019. doi: 10.1037/adb0000453 [DOI] [PubMed] [Google Scholar]
- 25.Bolla KI, Lesage SR, Gamaldo CE, et al. Sleep disturbance in heavy marijuana users. Sleep. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troxel WM, Ewing B, D’Amico EJ. Examining racial/ethnic disparities in the association between adolescent sleep and alcohol or marijuana use. Sleep Heal. 2015. doi: 10.1016/j.sleh.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010. doi: 10.1371/journal.pone.0009775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med (Baltim). 2011. doi: 10.1016/j.ypmed.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 29.Freeman D, Brugha T, Meltzer H, Jenkins R, Stahl D, Bebbington P. Persecutory ideation and insomnia: Findings from the second British National Survey Of Psychiatric Morbidity. J Psychiatr Res. 2010. doi: 10.1016/j.jpsychires.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008. [PMC free article] [PubMed] [Google Scholar]
- 31.Wong MM, Brower KJ, Zucker RA. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Med. 2009. doi: 10.1016/j.sleep.2008.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohnert KM, Bonar EE, Arnedt JT, Conroy DA, Walton MA, Ilgen MA. Utility of the comprehensive marijuana motives questionnaire among medical cannabis patients. Addict Behav. 2018. doi: 10.1016/j.addbeh.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayaki J, Herman DS, Hagerty CE, de Dios MA, Anderson BJ, Stein MD. Expectancies and self-efficacy mediate the effects of impulsivity on marijuana use outcomes: An application of the acquired preparedness model. Addict Behav. 2011. doi: 10.1016/j.addbeh.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner JD. College cannabis use: The unique roles of social norms, motives, and expectancies. J Stud Alcohol Drugs. 2013. doi: 10.15288/jsad.2013.74.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gates P, Albertella L, Copeland J. Cannabis withdrawal and sleep: A systematic review of human studies. Subst Abus. 2016. doi: 10.1080/08897077.2015.1023484 [DOI] [PubMed] [Google Scholar]
- 36.Groce E The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. J Med Regul. 2018. doi: 10.30770/2572-1852-104.4.32 [DOI] [PubMed] [Google Scholar]
- 37.Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int J Drug Policy. 2016. doi: 10.1016/j.drugpo.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use – basic prevalence and related health outcomes: A scoping review and synthesis. Int J Drug Policy. 2018. doi: 10.1016/j.drugpo.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 39.Lemberger L, Weiss JL, Watanabe AM, Galanter IM, Wyatt RJ, Cardon PV. Delta-9-Tetrahydrocannabinol: Temporal Correlation of the Psychologic Effects and Blood Levels after Various Routes of Administration. N Engl J Med. 1972. doi: 10.1056/NEJM197203302861303 [DOI] [PubMed] [Google Scholar]
- 40.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. i. absorption of thc and formation of 11-oh-thc and thccooh during and after smoking marijuana. J Anal Toxicol. 1992. doi: 10.1093/jat/16.5.276 [DOI] [PubMed] [Google Scholar]
- 41.Hutchison KE, Bidwell LC, Ellingson JM, Bryan AD. Cannabis and Health Research: Rapid Progress Requires Innovative Research Designs. Value Heal. 2019. doi: 10.1016/j.jval.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 42.Murillo-Rodríguez E, Budde H, Veras AB, et al. The Endocannabinoid System May Modulate Sleep Disorders In Aging. Curr Neuropharmacol. 2019. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodges EL, Ashpole NM. Aging circadian rhythms and cannabinoids. Neurobiol Aging. 2019. doi: 10.1016/j.neurobiolaging.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaojun Shi UK. Age-Related Changes in PharmacokineticsNo Title. Curr Drug Metab. 2011. [DOI] [PubMed] [Google Scholar]
- 45.Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br J Clin Pharmacol. 2004. doi: 10.1046/j.1365-2125.2003.02007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowling GJ, Weiss SRB, Condon TP. Drugs of abuse and the aging brain. Neuropsychopharmacology. 2008. doi: 10.1038/sj.npp.1301412 [DOI] [PubMed] [Google Scholar]
- 47.Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005. doi: 10.1592/phco.2005.25.10.1396 [DOI] [PubMed] [Google Scholar]
- 48.Corsonello A, Pedone C, Incalzi R. Age-Related Pharmacokinetic and Pharmacodynamic Changes and Related Risk of Adverse Drug Reactions. Curr Med Chem. 2010. doi: 10.2174/092986710790416326 [DOI] [PubMed] [Google Scholar]
- 49.Beauchet O Medical cannabis use in older patients: Update on medical knowledge. Maturitas. 2018. doi: 10.1016/j.maturitas.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 50.Van den Elsen GAH, Ahmed AIA, Lammers M, et al. Efficacy and safety of medical cannabinoids in older subjects: A systematic review. Ageing Res Rev. 2014. doi: 10.1016/j.arr.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Ahmed AIA, Van Den Elsen GAH, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl). 2015. doi: 10.1007/s00213-015-3889-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mokrysz C, Freeman TP, Korkki S, Griffiths K, Curran HV. Are adolescents more vulnerable to the harmful effects of cannabis than adults? A placebo-controlled study in human males. Transl Psychiatry. 2016. doi: 10.1038/tp.2016.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators - National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill Summ. 2016. doi: 10.15585/mmwr.ss6511a1 [DOI] [PubMed] [Google Scholar]
- 54.Rotermann M Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Heal Reports. 2019. doi: 10.25318/82-003-x201900600001-eng [DOI] [PubMed] [Google Scholar]
- 55.Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, Bonn-Miller MO. Cannabis use patterns and motives: A comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav. 2017. doi: 10.1016/j.addbeh.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther. 1979. doi: 10.1016/0005-7967(79)90025-1 [DOI] [PubMed] [Google Scholar]
- 57.Martin-Willett R, Helmuth T, Abraha M, et al. Validation of a multisubstance online Timeline Followback assessment. Brain Behav. 2020. doi: 10.1002/brb3.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 59.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006. doi: 10.1097/01.mcp.0000245705.69440.6a [DOI] [PubMed] [Google Scholar]
- 60.Reyner LA, Horne JA, Reyner A. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults [published erratum appears in Sleep 1995 Jun;18(5):391]. Sleep. 1995. [PubMed] [Google Scholar]
- 61.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001. doi: 10.1053/smrv.2001.0162 [DOI] [PubMed] [Google Scholar]
- 62.Vollrath M, Wicki W, Angst J. The Zurich study - VIII. Insomnia: Association with Depression, Anxiety, Somatic Syndromes, and Course of Insomnia. Eur Arch Psychiatry Neurol Sci. 1989. doi: 10.1007/BF01759584 [DOI] [PubMed] [Google Scholar]
- 63.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005. doi: 10.4088/JCP.v66n1008 [DOI] [PubMed] [Google Scholar]
- 64.SAUNDERS JB, AASLAND OG, BABOR TF, DE LA FUENTE JR, GRANT M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 65.Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995. doi: 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- 66.Gray JB, Woodall WH. The maximum size of standardized and internally studentized residuals in regression analysis. Am Stat. 1994. doi: 10.1080/00031305.1994.10476035 [DOI] [Google Scholar]
- 67.R Foundation for Statistical Computing. R. Development Core Team: R: A language and environment for statistical computing. ISBN 3-900051-07-0, http://www.R-project.org. 2015.
- 68.Schmidt AF, Finan C. Linear regression and the normality assumption. J Clin Epidemiol. 2018. doi: 10.1016/j.jclinepi.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 69.West, Finch, Curran. Structural equation models with non- normal variables: Problems and remedies. In: Structural Equation Modeling : Concepts, Issues, and Applications. ; 1995. [Google Scholar]
- 70.Hosmer DW Jr, & Lemeshow S Applied logistic regression John Wiley & Sons; Contemp Sociol. 2004. [Google Scholar]
- 71.Jessen HC, Menard S. Applied Logistic Regression Analysis. Stat. 1996. doi: 10.2307/2988559 [DOI] [Google Scholar]
- 72.Judd CM, McClelland GH, Ryan CS. Data Analysis : A Model Comparison Approach to Regression, ANOVA, and Beyond.; 2009.
- 73.Toothaker LE, Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. J Oper Res Soc. 1994. doi: 10.2307/2583960 [DOI] [Google Scholar]
- 74.Benjamini Yoav ; Hochberg Y Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological 1995.pdf. J R Stat Soc Ser B. 1995. doi: 10.2307/2346101 [DOI] [Google Scholar]
- 75.Ogborne AC, Smart RG, Adlaf EM. Self-reported medical use of marijuana: A survey of the general population. CMAJ. 2000. [PMC free article] [PubMed] [Google Scholar]
- 76.Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015. doi: 10.1186/s13722-015-0032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen B, Chen J, Furnish T, Wallace M. Medical Marijuana and Chronic Pain: a Review of Basic Science and Clinical Evidence. Curr Pain Headache Rep. 2015. doi: 10.1007/s11916-015-0524-x [DOI] [PubMed] [Google Scholar]
- 78.Altman BR, Mian MN, Slavin M, Earleywine M. Cannabis Expectancies for Sleep. J Psychoactive Drugs. 2019. doi: 10.1080/02791072.2019.1643053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maple KE, McDaniel KA, Shollenbarger SG, Lisdahl KM. Dose-dependent cannabis use, depressive symptoms, and FAAH genotype predict sleep quality in emerging adults: a pilot study. Am J Drug Alcohol Abuse. 2016. doi: 10.3109/00952990.2016.1141913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Δ−9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004. doi: 10.1097/01.jcp.0000125688.05091.8f [DOI] [PubMed] [Google Scholar]
- 81.Hsiao YT, Yi PL, Li CL, Chang FC. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. In: Neuropharmacology. ; 2012. doi: 10.1016/j.neuropharm.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 82.Chagas MHN, Crippa JAS, Zuardi AW, et al. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J Psychopharmacol. 2013. doi: 10.1177/0269881112474524 [DOI] [PubMed] [Google Scholar]
- 83.Zuardi AW, Cosme RA, Graeff FG, Guimaraes FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993. doi: 10.1177/026988119300700112 [DOI] [PubMed] [Google Scholar]
- 84.CARLINI EA, CUNHA JM. Hypnotic and Antiepileptic Effects of Cannabidiol. J Clin Pharmacol. 1981. doi: 10.1002/j.1552-4604.1981.tb02622.x [DOI] [PubMed] [Google Scholar]
- 85.Linares IMP, Guimaraes FS, Eckeli A, et al. No acute effects of Cannabidiol on the sleep-wake cycle of healthy subjects: A randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol. 2018. doi: 10.3389/fphar.2018.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999. doi: 10.1046/j.1365-2869.1999.00155.x [DOI] [PubMed] [Google Scholar]
- 87.Fernandez-Mendoza J The insomnia with short sleep duration phenotype: An update on it’s importance for health and prevention. Curr Opin Psychiatry. 2017. doi: 10.1097/YCO.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 88.Lemola S, Ledermann T, Friedman EM. Variability of Sleep Duration Is Related to Subjective Sleep Quality and Subjective Well-Being: An Actigraphy Study. PLoS One. 2013. doi: 10.1371/journal.pone.0071292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winiger EA, Huggett SB, Hatoum AS, Stallings MC, Hewitt JK. Onset of regular cannabis use and adult sleep duration: Genetic variation and the implications of a predictive relationship. Drug Alcohol Depend. 2019. doi: 10.1016/j.drugalcdep.2019.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winiger E, Hugget S, Hatoum A, Stallings M & H J. The Relationship Between Early Regular Cannabis Use & Adult Sleep Duration: Genetic Variation and the Implications of a Predictive Relationship. Drug Alcohol Depend. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep Problems in Early Childhood and Early Onset of Alcohol and Other Drug Use in Adolescence. Alcohol Clin Exp Res. 2004. doi: 10.1097/01.ALC.0000121651.75952.39 [DOI] [PubMed] [Google Scholar]
- 92.Pasch KE, Latimer LA, Cance JD, Moe SG, Lytle LA. Longitudinal Bi-directional Relationships Between Sleep and Youth Substance Use. J Youth Adolesc. 2012. doi: 10.1007/s10964-012-9784-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasler BP, Kirisci L, Clark DB. Restless Sleep and Variable Sleep Timing During Late Childhood Accelerate the Onset of Alcohol and Other Drug Involvement. J Stud Alcohol Drugs. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller MB, Janssen T, Jackson KM. The Prospective Association Between Sleep and Initiation of Substance Use in Young Adolescents. J Adolesc Heal. 2017. doi: 10.1016/j.jadohealth.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]


