Abstract
Background
Given the low retention and lack of persistent support by traditional tobacco cessation programs, evidence-based smartphone app-supported interventions can be an important tobacco control component. The objective of this systematic review was to identify and evaluate the types of studies that use smartphone apps for interventions in tobacco cessation.
Methods
We conducted a systematic review of PubMed (1946–2019), EMBASE (1974–2019), and PsycINFO (1806–2019) databases with keywords related to smartphone-supported tobacco cessation. Included articles were required to meet 3 baseline screening criteria: 1) be written in English, 2) include an abstract, and 3) be a full, peer-reviewed manuscript. The criteria for the second level of review were: 1) primary outcome of tobacco cessation, 2) intervention study, and 3) smartphone app as primary focus of study.
Results
Of 1973 eligible manuscripts, 18 met inclusion criteria. Most studies (n = 17) recruited adult participants (18+ years); one included teens (16+ years). Tobacco cessation was usually self-reported (n = 11), compared to biochemical verification (n = 3) or both (n = 4). There were 11 randomized controlled trials, 4 of which reported statistically significant results, and 7 single-arm trials that reported a mean abstinence rate of 33.9%.
Discussion
The majority of studies that use tobacco cessation apps as an intervention delivery modality are mostly at the pilot/feasibility stage. The growing field has resulted in studies that varied in methodologies, study design, and inclusion criteria. More consistency in intervention components and larger randomized controlled trials are needed for tobacco cessation smartphone apps.
Keywords: Tobacco, Apps, Cessation, Smoking, Smartphone, Systematic literature review
1. INTRODUCTION
Tobacco continues to be the leading cause of preventable death in the United States (WHO, 2017). Within 10 years, smokers who successfully quit reduce their risk of death from lung cancer by half (Yamaguchi et al., 1991); within 15 years, risk of coronary heart disease is equal to that of a nonsmoker (World Health Organization, 2016). Although 30 million smokers contact a healthcare provider each year (Fiore et al., 2008), and more than half attempt to quit, most are not successful (Shiffman et al., 2008). After one year, the success rates of cessation were 5% when patients tried to quit without support, 16% with behavioral intervention, and 24% when the patient used pharmacological treatment in addition to behavioral support (Castaldelli-Maia et al., 2013; Khan et al., 2012; Laniado-Laborín, 2010). Limitations in traditional cessation interventions include low utilization (Fiore et al., 2008; Soon et al., 2014) and intermittent interactions between treatment experts (e.g., cessation counselors) and smokers (Martinez et al., 2018). It is also difficult to provide real-time responses to smoking urges and related cues, which are known to be important factors in relapse (Fiore et al, 2008).
Smartphones are ubiquitous in the United States with 77% of Americans owning a smartphone (Pew Research Center, 2018). Providing real-time help and information to patients can be achieved with the use of mobile health (mHealth) smartphone apps. While short message service (SMS), or text-messages, successfully provide support for cessation (Scott-Sheldon et al., 2016), apps afford the functionality (e.g., visual aids, interactive components, advanced multimedia) to develop more complex interventions based on health behavioral theories that might not be implementable in an SMS-only platform. Given that smartphone apps are able to reach and engage a larger population of individuals, it is necessary to identify evidence-based tobacco cessation apps, understand the theories that support the intervention components they deliver, and collect information on their effectiveness.
Therefore, the objective of this systematic review was to identify and evaluate the current evidence on smartphone apps used as an intervention for tobacco cessation. The most important factors considered were a primary outcome of tobacco cessation and the explicit use of a smartphone app as the source of intervention. Previous related reviews were content analyses that focused on apps that were commercially available in Android, Apple, Blackberry, and Windows online stores (Abroms et al., 2011; Hoeppner et al., 2016; Ubhi et al., 2016); they reported on the lack of scientific evidence for most commercially available apps, and all tobacco cessation outcomes were self-reported. Those reviews rated the apps based on strength of evidence, which showed that only one commercially available app was supported by high quality evidence. Rather than identify potential apps through commercial venues (e.g., Apple app store), this review searched exclusively through several scientific databases. Previous reviews are also out-of-date; given the speed of changes in mobile technologies, there have been many new advances, developed by both industry and academic institutions, since 2016. This review focused on all smartphone apps reported in the scientific literature, including those outside commercial app stores, and include more information on the effectiveness of each study.
2. METHODS
2.1. Design
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed to report the results of this systematic review (see Appendix A). The protocol was registered with PROSPERO International Prospective Register of systematic reviews (CRD42018096567).
2.2. Data sources
Studies were located from PubMed/MEDLINE (1946–2019), EMBASE.com (1974–2019) and PsycINFO, Ovid® (1806–2019) using formulated search strategies developed by a medical librarian. Search strategies used appropriate and broad controlled vocabulary, phrases and keywords representative for the concepts of smartphones (e.g., smartphone, cellphone, iPhone), smoking (e.g., smoking, tobacco, nicotine), and smoking cessation (e.g., abstinence, quitting, intervention). Searches have no specific restrictions and include all languages and publication years (see Appendix B for search strategy details). Downloading database records, importing into EndNote, and de-duplicating records occurred on February 26, 2019. The web-based software DistillerSR aided in screening and data extraction.
2.3. Eligibility criteria
This systematic review proceeded through two levels of eligibility screening. First, articles were required to meet the following three baseline criteria: the articles 1) were written in English, 2) included text in the abstract, and 3) published as a full manuscript from a peer-reviewed journal. These requirements disqualified any dissertations, abstracts without manuscripts, book chapters, and other similar publications.
Articles then underwent a second level of review and had to meet the following criteria: the study must 1) have had tobacco cessation as a primary outcome, 2) was an intervention, and 3) primarily focused on a smartphone. For the purposes of this review, an app-based intervention was defined as using a smartphone application to support the user in stopping the use of tobacco. Studies that only used tablets (e.g., iPads) or websites accessed by smartphones—techniques that provide a different user experience (e.g., smartphone interventions might support outdoor locations where smokers feel the urge to have a cigarette, addressing a scenario that is not suitable for tablets)—were not considered. Given the ubiquity of smartphones, this systematic review excluded tablet apps in favor of smartphone apps that reach a more widespread audience.
2.4. Analysis
Before conducting this review, 5 researchers independently reviewed a preliminary group of 500 abstracts to establish a baseline understanding of the screening criteria. One adjudication meeting was held per level of review to better establish definitions and guidelines for including and excluding articles. If coders could not agree, a final determination was made by the lead author. Following adjudication, researchers began to independently review articles from the final dataset. An assessment of bias was also conducted for each study. Because there were a wide range of study designs as well as an expectation of small or underpowered studies, we applied several metrics from STROBE and CONSORT guidelines that could be considered for multiple types of studies (e.g., single and multiple arms). The data assessed included: 1) setting and location of study, 2) eligibility criteria, 3) recruitment source, 4) study size justification, 5) baseline participant demographics, 6) discussion of loss of participants, 7) discussion and justification on generalizability, and 8) funding source. Inter-rater reliability (Cohen’s Kappa) for the basic data extraction from two coders (CGEV and SJM) ranged from κ=0.78 to κ=1.0.
3. RESULTS
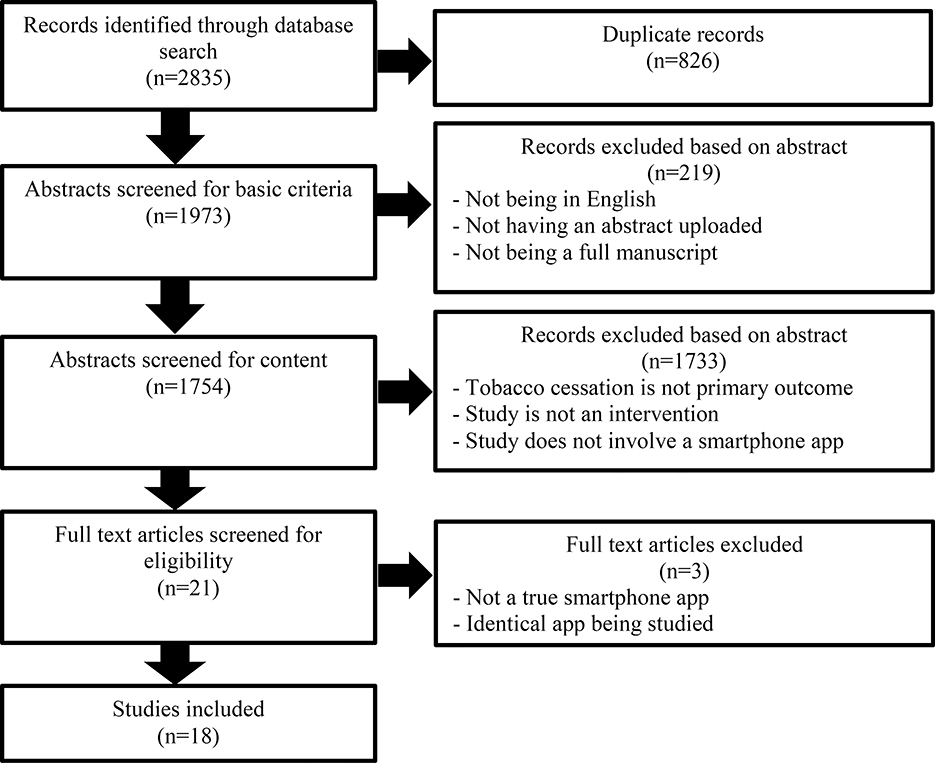
The searches retrieved 2835 database records containing 826 duplicate records, leaving 1973 records to screen (duplicates can occur more than once). In the initial screening, if the text was not written in English, did not have an abstract, or was not a full peer-reviewed manuscript, the record was excluded (n = 219). In the second screening, if tobacco cessation was not an outcome, the study was not an intervention, or if a smartphone app was not used, the record was excluded (n = 1733). Finally, after 21 manuscripts were selected for full text review, two additional manuscripts were deemed ineligible because they did not use an actual smartphone app (e.g., use of a smartphone to access a website); a third was disqualified because it was a secondary analysis of an app already included. A total of 18 manuscripts were included in the final review (Figure 1). There were two studies that used a different version of a similar app, but as the trial designs were also different, both studies were independently considered. Almost every study (n = 17) recruited adult participants (18+); 1 app included teens as young as 16 years old. Confirmation of cessation was usually self-reported (n = 11), compared to biochemical verification (n = 3) or both (n = 4).
Figure 1.
PRISMA Flow Diagram.
The length of study ranged from 1 to 12 months from enrollment to the final survey or assessment (mean=4.18), participants from 11 to 28,112 (mean=1953, median=235), and retention from 8% to 100% (mean=71.55%, median=81%). Participant eligibility criteria was highly variable in the inclusion criteria which comprised a mixture of smoking habits, location, smartphone ownership, gender, health status, and literacy. The behavioral theories applied in each study also showed heterogeneity, with 12 different theories or techniques applied (Table 1). Study designs split between randomized 2-arm (n = 11) or 1-arm (n = 7) trials. In the 2-arm trials, most had a different control condition: relapse training, counseling and medication, a different app, a text-messaging intervention, a self-help guide, experience training, brief advice, and a yoked condition (participants in the experimental arm were compensated specifically for low carbon monoxide (CO) readings, while participants in the control/yoked arm were compensated for any CO reading). In 3 of the 2-arm trials, the control arm was a reduced or downgraded version of the app (Table 2). Primary outcomes of point prevalence abstinence (PPA) for each study differed (e.g., 7-day PPA or 30-day PPA) based on study design as listed in Table 2. In the trials with a comparison group, 4 reported significantly higher rates of abstinence in the experimental app arm, 5 reported non-significant higher abstinence than control, and 2 reported no difference. In the 1-arm trials, the mean abstinence rate was 33.9%. In randomized-control trials, the mean abstinence rate was 22.9% for the app group. In total, the 18 studies reported an average of 6.1 out of the 8 categories that were assessed for bias (Table 3). The most common underreported categories were justification of sample size (39% of studies reported) and discussing loss of participants (39%).
Table 1.
Tobacco Cessation Smartphone App Studies, Overall Descriptions.
| Author & Year | App Name | Theories or Techniques | Phone Platforms | Participants | Completed | % Retained | Smoking Status | Location | Other Conditions |
|---|---|---|---|---|---|---|---|---|---|
| BinDhim 2018 | Smartphone Smoking Cessation Application (SSC App) | Ottawa decision support | Apple | 684 | 583 | 85% | Daily smoker | US, UK, Australia, Singapore | |
| Bricker 2014 | SmartQuit | Acceptance and commitment therapy (ACT) | Apple + Android | 340 | 196 | 58% | 5 cigs/day >1 year willing to quit in next 30 days | English speaking | |
| Bricker 2017 | SmartQuit 2.0 | Acceptance and commitment therapy (ACT) | Apple + Android | 99 | 84 | 85% | 5 cigs/day >1 year willing to quit in next 30 days | Not using other cessation | |
| Buller 2014 | REQ_mobile | Quit Coach theoretical framework | Android + Windows | 102 | 68 | 67% | Smokers | English proficient | |
| Businelle 2016 | Smart-T | EMA/tailored messages | Android | 61 | 59 | 97% | 5 cigs/day current smokers willing to quit within 7 days | 6th grade literacy, clinic visit once/week for 6 months | |
| Gordon 2017 | See me smoke-free | Guided imagery | Android | 151 | 73 | 48% | Smoked in last 30 days | US | Female, English speaking |
| Hassandra 2017 | Physical Activity over Smoking (PhoS) | Multiple | Android | 44 | 34 | 77% | Regular smokers (10+ years) motivated to quit | No mental health conditions | |
| Hertzberg 2013 | Mobile Contingency Management (mCM) | Contingency management | Apple + Android | 22 | 22 | 100% | 10 cigs/day >1 year, no other tobacco products | PTSD, not pregnant or schizophrenic | |
| Hicks 2017 | Stay Quit Coach | Contingency management | Apple + Android | 11 | 11 | 100% | 10 cigs/day >1 year willing to quit | US | VA PTSD patients, speak/write English |
| Iacoviello 2017 | Clickotine | Motivational interviewing | Apple | 416 | 365 | 88% | 5 cigs/day wants to quit | US | English speaking |
| Ubhi 2015 | SmokeFree28 (SF28) | PRIME theory | Apple | 1977 | 1170 | 59% | Smoked at time of registration, set quit date | ||
| Tombor 2019 | SmokeFree Baby | Behavioral Change Theories | Apple + Android | 565 | 247 | 44% | Weekly smoker | Pregnant, 18+, interested in cessation and set a quit date in the app | |
| Masaki 2019 | CureApp Smoking Cessation (CASC) | Cessation Counseling | Apple + Android | 56 | 51 | 91% | Nicotine dependence patients | ||
| Marler 2019 | Pivot | Multiple | Apple + Android | 319 | 272 | 85% | 5+ cigs/day current smokers | 18–65 years old | |
| Baskersville 2018 | Crush the Crave | Contingency Management | Apple + Android | 1599 | 725 | 45% | Smoker considering quitting in the next 30 days | Canada | |
| Crane 2019 | Smoke Free | Behavioral change techniques | Apple + Android | 28112 | 2114 | 8% | Current smokers | App users, 18+, set one quit date | |
| Krishnan 2019 | Coach2Quit | Biomarker Feedback | Android | 102 | 89 | 87% | Daily smoker | US | Willing to set a quit date within 2 weeks |
| Garrison 2020 | Craving to Quit | Mindfulness | Apple + Android | 505 | 325 | 64% | 5+ cigs/day current smokers | 18–65, less than 3 months past-year abstinence, 8 or great on the contemplation ladder and 4 or greater on the readiness to change questionnaire |
Table 2.
Tobacco Cessation Smartphone App Studies, Study Designs, and Outcomes.
| Author & Year | Study Design | Comparison or Control | Length | Abstinence Report | Primary Outcome | Difference with Control | Other Outcomes |
|---|---|---|---|---|---|---|---|
| BinDhim 2018 | Automated double blind RCT | Downgraded app (no motivational messages) | 6 months | Self-report | 28.5% abstinent | Significant difference (p<.001) | Quit attempts, app feasibility |
| Bricker 2014 | Double blind RCT pilot trial | QuitGuide | 2 months | Self-report | 13% abstinent | Significant difference (p<.001) | Receptivity to app, smoking reduction |
| Bricker 2017 | Single arm pilot | NA | 2 months | Self-report | 21% 7-day point-prevalence abstinent (PPA) | NA | Receptivity to app, smoking reduction |
| Buller 2014 | Randomized pre/post feasibility | onQ text messaging | 3 months | Self-report | 37% 7-day PPA | Significant difference (p=.04) | App usage |
| Businelle 2016 | Nonrandomized feasibility | NA | 12 weeks | Biochemical | 20% abstinent | NA | Acceptability |
| Gordon 2017 | Single arm pilot | NA | 90 days | Self-report | 47% 7-day PPA | NA | Weight, diet, exercise, app satisfaction and acceptability |
| Hassandra 2017 | RCT feasibility | Relapse prevention training | 6 months | Both | 36% 7-day PPA | No significant difference | App engagement, physical activity |
| Hertzbeg 2013 | Randomized feasibility | Yoked condition | 3 months | Biochemical | 50% abstinent | No significant difference | |
| Hicks 2017 | Two arm RCT feasibility | Counseling and medication | 6 months | Both | 40% 7-day PPA | 100% abstinent in Combined Contact 60% abstinent in QUIT4EVER | Participant satisfaction, app compliance |
| Iacoviello 2017 | Single arm feasibility | NA | 8 weeks | Self-report | 45% 7-day PPA | NA | App engagement, medical monitoring of other health events |
| Ubhi 2015 | Single arm observational prospective cohort | NA | 28 days | Self-report | 19% 28-day PPA | NA | App usage |
| Tombor 2019 | RCT | Different versions of the app | 6 weeks | Self-report | N/A | No difference | App engagement |
| Masaki 2019 | Single-arm | N/A | 52 weeks | Both | 58% continues abstinence rate at 52 weeks | NA | Withdrawal and craving symptoms |
| Marler 2019 | Open-label single arm | N/A | 18.5 weeks | Both | 27.6% 30-day PPA | NA | Module completion rate, increased confidence to quit |
| Baskersville 2018 | Parallel double-blind RCT | Evidence informed self-help guide OnRQ (usual care) | 6 months | Self-report | 14.4% 30-day PPA at six months | No significant difference | Frequency of use |
| Crane 2019 | Two-arm exploratory RCT | Reduced version (no daily ‘missions’) | 3 months | Self-report | 19.3% abstinent | Significant difference (p<.001) | CPD, quit date designation |
| Krishnan 2019 | RCT | Brief advice only | 30 days | Self-report | 3% 30 day PPA | No difference | Carbon monoxide levels, CPD |
| Garrison 2020 | RCT | Experience training | 6 months | Biochemical | 11.1% 7 day PPA | No significant difference | Craving strength and frequency, mindfulness |
Table 3.
Assessment of bias, including: aselection bias, breporting bias, cattrition bias.
| Author & Year | Study settinga | Eligibility criteriaa | Source of recruitmenta | Justify sample size | Baseline data in resultsb | Discuss loss of participantsc | Discuss generalizability | Funding source |
|---|---|---|---|---|---|---|---|---|
| BinDhim 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Bricker 2014 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Bricker 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Buller 2014 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Businelle 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Gordon 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Hassandra 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Hertzberg 2013 | ✓ | ✓ | ✓ | ✓ | ||||
| Hicks 2017 | ✓ | ✓ | ✓ | |||||
| Iacoviello 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Ubhi 2015 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Tombor 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Masaki 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Marler 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Baskersville 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Crane 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Krishnan 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Garrison 2020 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
4. DISCUSSION
This systematic review identified 18 articles that focused on smartphone apps for delivering theory-driven tobacco cessation intervention. Overall, there was heterogeneity in the design of testing each app, for example, many of the 2-arm trials had a different control condition. Additionally, studies varied in length of study duration, participant eligibility criteria, and behavioral theories. Unfortunately, the amount of variability in the number of theories and models applied by the different studies prevented identifying patterns to understand if any particular model or design can be more effective for tobacco cessation. Similarly, given the amount of variation in protocols, measurements of outcome, and control groups, a meta-analysis was not feasible. However, this review identified new apps and research developments since the most recently available analyses (Hoeppner et al., 2016; Ubhi et al., 2016). Additionally, this review searched exclusively for studies available through scientific databases rather than commericial app stores, allowing us to provide more detailed information on developing projects.
An important challenge to conducting mHealth studies is participant attrition (Lane et al., 2015; O’Connor et al., 2015; Pfammatter et al., 2017). This might be explained in part by app user fatigue or limited resources; unfortunately, only 39% (n=7) studies included any discussion regarding participant retention (Table 3). Gordan (2017) suggested the pragmatic nature of their trial, with little to no contact with participants, contributed to the high attrition; Hassandra (2017) found that the most common reason for dropping out was relapse back to smoking; Garrison (2020) believed their study was able to achieve high retention with proactive methods that included obtaining contact information for three additional referrals. Overall, there was no pattern of app features, participant compensation, or other retention strategies that demonstrated consistently high retention. It would also be beneficial to disentangle the issue of attrition with user engagement, leaving an opportunity for future research. For example, mHealth studies in other fields have found that gamification and interactive components help improve engagement and retention (Muessig et al., 2013), which could be applied for tobacco cessation. Another potential effect of scope and resources is that the majority of studies (61%) relied on self-report as the source of the cessation outcome, even though biochemical validation is the gold-standard for traditional tobacco cessation studies. However, mHealth studies are inherently based on remote use and/or monitoring. It is possible that participants could be more truthful reporting the amount they smoke to an app rather than a person that could be perceived as judgmental. Further studies are needed to understand whether the same reporting standards are necessary.
Single-arm trials had a mean abstinence rate of 33.9%, showing promise compared to rates of existing recommended therapies; as a frame of reference, 24% of patients successfully quit smoking when using pharmacological treatment in addition to behavioral support (Laniado-Laborín, 2010). Of the 11 trials with a control group, only 4 demonstrated significant improvement in intervention group over the control group. Only the Bindhim study (BinDhim, McGeechan, & Trevena, 2018) was both sufficiently powered and detected a significant difference in cessation outcomes (80% power at alpha=0.05).
Most of the studies underreported information or data that could lead to potential biases (Table 3). As most of the studies were limited in sample size and power, acknowledgement and discussion of sample sizes and retention rates would be beneficial to inform future trials and larger studies. Overall, a stronger adherence to reporting guidelines such as CONSORT or STROBE could help smaller pilot studies have a larger impact.
Only 10 studies (56%) had developed versions of their app for both Android and Apple, limiting their potential reach. Researchers likely constrained by time and budget focused their development time on a single platform. These design decisions affect their reach because only releasing the app on a single major platform limits the generalizability of the study to only users of that platform (StatCounter Global Stats, 2018). Additionally, the average price of an iPhone is more than double an average Android phone (The Washington Post, 2018), potentially limiting the reach of different apps based on socioeconomics.
Although traditional tobacco cessation studies often report outcomes at 12 months (Lemmens et al., 2008), the study periods in this review had a mean of 4.18 months, with only a single study reaching 12 months; this is likely due to the nature of pilot studies and limited resources. While there was a variation in participant eligibility criteria, we did not find studies focused on specific groups. Research has identified important disparities in tobacco products use among racial, ethnic, and sexual and gender minorities (Bränström and Pachankis, 2018; Drope et al., 2018; Margerison-Zilko and Cubbin, 2013; McQuoid et al., 2018). Future mHealth research should have a focus on studying feasibility, acceptability, and effectiveness of apps for all tobacco users, with an awareness for design features that are tailored and culturally appropriate for minority groups.
5. LIMITATIONS
This work has several limitations. We included studies with different methods and settings; there was also no scoring or review of the methodological quality of the included studies. However, these differences were explicitly reported, and a meta-analysis was not conducted due to the heterogeneity in study designs. The studies chosen for review were limited by the selected search terms (Appendix B). We also did not include studies that were not published, possibly due to null findings, raising the risk of publication bias. Despite these limitations, this systematic review was able to identify and summarize the peer-review literature of smartphone apps for delivering theory-driven tobacco cessation interventions. Using DistillerSR also provided rigor and consistency to the review. These findings are important for the future of research in this field. There is a need for more consistency in intervention components, which could be supported by a registry or consistent set of guidelines for mHealth tobacco trials. Larger, randomized controlled trials are also needed to better elucidate how smartphone apps can be effective for tobacco cessation.
6. CONCLUSIONS
Studies have consistently shown a significant decrease in cancer and heart disease mortality for people who quit tobacco use (World Health Organization; 2016; Yamaguchi et al., 1991). However, given the low retention and lack of consistent support in traditional evidence-based treatment programs (Fiore et al., 2008; Martinez et al., 2018; Soon et al., 2014), theory-driven app-supported interventions serve as a promising component in tobacco control strategies. Over the past several years, tobacco researchers have leveraged the engagement of smartphone users to develop and deliver app-supported cessation efforts. This study shows that more effort is needed to design studies that can be generalizable as well as better retain participants. Mobile apps provide the real-time, persistent, and cost-effective ability to support tobacco cessation and cancer prevention. If effective, they can reduce various negative health outcomes by providing prevention practitioners with a valuable tool to reduce smoking.
Supplementary Material
Highlights.
Identified 18 studies using apps for evidence-based tobacco cessation intervention
Most interventions using tobacco cessation apps were pilot/feasibility studies
Studies had varied methodology, study design, inclusion criteria
More effort is needed to design studies that can be generalizable as well as retain participants
Consistent app interventions and larger randomized controlled trials needed
7.
ROLE OF THE FUNDING SOURCE
Research in this publication was supported by the National Cancer Institute (K07CA222338, PI: Chu). The funders had no role in the design and conduct of this study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abroms LC, Padmanabhan N, Thaweethai L, & Phillips T (2011). iPhone apps for smoking cessation: a content analysis. American Journal of Preventive Medicine, 40(3), 279–285. 10.1016/j.amepre.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville NB, Struik LL, Guindon GE, Norman CD, Whittaker R, Burns C, Hammond D, Dash D, & Brown KS (2018). Effect of a mobile phone intervention on quitting smoking in a young adult population of smokers: Randomized controlled trial. JMIR MHealth and UHealth, 6(10), e10893 10.2196/10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BinDhim NF, McGeechan K, & Trevena L (2018). Smartphone Smoking Cessation Application (SSC App) trial: a multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid “app”. BMJ Open, 8(1), e017105 10.1136/bmjopen-2017-017105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränström R, & Pachankis JE (2018). Sexual orientation disparities in the co-occurrence of substance use and psychological distress: a national population-based study (2008–2015). Social Psychiatry and Psychiatric Epidemiology, 53(4), 403–412. 10.1007/s00127-018-1491-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Copeland W, Mull KE, Zeng EY, Watson NL, Akioka KJ, & Heffner JL (2017). Single-arm trial of the second version of an acceptance & commitment therapy smartphone application for smoking cessation. Drug and Alcohol Dependence, 170(2017), 37–42. 10.1016/j.drugalcdep.2016.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Mull KE, Kientz JA, Vilardaga R, Mercer LD, Akioka KJ, & Heffner JL (2014). Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug and Alcohol Dependence, 143, 87–94. 10.1016/j.drugalcdep.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller DB, Borland R, Bettinghaus EP, Shane JH, & Zimmerman DE (2014). Randomized Trial of a Smartphone Mobile Application Compared to Text Messaging to Support Smoking Cessation. Telemedicine and E-Health, 20(3), 206–214. 10.1089/tmj.2013.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Frank SG, Vidrine DJ, & Wetter DW (2016). An Ecological Momentary Intervention for Smoking Cessation: Evaluation of Feasibility and Effectiveness. Journal of Medical Internet Research, 18(12), e321 10.2196/jmir.6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldelli-Maia JM, Carvalho CFC, Armentano F, Frallonardo FP, Alves TC de TF, Andrade, de AG, … Nicastri S (2013). Outcome predictors of smoking cessation treatment provided by an addiction care unit between 2007 and 2010. Brazilian Journal of Psychiatry, 35(4), 338–346. 10.1590/1516-4446-2012-0907 [DOI] [PubMed] [Google Scholar]
- Crane D, Ubhi HK, Brown J, & West R (2019). Relative effectiveness of a full versus reduced version of the ‘smoke free’ mobile application for smoking cessation: An exploratory randomised controlled trial. F1000Research, 7 10.12688/f1000research.16148.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drope J, Liber AC, Cahn Z, Stoklosa M, Kennedy R, Douglas CE, … Drope J (2018). Who’s still smoking? disparities in adult cigarette smoking prevalence in the United States. CA: A Cancer Journal for Clinicians, 68(2), 106–115. 10.3322/caac.21444 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Bennett G, Benowitz N, … Williams C (2008). A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. public health service report. American Journal of Preventive Medicine, 35(2), 158–176. 10.1016/j.amepre.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison K, O’Malley S, Pittman B, Gueorguieva R, Rojiani R, Scheinost D, Dallery J, & Brewer J (2020). Craving to Quit: A Randomized Controlled Trial of Smartphone App-Based Mindfulness Training for Smoking Cessation. Nicotine & Tobacco Research, 22(3), 324–331. 10.1093/ntr/nty126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Armin J, Hingle D, M., Giacobbi P, Cunningham JK, Johnson T, Abbate K, Howe CL, & Roe DJ (2017). Development and evaluation of the See Me Smoke-Free multi-behavioral mHealth app for women smokers. Translational Behavioral Medicine, 7(2), 172–184. 10.1007/s13142-017-0463-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassandra M, Lintunen T, Hagger MS, Heikkinen R, Vanhala M, & Kettunen T (2017). An mHealth App for Supporting Quitters to Manage Cigarette Cravings With Short Bouts of Physical Activity: A Randomized Pilot Feasibility and Acceptability Study. JMIR MHealth and UHealth, 5(5), e74 10.2196/mhealth.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, Dennis PA, Dedert EA, & Beckham JC (2013). Mobile Contingency Management as an Adjunctive Smoking Cessation Treatment for Smokers With Posttraumatic Stress Disorder. Nicotine & Tobacco Research, 15(11), 1934–1938. 10.1093/ntr/ntt060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks TA, Thomas SP, Wilson SM, Calhoun PS, Kuhn ER, & Beckham JC (2017). A Preliminary Investigation of a Relapse Prevention Mobile Application to Maintain Smoking Abstinence Among Individuals With Posttraumatic Stress Disorder. Journal of Dual Diagnosis, 13(1), 15–20. 10.1080/15504263.2016.1267828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeppner BB, Hoeppner SS, Seaboyer L, Schick MR, Wu GWY, Bergman BG, & Kelly JF (2016). How smart are smartphone apps for smoking cessation? a content analysis. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco, 18(5), 1025–1031. 10.1093/ntr/ntv117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoviello BM, Steinerman JR, Klein DB, Silver TL, Berger AG, Luo SX, & Schork NJ (2017). Clickotine, A Personalized Smartphone App for Smoking Cessation: Initial Evaluation. JMIR MHealth and UHealth, 5(4), e56 10.2196/mhealth.7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Anderson JR, Du J, Tinker D, Bachyrycz AM, & Namdar R (2012). Smoking cessation and its predictors: results from a community-based pharmacy tobacco cessation program in New Mexico. Annals of Pharmacotherapy, 46(9), 1198–1204. 10.1345/aph.1P146 [DOI] [PubMed] [Google Scholar]
- Krishnan N, Elf J, Chon S, & Golub J (2019). COach2Quit: A Pilot Randomized Controlled Trial of a Personal Carbon Monoxide Monitor for Smoking Cessation. Nicotine & Tobacco Research, 21(11), 1573–1577. 10.1093/ntr/nty182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TS, Armin J, & Gordon JS (2015). Online recruitment methods for web-based and mobile health studies: a review of the literature. Journal of Medical Internet Research, 17(7), e183 10.2196/jmir.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado-Laborín R (2010). Smoking cessation intervention: an evidence-based approach. Postgraduate Medicine, 122(2), 74–82. 10.3810/pgm.2010.03.2124 [DOI] [PubMed] [Google Scholar]
- Lemmens V, Oenema A, Knut IK, & Brug J (2008). Effectiveness of smoking cessation interventions among adults: a systematic review of reviews. European Journal of Cancer Prevention, 17(6), 535–544. 10.1097/CEJ.0b013e3282f75e48 [DOI] [PubMed] [Google Scholar]
- Margerison-Zilko C, & Cubbin C (2013). Socioeconomic disparities in tobacco-related health outcomes across racial/ethnic groups in the United States: national health interview survey 2010. Nicotine & Tobacco Research, 15(6), 1161–1165. 10.1093/ntr/nts256 [DOI] [PubMed] [Google Scholar]
- Marler JD, Fujii CA, Utley DS, Tesfamariam LJ, Galanko JA, & Patrick H (2019). Initial assessment of a comprehensive digital smoking cessation program that incorporates a mobile app, breath sensor, and coaching: Cohort study. Journal of Medical Internet Research, 21(2), e12609 10.2196/12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E, Tatum KL, Weber DM, Kuzla N, Campbell K, Ridge JA, … Schnoll RA (2018). Issues related to implementing a smoking cessation clinical trial for cancer patients. Cancer Causes & Control, 20(1), 97–104. 10.1007/s10552-008-9222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki K, Tateno H, Kameyama N, Morino E, Watanabe R, Sekine K, Ono T, Satake K, Suzuki S, Nomura A, Betsuyaku T, & Fukunaga K (2019). Impact of a novel smartphone app (cureapp smoking cessation) on nicotine dependence: Prospective single-arm interventional pilot study. Journal of Medical Internet Research, 21(2), e12694 10.2196/12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuoid J, Thrul J, & Ling P (2018). A geographically explicit ecological momentary assessment (GEMA) mixed method for understanding substance use. Social Science & Medicine (1982), 202, 89–98. 10.1016/j.socscimed.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muessig KE, Pike EC, LeGrand S, & Hightow-Weidman LB (2013). Mobile phone applications for the care and prevention of HIV and other sexually transmitted diseases: a review. Journal of Medical Internet Research, 15(1). 10.2196/JMIR.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Mair FS, McGee-Lennon M, Bouamrane M-M, & O’Donnell K (2015). Engaging in large-scale digital health technologies and services. what factors hinder recruitment? Studies in Health Technology and Informatics, 210, 306–310. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25991155 [PubMed] [Google Scholar]
- Pew Research Center. (2018). Mobile fact sheet. Retrieved June 12, 2019, from http://www.pewinternet.org/fact-sheet/mobile/
- Pfammatter AF, Mitsos A, Wang S, Hood SH, & Spring B (2017). Evaluating and improving recruitment and retention in an mHealth clinical trial: an example of iterating methods during a trial. MHealth, 3, 49 10.21037/mhealth.2017.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LAJ, Lantini R, Jennings EG, Thind H, Rosen RK, Salmoirago-Blotcher E, & Bock BC (2016). Text messaging-based interventions for smoking cessation: a systematic review and meta-analysis. JMIR MHealth and UHealth, 4(2), e49 10.2196/mhealth.5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, & Gitchell JG (2008). Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine, 34(2), 102–111. 10.1016/j.amepre.2007.09.033 [DOI] [PubMed] [Google Scholar]
- Soon P, Bastian B, Anderson RN, Collins JL, & Jaffe HW (2014). Potentially preventable deaths from the five leading causes of death — United States, 2008–2010. Morbidity and Mortality Weekly Report, 63(17), 369–374. [PMC free article] [PubMed] [Google Scholar]
- StatCounter Global Stats. (2018). Mobile operating system market share United States of America. Retrieved June 12, 2019, from StatCounter Global Stats website: http://gs.statcounter.com/os-market-share/mobile/united-states-of-america/2016 [Google Scholar]
- The Washington Post. (2018). Are Apple products overpriced? The Washington Post. [Google Scholar]
- Tombor I, Beard E, Brown J, Shahab L, Michie S, & West R (2019). Randomized factorial experiment of components of the SmokeFree Baby smartphone application to aid smoking cessation in pregnancy. Translational Behavioral Medicine, 9(4), 583–593. 10.1093/tbm/iby073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi HK, Michie S, Kotz D, Wong WC, & West R (2015). A mobile app to aid smoking cessation: preliminary evaluation of SmokeFree28. Journal of Medical Internet Research, 17(1), e17 10.2196/jmir.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi HK, Kotz D, Michie S, van Schayck OCP, Sheard D, Selladurai A, & West R (2016). Comparative analysis of smoking cessation smartphone applications available in 2012 versus 2014. Addictive Behaviors, 58, 175–181. 10.1016/j.addbeh.2016.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Tobacco fact sheet. Retrieved October 18, 2018, from http://www.who.int/mediacentre/factsheets/fs339/en/
- World Health Organization. (2017). WHO report on the global tobacco epidemic 2017. In World Health Organization. Retrieved from World Health Organization website: https://apps.who.int/iris/bitstream/handle/10665/255874/9789241512824-eng.pdf;jsessionid=E4B96422129BEF7744D335D5705D0217?sequence=1 [Google Scholar]
- Yamaguchi N, Tamura Y, Sobue T, Akiba S, Ohtaki M, Baba Y, … Watanabe S (1991). Evaluation of cancer prevention strategies by computerized simulation model: an approach to lung cancer. Cancer Causes & Control, 2(3), 147–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.