Abstract
Rationale:
Genetic and non-genetic factors influence substance use disorders. Our previous work in genetic mouse models focused on genetic factors that influence methamphetamine (MA) intake. The current research examined several non-genetic factors for their potential influence on this trait.
Objectives:
We examined the impact on MA intake of several non-genetic factors, including MA access schedule, prior forced MA exposure, concomitant ethanol (EtOH) access, and gamma-aminobutyric acid type B (GABAB) receptor activation. Selectively bred MA high drinking (MAHDR) and low drinking (MALDR) mice participated in this research.
Results:
MAHDR, but not MALDR, mice increased MA intake when given intermittent access, compared to continuous access, with a water choice under both schedules. MA intake was not altered by previous exposure to forced MA consumption. Male MAHDR mice given simultaneous access to MA, EtOH, and an EtOH+MA mixture exhibited a strong preference for MA over EtOH and EtOH+MA; MA intake was not affected by EtOH in female MAHDR mice. When independent MAHDR groups were given access to MA, EtOH, or EtOH+MA vs. water in each case, MA intake was reduced in the water vs. EtOH+MA group, compared to the water vs. MA group. The GABAB receptor agonist R(+)-baclofen (BAC) reduced MA intake, but also reduced water intake and locomotor activity in MAHDR mice. There was a residual effect of BAC, such that MA intake was increased after termination of BAC treatment.
Conclusions:
These findings demonstrate that voluntary MA intake is influenced by non-genetic factors related to MA access schedule and co-morbid EtOH exposure.
Keywords: methamphetamine, ethanol, intake, GABA, substance use disorder, addiction
1. INTRODUCTION
Genetic and non-genetic factors and their interactions influence substance use disorders (SUDs). Data from adult twin studies have demonstrated that SUDs are heritable (Goldman et al. 2005; Kendler et al. 1999, 2000; Tsuang et al. 1996; van den Bree et al. 1998), and that large numbers of genes contribute to psychiatric disorders like SUDs (Dick et al. 2010; Manolio et al. 2009). Non-genetic factors that contribute can be intrinsic (age, sex, co-morbid SUD or other mental illness), extrinsic (drug availability, peer and parental influences), or related to characteristics of the drug (pharmacokinetics, route of administration) (Ahmed et al. 2020; Ducci and Goldman 2012; Egervari et al. 2018; Vink 2016). It is important to understand both genetic and non-genetic contributors to SUDs to discover better treatment options.
One strategy for identifying genetic contributions to drug-related phenotypes is through the use of selectively bred rodent lines. We developed a model of differential genetic risk for methamphetamine (MA) consumption using bidirectional selective breeding to create MA high drinking (MAHDR) and MA low drinking (MALDR) lines of mice, collectively known as the MA drinking (MADR) lines (Hitzemann et al. 2019; Shabani et al. 2011; Wheeler et al. 2009). Quantitative trait locus (QTL) mapping (Belknap et al. 2013), along with assessment of MA intake in multiple genetic mouse models (Harkness et al. 2015; Reed et al. 2018; Stafford et al. 2019), established a causal role for the trace amine-associated receptor 1 (Taar1) gene in variation in MA intake. A spontaneous mutation (Taar1m1J) that codes for a non-functional receptor (TAAR1) and is associated with higher levels of MA intake arose in the DBA/2J (D2) inbred strain progenitor of the MADR lines between 2001 and 2003 (Harkness et al. 2015; Reed et al. 2015; Stafford et al. 2019). The other progenitor is the C57BL/6J (B6) inbred strain that possesses the reference allele also found in the 27 other strains (including 4 wild-derived) that have been genotyped (Shi et al. 2016). It is clear from multiple sets of MADR lines in which Taar1 has been confirmed as a quantitative trait gene that accounts for 60% of the genetic variance (Belknap et al. 2013) that there is a strong genetic influence on level of MA intake.
However, even after strong directional selection resulting in complete fixation of the Taar1m1J allele, residual variation in MA intake remains high within the MAHDR line and also exists within the inbred D2 strain (Reed et al. 2018). Although some of the variation in MA intake in the MAHDR line is likely due to remaining variation among individuals in frequency of causal alleles or background genetic differences interacting with causal alleles, variation in MA intake among individuals of the inbred D2 strain suggests that non-genetic factors play some role. Furthermore, trait heritability is ~.36 in the MADR lines, indicating that only 36% of the variance in MA intake between the selected lines can be attributed to genetic differences. Therefore, non-genetic factors contribute to variation in MA intake and are the focus of the current studies.
Human and animal studies indicate that intermittent drug access, interspersed with cycles of abstinence, can affect drug intake and development of SUDs (Allain et al. 2015; Becker 2012; Cohen et al. 2012; Carnicella et al. 2014). Our standard two-bottle choice MA drinking procedure for measuring voluntary MA intake levels provides mice with intermittent access to MA, so that water and MA are offered for 18h/day, followed by 6h of forced abstinence (water only). For selective breeding, this access cycle is repeated over the course of 8 days (Hitzemann et al. 2019; Shabani et al. 2011; Wheeler et al. 2009), although we have performed studies in which access was provided for up to 28 days (Shabani et al. 2016, 2019). Using this intermittent access procedure, we have repeatedly observed escalation of voluntary MA intake in coordination with increasing MA concentration in MAHDR mice, and steady low levels of intake in MALDR mice, regardless of MA concentration (Harkness et al. 2015; Hitzemann et al. 2019; Shabani et al. 2011; 2016; Wheeler et al. 2009). Furthermore, when the forced abstinence period was extended to 30h or 78h, MAHDR mice maintained high levels of MA intake (Shabani et al. 2016). Unknown is how voluntary MA intake under an intermittent access schedule compares to intake under a continuous access schedule in our model of high vs. low genetic risk for MA intake. Thus, in the first study reported here, we compared MA intake in the MADR lines under these two conditions to determine whether the intermittent schedule elevates intake in this model.
Psychostimulant exposure history contributes to subsequent drug intake in rodent and non-human primate models. In animals, passive injections of amphetamines, cocaine, or nicotine result in an enhancement in subsequent self-administration of these drugs (Vezina 2004; Vezina and Leyton 2009). Further, voluntary ethanol (EtOH) intake was increased in EtOH-preferring B6 mice, as well as EtOH-avoiding D2 mice, that were given prior passive EtOH exposure by injection or intragastric infusion (Camarini and Hodge 2004; Fidler et al. 2012). We examined the impact of prior MA exposure on levels of voluntary MA intake in the MADR mice using a no-choice, followed by two-bottle choice, MA drinking procedure.
Finally, co-morbid MA and alcohol use are prevalent (UN Office of Drugs and Crime 2015), but few preclinical studies have studied their combined use. Human data indicate that MA users also consume large amounts of alcohol, a prior history of alcohol use disorder is a major risk factor for MA addiction, alcohol drinking increases the chance of same-day MA use, and MA-dependent individuals who also use alcohol are at higher risk for treatment discontinuation and non-compliance (Brecht et al. 2005, 2007, 2008; Bujarski et al. 2014; Furr et al. 2000; Herbeck et al. 2013; O’Grady et al. 2008). In the current studies, we used MAHDR mice to examine EtOH intake and its impact on MA intake. We then tested the effect of R(+)-baclofen (BAC) on MA intake in MAHDR mice. BAC is a gamma-aminobutyric acid type B (GABAB) receptor agonist that has shown some promise in reducing EtOH use, although efficacy is likely subject to individual differences, and also the use of other drugs, including amphetamines (for reviews, see Agabio and Colombo 2014; Phillips and Reed 2014). Based on studies showing that operant responding for d-amphetamine and MA was reduced in response to BAC in rats (Brebner et al. 2005; Ranaldi and Poeggel 2002), we hypothesized that BAC would reduce MA intake in MAHDR mice.
2. MATERIALS AND METHODS
2.1. Animals
Male and female MAHDR and MALDR mice bred within the Veterans Affairs Portland Health Care System animal facility (Portland, OR) participated in these experiments. The MADR lines were derived from the F2 cross of the B6 and D2 strains that were tested in a two-bottle choice MA drinking procedure, as detailed in our published papers (Hitzemann et al. 2019; Shabani et al. 2011; Wheeler et al. 2009). Briefly, singly housed mice were offered two graduated volumetric drinking tubes that contained water on the first 2 days, then water vs. 20 mg/l MA for 4 days, and then water vs. 40 mg/l MA for 4 days. Mice had access to water for 24h/day, and to MA for 18h/day. The mice that consumed the highest and lowest average amounts of 40 mg/l MA were chosen to produce offspring that established the MAHDR and MALDR lines, respectively. Bidirectional selective breeding continued for 5 selection generations each, and we have repeated this selection 5 times at a 2-year interval, with virtually identical outcomes (see Hitzemann et al. 2019; Shabani et al. 2011; Wheeler et al. 2009). MAHDR and MALDR mice used in the current experiments were second or later litter offspring of the fifth selection generation from replicates 2, 4, or 5.
After weaning, all mice used in these studies were initially group-housed (2-5 per cage) in a common colony room in polycarbonate shoebox cages (28.5 x 17.5 x 12 cm) lined with Bed-o’Cobs bedding (The Andersons, Inc., Maumee, OH) and fitted with wire tops. Temperature in the colony room was 22±1 °C with a 12-h light:dark schedule and lights on at 0600 h. Mice were moved to an experiment room at least 2 weeks prior to study initiation to acclimate to the new room. Temperature in all experiment rooms was 22±1 °C, with a 12-h light:dark schedule. The time at which the lights turned on varied across experiments, occurring at 0400 to 0700 h, depending upon experimenter schedule. The one exception was for Experiment 5, during which mice were on a reverse 12-h light:dark schedule, with lights on at 2200 h. Upon initiation of each experiment, mice were individually housed in the same type of caging, and provided with a cotton fiber nestlet for enrichment. All mice had free access to laboratory rodent block food (PicoLab Laboratory Rodent Diet 5LOD, 4.5% fat content; Animal Specialties, Woodburn, OR) and tap water, except when specified.
2.2. Drugs and Reagents
(+)-MA hydrochloride (Sigma-Aldrich, St. Louis, MO, USA; Cat. No. M8750) was dissolved in tap water for oral consumption. R(+)-BAC hydrochloride was purchased from Sigma-Aldrich (Cat. No. G013) and dissolved in sterile .9% saline (Baxter Healthcare Corp., Deerfield, IL, USA) for intraperitoneal (IP) injection at a volume of 10 ml/kg body weight. We chose R(+)-BAC because it has been shown to be more potent than S(−)-BAC (Falch et al. 1986; Witczuk et al. 1980), and one lab demonstrated enantioselective effects of BAC, in which R(+)-BAC reduced EtOH intake, while S(−)-BAC increased EtOH intake (Kasten and Boehm 2014; Kasten et al. 2015).
2.3. Behavioral Procedures
Prior to drug access phases of each study, mice were singly housed and offered graduated volumetric drinking tubes that contained water to give them experience with drinking from ball-bearing sippers. For most studies, drinking solutions were offered in 25 ml tubes, and volume consumed from each tube was measured in ml (accuracy = .2 ml). The exception was for Experiment 5, during which 10 ml drinking tubes (accuracy = .1 ml) were used, as appropriate for the shorter 6h drinking period. EtOH and MA consumption volumes were converted to g/kg and mg/kg intake, respectively, based on body weights measured every other day, except in Experiment 2, during which mice were weighed every day. When water and MA were available, the relative positions of the drinking tubes were alternated every 2 days to account for side bias, unless specified.
2.3.1. Experiment 1: Impact of continuous vs. intermittent MA access on MA intake
Female MAHDR and MALDR mice were tested for MA consumption in a two-bottle choice MA drinking procedure during 18h or 24h MA access periods to determine whether MA intake differs when MA access is intermittent vs. continuous (see Table 1). The 12-h light:dark schedule was set for lights on at 0400 h. We have not found consistent sex differences in MA intake in our previous studies (Eastwood and Phillips 2014a; Harkness et al. 2015; Reed et al. 2018; Shabani et al. 2011, 2016; Stafford et al. 2019; Wheeler et al. 2009); females were used for this study because they were available. Mice were divided into 2 groups. Half of the mice had access to a water tube and an MA tube for 24h/day (continuous) and the other half had access to water for 24h/day and a MA tube for 18h/day (intermittent). For the intermittent access group, the MA tube was present beginning 3h before dark onset through 3h after dark offset. MA concentration was 20 mg/l for 4 days, and then 40 mg/l for 4 days; concentrations and number of days were consistent with procedures used during selective breeding. For the continuous access group, water and MA intake were measured every 24h, as well as for the 18h period during which the intermittent group had access to MA, in order to collect comparable total volume data in both groups. Data from the second and fourth day of access to each MA concentration were averaged to provide indices of MA intake (mg/kg) and volume (ml) consumed. These are the days after a tube position switch, when mice should be most familiar with the relative location of the water and MA tubes. These measures, MA concentrations, and the 18h/day access period were used for selective breeding of the MADR lines (Hitzemann et al., 2019; Shabani et al., 2011; Wheeler et al., 2009). Mice were from the second replicate set of MADR lines. Final group sizes were: MAHDR-18h group, n=11; MAHDR-24h group, n=11; MALDR-18h group, n=10 (after exclusion of data from 1 mouse due to outlier status of >2.5 SD from the mean for MA intake from the 40 mg/l MA concentration); MALDR-24h group, n=12.
Table 1.
Experiment 1 timeline
| Group | Days 1-4 | Days 5-8 |
|---|---|---|
| Intermittent access | water vs. 20 mg/l MA (18h/day) | water vs. 40 mg/l MA (18h/day) |
| Continuous access | water vs. 20 mg/l MA (24h/day) | water vs. 40 mg/l MA (24h/day) |
MA, methamphetamine
2.3.2. Experiment 2: Impact of forced MA consumption on subsequent voluntary MA intake
Male and female MAHDR and MALDR mice were tested for MA intake in a no-choice MA drinking procedure (Phase I) followed by a two-bottle choice MA drinking procedure (Phase II) to determine if forced MA consumption alters voluntary MA intake (see Table 2). The 12-h light:dark schedule was set for lights on at 0600 h for the entirety of this two-phase experiment. During Phase I, mice were given 24h access to 2 water tubes (water control group) or 2 MA tubes for 12 days. Three independent groups of mice were offered different MA concentrations across days; group 1: 10 mg/l MA for 8 days, then 20 mg/l MA for 4 days, group 2: 10 mg/l MA for 4 days, then 20 mg/l for 8 days, and group 3: 20 mg/l MA for all 12 days. This allowed us to examine the potential impact of retaining the same MA concentration and phasing in higher concentrations of MA over time on MA intake under this forced condition, and on voluntary MA intake in Phase II. During Phase II, starting on Day 13, all groups of mice had 24h access to a water tube and a 20 mg/l MA tube for 8 days. The 24h access period was used to maintain daily tube reading and manipulation procedures from Phase I. The lower MA concentration was offered, because in two-bottle choice studies, the MALDR mice exhibit strong avoidance of MA solutions and we considered that a potential increase in MA intake in MALDR mice would be more likely to be detected for a weaker MA concentration. MA intake and total volume consumed were indexed for each 4-day period as described for Experiment 1. An exception, necessitated by data loss due to experimenter error, was for the day 5-8 period (time period 2; see Table 2). Instead of the average of days 6 and 8, intake and total volume consumed for this period were represented by day 6 data alone. This is further justified in Results. Mice were from MADR replicate sets 4 and 5, and tested in 3 cohorts of 24-44 mice/cohort. For the MAHDR line, final group sizes were: water group, n=6 female and 5 male; 10-10-20 MA group, n=6/sex; 10-20-20 MA group, n=6 female and 7 male; 20-20-20 MA group, n=5 female and 6 male. For the MALDR line, the final group sizes were: water group, n=7 female and 5 male; 10-10-20 MA group, n=5/sex; 10-20-20 MA group, n=5/sex; 20-20-20 MA group, n=5/sex. Data were lost due to drinking tube leaks or incorrect measurements (i.e., the recorded measurement indicated an increase in volume, rather than a decrease) from 1 MAHDR 10-20-20 MA group female and 2 MALDR females, 1 each from the 10-10-20 and 20-20-20 MA groups. No data were included from these mice in the analyses and they are not included in the final group sizes.
Table 2.
Experiment 2 timeline
| Group | Phase I: Day 1-4 | Phase I: Day 5-8 | Phase I: Day 9-12 | Phase II: Day 13-20 |
|---|---|---|---|---|
| Water | water | water | water | water vs. 20 mg/l MA |
| 10-10-20 | 10 mg/l MA | 10 mg/l MA | 20 mg/l MA | water vs. 20 mg/l MA |
| 10-20-20 | 10 mg/l MA | 20 mg/l MA | 20 mg/l MA | water vs. 20 mg/l MA |
| 20-20-20 | 20 mg/l MA | 20 mg/l MA | 20 mg/l MA | water vs. 20 mg/l MA |
MA, methamphetamine
2.3.3. Experiment 3: Three-bottle choice EtOH, MA, and EtOH+MA consumption and the impact on subsequent voluntary MA intake
Male and female MAHDR mice were tested in a 2-phase study (see Table 3). The 12-h light:dark schedule was set for lights on at 0700 h for the entirety of this two-phase experiment. In Phase I, half of the mice had access to 3 water tubes (water control), and the other half (EtOH/MA) had access to 3 drug-containing tubes: an EtOH tube, a MA tube, and an EtOH+MA tube. Drug access was for 18h/day, and all mice had access to a single water tube for the remaining 6h of each day (beginning 3 hours after lights on). The concentration of EtOH was increased every 4 days, from 3 to 6 to 10%, a procedure commonly used to reduce initial aversion that is more likely with higher EtOH concentrations (Crabbe et al. 2011; Elmer et al. 1986). A constant concentration of 20 mg/l MA was offered. The relative positions of the EtOH, MA, and EtOH+MA tubes were counterbalanced across mice, but remained constant for each mouse for the duration of Phase I. During Phase II (days 13-24), all mice were tested for two- bottle choice MA intake, with an 18h/day MA access period and increasing concentrations of MA every 4 days, from 20 to 40 to 80 mg/l. These concentrations were previously used to examine the effect of MA concentration on intake in MAHDR mice (Shabani et al. 2016). EtOH and MA intake and total volume consumed were indexed for each 4-day period as described for Experiment 1. Mice were from the fifth replicate set of MADR lines. Final group sizes were: water group, n=8/sex; EtOH/MA group, n=7 male and 8 female. Data were lost due to a drinking tube leak for 1 male mouse from the EtOH/MA group. No data were included from this mouse in the analyses and it is not included in the final group size.
Table 3.
Experiment 3 timeline
| Group | Phase I: Day 1-4 |
Phase I: Day 5-8 |
Phase I: Day 9-12 |
Phase II: Day 13-16 |
Phase II: Day 17-20 |
Phase II: Day 21-24 |
|---|---|---|---|---|---|---|
| Water | 3 tubes of water | 3 tubes of water | 3 tubes of water | water vs. 20 mg/l MA | water vs. 40 mg/l MA | water vs. 80 mg/l MA |
| EtOH/MA | 3% EtOH vs. 20 mg/l MA vs. 3% EtOH+ 20 mg/l MA | 6% EtOH vs. 20 mg/l MA vs. 6% EtOH+ 20 mg/l MA | 10% EtOH vs. 20 mg/l MA vs. 10% EtOH+ 20 mg/l MA | water vs. 20 mg/l MA | water vs. 40 mg/l MA | water vs. 80 mg/l MA |
EtOH, ethanol; MA, methamphetamine
2.3.4. Experiment 4: Two-bottle choice water vs. EtOH, MA, or EtOH+MA consumption and the impact on subsequent voluntary MA intake
This study examined the independent effects of prior voluntary EtOH, MA or EtOH+MA consumption on subsequent voluntary MA intake (see Table 4). The 12-h light:dark schedule was set for lights on at 0600 h for the entirety of this two-phase experiment. Independent groups of male and female MAHDR mice were tested in Phase I in a two-bottle choice drinking procedure, in which mice were offered 2 water tubes (group 1), water vs. EtOH (group 2), water vs. MA (group 3), or water vs. EtOH+MA (group 4). In Phase II, two-bottle choice MA drinking was measured in all mice. The intent was to 1) determine intake of the different types of drug solutions when offered vs. water, rather than vs. each other, and 2) determine the impact of the intake of each drug solution within the independent groups on subsequent MA intake. Mice were from the fifth replicate set of MADR lines. Final group sizes were: water group, n=6 female and 5 male; EtOH group, n=6/sex; MA group, n=6/sex; EtOH+MA group, n=6/sex. Data were lost due to drinking tube leaks from 2 male mice (1 each from the water and EtOH/MA groups). No data were included from these mice in the analyses and they are not included in the final group sizes.
Table 4.
Experiment 4 timeline
| Group | Phase I: Day 1-4 |
Phase I: Day 5-8 |
Phase I: Day 9-12 |
Phase II: Day 13-16 |
Phase II: Day 17-20 |
Phase II: Day 21-24 |
|---|---|---|---|---|---|---|
| Water | 2 tubes of water | 2 tubes of water | 2 tubes of water | water vs. 20 mg/l MA | water vs. 40 mg/l MA | water vs. 80 mg/l MA |
| EtOH | water vs. 3% EtOH | water vs. 6% EtOH | water vs. 10% EtOH | water vs. 20 mg/l MA | water vs. 40 mg/l MA | water vs. 80 mg/l MA |
| MA | water vs. 20 mg/l MA | water vs. 20 mg/l MA | water vs. 20 mg/l MA | water vs. 20 mg/l MA | water vs. 40 mg/l MA | water vs. 80 mg/l MA |
| EtOH+MA | water vs. 3% EtOH+ 20 mg/l MA | water vs. 6% EtOH+ 20 mg/l MA | water vs. 10% EtOH+ 20 mg/l MA | water vs. 20 mg/l MA | water vs. 40 mg/l MA | water vs. 80 mg/l MA |
EtOH, ethanol; MA, methamphetamine
2.3.5. Experiment 5: Effect of BAC on established voluntary MA intake and locomotor activity
The study timeline is summarized in Table 5. Mice were maintained on a reverse 12-h light:dark schedule with lights on at 2200 h. Voluntary MA consumption was established in male and female MAHDR mice using a modified two-bottle choice MA drinking procedure, and then mice were pretreated with saline or BAC to determine if GABAB receptor activation alters MA intake. Mice were offered a water tube and a MA tube for 6h/day, during the initial 6h of their dark phase. To assess MA intake in intervals, so that the time course of BAC effects could be evaluated, tube volumes were read every 2 hours during the 6h period on each day. Mice had access to a single water tube for the remaining 18h of each day. The MA concentration was 20 mg/l for 4 days, and then 40 mg/l for 4 days, and then on the next 2 days, mice were pretreated with an IP injection of saline, immediately before 40 mg/l MA access. The first saline pretreatment day served to acclimate mice to handling and injection; the second day provided baseline MA intake data following saline injection, and intake data from this day were used for analysis of changes in MA intake. Mice were assigned to BAC dose groups based on MA intake after saline injection, so that baseline MA intake levels would be matched across groups. BAC doses given on the next 2 days were 0, 2.5, 5, or 7.5 mg/kg. These doses were chosen based on the dose range effective at reducing self-administration of MA and d-amphetamine in rats (Brebner et al. 2005; Ranaldi and Poeggel 2002), as well as studies showing that these doses had non-significant or waning effects on locomotor activity in B6 or D2 mice (Bortolato et al. 2010; Broadbent and Harless 1999; Chester and Cunningham 1999; Korkosz et al. 2006). Finally, mice were given a final day of MA access in the absence of any pretreatment to test for potential residual effects of prior treatment. The absence of injection also allowed for determination of possible prior saline injection effects on MA intake. The relative positions of the water and MA tubes were counterbalanced across mice, but tube positions remained constant for individual mice for the duration of the experiment so that location would remain familiar. During the 6-h MA access period, MA intake (mg/kg) and total volume consumed (ml) were recorded every 2 hours. These methods are consistent with our prior studies of drug pretreatment effects on MA intake (Eastwood and Phillips 2014a; Eastwood et al., 2018). Mice were MAHDR mice from the fifth replicate set of MADR lines, and tested in 2 cohorts of 47-48 mice/cohort. Final group sizes were: 0 mg/kg BAC group, n=11/sex; 2.5 mg/kg BAC group, n=11/sex; 5 mg/kg BAC group, n=11 female and 13 male; 7.5 mg/kg BAC group, n=11/sex. Data were lost due to a drinking tube leak for 1 male mouse (dose group 0 mg/kg), misinjection for 2 males (1 each from dose groups 2.5 and 7.5 mg/kg), and for health reasons of undetermined origin for 2 males (1 from dose group 7.5 mg/kg, and 1 before dose groups were assigned). No data were included from these mice in the analyses and they are not included in the final group sizes.
Table 5.
Experiment 5 timeline
| Group (BAC dose) |
Day 1-4 | Day 5-8 | Day 9-10 | Day 11-12 | Day 13 |
|---|---|---|---|---|---|
| 0 mg/kg | water vs. 20 mg/l MA | water vs. 40 mg/l MA | Saline treatment then water vs. 40 mg/l MA | Saline treatment then water vs. 40 mg/l MA | No treatment water vs. 40 mg/l MA |
| 2.5 mg/kg | water vs. 20 mg/l MA | water vs. 40 mg/l MA | Saline treatment then water vs. 40 mg/l MA | 2.5 mg/kg BAC treatment then water vs. 40 mg/l MA | No treatment water vs. 40 mg/l MA |
| 5 mg/kg | water vs. 20 mg/l MA | water vs. 40 mg/l MA | Saline treatment then water vs. 40 mg/l MA | 5 mg/kg BAC treatment then water vs. 40 mg/l MA | No treatment water vs. 40 mg/l MA |
| 7.5 mg/kg | water vs. 20 mg/l MA | water vs. 40 mg/l MA | Saline treatment then water vs. 40 mg/l MA | 7.5 mg/kg BAC treatment then water vs. 40 mg/l MA | No treatment water vs. 40 mg/l MA |
BAC, R(+)-baclofen; MA, methamphetamine
Observations of the mice during the drinking study suggested potential behavioral depressant effects of BAC that might have interfered with drinking. Therefore, 82% of the mice from the drinking study were provided free access to water for several days (3-9) to allow complete clearance of MA and BAC, and then were tested for locomotor effects of BAC using automated activity monitors (40 cm W x 40 cm L x 30 cm H; Omnitech Electronics, Columbus, OH). The remaining 18% (16 mice) were not tested due to an unexpected equipment scheduling conflict. Each monitor was enclosed in an Environmental Control Chamber (Accuscan, Columbus, OH) equipped with a fan to provide ventilation and shielding from external noise, and 3.3 Watt incandescent light bulb that was illuminated during testing. Activity was measured using photocell beams located 2 cm above the floor, and beam interruptions were converted into horizontal distance (cm) using Fusion software (Omnitech Electronics). A 2-day protocol was used, during which locomotor activity was tested for 6h, starting at the same time MA access was given during the drinking experiment. The 3-9 day range between the end of MA drinking and testing was necessary, because we were limited by the number of test chambers (16) and had to test mice in 5 cohorts (13-16 mice/cohort). On activity test day 1, all mice received an IP injection of saline immediately prior to activity testing. On day 2, each mouse was treated with the same dose of BAC (0, 2.5, 5, or 7.5 mg/kg) that it had received during MA drinking. Mice were MAHDR mice from the fifth replicate set of MADR lines, and final group sizes were: 0 mg/kg BAC group, n=9 female and 8 male; 2.5 mg/kg BAC group, n=9 female and 8 male; 5 mg/kg BAC group, n=9 female and 10 male; 7.5 mg/kg BAC group, n=8 female and 9 male, representing 78% of the mice tested in the drinking phase of the study. Data were lost due to misinjection for 2 female mice (both from dose group 7.5 mg/kg) and for health reasons of undetermined origin for 2 male mice (1 from each of dose groups 2.5 and 5 mg/kg). No data were included from these mice in the analyses and they are not included in the final group sizes.
2.4. Statistical Analysis
Statistica 13.3 (TIBCO Software, Inc, Palo Alto, CA, USA) was used for statistical analyses. MA intake (mg/kg), EtOH intake (g/kg), preference ratio, and total volume consumed (ml) data were analyzed using repeated measures ANOVA, with drug concentration, day, and time as possible repeated measures, and line, sex, group, drug tube, and BAC dose as possible independent variables. Locomotor data were analyzed using repeated measures ANOVA, with day and time as repeated measures, and sex and BAC dose as independent variables. Significant main effects were interpreted using Newman-Keuls post hoc tests when appropriate. 3-way interactions were simplified by ANOVAs at the level of a particular factor, and 2-way interactions were followed up with simple main effects analysis and Newman-Keuls post hoc tests when appropriate. In Experiment 2, a 2-tailed t-test was used for a priori mean comparisons. Effects were considered significant when p≤05. Sample sizes for these studies were based on previous MA drinking studies in the MADR lines of mice (e.g., Eastwood and Phillips 2014a; Reed et al. 2018; Shabani et al. 2016). To reduce animal usage, data were analyzed when an equal number of each sex had been tested, accumulating to a total group size adequate for detecting group differences (based on known error variance for these traits from past studies; i.e., a total group size of 10-12). When significant sex effects or statistical trends toward sex effects were found, additional mice were tested. With the exception of Experiment 3, there were no significant sex differences, nor statistical trends toward sex differences. Group size for each sex was increased for Experiment 3.
3. RESULTS
3.1. Experiment 1: Impact of continuous vs. intermittent MA access on MA intake
MA intake data for MAHDR and MALDR mice offered intermittent (18h/day) or continuous (24h/day) access to MA are presented in Fig. 1a. There was a significant MA concentration x line x MA access period interaction (F(1,40)=25.8, p<.001). The 3-way interaction was first examined for the effects of line and MA access period within each MA concentration. When 20 mg/l MA was offered, there was a significant main effect of line (F(1,40)=89.3, p<.001), with MAHDR mice consuming significantly more MA than MALDR mice, regardless of MA access period. When 40 mg/l MA was offered, there was a significant line x MA access period interaction (F(1,40)=20.9, p<.001), with MAHDR mice of both the intermittent and continuous MA access groups consuming significantly more MA than MALDR mice, and additionally, consuming more MA when it was offered intermittently vs. continuously. When the impacts of MA concentration and access period were examined within each of the MADR lines, there was a significant interaction (F(1,20)=27.9, p<.001) in the MAHDR line, with greater MA intake when the MA concentration was increased only in the intermittent access group. For the MALDR line, there was a main effect of MA concentration (F(1,20)=8.7, p=.008), with reduced MA intake when the 40 mg/l concentration was offered, compared to the 20 mg/l concentration.
Fig. 1.
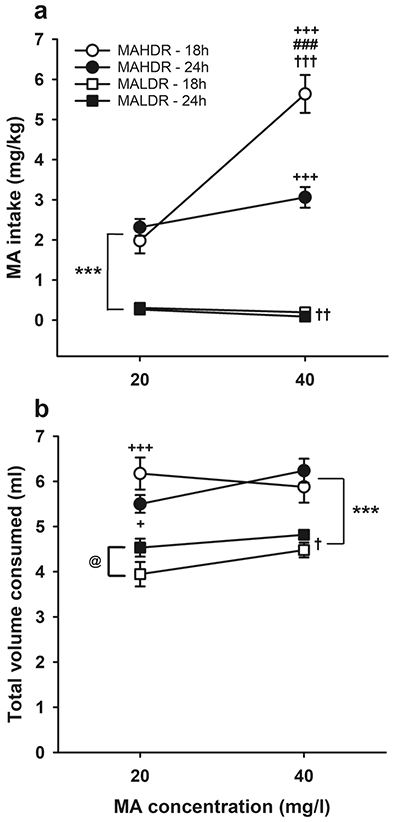
Intermittent MA access results in an escalation of MA intake in MAHDR mice. (a) MA intake and (b) total volume consumed, when MA was offered vs. water for 18h/day (intermittent) or 24h/day (continuous). Data are means ± SEM. MAHDR-18h group, n=11; MAHDR-24h group, n=11; MALDR-18h group, n=10; MALDR-24h group, n=12. ***p<.001 for the main effect of line; +p<.05, +++p<.001 vs. MALDR mice of the same MA access time at the indicated MA concentration; ###p<.001 vs. 24h within the MAHDR line; †p<.05, ††p<.01, †††p<.001 for the effect of concentration; @p<.05 for the main effect of MA access time within the MALDR line, regardless of MA concentration. MA, methamphetamine; MAHDR, MA high drinking mice; MALDR, MA low drinking mice
Total fluid consumption was examined during the time that MA was available (Fig. 1b). For 18h or 24h total fluid consumption (depending on group) from the water and MA tubes, there was a significant MA concentration x line x MA access period interaction (F(1,40)=4.4, p=.04). Follow up analyses identified a significant line x MA access period interaction (F(1,40)=5.8, p=.02) for the 20 mg/l MA concentration. MAHDR mice consumed significantly more total fluid than MALDR mice under both MA access conditions, but the mean difference was larger for intermittent than for continuous access (2.2 ml greater for MAHDR vs. MALDR vs. 1 ml greater for MAHDR vs. MALDR, respectively). For the 40 mg/l MA concentration, there was only a significant main effect of line (F(1,40)=34.4, p<.001), with MAHDR mice consuming significantly more total fluid than MALDR mice, independent of MA access period. When data were examined for the effects of MA concentration and access period within each of the MADR lines, there were no significant results for MAHDR mice. For MALDR mice, there were significant main effects of MA access period (F(1,20)=4.9, p=.04) and MA concentration (F(1,20)=5.7, p=.03), with greater volume consumed at the higher MA concentration, and during continuous access. Similar results were obtained when data for the 18h access period for the continuous access group were compared to the 18h access period for the intermittent group (data not shown).
3.2. Experiment 2: Impact of forced MA consumption on subsequent voluntary MA intake
Due to the inclusion of the water drinking group in this study, which was critical for addressing the question of whether prior forced MA consumption vs. water consumption would impact subsequent MA intake, data from all 4 groups could not be included in an analysis across both phases of the study (i.e., all values for MA intake are 0 for the water group). Therefore, data were examined separately for each phase and then a comparison across phases was conducted that included only the MA groups. MA intake and total volume consumed for time period 2 are represented by day 6 data instead of the average of days 6 and 8, due to lost data on day 8 in one cohort of mice (n=44). For the other two cohorts of mice (n=22 and n=23), day 8 data were averaged with day 6, as is our usual practice. To ascertain whether day 6 provided a strong estimate of the average of days 6 and 8, data from these 2 cohorts were collapsed and examined for correlation between the single day and averaged values. The correlation was r=.94 (p<.00001), indicating that MA intake based on the average of days 6 and 8 was well represented by MA intake on day 6.
3.2.1. Phase I – Forced MA Drinking
MA intake data for MAHDR and MALDR mice during the 12-day course of the no-choice (forced) phase of the study (periods 1-3) are presented in Fig. 2a,b (data to the left of the vertical dashed line). The water group is included in Fig. 2a,b for illustration only, since they did not consume MA during Phase I. Mice in the 3 MA groups had increasing MA concentrations in their drinking water or a constant 20 mg/l concentration during the 3 time periods, as described in Table 2. There was a significant time period x group x line interaction (F(4,108)=3.4, p=.01) for MA intake, but no significant effects of sex. When data were examined for the effects of time period and line within each group, there was a significant time period x line interaction for the 10-10-20 (p<.001) and 10-20-20 (p=.02) groups, and only a main effect of line (p=.002) for the 20-20-20 group. In all cases, MAHDR mice consumed more MA than MALDR mice (all ps<.02; compare data in Fig. 2a to Fig. 2b).
Fig. 2.
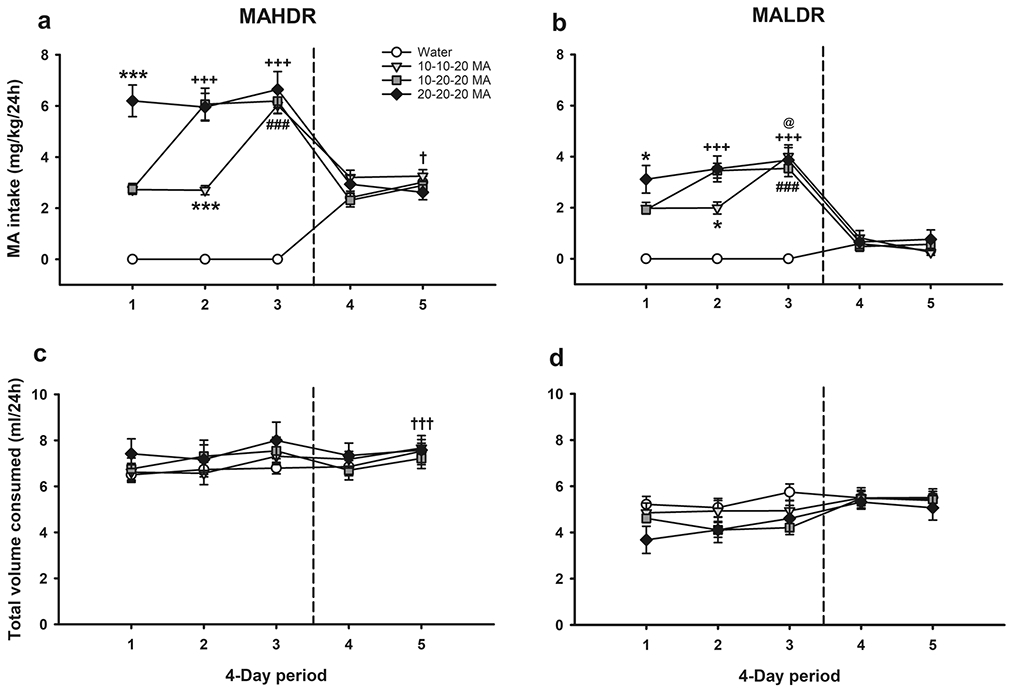
Forced MA consumption had no significant effect on voluntary MA intake in MAHDR or MALDR mice. MA intake data for (a) MAHDR and (b) MALDR mice under the no-choice condition are illustrated to the left of the vertical dashed line and for the two-bottle choice condition to the right of the vertical dashed line. Total volume consumed for (c) MAHDR and (d) MALDR mice under the no-choice condition (left of the vertical dashed line) and the two-bottle choice condition (right of the vertical dashed line). Data are shown as 5, 4-day periods, represented along the x-axis. Periods 1-3 represent specific MA concentrations (i.e., 10, 10 and 20 mg/l in the 10-10-20 group; 10, 20 and 20 mg/l in the 10-20-20 group; and 20, 20 and 20 mg/l in the 20-20-20 group); periods 4-5 represent two-bottle choice for water vs. 20 mg/l MA in comparable 4-day periods. Data are means ± SEM for the sexes combined, as there were no sex differences. MAHDR water group, n=11; MAHDR 10-10-20 MA group, n=12; MAHDR 10-20-20 MA group, n=13; MAHDR 20-20-20 group, n=11; MALDR water group, n=12; MALDR 10-10-20 MA group, n=10; MALDR 10-20-20 MA group, n=10; MALDR 20-20-20 group, n=10. *p<.05, ***p<.001 for the difference from the other 2 MA groups at the indicated time period; +++p<.001 vs. time period 1 for group 10-20-20 MA; ###p<.001 vs. time periods 1 and 2 for group 10-10-20 MA; †p<.05 vs. time period 4 for the water and 10-20-20 MA groups; †††p<.001 vs. time period 4 for the main effect of time; @p<.05 vs. time period 1 for group 20-20-20 MA. MA, methamphetamine; MAHDR, MA high drinking mice; MALDR, MA low drinking mice
Next, data were examined within each line. For the MAHDR line (Fig. 2a), there was a significant time period x group interaction (F(4,66)=15.1, p<.001). The effect of group was significant for the first 2 time periods (ps<.001). During both periods, MA intake was significantly greater in mice offered 20 mg/l MA (20-20-20 group), compared to mice offered 10 mg/l MA (10-20 and 10-20-20 groups). There was also a significant effect of time period within each of the 10-10-20 and 10-20-20 (ps<.001) groups, with escalation of MA intake when MA concentration was increased from 10 to 20 mg/l.
Results for the MALDR line were similar (Fig. 2b). There was a significant time period x group interaction (F(4,54)=12.2, p<.001), and the effect of group was significant for the first 2 time periods (ps<.05). During both time periods, MA intake was significantly greater in mice offered 20 mg/l MA (20-20-20 group), compared to mice offered 10 mg/l MA (10-10-20 and 10-20-20 groups), with a strong statistical trend for greater intake in the 20-20-20, compared to the 10-20-20 group for the first time period (p=.06). There was also a significant effect of time period within each of the 10-10-20 (p<.001), 10-20-20 (p<.001) and 20-20-20 (p=.01) groups. MA intake escalated when MA concentration was increased from 10 to 20 mg/l in the 10-10-20 and 10-20-20 groups. There was a smaller but significant increase in MA intake between the first and third time period in the 20-20-20 group.
Total fluid consumption data are shown in Fig. 2c,d (data to the left of the vertical dashed line). There were significant main effects of line (F(1,73)=58.8, p<.001) and time period (F(2,146)=5.5, p=.005), with greater volume consumed by MAHDR than MALDR mice (compare data in Fig. 2c to Fig. 2d), and more volume consumed overall during the third time period, compared to the first two, regardless of group or line.
3.2.2. Phase II – Two-bottle Choice MA Drinking
Voluntary MA intake was examined in Phase II, following the Phase I period of no-choice MA or water consumption. Data are presented in Fig. 2a,b (data to the right of the vertical dashed line). There was a significant time period x group x line interaction (F(3,73)=3.4, p=.02) for MA intake. First, data were examined for the effects of time period and line within each group. There was a main effect of line in each case (ps<.001), with MAHDR mice consuming more MA than MALDR mice (compare data in Fig. 2a to Fig. 2b). Data were next examined within each line. For MAHDR mice, there was a significant time period x group interaction (F(3,43)=3.1, p=.04), with greater MA intake during the final drinking period of Phase II than during the initial drinking period for the water and 10-20-20 groups. For MALDR mice, there were no significant statistical findings for Phase II.
For total fluid consumption (Fig. 2c-d; data to the right of the vertical dashed line), there was a significant time period x sex interaction (F(1,73)=9.3, p=.003). For male mice, total fluid consumption was greater during the final drinking period, compared to the initial period by .5 ml (p<.001), but there was no difference for female mice. Total fluid consumption was greater by .8 ml for males than females for the final (p=.04), but not initial period. Sex did not interact with line, and thus did not determine the pattern of fluid consumption across time for the MAHDR and MALDR mice, so the data presented are for the sexes combined. There was, however, a significant time period x line interaction (F(1,73)=12.7, p<.001), with MAHDR mice consuming more total fluid than MALDR mice during both periods of Phase II (ps<.001; compare data in Fig. 2c to Fig. 2d). In addition, total fluid consumption was greater during the final drinking period of Phase II, compared to the initial period, in MAHDR mice only.
3.2.3. Transition from forced MA drinking to two-bottle choice MA drinking
To address whether prior no-choice MA intake impacted MA intake during two-bottle choice, we compared MA intake during the last no-choice drinking period (the third drinking period in Fig. 2a,b) to the first two-bottle choice drinking period (the fourth drinking period in Fig. 2a,b). Because there were no differences among the MA groups during either of these drinking periods, we performed a 2-tailed t-test for each line with data collapsed on group. As is apparent in Fig. 2a,b, for both MAHDR and MALDR mice, there was a significant reduction in MA intake, corresponding with the transition from no-choice to two-bottle choice (MAHDR: t(35)=13.8, p<.001; MALDR: t(29)=17.6, p<.001).
3.3. Experiment 3: Three-bottle choice EtOH, MA, and EtOH+MA consumption and the impact on subsequent voluntary MA intake
Phase I and II data were examined in separate analyses, to address the question of whether prior consumption of MA, EtOH and EtOH+MA, resulted in differences among groups in subsequent MA intake.
3.3.1. Phase I – Three-bottle Choice Drinking
During Phase I, mice had simultaneous access to drinking tubes containing EtOH, MA, and EtOH+MA (see Table 3). For EtOH intake from each of the EtOH and EtOH+MA tubes (Fig. 3a), there was a significant EtOH concentration x sex interaction (F(2,52)=3.4, p=.04). There was a significant effect of EtOH concentration in females (p<.001), but not in males. For females, EtOH intake was significantly greater from the 6 and 10% EtOH solutions, compared to the 3% solution. Further, female mice consumed significantly more EtOH at all concentrations compared to male mice. There were no significant main or interaction effects involving tube type (EtOH vs. EtOH+MA). Total EtOH intake from the 2 EtOH-containing tubes is shown in Fig. 3b. There were significant main effects of EtOH concentration (F(2,26)=11.1, p<.001) and sex (F(1,13)=7, p=.02). EtOH intake was greater for the 6% and 10% solutions, compared to the 3% solution, and females consumed more EtOH than males.
Fig. 3.
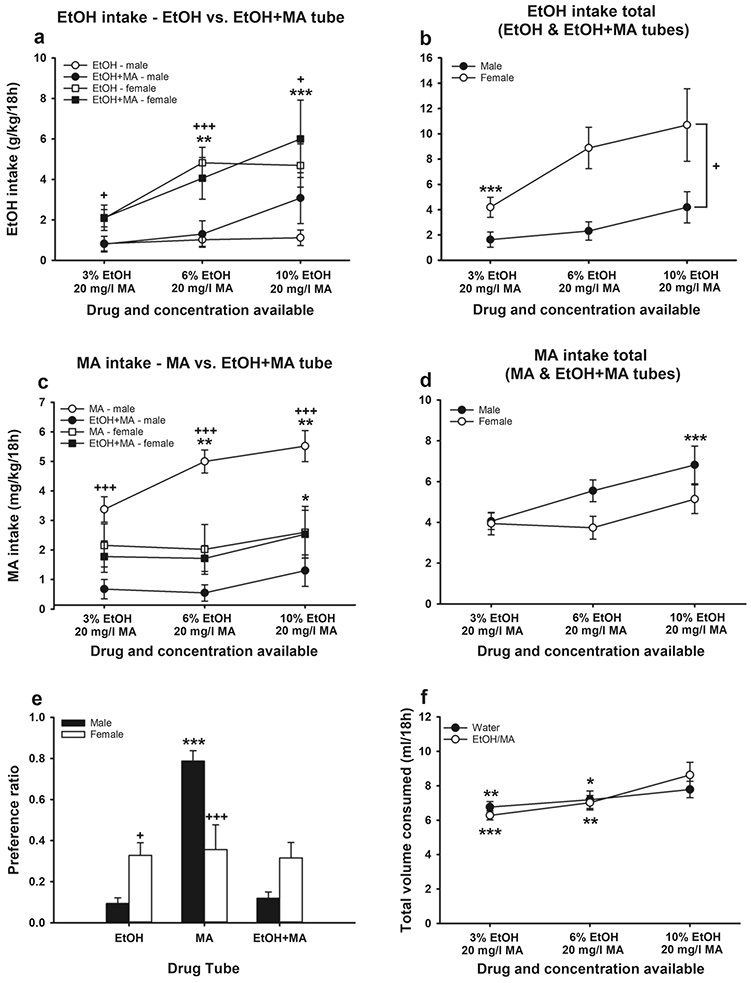
The effect on MA intake and preference of simultaneous access to EtOH, MA, and EtOH+MA in a three-bottle choice drinking procedure is sex-dependent in MAHDR mice. (a) EtOH intake from the EtOH and EtOH+MA tubes. **p<.01, ***p<.001 vs. 3% EtOH/20 mg/l MA in females, collapsed on tube type; +p<.05, +++p<.001 vs. males at the indicated concentration, collapsed on tube type. (b) Total EtOH intake from the EtOH and EtOH+MA tubes. ***p<.001 vs. the other 2 EtOH concentrations, for the sexes combined; +p<.05 for the main effect of sex. (c) MA intake from the MA and EtOH+MA tubes. *p<.05 vs. 3 and 6% EtOH, for females collapsed on tube type; **p<.01 vs. 3% EtOH in males for intake from the MA tube; +++p<.001 vs. EtOH+MA for males. (d) Total MA intake from the MA and EtOH+MA tubes. ***p<.001 vs. the other 2 EtOH concentrations, for the sexes combined. (e) Preference ratio for the EtOH, MA, and EtOH+MA tubes. ***p<.001 vs. EtOH and EtOH+MA for males; +p<.05, +++p<.001 vs. males. (f) Total volume consumed by mice offered 3 drug tubes (EtOH, MA, and EtOH+MA) or 3 water tubes. *p<.05, **p<.01, ***p<.001 vs. 10% EtOH. Data are means ± SEM. Water group, n=8/sex; EtOH/MA group, n=7 male, 8 female. EtOH, ethanol; MA, methamphetamine
For MA intake from each of the MA and EtOH+MA tubes during Phase I (Fig. 3c), there was a significant EtOH concentration x sex x tube type interaction (F(2,52)=3.3, p=.047). Data were examined for the effects of concentration and tube type, within each sex. For male mice, there was a significant EtOH concentration x tube type interaction (F(2,24)=4.1, p=.03). There was a significant effect of EtOH concentration for MA intake from the MA tube (p<.001), but not from the EtOH+MA tube. Males consumed more MA from the MA tube when the EtOH concentration in the EtOH+MA tube was 6% or 10%, compared to when it was 3%. Further, MA intake was reduced when EtOH was present in the MA tube at all EtOH concentrations. For females (Fig. 3c), there was a significant main effect of EtOH concentration (F(2,28)=4.5, p=.02). Mean MA intake was greater when the EtOH concentration was 10%, compared to 3 and 6%, independent of tube. For total MA intake from the 2 MA-containing tubes (Fig. 3d), there were no significant outcomes involving sex, but there was a significant main effect of EtOH concentration (F(2,26)=13.3, p<.001), with more MA consumed when the EtOH concentration was 10%, compared to 3 and 6%.
To compare our results to the results of a previous study that found B6 mice preferred EtOH+MA over EtOH or MA alone (Fultz et al. 2017), preference ratios were calculated (volume consumed from each tube divided by total volume consumed from all tubes). There was a significant tube type x sex interaction (F(2,39)=13.4, p<.001), but no significant effects involving EtOH concentration; therefore, data are presented collapsed on concentration (Fig. 3e). There was no significant effect of tube type in female mice; however, male mice exhibited significantly greater preference for the MA tube, compared to the EtOH and EtOH+MA tubes (p<.001). Compared to males, females had a significantly larger preference ratio for the EtOH tube, with a strong statistical trend for a larger preference ratio for the EtOH+MA tube (p=.06), and a significantly smaller preference ratio for the MA tube.
Total fluid consumption from the 3 tubes was examined, comparing mice that had access to tubes containing drugs vs. mice that had access only to water (Fig. 3f). There was a significant main effect of sex (F(1,27)=5.2, p=03), associated with less total fluid consumed by females, compared to males (mean differenc=.3 ml). Sex did not interact with time (i.e., EtOH concentration in the drug group) or group (drug vs. water) and thus, data are presented for the sexes combined. There was a significant time x group interaction (F(2,54)=4.1, p=.02) and a significant effect of time was found for both the water (p=.02) and the drug (p<.001) groups. Post-hoc mean comparisons indicated that total fluid consumption was greater during the third time period, whether the mice had access to drug or water, compared to the initial 2 periods, but there were no significant differences in total fluid consumed between the drug and water groups.
3.3.2. Phase II - Two-bottle Choice MA Drinking
MA was offered vs. water during two-bottle choice drinking Phase II, beginning the day after the completion of Phase I (see Table 3), to determine if prior access to EtOH/MA impacted voluntary MA intake, compared to access to water only. MA intake data are presented in Fig. 4a. There was a significant main effect of MA concentration (F(2,50)=114.1, p<.001), with increasing MA intake across increasing concentrations. However, intake was independent of Phase I exposure group and sex. There were no significant differences in total fluid consumption from the water and MA tubes during Phase II (Fig. 4b).
Fig. 4.
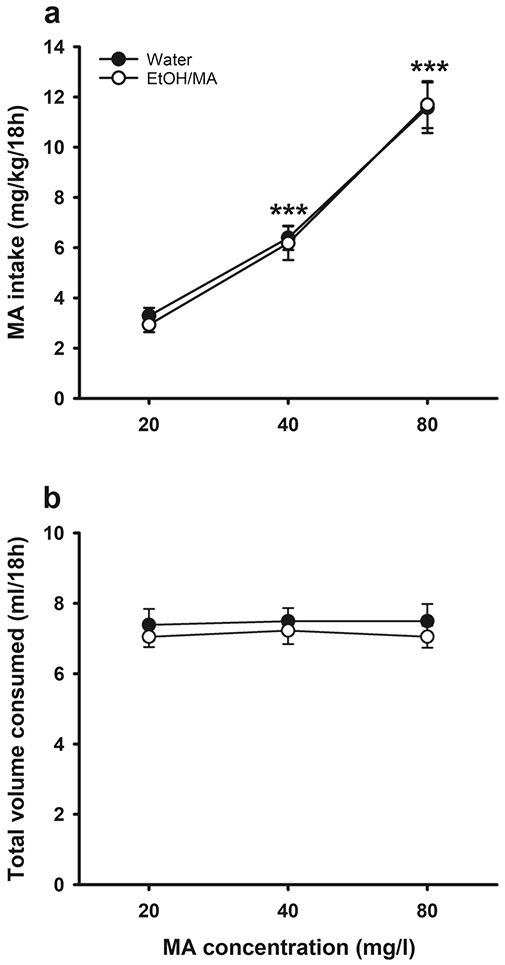
Prior consumption of EtOH and MA in a three-bottle choice procedure had no effect on subsequent voluntary MA intake in MAHDR mice. (a) MA intake and (b) total volume consumed, when MA was offered vs. water at concentrations of 20, 40, or 80 mg/l after previous three-bottle water or three-bottle EtOH, MA, and EtOH+MA consumption. Data are means ± SEM for the sexes combined, as there were no sex differences. Water group, n=16; EtOH/MA group, n=15. ***p<.001 vs. previous MA concentration, collapsed on group. EtOH, ethanol; MA, methamphetamine
3.4. Experiment 4: Two-bottle choice water vs. EtOH, MA, or EtOH+MA drinking and the impact on subsequent voluntary MA intake
3.4.1. Phase I - Two-Bottle Choice EtOH/MA Drinking
Phase I of this study examined EtOH and MA intakes in MAHDR mice when each drug was independently offered vs. water and when EtOH+MA was offered vs. water (see Table 4). There were no significant effects of sex, therefore, data for Phase I are presented in Fig. 5 for the sexes combined. For EtOH intake (Fig. 5a), there was a significant main effect of EtOH concentration (F(2,40)=5.7, p=.007). Intake was greater when the 10% concentration was offered, compared to when 3% was offered, regardless of whether MA was present in the solution or not. For MA intake (Fig. 5b), there was a significant main effect of MA vs. EtOH+MA group (F(1,20)=46.2, p<.001). Mice given access to MA alone consumed more MA compared to mice given access to EtOH+MA, independent of EtOH concentration. For total fluid consumed (Fig. 5c), the only significant effect was a main effect of time (F(2,78)=3.6, p=.03). Fluid consumption was greater by .3 ml during the second 4-day time period compared to the other 2 periods.
Fig. 5.
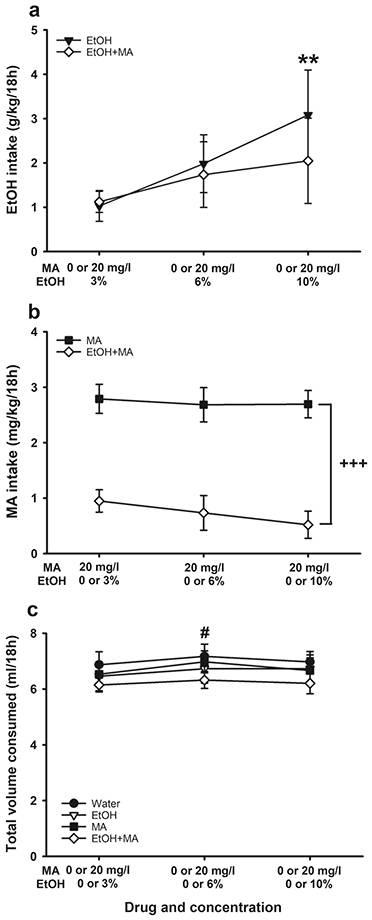
Voluntary two-bottle choice MA intake was reduced in MAHDR mice when EtOH was added to the MA solution. (a) EtOH intake when offered alone vs. water or mixed with MA (EtOH+MA) vs. water. (b) MA intake when offered alone vs. water or mixed with EtOH (EtOH+MA) vs. water. (c) Total volume consumed in mice offered 2 water tubes, or water vs. EtOH, MA, or EtOH+MA. Data are means ± SEM for the sexes combined, as there were no sex differences. Water group, n=11; EtOH group, n=12; MA group, n=12; EtOH+MA group, n=12. **p<.01 vs. 3% EtOH for data collapsed on group; +++p<.001 for the main effect of group; #p<.05 vs. consumption during the prior and latter time periods for data collapsed on group. EtOH, ethanol; MA, methamphetamine
3.4.2. Phase II – Two-bottle Choice MA Drinking
MA was offered vs. water during Phase II of this study, beginning the day after the completion of Phase I (see Table 4), to determine if prior access to any of the drug solutions impacted voluntary MA intake, compared to access to water only. MA intake data are presented in Fig. 6a. There was a significant main effect of MA concentration (F(2,76)=166.6, p<.001), with increasing MA intake across increasing concentrations, independent of Phase I exposure group. For total fluid consumption from the water and MA tubes (Fig. 6b), there was a significant main effect of time (F(2,78)=8.2, p<.001), with increased consumption when mice were transitioned to 40 mg/l MA, but no further increase at 80 mg/l.
Fig. 6.
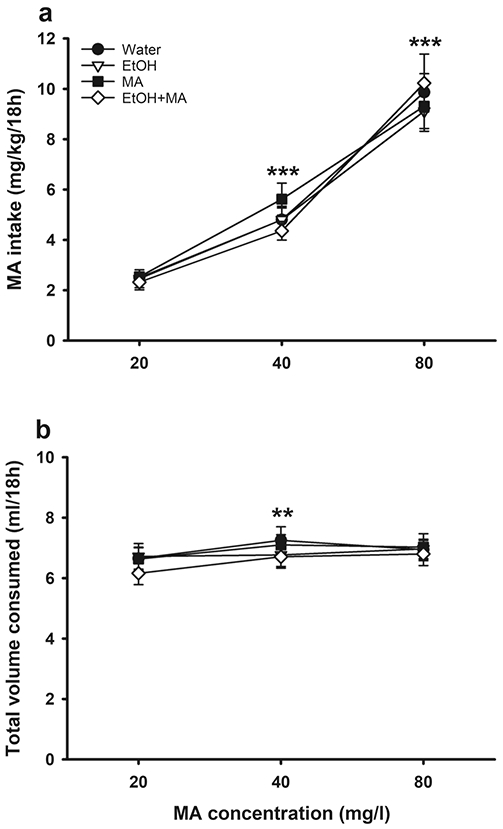
Prior consumption of EtOH and/or MA in a two-bottle choice procedure had no effect on subsequent voluntary MA intake in MAHDR mice. (a) MA intake and (b) total volume consumed, when MA was offered vs. water at concentrations of 20, 40, or 80 mg/l after previous two-bottle water or water vs. EtOH, MA, or EtOH+MA. Data are means ± SEM for the sexes combined, as there were no sex differences. Water group, n=11; EtOH group, n=12; MA group, n=12; EtOH+MA group, n=12. **p<.01, ***p<.001 vs. previous MA concentration, collapsed on group. EtOH, ethanol; MA, methamphetamine
3.5. Experiment 5: Effect of BAC on established voluntary MA consumption and locomotor activity
3.5.1. MA consumption prior to BAC treatment
MA intake data for each 2h interval during the 6h MA access periods and for the 6h period as a whole are summarized in Fig. 7a-d. No sex effects were detected in the initial MA concentration x sex x time x BAC dose ANOVA, and there were no differences among groups assigned to receive different doses of BAC in the next stage of the experiment. There was a significant MA concentration x time interaction (F(2,164)=8.2, p<.001), so data were examined for each 2h time interval. For each time interval, there was a significant effect of MA concentration (Fs (1,82)=27.6-42.6; all ps<.001), as indicated in Fig. 7a-d, which was also present in a separate analysis for the total 6h period (F1,82)=34.2, p<.001). In addition, there was a significant effect of time at both MA concentrations, in which MA intake during the first 2h period was lower compared to the second and third 2h periods (ps<.001; compare across Fig. 7a-c).
Fig. 7.
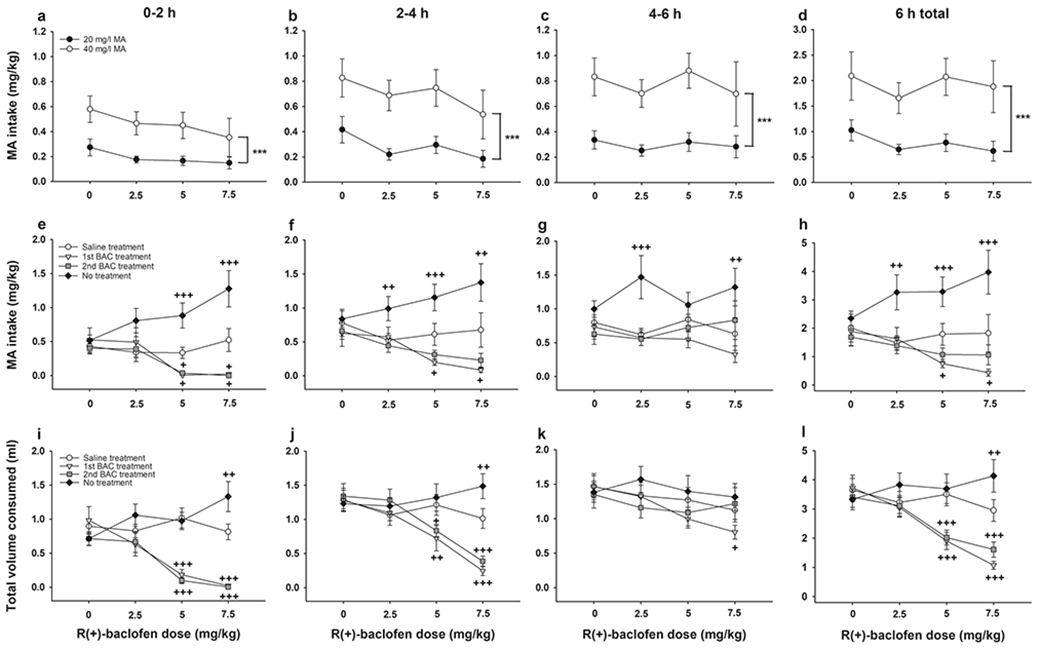
Baclofen dose-dependently reduced MA intake in MAHDR mice, but also reduced total volume consumed. (a-c) MA intake during the acquisition period in 2h time bins and (d) during the entire 6h drinking period; (e-g) MA intake during the treatment phase in 2h time bins and (h) during the entire 6h drinking period; and (i-k) total volume consumed during the treatment phase in 2h time bins and (l) during the entire 6h drinking period. Data are means ± SEM for the sexes combined, as there were no sex differences. 0 mg/kg BAC group, n=22; 2.5 mg/kg BAC group, n=22; 5 mg/kg BAC group, n=24; 7.5 mg/kg BAC group, n=22. ***p<.001 for the main effect of concentration; +p<.05, ++p<.01, +++p<.001 vs. saline treatment day. BAC, R(+)-baclofen; MA, methamphetamine
3.5.2. MA consumption during the BAC pretreatment phase
MA intake data are shown in Fig. 7e-h after saline or BAC pretreatment, and on the day after BAC treatment was terminated, for each 2h time interval and the total 6h period (see Table 5). There were no significant effects involving sex. There was a significant day x time x BAC dose interaction (F(18, 492)=2.9, p<.001), so data were examined for each 2h time interval. For each interval, there was a significant day x BAC dose interaction (0-2h: F(9,258)=4.3, p<.001; 2-4h: F(9,258)=3.2, p=.001; 4-6h: F(9,258)=1.9, p=.05). During the first 2h interval (Fig. 7e), pretreatment with 5 and 7.5 mg/kg BAC resulted in significant reductions in MA intake on both BAC pretreatment days, compared to MA intake on the initial saline pretreatment day for the same animals. Prior treatment with these BAC doses appeared to have residual effects on MA intake, as there was significantly greater MA intake the next day, in the absence of pretreatment, compared to intake on the initial saline pretreatment day. There were no significant differences in MA intake across days for the saline or 2.5 mg/kg BAC pretreatment groups.
During the second 2h interval (Fig. 7f), there were again significant dose-dependent reductions in MA intake after the first 5 or 7.5 mg/kg BAC pretreatment and strong trends for reductions by these doses after the second BAC pretreatment (ps=.06), compared to MA intake for the same mice on the saline pretreatment day. Again, there were apparent residual effects of all BAC doses, as indicated by significantly greater MA intake the next day, in the absence of pretreatment, compared to the initial saline pretreatment day. There were no significant differences in MA intake across days for mice treated with saline. During the third 2h interval (Fig. 7g), there were no significant differences in MA intake across days for mice treated with saline or 5 mg/kg BAC, but MA intake remained elevated on the final day in mice that had been treated with 2.5 or 7.5 mg/kg BAC. Finally, when data for the entire 6h period (Fig. 7h) were analyzed, the results supported the overall characterizations for the 2h intervals. There was a significant day x BAC dose interaction (F(9,258)=3.2, p=.001). The first 5 or 7.5 mg/kg BAC pretreatment produced significant reductions in MA intake compared to the saline pretreatment day, and there was a strong statistical trend for a reduction after the second pretreatment (p=.06) for the 5 mg/kg BAC group. There were elevations in MA intake on the no treatment day in the BAC pretreatment groups, but not in the saline pretreatment group.
For total fluid consumption (Fig. 7i-l), there was a significant day x time x BAC dose interaction (F(18, 492)=3.6, p<.001), so data were examined for each 2h time interval. For each 2h interval, there was a significant day x BAC dose interaction (0-2h: F(9,258)=9.1, p<.001; 2-4h: F(9,258)=7.8, p<.001; 4-6h: F(9,258)=1.9, p=.05). During the first and second 2h intervals (Fig. 7i,j), pretreatment with 5 or 7.5 mg/kg BAC produced significant reductions in total fluid consumption, compared to consumption on the saline pretreatment day. An apparent residual effect on total intake was observed on the no treatment day in mice that had been previously treated with 7.5 mg/kg BAC. There were no significant differences in total fluid consumption across days in mice treated with saline or 2.5 mg/kg BAC. By the third 2h interval (Fig. 7k), pretreatment effects were waning, as only the mice pretreated with 7.5 mg/kg BAC consumed significantly less total fluid and only after the first BAC pretreatment. Results for total fluid consumption (Fig. 7l) were comparable. There was a significant day x BAC dose interaction (F(9,258)=9.2, p<.001), and pretreatment with 5 or 7.5 mg/kg BAC produced significant reductions in fluid consumption. Total fluid consumption on the no treatment day was significantly increased only in the 7.5 mg/kg BAC group.
3.5.3. Locomotor activity
Based on existing literature demonstrating locomotor depressant effects of BAC that could have impacted drinking behavior, we tested the same mice for locomotor activity, measured as distance traveled (cm), 3-9 days after the MA drinking sessions concluded. Data are summarized in Fig. 8 after treatment with saline on locomotor test Day 1 (Fig. 8a), and BAC on locomotor test Day 2 (Fig. 8b). There was a significant day x time x BAC dose interaction (F(69,1426)=5, p<.001), so data were further examined for each day. On Day 1, there was a significant main effect of time (F(23,1518)=48, p<.001), but no effect of assigned Day 2 BAC treatment group on locomotor activity after saline treatment. Overall, activity declined at 30-75 min post-saline injection, compared to each previous time point, and then stabilized, with an increase in activity during the last 15-min period. On BAC treatment Day 2, there was a significant time x BAC dose interaction (F(69,1518)=5.8, p<.001). The effects of BAC on locomotor activity were dose-dependent (Fig. 8b), with reductions in activity, relative to the 0 mg/kg control group, lasting for 75 min post-treatment after 2.5 mg/kg BAC, 150 min after 5 mg/kg BAC, and 240 min after 7.5 mg/kg BAC.
Fig. 8.
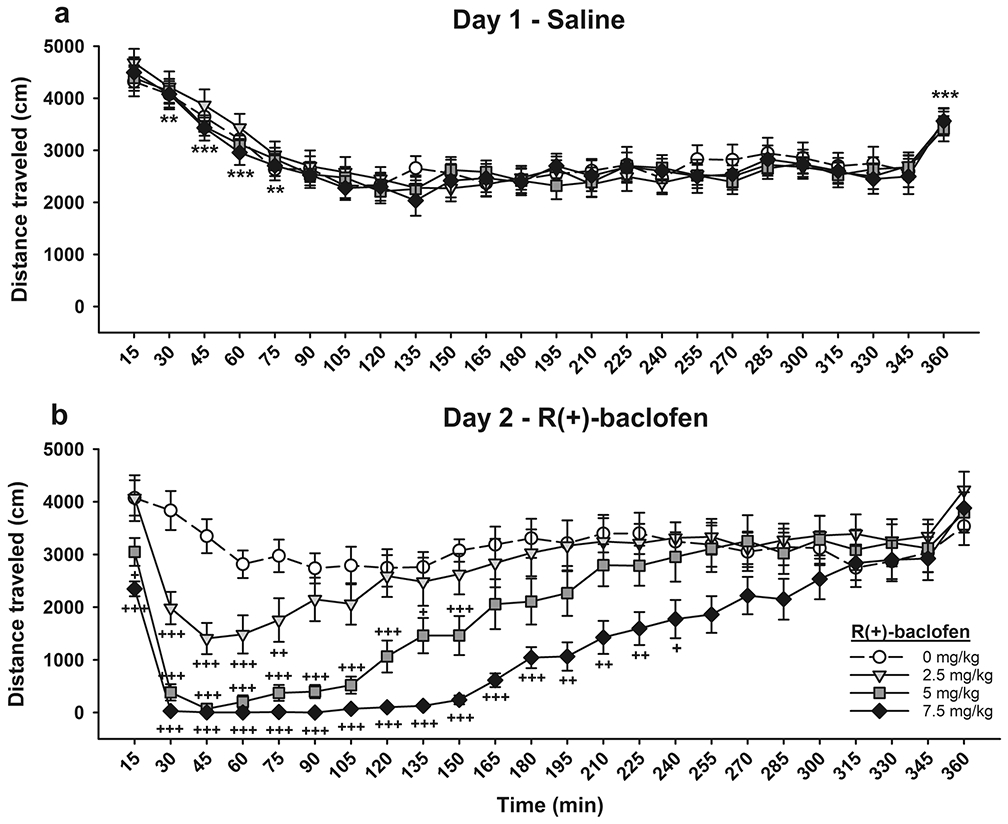
Baclofen dose-dependently reduced locomotor activity in MAHDR mice. Locomotor activity during 15-min time bins after (a) saline treatment and (b) 0, 2.5, 5, or 7.5 mg/kg BAC treatment. Data are means ± SEM for the sexes combined, as there were no sex differences. 0 mg/kg BAC group, n=17; 2.5 mg/kg BAC group, n=17; 5 mg/kg BAC group, n=19; 7.5 mg/kg BAC group, n=17. **p<.01, ***p<.001 vs. previous time point, collapsed on dose group; +p<.05, ++p<.01, +++p<.001 vs. 0 mg/kg
4. DISCUSSION
The present studies assessed the influence of several non-genetic factors on MA intake in a mouse model of high and low susceptibility to MA consumption. Previously, we determined that progressive increases in MA concentration or increasing the ratio of MA-containing to water-containing tubes to which the mice had access, increased MA intake in MAHDR, but not MALDR, mice (Shabani et al. 2016). Here, we demonstrate that intermittent MA access results in greater voluntary MA intake only in MAHDR mice, when compared to continuous access, whereas prior, forced consumption of MA had no effect on subsequent MA intake in MAHDR or MALDR mice. We also show that MAHDR mice decrease MA intake in the presence of EtOH in a sex-dependent and drinking procedure-dependent manner. Thus, EtOH reduced MA intake only in males when there was no water choice, but it reduced MA intake regardless of sex, when offered in the MA solution vs. water. Finally, BAC, which has been shown to reduce EtOH intake and MA operant self-administration, was examined for its effect on oral MA consumption, and the results indicated that BAC-associated reductions in MA intake were likely due to non-specific effects on behavior, but that prior BAC treatment had a residual increasing effect on MA intake. These results indicate that forced prior MA consumption does not impact MA intake, but continuous access to MA and the simultaneous presence of EtOH can reduce MA intake, in a mouse line with high genetic susceptibility to MA consumption.
We found that MAHDR mice consumed more MA under intermittent, compared to continuous, access conditions. This result is consistent with several EtOH studies in which temporal limitations on EtOH access increased voluntary EtOH intake in rodents (for review, see Crabbe et al. 2011; Spear 2020). The abstinence periods between EtOH access sessions in the published literature have typically been longer than the 6h abstinence period used in the current study. However, at least one study showed that a deprivation period as short as 12 hours resulted in increases in EtOH intake upon renewed access in rats with a history of EtOH consumption in a continuous two-bottle choice procedure (Sinclair and Li 1989). Greater MA consumption with intermittent access could reflect development of craving during the forced abstinence periods. A phenomenon known as “incubation of craving” has been demonstrated in rodents and humans alike, in which drug seeking increases after a period of withdrawal for many drugs of abuse, including EtOH,MA, heroin, nicotine, and cocaine (for review, see Pickens et al. 2011; Wolf 2016). However, in such studies, craving typically has been assessed after longer periods of abstinence (i.e., 1 week to 6 months; Pickens et al. 2011). Another possibility is that the MAHDR mice given intermittent MA access experience negative withdrawal symptoms during the abstinence periods that motivate an increase in MA intake. We observed an increase in depression-like symptoms after 6h of forced abstinence from MA in MAHDR mice with a 28-day history of binge-level MA intake (Shabani et al. 2019), but have not yet examined other potential symptoms in these mice, such as anxiety-like behaviors during periods of abstinence.
In MALDR mice, MA access schedule did not impact voluntary MA consumption. The finding that MA access schedule influences MA intake in MAHDR, but not MALDR mice demonstrates a genotype by environment interaction (Ducci and Goldman 2012). This is consistent with our previous studies in the MADR lines demonstrating gene by environment interactions, in which MAHDR mice increase MA intake when MA concentration or ratio of MA:water bottles is increased, whereas MALDR mice do not (Eastwood et al. 2014; Harkness et al. 2015; Reed et al. 2018; Shabani et al. 2011, 2016; Wheeler et al. 2009). Since a large difference between MAHDR and MALDR mice is trace amine-associated receptor 1 (TAAR1) functionality (Harkness et al. 2015; Shi et al. 2016), the Taar1 gene could be a player in the gene by environment interaction. Our previous studies have shown that sensitivity to the aversive effects of MA is associated with Taar1 genotype, so that mice with the Taar1+ allele coding for functional TAAR1 (i.e., MALDR mice) display MA-induced conditioned taste aversion (CTA) and place aversion, whereas mice with the Taar1m1J allele that codes for non-functional TAAR1 (i.e., MAHDR mice) are resistant (Reed et al., 2018; Shabani et al. 2011, 2012a; Wheeler et al. 2009). We have speculated that low MA intake in mice with functional TAAR1 results from their sensitivity to the aversive properties of MA. When TAAR1 function is absent, such aversive effects are not experienced, as also found in Taar1 knockout mice (Harkness et al., 2015). Thus, the difference in the effect of MA access schedule between MAHDR and MALDR mice is likely related to avoidance of MA by the MALDR line under all conditions, which may stem from their high sensitivity to aversive effects of MA. Whereas MAHDR mice may experience effects of MA abstinence that motivate MA intake under intermittent access, MALDR may not reach high enough levels of MA intake to experience any effect of abstinence.
In contrast to MA access schedule, prior forced exposure to MA does not appear to be a non-genetic factor with potential to influence voluntary MA intake in either MAHDR or MALDR mice. Thus, MA intake was similar in MA-naiïve mice, compared to mice previously exposed to an MA solution as their sole source of fluid. Further, when mice were transitioned from forced drinking to two-bottle choice drinking, 20 mg/l MA intake was reduced to levels equivalent to MA-naiïve mice that are typical of our previous two-bottle choice studies (ï3 mg/kg/18h; Harkness et al. 2015; Hitzemann et al. 2019; Reed et al. 2018; Shabani et al. 2011; Wheeler et al. 2009). These results are also comparable to our previous findings for MA intake in MAHDR mice after prolonged periods of abstinence (1-2 weeks) following voluntary MA intake of about 20 mg/kg/day on average (Shabani et al. 2019). However, these results are inconsistent with studies finding that prior, passive exposure to psychostimulants via injection produces increases in subsequent self-administration of these drugs in rats (for review, see Vezina 2004; Vezina and Leyton 2009). This could be explained by species, procedural and genetic differences among studies. Not only did we use mice, rather than rats, and a different method of prior MA exposure (oral vs. injection), we also tested the effect of prior exposure on a different MA-related behavior (two-bottle choice intake vs. operant self-administration) and used mice genetically prone to consume MA. However, we have not explored the effect of prior exposure to MA via injection or of prior exposure on operant responding for MA in our mice, which may yield different outcomes.
We have observed that MALDR and MAHDR mice consume similar amounts of MA on the first day of MA access, after which MALDR mice drastically reduce their MA intake levels, while MAHDR mice consume the same amount or increase their intake (Eastwood et al. 2014; Shabani et al. 2012b). These data, along with studies finding that the MAHDR and MALDR consume similar amounts of non-pharmacological tastants, including sweet, salty and bitter (Shabani et al. 2011; Wheeler et al. 2009), suggest that the lines have different interoceptive experiences during the first MA drinking experience. As discussed above, MALDR mice may reduce their MA intake after the first exposure because they are sensitive to the aversive effects of MA (Shabani et al. 2011, 2012a; Wheeler et al. 2009). In the forced exposure study, we reasoned that no-choice MA consumption might lead to increased voluntary intake in MALDR mice due to tolerance development to the aversive effects of MA. However, after 12 days of MA consumption, during which MALDR consumed an average of 2 to 4 mg/kg MA per day, depending on MA concentration, voluntary intake levels were lower (< 1 mg/kg) and similar to those of MA-naive mice that were exposed to water during the no-choice drinking phase, both of which consumed amounts typical for MALDR mice (Harkness et al. 2015; Hitzemann et al. 2019; Reed et al. 2018; Shabani et al. 2011; Wheeler et al. 2009). We have not directly tested the impact of forced MA exposure on sensitivity to aversive effects of MA, so cannot say whether tolerance was absent. However, the absence of an effect of forced consumption in either MAHDR or MALDR mice suggests that motivation for and/or interoceptive effects of MA were not altered.
These results for MA are different from findings for EtOH in a mouse strain genetically predisposed to consume low amounts of EtOH, the D2 strain (Belknap et al. 1993; Yoneyama et al. 2008). When D2 mice were pre-exposed to EtOH via passive injection or intragastic infusion, voluntary EtOH consumption was increased (Camarini and Hodge 2004; Fidler et al. 2012). Across multiple mouse strains, a negative association of EtOH intake and sensitivity to aversive effects of EtOH has been found (Broadbent et al. 2002; Cunningham 2014; 2019), and D2 mice exhibit high sensitivity to conditioned aversive effects of EtOH (Broadbent et al. 2002; Cunningham 2019). It is possible that forced EtOH exposure resulted in tolerance to aversive effects, whereas forced MA exposure in our MALDR mice did not result in tolerance. In fact, while there was no impact on conditioned reward, pre-exposure to EtOH by injection reduced both EtOH-induced conditioned place aversion (Cunningham et al. 2002) and taste aversion (Risinger and Cunningham 1995) in D2 mice, consistent with both a tolerance interpretation and the interpretation that the injection/EtOH association during pre-exposure interfered with learning in the conditioning tasks. Pre-exposure studies have not been conducted in MALDR mice to explore their impact on conditioned aversive effects of MA.
The final 3 studies of this series included only MAHDR mice, because the goal was to examine the effect of EtOH or BAC on MA intake in mice that have some avidity for MA and consume amounts of MA that could be either reduced or increased. In the initial EtOH study, mice were simultaneously offered 3 bottles containing EtOH, MA, and EtOH+MA without a water choice. In the second EtOH study, independent groups of mice were offered 2 bottles containing water vs. EtOH, water vs. MA, or water vs. EtOH+MA. A sex difference was found for both EtOH intake and preference ratio in the three-bottle choice procedure. Females consumed more EtOH than males and did not exhibit a preference for any 1 solution, whereas males exhibited a greater preference for the MA solution, compared to the EtOH-containing solutions.
In contrast to these results, a similar study found that B6 male mice showed the greatest preference for the EtOH+MA solution, and the lowest preference for the MA solution (Fultz et al. 2017). However, B6 mice are known to be both EtOH-preferring (Belknap et al. 1993; Yoneyama et al. 2008) and MA-avoiding (Eastwood and Phillips 2014b), and the difference in results may be associated with differences between MAHDR and B6 mice in proclivity to consume these drugs. We also note that there were differences in experimental procedure that could have contributed, as the Fultz et al (2017) study offered mice a choice between 20% EtOH, 10 mg/l MA, or a mixture for 2h/day for 3 days, whereas we offered mice a choice between escalating concentrations (3, 6, or 10%) of EtOH, 20 mg/l MA, or a mixture for 18h/day for 4 days at each concentration (12 days total).
In both the two- and three-bottle choice experiments, EtOH intake from the EtOH only and EtOH+MA tubes did not significantly differ. Therefore, regardless of whether mice were offered EtOH and EtOH+MA simultaneously, or one or the other in independent groups vs. water, EtOH intake was not altered by the presence of MA. In contrast, when EtOH and MA were mixed together, MA intake was reduced, with one exception, related to sex. When the 3 drug solutions were offered simultaneously, male mice consumed almost all of their MA from the MA only tube, whereas female mice drank similar amounts of MA from the MA only and EtOH+MA tubes. However, in the three-bottle choice study, total MA intake from both MA- containing tubes significantly increased with increasing EtOH concentration, independent of sex, suggesting an effect of EtOH presence on MA consumption. Whether the EtOH effect was driven by male mice avoiding the EtOH-containing solutions or EtOH increasing their preference for the pure MA solution is not known. To address a potential sex difference in sensitivity to the aversive effects of EtOH, studies using a conditioned taste or place aversion task could be conducted. There was no sex difference for MA consumption in the two-bottle choice experiment, and mice of both sexes offered MA alone consumed more MA compared to mice offered the EtOH+MA solution. The absence of consistent sex differences in these 2 studies comparing EtOH+MA co-consumption to consumption of each drug alone suggests that the difference between male and female mice observed in the three-bottle choice experiment is unlikely due to sex differences in factors such as gustatory function or stress response to the shift from group to single housing. We have found similar levels of MA intake and of the intake of sweet and bitter tasting substances in males and females in prior studies (Shabani et al. 2011; Wheeler et al. 2009).
Because GABAB receptor agonists have shown some efficacy in reducing operant (meth)amphetamine self-administration (Brebner et al. 2005; Ranaldi and Poeggel 2002), we investigated the effect of BAC on oral MA intake. Pretreatment with BAC reduced MA intake in MAHDR mice at the two highest administered doses (5 and 7.5 mg/kg), but these doses also robustly reduced total fluid consumption and locomotor activity. The BAC pretreatment doses that did not significantly impact MA intake did not reduce fluid consumption or locomotor activity. The BAC doses used for this study were chosen based on literature indicating that they had non-significant effects on locomotor activity or an effect only after the first administration in the D2 or B6 inbred strain progenitors of the MAHDR mice (Bortolato et al. 2010; Broadbent and Harless 1999; Chester and Cunningham 1999; Korkosz et al. 2006). The discrepancy between the previous studies and our current study, in which depressant effects of the higher doses of BAC were largely equivalent on both treatment days, could be due to a change in sensitivity to locomotor effects of BAC when the 2 strains were combined to produce the MAHDR line. Another potential explanation is the use of R(+)-BAC in the current study vs. other forms of BAC in the studies cited above, which used (±)-BAC or did not specify the enantiomer.
The temporal pattern of responses to the higher BAC doses overlapped for MA consumption, total fluid consumption, and locomotor activity; thus, activity levels began to recover around the same time that MA intake and total fluid consumption increased. Therefore, it seems likely that BAC-induced reductions in MA consumption were due to sedative effects, rather than to interference with a mechanism important for MA intake. It is possible that the EtOH consumed in the three-bottle choice study had behavioral effects that could have impacted MA intake. However, we found that MA intake increased with increasing EtOH concentration, suggesting that non-specific sedation is not a parsimonious explanation. On the other hand, EtOH has stimulatory effects at lower doses, which could have potentiated MA drinking behavior.
An apparent residual effect of previous BAC treatment was to elevate MA intake on the following day for all doses of BAC. It is possible that mice treated with the higher doses of BAC did not obtain a desired dose of MA during the 2 days of BAC treatment and compensated on the following day. However, this does not explain the effect in mice treated with the lower BAC dose for which MA intake was not significantly impacted by BAC pretreatment, compared to saline pretreatment. Therefore, it appears that BAC exposure during the time of MA intake had an impact resulting in increased MA intake on the following day. MA induces long-lasting depression of GABAB receptor signaling in the ventral tegmental area (Padgett et al. 2012; Munoz et al. 2016; Sharpe et al. 2014), and BAC is thought to inhibit reinforcing drug effects (Vlachou and Markou 2010), such as amphetamine-induced stimulation of dopamine in the striatum (Zhou et al. 2004). Thus, although the lower BAC dose did not significantly impact MA intake, perhaps the removal of GABAB stimulation during MA access resulted in MA being experienced as more reinforcing, resulting in increased intake. The effect of application of a GABAB receptor antagonist simultaneously with BAC could be examined and would be predicted to eliminate the post-BAC increase in MA intake.
We did not observe any effects of prior exposure to EtOH, MA, and/or EtOH+MA on subsequent voluntary MA consumption. These results are inconsistent with those from a study demonstrating that mice on a mixed B6 and 129 x 1/SvJ background with a prior 10-day history of EtOH drinking showed reduced MA consumption under operant conditions, whereas B6 mice with a 7-day history of EtOH drinking showed increases in home-cage MA intake (Fultz et al. 2017). Also, as mentioned above, some studies have found that animals with a psychostimulant exposure history subsequently self-administered more of these drugs (for review, see Vezina 2004; Vezina and Leyton 2009). One unique aspect of our studies is the genetic mouse model possessing the Taar1m1J/m1J genotype that eliminates sensitivity to aversive effects of MA and results in significantly higher MA intake levels than seen in mice without the mutant genotype, including the mice tested in Fultz et al. (2017). Our current results may diverge from previous outcomes, due to genotype, route of MA preexposure, and a different measurement of subsequent intake (voluntary two-bottle choice consumption vs. operant self-administration).
Overall, we demonstrated that while voluntary MA intake in the MADR lines is heavily influenced by genetic factors, there are also influential non-genetic factors. Thus, intermittent MA access increased MA intake, co-morbid EtOH exposure increased or decreased MA intake in mice at high genetic risk for MA consumption, depending upon choices of drinking solutions available, and BAC treatment increased MA intake 24 hours after cessation of treatment, potentially because MA was experienced as more reinforcing after attenuation of the reinforcing effects of MA during BAC treatment. On the other hand, prior forced exposure to MA had no effect on subsequent voluntary MA intake in MAHDR or MALDR mice. Future studies are needed to further explore mechanisms underlying these effects on MA intake.
Acknowledgements:
We thank Harue Baba, Andy Zu, David Madison, and Virginia Fuhrmark for assistance with data collection. Funding was provided by NIDA P50DA018165 (TJP), NIDA U01DA041579 (TJP), NIDA R01DA046081 (TJP), Department of Veterans Affairs Merit Review Grant I01BX002106 (TJP), the VA Research Career Scientist Program (TJP), NCATS TL1TR002371 (AMS), and NIAAA P60AA010760 (TJP). The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States government.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Compliance with ethical standards: All experiments were approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Portland Health Care System and were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Agabio R, Colombo G (2014) GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front Neurosci 8:140 doi: 10.3389/fnins.2014.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Badiani A, Miczek KA, Muller CP (2020) Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev 110:3–27 doi: 10.1016/j.neubiorev.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, Samaha AN (2015) How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–179 doi: 10.1016/j.neubiorev.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Becker HC (2012) Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res 34:448–458 [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER (1993) Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 112:503–510 doi: 10.1007/bf02244901 [DOI] [PubMed] [Google Scholar]
- Belknap JK et al. (2013) Genetic factors involved in risk for methamphetamine intake and sensitization. Mamm Genome 24:446–458 doi: 10.1007/s00335-013-9484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M et al. (2010) GABAB receptor activation exacerbates spontaneous spike-and-wave discharges in DBA/2J mice. Seizure 19:226–231 doi: 10.1016/j.seizure.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Brebner K, Ahn S, Phillips AG (2005) Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berl) 177:409–417 doi: 10.1007/s00213-004-1968-6 [DOI] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, Anglin MD (2005) Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992-2002). J Subst Abuse Treat 29:295–306 doi: 10.1016/j.jsat.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, Anglin MD (2007) Substance use pathways to methamphetamine use among treated users. Addict Behav 32:24–38 doi: 10.1016/j.addbeh.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Brecht ML, Huang D, Evans E, Hser YI (2008) Polydrug use and implications for longitudinal research: ten-year trajectories for heroin, cocaine, and methamphetamine users. Drug Alcohol Depend 96:193–201 doi: 10.1016/j.drugalcdep.2008.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Harless WE (1999) Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice. Psychopharmacology (Berl) 141:197–205 doi: 10.1007/s002130050825 [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL (2002) Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci 116:138–148 doi: 10.1037/0735-7044.116.1.138 [DOI] [PubMed] [Google Scholar]
- Bujarski S et al. (2014) The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug Alcohol Depend 142:127–132 doi: 10.1016/j.drugalcdep.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Hodge CW (2004) Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav 79:623–632 doi: 10.1016/j.pbb.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014) Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48:243–252 doi: 10.1016/j.alcohol.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL (1999) Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Behav 63:325–331 doi: 10.1016/s0091-3057(98)00253-6 [DOI] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O (2012) Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37:2153–2160 doi: 10.1038/npp.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF (2011) Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci 1216:24–40 doi: 10.1111/j.1749-6632.2010.05895.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crime UNOoDa (2015) World Drug Report. United Nations; publication, Sales No. E.15.XI.6. [Google Scholar]
- Cunningham CL (2014) Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav Neurosci 128: 430–445 doi: 10.1037/a0036459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL (2019) Genetic relationships between ethanol-induced conditioned place aversion and other ethanol phenotypes in 15 inbred mouse strains. Brain Sci 9:E209 doi: 10.3390/brainsci9080209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ (2002) Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 160:414–424 doi: 10.1007/s00213-001-0990-1 [DOI] [PubMed] [Google Scholar]
- Dick DM, Riley B, Kendler KS (2010) Nature and nurture in neuropsychiatric genetics: where do we stand? Dialogues Clin Neurosci 12:7–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D (2012) The genetic basis of addictive disorders. Psychiatr Clin North Am 35:495–519 doi: 10.1016/j.psc.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood EC, Barkley-Levenson AM, Phillips TJ (2014) Methamphetamine drinking microstructure in mice bred to drink high or low amounts of methamphetamine. Behav Brain Res 272:111–120 doi: 10.1016/j.bbr.2014.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood EC, Eshleman AJ, Janowsky A, Phillips TJ (2018) Verification of a genetic locus for methamphetamine intake and the impact of morphine. Mamm Genome 29:260–272 doi: 10.1007/s00335-017-9724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood EC, Phillips TJ (2014a) Morphine intake and the effects of naltrexone and buprenorphine on the acquisition of methamphetamine intake. Genes Brain Behav 13:226–235 doi: 10.1111/gbb.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood EC, Phillips TJ (2014b) Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict Biol 19:370–379 doi: 10.1111/adb.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egervari G, Ciccocioppo R, Jentsch JD, Hurd YL (2018) Shaping vulnerability to addiction – the contribution of behavior, neural circuits and molecular mechanisms. Neurosci Biobehav Rev 85:117–125 doi: 10.1016/j.neubiorev.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GI Elmer, Meisch RA George FR (1986) Oral ethanol reinforced behavior in inbred mice. Pharmacol Biochem Behav 24:1417–1421 doi: 10.1016/0091-3057(86)90204-2 [DOI] [PubMed] [Google Scholar]
- Falch E, Hedegaard A, Nielsen L, Jensen BR, Hjeds H, Krogsgaard-Larsen P (1986) Comparative stereostructure-activity studies on GABAA and GABAB receptor sites and GABA uptake using rat brain membrane preparations. J Neurochem 47:898–903 doi: 10.1111/j.1471-4159.1986.tb00695.x [DOI] [PubMed] [Google Scholar]
- Fidler TL, Powers MS, Ramirez JJ, Crane A, Mulgrew J, Smitasin P, Cunningham CL (2012) Dependence induced increases in intragastric alcohol consumption in mice. Addict Biol 17:13–32 doi: 10.1111/j.1369-1600.2011.00363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz EK, Martin DL, Hudson CN, Kippin TE, Szumlinski KK (2017) Methamphetamine-alcohol interactions in murine models of sequential and simultaneous oral drug-taking. Drug Alcohol Depend 177:178–186 doi: 10.1016/j.drugalcdep.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr CD, Delva J, Anthony JC (2000) The suspected association between methamphetamine ('ice') smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug Alcohol Depend 59:89–93 [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6:521–532 doi: 10.1038/nrg1635 [DOI] [PubMed] [Google Scholar]
- Harkness JH, Shi X, Janowsky A, Phillips TJ (2015) Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology 40:2175–2184 doi: 10.1038/npp.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck DM, Brecht ML, Lovinger K, Raihan A, Christou D, Sheaff P (2013) Poly-drug and marijuana use among adults who primarily used methamphetamine. J Psychoactive Drugs 45:132–140 doi: 10.1080/02791072.2013.785824 [DOI] [PubMed] [Google Scholar]
- Hitzemann R, lancu OD, Reed C, Baba H, Lockwood DR, Phillips TJ (2019) Regional Analysis of the Brain Transcriptome in Mice Bred for High and Low Methamphetamine Consumption. Brain Sci 9 doi: 10.3390/brainsci9070155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Blasingame SN, Boehm SL 2nd (2015) Bidirectional enantioselective effects of the GABAB receptor agonist baclofen in two mouse models of excessive ethanol consumption. Alcohol 49:37–46 doi: 10.1016/j.alcohol.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Boehm SL 2nd (2014) Intra-nucleus accumbens shell injections of R(+)- and S(−)-baclofen bidirectionally alter binge-like ethanol, but not saccharin, intake in C57Bl/6J mice. Behav Brain Res 272:238–247 doi: 10.1016/j.bbr.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA (2000) Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry 57:261–269 doi: 10.1001/archpsyc.57.3.261 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski L, Prescott CA (1999) Hallucinogen, opiate, sedative and stimulant use and abuse in a population-based sample of female twins. Acta Psychiatr Scand 99:368–376 doi: 10.1111/j.1600-0447.1999.tb07243.x [DOI] [PubMed] [Google Scholar]
- Korkosz A, Zatorski P, Taracha E, Plaznik A, Kostowski W, Bienkowski P (2006) Ethanol blocks nicotine-induced seizures in mice: comparison with midazolam and baclofen. Alcohol 40:151–157 doi: 10.1016/j.alcohol.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Manolio TA et al. (2009) Finding the missing heritability of complex diseases. Nature 461:747–753 doi: 10.1038/nature08494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz MB, Padgett CL, Rifkin R et al. (2016) A role for the GIRK3 subunit in methamphetamine-induced attenuation of GABAB receptor-acativated GIRK currents in VTA dopamine neurons. J Neurosci 36:3106–3114 doi: 10.1523/JNEUROSCI.1327-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady KE, Arria AM, Fitzelle DM, Wish ED (2008) Heavy Drinking and Polydrug Use among College Students. J Drug Issues 38:445–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, et al. (2012) Methamphetamine-evoked depression of GABAB receptor signaling in GABA neurons of the VTA. Neuron 73:978–89 doi: 10.1016/j.neuron.2011.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Reed C (2014) Targeting GABAB receptors for anti-abuse drug discovery. Expert Opin Drug Discov 9:1307–1317 doi: 10.1517/17460441.2014.956076. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34:411–420 doi: 10.1016/j.tins.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Poeggel K (2002) Baclofen decreases methamphetamine self-administration in rats. Neuroreport 13:1107–1110 doi: 10.1097/00001756-200207020-00007 [DOI] [PubMed] [Google Scholar]
- Reed C et al. (2018) A spontaneous mutation in Taar1 impacts methamphetamine-related traits exclusively in DBA/2 mice from a single vendor. Front Pharmacol 8:993 doi: 10.3389/fphar.2017.00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL (1995) Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol 12:535–539 doi: 10.1016/0741-8329(95)00040-2 [DOI] [PubMed] [Google Scholar]
- Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ (2012b) A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology 62:2169–2177 doi: 10.1016/j.neuropharm.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S et al. (2016) A mouse model for binge-level methamphetamine use. Front Neurosci 10:493 doi: 10.3389/fnins.2016.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Cunningham CL, Phillips TJ (2012a) Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology 62:1134–1141 doi: 10.1016/j.neuropharm.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ (2011) Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav 10:625–636 doi: 10.1111/j.1601-183X.2011.00700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, Schmidt B, Ghimire B, Houlton SK, Hellmuth L, Mojica E, Phillips TJ (2019) Depression-like symptoms of withdrawal in a genetic mouse model of binge methamphetamine intake. Genes Brain Behav 18:e12533 doi: 10.1111/gbb.12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ (2014) Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol 18 doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X et al. (2016) Genetic polymorphisms affect mouse and human trace amine-associated receptor 1 function. PLoS One 11:e0152581 doi: 10.1371/journal.pone.0152581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Li TK (1989) Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol 6:505–509 doi: 10.1016/0741-8329(89)90059-1 [DOI] [PubMed] [Google Scholar]
- Spear LP (2020) Timing eclipses amount: The critical importance of intermittency in alcohol exposure effects. Alcohol Clin Exp Res epub ahead of print doi: 10.1111/acer.14307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford AM et al. (2019) Taar1 gene variants have a causal role in methamphetamine intake and response and interact with Oprm1. Elife 8 doi: 10.7554/eLife.46472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT et al. (1996) Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet 67:473–477 doi: [DOI] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW (1998) Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend 52:231–241 doi: 10.1016/s0376-8716(98)00101-x [DOI] [PubMed] [Google Scholar]
- Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839 doi: 10.1016/j.neubiorev.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56 Suppl 1:160–168 doi: 10.1016/j.neuropharm.2008.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink (2016) Genetics of addiction: Future focus on gene x environment interaction? J Stud Alcohol Drugs 5:684–687 doi: 10.15288/jsad.2016.77.684 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371 doi: 10.1016/S1054-3589(10)58013-X [DOI] [PubMed] [Google Scholar]
- Wheeler JM et al. (2009) Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav 8:758–771 doi: 10.1111/j.1601-183X.2009.00522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witczuk B, Khaunina RA, Kupryszewski G (1980) 3-(p-Chlorophenyl)-4-aminobutanoic acid--resolution into enantiomers and pharmacological activity. Pol J Pharmacol Pharm 32:187–196 [PubMed] [Google Scholar]
- Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365 doi: 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA (2008) Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42:149–160 doi: 10.1016/j.alcohol.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Mailloux AW, Jung BJ, Edmunds HS, McGinty JF (2004) GABAB receptor stimulation decreases amphetamine-induced behavior and neuropeptide gene expression in the striatum. Brain Res 1004:18–28 doi: 10.1016/j.brainres.2003.11.007 [DOI] [PubMed] [Google Scholar]


