Abstract
Cells in a tumor are heterogeneous, often including a small number of tumor-initiating cells (TICs) and the majority of cancerous and non-cancerous cells. We have previously reported that constitutive activation of Notch signaling in adipocytes of mice leads to dedifferentiated liposarcoma (LPS), an aggressive LPS with no effective treatment. Here, we explored the role of Notch signaling in cellular heterogeneity of LPS. We performed serial transplantations to enrich for TICs, and derived cells exhibiting sustained Notch activation (mLPS1 cells) and cells with normal Notch activity (mLPS2 cells). Both mLPS1 and mLPS2 cells proliferated rapidly, and neither exhibited contact inhibition. However, only the mLPS1 cells exhibited tumorigenicity and gave rise to LPS upon engraftment into mice. The mLPS1 cells also highly expressed markers of cancer stem cells (Cd133), mesenchymal stem cells (Cd73, Cd90, Cd105, Dlk1) and the long non-coding RNA Rian. By contrast, the mLPS2 cells accumulated lipid droplets and expressed mature adipocyte markers when induced to differentiate. Most importantly, CRISPR-mediated disruption of Notch abrogated the tumorigenic properties of mLPS1 cells. These results reveal a key role of Notch signaling in maintaining TICs in LPS.
Keywords: Adipose Tissue, Dedifferentiation, Cancer stem cells, Tumor heterogeneity, Transplantation, Clustered regularly interspaced short palindromic repeats (CRISPR)
1. Introduction
Tumors are highly heterogenous in cellular composition, comprised of not only cancer cells but also various immune cells, stromal cells (interstitial fibroblasts and adipocytes), vessel-associated cells (endothelial cells, smooth muscle cells and pericytes), and undifferentiated progenitor cells such as mesenchymal stem cells [1]. Tumor-initiating cells (TICs), also known as cancer stem cells, are a subpopulation of cells found in almost all tumors [2]. TICs are highly tumorigenic and have the capacity to give rise to new tumors upon transplantation or metastasis. The high plasticity of TICs is essential for tumor heterogeneity, cancer cell survival and chemoresistance, and cancer relapse [3]. However, the molecular mechanisms that maintains TICs and drive their oncogenesis remain unclear.
Emerging studies in many different types of cancer have demonstrated that Notch signaling plays a key role in stem cell behavior and TIC function, as well as oncogenesis [4,5]. Classical Notch signaling is transmitted between two adjacent cells from the ligand-presenting cell to the receptor-expressing cell. Upon ligand (DLL and JAG) binding to Notch receptors on the neighboring cell, two proteolytic cleavage events lead to the release and nuclear translocation of the Notch intracellular domain (NICD) [6]. NICD subsequently recruits a transcriptional complex to activate the expression of HES and HEY family genes, which encode transcriptional factors that further regulate the expression of a myriad of tertiary genes [7]. Activation of Notch signaling has been reported to either promote or suppress oncogenesis depending on the types of tumor [8–11].
Liposarcoma (LPS) is a type of solid tumor thought to be derived from adipocytes [12]. LPS represents the most common type of soft tissue sarcoma, with approximately 10,000 cases diagnosed per year [13]. Based on histological features, LPS can be classified into four subtypes: well-differentiated, dedifferentiated, myxoid/round cell, and pleomorphic liposarcomas [14]. Among these, dedifferentiated LPS (DDLPS) is the most malignant subtype with a five-year survival rate of 40–60% and a local recurrence rate of over 80% [15]. Several studies have indicated that DDLPS harbors a larger population of TICs compared to well-differentiated LPS (WDLPD), potentially contributing to the lower survival rate of DDLPS patients [16,17]. In addition, tumor genotyping in human DDLPS specimens establishes an association with increased copy numbers of NOTCH1, implying a possible role of Notch signaling in the tumorigenesis of DDLPS [18]. Consistent with this notion, adipocyte-specific activation of Notch signaling in mice leads to the development of LPS resembling human DDLPS [19]. However, how Notch signaling drives LPS, especially its role in TICs, is unknown.
In this study, we test the hypothesis that constitutive activation of Notch signaling drives tumorigenesis of LPS through regulating TICs. We first derived through serial transplantation a couple of LPS cell lines from a primary tumor of adipocyte-specific NICD-overexpressing transgenic mice. We further characterized these cells in proliferation, differentiation, gene expression and tumorigenic capacities. The Notch-overexpressing TICs (mLPS1) represent the first murine LPS cell line with a clear genetic background driven by adipocyte-specific expression of an oncogene. We anticipate that the mLPS1 cells will serve as a valuable model with which to investigate the molecular mechanisms underlying LPS and to identify potential therapeutic targets to treat LPS.
2. Materials and methods
2.1. Animals
The following mice were purchased from the Jackson Laboratory: Adipoq-Cre [20], Rosa26LSL-N1ICD (abbreviated as RosaNICD) [21], Ptenf/f [22]. Mice were bred consecutively for at least 5 generations to derive Adipoq-Cre/RosaNICD/+/Ptenf/f compound mutant mice. Mice were housed in an animal facility with a 12 hours light/dark cycle in a temperature-controlled room. All procedures involving mice were approved by Purdue Animal Care and Use Committee.
2.2. Tumor grafting and LPS cell transplantation
Tumor grafting studies were performed using 2–3 months old immune-deficient NRG mice (NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ) as recipients. The NRG mice were provided by Purdue University Biological Evaluation Core Facility. The tumors collected from Adipoq-Cre/RosaNICD/+/Ptenf/f mice were washed with Dulbecco’s phosphate buffered saline (PBS, Gibco BRL #21300-025) for 1 min and transferred to RPMI-1640 medium (Corning #10-040-CV) supplemented with 10% fetal bovine serum (FBS, Corning #35-010-CV) and penicillin (100U/mL)/streptomycin (100 µg/mL)(Hyclone #SV30010) in 6-cm tissue culture plates. The tumor was minced on ice into small pieces in dimensions of approximately 0.5 X 0.5 X 0.5 mm3 using sterile scissors. To implant the tumor pieces, NRG mice were anesthetized by isoflurane, and the hairs around flanks were removed using a hair removal cream (Nair). A small opening was then made within the 1-cm diameter area free of hairs to allow transfer of a piece of minced tumor into the space under the skin. The opening was then closed with tissue adhesive (GLUture, Topical Adhesive #503763). Allografting of mLPS cells into NRG mice was performed by subcutaneously injecting 2 x 106 mLPS1 and mLPS2 cells resuspended in 50 μL PBS into left and right flanks of the same mouse, respectively. Tumor growth was monitored every 2–3 days and the tumor was collected once it reached a volume of 1,000 mm3. Tumor width and length were measured using a caliper and volume (V) calculated using the formula: width x width x length/2 = V (mm3). Tumors were dissected and fixed with 4% paraformaldehyde (PFA) for histology and immunohistochemical analysis.
2.3. Cell culture, growth analysis, and adipogenic differentiation
To derive LPS cells, tumor tissues were minced using scissors and cultured in 6-cm tissue culture plates in RPMI-1640 medium supplemented with 10% FBS and penicillin/streptomycin. The medium was changed on the third day, with the floating tissue removed. The tumor cells growing around the attached tumor tissue were passaged every 5–7 days. Cell proliferation was evaluated by counting cell numbers using a hemocytometer. Cells at a density of 5 x 104 cells/well were seeded onto 24-well plates and incubated at 37 °C in 5% CO2 for 1 – 4 days. Cells were dissociated using trypsin (0.25%), neutralized with FBS-containing culture medium, and cell viability was measured using Trypan Blue.
For adipogenic differentiation of LPS cells, cells were seeded onto 24-well culture plates at a density of 1X105 cells per well and cultured in RPMI-1640 medium at 37 °C and 5% CO2 until reaching 90% confluency. To induce adipogenic differentiation, the culture medium was replaced with the differentiation medium containing 33 μM Biotin, 0.5 μM human insulin, 17 μM Pantothenate, 0.1 μM Dexamethasone, 2 nM Triiodothyronine, 500 μM IBMX (3-isobutyl-1-methylxanthine), 30 μM Indomethacin, and 2% FBS. The cells were differentiated for eight days with the medium refreshed every three days. Adipogenic differentiation was confirmed by staining with BODIPY (Thermo Scientific #D6003), and Hoechst 33342 (Invitrogen #H3570) was used to counterstain nuclei. Briefly, cells were fixed with 4% PFA for 30 min at room temperature followed by 3 x 10 min washes with PBS. The fixed cells were incubated with BODIPY (1:5000) and Hoechst 33342 (1:10000) diluted in PBS at 37 °C for 15 minutes, followed by 2 x 10 min washes with PBS. Images were acquired using a fluorescence microscope (Leica DMI6000B).
2.4. Flow cytometric cell sorting and cell population identification
NICD overexpressing cells were sorted using fluorescence-activated cell sorting (FACS) based on green fluorescent protein (GFP) that is expressed after NICD following an internal ribosome entry site IRES (refer to Fig. 1B for illustration of the RosaNICD locus). Primary cells derived from serial transplanted LPS (after 3 consecutive transplantation) were sorted at Passage #15 to isolate GFP+ cells (mLPS1) and GFP− cells (mLPS2). The resulting GFP+ cells were continuously cultured and re-sorted at passages 30 and 45 to collect GFP+ (the brightest 20%) and to get rid of GFP− cells. The cell sorting and analysis were performed on BD FACS Aria III at the Purdue Flow Cytometry and Cell Separation Facility.
Figure 1. Establishment of mouse LPS cells.
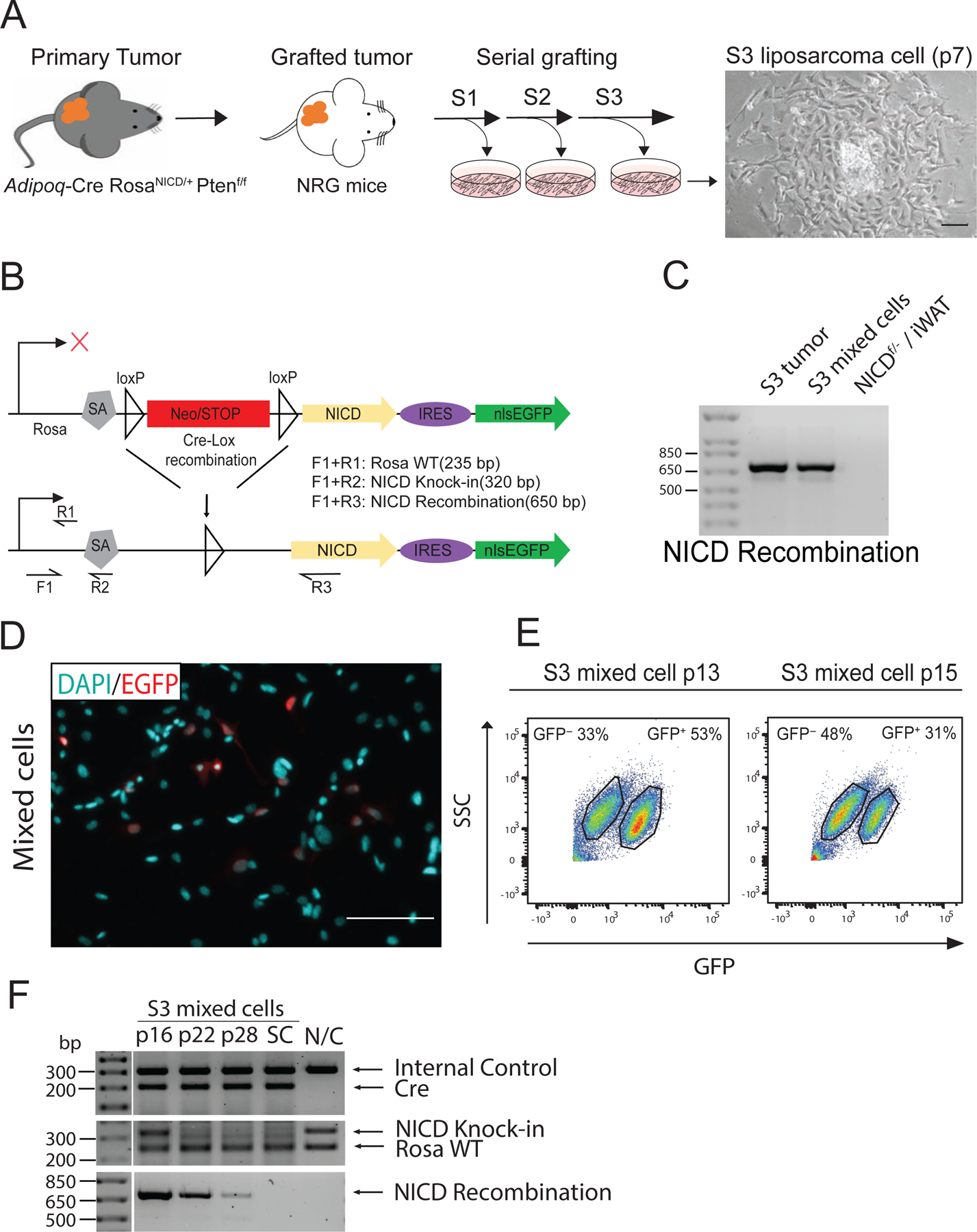
(A) The workflow for generating primary LPS cells by serial tumor grafting, and a phase contrast image of a clone-like primary mouse LPS cells derived from a tumor graft. S: tumor serial transplantation numbers, p: cell passage numbers. (B) Schematic representation of the targeted genetic locus in RosaNICD knock-in mice. The forward primer IMR0883(F1) and reverse primer IMR8038(R1) recognize the wild type Rosa locus sequence (Rosa WT) and amplify a 235 base pair sequence. The forward primer IMR0883(F1) and reverse primer IMR8039(R2) recognize the NICD knock-in region (NICD Knock-in) at the Rosa locus and amplify a 320 base pair sequence. The forward primer IMR0883(F1) and reverse primer NICD-5477(R3) recognize the activated NICD region (NICD Recombination) and amplify a 650 base pair sequence. (C) PCR-based genomic recombination analysis of RosaNICD regions in tumor tissue and mLPS mixed cells. Cre-mediated recombination of loxP sites resulted in a NICD recombination band at 650 bp. (D) Immunofluorescence staining of GFP protein (red) in mLPS, and nuclear stain by DAPI (cyan). Scale bars, 100 m. (E) Flow cytometry analysis of GFP+ cell population in mixed primary mLPS cells at p13 and p15. (F) Genomic recombination of RosaNICD regions in mixed mLPS cells at p16, p22, and p28. SC: cells in a single colony derived from mixed mLPS cells. N/C: Negative Control, from Adipoq-Cre− /NICDf/− white adipocyte tissues.
2.5. Transfection of sgRNAs and clonal isolation of CRISPR-edited cells
Gene editing was conducted by clustered regularly interspaced short palindromic repeats (CRISPR) method catalyzed by CRISPR-associated protein 9 (CAS9), directed by guide RNA (gRNA) that recognizes specific sequences of NICD. Briefly, LPS cells (1 × 10 6) were seeded in a 6-cm plate and co-transfected with 2 µg gRNA and CAS9, using Lipofectamine 2000 transfection reagent (ThermoFisher # 11668019). The gRNA is cloned in the pSpCas9(BB)-2A-Puro (PX459) V2.0 plasmid separately. Three NICD gRNAs were used to target different regions of the NICD cassette. The sequences were: sgRNA#1 (5’ to 3’ GACCAAGAAGTTCCGGTTTG), sgRNA#2 (5’ to 3’ GTGTCTTCCAGATCCTGCTC), sgRNA#3 (5’ to 3’ CGTGGATGACCTAGGCAAGT).
Forty-eight hours after transfection, cells were treated with 1.2 µg/ml puromycin in order to select cells expressing Cas9 for clonal selection. Puromycin selection was maintained for 2 days. After selected cells were recovered, single cells were isolated by FACS and grown in a 96-well plate. Cells that formed colonies were passaged several times until sufficient cells were available for analyzing genomic DNA and RNA.
2.6. PCR analysis of genomic DNA recombination
Cultured cells were detached by trypsin and centrifuged at 1000 rpm, and the cell pellets were washed by PBS and stored for further analysis. Tissue samples were minced by scissors and homogenized by sonication. Cell pellets or tissue homogenates were lysed using digestion buffer (100 mM MgCl, 10 mM Tris HCl pH 8, 25 mM EDTA pH 8, 0.5% SDS, 0.1 mg/ml proteinase K) and incubated at 50 °C for overnight. Genomic DNA was extracted using phenol/chloroform, and purified DNA was resuspended in 10 mM Tris-HCl buffer at pH 8.5. The recombination of RosaLSL-NICD alleles was analyzed by PCR with 800 ng of genomic DNA. The primers used for PCR detection of recombination of RosaNICD and Adipoq-Cre alleles were (5’ to 3’): Adipoq-Cre Transgene forward primer ACGGACAGAAGCATTTTCCA and reverse primer GGATGTGCCATGTGAGTCTG; Adipoq-Cre WT forward primer CTAGGCCACAGAATTGAAAGATCT and reverse primer GTAGGTGGAAATTCTAGCATCATCC; RosaNICD WT, IMR0883(F1) AAAGTCGCTCTGAGTTGTTAT and IMR8038(R1) TAAGCCTGCCCAGAAGACTC; non-recombined RosaNICD allele, IMR0883(F1) and IMR8039(R2) GAAAGACCGCGAAGAGTTTG; Recombined RosaNICD allele, IMR0883(F1) and NICD-5477(R3) GATTGTCGTCCATCAGAGCACCATCTGAGG.
2.7. RNA isolation and qRT-PCR
Cells were rinsed with PBS and scraped from cell culture plates, and total RNA was extracted using TriReagent (Sigma-Aldrich #T9424). RNA was reverse transcribed to cDNA using M-MLV reverse transcriptase (Invitrogen #28025-021). Quantitative PCR (qPCR) was performed on a LightCycler 96 (Roche) real-time PCR system (Roche), using LightCycler 480 SYBR Green I Mastermix (Roche). 18S was used as internal control gene, and fold changes of gene expression were calculated by the comparative Ct method(∆∆Ct). The following amplification primers (Sigma) were used (5’ to 3’): 18s (Forward, AGTCCCTGCCCTTTGTACACA; Reverse, CGATCCGAGGGCCTCACTA), NICD (Forward, TGGCCTGCCTGTCTGGAACAACAGTTCA; Reverse, ACCCTTGCCTCAGTTCAAACACAAGATAC), Hes1 (Forward, CCAGCCAGTGTCAACACGA; Reverse, AATGCCGGGAGCTATCTTTCT), Hey1 (Forward, TGAATCCAGATGACCAGCTACTGT; Reverse, TACTTTCAGACTCCGATCGCTTAC), Heyl (Forward, TCCTAGCCAGAGATTCAGTGTCAC; Reverse, GTTTGTCTGCAACACCCTAGAGTG), Pparg (Forward, GGAAGACCACTCGCATTCCTT; Reverse, GTAATCAGCAACCATTGGGTCA) Ppargc1a (Forward, TATGGAGTGACATAGAGTGTGCT; Reverse, CCACTTCAATCCACCCAGAAAG), Adipoq (Forward, TGTTCCTCTTAATCCTGCCCA; Reverse, CCAACCTGCACAAGTTCCCTT), Cebpa (Forward, CAAGAACAGCAACGAGTACCG; Reverse, GTCACTGGTCAACTCCAGCAC), Leptin (Forward, GAGACCCCTGTGTCGGTTC; Reverse, CTGCGTGTGTGAAATGTCATTG), Mdm2 (Forward, TGTCTGTGTCTACCGAGGGTG; Reverse, TCCAACGGACTTTAACAACTTCA). Cdk4 (Forward, AAGGTCACCCTAGTGTTTGAGC; Reverse, CCGCTTAGAAACTGACGCATTAG), Dlk1 (Forward, AGTGCGAAACCTGGGTGTC; Reverse, GCCTCCTTGTTGAAAGTGGTCA), Rian (Forward, CTGTTGTGCCCTCCCTGGATG; Reverse, CCAGCTAGGCTGTGTAAATCATC), CD133 (Forward, CCTTGTGGTTCTTACGTTTGTTG; Reverse, CGTTGACGACATTCTCAAGCTG), CD105 (Forward, CCCTCTGCCCATTACCCTG; Reverse, GTAAACGTCACCTCACCCCTT), CD90 (Forward, TGCTCTCAGTCTTGCAGGTG; Reverse, TGGATGGAGTTATCCTTGGTGTT), CD73 (Forward, GGACATTTGACCTCGTCCAAT; Reverse, GGGCACTCGACACTTGGTG).
2.8. Protein extraction and western blot analysis
Cells were lysed using NP-40 buffer supplemented with protease inhibitor (Sigma #P8340). Protein concentration was determined using the BCA assay (Thermo Scientific #23228). Protein samples were subjected to electrophoresis and transferred onto a PVDF membrane for immunoblot analysis. The membrane was blocked by 5% non-fat milk for 1 hour at room temperature followed by overnight incubation at 4 °C with the following specific primary antibodies: Actin (Sigma #A5441, 1:5000), NICD (Sigma-Millipore #MAB5352, 1:1000), GFP (Xu, L. et al. PLoS Pathog. 2010, 1:1000). The membrane was subsequently incubated with species-specific HRP conjugated secondary antibodies for 1 hour at room temperature, and the images were acquired using the FluorChem R image system (ProteinSimple).
2.9. Histology
Fixed tumors were embedded into paraffin and sectioned at a thickness of 7 μm. The paraffin sections were deparaffinized and stained with hematoxylin and eosin (H&E) to identify nuclei and cytoplasm, respectively. In brief, the tissue section was deparaffinized by 3 x 20 s washes in xylene and hydrated in successive solutions of 100%, 95%, 90%, and 70% ethanol for 20 s each. After staining in hematoxylin solution for 15 min, tissue slides were rinsed in water for 1 minute. Sections were then stained briefly in Eosin for 30 seconds, and finally dehydrated in 70%, 90%, 95%, and 100% alcohol followed by 100% xylene rinses. The slides were air dried for 5 min before sealed with mounting media (Source Medical Products #9277722).
2.10. Immunofluorescence staining of tumor sections and cells
Tumors were embedded into OCT immediately after isolation from mice and frozen in dry ice-chilled isopentane. Frozen tissues were sectioned at a thickness of 20 μm, fixed with 4% PFA for 30 min, and washed three times with PBS. The fixed sections were subsequently neutralized with 100 mM glycine for 10 min at room temperature, washed with PBS, and blocked with the blocking buffer containing 5% goat serum, 0.2% Triton X-100, and 0.1% sodium azide for two hours at room temperature. Sections were then incubated with primary antibodies diluted in blocking buffer for overnight at 4°C. The primary antibodies used were: GFP (Xu, L. et al. PLoS Pathog. 2010, 1:300), NICD (Sigma-Millipore #MAB5352, 1:300), and Ki67 (Abcam #ab16667, 1:300). Secondary antibodies, including Goat anti-Mouse Alexa Fluor 488 (Thermo Fisher #A28175) and Goat anti-Rabbit Alexa Fluor 594 (Thermo Fisher #A32740), were diluted in PBS at 1:1000 and incubated for 1 hour at room temperature in the dark followed by counterstaining with Hoechst 33342 (1:10000) for 10 min. The stained sections were mounted using Fluoromount (Diagnostic Biosystems #K024), and images were taken using a fluorescence microscope (Leica DMI6000 B).
Cells were seeded on 12 mm coverslips immersed in 24-well plates at a density of 1 x 105 cells/well. After attachment, cells were fixed in 4% PFA at room temperature for 15 min and permeabilized with blocking buffer containing 5% goat serum, 0.2% Triton X-100, and 0.1% sodium azide for 1 hour at room temperature. Next, cells were incubated with primary antibodies at a 1:1000 dilution in blocking buffer and incubated at 4 °C overnight. Alexa 488-conjugated or Alexa 594-conjugated secondary antibodies diluted in PBS (1:1000) were then applied for 1 hour at room temperature in the dark, followed by DAPI staining (1:1000) diluted in PBS.
2.11. Statistical analysis
Data were presented as mean ± SEM. Statistical analysis and graphs were generated using Excel and GraphPad Prism software. In vitro studies were repeated at least three times and replicate numbers were provided in figure legends. Statistical analysis was performed by two tailed, unpaired t-test, with the following significance indications for p values: *p< 0.05, **p< 0.001.
3. Results
3.1. Serial grafting of Notch-driven LPS enriches tumor cells
We have recently reported that constitutive activation of Notch signaling through overexpression of NOTCH1 intracellular domain (RosaNICD) in adipocytes leads to the development of LPS in mice [19]. To understand the cellular heterogeneity of the LPS and molecular mechanisms underlying tumorigenic transformation, we attempted to derive and grow cells from primary tumors of the RosaNICD knock-in mice. However, cells failed to thrive in culture and died upon passaging. We then adopted a serial transplantation protocol to enrich TICs in vivo [23]. We collected tissue implants (2 x 2 x 2 mm) from the high-density region of primary liposarcomas isolated from the RosaNICD knock-in mice, and transplanted them to the subcutaneous space near the back of immunodeficient NRG mice. After 35 days, 66% of implanted tumor pieces reached a volume greater than 1000 mm3. Tissue implants from the fastest growing grafted LPS were again collected and transplanted. Tumor growth was faster and more homogeneous upon re-grafting. After three consecutive grafts, tumors all grew rapidly, reaching at least 1000 mm3 in 21 days. These observations indicate that serial grafting of primary LPS tissues leads to enrichment of fast-growing cells with tumor-initiating capacity.
Cells from serial transplantation #3 (S3), defined as mLPS (for murine LPS) cells, were then cultured in tissue culture plates. The mLPS cells grew rapidly and formed distinct colonies with homogeneous cell morphology within each colony (Fig. 1A). Since tumors are known to be composed of multiple types of cells with heterogeneous properties [24], we evaluated the S3 mLPS cells to confirm the presence of transgenic recombination and GFP expression as indication of NICD overexpression (Fig. 1B). The genomic DNA recombination assay revealed the existence of loxP recombination (Fig. 1C), indicating RosaNICD expression in the mixture of S3 mLPS cells. We also evaluated the expression of GFP in S3 mLPS cells by immunofluorescence staining. Surprisingly, we found that the mixture of S3 mLPS cells contained both GFP+ and GFP− cells (Fig. 1D), indicating that the tumor cells are heterogeneous in NICD expression. We then performed flow cytometry analysis to assess the proportion of GFP+ and GFP− cells and found that the proportion of GFP+ cells decreased with successive passages (Fig.1E). Consistently, the intensity of NICD genomic DNA recombination bands (relative to non-recombined bands) also decreased with increasing number of passages (Fig. 1F). By passage #45 without FACS-enrichment of GFP+ cells, NICD recombination and GFP+ cells were completely lost (data not shown). These data suggest that the GFP− (NICD non-overexpressing, NICDWT) cells outgrew the GFP+ (NICD-overexpressing, NICDOE) cells when they were grown together. Collectively, we successfully derived a heterogeneous population of tumor cells from primary LPS of the RosaNICD mice after serial tumor grafting.
3.2. Establishing stable mLPS1 (NICDOE) and mLPS2 (NICDWT) LPS cells
As the S3 mLPS cells contained both GFP+ (NICDOE) and GFP− (NICDWT) cells, we used FACS to separate these cells based on GFP expression (Fig. 2A). At passage 15 (p15), 30.9% of the S3 mLPS cells were GFP+ (Fig. 2B). The sorted GFP+ cells expressed higher levels of NICD compared to the GFP− cells at p20 (Fig. 2C), confirming their Notch activation status. We therefore referred to the GFP+ cells as mLPS1 cells or NICDOE cells, and the GFP− cells as mLPS2 cells or NICDWT cells. However, the percentage GFP+ mLPS1 cells gradually declined with passages, down to 36.6% at p30 (Fig. 2B). We then re-sorted the mLPS1 cells at p30 and found that 86.5% of the cells remained GFP+ at p45 (Fig. 2B), suggesting that repeated sorting enriches GFP+ cells. Encouraged by this observation, we performed the third consecutive rounds of FACS at p45 to establish a pure and stable population of GFP+ (NICDOE) cells, which retained GFP expression in 98% of the cells at p60 (Fig. 2B). Genomic DNA recombination analysis (Fig. 2D) confirmed NICD recombination in the GFP+ cells, while the recombination band was not detected in the GFP− cells. The genomic DNA recombination analysis also revealed that both GFP+ and GFP− cells were Adipoq-Cre+, suggesting that they originate from the same primary tumor (NRG recipient mice do not carry the Adipoq-Cre transgene). To understand the stability of mLPS1 and mLPS2 cells, we used FACS analysis and immunofluorescence staining of mLPS1 cells at p75, which were continuously cultured for 30 passages after the last sorting (p45), and mLPS2 cells cultured for 37 passages after the last sorting. The mLPS1 cell population contained more than 95% of GFP+ cells, and the mLPS2 cells were more than 99% GFP− (Fig. 2E), indicating these cells are highly stable. Hence, we established stable subpopulations of NICDOE (mLPS1) and NICDWT (mLPS2) LPS cells through FACS-mediated serial enrichment.
Figure 2. Enrichment of GFP+ LPS cells from primary mLPS cells.
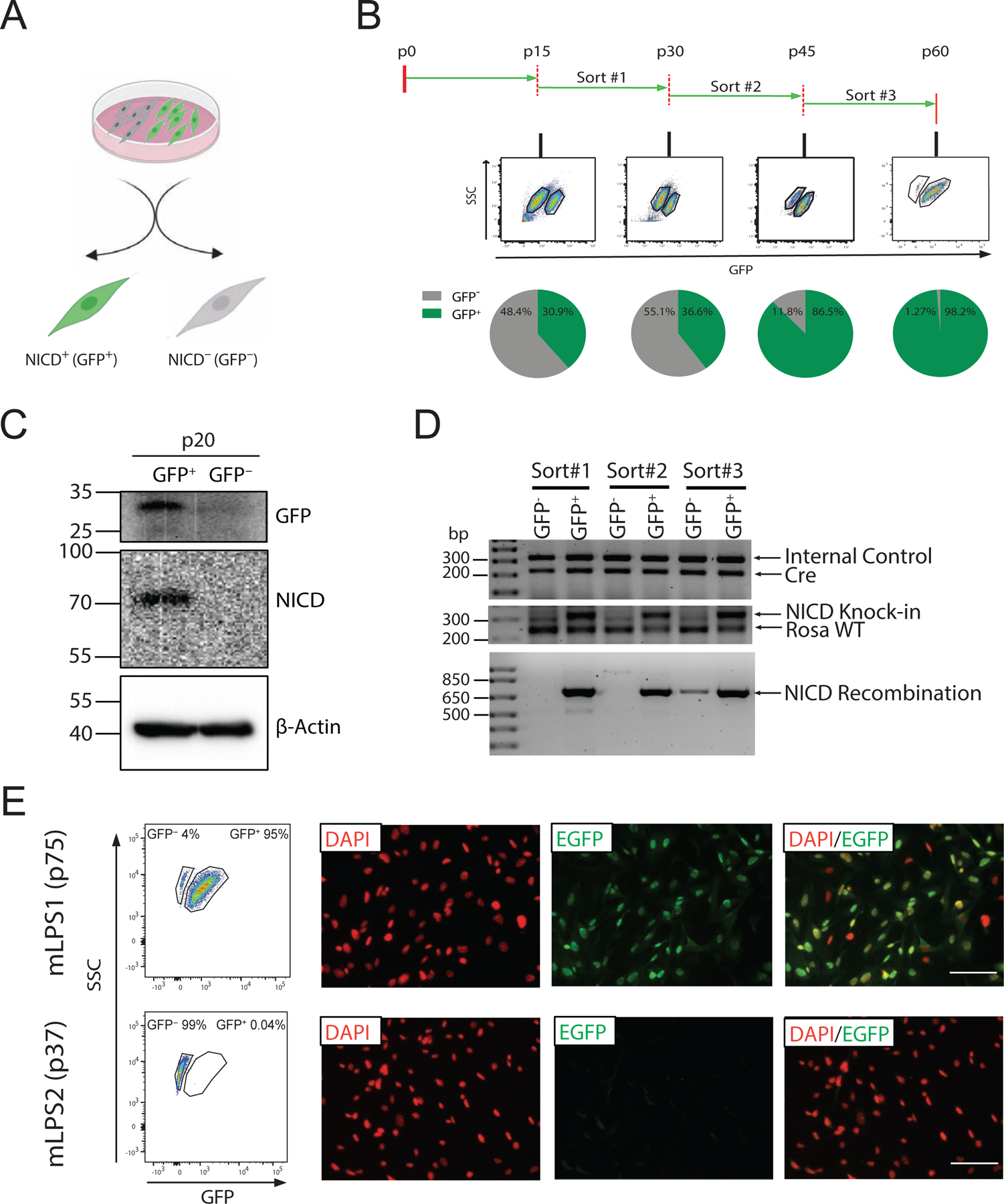
(A) Schematic representation of the subpopulations of mLPS mixed cells; GFP+ (NICD overexpressing, NICDOE) and GFP− (NICD non-overexpressing, NICDWT) cells. (B) Fluorescence activated cell sorting of GFP+ cells at p15, p30 and p45, sequentially. (C) Western blot analysis of GFP+ and GFP− mLPS cells showing NICD and GFP protein levels. (D) Genomic DNA analysis of different batch of sorted GFP+, GFP− cells at various passages. Cre represents Adipoq-Cre, Rosa WT represents wild type Rosa locus, NICD Recombination represent Adipoq-Cre mediated recombination at the NICD knock-in region. (E) Flow cytometry analysis of mLPS1 cells and mLPS2 cells at late passage by GFP fluorescence, and immune fluorescence staining of GFP protein (green) and nuclei by DAPI (red). Scale bars, 100 μm.
3.3. Characteristics of mLPS1 (NICDOE) and mLPS2 (NICDWT) LPS cells
Having established two subpopulations of mLPS cells exhibiting different levels of Notch signaling activity, we sought to characterize the growth and gene expression of these cells. We first evaluated the growth curves of mLPS1 and mLPS2 cells at early (p20), intermediate (p33), and late passages (p51). Overall all cells grew slowly in the first 24 h after passaging (Fig. 3A–C), and exponential growth started after 24 h in p20 and p33 cells (Fig. S1A–C). The mLPS2 cells at various passages exhibited very similar growth rates, reaching identical cell density after 3 days of culture (Fig. 3A–C). The proliferation of mLPS1 cells, however, was accelerated with serial FACS-enrichment (Fig. 3A–C). At p20 the mLPS1 cells proliferated more slowly than the mLPS2 cells based on the slope of exponential growth (Fig. 1A, Fig. S1A). This result explains the gradual reduction in the percentage of GFP+ mLPS cells at p30 prior to the 2nd sorting, as the FACS-purified GFP+ cells (after the 1st sorting) presumably contained few contaminating GFP− cells that overtook the GFP+ cells gradually. However, the proliferation rate of mLPS1 cells was faster after the second FACS-purification, exhibiting a similar rate to that of the mLPS2 cells (Fig. 3B, Fig. S1B). After the third FACS-purification, strikingly, the mLPS1 cells proliferated significantly faster than the mLPS2 cells (Fig. 3C, Fig. S1C). These results demonstrate that sequential FACS purification of GFP+ enriches a subset of fast-growing NICDOE cells.
Figure 3. Growth and gene expression of mLPS1 and mLPS2 cells.
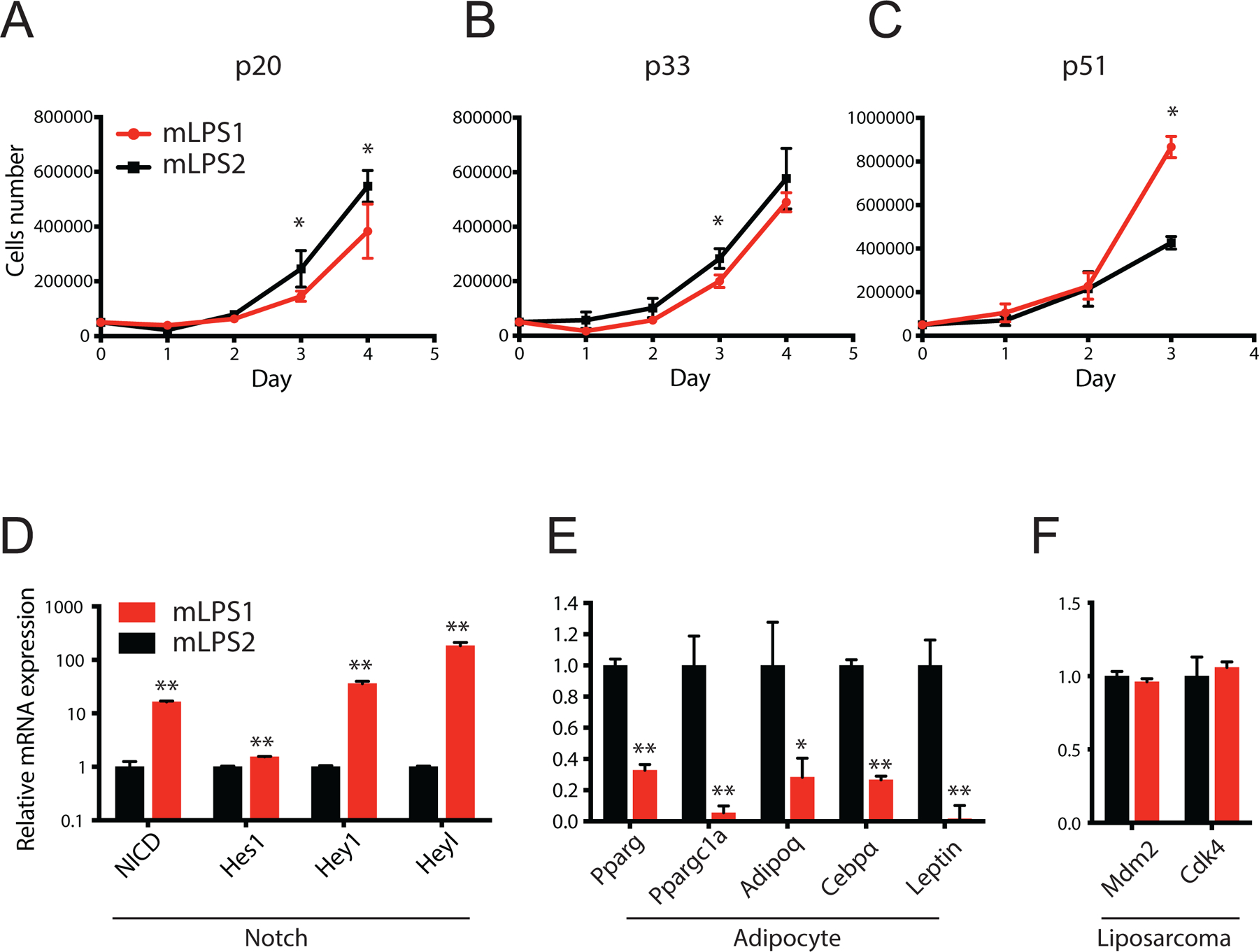
(A, B, C) Growth curves of mLPS1 and mLPS2 cells at p20, p33 and p51, respectively. Cells were seeded in 24-well plates at 5 x 104 cells per well and cultured for 4 days. Numbers of live cells were determined by cell counting with trypan blue staining. (D) Relative mRNA levels of NICD and Notch target genes (Hes1, Hey1, and Heyl) in mLPS1 and mLPS2 cells. (E) Relative mRNA levels of mature adipocyte markers (Pparg, Ppargc1a, Adipoq, Cebpα, Leptin) in mLPS1 and mLPS2 cells. (F) Relative mRNA levels of LPS markers (Mdm2, CDK4) in mLPS1 and mLPS2 cells. Data are presented as mean ± SEM, N=3. *p< 0.05, **p< 0.001.
To further characterize the mLPS1 and mLPS2 cells, we evaluated the expression of Notch signaling related genes, adipocyte markers, and LPS markers of these cells. Compared to mLPS2 cells, the mLPS1 cells expressed higher levels of Notch signaling genes (NICD, Hey1, Heyl) but lower levels of adipocyte makers, including Pparg, Ppargc1a, Adipoq, Cebpa, and Leptin (Fig. 3D, E). These results suggest that mLPS1 cells exhibit more active Notch signaling, associated with loss of adipocyte marker expression. Interestingly, both mLPS1 and mLPS2 cells showed comparable mRNA levels of Mdm2 and Cdk4 (Fig. 3F), two signature markers of human dedifferentiated LPS. These results indicate that mLPS1 and mLPS2 cells are distinct in their expression of Notch signaling and mature adipocyte genes but exhibit similar expression of DDLPS markers.
The differing expression levels of mature adipocyte markers in mLPS1 and mLPS2 led us to examine the adipogenic differentiation capacity of the two cell types. When induced by adipogenic culture media, both mLPS1 and mLPS2 cells underwent significant morphological changes (Fig. 4A), some cells accumulated lipid droplets in the cytoplasm as marked by BODIPY (Fig. 4B). However, the mLPS2 cells had more robust differentiation efficiency compared to mLPS1 cells. This observation suggests that mLPS2 cells may be more similar to human WDLPS cells that express higher levels of adipocyte markers and have a higher tendency to re-differentiate into adipocytes than DDLPS cells [25]. Furthermore, a substantial fraction of mLPS1 cells died and floated in response to adipogenic induction medium (Fig. 4A), suggesting that they are intrinsically resistant to adipogenic differentiation. As the mLPS1 have more active Notch signaling, these results indicate activation of Notch signaling inhibits re-differentiation of LPS cells.
Figure 4. Differentiation of mLPS1 and mLPS2 cells in culture.
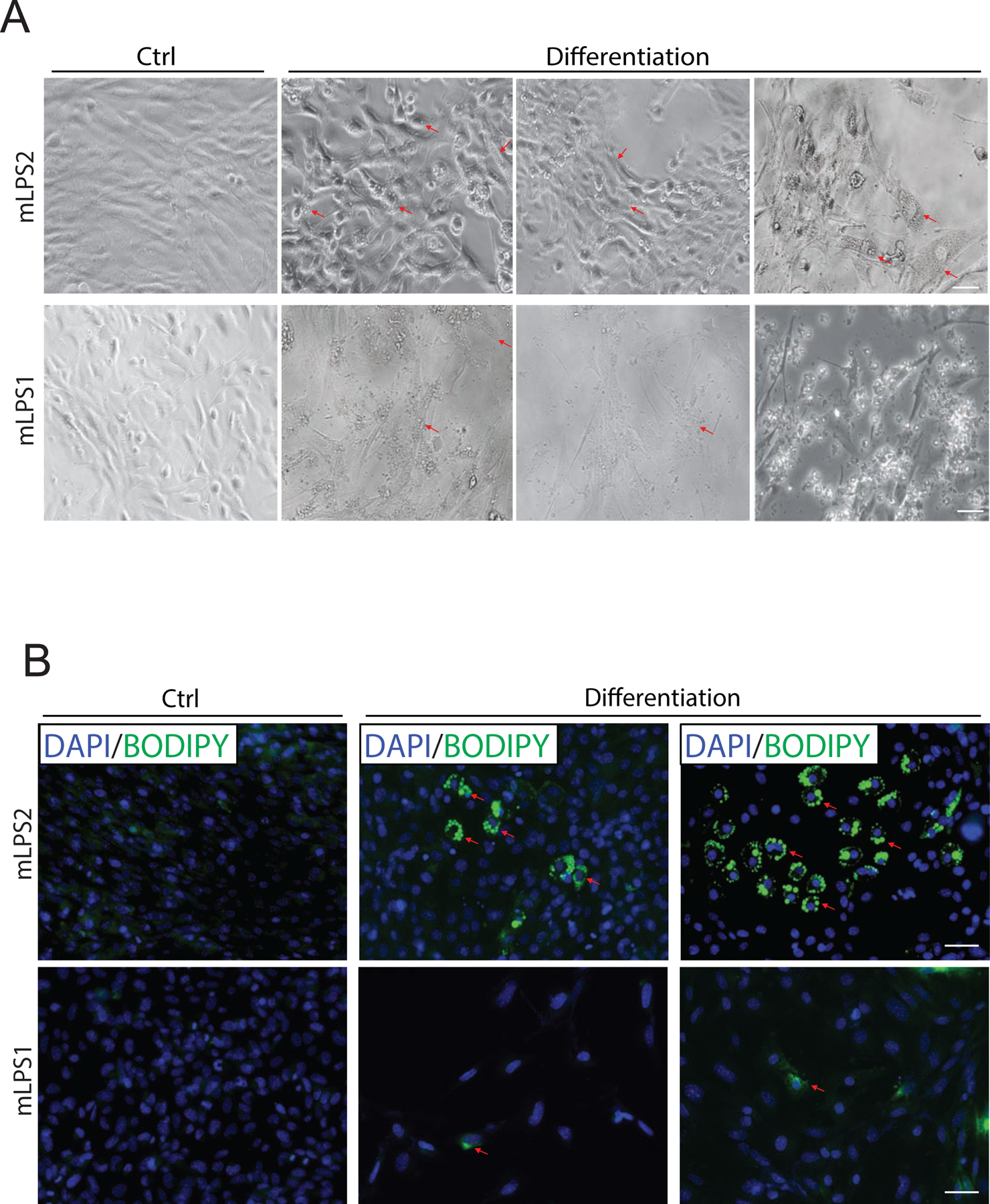
(A) Phase contrast images of mLPS1 cells and mLPS2 cells after treated with induction medium to induce adipogenesis for 8 days. The arrowheads indicate lipid droplets. (B) Fluorescent staining images of lipid droplets (labeled with BODIPY in green) after differentiation, visualized by immunofluorescence microscopy. Nuclei were counterstained with DAPI (blue). Scale bars, 100 μm. N=3.
3.4. mLPS1 cells express markers of TICs and are tumorigenic in vivo
As the mLPS1 cells are resistant to adipogenic differentiation, we further examined if they resemble TICs. Indeed, the mLPS1 cells expressed significantly higher levels of Cd133, an established marker of cancer stem cells, than mLPS2 cells (Fig. 5A). The mLPS1 cells also expressed higher levels of mesenchymal stem cell markers, including Cd90 and Cd105 (Fig. 5A). We previously reported that the Notch-driven primary LPS expressed high levels of the preadipocyte marker Dlk1 (Delta-like 1 homolog) [19]. Consistently, we found that mLPS1 expressed significantly higher levels of Dlk1 than the mLPS2 cells (Fig. 5B). Interestingly, expression of the non-coding RNA gene Rian, which is located in the Dlk1–Dio3 imprinting locus, is elevated by more than 20 times in mLPS1 compared to mLPS2 cells (Fig. 5B). This is consistent to previous reports that activation of Dlk1–Dio3 locus genes is implicated in reprogramming of induced pluripotent cells and deregulated in cancer [26].
Figure 5. Notch-activated mLPS1 cells contributes to tumorigenesis in vivo.
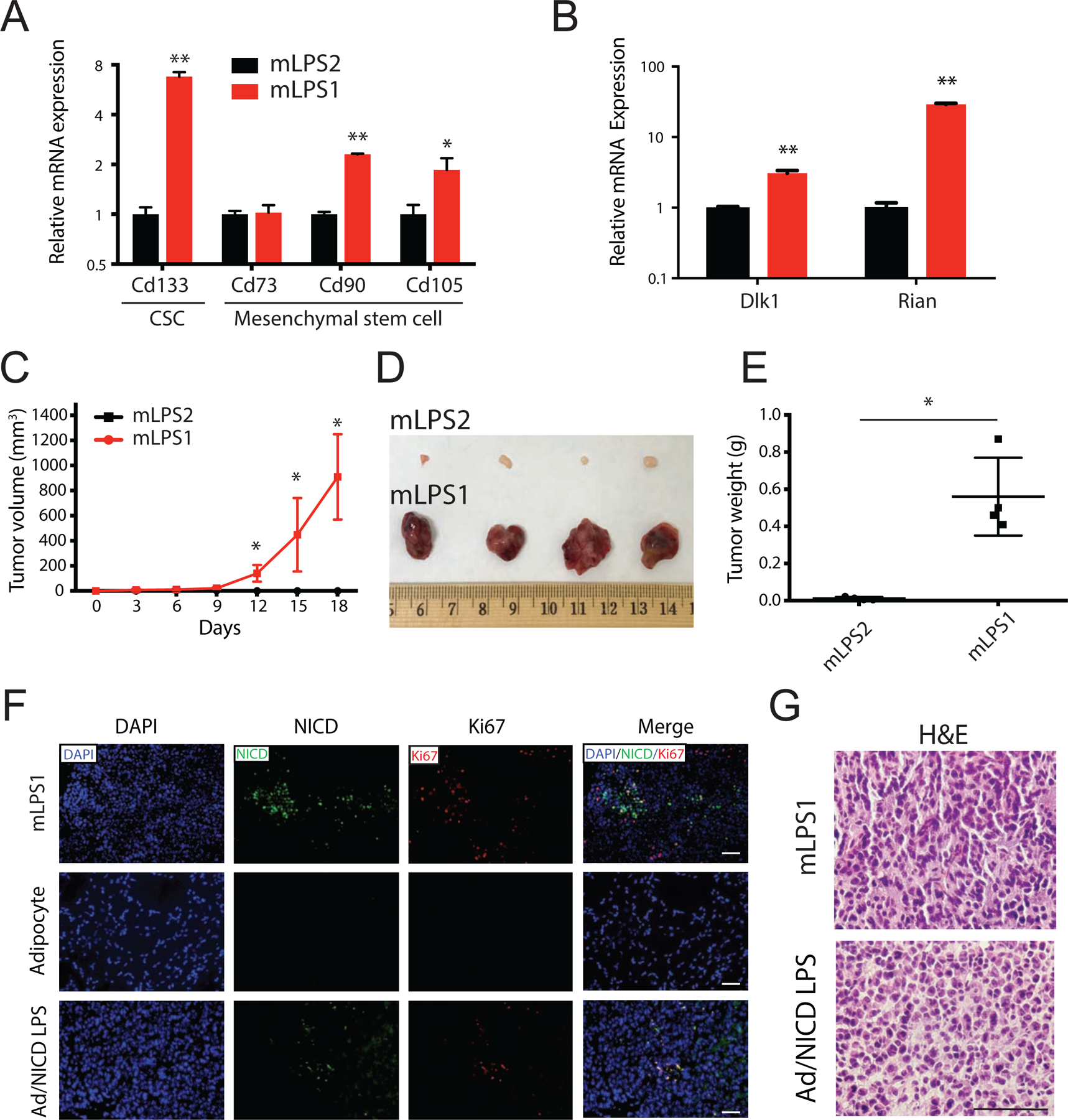
(A) Relative mRNA levels of cancer stem cell marker CD133 and several mesenchymal stem cell markers (CD73, CD90, and CD105) in mLPS1 and mLPS2 cells. (B) Relative mRNA levels of Dlk1 and long non-coding RNA Rian in the Dlk1–Dio3 imprinted region in mLPS1 and mLPS2 cells. Data are presented as mean ± SEM, N = 3. *p< 0.05, **p< 0.001. (C) Tumorigenicity of mLPS1 and mLPS2 cells examined by injecting 2 x 106 mLPS1 cells subcutaneously to the left flank and 2 x 106 mLPS2 cell to the right flank of NRG mice (N=4). The tumor volume was calculated based on caliper measurement of tumor width and length. (D) Images of the grafted tumors after surgical removal from the NRG recipient mice. (E) Average weights of the tumors shown in B. (F) Immunofluorescence staining showing relative expression of NICD (Green) and Ki67 (Red, proliferation marker) in mLPS1-derived allograft tumor, mouse inguinal adipose tissue and primary LPS tissue from Adipoq-Cre/RosaNICD mice. Nuclei were stained by DAPI. (G) H&E staining of tumor sections showing comparable pathology of mLPS1-derived allograft tumors and Notch-driven primary LPS tumors. Nuclei were stained with hematoxylin (blue), and extracellular matrix and cytoplasm were stained with Eosin (pink). Imaging captured using a Leica DMI 6000b microscope. Scale bars, 100 μm. Data is presented as mean ± SEM, N=4. *p< 0.05.
To directly examine the TICs function and the tumorigenic capacity of mLPS1 and mLPS2 cells, we performed cell transplantation by injecting cells subcutaneously into NRG mice. Strikingly, only NICDOE mLPS1 cells gave rise to tumors in vivo after transplantation (Fig. 5C–E ). Although the NICDWT mLPS2 cells were highly proliferative in culture and shared similarities with well-differentiated LPS, they failed to grow and lacked tumorigenic ability in vivo (Fig. 5C, D). In contrast, the NICDOE mLPS1 cells grew rapidly and reached 900 mm3 (0.5 g in weight) in 18 days after transplantation (Fig. 5C, E). The correlation of NICD expression with proliferating cancer cells was confirmed by immunofluorescence staining with the cell proliferation marker Ki67. Co-expression of NICD and Ki67 was detected in the tumor tissues derived from the mLPS1 cells and Adipoq-Cre/RosaNICD primary LPS, but not in normal mouse adipose tissue (Fig. 5F). The higher abundance of NICD and Ki67 staining in mLPS1-dervied tumors compared to Adipoq-Cre/RosaNICD primary tumors (Fig. 5F) indicates that the mLPS1 cells are more enriched with TICs after serial transplantations and in vitro selections. Moreover, H&E staining showed that both mLPS1-derived LPS and Adipoq-Cre/RosaNICD primary LPS possessed similar histological features of high grade tumor (Fig. 5G). Together, these data demonstrate a critical role for Notch signaling in promoting TICs potential and driving tumorigenesis of LPS cells in vivo.
3.5. Serial transplantation and culture enrich LPS and TIC markers
The observation that only mLPS1 cells give rise to LPS in vivo prompted us to further examine how serial grafting and culture lead to the gene signature in mLPS1 cells. We hypothesized that the selection pressure enriches cancer stem cells with the properties of self-renewal and capacity for tumor formation in serially grafted tumors [16,17]. To test the hypothesis we compared mLPS1 cells of different passages to inguinal while adipose tissue (iWAT) and primary LPS of Adipoq-Cre/RosaNICD mice, as well as serially grafted LPS (Fig. 6). We first confirmed that the grafted tumors and mLPS1 cells expressed high levels of NICD, over 100 times higher than iWAT and 10 times higher than primary LPS (Fig. 6A). Next, expression of markers of cancer stem cells (CD133), mesenchymal stem cells (CD90, CD105, CD73) and DDLPS (MDM2) were all robustly increased in grafted tumors than in primary tumors (Fig. 6B–F), suggesting that serial grafting enriches cells with stem cells and LPS properties. We also found that the mRNA levels of Cd133 increased robustly during serial transplantation and mLPS1 derivation, reaching the highest level in p21 mLPS1 cells (Fig. 6B). The serially grafted tumors also expressed higher levels of Cd90 and Cd73 than did the mLPS1 cells, but the levels of Cd105 and Mdm2 were comparable in grafted tumors and mLPS1 cells (Fig. 6C–F). We also examined expression of mitochondrial biogenesis and adipocyte markers, which were highly expressed in iWAT compared to LPS tissue and cells (Fig. 6G–H). Among LPS tissue and cells, the mLPS1 cells expressed significantly lower levels of Ppargc1a and Leptin than did the grafted tumors (Fig. 6G–H). Interestingly, the grafted tumors expressed higher levels of Ppargc1a and Leptin than did the primary tumors (Fig. 6G–H), suggesting that the grafted tumors may contain a population of well-differentiated LPS cells or adipocytes. These results together demonstrate that serial tumor transplantation and following cell culture selection enrich specific subtypes of cells, with mLPS1 cells recapitulating many gene signatures of serial grafted LPS tissue.
Figure 6. Expression of LPS, cancer stem cell, and adipogenic markers at various stages during derivation of mLPS1 cells.
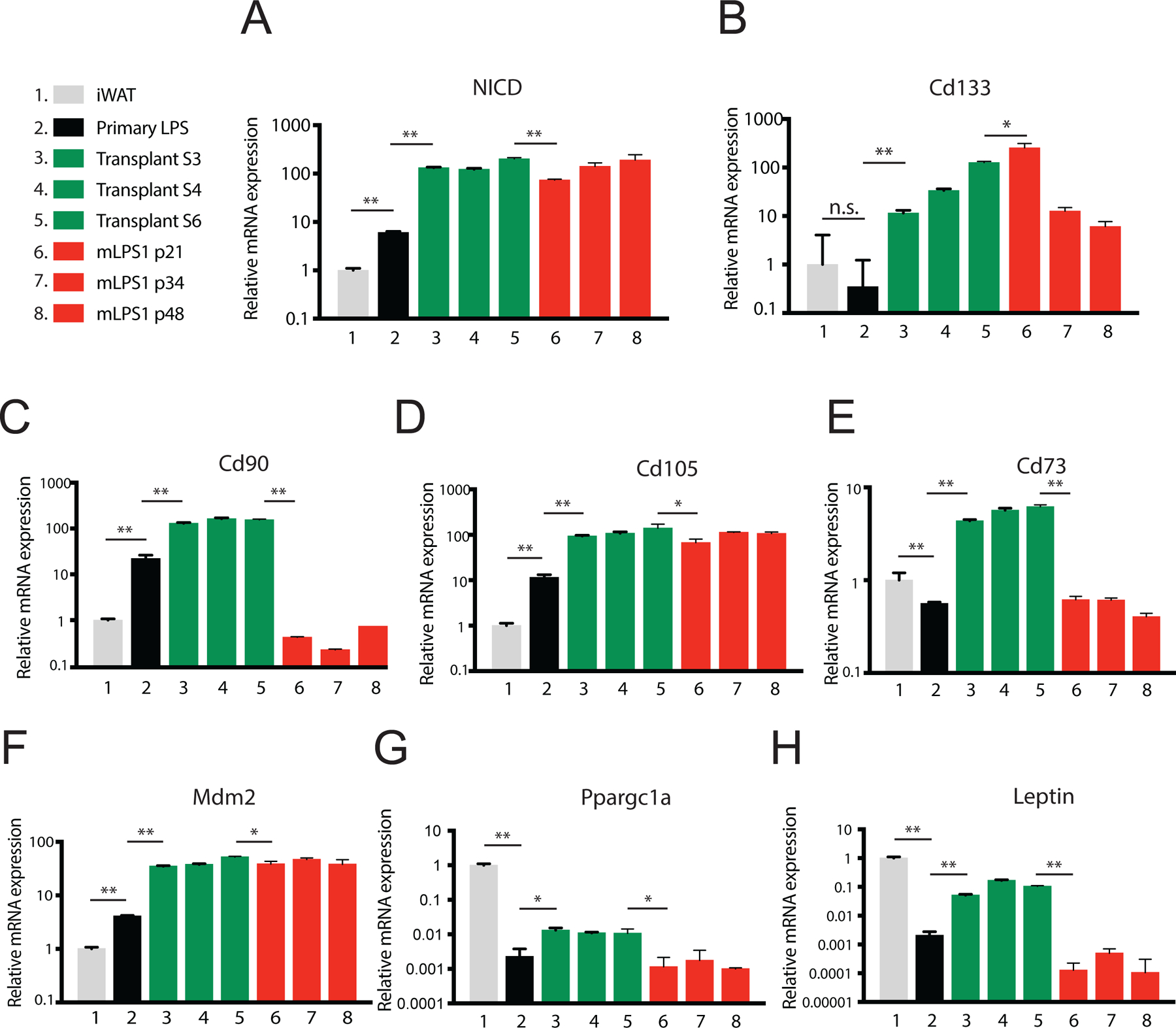
Relative mRNA levels of NICD (A); cancer stem cell marker CD133 (B); mesenchymal stem cell markers CD73, CD90, and CD105 (C-E); LPS marker Mdm2 (F); and adipogenic markers Ppargc1a and Letpin (G-H) at various stages were determined by qPCR. iWAT, inguinal white adipose tissue; S1-S3: serially transplanted LPS; p: cell passage numbers of mLPS1 cells. Data are presented as mean ± SEM, N=3. *p< 0.05, **p< 0.001.
3.6. Notch activation is necessary for tumorigenicity of mLPS1 cells
To directly confirm that NICD overexpression in mLPS1 cells underlies their TIC properties and function, we used CRISPR/CAS9 to disrupt the RosaNICD cassette in mLPS1 cells. Three gRNAs were designed to specifically target the RosaNICD sequence without affecting the endogenous Notch1 sequence (Fig. 7A). CRISPR targeting of NICD in mLPS1 cells (mLPS1-sgNICD) reduced the mRNA level of NICD by over 20-fold, down to a level comparable to that of mLPS2 cells (Fig. 7B), indicating endogenous Notch1 expression was unaltered. Accordingly, CRISPR-targeting dramatically decreased the level of Notch target genes Hes1, Hey, and Heyl in mLPS1 cells (Fig. 7C–E) and upregulated the expression of adipogenic genes Pparg and Ppargc1a (Fig. 7F, G). However, the expression of these Notch target and adipogenic genes in the mLPS1-sgNICD group was not fully restored to the levels of mLPS2 cells (Fig. 7C–G), suggesting that NICD overexpression may have led to other irreversible changes in mLPS1 cells. Nonetheless, disruption of RosaNICD in mLPS1 cells completely abrogated their tumorigenicity after transplanted into NRG mice, while the untargeted mLPS1 cells gave rise to LPS within 3 weeks in all four recipients (Fig. 7H–J). Immunofluorescence staining of tissues resulting from the grafted cells confirmed the loss of NICD and proliferation marker Ki67 in the mLPS1-sgNICD group (Fig. 7K). Lastly, H&E staining revealed that tissues derived from the grafted mLPS1-sgNICD cells exhibited a fibroblastic morphology distinct from that of the high-grade tumors derived from the untargeted mLPS1 cells (Fig. 7L). Taken together, these data provide compelling evidence for a causative role of Notch activity in tumorigenesis of mLPS1 cells.
Figure 7. NICD is responsible for the tumorigenic potential of mLPS1 cells.
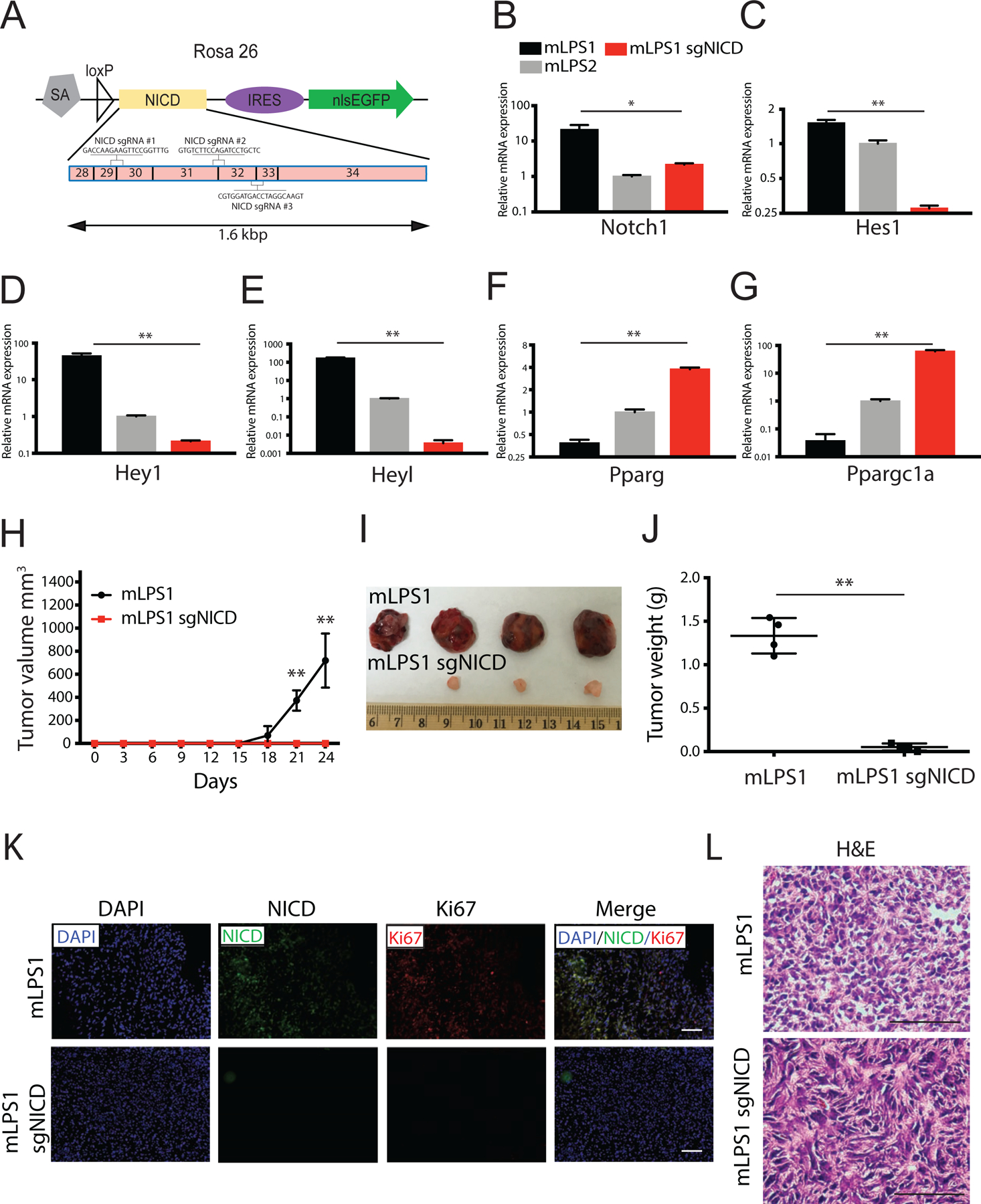
(A) Genomic structure of RosaNICD cassette. The NICD sequences from exon 28 to 34 were targeted by three single strand guide RNAs: sgRNAs #1, #2, and #3. All three gRNAs span two exons and were co-transfected into cells. (B, C, D, E) Relative mRNA levels of Notch signaling targeted genes (Notch1, Hes1, Hey1, Heyl) in mLPS1, mLPS2, and mLPS1-sgRNA cells (mLPS1 transfected with 2 ug guide RNA and CAS9). (F, G) Relative mRNA levels of mature adipocyte markers in mLPS1, mLPS2, and mLPS1-sgRNA cells. (H) Tumorigenicity of mLPS1 and mLPS1-sgRNA cells determined by injecting 2 x 106 mLPS1 cells subcutaneously to the left flanks and 2 x 106 mLPS1-sgRNA cells to right flanks of NRG mice (n=4). The tumor volume was calculated based on width and length measured with a caliper. (I) Images of the grafted tumors after surgical removal from the NRG recipient mice. (J) Average weights of the tumors shown in H. (K) Immunofluorescent staining showing relative expression of NICD and Ki67 in mLPS1-derived allograft tumor, and mLPS1-sgRNA tissue. Nuclei were stained by DAPI. (L) H&E staining of tumor sections. Nuclei were stained with hematoxylin (blue), and extracellular matrix and cytoplasm were stained with Eosin (pink). Images were captured under a Leica DMI 6000b microscope. Scale bars, 100 μm. Data is presented as mean ± SEM, N=4, *p< 0.05.
4. Discussion
Activation and inactivation of Notch signaling have been implicated in tumorigenesis of various cancer types and cancer stem cell populations [4,27]. However, there are few studies using constitutively activated Notch in animal and cell models to directly connect Notch activation to tumor-initiating ability [28–31]. Our previous study revealed that Notch activation drives tumorigenic transformation of adipocytes in mice [19]. However, it is unclear if all cells in the Notch-driven LPS have similar tumorigenic capacity, and, if not, which population represents TICs. In this study, we established from the same primary tumor two cell populations exhibiting contrasting Notch activities. One population of cells (mLPS1 cells) displays constitutively active Notch (NICD) and the other population (mLPS2 cells) has normal Notch activity. We provide behavioral, gene expression, tumorigenesis and functional rescue data to demonstrate that the NICDOE cells represent the TICs and give rise to tumors upon transplantation into NRG mice. In contrast, the fast growing mLPS2 cells exhibiting normal Notch signaling failed to give rise to LPS, and therefore likely represent the vast majority of tumor cells in vivo. These results provide direct evidence that Notch activation renders TIC properties in a subset of LPS cells and underscores the heterogeneity of cells found in the Notch-driven LPS.
Mesenchymal stem cells (MSCs) are undifferentiated multipotent cells that can differentiate into mature adipocytes [33,34]. In this study, we found that mLPS1 cells express high levels of MSC markers, which may function to promote TIC self-renewal and prevent their differentiation. Similar to our finding, a study revealed that Notch signaling prevents pancreatic epithelial cell differentiation and results in increased maintenance of undifferentiated pancreatic progenitor cells [35]. Also, in response to differentiation cues, breast cancer cells trans-differentiate into adipocyte cells [36]. These studies suggest that re-differentiation treatment could shift cancer cells to mature differentiated states and cease cell cycle progression [37,38]. Since NICD-overexpressing mLPS cells have TICs function, inhibition of Notch signaling may be necessary to diminish cancer stem cells’ self-renewal ability and induce them to differentiate terminally [39]. In this scenario, inhibition of Notch signaling in LPS cells may promote TICs to re-differentiate into well-differentiated cells.
Genes in the Dlk1-Dio3 imprinted locus plays an important role in maintaining mouse embryonic stem cells and regulating the pluripotency of induced pluripotent stem cells [40,41]. Our previous study indicated that Notch-driven LPS tissue has profoundly higher levels of Dlk1 (~ 2000-fold increase) than the pre-cancerous adipose tissues from the same mice [19]. In the current study, we show that the mLPS1 cells express higher levels of Dlk1 than do the mLPS2 cells, suggesting the DLK1 may represent a biomarker of TICs in LPS and function to maintain their pluripotency. Interestingly, the expression of Rian, a non-coding RNA in the Dlk1-Dio3 imprinted region, was also upregulated in the mLPS1 cells compared to the mPLS2 cells. DLK1 is reported as a negative regulator of Notch signaling in neighboring cells as it binds to the NOTCH receptors without activating them [42]. It is intriguing to examine if the mLPS1 TICs express high levels of DLK1 to inhibit Notch signaling in neighboring mLPS2 cells, generating a model of “lateral inhibition” underlying TIC maintenance. Although the current study did not reveal the detailed role of DLK1 in LPS, our data shed light on the cellular heterogeneity within LPS and molecular pathways downstream of Notch signaling involved in the maintenance of TICs.
Cancer stem cells employ different metabolic pathways to maintain stemness and support tumorigenic transformation [32]. Interestingly, the Notch overexpressing mLPS1 cells exhibited lower expression levels of Pparg and Ppargc1a, crucial regulators of fatty acid metabolism and mitochondria biogenesis. This observation is consistent with the strong aerobic glycolysis capacity and glucose metabolism reported in various cancer cells. Thus, Notch signaling may affect cancer stem cells through rewiring cell metabolic pathways.
Gene expression analysis also indicates that serial grafting further enriched expression of Mdm2, a marker for the human DDLPS that is already elevated in the primary LPS of Adipoq-Cre/RosaNICD mice. However, mLPS1 and mLPS2 cells have identical expression of Mdm2, indicating that MDM2 is highly expressed in both TICs and the bulk of LPS cells. Previous studies have shown that co-overexpression of MDM2 and CDK4 in human bone marrow stem cells transformed the cells into sarcoma [43,44]. However, direct evidence supporting tumorigenic driver function of MDM2 in vivo is lacking [45]. Conversely, loss-of-function studies indicate that Mdm2 knockdown in 3T3-L1 preadipocyte cells inhibits adipocyte differentiation [46] and knockout of Mdm2 in mouse adipocytes leads to lipodystrophy [47], suggesting a potential role of MDM2 in adipogenic differentiation. Therefore, the specific role of MDM2 in TICs and other LPS cells remain to be resolved.
The derivation of GFP− mLPS2 cells from the Notch-driven LPS is unexpected, as our previous study indicated that cells in the primary LPS are predominantly GFP+. Interestingly, the mLPS2 cells dominated the in vitro culture prior to the FACS-enrichment of mLPS1 cells. Although we did not fully characterize the lineage origin of the mLPS2 cells, we show that they come from the primary Notch-driven LPS based on Adipoq-Cre allele genotyping. Most cancers are a heterogeneous population of cells containing different gene mutations [48]. Thus, the mLPS2 cells may have arisen from normal adipocytes or LPS cells with different chromosomal aberrations [49]. Alternatively, the mLPS2 cells may have arisen from the microenvironment around tumor tissues, such as cancer-associated fibroblasts or cells transdifferentiated from adipocytes [50]. The mLPS2 cell population is highly stable in cell culture and co-exist in mLPS1 cell culture; they might represent a population of tumor-supporting cells in cancer biology [51], which functions to promote tumor cell proliferation.
Various cancer cell lines represent important experimental tools with which to study oncogenic signaling pathways and to screen for therapeutic candidates. However, the average success rate of generating primary cell lines is lower than 30% [52], as primary tumors are grown in a microenvironment surrounded by many supporting cells [1]. In this study, we took advantage of serial tissue grafting technique to enrich fasting growing cancer cells and TICs. This process selects for the fastest proliferating and enriches the most malignant subpopulation of LPS cells. These subpopulations of cells expressed sequentially higher levels cancer and mesenchymal stem cell markers during the serial grafting. This selection process may have enhanced the adaptability of the cancer cells and facilitated the generation of the primary cancer cell lines.
In summary, this study reveals that activation of Notch signaling is an oncogenic driver in LPS, and overexpressing NICD leads to enrichment of tumor-initiating cells. Future studies will focus on dissecting how Notch signaling pathway promotes the TICs phenotype and if it plays a similar role in other cancer types. Such knowledge will assist in the discovery of new therapeutic strategies to target Notch-driven cancers.
Supplementary Material
Supplemental Figure S1. Growth rates of mLPS1 and mLPS2 cells at various passages.
Growth regression curves of mLPS1 and mLPS2 cells at p20 (A), p33 (B) and p51 (C), shown in logarithmic scale, based on identical data presented in Figure 3A–C. Data are presented as mean from N=3. Data points at 0 h were removed in A and B due to significant loss of cells in the first 24 h after seeding 5 x 104 cells per well into 24-well plates. The slope of the regression curve indicates relative growth rate.
Highlights.
Liposarcomas are heterogeneous and contain tumor-initiating cells (TICs)
Serial transplantation of liposarcomas efficiently enrich TICs
Notch activation is a signature of TICs in liposarcoma
Liposarcoma TICs express genes of cancer and mesenchymal stem cells
5. Acknowledgements
We thank Dr. Laurent Le Cam (Montpellier Cancer Center, INSERM) for sharing the tumor grafting protocol, Dr. Castro Bohorquez Beatriz for assistance with animal surgery, Purdue University Biological Evaluation Core Facility for tumor grafting support, Purdue Flow Cytometry and Cell Separation Facility for assistance with FACS data collection and analysis, Stephanie Oprescu for critical reading of the manuscript, Dr. Feng Yue for advice on the study and Jun Wu for technical support. We also thank the Purdue University writing lab for assistance with academic English writing.
8. Funding
This work was supported by grants from the National Cancer Institute of US National Institutes of Health (R01CA212609), and Purdue University Center for Cancer Research (P30CA023168).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
The authors declare that they have no conflicts of interest with the contents of this article.
9. Reference
- [1].Frantz C, Stewart KM, Weaver VM, The extracellular matrix at a glance., J. Cell Sci 123 (2010) 4195–200. 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ciurea ME, Georgescu AM, Purcaru SO, Artene S-A, Emami GH, Boldeanu MV, Tache DE, Dricu A, Cancer stem cells: biological functions and therapeutically targeting., Int. J. Mol. Sci 15 (2014) 8169–85. 10.3390/ijms15058169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou B-BS, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB, Tumour-initiating cells: challenges and opportunities for anticancer drug discovery, Nat. Rev. Drug Discov 8 (2009) 806–823. 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- [4].Xiao W, Gao Z, Duan Y, Yuan W, Ke Y, Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma, J. Exp. Clin. Cancer Res 36 (2017) 41 10.1186/s13046-017-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fang S, Liu M, Li L, Zhang F-F, Li Y, Yan Q, Cui Y-Z, Zhu Y-H, Yuan Y-F, Guan X-Y, Lymphoid enhancer-binding factor-1 promotes stemness and poor differentiation of hepatocellular carcinoma by directly activating the NOTCH pathway, Oncogene 38 (2019) 4061–4074. 10.1038/s41388-019-0704-y. [DOI] [PubMed] [Google Scholar]
- [6].Bi P, Kuang S, Notch signaling as a novel regulator of metabolism, Trends Endocrinol. Metab 26 (2015) 248–255. 10.1016/J.TEM.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fischer A, Gessler M, Delta Notch and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors, Nucleic Acids Res 35 (2007) 4583–4596. 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nowell CS, Radtke F, Notch as a tumour suppressor, Nat. Rev. Cancer. 17 (2017) 145–159. 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- [9].Loganathan SK, Schleicher K, Malik A, Quevedo R, Langille E, Teng K, Oh RH, Rathod B, Tsai R, Samavarchi-Tehrani P, Pugh TJ, Gingras A-C, Schramek D, Rare driver mutations in head and neck squamous cell carcinomas converge on NOTCH signaling., Science. 367 (2020) 1264–1269. 10.1126/science.aax0902. [DOI] [PubMed] [Google Scholar]
- [10].Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, Wouters VM, Roper J, Kendall TJ, Roxburgh CS, Horgan PG, Nixon C, Nourse C, Gunzer M, Clark W, Hedley A, Yilmaz OH, Rashid M, Bailey P, Biankin AV, Campbell AD, Adams DJ, Barry ST, Steele CW, Medema JP, Sansom OJ, Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis, Cancer Cell. 36 (2019) 319–336.e7. 10.1016/J.CCELL.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu L, Xia K, Gao T, Chen J, Zhang Z, Sun X, Simões BM, Eyre R, Fan Z, Guo W, Clarke RB, The Notch Pathway Promotes Osteosarcoma Progression through Activation of Ephrin Reverse Signaling., Mol. Cancer Res 17 (2019) 2383–2394. 10.1158/1541-7786.MCR-19-0493. [DOI] [PubMed] [Google Scholar]
- [12].Fletcher CDM, Unni KK, Mertens F, World Health Organization., International Agency for Research on Cancer., Pathology and genetics of tumours of soft tissue and bone, IARC Press, 2002. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Pathology-And-Genetics-Of-Tumours-Of-Soft-Tissue-And-Bone-2002 (accessed July 16, 2020). [Google Scholar]
- [13].Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ, Cancer Statistics, 2007, CA. Cancer J. Clin (2007). 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- [14].Dalal KM, Antonescu CR, Singer S, Diagnosis and management of lipomatous tumors, J. Surg. Oncol 97 (2008) 298–313. 10.1002/jso.20975. [DOI] [PubMed] [Google Scholar]
- [15].Dodd LG, Update on Liposarcoma: A review for cytopathologists, Diagn. Cytopathol 40 (2012) 1122–1131. 10.1002/dc.21794. [DOI] [PubMed] [Google Scholar]
- [16].Stratford EW, Castro R, Wennerstrøm A, Holm R, Munthe E, Lauvrak S, Bjerkehagen B, Myklebost O, Liposarcoma cells with aldefluor and CD133 activity have a cancer stem cell potential, Clin. Sarcoma Res 1 (2011) 8 10.1186/2045-3329-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stratford EW, Castro R, Daffinrud J, Skårn M, Lauvrak S, Munthe E, Myklebost O, Characterization of Liposarcoma Cell Lines for Preclinical and Biological Studies, Sarcoma 2012 (2012) 1–9. 10.1155/2012/148614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Somaiah N, Beird HC, Barbo A, Song J, Mills Shaw KR, Wang W-L, Eterovic K, Chen K, Lazar A, Conley AP, Ravi V, Hwu P, Futreal A, Simon G, Meric-Bernstam F, Hong D, Targeted next generation sequencing of well-differentiated/dedifferentiated liposarcoma reveals novel gene amplifications and mutations., Oncotarget 9 (2018) 19891–19899. 10.18632/oncotarget.24924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bi P, Yue F, Karki A, Castro B, Wirbisky SE, Wang C, Durkes A, Elzey BD, Andrisani OM, Bidwell CA, Freeman JL, Konieczny SF, Kuang S, Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice, J. Exp. Med 213 (2016) 2019–2037. 10.1084/jem.20160157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED, Transcriptional control of adipose lipid handling by IRF4., Cell Metab 13 (2011) 249–59. 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murtaugh LC, Stanger BZ, Kwan KM, Melton DA, Notch signaling controls multiple steps of pancreatic differentiation., Proc. Natl. Acad. Sci. U. S. A 100 (2003) 14920–5. 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H, Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo., Science. 294 (2001) 2186–9. 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- [23].Hatchi E, Rodier G, Lacroix M, Caramel J, Kirsh O, Jacquet C, Schrepfer E, Lagarrigue S, Linares LK, Lledo G, Tondeur S, Dubus P, Sardet C, Le Cam L, E4F1 deficiency results in oxidative stress–mediated cell death of leukemic cells, J. Exp. Med 208 (2011) 1403–1417. 10.1084/jem.20101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fisher R, Pusztai L, Swanton C, Cancer heterogeneity: implications for targeted therapeutics, Br. J. Cancer. 108 (2013) 479–485. 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peng T, Zhang P, Liu J, Nguyen T, Bolshakov S, Belousov R, Young ED, Wang X, Brewer K, López-Terrada DH, Oliveira AM, Lazar AJ, Lev D, An experimental model for the study of well-differentiated and dedifferentiated liposarcoma; deregulation of targetable tyrosine kinase receptors, Lab. Investig 91 (2011) 392–403. 10.1038/labinvest.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu L, Luo G-Z, Yang W, Zhao X, Zheng Q, Lv Z, Li W, Wu H-J, Wang L, Wang X-J, Zhou Q, Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells., J. Biol. Chem 285 (2010) 19483–90. 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang J, Sullenger BA, Rich JN, Notch Signaling in Cancer Stem Cells, in: Springer, New York, NY, 2012: pp. 174–185. 10.1007/978-1-4614-0899-4_13. [DOI] [PubMed] [Google Scholar]
- [28].Yu L, Xia K, Gao T, Chen J, Zhang Z, Sun X, Simões BM, Eyre R, Fan Z, Guo W, Clarke RB, The Notch Pathway Promotes Osteosarcoma Progression through Activation of Ephrin Reverse Signaling, Mol. Cancer Res 17 (2019) 2383–2394. 10.1158/1541-7786.MCR-19-0493. [DOI] [PubMed] [Google Scholar]
- [29].Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H, Cholangiocarcinomas can originate from hepatocytes in mice., J. Clin. Invest 122 (2012) 2911–5. 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Luo J, Wang P, Wang R, Wang J, Liu M, Xiong S, Li Y, Cheng B, The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma., Oncotarget 7 (2016) 9525–37. 10.18632/oncotarget.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seo EJ, Kim DK, Jang IH, Choi EJ, Shin SH, Lee SI, Kwon S-M, Kim K-H, Suh D-S, Kim JH, Hypoxia-NOTCH1-SOX2 signaling is important for maintaining cancer stem cells in ovarian cancer., Oncotarget 7 (2016) 55624–55638. 10.18632/oncotarget.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Intlekofer AM, Finley LWS, Metabolic signatures of cancer cells and stem cells, Nat. Metab 1 (2019) 177–188. 10.1038/s42255-019-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chan YW, So C, Yau KL, Chiu KC, Wang X, Chan FL, Tsang SY, Adipose-derived stem cells and cancer cells fuse to generate cancer stem cell-like cells with increased tumorigenicity, J. Cell. Physiol (2020) jcp.29574. 10.1002/jcp.29574. [DOI] [PubMed] [Google Scholar]
- [34].Ghaben AL, Scherer PE, Adipogenesis and metabolic health, Nat. Rev. Mol. Cell Biol 20 (2019) 242–258. 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- [35].Abel EV, Kim EJ, Wu J, Hynes M, Bednar F, Proctor E, Wang L, Dziubinski ML, Simeone DM, The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer., PLoS One 9 (2014) e91983 10.1371/journal.pone.0091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ishay-Ronen D, Diepenbruck M, Kalathur RKR, Sugiyama N, Tiede S, Ivanek R, Bantug G, Morini MF, Wang J, Hess C, Christofori G, Gain Fat—Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis, Cancer Cell. 35 (2019) 17–32.e6. 10.1016/J.CCELL.2018.12.002. [DOI] [PubMed] [Google Scholar]
- [37].Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J, Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development, Dev. Biol 260 (2003) 426–437. 10.1016/S0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- [38].Matushansky I, Hernando E, Socci ND, Matos T, Mills J, Edgar MA, Schwartz GK, Singer S, Cordon-Cardo C, Maki RG, A Developmental Model of Sarcomagenesis Defines a Differentiation-Based Classification for Liposarcomas, Am. J. Pathol 172 (2008) 1069–1080. 10.2353/AJPATH.2008.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Thé H, Differentiation therapy revisited, Nat. Rev. Cancer. 18 (2018) 117–127. 10.1038/nrc.2017.103. [DOI] [PubMed] [Google Scholar]
- [40].Plasschaert RN, Bartolomei MS, Genomic imprinting in development, growth, behavior and stem cells., Development. 141 (2014) 1805–13. 10.1242/dev.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ferrón SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, Morante-Redolat JM, Laborda J, Guillemot F, Bauer SR, Fariñas I, Ferguson-Smith AC, Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis, Nature. 475 (2011) 381–385. 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Falix FA, Aronson DC, Lamers WH, Gaemers IC, Possible roles of DLK1 in the Notch pathway during development and disease, Biochim. Biophys. Acta - Mol. Basis Dis 1822 (2012) 988–995. 10.1016/J.BBADIS.2012.02.003. [DOI] [PubMed] [Google Scholar]
- [43].C. L, C. F, B. DA, Z. A, D. AA, F. P, L. G, W. M, S. C, L. JL, G. VP, L. D, C. CM, P. RE, MDM2 Derived From Dedifferentiated Liposarcoma Extracellular Vesicles Induces MMP2 Production From Preadipocytes, Cancer Res 79 (2019). 10.1158/0008-5472.CAN-19-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim YJ, Kim M, Park HK, Yu DB, Jung K, Song K, Choi Y-L, Co-expression of MDM2 and CDK4 in transformed human mesenchymal stem cells causes high-grade sarcoma with a dedifferentiated liposarcoma-like morphology, Lab. Investig 99 (2019) 1309–1320. 10.1038/s41374-019-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Codenotti S, Mansoury W, Pinardi L, Monti E, Marampon F, Fanzani A, Animal models of well-differentiated/dedifferentiated liposarcoma: utility and limitations., Onco. Targets. Ther 12 (2019) 5257–5268. 10.2147/OTT.S175710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hallenborg P, Siersbæk M, Barrio-Hernandez I, Nielsen R, Kristiansen K, Mandrup S, Grøntved L, Blagoev B, MDM2 facilitates adipocyte differentiation through CRTC-mediated activation of STAT3, Cell Death Dis 7 (2016) e2289–e2289. 10.1038/cddis.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu Z, Jin L, Yang J-K, Wang B, Wu KKL, Hallenborg P, Xu A, Cheng KKY, The Dysfunctional MDM2-p53 Axis in Adipocytes Contributes to Aging-Related Metabolic Complications by Induction of Lipodystrophy., Diabetes. 67 (2018) 2397–2409. 10.2337/db18-0684. [DOI] [PubMed] [Google Scholar]
- [48].Khatib S, Pomyen Y, Dang H, Wang XW, Understanding the Cause and Consequence of Tumor Heterogeneity, Trends in Cancer. 6 (2020) 267–271. 10.1016/j.trecan.2020.01.010. [DOI] [PubMed] [Google Scholar]
- [49].Dagogo-Jack I, Shaw AT, Tumour heterogeneity and resistance to cancer therapies, Nat. Rev. Clin. Oncol 15 (2018) 81–94. 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- [50].Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song Y-H, Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors, Cell. Oncol 34 (2011) 55–67. 10.1007/s13402-011-0012-1. [DOI] [PubMed] [Google Scholar]
- [51].LeBleu VS, Kalluri R, A peek into cancer-associated fibroblasts: origins, functions and translational impact., Dis. Model. Mech 11 (2018). 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].K. DP, F. AF, D. A, H. MA, D. L, F. L, von F. F, D. LJ, L. D, P. M, D. R, G. M, K. KE, R. AK, F. FJ, R. C, P. Z, S. AT, G. JF, S. LV, N. MJ, E. JA, B. CH, Primary Patient-Derived Cancer Cells and Their Potential for Personalized Cancer Patient Care, Cell Rep 21 (2017). 10.1016/J.CELREP.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Growth rates of mLPS1 and mLPS2 cells at various passages.
Growth regression curves of mLPS1 and mLPS2 cells at p20 (A), p33 (B) and p51 (C), shown in logarithmic scale, based on identical data presented in Figure 3A–C. Data are presented as mean from N=3. Data points at 0 h were removed in A and B due to significant loss of cells in the first 24 h after seeding 5 x 104 cells per well into 24-well plates. The slope of the regression curve indicates relative growth rate.


