Abstract
Background:
Chronic alcohol results in changes to stress biology and autonomic arousal contributing to acute alcohol withdrawal symptoms, neuroendocrine tolerance of the hypothalamic-pituitary-adrenal (HPA) axis responses, high stress-induced craving, and risk of alcohol relapse. Thus, stress coping and recovery from alcohol during early abstinence may be jeopardized by such stress system dysfunction. Significant preclinical evidence suggests that noradrenergic disruption may contribute to these alcohol-related stress arousal changes and that alpha-1 adrenergic antagonists, such as prazosin, may normalize these stress system adaptations and reduce alcohol intake. Thus, we hypothesized that prazosin would reduce stress-induced craving and improve neuroendocrine and autonomic response to stress- and alcohol cue exposure during early abstinence. We secondarily also assessed the role of lifetime anxiety disorders on these prazosin effects.
Methods:
Forty inpatient treatment-seeking alcohol dependent individuals were randomly assigned to receive placebo (n=18) or 16mg/day, T.I.D., prazosin (n=22) in a double-blind manner, titrated over 2 weeks. In week 3–4 after achieving full dose, patients were exposed to three 5-minute personalized guided imagery conditions (stress cue, alcohol cue, neutral/relaxing cue), on three consecutive days in a random, counterbalanced order. Alcohol craving, anxiety, heart rate, cortisol and ACTH levels were assessed at baseline, following imagery and at repeated recovery timepoints.
Results:
Prazosin reduced stress cue-induced alcohol craving (p<.05) and stress- and alcohol cue-induced anxiety (p<.05); and increased heart rate responses in all imagery conditions (p<.05). Prazosin lowered basal cortisol and ACTH (p’s<.05), and attenuated stress cue-induced rises in cortisol (p<.05) vs placebo. Finally, in those without lifetime anxiety disorder, the placebo group showed stress- and alcohol cue-induced increases in cortisol (p’s<.05), while the prazosin group did not.
Conclusions:
Prazosin may attenuate stress cue-induced alcohol craving and anxiety during early abstinence while improving adrenergic and stress system function, effects which are independent of a history of lifetime anxiety disorders.
Keywords: prazosin, alcohol use disorders, stress, craving, heart rate, anxiety
Introduction
Chronic alcohol misuse results in significant changes to stress biology and autonomic arousal which contribute to acute alcohol withdrawal symptoms, tolerance of the hypothalamic-pituitary adrenal (HPA) axis stress responses, stress-induced craving and high risk of relapse (Brady et al., 2006, Fox et al., 2007, Sinha et al., 2011). Thus, reducing the disruption of the stress response and stress-induced alcohol craving during early abstinence may be an important therapeutic target for alcohol relapse prevention.
There are currently three FDA-approved medications for alcohol use disorder (AUD), acamprosate, naltrexone and disulfiram, and there is no evidence that these approved medications reduce stress-induced alcohol craving and intake.
As alcohol use disorder is a heterogeneous illness characterized by a number of biobehavioral phenotypes, it is perhaps unsurprising that medications for AUD have demonstrated only modest efficacy thus far. Hence, there is an ongoing need to develop new targeted therapies for the prevention and treatment of alcoholism (Davies et al., 2012), particularly to specifically target stress-system psychopathology during early and protracted abstinence, known to underlie high craving, anxiety and excessive drinking and relapse risk (Blaine and Sinha, 2017, Milivojevic and Sinha, 2018, Koob, 2008). Significant preclinical evidence suggests that noradrenergic disruption may contribute to the stress arousal changes that are observed in chronic alcohol use and findings show that alpha-1 adrenergic antagonists, such as prazosin, may normalize these relapse-related stress system adaptations and reduce alcohol intake (Walker et al., 2008, Rasmussen et al., 2009, Le et al., 2011).
In humans, prazosin has been successfully used to treat nightmares in individuals with post-traumatic stress disorder (PTSD) (Raskind et al., 2007, Taylor et al., 2008). However, clinical trials using prazosin for AUD have demonstrated mixed findings, highlighting the need to discern for whom prazosin may be most beneficial. For example, in a sample of veterans with PTSD and comorbid AUD, prazosin had no effect on either PTSD symptoms or alcohol use outcomes over placebo (Petrakis et al., 2016). In another study, individuals with comorbid PTSD and AUD randomized to prazosin vs placebo demonstrated a greater reduction in percent days drinking per week and percent days heavy drinking per week in a 6-week outpatient regimen (Simpson et al., 2015). A more recent study in individuals with AUD found that prazosin did not significantly affect reduction in alcohol use compared to placebo, but did significantly improve reduction in drinks per week in an optimal treatment exposure subgroup with high diastolic blood pressure (Wilcox et al., 2018). In another 12-week clinical trial of prazosin for AUD, a modest decrease over time in drinking and heavy drinking in prazosin compared to placebo was observed (Simpson et al., 2018).
In a previous experimental study, we found that prazosin reduced alcohol craving, anxiety and negative emotion in response to provoked stress and alcohol cue exposure in the laboratory (Fox et al., 2012). However, given the mixed effects of prazosin clinically in the literature, we conducted a follow-up extension of the previous inpatient laboratory study. Moreover, as chronic alcohol use is known to alter the stress pathway, and increased anxiety is a key symptom of acute and protracted alcohol withdrawal (Koob et al., 2014), we also secondarily assessed whether co-morbid lifetime anxiety disorders played a role in prazosin effects. A sample of 40 treatment seeking patients with AUD were admitted to an inpatient clinical research unit and initiated on prazosin (16 mg t.i.d.) or placebo in a controlled double blind randomized manner. In week 3–4, they participated in the laboratory experiment to assess craving, anxiety and stress arousal responses after exposure to personalized guided imagery conditions of stress, alcohol cue, and neutral/relaxing situations. We hypothesized that prazosin would reduce stress- and alcohol cue-induced craving and improve neuroendocrine and autonomic adaptations in response to stress and alcohol cue provocation in a human laboratory experimental study, and that this effect would be more pronounced in individuals with lifetime anxiety disorders.
Methods
Participants
Forty treatment-seeking alcohol dependent individuals (31M/9F) who responded to local advertisements around the New Haven area participated in the study. Current alcohol dependence was determined using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV (SCID IV-TR - (First, 1997) as well as positive urine toxicology screens for alcohol metabolite (EtG – ethyl glucuronide) collected upon entry into inpatient treatment. Lifetime anxiety disorders were also assessed using the SCID IV-TR. Exclusion criteria included DSM-IV dependence for any drug other than alcohol or nicotine. Participants using prescribed medications for any psychiatric or medical disorders were also excluded, and all individuals underwent stringent medical assessments including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic and thyroid function. Written and verbal consent was obtained from all participants and the Human Investigation Committee of the Yale University School of Medicine approved the study.
Study Procedures
All participants were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for 4–5 weeks of inpatient treatment and study participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs and very limited access to visitors. Alcohol and drug testing was conducted regularly to ensure drug abstinence. As subjects were treatment seeking, they participated in group counseling treatment for alcohol dependence using the standard 12-Step based alcohol and drug counseling manual as a guide (Project Match 12-Step Manual). During the first week of inpatient stay, participants were administered structured baseline assessments measuring psychiatric and substance use history and initiated on study medication which involved a 2-week titration period. In the second week, scripts for the guided imagery induction were developed as described in previous studies (Sinha et al., 2009). A laboratory experiment involving participation in 3 testing sessions over 3 days was conducted after participants were at full dose between weeks 3–4 of admission. Research staff was blind to which imagery condition was presented on what day. Subjects also remained blind until imagery presentation.
Study Medication and Dosing
All prazosin pills (marketed by Watson Pharmaceuticals) were purchased through the pharmacy located at the CMHC and the research pharmacist ensured that both active and matching placebo capsules were also formulated. Upon inpatient admission, subjects were randomly assigned to matching placebo or prazosin medication and were initiated on a 2-week dose escalation schedule similar to previous studies (Raskind et al., 2007, Simpson et al., 2009). Briefly, dosing started at 1 mg taken at bed time on day 1, 1 mg in the morning and evening on days 2–3, 2 mg t.i.d. on days 4–6, and 3 mg for the morning and afternoon dose and 4 mg for the evening dose on days 7–9, 4 mg t.i.d. on days 10–13, followed by 5 mg, 5 mg and 6 mg dosing starting on days 14 to meet the target 16 mg/day dosing. Study medications were administered at 8 am, 3 pm and between 8–9 pm daily. Experimental laboratory sessions were conducted between weeks 3–4 once subjects were on full medication dose. Subjects were also offered the opportunity to continue on study medication in an outpatient phase for 7 more weeks and tapered in week 8 of the outpatient phase. Those not continuing in the outpatient phase were tapered down over 5 days following the laboratory sessions. Randomization and double-blind procedures were implemented by the Yale Stress Center Statistician using Urn randomization procedures (Stout et al., 1994) in coordination with the CMHC research pharmacist for formulation and dispensing of study medication.
Imagery Script Development Procedures
Imagery script development was conducted in approximately week 2 following inpatient admission in a session prior to the laboratory experiment. Procedures are based on methods developed by Lang and his colleagues (Lang et al., 1980, Lang et al., 1983), and further adapted and validated in our previous studies (Fox et al., 2005, Sinha et al., 2009, Sinha et al., 1992, Sinha et al., 2000, Sinha et al., 2003). Briefly, the stress cue imagery script was based on subjects’ descriptions of a recent “most stressful” adverse personal event that made them “sad, mad or upset”, that they were not able to control in the moment. “Most stressful” was determined by having the subjects rate their perceived stress on a 10-point Likert scale where 0= not at all stressful and 10= the most stress they felt recently in their life. Only situations rated as 8 or above were accepted as appropriate for script development (e.g. being fired from their job, marital conflict situation). The alcohol-related cue scripts were developed by having subjects identify a recent situation that included alcohol-related stimuli and resulted in subsequent alcohol use (e.g. walking by their favorite bar; watching others drink alcohol). Alcohol-related situations that were associated with negative affect or psychological distress were not allowed. A relaxing, non-physiologically arousing and non-alcohol related (neutral cue) script was developed from the subjects’ description of a personal, relaxing situation (e.g., being at the beach; fall afternoon reading at the park). In addition to the script development, on the day prior to the laboratory sessions, subjects were brought into the testing room in order to acclimatize them to specific aspects of the study procedures including the subjective rating forms and training in relaxation and imagery procedures, as previously described in Sinha et al. (2009).
Laboratory Sessions (conducted across three consecutive days)
Subjects were brought into the testing room at 7:45 AM. All subjects were allowed an initial smoke break at 7:30 AM in order to reduce potential nicotine withdrawal during the session. After settling in a sitting position on a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject’s non-preferred arm, in order to periodically obtain blood samples. A pulse sensor was placed on the subject’s forefinger. Self–reports of craving, anxiety and emotion were completed immediately after set-up. This was followed by a one-hour adaptation period during which the subjects were instructed to practice relaxation. At 9:00 AM, subjects were provided headphones and given the following instructions for the 5-minute imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”. Alcohol craving and subjective ratings, heart rate (HR), and blood samples for cortisol and adrenocorticotropic hormone (ACTH) were collected at baseline (−20 and −5 minutes prior to imagery), immediately following imagery presentation (0) and every 15 minutes after the imagery period, up to 75 minutes (+15, +30, +45, +60, +75). Subjects were then free to leave the testing room and eat breakfast.
Laboratory Assessments
Alcohol Craving:
The desire for using alcohol was assessed using a 10-point visual analog scale (VAS) in which 0=“not at all” and 10=“extremely high”.
Anxiety:
Study participants were asked to rate how tense, anxious, and/or jittery they feel using a 10-point visual analog scale (VAS) in which 0=“not at all” and 10=“more than ever”.
Heart Rate:
A Critikon Dinamap 120 Patient Monitor (GE Medical Systems, Tampa, FL) was used to assess heart rate at the specific timepoints outlined above in the Laboratory Sessions section.
HPA Axis:
Blood samples for measurement of adrenocorticotropic hormone (ACTH) and cortisol were obtained in heparinized tubes. All tubes were placed on ice immediately after drawing, and then aliquoted after being centrifuged at 4C within 30 minutes of collection. Blood samples for HPA axis measures were stored at −80C and processed at the Yale Center for Clinical Investigation Core Laboratories using commercially available cortisol and ACTH radio-immuno-assay (RIA) kits from MP Biomedicals, LLC (Solon, OH, USA). For cortisol, the Corti-Cote Cortisol Solid Phase RIA kit was used, which has a sensitivity of 0.07 μg/dL and a 100.0% antiserum specificity for cortisol. For ACTH, the ImmuChem Double Antibody hACTH RIA kit was used, with a sensitivity of 5.7 pg/mL and a 100.0% specificity for ACTH.
Data and Statistical Analysis
All statistical analyses were performed using SPSS software (SPSS Inc., Version 24, Chicago, IL, USA) and using Linear Mixed Effects models. The statistical analysis included Within-subject factors of Imagery Condition and Timepoint and the Between-subjects factors of Medication Group and presence or absence of Lifetime Anxiety disorder as the fixed effects and Subjects was the random effect. The Bonferroni test for multiple comparisons was used to analyze simple effects. Independent t-tests were used to compare medication groups on baseline levels of cortisol and ACTH. T-tests and Chi-square analyses using were used to compare the medication groups on demographic variables. Figures were created with GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA). As significant main effect of Condition and Timepoint and significant interaction of Condition X Timepoint were expected, given our previous reports on validation of the experimental paradigm (Fox et al., 2005, Sinha et al., 2009, Sinha et al., 1992, Sinha et al., 2000, Sinha et al., 2003, Sinha et al., 2011), these effects are not specifically reported here unless they were non-significant.
Results
Participants
Both placebo and prazosin medication groups were statistically matched on all demographic characteristics (Table 1). The groups did not differ in sex or race distribution. Participants in both groups were similar in age and majority of participants in both groups were regular smokers. There were no group differences in years of alcohol use or prevalence of lifetime anxiety disorders.
TABLE 1.
Demographic and Clinical Characteristics
| Placebo (n=18) | Prazosin (n=22) | |
|---|---|---|
| Gender – no. of males | 12 (67%) | 19 (86%) |
| Race | ||
| Caucasian | 10 (56%) | 11 (50%) |
| African American | 8 (44%) | 9 (41%) |
| Hispanic | 0 | 1 (.05%) |
| Other | 0 | 1 (.05%) |
| Agea | 38.2 ± 9.0 | 40.8 ± 9.7 |
| Years of Educationa | 13.4 ± 1.6 | 11.2 ± 3.2 |
| No. of regular smokers | 16 (89%) | 19 (86%) |
| Years of alcohol usea | 18.0 ± 10.5 | 24.4 ± 9.6 |
| Past 30 days alcohol usea | 22.8 ± 9.2 | 19.4 ± 9.7 |
| Lifetime Anxiety Disorders | 9 (50%) | 5 (23%) |
Data indicate means and standard deviations.
All variables: p>.05.
Alcohol Craving
A significant medication group x imagery condition interaction (F(2,866)= 3.5); p=0.03) showed that alcohol craving was significantly higher in the alcohol cue and stress cue conditions compared to neutral cue in the placebo group ((p<0.0001), whereas in the prazosin group this significant difference was only seen in the alcohol cue (p<0.0001) and not in the stress cue compared to neutral cue condition (p<n.s.). Thus, the stress cue-induced increase in alcohol craving was suppressed in the prazosin group (see Fig. 1). A significant timepoint x imagery condition effect (F(14, 866)= 7.76, p=0.003) indicated that alcohol craving in both the placebo and the prazosin groups increased significantly after stress cue and alcohol cue imagery exposure (p< 0.0001) and decreased significantly from the initial peak response compared with all other recovery time points in the stress cue and alcohol cue conditions (p< 0.0001, in all cases) but not the neutral cue condition. No significant medication group x timepoint or medication group x imagery condition x timepoint effects were observed. There were no interaction effects of medication group and lifetime anxiety on alcohol craving.
Figure 1.
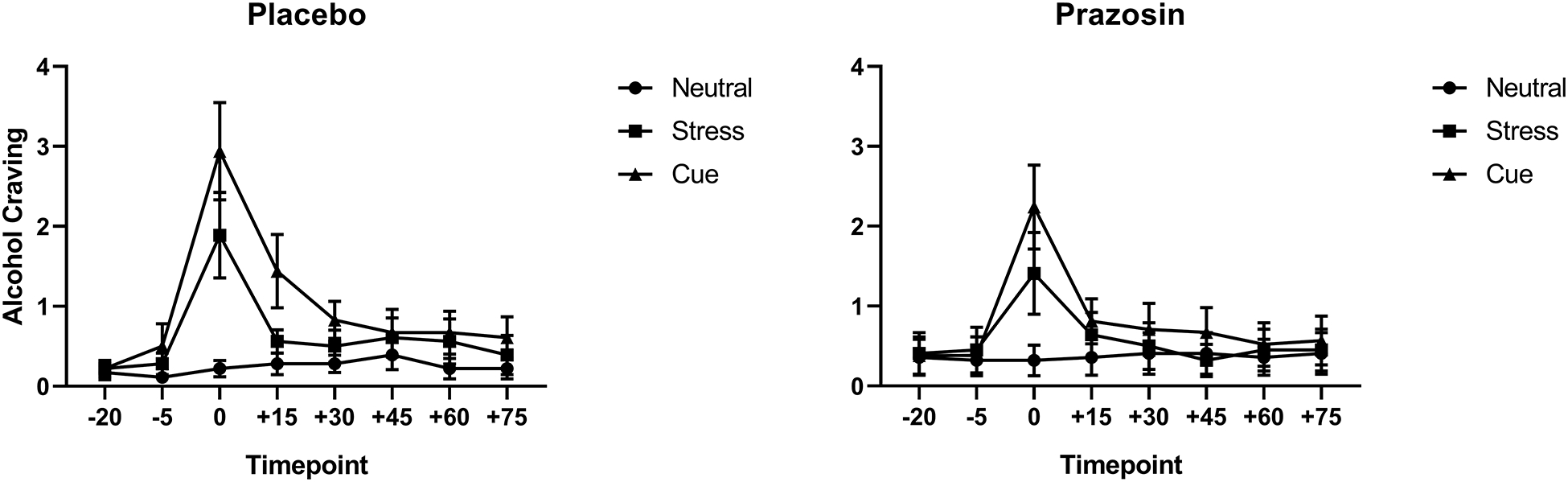
Alcohol craving as a function of placebo (left) vs prazosin (right). Alcohol craving was significantly higher in the stress cue (p<.05) and the alcohol cue (p<.05) compared to the neutral cue condition in the placebo group (left). In the prazosin group, alcohol craving was higher in the alcohol cue condition (p<.05) but was not significantly higher than neutral cue in the stress cue condition (p<n.s.) (right). All data are displayed as mean ± S.E.M.
Anxiety
A significant medication group x imagery condition interaction (F(2,865)= 2.97); p<0.05) showed that significantly higher anxiety was reported following stress cue (p<0.0001) and alcohol cue (p<0.0001) compared to the neutral cue condition only in the placebo but not the prazosin group y (Fig. 2). A main effect of lifetime anxiety (F(1,36)= 3.9); p=0.05) also showed that individuals with lifetime anxiety disorder reported higher provoked subjective anxiety compared with individuals without lifetime anxiety disorder across all three imagery conditions (data not shown). However, no significant interactions with medication were observed.
Figure 2.
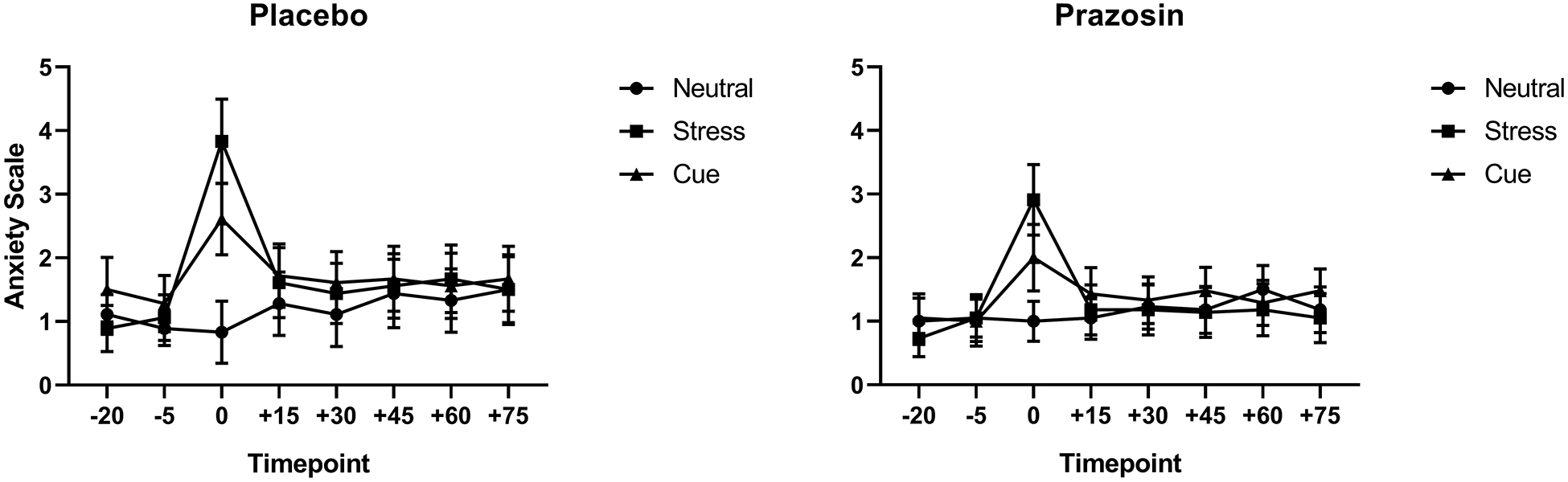
Anxiety rating as a function of placebo (left) vs prazosin (right). Anxiety was significantly higher in the stress cue (p<.05) and alcohol cue (p<.05) compared to the neutral cue condition in the placebo group, but no differences in stress cue or alcohol cue-induced anxiety was seen in the prazosin group (p<n.s.). All data are displayed as mean ± S.E.M.
Heart Rate
A significant main effect of medication group (F(1,53)= 5.1); p=0.028) was observed, where prazosin increased heart rate in all imagery conditions compared to placebo (Fig. 3). There were no interaction effects of medication group and lifetime anxiety on heart rate responses.
Figure 3.
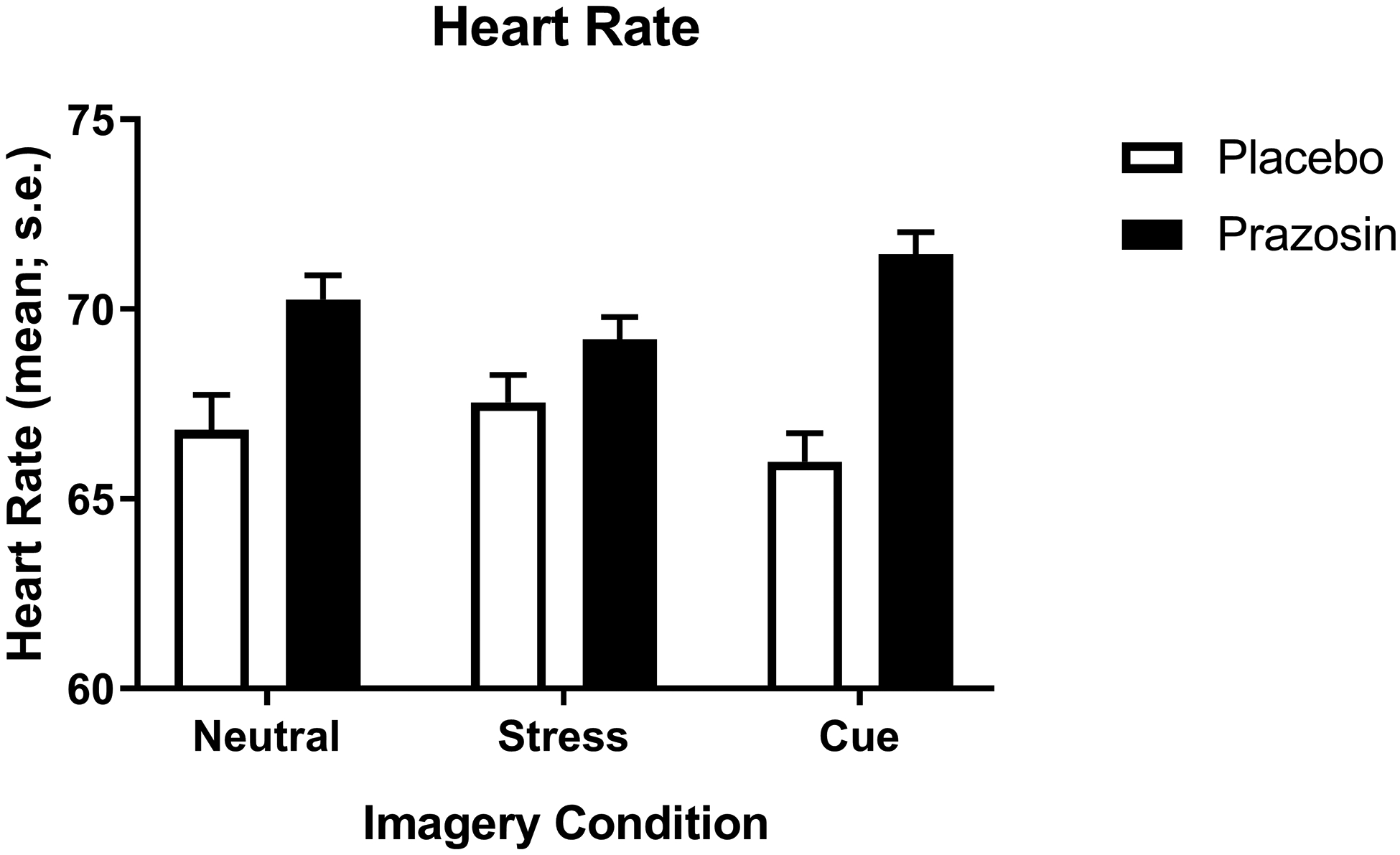
Change in average heart rate across timepoints as a function of imagery condition and prazosin vs placebo. Heart rate, averaged across all timepoints, was higher in all three conditions in the prazosin group compared to the placebo group (p<.05). Data are displayed as mean ± S.E.M.
Cortisol and ACTH Levels
At baseline, a group comparison showed that levels of cortisol (F(1,211)= 7.1); p=0.008) and ACTH (F(1,211)= 22.7); p<0.001), averaged across all three days, were reduced in the prazosin group compared to the placebo group (Fig. 4A and B).
Figure 4.
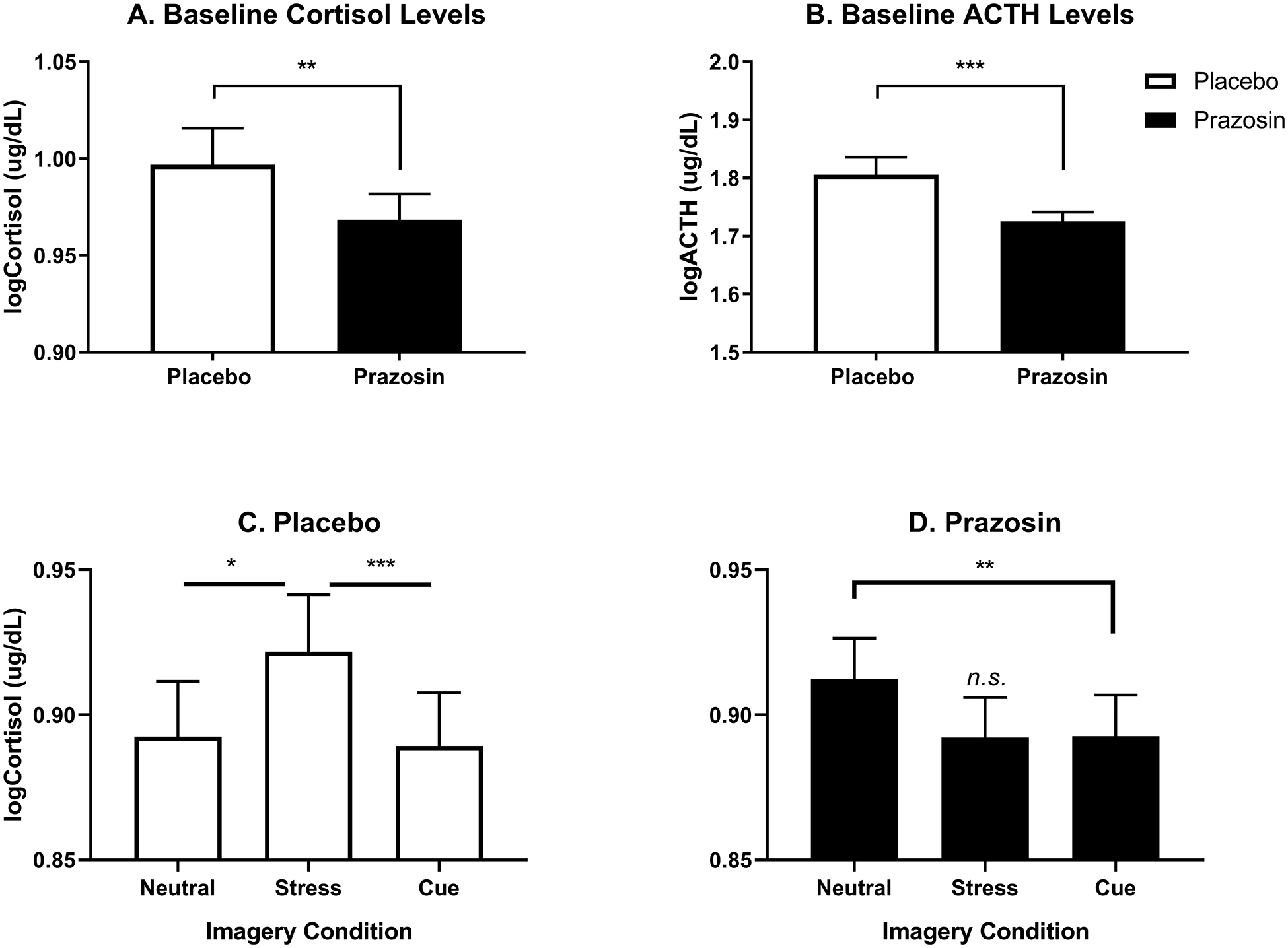
Log-transformed cortisol and ACTH levels as a function of prazosin vs placebo. Prazosin reduced baseline levels of cortisol (p<0.01) (A) and ACTH (p<0.001) (B) compared to placebo across all imagery days. After imagery, cortisol response to stress cue compared to neutral cue (p<.05) and alcohol cue imagery (p<.001) was higher in placebo (C), and in the prazosin group cortisol levels were significantly lower in the alcohol cue compared to the neutral cue condition (p<.01) but did not differ between the stress cue imagery and neutral cue condition (p<n.s.) (D). Data are displayed as mean ± S.E.M.
After exposure to imagery conditions, a significant medication group x imagery condition interaction (F(2,763)= 3.2); p=0.04) showed that cortisol levels were significantly higher in the stress cue relative to neutral cue (p=0.03) and the alcohol cue (p=0.001) conditions in the placebo group (Fig. 4C). This was reversed in the prazosin group where cortisol levels were significantly lower in the alcohol cue condition compared with the neutral cue condition (p=0.008) and did not differ in the stress cue compared to neutral cue condition (Fig. 4D).
A significant medication group x anxiety group x imagery condition interaction (F (2, 719) = 3.5, p=.03) showed that cortisol response was significantly higher in the stress cue compared to the neutral cue condition (S>N, p=0.04) and in the alcohol cue compared to the neutral cue condition (C>N, p=0.01), but only in individuals with no lifetime anxiety in the placebo group. This stress- and alcohol cue-related increase in cortisol was not observed in individuals with no lifetime anxiety in the prazosin group (Fig. 5). There was no effect of lifetime anxiety disorders on ACTH levels.
Figure 5.
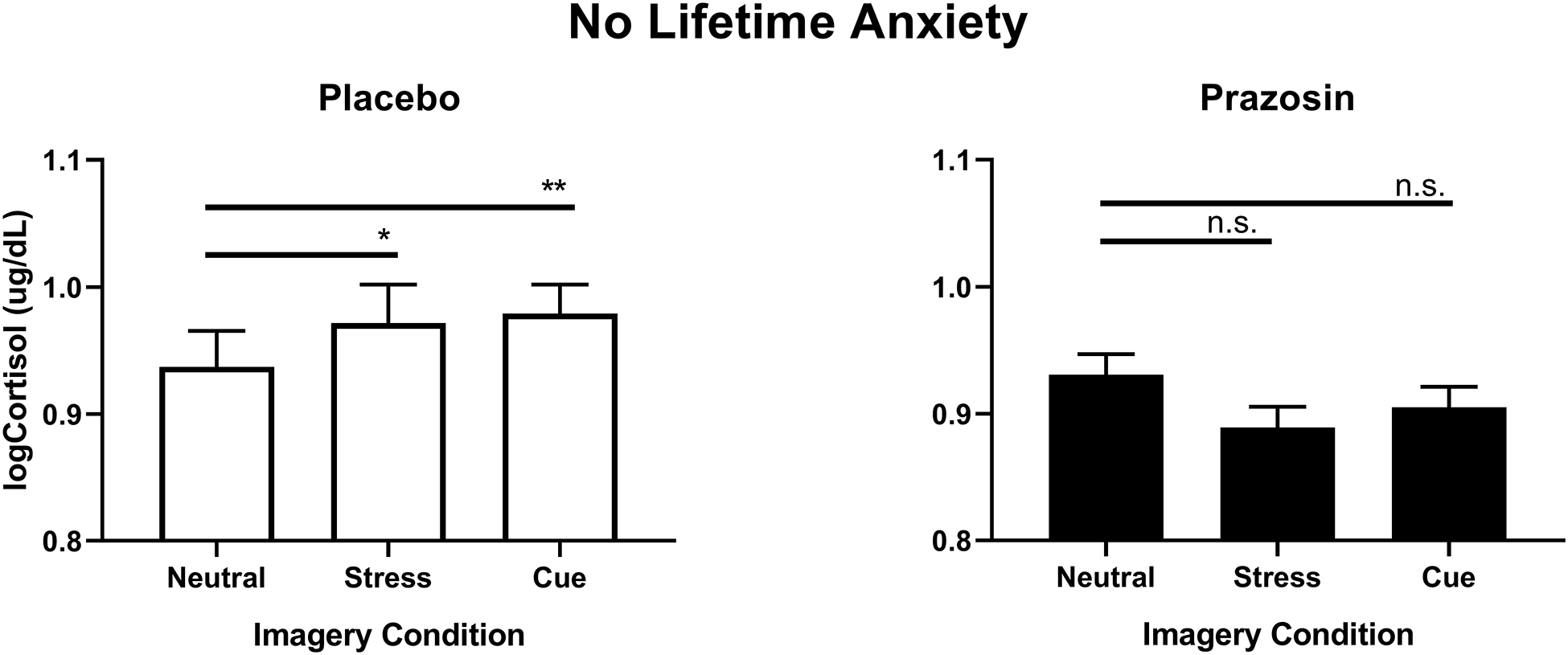
Log-transformed cortisol levels as a function of prazosin vs placebo in individuals without lifetime anxiety. Cortisol response to stress cue compared to neutral cue (p<.05) and alcohol cue compared to neutral (p<.01) imagery condition was higher in placebo (left) but not in prazosin (p<n.s.) (right). Data are displayed as mean ± S.E.M.
Discussion
The current findings indicate that the alpha-1 adrenergic antagonist prazosin reduced stress cue-induced alcohol craving and stress- and alcohol cue-provoked anxiety in early abstinent alcohol dependent men and women. Prazosin increased heart rate responses to all imagery conditions, thereby normalizing the blunted autonomic output observed in early abstinent alcohol dependent individuals. Moreover, the prazosin group showed lower levels of cortisol and ACTH at baseline (before imagery exposure) across all three days, and reduced cortisol response to stress cue compared to neutral cue imagery. These results are consistent with previous findings showing reductions in stress-induced alcohol craving, anxiety and negative mood in the prazosin vs placebo group (Fox et al., 2012). We also secondarily assessed the role of lifetime anxiety disorders co-morbidity on prazosin effects on laboratory provoked craving and stress responses. Contrary to our hypotheses, prazosin’s effects were not observed in those with lifetime anxiety disorders, but was more prominent in those without lifetime anxiety disorders. In summary, these findings of decreased craving and anxiety, and normalization of tonic and phasic neuroendocrine and autonomic stress responsivity supports further assessment of prazosin for improvement in adrenergic and stress system function, provoked alcohol craving responses and heavy drinking outcomes, particularly in those with disrupted stress system functioning.
Our findings provide evidence that prazosin reduces stress-induced craving in early abstinent alcohol dependent individuals in a laboratory experiment of stress cue and alcohol cue provocation, and stress and alcohol craving are known to promote continued alcohol use and relapse during treatment (Wemm et al., 2019, Sinha et al., 2011). These results replicate our previous finding that prazosin vs placebo showed significantly lower alcohol craving in response to stress (Fox et al., 2012). This finding is also consistent with preclinical studies which show that prazosin has potent effects on alcohol seeking and intake. For example, rats selectively bred for alcohol preference showed a reduction in alcohol intake for up to 5 consecutive days following prazosin administration (Rasmussen et al., 2009) as well as suppressed responding for ethanol during acute withdrawal (Walker et al., 2008). Similarly, prazosin significantly decreased alcohol seeking in alcohol-preferring (P) rats (Verplaetse et al., 2012). Another study also found prazosin to significantly decrease stress-induced reinstatement of yohimbine-induced and footshock-induced alcohol seeking in laboratory animals (Le et al., 2011). In clinical samples, there is some evidence that prazosin may decrease craving but only in individuals who were able to initiate abstinence from alcohol versus those who were not (Hallgren et al., 2018). Other a1-adrenergic antagonists have been examined for their effects on craving. For example, doxazosin was shown to decrease tobacco craving in a laboratory study (Verplaetse et al., 2017), and alcohol craving and alcohol use in an outpatient study, although alcohol intake was only decreased on individuals with a high density of family history of alcoholism (Kenna et al., 2016). In a preclinical model, doxazosin significantly reduced alcohol intake in alcohol-preferring (P) rats over repeated trials (O’Neil et al., 2013).
We observed selective effects of lifetime anxiety disorders in the current study. Anxiety ratings were significantly higher overall in those with lifetime anxiety disorders, but prazosin did not interact with this response. That is, the expected significant stress- and alcohol cue-induced anxiety increases were only seen in the placebo relative to prazosin group, and contrary to our hypothesis, this effect was not moderated by co-morbid lifetime anxiety disorders. These findings suggest that increased subjective anxiety responses are more directly a result of chronic alcohol use disorder related effects on stress system dysfunction, rather than due to an underlying primary anxiety disorder. Chronic alcohol use disorder is known to increase anxiety and stress due to dysfunction of the stress pathways, and increased anxiety is a key symptom of acute and protracted alcohol withdrawal (Koob et al., 2014). Prazosin’s effects in reducing anxiety suggests it may have specific benefit for those with alcohol related anxiety and withdrawal symptoms during early abstinence. However, an important caveat is that the sample had low numbers of individuals with anxiety disorders and those on medications for their anxiety disorders were excluded. Thus, the generalizability of the findings to that group is limited and needs further assessment in future studies.
While Prazosin appeared to normalize the HPA axis responses, there was a prazosin interaction with lifetime anxiety, where cortisol responses to stress cue and alcohol cue were lower in the prazosin group than placebo, but this was only observed in individuals without lifetime anxiety disorders. Interestingly, lifetime anxiety had no effects on alcohol craving or heart rate responses. These findings suggest that prazosin’s therapeutic effects on craving and neuroendocrine responses are likely independent of a history of anxiety disorders and more specific to chronic alcohol-related effects on subjective and physiological stress system changes. Previous preclinical studies clearly document significant sympathoadrenal and neuroendocrine changes as a result of chronic alcohol exposure (Rasmussen et al., 2000, Rasmussen et al., 2006) and some evidence of reversal by prazosin (Fox et al., 2012). These findings suggest that in humans, prazosin may be effective among those with significant alcohol-related changes in stress pathways, that is, in those with greater alcohol dependence severity, as shown in those with higher blood pressure (Haass-Koffler et al., 2017) or perhaps in those with alcohol withdrawal as alcohol withdrawal is associated with higher noradrenergic dysregulation (Krystal et al., 1996). These aspects may contribute to the mixed clinical findings in the literature on prazosin’s efficacy in the treatment of alcohol dependence (Petrakis et al., 2016, Wilcox et al., 2018, Simpson et al., 2009, Simpson et al., 2015, Simpson et al., 2018).
Prazosin increased HR responses in all imagery conditions. This is an important finding, as it has been repeatedly found that chronic alcohol use leads to adaptations in the autonomic nervous system. The autonomic nervous system is an important pathway that mediates the biological response to stress, in which the sympathetic component mobilizes arousal by increasing heart rate (HR) (Sinha, 2008). Chronic alcohol use dysregulates the autonomic nervous system and is known to blunt the HR response to alcohol/cue and stress cue exposure (for review see (Milivojevic and Sinha, 2018)). For example, previous work has found that inpatient treatment-engaged, recovering alcohol-dependent individuals displayed reduced HR responses to the stress provocation relative to healthy controls (Fox et al., 2008, Sinha et al., 2009). Current findings suggest that prazosin may be reversing these blunted autonomic adaptations and restoring increased HR responses observed in the placebo group. On the other hand, we did not find any prazosin effects on stress- or alcohol cue- induced HR responses, suggesting that its effects were more tonic in nature and not in any phasic arousal due to acute challenge.
Extensive evidence has demonstrated that chronic exposure to alcohol leads to significant HPA axis adaptations, marked by high levels of basal cortisol and ACTH (Sinha et al., 2009, Sinha et al., 2011). Importantly, high basal or neutral-relaxed state cortisol has been associated with alcohol relapse outcomes (Adinoff et al., 2005, Brady et al., 2006, Junghanns et al., 2003, Sinha et al., 2011). Thus, treatment targets that normalize these adaptations hold promise in increasing abstinence and reducing relapse rates (Milivojevic and Sinha, 2018). The findings in the current study show that prazosin may indeed normalize these HPA axis disruptions by decreasing basal levels of cortisol and ACTH and also overall cortisol response to stress cue and alcohol cue, thereby potentially restoring select components of the HPA dysregulation observed with chronic alcohol use. Moreover, prazosin reduced stress- and alcohol cue-induced levels of cortisol in individuals without lifetime anxiety, whereas it had no effect in those with presence of lifetime anxiety disorders. Individuals with anxiety disorders and those with PTSD are known to have blunted neuroendocrine levels (Morris et al., 2012) and our findings are consistent with these earlier reports. These data suggest specificity of prazosin’s positive effects on chronic alcohol-related stress adaptations rather than a generalized non-specific effect on anxiety symptomatology.
The present study has a number of strengths. The study had a well-controlled human laboratory design that assessed chronic dosing of prazosin versus placebo in a human laboratory model of stress cue and alcohol cue-induced alcohol craving and reactivity. All participants were treatment-seeking and completed all study-related prazosin/placebo administrations, laboratory challenges and assessments whilst in an inpatient clinical research unit. Limitations of the study include recruitment of low numbers of women, preventing us from assessing sex differences as well as low number of individuals with lifetime/current anxiety disorders which may have limited our ability to fully assess the impact of current co-morbid anxiety disorders on laboratory-based alcohol craving and stress responses. Also due to small numbers, we were not able to examine potential racial differences in HPA axis activation which have been recently documented elsewhere (Price et al., 2019), and future studies need to assess race effects on prazosin’s modulation of the HPA axis. Despite these limitations, current findings replicate and further extend previous preliminary data in a larger sample and indicate that prazosin reduced subjective alcohol craving and anxiety while also improving heart rate and cortisol responses, and that these effects may be independent of comorbid anxiety disorders.
Acknowledgements
We would like to thank the staff at the Clinical Neuroscience Research Unit (CNRU) and the Yale Center for Clinical Investigation (YCCI) for their assistance in completing this study.
This study was supported by Grants R01-AA020504 (Sinha), K02-AA023566 (Fox), K01-DA046561 (Milivojevic) and the NIH/NCRR/CTSA Program Grant no.1 UL1 RR024139 (Yale Center for Clinical Investigation).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- ADINOFF B, JUNGHANNS K, KIEFER F & KRISHNAN-SARIN S 2005. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res, 29, 1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINE SK & SINHA R 2017. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 122, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY KT, WALDROP AE, MCRAE AL, BACK SE, SALADIN ME, UPADHYAYA HP, ANTON RF & RANDALL PK 2006. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol, 67, 700–6. [DOI] [PubMed] [Google Scholar]
- DAVIES DL, BORTOLATO M, FINN DA, RAMAKER MJ, BARAK S, RON D, LIANG J & OLSEN RW 2012. Recent Advances in the Discovery and Preclinical Testing of Novel Compounds for the Prevention and/or Treatment of Alcohol Use Disorders. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRST MB, SPITZER RL, GIBBON M, WILLIAMS JBW 1997. Structured Clinical Interview for the DSM-IV Axis I Disorders—Patient Edition (SCID-I ⁄ P, Version 2.0, 4 ⁄ 97 revision) New York State Psychiatric Institute, New York. [Google Scholar]
- FOX HC, ANDERSON GM, TUIT K, HANSEN J, KIMMERLING A, SIEDLARZ KM, MORGAN PT & SINHA R 2012. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res, 36, 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX HC, BERGQUIST KL, HONG KI & SINHA R 2007. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res, 31, 395–403. [DOI] [PubMed] [Google Scholar]
- FOX HC, HONG KI, SIEDLARZ K & SINHA R 2008. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology, 33, 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX HC, TALIH M, MALISON R, ANDERSON GM, KREEK MJ & SINHA R 2005. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology, 30, 880–91. [DOI] [PubMed] [Google Scholar]
- HAASS-KOFFLER CL, GOODYEAR K, ZYWIAK WH, MAGILL M, ELTINGE SE, WALLACE PM, LONG VM, JAYARAM-LINDSTROM N, SWIFT RM, KENNA GA & LEGGIO L 2017. Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend, 177, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLGREN KA, DELKER BC & SIMPSON TL 2018. Effects of Initiating Abstinence from Alcohol on Daily Craving and Negative Affect: Results from a Pharmacotherapy Clinical Trial. Alcohol Clin Exp Res, 42, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGHANNS K, BACKHAUS J, TIETZ U, LANGE W, BERNZEN J, WETTERLING T, RINK L & DRIESSEN M 2003. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol, 38, 189–93. [DOI] [PubMed] [Google Scholar]
- KENNA GA, HAASS-KOFFLER CL, ZYWIAK WH, EDWARDS SM, BRICKLEY MB, SWIFT RM & LEGGIO L 2016. Role of the alpha1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol, 21, 904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF 2008. A role for brain stress systems in addiction. Neuron, 59, 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF, BUCK CL, COHEN A, EDWARDS S, PARK PE, SCHLOSBURG JE, SCHMEICHEL B, VENDRUSCOLO LF, WADE CL, WHITFIELD TW JR. & GEORGE O 2014. Addiction as a stress surfeit disorder. Neuropharmacology, 76 Pt B, 370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRYSTAL JH, WEBB E, COONEY NL, KRANZLER HR, SOUTHWICK SW, HENINGER GR & CHARNEY DS 1996. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry, 153, 83–92. [DOI] [PubMed] [Google Scholar]
- LANG PJ, KOZAK MJ, MILLER GA, LEVIN DN & MCLEAN A JR. 1980. Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology, 17, 179–92. [DOI] [PubMed] [Google Scholar]
- LANG PJ, LEVIN DN, MILLER GA & KOZAK MJ 1983. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol, 92, 276–306. [DOI] [PubMed] [Google Scholar]
- LE AD, FUNK D, JUZYTSCH W, COEN K, NAVARRE BM, CIFANI C & SHAHAM Y 2011. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl), 218, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILIVOJEVIC V & SINHA R 2018. Central and Peripheral Biomarkers of Stress Response for Addiction Risk and Relapse Vulnerability. Trends Mol Med, 24, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS MC, COMPAS BE & GARBER J 2012. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev, 32, 301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’NEIL ML, BECKWITH LE, KINCAID CL & RASMUSSEN DD 2013. The alpha1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcohol Clin Exp Res, 37, 202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRAKIS IL, DESAI N, GUEORGUIEVA R, ARIAS A, O’BRIEN E, JANE JS, SEVARINO K, SOUTHWICK S & RALEVSKI E 2016. Prazosin for Veterans with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence: A Clinical Trial. Alcohol Clin Exp Res, 40, 178–86. [DOI] [PubMed] [Google Scholar]
- PRICE JL, FRAZIER IR, LEWIS B, WALKER R, JAVORS MA, NIXON SJ & ADINOFF B 2019. Differences in pituitary-adrenal reactivity in Black and White men with and without alcohol use disorder. Psychoneuroendocrinology, 100, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASKIND MA, PESKIND ER, HOFF DJ, HART KL, HOLMES HA, WARREN D, SHOFER J, O’CONNELL J, TAYLOR F, GROSS C, ROHDE K & MCFALL ME 2007. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry, 61, 928–34. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN DD, ALEXANDER LL, RASKIND MA & FROEHLICH JC 2009. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res, 33, 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN DD, BOLDT BM, BRYANT CA, MITTON DR, LARSEN SA & WILKINSON CW 2000. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res, 24, 1836–49. [PubMed] [Google Scholar]
- RASMUSSEN DD, WILKINSON CW & RASKIND MA 2006. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol, 38, 173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON TL, MALTE CA, DIETEL B, TELL D, POCOCK I, LYONS R, VARON D, RASKIND M & SAXON AJ 2015. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res, 39, 808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON TL, SAXON AJ, MEREDITH CW, MALTE CA, MCBRIDE B, FERGUSON LC, GROSS CA, HART KL & RASKIND M 2009. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res, 33, 255–63. [DOI] [PubMed] [Google Scholar]
- SIMPSON TL, SAXON AJ, STAPPENBECK C, MALTE CA, LYONS R, TELL D, MILLARD SP & RASKIND M 2018. Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder. Am J Psychiatry, 175, 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R 2008. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci, 1141, 105–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R, FOX HC, HONG KA, BERGQUIST K, BHAGWAGAR Z & SIEDLARZ KM 2009. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology, 34, 1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R, FOX HC, HONG KI, HANSEN J, TUIT K & KREEK MJ 2011. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry, 68, 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R, FUSE T, AUBIN LR & O’MALLEY SS 2000. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl), 152, 140–8. [DOI] [PubMed] [Google Scholar]
- SINHA R, LOVALLO WR & PARSONS OA 1992. Cardiovascular differentiation of emotions. Psychosom Med, 54, 422–35. [DOI] [PubMed] [Google Scholar]
- SINHA R, TALIH M, MALISON R, COONEY N, ANDERSON GM & KREEK MJ 2003. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl), 170, 62–72. [DOI] [PubMed] [Google Scholar]
- STOUT RL, WIRTZ PW, CARBONARI JP & DEL BOCA FK 1994. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl, 12, 70–5. [DOI] [PubMed] [Google Scholar]
- TAYLOR HR, FREEMAN MK & CATES ME 2008. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm, 65, 716–22. [DOI] [PubMed] [Google Scholar]
- VERPLAETSE TL, RASMUSSEN DD, FROEHLICH JC & CZACHOWSKI CL 2012. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res, 36, 881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERPLAETSE TL, WEINBERGER AH, OBERLEITNER LM, SMITH KM, PITTMAN BP, SHI JM, TETRAULT JM, LAVERY ME, PICCIOTTO MR & MCKEE SA 2017. Effect of doxazosin on stress reactivity and the ability to resist smoking. J Psychopharmacol, 31, 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER BM, RASMUSSEN DD, RASKIND MA & KOOB GF 2008. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol, 42, 91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEMM SE, LARKIN C, HERMES G, TENNEN H & SINHA R 2019. A day-by-day prospective analysis of stress, craving and risk of next day alcohol intake during alcohol use disorder treatment. Drug Alcohol Depend, 204, 107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX CE, TONIGAN JS, BOGENSCHUTZ MP, CLIFFORD J, BIGELOW R & SIMPSON T 2018. A Randomized, Placebo-controlled, Clinical Trial of Prazosin for the Treatment of Alcohol Use Disorder. J Addict Med, 12, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]


