Abstract
Alcoholic hepatitis (AH) is a clinical syndrome characterized by jaundice and progressive inflammatory liver injury in patients with a history of prolonged periods of excess alcohol consumption and recent heavy alcohol abuse. Severe AH is a life-threatening form of alcohol-associated liver disease with a high short-term mortality rate around 30–50% at one month from the initial presentation. A large number of pro-inflammatory mediators, metabolic pathways, transcriptional factors and epigenetic factors have been suggested to be associated with the development and progression of AH. Several factors may contribute to liver failure and mortality in patients with severe AH including hepatocyte death, inflammation, and impaired liver regeneration. Although the pathogeneses of AH have been extensively investigated and many therapeutic targets have been identified over the last five decades, no new drugs for AH have been successfully developed. In this review, we discuss interleukin-22 (IL-22) biology and its roles of anti-apoptosis, anti-fibrosis, anti-oxidation, anti-bacterial infection and regenerative stimulation in protecting against liver injury in many preclinical models including several recently developed models such as chronic-plus-binge ethanol feeding, acute-on-chronic liver failure (ACLF), C-X-C motif chemokine ligand 1 (CXCL1) plus high-fat diet (HFD) (HFD+Cxcl1)-induced nonalcoholic steatohepatitis (NASH). Finally, clinical trials of IL-22 for the treatment of AH are also discussed, which showed some promising benefits for AH patients.
Keywords: alcoholic liver disease, liver regeneration, inflammation, acute-on-chronic liver failure, nonalcoholic steatohepatitis
Alcohol-associated liver disease (ALD) is a crucial cause of liver damage, liver cirrhosis, hepatocellular carcinoma and end-stage liver diseases worldwide [1–4]. There are several major stages of ALD including alcoholic fatty liver, alcoholic steatohepatitis (ASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma [1, 5]. Alcoholic hepatitis (AH) is a clinical syndrome characterized by jaundice and progressive inflammatory liver injury in patients with a history of prolonged periods of excess alcohol consumption and recent heavy alcohol abuse [5–8]. Both chronic ALD with active alcohol binge and systemic inflammatory response syndrome (SIRS) caused by bacterial infection are the most frequent precipitating factors for progressing to acute-on-chronic liver failure (ACLF), a newly recognized syndrome that occurs in patients with chronic liver diseases and is characterized by acute decompensation, organ failures and a high risk of short-term mortality [9–11]. Severe alcoholic hepatitis (SAH), characterized by a modified Maddrey’s Discriminant Function (mDF) score ≥32, is a life-threatening form of ALD with a high short-term mortality rate around 30–50% at one month from the initial presentation [12, 13]. Nearly 50% of liver-related deaths in North American and Europe are implicated with ALD, which has recently represented the most common indication for liver transplantation [3, 14, 15].
Unfortunately, no new drugs for AH have been successfully developed over the last five decades [16, 17]. So far, corticosteroids are still the backbone drugs for treating SAH since first introduced in 1971, and there are many contraindications such as infection, gastrointestinal bleeding, pancreatitis, and viral hepatitis for using corticosteroids [16]. Moreover, the therapeutic benefit of corticosteroids is limited in AH. A recent meta-analysis of clinical trials including the STOPAH study has confirmed that treatment with corticosteroids in SAH only reduces risk of death within 28 days but not the following 6 months [18]. Therefore, there is an urgent need for development of novel therapeutic strategies for treating AH.
Pathogenesis of AH
The pathogeneses of AH have been extensively investigated over the last five decades [3, 19]. A large number of pro-inflammatory mediators, metabolic pathways, transcriptional factors, epigenetic factors have been found associated with the development and progression of AH [1, 3, 19, 20]. Liver is the major organ to convert ethanol into acetaldehyde, then into acetate via several enzymatic systems, such as alcohol dehydrogenase (ADH), cytochrome P450 isozyme CYP2E1, and aldehyde dehydrogenase (ALDH) [21]. In the process of ethanol metabolism, ethanol-induced liver damage develops via direct hepatocyte injury and inflammation caused by the cytotoxic effect of ethanol and its metabolites [19]. Meanwhile, the carbohydrate and lipid metabolism in hepatocytes are also influenced due to the changes of hepatic redox state which is conductive to generate hepatic steatosis [20]. Excessive alcohol consumption that exceeds the limit of ethanol metabolic ability of the liver would cause clinical symptoms such as anorexia, nausea, vomiting, headache, upper abdominal pain, hepatomegaly and fever [22]. Ethanol and its metabolites such as acetaldehyde would increase the susceptibility of hepatocytes to free radical damage by activating CYP2E1 isozyme, inducing mitochondrial dysfunction, depleting anti-oxidant stores, and recruiting inflammatory cells, exacerbating oxidative stress and cellular injury [17, 22].
Besides direct hepatocyte injury during ethanol metabolism, liver injury caused by inflammation is also very important [20, 23] (Figure 1). Damaged hepatocytes release a large amount of cellular components as sterile inflammatory mediators, which are recognized as damage-associated molecular patterns (DAMPs) and enter the circulation to activate neutrophils [24, 25]. Alcohol consumption also damages the gastrointestinal mucosa and changes the intestinal flora balance leading to increased gut permeability and subsequent elevated bacterial products and aggravated inflammatory response in the liver [26–28]. These bacterial products such as lipopolysaccharide (LPS) were recognized as pathogen-associated molecular patterns (PAMPs) [24, 29]. Innate immune responses are activated by the rapid recognition of PAMPs or DAMPs via pattern recognition receptors (PRRs) on immune cells, including toll-like receptors (TLRs), nucleotide-binding and oligomerization domain (NOD)-like receptors, and retinoid X receptors (RXRs). Multiple types of cells in the liver, including immune cells, hepatocytes, and liver non-parenchymal cells, can be activated by PAMPs or DAMPs to release chemokines, cytokines, acute phase response proteins, and extracellular vesicles that play important roles in regulating inflammatory responses in AH [20]. Hepatic neutrophil infiltration, a pathological feature of AH, is attributed to cytokine-mediated and chemokine-mediated attraction of peripheral neutrophils and monocytes into the liver [30–32]. Moreover, adaptive immune responses mediated by B cells, T cells, natural killer (NK) cells, and NK T cells also have crucial roles in inflammatory response in the liver [17, 33]. Excessive binge drinking is an important precipitating factor to trigger liver inflammation in AH patients, which is in accordance with the findings in a mouse model of chronic plus binge ethanol feeding model (NIAAA model) showing that acute ethanol gavage (binge) induces marked increases in neutrophil infiltration and liver injury in mice chronically fed an ethanol diet [34]. Recent studies suggest that several new factors contribute to liver inflammation in ALD, including adipocyte death and inflammation in adipose tissues [35, 36], mitochondrial RNA [37], intestinal fungal dysbiosis [27], etc. Some of these factors could be used as therapeutic targets to treat ALD.
Figure 1: Mechanisms causing inflammation in ALD.
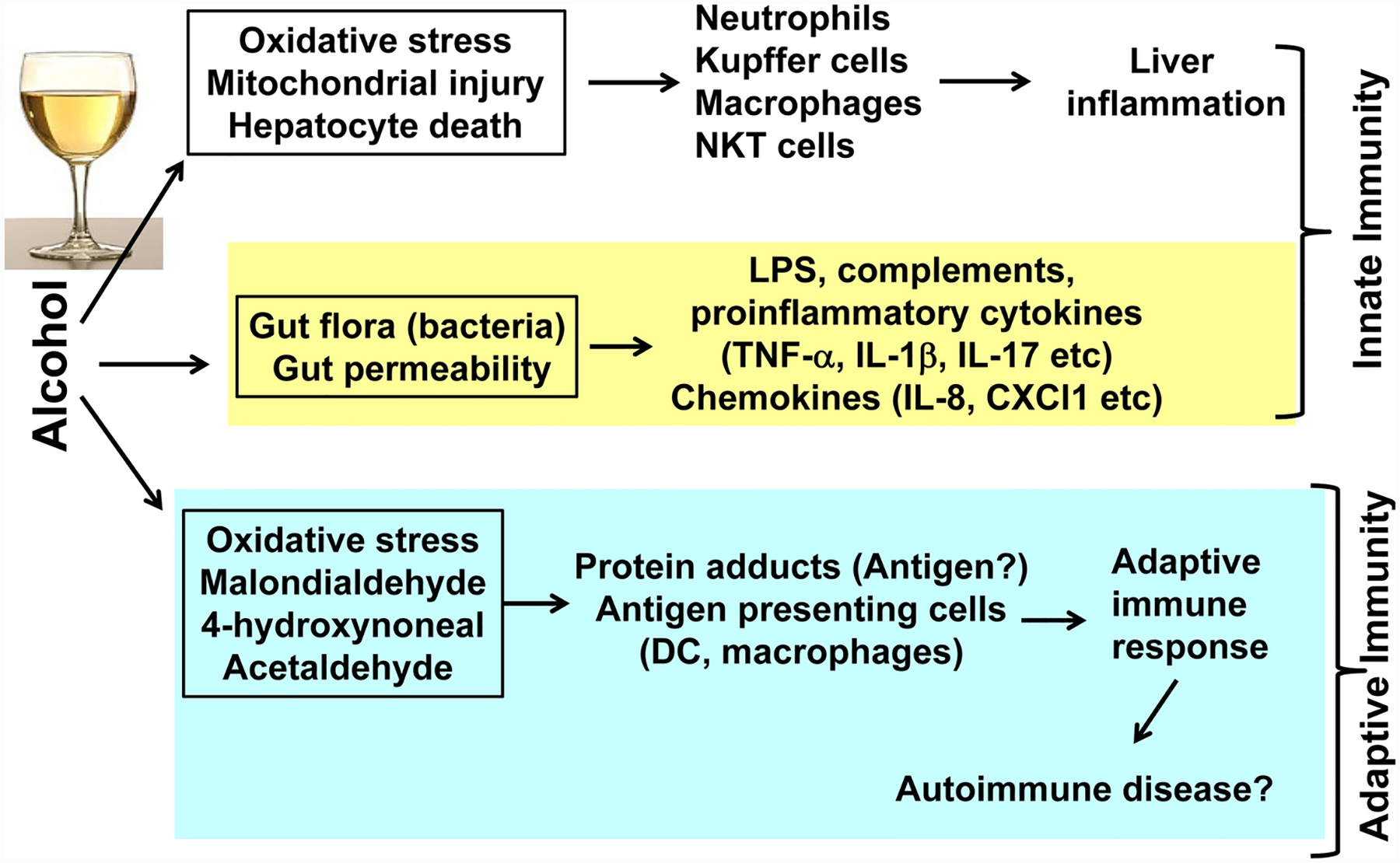
Inflammation plays a key role in inducing ALD progression. Activation of innate immunity is well documented and contributes to inflammation in ALD. Activation of adaptive immunity has also been implicated in the pathogenesis of ALD, but more studies are required to support this notion.
The liver is an organ with great ability to regenerate after injury or resection [38]. Early studies in a rat partial hepatectomy model showed that liver regeneration is impaired by chronic ethanol feeding [39]. In a novel mouse model of ACLF developed recently by our group, it was found that acute-on-chronic liver injury or bacterial infection is associated with impaired liver regeneration due to a shift from a pro-regenerative to an anti-regenerative pathway [40]. Our group has previously demonstrated that alcohol or alcohol plus hepatitis C virus (HCV) infection related cirrhotic livers have significantly fewer Ki67+ and phospho-STAT3+ hepatocytes and bile duct cells than HCV cirrhotic livers, suggesting that hepatocyte proliferation is suppressed in alcoholic cirrhosis compared with HCV cirrhosis [41]. In the AH related ACLF patients, we also found lower numbers of Ki67+ and PCNA+ hepatocytes suggesting impaired liver regeneration in these patients [40]. Other studies demonstrated that the elevation of liver progenitor cell markers is correlated with suppressed hepatocyte proliferation and short-term mortality in AH patients [42, 43]. suggesting hepatocyte regeneration is attenuated in AH.
Clearly, hepatocyte death, inflammation, and impaired liver regeneration are the three important factors in the pathogenesis of AH (Figure 2). Identification of some of these factors as therapeutic targets may help design better strategies for the treatment of AH.
Figure 2: Pathogenesis of alcoholic hepatitis.
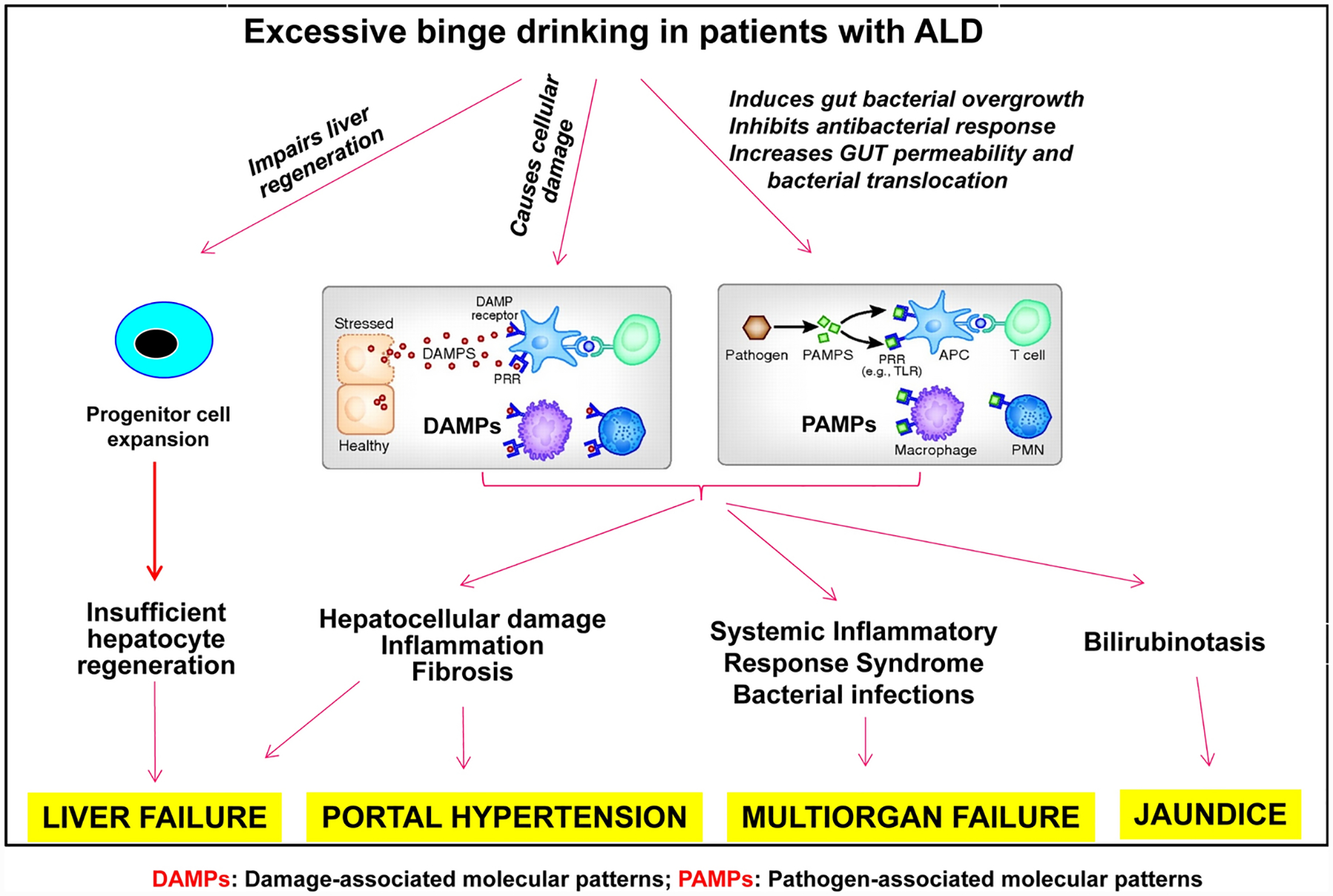
Damaged hepatocytes release various types of cellular components as sterile inflammatory mediators, which act as damage-associated molecular patterns (DAMPs). The bacterial products such as lipopolysaccharide (LPS) are elevated after alcohol consumption and act as pathogen-associated molecular patterns (PAMPs). Both DAMPs and PAMPs activate hepatic and systemic inflammation, causing systemic inflammatory response syndrome. Liver regeneration is impaired in AH with massive proliferation of liver progenitor cells, causing liver failure. Adapted from Mandrekar et al.: Hepatology. 2016;64:1343–55.
Interleukin-22 Biology in the Liver
The receptors and signal pathway of interleukin-22:
Interleukin-22 (IL-22), a cytokine first cloned in 2000, is one of the best-studied members in IL-10 family [44, 45]. The function of IL-22 is mainly mediated via the activation of the Jakl/Tyk2-STAT3 pathway by binding to the heterodimeric receptors of IL-22R1 and IL-10R2 [46]. IL-22 also activates many other signaling pathways but to a much lesser extent, such as STATI, STAT5, Akt, MAPK etc. Activation of STAT3 induces transcription of many genes in hepatocytes that play an important role in promoting acute phase response, hepatocyte survival, liver regeneration, lipid metabolism, and anti-bacterial responses (Figure 3). IL-10R2 is ubiquitously expressed in many types of cells, whereas IL-22R1 expression is mainly restricted on epithelial cells, on the ‘out-body barrier’, such as skin, liver, gut, kidney and lung, while not on immune cells [47]. This indicates that IL-22 specifically targets epithelial cells without affecting immune cells, which makes us predicate the minimal side effects of IL-22 therapy [45]. Interestingly, IL-6 is also a well-documented hepatoprotective cytokine via the activation of STAT3 in hepatocytes, but IL-6 receptor and its signal chain gpl30 are expressed in many types of cells including immune cells [48] (Figure 3). Therefore, IL-6 has much broader effects by targeting many types of cells than IL-22 that mainly targets epithelial cells.
Figure 3: IL-22 and IL-6 mainly activates STAT3 in hepatocytes.
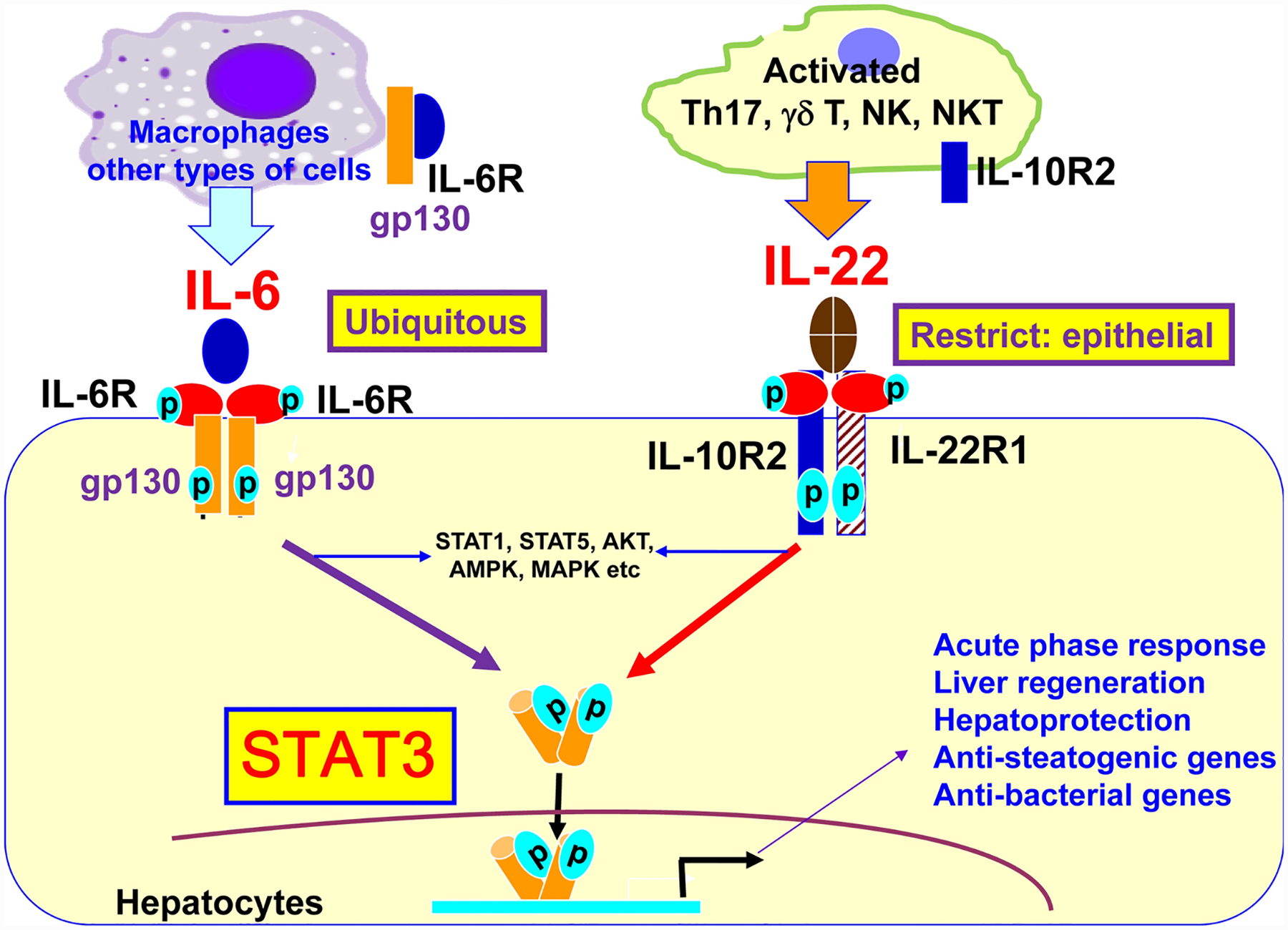
Both IL-6 and IL-22 mainly induces STAT3 in hepatocytes and subsequently induces transcription of many genes that exert many important functions as indicated in the figure. IL-6 and IL-22 also activate many other signaling pathways (such as STATI, STAT5, AKT, MAPK, AMPK etc) but to a much lesser extent. The action of IL-22 is mediated by binding to the heterodimeric receptors of IL-10R2 and IL-22R1 with the latter one mainly expressed on epithelial cells. The function of IL-6 is mediated by binding to the IL-6R and its signal chain gpl30, both of receptor and signal protein are ubiquitously expressed. Thus IL-6 has much broader effects than IL-22 that mainly targets epithelial cells including hepatocytes.
The producing and target cells of IL-22:
IL-22 is mainly secreted by various immune cells including Thl, Th2, Thl7, Th22, NK, NKT, innate lymphoid cells (ILCs), and mucosal-associated invariant T cells (MAIT cells) [47, 49, 50]. Although immune cells produce IL-22 but they do not express IL-22R1, thus IL-22 does not target immune cells instead IL-22 targets several types of liver cells that express IL-22R1 and IL-10R2 (Figure 4). Hepatocytes and liver progenitor/stem cells express high levels of IL-22R1 and IL-10R2 [51, 52]. By targeting these receptors, IL-22 promotes survival and proliferation of hepatocytes and liver progenitor cells, thereby promoting liver repair [51, 52]. Hepatic stellate cells (HSC) are mesodermal in origin but they also express IL-22R1 and IL-10R2 [53]. IL-22 can promote HSC survival and proliferation but also induce its senescence, thereby inhibiting liver fibrosis [53]. Hepatocellular carcinoma cells (HCCs), which are derived from hepatocytes, express high levels of IL-22R1 and IL-10R2 [51, 54]. Studies from IL-22 transgenic mice and in vitro experiments suggest that IL-22 does not induce hepatocyte transformation into cancer cells but can stimulate HCC survival and proliferation [51, 54], Kupffer cells express IL-10R2butnot IL-22R1 [53], so they do not respond to IL-22 stimulation. Finally the functions of IL-22 in biliary epithelial cells have not been explored.
Figure 4: IL-22 functions in the liver.
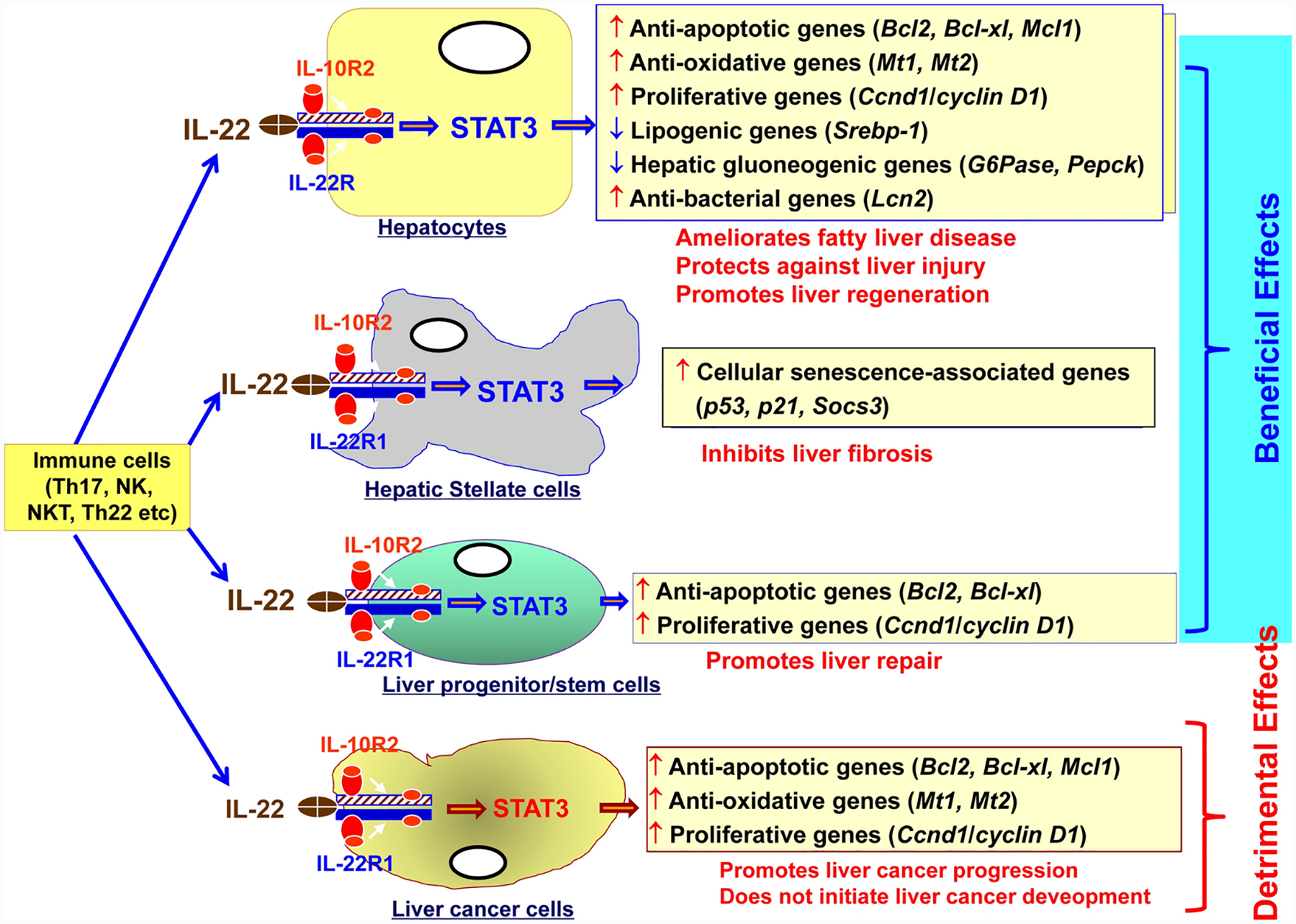
IL-22R1 and IL-10R2 are expressed in hepatocytes, HSCs, liver progenitor cells, and liver cancer cells. By binding to these receptors, IL-22 exert many important beneficial functions in the liver but also some detrimental functions such as promoting liver cancer progression. However, IL-22 does not initiate liver cancer development.
The role of IL-22 binding protein (IL-22BP):
IL-22BP (also called IL-22Ra2) is a soluble protein that is believed to inhibit IL-22 activity; however, its function is less clear. It has been reported that serum IL-22BP levels are very high (40,000–70,000 pg/ml) in humans [55] but low (~40 pg/ml) in mice [40]. It is not clear why there is a huge difference in serum IL-22BP levels between humans and mice, and whether this difference was due to different ELISA kits used for the measurement. Interestingly, Il22bp-deficient mice were found to be more susceptible to acute liver damage induced by ischemia reperfusion and acetaminophen (APAP) administration, which suggests that IL-22BP plays a protective role in acute liver damage, via controlling IL-22-induced Cxcl10 expression [56]. A recent study reported that ACLF patients are associated with elevated IL-22 and IL-22BP [55], suggesting IL-22BP may affect the severity of ACLF via the regulation of IL-22 activity. More studies are required to clarify the functions of IL-22BP in the pathogenesis of liver diseases.
Interleukin-22 study in preclinical models of liver injury
Since we first reported the hepatoprotective functions of IL-22 in 2004 [51, 57], IL-22 has been extensively examined and found to protect against liver injury in a wide variety of preclinical models of liver injury (Table 1). Subsequently, the protections of IL-22 against injury of epithelial cells in many other organs (pancreas, gut, kidney, and lung) have been reported in animal models by our laboratory and other groups [44, 47]. Clinical studies also suggest the protective effects of IL-22 in patients with liver diseases such as chronic hepatitis B [58], drug-induced liver injury (DILI) [59] and ACLF patients [60]. In addition, the role of host defense against bacterial infection of IL-22 was also demonstrated [40, 44, 61].
Table 1:
Major studies of IL-22 in preclinical models of liver injury and in patients
| The year the study was conducted [Ref] | Targeting cells Or disease | Liver injury models and results |
|---|---|---|
| 2004 [51, 57] 2007 [79]; 2011 [54] |
Hepatocytes | IL-22 protects again liver injury induced by Con A, CCl4, Fas ligand, APAP; also tested in IL-22 KO and IL-22 transgenic mice, |
| 2004 [51] 2011 [54, 78] |
HCC | IL-22 promotes HCC survival and proliferation in vitro, and growth in vivo; but IL-22 does not initiate liver cancer development |
| 2010 [62]; 2019 [80]; 2020 [74] | ALD | IL-22 protects against chronic-plus-binge ethanol-induced liver injury in mouse models. IL-22 improves AH in patients in a phase IIb trial |
| 2010 [52]; 2014 [81] 2020 [70] |
Fatty liver, NASH | IL-22 protects against HFD-induced fatty liver and HFD+Cxcll-induced NASH |
| 2012 [53]; 2016 [82] | HSCs, liver fibrosis | IL-22 induces HSC senescence and inhibits liver fibrosis |
| 2012 [52] | Liver progenitor/stem cells | IL-22 promotes HSC survival and proliferation, promoting liver repair |
| 2014 [64]; 2018 [65] | Drug-induced liver injury | IL-22 protects against APAP-induced acute liver injury |
| 2016 [61]; 2020 [40] | Bacterial infection | IL-22 promotes hepatocytes to produce anti-bacterial proteins, thereby inhibiting bacterial infection |
| 2016 [83] | Ischemia/reperfusion | IL-22 protects against ischemia/reperfusion-induced acute liver injury |
| 2020 [40] | ACLF | IL-22 protects against ACLF in a new model induced by chronic and acute CCl4 treatment plus bacterial infection |
| 2019 [76, 77] | Phase I trial | Well-tolerance with favorable pharmacokinetics (PK) and pharmacodynamics (PD) properties |
| 2020 [74] | Phase IIb trial | IL-22 improves Lille and MELD scores, reduces inflammatory markers and increases regenerative markers in AH patients |
In a murine model of chronic-plus-binge ethanol feeding simulating alcoholic liver injury (NIAAA model), treatment with IL-22 activates hepatic STAT3 and ameliorates alcoholic fatty liver, liver injury, and hepatic oxidative stress, indicating the potential therapeutic effect of IL-22 for alcoholic liver injury [62]. Considering the condition of hepatocyte injury, inflammation, and impaired liver regeneration in AH patients, theoretically, IL-22 combined with the most commonly used glucocorticoid might be a promising strategy for treating these patients.
Besides alcoholic liver injury, IL-22 has been demonstrated to protect mice from DILI. Several studies revealed that IL-22 protected mice from APAP-induced liver injury, the most widely used model for studying DILI in mice, mainly via the activation of STAT3 [63, 64]. Recently, activation of autophagy has been reported to be also responsible for the ability of IL-22 to protect against APAP-induced liver injury [65]. In addition, IL-22 also protects mice from T cell-mediated liver injury induced by injection of Concanavalin A (Con A), a T cell mitogen. IL-22 reduces Con A-induced liver injury primarily through the activation of STAT3 and its downstream anti-apoptotic genes such as Bcl2, Bclxl, and Mcl1 [51, 54, 57, 66, 67].
While IL-22 has been intensively studied in the preclinical models of various liver injury, the effect of IL-22 has been elusive in acute-on-chronic liver failure (ACLF) and nonalcoholic steatohepatitis (NASH), in part due to the absence of appropriate animal models that recapitulate the pathophysiology of these diseases. In our newly developed ACLF mouse model combining chronic liver injury, acute hepatic insult and bacterial infection which recapitulates some of the key features of acute-on-chronic liver failure in patients, IL-22 therapy improved survival rate in ACLF mice by reprogramming impaired regenerative pathways and attenuating bacterial infection [40].
Although fatty liver is readily induced by chronic high-fat diet (HFD) feeding in mice, severe liver injury that can cause fatty liver-to-NASH promotion is usually not observed in HFD-fed mice. IL-22 has been shown to protect mice from fatty liver and mild liver injury caused by HFD feeding [68, 69]. NASH has become the leading cause of chronic liver disease due to the recent prevalence of metabolic syndrome. Effort has been made to elucidate the NASH pathogenesis; however, there has been no FDA-approved medications for NASH, and existing preclinical models fail to reflect the molecular pathogenesis and pathophysiology of human NASH. Since remarkable neutrophil infiltration and increased expression of neutrophil-recruiting chemokines (e.g., CXCL1 and IL-8) in the liver are key features of human NASH as compared with fatty liver, our group has recently established a new preclinical NASH model through the hepatic overexpression of Cxcl1 in the liver of 3-month HFD-fed mice (HFD+Cxcl1-induced NASH model) [70]. Hepatic Cxcl1 overexpression markedly elevated hepatic neutrophil infiltration in HFD-fed mice, and reactive oxygen species produced by infiltrating neutrophils promoted hepatocyte death through the activation of p38 mitogen-activated protein kinase which regulated factors involved in apoptosis and ER stress such as BCL2, CHOP, and CASP3 [70]. HFD+Ctcl1-induced NASH was ameliorated by IL-22 administration primarily through the STAT3-dependent activation of potent anti oxidant enzymes, metallothionein (MT)-l and MT-2 [70].
These promising findings obtained from the preclinical models of liver injury have highlighted the potential of IL-22 to be developed as a therapeutic option in the clinical setting, which has motivated researchers to investigate the feasibility of the IL-22 therapy in clinical trials as discussed below.
Clinical trials of IL-22 in AH
Over the last 10 years, many therapeutic targets have been identified for the treatment of AH and some of them are currently being tested in clinical trials (Table 2)[71–73] with some positive preliminary data reported [74, 75]. Among them, IL-22 is one drug with multiple targets for severe alcoholic hepatitis with limited side effects due to its unique features as described above (Fig. 5). Two phase I clinical trials of IL-22Fc (recombinant human IL-22 IgG2-Fc) have been performed and demonstrated the well-tolerance with favorable pharmacokinetics (PK) and pharmacodynamics (PD) properties via intravenous administration in healthy volunteers [76, 77]. Because delayed injection site reactions were observed via subcutaneous injection of IL-22Fc, intravenous administration was adopted. No severe adverse events were observed via intravenous injection at the dose up to 45 μg/kg [76, 77].
Table II:
Current registered and ongoing trials for AH
| AH targets | Drug types | Trial design | Title/trial number |
|---|---|---|---|
| Lipid metabolism, inflammation | DUR-928 (sulfated oxysterol) | DUR-928 dose-escalation | DUR-928 in patients with AH/ NCT03917407 |
| Lipid metabolism, inflammation | 5-lipoxygenase inhibitor (DS102) | DS102 versus placebo | Efficacy and safety of orally administered DS102 in patients with acute AH/ NCT03452540 |
| Inflammation, IL-1 | IL-1 monoclonal antibody (canakinumab) | Canakinumab versus placebo | IL-1 signal inhibition in AH/ NCT03775109 |
| Neutrophils, Hepatic regeneration | Anakinra (plus zinc) and G-CSF (also known as filgrastim) | Anakinra (plus zinc) versus prednisone or placebo; G-CSF versus prednisone or placebo | Trial of anakinra (plus zinc), G-CSF, or prednisone in patients with SAH/ NCT04072822 |
| G-CSF | G-CSF versus steroid or placebo | Efficacy and safety of G-CSF in patients with SAH with null or partial response to steroid/ NCT02442180 | |
| G-CSF | G-CSF + standard medical therapy versus standard medical therapy | GCSF in AH/ NCT03703674 | |
| Pegfilgrastim (pegylated form of filgrastim) | Pegfilgrastim + standard of care versus standard of care | Pegfilgrastim in patients with AH/ NCT02776059 | |
| Hepatic regeneration | IL-22 (F-652) | F-652 dose escalation | Use of F-652 in patients with AH/ NCT02655510 |
| Gut microbiota | Probiotics | Probiotic Lactobacillus rhamnosus GG versus placebo | Novel therapies in moderately severe acute AH/ NCT01922895 |
| Immunomodulation, Gut permeability | Bovine colostrum (IMM-124E) | IMM-124E versus placebo | Comparison of bovine colostrum versus placebo in treatment of SAH: A randomized double-blind controlled trial/ NCT02473341 |
| Oxidative stress, Infection | Antioxidant (N-acetylcysteine, NAC) | NAC + prednisolone versus prednisolone | N-acetylcysteine to reduce infection and mortality for AH/ NCT03069300 |
Figure 5: IL-22: one drug with multiple targets for severe alcoholic hepatitis with limited side effects.
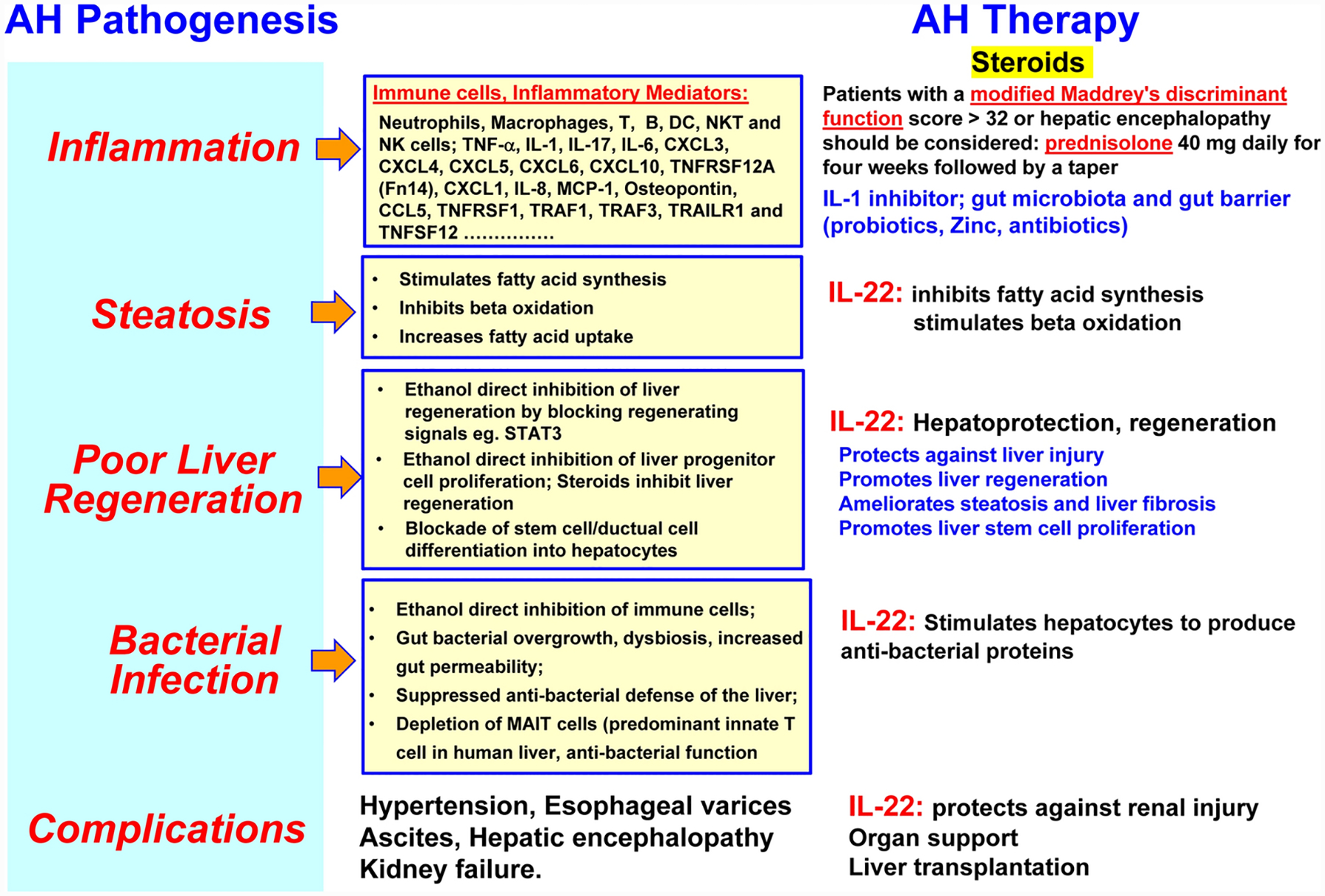
Due to its anti-apoptosis, anti-fibrosis, anti-oxidation, anti-bacterial infection and regenerative stimulation functions, IL-22 therapy may generate multiple benefits for AH patients.
Recently, an open-label, cohort dose-escalation phase Ha study to assess the safety and efficacy of IL-22 in patients with moderate and severe AH was performed (Fig. 6), showing improved clinical manifestations treating with IL-22Fc plus standard of care which included corticosteroids in some patients [74]. In detail, 18 patients (9 moderate and 9 severe AH) were enrolled, and three doses of IL-22Fc (10, 30, 45 μg/kg) were administered. IL-22Fc injection was safe in all three doses in these AH patients. Patients showed a high rate of improvement determined by Lille and MELD scores compared to propensity matched controls with reductions in markers of inflammation and increases in markers of hepatic regeneration. Infection is a major factor contributing to death in severe AH patients; however, none of the patients in this study developed an infection requiring antibiotic treatment, which theoretically could be related to the anti-microbial effect of IL-22 in addition to hepatoprotective and regenerative functions. Because only a very small number of patients (18 patients) were enrolled in this pilot cohort dose-escalation phase Ha study, the positive results acquired from this study should be interpreted cautiously until multi-center and randomized placebo-controlled trials can be performed to confirm the benefits of IL-22Fc treatment for AH.
Figure 6:
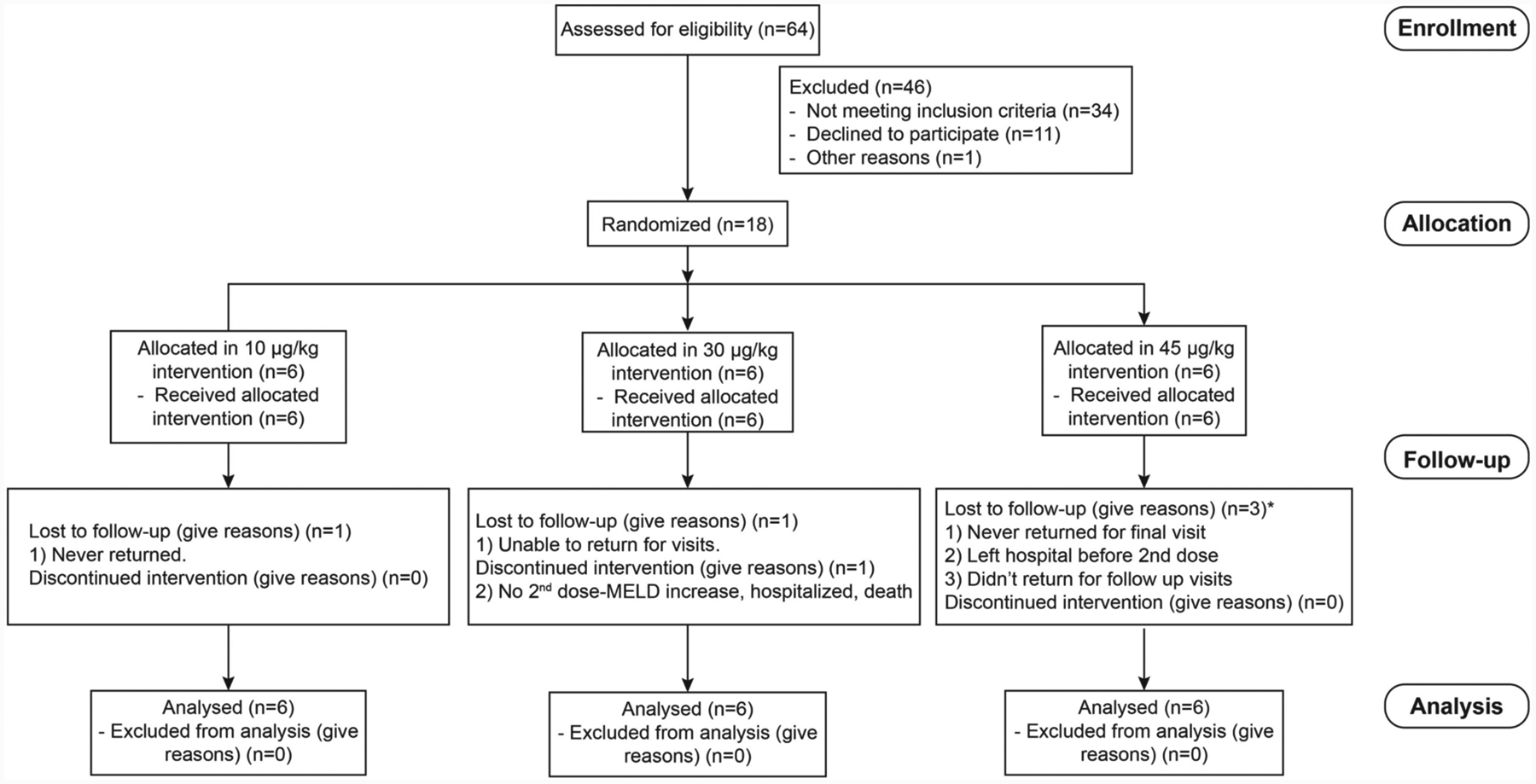
Consolidated Standards of Reporting Trials (CONSORT) flowchart from enrolled patients. An open-label, cohort dose-escalation phase Ha study to assess the safety and efficacy of IL-22 in patients with moderate and severe AH was performed. In detail, 18 patients (9 moderate and 9 severe AH) were enrolled, and three doses of IL-22Fc (10, 30, 45 μg/kg) were administered. Adapted from Arab et al.: Hepatology 2019 Nov 27. doi: 10.1002/hep.31046
Although humans have high serum IL-22BP levels (40,000–70,000 pg/ml) [55], which is believed to inhibit IL-22 activity, emerging evidence suggests that IL-22Fc therapy is effective in humans. First, healthy individuals responded very well to IL-22Fc injection, as evidenced by marked elevation of acute phase proteins [76, 77]. Second, patients with AH also responded to IL-22Fc injection as described above [74]. One of reasons for the effectiveness of IL-22Fc therapy is probably because administration of pharmacological doses of IL-22Fc elevated serum IL-22Fc levels up to 700,000 pg/ml [74], which is a thousand-fold higher compared to endogenous IL-22 levels (~200 pg) in patients, and is also a 10-fold higher compared to endogenous IL-22BP levels. In addition, how potently IL-22BP inhibits IL-22 activity in vivo still needs to be clarified. Collectively, endogenous IL-22BP is not enough to block the pharmacologic effects of IL-22Fc therapy.
Another concern for IL-22Fc therapy is HCC growth because IL-22 promotes HCC survival and proliferation [51, 54, 78]. However, transgenic mice with high levels of IL-22 (~ 6000 pg/ml) do not have higher incidence of spontaneous tumor development compared to wild-type mice, suggesting that IL-22 itself does not initiate any tumor development including HCC [54]. Thus, human studies have focused on short-term (such as 4 weeks) treatment with IL-22 in patients without obvious liver cancer to avoid concerns between IL-22 and HCC.
Conclusions and Future perspectives
IL-22 biology in the liver has been extensively studied over the last 15 years, and multiple beneficial effects of IL-22 have been identified in many preclinical models of liver injury including ALD. An open-label, cohort dose-escalation phase Ha study demonstrated that treatment with IL-22 is safe in patients with moderate and severe AH [74], This pilot trial study with a very small number of patients revealed promising results that IL-22 treatment improved Lille and MELD scores, reduced inflammatory markers but increased hepatic regeneration markers [74]. supporting the need for randomized placebo-controlled multi-center trials of IL-22 the test the efficacy of IL-22 in AH. In addition, IL-22 treatment improves several types of liver injury in preclinical models including ACLF [40] and NASH [70], suggesting IL-22 may also have therapeutic potential for the treatment of these maladies.
Serum IL-22BP levels are very high (40,000–70,000 pg/ml) in humans [55] and IL-22BP is believed to inhibit IL-22 activity; however, how IL-22BP affects IL-22 signaling in the liver and IL-22 therapy remains unknown, which should be evaluated in the future.
Grant support:
The work described from Dr. Bin Gao’s laboratory was supported by the intramural program of NIAAA, NIH. Dr. Xiaogang Xiang was a visiting scholar at the NIAAA between 2017-2019.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Requirements:
This article does not contain any studies with human or animal subjects
Reference
- 1.Gao B and Bataller R, Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011; 141(5):1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabo G, et al. , Alcohol-Related Liver Disease: Areas of Consensus, Unmet Needs and Opportunities for Further Study. Hepatology. 2019; 69(5):2271–2283. [DOI] [PubMed] [Google Scholar]
- 3.Avila MA, et al. , Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting. Gut. 2020; 69(4):764–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabb DW, et al. , Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020; 71(1): 306–333. [DOI] [PubMed] [Google Scholar]
- 5.Lucey MR, Mathurin P, and Morgan TR, Alcoholic hepatitis. N Engl J Med. 2009; 360(26):2758–69. [DOI] [PubMed] [Google Scholar]
- 6.Sandahl TD, et al. , Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. J Hepatol. 2011; 54(4):760–4. [DOI] [PubMed] [Google Scholar]
- 7.Shah ND, et al. , Alcohol-Related Liver Disease Is Rarely Detected at Early Stages Compared With Liver Diseases of Other Etiologies Worldwide. Clin Gastroenterol Hepatol. 2019; 17(11):2320–2329 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabb DW, et al. , Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NlAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016; 150(4):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustot T and Jalan R, Acute-on-chronic liver failure in patients with alcohol-related liver disease. J Hepatol. 2019; 70(2):319–327. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj JS, et al. , Acute-on-Chronic Liver Failure: Getting Ready for Prime Time? Hepatology. 2018; 68(4) : 1621–1632. [DOI] [PubMed] [Google Scholar]
- 11.Singal AK, et al. , Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014; 12(4):555–64; quiz e31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thursz M and Morgan TR, Treatment of Severe Alcoholic Hepatitis. Gastroenterology. 2016; 150(8):1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathurin P, et al. , Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011; 60(2):255–60. [DOI] [PubMed] [Google Scholar]
- 14.Singal AK, et al. , ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018; 113(2): 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BP, et al. , National Trends and Long-term Outcomes of Liver Transplant for Alcohol-Associated Liver Disease in the United States. JAMA Intern Med. 2019; 179(3):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesesne HR, Bozymski EM, and Fallon HJ, Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology. 1978; 74(2 Pt 1): 169–73. [PubMed] [Google Scholar]
- 17.Sehrawat TS, Liu M, and Shah VH, The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020; 5(5):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louvet A, et al. , Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology. 2018; 155(2):458–468 e8. [DOI] [PubMed] [Google Scholar]
- 19.Seitz HK, et al. , Alcoholic liver disease. Nat Rev Dis Primers. 2018; 4(1): 16. [DOI] [PubMed] [Google Scholar]
- 20.Gao B, et al. , Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019; 70(2):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandrekar P, et al. , Alcoholic hepatitis: Translational approaches to develop targeted therapies. Hepatology. 2016; 64(4): 1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber CS, Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004; 34(1):9–19. [DOI] [PubMed] [Google Scholar]
- 23.Gao B and Tsukamoto H, Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology. 2016; 150(8): 1704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnabl B and Brenner DA, Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014; 146(6):1513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubes P and Mehal WZ, Sterile inflammation in the liver. Gastroenterology. 2012; 143(5): 1158–1172. [DOI] [PubMed] [Google Scholar]
- 26.Zhong W, et al. , Paneth Cell Dysfunction Mediates Alcohol-related Steatohepatitis Through Promoting Bacterial Translocation in Mice: Role of Zinc Deficiency. Hepatology. 2020; 71(5):1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang S, et al. , Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology. 2020; 71(2):522–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smirnova E, et al. , Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo G, Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015; 148(1):30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez M, et al. , Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009; 136(5): 1639–50. [DOI] [PubMed] [Google Scholar]
- 31.Altamirano J, et al. , A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014; 146(5): 1231–9 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha B, et al. , Biomarkers of Macrophage Activation and Immune Danger Signals Predict Clinical Outcomes in Alcoholic Hepatitis. Hepatology. 2019; 70(4):1134–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louvet A and Mathurin P, Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015; 12(4):231–42. [DOI] [PubMed] [Google Scholar]
- 34.Bertola A, et al. , Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013; 8(3):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker R, Kim SJ, and Gao B, Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol. 2018; 15(1):50–59. [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, et al. , Adipocyte Death Preferentially Induces Liver Injury and Inflammation Through the Activation of Chemokine (C-C Motif) Receptor 2-Positive Macrophages and Lipolysis. Hepatology. 2019; 69(5):1965–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JH, et al. , Mitochondrial double-stranded RNA in exosome promotes interleukin-17 production through toll-like receptor 3 in alcoholic liver injury. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalopoulos GK, Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017; 65(4):1384–1392. [DOI] [PubMed] [Google Scholar]
- 39.Wands JR, et al. , Inhibition of hepatic regeneration in rats by acute and chronic ethanol intoxication. Gastroenterology. 1979; 77(3):528–31. [PubMed] [Google Scholar]
- 40.Xiang X, et al. , Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol. 2020; 72(4):736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horiguchi N, Ishac EJ, and Gao B, Liver regeneration is suppressed in alcoholic cirrhosis: correlation with decreased STAT3 activation. Alcohol. 2007; 41(4):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubuquoy L, et al. , Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015; 64(12):1949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancho-Bru P, et al. , Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012; 55(6):1931–41. [DOI] [PubMed] [Google Scholar]
- 44.Dudakov JA, Hanash AM, and van den Brink MR, Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015; 33:747–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao B and Xiang X, Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019; 16(7):666–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolk K, et al. , Biology of interleukin-22. Semin Immunopathol. 2010; 32(1): 17–31. [DOI] [PubMed] [Google Scholar]
- 47.Sabat R, Ouyang W, and Wolk K, Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014; 13(1):21–38. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt-Arras D and Rose-John S, IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. 2016; 64(6):1403–15. [DOI] [PubMed] [Google Scholar]
- 49.Gao B, Ma J, and Xiang X, MAIT cells: a novel therapeutic target for alcoholic liver disease? Gut. 2018; 67(5):784–786. [DOI] [PubMed] [Google Scholar]
- 50.Lu Z, et al. , MicroRNA 15a/16–1 suppresses aryl hydrocarbon receptor-dependent interleukin-22 secretion in CD4(+) T cells and contributes to immune-mediated organ injury. Hepatology. 2018; 67(3):1027–1040. [DOI] [PubMed] [Google Scholar]
- 51.Radaeva S, et al. , Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004; 39(5):1332–42. [DOI] [PubMed] [Google Scholar]
- 52.Feng D, et al. , Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012; 143(1): 188–98 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong X, et al. , Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012; 56(3):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park O, et al. , In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011; 54(1):252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarzkopf K, et al. , IL-22 and IL-22-Binding Protein Are Associated With Development of and Mortality From Acute-on-Chronic Liver Failure. Hepatol Commun. 2019; 3(3):392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleinschmidt D, et al. , A Protective Function of IL-22BP in Ischemia Reperfusion and Acetaminophen-Induced Liver Injury. J Immunol. 2017; 199(12):4078–4090. [DOI] [PubMed] [Google Scholar]
- 57.Pan H,et al. , Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004; 1(1): 43–9. [PubMed] [Google Scholar]
- 58.Xiang X, et al. , IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol Cell Biol. 2012; 90(6):611–9. [DOI] [PubMed] [Google Scholar]
- 59.Lai R, et al. , Protective effect of Th22 cells and intrahepatic IL-22 in drug induced hepatocellular injury. J Hepatol. 2015; 63(1):148–55. [DOI] [PubMed] [Google Scholar]
- 60.Mo R, et al. , Persistently elevated circulating Th22 reversely correlates with prognosis in HBV-related acute-on-chronic liver failure. J Gastroenterol Hepatol. 2017; 32(3):677–686. [DOI] [PubMed] [Google Scholar]
- 61.Zheng M, et al. , Therapeutic Role of Interleukin 22 in Experimental Intra-abdominal Klebsiella pneumoniae Infection in Mice. Infect Immun. 2016; 84(3):782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ki SH, et al. , Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010; 52(4):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheiermann P, et al. , Application of interleukin-22 mediates protection in experimental acetaminophen-induced acute liver injury. Am J Pathol. 2013; 182(4): 1107–13. [DOI] [PubMed] [Google Scholar]
- 64.Feng D, et al. , Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014; 193(5):2512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mo R, et al. , Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics. 2018; 8(15):4170–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abe H, et al. , Aryl hydrocarbon receptor plays protective roles in ConA-induced hepatic injury by both suppressing IFN-gamma expression and inducing IL-22. Int Immunol. 2014; 26(3):129–37. [DOI] [PubMed] [Google Scholar]
- 67.Park O, et al. , Biologically active, high levels of interleukin-22 inhibit hepatic gluconeogenesis but do not affect obesity and its metabolic consequences. Cell Biosci. 2015; 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang L, et al. , Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010; 53(2):339–47. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, et al. , Interleukin-22 drives a metabolic adaptive reprogramming to maintain mitochondrial fitness and treat liver injury. Theranostics. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang S, et al. , Interleukin-22 Ameliorates Neutrophil-Driven Nonalcoholic Steatohepatitis Through Multiple Targets. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo G, Clinical Trial Design for Alcoholic Hepatitis. Semin Liver Dis. 2017; 37(4):332–342. [DOI] [PubMed] [Google Scholar]
- 72.Xu M, et al. , New drug targets for alcoholic liver disease. Hepatol Int. 2014; 8 Suppl 2:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singal AK and Shah VH, Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol. 2019; 70(2):305–313. [DOI] [PubMed] [Google Scholar]
- 74.Arab JP, et al. , An Open Label, Dose Escalation Study To Assess The Safety And Efficacy Of IL-22 Agonist F-652 In Patients With Alcoholic Hepatitis. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shasthry SM, et al. , Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial. Hepatology. 2019; 70(3):802–811. [DOI] [PubMed] [Google Scholar]
- 76.Tang KY, et al. , Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol. 2019; 16(5):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothenberg ME, et al. , Randomized Phase I Healthy Volunteer Study of UTTR1147A (IL-22Fc): A Potential Therapy for Epithelial Injury. Clin Pharmacol Ther. 2019; 105(1): 177–189. [DOI] [PubMed] [Google Scholar]
- 78.Jiang R, et al. , Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011; 54(3):900–9. [DOI] [PubMed] [Google Scholar]
- 79.Zenewicz LA, et al. , Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007; 27(4):647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hendrikx T, et al. , Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019; 68(8):1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, et al. , Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014; 514(7521):237–41. [DOI] [PubMed] [Google Scholar]
- 82.Hu BL, et al. , Interleukin-22 ameliorates liver fibrosis through miR-200a/beta-catenin. Sci Rep. 2016; 6:36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eggenhofer E, et al. , RORgammat(+) IL-22-producing NKp46(+) cells protect from hepatic ischemia reperfusion injury in mice. J Hepatol. 2016; 64(1):128–34. [DOI] [PubMed] [Google Scholar]


