Abstract
Objectives
Loeys–Dietz syndrome (LDS) and vascular Ehlers–Danlos Syndrome (vEDS) are genetically heterogeneous heritable disorders of connective tissue. Both are multi-system disorders with dominant vascular pathology and associated gastrointestinal manifestations.
Aim
To summarize the abdominal manifestations found in these two disorders in a cohort of patients seen at Mayo Clinic during a period of 25 years.
Methods
Data were collected via the advanced cohort explorer (ACE) of Mayo Clinic records from 1994 to 2018 in patients with vEDS or LDS confirmed by genetic testing and/or medical genetics consultation. We extracted information concerning gastrointestinal symptoms, abdominal hernias, and vascular manifestations or complications.
Results
We identified and reviewed records of 68 vEDS and 13 LDS patients. Patients were similar in age at diagnosis and gender distribution. Gastrointestinal symptoms were frequently reported in both disorders and largely similar, although altered bowel function was more prevalent in LDS patients. Hernias were present in similar proportions of patients with vEDS and LDS; however, ventral hernias were more frequent and more likely to be postoperative in vEDS than LDS. LDS patients had more arterial aneurysms overall (76.9% LDS vs. 58% vEDS, p = 0.02) and a higher proportion required arterial repair (69.2% LDS vs. 32.7% vEDS S, p = 0.03). Co-morbidities of autonomic dysfunction, psychopathology (most commonly anxiety, depression, adjustment disorder), and allergy were more prevalent in LDS than vEDS.
Conclusion
Patients with vEDS and LDS had a propensity for gastrointestinal symptoms, abdominal hernias, and aneurysm formation, but repair for arterial rupture was more prevalent in LDS than EDS.
Keywords: Angiography, Genetics, Type IV
Introduction
Loeys–Dietz syndrome (LDS) and vascular Ehlers–Danlos syndrome (vEDS) are autosomal dominant heritable disorders of connective tissue which have multi-system involvement that includes vascular and gastrointestinal manifestations. These patients can be referred to gastroenterology clinics for symptom evaluation either before or after the diagnosis of LDS or vEDS is established. In either situation, it is essential to identify these disorders, as the patients have increased vascular comorbidities and carry increased risk for complications such as perforation during routine endoscopic evaluations.
Ehlers–Danlos syndrome (EDS) is a group of clinically and genetically heterogeneous, heritable connective tissue disorders affecting skin, bones, and other organs and systems [1]. The combined prevalence of all 13 types of EDS is approximately 1 in 5000 individuals, with the hypermobility type (hEDS) being the most common; other forms of the syndrome are relatively rare [1]. Joint laxity is a prevalent problem in most EDS types, but life-threatening complications such as arterial aneurysms and ruptures are mostly limited to vEDS [2]. Mutations in at least 19 collagen genes have been associated with the diverse subtypes of EDS. The hypermobility subtypes are associated with gastrointestinal symptoms including hiatal hernia, gastritis, abdominal pain and distention, nausea, vomiting, and gastroesophageal reflux disease, constipation, and evacuation disorders [3]. Most cases of vEDS are caused by autosomal dominant mutations of the COL3A1 gene [4] leading to defective type III collagen in the lungs, skin, intestines, and blood vessels resulting in severe tissue fragility [1]. Some patients with vEDS have a characteristic facies, thin or translucent skin, and easy bruising, but many have a normal phenotype. Vascular complications include arterial aneurysm and dissection. Dissection and rupture occur in both small and large aneurysms (Fig. 1). Severe tissue fragility also causes spontaneous hollow internal organ rupture, most commonly of the colon and uterus [2].
Fig. 1.
Forty-four year-old woman with vascular Ehlers–Danlos syndrome with abdominal aortic aneurysm, right common iliac artery aneurysm with dissection, and focal dissection of the distal left external iliac artery
According to the 2017 diagnostic criteria established by the Ehlers–Danlos Society, evaluation for vEDS should be triggered by a positive family history or suggestive clinical features [2]. Major and minor criteria, as well as minimal criteria for diagnostic evaluation, are listed in Tables 1 and 2. Diagnosis is made by identification of a known pathogenic variant of the COL3A1 gene [2].
Table 1.
Diagnostic criteria of vascular Ehlers–Danlos syndrome [2]
| Major criteria | Family history of vEDS with documented causative variant in COL3A1 Arterial rupture at a young age Spontaneous sigmoid colon perforation in the absence of bowel pathology Uterine rupture during the third trimester in the absence of prior injury Carotid-cavernous sinus fistula (CCSF) formation in the absence of trauma |
| Minor criteria | Bruising unrelated to identified trauma and/or in unusual sites such as cheeks and back Thin, translucent skin with increased venous visibility Characteristic facial appearance Spontaneous pneumothorax Acrogeria Talipes equinovarus Congenital hip dislocation Hypermobility of small joints Tendon and muscle rupture Keratoconus Gingival recession and gingival fragility Early-onset varicose veins (under age 30 and nulliparous if female) |
| Minimal suggestive criteria* | Family history of the disorder Arterial rupture or dissection in individuals < 40 years of age Unexplained sigmoid colon rupture Spontaneous pneumothorax in the presence of other features consistent with vEDS Combination of the other ‘minor’ criteria |
| Diagnostic confirmation | Identification of a causative variant in one allele of COL3A1 |
Should trigger diagnostic studies for confirmation
Table 2.
Diagnostic criteria of Loeys–Dietz syndrome [5]
| Compatible systemic features | |
| Vascular | Dilation or dissection of the aorta and other arteries Other arterial aneurysms and tortuosity |
| Skeletal | Pectus excavatum or pectus carinatum Scoliosis Joint laxity or contracture (typically involving the fingers) Arachnodactyly Talipes equinovarus Cervical spine malformation and/or instability Osteoarthritis |
| Craniofacial | Widely spaced eyes Bifid uvula/cleft palate Craniosynostosis, in which any sutures can be involved |
| Cutaneous | Soft and velvety skin Translucent skin with easily visible underlying veins Easy bruising Dystrophic scars Milia, prominently on the face |
| Allergic/inflammatory disease | Food allergies Seasonal allergies Asthma/chronic sinusitis Eczema Eosinophilic esophagitis/gastritis Inflammatory bowel disease |
| Ocular | Blue or dusky sclera |
| Diagnostic CONFIRMATION | |
| Heterozygous pathogenic variant in SMAD2, SMAD3, TGFB2, TGFB3, TGFBR1, or TGFBR2 AND: Aortic root enlargement (Aortic root z-score ≥ 2.0) or type A dissection OR Compatible systemic features in combination, particularly vascular malformations | |
LDS is an autosomal dominant connective tissue disorder caused by a mutation in several genes along the transforming growth factor beta (TGF-β) pathway, most commonly TGFBR1 or TGFBR2. These mutations lead to over-activation of TGF-β signaling and disruption of the extracellular matrix [6] and, as a result, there are changes in the integrity of diverse tissues. Characteristic features of LDS include a marfanoid body build and craniofacial anomalies, especially hypertelorism and bifid uvula [5]. Vascular findings include severe arterial tortuosity, and aneurysm formation in both the aorta but also in other arterial distributions. There is a high risk of arterial dissection (Fig. 2) [7]. There are five subtypes of LDS based on the specific TGF-β pathway mutation. There can be phenotypic overlap with both Marfan syndrome and vEDS, and genetic testing is needed for an accurate diagnosis.
Fig. 2.

Loeys–Dietz syndrome with thoracic and abdominal aortic tortuosity and abdominal aortic aneurysm in a patient evaluated at Mayo Clinic
The TGF-β pathway regulates immune homeostasis, and the receptor mutations in LDS patients lead to a strong predisposition to allergic diseases. Laboratory findings include increased plasma IgE levels, elevated eosinophil counts, and T-helper2 cytokines. There is increased prevalence of eosinophilic esophagitis, gastritis and colitis [8], and early onset and severe forms of inflammatory bowel disease reported in the literature [9].
LDS should be suspected in patients with suggestive systemic and vascular features. The diagnosis is established when a disease-causing mutation is found in a TGF-β pathway gene. The TGFBR1 and TGFBR2 genes encode for receptor proteins. The TGFB2 and TGFB3 genes encode for ligand proteins, and the SMAD2 and SMAD3 genes [10] encode for cytoplasmic signaling proteins (Table 2).
Our aim was to audit the medical records at a tertiary referral center of patients diagnosed with these two rare genetic connective tissue disorders (LDS and vEDS) and to compare abdominal wall, gastrointestinal tract, and vascular manifestations, as well as co-morbid conditions observed in patients with LDS and/or vEDS and in patients with overlapping phenotypes. Nineteen of the vEDS patients were included in our group’s prior description of GI manifestations in all subgroups of EDS [3].
Methods
Design
We conducted a retrospective analysis of the medical records of patients with vEDS and LDS from 1994 to 2018 at Mayo Clinic, Rochester, MN. The study was exempt from requiring patient consent by the Mayo Clinic Institutional Review Board. Only patients who had previously authorized consent to use their records for research purposes were included. Databases containing the electronic medical records were screened, and data were collected using the Mayo Clinic Advanced Cohort Explorer (ACE) in order to identify patients with vEDS or LDS. ACE is a clinical data repository maintained by the Unified Data Platform at Mayo Clinic and uses natural language processing for data retrieval.
Information was extracted to identify patient demographics, gastrointestinal symptoms, prevalence of abdominal hernias, co-morbid conditions, vascular complications, and diagnostic investigations undertaken to assess these abdominal manifestations. Comorbid conditions were diagnosed by a combination of clinical features and testing as identified in the physician-recorded notes. We also documented the genetic abnormalities identified in the patient cohorts included in our studies. As the clinical course for patients with heterozygosity for COL3A1 null mutations appears to be less severe compared with exon-skipping or missense alterations resulting in abnormal type 3 collagen structure [11], we appraised the manifestations for those with null mutations as well as missense alterations in the patients with vEDS.
Patients were included if diagnoses were confirmed by genetic testing and/or clinical review through the specialized medical genetics clinic. Patients who were diagnosed with other subtypes or indeterminate subtypes of EDS were excluded. Patients who were initially referred for vEDS or LDS evaluation based on concerning symptoms but who were not ultimately deemed to have those disorders on clinical review and/or genetic testing were also excluded.
Statistical Analysis
Descriptive statistics were utilized to provide summary data. The LDS and vEDS groups were compared using t test for continuous variables and Chi square and Fisher exact tests for nominal variables. Nonparametric tests were utilized in comparisons that involved small sample size.
Results
Patients Demographics
The ACE software identified records of 236 EDS (all subtypes) and 13 LDS patients that matched either by diagnostic code or had these terms in the clinical notes. After manual review, 68 patients with vEDS (having excluded those with other or indeterminate subtypes of EDS) and 13 patients with LDS were identified for inclusion in the study.
The patients were primarily Caucasian (92.5%) of non-Hispanic, non-Latino ethnicity. The two cohorts were similar in age at diagnosis and gender distribution, with 59% of the vEDS patients being female with a mean age at diagnosis of 38.1 ± 16.6 years, and 38.5% of the LDS patients being female with a mean age at diagnosis of 33.1 ± 19.9 years (Table 3).
Table 3.
Summary of demographics, hernias, co-morbidity history
| Vascular EDS (n = 68) | LDS (n = 13) | p value | |
|---|---|---|---|
| Demographics | |||
| Female (N, %) | 40 (58.8%) | 5 (38.5%) | 0.16 |
| Age at diagnosis (mean ± SD) | 38.18 ± 16.5 | 33.08 ± 19.9 | 0.42 |
| Prior abdominal surgery | 57.1% | 33.33% | 0.13 |
| Hernias present | 49.3% | 46.2% | 0.84 |
| Hernia type (% of all hernias) | |||
| Ventral (incl. parastomal, incisional) | 29.4% | 16.67% | |
| Hiatal/non-hiatal diaphragmatic | 38.24% | 33.3 | |
| Inguinal | 17.7% | 16.7% | |
| Umbilical | 14.7% | 33.3% | |
| Postoperative hernia (% of all hernias) | 65.6% | 33.3% | 0.14 |
| Recurrent hernia (% of all hernias) | 21.9% | 33.3% | 0.55 |
| Co-morbidity | |||
| Skin conditions | 28.4% | 8.3% | 0.10 |
| Arthralgias | 28.4% | 50% | 0.15 |
| Joint dislocations | 13.4% | 8.3% | 0.61 |
| Autonomic dysfunction (syncope, orthostatic intolerance) | 8.96% | 30.8% | 0.03 |
| Fibromyalgia | 9.0% | 16.7% | 0.44 |
| Psychopathology | 37.3% | 84.6% | 0.002 |
| Chronic fatigue | 20.9% | 7.69% | 0.16 |
| Sleep disturbance | 22.4% | 46.1% | 0.08 |
| Migraine headaches | 19.4% | 23.1 | 0.77 |
| Peripheral blood eosinophilia | 17.1% | 23% | 0.63 |
| Allergy | 38.8% | 76.92% | 0.01 |
Bolded p values are statistically significant
Genetic Analysis
Generally accepted genes for vEDS are only COL3A1 and COL1A1, and that for LDS genes are SMAD2, SMAD3, TGFB2, TGFB3, TGFBR1, or TGFBR2. Fifty-eight out of 68 vEDS patients underwent genetic testing (85.3%) with 75% (51 of 68) showing mutation in the COL3A1 gene. There were patients with overlapping phenotypes that resembled vEDS, and these were included, and their specific genetic variants documented in Table 3. We identified two patients with COL5A1 and 2 others with COL5A2 mutations, whose phenotype was clinically similar, though these mutations are typically associated with classical EDS. One vEDS patient with COL3A1 mutation had concomitant pathologic variant of the MYH11 gene, which encodes for myosin heavy chain and influences smooth muscle tension or contraction, as in blood vessels or the gastrointestinal tract. Another vEDS patient with concomitant developmental delay had a chromosomal microdeletion at 2q32.2 which involved COL3A1, COL5A2, and SLC40A1 (Solute Carrier Family 40 Member 1) genes. Mutations found as well as their expected functions are included in Table 4.
Table 4.
Genetic mutations of vEDS and LDS functions, and clinical manifestations
| Gene | # with muta tion (% of tested) |
Gene product* | Disease associations* |
|---|---|---|---|
| vEDS [N = 58 (85.3% of entire vEDS cohort) tested] | |||
| COL3A1 | 51 (87.9%) | Type III collagen | vEDS |
| COL5A1 | 2 (3.4%) | Pro-α1(V) chain of type V collagen | Classical EDS^ Carpal tunnel syndrome Keratoconus |
| COL5A2 | 2 (3.4%) | Pro-α2(V) chain of type V collagen | Classical EDS |
| MYH11 | 1 (1.7%) | Smooth muscle myosin heavy chain 11 | Core binding factor acute myeloid leukemia Familial thoracic aortic aneurysm and dissection Intestinal pseudo-obstruction Megacystis-microcolon-intestinal hypoperistalsis syndrome |
| MYLK | 1(1.7%) | Myosin light chain kinase Telokin (stabilizes myosin filaments) |
Familial thoracic aortic aneurysm and dissection Intestinal pseudo-obstruction Megacystis-microcolon-intestinal hypoperistalsis syndrome |
| SLC40A1 | 1 (1.7%) | Ferroportin (basolateral iron transporter on enterocytes) | Hereditary hemochromatosis African iron overload |
| NOTCH1 | 1 (1.7%) | Notch1 (receptor signaling determines cell fate determination, proliferation, differentiation and apoptosis) | Adams–Oliver syndrome Critical congenital heart disease# Bicuspid aortic valves Thoracic aortic aneurysms T cell acute lymphoblastic leukemia Chronic lymphocytic leukemia Head and neck squamous cell carcinoma Non-small cell lung cancer |
| LDS or related phenotype [N = 13 (100%) tested] | |||
| TGFBR1 | 4 (30.8%) | Transforming growth factor-beta (TGF-β) receptor type 1 |
LDS; familial thoracic aortic aneurysm and dissection Prostate cancer Multiple self-healing squamous epithelioma |
| TGFBR2 | 7 (53.9%) | TGF-β receptor type 2 | LDS; familial thoracic aortic aneurysm and dissection Cancers of the colon, rectum and esophagus |
| SMAD 3 | 1 (7.7%) | SMAD3 (TGF-β signaling pathway) | LDS; familial thoracic aortic aneurysm and dissection |
| FBN2 | 1 (7.7%) | Fibrillin-2 (forms microfibrils in extracellular matrix) | Congenital contractural arachnodactyly |
| LOX | 1 (7.7%) | Lysyl oxidase protein family | Familial thoracic aortic aneurysm Keratoconus |
Generally accepted genes for vEDS are only COL3A1 and COL1A1, and for LDS genes are SMAD2, SMAD3, TGFB2, TGFB3, TGFBR1, or TGFBR2
U.S. National Library of Medicine, Genetics Home Reference
Classical EDS, or type I and II EDS, is characterized by hyperextensible skin, generalized joint hypermobility, atrophic scars, fragile skin, increased bruisability, and doughty/velvety skin
Critical congenital heart disease refers to a group of serious congenital heart defects resulting for one or more abnormalities in embryonic cardiac development. These include coarctation of the aorta, double-outlet right ventricle, D-transposition of the great arteries, Ebstein anomaly, hypoplastic left heart syndrome, interrupted aortic arch, pulmonary atresia with intact septum, single ventricle, total anomalous pulmonary venous connection, tetralogy of Fallot, tricuspid atresia, and truncus arteriosis
Two of the vEDS patients were found to have heterozygosity for the COL3A1 gene resulting in a null mutation for vEDS; this was confirmed by genetic testing and analysis. The biological significance of the heterozygous null mutation for COL3A1 in these two patients was confirmed by collagen testing in dermal fibroblasts; these two patients showed different clinical courses. One patient had milder clinical manifestation and had no major arterial ruptures or dissections and has had no vascular events or interventions to date. However, the second patient with the null mutation for COL3A1 has had quite a severe clinical course including ventral hernia with multiple failed surgical repairs; aortic aneurysm dissection; and stable aneurysms of the celiac, common hepatic, and left external iliac arteries.
In the LDS cohort, all 13 (100%) patients underwent gene analysis, with 7 of the 13 patients showing a mutation in TGFBR2 (53.8%), 4 out of the 13 in TGFBR1 (30.8%), 1 out of the 13 showing mutation in SMAD3, and 1 out of the 13 showing mutation inLOX along with a variant of uncertain significance in the FBN2 gene with clinical features suggestive of LDS (Table 4).
Gastrointestinal Manifestations
Overall, 89.7% (61/68) and 92.3% (12/13) of LDS patients reported at least one gastrointestinal symptom. The most common gastrointestinal symptoms reported by patients with vEDS and LDS are summarized in Table 5. Abdominal pain was the most common symptom in vEDS patients (66.7%) followed by nausea (40.9%), heartburn or regurgitation (40.3%), and constipation (37.3%). The most common gastrointestinal symptom for LDS patients was constipation (76.9%) followed by heartburn or regurgitation (61.5%), nausea (53.8%), and diarrhea (46.2%). While gastrointestinal symptoms were prevalent in both disorders at largely the same rates, LDS patients had significantly higher prevalence of diarrhea and constipation (respectively, 46.2% and 76.9% for LDS and 36% and 16% for vEDS). There were no LDS patients with symptoms suggestive of eosinophilic esophagitis/gastritis or eosinophilic gastroenteritis.
Table 5.
Percentages of patients with gastrointestinal symptoms
| Vasc lar EDS (n = 67) |
Loeys–Dietz Syndrome(n = 13) |
p value | |
|---|---|---|---|
| Upper GI symptoms (%) | |||
| Nausea | 40.9 | 53.8 | 0.40 |
| Vomiting | 28.4 | 38.5 | 0.47 |
| Globus sensation | 3.0 | 15.4 | 0.06 |
| Heartburn/reflux | 40.3 | 61.5 | 0.16 |
| Retrosternal chest pain | 17.9 | 0 | |
| Abdominal pain | 66.7 | 46.2 | 0.16 |
| Dysphagia | 11.9 | 23.1 | 0.29 |
| Dyspepsia | 10.5 | 0 | |
| Regurgitation | 10.5 | 0 | |
| Belching | 11.94 | 0 | |
| Bloating | 19.4 | 38.5 | 0.15 |
| Postprandial fullness | 3.0 | 15.4 | 0.11 |
| Eosinophilic esophagitis | 1.5 | 0 | |
| Lower GI symptoms (%) | |||
| IBS | 9.0 | 15.4 | 0.50 |
| Constipation | 37.3* | 76.9 | 0.008 |
| Diarrhea | 19.4* | 46.2 | 0.038 |
| Fecal incontinence | 6.0 | 15.4 | 0.28 |
| Excessive straining | 10.6 | 7.7 | 0.74 |
| Incomplete evacuation | 9.0 | 0 | |
1 patient with Crohn’s disease had alternating bowel movements. Bolded p values are statistically significant
Comorbid Conditions
Several co-morbid conditions were documented as shown in Table 3. Those co-morbidities more prevalent in LDS than vEDS were autonomic dysfunction (30.8% LDS vs. 9.0% vEDS, p = 0.03), psychopathology (84.6% LDS vs. 37.3% vEDS, p = 0.002), and allergy (76.9% LDS vs. 38.8% vEDS, p = 0.01). Autonomic dysfunction included patients with orthostatic intolerance, unexplained presyncope/syncope, or both. Diagnosis was provided by episodes of presyncope/syncope documented in physician notes, or by autonomic reflex testing. Those with documented episodes of syncope due to known cause (pain, bleeding, etc.) were excluded. The most common psychopathologies, as diagnosed by chart review, were affective disorders including anxiety (generalized anxiety disorder, panic disorder, and post-traumatic stress disorder), depression, major depressive disorder, dysthymia, and bipolar disorder. Patients also experienced adjustment disorder often secondary to the diagnosis of EDS/LDS and associated co-morbidities or complications.
Gastrointestinal Investigations
Despite the relatively high prevalence of gastrointestinal symptoms, investigations of the gastrointestinal tract were limited in both disorders and not significantly different (Table 6).
Table 6.
Percentages of patients who underwent gastrointestinal tests and procedures
| Vascular EDS (n = 67) |
Loeys–Dietz syndrome (n = 13) |
|||
|---|---|---|---|---|
| Number (%) tested | Number (%) abnormal* |
Number (%) tested | Number (%) abnor mal* |
|
| Imaging (US, CT, MRI of abdomen or thorax) | 54 (81.8%) | 49 (90.7%) | 12 (92.3%) | 12 (100%) |
| Upper endoscopy | 17 (25%) | 9 (52.9%) | 4 (30.8%) | 2 (50%) |
| Colonoscopy | 21 (30.9%) | 10 (47.6%) | 3 (23.1%) | 1 (33.3%) |
There were no statistically significant differences in the proportions undergoing GI tests
% of abnormal results among patients who underwent testing
Endoscopy and Imaging
Upper gastrointestinal endoscopy was performed in 17 vEDS patients. There were 9 patients (53%) with abnormal findings including: gastritis (5), pre-pyloric antral stricture 2–3 cm proximal to the pylorus (1), esophageal varices (1), giardiasis (1), and eosinophilic esophagitis confirmed on mucosal biopsies (1). Among 4 LDS patients who underwent upper gastrointestinal endoscopy, two had abnormal findings: congestive gastropathy and esophageal candidiasis.
Colonoscopy was performed in 21 vEDS patients with 10 (42.9%) yielding an abnormal result or pathology. Together, the colonoscopy and abdominal radiological imaging studies showed abnormal findings in these patients including: diverticulosis (15), colonic polyp/adenomas (2), dilated left colon (2), dilated small bowel (1), ulceration (1), Crohn’s disease (1), and sigmoid volvulus (1). Of the three LDS patients who underwent colonoscopy, one was abnormal showing six benign polyps (two tubular adenomas without high-grade dysplasia, one hyperplastic polyp, two benign lymphoid aggregates, and one diminutive rectal polyp with colonic mucosa).
Hernias
Hernias were present in similar proportions in both groups of patients with vEDS and LDS (Tables 1 and 2): 49.3% (33/67) of vEDS patients and 46.2% (6/13) of LDS patients; p = 0.84. Among vEDS patients, hiatal and non-hiatal (1 Morgagni) diaphragmatic hernias were most prevalent accounting for 38.3% of hernias, followed by ventral hernias accounting for 29.4%. In LDS patients, there was similar prevalence of hiatal and non-hiatal (1 Bochdalek and 1 Morgagni) diaphragmatic hernias as well as umbilical hernias, both accounting for 33.3% of hernias identified. Non-hiatal diaphragmatic hernias consisted predominantly of Morgagni hernias.
Hernias were more likely to be diagnosed postoperatively in vEDS patients accounting for 65.6% of hernias in vEDS; only 33.3% of hernias in LDS patients were diagnosed postoperatively. There was a higher rate of prior abdominal surgeries in vEDS patients (57.1%) compared to LDS patients (33.3%). Hernias were recurrent in 21.9% of vEDS patients and in 33.3% of LDS patients.
Vascular Manifestations
Vascular imaging studies were undertaken in 81.8% of the 63 vEDS patients and in 92.3% of the 13 LDS patients. Among these patients, abnormalities were found in 90.7% of vEDS patients (49 of 54) and 100% of LDS patients (12 of 12) who underwent imaging (Table 6). Aneurysms were identified in 63.1% of vEDS patients and 76.9% of LDS patients (Table 7). While the prevalence of aneurysms was similar in vEDS and LDS patients, the locations of aneurysms differed between the two groups, with abdominal aortic aneurysms being more common in LDS patients (60% in LDS vs. 33.3% in vEDS). In addition, 10% of LDS patients had multiple aneurysms compared to 2.8% of patients with vEDS. Rates of vascular dissection (and vascular repair) were similar between the cohorts: 55.4% in vEDS and 53.8% in LDS.
Table 7.
Summary of vascular manifestations and history of vascular surgery
| Vascular EDS (n = 67) | Loeys–Dietz syndrome (n = 13) |
p value | |
|---|---|---|---|
| Aneurysms | 63.1% (n = 41) | 76.9% (n = 10) | 0.33 |
| Location (% total) | 0.008* | ||
| Thoracic aorta | 11.9% | 30% | |
| Abdominal aorta | 33.33% | 60% | |
| Others | 50.0% | 0% | |
| Multiple aneurysms | 2.8% | 10% | |
| Vascular dissections (N, %) | 55.4% | 53.8% | 0.83 |
| Surgical history for vascular or gastrointestinal complications | |||
| Arterial repair | 29.7% | 69.2% | 0.007 |
| Colectomy | 12.3% | 0 | 0.20 |
| Small bowel surgery | 12.3% | 16.7% | 0.68 |
| Cholecystectomy | 21.2% | 15.4% | 0.62 |
Based on Chi square likelihood ratio test. Bolded p values are statistically significant
Surgical (Non-vascular) Interventions
Patients in both the vEDS and LDS cohort underwent non-vascular surgical interventions. Eight vEDS patients and none of the LDS patients underwent partial or total colectomy: four of these were for spontaneous colon perforation, one of which was due to diverticulitis, and four were for severe pelvic floor dysfunction causing fecal incontinence (3) or constipation (1) with formation of colostomy or ileostomy. Eight vEDS patients and two LDS patients underwent small bowel resections. Of the eight vEDS patients who underwent partial or total colectomy, three were for small bowel obstructions due to adhesive disease from prior abdominal surgeries, two were for incarcerated bowel in parastomal hernias after stoma creation, two were for small bowel perforations (1 spontaneous, 1 iatrogenic), and one was for bowel adhesions followed by colonic restoration. A single small bowel perforation in this cohort of patients occurred in a patient 2 days following a through-the-scope balloon dilation of an area of pre-pylorus antral stenosis. Surgical evaluation found a perforation in the second portion of the duodenum as well as significant pyloric stenosis. This same patient also had a separate history of spontaneous colonic perforation leading to colectomy. In total, 5 (7.5%) vEDS patients experienced spontaneous perforation of their gastrointestinal tract.
Of the two small bowel resections performed on LDS patients, one was due to incarcerated and necrotic small bowel in a Morgagni hernia in a patient who underwent a Ladd’s procedure for intestinal malrotation, and the other resection was for a stromal tumor. Fourteen vEDS and 2 LDS patients underwent cholecystectomy (Table 7).
Discussion
This study has documented the prevalence of gastrointestinal symptoms, abdominal herniation, generalized vascular manifestations, and co-morbid conditions found in a cohort of vEDS and LDS patients over a period of 24 years at a single tertiary referral center. LDS patients had a higher rate of constipation and diarrhea and lower rates of postoperative herniation compared to vEDS. Overall rates of herniation were similar between the two groups, with hiatal or diaphragmatic hernias being the most prevalent hernias in both cohorts. LDS patients underwent more arterial repairs, but there were no colonic perforations or colectomy in LDS as compared to vEDS. When compared to patients with vEDS, those with LDS had increased rates of autonomic dysfunction, psychopathology, and allergy. These observations are consistent with the potential role of TGF-β in these disease processes, particularly allergies. Patients demonstrated several of the characteristic clinical manifestations as outlined in Tables 1 and 2.
Our audit has identified the frequent occurrence of gastrointestinal symptoms in patients with these two syndromes, LDS and vEDS. The frequency of these symptoms in vEDS is significantly increased compared to the whole cohort of the EDS population where only 43.0% reported GI symptoms. This increase may be related to elevated awareness of these associations, increasing patient reports and physician documentation. In addition, longitudinal follow-up of the patients in the initial cohort [3] may have shown development of symptoms that were previously not present. Some of the gastrointestinal and abdominal manifestations occur commonly in the general population and may not be caused by connective tissue disease. For example, propensity to heartburn, acid regurgitation, constipation, and rectal evacuation disorder may reflect the high prevalence of these symptoms in the community at large. Similarly, psychopathology is also common in the community, particularly in the setting of chronic illness. Structural findings such as abdominal wall herniation (especially if recurrent), non-hiatal diaphragmatic hernias, segmental intestinal dilatation, colonic diverticulosis, or intestinal ischemia are more likely to be caused or aggravated by the underlying connective tissue disorder. Certain findings, such as bowel perforation and intestinal ischemia, are highly suggestive of intestinal fragility and mesenteric vascular complications, and their presence should trigger a search for LDS or vEDS.
The medical literature has shown that vEDS patients have an increased risk of catastrophic gastrointestinal events, with 27% incidence of spontaneous intestinal perforation [12] and 47% presenting with colon perforation as a first gastrointestinal event [13]. Among those with vEDS and perforations, re-perforation rates range from 41 to 53% [13, 14]. The risk of perforation during endoscopic procedures, which has been described as high as 9.4% [12], requires consideration of the benefit-to-risk ratio. For example, screening for colon cancer in patients with vEDS who have a high-risk mutation can be done with non-endoscopic colon cancer screening procedures such as stool DNA testing. Screening with CT colonography may be considered, but this often also includes insufflation of the bowels with air and contrast, potentially leading to elevated perforation risk.
Endoscopy can involve other risks in addition to the risk of perforation. For example, LDS patients often have cervical spine instability which should be considered when performing upper endoscopy or endotracheal intubation. Preoperative flexion and extension cervical spine X-rays and the use of fiberoptic visualization during intubation can help to protect the cervical spine [7]. vEDS patients are at increased risk for soft tissue and intramural hematomas during endoscopy.
While there are no current reports of the risk of aneurysm rupture during routine endoscopic procedures, it is important to note that LDS patients tend to have ruptures of aneurysms that are of smaller sizes compared to patients with other connective tissue disorders [14]. Patients who are found to have aneurysms should be referred for early evaluation by a vascular surgeon.
The findings in our study are generally concordant with previous reports of gastrointestinal symptoms in vEDS [3]. It is interesting to note that there were 15 (22.1%) vEDS patients with diverticulosis in the left colon, one of which progressed to diverticulitis with rupture, eventually requiring surgical management. In the past, there has been limited research to establish the occurrence and consequences of diverticulosis in EDS patients, other than noting the increased occurrence of overall diverticular events in EDS patients compared to average populations (2% in EDS vs. 0.68% in matched controls); however, this work did not specifically evaluate diverticulosis in vEDS [15]. Only 12.3% of our vEDS cohort underwent colectomy in contrast to rates of colonic perforation and colectomy reported in previous cohorts [6, 7].
While there has been limited prior literature on gastrointestinal manifestations in LDS, we did not find any cases of inflammatory bowel disease, eosinophilic disease [16], or peripheral blood eosinophilia in our group of patients; we did find increased rates of allergy. Overall, the relatively small number of patients with LDS in our series, which is consistent with the rarity of the disease, and the small percentage of patients who underwent gastrointestinal evaluations such as endoscopic procedures with biopsy (reflecting concerns regarding the risk of perforation in association with such diseases characterized by collagen deficiencies) may have reduced our ability to comprehensively assess gastrointestinal manifestations in LDS.
In addition to its impact on immune homeostasis, TGF-β signaling is important in neuronal development in both the central and enteric nervous systems. Human colonic samples have shown abundant mRNA expression of the TGF-βs in the colonic wall and myenteric ganglia [17]. Thus, it is conceivable that the TGF-β mutations may contribute to the high prevalence of altered bowel functions observed in LDS patients.
Current guidelines for gastroenterology and nutrition management in LDS patients recommend monitoring for nutritional deficiencies with low threshold for caloric supplementation including consideration of nasogastric or gastrostomy tube placement to assist with caloric intake. Food allergy needs to be considered in this cohort and constipation needs to be managed if present before commencing maintenance nutritional therapy [7]. We noted that none of the Mayo Clinic patients with LDS required feeding supplementation at the time of evaluation.
Strengths and Limitations
One of the major strengths of this study is that 82% of the patients with vEDS had undergone genetic testing to confirm the clinical presentation of vEDS, and all 13 LDS patients had undergone genetic testing which confirmed the diagnosis. Conversely, this audit study is limited by the relatively small number of patients with LDS, which is a rare genetic disorder. We did not have enough patient numbers to determine if the risk of interventions is lower in patients with null vEDS mutations. In addition, many patients did not undergo further investigations for their gastrointestinal symptoms, possibly because of the known increased risk associated with endoscopic examination in these connective tissue disorders. The data reviewed do not allow an exhaustive assessment of the efficacy of treatment. Lastly, this is a retrospective study of patients referred to a tertiary care center and may differ from the general vEDS/LDS populations who have not been sent for tertiary evaluation. Multicenter studies with larger patient numbers will be needed to describe the natural history of these disorders and the outcomes of treatments [18].
Conclusions
Vascular Ehlers–Danlos Syndrome
Patients with major criteria of vEDS or a mixture of minor criteria should prompt further genetic evaluation for pathogenic variants of the COL3A1 gene. These patients can present with catastrophic gastrointestinal organ perforations as a first gastrointestinal event with high rates of recurrent perforation. Further study is needed to address the hypermobility component of EDS which can manifest as descending perineum syndrome leading to rectal evacuation disorder [19].
Loeys–Dietz Syndrome
Patients with compatible systemic features, particularly those with aortic root dilation, type A dissection, or arterial aneurysms and tortuosity (Fig. 3), should undergo genetic evaluation for LDS. Our study shows that the prevalence of abdominal (other than postoperative) hernias, aneurysm formation, risk of arterial vessel rupture, autonomic dysfunction, psychopathology, and allergy are greater in LDS than EDS. It appears that LDS patients experience more constipation and diarrhea than vEDS patients and the mechanism is unclear. Future studies should focus on investigation of colonic dysmotility which may conceivably result from the effect of TGF-β on myenteric neuronal function.
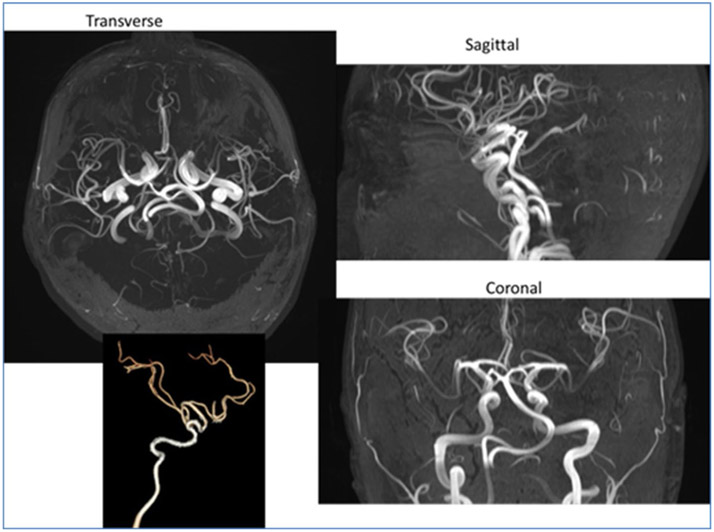
Fig. 3.
Tortuosity of internal carotid arteries in a different patient with Loeys–Dietz syndrome
Summary
vEDS and LDS are inherited connective tissue disorders with vascular manifestations and propensity for gastrointestinal symptoms. While several of the manifestations we evaluated appear to be specific to the genetic connective tissue disorder, other frequently encountered conditions including heartburn and constipation may simply represent symptoms that are commonly encountered in the community and require standard evaluation and treatment with the additional precaution that endoscopic approaches have to be selected judiciously because of the risk of perforation in these disorders. Noninvasive screening measures should be considered in patients for colorectal cancer screening as much as possible.
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding M. Camilleri’s research is supported by Grants RO1-DK115950 and RO1-DK67071 from National Institutes of Health.
Abbreviations
- LDS
Loeys–Dietz syndrome
- vEDS
Vascular Ehlers–Danlos syndrome
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Compliance with Ethical Standards
Conflict of interest The authors have no conflict of interest.
References
- 1.Steinmann B, Royce P, Superti-Furga A. The Ehlers-Danlos syndrome. In: Royce PM, Steinmann B, eds. Connective tissue and its heritable disorders. 2nd ed. New York: Wiley-Liss Inc; 2002. [Google Scholar]
- 2.Matfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26. [DOI] [PubMed] [Google Scholar]
- 3.Nelson AD, Mouchli MA, Valentin N, et al. Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. Neurogastroenterol Motil. 2015;27:1657–1666. [DOI] [PubMed] [Google Scholar]
- 4.Lum YW, Brooke BS, Black JH 3rd. Contemporary management of vascular Ehlers–Danlos syndrome. Curr Opin Cardiol. 2011;26:494–501. [DOI] [PubMed] [Google Scholar]
- 5.Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. [DOI] [PubMed] [Google Scholar]
- 6.Loeys BL, Dietz HC. Loeys-Dietz syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2019. [PubMed] [Google Scholar]
- 7.MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys–Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, et al. TGF-beta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5:195ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naviglio S, Arrigo S, Martelossi S, et al. Severe inflammatory bowel disease associated with congenital alteration of transforming growth factor beta signaling. J Crohns Colitis. 2014;8:770–774. [DOI] [PubMed] [Google Scholar]
- 10.Richter JE Jr, Samreen A, Vadlamudi C, et al. Genomic observations of a rare/pathogenic SMAD3 variant in Loeys–Dietz syndrome 3 confirmed by protein informatics and structural investigations. Medicina (Kaunas). 2019;55:E137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leistritz DF, Pepin MG, Schwarze U, et al. COL3A1 haploinsufficiency results in a variety of Ehlers–Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet Med. 2011;13:717–722. [DOI] [PubMed] [Google Scholar]
- 12.Kilaru SM, Mukamal KJ, Nee JW, Oza SS, Lembo AJ, Wolf JL. Safety of endoscopy in heritable connective tissue disorders. Am J Gastroenterol. 2019;114:1343–1345. [DOI] [PubMed] [Google Scholar]
- 13.Frank M, Adham S, Zinzindohoué F, et al. Natural history of gastrointestinal manifestations in vascular Ehlers–Danlos syndrome: a 17-year retrospective review. J Gastroenterol Hepatol. 2019;34:857–863. [DOI] [PubMed] [Google Scholar]
- 14.Adham S, Zinzindohoué F, Jeunemaitre X, et al. Natural history and surgical management of colonic perforations in vascular Ehlers–Danlos syndrome: a retrospective review. Dis Colon Rectum. 2019;62:859–866. [DOI] [PubMed] [Google Scholar]
- 15.Leganger J, Søborg MK, Mortensen LQ, et al. Association between diverticular disease and Ehlers–Danlos syndrome: a 13-year nationwide population-based cohort study. Int J Colorectal Dis. 2016;31:1863–1867. [DOI] [PubMed] [Google Scholar]
- 16.Abonia JP, Wen T, Stucke EM, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagl C, Schäfer KH, Hellwig I, et al. Expression and function of the transforming growth factor-b system in the human and rat enteric nervous system. Neurogastroenterol Motil. 2013;25:601–e464. [DOI] [PubMed] [Google Scholar]
- 18.Jondeau G, Ropers J, Regalado E, et al. International registry of patients carrying TGFBR1 or TGFBR2 mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayvargiya P, Camilleri M, Cima R. COL1A1 mutations presenting as descending perineum syndrome in a young patient with hypermobility syndrome. Mayo Clin Proc. 2018;93:386–391. [DOI] [PubMed] [Google Scholar]