Abstract
The present study tested cooperation in rats playing a 2×2 game (2 players, 2 responses) in an operant chamber, where players choose to cooperate or defect without knowledge of their partner’s choice. We evaluated cooperative responses in rats (Subjects) playing different games [iterated Prisoner’s Dilemma (IPD), Stag Hunt] with a Stooge partner utilizing different response strategies [Tit-for-tat (TFT), Win-stay, Lose-shift (WSLS), Random], and we determined the effects of oxytocin (OT). IPD trial outcomes and payoffs included mutual cooperation (reward, R, 3 sugar pellets each), mutual defection (punishment, P, 1 pellet each), or unilateral defection (temptation, T, 5 pellets) and cooperation (sucker, S, 0 pellets). Stag Hunt was similar, except that T=2 pellets. We hypothesized that Subjects would make more cooperative responses when playing Stag Hunt vs IPD, when playing IPD with a Stooge using TFT vs WSLS or Random, and when treated with OT. At baseline, Subjects’ overall likelihood of cooperation was unaffected by the game (IPD vs SH) or by the Stooges’ response strategy (TFT, WSLS, Random). Cooperative responses earned Subjects more pellets, except when playing with a Stooge using a random strategy. Trial outcomes (R, T, S or P) also varied by game and strategy, although the mutual defection (P) was the most common. Systemic pretreatment with OT increased Subjects’ cooperative responses, resulting in fewer P and more R outcomes. In particular, IPD-Random Subjects were more cooperative, even at the expense of earning fewer pellets. These results demonstrate that OT increases cooperative behavior in rats playing 2×2 games.
Keywords: animal behavior, cooperative behavior, operant behavior, oxytocin, prisoner’s dilemma, social behavior, animal
1. INTRODUCTION
Social interactions among unrelated conspecifics present opportunities for decision-making, pitting competition and individual gain against cooperation and social reward. Game theory has been used to model interactions among participants (humans, animals, organizations, governments) to understand social decision-making (Axelrod, 2006). In studies of social behavior using humans and animals, participants do not necessarily act in their own best interests as ‘rational agents’ (Fawcett et al, 2012). In part, this reflects the reinforcing effects of social interaction in humans and other social animals, including rodents. Rats prefer an environment that is associated with social play (Calcagnetti and Schechter, 1992), and prefer social over non-social play opportunities (e.g. a ball; Peartree et al, 2012). However, when participants incur costs from social interaction (loss of resources or risk of harm), they must decide whether the costs are worth the potential benefits. In circumstances where participants interact repeatedly, mutual cooperation can offer long-term benefits to overcome short-term costs (Fehr and Camerer, 2007). The present study explored social decision-making in rats, and the role of oxytocin (OT) to promote cooperation.
Laboratory investigations of social decision-making often simplify social interactions to pairs of conspecifics (Axelrod, 2006). Classic 2×2 games include the Prisoner’s Dilemma and Stag Hunt. Prisoner’s Dilemma contrasts cooperation vs self-interest, while Stag Hunt compares cooperation vs safety (Skyrms, 2004). Both of these games are symmetric and simultaneous, where each player chooses to cooperate or defect without knowledge of the actions of their partner. Testing participants in repeated trials, as with iterated Prisoner’s Dilemma (IPD), allows for development and expression of cooperative response strategies (Raihani & Bshary, 2011). Successful response strategies in IPD include Tit-for-tat (TFT; reviewed in Axelrod 2006) and Win-Stay/Lose-Shift (WSLS; Nowak and Sigmund, 1993). TFT cooperates on the first trial, and then replicates the partner’s response from the previous trial (Rapoport and Chammah, 1965). TFT performs well against other strategies because it is nice (cooperates on the first trial), provocable, forgiving and clear (Axelrod, 2006). TFT won the first two IPD tournaments organized by Robert Axelrod in 1979 and 1980. The WSLS strategy relies on the outcome of the previous round, categorized as a win or loss. After a win, the participant repeats their response from the previous trial, but switches responses after a loss (Nowak and Sigmund, 1993). Like TFT, WSLS also favors mutual cooperation, but has the advantage that it tolerates accidental defection (Imhof et al, 2008). In situations where participants make mistakes, WSLS can outperform TFT.
Recently, our laboratory developed a rat model of IPD in which pairs of rats in an operant chamber separated by a metal screen have the opportunity to press a lever to receive sucrose pellets (Wood et al, 2016). As with the classic IPD game, our model requires that participants quickly make a decision to cooperate or defect without information about their partner’s choice. We found that rats adjusted their operant behavior according to the response requirements for cooperation (pressing a lever vs withholding a response), and according to their motivation for food (hungry vs fed ad libitum). As with previous studies in birds (Stevens and Stephens, 2004), the rats did not employ TFT or WSLS strategies to determine cooperative responses. Instead, they tended to follow a “cooperate after cooperating” strategy. In other words, the choice to cooperate or defect was relatively stable. Cooperation on the previous trial was the best predictor of cooperation on a subsequent trial, regardless of the outcome. The present study extends these findings. Specifically, to foster the development of consistent response strategies, we tested rats with a Stooge partner, whose responses were controlled by computer according to a consistent rule: TFT, WSLS or random. The hypothesis was that playing IPD with a Stooge using TFT or WSLS would increase rats’ cooperative responses, compared to playing with a Stooge responding randomly. We also determined if cooperative responses vary by game, comparing IPD and Stag Hunt. IPD offers the highest payoff for unilateral defection (Temptation), while Stag Hunt rewards mutual cooperation (Reward). The hypothesis was that rats would make more cooperative responses when playing Stag Hunt vs IPD.
Lastly, we determined the effects of the nonapeptide hormone oxytocin (OT) on cooperative responses. Although OT is most well-known for its role in parturition and milk letdown, it also promotes social behavior and social bonding (Lim and Young, 2006; Ebstein et al, 2012). In humans, OT has calming effects in stressful settings similar to those of social support, reduces fear-related amygdala activity, and increases both trust and generosity (Macdonald and Macdonald, 2010). In the female prairie vole, OT receptor expression in the nucleus accumbens is strongly correlated with the formation of partner preference (Ross et al, 2009), and OT disruption prevents the formation of social attachments (Young et al, 2001). Moreover, social recognition is absent in mice without OT, and is facilitated in rats that receive exogenous OT (Popik et al, 1992; Carter et al, 2008). Accordingly, we predicted that OT would promote rats’ cooperative responses.
2. MATERIALS AND METHODS
2.1. Animals
80 adolescent male Long-Evans rats (6 weeks of age, ca. 200 g BW at the start of the study, Charles River Laboratories, MA) were pair-housed under a reversed 14L:10D photoperiod with access to food and water ad libitum. One rat from each pair was designated as the Subject. His cage-mate was the Stooge, whose responses were controlled by computer. Behavior was tested daily (5 d/wk) during the first 4 hours of the dark phase when activity peaks. Experimental procedures were approved by USC’s Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Ed (National Research Council, National Academies Press, Washington DC; 2011).
The present study tested male rats because males are more likely to adjust their response according to the response of their partner in a 2×2 game. In our previous study, cooperative responses in female rats playing IPD did not vary with dominance status or feeding condition (Wood et al, 2016). Likewise, females’ responses for a partner in a test of direct reciprocity did not depend on the respond of their partner. In a related study, female rats showed generalized reciprocity, in which their willingness to work on behalf of an unfamiliar partner was increased if they had previously benefited from work by another rat (Rutte and Taborsky, 2007). For these reasons, males are a better model to explore the flexibility of cooperative response strategies.
The 40 pairs of rats were divided into 4 groups of 10 pairs each. Three groups played IPD; the remaining group played Stag Hunt. To determine if rats playing IPD adjust their cooperative responses according to the strategy of their partner, Stooges followed a strategy of TFT (IPD-TFT; Axelrod, 2006) or WSLS (IPD-WSLS; Nowak & Sigmund, 1993), or responded randomly (IPD-Random). To determine if rats adjust their cooperative responses to different games, we compared cooperative responses in Subjects playing IPD-TFT with Subjects playing Stag Hunt against a Stooge using the same TFT strategy (SH-TFT). Lastly, to determine if OT promotes cooperation (Soares et al, 2010), Subjects were retested for two days following pretreatment with saline or OT by systemic injection.
2.2. Operant Chambers
Training and testing were conducted in operant conditioning chambers controlled by WMPC software (Med Associates, VT), and enclosed in sound-attenuating boxes with fans for ventilation. As in our previous studies (Wood et al, 2016; Li and Wood, 2017), operant chambers were divided in half by a removable mesh screen (0.5 cm grid). Each side of the chamber was equipped with a retractable lever (ENV-112CM, Med Associates) and stimulus light (ENV-229M) adjacent to a food trough (ENV-200R2M) connected to a pellet dispenser (ENV-203–45). For Stooges, the levers extended and retracted, but responses on the levers had no consequence and were not recorded. Instead, Stooge responses were controlled by computer according to the TFT, WSLS, or random strategy, and pellets were delivered to Subject and Stooge according to the payoff matrix of the game (IPD or Stag Hunt). A house light (ENV-215M) and clicker (ENV-135M) were mounted in the center of the ceiling.
2.3. Training
Initially, Subjects were trained to respond on the lever to receive 45 mg sucrose pellets (Bio-Serv Inc., Frenchtown, NJ). They were then habituated to lever insertion in daily 20-minute sessions. Each trial began in darkness with the lever retracted in the inter-trial interval (ITI) state. The stimulus light was illuminated 2 seconds before the lever was inserted into the chamber. Subjects were required to press the lever within 10 seconds to receive a sucrose pellet, after which the lever retracted, the stimulus light turned off, and the house-light was illuminated for 30 seconds. If the Subject failed to respond within 10 seconds, the chamber reverted to ITI and the trial counted as an omission. The response time was gradually decreased to 5 seconds, and then to 2 seconds. Subjects met criteria of 25 responses per 20-minute session (35 trials) for 2 consecutive days before behavioral testing began.
2.4. Testing
Subjects and Stooges were tested as pairs in 10 daily sessions of 24 trials each, according to modifications of Wood et al (2016; see Fig 1A). Briefly, at the start of each trial, stimulus lights were illuminated for 2 seconds before 1 lever was inserted on each side of the chamber. Levers were positioned on opposite sides of the chamber, and were presented for only 2 seconds. The location and brief presentation of the levers required the Subject quickly make a decision to cooperate or defect with minimal information about the Stooge’s response. After both levers retracted, pellets were dispensed, and the house light came on for 30 seconds. Pellets were dispensed every 0.5 seconds, and an audible clicker on the cage top signified each pellet entry into a food trough so that both rats could recognize when pellets were delivered.
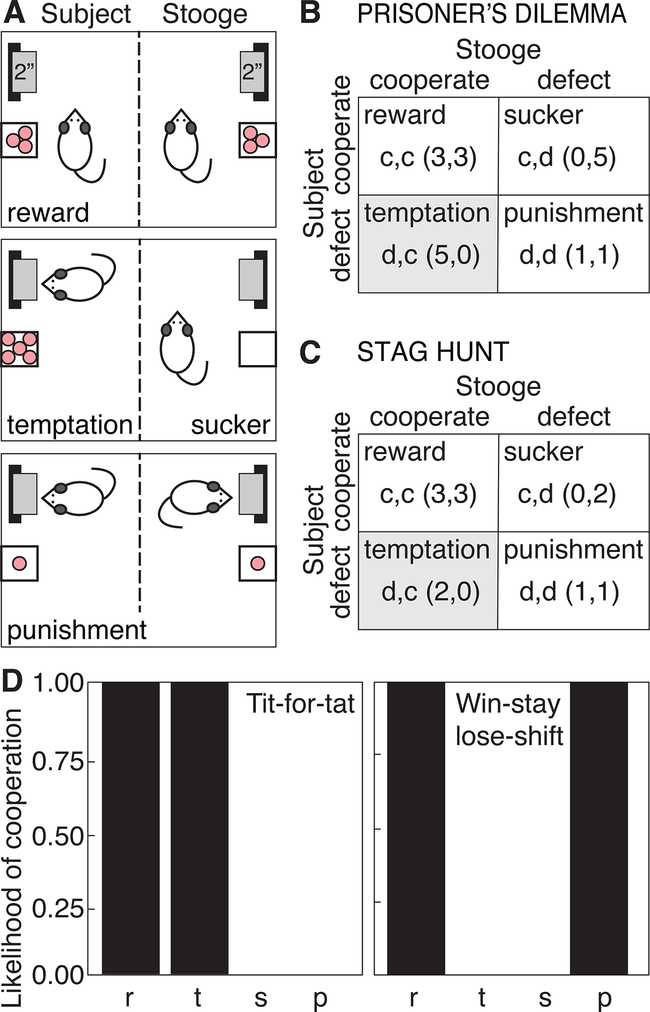
Figure 1:
A. Experimental model testing Prisoner’s dilemma in pairs of rats responding for food reward in an operant chamber bisected by a metal screen. In each pair, one rat is designated as the Subject. His partner is the Stooge, whose responses are controlled by computer. During a 2-second lever presentation, Subjects choose to cooperate (c, withhold a response) or defect (d, respond on the lever), resulting in outcomes of mutual cooperation (reward, R), mutual defection (punishment, P) or unilateral cooperation (sucker, S/temptation, T). B. Payoff matrix for Prisoner’s Dilemma (numbers in parentheses represent sugar pellets to each partner). C. Payoff matrix for the Stag Hunt game. D. Tit-for-tat (TFT) and win-stay/lose-shift (WSLS) response strategies according to transition vectors r, t, s and p, which reflect the probability of cooperation when the previous trial resulted in outcomes of R, T, S or P, respectively.
For each trial, the Subject could cooperate or defect. As defined by Rapoport and Chammah (1965), mutual cooperation is represented as Reward (R), unilateral defection is Temptation (T), unilateral cooperation is Sucker (S), and mutual defection is Punishment (P). In the present study, withholding a response on the lever signified cooperation, while responding on the lever counted as defection (Fig. 1A). Because rats were initially trained to respond on the lever to receive pellets, cooperation required suppression of a lever response. Previously, we determined that rats make similar numbers of cooperative responses when cooperation requires pressing a lever or withholding a response (Wood et al, 2016).
2.4.1. IPD
The payoff matrix for IPD followed the classic model of Axelrod (2006; Fig. 1B). Each rat received 3 pellets for R, and 1 pellet each for P. Unilateral defection (T) earned 5 pellets, while unilateral cooperation (S) earned none. This meets the definition of a strong IPD, where T > R > P > S, and 2R > T+S (Axelrod, 2006).
In the IPD-TFT group, Stooges followed the TFT strategy (Rappaport, 2015), which achieved the highest score in the original Computer Prisoner’s Dilemma Tournament (Axelrod, 2006). TFT cooperates in the first trial (nice). In subsequent trials, it repeats the opponent’s response from the previous trial. In this manner, TFT retaliates after defection, and forgives after cooperation. In the IPD-WSLS group, Stooges followed the WSLS strategy of Nowak and Sigmund (1993). On the first trial, WSLS randomly chooses to cooperate or defect. If this results in a win (R or T), it repeats the same response in the subsequent trial (Win-Stay). If the outcome is a loss (P or S), it switches the response in the next trial (Lose-Shift). Stooges in the IPD-Random group chose cooperation or defection randomly on each trial.
2.4.2. Stag Hunt
In Stag Hunt, trial outcomes (R, P, T, S) are as in IPD. However, the Stag Hunt payoff matrix has R > T > P > S. This offers Nash equilibria for Reward and Punishment outcomes (Skyrms, 2004), whereas Punishment is the only Nash equilibrium in IPD (Axelrod, 2006). In the present study, the Stag Hunt payoff matrix matched that for IPD (R = 3 pellets, P = 1, S = 0), except for T, which delivered 2 pellets (Fig. 1C).
2.5. Oxytocin
Once cooperative behavior was stable after 10 days of testing, Subjects were retested for 2 more days after injection i.p. of saline vehicle or OT (0.1 mg/kg, Spectrum Chemical, Gardena, CA). This OT dose has been shown previously to induce brain activation in rats by fMRI (Dumais et al 2017) without influencing locomotor activity, operant responding, or sucrose pellet intake (Zhou et al, 2015). OT was prepared fresh and injected at a volume of 1 ml/kg 40 minutes before testing, as in Zhou et al (2015).
Nonetheless, to control for potential sedative or anorexigenic effects of OT that could reduce operant responding and thereby confound measures of cooperation, Subjects were retested without their Stooge partner for operant responding on an FR1 schedule of reinforcement after injections of vehicle or OT. Subjects had 2 days to respond for pellets on an FR1 schedule in 20-minute daily sessions, followed by 2 days of testing with saline and OT, as above.
2.6. Data Analysis
To determine cooperative responses at baseline, data were averaged from 24 trials/day in the last 4 days of testing (96 trials total). For each Subject, we calculated pellets earned, cooperative responses, trial outcomes (R, T, S, and P), and nice responses (cooperation on the first trial). To evaluate Subjects’ decision rules, we determined transition vectors r, t, s and p, which reflect the probability of cooperation when the previous trial resulted in outcomes of R, T, S or P, respectively (Stevens & Stephens, 2004). As illustrated in Figure 1D, the TFT strategy produces high values for r and t, and low values for s and p. For WSLS, values for r and p are high, while t and s are low. Mean data from each Subject were averaged for all Subjects in each experimental group (IPD-TFT, IPD-WSLS, IPD-Random, SH-TFT). Trial outcomes were subjected to arcsine transformation to limit the effect of an artificially-imposed ceiling (Ferland et al, 2014). Pellets earned and nice responses in each group were compared by a Kruskal-Wallis non-parametric test because of non-homogeneity of variance. Trial outcomes and transition vectors were compared by repeated measures ANOVA (RM-ANOVA) with group as the between-subjects factor, and outcome or vector as the repeated measure. For RM-ANOVA, Mauchly’s test of sphericity was applied and the degrees of freedom were corrected to more conservative values using the Greenhouse-Geisser ε whenever the sphericity assumption was violated. Corrected degrees of freedom were presented rounded to the nearest integer. If a significant effect was found, estimate of effect size was calculated and reported as ηp2 or g, using the appropriate test as specified in Lakens (2013). Data were analyzed using JMP Pro 12 statistical software (SAS Institute, Cary, NC, U.S.A.), and p<0.05 was considered significant.
To determine the effect of OT on cooperative responses, we compared pellets earned, cooperative responses, trial outcomes, and nice responses after saline and OT. Due to missing values, it was not possible to evaluate effects of OT on transition vectors. Data were analyzed by RM-ANOVA with group as the between-subjects factor, and OT and trial outcome as within-subjects factors.
3. RESULTS
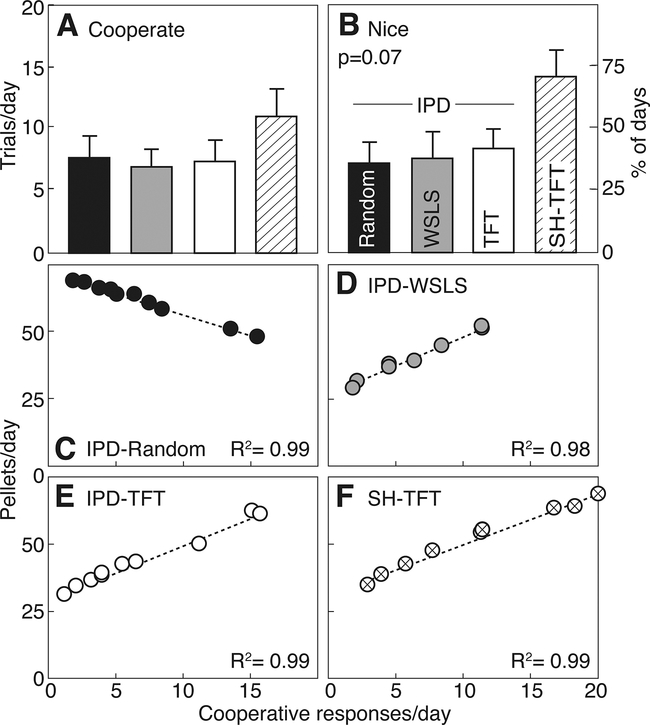
Figure 2 illustrates cooperative responses and pellets earned at baseline in pairs of rats playing IPD-TFT, IPD-WSLS, IPD-Random, or SH-TFT. The overall likelihood of cooperation did not differ between groups (Fig. 2A, H=3.34, N.S), and the probability of cooperation on the first trial of each session (Fig. 2B, nice) just missed significance (H=6.78, p=0.08). SH-TFT Subjects cooperated on 45.6±9.3% of trials, and were nice on 72.5±11.3% of first trials. By contrast, IPD-WSLS rats cooperated on only 26.6±5.6% of all trials, and 35.0±11.2% of first trials. Even though there was no overall difference in cooperation, Subjects earned significantly different numbers of pellets according to the game and to the strategy of their Stooge (H=16.22, p<0.05, ηp2=0.43). Subjects paired with an IPD-Random Stooge earned significantly more pellets (61.4±2.5 per 24 trials, p<0.05) than those paired with an IPD-TFT (43.5±3.4) or IPD-WSLS Stooge (40.4±2.9). Subjects playing SH-TFT were not significantly different from any group (51.9±4.2 pellets per 24 trials). For Subjects playing with a Stooge using a successful strategy (TFT or WSLS), there was a significant positive correlation between cooperative responses and pellets earned (Fig 2D–F, R2=0.98 or 0.99). By contrast, when playing IPD with a Random Stooge, Subjects earned more pellets when they made fewer cooperative responses (Fig. 2C, R2=0.99).
Figure 2:
A. Cooperative responses in 24 trials/day (mean±SEM) from rats playing iterated Prisoner’s Dilemma (IPD) or Stag Hunt (SH) with a Stooge who follows a random response strategy (IPD-Random, closed symbols), Win-stay/Lose-shift (IPD-WSLS, gray symbols), or Tit-for-tat (IPD-TFT, open symbols; SH-TFT, striped symbols). See Figure 1 for experimental details. B. Cooperative responses on the first trial each day (Nice). Cooperative responses vs pellets earned for individual rats playing IPD-Random (C), IPD-WSLS (D), IPD-TFT (E), or SH-TFT (F).
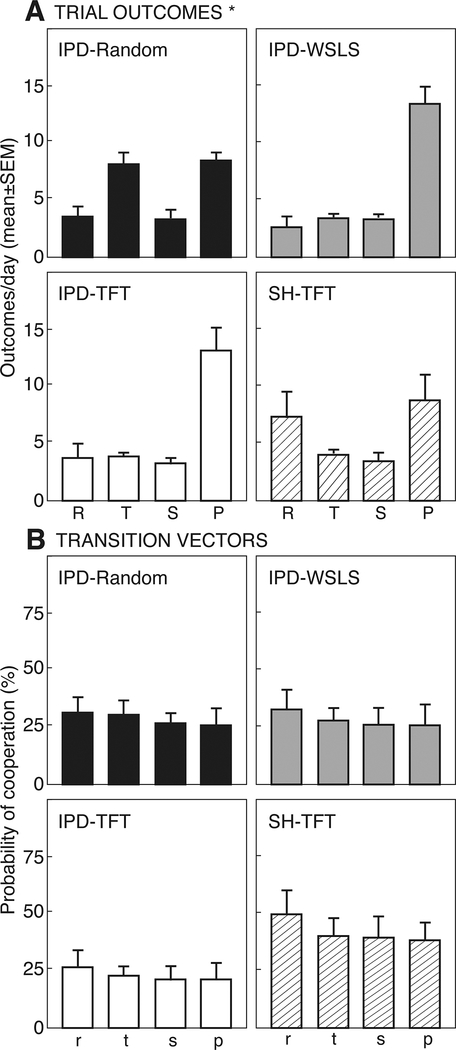
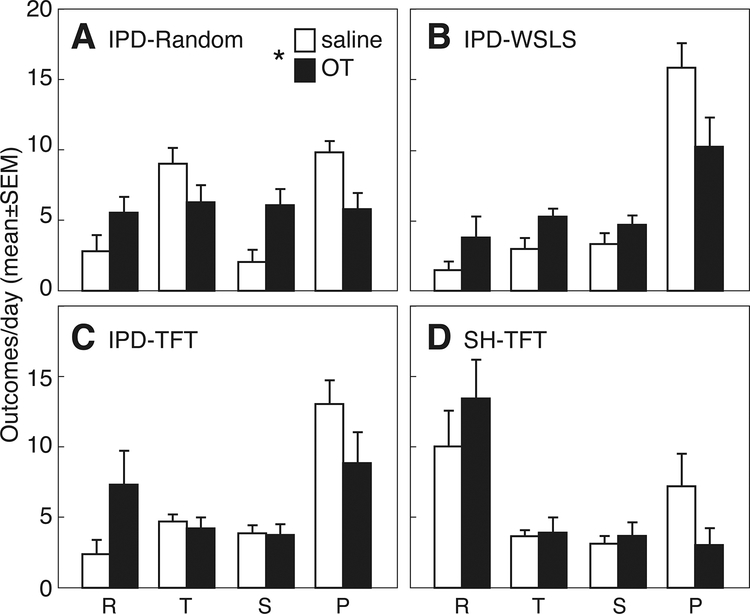
Figure 3 presents trial outcomes (Fig. 3A, R, T, S, P) and decision vectors (Fig. 3B, r, t, s, p) at baseline. Across all groups, Punishment was the most common outcome, and accounted for >50% of all trials in the IPD-TFT and IPD-WSLS groups. In the IPD-Random group, lower levels of mutual defection (P: 8.6±0.7 per 24 trials) were balanced by high levels of unilateral defection (T: 8.3±0.9). For SH-TFT Subjects, lower levels of mutual defection (P: 8.9±2.2 per 24 trials) were balanced by high levels of mutual cooperation (R: 7.4±2.2). Thus, there was a significant interaction of group by trial outcome (F4,42=3.06, p<0.05, ηp2=0.31). For decision vectors r, t, s, and p, there was no significant variation among vectors, no effect of group, and no group by vector interaction. Subjects did not follow either a TFT strategy (r+t > s+p) or a WSLS strategy (r+p > s+t). However, across all 4 groups, r+s was significantly greater than t+p (p<0.05 by Wilcoxon signed rank test, g=0.22), suggesting that rats use a ‘cooperate after cooperating’ strategy, as reported previously (Wood et al, 2016; Stephens and Stevens, 2004).
Figure 3:
A. Trial outcomes (reward, R; temptation, T; sucker, S; punishment, P) in 24 trials/day (mean±SEM) from rats playing iterated Prisoner’s Dilemma (IPD) or Stag hunt (SH) with a Stooge who follows a random response strategy (IPD-Random, closed bars), Win-stay/Lose-shift (IPD-WSLS, gray bars), or Tit-for-tat (IPD-TFT, open bars; SH-TFT, striped bars). See Figure 1 for experimental details. B. Transition vectors, reflecting the probability of cooperation when the previous trial resulted in outcomes of R, T, S or P, respectively. Asterisk indicates significant effect of trial outcome.
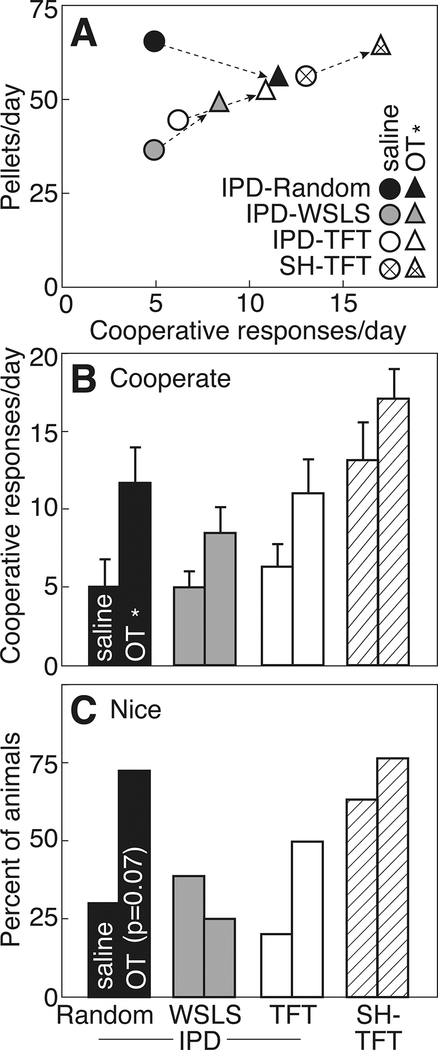
Pretreatment with 0.1 mg/kg OT caused a substantial increase in cooperation. Figure 4 compares cooperative (Fig. 4B) and nice responses (Fig. 4C), and pellets earned (Fig. 4A) after saline or OT. OT significantly increased cooperative responses (F1,33=21.08, p<0.05, ηp2=0.39), and the effect on nice responses just missed significance (F1,33=3.47, p=0.07). OT also caused a significant increase in the number of pellets earned (F1,33=5.75, p<0.05, ηp2=0.15), but also an interaction of group x OT (F3,33=7.19, p<0.05, ηp2=0.40). When the Stooge used a TFT or WSLS strategy, the increase in cooperation prompted an increase in the number of pellets received. Importantly, OT increased cooperation in Subjects paired with an IPD-Random Stooge, even though they received fewer pellets as a result (saline: 64.1±2.9 vs OT: 54.7±3.5, p<0.05).
Figure 4:
A. Cooperative responses vs pellets earned for rats playing iterated Prisoner’s Dilemma (IPD) or Stag Hunt (SH) with a Stooge who follows a random response strategy (IPD-Random, closed symbols), Win-stay/Lose-shift (IPD-WSLS, gray symbols), or Tit-for-tat (IPD-TFT, open symbols; SH-TFT, striped symbols). See Figure 1 for experimental details. Rats were pre-treated i.p. with saline (circles) or 0.1 mg/kg oxytocin (OT, triangles). B. Cooperative responses in 24 trials/day (mean±SEM) after pre-treatment with saline (left bars) or OT (right bars). C. Cooperative responses on the first trial each day (Nice). Asterisks indicate significant effect of OT.
Trial outcomes after saline and OT pretreatment are shown in Figure 5. Decision vectors r, t, s, and p could not be computed due to missing values. There was a significant effect of OT on trial outcome (F1,33=4.67, p<0.05, ηp2=0.12). This was reflected by significantly fewer trials resulting in mutual defection (P, saline: 11.4±1.0 vs OT: 6.9±0.9, p<0.05, g=0.71), with a corresponding increase in mutual cooperation (R, saline: 4.2±0.9 vs OT: 7.5±1.1, p<0.05).
Figure 5:
Trial outcomes (reward, R; temptation, T; sucker, S; punishment, P) in 24 trials/day (mean±SEM) from rats playing iterated Prisoner’s Dilemma (IPD) or Stag hunt (SH) with a Stooge who follows a random response strategy (IPD-Random, A), Win-stay/Lose-shift (IPD-WSLS, B), or Tit-for-tat (IPD-TFT, C; SH-TFT, D). See Figure 1 for experimental details. Rats were pre-treated i.p. with saline (open bars) or 0.1 mg/kg oxytocin (OT, closed bars). Asterisk indicates significant effect of OT.
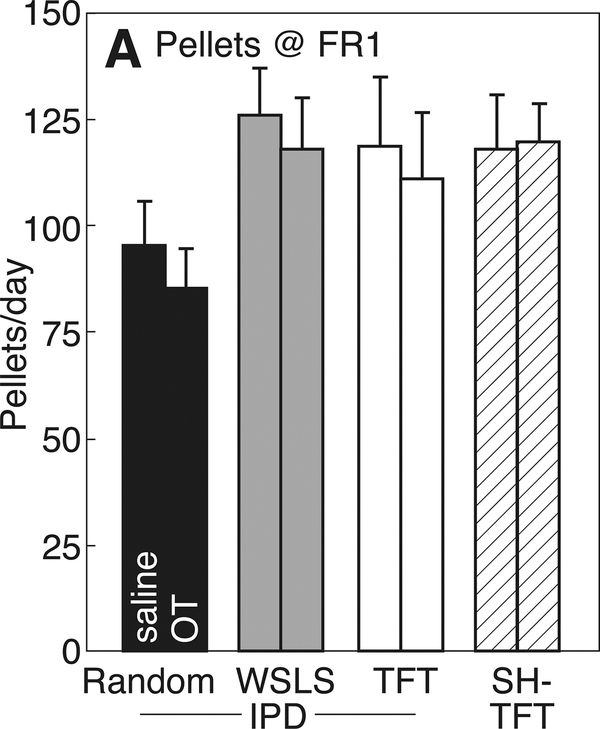
Lastly, Figure 6 presents pellets earned on an FR1 schedule of reinforcement following pretreatment with saline or OT. Subjects responded vigorously for pellets, averaging >100 pellets per 20 min. There was no effect of pretreatment OT on operant responses (p>0.05 by paired t-test).
Figure 6:
Operant responses/20 minutes on an FR1 schedule of reinforcement in rats pre-treated i.p. with saline (left bars) or 0.1 mg/kg oxytocin (OT, right bars). Rats had previously been tested for cooperative responses when playing iterated Prisoner’s Dilemma (IPD) or Stag hunt (SH) with a Stooge who follows a random response strategy (IPD-Random), Win-stay/Lose-shift (IPD-WSLS), or Tit-for-tat (IPD-TFT, SH-TFT). See Figure 1 for experimental details.
4. DISCUSSION
The present study examined cooperative behavior in male rats working for food reward in an operant model of a 2×2 game, where Subjects were paired with a cage-mate Stooge whose responses were computer-controlled. At baseline, Subjects’ overall likelihood of cooperation was unaffected by the game (IPD vs SH) or by the Stooges’ response strategy (TFT, WSLS, Random). However, there was a tendency for Subjects to be nicer when their Stooge partner was also nice. Cooperative responses earned Subjects more pellets, except when playing with a Stooge using a random strategy. Trial outcomes (R, T, S or P) also varied by group, although mutual defection (P) was the most common outcome. Systemic pretreatment with OT increased Subjects’ cooperative responses, resulting in fewer P and more R outcomes. In particular, IPD-Random Subjects were more cooperative, even at the expense of earning fewer pellets. These results demonstrate that OT increases cooperative behavior in rats playing 2×2 games.
The present study extends our operant model testing cooperative behavior in pairs of rats. Previously, we found similar levels of cooperative responses when cooperation was signified by responding on a lever or by withholding a response (Wood et al, 2016). Male rats made more cooperative responses during ad libitum feeding vs food restriction, and subordinate males were more cooperative than dominant males. However, there were 2 key limitations of that previous study. First, the payoff matrix (R=3, T=5, S/P=0) in Wood et al (2016) was a weak Prisoner’s Dilemma. In the present study, the payoff matrix was adjusted to correct this. Secondly, both rats in Wood et al (2016) played IPD, and the variable responses among individual rats might limit the expression of cooperative strategies. The present study used Stooges to determine if Subjects would utilize a consistent response strategy when playing with a partner applying a consistent strategy.
In our previous study where both rats of each pair played IPD (Wood et al, 2016), average cooperative responses (56%) were nearly twice that of Subjects in the present study (29% for IPD-TFT). It is unlikely that this was due to the strategy of the Stooge, since Subjects playing with an IPD-Random Stooge showed similar rates of cooperation (30%). Instead, the low level of cooperation in the present study likely reflects differences in the Punishment payoff (0 vs 1 pellet). The suggestion is that rats are more sensitive to the payoff matrix than to the response strategy of their partner.
If so, we might have expected a significant increase in cooperation for Subjects playing Stag Hunt vs those playing IPD. Rats are particularly motivated to obtain large payoffs (Wallin-Miller et al, 2018), and Stag Hunt delivers the greatest number of pellets (3) for Reward outcomes (vs 5 pellets for Temptation in IPD). Instead, although the overall likelihood of cooperation was marginally greater in rats playing SH-TFT (45%) vs IPD-TFT, the effect was not significant. In part, this reflects the variability of individual responses. Average rates of cooperation in 24 trials ranged from 2.2 to 15.8 for IPD-TFT and from 3.0 to 20.0 for Stag Hunt-TFT. Low levels of cooperation can happen when participants playing a 2×2 game with a TFT partner get caught in cycles of repeated defection (Axelrod, 2006). On the other hand, cooperation was not improved in Subjects playing IPD with a WSLS partner. Variable levels of cooperation in the present study may reflect individual differences in cognitive flexibility, since cooperation was denoted by withholding a previously-learned lever response.
It is interesting that few studies have tested animals in a Stag Hunt game, because Stag Hunt favors mutual cooperation. Furthermore, it has been argued that many situations that are described as Prisoners Dilemmas are, in fact, Stag Hunts (Skyrms, 2004). A recent study tested pairs of chimpanzees or human children working for food reward in a Stag Hunt game (Duguid et al, 2014). The chimpanzees were less successful than the children in coordinating a cooperative response when observing their partner was difficult. Duguid et al (2014) argue that human skills in communication and coordination have facilitated our cooperative abilities. In the present study, Subjects playing Stag Hunt also failed to cooperate consistently, perhaps in part because they could not observe the cooperative response of their Stooge. When social communication is permitted, rats are able to cooperate in a task requiring simultaneous nose-poking (Lopuch and Popik, 2011).
In the present study, we were also surprised that Subjects did not match their response strategy to the successful response strategy of their Stooge partner (TFT or WSLS). Instead, our rats favored the same strategy (“cooperate after cooperation”: average of r and s less average of t and p) as rats in Wood et al (2016) and blue jays Cyanocitta cristata in Stevens and Stephens (2004). In this regard, TFT is a simple strategy, but it is also cognitively demanding, and rats may lack the capacity to use a strict bookkeeping strategy. Although rats do adjust their cooperative responses when sated or hungry (Wood et al, 2016), it is also possible that cooperative responses in individuals reflect personality traits in rats, as they do in humans (Mao et al, 2017).
The present study investigated cooperative strategies in male rats because cooperation in males is more sensitive to partner responses. Male Norway rats trained to pull a stick to bring food to a partner are more likely to work on behalf of a familiar cooperative partner (direct reciprocity), compared with a non-cooperative partner or an unfamiliar male (generalized reciprocity; Schweinfurth et al, 2019). By contrast, females show generalized reciprocity, and will work on behalf of an unfamiliar female (Rutte and Taborsky, 2008). Furthermore, females are more likely to work for a partner that was underfed, suggesting that responses in females take into account the needs of the partner (Schneeberger et al, 2012). Likewise, in a test of direct reciprocity, females respond vigorously on behalf of their partner (Wood et al, 2016). Unlike males, females’ responses do not depend on the assistance received from their partner. Thus, although females are more likely to cooperate, their behavior is less dependent on the response strategy of their partner.
Even though rats did not adjust their level of cooperation in accordance with the strategy of their partner, they did demonstrate a substantial increase in cooperation in response to OT. They also were more likely to be nice, to cooperate on the first trial. OT has received a great deal of attention recently for its ability to promote pair-bonding in voles (Tabbaa et al, 2016), and trust and cooperation in humans (Kendrick et al, 2018). However, the role of OT in human cooperation is complex. OT reduces competitive responses towards in-group members in a 2×2 game (Ten Velden et al, 2014), and increases protection of in-group members (De Dreu et al, 2012). At the same time, OT reduces cooperation with out-group members (De Dreu et al, 2011). Similar to our findings in rats, intranasal OT in humans playing Prisoners Dilemma increases cooperation if participants have had prior contact with their partner (Declerck et al, 2014). Without prior contact, OT decreases cooperation. From a game theory perspective, this makes sense. It is not advantageous for OT to induce unrestrained cooperation. A strategy of ‘always cooperate’ is susceptible to exploitation by those with a stronger self-interest (Axelrod, 2006). Nonetheless, it does make sense for OT to promote cooperation with kin or pair-bonded partners. Kin selection favors cooperation when the benefit to the recipient increases the evolutionary fitness of the donor (Rapoport and Chammah, 1965). However, kin selection depends on kin recognition and/or limited dispersal (Grafen, 2007). Where kinship is uncertain, familiarity becomes a proxy. Such ‘nurture kinship’ may explain why OT promotes in-group cooperation, while also protecting against potential defection from out-group partners. Whether OT would reduce Subjects’ cooperative responses when playing a 2×2 game with an unfamiliar Stooge in our model has not been tested.
It is unlikely that the effects of OT at 0.1 mg/kg to stimulate cooperation in the present study are due to reduced motivation for food. Indeed, except for those paired with an IPD-Random Stooge, Subjects earned significantly more sucrose pellets with OT pretreatment, due to their increased cooperation. Subjects playing with IPD-Random partner also made more cooperative responses but earned fewer pellets. However, there is a literature detailing anorexigenic effects of OT at higher doses when administered to hungry rats. At 0.375 mg/kg, OT reduces chow intake in rats food-deprived for 21 hr (Arletti et al 1989). On the other hand, at doses up to 2 mg/kg, OT has no effect on operant responding for sucrose pellets in male rats without food restriction (Zhou et al, 2015; Cox et al, 2013). Similarly, when Subjects in the present study were retested for operant responding for sucrose pellets on an FR1 schedule, there was no effect of pretreatment with OT. Instead, our results support the concept that OT promotes prosocial interactions with unrelated, non-sexual conspecifics (Lim and Young, 2006), including food sharing (vampire bats: Carter & Wilkinson, 2015; pinyon jays: Duque et al 2017).
In conclusion, the present study extends our understanding of cooperation in pairs of male rats. Rats are a social species, and males have a strong dominance hierarchy (Blanchard et al. 1988). Even so, they will cooperate according to rules for direct reciprocity (Schweinfurth et al, 2019). In the present study using a 2×2 game, males do not adjust their response strategy to mirror that of their partner. Instead, they tend to follow a stable response strategy (cooperate after cooperating). However, OT increases cooperative responses, even at the expense of reducing the rewards obtained. These results are in line with studies showing that OT enhances in-group cooperation in humans (Ten Velden et al, 2014).
Highlights.
Rats adjust their cooperative responses when playing a 2×2 game (iterated Prisoner’s Dilemma or Stag Hunt) according to the payoffs and the strategy of their opponent.
Rats use a strategy of ‘cooperate after cooperation’.
Oxytocin increases cooperation, even at the expense of earning fewer rewards.
5. ACKNOWLEDGEMENTS
We thank Ms. Grace Li for assistance with programming, and Ms. Rebecka Serpa for behavioral testing and data analysis. This work supported by a grant from the NIH (R01-DA029613 to RIW).
Footnotes
6. DECLARATION OF INTERESTS
None
6. REFERENCES
- Arletti R, Benelli A, Bertolini A, 1989. Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93. [DOI] [PubMed] [Google Scholar]
- Axelrod R, 2006. The evolution of cooperation: Revised edition. Basic Books, Cambridge, MA. [Google Scholar]
- Blanchard R,J, Flannelly K,J, Blanchard D,C, 1988. Life-span studies of dominance and aggression in established colonies of laboratory rats. Physiol. Behav 43, 1–7. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD, 1992. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol. Behav 51(4), 667–672. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW, 2008. Oxytocin, vasopressin and sociality. Prog. Brain Res 170, 331–336. [DOI] [PubMed] [Google Scholar]
- Carter GG, Wilkinson GS, 2015. Intranasal oxytocin increases social grooming and food sharing in the common vampire bat Desmodus rotundus. Horm. Behav 75, 150–153. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM, 2013. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology 38(10), 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T, 2014. The effect of oxytocin on cooperation in a prisoner’s dilemma depends on the social context and a person’s social value orientation. Soc. Cogn. Affect. Neurosci 9(6), 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ, 2011. Oxytocin promotes human ethnocentrism. Proc. Natl. Acad. Sci 108, 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Shalvi S, Greer LL, Van Kleef GA, Handgraaf MJ, 2012. Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PLoS ONE 7(11), e46751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid S, Wyman E, Bullinger AF, Herfurth-Majstorovic K, Tomasello M, 2014. Coordination strategies of chimpanzees and human children in a Stag Hunt game. Proc. R. Soc. Lond, B Biol. Sci 281(1796), 20141973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Kulkarni PP, Ferris CF, Veenema AH, 2017. Sex differences in neural activation following different routes of oxytocin administration in awake adult rats. Psychoneuroendocrinology 81, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque JF, Leichner W, Ahmann H, Stevens JR, 2018. Mesotocin influences pinyon jay prosociality. Biol. Lett 14(4), 20180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS, 2012. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm. Behav 61(3), 359–379. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Hamblin S, Giraldeau L-A, 2013. Exposing the behavioral gambit: the evolution of learning and decision rules. Behav. Ecol 24 (1), 2–11. [Google Scholar]
- Fehr E, Camerer CF, 2007. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn. Sci 11(10), 419–427. [DOI] [PubMed] [Google Scholar]
- Grafen A, 2007. Detecting kin selection at work using inclusive fitness. Proc. R. Soc. Lond, B Biol. Sci 274(1610), 713–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof LA, Fudenberg D, Nowak MA, 2008. Tit-for-tat or Win-stay, Lose-shift? J. Theor. Biol 247(3), 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D, 2013. Calculating reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVSs. Front. Psychol 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wood RI, 2017. Male rats play a repeated donation game. Physiol. Behav 174, 95–103. doi: 10.1016/j.physbeh.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ, 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav 50(4), 506–517. [DOI] [PubMed] [Google Scholar]
- Lopuch S, Popik P, 2011. Cooperative behavior of laboratory rats (Rattus norvegicus) in an instrumental task. J. Comp. Psychol 125(2), 250–253. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guastella AJ, Becker B, 2018. Overview of human oxytocin research. Curr. Top. Behav. Neurosci 35, 321–348. doi: 10.1007/7854_2017_19. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM, 2010. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiatry 18(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Mao A, Dworkin L, Suri S, Watts DJ, 2017. Resilient cooperators stabilize long-run cooperation in the finitely repeated Prisoner’s Dilemma. Nature Commun. 8(1), 13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K, 1993. A strategy of win-stay, lose-shift that outperforms tit-for-tat in the Prisoner’s Dilemma game. Nature. 364, 56–58. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL, 2012. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol. Behav 105(3), 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Vetulani J, van Ree JM, 1992. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 106(1), 71–74. [DOI] [PubMed] [Google Scholar]
- Raihani NJ, Bshary R, 2011. Resolving the iterated prisoner’s dilemma: theory and reality. J. Evol. Biol 24(8), 1628–1639. [DOI] [PubMed] [Google Scholar]
- Rapoport A, Chammah AM, 1965. Prisoner’s Dilemma, a study in conflict and cooperation. University of Michigan Press, Ann Arbor, MI. [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ, 2009. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 29(5), 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, 2007. Generalized reciprocity in rats. PLoS Biology, 5(7), e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Dietz M, Taborsky M, 2012. Reciprocal cooperation between unrelated rats depends on cost to donor and benefit to recipient. Evolutionary Biology, 12, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfurth MK, Aeschbacher J, Santi M, Taborsky M, 2019. Male Norway rats cooperate according to direct but not generalized reciprocity rules. Animal Behav. 152, 93–101. [Google Scholar]
- Skyrms B, 2004. The stag hunt and the evolution of social structure. Cambridge University Press, Cambridge. [Google Scholar]
- Soares MC, Bshary R, Fusani L, Goymann W, Hau M, Hirschenhauser K, Oliveira RF, 2010. Hormonal mechanisms of cooperative behavior. Phil. Trans. R. Soc. B 365, 2737–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Stephens DW, 2004. The economic basis of cooperation: tradeoffs between selfishness and generosity. Behav. Ecol 15(2), 255–261. [Google Scholar]
- Tabbaa M, Paedae B, Liu Y, Wang Z, 2016. Neuropeptide regulation of social attachment: The prairie vole model. Compr. Physiol 7(1), 81–104. doi: 10.1002/cphy.c150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Velden FS, Baas M, Shalvi S, Kret ME, De Dreu CK, 2014. Oxytocin differentially modulates compromise and competitive approach but not withdrawal to antagonists from own vs. rivaling other groups. Brain Res. 1580:172–179. [DOI] [PubMed] [Google Scholar]
- Wallin-Miller KG, Li G, Kelishani D, Wood RI, 2018. Anabolic-androgenic steroids alter decision making in a balanced rodent model of the Iowa gambling task. Behav. Neurosci 132(3), 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Kim JY, Li GR, 2016. Cooperation in rats playing the iterated Prisoner’s Dilemma game. Anim. Behav 114, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR, 2001. Cellular mechanisms of social attachment. Horm. Behav 40(2), 133–138. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM, 2015. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav. Brain Res 283, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]