Abstract
Rationale:
Atypical dopamine (DA) transport blockers such as modafinil and its analogs may be useful for treating motivational symptoms of depression and other disorders. Previous research has shown that the DA depleting agent tetrabenazine can reliably induce motivational deficits in rats, as evidenced by a shift towards a low-effort bias in effort-based choice tasks. This is consistent with human studies showing that people with major depression show a bias towards low-effort activities.
Objectives:
Recent studies demonstrated that the atypical DA transport (DAT) inhibitor (S)-CE-123 reversed tetrabenazine-induced motivational deficits, increased progressive ratio (PROG) lever pressing, and increased extracellular DA in the nucleus accumbens. In the present studies, a recently synthesized modafinil analog, (S, S)-CE-158, was assessed in a series of neurochemical and behavioral studies in rats.
Results:
(S, S)-CE-158 demonstrated the ability to reverse the effort-related effects of tetrabenazine, and increase selection of high-effort PROG lever pressing in rats tested on PROG/chow feeding choice task. (S, S)-CE-158 showed a high selectivity for inhibiting DAT compared to other monoamine transporters, and systemic administration of (S, S)-CE-158 increased extracellular DA in the nucleus accumbens during the behaviorally active time course, which is consistent with the effects of (S)-CE-123 and other DAT inhibitors that enhance high-effort responding.
Conclusions:
These studies provide an initial neurochemical characterization of a novel atypical DAT inhibitor, and demonstrate that this compound is active in models of effort-related choice. This research could contribute to the development of novel compounds for the treatment of motivational dysfunctions in humans.
Keywords: dopamine, transport, synthesis, motivation, depression, fatigue, anergia, modafinil
INTRODUCTION
Motivational symptoms such as anergia, fatigue, and effort-related dysfunctions are common and debilitating features of major depressive disorder, schizophrenia, Parkinson’s disease and other psychiatric and neurological disorders (Tylee et al. 1999; Demyttenaere et al. 2005; Treadway et al. 2012; Barch et al. 2014; Chong et al. 2015). Motivational symptoms are not well treated by many of the most commonly prescribed antidepressant drugs (Tylee et al. 1999; Demyttenaere et al. 2005; Cooper et al. 2014; Fava et al. 2014; Ghanean et al. 2018). Patients treated with drugs that block the serotonin transporter (SERT), such as fluoxetine, sertraline, paroxetine or escitalopram, often suffer from residual symptoms of apathy, fatigue, and a lack of energy (Katz et al. 2004; Padala et al. 2012; Rothschild et al. 2013; Cooper et al. 2014; Ferguson et al. 2014), which can be detrimental to daily functioning. Clinical reports have revealed a critical link between symptoms related to fatigue and energy expenditure and the overall severity of depressive symptoms (Gullion and Rush 1998; Fava et al. 2014; Chung et al. 2015; Ghanean et al. 2018). Moreover, self-reported fatigue is correlated with global functioning deficits and reduced likelihood of remission (Ferguson et al. 2014). The array and prevalence of symptoms left unabated by antidepressant medications emphasizes the importance of utilizing animal models of fatigue and anergia to aid in the development of new and more effective treatments.
In order to conduct preclinical studies of motivational dysfunctions using animal models, tasks have been developed to evaluate effort-based decision making in rodents (Salamone et al. 2006, 2007, 2016a,b, 2018; Mai et al. 2012; Nunes et al. 2013; Sommer et al. 2014; Winstanley and Floresco 2016; Bailey et al. 2016, 2020; Hart et al. 2017; Stutz et al. 2019). With these tasks, animals are offered the choice between lever pressing for a highly valued reinforcer vs. approaching and consuming a freely available but less preferred reinforcer. Tasks such as the concurrent fixed ratio 5 (FR5)/chow feeding choice task and the concurrent progressive ratio (PROG)/feeding choice task are useful for measuring effort-related choice behavior (Salamone et al. 2002, 2007, 2016a; Salamone and Correa, 2012; Randall et al. 2012). Several studies have shown that selection of the high-effort alternative is reduced when animals are exposed to manipulations associated with depression and negative motivational symptoms, such as stress (Shafiei et al. 2012; Bryce and Floresco, 2016; Dieterich et al. 2020), proinflammatory cytokine administration (Nunes et al. 2014; Yohn et al. 2015; Rotolo et al. 2020, in prep), and dopamine (DA) depletion or antagonism (Nunes et al. 2013; Randall et al. 2012, 2014; Yohn et al. 2015a; Hosking et al. 2015; Pardo et al. 2015; Yohn et al. 2016a,b,c; Rotolo et al. 2019; Yang et al. 2020; Bailey et al. 2020). For example, rats treated with the vesicular monoamine transport (VMAT-2) inhibitor tetrabenazine (TBZ) demonstrate a low-effort bias across several tasks, shifting choice away from the high-effort alternative (Nunes et al. 2013; Randall et al. 2014; Yohn et al. 2015a; Pardo et al. 2015). When administered to humans, TBZ induces depressive symptoms including fatigue (Chitnis and Karunapuzha, 2009; Frank, 2010; Chen et al. 2012). Importantly, these effort-related dysfunctions in animals are not reversed by co-administration of inhibitors of SERT or norepinephrine transport (NET) (Yohn et al. 2016a,b). Unlike drugs that inhibit SERT or NET, several inhibitors of the DA transporter (DAT), including bupropion, vanoxerine (GBR12909), lisdexamfetamine, PRX-14040, methylphenidate, and modafinil, can reverse the low-effort bias induced by TBZ (Nunes et al. 2013, Yohn et al. 2016a,b,c; Salamone et al. 2016b) and by pro-inflammatory cytokines (Yohn et al. 2016b,d). Moreover, several DAT inhibitors have demonstrated the ability to increase motivation to exert high levels of effort, increasing selection of lever pressing in rats tested on the PROG/chow feeding choice task when administered alone (Sommer et al. 2014; Randall et al., 2015; Yohn et al., 2016b,c,d).
The ability of drugs that block DAT to reverse effort-related impairments and increase selection of high-effort activities in rodents is consistent with clinical studies showing that DAT inhibitors, including bupropion, amphetamine, and methylphenidate, can improve motivational function in people (Stotz et al. 1999; Papakostas et al. 2006; Pae et al. 2007; Blockmans and Persoons 2016). The wakefulness agent modafinil, which inhibits DAT (Schmitt and Reith 2011), and elevates extracellular DA as measured by microdialysis (Mereu et al. 2017) and human imaging studies (Volkow et al. 2009), has been shown to improve motivational function in people with depression (Fava et al. 2007; Lam et al. 2007) and multiple sclerosis (Shangyan et al. 2018). Moreover, modafinil reduces perception of physical task demands during exercise (Rattray et al. 2019), and increases motivation and enhances task engagement in humans at doses that do not produce a powerful euphoria or ‘high’ (Müller et al. 2013). Modafinil has atypical DAT binding characteristics and behavioral effects (Schmitt and Reith 2011; Mereu et al. 2013, 2017; Cao et al. 2016; Nikiforuk et al. 2017), and is part of an emerging class of drugs (e.g. benztropine and GBR12909 analogs) that have binding properties and behavioral effects that are different from cocaine. Recently, various stereoisomers of the novel thiazole-based modafinil analog CE-123 were demonstrated to have pro-cognitive effects in both rats and mice (Nikiforuk et al. 2017; Kristofova et al. 2018; Camats-Perna et al. 2019), as well as the ability to reverse the effort-related effects of TBZ in rats at doses that increase extracellular DA in nucleus accumbens core (Rotolo et al. 2019). While CE-123 has shown preclinical effectiveness across a variety of rodent behavioral tasks without adverse toxic effects (Kalaba et al. 2017), and has a higher specificity for the DAT than other commercially available drugs (Kalaba et al. 2020), it is important to provide a detailed characterization of a broad range of modafinil analogs to identify the most promising candidate for treating motivational dysfunctions and fatigue. The present study investigated a novel modafinil analog, (S, S)-CE-158, for its ability to bind to DAT, the specificity of its inhibition of DAT, and its ability to reverse the effort-related effects of TBZ, enhance high-effort PROG responding, and increase accumbens DA transmission.
METHODS
Animals
Adult male, drug-naïve, Sprague Dawley rats (Envigo, Indianapolis, IN, USA) were housed in a colony maintained at 23 °C with 12-h light/dark cycles (lights on 07:00). Rats (n=23 for the behavioral pharmacology experiments; n=9 for the microdialysis experiment) weighed 275–299 g at the beginning of the study, and were initially food restricted to 85% of their free-feeding body weight for operant training. Rats were fed supplemental chow to maintain weight throughout the study, with water available ad libitum. Rats were allowed modest weight gain throughout the experiment. Animal protocols were approved by the University of Connecticut animal care and use committee, and followed NIH guidelines.
In vitro binding experiment
To determine binding specificity, in vitro binding assays were conducted using transfected HEK293 cells with stable expression of human DAT with [N-Methyl-3H]-WIN35,428 = [3H]CFT = 2ß-carbomethoxy-3ß-(4-fluorophenyl) tropane. Cells were seeded in poly-D-lysine-coated 24-well plates (3 × 104 HEK293 cells/well) and, 2 days later, binding on whole cells was performed as described previously (Pifl et al., 2009). Cells were incubated on ice for 2 h in 0.25ml buffer (mmol/l: 4 Tris–HCl; 6.25 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES); 120 NaCl; 5 KCl; 1.2 CaCl2; 1.2 MgSO4; 5 D-glucose; 0.5 ascorbic acid; pH 7.1) containing 6 nM [3H]CFT and various concentrations of (S, S)-CE-158. Non-specific binding was measured in the presence of 10 μM mazindol.
In vitro monoamine transporter reuptake inhibition
Monoamine transporter reuptake inhibition was outsourced to Eurofins DiscoverX Corporation (Fremont, CA) and performed according to the standard company protocol for the Neurotransmitter Transporter Uptake Assay (Molecular Devices). In short, (S, S)-CE-158 was tested for monoamine reuptake inhibition using a fluorescence-based assay. Fluorescent substrates mimicking amine neurotransmitters were taken up into HEK293 cells with stable expression of DAT, NET, and SERT, and intensity of intracellular fluorescence was quantified.
Pharmacological agents and selection of doses
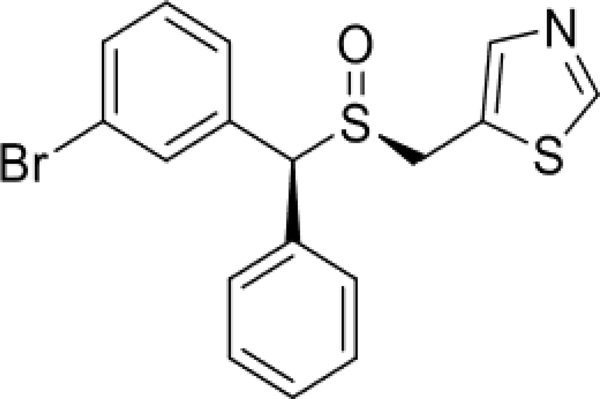
(S, S)-CE-158 (5-(((S)-((S)-(3-bromophenyl)(phenyl)methyl)sulfinyl)methyl)thiazole) (Figure 1) was obtained from the Lubec Laboratory (University of Vienna, Austria) and dissolved in dimethyl sulfoxide (DMSO), Tween 80, and 0.9% saline. The DMSO/Tween 80/saline solution was administered as the vehicle control. TBZ (9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7, 11b hexahydrobenzo[a]quinolizin-2-one) was obtained from Tocris Bioscience (Ellisville, MO) and was dissolved in DMSO, 0.9% saline, and titrated with HCl. The DMSO/saline solution was administered as the vehicle control. The dose of 1.0 mg/kg TBZ was based on extensive piloting in our laboratory. The doses of (S, S)-CE-158 were selected based on extensive pilot studies and information about its relative affinity for DAT.
Fig. 1.
Chemical structure of (S, S)-CE-158 (5-(((S)-((S)-(3-bromophenyl)(phenyl)methyl)sulfinyl)methyl)thiazole)
Behavioral procedures
Concurrent FR5/chow feeding choice task
Behavioral sessions were conducted in operant chambers (28 × 23 × 23 cm; Med Associates, Fairfax, VT). Sessions lasted 30 minutes a day for 5 days/week. First, rats were trained to lever press on a continuous reinforcement schedule to receive 45 mg highcarbohydrate pellets (Bio-Serv; Frenchtown, NJ, USA) for one week, then were shifted to the FR5 schedule. After 5 weeks of FR5 training, chow was introduced. During each FR5/chow feeding choice task session, 15–20 g of lab chow (Laboratory Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO) was concurrently available on the floor of the chamber. Rats were trained on this FR5/chow feeding choice procedure for 5 weeks, after which drug testing began. On baseline and drug treatment days, rats consumed all of the operant pellets that were delivered during each session.
Concurrent PROG/chow feeding choice task
A second behavioral experiment was conducted to determine if (S, S)-CE-158 had an effect on rats’ behavior on the PROG/chow feeding choice task when administered alone. Sessions were conducted in operant chambers (28 × 23 × 23 cm; Med Associates, Fairfax, VT) with 30-minute sessions 5 days/week. Rats were initially trained to lever press on a continuous reinforcement FR1 schedule (high-carbohydrate 45 mg pellets, Bio-Serv) and then shifted to the PROG schedule (Randall et al. 2012, 2014, 2015). For PROG sessions, the ratio started at FR1 and was increased by 1 additional response every time 15 reinforcements were obtained (FR1×15, FR2×15, etc.). A “time-out” feature deactivated the response lever for the rest of the session whenever 2 minutes elapsed without a completed ratio. After 9 weeks of training on the PROG schedule, chow was introduced and was concurrently available on the floor of the chamber during the PROG/chow feeding choice task sessions as previously described. Rats were trained on the PROG/chow feeding choice procedure for 5 weeks, after which drug testing began. On baseline and drug treatment days, rats consumed all of the operant pellets that were delivered during each session.
Surgical implantation of dialysis guide cannulae
Animals were anesthesized with an intraperitoneal injection of 100.0 mg/kg ketamine hydrochloride and 10.0 mg/kg xylazine prior to placement in a stereotaxic device (incisor bar 5.0 mm above interaural line). Microdialysis guide cannulae (Bioanalytical Systems) were implanted 2.0 mm dorsal to the accumbens core (AP +2.8 mm, ML +1.8 mm, DV −6.8 mm from bregma). Cannulae were surgically implanted in nine drug-naïve adult male Sprague Dawley rats, and placements were counterbalanced, with four rats implanted on the left and five rats implanted on the right. After placement, guide cannulae were secured to the skull by stainless steel screws and cranioplastic cement. A stainless-steel stylet was inserted through each cannula while not in use. Rats were allowed 7 days to recover from surgery before undergoing dialysis procedures.
DA microdialysis and high performance liquid chromatography
On sample collection days, dialysis probes (Bioanalytical Systems; 2.0 mm active surface) were inserted through the microdialysis guide cannulae. Artificial CSF (aCSF; 147.2 mm NaCl, 2.4 mm CaCl2, 4.0 mm KCl) was continuously perfused through the probe at a rate of 2.0 μL/min. Neurochemical samples were collected every 30 min in tubes containing 2.0 μl of ascorbic acid and sodium metabisulfite to prevent oxidation of DA. Up to 7 baseline samples were collected before an intraperitoneal injection of 8.0 mg/kg (S, S)-CE-158 to establish a stable DA level. The last three of those baseline samples were used as the statistical baseline. Samples were either immediately frozen or analyzed fresh using reverse-phase high-performance liquid chromatography (HPLC) with electrochemical detection (ESA Coulochem II system). The electrochemical parameters were as follows: channel 1 = −100 mV, channel 2 = +200 mV, and guard cell = +350 mV. Each liter of mobile phase contained 27.5 g sodium phosphate monobasic, 7.0 % methanol, 750 μl of 0.1 m EDTA, and 2200 μl of 0.4 m sodium octyl sulfate dissolved in deionized ultrapure H2O with a final pH of 4.5. The flow rate was 1.0 ml/min. DA standards were run each day before the dialysate samples. Probe placements were verified with histological analyses and only probes with placement in the nucleus accumbens core were used for analyses.
Nissl staining for identifying probe placements
At the completion of behavioral testing in the intracranial injection and microdialysis experiments, each animal was anesthetized with CO2 and then perfused intracardially with physiological saline followed by a 3.7 % formaldehyde solution. The brains were removed and stored in formaldehyde and then sliced with a vibratome in 60 μm sections, which were mounted on glass microscope slides. After mounting, slides were stained with cresyl violet for microscopic observation by a blind observer. Any animal with improper cannulae placement or significant damage around the injection site was excluded from the statistical analyses of behavioral data.
Experimental procedures
General design features for behavioral pharmacology experiments
The effort-related choice experiments used a within-groups design, with each rat receiving each drug treatment in a randomly varied order, once per week over the course of four weeks (no-drug baseline days were conducted four days per week). Rats were generally tested five days per week, with four baseline days and one drug treatment day. In most cases, rats were not tested the day after drug treatment, so that body weights could be adjusted with supplemental food. In the small number of cases in which rats were tested the day after treatment, behavior largely recovered to pre-treatment baseline levels.
Effects of systemic administration of TBZ on the concurrent FR5/chow feeding choice procedure and reversal with (S, S)-CE-158
Trained rats (n=7) were administered either TBZ (1.0 mg/kg) or vehicle, and (S, S)-CE158 (2.0, 4.0, 8.0 mg/kg) or vehicle, via intraperitoneal (IP) injections on drug testing days. Rats received TBZ or vehicle 120 minutes before testing and (S, S)-CE-158 or vehicle 30 minutes before testing. The experiment used a within-groups design, with each rat receiving each drug treatment in a randomly varied order. The following five treatment combinations were given: TBZ vehicle + (S, S)-CE-158 vehicle; 1.0 mg/kg TBZ + (S, S)-CE-158 vehicle; 1.0 mg/kg TBZ + 2.0 mg/kg (S, S)-CE-158; 1.0 mg/kg TBZ + 4.0 mg/kg (S, S)-CE-158; 1.0 mg/kg TBZ + 8.0 mg/kg (S, S)-CE-158.
Effects of systemic administration of (S, S)-CE-158 on the concurrent PROG/chow feeding choice procedure
Thirty minutes prior to the testing session, trained rats (n=16) were administered either vehicle or 2.0, 4.0, or 8.0 mg/kg (S, S)-CE-158. This experiment used a within-groups design, with each rat receiving each drug treatment in a randomly varied order, once per week over the course of four weeks.
Effects of (S, S)-CE-158 on extracellular DA in the nucleus accumbens
Rats were implanted with dialysis probes in nucleus accumbens core as described earlier. On the test day, after baseline dialysis samples were collected, rats received intraperitoneal injections of either vehicle (n=4) or 8.0 mg/kg (S, S)-CE-158 (n=5). Seven post-injection samples were collected.
Statistical Analysis
Repeated measures ANOVA was used to determine the effect of drug treatment on lever pressing and chow intake in the behavioral pharmacology experiments. Since there were significant overall F values for the two behavioral measures being used, nonorthogonal planned comparisons were performed, using the overall error term to assess differences between each treatment and the control condition. The number of comparisons was restricted to the number of treatments minus one (Keppel, 1991). Statistical outliers were predefined as any point that is more than two standard deviations from the mean. No data from this study were excluded as outliers.
Changes in extracellular DA levels in the microdialysis experiment were calculated as the percent change from baseline, with the mean of the three samples immediately preceding the drug injections serving as the 100% baseline level. A 2 × 7 factorial ANOVA with the treatment (drug vs. vehicle) factor being between groups, and the sample factor (samples collected after drug injection) being repeated measures, was used to test for post-injection differences in extracellular levels of DA. The raw DA levels of the baseline samples were analyzed using t test to verify that the baseline DA levels were not different between conditions. Nonorthogonal planned comparisons were performed using the error term from the between-subjects analysis to assess differences between the two treatments at each particular sample.
RESULTS
Binding experiments on DAT expressing cells and inhibition of monoamine transporters (DAT, NET, SERT) by (S, S)-CE-158
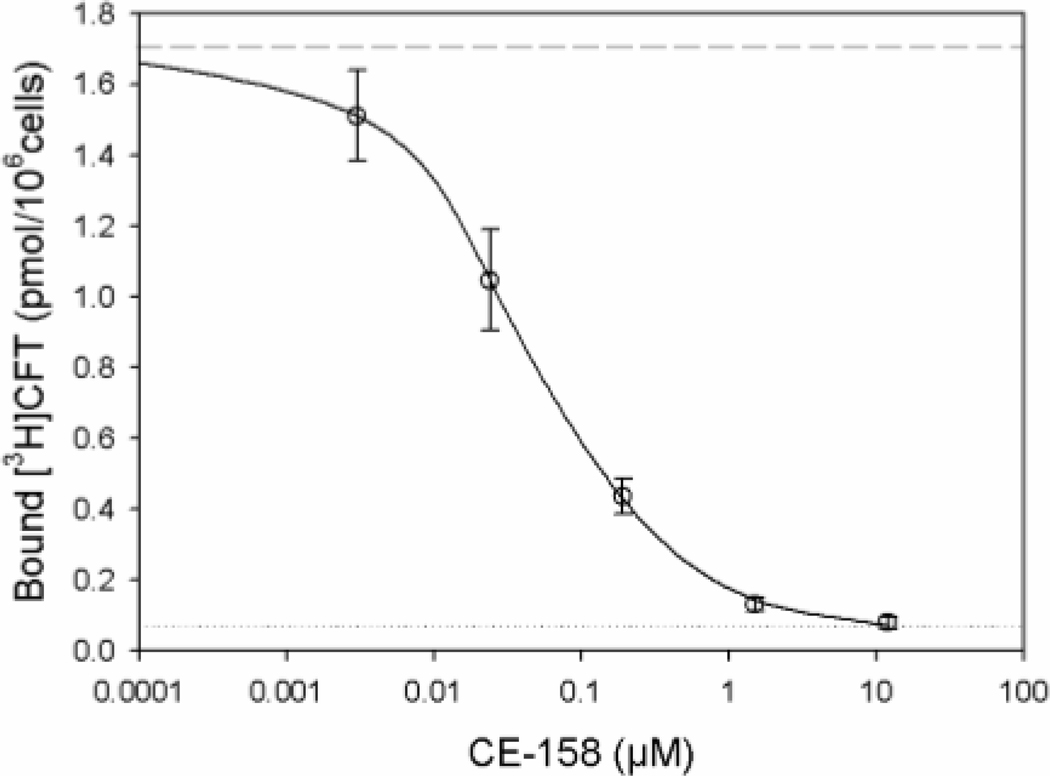
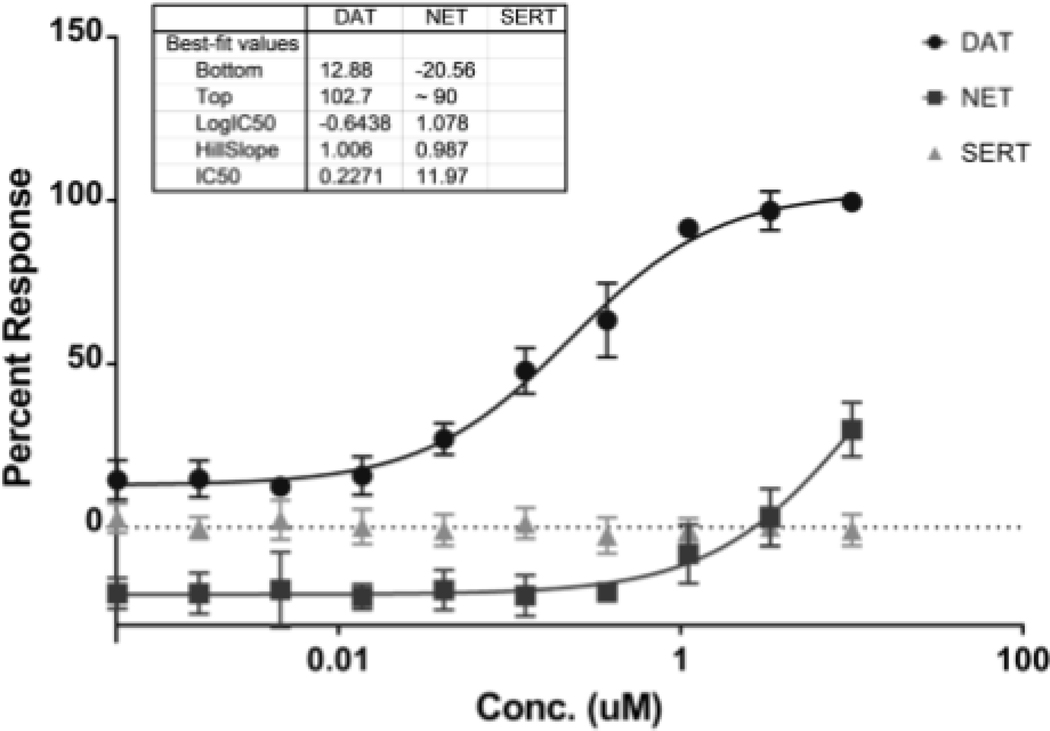
(S, S)-CE-158 concentration-dependently displaced [3H]CFT from human DAT with an IC50 of 0.052 ± 0.017 μM (Figure 2). As shown in Figure 3, (S, S)-CE-158 selectively and potently blocked DAT (IC50 = 0.2271 μM), while having a weak effect on NET (IC50 = 11.97 μM) and no effect on SERT.
Fig. 2.
Effect of (S, S)-CE-158 on binding to DAT expressing cells. HEK293 cells with stable expression of the human DAT and seeded in 24-well plates were incubated with [3H]CFT for 2 hrs in the absence (dashed line) or presence of (S, S)-CE-158 at the concentration indicated (open circles, solid line) or 10 μM mazindol (dotted line) at 4°C, and binding of tritium was determined as described under “Experimental procedures”. Symbols represent means of binding ± standard error of four independent experiments, each performed in duplicates
Fig. 3.
In vitro monoamine transporter reuptake inhibition of (S, S)-CE-158 using HEK293 cells with stable expression of DAT, NET, and SERT. (S, S)-CE-158 selectively and potently blocked DAT (IC50 = 0.2271 μM), while having a weak effect on NET (IC50 = 11.97 μM) and no effect on SERT. Symbols represent means of uptake inhibition ± standard error of three independent experiments, each performed in duplicates. The percent response was calculated using the following formulas: % Control = 100 x (mean relative luminescence units (RLU) of test sample - mean RLU of DAT, NET, or SERT blocker control) / (mean RLU of vehicle control - mean RLU of DAT, NET, or SERT blocker control). Percent of Control was then converted to percent response using formula: Percent Response = (100 - Percent Control). The percent response for NET came out to be negative is because the mean RLU of test sample was higher than the mean RLU of vehicle control.
Ability of (S, S)-CE-158 to reverse the effects of TBZ on the concurrent FR5/chow feeding choice procedure
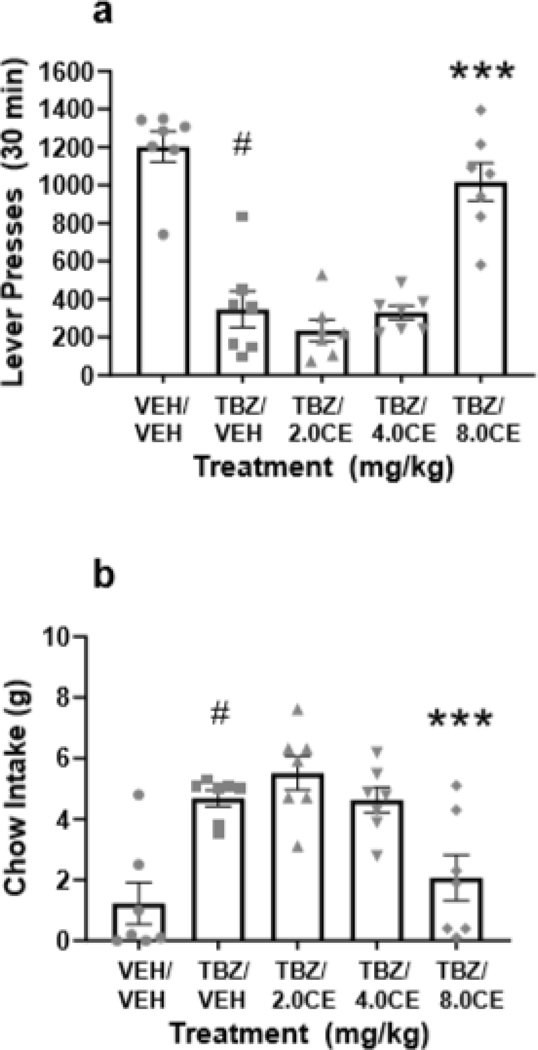
As shown in Figure 4, a repeated measures ANOVA revealed that there was an overall significant effect of drug treatment on lever pressing [F(4,24)=34.940, p<0.001; effect size ηp2 = 0.853]. Planned comparisons showed that TBZ significantly decreased lever pressing compared to vehicle treatment [F(1,24)=63.675, p<0.001], and that co-administration of the dose of 8.0 mg/kg (S, S)-CE-158 with TBZ significantly attenuated the effects of TBZ on lever pressing [F(1,24)=39.06, p<0.001]. A two-tailed t-test revealed no difference in lever pressing between the vehicle plus vehicle and TBZ plus (S, S)-CE-158 8.0 mg/kg treatments (p=0.17) (Figure 4a). There was also a significant overall effect of drug treatment on chow intake [F(4,24)=22.086, p<0.001; effect size ηp2 = 0.786]. Additional planned comparisons revealed that TBZ alone significantly increased chow intake relative to vehicle treatment [F(1,24)=38.098, p<0.001], and that co-administration of 8.0 mg/kg (S, S)-CE-158 with TBZ significantly reduced chow intake compared to the TBZ plus vehicle condition [F(1,24)=21.786, p<0.001]. Furthermore, a two-tailed t-test revealed no difference in chow intake between the vehicle plus vehicle and TBZ plus 8.0 mg/kg (S, S)-CE-158 treatment groups (p=0.42) (Figure 4b).
Fig. 4.
The effects of the DAT blocker (S, S)-CE-158 on TBZ-induced changes in performance on the concurrent lever pressing/chow-feeding choice procedure. (a) Mean (± SEM) number of FR5 lever presses in 30 min across drug treatment conditions. There was an overall significant effect of drug treatment on lever pressing. (b) Mean (± SEM) gram quantity of chow intake in 30 min across drug treatment conditions. There was an overall significant effect of drug treatment on chow intake. VEH= vehicle control, TBZ= tetrabenazine, CE= (S, S)-CE-158 # p < 0.001, TBZ/VEH different from VEH/VEH; *** p < 0.001, TBZ/8.0 mg/kg (S, S)-CE-158 different from TBZ/VEH
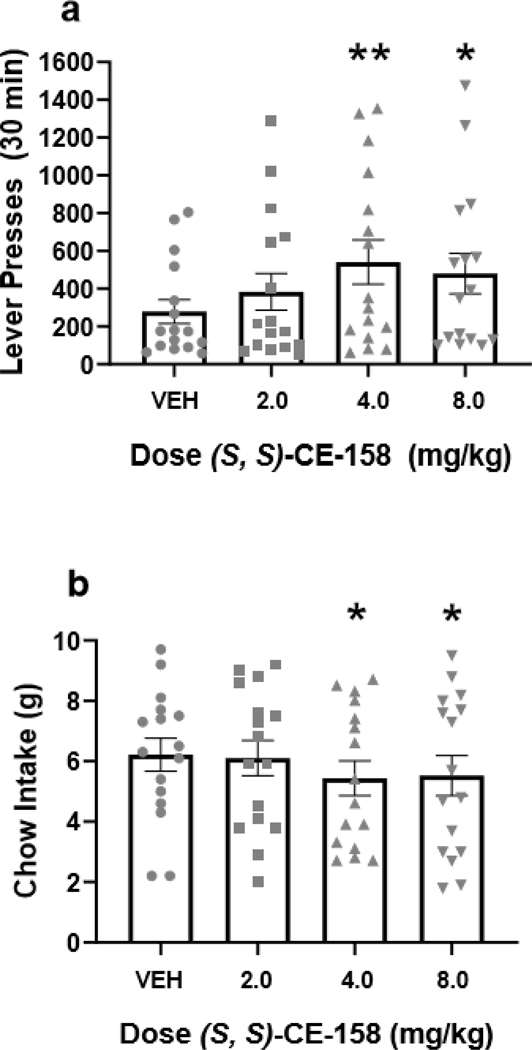
Ability of (S, S)-CE-158 to increase high-effort responding on the concurrent PROG/chow feeding choice procedure
Repeated measures ANOVA revealed an overall main effect of drug treatment on lever pressing [F(3,45)=3.583, p<0.05; effect size ηp2 = 0.193], and planned comparisons demonstrated that lever presses were significantly increased at 4.0 mg/kg (S, S)-CE-158 [F(1,45)=9.433, p<0.01] and at 8.0 mg/kg (S, S)-CE-158 [F(1,45)=5.54, p<0.05] compared to vehicle treatment (Figure 5a). Drug treatment with (S, S)-CE-158 also had a significant effect on chow intake during the PROG/chow session [F(3,45)=3.686, p<0.05; effect size ηp2 = 0.197], with a significant reduction in chow intake at the 4.0 mg/kg dose [F(1,45)=7.118, p<0.05] and at the 8.0 mg/kg dose [F(1,45)=5.613, p<0.05] compared to vehicle (Figure 5b).
Fig. 5.
The effects of the DAT blocker (S, S)-CE-158 on performance on the concurrent PROG lever pressing/chow-feeding choice procedure. (a) Mean (± SEM) number of lever presses in 30 min across drug treatments with vehicle (VEH) and various doses of (S, S)-CE-158. There was an overall significant effect of drug treatment on lever pressing. (b) Mean (± SEM) gram quantity of chow intake in 30 min across drug treatment conditions. There was an overall significant effect of drug treatment on chow intake.
*p < 0.05, different from VEH; ** p < 0.01, different from VEH
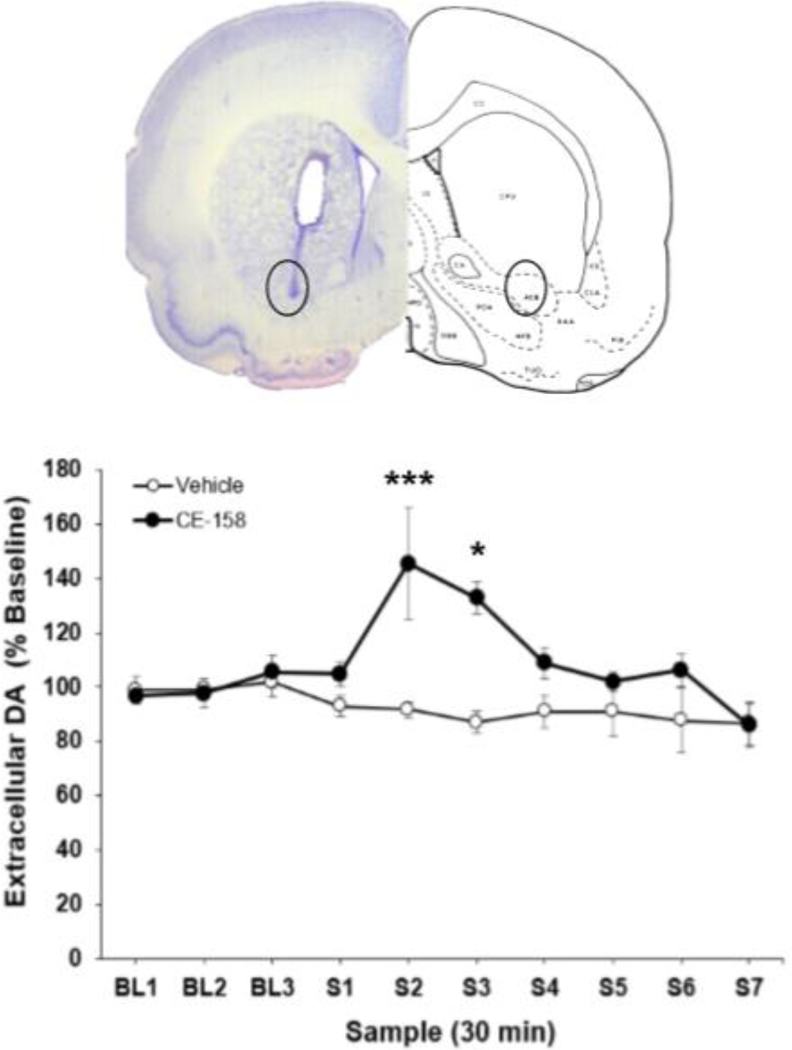
Systemic administration of (S, S)-CE-158 increased extracellular DA in the nucleus accumbens
Administration of 8.0 mg/kg (S, S)-CE-158 significantly increased extracellular DA content in the nucleus accumbens (Figure 6). Factorial ANOVA with repeated measures on the sample factor demonstrated that there was a significant overall difference across samples [F(6,42)=3.339, p<0.01] and a significant sample x treatment interaction [F(6,42)=3.011, p<0.05]. The factorial ANOVA also revealed a significant quadratic trend for the sample x treatment interaction [F(1,7)=912.422, p<0.001], which statistically characterized the tendency for DA levels to show a transient increase, followed by a decrease, after injection of (S, S)-CE158. In addition, there was a significant overall difference between treatment groups across the seven samples [F(1,7)=703.162, p<0.001], showing that (S, S)-CE-158 produced a significant overall difference in DA across samples relative to vehicle injection. Nonorthogonal planned comparisons assessing the difference between the two treatments at each sample revealed significant differences at sample 2 (S2) [F(1,7)=14.376, p<0.01] and sample 3 (S3) [F(1,7)=10.509, p<0.05].
Fig. 6.
Effect of (S, S)-CE-158 on extracellular DA in the nucleus accumbens. TOP: Photomicrograph and schematic showing a representative probe placement in nucleus accumbens. Abbreviations: acb: nucleus accumbens; cpu: caudate nucleus-putamen (neostriatum) (Pellegrino et al. 1979). BOTTOM: Mean (± SEM) extracellular DA (expressed as percent baseline) in 30-min samples. Three baseline (BL) samples were collected prior to injection of vehicle or 8 mg/kg (S, S)-CE-158, followed by seven post-drug samples (S1–7). There was a significant overall between-groups difference between vehicle and (S, S)-CE-158 across the seven post-drug samples
*p<0.05, (S, S)-CE-158 significantly differs from vehicle at S3; **p<0.001, (S, S)-CE-158 significantly differs from vehicle at S2
DISCUSSION
DAT inhibitors with atypical binding characteristics are being studied for their ability to ameliorate effort-related symptoms of fatigue and motivational dysfunction in rodents. The present work focused on the pharmacodynamic, neurochemical, and behavioral profile of the recently synthesized modafinil analog, (S, S)-CE-158. To determine the selectivity of (S, S)-CE158 for the DAT, in vitro binding and monoamine transporters reuptake inhibition experiments were conducted using transfected HEK293 cells expressing human isoforms of DAT, NET, and SERT. In the present study, the IC50 of (S, S)-CE-158 binding to the DAT was reported to be 0.052 μM, while its DAT inhibition was greater than 50-fold selective relative to NET (SERT reuptake inhibition was undetected; Figures 2 and 3). These patterns are consistent with results from inhibition assays measuring the EC50 for inhibition of monoamine transporters of the previously synthesized compound, (S)-CE-123, which revealed a 30-fold selective inhibition of DAT relative to NET and more than 400-fold selective compared to SERT (Nikiforuk et al. 2017). By direct comparison, (S, S)-CE-158 is even more selective for the DAT than (S)-CE-123, supporting its potential use as a DAT inhibitor with limited off-target effects.
The FR5/chow feeding choice experiment was performed to investigate the effects of (S, S)-CE-158 for its ability to reverse the effort-related effects of TBZ. Consistent with previous findings (Nunes et al. 2013; Randall et al. 2014; Yohn et al. 2015a,b,c; Rotolo et al. 2019), administration of 1.0 mg/kg TBZ shifted choice behavior, decreasing high effort lever pressing and increasing low-effort chow intake (Figure 4). TBZ was used to induce a low effort bias because this drug has been shown to induce depressive symptoms including fatigue in humans (Chitnis and Karunapuzha, 2009; Frank 2010; Chen et al. 2012). In addition, previous research has shown that doses of TBZ that alter effort-related choice do not suppress intake of Bio-serv pellets or chow in free-feeding preference tests and do not alter preference between the two foods used in the FR5/choice task (Nunes et al. 2013), do not affect discrimination of reinforcer magnitude or reference memory in a T-maze task (Yohn et al. 2015a), and fail to alter sucrose preference or hedonic reactivity (Pardo et al. 2015). Furthermore, the effects of TBZ on effort-based tasks do not resemble the effects of reinforcer devaluation by reduction in food motivation, or appetite suppressant drugs (Randall et al. 2012, 2014; Yang et al. 2020).
Co-administration of 8.0 mg/kg (S, S)-CE-158 with TBZ resulted in a significant reversal of the effects of TBZ on both lever pressing and chow intake. There was a very large effect size for the overall ANOVA, and the magnitude of the reversal effect can be observed by comparing the average number of lever presses at the dose that produced a significant reversal to the number of lever presses in the vehicle group. Here, the co-administration of 8.0 mg/kg (S, S)-CE-158 with TBZ yielded a lever pressing average which was 85% of the vehicle/vehicle average (Figure 4a), whereas Rotolo et al. (2019) reported only a 40% restoration of the vehicle/vehicle lever pressing average when the highest dose of (S)-CE-123 (24.0 mg/kg) was co-administered with TBZ. The ability of (S)-CE-123 to only partially reverse the effects of TBZ was attributed to possible appetite suppressant actions, which were not observed in the present experiment. Moreover, the efficacy of (S, S)-CE-158 for reversing the effort-related motivational dysfunction induced by TBZ is comparable to a group of DAT inhibitors including GBR12909, lisdexamfetamine, and methylphenidate in terms of their relatively large effects in terms of reversing the effects of TBZ (Salamone et al., 2016a; Yohn et al., 2016a,b,c).
To measure its effect when administered alone, (S, S)-CE-158 was administered to rats trained on the concurrent PROG/chow feeding choice task. This task is well suited for assessing the effects of a drug when administered alone, in terms of increasing the motivation to exert high levels of effort on a more highly demanding task. In the present study, (S, S)-CE-158 modestly but significantly increased the selection of the high-effort option (lever pressing) and reduced selection of the low-effort option (chow intake) at both the 4.0 mg/kg and 8.0 mg/kg doses (Figure 5). These results are consistent with previous experiments demonstrating that DAT inhibitors such as bupropion, lisdexamfetamine, MRZ-9547, and PRX-14040 significantly increased PROG lever pressing and decreasing chow consumption (Sommer et al. 2014; Randall et al., 2015; Yohn et al., 2016b,c,d), whereas drugs that inhibit NET and SERT did not (Yohn et al. 2016d). These results also are consistent with the findings of Cagniard et al. (2006), who reported that DAT knockdown increased selection of lever pressing in mice tested on an effort-based choice task, and with Wardle et al. (2011), who reported that amphetamine increased selection of high-effort activity in human participants.. Furthermore, it was shown that systemic administration of (S, S)-CE-158 at a dose of 8.0 mg/kg significantly increased extracellular DA in the nucleus accumbens core as measured by microdialysis (Figure 6). The peak increase in DA at 30–90 minutes post-injection is aligned with the time course used for the behavioral pharmacology experiments (i.e. 30 min injection lead time prior to a 30 min task). Systemic administration of DAT inhibitors in rodents has been reported to increase extracellular DA in the nucleus accumbens core, which has been associated with increased motivation (Yohn et al. 2016d; Rotolo et al. 2019) and pro-cognitive effects such as enhanced recognition memory (Carnats-Perna et al. 2019). The magnitude of the increases in extracellular DA induced by CE-123 (approximately 200% of baseline; Rotolo et al. 2019) and by CE-158 in the present study (145.4 ± 20.5 % of baseline) are in the low end of what is generally seen for cocaine microdialysis studies (150–1500% of baseline) as reported in a meta-analysis by Frank et al. (2008).
The ability of (S)-CE-123 and (S, S)-CE-158 to increase motivation and ameliorate effort-related impairments can be seen as complimentary to the known effects of their parent compound, modafinil. In addition to its prescribed indication as a treatment for narcolepsy, modafinil is frequently used by many individuals as a nootropic, for off-label effects such as cognitive enhancement (Sahakian and Morein-Zamir, 2011; Battleday and Brem, 2015; Sousa and Dinis-Oliveira, 2020; Teodorini et al. 2020). Memory and cognitive flexibility have been described as effects of both the S-configuration and the racemic mixture of the thiazolecontaining modafinil analog, CE-123 (Nikiforuk et al. 2017; Kristofova et al. 2018; CarnatsPerna et al. 2019). Interestingly, it has been suggested that modafinil’s effects related to cognitive processes and task engagement may be dependent on fronto-striatal circuitry, rather than hippocampal (Müller et al. 2013), which further supports the utility of these drugs as treatments for effort-related dysfunction. Moreover, it has been suggested that modafinil and its derivatives possess anti-inflammatory properties in vitro (Jung et al. 2012) and in vivo (Zager et al. 2017; Han et al. 2018; Brandão et al. 2019), and may reduce multiple sclerosis fatigue in humans (Shangyan et al. 2018), underlying possible utility of these compounds to attenuate symptoms of various disorders that are propagated by inflammatory cascades. Therefore, results from the current studies may be useful to guide future assessments of atypical DAT inhibitors to expand the clinical indications for modafinil and similar compounds.
In summary, these experiments contributed to the characterization of a recently developed group of atypical DAT inhibitor compounds, by demonstrating the ability of (S, S)CE-158 to reverse the effort-related effects of TBZ, and increase high-effort responding in tasks measuring effort-based choice. Additionally, it was shown that systemic administration of (S, S)CE-158 increased extracellular DA in the nucleus accumbens during the behaviorally active time course. The behavioral assessment of atypical DAT inhibitors such as analogs of benztropine, GBR12909 and modafinil has led to the discovery that not all inhibitors of DA uptake show signs of abuse liability to the same extent as the classical DAT inhibitors that are major psychostimulants such as cocaine (Sogaard et al. 1990; Preti 2000; Woolverton et al. 2001; Loland et al. 2008; Schmitt et al. 2008; Esumi et al. 2012; Tanda et al. 2013; Mereu et al. 2013). These results suggest it is possible that atypical DAT inhibitors may be effective at reducing motivational impairments with minimal induction of major psychomotor side effects or abuse liability. Ultimately, this research will guide future studies to advance target identification for treatments of motivational dysfunction. These and other similar findings could lead to the development of atypical DA uptake blockers that could potentially treat motivational symptoms of depression, chronic fatigue syndrome, or other disorders, while reducing undesirable side effects.
Acknowledgements
The authors would like to acknowledge Eurofins DiscoverX Corporation (Fremont, CA).
Footnotes
Conflict of Interests: This research was supported by grants the University of Connecticut Research Foundation (JS), University of Vienna (JS), and to MC from MINECO (PSI2015–68497-R) Spain. JS has received grants from, and done consulting work for, Pfizer, Roche, Shire, Prexa, Chronos, Blackthorn, Lundbeck and Acadia. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Bailey MR, Simpson EH, Balsam PD (2016) Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol Learn Mem 133:233–256 doi: 10.1016/j.nlm.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Chun E, Schipani E, Balsam PD, Simpson EH (2020) Dissociating the effects of dopamine D2 receptors on effort-based versus value-based decision making using a novel behavioral approach. Behav Neurosci, 134(2): 101–118 doi: 10.1037/bne0000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N (2014) Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. J Abnormal Psychol 123: 387–397 doi: 10.1037/a0036299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battleday RM, Brem AK (2015) Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol 25, 1865–1881 doi: 10.1016/j.euroneuro.2015.07.028 [DOI] [PubMed] [Google Scholar]
- Blockmans D, Persoons P (2016) Long-term methylphenidate intake in chronic fatigue syndrome. Acta Clin Belg 71(6):407–414 doi: 10.1080/17843286.2016.1200816 [DOI] [PubMed] [Google Scholar]
- Brandão WN, Andersen ML, Palermo-Neto J, Peron JP, Zager A (2019) Therapeutic treatment with Modafinil decreases the severity of experimental autoimmune encephalomyelitis in mice. Int Immunopharmacol 75:105809 doi: 10.1016/j.intimp.2019.105809 [DOI] [PubMed] [Google Scholar]
- Bryce CA, Floresco SB (2016) Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor. Neuropsychopharmacology 41(8):2147–2159 doi: 10.1038/npp.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Balsam P, Brunner D, Zhuang X (2006) Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 31:1362–1370 doi. 10.1038/sj.npp.1300966 [DOI] [PubMed] [Google Scholar]
- Camats-Perna J, Kalaba P, Ebner K, Sartori SB, Vuyyuru H, Aher NY, Dragačević V, Singewald N, Engelmann M and Lubec G (2019) Differential effects of novel dopamine reuptake inhibitors on interference with long-term social memory in mice. Front Behav Neurosci 13:63 doi: 10.3389/fnbeh.2019.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, Slusher BS et al. (2016). Novel and high affinity 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues as atypical dopamine transporter inhibitors. J Med Chem 59:10676–10691 doi: 10.1021/acs.jmedchem.6b01373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Ondo WG, Dashtipour K, Swope DM (2012) Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther 34(7):1487–1504 doi: 10.1016/j.clinthera.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Chitnis S, Karunapuzha CA (2009) Tetrabenazine in Huntington’s disease chorea. Clin Med Ther 1:669–681 10.4137/CMT.S2134 [DOI] [Google Scholar]
- Chong TT, Bonnelle V, Manohar S, Veromann KR, Muhammed K, Tofaris GK, Hu M, Husain M (2015) Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex 69:40–46 doi: 10.1016/j.cortex.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF, Yu YM, Yeung WF (2015) Correlates of residual fatigue in patients with major depressive disorder: The role of psychotropic medication J Affect Disord 186:192–197. doi: 10.1016/j.jad.2015.07.026 [DOI] [PubMed] [Google Scholar]
- Cooper JA, Tucker VL, and Papakostas GI (2014) Resolution of sleepiness and fatigue: a comparison of bupropion and selective serotonin reuptake inhibitors in subjects with major depressive disorder achieving remission at doses approved in the European Union. J Psychopharmacol 28, 118–124 doi: 10.1177/0269881113514878 [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM (2005) The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacology 8, 93–105 doi: 10.1017/S1461145704004729 [DOI] [PubMed] [Google Scholar]
- Dieterich A, Stech K, Srivastava P, Lee J, Sharif A, Samuels BA (2020) Chronic corticosterone shifts effort-related choice behavior in male mice. Psychopharm [Online ahead of print] doi: 10.1007/s00213-020-05521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi S, Sagara H, Nakamoto A, Kawasaki Y, Gomita Y, Sendo T (2013) Effect of GBR12909 on affective behavior: distinguishing motivational behavior from antidepressant-like and addiction-like behavior using the runway model of intracranial self-stimulation. Behav Brain Res 243: 313–321 doi: 10.1016/j.bbr.2012.10.051 [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C, Doghramji K, Arora S, Hughes RJ (2007) Modafinil augmentation of selective serotonin reuptake inhibitor therapy in MDD partial responders with persistent fatigue and sleepiness. Ann Clin Psychiatry 19(3):153–9 doi: 10.1080/10401230701464858 [DOI] [PubMed] [Google Scholar]
- Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P et al. (2014) Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety 31(3), 250257 doi: 10.1002/da.22199 [DOI] [PubMed] [Google Scholar]
- Ferguson M, Dennehy EB, Marangell LB, Martinez J, Wisniewski SR (2014) Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: secondary analysis of STAR*D, Current Medical Research and Opinion 30:10, 2109–2118 doi: 10.1185/03007995.2014.936553 [DOI] [PubMed] [Google Scholar]
- Frank S (2010) Tetrabenazine: the first approved drug for the treatment of chorea in US patients with Huntington disease. Neuropsychiatr Dis Treat 6: 657–665 doi: 10.2147/NDT.S6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ST, Krumm B, Spanagel R (2008) Cocaine-induced dopamine overflow within the nucleus accumbens measured by in vivo microdialysis: a meta-analysis. Synapse 62(4): 243–252 doi: 10.1002/syn.20489 [DOI] [PubMed] [Google Scholar]
- Ghanean H, Ceniti AK, Kennedy SH (2018) Fatigue in patients with major depressive disorder: prevalence, burden and pharmacological approaches to management. CNS Drugs 32:6574 10.1007/s40263-018-0490-z [DOI] [PubMed] [Google Scholar]
- Gullion CM, Rush AJ (1998) Toward a generalizable model of symptoms in major depressive disorder. Biol Psychiatry 44: 959–972 doi: 10.1016/s0006-3223(98)00235-2 [DOI] [PubMed] [Google Scholar]
- Han J, Chen D, Liu D, Zhu Y (2018) Modafinil attenuates inflammation via inhibiting Akt/NFκB pathway in apoE-deficient mouse model of atherosclerosis. Inflammopharmacology 26(2):385–393 doi: 10.1007/s10787-017-0387-3 [DOI] [PubMed] [Google Scholar]
- Hart EE, Gerson JO, Zoken Y, Garcia M, Izquierdo A (2017) Anterior cingulate cortex supports effort allocation towards a qualitatively preferred option. Eur J Neurosci 46(1): 1682–1688 doi: 10.1111/ejn.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Floresco SB, Winstanley CA (2015) Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology 40(4):1005–1015 doi: 10.1038/npp.2014.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-C, Lee Y, Son J-Y, Lim E, Jung M, Oh S (2012) Simple synthesis of modafinil derivatives and their anti-inflammatory activity. Molecules 17(12), 10446−10458 doi: 10.3390/molecules170910446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaba P, Aher NY, Ilic M, et al. (2017) Heterocyclic analogues of modafinil as novel, atypical dopamine transporter inhibitors. J Med Chem 60(22):9330–9348 doi: 10.1021/acs.jmedchem.7b01313 [DOI] [PubMed] [Google Scholar]
- Kalaba P, Ilić M, Aher NY, Dragačević V, Wieder M, Zehl M, Wackerlig J, Beyl S, Sartori SB, Ebner K, Roller A, Lukic N, Beryozkina T, Gonzalez ERP, Neill P, Khan JA, Bakulev V, Leban JJ, Hering S, Pifl C, Singewald N, Lubec J, Urban E, Sitte HH, Langer T, Lubec G (2020) Structure-activity relationships of novel thiazole-based modafinil analogues acting at monoamine transporters. J Med Chem 63(1): 391–417 doi: 10.1021/acs.jmedchem.9b01938 [DOI] [PubMed] [Google Scholar]
- Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, Frazer A (2004) Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology 29: 566–579 doi: 10.1038/sj.npp.1300341 [DOI] [PubMed] [Google Scholar]
- Keppel G (1991) Design and analysis a researcher’s handbook. 3rd ed. Englewood Clifts, NY: Prentice Hall. [Google Scholar]
- Kristofova M, Aher YD, Ilic M, Radoman B, Kalaba P, Dragacevic V et al. (2018) A daily single dose of a novel modafinil analogue CE-123 improves memory acquisition and memory retrieval. Behavioral Brain Research 343: 83–94 doi: 10.1016/j.bbr.2018.01.032 [DOI] [PubMed] [Google Scholar]
- Lam JY, Freeman MK, Cates ME (2007) Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother 41: 1005–1012 doi: 10.1345/aph.1H526 [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73:813–823 doi: 10.1124/mol.107.039800 [DOI] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W (2012) Motivational states influence effort-based decision making in rats: The role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci 12:74–84 doi: 10.3758/s13415-011-0068-4 [DOI] [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G (2013) The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology 229, 415–434 doi: 10.1007/s00213-013-3232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu M, Chun LE, Prisinzano TE, Newman AH, Katz JL, Tanda G (2017) The unique psychostimulant profile of (±)-modafinil: investigation of behavioral and neurochemical effects in mice. Eur J Neurosci 45(1): 167–174 doi: 10.1111/ejn.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ (2013) Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology 64, 490–495 doi: 10.1016/j.neuropharm.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Kalaba P, Ilic M, Korz V, Dragacevic V, Wackerlig J et al. (2017) A novel dopamine transport inhibitor CE-123 improves cognitive flexibility and maintains impulsivity in healthy male rats. Front Behav Neurosci 11: 222 doi: 10.3389/fnbeh.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn SE, Baqi Y, Müller CE, López-Cruz L, Correa M, Salamone JD (2013) Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: implications for animal models of the motivational symptoms of depression. J Neurosci 33(49):19120–30 doi: 10.1523/JNEUROSCI.2730-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA et al. (2014) Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/ chow feeding choice task. Psychopharmacology (Berl) 231(4), 727–736 doi: 10.1007/s00213-013-3285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padala PR, Padala KP, Monga V, Ramirez DA, Sullivan DH (2012) Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. Ann Pharmacother 46: e8. doi: 10.1345/aph.1Q656 [DOI] [PubMed] [Google Scholar]
- Pae CU, Lim HK, Han C, Patkar AA, Steffens DC, Masand PS, Lee C (2007) Fatigue as a core symptom in major depressive disorder: overview and the role of bupropion. Expert Rev Neurother 7(10): 1251–1263 doi: 10.1586/14737175.7.10.1251 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M (2006) Resolution of sleepiness and fatigue in major depressive disorder: A comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry 60(12): 1350–1355 doi: 10.1016/j.biopsych.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Pardo M, López-Cruz L, San Miguel N, Salamone JD, Correa M (2015) Selection of sucrose concentration depends on the effort required to obtain it: studies using tetrabenazine, D1, D2, and D3 receptor antagonists. Psychopharmacology 232(13):2377–91 doi: 10.1007/s00213-015-3872-7 [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ (1979) A stereotaxic atlas of the rat brain, Ed 2 Penlum Press, New York. [Google Scholar]
- Pifl C, Wolf A, Rebernik P, Reither H, Berger ML (2009) Zinc regulates the dopamine transporter in a membrane potential and chloride dependent manner. Neuropharmacology 56: 531–540 doi: 10.1016/j.neuropharm.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Preti A (2000) Vanoxerine National Institute on Drug Abuse. Curr Opin Investig Drugs 1(2), 241–251 PMID: 11249581 [PubMed] [Google Scholar]
- Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B et al. (2014) The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs. PLoS One 9 (6): e99320 doi: 10.1371/journal.pone.0099320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M et al. (2015) Bupropion increases selection of high effort activity in rats tested on a progressive/ratio chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int. J. Neuropsychopharmacol. 18 (2): 1–11 doi: 10.1093/ijnp/pyu017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A et al. (2012) Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7 (10): e47934 doi: 10.1371/journal.pone.0047934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray B, Martin K, Hewitt A, Cooper G, McDonald W (2019) Effect of acute modafinil ingestion on cognitive and physical performance following mental exertion. Hum Psychopharmacol 34(4):e2700 doi: 10.1002/hup.2700. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ, Raskin J, Wang CN, Marangell LB, Fava M (2014) The relationship between change in apathy and changes in cognition and functional outcomes in currently nondepressed SSRI-treated patients with major depressive disorder. Compr Psychiatry 55:1–10 doi: 10.1016/j.comppsych.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Rotolo RA, Dragacevic V, Kalaba P, Urban K, Zehl M, Roller A, Wackerlig J, Langer T, Pistis M, De Luca MA, Caria F, Schwartz R, Presby RE, Yang JH, Samels S, Correa M, Lubec G, Salamone JD (2019) The novel atypical dopamine uptake inhibitor (S)-CE-123 partially reverses the effort-related effects of the dopamine depleting agent tetrabenazine and increases progressive ratio responding. Front Pharm 10: 682 doi: 10.3389/fphar.2019.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Morein-Zamir S (2011) Neuroethical issues in cognitive enhancement. J Psychopharmacol 25: 197e204 doi: 10.1016/j.comppsych.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2002) Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137: 3–25 doi: 10.1016/s0166-4328(02)00282-6 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76(3): 470–485 doi: 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191: 461–482 doi: 10.1007/s00213-006-0668-9 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM (2006) Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Current Psychiatry Reviews 2: 267–280 doi: 10.2174/157340006776875914 [DOI] [Google Scholar]
- Salamone JD, Yohn S, Lopez-Cruz L, San Miguel N, Correa M (2016a) Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology. Brain 139(Pt 5), 1325–1347 doi: 10.1093/brain/aww050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yohn S, Lopez-Cruz L, San Miguel N, Alatorre L (2016b) The pharmacology of effort-related choice behavior: dopamine, depression, and individual differences. Behav Processes 127: 3–17 doi: 10.1016/j.beproc.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Ferrigno S, Yang J-H, Rotolo RA, Presby RE (2018a) The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation. Pharmacol Rev 70:747–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME (2011) The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PloS One 6(10): e25790 10.1371/journal.pone.0025790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith ME (2008) Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J Neurochem 107(4): 928–940 doi: 10.1111/j.14714159.2008.05667.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Saft S, Hauber W (2005) Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci 119: 1687–1692. doi: 10.1037/0735-7044.119.6.1687 [DOI] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB (2012) Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37: 2194–2209. doi: 10.1038/npp.2012.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangyan H, Kuiqing L, Yumin X, Jie C, Weixiong L (2018) Meta-analysis of the efficacy of modafinil versus placebo in the treatment of multiple sclerosis fatigue. Mult Scler Relat Disord 19:85–89 doi: 10.1016/j.msard.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Sogaard U, Michalow J, Butler B, Lund Laursen A, Ingersen SH, Skrumsager BK et al. (1990) A tolerance study of single and multiple dosing of the selective dopamine uptake inhibitor GBR 12909 in healthy subjects. Int Clin Psychopharmacol 5(4): 237–251. doi: 10.1097/00004850-199010000-00001 [DOI] [PubMed] [Google Scholar]
- Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W (2014) The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol 17: 2045–2056 doi: 10.1017/S1461145714000996 [DOI] [PubMed] [Google Scholar]
- Sousa A, Dinis-Oliveira RJ (2020) Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: Relevant clinical and forensic aspects. Subst Abus 41(2):155–173 DOI: 10.1080/08897077.2019.1700584 [DOI] [PubMed] [Google Scholar]
- Stotz G, Woggon B, Angst J (1999) Psychostimulants in the therapy of treatment-resistant depression review of the literature and findings from a retrospective study in 65 depressed patients. Dialogues Clin Neurosci 1(3): 165–174 PMID: 22034135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz PV, Golani LK, Witkin JM (2019) Animal models of fatigue in major depressive disorder. Physiology & Behavior 199, 300–305. 10.1016/j.physbeh.2018.11.042 [DOI] [PubMed] [Google Scholar]
- Tanda G, Li SM, Mereu M, Thomas AM, Ebbs AL, Chun LE, Tronci V, Green JL, Zou MF, Kopajtic TA, Newman AH, Katz JL (2013) Relations between stimulation of mesolimbic dopamine and place conditioning in rats produced by cocaine or drugs that are tolerant to dopamine transporter conformational change. Pharmacology (Berl) 229(2):307–321 doi: 10.1007/s00213-013-3109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorini RD, Rycroft N, Smith-Spark JH (2020) The off-prescription use of modafinil: An online survey of perceived risks and benefits. PLoS ONE 15(2): e0227818 10.1371/journal.pone.0227818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012) Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121(3): 553–558 doi: 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J (1999) DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. Int Clin Psychopharmacol 14: 139–151 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K (2009) Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA 301(11):1148–1154 doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H (2011) Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci 31(46): 16597–16602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Floresco SB (2016) Deciphering decision making: variation in animal models of effort- and uncertainty-based choice reveals distinct neural circuitries underlying core cognitive processes. J Neurosci 36(48):12069–12079 doi: 10.1523/JNEUROSCI.1713-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, Newman AH (2001) Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology 154(4): 375–382 doi: 10.1007/s002130000616 [DOI] [PubMed] [Google Scholar]
- Yang JH, Presby RE, Jarvie AA, Rotolo RA, Fitch RH, Correa M, Salamone JD (2020) Pharmacological studies of effort-related decision making using mouse touchscreen procedures: effects of dopamine antagonism do not resemble reinforcer devaluation by removal of food restriction. Psychopharmacology 237: 33–43 doi: 10.1007/s00213-01905343-8 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Thompson C, Randall PA, Lee CA, Müller CE, Baqi Y et al. (2015a) The VMAT-2 inhibitor tetrabenazine alters effort-related decision making as measured by the T-maze barrier choice task: reversal with the adenosine A2A antagonist MSX-3 and the catecholamine uptake blocker bupropion. Psychopharmacology 232(7): 1313–1323 doi: 10.1007/s00213-014-3766-0 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M, Salamone JD (2015b) The role of dopamine D1 receptor transmission in effort-related choice behavior: effects of D1 agonists. Pharmacol Biochem Behav 135: 217–226 doi: 10.1016/j.pbb.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Collins SL, Contreras-Mora HM, Errante EL, Rowland MA, Correa M, Salamone JD (2016a) Not all antidepressants are created equal: differential effects of monoamine uptake inhibitors on effort-related choice behavior. Neuropsychopharmacology 41(3): 686–694 doi: 10.1038/npp.2015.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Lopez-Cruz L, Hutson PH, Correa M, Salamone JD (2016b) Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat. Psychopharmacology 233(6): 949–960 doi: 10.1007/s00213-015-4176-7 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Gogoj A, Haque A, Lopez-Cruz L, Haley A, Huxley P et al. (2016c) Evaluation of the effort-related motivational effects of the novel dopamine uptake inhibitor PRX-14040. Pharmacol Biochem Behav 148: 84–91 doi: 10.1016/j.pbb.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Errante EL, Rosenbloom-Snow A, Sommerville M, Rowland MA, Tokarski K et al. (2016d) Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: implications for treatment of effort-related motivational symptoms in psychopathology. Neuropharmacology 109: 270–280 doi: 10.1016/j.neuropharm.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Müller CE, Miguel NS, Correa M, Salamone JD (2016d) Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology 233(19–20): 3575–3586 doi: 10.1007/s00213-016-4392-9 [DOI] [PubMed] [Google Scholar]
- Zager A, Brandão WN, Margatho RO, et al. (2018) The wake-promoting drug Modafinil prevents motor impairment in sickness behavior induced by LPS in mice: Role for dopaminergic D1 receptor. Prog Neuropsychopharmacol Biol Psychiatry 81: 468–476 doi: 10.1016/j.pnpbp.2017.05.003 [DOI] [PubMed] [Google Scholar]