Abstract
Background:
We evaluated the diagnostic utility of procalcitonin (PCT) in predicting bacterial bloodstream infections (BSI) in critically ill cancer patients with and without neutropenia. We also investigated the role of PCT as a prognostic marker of supportive modalities (vasopressors, invasive mechanical ventilation, and renal replacement therapy (RRT)) in the intensive care unit (ICU).
Methods:
We retrospectively analyzed 2,200 PCT and blood cultures from adult cancer patients with suspected sepsis. Primary outcome was BSI, defined by positive blood culture, collected within 72 h of PCT collection.
Results:
Median PCT values were higher in encounters with BSI (3.2 vs 0.5 ng/ml, p<0.001). The area under the ROC curve (AUC) was 0.726 (95%CI 0.698, 0.754). PCT>2.0 ng/ml was significantly associated with greater likelihood of BSI and this effect was significantly stronger for neutropenic (OR 9.09, 95%CI: 4.39, 18.79) compared with non-neutropenic patients (OR 4.00 (95% CI: 3.13, 5.10), interaction p=0.036). PCT >2.0 was associated with vasopressor requirement on ICU admission (OR 1.82 (95% CI 1.31, 2.53), p<0.001) and RRT (OR 2.20 (95% CI 1.24, 3.91), p=0.007).
Conclusions:
Procalcitonin is a fair discriminator of BSI in critically ill cancer patients with and without neutropenia and a PCT >2.0 ng/ml was significantly more likely to require vasopressors and RRT in the ICU.
1. INTRODUCTION
Despite significant advances in the management of sepsis, early diagnosis remains the most important factor for patient survival and outcome. Current obstacles to rapid diagnosis include time required for microbiology methods (culture turnaround time usually takes several days) and low sensitivity for some organisms. Culture-negative sepsis can account for up to 50% of severe sepsis cases [1].
Biomarkers that can facilitate rapid, accurate diagnosis of bacterial bloodstream infections (BSI) would enhance early sepsis diagnosis. Although several biomarkers for sepsis have shown promising results, single markers have not been effective across all patient populations. Procalcitonin (PCT), a host-response marker that is upregulated by microbial toxins and proinflammatory cytokines, has demonstrated moderate sensitivity for identifying patients at risk of sepsis. The diagnostic value of PCT in sepsis is well-established in non-cancer patients. PCT concentration less than 0.5 ng/ml is generally associated with a low risk of sepsis, whereas PCT concentration greater than 2 ng/ml is associated with high risk [2]. However, the utility of PCT in the diagnosis of BSI and sepsis in immunocompromised patients including neutropenic cancer patients has been questioned [3–9]. Some studies suggest PCT lacks both specificity and positive predictive value for diagnosing sepsis in patients with cancer [10]. PCT elevations have been described in progression of cancer, in the absence of infection [11]. Neutropenia, frequently due to underlying malignancy or chemotherapy places cancer patients at an increased risk of infection, which can be difficult to diagnose and distinguish from noninfectious etiologies. Neutrophils have also been proposed as the source of PCT in the inflammatory response during infection, introducing the possibility that the PCT response in neutropenic patients may be impaired or different than what is seen in the immunocompetent patients[5]. Published studies and meta-analyses of PCT as a diagnostic and prognostic marker of BSI and sepsis in neutropenic patients have shown conflicting results [10,12].
In the setting of these conflicting findings, we investigated the role of PCT in the evaluation of suspected sepsis in our cancer patient population. Specifically, we sought to evaluate the diagnostic accuracy of PCT for bacterial BSI in critically ill cancer patients with and without neutropenia. Additionally, we investigated the role of PCT as a prognostic marker of supportive treatment modalities (vasopressors, invasive mechanical ventilation, and renal replacement therapy) in the intensive care unit (ICU).
2. MATERIALS AND METHODS:
2.1. Study Design
We performed a retrospective review of clinically ordered serum procalcitonin (PCT) levels between December 2015 and June 2017 and corresponding blood culture results (collected within 72 hours of a PCT) in adult patients (> 18 y) with cancer who were treated in the Intensive Care Unit (ICU) and Urgent Care Center (UCC) at Memorial Sloan Kettering Cancer Center (MSKCC) in New York City. This study was approved by the institutional review board of MSKCC and granted a waiver of informed consent.
Only the initial PCT value from each ICU or UCC encounter within a 6-day window was included. PCTs ordered on encounters/admissions greater than 6 days apart were considered separate events because we deemed them to be prompted by a change in clinical condition and were therefore analyzed accordingly. In total, 2,200 PCT results from 1,886 unique patients were included for analysis.
Our primary outcome was bloodstream infection, as defined by a positive blood culture result. Blood culture results were considered if collected within 72 h of a PCT result. Immune status (neutropenic vs non-neutropenic) for purposes of categorization was determined by the closest absolute neutrophil count (ANC) result within 24 h of the PCT sample collection time. Neutropenia was defined as ANC <500/ul.
In the cohort of patients admitted to the ICU (n=697), we collected demographic and clinical data from our ICU database. This included age, gender, type of malignancy (solid vs hematologic), Mortality Probability Model II score on ICU admission (MPM II0), treatment modalities used in the ICU including vasopressor agents, mechanical ventilation, hemodialysis or renal replacement therapy and ICU and Hospital length of stay (LOS) and mortality. The MPM II0 score is the severity of illness scoring system for critically ill patients utilized by our institution during the timeframe of this study. Patients with multiple ICU admissions within the same hospitalization were considered independent with respect to the need for treatment modalities.
2.2. Laboratory Methods
Serum PCT concentrations were measured by an enzyme-linked fluorescent assay, the VIDAS ® B-R-A-H-M-S PCT ™ (bioMerieux) according to manufacturer’s instructions. The analytical measurement range verified in our laboratory was 0.05 – 200.00 ng/ml.
2.3. Statistical methods
The primary objective was to investigate the diagnostic accuracy of PCT for discriminating bloodstream infection results in a cancer population with neutropenic and non-neutropenic subpopulations. Patient-level and encounter-level characteristics were first summarized with medians and interquartile ranges (IQR: 25th and 75th percentiles) or frequencies and percentages for continuous and categorical variables, respectively. The association of PCT and ANC with BSI was summarized and assessed using logistic regression. Receiver operator characteristics (ROC) curves were used to evaluate diagnostic accuracy of PCT for BSI and the area under the ROC curve (AUC) was estimated for our cancer population and separately by ANC status. The AUCs were compared by ANC status using an unpaired t-test with estimated variances based on DeLong’s method [13]. A best threshold was calculated using Youden’s index, which is based on the maximum sum of sensitivity and specificity.
In the ICU population, we further investigated the association of PCT with the severity of illness score on ICU admission (MPM-II0) and need for supportive modalities (vasopressor agents, mechanical ventilation, hemodialysis or renal replacement therapy) as surrogates for the severity and treatment of sepsis, and with hospital discharge status.
Binary outcomes were analyzed using logistic regression models and continuous outcomes were analyzed using linear regression. Multivariable analysis (MVA) using the respective regression models were performed to control for potential confounding effect of neutropenic status.
A 2-sided p<0.05 was considered significant. All statistical analyses were performed using R ver 3.5 (The R foundation for Statistical Computing).
3. RESULTS:
3.1. Clinical Characteristics
A total of 2,200 encounters from 1,886 unique patients were analyzed. The demographic and clinical characteristics of the patients are listed in Table 1. Of the 2,200 encounters, 399 (18%) had at least one positive blood culture result. Median PCT values were significantly higher in encounters with positive blood cultures (3.2 ng/ml) vs encounters with no positive blood cultures (0.5 ng/ml) (p <0.001). Using the current recommended cut-off of 2.0 ng/ml, encounters with a PCT > 2 ng/ml had higher odds of positive blood culture than encounters with a PCT ≤ 2 ng/ml (OR: 4.6, 95%CI: 3.7–5.8). While neutropenic encounters made up only 16.8% of encounters with a positive blood culture, neutropenic encounters also had higher odds of positive culture than non-neutropenic encounters (OR 3.4, 95%CI: 2.4–4.7). In both neutropenic and non-neutropenic subgroups, a PCT >2.0 ng/ml was significantly more likely to be associated with a positive blood culture result; however, the likelihood was higher for neutropenic patients (OR 9.09, 95%CI: 4.39–18.79) than non-neutropenic patients (OR 4.00, 95%CI: 3.13–5.10, interaction p=0.036) (Supplemental Table 1). In our cohort, the prevalence of BSI in neutropenic patients was 39.6% (67/169 patients). In the nonneutropenic patients, the prevalence was 16.3% (332/2031).
Table 1:
Demographic and Clinical Characteristics of Patients
| Patient Characteristics | Patients (n=1,886) |
|---|---|
| Male, n (%) | 999 (53%) |
| Female, n (%) | 887 (47%) |
| Age, mean in years (range) | 62 (19–96) |
| Neutropenia at time of initial PCT, n (%) | 169 (7.7%) |
| Encounters per patient | |
| 1 | 1643 (87%) |
| 2 | 197 (10%) |
| 3+ | 46 (2.4%) |
| PCT, overall | Encounters (N=2,200) |
| PCT, median ng/mL | 0.61 |
| <0.5 | 1017 (46%) |
| 0.5–2.0 | 527 (24%) |
| >2.0 | 656 (30%) |
| PCT, ANC ≥ 0.5 | N = 2,031 (92%) |
| PCT, median ng/mL | 0.55 |
| <0.5 | 960 (47%) |
| 0.5–2.0 | 498 (25%) |
| >2.0 | 573 (28%) |
| PCT, ANC < 0.5 | N = 169 (7.7%) |
| PCT, median ng/mL | 1.93 |
| <0.5 | 57 (34%) |
| 0.5–2.0 | 29 (17%) |
| >2.0 | 83 (49%) |
3.2. Accuracy of PCT in neutropenic and non-neutropenic patients
ROC analysis was performed to examine the accuracy of PCT in the prediction of BSI (Fig. 1). The area under the ROC curve (AUC) was 0.726 (95%CI 0.698–0.754), considered “fair” in discriminating between culture positive and negative results. Our data suggest that the best threshold for PCT in discriminating blood culture results was 1.7 ng/ml, with a sensitivity and specificity of 61% and 74%, respectively. The AUC of the neutropenic group was higher compared to the non-neutropenic group (AUCs: 0.792 vs. 0.708, p=0.030). Within the neutropenic group, the best threshold was higher, >2.05 ng/ml, with an increased sensitivity of 79%. Within the non-neutropenic group, the best threshold was the same as the overall group, >1.695 ng/ml, with a slightly lower sensitivity of 58%. The positive predictive value of PCT in neutropenic patients was also higher than in nonneutropenic patients (64% vs 31%). The negative predictive value of PCT was 84% in neutropenic patients and 90% in nonneutropenic patients.
Fig. 1. Receiver operating characteristics (ROC) analysis of PCT Predictive Performance of BSI Diagnosis.
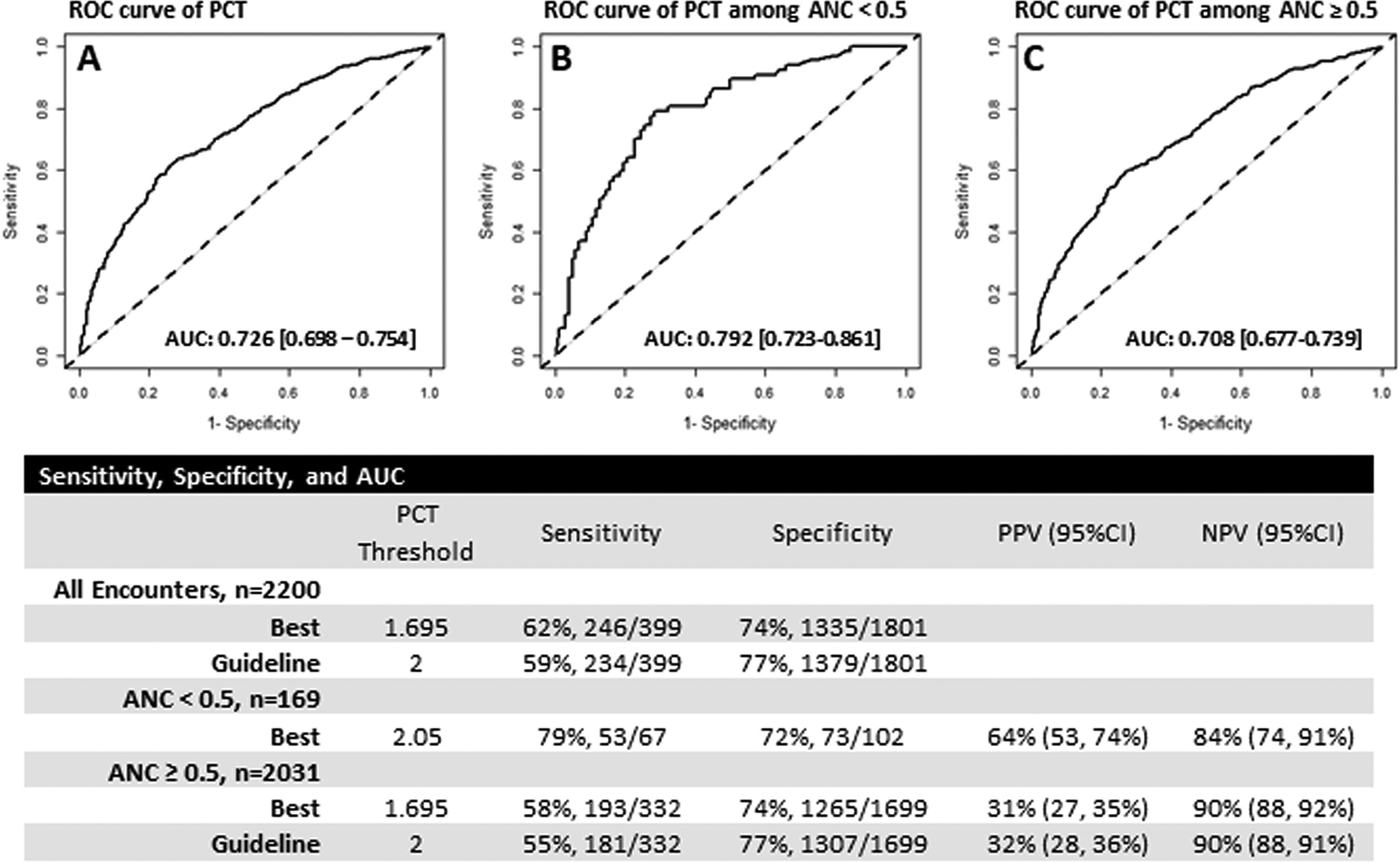
ROC curves for PCT test for BSI diagnosis in All Encounters (A), Encounters with ANC < 0.5 K/ul (B), and Encounters with ANC ≥ 0.5 K/ul (C).
3.3. PCT in the ICU subset
Of the 735 encounters in our ICU population (Table 2), 183 (24.9%) had at least one positive blood culture result. Encounters with a PCT > 2 ng/ml, had higher odds of positive blood culture than encounters with a PCT ≤ 2 ng/ml (OR: 3.1, 95%CI: 2.1–4.3). While neutropenic encounters made up only 19.7% of encounters with a positive blood culture, neutropenic encounters also had higher odds of positive culture than non-neutropenic encounters (OR 4.3, 95%CI: 2.5–7.2).
Table 2:
Demographic and Clinical Characteristics of ICU Patients
| Patient Characteristics | Patients (n=697) | |
|---|---|---|
| Male, n (%) | 364 (52%) | |
| Female, n (%) | 333 (48%) | |
| Age, median in years (range) | 64 (21,95) | |
| Encounters per patient | 1 | 565 (81%) |
| 2 | 100 (14%) | |
| 3+ | 32 (4.6%) | |
| Hospital Discharge Status, n (%) | ||
| ALIVE | 451 (65%) | |
| DEAD | 246 (35%) | |
| Hospital Length of Stay, median (range) | 17 (1,308) | |
| Encounter Characteristics | Encounters (n=735) | |
| ICU Discharge Status, n (%) | ||
| ALIVE | 564 (77%) | |
| DEAD | 171 (23%) | |
| Pre-ICU LOS, days, median (range) | 3 (1,173) | |
| ICU LOS, days, median (range) | 5 (1,72) | |
| MPM II0 score, median (range) | 41 (2,98) | |
| Malignancy Type, n (%) | ||
| Heme | 239 (33%) | |
| Solid | 496 (67%) | |
| Vasopressor Requirement on ICU admission, n patients (%) | 209 (28%) | |
| Mechanical Ventilation Requirement on admission, n patients (%) | 241 (33%) | |
| Hemodialysis Requirement, n patients (%) | 23 (3.1%) | |
| Continuous Renal Replacement Therapy Requirement, n patients (%) | 42 (5.7%) | |
Mean MPM II0 score was higher in encounters with a PCT >2.0 compared to PCT≤2.0 (Mean difference 11.8, p<0.001) (Table 3). Neutropenia was not associated with MPM II0 score. Univariate analysis demonstrated that PCT >2.0 was associated with requirement for vasopressors on admission (OR 1.76 (95%CI 1.27, 2.34)), Table 3. In a multivariable model controlling for ANC this association remained significant (OR 1.82 (95%CI 1.31, 2.53)). Neutropenia was not associated with vasopressor requirements (OR 0.86 (0.48, 1.53)). Interestingly, both initial PCT >2.0 and neutropenia had lower likelihood of ventilator requirements on admission (OR 0.71 and OR 0.48, respectively on MVA). Initial PCT concentrations >2.0 were associated with renal replacement therapy (RRT) requirements (OR 2.20 (1.24–3.91)). However, ANC was not associated with RRT requirement during an ICU encounter (OR 0.63 (0.22–1.82)). We also examined the association of PCT with death at hospital discharge. In multivariable analysis controlling for ANC, PCT>2.0 was associated with increased odds of death at hospital discharge (OR 1.38 (95% CI 1.01–1.89, p=0.046).
TABLE 3:
Analysis of PCT with Supportive Modalities and Hospital Discharge Status in the ICU Cohort
| Encounters, N | Vasopressor at ICU admission, N (%) | Univariable OR (95%CI) | p | Multivariable OR (95%CI) | p | ||
|---|---|---|---|---|---|---|---|
| PCT | ≤2.0 | 411 | 96 (23) | ||||
| >2.0 | 324 | 113 (35) | 1.76 (1.27, 2.43) | 0.001 | 1.82 (1.31, 2.53) | <.001 | |
| ANC | ≥0.5 | 669 | 192 (29) | ||||
| <0.5 | 66 | 17 (26) | 0.86 (0.48, 1.53) | 0.613 | 0.71 (0.39, 1.28) | NS | |
| Encounters, N | Ventilator at ICU admission, N (%) | Univariable OR (95%CI) | p | Multivariable OR (95%CI) | p | ||
| PCT | ≤2.0 | 411 | 151 (37) | ||||
| >2.0 | 324 | 90 (28) | 0.66 (0.48, 0.91) | 0.01 | 0.71 (0.51, 0.98) | 0.035 | |
| ANC | ≥0.5 | 669 | 229 (34) | ||||
| <0.5 | 66 | 12 (18) | 0.43 (0.22, 0.81) | 0.01 | 0.48 (0.25, 0.92) | 0.027 | |
| Encounters, N | HD or CRRT, N (%) | Univariable OR (95%CI) | p | Multivariable OR (95%CI) | p | ||
| PCT | ≤2.0 | 411 | 21 (5) | ||||
| >2.0 | 324 | 33 (10) | 2.11 (1.19, 3.72) | 0.01 | 2.2 (1.24, 3.91) | 0.007 | |
| ANC | ≥0.5 | 669 | 50 (7) | ||||
| <0.5 | 66 | 4 (6) | 0.8 (0.28, 2.29) | 0.675 | 0.63 (0.22, 1.82) | NS | |
| MPMII0 Score, Mean (SD) | Estimate (SE) | p | Estimate (SE) | p | |||
| PCT | ≤2.0 | 38.9 (23.8) | |||||
| >2.0 | 50.1 (24.7) | 11.1 (1.8) | <.001 | 11.8 (1.8) | <.001 | ||
| ANC | ≥0.5 | 44 (24.6) | |||||
| <0.5 | 42.4 (27) | −1.6 (3.2) | 0.615 | −5.5 (3.2) | NS | ||
| Patients, N | Death at hospital discharge (%) | Univariable OR (95%CI) | p | Multivariable OR (95%CI) | p | ||
| PCT | ≤2.0 | 391 | 129 (33) | ||||
| >2.0 | 306 | 123 (40) | 1.37 (1, 1.86) | 0.05 | 1.38 (1.01, 1.89) | 0.046 | |
| ANC | ≥0.5 | 636 | 230 (36) | ||||
| <0.5 | 61 | 22 (36) | 1 (0.58, 1.72) | 0.988 | 0.9 (0.52, 1.57) | NS | |
4. DISCUSSION
This study provides insight into the diagnostic accuracy of PCT for bacterial BSI in critically ill cancer patients. Similar to previous studies conducted in oncology patients [14–19], we demonstrate that BSI is associated with elevated PCT. The optimal cut-off to maximizing sensitivity and specificity in our study was 1.7 ng/ml. Overall, our data indicate that PCT is a fair predictor of BSI in critically cancer patients, including those who are neutropenic. Bele and colleagues [4] similarly found that in critically ill immunocompromised patients, PCT concentrations on Day 1 of ICU stay were significantly associated with bacterial infection (microbiologically or clinically documented) with an AUC of 0.851 (95% CI 0.782–0.919). A cutoff of 2 ng/ml had a sensitivity of 0.67 ± 0.12 and a specificity of 0.82 ± 0.08 [4]. The higher AUC and improved performance of PCT in their study may be related to the differences in population (the definition of “immunocompromised” included non-cancer patients) and inclusion criteria (neutropenia was defined at ANC <1000/uL), or the difference in primary endpoint: clinically- or microbiologically-documented infection compared to the more restrictive BSI as the endpoint in our study.
Although the association of PCT and bacteremia or sepsis is well documented [2,10], a challenge to its widespread clinical use is its low specificity. This may be explained by low sensitivity of BSI as an indicator of infection or sepsis. Additionally, PCT elevations may be explained by severe local infections, as reported in one study of patients with hematological malignancies [20].
We observed a stronger association between PCT and BSI in neutropenic patients which may be due to the higher prevalence of BSI and exaggerated systemic inflammatory response in this population. This is consistent with other published studies showing that neutropenic patients have a significantly higher rate of bacteremia, potentially resulting in sepsis [21–24]. We also found associations of initial PCT values with supportive treatment modalities in the ICU in our ICU patient cohort. Controlling for ANC status, PCT was significantly associated with the requirement for vasopressors on admission. However, PCT was not found to be associated with an increased likelihood for requirement for invasive mechanical ventilation. This contrasts with previous studies where PCT elevations were associated with need for mechanical ventilation (considered as treatment failure) in patients with pneumonia [25, 26]. Yet, in an examination of the prognostic value of PCT in respiratory tract infections across clinical settings (primary care vs emergency department vs ICU) Kutz et al. [27] found significant association between PCT and treatment failure in the emergency department setting, but not for the ICU or primary care patients. The conflicting findings suggest that the prognostic value of PCT may vary among differing patient populations or clinical settings.
PCT values were also associated with severity of illness and mortality using MPM II0 scores and death at hospital discharge. PCT >2.0 ng/ml was associated with higher mean MPM II0 scores. Furthermore, controlling for ANC, PCT >2.0 ng/ml was significantly associated with increased odds of death. The latter finding is in agreement with prior studies [4,28,29] and in a study of ICU patients with hematologic malignancy [30].
To our knowledge, our study is one of the largest studies of PCT and BSI in patients with cancer. Chaftari et al. [11] examined PCT concentrations in 985 cancer patients. While the overall focus was PCT association with cancer progression, their analysis included a comparison of febrile cancer patients with bacteremia or sepsis vs non-febrile cancer patients. They reported that PCT was a good marker for bacteremia or sepsis in febrile cancer patients with an optimal cut-off point of 0.17 ng/ml with 81% sensitivity and 69% specificity. A recent meta-analysis demonstrated the high discriminative power of PCT to differentiate bacterial infection from other causes of fever in patients with febrile neutropenia [10].
Our study has several limitations. First, our primary endpoint was BSI, which does not impute sepsis. Such laboratory results can be less subjective than clinical measurements, and this approach made it logistically possible to perform this large study. However, this approach misses “culture negative sepsis” patients; patients with viral infections, and patients with severe localized culture-proven infections (i.e., pneumonia) and sepsis but without BSI. Second, our analysis does not account for treatment at the time of (or before) the first PCT draw. Additionally, our analysis does not differentiate patients by cancer stage (others have shown that cancer stage can be associated with PCT concentration [11]). Furthermore, our dataset represents clinically ordered PCTs in the care of cancer patients in our UCC and ICU. Although we have largely standardized our clinical approach for sepsis work-up and Clinical Decision Support tools to assist in test ordering, there is an assumption that these orders were placed on patients meeting certain criteria for concern of sepsis. However, there may be encounters in our dataset that did not meet these criteria (PCT ordered outside of the concern for sepsis).
In conclusion, procalcitonin is predictive of bacterial BSI in critically ill cancer patients with and without neutropenia but should not replace clinical decision making regarding the initiation of antibiotic therapy. Prognostically, PCT is associated with requirement for vasopressors and renal replacement therapy, but not for mechanical ventilation. Given the lack of a consensus cut-off and the differing primary endpoint used in previous studies, a re-evaluation of the cut-offs for PCT values is warranted and may require population-specific decision levels.
Supplementary Material
HIGHLIGHTS:
Procalcitonin is predictive of bloodstream infections in critically ill cancer patients
PCT >2.0 ng/mL was more likely to be associated with BSI particularly for neutropenic (OR 9.09, 95%CI: 4.39, 18.79) compared with non-neutropenic patients (OR 4.00 (95% CI: 3.13, 5.10), interaction p=0.036).
Prognostically, PCT was associated with requirements for vasopressors but not mechanical ventilation
Although PCT predicts BSI, it should not replace clinical decision making.
ACKNOWLEDGEMENTS
The authors acknowledge Natalie Kostelecky, RN and Terrence Newton for their assistance in the data collection for this study. This work was supported, in part, by the Core Grant and the Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB, Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes, Chest 150(6) (2016) 1251–1259. [DOI] [PubMed] [Google Scholar]
- [2].Wacker C, Prkno A, Brunkhorst FM, Schlattmann P, Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis, Lancet Infect Dis 13(5) (2013) 426–35. [DOI] [PubMed] [Google Scholar]
- [3].Russwurm S, Oberhoffer M, Zipfel PF, Reinhart K, Procalcitonin--a novel biochemical marker for the mediator-directed therapy of sepsis, Mol Med Today 5(7) (1999) 286–7. [DOI] [PubMed] [Google Scholar]
- [4].Bele N, Darmon M, Coquet I, Feugeas JP, Legriel S, Adaoui N, Schlemmer B, Azoulay E, Diagnostic accuracy of procalcitonin in critically ill immunocompromised patients, BMC Infect Dis 11 (2011) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim DY, Lee YS, Ahn S, Chun YH, Lim KS, The usefulness of procalcitonin and C-reactive protein as early diagnostic markers of bacteremia in cancer patients with febrile neutropenia, Cancer Res Treat 43(3) (2011) 176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sakr Y, Sponholz C, Tuche F, Brunkhorst F, Reinhart K, The role of procalcitonin in febrile neutropenic patients: review of the literature, Infection 36(5) (2008) 396–407. [DOI] [PubMed] [Google Scholar]
- [7].de Bont ES, Vellenga E, Swaanenburg J, Kamps W, Procalcitonin: a diagnostic marker of bacterial infection in neutropenic cancer patients with fever?, Infection 28(6) (2000) 398–400. [DOI] [PubMed] [Google Scholar]
- [8].Koivula I, Juutilainen A, Procalcitonin is a useful marker of infection in neutropenia, Leuk Res 35(10) (2011) 1288–9. [DOI] [PubMed] [Google Scholar]
- [9].Robinson JO, Lamoth F, Bally F, Knaup M, Calandra T, Marchetti O, Monitoring procalcitonin in febrile neutropenia: what is its utility for initial diagnosis of infection and reassessment in persistent fever?, PLoS One 6(4) (2011) e18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu CW, Wu JY, Chen CK, Huang SL, Hsu SC, Lee MT, Chang SS, Lee CC, Does procalcitonin C -reactive protein, or interleukin-6 test have a role in the diagnosis of severe infection in patients with febrile neutropenia? A systematic review and meta-analysis, Support Care Cancer 23(10) (2015) 2863–72. [DOI] [PubMed] [Google Scholar]
- [11].Chaftari AM, Hachem R, Reitzel R, Jordan M, Jiang Y, Yousif A, Garoge K, Deshmukh P, Al Hamal Z, Jabbour J, Hanania A, Raad S, Jamal M, Raad I, Role of Procalcitonin and Interleukin-6 in Predicting Cancer, and Its Progression Independent of Infection, PLoS One 10(7) (2015) e0130999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Debiane L, Hachem RY, Al Wohoush I, Shomali W, Bahu RR, Jiang Y, Chaftari AM, Jabbour J, Al Shuaibi M, Hanania A, Pravinkumar SE, Schuetz P, Raad I, The utility of proadrenomedullin and procalcitonin in comparison to C-reactive protein as predictors of sepsis and bloodstream infections in critically ill patients with cancer*, Crit Care Med 42(12) (2014) 2500–7. [DOI] [PubMed] [Google Scholar]
- [13].DeLong ER, DeLong DM, Clarke-Pearson DL, Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach, Biometrics 44(3) (1988) 837–45. [PubMed] [Google Scholar]
- [14].Shomali W, Hachem R, Chaftari AM, Jiang Y, Bahu R, Jabbour J, Raad S, Al Shuaibi M, Al Wohoush I, Raad I, Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients?, Cancer 118(23) (2012) 5823–9. [DOI] [PubMed] [Google Scholar]
- [15].Sandri MT, Passerini R, Leon ME, Peccatori FA, Zorzino L, Salvatici M, Riggio D, Cassatella C, Cinieri S, Martinelli G, Procalcitonin as a useful marker of infection in hemato-oncological patients with fever, Anticancer Res 28(5B) (2008) 3061–5. [PubMed] [Google Scholar]
- [16].Wang XJ, Tan TT, Lim ST, Farid M, Tao M, Quek R, Chan A, Tang T, Role of Procalcitonin in Differentiating between Infectious and Noninfectious Fevers among Patients with Lymphoma, Pharmacotherapy 37(8) (2017) 908–915. [DOI] [PubMed] [Google Scholar]
- [17].Meidani M, Khorvash F, Abolghasemi H, Jamali B, Procalcitonin and quantitative C-reactive protein role in the early diagnosis of sepsis in patients with febrile neutropenia, South Asian J Cancer 2(4) (2013) 216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Juutilainen A, Hamalainen S, Pulkki K, Kuittinen T, Nousiainen T, Jantunen E, Koivula I, Biomarkers for bacteremia and severe sepsis in hematological patients with neutropenic fever: multivariate logistic regression analysis and factor analysis, Leuk Lymphoma 52(12) (2011) 2349–55. [DOI] [PubMed] [Google Scholar]
- [19].Koivula I, Hamalainen S, Jantunen E, Pulkki K, Kuittinen T, Nousiainen T, Juutilainen A, Elevated procalcitonin predicts Gram-negative sepsis in haematological patients with febrile neutropenia, Scand J Infect Dis 43(6–7) (2011) 471–8. [DOI] [PubMed] [Google Scholar]
- [20].Ebihara Y, Kobayashi K, Ishida A, Maeda T, Takahashi N, Taji Y, Asou N, Ikebuchi K, Diagnostic performance of procalcitonin, presepsin, and C-reactive protein in patients with hematological malignancies, J Clin Lab Anal 31(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gustinetti G, Mikulska M, Bloodstream infections in neutropenic cancer patients: A practical update, Virulence 7(3) (2016) 280–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Patel A, Gruber P, Severe infections in neutropenic patients, Current opinion in critical care 21(6) (2015) 586–92. [DOI] [PubMed] [Google Scholar]
- [23].Rolston KV, Management of infections in the neutropenic patient, Annual review of medicine 55 (2004) 519–26. [DOI] [PubMed] [Google Scholar]
- [24].Bodey GP, Buckley M, Sathe YS, Freireich EJ, Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia, Ann Intern Med 64(2) (1966) 328–40. [DOI] [PubMed] [Google Scholar]
- [25].Nobre V, Borges I, Prognostic value of procalcitonin in hospitalized patients with lower respiratory tract infections, Revista Brasileira de terapia intensiva 28(2) (2016) 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morley D, Torres A, Cilloniz C, Martin-Loeches I, Predictors of treatment failure and clinical stability in patients with community acquired pneumonia, Annals of translational medicine 5(22) (2017) 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kutz A, Briel M, Christ-Crain M, Stolz D, Bouadma L, Wolff M, Kristoffersen KB, Wei L, Burkhardt O, Welte T, Schroeder S, Nobre V, Tamm M, Bhatnagar N, Bucher HC, Luyt CE, Chastre J, Tubach F, Mueller B, Schuetz P, Prognostic value of procalcitonin in respiratory tract infections across clinical settings, Critical care (London, England) 19 (2015) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kelly BJ, Lautenbach E, Nachamkin I, Coffin SE, Gerber JS, Fuchs BD, Garrigan C, Han X, Bilker WB, Wise J, Tolomeo P, Han JH, Centers for Disease C, Prevention Prevention Epicenters P, Combined Biomarkers Predict Acute Mortality Among Critically Ill Patients With Suspected Sepsis, Crit Care Med 46(7) (2018) 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Murat Sedef A, Kose F, Taner Sumbul A, Dogan O, Kursun E, Yurdakul Z, Sumbul Gultepe B, Mertsoylu H, Sezer A, Ozyilkan O, Prognostic value of procalcitonin in infection-related mortality of cancer patients, Journal of B.U.ON. : official journal of the Balkan Union of Oncology 21(3) (2016) 740–4. [PubMed] [Google Scholar]
- [30].Ferra C, Lacoma A, Garcia O, Marcos P, Dominguez J, Ribera JM, [Relationship between procalcitonin serum levels and complications and outcome of patients with hematological malignancy admitted to Intensive Care Unit], Med Clin (Barc) 138(9) (2012) 385–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


