Abstract
Pulmonary hypertension (PH) is a complex condition that arises due to pulmonary vascular disease, heart disease, lung disease, chronic thromboembolism, or several rare causes. Regardless of underlying cause, PH increases mortality, yet there are no directed treatments for the most common forms of PH due to left heart or lung disease. Because metabolic factors have been implicated in the pathogenesis of PH, we used a large administrative cohort to assess diabetes and weight, potentially modifiable risk factors, on PH outcome. We analyzed 110,495 veterans diagnosed with PH from 01/01/2003 to 09/30/2015 in the Veterans Health Affairs system. Veterans with PH survived an average of 3.88 [IQR 3.85, 3.92] years after PH diagnosis. Diabetes occurred in 36% and increased risk of death by 31% (95% CI 28–33%, multivariate adjusted). Higher body mass index (BMI) was associated with lower mortality in a J-shaped pattern with highest risk in underweight and normal weight veterans. Improved survival in obesity has been referred to as the obesity paradox in heart failure and other diseases. These data show that lower weight and diabetes are strong risk factors for mortality in PH. Our results underscore the importance of systemic conditions on outcome in PH.
Keywords: pulmonary hypertension, metabolic syndrome, body mass index, retrospective cohort study, survival, risk assessment
Pulmonary hypertension (PH) is common in the U.S., affecting more than 5 million adults and has increasing prevalence among older individuals.1 Common forms of PH include those due to underlying left heart disease (Group 2) and lung diseases (Group 3), while Group 1 pulmonary arterial hypertension (PAH) is rare.1 It is increasingly recognized that patients with Group 2 and 3 PH have poorer survival than patients with PAH and do not benefit from pulmonary vasodilator medications approved for PAH.2,3,4 However, ongoing efforts to more specifically define and therapeutically target underlying pathogenic mechanisms in PH may improve outcomes in the common forms of PH as well as PAH. Pre-clinical studies have demonstrated an important role for altered glucose and lipid metabolism in pulmonary vascular regulation, hypoxia-mediated PH, and PAH.5–7 Diabetes mellitus (DM) is enriched in cohorts of PAH and is a major risk factor for the development of left heart disease, the most common cause of PH.8–10 Our group has previously noted that U.S. veterans with PH have a high prevalence of DM.2 Acknowledging these epidemiologic and pre-clinical findings, we investigated the relationship between potentially modifiable metabolic factors including DM and weight on patient outcome using the largest reported patient-level cohort with a case mix of PH etiologies.
Methods
A retrospective cohort study was designed to assess the effects of weight and DM on risk of death in veterans diagnosed with PH. The Emory University Institutional Review Board and the Atlanta VA Medical Center Research & Development Committee approved the study with waiver of informed consent. The design and description of the cohort used in the current study has been previously reported.2 Briefly, using national Veterans Health Affairs (VHA) data, veterans were included if they newly received an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for PH (416.0, 416.2, 416.8, 416.9) between 01/01/2003 and 09/30/2015. Because diagnosis codes may be used to “rule out” a disorder, veterans were excluded if the diagnosis of PH did not occur during: (i) ≥1 inpatient visit, or (ii) ≥2 outpatient visits spanning ≥30 days. Subjects were also excluded if they had no follow up time available after the date of PH diagnosis. The initial diagnosis of PH was considered the baseline date for collection of other covariates and for outcome analyses. All data were obtained from VHA data sources.
Exposures and covariates were selected based on presumed relevance to development, progression, and/or outcome of PH as well as to control for confounding in analyses of the main exposures of interest (DM and weight). Presence of DM was defined using a validated method which required (i) ≥1 use of ICD-9 diagnosis code 250.xx in conjunction with a face to face outpatient primary care provider visit; or (ii) ≥2 outpatient uses of code 250.xx; and (iii) use of a medication to treat DM.11 Baseline weight was defined as the median of values obtained 6 months before and after the date of PH diagnosis. Weights were also collected longitudinally for each subject, through the extent of data available. Because height varies less over time than weight, height was defined as the median height value from 2 years before and after the diagnosis of PH. Body mass index (BMI) was calculated using this baseline height.
Covariates included demographics, comorbid conditions, medication use, measures of PH disease severity, hospitalizations and death. Comorbidities (other than DM; see above) were defined using ICD-9 diagnosis codes used in the Elixhauser comorbidity index12 and considered present if they existed prior to a date 6 months following the PH diagnosis date. The subtype of PH was classified for each veteran based on comorbid diagnoses using a modification of the 2015 clinical classification of PH as we previously reported2,13. Because some patients have multimorbidity and their underlying conditions may predispose to PH of differing types (e.g. both systolic heart failure and chronic obstructive lung disease), such cases were classified as “Multiple causes of PH”. Patients were only considered to have PAH if they did not have comorbid conditions expected to predispose to other forms of PH.
The outcome for this study was time from the diagnosis of PH to death from any cause. VHA death data have been validated against the National Death Index with a sensitivity of 98.3% and specificity of 99.8%.14 Vital status was collected through one additional year following the inclusion period (through 09/30/2016). Veterans were censored at their last VHA encounter (loss to follow up), or if they were alive after 09/30/2016 (administratively censored).
Statistical analyses were performed using SAS (Enterprise Guide v7.1, SAS Institute, Cary, NC) and R (R Core Team, 2019). Continuous variables are reported as means with standard deviation (SD) or median with interquartile range [IQR]. Categorical variables are reported as the number and proportion. For variables used in analyses, missing data were infrequent and are given in tables. Univariate and multivariable Cox proportional hazards regression analysis were used and resulting hazard ratios for death with 95% confidence intervals (HR, 95% CI) are reported with two-sided P values. Complete case analysis was used in models assessing effect of weight and BMI and a missing category was used for race.
Several modeling approaches were used to assess the impact of weight and BMI on survival. To assess the linear nature of these variables, multivariable models with weight or BMI as restricted cubic spline terms were developed. Separately, to investigate more fully the association between weight/BMI and survival and whether there was a time-dependent nature of that association, sensitivity analyses were performed. In addition to unadjusted analyses, we report: Model 1) adjusted for age, race, gender, PH type, and DM; Model 2) adjusted for pre-PH weight trend in addition to covariates in Model 1; Model 3) adjusted for Elixhauser comorbidities in addition to covariates in Model 1; and Model 4) multivariable model of weight/BMI as a time-varying covariate and adjusted for age, gender, race, PH type, DM and pre-PH weight trend. For Model 4, the cohort was restricted to veterans with consistent VHA healthcare exposure in the 5 years prior to PH diagnosis and those with missing longitudinal weight data were excluded.
Results
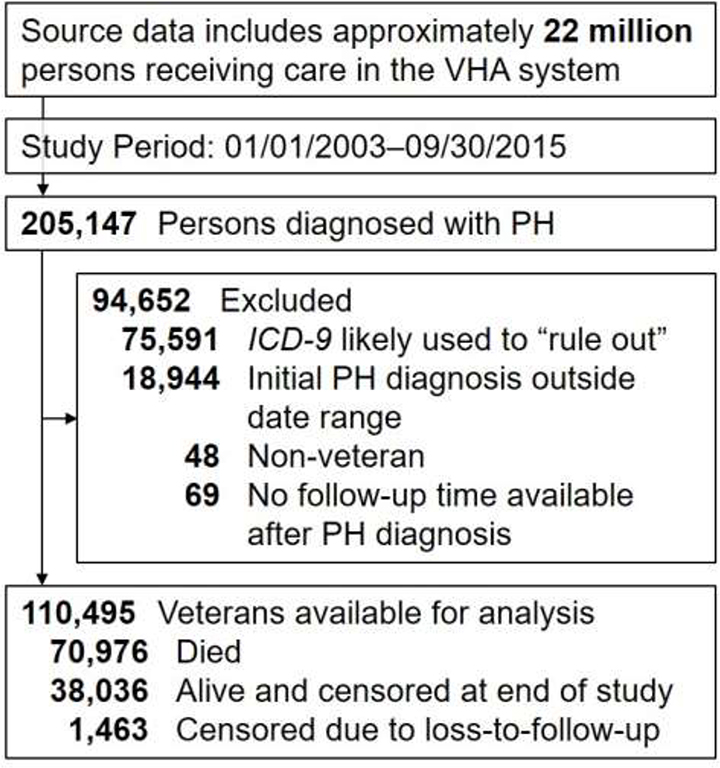
A data query of VHA-CDW identified 205,147 veterans with a diagnosis of PH between 01/01/2003 and 09/30/2015. After exclusions, 110,495 veterans were available for all analyses (Figure 1). The subjects were majority male with median age 70.2 years and frequently had comorbid conditions (Table 1). Left heart disease and lung disease were each highly prevalent in this cohort. Veterans in the cohort had a median of 8.0 [IQR 5.0, 11.1] years of healthcare data available prior to their diagnosis of PH. The median length of follow-up after PH diagnosis was 2.9 [IQR 1.2, 5.5] years. Within the cohort, 71,045 (64.3%) died with median survival of 3.88 [IQR 3.85, 3.92] years; 38,036 were alive at the end of the study period and 1,463 were lost to follow up.
Figure 1. Cohort flow diagram.
Flow diagram for cohort development. Cohort generation query was performed 09/08/2017. Note that if a patient had the diagnosis code used as an outpatient only and for a duration < 30 days, the patient was excluded on suspicion that the diagnosis code was used to evaluate for or “rule out” the condition. CDW, corporate data warehouse; ICD-9, International Classification of Diseases 9th version; PH, pulmonary hypertension; VHA, Veterans Health Affairs.
Table 1:
Baseline characteristics of veterans diagnosed with pulmonary hypertension.
| Variable | Total Cohort (n=110,495) | Diabetes Absent (n=70,455; 63.8%) | Diabetes Present (n=40,040; 36.2%) |
|---|---|---|---|
| Age at PH diagnosis (years) | 70.2 [62.1, 79.6] | 71.2 [61.9, 80.7] | 68.9 [62.4, 77.5] |
| Age categories (years) | |||
| <60 | 20,900 (18.9%) | 14,085 (20.0%) | 6,815 (17.0%) |
| 60–70 | 33,825 (30.6%) | 19,259 (27.3%) | 14,566 (36.4%) |
| 70–80 | 29,414 (26.6%) | 17,931 (25.5%) | 11,483 (28.7%) |
| >80 | 26,356 (23.9%) | 19,180 (27.2%) | 7,176 (17.9%) |
| Male | 106,562 (96.4%) | 67,556 (95.9%) | 39,006 (97.4%) |
| Race | |||
| White | 82,236 (74.4%) | 52,435 (74.4%) | 29,801 (74.4%) |
| Black | 18,558 (16.8%) | 11,397 (16.2%) | 7,161 (17.9%) |
| Other | 1,866 (1.7%) | 1,111 (1.6%) | 755 (1.9%) |
| Missing | 7,835 (7.1%) | 5,512 (7.8%) | 2,323 (5.8%) |
| Presumed PH group | |||
| PAH | 8,835 (8.0%) | 6596 (9.4%) | 2,239 (5.6%) |
| PH, left heart disease | 17,813 (16.1%) | 10,735 (15.2%) | 7,078 (17.7%) |
| PH, lung disease | 18,375 (16.6%) | 14,189 (20.1%) | 4,186 (10.5%) |
| CTEPH | 1,561 (1.4%) | 1,288 (1.8%) | 273 (0.7%) |
| PH, misc causes | 309 (0.3%) | 218 (0.3%) | 91 (0.2%) |
| Multiple causes of PH | 63,602 (57.6%) | 37,429 (53.1%) | 26,173 (65.4%) |
| Any heart disease | 78,135 (70.7%) | 45,653 (64.8%) | 32,482 (81.1%) |
| Any lung disease | 80,112 (72.5%) | 50,407 (71.5%) | 29,705 (74.2%) |
| Weight (kg) | 89.5 [75.3, 108] | 84.5 [71.7, 101] | 99.7 [83.8, 119] |
| Missing | 494 (0.4%) | 385 (0.5%) | 109 (0.3%) |
| BMI (kg/m2) | 28.9 [24.7, 34.5] | 27.4 [23.7, 32.2] | 32.0 [27.3, 37.8] |
| Missing | 3,265 (3.0%) | 2,405 (3.4%) | 860 (2.1%) |
| BMI category (kg/m2) | |||
| < 18.5 | 2,570 (2.3%) | 2,376 (3.4%) | 194 (0.5%) |
| 18.5–24.9 | 25,930 (23.5%) | 20,721 (29.4%) | 5,209 (13.0%) |
| 25.0–29.9 | 31,331 (28.4%) | 21,314 (30.3%) | 10,017 (25.0%) |
| ≥ 30 | 47,399 (42.9%) | 23,639 (33.6%) | 23,760 (59.3%) |
| Missing | 3,265 (3.0%) | 2,405 (3.4%) | 860 (2.1%) |
| Weight change in 3 years prior to PH | |||
| >5% loss | 22,598 (20.5%) | 13,797 (19.6%) | 8,801 (22.0%) |
| 1–5% loss | 19,250 (17.4%) | 11,687 (16.6%) | 7,563 (18.9%) |
| <1% change | 10,307 (9.3%) | 6,164 (8.7%) | 4,143 (10.3%) |
| 1–5% gain | 15,782 (14.3%) | 9,054 (12.9%) | 6,728 (16.8%) |
| >5% gain | 15,779 (14.3%) | 8,640 (12.3%) | 7,139 (17.8%) |
| Missing | 26,779 (24.2%) | 21,113 (30.0%) | 5,666 (14.2%) |
| Weight change in 3 years prior to PH (%) | −1.0 [−5.4, 3.5] | −1.2 [−5.6, 3.1] | −0.6 [−5.2, 4.0] |
| Missing | 26,779 (24.2%) | 21,113 (30.0%) | 5,666 (14.2%) |
| BNP (pg/mL) | 368 [127, 886] | 369 [120, 911] | 368 [137, 860] |
| Missing | 61,983 (56.1%) | 41,671 (59.1%) | 20,312 (50.7%) |
| Creatinine (mg/dL) | 1.20 [0.92, 1.50] | 1.10 [0.90, 1.40] | 1.30 [1.00, 1.77] |
| Missing | 5,627 (5.1%) | 4,012 (5.7%) | 1,615 (4.0%) |
| Sodium (mEq/L) | 139 [136, 141] | 139 [137, 141] | 139 [136, 141] |
| Missing | 4,762 (4.3%) | 3,590 (5.1%) | 1,172 (2.9%) |
| Hemoglobin A1c (%) | |||
| Mean (SD) | 6.73 (1.37) | 5.98 (0.844) | 7.34 (1.41) |
| Median [IQR] | 6.40 [5.80, 7.35] | 5.90 [5.50, 6.30] | 7.10 [6.36, 8.03] |
| Missing | 42,633 (38.6%) | 40,168 (57.0%) | 2,465 (6.2%) |
| Died | 70,976 (64.2%) | 44,393 (63.0%) | 26,583 (66.4%) |
BMI: body mass index; BNP: brain natriuretic peptide; CTEPH: chronic thromboembolic pulmonary hypertension; PH: pulmonary hypertension; PAH: pulmonary arterial hypertension. Notes: 1. Comorbid diagnoses listed are those present prior to or within 6 months after PH diagnosis. Laboratory, weight and BMI data are those values present within 6 months before to 6 months after PH diagnosis and if multiple values were available for a given patient, the median was taken. Values given are the median [25th, 75th percentile] unless specifically noted. Comparison between those with and without diabetes was performed using t-tests and Chi-square tests for continuous and categorical data, respectively. Comparing the groups with and without diabetes, all variables were statistically different (P<0.001) except for BNP (P=0.25).
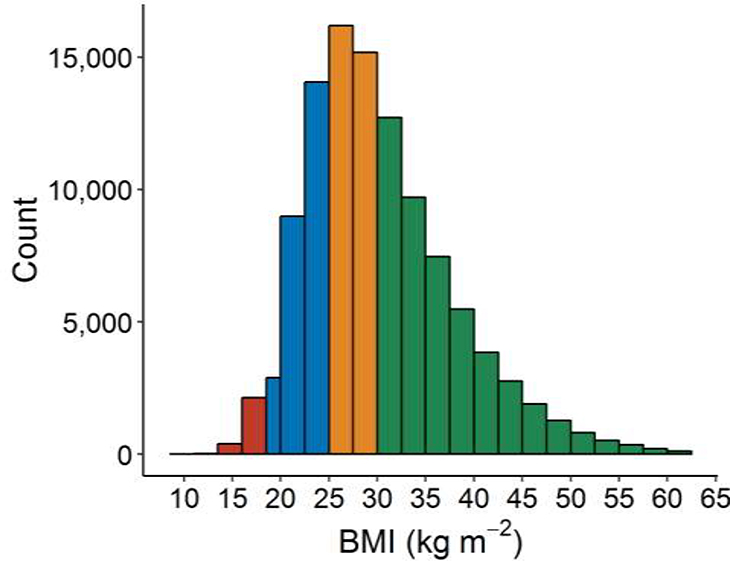
At the time of PH diagnosis, 36.2% of veterans had DM. Prevalence of DM varied by PH subtype: 25% in presumed PAH, 40% in PH due to left heart disease, 23% in PH due to lung disease, and 41% in PH due to multiple causes. BMI at the time of PH diagnosis was available in 97.0% of veterans; 73% were either overweight or obese (Figure 2). Veterans with DM had baseline characteristics dis-similar to those without DM. In general, they were more likely to be younger, male, have higher BMI, and have left heart disease (Table 1; all P<0.0001 compared to veterans without DM). Compared to those not meeting criteria for DM, DM was positively associated with death with unadjusted HR 1.14 (95% CI 1.13–1.16; Table 2, Supplemental Figure 1). When adjusted for age, gender, race, type of PH, and baseline BMI, the presence of DM was associated with death more strongly (HRadj 1.31, 95% CI 1.28–1.33).
Figure 2: Distribution of body mass index in veterans with pulmonary hypertension.
BMI was assessed at baseline, defined as ±6 months from the first diagnosis of PH. BMI values were missing in 3,265 (3.0%) of subjects. Median BMI was 28.9 [24.7, 34.5]. Note the histogram bar at 18.5–20 kg m−2 is a different width than all others to be consistent with accepted BMI category cutoffs.
Table 2:
Association of clinical variables with risk of death in veterans with pulmonary hypertension
| Variable | HR (univariable) | HR (multivariable) |
|---|---|---|
| Age (per decade) | 1.46 (1.45–1.47, p<0.001) | 1.39 (1.38–1.40, p<0.001) |
| Race | ||
| White | Ref | Ref |
| Black | 0.91 (0.89–0.93, p<0.001) | 0.97 (0.95–0.99, p=0.005) |
| Other | 0.86 (0.81–0.91, p<0.001) | 0.94 (0.88–1.00, p=0.043) |
| Unknown | 1.51 (1.47–1.55, p<0.001) | 1.44 (1.40–1.48, p<0.001) |
| Male (vs Female) | 1.73 (1.65–1.82, p<0.001) | 1.37 (1.31–1.44, p<0.001) |
| PH Group | ||
| PAH | Ref | Ref |
| PH, left heart disease | 2.02 (1.95–2.09, p<0.001) | 1.90 (1.83–1.97, p<0.001) |
| PH, lung disease | 1.53 (1.47–1.58, p<0.001) | 1.57 (1.51–1.63, p<0.001) |
| CTEPH | 0.75 (0.68–0.83, p<0.001) | 0.96 (0.87–1.07, p=0.490) |
| PH, misc causes | 0.91 (0.76–1.08, p=0.278) | 1.03 (0.87–1.23, p=0.708) |
| Multiple causes of PH | 2.49 (2.41–2.57, p<0.001) | 2.54 (2.46–2.63, p<0.001) |
| Diabetes (vs no diabetes) | 1.14 (1.13–1.16, p<0.001) | 1.31 (1.28–1.33, p<0.001) |
| BMI category | ||
| Underweight | 1.54 (1.47–1.61, p<0.001) | 1.73 (1.66–1.81, p<0.001) |
| Normal | Ref | Ref |
| Overweight | 0.71 (0.70–0.72, p<0.001) | 0.71 (0.70–0.72, p<0.001) |
| Obese | 0.52 (0.51–0.53, p<0.001) | 0.56 (0.55–0.57, p<0.001) |
BMI: body mass index; CTEPH: chronic thromboembolic pulmonary hypertension; PH: pulmonary hypertension; PAH: pulmonary arterial hypertension.
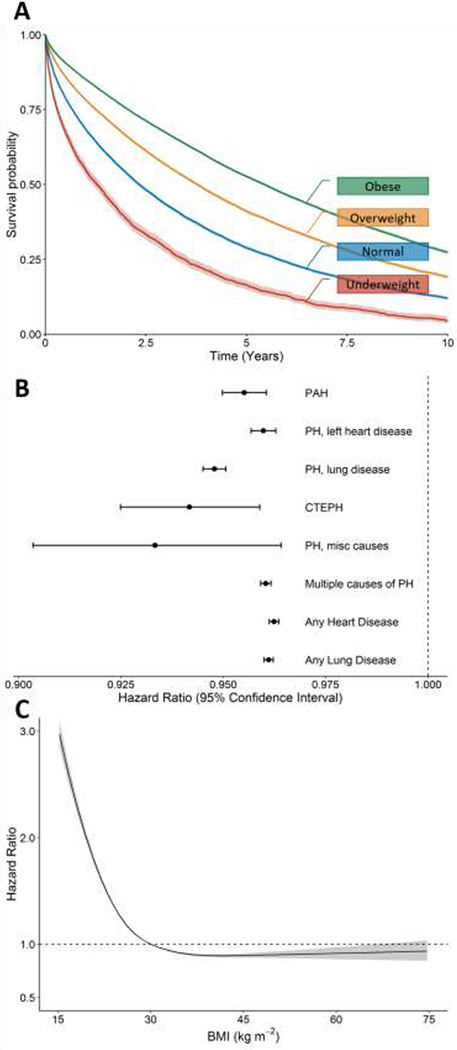
Overweight or obesity, despite being established risk factors for DM, were associated with improved outcome (Table 2 and Figure 3a). Compared to normal weight, veterans who were overweight or obese at PH diagnosis were at 29% and 48% lower risk of death, respectively; underweight veterans with PH had 54% increased risk of death. Multivariable adjusted analyses yielded similar results with slightly higher effect measures. Models showed a statistically significant interaction between age and BMI, with the effect of BMI being more pronounced as age increased. However, the magnitude of hazard ratios were comparable in the interaction and the no-interaction models and support the same conclusion that higher BMI is associated with improved outcome (data not shown). The association of improved outcome with higher BMI was present in veterans with all presumed PH types (Figure 3b). In subgroup analyses, the effect of BMI was noted to be no different in a cohort under age 70, and in those with and without lung disease.
Figure 3: Effect of BMI on survival in veterans diagnosed with pulmonary hypertension.
A) Kaplan-Meier survival probability by BMI category. Higher BMI classes were associated with improved survival (log-rank test P < 0.0001). B) BMI is associated with lower risk of death (hazard ratio) in all types of pulmonary hypertension and irrespective of the presence of underlying heart or lung disease. C) The association of BMI with survival is non-linear across the BMI range with sharply higher risk of death seen at BMI values in the normal to underweight range.
The effect of BMI was found to be non-linear. Model fit was improved with incorporating BMI as a restricted cubic spline. With that approach, the relationship between BMI and risk, adjusted for confounders, was shown to be more complex with an increased effect of BMI in the normal and underweight ranges (Figure 3c). The same pattern was noted when analyses were restricted by PH type or DM status (Supplemental Figure 2).
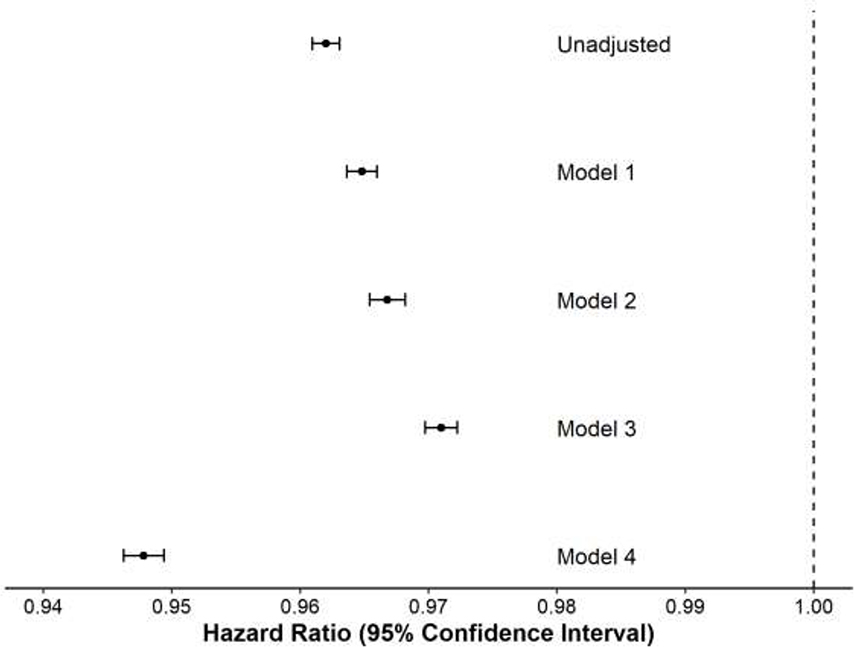
Because this protective effect of obesity, described elsewhere as the “obesity paradox” could be explained by index diagnosis bias15, additional sensitivity analyses were performed. For these analyses, the dataset was restricted to subjects with robust follow-up within the VHA system who had weight data recorded in at least half of ten sequential 6-month intervals prior to the diagnosis of PH (n=83,769, 75.8% of original cohort). Each veteran’s weight trend in the 3 years prior to PH diagnosis was calculated. DM and other comorbid conditions known to be independently associated with mortality12 were included in survival models as specified. These adjusted analyses suggested that the protective effect of increased BMI was partially but minimally reduced by adjustment for the presence of those factors (Figure 4, hazard for weight in unadjusted analysis and multivariable Models 1–3).
Figure 4: Sensitivity analysis of the effect of BMI on survival in veterans diagnosed with pulmonary hypertension.
The effect of BMI (hazard ratio, HR, and 95% confidence intervals for a 1-unit change in BMI) on death using various Cox modeling approaches is shown. In the unadjusted model, the effect is estimated for BMI (as a continuous variable) on death. Model 1 is adjusted for age, race, gender, PH type and presence of DM. Model 2 is the same as Model 1 except also includes the weight trend in the 3 years prior to PH diagnosis as a covariate. Model 3 is the same as Model 1 except includes the presence/absence of all Elixhauser comorbidities as covariates. Model 4 incorporates BMI as a time-varying covariate and otherwise use covariates from Model 2.
The protective effect of higher weight was consistently seen in analyses designed to adjust for potential bias. Weight was measured at most clinical encounters enabling longitudinal weight data to be incorporated into a multivariable model as a time-varying covariate. In that model, the association of BMI with death was more pronounced than when a single value of BMI from baseline was used, (Figure 4, Model 4). This indicates that weight is an important marker of mortality risk, both when measured at one point in time and longitudinally.
Discussion
This study reports that in veterans with PH, DM and obesity have opposing effects on risk of death. Compared to PH patients without DM, those with DM had 28–33% higher risk of death, accounting for comorbidities, age, cause of PH, and BMI. Being overweight or obese was associated with improved survival compared to normal or underweight. These findings are of significant relevance to VHA and likely other patient populations with multimorbidity and advanced age for several reasons. PH and the comorbidities investigated were common – the cohort included 0.5% of the source veteran population and both DM and obesity were prevalent. Most patients had PH due to left heart disease, lung disease or both, subgroups of PH for which no direct treatment options currently exist and known to have high risk of death.2,3
The overall prevalence of DM in this PH cohort was 36.2%, higher than that reported in the U.S. population (9.3%)16, in the VHA system (25%)17, and in a large U.S.-based, prospectively-enrolled PAH registry (12%)18. The reported prevalence of DM in PAH patients at academic medical centers across the U.S. has varied from 17–26%9,19–21 and studies from those centers demonstrate an association between DM and PAH severity, morbidity and/or mortality.18–22 Complementary to those earlier reports, our analysis reaffirms that DM is strongly associated with risk of death in PH and extends that risk to other more common PH subtypes.
While our findings that DM increases risk of death in PH was expected, the protective effect of obesity was not anticipated. Previous reports from small cohorts with PAH23,24 or PH related to lung or heart disease25 suggested that obesity might be protective. The current analysis demonstrates that higher weight is associated with improved outcome in a much broader population with all types of PH. We believe that the preponderance of data from our analyses and those of other authors supports a protective effect of increased weight in PH.
The current results must be interpreted with caution and do not: a) confirm a causal relationship between weight and risk of death in PH, b) provide evidence that weight affects the risk of PH (since only PH subjects were included), or c) suggest that weight gain is healthy or leads to disease improvement in PH. Although our study cannot address underlying biological explanations for the association between increased weight and reduced PH mortality, it does invite opportunities for hypothesis generation. Here we will pose four possible mechanisms. (1) Our data show that low BMI is strongly associated with mortality. While a case of intentional weight loss in PAH was shown beneficial26, weight loss may often accompany declining health and lead to poor tolerance of chronic disease. Lower BMI is associated with less energy stored in fat and lean mass25 which may worsen known maladaptive increases in glycolytic metabolism in the failing heart.27 Supporting the plausibility of this mechanism, BMI was noted to have a stronger association with outcome when incorporated as a time-varying covariate, indicating that low BMI at any time during the disease course is a strong indicator of mortality risk. (2) Adipose tissue can exert beneficial endocrine and physiologic effects. Potential mechanisms are complex and beyond the scope of this report, but we note that adipokines including apelin promote hemodynamic features plausibly beneficial in PH.28 (3) Subjects with obesity and heart failure are known to have higher cardiac output and systemic blood pressure, which may allow more liberal use of cardio-beneficial medications such as beta-blockers or inhibitors of the renin-angiotensin-aldosterone system.25 (4) Adipose tissue also exerts paracrine effects in the perivascular space, primarily reducing vasoconstriction, inflammation and proliferation in systemic vessels. These beneficial features are lost in obesity with DM due to a pro-inflammatory adipocyte phenotype.29 The complex previously reported interdependent and sometimes opposing influences of adipose tissue and DM could explain the observed opposing effects of these factors on mortality in our study although their relevance in the pulmonary vasculature is unknown. Supplemental Figure 3 demonstrates predicted survival probabilities for various BMI levels and with and without DM, showing that DM has a larger impact than differences in weight at higher BMI ranges, but not at lower BMI ranges. Collectively, the current report and existing literature on the pathobiology of metabolic changes in PH reinforce the importance of recognition and optimal management of metabolic comorbidities in PH as their management may have significant impact on outcome.
The current study has several limitations. By design, our study used ICD-9 codes to identify veterans with PH, comorbidities, and the presumed cause of PH. While this strategy could lead to misclassification of PH, the makeup and outcome by PH type of our cohort was similar to other studies.3 The observational healthcare dataset used did not allow us to account for some factors known to be important in PH risk and outcome such as NYHA/WHO Functional Classification.4 Further, the data source was able to robustly determine longitudinal weight, but we recognize that specific obesity phenotypes, such as predominantly visceral versus subcutaneous fat, may provide additional insight. Because our study included only veterans receiving care in the VHA our findings may not be generalizable to other populations. We have attempted to address bias such as index diagnosis bias15 by including subjects with consistent long-term follow-up in the VHA system and including numerous biological confounders in various multivariable models. While possible residual confounding cannot be eliminated, the magnitude of the effect of weight on mortality would be unlikely to be explained on this basis.
Our study demonstrates that, in veterans with PH, DM increases risk of death by 28–33% while overweight and obese veterans are protected compared to those underweight or of normal weight. These findings, observed irrespective of underlying type of PH, have important implications for clinicians caring for PH. PH due to left heart or lung diseases are common and have poor survival not effectively treated with current PAH therapies. Therefore, treatment or management directed at other factors, such as DM or weight, may have magnified importance. Future study of weight management strategies including ways to maintain lean mass would extend the findings from our study. Additionally, the findings in this study along with recognized links between dysregulated glucose and lipid metabolism and PH pathogenesis emphasize the need for deeper understanding of how DM and its treatment strategies may impact the development and course of PH. The high prevalence of overweight, obesity, and DM in subjects with PH underscores that new insights into how these metabolic factors contribute to the course and management of PH could exert considerable impact.
Supplementary Material
Supplemental Figure 1: All cause survival by presence of diabetes at baseline in veterans diagnosed with PH.
The Kaplan-Meier survival of diabetics (blue) when compared to non-diabetics (red) showed higher mortality in the presence of diabetes (log-rank test P <0.0001).
Supplemental Figure 2: Non-linear effect of BMI on risk of death: Stratified analysis for major clinical PH groups and for diabetics and non-diabetics.
The effect of BMI on risk of death is non-linear across the BMI range. The pattern of an increased effect at lower BMI ranges is consistent in various types of PH as well as in diabetics
Supplemental Figure 3: Predicted survival for patients at various BMI levels and with/without diabetes.
Using multivariable Cox modeling (age, gender, race, PH type, BMI, and diabetes included as covariates), the predicted survival probability (and 95% confidence interval) is displayed for patients at BMI 20, 30, and 40 and with/without DM. Strata of BMI are represented by colors, and presence of DM by the line pattern as indicated in the graphic. Other than BMI and DM, which varied, the prediction assumes patients are 70 years old, male gender, white race, and have presumed Group 2 PH. Continuous variables (age and BMI) were modeled using restricted cubic splines with 4 knots.
Acknowledgements
This work is supported by NIH UL1TR002378 (training for AWT), KL2TR002381 (AWT), P01HL108800-03 (ARH), R01HL122417-03 (ARH), T32HL116271 (training for VT), K23HL127251 (AJS), R03HL146879 (AJS), R03 AI133172 (LSP), U01DK098246 (LSP). Veterans Affairs, VA BLR&D Merit Review Award I01 BX004263-01A2 (CMH) and VA CSR&D Merit Review Awards IK2 CX001907 (LSP), I01 CX001737 (LSP).
The authors would like to thank Christine Jasien for her assistance in data retrieval and curation instrumental to this work.
This material is the result of work supported with resources and the use of facilities at the Atlanta VA Medical Center. The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. This work has been supported by funding provided by the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AWT, VT, AJS and CMH declare no potential conflicts of interest. ARH has served as a consultant for Actelion, Bayer, Complexa, and United Therapeutics. LSP has received grant funding from the CMREF and the NIH/NHLBI. She holds stock options in PHPreMedicine. LSP has served on Scientific Advisory Boards for Boehringer Ingelheim and Janssen, and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, Abbvie, Vascular Pharmaceuticals, Janssen, Glaxo SmithKline, and the Cystic Fibrosis Foundation. LSP is a cofounder, Officer and Board member and stockholder of a company, Diasyst, Inc., which is developing software aimed to help improve diabetes management.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Jing ZC, Gibbs JS. A global view of pulmonary hypertension. Lancet Respir Med 2016;4:306–322. [DOI] [PubMed] [Google Scholar]
- 2.Trammell AW, Shah AJ, Phillips LS, Michael Hart C. Mortality in US veterans with pulmonary hypertension: a retrospective analysis of survival by subtype and baseline factors. Pulm Circ 2019;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017;36:957–967. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Channick RN, Frantz RP, Grunig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemnes AR, Luther JM, Rhodes CJ, Burgess JP, Carlson J, Fan R, Fessel JP, Fortune N, Gerszten RE, Halliday SJ, Hekmat R, Howard L, Newman JH, Niswender KD, Pugh ME, Robbins IM, Sheng Q, Shibao CA, Shyr Y, Sumner S, Talati M, Wharton J, Wilkins MR, Ye F, Yu C, West J, Brittain EL. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight 2019;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeligar SM, Kang BY, Bijli KM, Kleinhenz JM, Murphy TC, Torres G, San Martin A, Sutliff RL, Hart CM. PPARgamma Regulates Mitochondrial Structure and Function and Human Pulmonary Artery Smooth Muscle Cell Proliferation. Am J Respir Cell Mol Biol 2018;58:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SY, Rubin LJ. Metabolic dysfunction in pulmonary hypertension: from basic science to clinical practice. Eur Respir Rev 2017;26:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879–1884. [DOI] [PubMed] [Google Scholar]
- 9.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011;30:904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 11.Twombly JG, Long Q, Zhu M, Fraser LA, Olson DE, Wilson PW, Narayan KM, Phillips LS. Validity of the primary care diagnosis of diabetes in veterans in the southeastern United States. Diabetes Res Clin Pract 2011;91:395–400. [DOI] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Lee KM, Freiman MR, Powell WR, Klings ES, Rinne ST, Miller DR, Rose AJ, Wiener RS. Phosphodiesterase-5 Inhibitor Therapy for Pulmonary Hypertension in the United States. Actual versus Recommended Use. Ann Am Thorac Soc 2018;15:693–701. [DOI] [PubMed] [Google Scholar]
- 14.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanders WD, Eldridge RC, McClellan W. A nearly unavoidable mechanism for collider bias with index-event studies. Epidemiology 2014;25:762–764. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control & Prevention. National diabetes statistics report, 2017. [Google Scholar]
- 17.U.S. Department of Veterans Affairs, Office of Research & Development. Diabetes in veterans, 2019.
- 18.Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013;144:169–176. [DOI] [PubMed] [Google Scholar]
- 19.Benson L, Brittain EL, Pugh ME, Austin ED, Fox K, Wheeler L, Robbins IM, Hemnes AR. Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ 2014;4:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitaker ME, Nair V, Sinari S, Dherange PA, Natarajan B, Trutter L, Brittain EL, Hemnes AR, Austin ED, Patel K, Black SM, Garcia JGN, Yuan Md Ph DJ, Vanderpool RR, Rischard F, Makino A, Bedrick EJ, Desai AA. Diabetes Mellitus Associates with Increased Right Ventricular Afterload and Remodeling in Pulmonary Arterial Hypertension. Am J Med 2018;131:702.e707–702.e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abernethy AD, Stackhouse K, Hart S, Devendra G, Bashore TM, Dweik R, Krasuski RA. Impact of diabetes in patients with pulmonary hypertension. Pulm Circ 2015;5:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009;33:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazimba S, Holland E, Nagarajan V, Mihalek AD, Kennedy JLW, Bilchick KC. Obesity paradox in group 1 pulmonary hypertension: analysis of the NIH-Pulmonary Hypertension registry. Int J Obes (Lond) 2017;41:1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafrir B, Adir Y, Shehadeh W, Shteinberg M, Salman N, Amir O. The association between obesity, mortality and filling pressures in pulmonary hypertension patients; the “obesity paradox”. Respir Med 2013;107:139–146. [DOI] [PubMed] [Google Scholar]
- 25.Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag 2019;15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh ME, Newman JH, Williams DB, Brittain E, Robbins IM, Hemnes AR. Hemodynamic improvement of pulmonary arterial hypertension after bariatric surgery: potential role for metabolic regulation. Diabetes Care 2013;36:e32–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019;53:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, Sharma S, Neilson I, Webb DJ, Megson IL, Flapan AD, Newby DE. Vascular effects of apelin in vivo in man. J Am Coll Cardiol 2008;52:908–913. [DOI] [PubMed] [Google Scholar]
- 29.Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, Pemberton PW, Ammori B, Malik RA, Soran H, Heagerty AM. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol 2013;62:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: All cause survival by presence of diabetes at baseline in veterans diagnosed with PH.
The Kaplan-Meier survival of diabetics (blue) when compared to non-diabetics (red) showed higher mortality in the presence of diabetes (log-rank test P <0.0001).
Supplemental Figure 2: Non-linear effect of BMI on risk of death: Stratified analysis for major clinical PH groups and for diabetics and non-diabetics.
The effect of BMI on risk of death is non-linear across the BMI range. The pattern of an increased effect at lower BMI ranges is consistent in various types of PH as well as in diabetics
Supplemental Figure 3: Predicted survival for patients at various BMI levels and with/without diabetes.
Using multivariable Cox modeling (age, gender, race, PH type, BMI, and diabetes included as covariates), the predicted survival probability (and 95% confidence interval) is displayed for patients at BMI 20, 30, and 40 and with/without DM. Strata of BMI are represented by colors, and presence of DM by the line pattern as indicated in the graphic. Other than BMI and DM, which varied, the prediction assumes patients are 70 years old, male gender, white race, and have presumed Group 2 PH. Continuous variables (age and BMI) were modeled using restricted cubic splines with 4 knots.