Abstract
Lung inflammation is tightly controlled to balance microbial clearance with the tissue damage that accompanies this response. Bacterial pathogens including Streptococcus pneumoniae modulate immune regulation by promoting secretion of the anti-inflammatory cytokine IL-10. The important cellular sources of IL-10 that impact protection against different bacterial infections are not well characterized. We find that S. pneumoniae activates IL-10 secretion from natural killer (NK) cells in the lung, which restrict host protection in a mouse model of sublethal infection. Direct transfer of wild-type NK cells into the lungs of IL-10-deficient mice drives bacterial expansion, identifying NK cells as a critical source of IL-10 promoting S. pneumoniae infection. The S. pneumoniae virulence protein Spr1875 was found to elicit NK cell IL-10 production in purified cells and in the lungs of live animals. These findings reveal therapeutic targets to combat bacterial-driven immune regulation in the lung.
Introduction
Streptococcus pneumoniae (the pneumococcus) contributes to a major burden of disease. Despite widespread vaccine use, S. pneumoniae remains the number one cause of pneumonia and is a major source of bacterial otitis media (ear infections) and meningitis1,2. Community acquired pneumonia is the leading cause of death in children worldwide and is the most common infectious cause of death in the elderly3,4. Pneumonia is characterized by inflammation of the air sacs in the lung, leading to fluid accumulation and making gas exchange more difficult5. Innate immune cells including alveolar macrophages, neutrophils and inflammatory monocytes are critical for bacterial clearance but also cause tissue damage through the secretion of pro-inflammatory cytokines6,7. These issues highlight the importance of understanding the host and microbial factors that drive the regulation of lung inflammation during bacterial infection.
S. pneumoniae has evolved several mechanisms to modulate the immune response. During colonization, which is typically asymptomatic, S. pneumoniae promotes inflammation, which improves transmission and bacterial growth in the nasopharynx8. In contrast, S. pneumoniae invasion to the lung is associated with host production of the anti-inflammatory cytokine IL-109,10. The outcome of this response is dependent on the severity of the infection. In the absence of IL-10, neutrophil recruitment is improved along with survival for systemic infection9, but only up to a point. At higher infectious doses, increased tissue damage reduces survival in IL-10-deficient animals10. Regardless of infectious dose, IL-10 restricts bacterial clearance in the lung and systemic tissues during lethal infection9,10. The cellular sources of immune regulatory IL-10 during S. pneumoniae infection, and the bacterial products that stimulate this response, are not clear. While almost all leukocytes are capable of IL-10 secretion, diverse signaling pathways regulate IL-10 production by different cell types11.
Natural killer (NK) cells are innate lymphoid cells (ILC) that serve as an early source of IL-10 in several animal models of microbial infection12–14. Human NK cells secrete IL-10 in response to viral and bacterial products14–16, and circulating IL-10+ NK cells are found in people with chronic viral infections17–19. While NK cells improve resistance against tumors and acute viral infection20,21, they instead limit protection against some bacterial, parasite, and chronic viral infections12,14,22,23. The lung contains the highest proportion of NK cells in the body24. However, it is unclear how NK cells in the lung regulate the immune response to microbial infection. In one study, NK cell depletion resulted in lower burdens of S. pneumoniae in the lungs25, but the mechanism for this effect was not established. Circulating IL-10+ NK cells have been observed in the context of systemic infections12, but it has been unclear whether respiratory tract infections activate NK cell IL-10 production in the lung.
Previously, we found that intravenous infection with the bacterial pathogen Listeria monocytogenes stimulates a systemic NK cell IL-10 response that drives bacterial expansion in the liver and spleen14. L. monocytogenes secretes a virulence protein containing a LysM domain, p60, which induces mouse and human NK cell secretion of IL-10 in purified cell co-cultures with dendritic cells (DCs). Similarly, S. pneumoniae expresses a virulence protein, Spr1875, that contains a LysM domain26. This led us to investigate whether S. pneumoniae activates IL-10 production by NK cells in the lung, how this response impacts bacterial clearance, and the potential for Spr1875 to promote NK cell IL-10 production.
Material and Methods
Animals
All mice used in this study were purchased or generated on the C57BL/6J background, including the following strains: C57BL/6J (WT), CD45.1+ B6.il10−/− (il10−/−), and B6.il10-GFP reporter (Tiger) mice. Male and female mice between 8–12 weeks of age were used in all experiments. Animals were maintained in specific pathogen-free conditions in the University of Colorado Office of Laboratory Animal Resources.
Infections and NK cell depletion
Type 2 S. pneumoniae strain D39 was a kind gift from Dr. Edward N. Janoff. S. pneumoniae was thawed from frozen starter cultures for dilution in Todd-Hewitt Broth with 0.5% yeast extract (MP Biomedicals) and grown to mid-log phase in static cultures at 37°C in a CO2-enriched environment. Mid-log phase bacterial cultures were diluted in PBS and used to inoculate isoflurane anesthetized mice intranasally (i.n.) at a sublethal dose of 107 colony forming units (CFU) per mouse in a volume of 50 µL. For cell depletions, mice were treated intraperitoneally (i.p.) with PBS containing 200 µg of isotype control IgG2a antibody (clone C1.18, Bio X Cell), αNK1.1 antibody (clone PK136, Bio X Cell), or antisera to the ganglioside asialo-GM1 (anti-GM1, Wako USA). Mice were treated with antibodies 24 h prior to infection, and depletions were confirmed at the time of sacrifice by flow cytometry. Organs harvested for CFU enumeration were homogenized in PBS with a tissue homogenizer (IKA Works) and serial dilutions were plated on Tryptic Soy agar plates supplemented with neomycin (5 µg/mL) and catalase (5,000 Units per plate, Worthington) and grown overnight at 37°C in a CO2-enriched environment.
Flow Cytometry
Lungs harvested for the preparation of single cells for flow cytometry were perfused by transcardial injection of 10 mL PBS prior to collection. Perfused lungs were harvested into HBSS plus cations media (Invitrogen) supplemented with DNAseI (30 µg/mL, Sigma) and type 4 collagenase (1 mg/mL, Worthington). Minced lungs were incubated for 25 min at 37°C, and digested tissue was passaged through a 70 µM strainer. Remaining red blood cells were removed by rapid RBC lysis buffer treatment (30 s). Cells were incubated in anti-CD16/32 (2.4G2 hybridoma supernatant) for 30 min at 4°C to block Fc receptors. Cells were stained in FACS buffer (1% BSA, 0.01% NaN3, PBS) and fixed in 1% paraformaldehyde. Antibodies for staining included anti-CD3 (clone 1452C11), anti-NK1.1 (clone PK136), anti-NKp46 (clone 29A1.4), anti-CD49b (clone HMα2), anti-CD45.2 (clone 104), anti-CD45.1 (clone A20), anti-Ly6C (clone KH1.4), anti-CD11b (clone M1/70), anti-Ly6G (clone 1A8), anti-CD11c (clone N418), and anti-Siglec F (clone E50–2440). Antibodies were purchased from eBioScience, BioLegend and BD Biosciences. For GFP (IL-10) analysis in GFP reporter (Tiger) mice, cells were treated with saponin and IL-10-GFP signal was amplified by staining with rabbit monoclonal anti-GFP followed by goat anti-rabbit IgG Alexa Fluor 488 (Life Technologies). A minimum of 100,000 events per sample were collected on an LSRII (BD Biosciences) using BD FACSDiva and FlowJo software (Tree Star Technologies) for data analysis.
Cell Isolation and Stimulations
NK cells were purified for cell transfers and cell cultures by negative selection from the spleens or lungs of mice using the EasySep Mouse NK cell Isolation Kit (StemCell Technologies). Purified NK cell populations were >80% NK1.1+CD3− cells. For in vivo cell transfers, 1–2 × 106 cells were injected intratracheally (i.t.) into mice anesthetized with 150 µL i.p. of 100 mg/kg ketamine + 10 mg/kg xylazine (MWI Vet Supply). For co-culture experiments, il10−/− BMDCs were cultured in GM-CSF for 6 d prior to plating 3 × 105 cells/well (>90% CD11c+) overnight in 24-well plates. For cell stimulations, 10 ng/mL LPS (L8294 Sigma) and 30 µg/mL purified Spr1875 protein were added to BMDCs for 1 h, as indicated. The Spr1875 protein was expressed in Escherichia coli in a pTrcHis construct (Invitrogen) for purification in a Nickel column by affinity purification as previously described27. Protein purity was assessed by running fractions on a 10% SDS-PAGE gel under reducing conditions and staining with Coomassie Blue and by Western Blotting following protein transfer to a nitrocellulose membrane and probing with anti-His antibody (Invitrogen). The nucleotide sequence used for Spr1875 expression was obtained from the full genome sequence for S. pneumoniae strain R6, GenBank accession number AE007317.1 (positions 1844525–1845667, locus tag ‘spr1875’). For co-culture infections, mid-log phase S. pneumoniae was added at a multiplicity of 1 bacterium per BMDC for 1 h. BMDCs were washed and gentamycin was added to fresh media at 10 µg/mL prior to the addition of purified NK cells at a ratio of 1:10 (NK cells:BMDCs). Supernatants were harvested at 72 h for analysis of IL-10 using an ELISA kit (BD Biosciences).
Study Approval
These studies were approved by the Animal Care and Use Committee (protocol #00313) and the Institutional Biosafety Committee of the University of Colorado School of Medicine.
Statistical Analysis
Prism (GraphPad) software was used for graphing and statistical analysis. Significance was determined using t-tests and ANOVA analysis. p < 0.05 was considered significant.
Results
IL-10 is produced by lung NK cells and increases S. pneumoniae burdens
NK cell IL-10 production peaks by day 3–4 post-infection in several animal models of systemic microbial infection12,14,28. Tiger IL-10 GFP reporter mice were used to determine whether S. pneumoniae infection of the respiratory tract similarly stimulates IL-10 production by NK cells in the lung and periphery. In contrast to other IL-10 reporter strains including Vert-X mice, post-transcriptional regulation of il10 is preserved in Tiger reporter mice due to an unaltered 3’UTR, permitting an accurate readout for IL-10 production without re-stimulation ex vivo29. Mice were intranasally infected with S. pneumoniae (type 2 strain D39, 107 CFU/mouse), and cells from the lung and blood were collected at 72 hours post-infection (hpi) for analysis of GFP (IL-10) expression by flow cytometry. NK cells (NK1.1+CD3−) from both the lung and blood were found to express GFP (IL-10) in mice infected with S. pneumoniae, including approximately 50% of the total NK cell population in the lung (Figure 1A–B). In contrast, we found no detectable GFP (IL-10) in NK cells from naïve mice. While NK cells are an early source of IFNγ at 24 in several microbial infections including S. pneumoniae [Horowitz 2013, Baranek 2017], by 72 hpi NK cells in the lung and blood were not producing detectable amounts of IFNγ (Supplemental Figure 1A–B). Other lung cells, including alveolar macrophages, neutrophils, DCs and T cells, did not have detectable GFP (IL-10) expression at 72 hpi (Figure 1C). These results indicate that respiratory tract infection with S. pneumoniae stimulates both mucosal lung and circulating NK cells to produce IL-10.
Figure 1. IL-10 is produced by lung NK cells and increases S. pneumoniae burdens.
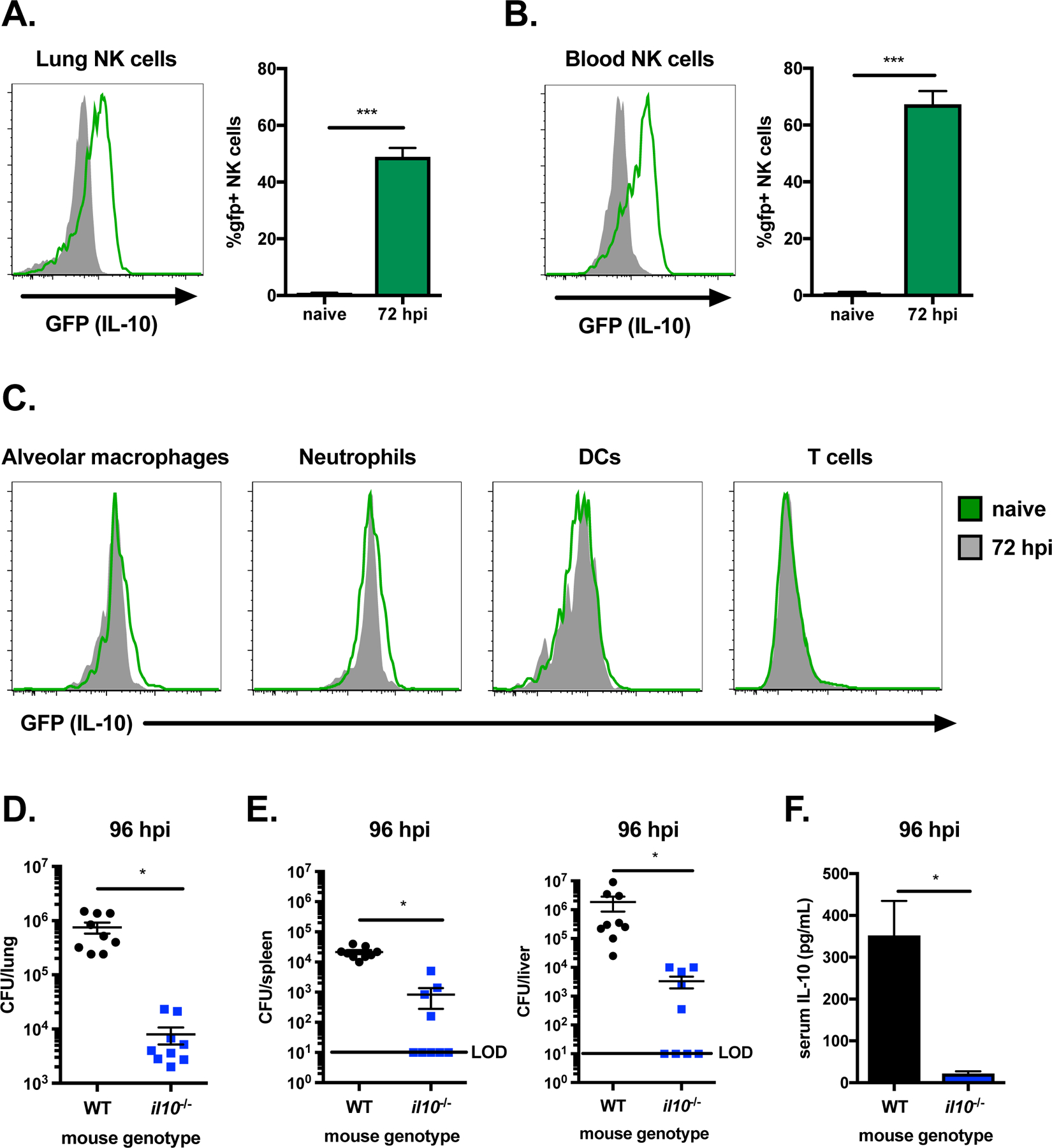
Histograms of GFP (IL-10) expression in NK cells (NK1.1+CD3−) from the lung (A) and blood (B) of naïve IL-10 GFP reporter mice (solid grey) compared to those at 72 hpi (green) with S. pneumoniae (107 CFU/mouse i.n.). Histograms of GFP (IL-10) expression are also shown for lung alveolar macrophages (SiglecF+CD11c+), neutrophils (Ly6G+CD11b+), DCs (SiglecF− CD11c+) and T cells (CD3+) in naïve (solid grey) IL-10 GFP reporter mice compared to those at 72 hpi (green) (C). Bacterial burdens in the lung (D), spleen and liver (E) and serum IL-10 (F) in WT and il10−/− mice at 96 hpi with S. pneumoniae. Data are pooled from three independent experiments with n = 3 mice/group, *p<.05, ***p<.001 as measured by t-test.
IL-10 improves resistance to lethal S. pneumoniae infection but also hinders bacterial clearance at early timepoints10. IL-10-deficient mice (il10−/−) were used to determine whether IL-10 restricts bacterial clearance in the lung and systemic tissues during sublethal S. pneumoniae infection. Bacterial burdens were compared following intranasal infection (107 CFU/mouse) at 96 hpi in wild-type (WT) and IL-10-deficient mice. We found that bacterial burdens were significantly reduced in the lungs of IL-10-deficient mice compared to WT mice, where burdens were ~2 logs higher (Figure 1D). IL-10 also promoted bacterial invasion to other tissues, as fewer IL-10-deficient mice had detectable burdens in the spleen and liver (Figure 1E). Systemic IL-10 production at this timepoint was confirmed by detection of IL-10 in the serum of WT, but not IL-10-deficient, mice (Figure 1F). These data indicate that IL-10 restricts bacterial clearance during sublethal S. pneumoniae infection, promoting bacterial growth in the lungs and enhancing systemic infection.
NK cells restrict clearance of S. pneumoniae in the lung and systemic tissues
NK cells are a source of both pro-inflammatory IFNγ and anti-inflammatory IL-10, which can play opposing roles in the immune response to microbial infection30. To determine whether NK cells negatively regulate sublethal S. pneumoniae infection, we examined bacterial burdens and IL-10 production in NK cell depleted mice. NK cells were depleted 24 h prior to infection by injection of a purified monoclonal αNK1.1 antibody and compared to mice injected with an isotype control antibody, IgG2a. Because NK1.1 is expressed by both NK cells and NKT cells, we treated a separate group of mice with antisera specific for the ganglioside asialo-GM1, which depletes NK cells but not conventional NKT cells31. Specific depletion of CD3−NK1.1+NKp46+ NK cells in the lungs of mice treated with αNK1.1 and asialo-GM1 antisera was sustained up to 96 hpi, as confirmed by flow cytometry (Supplemental Figure 2A). Depleted cells included CD49b+ traditional NK cells, but not non-NK innate lymphoid cells (ILC1) (Supplemental Figure 2B). Bacterial clearance was significantly improved in the lungs of both groups of NK cell depleted mice compared isotype control treated mice at 96 hpi (Figure 2A). IL-10 in lung tissue homogenates was also much lower in NK cell depleted mice compared to isotype control treated animals (Figure 2B). These results indicate that NK cells have an overall detrimental impact on clearance of S. pneumoniae from the lung. Further, these findings demonstrate that NK cells are a major source of IL-10 in the lung during S. pneumoniae infection.
Figure 2. NK cells restrict clearance of S. pneumoniae in the lung and systemic tissues.
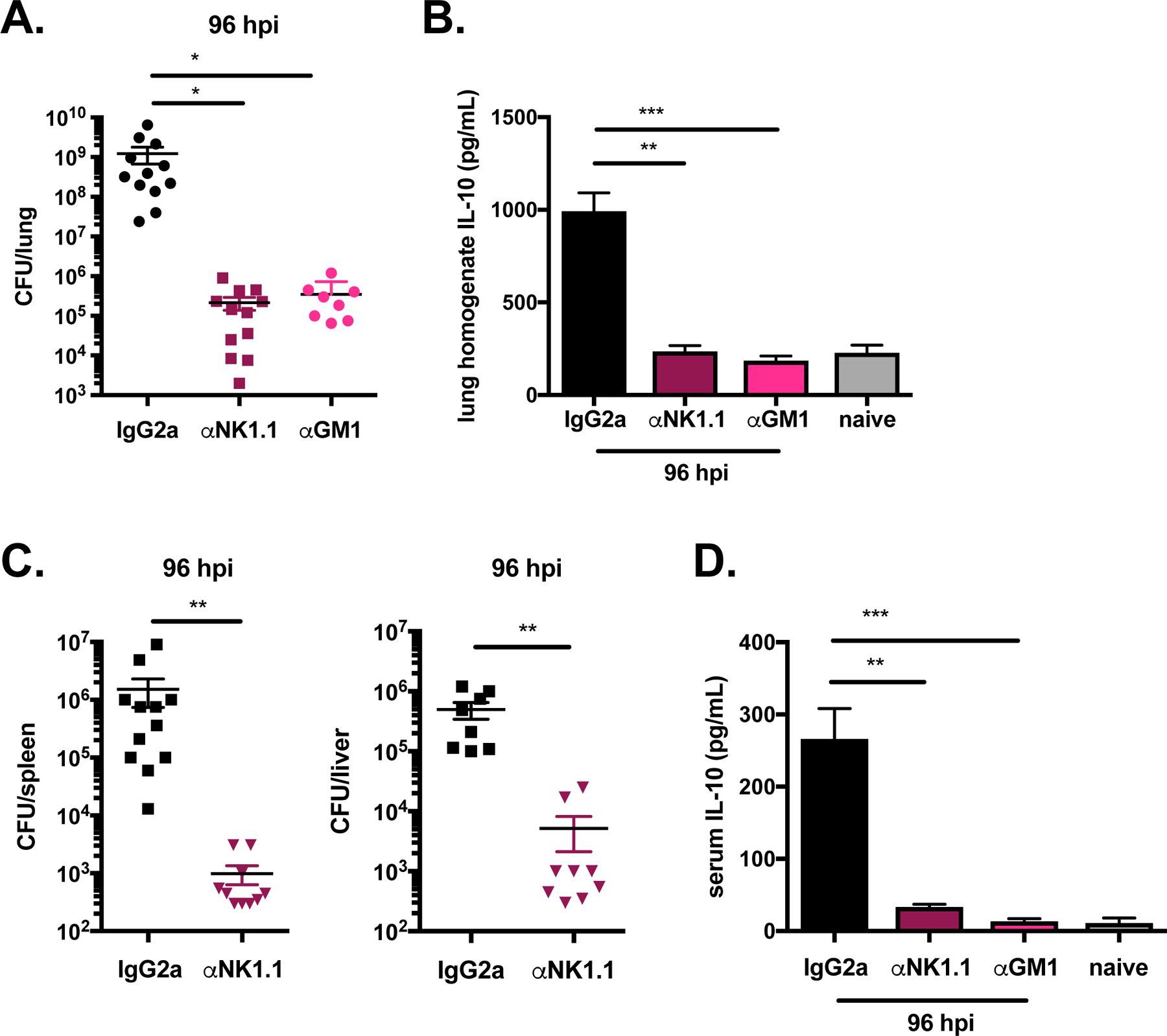
Lung bacterial burdens (A) and lung homogenate IL-10 (B) in WT naïve mice and 96 hpi in WT mice treated with isotype control antibody (anti-IgG2a), αNK1.1 antibody, or αGM1 serum and infected 24 h later with S. pneumoniae (107 CFU/mouse i.n.). Bacterial burdens in the spleen and liver (C) and serum IL-10 (D) in WT naïve mice and 96 hpi in WT mice treated with isotype control antibody, αNK1.1 antibody, or αGM1 serum and infected 24 h later with S. pneumoniae. Data are pooled from three experiments with n = 3–5 mice/group, *p<.05, **p<.01, ***p<.001 as measured by t-test.
The impact of NK cell depletion on bacterial invasion to other host tissues was also determined. S. pneumoniae burdens in the spleen and liver at 96 hpi were significantly reduced in NK cell depleted mice compared to isotype control treated mice (Figure 2C), indicating that NK cells promote bacterial growth in sites beyond the lung. We also observed an almost complete loss of systemic IL-10 in NK cell depleted mice relative to isotype treated controls (Figure 2D). These results suggest that NK cells both contribute to IL-10 production in the lung and constitute the major circulating source of IL-10 during S. pneumoniae infection. NK cells, rather than NKT cells, are predominantly responsible for restricting bacterial clearance and producing IL-10, as lung bacterial burdens and IL-10 were similarly reduced in mice treated with αNK1.1 antibody and anti-GM1 sera. Based on these findings we conclude that NK cells promote bacterial growth in the lungs and invasion to other host sites during sublethal respiratory tract infection.
NK cells and IL-10 limit innate immune cell recruitment to the lung
NK cells were previously shown to restrict infiltration of inflammatory myeloid cells to the spleen during systemic infection with L. monocytogenes14. Similarly, IL-10-deficient mice have increased neutrophils in the lungs at early timepoints during lethal S. pneumoniae infection10. We next compared the impact of NK cells and IL-10 on the accumulation of myeloid cells in the lung during sublethal S. pneumoniae infection. WT mice were treated with either αNK1.1 antibody or isotype control antibody 24 h prior to S. pneumoniae infection, and innate immune cell populations in the lung were compared to those in IL-10-deficient mice at 96 hpi. Neutrophil (Ly6G+CD11b+), inflammatory monocyte (Ly6G−Ly6ChiCD11b+), and alveolar macrophage (Siglec F+CD11c+) populations in the lung were identified by flow cytometry (Supplemental Figure 3A–B). NK cell depletion significantly increased the percentages and total cell numbers of neutrophils and inflammatory monocytes compared to isotype control treated mice infected with S. pneumoniae (Figure 3A–B). Increased total numbers, but not percentages, of alveolar macrophages in the lungs (Figure 3C) and bronchoalveolar lavage fluid (BALF) (Supplemental Figure 3C) was also observed for NK cell depleted mice compared to controls. These data indicate that NK cells limit innate immune cell accumulation in the lung tissue of S. pneumoniae infected mice.
Figure 3. NK cells and IL-10 limit innate immune cell recruitment to the lung.
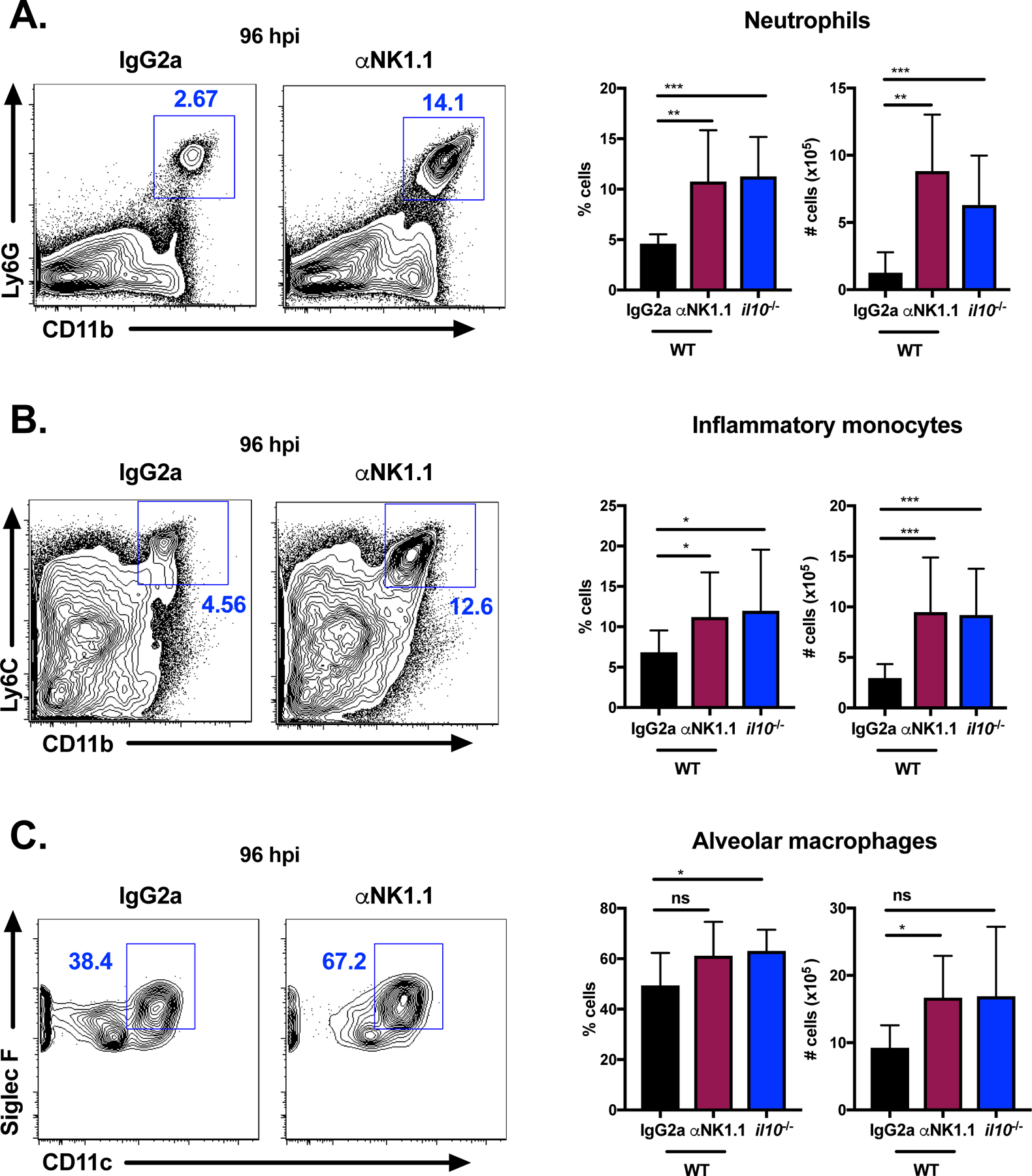
Representative flow plots of neutrophils (Ly6G+CD11b+) (A), inflammatory monocytes (Ly6G− Ly6ChiCD11b+) (B), and alveolar macrophages (Siglec F+CD11c+) (C) at 96 hpi in the lungs of WT mice treated with isotype control (IgG2a) antibody or αNK1.1 antibody 24 h prior to infection or il10−/− (untreated) mice infected with S. pneumoniae (107 CFU/mouse i.n.). Summary of cell percentages and total numbers for each group is also shown. Data are pooled from three independent experiments with n = 3–5 mice/group, *p<.05, **p<.01, ***p<.001 as measured by t-test.
Analysis of the innate immune cell populations in the lungs of IL-10-deficient mice infected with S. pneumoniae revealed a similar phenotype to NK cell depleted mice. IL-10-deficient mice had significantly increased percentages and total cell numbers of neutrophils and inflammatory monocytes compared to WT mice at 96 hpi (Figure 3A–B). There was also a slight increase in the percentage (but not total cell number) of alveolar macrophages in the lungs of IL-10-deficient animals (Figure 3C). These findings indicate that NK cells and IL-10 similarly restrict the recruitment of myeloid cells including neutrophils and inflammatory monocytes to the lung during sublethal S. pneumoniae infection. The reduced presence of such cell types is anticipated to directly impact bacterial clearance, as neutrophils and monocytes are important mediators of bacterial phagocytosis and killing in the lung32,33. Alveolar macrophages, which were also slightly increased in the absence of NK cells and IL-10, also contribute to bacterial clearance at early timepoints34. Together, these data indicate that NK cells and IL-10 similarly restrict the recruitment of protective myeloid cell populations to the lung during sublethal S. pneumoniae infection.
NK cells are the critical cellular source of IL-10 limiting protection against S. pneumoniae
IL-10 is produced by diverse immune cells, making it unclear which cell types mediate IL-10-dependent susceptibility to S. pneumoniae infection. Our data thus far indicated that NK cells are a major source of lung and systemic IL-10 during S. pneumoniae infection, but do not rule out a contribution from other cell types. To address this, we conducted a cell transfer experiment using WT and IL-10-deficient mice. NK cells were purified from the lungs of naïve IL-10 sufficient (WT) and deficient (il10−/−) mice. Donor NK cells were injected directly into the lungs of IL-10-deficient recipient mice infected with S. pneumoniae at 24 hpi (Figure 4A). Bacterial burdens, IL-10, and lung myeloid cell populations were analyzed at 96 hpi. In this system, donor NK cells are the only source of IL-10 in S. pneumoniae infected mice. We confirmed the presence of a small population of transferred NK cells (CD45.2+ donor cells) in the lungs of recipient mice (CD45.1+) at 96 hpi (Figure 4B). Lung bacterial burdens were similar between IL-10-deficient mice that received no cells and recipients of IL-10-deficient NK cells, as expected (Figure 4C). Strikingly, mice that received WT NK cells had increased bacterial burdens in the lung relative to recipients of no cells or IL-10-deficient NK cells (Figure 4C). IL-10 was also only detected in the lung tissue of mice that received WT NK, but not IL-10-deficient, donor NK cells (Figure 4D). These results demonstrate a critical role for IL-10 from NK cells in promoting bacterial growth in the lung.
Figure 4. NK cells are the critical cellular source of IL-10 limiting protection against S. pneumoniae.
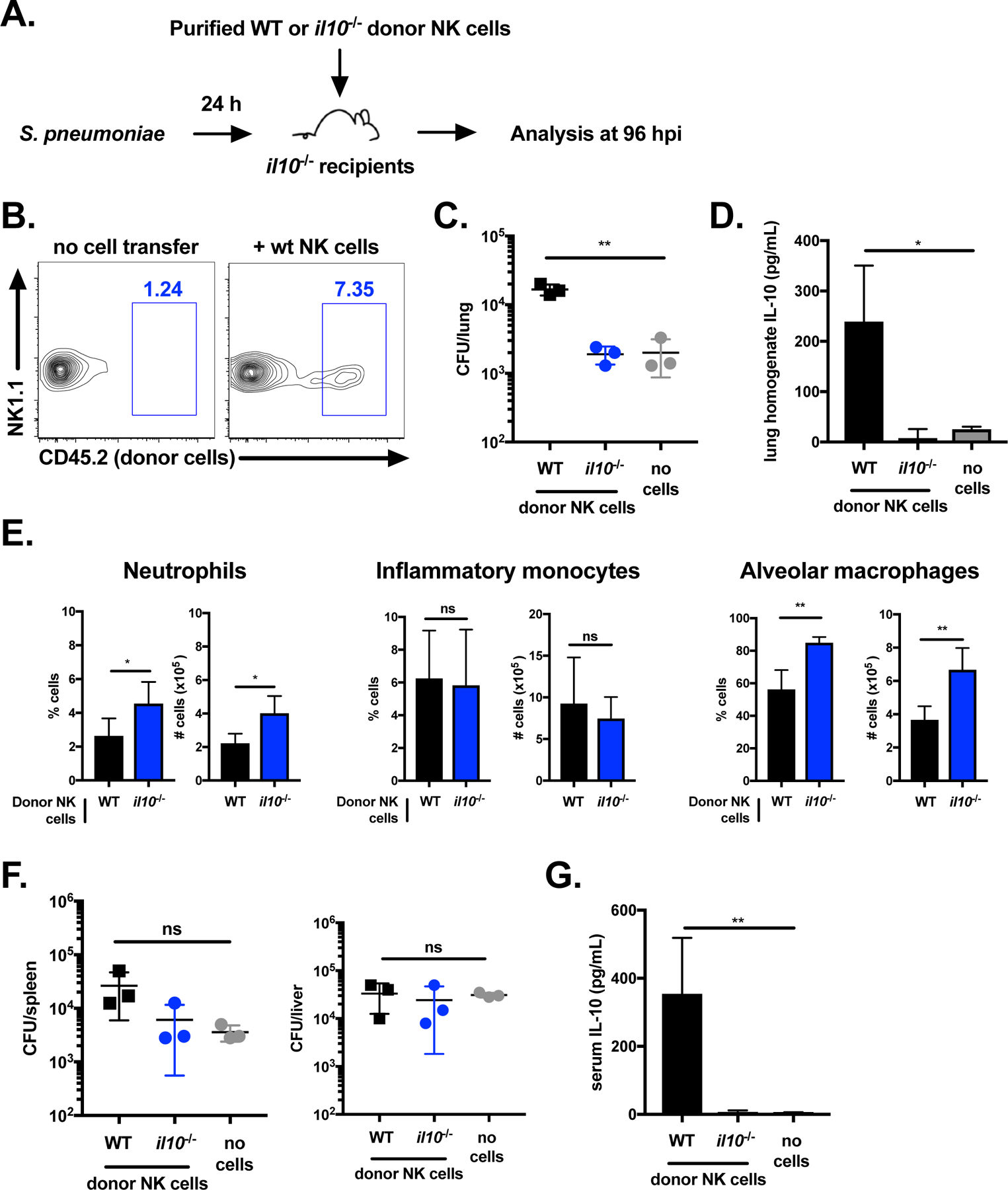
Schematic of NK cell transfer experiment, where NK cells were purified from naïve donor mice and transferred i.t. to IL-10-deficient recipients (A). Dot plot of transferred WT NK cells (CD45.2+) in il10−/− recipients (CD45.1+) (B). Lung bacterial burdens (C) and homogenate IL-10 (D) in il10−/− recipients of no cells, il10−/− NK cells, or WT NK cells 24 hpi with S. pneumoniae (107 CFU/mouse i.n.). Summary of percentage and total cell numbers of neutrophils (Ly6G+CD11b+), inflammatory monocytes (Ly6G−Ly6ChiCD11b+), and alveolar macrophages (Siglec F+CD11c+) at 96 hpi in the lungs of il10−/− recipients of il10−/− or WT NK cells 24 hpi with S. pneumoniae (E). Bacterial burdens in the liver and spleen (F) and serum IL-10 (G) at 96 hpi in il10−/− recipients of no cells, il10−/− NK cells, or WT NK cells 24 hpi with S. pneumoniae. Data are representative of three independent experiments with n = 3 mice/group, *p<.05, **p<.01 as measured by t-test.
Comparison of the innate immune cell populations in infected recipients revealed a higher percentage and total cell number of neutrophils and alveolar macrophages in mice that received IL-10-deficient NK cells compared to WT NK cells (Figure 4E). In contrast, inflammatory monocytes were similar between both groups. These findings demonstrate that IL-10 expression in NK cells alone restricts neutrophil accumulation and alveolar macrophage recovery in the lung during S. pneumoniae infection. The corresponding increase in lung bacterial burdens for recipients of WT NK cells, but not IL-10-deficient NK cells, further implicates NK cell dependent IL-10 in the suppression of bacterial clearance.
In contrast to the lung, bacterial burdens in systemic tissues were not significantly altered following WT NK cell transfer (Figure 4F). However, systemic IL-10 was detected in mice that received WT, but not IL-10-deficient, NK cells (Figure 4G). Thus, circulating IL-10 was not sufficient to increase bacterial burdens beyond the lung. Together, these findings indicate that NK cells are the important immune restricting source of IL-10 in the lung during sublethal S. pneumoniae infection. Further, NK cell production of IL-10 limits recruitment of myeloid cells including neutrophils and alveolar macrophages to this tissue.
The S. pneumoniae virulence protein Spr1875 elicits IL-10 production by NK cells
We used a defined co-culture system comprised of bone marrow-derived dendritic cells (BMDCs) and purified NK cells to examine the host and bacterial signals that drive the production of IL-10 by lung NK cells. This co-culture system was previously developed to define the requirements for NK cell responses to L. monocytogenes14,35,36. BMDCs were prepared from IL-10-deficient mice to isolate IL-10 production by NK cells. We first compared IL-10 production in co-cultures of NK cells purified from the lung and spleen following BMDC infection with S. pneumoniae. BMDCs were infected for 1 h and then treated with antibiotics to remove all extracellular bacteria prior to the addition of purified NK cells (Figure 5A). Measurement of IL-10 in co-culture supernatants at 72 hpi revealed that NK cells from both the lung and spleen produced IL-10 in response to BMDC infection with S. pneumoniae (Figure 5B). These data indicate that DCs are sufficient to promote NK cell secretion of IL-10 in response to S. pneumoniae infection in the absence of other cells or soluble signals in the lung or periphery.
Figure 5. The S. pneumoniae virulence protein Spr1875 elicits IL-10 production by NK cells.
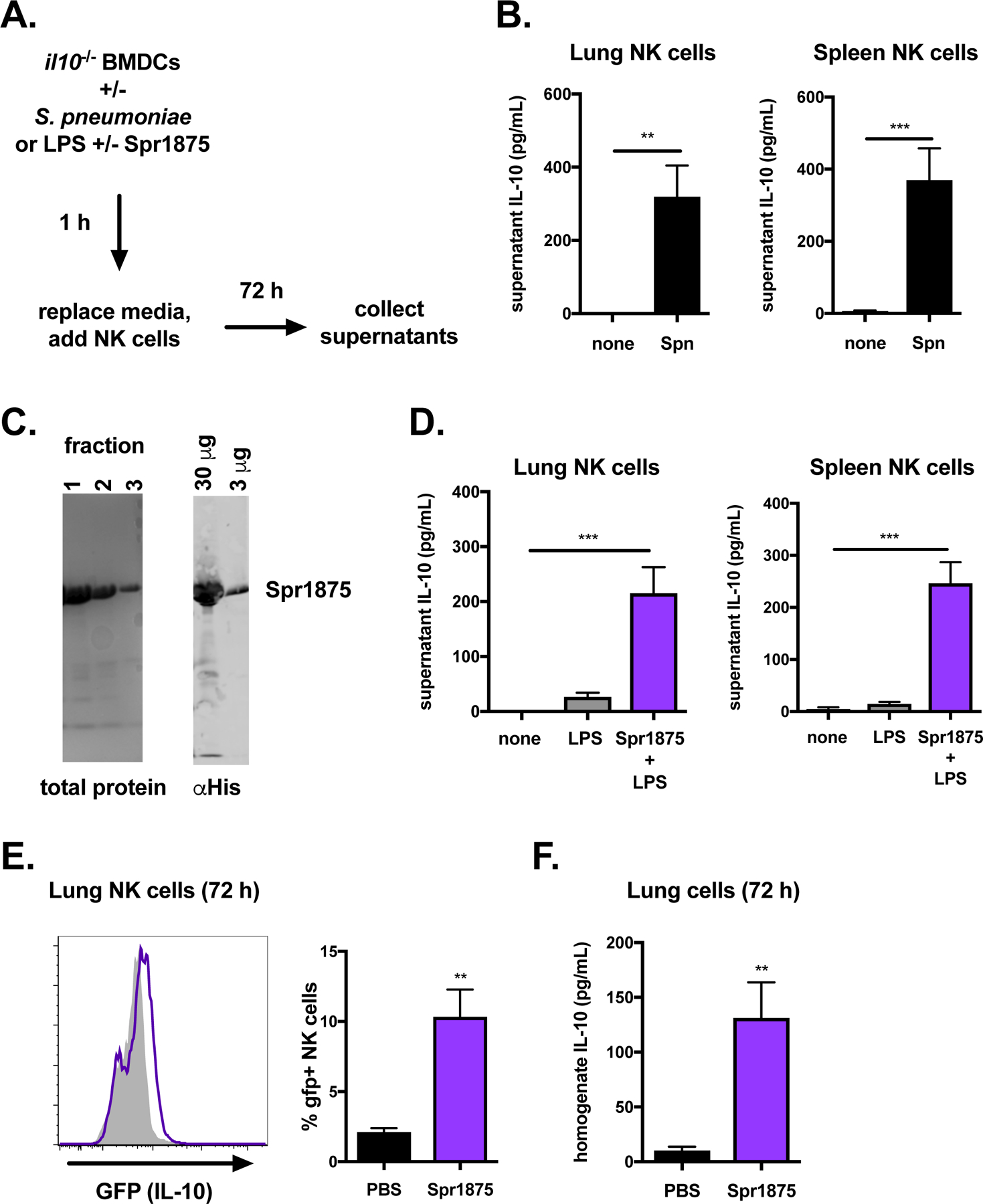
Schematic of co-culture experiment with IL-10-deficient BMDCs, where BMDCs were infected or stimulated with protein prior to the addition of NK cells purified from the lungs or spleens of naïve mice (A). Supernatant IL-10 in 72 h cultures of NK cells purified from the lungs or spleens following co-culture with IL-10-deficient BMDCs infected for 1 h with S. pneumoniae (Spn) (B). Representative gel of Spr1875 protein fractions stained with Coomassie (left) and anti-His immunoblot of pooled protein fractions (right) (C). Supernatant IL-10 in 72 h cultures of NK cells purified from the lungs or spleens following co-culture with IL-10-deficient BMDCs stimulated with LPS or LPS + Spr1875 for 1 h (D). Histogram of GFP (IL-10) expression in NK cells (NK1.1+CD3−) from the lungs of IL-10 GFP reporter mice 72 h following injection with either PBS (solid grey) or Spr1875 i.t. (purple) (E). Summary of % GFP+ NK cells for each group is also shown. Lung homogenate IL-10 in PBS versus Spr1875 treated mice is compared at 72 h (F). In vitro data (B, D) are pooled from three independent experiments with technical replicates in triplicate, **p<.01, ***p<.001 as measured by t-test. In vivo data (E-F) are pooled from three independent experiments with n = 3 mice/group, **p<.01 as measured by t-test.
The bacterial products that induce IL-10 production by NK cells are not well characterized. We previously found that the Listeria monocytogenes virulence protein p60 is sufficient to stimulate NK cell activation in co-cultures14,36. The S. pneumoniae virulence protein Spr1875 contains a LysM domain with homology to the critical LysM domain required for p60 activation of NK cells36. To determine the potential for Spr1875 to induce IL-10 production by lung NK cells, full length (His tagged) protein was expressed in E. coli and isolated by affinity purification (Figure 5C). We found that 1 h stimulation of BMDCs with Spr1875 was sufficient to induce IL-10 secretion by purified lung or spleen NK cells (Figure 5A, D). The toll-like receptor (TLR) agonist lipopolysaccharide (LPS) was included in these experiments to promote DC priming and did not itself stimulate NK cell activation. Thus, both lung and spleen NK cells co-cultured with S. pneumoniae-infected or Spr1875-treated BMDCs were stimulated to produce IL-10.
We next assayed if purified Spr1875 could induce NK cell IL-10 production in the lung. Spr1875 was administered intratracheally (i.t.) into naïve (Tiger) IL-10 GFP reporter mice. Upon harvest 72 h later, GFP (IL-10) expression was detected in NK cells in mice treated with Spr1875 but not PBS (Figure 5E). These GFP (IL-10)+ cells comprised approximately 10% of the total NK cell population in the lung (Figure 5E). IL-10 protein could also be detected in lung homogenates from Spr1875 treated, but not PBS treated, mice (Figure 5F). These data indicate that the S. pneumoniae virulence protein Spr1875 suffices to elicit IL-10 production by lung NK cells both in vitro and when administered in vivo.
Discussion
With the rising clinical relevance of immune therapy, understanding how pathogens exploit immune regulatory pathways is of increasing importance. A prominent example is microbial activation of host cell IL-10 production, which can suppress otherwise protective immune responses37. While diverse signaling pathways regulate IL-10 expression in different cell types, the bacterial molecules that initiate this response and the critical sources of immune restricting IL-10 are not well defined. Here, we report that S. pneumoniae infection or treatment with the virulence protein Spr1875 can stimulate IL-10 production by NK cells in the lung. NK cell dependent IL-10 limits myeloid cell recruitment, exacerbating S. pneumoniae infection in the lung and invasion to other host tissues.
NK cells have a dual role during microbial infections. In addition to IL-10, NK cells produce the pro-inflammatory cytokine IFNγ, which contributes to host resistance against some lung infections38–40. However, our data show that NK cells impair bacterial clearance from the lung during sublethal S. pneumoniae infection. This finding is consistent with earlier work demonstrating that the absence of NK cells improved S. pneumoniae clearance25,41, despite their production of IFNγ41,42. One prior study further showed that NK cell depletion reduced burdens in the lung, but not liver25. We similarly observed a negative impact on pulmonary, but not systemic, infection following transfer of donor NK cells into IL-10-deficient mice. These findings together suggest that in the context of pulmonary S. pneumoniae infection, NK cell IL-10 production has a dominant negative impact on host resistance. Targeting the signaling pathways that promote NK cell IL-10 in the lung may therefore improve outcome of this pulmonary infection.
Previously, systemic infections including L. monocytogenes, Toxoplasma gondii, and Leishmania major were found to promote a detrimental NK cell IL-10 response12–14. In contrast, NK cell IL-10 was not seen during lung infections by influenza A virus and attenuated Yersinia pestis12. It was therefore proposed that only systemic infections elicit IL-10 production by NK cells. Our findings here argue against this interpretation and instead suggest lung mucosal infections can promote NK cell IL-10 production. The failure to observe NK cell IL-10 production in the context of influenza A or attenuated Y. pestis lung infections may thus be due to a failure of these pathogens to stimulate IL-10 production by NK cells. The IL-10 production reported in these earlier studies was dependent on STAT4 activation and attributed to IL-1212, while we previously found that L. monocytogenes protein p60 activation of NK cell IL-10 requires STAT3 and is independent of the IL-12/STAT4 pathway43,44. It remains to be determined if NK cell activation by S. pneumoniae (and Spr1875) requires STAT3 or STAT4. However, it is worth noting that exogenous IL-12 increases protection against S. pneumoniae45, suggesting that IL-12 also fails to drive the NK cell IL-10 response in this infection.
Our finding that IL-10 restricts neutrophil recruitment during S. pneumoniae infection is consistent with previous reports demonstrating increased neutrophil accumulation at 48 hpi in IL-10-deficient mice10,46. We further observed that the suppressive effect of IL-10 on neutrophil infiltration is sustained up to 96 hpi, at which time inflammatory monocyte populations are similarly reduced in WT relative to IL-10-deficient mice, with a more modest reduction in alveolar macrophages. This phenotype in IL-10-deficient mice is mirrored in mice depleted of NK cells, arguing that NK cells are important for coordinating myeloid cell recruitment in the critical first few days following S. pneumoniae infection. However, the transfer of WT NK cells reduced the recruitment of neutrophils, but not inflammatory monocytes, into the lungs of IL-10-deficient mice. These findings suggest that acute NK cell IL-10 production preferentially restricts neutrophil infiltration into S. pneumoniae infected lungs. S. pneumoniae expression of bacterial capsule and pneumolysin, two key virulence factors, contribute to the evasion of neutrophil phagocytosis and killing47. Our data suggest that bacterial activation of NK cell IL-10 to dampen neutrophil influx may be another critical mechanism for evasion of neutrophil mediated protection. Whether IL-10 directly impacts neutrophil activation and phagocytosis of S. pneumoniae remains an important area for further investigation. Under normal circumstances, resolution of S. pneumoniae infection requires the participation of additional cell types beyond neutrophils for complete bacterial clearance48. Hence, interventions to increase neutrophil recruitment and activation could improve the anti-bacterial efficacy of these cells.
New vaccine strategies for S. pneumoniae mucosal infections are critical for reducing the widespread disease burden. While S. pneumoniae vaccines have dramatically reduced invasive infections, pulmonary infections remain prevalent1. Vaccines targeting virulence factors that support bacterial growth in the lung may therefore improve protection against pneumonia caused by S. pneumoniae. Our finding that Spr1875 activates IL-10 production by NK cells in the lung suggests that this protein, either alone or in combination with others inducing this response, promotes S. pneumoniae expansion during pulmonary infection. Spr1875, which is widely conserved among S. pneumoniae clinical isolates, was originally identified in a screen for protein fragments recognized by antibodies from people who had recovered from S. pneumoniae infection26. Specifically, the LysM domain of Spr1875 is critical for protection by protein immunization in mice26. The swine pathogen Streptococcus suis also expresses an immunogenic LysM protein that contributes to bacterial virulence49, and the LysM domain of the L. monocytogenes virulence protein p60 is required for both virulence and activation of NK cell responses14,36. Therefore, vaccination against the LysM region of these proteins could be used to elicit protective antibodies that interfere with bacterial stimulation of NK cell IL-10. Indeed, immunization with a LysM protein made by group B Streptococcus generates protective immunity50. Further work may thus demonstrate the potential value of targeting bacterial LysM proteins in vaccines.
In people, higher levels of IL-10 correlate with increased mortality from S. pneumoniae infection51. Individuals infected with human immunodeficiency virus (HIV) have increased circulating IL-10+ NK cells compared to those without HIV19, together with a well-documented increased susceptibility to S. pneumoniae52. Similarly, individuals with chronic hepatitis C virus (HCV) infection produce higher amounts of IL-10 compared to HCV negative controls17, and these patients had a higher mortality rate in the context of invasive S. pneumoniae co-infection53. Circulating lymphocytes from children with Down’s syndrome secrete increased IL-10 following stimulation with S. pneumoniae compared to children without Down’s syndrome54 and suffer increased mortality from respiratory tract infections55. Together, these findings emphasize the impact of IL-10 on susceptibility to S. pneumoniae infection in humans and highlight the potential for the therapeutic impact of targeting NK cell IL-10 production.
Supplementary Material
Brief Commentary.
Background
While Natural killer (NK) cells are best known for their anti-viral activity, diverse infections promote immune regulatory NK cells that secrete IL-10. It was unclear whether this response, which restricts clearance of systemic infections, is activated in mucosal tissues.
Translational Significance
We find that IL-10 production by NK cells in the lung dampens the immune response to Streptococcus pneumoniae, increasing bacterial growth and systemic invasion. We also identify a bacterial protein, Spr1875, that is sufficient to drive NK cell IL-10 production in the lung. These findings reveal NK cell IL-10 as a target for S. pneumoniae vaccine and immunotherapy strategies.
Acknowledgements
We thank Edward N. Janoff for the kind gift of the S. pneumoniae strain used in these studies. We are also grateful to current and former lab members for their helpful feedback and discussions concerning this project. This work was funded by the National Institute of Allergy and Infectious Diseases grants R01-AI131662 and R01-AI06563 (LL) and K22-AI143922 (SC).
Abbreviations:
- NK cell
Natural killer cell
- IL-10
Interleukin-10
- Spn
Streptococcus pneumoniae
- WT
Wild-type
- GFP
Green fluorescent protein
- CFU
Colony forming unit
- i.n.
Intranasal
- i.p.
Intraperitoneal
- i.t.
Intratracheal
- IFNγ
Interferon-gamma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have read the authorship agreement and policy on disclosure of potential conflicts of interest for this journal and declare no conflicts of interest.
References
- 1.Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. Borrow R, ed. PLoS ONE. 2017;12(5):e0177113–e0177120. 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koelman DLH, Brouwer MC, van de Beek D. Resurgence of pneumococcal meningitis in Europe and North America. Clin Microbiol Infect. 2019;19:30210–30211. [DOI] [PubMed] [Google Scholar]
- 3.Broulette J, Yu H, Pyenson B, Iwasaki K, Sato R. The Incidence Rate and Economic Burden of Community-Acquired Pneumonia in a Working-Age Population. Am Health Drug Benefits. 2013;6:494–503. [PMC free article] [PubMed] [Google Scholar]
- 4.Deardorff KV, McCollum ED, Ginsburg AS. Pneumonia Risk Stratification Scores for Children in Low-Resource Settings. Pediatr Infect Dis J. 2018;37(8):743–748. 10.1097/INF.0000000000001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullers JA. Molecular pathogenesis of pneumococcal pneumonia. Front Biosci. 2001;6(3):d877–d889. 10.2741/A649. [DOI] [PubMed] [Google Scholar]
- 6.Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on Neutrophil Function in Severe Inflammation. Front Immunol. 2018;9:173–14. 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baharom F, Rankin G, Blomberg A, Smed-Sörensen A. Human Lung Mononuclear Phagocytes in Health and Disease. Front Immunol. 2017;8:121–16. 10.3389/fimmu.2017.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018:1–13. 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed]
- 9.van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interieukin-10 Impairs Host Defense in Murine Pneumococcal Pneumonia. Journal Infect Dis. 1996;174:994–1000. [DOI] [PubMed] [Google Scholar]
- 10.Peñaloza HF, Nieto PA, Muñoz-Durango N, et al. Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection by Streptococcus pneumoniae. Immunology. 2015;146(1):100–112. 10.1111/imm.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer SS, Cheng G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit Rev Immunol. 2012;32:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perona-Wright G, Mohrs K, Szaba FM, et al. Systemic but Not Local Infections Elicit Immunosuppressive IL-10 Production by Natural Killer Cells. Cell Host Microbe. 2009;6(6):503–512. 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maroof A, Beattie L, Zubairi S, Svensson M, Stäger S, Kaye PM. Posttranscriptional Regulation of Il10 Gene Expression Allows Natural Killer Cells to Express Immunoregulatory Function. Immunity. 2008;29(2):295–305. 10.1016/j.immuni.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark SE, Filak HC, Guthrie BS, et al. Bacterial Manipulation of NK Cell Regulatory Activity Increases Susceptibility to Listeria monocytogenes Infection. Coers J, ed. PLoS Pathog. 2016;12(6):e1005708–e1005721. 10.1371/journal.ppat.1005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudnicka K, Miszczyk E, Matusiak A, et al. Helicobacter pylori-driven modulation of NK cell expansion, intracellular cytokine expression and cytotoxic activity. Innate Immun. 2015;21:127–139. 10.1177/1753425913518225. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zhai N, Wang Z, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut. 2018;67(11):2035–2044. 10.1136/gutjnl-2017-314098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Maria A, Fogli M, Mazza S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37(2):445–455. 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 18.Conroy MJ, Mac Nicholas R, Grealy R, et al. Circulating CD56dim natural killer cells and CD56+ T cells that produce interferon-gamma or interleukin-10 are expanded in asymptomatic, E antigen-negative patients with persistent hepatitis B virus infection. J Viral Hepat. 2015;22:335–345. 10.1111/jvh.12299. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Yang M, Sun X, et al. IL-10+ NK and TGF-β+ NK cells play negative regulatory roles in HIV infection. BMC Infect Dis 2018;18:80 10.1186/s12879-018-2991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesker PR, Krupnick AS. The role of natural killer cells in pulmonary immunosurveillance. Front Biosci. 2015;5:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–525. 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ejrnaes M, Filippi CM, Martinic MM, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook LC, LaSarre B, Federle MJ. Interspecies Communication among Commensal and Pathogenic Streptococci. mBio. 2013;4:e00382–13. 10.1128/mBio.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128(2):151–163. 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christaki E, Diza E, Giamarellos-Bourboulis EJ, et al. NK and NKT Cell Depletion Alters the Outcome of Experimental Pneumococcal Pneumonia: Relationship with Regulation of Interferon-gamma Production. J Immunol Res. 2015;2015:532717 10.1155/2015/532717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardaci A, Papasergi S, Midiri A, et al. Protective Activity of Streptococcus pneumoniae Spr1875 Protein Fragments Identified Using a Phage Displayed Genomic Library. Kaufmann GF, ed. PLoS ONE. 2012;7(5):e36588–e36588. 10.1371/journal.pone.0036588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortiz AL, Lenz LL. A Listeria-Derived Polypeptide Promotes In Vivo Activation of NK Cells for Antitumor Therapy. IH. 2017;1(4):53–62. 10.4049/immunohorizons.1700013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarrio ML, Lee S-H, Fragoso MF, et al. Proliferation conditions promote intrinsic changes in NK cells for an IL-10 response. J Immunol. 2014;193(1):354–363. 10.4049/jimmunol.1302999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouabe H Cytokine Reporter Mice: The Special Case of IL-10. Scand J Immunol. 2012;75(6):553–567. 10.1111/j.1365-3083.2012.02695.x. [DOI] [PubMed] [Google Scholar]
- 30.Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon J-M. Natural Killer (NK) Cells in Antibacterial Innate Immunity: Angels or Devils? Mol Med. 2011;18(2):270–285. 10.2119/molmed.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–463. [DOI] [PubMed] [Google Scholar]
- 32.Pechous RD PhD. With Friends Like These: The Complex Role of Neutrophils in the Progression of Severe Pneumonia. Front Cell Infect Microbiol. 2017;7:119–11. 10.3389/fcimb.2017.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter C, Taut K, Srivastava M, et al. Lung-Specific Overexpression of CC Chemokine Ligand (CCL) 2 Enhances the Host Defense to Streptococcus pneumoniae Infection in Mice: Role of the CCL2-CCR2 Axis. J Immunol. 2007;178(9):5828–5838. 10.4049/jimmunol.178.9.5828. [DOI] [PubMed] [Google Scholar]
- 34.Preston JA, Bewley MA, Marriott HM, et al. Alveolar Macrophage Apoptosis–associated Bacterial Killing Helps Prevent Murine Pneumonia. Am J Respir Crit Care Med. 2019;200(1):84–97. 10.1164/rccm.201804-0646OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humann J, Lenz LL. Activation of Naive NK Cells in Response to Listeria monocytogenes Requires IL-18 and Contact with Infected Dendritic Cells. J Immunol. 2010;184(9):5172–5178. 10.4049/jimmunol.0903759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt RL, Filak HC, Lemon JD, Potter TA, Lenz LL. A LysM and SH3-Domain Containing Region of the Listeria monocytogenes p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells. O’Riordan MXD, ed. PLoS Pathog. 2011;7(11):e1002368–13. 10.1371/journal.ppat.1002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cyktor JC, Turner J. Interleukin-10 and Immunity against Prokaryotic and Eukaryotic Intracellular Pathogens. Maurelli AT, ed. Infect Immun. 2011;79(8):2964–2973. 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sporri R, Joller N, Hilbi H, Oxenius A. A Novel Role for Neutrophils As Critical Activators of NK Cells. J Immunol. 2008;181:7121–7130. 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 39.Small C-L, McCormick S, Gill N, et al. NK Cells Play a Critical Protective Role in Host Defense against Acute Extracellular Staphylococcus aureus Bacterial Infection in the Lung. J Immunol. 2008;180(8):5558–5568. 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 40.Weiss ID, Wald O, Wald H, et al. IFN-gamma treatment at early stages of Influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res. 2010;30(439–449):439–449. [DOI] [PubMed] [Google Scholar]
- 41.Kerr AR, Kirkham LAS, Kadioglu A, et al. Identification of a detrimental role for NK cells in pneumococcal pneumonia and sepsis in immunocompromised hosts. Microbes Infect. 2005;7:845–852. 10.1016/j.micinf.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AJ, Yau B, McQuillan JA, et al. Inflammasome-Dependent IFN-γ Drives Pathogenesis in Streptococcus pneumoniae Meningitis. J Immunol. 2012;189(10):4970–4980. 10.4049/jimmunol.1201687. [DOI] [PubMed] [Google Scholar]
- 43.Clark SE, Schmidt RL, McDermott DS, Lenz LL. A Batf3/Nlrp3/IL-18 Axis Promotes Natural Killer Cell IL-10 Production during Listeria monocytogenes Infection. Cell Reports. 2018;23(9):2582–2594. 10.1016/j.celrep.2018.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark SE, Burrack KS, Jameson SC, Hamilton SE, Lenz LL. NK Cell IL-10 Production Requires IL-15 and IL-10 Driven STAT3 Activation. Front Immunol. 2019;10:129–16. 10.3389/fimmu.2019.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 Promotes Gamma Interferon-Dependent Neutrophil Recruitment in the Lung and Improves Protection against Respiratory Streptococcus pneumoniae Infection. Infect Immun. 2007;75(3):1196–1202. 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peñaloza HF, Salazar-Echegarai FJ, Bueno SM. Interleukin 10 modulation of neutrophil subsets infiltrating lungs during Streptococcus pneumoniae infection. Biochem Biophys Rep. 2018;13:12–16. 10.1016/j.bbrep.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martner A, Dahlgren C, Paton JC, Wold AE. Pneumolysin Released during Streptococcus pneumoniae Autolysis Is a Potent Activator of Intracellular Oxygen Radical Production in Neutrophils. Infect Immun. 2008;76(9):4079–4087. 10.1128/IAI.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;2:e15–11. 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Z, Shao J, Ren H, et al. A Streptococcus suis LysM domain surface protein contributes to bacterial virulence. Vet Microbiol. 2016;187:64–69. 10.1016/j.vetmic.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Martin D, Rioux S, Gagnon E, et al. Protection from Group B Streptococcal Infection in Neonatal Mice by Maternal Immunization with Recombinant Sip Protein. Infect Immun. 2002;70(9):4897–4901. 10.1128/IAI.70.9.4897-4901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez R, Menendez R, Reyes S, et al. Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. Eur Respir J. 2011;37(2):393–399. 10.1183/09031936.00040710. [DOI] [PubMed] [Google Scholar]
- 52.Grau I, Pallares R, Tubau F, et al. Epidemiologic Changes in Bacteremic Pneumococcal Disease in Patients With Human Immunodeficiency Virus in the Era of Highly Active Antiretroviral Therapy. Arch Intern Med. 2005;165:1533–1540. [DOI] [PubMed] [Google Scholar]
- 53.Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Concurrent Infection with Hepatitis C Virus and Streptococcus pneumoniae. Emerg Infect Dis. 2017;23(7):1118–1123. 10.3201/eid2307.161858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broers CJM, Gemke RJBJ, Morré SA, Weijerman ME, van Furth AM. Increased production of interleukin-10 in children with Down syndrome upon ex vivo stimulation with Streptococcus pneumoniae. Pediatr Res. 2013;75(1):109–113. 10.1038/pr.2013.173. [DOI] [PubMed] [Google Scholar]
- 55.Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol. 2011;164(1):9–16. 10.1111/j.1365-2249.2011.04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


