Abstract
Objectives/Hypothesis:
To describe the histopathology of the invasion patterns of advanced-stage external auditory canal (EAC) squamous cell carcinoma (SCC).
Study Design
Retrospective cohort study.
Methods:
Retrospective analysis of medical records of patients diagnosed with EAC SCC available at the Massachusetts Eye and Ear temporal bone (TB) collection. TBs underwent processing for histologic examination. Hematoxylin and eosin–stained slides were examined. Histologic findings were compared to premortem clinical data.
Results:
Nine TBs were identified. Male:female ratio was 6:3. The average age of diagnosis and duration of survival was 64 (46–80 years) and 2.3 years (1–50 months), respectively. All presented with T4 disease, most commonly due to petrous apex (PA) invasion and facial nerve (FN) weakness. The mastoid air cells system served as a tumor conduit to the tegmen mastoideum and overlying dura in four patients, posterior fossa dura in one patient, vertical segment of FN in four patients, and middle ear (ME) and lateral semicircular canal in five patients. The tumor did not penetrate the tympanic membrane, oval window membrane (fenestra vestibule), or round window (RW) membrane. Supra- and infralabyrinthine pneumatization patterns allowed direct routes to the PA. Translabyrinthine PA invasion was seen in two patients. The most common locus of otic capsule invasion was the cochlea. One patient had FN paralysis due to compression rather than invasion.
Conclusions:
SCC does not tend to extend from the ME to the inner ear through the RW and vestibule-stapedial ligament. Tumors tend to spread along the preexisting TB air-tract routes. Well-aerated TB, may facilitate extension to the PA.
Keywords: Squamous cell carcinoma of the external auditory canal, temporal bone histopathology, otopathology, temporal bone histology, temporal bone malignancy
INTRODUCTION
Temporal bone (TB) malignant neoplasms are rare, occurring in 1:5,000 to 20,000 ear disorders1 and account for no more than 0.2% of all head and neck tumors.2 These malignant tumors can be categorized as primary or secondary. Primary TB malignant tumors originate in the TB, most commonly in the external auditory canal (EAC). Secondary tumors arise from extra-TB tissue and invade it by means of local extension or metastatic spread. The most common primary TB cancer is sun-induced basal cell carcinoma, but the most common advanced-stage malignancy is squamous cell carcinoma (SCC) of the EAC.3
EAC SCC tends to expand through consistent patterns such as invasion of compact bone, along blood vessels, cranial nerves, and areas of osseous weaknesses like sutures and along the TB air cells tracks. Staging is of paramount importance in determining the prognosis and treatment plan. Several TB malignancy staging systems exist,2,4–10 none of which is accepted by the American Joint Committee on Cancer.11 The most commonly used staging system is the modified Pittsburgh staging system.2
Conjugation of clinical premortem clinical data with postmortem TB histopathology is a powerful tool for understanding pathophysiology of TB disease.12 The aim of this study was to examine the invasion patterns of advanced-stage SCC of the EAC and to compare the histological findings to clinical data. To the best of our knowledge, this is the first analysis of a series of TBs of patients with primary SCC of the TB.
MATERIALS AND METHODS
All cases in the Massachusetts Eye and Ear TB collection with a primary SCC of the EAC were identified. The clinical history was collected during life through enrollment in the National Institute on Deafness and Other Communication Disorders, National Temporal Bone, Hearing and Balance Pathology Resource Registry.
After death, all TBs were prepared for light microscopy by fixation in formalin or Heidenhain’s-Susa (HS), followed by standard processing for histologic examination, including decalcification with ethylenediaminetetraacetic acid (EDTA) or trichloroacetic acid (TCA) and celloidin embedding.13 Specimens were sectioned serially in the horizontal or vertical planes at a section thickness of 20 μm. Every tenth section was stained with hematoxylin and eosin and mounted on a glass slide. The slides were examined by light microscopy.
Each subject was randomly numbered (1–9). These numbers are kept throughout the text, tables, and figures. Each TB was examined individually for malignant violation and associated conditions.
Categorical variables were described as frequency and percentage and continuous variables as median and range. All the statistical analyses were two-tailed. A P value <.05 was considered statistically significant. SPSS version 22.0 (IBM, Armonk, NY) was used for all the statistical analyses.
RESULTS
Nine TBs from nine patients were included in the cohort. The contralateral TB of each patient was used as a control. The fixating agent was formalin 10% or HS (five and four TBs, respectively) for an average period of 11 days (range, 1–26 days). Decalcification was performed by TCA or EDTA (seven and two TBs, respectively) for an average period of 108 days (range, 31–334 days).
Patients were diagnosed from 1968 to 2010 with non-normal distribution; the median year of diagnosis was 1980. The male:female distribution was 2:1. The average age of diagnosis was 64 years (range, 46–80 years). The initial biopsy upon presentation was positive for SCC in six (66%) of the patients. The other three biopsies were interpreted as squamous papilloma, cholesteatoma, and epithelial debris each, respectively. The most common presenting symptom was hearing loss (seven patients); in the majority it was severe to profound (five patients). Five patients had tinnitus, the nature of which was not available. Four patients had vertigo. Otalgia was not mentioned in the medical records. Facial paresis was present in six patients, three of whom had complete paralysis (House-Brackmann grade 614). The most common otoscopic finding was obstructing mass EAC (five patients), followed by draining ear, bleeding mass. and postauricular mass (one each) (Table I).
TABLE I.
Demographic Data and Premortem Reported Symptoms
| Presenting Symptoms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Gender | Side | Age at Diagnosis, yr | Result of Biopsy | Hearing Loss | Vertigo | Tinnitus | CNVII Palsy, HB Scale | Otoscopy | Age of Death, yr | Cause of Death |
| 1 | F | R | 62 | SCC | Moderate | − | − | 1 | Blocked EAC | 66 | Distant metastasis |
| 2 | M | R | 70 | SCC | Profound | − | + | 6 | Blocked EAC | 70 | Brain extension |
| 3 | M | R | 73 | Epithelial debris | Moderate | + | − | 6 | Blocked EAC | 79 | NA |
| 4 | F | R | 56 | SCC | Profound | − | + | 3 | NA | 57 | Cerebellar abscess |
| 5 | F | L | 55 | SCC | Severe | + | + | 6 | NA | 56 | NA |
| 6 | F | L | 46 | SCC | Profound | − | + | 2 | Blocked EAC | 49 | Massive MI |
| 7 | M | L | 51 | Infected cholesteatoma | Severe | NA | + | 1 | Post auricular mass + Blocked EAC | 52 | Brain extension |
| 8 | F | R | 80 | Squamous papilloma | NA | + | NA | 3 | Bleeding from EAC | 81 | Brain extension |
| 9 | F | L | 80 | SCC | No | + | NA | NA | Draining ear | 84 | Pneumonia |
CNVII = cranial nerve 7; EAC = external auditory canal; F = female; HB =House-Brackmann; L = left; M = male; MI = myocardial infarction; NA = not available; R = right; SCC = squamous cell carcinoma.
All cases presented with T4-stage disease according to the modified Pittsburgh staging system,2 due to one of the following clinical or histological findings: cochlear erosion (five patients), petrous apex (PA) involvement (six patients), medial middle ear (ME) wall invasion (six patients), carotid canal invasion (six patients), dural invasion (four patients), and any degree of facial nerve (FN) paresis (six patients) (Fig. 1A–I, Table II]. One patient had locoregional (cervical) lymph node metastasis upon presentation (N2). The rest of the cohort either did not have cervical spread or the status of the neck was undetermined (five and three patients, respectively). Distant metastasis was not found/reported in any patient. The average age at death was 66 years (range, 49–84 years), 2.3 years (range, 1–50 months) from initial diagnosis (Table I). The cohort is too small to determine if any presenting symptoms or signs are associated with improved survival.
Fig. 1.
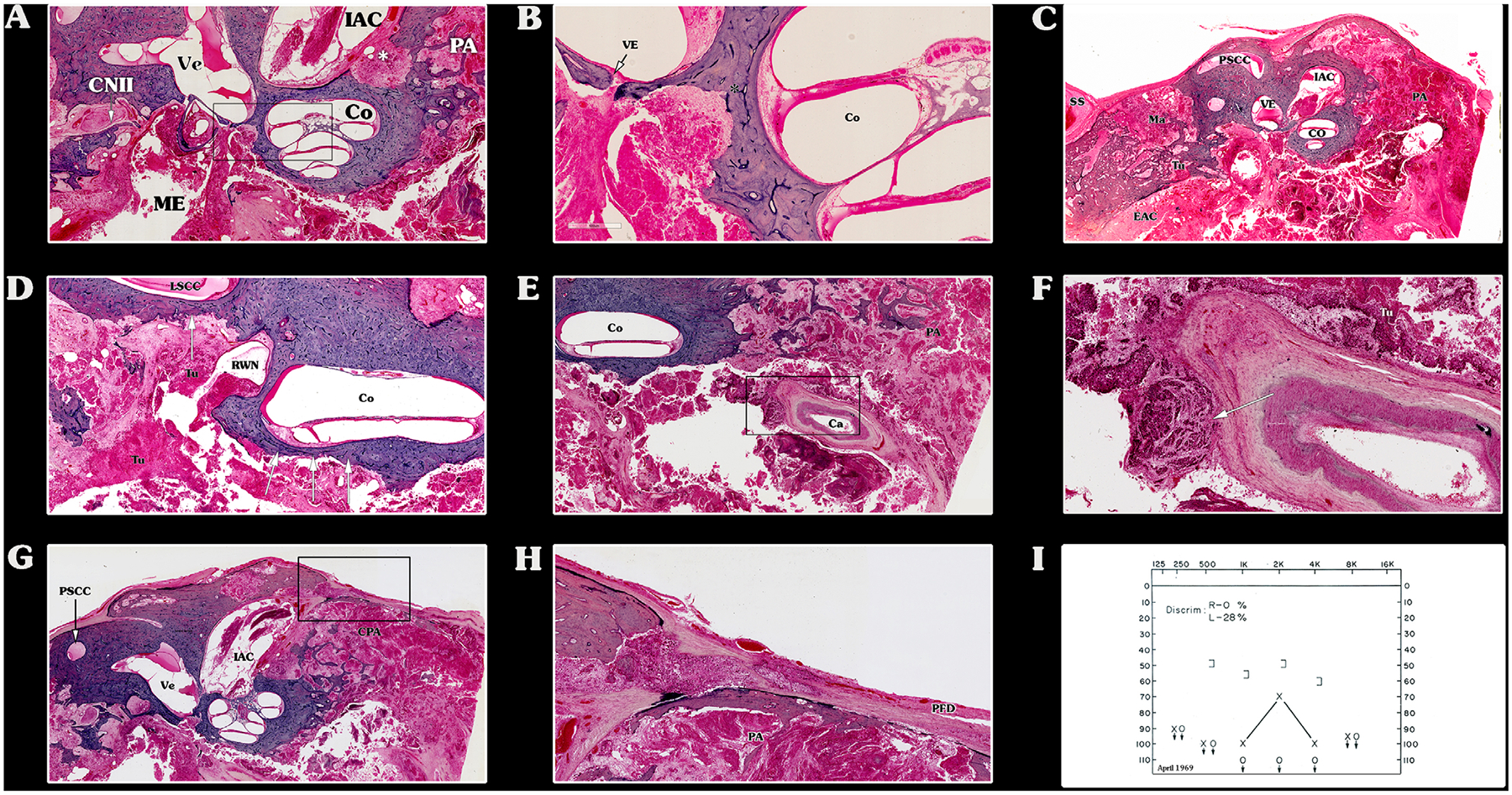
Malignant invasion throughout the temporal bone (TB), indicating stage T4. (A–I) These hematoxylin and eosin horizontally oriented histological preparations belong to patient 2. (A) Middle ear (ME) and petrous apex (PA) air cells are filled with the tumor (Tu). The stapes is partially subluxated. The otic capsule is invaded near the basal turn of the cochlea (Co) and internal auditory canal (asterisk). (B) High-power magnification of (A). The vestibular-stapedial (annular) ligament is intact, limiting tumor (Tu) invasion to the vestibule (Ve) (arrow). The tumor (Tu) invades the otic capsule toward the cochlea (Co) and vestibule (asterisk). (C) Low-power magnification of the same TB. The tumor (Tu) fills the external ear canal, mastoid (Ma), toward the sigmoid sinus (SS) and PA. The posterior fossa dura (PFD) over the mastoid is partially dehiscent. (D) The tumor (Tu) invades the round window niche (RWN) and the otic capsule overlying the lateral semicircular canal (LSCC) and cochlea (Co) (arrows). (E) Low-power magnification of the PA. The Tu encases the carotid (Ca) canal, which is partially dehiscent. (F) High-power magnification of (E). The tumor (Tu) invades the tunica externa of the petrous segment of the Ca artery (white arrow). (G) PA air cells and internal auditory canal are filled with tumor (Tu). (H) High-power magnification of (G). The internal auditory canal (IAC) is partially invaded by the tumor (Tu), as well as the PFD. (I) Pure-tone audiogram of the same patient showing profound conductive hearing loss, with reduced discrimination. EAC = external auditory canal; PSCC = posterior semicircular canal; Ve = vestibule.
TABLE II.
Tumor Spread and Staging for each of the Temporal Bones
| Patient No. | Cochlear Erosion | Medial ME Wall Invasion | Carotid Canal Invasion | Dural Invasion | PA | Tracts to the PA | T | N | M | Neoadjuvant | Definite | Adjuvant | Additional |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | • | • | • | Supra- and infralabyrinthine | 4 | 2 | 0 | 60 Gy | TTBR | 60 Gy | RND + 60 Gy* | ||
| 2 | • | • | • | • | • | Supralabyrinthine, subarcuate | 4 | X | 0 | 29.5Gy† | |||
| 3 | • | • | Supralabyrinthine | 4 | 0 | 0 | 60 Gy | ||||||
| 4 | • | • | 4 | 0 | X | 60 Gy | |||||||
| 5 | • | • | • | Supralabyrinthine, peritubal | 4 | X | 0 | 45 Gy | |||||
| 6 | • | • | Supra labyrinthine, subarcuate | 4 | X | X | STTBR | 50 Gy | |||||
| 7 | • | • | 4 | 0 | X | STTBR | 60 Gy | ||||||
| 8 | • | • | • | 4 | 0 | 0 | STP | 60 Gy | |||||
| 9 | • | • | • | Supralabyrinthine | 4 | 0 | X | 55 Gy | STTBR |
This dose was directed to the neck.
A 60-Gy dose was recommended. The patient died before completing the radiative regimen.
ME = middle ear; PA = petrous apex; T,N,M - Tumor, Node, Metastases, RND = radical neck dissection; STP = subtotal petrosectomy; STTBR = subtotal temporal bone resection; TTBR = total temporal bone resection.
All subjects were treated with external beam radiation (XRT), either as a neoadjuvant therapy before surgery (two subjects), adjuvant therapy after surgical intervention (three subjects), or as definite treatment (four subjects). The radiation dose ranged from 45–60 Gy, except subject 2, who received 29.5 Gy and died before completion of the XRT dose recommended (60 Gy). Surgery alone was not used as a treatment protocol, probably due to the advanced-stage disease at presentation (Table II). One patient (subject 1) was treated with additional radical neck dissection due to locoregional spread. The surgical pathologies of the specimens removed from the TB donors were not available for analysis.
Several soft tissue elements within the TB were found to serve as barriers, limiting tumor invasion. The tympanic membrane was found to limit tumor extension medially in four patients (Fig. 2A). In these patients, the pathway of spread from the EAC to the ME was through the bony posterior EAC wall, to the mastoid air cells system (MACS) and the antrum (Fig. 2B, C). In five patients, the tumor invaded the ME from the EAC directly, through the tympanic membrane (TM). The vestibulo-stapedial (annular) ligament was found to be a significant anatomic barrier for tumor spread from the middle to the inner ear. The resistance against tumor spread was so effective, that otic capsule invasion was seen adjacent to intact vestibulo-stapedial ligament (VSL) (Fig. 2D, E]. The round window membrane (RWM) was not invaded, although its niche was filled with tumor in three subjects (Fig. 2F). Examples of the relationship between these soft tissue barriers and tumor extension are shown in Figure 2.
Fig. 2.

Soft tissue barriers limit squamous cell carcinoma (SCC) spread throughout the temporal bone (TB). (A) Hematoxylin and eosin (H&E) horizontally oriented histological preparations of patient 1. This patient was treated with a high dose of external beam radiation (120 Gy) to the TB, replacing the SCC by fibrotic mass. The external auditory canal (EAC) is filled with fibrotic mass. The tympanic membrane is intact, effectively preventing medial invasion. The mastoid is invaded through the EAC posterior wall. The facial nerve is intact. (B–E) H&E preparation from the TB described in Figure 1. (B) H&E preparation from the TB described in Figure 1. The mastoid is invaded by the tumor (TU), totally destroying the air cells system. (C) Low-power magnification of (B). The dura is invaded, as well as the otic capsule around the vestibule (Ve), which is partially dehiscent. (D) The vestibular-stapedial ligament limits medial TU extension to the vestibule. The TU invades the otic capsule around the basal and medium cochlear turns (asterisk). (E) High-power magnification of (D). (F) The TU fills the round window niche. The round window is intact, preventing tumor extension to the inner ear spaces. ASC = anterior stapedial crus; Co = cochlea; IAC = internal auditory canal; ME = middle ear; PA = petrous apex; RWM = round window membrane; S = stapes; SS = sigmoid sinus; ST = sinus tympani. CNVII - facial nerve.
The bone overlying the middle and posterior fossae dura was dehiscent in five subjects, with involvement of the dura in four patients (Fig. 1G, H].
The anterior EAC wall was invaded in three patients, and in one patient tumor extended to the man-dibular fossa. The horizontal orientation of the slides made valuation of the roof of the EAC less reliable. The inferior wall of the EAC could not be analyzed, because it was not included in most specimens.
Several patterns of tumor spread were identified. Besides serving as a route to the ME, the MACS was found to serve as a tumor conduit to the tegmen mastoideum and overlying dura (four patients), the posterior fossa dura (one patient), the vertical segment of the FN (four patients), the ME (five patients), and the lateral semicircular canal (LSCC) (two patients). The supra and infralabyrinthine pneumatization patterns allowed direct routes of tumor spread to the PA, leaving the otic capsule intact and most easily demonstrated in the vertically oriented histological preparations (Fig. 3). The petromastoid canal served as an additional route to the PA via the subarcuate route (Fig. 4). Translabyrinthine PA invasion was seen in two patients. Once in the ME (nine patients), tumors tended to spread to the anterior and posterior attic (seven and five patients, respectively). The tegmen tympani was invaded in three patients, with middle fossa dural involvement in two of them. We found no evidence that surgical modification of the TB anatomy created iatrogenic pathways for tumor spread. The otic capsule itself was involved in six patients. The most common locus of otic capsule invasion was the cochlea, followed by the LSCC and vestibule (four, two, and two patients, respectively).
Fig. 3.

(A, B) Vertical sections of the temporal bone of patient 3. (A) Multiple nests of viable, well- differentiated squamous cell carcinoma within the middle fossa dura and in the surrounding bone. (B) The basal turn of the cochlea is dehiscent (black arrows). Co = cochlea; D = posterior fossa dura; IAC = internal auditory canal; Ma = mastoid; ME = middle ear; SS = sigmoid sinus; Ve = vestibule. CNVII - facila nerve.
Fig. 4.

Hematoxylin and eosin horizontally oriented histological preparations of patient 1 throughout this article. Tumor (TU) extension to the petrous apex between the superior semicircular canal crura is seen in the subarcuate route. A-SSCC = ampullary crus of the superior semicircular canal; LSCC = lateral semicircular canal; MFD = middle fossa dura; NA-SSCC = nonampullary crus of the superior semicircular canal.
The tympanic segment of FN was involved in five subjects, and the vertical segment was involved in three, resulting in varying degrees of FN weakness. Wallerian degeneration was present distal to the proximal-most site of neural invasion (Fig. 5). FN weakness did not mandate histologic perineural invasion (PNI). In one patient (patient 5 in the tables throughout this article) who presented with complete paralysis, FN weakness resulted from external compression of the nerve by tumor and fibrosis, without PNI. Nerve injury was associated with Wallerian degeneration (Fig. 6).
Fig. 5.

Facial nerve (FN) invasion. (A, B) Hematoxylin and eosin horizontally oriented histological preparations belong to patient 3. The meatal segment of the FN is invaded by the tumor (Tu), and distal valerian degeneration is seen. (C) Schematic drawing of the FN of the same patient. The perineural invasion occurred in the internal auditory canal (black), resulting in distal Wallerian degeneration (gray). GSPN = greater superficial petrosal nerve; Lab = labyrinth; SMF = stylomastoid foramen; Tu = tumor; Ty = tympanic segment.
Fig. 6.

(A, B) Squamous cell carcinoma of the right temporal bone. The facial nerve is compressed in its labyrinthine segment, resulting in HB 6/6 paralysis. (C) Profound sensorineural hearing loss is a result of compression of the vestibule-cochlear nerve. AS = auris sinistra (left ear); Co = cochlea; GSPN = greater superficial petrosal nerve; HB = House-Brackmann; IAC = internal auditory canal; Me = middle ear; La = labyrinthine segment of the facial nerve; SMF = stylomastoid foramen; Ty = tympanic segment; Ve = vestibule.
DISCUSSION
Primary TB SCC is a rare, aggressive tumor, comprising 80% of primary TB malignancies.15,16 This tumor carries a substantial risk for morbidity and mortality because of its aggressive nature, location at the skull base, and the common delay in diagnosis.17 Early symptoms can be easily attributed to more common conditions such as otitis media and externa. This delay is likely to have a detrimental effect on treatment and outcome. Persistent symptoms often attributable to unremitting infection, should raise a high index of suspicion for cancer. Although early publications reported chronic suppurative otitis media and chlorinated disinfectants as an independent risk factor for TB SCC,18,19 the association was later refuted. Currently, ultraviolet and ionizing radiation are the only evidence-based supported risk factors. The average age of diagnosis of our cohort was 64 years, comparable with published data.20,21 TB SCC was reported to have a higher incidence among women.22 Our cohort supports this observation, even though this finding may be the result of selection bias due to the uneven gender distribution in our TB collection (M:F = 624:545, with more males donating both TBs than women).23 More recently, equal distribution was reported.20
Symptoms can point to TB sites with tumor involvement. The triad of pain, bleeding, and otorrhea is the classical presentation of TB cancer.24 Symptoms associated with TB cancer are nonspecific and can be seen in other pathologies. Cancer patients present with bleeding as from the EAC (33%), otorrhea (72%–75%), otalgia (35%–81%), hearing loss (22%–67%), and FN palsy (17%–41%).2,18,20,24,25 These data are taken from patients diagnosed with TB SCC at any stage, and more than 50% of newly diagnosed TB SCC are presented in T1 to T3 stage.25–27 The current cohort presented with a higher incidence of a variety of signs and symptoms, which is probably due to the fact that all patients presented with T4 advanced-stage disease. An obstructing mass in the EAC is probably the most specific sign, especially when combined with FN palsy. Otalgia is the second most common presenting symptom26 but is highly nonspecific. It was found to be the leading symptom of occult TB SCC, falsely diagnosed as benign pathology (i.e., cholesteatoma, EAC atresia, EAC furuncle, preauricular fistula).17
Tissue diagnosis is imperative for optimizing therapy, but initial biopsy can be misleading; TB SCC can be misdiagnosed as cholesteatoma and pseudoepitheliomatous hyperplasia (PEH).28,29 In the current cohort, initial biopsy yielded the correct diagnosis only in 66% of patients. One patient was diagnosed with cholesteatoma and another with PEH. It is impossible to determine whether the diagnosis of cholesteatoma was a misdiagnosed SCC or represented a concomitant cholesteatoma with TB SCC. Concomitant cholesteatoma and TB SCC were reported in the literature30,31; it was hypothesized that the former may trigger the latter.32 PEH may occur in the setting of acute and chronic inflammation of the EAC.21,29 The biopsy of the lesion may be indistinguishable from SCC, even when examined by an experienced pathologist.33 Due to the ubiquitous secondary infection and edema of the periphery of TB SCC within the EAC, deep biopsy is necessary, sometimes under general anesthesia.21 Computed tomography (CT)-guided deep tissue biopsy was proven to increase the probability of conclusive histologic result in selected cases.34
Several anatomical routes and barriers for tumor spread were identified. Routes of cancer spread through the TB are highly dependent on TB pneumatization. Most of the published literature relates to cholesteatoma patterns of spread. However, there are several major differences between SCC and cholesteatoma, including cancer propensities to spread along nerves and the site of origin of the disease. Cholesteatoma originates from the TM (except when congenital, iatrogenic, and blast induced), whereas SCC may originate anywhere in the EAC.21,35 From the EAC, tumor approaches the ME via the TM, whether intact or perforated, or by means of invasion to the MACS through the posterior EAC wall. The intact TM was found to serve as a reliable tumor expansion barrier in three cases among our cohort. In several specimens, it was impossible to determine if the tumor invaded and perforated a previously intact TM or via a previously perforated TM. Another soft tissue barrier was found to be the VSL. The VSL limited SCC invasion to the vestibule in two cases. An intact VSL was seen adjacent to the otic capsule (promontory) invasion. The RWM was the second soft tissue barrier for inner ear cavity penetration. The clinical importance of these anatomical barriers is the difference in surgical extent indicated in case of intact versus invaded barriers. Tumor confined to the EAC, with some bone erosion (T1–T2), can be treated effectively with a lateral TB resection (LTBR).9 In these cases LTBR with free pathological margins can save a patient from the need to be irradiated. Historically, extensive en bloc surgery (subtotal and total TB resections) has been offered to patients with more advanced-stage disease. However, en bloc resection has not proven to have superior results as compared to procedures involving piecemeal dissection. These advanced-stage disease ears usually requires the addition of radiation therapy. Once the TM is invaded and tumor extends to the ME, LTBR may be considered, and subtotal TB resection (STTBR) or extended canal wall down tympanomastoidectomy may be used as more radical alternatives with similar cure rates.18,36 Although the inner ear is involved through otic capsule invasion or through one of the inner ear windows, STTBR is indicated. Total TB resection is rarely indicated or performed nowa-days. Defining the typical routes of SCC spread in the TB can help plan surgery and direct efforts to TB subsites involved and anticipate possible location of disease not depicted by preoperative imaging.
Comparably, soft tissue serving as a barrier for tumor spread is seen in the laryngeal ligamental frame-work. These ligaments are composed of ground substance, elastic fibers, and dense collagen. They may serve as a strong anatomic barrier to laryngeal tumors. For example, the vocal ligament tendon, also known as Broyles ligament, is a strong barrier to tumor invasion.37–39
TB pneumatization is generally divided into five regions: ME, mastoid, perilabyrinthine air cells, PA, and accessory cells.40,41 The accessory cells are further divided into zygomatic, squamous, occipital, and styloid. The ME space and its associated air cells system are divided into five areas, relative to the mesotympanum: epitympanic, hypotympanic, protympanic, posterior tympanic, and mesotympanic.42 An extensively pneumatized TB may facilitate tumor extension medially from the ME and mastoid to the PA, via the posteromedial tract, post-erosuperior tract, and the subarcuate tract. SCC of the TB can spread along any of these tracts exactly as cholesteatoma or suppurative disease approaches the PA. There is a notion that surgical modification of the TB anatomy may create new and iatrogenic pathways for tumor spread (i.e., from the drilled mastoid process to the neck). However, we found no evidence that this phenomenon actually occurred in the series. The neurotrophic nature of SCC can make the FN an additional route for tumor spread. The vertical (mastoid) segment is the most commonly involved, followed by the tympanic segment. TB malignancies presenting with FN palsy doe not necessarily mean PNI.43 In one case, facial palsy resulted from compression of the vertical segment by tumor and fibrosis. Therefore, at least in theory, it may be possible in selected cases to anatomically preserve the FN during tumor extirpation. However, no reliable preoperative and intraoperative method to differentiate between PNI and compression is available.
Advances in surgical and reconstructive techniques have allowed adequate tumor ablation and restoration of cosmesis and function. Radiotherapy has an important role. Treatment of these uncommon neoplasms is best managed by a multidisciplinary team comprising a otolar-yngologist, reconstructive surgeon, neurosurgeon, and oncologist. The complex TB anatomy makes curing TB SCC a challenging mission. The specimens in our cohort that were not operated upon can serve as a valuable predictor to surgical success in terms of oncological margins. We found no single preoperative sign or symptom that can guide the extent of the oncological surgical intervention. Surgery should be tailored to the patient according to physical examination and imaging. The number of options for hearing rehabilitation is growing, including a variety of bone-vibrating hearing aids.
Radiation is known to have extensive effect on the TB and its content, both in the early and late postradiation periods. The early reaction consists of vasculitis, inflammatory changes of ME mucosa, which was seen in all of our specimens, and focal osteoradionecrosis (ORN).44,45 ORN of the otic capsule, tympanic, mastoid, and petrous bones are associated with the late effects as well46 and was not detected in our series. Histologic postirradiation changes in bone architecture consist of osteocyte death, resulting in empty lacunae, and infiltration of bone by fibrillar connective tissue around dead bony spicules. Another late consequence is atrophy of the vestibule-stapedial (annular) ligament, with resultant fistulization of the oval window.41 Each of these osseous changes may be associated with reduced resistance to malignant invasion. Subluxation of the vestibule-stapedial ligament, without oval window fistulization, was seen in one patient (Fig. 1A). This should be taken into account while analyzing irradiated TB histology.
The limitation of the study is derived from its retrospective nature. All patients presented with advanced-stage disease, limiting the application of the finding to early-stage SCC. In clinical practice, imaging is of paramount importance, for disease extent assessment and surgical tailoring. Unfortunately, our cohort lacks axial imaging (CT or magnetic resonance imaging), due to one of two reasons: most subjects (five) were diagnosed when the technology was not available, and four medical records do not include imaging (if performed). This fact does not allow us to correlate clinical presentation, imaging findings, and histological disease extent.
CONCLUSION
TB histopathology can elucidate the extension routes of primary SCC carcinoma. The TM may serve as an incomplete barrier for tumor extension from the EAC to the ME. SCC does not tend to extend from the ME to the inner ear through the round window and vestibule-stapedial ligament. Tumors do tend to spread along the preexisting TB air-tract routes. A well-aerated TB may facilitate easier extension to the PA. In one case, facial paresis resulted from nerve compression and not invasion.
ACKNOWLEDGMENTS
The authors are grateful to Meng Yu Zhu, Barbara Burgess, Diane Jones, and Jennifer T. O’Malley for technical assistance.
This work was supported by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders (U24DC013983-01).
Footnotes
Editor’s Note: This Manuscript was accepted for publication on March 20, 2020.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Lewis JS. Surgical management of tumors of the middle ear and mastoid. J Laryngol Otol 1983;97:299–312. [DOI] [PubMed] [Google Scholar]
- 2.Moody SA, Hirsch BE, Myers EN. Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Otol Neurotol 2000;21: 582–588. [PubMed] [Google Scholar]
- 3.Koriwchak M Temporal bone cancer. Am J Otol 1993;14:623–626. [PubMed] [Google Scholar]
- 4.Crabtree JA, Hill Britton B, Pierce MK. Carcinoma of the external auditory canal. Laryngoscope 1976;86:405–415. [DOI] [PubMed] [Google Scholar]
- 5.Stell PM, McCormick MS. Carcinoma of the external auditory meatus and middle ear: prognostic factors and a suggested staging system. J Laryngol Otol 1985;99:847–850. [DOI] [PubMed] [Google Scholar]
- 6.Kinney SE. Squamous cell carcinoma of the external auditory canal. Am J Otol 1989;10:111–116. [PubMed] [Google Scholar]
- 7.Shih L, Crabtree JA. Carcinoma of the external auditory canal: an update. Laryngoscope 1990;100:1215–1218. [DOI] [PubMed] [Google Scholar]
- 8.Spector JG. Management of temporal bone carcinomas: a therapeutic analysis of two groups of patients and long-term followup. Otolaryngol Head Neck Surg 1991;104:58–66. [DOI] [PubMed] [Google Scholar]
- 9.Pensak ML, Gleich LL, Gluckman JL Shumrick KA. Temporal bone carcinoma: contemporary perspectives in the skull base surgical era. Laryngoscope 1996;106:1234–1237. [DOI] [PubMed] [Google Scholar]
- 10.Manolidis S, Pappas JD, Von PD, Jackson CG. Temporal bone and lateral skull base malignancy: experience and results with 81 patients. Am J Otol 1998;19:S1–S15. [PubMed] [Google Scholar]
- 11.Gidley PW. Managing malignancies of the external auditory canal. Expert Rev Anticancer Ther 2009;9:1277–1282. [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht HF. Temporal bone collections in Europe and the United States observations on a productive laboratory, pathologic findings of clinical relevance, and recommendations. Ann Otol Rhinol Laryngol 1987;96:3–19. [PubMed] [Google Scholar]
- 13.Schuknecht HF, Merchant SN, Nadol JB. Schuknecht’s Pathology of the Ear. Shelton, CT: People’s Medical Publishing House-USA; 2010. [Google Scholar]
- 14.House WE. Facial nerve grading system. Otolaryngol Head Neck Surg 1985; 93:184–193. [DOI] [PubMed] [Google Scholar]
- 15.Morton RP, Stell PM, Derrick PP. Epidemiology of cancer of the middle ear cleft. Cancer 1984;53:1612–1617. [DOI] [PubMed] [Google Scholar]
- 16.Kuhel WI, Hume CR, Selesnick SH. Cancer of the external auditory canal and temporal bone. Otolaryngol Clin North Am 1996;29:827–852. [PubMed] [Google Scholar]
- 17.Zhang T, Dai C, Wang Z. The misdiagnosis of external auditory canal carcinoma. Eur Arch Otorhinolaryngol 2013;270:1607–1613. [DOI] [PubMed] [Google Scholar]
- 18.Moffat DA, Grey P, Ballagh RH, Hardy DG. Extended temporal bone resection for squamous cell carcinoma. Otolaryngol Head Neck Surg 1997; 116:617–623. [DOI] [PubMed] [Google Scholar]
- 19.Monem SA, Moffat DA, Frampton MC. Carcinoma of the ear: a case report of a possible association with chlorinated disinfectants. J Laryngol Otol 1999;113:1004–1007. [DOI] [PubMed] [Google Scholar]
- 20.Nyrop M, Grøntved A. Cancer of the external auditory canal. Arch Otolaryngol Head Neck Surg 2002;128:834–837. [DOI] [PubMed] [Google Scholar]
- 21.Ouaz K, Robier A, Lescanne E, Bobillier C, Moriniere S, Bakhos D. Cancer of the external auditory canal. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130:175–182. [DOI] [PubMed] [Google Scholar]
- 22.Kwok HC, Morton RP, Chaplin JM, McIvor NP, Sillars HA. Quality of life after parotid and temporal bone surgery for cancer. Laryngoscope 2002; 112:820–833. [DOI] [PubMed] [Google Scholar]
- 23.Ungar OJ, Franck M, Nadol JB, Santos F. Arachnoid cysts of the internal auditory canal: an underappreciated entity? Laryngoscope 2019;129:1667–1674. [DOI] [PubMed] [Google Scholar]
- 24.Moffat DA, Wagstaff SA, Hardy DG. The outcome of radical surgery and postoperative radiotherapy for squamous carcinoma of the temporal bone. Laryngoscope 2005;115:341–347. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie MB, Francis HW, Chee N, Eisele DW. Squamous cell carcinoma of the temporal bone: a radiographic-pathologic correlation. Arch Otolaryngol Head Neck Surg 2001;127:803–807. [PubMed] [Google Scholar]
- 26.Gidley PW, Roberts DB, Sturgis EM. Squamous cell carcinoma of the temporal bone. Laryngoscope 2010;120:1144–1151. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Tu G, Xu G, Tang P, Hu Y. Squamous cell carcinoma of temporal bone: reported on 33 patients. Head Neck 1999;21:461–466. [DOI] [PubMed] [Google Scholar]
- 28.Chee G, Mok P, Sim R. Squamous cell carcinoma of the temporal bone: diagnosis, treatment and prognosis. Singapore Med J 2000;41:441–451. [PubMed] [Google Scholar]
- 29.Sauerwein W, Feldmann HJ. Radiotherapy of carcinoma of the auditory canal. Strahlenther Onkol 1988;164:567–573. [PubMed] [Google Scholar]
- 30.Takahashi K, Yamamoto Y, Sato K, Sato Y, Takahashi S. Middle ear carcinoma originating from a primary acquired cholesteatoma: a case report. Otol Neurotol 2005;26:105–108. [DOI] [PubMed] [Google Scholar]
- 31.Westerman ST, Sylvia LC, Tepper E. Carcinoma arising out of a primary acquired cholesteatoma. J Med Soc N J 1981;78:600–602. [PubMed] [Google Scholar]
- 32.Rothschild S, Ciernik IF, Hartmann M, Schuknecht B, Lütolf UM, Huber AM. Cholesteatoma triggering squamous cell carcinoma: case report and literature review of a rare tumor. Am J Otolaryngol 2009;30:256–260. [DOI] [PubMed] [Google Scholar]
- 33.Nadol JB, McKenna MJ, eds. Surgery of the Ear and Temporal Bone. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 34.Zaharopoulos P Carcinoma of temporal bone, base of the skull: diagnosis by needle aspiration cytology. Diagn Cytopathol 2001;24:356–360. [DOI] [PubMed] [Google Scholar]
- 35.Breau RL, Gardner EK, Dornhoffer JL. Cancer of the external auditory canal and temporal bone. Curr Oncol Rep 2002;4:76–80. [DOI] [PubMed] [Google Scholar]
- 36.Prasad S, Janecka IP. Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone: a literature review. Otolaryngol Head Neck Surg 1994;110:270–280. [DOI] [PubMed] [Google Scholar]
- 37.Tucker GF Jr, Smith HR Jr. A histological demonstration of the development of laryngeal connective tissue compartments. Trans Am Acad Ophthalmol Otolaryngol 1962;66:308. [PubMed] [Google Scholar]
- 38.Beitler JJ, Mahadevia PS, Silver CE, et al. New barriers to ventricular invasion in paraglottic laryngeal cancer. Cancer 1994;73:2648–2652. [DOI] [PubMed] [Google Scholar]
- 39.Bridger GP, Nassar VH. Cancer spread in the larynx. Arch Otolaryngol 1972;95:497–505. [DOI] [PubMed] [Google Scholar]
- 40.Allam AF. Pneumatization of the temporal bone. Ann Otol Rhinol Laryngol 1969;78:49–64. [DOI] [PubMed] [Google Scholar]
- 41.Merchant SN. Schuknecht’s Pathology of the Ear. Shelton, CT: People’s Medical Publishing House-USA; 2010. [Google Scholar]
- 42.Eby TL, Nadol JB Jr. Postnatal growth of the human temporal bone: implications for cochlear implants in children. Ann Otol Rhinol Laryngol 1986; 95:356–364. [DOI] [PubMed] [Google Scholar]
- 43.Ungar OJ, Nadol JB, Faquin WC, Carey JP, Handzel O, Santos F. Histological characteristics of intra-temporal facial nerve paralysis in temporal bone malignancies [published online August 1, 2019]. Laryngoscope. 10.1002/lary.28212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borsanyi S, Blanchard CL, Thorne B. XXI the effects of ionizing radiation on the ear. Ann Otol Rhinol Laryngol 1961;70:255–262. [Google Scholar]
- 45.Frey JG. Über die Kombinationsbehandlung von Röntgenspätschäden der Haut mit Kurzwellen und Vitamin E. Strahlentherapie 1954;95: 440–443. [PubMed] [Google Scholar]
- 46.Wurster CF, Krespi YP, Curtis AW. Osteoradionecrosis of the temporal bone. Otolaryngol Head Neck Surg 1982;90:126–129. [DOI] [PubMed] [Google Scholar]


