Abstract
Background
Perceived stress can lead to dysregulated cortisol patterns, including blunted peaks and flatter slopes, which are associated with increased morbidity and mortality risks. Couples’ interdependence provides a prime opportunity for partners’ stress to disrupt a healthy cortisol pattern. This study examined how individuals’ own perceived stress and their partners’ perceived stress shape cortisol levels and slopes across the day, as well as how positive and negative behaviors during conflict discussions impact associations between stress and cortisol.
Methods
Both partners of a married couple (n = 43 couples, 86 individuals) completed a full day in-person visit. Each partner completed the Perceived Stress Scale, and all couples engaged in a 20-min marital problem discussion which was recorded and later coded for positive and negative behaviors using the Rapid Marital Interaction Coding System (RMICS). Partners also provided five salivary cortisol samples across the day, two samples before the conflict and three after the conflict. The dyadic design and analyses provided a way to account for the interdependent nature of married couples’ data, as well as to use the Actor-Partner Interdependence Model (APIM) to assess the mutual influence of spouses’ stress on cortisol.
Results
Individuals with more stressed partners had flatter cortisol slopes than individuals with less stressed partners, who showed steeper and thus healthier declines across the day. Individuals’ cortisol levels at the beginning of the day were similar regardless of their partners’ perceived stress, but individuals with more stressed partners had higher cortisol levels 30-min, 1 hr, and 4 hr after the conflict discussion than those with less stressed partners. Couples’ behavior during the conflict moderated the relationship between partner perceived stress and average cortisol; when couples used more negative and less positive behaviors, individuals with more stressed partners had higher average cortisol levels than those with less stressed partners.
Conclusion
On a day couples experienced conflict, having a partner with higher perceived stress is associated with dysregulated cortisol patterns, including higher levels and flatter slopes, but having a partner with lower perceived stress is linked to steeper and thus healthier cortisol declines. A partner’s stress was particularly consequential for one’s own cortisol when couples used more negative and fewer positive behaviors during a conflict discussion. This research adds to the growing literature on pathways connecting marital interactions to important biorhythms and health.
Keywords: marriage, stress, cortisol, conflict, health, romantic relationships
1. Introduction
Although being in a high quality marriage is consistently linked to better health, married people can experience stress that has negative health consequences (Robles et al., 2014). Perceived stress can alter the hypothalamic-pituitary-adrenal axis and its end product, cortisol (Chrousos, 2009). Cortisol follows a diurnal rhythm, with a healthy diurnal rhythm peaking within an hour of waking and gradually declining across the day. However, chronically elevated stress can dysregulate the diurnal rhythm and lead to blunted cortisol peaks and flattened slopes (Gunnar and Vazquez, 2001). These dysregulated patterns affect multiple regulatory systems, such as immune, metabolic, and autonomic function, which can increase morbidity and mortality risks (Adam et al., 2017; Kumari et al., 2011, 2009).
Couples’ interconnectedness provides a prime opportunity for one partner’s stress perceptions to influence the health of the other partner (Kiecolt-Glaser and Wilson, 2017). Indeed, interdependence theory highlights how people’s own experiences and their partners’ experiences affect their own outcomes including their health (Kelley and Thibaut, 1978; Lewis et al., 2006). For instance, the more people and their partners felt stressed, the worse their own health and well-being (Falconier et al., 2015; Shrout, 2019). Initial research about physiological outcomes supports interdependence theory. For example, the more caregivers perceived their spouses were stressed, the higher their own systolic blood pressure and heart rate (Monin et al., 2010). In addition, a dyadic study on middle age and older couples showed that greater perceived stress in one partner was associated with higher blood pressure in the other partner (Birditt et al., 2016).
Spouses’ cortisol levels and diurnal rhythms are also connected. Couples’ cortisol can synchronize or covary over time, meaning partners’ cortisol changes in unison (Timmons et al., 2015). For example, couples had similar cortisol levels at various points throughout the day (Papp et al., 2013), and their diurnal cortisol declined at similar rates (Liu et al., 2013). A daily diary study showed that on days that spouses were more physically intimate, such as when they hugged, held hands, and touched, they had lower average cortisol levels (Ditzen et al., 2008). Longitudinal research has demonstrated that partners might impact each other’s diurnal rhythms over time. The more people felt understood, cared for, and appreciated by their partners, the steeper (i.e., healthier) their diurnal cortisol slopes were 10 years later (Slatcher et al., 2015). Despite these health benefits, spouses can have negative effects on each other’s cortisol levels and slopes. Indeed, strained spouses had similarly low cortisol levels upon awakening (Liu et al., 2013), and the more dissatisfied spouses were in their relationships, the more likely their cortisol slopes were to change in unison (Saxbe and Repetti, 2010). In addition, women’s cortisol increased when watching their male partners complete the Trier Social Stress Test, and the more similar their own cortisol increase was to their partners’ increase, the more likely the couples’ cortisol was to covary over two subsequent days (Engert et al., 2018). Despite previous research stating the importance of perceived stress in couples’ physiology (Timmons et al., 2015), the roles of individuals’ own and their partners’ perceived stress on cortisol have not been examined. Studies linking both spouses’ stress to one’s own cortisol would help to understand how partners “get under the skin” to influence stress-related health risk.
Spouses’ conflict, and the way they communicate with each other, also have important implications for their cortisol. The more people received affectionate communication from their spouses, the higher their own waking cortisol levels and the steeper their own cortisol declines across the day (Floyd and Riforgiate, 2008). People who were objectively rated as using more negative and hostile behaviors during conflict, such as demanding too much from each other and withdrawing from the conversation, had higher cortisol levels than their less negative counterparts (Kiecolt-Glaser et al., 1997, 1996; Miller et al., 1999). Likewise, spouses who reported that they used more negative behaviors during conflict also had higher cortisol than those who reported using less negative behaviors (Heffner et al., 2006). This work shows that people’s cortisol can be susceptible to unhealthy changes during conflict, particularly when they use more negative and less positive behaviors; however, less is known about how fast or slow spouses’ cortisol declines after conflict. Assessing spouses’ cortisol levels across the day and after conflict—a common and yet stressful occurrence (Shrout et al., 2019; Wright and Loving, 2011)—may offer new insight into marriage’s health impact.
Given the potent effects spouses have on each other’s cortisol, and that individuals’ stress perceptions are associated with their own and their partners’ health outcomes (Shrout, 2019; Slatcher et al., 2015), it is important to assess how perceived stress relates to cortisol through a dyadic lens. Although individuals’ own perceived stress is associated with dysregulated cortisol patterns (Chrousos, 2009; Gunnar and Vazquez, 2001), spouses are interdependent and affect one another (Kelley and Thibaut, 1978; Kiecolt-Glaser and Wilson, 2017). For instance, people with stressed partners might have flatter, less healthy cortisol slopes. In contrast, spouses’ interdependence may be beneficial; people with less stressed spouses may have healthy cortisol declines across the day. In addition, because conflict is associated with spouses’ cortisol (Kiecolt-Glaser et al., 1997, 1996), individuals might have higher cortisol levels and slower declines if they or their partners feel stressed and use more negative and less positive behaviors during conflict. Cortisol’s effects on multiple biological and regulatory systems make it a key mechanism to test how spouses pose risks to their own and their partners’ long-term health (Adam et al., 2017; Kumari et al., 2011, 2009). Thus, the effects of spouses’ perceived stress and their conflict behaviors on each other’s cortisol levels and slopes may provide new pathways linking marriage to health.
Accordingly, this study assessed whether individuals’ own perceived stress and their partners’ perceived stress was linked to their own cortisol levels and trajectories across the day, as well as how their behavior during a marital conflict moderated the associations between stress and cortisol. We accounted for the interdependence of married couples and links between both one’s own and one’s partner’s perceived stress on cortisol using the Actor-Partner Interdependence Model (APIM). We hypothesized that individuals’ own perceived stress, as well as their partners’ perceived stress, would be associated with higher average cortisol levels and less healthy (i.e., flatter) cortisol slopes across the day. We also expected that the negative effects of individuals’ own and their partners’ perceived stress on cortisol would be exacerbated when couples used more negative behaviors and fewer positive behaviors during the conflict discussion.
2. Method
2.1. Participants
Couples (n = 43 couples, 86 participants) were recruited for a parent study on metabolic responses to high-fat meals (Kiecolt-Glaser et al., 2015b). Interested couples completed online and in-person screens to determine eligibility. Exclusions included couples married fewer than 3 years, as well as those with sensory impairments that would interfere with study completion, chronic health problems or behaviors (e.g., diabetes, anemia, smoking, alcohol/drug abuse), and prescription medications other than birth control (n = 5) or levothyroxine (n = 3). To address the parent study’s aims, we prioritized recruiting couples who were sedentary and overweight. A total of 350 interested individuals were excluded because they or their spouse did not meet the stringent health criteria. Participants’ average age was 38.22 years (SD = 8.18, range = 24–61), and most participants were white (81%). All couples were married with an average duration of 11.49 years (SD = 6.64, range = 3–27). Most participants were college educated (67%) and worked full-time (70%).
2.2. Procedure
Participants completed two full-day visits at the Clinical Research Center (CRC), a hospital research unit; we used data from the second visit when perceived stress was assessed. During this double-blind randomized crossover study, spouses ate either a high saturated or a high oleic sunflower oil meal during the second visit (to test the parent study’s key aims). Couples were told to avoid alcohol and caffeine use within 1 day prior and strenuous physical activity within 2 days before the study visit. Participants were also instructed to stop taking aspirin, vitamins (except multivitamins), antioxidants, and any other dietary supplements for 7 days before admission. On the day before their second visit, participants received three standardized meals from the CRC’s metabolic kitchen, reducing any variability in physiology associated with recent food intake. They began a 12-h fast at 7:30 p.m. the evening before the visit and then couples arrived at 7:30 a.m. Following a brief resting period, participants ate either the high saturated fat or high oleic sunflower oil meal; spouses received the same meal, and they were required to eat the entire meal. Spouses then completed several self-report questionnaires including perceived stress. Couples engaged in a 20-min marital problem discussion later that morning, about 2 h after the meal. Salivary cortisol was sampled before the meal (8:30 a.m.), 2 hr after the meal/1 h before the conflict (11:00 a.m.), and approximately 30 min (12:30 p.m.), 1 hr (1:00 p.m.), and 4 hr (4:45 p.m.) after the conflict discussion. Study procedures were approved by the Ohio State University Institutional Review Board; participants provided written informed consent before participating.
2.3. Perceived stress
Each partner completed the 4-item Perceived Stress Scale (PSS-4), a widely used psychological instrument for measuring stress perceptions (Cohen et al., 1983). The 4-item version provides researchers the opportunity to assess perceived stress relatively easily in situations where short questionnaires are required. Cronbach’s α was .74 in the present study, which is consistent with prior research (e.g., α = 0.72) (Cohen et al., 1983).
2.4. Cortisol
Saliva was collected using a salivette (Sarstedt, Newton, NC), an untreated sterile cotton roll that was placed in the participant’s mouth for approximately 2 min or upon saturation. Each subject’s samples were frozen after collection and analyzed within the same assay using the Cortisol Coat-A-Count radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles, CA).
2.5. Marital conflict behavior
To initiate the couples’ marital disagreement discussions, an experimenter first conducted a 10–20-min interview to identify the most contentious topics within the marriage for both spouses (Kiecolt-Glaser et al., 2005; Kiecolt-Glaser and Newton, 2001). These topics were selected from an inventory each spouse completed about their relationship problems. Couples were then asked to discuss and try to resolve one or more marital issues that the experimenter judged to be the most conflict-producing (e.g., money, communication, or in-laws). The research team remained out of sight while videotaping the subsequent 20-min problem discussion.
Couples’ behavior during the marital disagreement discussion was coded using the Rapid Marital Interaction Coding System (RMICS) which discriminates well between distressed and nondistressed couples (Heyman, 2004). RMICS is designed to code dyadic behavior and interaction patterns, and thus both partners’ behaviors are coded simultaneously to create individual- and couple-level ratings. Consistent with prior research (Kiecolt-Glaser et al., 2018, 2015b), this study focused on couple-level ratings and how couples’ behaviors related to each partner’s cortisol levels.
A composite index for couples’ negative conflict behavior summed four RMICS codes: psychological abuse (e.g., verbal statements that indicate disgust, contempt, belligerence, or devaluing, such as “That’s a stupid idea,” as well as nonverbal behaviors like glowering or smacking fists into hand); distress-maintaining attributions (e.g., negative causal explanations, “You’re only being nice so I’ll have sex with you tonight” or “You were being mean on purpose”); hostility (e.g., verbal indications such as criticism or hostile voice tone [“You never listen to me”], along with nonverbal behaviors like rolling the eyes dramatically or quickly turning away from the partner); and withdrawal (behaviors that suggest pulling back from the interaction or not listening, including verbal statements like “I don’t want to discuss it anymore!” and nonverbal behaviors such as not listening or moving chair away from the partner).
A composite index for couples’ positive conflict behavior summed five RMICS codes: acceptance (e.g., verbal and nonverbal attempts at active listening and expressing concern, “I could imagine that you would be sad now” and holding the partner’s hand); relationship-enhancing attributions (e.g., negative behaviors explained by circumstances or to involuntary or unintentional causes, “You’re short with me because you’ve had a hard day”); self-disclosure (e.g., any verbal expression of feelings, wishes, or beliefs not considered hostile toward the partner, “I felt uncomfortable at your parents’ house” or “I think children should respect their parents”); humor (e.g. playful joking, teasing, or sarcasm, such as “Let’s shave our heads and sell flowers at the airport for extra income”); and constructive problem discussion (e.g., constructive approaches to discussing or solving problems, “Let’s stop eating out so often” or “I think you’re right about that,” as well as nonverbal signs of agreement).
All conflict interactions were coded by at least one trained coder; approximately 25% of all conflict interactions were dual coded to establish interrater agreement. Holley and Gilford’s G was used to quantify interrater agreement for the RMICS positive and negative behavior composites (Holley and Guilford, 1964; Xu and Lorber, 2014). Interrater agreement was high, with a value of 0.97 averaged over G indices for negative behaviors and 0.87 averaged over positive behaviors.
2.6. Covariates
Participant age, gender, and trunk fat served as covariates given their associations with cortisol levels and slopes (Kirschbaum et al., 1992; Larsson et al., 2009; Roelfsema et al., 2017). Meal type was controlled for because of its potential effects on cortisol (Kiecolt-Glaser et al., 2015a). Marital satisfaction was also included as a covariate to account for the relationship between general relationship quality and cortisol levels (Ditzen et al., 2011). Marital satisfaction was assessed with the Couples Satisfaction Index, a measure that can discriminate between satisfied and dissatisfied couples with greater precision that other commonly used marital scales (Funk and Rogge, 2007).
2.7. Analytic plan
Multilevel modeling (MLM) was used to conduct APIMs testing the study hypotheses (Kenny et al., 2006). This analytical approach allowed for explicit modeling of the non-independence in married couples’ data. We specified that individuals were nested within couples and that time was a repeated factor across couples (i.e., that we had observations for both partners on each sample time; Kenny et al., 2006). We accounted for the similarity in the spouses’ average outcomes by including a random intercept and a random slope for time using a variance components covariance structure. We also accounted for the similarity in the residuals of the spouses’ outcomes across the specific time points using an unstructured covariance matrix. An additional strength of using MLM is that it accounts for missing data by maximizing the use of existing data. MLM analyses were performed using the MIXED MODELS procedure with restricted maximum likelihood estimation in SPSS version 25. Cortisol data were natural-log (ln) transformed to better approximate normality of residuals. All analyses used the transformed data; MLM coefficients in Tables 2, 4, and 5 represent natural-log transformed salivary cortisol. Cortisol numbers in Tables 1 and 3 and Figure 1 represent back transformed geometric means (anti-log) for clinical interpretability. Initial exploratory data analysis revealed that the cortisol trajectories were approximately linear; thus, the MLMs included a linear fixed effect of time. Prior to the main analyses, the independent variables (own stress, partner stress, time, positive conflict behavior, and negative conflict behavior) and continuous covariates (age, trunk fat, and marital satisfaction) were grand mean-centered to improve the interpretability of the intercepts. Dichotomous covariates were effects coded (gender: female = −1, male = 1; meal: high oleic sunflower oil = −1, high saturated fat = 1).
Table 2.
Multilevel model coefficients for own perceived stress and partner perceived stress predicting salivary cortisol (log nmol/l)
| Model 1a |
Model 1b |
|||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| Intercept | 1.116 | 0.042 | <0.001 | 1.115 | 0.042 | <0.001 |
| Time | −0.103 | 0.013 | <0.001 | −0.103 | 0.013 | <0.001 |
| Age | −0.008 | 0.005 | 0.169 | −0.008 | 0.005 | 0.168 |
| Gender | 0.086 | 0.026 | 0.001 | 0.085 | 0.026 | 0.001 |
| Meal | 0.010 | 0.044 | 0.817 | 0.010 | 0.044 | 0.812 |
| Trunk fat | −0.000 | 0.000 | <0.001 | −0.000 | 0.000 | <0.001 |
| Marital satisfaction | −0.000 | 0.001 | 0.976 | −0.000 | 0.001 | 0.985 |
| Own perceived stress | 0.021 | 0.013 | 0.112 | 0.021 | 0.013 | 0.110 |
| Partner perceived stress | 0.034 | 0.012 | 0.008 | 0.034 | 0.012 | 0.008 |
| Time*Own stress | −0.001 | 0.004 | 0.878 | |||
| Time*Partner stress | 0.008 | 0.004 | 0.039 | |||
Note. Participant gender coded 1 = male, −1 = female. Meal coded 1 = high saturated fat, −1 = high oleic sunflower oil. Numbers represent natural-log transformed salivary cortisol. Higher order interactions were not significant and thus were removed in constructing the final models.
Table 4.
Multilevel model coefficients for own perceived stress and partner perceived stress by couples’ negative conflict behavior predicting salivary cortisol (log nmol/l)
| Model 2a |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| Intercept | 1.106 | 0.040 | <0.001 | 1.105 | 0.040 | <0.001 |
| Time | −0.103 | 0.013 | < 0.001 | −0.103 | 0.013 | <.001 |
| Age | −0.009 | 0.006 | 0.118 | −0.009 | 0.006 | 0.117 |
| Gender | 0.084 | 0.026 | 0.002 | 0.083 | 0.026 | 0.002 |
| Meal | 0.009 | 0.043 | .838 | 0.009 | 0.043 | 0.840 |
| Trunk fat | −0.000 | 0.000 | <0.001 | −0.000 | 0.000 | <0.001 |
| Marital satisfaction | 0.000 | 0.001 | 0.758 | 0.000 | 0.001 | 0.759 |
| Own perceived stress | 0.026 | 0.013 | 0.049 | 0.026 | 0.013 | 0.049 |
| Partner perceived stress | 0.035 | 0.012 | 0.006 | 0.035 | 0.012 | 0.007 |
| Couple negative conflict behavior | −0.002 | 0.002 | 0.324 | −0.002 | 0.002 | 0.323 |
| Couple negative conflict behavior*Own stress | 0.000 | 0.001 | 0.374 | 0.000 | 0.001 | 0.367 |
| Couple negative conflict behavior*Partner stress | 0.001 | 0.001 | 0.007 | 0.001 | 0.001 | 0.007 |
| Time*Own stress | −0.001 | 0.004 | 0.891 | |||
| Time*Partner stress | 0.009 | 0.004 | 0.037 | |||
| Time*Couple negative conflict behavior | 0.000 | 0.001 | 0.646 | |||
Note. Participant gender coded 1 = male, −1 = female. Meal coded 1 = high saturated fat, −1 = high oleic sunflower oil. Numbers represent natural-log transformed salivary cortisol. Higher order interactions were not significant and thus were removed in constructing the final models.
Table 5.
Multilevel model coefficients for own perceived stress and partner perceived stress by couples’ positive conflict behavior predicting salivary cortisol (log nmol/l)
| Model 3a |
Model 3b |
|||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| Intercept | 1.099 | 0.041 | <0.001 | 1.097 | 0.041 | <0.001 |
| Time | −0.103 | 0.013 | <0.001 | −0.103 | 0.013 | <0.001 |
| Age | −0.006 | 0.005 | 0.233 | −0.007 | 0.005 | 0.227 |
| Gender | 0.089 | 0.026 | 0.001 | 0.088 | 0.026 | 0.001 |
| Meal | 0.024 | 0.043 | 0.576 | 0.024 | 0.043 | 0.574 |
| Trunk fat | −0.000 | 0.000 | <0.001 | −0.000 | 0.000 | <0.001 |
| Marital satisfaction | −0.001 | 0.001 | 0.610 | −0.001 | 0.001 | 0.619 |
| Own perceived stress | 0.015 | 0.013 | 0.255 | 0.015 | 0.013 | 0.251 |
| Partner perceived stress | 0.031 | 0.012 | 0.014 | 0.031 | 0.012 | 0.014 |
| Couple positive conflict behavior | −0.000 | 0.001 | 0.922 | −0.000 | 0.001 | 0.915 |
| Couple positive conflict behavior*Own stress | 0.000 | 0.000 | 0.648 | 0.000 | 0.000 | 0.647 |
| Couple positive conflict behavior*Partner stress | −0.001 | 0.000 | 0.011 | −0.001 | 0.000 | 0.011 |
| Time*Own stress | 0.000 | 0.004 | 0.950 | |||
| Time*Partner stress | 0.009 | 0.004 | 0.032 | |||
| Time*Couple positive conflict behavior | 0.000 | 0.000 | 0.380 | |||
Note. Participant gender coded 1 = male, −1 = female. Meal coded 1 = high saturated fat, −1 = high oleic sunflower oil. Numbers represent natural-log transformed salivary cortisol. Higher order interactions were not significant and thus were removed in constructing the final models.
Table 1.
Average salivary cortisol levels (nmol/l)
| M (SD) | |
|---|---|
| 8:30 AM (baseline/4 hr before conflict) | 4.67 (1.52) |
| 11:00 AM (2 hr post-meal/1 hr before conflict) | 3.35 (1.61) |
| 12:30 PM (30 min post-conflict) | 3.05 (1.79) |
| 1:00 PM (1 hr post-conflict) | 2.47 (1.90) |
| 4:45 PM (4 hr post-conflict) | 1.99 (2.13) |
Note. Numbers represent back transformed geometric means (anti-log). To convert cortisol from nmol/l to mg/dl, divide by 27.59.
Table 3.
Estimated marginal means (standard errors) of salivary cortisol (nmol/l) by partner perceived stress
| High partner stress | Low partner stress | t | p | |
|---|---|---|---|---|
| 8:30 AM (baseline/4 hr before conflict) | 4.52 (1.09) | 4.48 (1.09) | 0.07 | 0.943 |
| 11:00 AM (2 hr post-meal/1 hr before conflict) | 3.70 (1.06) | 3.26 (1.06) | 1.66 | 0.100 |
| 12:30 PM (30 min post-conflict) | 3.29 (1.06) | 2.70 (1.06) | 2.82 | 0.006 |
| 1:00 PM (1 hr post-conflict) | 3.16 (1.06) | 2.53 (1.06) | 3.09 | 0.003 |
| 4:45 PM (4 hr post-conflict) | 2.36 (1.10) | 1.60 (1.10) | 3.28 | 0.001 |
Note. Numbers represent back transformed geometric means (anti-log). To convert cortisol from nmol/l to mg/dl, divide by 27.59.
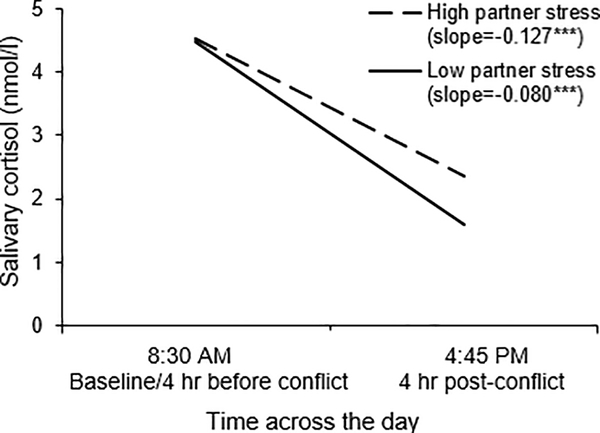
Figure 1.
A visual representation of the Partner perceived stress X Time interaction. Cortisol level is graphed as a function of time across the day, separately for individuals whose partners reported high stress (1 SD above the mean) and for individuals whose partners reported low stress (1 SD below the mean). Numbers represent back transformed geometric means (anti-log). To convert cortisol from nmol/l to mg/dl, divide by 27.59.
*p < .05; **p < .01; ***p < .001.
Hypotheses were tested in two-model sequences to examine the effects on average cortisol levels and then on cortisol trajectories across the day (i.e., adding two- and three-way interactions by time). We first specified a model with the main effects of own stress and partner stress on average cortisol, and then we added two-way interactions by time to assess whether cortisol trajectories differed by own stress and partner stress. To test whether couples’ conflict behaviors altered the effects of own stress and partner stress on average cortisol, we first specified a model with two-way interactions between couples’ behaviors and their own stress and their partners’ stress. Then we included two- and three-way interactions with time to examine whether conflict behaviors moderated cortisol trajectories; positive conflict behaviors and negative conflict behaviors were tested in separate models for all analyses. Three-way interactions with own stress/partner stress, positive/negative conflict behavior, and time were not significant (ps > 0.06) and thus removed in constructing the final models. We also tested lower- and high-order interactions between own stress and partner stress, but they were not significant (ps > 0.27) and thus removed in constructing the final models. Finally, we specified interactions with gender to test for potential differences, which revealed a significant interaction between negative conflict behavior and gender; (p = .002); however, the simple slopes were not significant for men (p = .17) or women (p = .42). Therefore, interactions with gender were not included in the subsequent analyses. Interaction terms were computed as the product of the mean-centered variables (Aiken and West, 1991). Significant interacting effects were probed at one standard deviation above and below the means for each interacting variable.
3. Results
3.1. Descriptives
Table 1 shows means and SDs for cortisol across the day. The mean score on the PSS-4 was 4.40 (SD = 2.82) and ranged from 0 to 11, which is slightly lower than means in prior research (Ms = 5.6 to 5.9, SDs = 3.6 to 4.0) (Cohen et al., 1983). According to zero-order correlations, spouses’ scores on the PSS-4 were positively related to each other; the more individuals felt stressed, the more stressed their spouses felt (r = 0.39, p < 0.001). Spouses’ stress was not correlated with the composites for couples’ positive or negative conflict behaviors (rpositive = −0.15, p = 0.17; rnegative = 0.08, p = 0.47). The correlation between positive and negative conflict behaviors was not significant (r = 0.16, p = 0.14).
3.2. Own and partner perceived stress on cortisol
As shown in Table 2, results from Model 1a with the main effects of own and partner perceived stress demonstrated that although individuals’ own stress was not associated with their own average cortisol levels across the day (p = 0.112), the more their partners felt stressed, the higher their own average cortisol levels (p = 0.008). Cortisol levels also decreased across the day (p < 0.001). Model 1b with the interactions by time showed that one’s own perceived stress did not impact the decline in cortisol (p = 0.878); however, one’s partner’s perceived stress altered how fast or slow cortisol declined across the day (p = 0.039). As illustrated in Figure 1, simple slopes analyses for the Time X Partner perceived stress interaction demonstrated that individuals whose partners reported higher stress had less steep cortisol slopes across the day (b = −0.080, SE = 0.017, p < 0.001) compared to individuals whose partners reported lower stress (b = −0.127, SE = 0.017, p < 0.001). Thus, individuals with stressed partners had flatter cortisol trajectories, whereas individuals with less stressed partners showed steeper and thus healthier declines across the day.
Table 3 shows the estimated marginal means across the day by high and low partner perceived stress. Individuals’ cortisol did not differ based on their partners’ perceived stress levels at either assessment before the conflict (ps > 0.10). Instead, differences in cortisol levels emerged after the conflict and remained significant the rest of the day (ps < 0.01); individuals with more stressed partners had higher average cortisol levels 30 min, 1 h, and 4 hr after the conflict compared to those with less stressed partners.
3.3. Associations between own and partner perceived stress and cortisol based on conflict behaviors
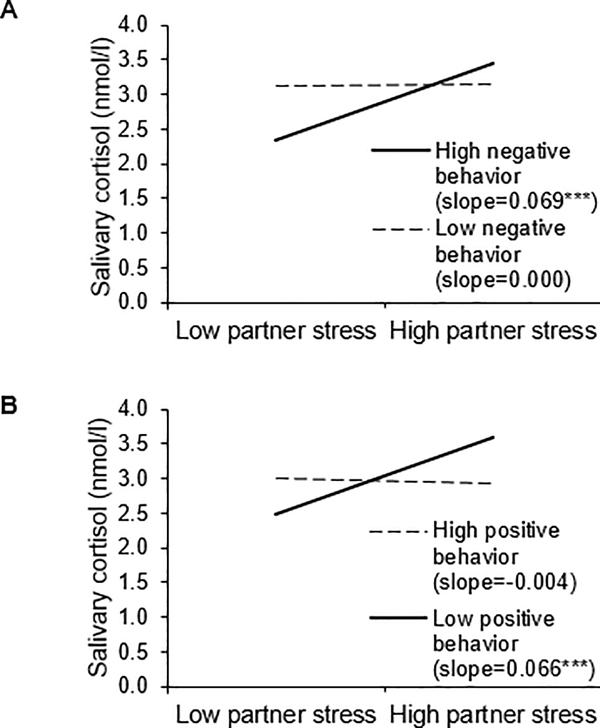
Next we assessed whether couples’ negative conflict behavior moderated the effects of own and partner perceived stress on cortisol (see Table 4 for coefficients). Results from Model 2a showed that the main effect of couples’ negative conflict behaviors on cortisol was not significant (p = .324), but the interaction with partner perceived stress was significant (p = .007). As shown in Figure 2A, simple slopes analyses for the Negative conflict behavior X Partner perceived stress interaction indicated that when couples used more negative conflict behaviors, individuals with stressed partners had higher average cortisol levels than those with less stressed partners (b = .069, SE = .018, p < .001). In contrast, when couples used fewer negative conflict behaviors, individuals’ partners’ perceived stress was unrelated to their average cortisol levels (b = .000, SE = .017, p = .979). Thus, when couples used more negative behaviors, individuals with more stressed partners had higher average cortisol levels than those with less stressed partners. The interaction between negative conflict behavior and own perceived stress was not significant (p = .374). Model 2b with interactions by time showed that couples’ negative conflict behaviors did not alter how fast or slow cortisol declined across the day (p = .646).
Figure 2.
Visual representations of the (A) Partner perceived stress X Negative conflict behavior and (B) Partner perceived stress X Positive conflict behavior interactions. Cortisol level is graphed as a function of partner perceived stress, separately for high (1 SD above the mean) and low (1 SD below the mean) negative and positive conflict behaviors. Numbers represent back transformed geometric means (anti-log). To convert cortisol from nmol/l to mg/dl, divide by 27.59.
*p < .05; **p < .01; ***p < .001.
Results from the models with couples’ positive conflict behaviors were similar to those in the models with negative conflict behavior. As shown in Table 5 (Model 3a), the main effect of positive conflict behavior was not significant (p = 0.922); however, the interaction between positive conflict behavior and partner stress was significant (p = .011). Simple slopes analyses for the Positive conflict behavior X Partner stress interaction revealed that individuals with stressed partners had higher average cortisol levels than those with less stressed partners when couples used fewer positive behaviors during their discussion (b = .066, SE = .017, p < .001; see Figure 2B). Thus, individuals who used fewer positive behaviors with a stressed partner had higher average cortisol levels than those who used fewer positive behaviors but were with a less stressed partner. When couples used more positive conflict behaviors, individuals’ partners’ perceived stress was not associated with their own average cortisol levels (b = −.004, SE = .019, p = .853). The interaction between individuals’ own perceived stress and positive conflict behavior was not significant (p = .648). Model 3b with interactions by time showed that positive conflict behavior did not alter cortisol slopes across the day (p = .380).
4. Discussion
As expected, a partner’s perceived stress had significant implications for cortisol trajectories on a day couples experienced conflict. Individuals whose partners felt stressed had flatter cortisol slopes than individuals with less stressed partners, who showed steeper declines across the day. Individuals’ own perceived stress was not consistently related to their own cortisol; although unexpected, this finding is consistent with previous work demonstrating that a person’s own stress was not related to the couples’ physiological linkage (Hubler, 2012). Individuals’ own stress may be less closely tied to how fast or slow their cortisol declines on a day where they experience conflict with their spouse, particularly given the importance of a partner’s perceptions and behaviors on an individual’s own cortisol (Ditzen et al., 2008; Doerr et al., 2018). Thus, spending the day and interacting with a stressed spouse might have had a greater impact on their own cortisol levels and slopes, particularly because couples discussed a marital problem in the middle of the day. Indeed, individuals’ cortisol levels at the beginning of the day were similar regardless of their partners’ perceived stress. However, differences in cortisol levels emerged after couples discussed a problem in their marriage. Individuals who were with a stressed partner had higher cortisol after the conflict and four hours later compared to those who were with less stressed partners. These results suggest that, although having a partner who feels stressed is associated with dysregulated cortisol patterns, including higher levels and flatter slopes, a partner who feels less stressed is linked to steeper and thus healthier cortisol declines across a day the couple experienced conflict.
This study also showed that a partner’s perceived stress was particularly consequential for one’s own cortisol when couples used more negative behaviors and fewer positive behaviors during the conflict. That is, when couples used more negative and less positive behaviors, individuals with more stressed partners had higher average cortisol levels than those with less stressed partners. Thus, the combination of negative conflict behaviors and a stressed partner, as well as fewer positive behaviors and a stressed partner, was linked to higher average cortisol levels. In contrast, a partner’s perceived stress was not associated with average cortisol levels when couples used fewer negative behaviors or more positive behaviors during the conflict discussion. In this way, couples’ relationship-promoting behaviors helped protect people from their partners’ higher stress.
These findings are consistent with prior research showing that spouses impact each other’s cortisol levels and diurnal rhythms. For instance, partners’ physical intimacy (Ditzen et al., 2008), conflict behaviors (Heffner et al., 2006; Kiecolt-Glaser et al., 1997, 1996), affection (Floyd and Riforgiate, 2008), and general feelings that they are understood, cared for, and appreciated (Slatcher et al., 2015) were associated with cortisol levels and slopes over time. The present study extends this work by providing evidence that a partner’s perceived stress is associated with how fast or slow a person’s own cortisol declines across a day the couple experienced conflict. Moreover, this study demonstrates the importance of the behaviors that couples use during conflict in understanding how a stressed partner contributes to a person’s own cortisol. According to interdependence theory, partners are interconnected, and their experiences affect each other’s outcomes (Kelley and Thibaut, 1978). Our findings fit within interdependence theory and demonstrate how partners can “get under the skin” to influence stress-related health risk.
This study also revealed that a person’s perceived stress level can be both helpful and harmful for their spouse on a conflictual day: stressed partners were linked to higher cortisol levels and flatter slopes, whereas less stressed partners were associated with lower levels and steeper declines across the day. Individuals may only promote healthier diurnal rhythms in their partners when they feel less stressed themselves; consequently, interacting and discussing a marital problem with a stressed partner may contribute to unhealthy patterns, and thus do more harm than good. Indeed, one study showed wives had higher cortisol levels when their husbands responded negatively to their own hostile behavior during conflict compared to wives whose husbands responded less negatively (Kiecolt-Glaser et al., 1996). Another study on how couples’ stress perceptions change together revealed that the linkage between partners’ perceived stress was stronger when they interacted together than when they were apart (Doerr et al., 2018). It is possible that spouses who felt stressed might not have been able to console each other, or that they might have had a shorter fuse, during their conflict discussion, reducing their relationship’s typical salutary effects. These findings demonstrate the importance of understanding both partners’ perceived stress levels when assessing marriage’s health impact.
This research also has implications for couples’ health. Cortisol production during stress is a key part of the body’s stress response. Still, dysregulated cortisol patterns are associated with poor immune, metabolic, and autonomic function, which increase the risks for disease development and mortality (Adam et al., 2017; Kumari et al., 2011, 2009). In marriage, stress can alter spouses’ health through several biological pathways including endocrine function (Robles and Kiecolt-Glaser, 2003). For example, wives whose negative behaviors escalated while interacting with their husbands had greater cortisol, adrenocorticotropic hormone, and norepinephrine (Kiecolt-Glaser et al., 1997), each of which has consequences for their immune function (Glaser and Kiecolt-Glaser, 2005). Spouses’ perceived stress may therefore not only contribute to their cortisol levels and slopes but may also pose risks to each other’s long-term health.
The strengths of this study include the dyadic approach to understanding the effects of perceived stress and conflict behavior on cortisol. By recruiting and examining married couples, we were able to examine how people’s own and their partners’ perceived stress related to their own cortisol slopes across the day. Likewise, having both partners of a married couple allowed us to assess gender differences. The links between perceived stress, conflict behaviors, and cortisol were similar for men and women, but average cortisol was higher in men than women, which is consistent with previous research (Roelfsema et al., 2017). In addition, the 20-minute marital problem-solving task allowed us to code couples’ conflict behaviors, which are reliable indicators of distressed and nondistressed couples (Heyman, 2004, 2001). The dyadic sample and modeling technique allowed us to control for the interdependent nature of married couples’ data while also accounting for the non-independence in spouses’ cortisol assessments throughout the day. In addition, the repeated measurements of spouses’ salivary cortisol across an 8-hour study visit permitted examination of cortisol slopes, which is necessary to capture rates of change throughout the day.
A limitation of the study was that the sample was primarily white and educated and included only heterosexual couples. Accordingly, researchers should assess the link between spouses’ perceived stress and cortisol slopes in more diverse samples. Relatedly, participants’ average age was 38, and thus future work is necessary to capture experiences of older and younger adults. In addition, although the parent study included two in-person visits, perceived stress was assessed at the second visit. Thus, we could not examine whether these findings were evident at both visits. In addition, because all couples discussed a relationship problem and we did not include a control group, it is possible that the discussion along with typical diurnal patterns contributed to cortisol slopes and levels across the day. Additional research comparing associations in couples who engage in a problem versus a control discussion is warranted to tease apart their effects.
This study demonstrated the importance of both members of a couple in predicting cortisol levels and slopes across a day couples experienced conflict. When people’s partners felt stressed, their own cortisol declined at a slower rate across the day compared to people whose partners felt less stressed. Thus, having a less stressed partner was link to steeper and thus healthier cortisol slopes. In addition, individuals whose partners reported higher stress also had higher cortisol after the conflict and four hours later compared to those whose partners reported lower stress. When couples used more negative and less positive behaviors during the conflict, individuals with more stressed partners had higher average cortisol levels than those with less stressed partners. This research contributes to the growing literature on pathways connecting marital interactions to important biorhythms and health.
Highlights.
Married couples provided 5 salivary cortisol samples during a 9.5 h visit
Coding of couples’ conflicts provided data on positive and negative behavior
A stressed spouse was associated with slower declines in one’s own cortisol
Negative and less positive behaviors and a stressed spouse related to higher cortisol
We show how partners “get under the skin” to influence stress-related health risk
Acknowledgments
Funding
This work was supported in part by NIH grants R21 CA158868, UL1TR001070, K05 CA172296, T32 CA229114; a Pelotonia Postdoctoral Fellowship from Ohio State University’s Comprehensive Cancer Center; and American Cancer Society Postdoctoral Fellowship Grant 121911-PF-12–040-01-CPPB.
Footnotes
The authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE, 2017. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG, 1991. Multiple regression: Testing and interpreting interactions., Multiple regression: Testing and interpreting interactions. Sage Publications, Inc, Thousand Oaks, CA, US. [Google Scholar]
- Birditt KS, Newton NJ, Cranford JA, Ryan LH, 2016. Stress and negative relationship quality among older couples: Implications for blood pressure. Journals Gerontol. - Ser. B Psychol. Sci. Soc. Sci 71, 775–785. 10.1093/geronb/gbv023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, 2009. Stress and disorders of the stress system. Nat. Rev. Endocrinol 5, 374–381. 10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav 24, 385–396. [PubMed] [Google Scholar]
- Ditzen B, Hahlweg K, Fehm-Wolfsdorf G, Baucom D, 2011. Assisting couples to develop healthy relationships: Effects of couples relationship education on cortisol. Psychoneuroendocrinology 36, 597–607. 10.1016/j.psyneuen.2010.07.019 [DOI] [PubMed] [Google Scholar]
- Ditzen B, Hoppmann C, Klumb P, 2008. Positive couple interactions and daily cortisol: On the stress-protecting role of intimacy. Psychosom. Med 70, 883–889. 10.1097/PSY.0b013e318185c4fc [DOI] [PubMed] [Google Scholar]
- Doerr JM, Nater UM, Ehlert U, Ditzen B, 2018. Co-variation of fatigue and psychobiological stress in couples’ everyday life. Psychoneuroendocrinology 92, 135–141. 10.1016/j.psyneuen.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Engert V, Ragsdale AM, Singer T, 2018. Cortisol stress resonance in the laboratory is associated with inter-couple diurnal cortisol covariation in daily life. Horm. Behav 98, 183–190. 10.1016/j.yhbeh.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Falconier MK, Nussbeck F, Bodenmann G, Schneider H, Bradbury T, 2015. Stress from daily hassles in couples: Its effects on intradyadic stress, relationship satisfaction, and physical and psychological well-being. J. Marital Fam. Ther 41, 221–235. 10.1111/jmft.12073 [DOI] [PubMed] [Google Scholar]
- Floyd K, Riforgiate S, 2008. Affectionate communication received from spouses predicts stress hormone levels in healthy adults. Commun. Monogr 75, 351–368. 10.1080/03637750802512371 [DOI] [Google Scholar]
- Funk JL, Rogge RD, 2007. Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the couples satisfaction index. J. Fam. Psychol 21, 572–583. 10.1037/0893-3200.21.4.572 [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, 2005. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol 5, 243–251. 10.3390/nu5041241 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM, 2001. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev. Psychopathol 13, 515–538. 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Heffner KL, Loving TJ, Kiecolt-Glaser JK, Himawan LK, Glaser R, Malarkey WB, 2006. Older spouses’ cortisol responses to marital conflict: Associations with demand/withdraw communication patterns. J. Behav. Med 29, 317–325. 10.1007/s10865-006-9058-3 [DOI] [PubMed] [Google Scholar]
- Heyman RE, 2004. Rapid Marital Interaction Coding System (RMICS), in: Kerig PK, Baucom DH (Eds.), Rapid Marital Interaction Coding System (RMICS). Lawrence Erlbaum, Mahway, NJ, pp. 67–93. [Google Scholar]
- Heyman RE, 2001. Observation of couple conflicts: Clinical assessment applications, stubborn truths, and shaky foundations. Psychol. Assess 13, 5–35. 10.1037//1040-3590.13.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley JW, Guilford JP, 1964. Note on the G index of agreement. Educ. Psychol. Meas 24, 749–753. [Google Scholar]
- Hubler DS, 2012. Testing nested associations between microdimensional and global indicators of relationship outcomes (Doctoral dissertation). Retrieved from ProQuest Inf. Learn. (Accession Order No. AAI3512985). [Google Scholar]
- Kelley HH, Thibaut JW, 1978. Interpersonal relations: A theory of interdependence. Wiley, New York, NY. [Google Scholar]
- Kenny DA, Kashy DA, Cook WL, 2006. Dyadic data analysis, Dyadic data analysis., Methodology in the social sciences (Kenny David A., Series Editor). Guilford Press, New York, NY, US. [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, Malarkey WB, 1997. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosom. Med 59, 339–351. 10.1097/00006842-199707000-00001 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, Belury MA, 2015a. Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biol. Psychiatry 77, 653–660. 10.1016/j.biopsych.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA, 2015b. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology 52, 239–250. 10.1016/j.psyneuen.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R, 2005. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatry 62, 1377–1384. 10.1001/archpsyc.62.12.1377 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB, 1996. Marital conflict and endocrine function: Are men really more physiologically affected than women? J. Consult. Clin. Psychol 64, 324–332. 10.1037/0022-006X.64.2.324 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL, 2001. Marriage and health: His and hers. Psychol. Bull 127, 472–503. 10.1037/0033-2909.127.4.472 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson S, 2017. Lovesick: How couples’ relationships influence health. Annu. Rev. ofClinical Psychol 10.1146/annurev-clinpsy-032816-045111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, Fagundes CP, Malarkey WB, Laskowski B, Belury MA, 2018. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 98, 52–60. 10.1016/j.psyneuen.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D, 1992. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med 54, 648–657. 10.1097/00006842-199211000-00004 [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kirschbaum C, Kivimaki M, 2009. Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology 34, 1476–1485. 10.1016/j.psyneuen.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M, 2011. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 96, 1478–1485. 10.1210/jc.2010-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson CA, Gullberg B, Råstam L, Lindblad U, 2009. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: A cross-sectional study. BMC Endocr. Disord 9, 1–11. 10.1186/1472-6823-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM, 2006. Understanding health behavior change among couples: An interdependence and communal coping approach. Soc. Sci. Med 62, 1369–1380. 10.1016/j.socscimed.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Klein LC, Almeida DM, 2013. Synchrony of diurnal cortisol pattern in couples. J. Fam. Psychol 27, 579–588. 10.1037/a0033735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Dopp JM, Myers HF, Stevens SY, 1999. Psychosocial predictors of natural killer cell mobilization during marital conflict. Heal. Psychol 18, 262–171. [DOI] [PubMed] [Google Scholar]
- Monin JK, Schulz R, Martire LM, Jennings JR, Lingler JH, Greenberg MS, 2010. Spouses’ cardiovascular reactivity to their partners’ suffering. Journals Gerontol. - Ser. B Psychol. Sci. Soc. Sci 65 B, 195–201. 10.1093/geronb/gbp133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Simon CD, Adam EK, 2013. Spouses’ cortisol associations and moderators: Testing physiological synchrony and connectedness in everyday life. Fam. Process 52, 284–298. 10.1111/j.1545-5300.2012.01413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK, 2003. The physiology of marriage: Pathways to health. Physiol. Behav 79, 409–416. 10.1016/S0031-9384(03)00160-4 [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM, 2014. Marital quality and health: A meta-analytic review. Psychol. Bull 140, 140–187. 10.1037/a0031859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema F, Van Heemst D, Iranmanesh A, Takahashi P, Yang R, Veldhuis JD, 2017. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr. Connect 6, 500–509. 10.1530/EC-17-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D, Repetti RL, 2010. For better or worse? Coregulation of couples’ cortisol levels and mood states. J. Pers. Soc. Psychol 98, 92–103. 10.1037/a0016959 [DOI] [PubMed] [Google Scholar]
- Shrout MR, 2019. Couples and nonvisible chronic illness: An integrated model of dyadic Coping. Dissertation 23, 2019. [Google Scholar]
- Shrout MR, Brown RD, Orbuch TL, Weigel DJ, 2019. A multidimensional examination of marital conflict and subjective health over 16 years. Pers. Relatsh 26, 490–506. 10.1111/pere.12292 [DOI] [Google Scholar]
- Slatcher RB, Selcuk E, Ong AD, 2015. Perceived partner responsiveness predicts diurnal cortisol profiles 10 years later. Psychol. Sci 26, 972–982. 10.1177/0956797615575022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons AC, Margolin G, Saxbe DE, 2015. Physiological linkage in couples and its implications for individual and interpersonal functioning: A literature review. J. Fam. Psychol 29, 720–731. 10.1037/fam0000115.supp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BL, Loving TJ, 2011. Health implications of conflict in close relationships. Soc. Personal. Psychol. Compass 5, 552–562. 10.1111/j.1751-9004.2011.00371.x [DOI] [Google Scholar]
- Xu S, Lorber MF, 2014. Interrater agreement statistics with skewed data: Evaluation of alternatives to Cohen’s kappa. J. Consult. Clin. Psychol 82, 1219–1227. 10.1037/a0037489 [DOI] [PubMed] [Google Scholar]