Abstract
This study was designed to assess whether nicotine can acquire additional reinforcing properties through associations with other rewards. To this end, rats self-administered nicotine-alone (0.01 mg/kg) or nicotine paired with access to sucrose during the conditioning phase. In the subsequent challenge phase, we tested the effect of nicotine-sucrose pairings on the reinforcing effects of nicotine using a progressive ratio schedule of reinforcement. Using this approach, we show that (a) rats in both paired and nicotine-alone conditions self-administered similar amounts of nicotine in the initial conditioning phase of the study when intake was limited to 10 infusions per session, (b) nicotine rapidly acquired control over goal-tracking behavior in the paired condition, (c) rats that had a history of nicotine and sucrose pairings worked harder and took more nicotine as measured on a progressive ratio using a distinct response form, and (d) conditioned goal-tracking evoked by nicotine did not show extinction when sucrose was no longer paired with nicotine over the 11 days of nicotine self-administration on a progressive ratio schedule of reinforcement. Overall, our results demonstrate that in addition to the multifaceted nature of nicotine stimulus that includes primary reinforcing effects, conditioned reinforcing effects, and reward enhancing effects, nicotine can also acquire additional reinforcing properties through associations with other rewards. This ability to acquire additional reinforcing properties through associative learning may contribute to the development and perpetuation of tobacco use disorder.
Keywords: self-administration, smoking, e-cigarettes, rats, Pavlovian conditioning
For an individual, chronic tobacco use is associated with marked increases in heart disease and many forms of cancer. Further, the health of nontobacco users is negatively affected by second-hand smoke. The estimated annual economic cost in the United States is around $400 billion, and more than 7 million deaths each year globally (United States Department of Health & Human Services, 2014; World Health Organization, 2017). The rising popularity of the mostly unregulated e-cigarettes with their yet unknown health impact is a further reason for concern (Levy et al., 2017). The causes of this major public health issue are complex and include cultural, social, behavioral, and biological factors. Basic science advances in each of these realms are needed to make inroads on solving this major health problem and to decrease the 70% to 80% relapse rate reported with interventions (Cahill, Stevens, Perera, & Lancaster, 2013).
Nicotine is a stimulant that has been identified as the primary constituent of tobacco products responsible for the smoking habit and is the key active ingredient in e-cigarettes. Many stimulants abused by humans have potent reinforcing effects. Such reinforcing effects in preclinical studies are often assessed using the self-administration (SA) task. In general, subjects (e.g., rats or mice) are implanted with a jugular catheter. Following recovery from surgery, subjects are placed into an experimental chamber and given a chance to take a drug. A response (press) on the so-called active lever is followed immediately by an intravenous (IV) infusion of the drug. Conversely, a press on the opposite, inactive lever, has no programmed consequence. The reinforcing effects of a drug are evidenced by responding being maintained on the active lever, and little to no responding on the inactive lever (Corrigall & Coen, 1989; Stolerman, 1992). The potent reinforcing effects of some abused stimulants (e.g., meth or cocaine) are considered dogma in the field; this status has not been conferred to nicotine (Dougherty, Miller, Todd, & Kostenbauder, 1981). Indeed, the relatively weak reinforcing effects of nicotine were recognized over 35 years ago. For example, Dougherty and colleagues (1981) noted that the extant research at the time indicated that the reinforcing properties of nicotine can be considered relatively weak when comparing to drugs like psychomotor stimulants, opiates, and sedative-hypnotic classes. This conclusion was drawn based on the fact that the self-infusion rates for nicotine are generally only 2–3 times higher than saline and are often insensitive to the nicotine dose. Although, with few exceptions, the literature still supports this conclusion (Huynh, Fam, Ahmed, & Clemens, 2017; Kohut & Bergman, 2016) it is important to point out that responding for nicotine, like responding for other reinforcers, vary according to schedules of reinforcement with variable or progressive schedules typically controlling higher rates of responding than fixed ratio schedules (Charntikov et al., 2013; Donny et al., 1999, 2003; Kazan & Charntikov, 2019; Killeen, Posadas-Sanchez, Johansen, & Thrailkill, 2009). Given the tenacity of the smoking habit and its associated nicotine dependence, there is a need to identify other factors along with the reinforcing effects of nicotine contributing to nicotine use if our theoretical models are to better describe this addiction.
One contributing factor that has changed theory and research on nicotine addiction was first studied in rats by Caggiula, Donny, and colleagues. Specifically, nicotine can enhance the reinforcing value of other stimuli present in the environment (Barret & Bevins, 2013; Donny et al., 2003). The consequence of this enhancement is to increase the intake of nicotine in an unconditional manner [that is, the inherent effect of nicotine; see Caggiula et al. (2008)]. Notably, this effect was well described in rats before it was identified in humans (Perkins & Karelitz, 2013). Another behavioral factor within our conceptual model that has led to important advances in understanding drug use in general, and nicotine dependence specifically, is the study of Pavlovian conditioning processes (Bevins et al., 2012; Bevins & Besheer, 2014). From this perspective, nicotine is typically considered the unconditioned stimulus (US) that becomes associated with other stimuli (e.g., cigarette, the smell of tobacco, situational cues) that reliably co-occur with its effects. These drug-associated stimuli can control drug-seeking, as well as induce relapse. The nonreinforced presentation (i.e., extinction) of some of the environmental (exteroceptive) stimuli, along with therapist guided imagery, form the basis of cue-exposure therapy. This approach to smoking cessation has met with mixed success (Conklin & Tiffany, 2002). There are a host of reasons for its limited success. Some examples include not using smoker-relevant stimuli, not extinguishing stimuli in several drug contexts, and not considering the import of internal stimuli and interoceptive conditioning.
The list of internal stimuli is extensive and includes thoughts, anxiety, pain, hunger, satiety, stress, intoxication, and so forth Learning that modifies behavioral and physiological processes controlled by internal stimuli has been implicated in such health issues as cancer treatment, cardiovascular disease, obesity, mental health disorders, and drug addiction (Ceunen, Vlaeyen, & Van Diest, 2016; Davidson, 1993; Fadel & Burk, 2010; Koroboki et al., 2010; Meagher, 2010; Murray & Bevins, 2011; Oldershaw et al., 2011; Paulus & Stein, 2010; Wylie & Tregellas, 2010). Of particular relevance here is the research on how learning processes modify the behavior controlled by drug stimuli (Bevins & Besheer, 2014). There have only been a handful of studies on interoceptive drug conditioning in humans. One such thought-provoking study was conducted by Alessi, Roll, Reilly, and Johanson (2002). In that study, participants that preferred placebo over diazepam had diazepam stimulus paired with a high monetary payoff in a computer task (the US); placebo was paired with a low payoff. The payoff was not contingent on the person’s behavior. Following this conditioning, all individuals preferring the placebo switched their preference to diazepam. Subjective reports of liking also switched, suggesting an acquired association between the diazepam stimulus and high monetary payoff.
We previously showed that intravenous nicotine can serve as a conditioned stimulus and acquire control of behavior after a period of learning (Murray & Bevins, 2009). In that study, rats received intravenous infusions of nicotine (i.e., the stimulus; 0.03 mg/kg) paired or unpaired with access to liquid sucrose. In the paired condition, each nicotine infusion was followed 30 s later by 4-s access to liquid sucrose and each nicotine-sucrose pairing was separated by an average of 11 min. The unpaired group received the same number of nicotine infusions and sucrose deliveries, but the nicotine infusions never occurred in close temporal proximity to sucrose (always ≥240 s). In comparison to rats in the unpaired condition, rats in the paired condition showed an increased number of snout entries into the area where sucrose has been previously delivered (i.e., dipper entries) in the 30 s following the nicotine infusion (i.e., conditioned responding). Importantly, this increase in dipper entries in the paired condition decreased across sessions when sucrose was withheld thus eliminating nonassociative accounts such as motor simulation or sensitization by nicotine. Thus, the study Murray and Bevins (2009) demonstrated that intravenous nicotine can serve as an appetitive conditioned stimulus and that this effect is mediated by centrally localized nicotinic acetylcholine receptors. Notably, these previous findings from our laboratory demonstrate that learning with intravenous nicotine stimulus follows traditional tenets of Pavlovian conditioning and allow for further investigation of associative effects in a context of the self-administration task. One of the questions that has not been answered is whether nicotine can acquire additional reinforcing effect after an appetitive conditioning history. Previous research has firmly established that stimuli associated with primary reinforcers acquire reinforcing properties and it is generally accepted that these effects are the product of Pavlovian conditioning (Bouton, 2007; Mazur, 2006). This effect has not been previously extended to the nicotine stimulus. To test the possibility that the intravenous nicotine stimulus can acquire additional reinforcing effects when it is explicitly paired with sucrose reward, we designed a protocol where nicotine is paired in a predictive manner with access to liquid sucrose in the first phase of the study and then changed the response type for nicotine alone in the subsequent phase of the study. Thus, in the first conditioning phase of our study, some rats self-administer nicotine paired with sucrose in the manner described above (Murray & Bevins, 2009) while other rats self-administer nicotine alone without access to sucrose. During this initial phase, rats in both conditions self-administered nicotine using levers as manipulanda. In the next challenge phase of the study, all rats were allowed to self-administer nicotine alone without access to sucrose on a progressive ratio of reinforcement using nosepokes as manipulanda. The switch from levers to nosepokes is important in order to rule out in the second challenge phase the conditioned enhancement of reinforcing effects associated with manipulanda itself that were previously paired with the sucrose stimulus. In the challenge phase, the number of active nosepoke responses served as a measure of the effort to earn nicotine infusions. With this in mind, we hypothesized that self-administered nicotine paired with access to sucrose will acquire additional reinforcing effects that can be measured in the challenge phase as elevated active nosepoke responses when compared to rats without a history of nicotine-sucrose pairings.
Method and Materials
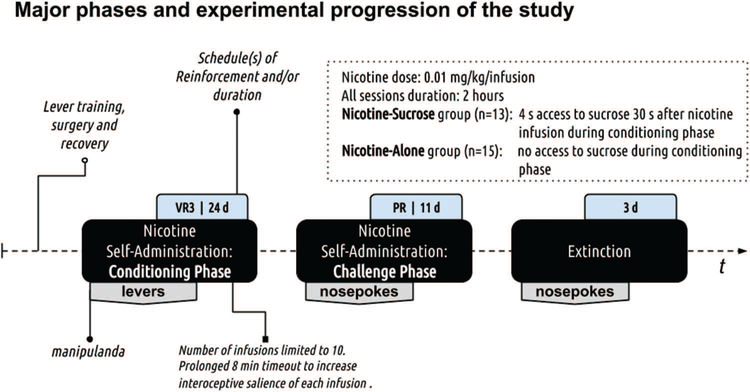
Experimental progression is outlined in Figure 1.
Figure 1.
Experimental progression. The study used the between-subjects design (N = 30; 2 rats were excluded) with three distinct experimental phases as outlined in the figure above (refer to black filled rounded rectangles). The first phase was used to establish a nicotine-sucrose association. The second phase was used to test the reinforcing effects of nicotine using the PR schedule of reinforcement (sucrose was no longer available). The third phase was used to assess responding in extinction when neither nicotine nor sucrose were available.
Animals
Thirty male Sprague–Dawley rats (275–299g), purchased from Harlan (Indianapolis, Indiana), were housed individually in clear polycarbonate cages (48.3 × 26.7 × 20.3 cm) lined with wood shavings. The temperature- and humidity-controlled colony was on a 12-h light/dark schedule (lights on at 0600); experiments were conducted during the light cycle. Water was freely available in the home cage except for the lever response shaping phase (see below). Chow was available ad libitum for the day before surgery and the 7 days following surgery. Otherwise, rats were maintained at 90% of their free-feeding body weight. Four weeks into the self-administration study, the target weight was increased by 2g to adjust for the average growth pattern provided by the supplier. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
The conditioning chambers (ENV-008CT; Med Associates, Inc.; St. Albans, Vermont) measuring 30.5 × 24.1 × 21.0 cm (l × w × h), were enclosed in a sound- and light-attenuating cubicle equipped with an exhaust fan. Each chamber had aluminum sidewalls, metal rod floors with polycarbonate front, back, and ceiling. A recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) was centered on one sidewall. A dipper arm, when raised, provided access to 100 μl of 26% (wt/vol) sucrose solution or tap water in the receptacle. Access to the dipper was monitored by an infrared beam mounted 1.2 cm into the receptacle and 3 cm above the floor. A second infrared beam that monitored chamber activity was located 4 cm above the floor and 14.5 cm from the sidewall containing the receptacle. Two retractable levers (147 nN required for microswitch closure) were mounted on each side of the receptacle. A white cue-light (2.54 cm diameter; 28V, 100 mA) was mounted 7 cm above each lever. A house light (two white 28V, 100 mA lamps) was located 10 cm above the conditioning chamber ceiling. When manipulandum was switched, two nosepokes1 were installed on the sidewall opposite from levers and levers were removed from the chamber. The nosepoke hole (2.5 cm in diameter) had a yellow LED mounted inside and the infrared beam monitored the entry. The infusion pump (PMH-100VS; Med Associates; St. Albans, Vermont) for each chamber was located outside the sound-attenuating cubicle. A 5 mL syringe mounted on the infusion pump was connected with Tygon tubing (AAQ04103; VWR; West Chester, Pennsylvania). The tubing was attached to a swivel coupled with a spring leash (C313C; Plastics One; Roanoke, Virginia) that was suspended over the ceiling of the chamber on a balanced metal arm. Med Associates interface and software (Med-PC for Windows, version IV) were used to collect data and present programmed events.
Drugs
(–)-Nicotine hydrogen tartrate (Sigma; St. Louis, Missouri) was dissolved in 0.9% saline and the pH of the solution was adjusted to 7.0 ± 0.2 with a dilute NaOH. The nicotine dose is reported as a base and was infused intravenously at 35.74 μl per infusion across 1 s.
Preliminary Lever Training
Before the start of any experimental manipulations, rats (n = 30) were handled for a minimum of 2 min per each of three consecutive days. Rats were then trained to lever press as previously described (Charntikov et al., 2015; Kazan & Charntikov, 2019; Stafford et al., 2019). Briefly, 23 h prior to the first training session, water was removed from the home cages. The start of each training session was signaled by illumination of the house light and insertion of a randomly selected lever (right or left). A lever press or a lapse of 15 s resulted in 4 s access to water, retraction of the lever, and commencement of a timeout that lasted on average 60 s (range = 30 to 89 s). Following the timeout, a randomly selected lever was again inserted into the chamber with the condition that the same lever could not be presented more than two times in a row. This protocol was repeated for 60 water deliveries. Daily sessions ranged from 65–80 min, depending on individual performance and were continued until a lever press was made on at least 80% of the lever insertions for two consecutive days (i.e,, three to five training sessions). Immediately after completion of each training session, rats were given 1 h of free access to water in their home cage after which water bottles were removed until the end of the following session. After meeting the training requirement, all rats were given ad libitum water access.
Catheter Implantation Surgery
Following at least 24 h after preliminary training, rats were anesthetized with 1 ml/kg ketamine (100 mg/ml) and xylazine (20 mg/ml) mixture (2:1 ratio; administered intramuscularly; Sigma; St. Louis, Missouri). Polyurethane catheter (RJVR-23; Strategic Applications Inc.; Lake Villa, IL, U.S.A.) with a rounded tip and double suture beads (one secured internally and other externally) was implanted into the right external jugular vein. The other end of the catheter was subcutaneously placed around the shoulder and exited below the scapula via subcutaneously implanted polycarbonate back-mount access port (313–000BM; Plastics One Inc.; Roanoke, Virginia). Immediately following surgery, catheters were flushed with 0.2 ml of streptokinase (2 mg/ml; Sigma; St. Louis, Missouri) diluted in sterile heparinized saline (30 U/ml; Midwest Veterinary Supply; Lincoln, Nebraska). Atipamezole hydrochloride (0.5 mg/kg; IM; Sigma; St. Louis, Missouri) diluted in saline was used to terminate anesthesia. To manage postsurgical pain, buprenorphine hydrochloride (0.1 mg/kg; SC) was administered immediately after the surgery and daily for the next two recovery days. Starting from the day after surgery, catheters were flushed daily with heparinized saline (30 U/ml). Catheter patency was assessed 6 days after recovery (prior to the retraining of lever response) and right after the completion of the nicotine self-administration phase using nosepoke manipulandum (see below). Patency was assessed using an infusion of 0.05 mL xylazine (20 mg/ml; IV). This xylazine concentration produces motor ataxia within 5 s (Charntikov et al., 2015; Kazan & Charntikov, 2019; Reichel, Murray, Grant, & Bevins, 2009; Stafford et al., 2019). One rat did not recover from the surgery and one rat was removed from the statistical analysis due to loss of patency.
Nicotine Self-Administration: Conditioning Phase
Following 7 days of recovery from surgery, rats were trained for 3 consecutive daily sessions to lever press for water on a variable ratio (VR3) schedule of reinforcement (i.e., on average every third response was followed by access to water; range = 1 to 5 presses). Rats were water-deprived for each session as described earlier. This training was similar to the presurgery training except that pressing a lever was now required to access water. Across the 3 daily sessions, all rats earned 80% or more of the 60 available water deliveries. This protocol ensured a high baseline level of lever pressing with both levers having a similar reinforcement history. Before the start of each 120 min self-administration session (range based on performance 80.3 to 120 min), catheters were flushed with 0.2 mL of heparinized saline. The start of each session was signaled by turning the house light off, priming the catheter with nicotine (31 μl or 90% of internal catheter volume), and insertion of both levers. Which lever served as the active lever was pseudorandomly assigned to ensure counterbalancing in each group as much as allowed by sample size. The active lever was reinforced using VR3 schedule of reinforcement (range 1–5; Charntikov et al., 2013, 2015; Kazan & Charntikov, 2019; Stafford et al., 2019). Upon meeting the schedule requirement, there was a 1 s infusion of nicotine (0.01 mg/kg/infusion), retraction of both levers, and the illumination of the house lights for 5 s. Thirty seconds after infusion rats in Nicotine-Sucrose condition (n = 13) received 4 s access to liquid sucrose (100 μl; 26% v/w) while rats in Nicotine-Alone condition (n = 15) had no access to sucrose throughout the study. Levers were inserted back into a chamber after an 8 min timeout. This prolonged timeout was instituted to ensure the salience of each successive infusion with the goal of strengthening the nicotine-sucrose association among rats in the Nicotine-Sucrose condition (Murray & Bevins, 2009). The number of infusions was limited to 10 for all subjects to equate nicotine intake between the groups (i.e., we assumed a ceiling effect would occur). There were 24 nicotine self-administration sessions using levers as a manipulandum. Inactive lever responding was recorded but had no programmed consequence. Immediately after each self-administration session, catheters were flushed with the antibiotic cefazolin (10 mg) diluted in 0.2 mL of heparinized saline (30 U/mL).
Nicotine Self-Administration: Challenge Phase
The challenge phase using a progressive ratio (PR) schedule of reinforcement and nosepokes instead of lever presses on a VR3 commenced a day after the last conditioning self-administration session. No sucrose was available from this point onward to any rat in any condition. The manipulandum was switched to eliminate the conditioned reinforcement effect of levers as a possible explanation for the differences in responding between the two conditions during the challenge phase. We also moved nosepokes to the opposite side of the chamber and changed the active nosepoke side to further dissociate the manipulandum from the previous location and the sucrose dipper. With the same reasoning, the house light-on cue used in the previous phase was switched to both nosepoke lights-off (see below). Before and after session procedures were identical to the previous phase. The start of each 120 min session was signaled by turning the house lights-off, turning nosepoke LED lights on, and priming the catheter with nicotine as described earlier. The PR schedule was adopted from Donny et al. (1999) using {5 × EXP(0.2 × infusion number)–5} formula with the exception that we added 1 as an initial step in the schedule. This resulted in the following progression: 1, 3, 6 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 179, 219, 268, 328, 402, 492. After meeting the response requirement on the active nosepoke, rats received an infusion of nicotine (1 s; 0.01 mg/kg/infusion), both nosepoke LED lights were turned off for 2 s, and there was no response timeout on the active nosepoke.
Extinction
Extinction sessions were identical to self-administration sessions, except that nosepoke entries had no programmed consequences. There were a total of three daily extinction sessions.
Dependent Measures and Statistical Analyses
Lever presses, nosepoke entries, and dipper entries were used as primary dependent measures. Analysis of variance (ANOVA) was used across all data (see Results). Lever presses during conditioning phase were analyzed using mixed-effects ANOVA with Condition as a between-subjects factor, Lever as a within-subjects factor, and Sessions as a repeated measure. Significant effect of Condition was followed up with separate ANOVAs for each Condition. To assess nosepoke discrimination for each Condition, nosepoke entries during the challenge phase were analyzed using mixed-effects ANOVA for each condition separately with Nosepoke as a within-subjects factor and Session as a repeated measure. To assess differences in responding on the active or inactive nosepokes during the challenge phase of the experiment, active or inactive nosepoke entries were analyzed using mixed-effects ANOVA with Condition as a between-subjects factor and Session as a repeated measures. Dipper entries during all phases of the experiment were analyzed using mixed-effects ANOVAs with Condition as a between-subjects factor and Sessions as a repeated measure. All analyses included interaction terms. Significant interactions were followed by the Fisher LSD comparisons tests. Statistical significance was set at p < .05. Statistical analyses were performed using R (3.4.2) software environment for statistical computing.
Results
Nicotine Self-Administration: Conditioning Phase
Lever presses
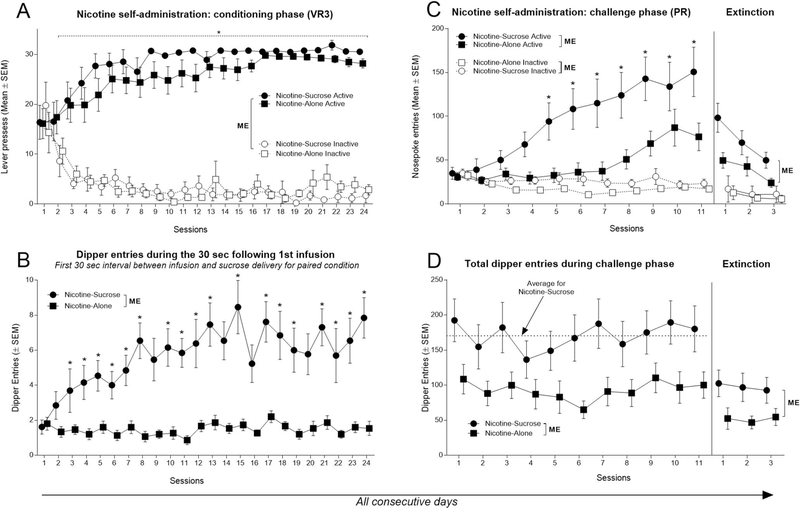
Omnibus ANOVA revealed no effect of Condition [F(1, 26) = 1.89, p = .18], a main effect of Lever [active or inactive; F(1, 26) = 572.92, p < .001], and significant Lever × Session interaction [F(23, 598) = 18.39, p < .001]. A separate ANOVA assessing data from Nicotine-Sucrose condition revealed no effect of Session [F(23, 276) = 1.01, p = .44], main effect of Lever [F(1, 12) = 690.7, p < .001], and Lever × Session interaction [F(23, 276) = 11.59, p < .001]. This difference between active and inactive lever pressing was evident from Session 2 to Session 24 (Figure 2A). Similarly, an ANOVA assessing data from the Nicotine-Alone condition revealed no effect of Session [F(23, 322) = 1.21, p = .23], a main effect of Lever [F(1, 14) = 183.9, p < .001], and their interaction [F(23, 322) = 7.77, p < .001]. This difference was evident from Session 2 to Session 24 (Figure 2A). Rats in both conditions rapidly acquired nicotine self-administration and had a similar pattern of lever discrimination during the conditioning self-administration phase.
Figure 2.
Visualization of responding for nicotine and dipper entries over 38 consecutive daily sessions. (A) Rats in both conditions had higher active lever responding starting from Session 3. ME Indicates significant main effects. * Indicates significant difference from inactive lever responding. (B) Rats in a Nicotine-Sucrose condition had significantly higher dipper entries throughout the conditioning phase. ME Indicates significant main effects. * Indicates significant difference from Nicotine-Alone condition. (C) Rats in a Nicotine-Sucrose condition had significantly higher nosepoke entries than Nicotine-Alone control starting from Session 5. Rats in a Nicotine-Sucrose condition had significantly higher nosepoke entries than Nicotine-Alone control over three extinction sessions. ME Indicates significant main effects. * Indicates significant difference from active nosepoke entries of Nicotine-Alone condition. (D) Rats in a Nicotine-Sucrose condition had consistently higher dipper entries than rats in nicotine alone-condition throughout the challenge phase. Rats in a Nicotine-Sucrose had significantly higher dipper entries than Nicotine-Alone condition over three extinction sessions. ME Indicates significant main effects.
Dipper entries
Figure 2B shows total dipper entries during the 30 s interval following the first nicotine infusion. Importantly, for the rats in the Nicotine-Sucrose condition, this interval reflects the initial experience with the nicotine stimulus before any influence of sucrose for that session. Differential goal-tracking or anticipatory approach to the location of the sucrose provides a conservative estimate of conditioned responding evoked by the interoceptive nicotine stimulus (Farwell & Ayres, 1979; Murray & Bevins, 2009). There were significant main effects of Condition [F(1, 26) = 56.57, p < .001] and Session [F(23, 598) = 2.96, p < .001] as well as their interaction [F(23, 598) = 2.68, p < .001]. This difference in goal-tracking was evident on Sessions 8, 10 to 15, and 17 to 24 (Figure 2B). Thus, for rats in the Nicotine-Sucrose condition, the nicotine stimulus acquired control of goal-tracking behavior. An outcome that suggests that nicotine acquired additional reinforcing value.
Nicotine Self-Administration: Challenge Phase
Nosepoke entries
Analysis of nosepoke entries from rats in the Nicotine-Sucrose condition revealed significant effects of Nosepoke [active or inactive; F(1, 12) = 24.37, p < .001], Session [F(10, 120) = 5.82, p < .001], and their interaction [F(10, 120) = 11.64, p < .001]. There were significantly more active nosepoke entries on Sessions 5 to 11 (Figure 2C; left panel). Analysis of data from Nicotine-Alone condition revealed a main effect of Nosepoke [F(1, 14) = 14.78, p < .01], a main effect of Session [F(10, 140) = 6.28, p < .001], and their interaction [F(10, 140) = 9.38, p < .001]. There were significantly more active nosepoke entries on Sessions 6 to 11 (Figure 2C; left panel). Thus, both groups learned to self-administer nicotine on the PR schedule of reinforcement and to discriminate between active and inactive nose-poke manipulandum.
A comparison of active nosepoke entries between the two conditions revealed a main effect of Condition [F(1, 26) = 7.64, p < .05] and Session [F(10, 260) = 14.17, p < .001] as well as their interaction [F(10, 260) = 3.20, p < .001]. Rats in the Nicotine-Sucrose condition had a higher number of nosepoke entries than rats in the Nicotine-Alone condition on Sessions 5 to 11 (Figure 2C; left panel). A separate ANOVA of the inactive lever responses revealed a main effect of Group [F(1, 26) = 4.37, p = .04], a main effect of Session [F(10, 260) = 3.59, p < .001], but no interaction [F(10, 260) = 1.22, p = .28]. Overall, inactive nosepoke entries were slightly higher in the Nicotine-Sucrose condition.
Dipper entries
A comparison of total dipper entries across sessions during the PR challenge phase of nicotine self-administration revealed a main effect of Condition [F(1, 26) = 5.33, p < .05], main effect of Session [F(10, 260) = 3.49, p < .001], and no interaction [F(10, 260) = 1.22, p = .27]. Interestingly, there was no evidence of the extinction of dipper entries, as would be otherwise expected because sucrose was never available during this phase of the experiment (Figure 2D; left panel; compare dipper entries rates on each session to the average responding during this phase as visualized by a horizontal dashed line).
Extinction
Nosepoke entries
Nosepoke entries in the Nicotine-Sucrose condition decreased from the mean of 150.6 observed on the last PR self-administration session to the mean of 98.3 (35% reduction) on the initial extinction session, mean of 69.9 (53% reduction) on extinction Session 2, and the mean of 49.8 (67% reduction) on the last extinction session (Figure 2C; right panel). Nosepoke entries in the Nicotine-Alone condition decreased from the mean of 76.5 observed on the last PR self-administration session to the mean of 49.6 (35% reduction) on the extinction Session 1, mean of 42.7 (44% reduction) on extinction Session 2, and the mean of 24.1 (68% reduction) on the last extinction session (Figure 2C; right panel). Analysis of active nosepoke entries between the two conditions revealed significant main effects of Condition [F(1, 26) = 5.62, p < .05] and Session [F(2, 52) = 22.81, p < .001] but no interaction [F(2, 52) = 2.85, p = .06]. In the absence of nicotine and the cues, rats in both conditions decreased active nosepoke entries by more than 60% over the course of 3 extinction sessions. Importantly, rats in the Nicotine-Sucrose condition persisted in having higher active nosepoke entries than rats in the Nicotine-Alone condition over the course of extinction.
Dipper entries
Entries into the dipper receptacle that was previously associated with the sucrose delivery in the Nicotine-Sucrose condition decreased from the mean of 180.15 observed on the last PR self-administration session to the mean of 102.5 (43% reduction) on the extinction Session 1, mean of 96.8 (46% reduction) on extinction Session 2, and mean of 92.6 (48% reduction) on the last extinction session. Dipper entries among the rats in the Nicotine-Alone condition that have never received sucrose in that dipper decreased from the mean of 100.1 observed on the last PR self-administration session, to the mean of 52.4 (48% reduction) on the extinction Session 1, mean of 46.8 (53% reduction) on extinction Session 2, and mean of 54.5 (46% reduction) on the last extinction session (Figure 2D; right panel). Analysis of dipper entries between the two conditions revealed a significant main effect of Condition [F(1, 26) = 4.75, p < .05] but no effect of Session [F(2, 52) = 0.44, p = .64] or interaction [F(2, 52) = 0.65, p = .52]. Overall, rats in both conditions decreased their dipper entries by more than 40% in the absence of nicotine and the associated cues. Rats in the Nicotine-Sucrose condition persisted to have higher dipper entries than rats in the Nicotine-Alone condition over the course of extinction. Importantly, this decrease in responding among rats in the Nicotine-Sucrose condition from relatively stable levels of dipper entries during the PR challenge phase to a much lower responding during the extinction phase indicates that a primary driver of that behavior during the challenge phase is the nicotine stimulus (see Discussion for more).
Discussion
For years we have been studying the interoceptive stimulus effects of nicotine and how behavioral, pharmacological, and neural manipulations alter nicotine’s control of conditioned responding (Besheer, Palmatier, Metschke, & Bevins, 2004; Bevins & Besheer, 2014; Charntikov, Pittenger, Swalve, Li, & Bevins 2017; Murray & Bevins, 2009). Underlying much of this research was our assumption that nicotine had acquired conditioned reinforcing effect when paired with an appetitive outcome such as sucrose. Until now, this assumption was inferred from acquired control of goal-tracking by the nicotine stimulus and that, in general, behavioral manipulations followed the laws of learning (Murray & Bevins, 2009). In the present work, we developed a task that wedded nicotine self-administration with our earlier work on intravenous nicotine as a stimulus paired with an appetitive outcome to test whether such conditioning history altered the later reinforcing effects of nicotine. Using this approach, we show that (a) rats in both Nicotine-Sucrose and Nicotine-Alone conditions self-administered similar amounts of nicotine in the initial conditioning phase of the study when intake was limited to 10 infusions per session, (b) nicotine rapidly acquired control over goal-tracking behavior in the Nicotine-Sucrose condition, (c) rats that had a history of nicotine and sucrose pairings worked harder and took more nicotine as measured on a progressive ratio using a distinct response form, and (d) conditioned goal-tracking evoked by nicotine did not show extinction when sucrose was no longer paired with nicotine over the 11 days of nicotine self-administration on a PR schedule of reinforcement.
It is widely accepted that nicotine is the primary psychoactive and reinforcing component responsible for the addictive effects of various tobacco products (Rupprecht et al., 2015; Stolerman & Jarvis, 1995). However, converging evidence suggests that the primary reinforcing properties of nicotine alone are weak. For example, most preclinical studies investigating the behavioral and neural mechanisms associated with nicotine reinforcement via self-administration use cued protocols where rats’ responding is not just followed by nicotine infusions but also included other stimulus changes like lights, sounds, or lever movement (Caggiula et al., 2008). The few studies that examine nicotine self-administration without such support stimuli find that rats can acquire nicotine taking behavior but the rate of self-administration is typically low and the behavior does not seem to be negatively sensitive to schedule of reinforcement, and only a narrow range of doses could support this behavior (Chaudhri et al., 2007; Donny et al., 2003; Palmatier et al., 2006; Sorge, Pierre, & Clarke, 2009). For example, Sorge and colleagues (2009) found that rats on average will earn fewer than five uncued nicotine infusions (0.015 mg/kg infused over 30 s) in a 2 h self-administration session using a fixed-ratio schedule of reinforcement where one lever press on the active lever is required to earn an infusion (FR1). In that study, increasing the response requirement to an FR2 or PR decreased average infusions earned to below ∼2.5. This decrease in intake was accompanied by a loss of lever discrimination (i.e., no differences between active and inactive lever presses). With this example in mind, it is important to note that the use of cues in most preclinical studies does not necessarily suggest a flaw in the model because humans often consume nicotine-containing products in the presence of other stimuli that vary from environmental contexts to foods, drinks, or social interactions to name a few. These environmental stimuli can, in turn, acquire additional conditional properties through pairings with the effects of nicotine and subsequently affect substance use behavior.
As stated above, the cues that are often paired with nicotine infusions in the majority of preclinical studies can facilitate higher levels of nicotine consumption and lever discrimination which is important for the assessment of many behavioral, pharmacological, or biological effects underlying nicotine use. Notably, stimuli used in the self-administration task can function as sensory reinforcers and maintain active lever responding and lever discrimination by themselves (Barrett & Bevins, 2012; Chaudhri et al., 2006; Palmatier et al., 2006). In fact, rats will lever press more for a visual stimulus consisting of 1 s illumination of the cue light followed by house light-off for 60 s than for a 0.06 mg/kg nicotine alone infusion (Palmatier et al., 2006). The prevailing idea for why these support stimuli are needed to demonstrate more robust self-administration is that nicotine enhances the reinforcing effects of other stimuli such as the sensory stimuli in the Palmatier et al. (2006) study just described. Indeed, contingent or noncontingent (i.e., response independent or experimenter administered) nicotine can enhance responding for weakly reinforcing visual stimuli (Barrett & Bevins, 2012; Chaudhri et al., 2006; Palmatier et al., 2006). Our laboratory has accumulated a large body of evidence disentangling the nature of nicotine stimulus that includes behavioral, pharmacological, and neural processes (Besheer et al., 2004; Bevins et al., 2012; Bevins & Besheer, 2014; Charntikov, Falco, Fink, Dwoskin, & Bevins, 2017; Charntikov, Pittenger, et al., 2017). In addition to nicotine’s weak primary reinforcing and reinforcer enhancing effects, there is a large body of research demonstrating that the interoceptive stimulus effects of nicotine can be associated with appetitive or rewarding outcomes as evidenced by the acquisition of a conditioned response (i.e., goal-tracking). We take this response to indicate that the nicotine stimulus can enter into an association with sucrose and can come to guide responding toward the area associated with reward (cf. Pittenger & Bevins, 2013). This associative learning model predicts that repeated nicotine-sucrose pairings should imbue nicotine with acquired or conditioned reinforcing value. If so, rats should respond greater to take nicotine and may even consume more nicotine. We confirmed both predictions in the present study. After IV nicotine was repeatedly paired with sucrose in the initial conditioning phase, rats had greater responding on a PR schedule and earned more infusions on that schedule than rats that had a similar history of nicotine self-administration without sucrose. Thus, we offer that another potential factor that may contribute to abuse liability of nicotine is its associative learning history through which stimulus effects of nicotine may have accrued additional conditioned reinforcing properties.
There are a number of methodological details that deserve some discussion. First, the levers and or the timeout cues in the training phase may acquire conditioned reinforcing effects by being paired with the mild reinforcing effects of nicotine and/or the later sucrose in the Nicotine-Sucrose condition. Therefore, we decided that it was important that these stimuli are not a part of the critical test assessing the reinforcing effects of nicotine. To this end, we switched the manipulandum from levers to nosepokes during our challenge phase and changed the visual cues from house light-on to nosepoke lights-off to signal nicotine infusion. Further, we moved our manipulandum to the opposite side of the conditioning chamber and moved the active manipulandum to the opposite side (e.g., if it was left lever then the right nosepoke hole was active and vice versa). A second and also critical methodological detail was the need to equate overall nicotine exposure during the initial phase for both conditions in the study. To do this, we limited the number of nicotine infusions to 10 in a 2 h session and instituted a variable ratio schedule of reinforcement (VR3) that facilitates robust acquisition of nicotine self-administration with relatively high nicotine intake (Charntikov et al., 2013, 2015; Kazan & Charntikov, 2019). Stated in another way, given our experience with nicotine self-administration, we selected a nicotine dose, a long duration time out, a schedule of reinforcement, and so forth that we expected would produce a ceiling effect in the initial phase. It worked. Using this experimental design, we found that rats in the Nicotine-Alone and Nicotine-Sucrose conditions rapidly acquired nicotine self-administration and lever discrimination. Importantly, rats in both conditions self-administered a comparable amount of nicotine throughout the conditioning phase and rats in both conditions earned the maximum or close to the maximum number of infusions at the end of that initial phase. Thus, the difference in PR performance in the challenge phase with nosepoke responding cannot be attributed to differences in nicotine exposure. For the first time, we show that an appetitive conditioning history involving the nicotine stimulus will imbue nicotine with additional reinforcing value as measured by greater responding and nicotine intake on a PR schedule of reinforcement as well as continued persistence of responding in the face of nonreinforcement (i.e., extinction). Some researchers have used PR schedules to assess differences in the pleasurable or hedonic effects of a drug (McGregor & Roberts, 1995; Mendrek, Blaha, & Phillips, 1998) while others suggest that PR schedules inform one about the incentive salience of a drug (Arnold & Roberts, 1997; Markou et al., 1993). Although the interpretation of increased responding on the PR schedule of reinforcement may vary, our study clearly demonstrates that the appetitive conditioning history increased overall responding for nicotine infusions by nearly 30%.
Consistent with previous reports from our laboratory, we show in the present study that rats in the Nicotine-Sucrose condition gradually increased entries into the receptacle where sucrose was delivered (dipper entries) during the first 30 s interval following first nicotine infusion throughout the conditioning phase. This increase in dipper entries indicates that nicotine acquired control of a new behavioral response and demonstrates the development and establishment of the association between nicotine stimulus and sucrose. This increase in dipper entries evoked by the contingent nicotine infusions further extends our previous report where we showed that noncontingent nicotine infusions paired with the access to sucrose 30 s later can also acquire control of behavior in a similar manner (Murray & Bevins, 2009). For example, when noncontingent nicotine infusions were paired with access to sucrose 30 s later, rats showed similar magnitude and escalation of dipper entries following nicotine infusion over the course of the training (Murray & Bevins, 2009). In that study, rats in the unpaired condition, those that received both nicotine and sucrose throughout each session but the sucrose was never presented in the temporal proximity to the nicotine infusion (always ≥240 s), showed significantly lower entries into the sucrose receptacle, no escalation of entries into the sucrose receptacle, and significantly lower general chamber activity (the number of times a single infrared beam dissecting the chamber in half was interrupted during the session) than rats in the paired condition. The fact that rats in the paired condition showed higher general chamber activity indicates that either nicotine alone or a combination of nicotine and the environmental context acquired additional conditioned excitation properties by association with the sucrose throughout the training. In contrast, the fact that rats in the unpaired condition showed lower general activity and no escalation indicates that unconditioned effects of sucrose do not affect relevant behaviors in this task as evident by the lack of conditioned context excitation. This suggests that unconditioned effects of sucrose play a nominal role in our study and that the increase in workload for nicotine in the challenge phase of the study can be mostly explained by the conditioning history in the early phase. Overall, our results demonstrate that in addition to the multifaceted nature of nicotine stimulus that includes primary reinforcing effects, conditioned reinforcing effects, and reward enhancing effects, nicotine can also acquire additional reinforcing properties through associations with other rewards that may contribute to the development and perpetuation of the tobacco use.
Public Health Significance.
This study suggests that nicotine stimulus can acquire additional reinforcing properties through associations with other rewards. This ability to acquire additional reinforcing properties through associative learning may contribute to the development and perpetuation of tobacco use disorder.
Acknowledgments
Sergios Charntikov was partially supported by GM113131 (CIBBR, P20) while preparing this article for publication and Rick A. Bevins was in part supported by DA034389 and DA046109.
Footnotes
The authors report no conflicts of interest.
Disambiguation: “nosepoke”— a manipulandum used in the operant chambers; “nosepoke entry”—an act of inserting a rat’s snout into a nosepoke hole.
Contributor Information
Sergios Charntikov, University of New Hampshire.
Steven T. Pittenger, National Institute of Health, Bethesda, Maryland
Natashia Swalve, Alma College.
Scott T. Barrett, University of Nebraska.
Rick A. Bevins, University of Nebraska.
References
- Alessi SM, Roll JM, Reilly MP, & Johanson C-E (2002). Establishment of a diazepam preference in human volunteers following a differential-conditioning history of placebo versus diazepam choice. Experimental and Clinical Psychopharmacology, 10, 77–83. 10.1037/1064-1297.10.2.77 [DOI] [PubMed] [Google Scholar]
- Arnold JM, & Roberts DC (1997). A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology, Biochemistry and Behavior, 57, 441–447. 10.1016/S0091-3057(96)00445-5 [DOI] [PubMed] [Google Scholar]
- Barret ST, & Bevins RA (2013). Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacology, Biochemistry and Behavior, 114–115, 9–15. 10.1016/j.pbb.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, & Bevins RA (2012). A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behavioural Pharmacology, 23, 781–789. 10.1097/FBP.0b013e32835a38d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, & Bevins RA (2004). Nicotine as a signal for the presence or absence of sucrose reward: A Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology, 172, 108–117. 10.1007/s00213-003-1621-9 [DOI] [PubMed] [Google Scholar]
- Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, & Charntikov S (2012). Disentangling the nature of the nicotine stimulus. Behavioural Processes, 90, 28–33. 10.1016/j.beproc.2011.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, & Besheer J (2014). Interoception and learning: Import to understanding and treating diseases and psychopathologies. ACS Chemical Neuroscience, 5, 624–631. 10.1021/cn5001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2007). Learning and behavior: A contemporary synthesis. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, & Sved AF (2008). The role of nicotine in smoking: A dual-reinforcement model. Nebraska Symposium on Motivation, 55, 91–109. 10.1007/978-0-387-78748-0_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, & Lancaster T (2013). Pharmacological interventions for smoking cessation: An overview and network meta-analysis. Cochrane Database of Systematic Reviews. Retrieved from 10.1002/14651858.CD009329.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceunen E, Vlaeyen JWS, & Van Diest I (2016). On the origin of interoception. Frontiers in Psychology, 7, 743. 10.3389/fpsyg.2016.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Falco AM, Fink K, Dwoskin LP, & Bevins RA (2017). The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology, 113, 354–366. 10.1016/j.neuropharm.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Swalve N, Li M, & Bevins RA (2017). Double dissociation of the anterior and posterior dorsomedial caudate-putamen in the acquisition and expression of associative learning with the nicotine stimulus. Neuropharmacology, 121, 111–119. 10.1016/j.neuropharm.2017.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Thapa I, Bastola DR, Bevins RA, & Pendyala G (2015). Ibudilast reverses the decrease in the synaptic signaling protein phosphatidylethanolamine-binding protein 1 (PEBP1) produced by chronic methamphetamine intake in rats. Drug and Alcohol Dependence, 152, 15–23. 10.1016/j.drugalcdep.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, … Bevins RA (2013). Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology, 75, 138–144. 10.1016/j.neuropharm.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, … Sved AF (2006). Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology, 189, 27–36. 10.1007/s00213-006-0522-0 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, … Sved AF (2007). Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: Impact of nicotine dose and reinforcement schedule. Psychopharmacology, 190, 353–362. 10.1007/s00213-006-0454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, & Tiffany ST (2002). Applying extinction research and theory to cue-exposure addiction treatments. Addiction, 97, 155–167. 10.1046/j.1360-0443.2002.00014.x [DOI] [PubMed] [Google Scholar]
- Corrigall WA, & Coen KM (1989). Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology, 99, 473–478. 10.1007/BF00589894 [DOI] [PubMed] [Google Scholar]
- Davidson TL (1993). The nature and function of interoceptive signals to feed: Toward integration of physiological and learning perspectives. Psychological Review, 100, 640–657. 10.1037/0033-295X.100.4.640 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, … McCallum SE (1999). Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology, 147, 135–142. 10.1007/s002130051153 [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, … Sved AF (2003). Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology, 169, 68–76. 10.1007/s00213-003-1473-3 [DOI] [PubMed] [Google Scholar]
- Dougherty J, Miller D, Todd G, & Kostenbauder HB (1981). Reinforcing and other behavioral effects of nicotine. Neuroscience and Biobehavioral Reviews, 5, 487–495. 10.1016/0149-7634(81)90019-1 [DOI] [PubMed] [Google Scholar]
- Fadel J, & Burk JA (2010). Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Research, 1314, 112–123. 10.1016/j.brainres.2009.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, & Ayres JJB (1979). Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (“goal tracking”) in rats. Learning and Motivation, 10, 295–312. 10.1016/0023-9690(79)90035–3 [DOI] [Google Scholar]
- Huynh C, Fam J, Ahmed SH, & Clemens KJ (2017). Rats quit nicotine for a sweet reward following an extensive history of nicotine use. Addiction Biology, 22, 142–151. 10.1111/adb.12306 [DOI] [PubMed] [Google Scholar]
- Kazan T, & Charntikov S (2019). Individual differences in responding to bupropion or varenicline in a preclinical model of nicotine self-administration vary according to individual demand for nicotine. Neuropharmacology, 148, 139–150. 10.1016/j.neuropharm.2018.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR, Posadas-Sanchez D, Johansen EB, & Thrailkill EA (2009). Progressive ratio schedules of reinforcement. Journal of Experimental Psychology: Animal Behavior Processes, 35, 35–50. 10.1037/a0012497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, & Bergman J (2016). Reinforcing effectiveness of nicotine in nonhuman primates: Effects of nicotine dose and history of nicotine self-administration. Psychopharmacology, 233, 2451–2458. 10.1007/s00213-016-4293-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroboki E, Zakopoulos N, Manios E, Rotas V, Papadimitriou G, & Papageorgiou C (2010). Interoceptive awareness in essential hypertension. International Journal of Psychophysiology, 78, 158–162. 10.1016/j.ijpsycho.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Levy DT, Fong GT, Cummings KM, Borland R, Abrams DB, Villanti AC, & Niaura R (2017). The need for a comprehensive framework. Addiction, 112, 22–24. 10.1111/add.13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, & Koob GF (1993). Animal models of drug craving. Psychopharmacology, 112, 163–182. 10.1007/BF02244907 [DOI] [PubMed] [Google Scholar]
- Mazur JE (2006). Learning and behavior (6th Ed.). New York, NY: Psychology Press. [Google Scholar]
- McGregor A, & Roberts DCS (1995). Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behavioural Brain Research, 67, 75–80. 10.1016/0166-4328(94)00106-P [DOI] [PubMed] [Google Scholar]
- Meagher MW (2010). Developing translational animal models of cancer-related fatigue. In Cleeland CS, Fisch MJ, & Dunn AJ (Eds.), Cancer symptom science: Measurement, mechanisms, and management (pp. 124–141). 10.1017/CBO9780511780868.016 [DOI] [Google Scholar]
- Mendrek A, Blaha CD, & Phillips AG (1998). Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology, 135, 416–422. 10.1007/s002130050530 [DOI] [PubMed] [Google Scholar]
- Murray JE, & Bevins RA (2009). Acquired appetitive responding to intravenous nicotine reflects a Pavlovian conditioned association. Behavioral Neuroscience, 123, 97–108. 10.1037/a0013735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, & Bevins RA (2011). Excitatory conditioning to the interoceptive nicotine stimulus blocks subsequent conditioning to an exteroceptive light stimulus. Behavioural Brain Research, 221, 314–319. 10.1016/j.bbr.2011.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw A, Hambrook D, Stahl D, Tchanturia K, Treasure J, & Schmidt U (2011). The socio-emotional processing stream in Anorexia Nervosa. Neuroscience and Biobehavioral Reviews, 35, 970–988. 10.1016/j.neubiorev.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, … Sved AF (2006). Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology, 184, 391–400. 10.1007/s00213-005-0183-4 [DOI] [PubMed] [Google Scholar]
- Paulus MP, & Stein MB (2010). Interoception in anxiety and depression. Brain Structure & Function, 214, 451–463. 10.1007/s00429-010-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (2013). Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology, 228, 479–486. 10.1007/s00213-013-3054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, & Bevins RA (2013). Interoceptive conditioning with a nicotine stimulus is susceptible to reinforcer devaluation. Behavioral Neuroscience, 127, 465–473. 10.1037/a0032691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, & Bevins RA (2009). Bupropion attenuates methamphetamine self-administration in adult male rats. Drug and Alcohol Dependence, 100, 54–62. 10.1016/j.drugalcdep.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, & Donny EC (2015). Behavioral mechanisms underlying nicotine reinforcement. Current Topics in Behavioral Neurosciences, 24, 19–53. 10.1007/978-3-319-13482-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Pierre VJ, & Clarke PBS (2009). Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology, 207, 191–200. 10.1007/s00213-009-1647-8 [DOI] [PubMed] [Google Scholar]
- Stafford NP, Kazan TN, Donovan CM, Hart EE, Drugan RC, & Charntikov S (2019). Individual vulnerability to stress is associated with increased demand for intravenous heroin self-administration in rats. Frontiers in Behavioral Neuroscience, 13, 134. 10.3389/fnbeh.2019.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman I (1992). Drugs of abuse: Behavioural principles, methods and terms. Trends in Pharmacological Sciences, 13, 170–176. 10.1016/0165-6147(92)90059-F [DOI] [PubMed] [Google Scholar]
- Stolerman IP, & Jarvis MJ (1995). The scientific case that nicotine is addictive. Psychopharmacology, 117, 2–10. 10.1007/BF02245088 [DOI] [PubMed] [Google Scholar]
- United States Department of Health & Human Services. (2014). The health consequences of smoking-50 years of progress. A report of the surgeon general. Atlanta, GA: USDHHS. [Google Scholar]
- World Health Organization. (2017). WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. Geneva, Switzerland: Author. Retrieved from https://www.who.int/tobacco/global_report/2017/en/ [Google Scholar]
- Wylie KP, & Tregellas JR (2010). The role of the insula in schizophrenia. Schizophrenia Research, 123, 93–104. 10.1016/j.schres.2010.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]