Abstract
Background:
Early-life phthalate exposures may adversely influence neurodevelopment by disrupting thyroid hormone homeostasis, altering brain lipid metabolism, or reducing gonadal hormone concentrations. Previous literature examining gestational phthalate exposure and child behavior were inconclusive and few prospective studies have examined childhood phthalate exposure, particularly phthalate mixtures. We investigated whether gestational and childhood phthalate exposures were associated with child behavior.
Methods:
We used data from 314 mother-child pairs in the HOME Study, a longitudinal pregnancy and birth cohort that enrolled pregnant women from Cincinnati, Ohio. We quantified urinary concentrations of 11 phthalate metabolites in samples collected twice during gestation from women and six times from their children when they were ages 1,2,3, 4, 5, and 8 years. We assessed children’s behavior at ages 2, 3, 4, 5, and 8 years using the Behavioral Assessment System for Children-2. Using linear mixed models, we estimated covariate-adjusted associations of measurement-error-corrected gestational and childhood phthalate metabolite concentrations (per interquartile range increase) with repeated child behavior assessments. We used Weighted Quantile Sum (WQS) regression to estimate the association of phthalate mixtures with child behavior.
Results:
Gestational mono(3-carboxypropyl) phthalate (MCPP) concentrations were associated with more problem behaviors (internalizing: β=0.9, 95% confidence interval [CI]=−0.1, 1.9; externalizing: β= 1.0, 95%CI=−0.1, 2.0; behavioral symptoms index [BSI]: β= 1.1, 95%CI=0.1, 2.1). Higher childhood monobenzyl phthalate (MBzP) (β=1.4; 95%CI=0.0, 2.7), monocarboxynonyl phthalate (MCNP) (β=3.2; 95%CI=1.6, 4.8), monocarboxyoctyl phthalate (MCOP) (β=0.9; 95%CI=0.0, 1.7), MCPP (β=1.8; 95%CI=0.2, 3.5), and monoethyl phthalate (MEP) (β=1.6; 95%CI=0.1, 3.1) concentrations were associated with higher BSI composite scores. Consistent with this, the weighted childhood phthalate index was associated with more problem behaviors (internalizing: β= 1.5, 95%CI=−0.2, 3.1; externalizing: β=1.7, 95%CI=0.1, 3.5; BSI: β= 1.7, 95%CI=0.2, 3.2); MBzP, MCNP, and MEP largely contributed to these associations.
Conclusion:
Our findings suggest that childhood exposure to phthalate mixtures may be associated with children’s problem behaviors.
Keywords: phthalates, children, behavior
1. Introduction
Phthalates are a class of endocrine disrupting chemicals that are widely used as plasticizers and additives in some plastic containers, food packaging, children’s toys, medical equipment, building materials, and personal care products (Braun et al., 2014; Carlstedt et al., 2013; Hauser and Calafat, 2005; Hauser et al., 2004; Lyche et al., 2009; Rudel et al., 2011). Because phthalates are not covalently bound to materials, they leach into the environment, foods and consumer products, exposing humans via inhalation, ingestion, and dermal absorption (Bornehag et al., 2005; Braun et al., 2014; Braun et al., 2013; Meeker et al., 2009; Rudel et al., 2011). Phthalates are rapidly metabolized in the body and eliminated in urine (Heudorf et al., 2007; Wittassek and Angerer, 2008). Thus, measuring urinary phthalate metabolite concentrations is the most common, reliable, and non-invasive means of assessing phthalate exposure (Braun et al., 2012; Calafat, 2016; Hauser and Calafat, 2005). Although phthalates have relatively short biological half-lives (<24 hours) in the human body, humans are chronically exposed to phthalates due to their widespread use (Braun et al., 2012; Calafat, 2016; Hauser and Calafat, 2005). Exposure to phthalates are ubiquitous, even among pregnant women, infants, and children (Braun et al., 2014; Carlstedt et al., 2013; Lewis et al., 2013; Rudel et al., 2011; Silva et al., 2004; Watkins et al., 2014). Some phthalates and their metabolites (e.g., di-2-ethylhexyl phthalate [DEHP], diethyl phthalate [DEP], and monoethyl phthalate [MEP], etc.) can cross the placenta and possibly affect the developing fetus (Mose et al., 2007; Singh et al., 1975). Moreover, some phthalates may affect the hormonal milieu of the mothers, which may in turn disrupt fetal development (Johns et al., 2015; Sathyanarayana et al., 2017). Finally, due to children’s unique physiology and behaviors, urinary concentrations of some phthalate metabolites are higher in children compared to adults (Koch et al., 2004; Koch et al., 2005; Silva et al., 2004; Sørensen, 2006; Watkins et al., 2014).
Phthalates may influence neurodevelopment by disrupting thyroid hormone homeostasis, altering brain lipid metabolism, or reducing gonadal hormone levels (Akaike et al., 1991; Andrade et al., 2006; Boas et al., 2012; Breous et al., 2005; Howdeshell et al., 2008; Huang et al., 2007; Meeker et al., 2007; Miodovnik et al., 2014; Moog et al., 2017; Stein et al., 1991; Xu et al., 2007). The developing fetus, infant, and child are especially susceptible to the potential neurotoxicity of phthalate exposure due to their increased sensitivity to environmental factors and incompletely developed biologically protective mechanisms (Huen et al., 2010; Selevan et al., 2000). Rodent studies have shown that gestational or perinatal exposures to phthalate mixtures, di-n-butyl phthalate (DBP, the parent chemical of mono-n-butyl phthalate [MnBP]), and DEHP were associated with decreased social play behavior, decreased grooming behavior, and elevated anxiety in offspring, respectively (Barakat et al., 2018; Hoshi and Ohtsuka, 2009; Kougias et al., 2018).
Previous epidemiologic studies examining early life phthalate exposure and child behavior are inconclusive in terms of particular phthalate metabolites and distinctive behavior profiles (Engel et al., 2010; Engel et al., 2018; Gascon et al., 2015; Huang et al., 2019; Hyland et al., 2019; Jankowska et al., 2019; Kobrosly et al., 2014; Ku et al., 2019; Lien et al., 2015; Messerlian et al., 2017; Minatoya et al., 2018; Philippat et al., 2017; Whyatt et al., 2012). Besides differences in the timing of and instruments used for behavior assessment, failure to account for exposure misclassification, periods of heightened susceptibility, and the effects of phthalate mixtures may also contribute to discrepancies (Radke et al., 2020). Due to their short half-life and the episodic nature of phthalate exposures, urinary concentrations of phthalate metabolite vary within individuals (Koch and Angerer, 2007; Koch et al., 2013; Watkins et al., 2014). Most previous studies examining gestational or childhood phthalate exposure only collected one urine during these periods of development, which may not accurately reflect phthalate exposure. This potential exposure misclassification could attenuate associations between urinary phthalate metabolite concentrations and child behavior. Moreover, only three prospective cohorts have examined the impact of childhood phthalate exposure on child behavior (Huang et al., 2019; Jankowska et al., 2019; Ku et al., 2019).
Mixture effects of phthalate exposures have not been considered in these studies. Individuals are chronically exposed to multiple phthalates across their lifespan. However, most previous studies used the “one chemical at a time” and “one exposure period at a time” approaches, and have not considered human exposure to these phthalate mixtures (aggregate effects) or the potential for phthalates to have cumulative effects on human health.
To address these research gaps and better understand the potential influence of phthalate exposure on child behavior, we investigated the associations of repeatedly measured gestational (up to twice) and childhood (up to six times) urinary phthalate metabolite concentrations with parent-reported child behavior using a longitudinal pregnancy and birth cohort, accounting for measurement error in urinary phthalate metabolite concentrations.
2. Materials and Methods
2.1. Study Participants
We used data from a longitudinal pregnancy and birth cohort, the Health Outcomes and Measures of the Environment (HOME) Study, which recruited pregnant women from the greater Cincinnati, Ohio area between 2003-2006, and conducted follow-up visits with the mothers and their children through age 8 years (Braun et al., 2017). Details regarding the HOME Study eligibility criteria, recruitment, and follow up are available elsewhere (Braun et al., 2017). Briefly, inclusion criteria included: ≥18 years of age; 16±3 weeks of gestation; living in the Cincinnati, Ohio area in a home built before 1978; not taking any medications for seizure or thyroid disorders; no history of HIV infection; and no diagnosis of diabetes, schizophrenia, bipolar disorder, or cancer. Of 1,263 eligible women, 468 agreed to participate in the study; the relatively low participation rate may be due to the high frequency and intensity of the study visits. Of the 401 mothers who remained in the study until delivery, 389 had live singleton births. A total of 314 mother-child pairs who had at least one urinary phthalate metabolite concentration measurement during pregnancy or childhood, at least one behavior problem assessment, and relevant covariate data were included in the current analysis.
The institutional review boards (IRBs) at Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals approved the HOME Study protocols. Brown University and the Centers for Disease Control and Prevention IRBs deferred to the CCHMC IRB. All mothers provided informed consent for themselves and their children.
2.2. Phthalate Exposure Assessment
We collected maternal urine samples twice, at approximately 16- and 26-week gestation, using polypropylene specimen cups. We collected child urine samples annually from ages 1-5 years and at age 8 years. We used polypropylene specimen cups to collect urine samples for children who were toilet-trained. For children who were not toilet-trained, we placed surgical inserts into a clean diaper; for children who were in the process of being toilet-trained, we lined a training potty with inserts to collect urine samples. Urine was then expressed from the insert into a urine collection cup with a syringe in the laboratory. All urine samples were refrigerated for <24 hours prior to processing and stored at ≤−20 °C until phthalate metabolites quantification.
Using previously described analytic chemistry methods (Silva et al., 2004), we measured urinary concentrations of four DEHP metabolites: mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), and mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP). We also measured urinary concentrations of MnBP, MBzP, monocarboxynonyl phthalate (MCNP), monocarboxyoctyl phthalate (MCOP), mono(3-carboxypropyl) phthalate (MCPP), MEP, and mono-isobutyl phthalate (MiBP). We were unable to quantify gestational urinary MCNP or MCOP concentrations because the method had not yet been developed. In addition, we detected some phthalate contamination in the diaper inserts, and thus we were unable to quantify urinary concentrations of MEHP, MnBP, and MiBP in the children’s urine samples collected at ages 1-3 years (Watkins et al., 2014). For both gestational and childhood samples, we used three oxidative DEHP metabolites to create a summary DEHP metabolite measure (i.e., ΣDEHP). To calculate ΣDEHP concentrations, we divided each DEHP metabolite concentration by its molar mass, summed the individual concentrations, and then multiplied the molar sum by the molar mass of MECPP, using the following formula: ΣDEHP (ng/mL) = [MEOHP (ng/mL) / 292.331 g/mol + MECPP (ng/mL) / 308.33 g/mol + MEHHP (ng/mL) / 294.347 g/mol] * 308.33 g/mol. The range of limits of detection (LODs) for the urinary phthalate metabolites was 0.1 to 1 ng/mL. Less than 3% of the values were below the LOD, and were replaced with the LOD/√2 (Hornung and Reed, 1990). We measured urinary creatinine concentrations to account for urine dilution (Larsen, 1972). Urinary phthalate metabolite concentrations (ng/mL) were creatinine-standardized (dividing metabolite concentrations by creatinine concentrations) and log10-transformed in statistical analyses. Finally, we standardized the concentrations of each phthalate metabolite to its interquartile range (IQR) across visits to make effect sizes more comparable across different metabolites (Buckley et al., 2016).
2.3. Child Behavioral Outcomes
Parents or caregivers rated their children’s behavior at ages 2, 3, 4, 5, and 8 years using the Behavioral Assessment System for Children-2 (BASC-2) (Reynolds and Kamphaus, 2004). The BASC-2 is a valid and reliable tool for assessing children’s adaptive and problem behaviors in both home and community settings (Reynolds and Kamphaus, 2004). We analyzed three behavior problem composite scales (internalizing problems, externalizing problems, and Behavioral Symptoms Index [BSI]) and nine clinical subscales (anxiety, depression, somatization, aggression, conduct problems, hyperactivity, attention problems, atypicality, and withdrawal). The internalizing problem composite scale reflects inwardly directed behaviors (i.e., anxiety, depression, somatization); the externalizing problem composite scale measures disruptive behavior problems (i.e., aggression, conduct problems, hyperactivity); and the BSI assesses the child’s overall level of problem behaviors (i.e., aggression, atypicality, attention problems, depression, hyperactivity, and withdrawal). Conduct problems were only assessed at age 8 years. We used software provided by the test publishers and U.S. population-based normative referent data to calculate T-scores (mean: 50, standard deviation [SD]: 10) for each composite and clinical scale. Higher scores on these scales indicate more problem behaviors.
2.4. Covariate Assessment
We used previous literature and directed acyclic graphs to identify potential confounders that may be associated with both phthalate exposure and child behavior, but not causal intermediates or colliders (Supplemental Figures S1–S2). Trained interviewers administered standardized questionnaires to assess maternal age, education, household income, marital status, parity, gestational alcohol consumption, and children’s race. We abstracted data on maternal pregnancy-induced hypertension and child sex from hospital medical charts. We used self-reported height and weight (or imputed weight using the Super Learner method if data were missing) to calculate pre-pregnancy body mass index (BMI) (van der Laan et al., 2007). We assessed maternal depressive symptoms during the 2nd trimester using the Beck Depression Inventory-II and attention-deficit/hyperactivity disorder (ADHD) behaviors at the 8-year study visit using Conners’ Adult ADHD Rating Scale, with higher scores indicating more depressive and ADHD behavior problems, respectively (Beck et al., 1997; Conners et al., 1999). We used the average of the log10-transformed serum cotinine concentrations measured at 16- and 26-week gestation to assess gestational tobacco smoke exposure. Similar to phthalates, urinary bisphenol A (BPA) and triclosan concentrations and blood lead concentrations were measured up to twice during gestation and six times during childhood. We used the average of the natural log-transformed blood lead concentrations measured at 16- and 26-week gestation to assess gestational lead exposure. We assessed the caregiving environment when children were 1 year old using the Home Observation for Measurement of the Environment (Caldwell and Bradley, 2003).
2.5. Statistical Analyses
We began by calculating univariate statistics and creating violin plots to examine the distribution of observed and measurement error-corrected urinary phthalate metabolite concentrations across visits (Hintze and Nelson, 2012). Then we calculated the mean and SD of the three BASC-2 composite scale scores by strata of the key covariates and compared the scores across these strata using the Kruskal-Wallis test. We calculated the intraclass correlation coefficients of BASC-2 scores assessed from ages 2-8 years. We also compared the covariate distribution between the HOME Study children included and excluded in this analysis using Chi-square test or Fisher’s exact test.
2.5.1. Exposure Measurement Error.
We used two distinct regression-calibration approaches to account for exposure measurement error of gestational and childhood phthalate metabolite measures, depending on whether urinary phthalate metabolite concentrations showed time trends across measurements. Details of the approaches have been previously described (Jackson-Browne et al., 2019).
For gestational exposure, we used a random intercept model (Carroll et al., 2006), where subject-specific replicates were used to estimate true geometric mean urinary phthalate metabolite concentrations (log10-transformed and creatinine standardized) across the two measurements during gestation (16 and 26 weeks). Our model is based on the assumption that phthalate metabolite concentrations collected from the ith mother at the jth measurement during gestation are distributed as Yij = μi +εij, where and . Thus, the reliability of observed geometric mean phthalate metabolite concentration () for the ith mother across gestation is given by , where and are the between-subject and within-subject variance components respectively, and ni, is the number of available phthalate metabolite measures. In our regression model for the BASC-2 outcomes, using the NLME package in R (Pinheiro and Bates, 2000), we replaced by its best linear unbiased predictor (BLUP) from a linear mixed effects model, . Under a random intercept model, the BLUP has a closed form given by , where is the generalized least squares estimate of the geometric mean gestational phthalate metabolite concentration across the two gestational measurement occasions for the entire HOME Study sample. Because of their lower reliability, observed geometric mean concentrations from mothers with one gestational measure are shrunk more towards the grand mean than mothers with two measures.
For childhood exposure, a more complex linear mixed effects model was used to account for measurement error because childhood urinary phthalate metabolite concentrations changed non-linearly with child age. Models included random intercepts, random linear time slopes, and linear, quadratic and cubic fixed effects of time. We specified a spherical covariance matrix for the within-subject residuals and an unstructured between-subject covariance matrix for the random effects. The scalar reliability measure for the gestational geometric mean is replaced by a reliability matrix that differentially shrinks the observed urinary phthalate metabolite concentrations towards the occasion-specific grand means depending on both the number and timing of measurement occasions for each participant. Based on the subject-specific urinary phthalate metabolite concentration trajectory we estimated for each HOME Study participant, we calculated the exposure intensity of the urinary phthalate metabolite concentration during the childhood period by integrating the resulting quadratic polynomial in closed form and dividing the area under the curve by the length of exposure for each subject. For example, the cumulative MEP exposure up to age 4 years was used to calculate exposure intensity of urinary MEP concentrations at age 4 years.
2.5.2. Periods of Susceptibility Analyses.
First, we used a previously described multiple informant method, which utilizes generalized estimating equations, to jointly estimate the associations of repeated gestational and childhood urinary phthalate metabolite concentrations at each exposure period with the BASC-2 measurements (Jackson-Browne et al., 2018; Sanchez et al., 2011; Stacy et al., 2017). This method also allowed us to test if the associations were equal across gestational (16- and 26-week) or childhood (ages 1, 2, 3, 4, 5, and 8 years) periods. We used the measurement-error-corrected log10-transformed creatinine-standardized urinary phthalate metabolite concentrations as our exposure variables, and three composite scales as distinctive outcomes. The multiple informant model included visit, phthalate x visit, and covariates. A P-value of the phthalate x visit interaction term <0.05 was considered evidence that at least one of the phthalate-BASC-2 associations differed from the rest. If we found no evidence that the associations differed across gestation/childhood exposure periods, we used the geometric mean measurement-error-corrected gestational phthalates and the measurement-error-corrected exposure intensity of childhood phthalates as the exposure variables for the rest of our analyses.
2.5.3. Main Analyses.
We used random intercept linear mixed models (with empirical standard errors, as implemented in SAS Proc Mixed version 13.2) to estimate the associations of gestational and childhood urinary phthalate metabolite concentrations with children’s repeated BASC-2 measurements. BASC-2 was measured in 261 children at age 2 years, 244 children at age 3 years, 183 children at age 4 years, 206 children at age 5 years, and 228 children at age 8 years. Because BASC-2 measurements were relatively stable during childhood (intraclass correlation coefficient ranged from 0.5 to 0.7, moderately correlated, Supplemental Table S1), we used all BASC-2 measurements as the outcome to increase our sample size and effect estimate precision. The three problem behavior composite scales and nine clinical subscales were examined separately as outcomes. Geometric mean measurement-error-corrected log10-transformed gestational creatinine-standardized urinary phthalate metabolite concentrations and measurement-error-corrected exposure intensity of log10-transformed childhood creatinine-standardized urinary phthalate metabolite concentrations were used as exposure variables. The childhood exposure variable was characterized as a time-varying variable to avoid reverse causation. For example, to estimate the association with BASC-2 assessment at age 8 years, exposure intensity of childhood MEP concentrations during the first 8 years of life were used; whereas to estimate the association with BASC-2 assessment at age 4 years, exposure intensity of childhood MEP concentrations during the first 4 years of life were used. We adjusted for maternal age at delivery, education, marital status, pre-pregnancy BMI, depression score at enrollment, serum cotinine concentrations during pregnancy, alcohol consumption during pregnancy, and child’s sex, race, and age at outcome assessment.
In addition, we specified a weighted quantile sum (WQS) regression model to estimate the influence of our eight-component phthalate mixture on child behavior (Czarnota et al., 2015). Briefly, a WQS regression model constructs a weighted index within a set of correlated exposures and tests its overall association with an outcome. The relative variable importance (RVI) of each individual exposure can be then assessed by the weights that the model assigns to it within the index (Czarnota et al., 2015). WQS regression is typically employed in a non-iterative fashion, thus failing to capture weight uncertainty. Thus, we chose to implement WQS regression in an iterative fashion within a parallel-processing computing environment (Tanner et al., 2019).
In each of N=1,000 iterations, we randomly sampled 40% of the observations and used them to estimate a set of weights that maximized the association between childhood phthalate exposure and children’s BASC-2 scores across B=100 bootstrap sub-samples. Because WQS regression is constrained to model associations between individual exposures and outcome that are all in the same direction, we assumed that these weights were non-negative and summed to one across index components. Given that the optimally-weighted index varied over its iteration-specific interquartile range, we then used the remaining 60% of the observations to fit a linear regression model to estimate a standardized coefficient that assessed the association of childhood phthalate concentrations on children’s BASC-2 scores. As a result, percentile confidence intervals for β across iterations fully capture uncertainty in both the RVI of individual phthalate exposures and in the strength of the association of overall childhood phthalate exposure with children’s BASC-2 scores. In addition to examining box plots of weights across iterations, we also assessed RVI by the proportion of iterations for which the corresponding weights exceeded the threshold expected by chance, 1/c, where c=8 is the number of index components. Certain index components may appear relatively unimportant based on their average weight across iterations, but still have high inclusion probabilities, due to large weight variability across iterations. Phthalate metabolites with inclusion probabilities >0.125 (1/8) were considered as important components of the mixture. Based on our initial analyses described above and given that WQS cannot currently accommodate repeated measurements, we used exposure intensity of urinary childhood phthalate metabolite concentrations through age 8 years as the exposure and BASC-2 scores at age 8 years as the outcome for this analysis.
2.5.4. Secondary Analyses.
We examined whether the phthalate-behavior associations differ by child sex by including a phthalate x sex product interaction term in the model; a P value for this term <0.05 was considered as evidence for interaction.
2.5.5. Sensitivity Analyses.
Because mothers and children may share common sources of exposure over time, we mutually adjusted for gestational and childhood phthalate metabolites in the model to reduce potential co-pollutant confounding and test the robustness of our results. Because our group previously reported sex-specific associations of gestational urinary BPA and gestational/childhood triclosan concentrations with child behavior, we additionally adjusted for gestational urinary BPA and triclosan concentrations in our sex-specific prenatal exposure models, and childhood triclosan concentrations in our sex-specific postnatal exposure models to reduce potential confounding and test the robustness of our results (Jackson-Browne et al., 2019; Stacy et al., 2017). The geometric mean measurement-error-corrected log10-transformed creatinine-standardized gestational urinary BPA and triclosan concentrations, as well as measurement-error-corrected exposure intensity of the childhood urinary BPA and triclosan concentrations were used in the analysis. In addition, because all pregnant woman subjects were living in a home built before 1978, which would increase the risk of lead exposure, we additionally adjusted for gestational and childhood blood lead concentrations in the prenatal and postnatal exposure models, respectively, to test the robustness of our results. The average of gestational blood lead concentrations was adjusted in the prenatal model, and the childhood blood lead concentrations was used as a time-varying covariate to be consistent with our phthalate biomarker parameterization. We also additionally adjusted for household income, pregnancy-induced hypertension, parity, maternal ADHD behavior, and caregiving environment given that the presence or directionality of the associations of some of these covariates with exposure and outcome is unclear.
We performed all analyses using R (R Development Core Team, 2015) and SAS version 9.4 (SAS Institute Inc., USA). Consistent with the recommendations of the American Statistical Association and other epidemiologists that we should not conclude there is ‘no association’ just because a P value is larger than a threshold such as 0.05 or a confidence interval does not include the null (Amrhein et al., 2019; Wassersteina and Lazar, 2016), we focused on the pattern of our results and not just the statistical significance of our estimates in this study. If an association showed a pattern, that is, the upper or lower confidence limit closes to 0, we consider it a potentially important association.
3. Results
Our analysis included 314 mother-child pairs who had at least one urinary phthalate metabolite measurement, at least one BASC-2 assessment at ages 2-8 years, and complete covariate data (Supplemental Table S2). The creatinine-standardized urinary concentrations of most phthalate metabolites decreased with children’s age, except for MCOP concentrations which changed non-monotonically with age (Supplemental Table S3 and Supplemental Figure S3). For measurement-error-corrected gestational phthalate metabolite concentrations, MnBP was moderately correlated with MBzP, MCPP, and MiBP (Pearson’s r ranges 0.41-0.45). For measurement-error-corrected exposure intensity of childhood phthalate metabolite concentrations, the correlations of MnBP with MBzP, MCPP, and MiBP (Pearson’s r ranges 0.40-0.62), and the correlations of MCOP with MCNP and MCPP (Pearson’s r ranges 0.57-0.67) were moderate as well (Supplemental Figure S4).
Mean ± SD of internalizing problem, externalizing problem, and BSI scores were 48 ± 9, 49 ± 9 and 49 ± 9, respectively, which are close to the U.S. population-based normative referent data (Table 1). Below we highlighted BASC-2 scores that were statistically significantly different based on the Kruskal-Wallis test. On average, children’s mean internalizing problem scores were higher among those who were born to mothers who had higher depression scores at enrollment (47 for BDI scores 0-14; 50 for BDI scores >14-19; and 51 for BDI scores≥19, P=0.02). Children’s mean externalizing problem and BSI scores were higher among males, or those born to mothers who had higher (compared to lower) depression scores at enrollment (P values <0.05). The distributions of most covariates were similar between the HOME Study children included and excluded in this analysis, except for maternal education, maternal age, marital status, and gestational smoking (Supplemental Table S4).
Table 1.
Behavior Assessment System for Children-2 (BASC-2) Scores According to Covariates among Children from the Health Outcomes and Measures of the Environment Study (N=314)
| N | Internalizing Problemsa | P-value | Externalizing Problemsa | P-value | Behavioral Symptoms Indexa | P-value | |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Overall | 314 | 48 ±9 | 49 ±9 | 49 ±9 | |||
| Child Sex | 0.37 | 0.01 | 0.01 | ||||
| Female | 171 | 48 ±9 | 47 ±9 | 48 ±9 | |||
| Male | 143 | 47 ±8 | 50 ±9 | 50 ±9 | |||
| Child Race | 0.60 | 0.84 | 0.37 | ||||
| Non-Hispanic White | 197 | 48 ±8 | 49 ±8 | 49 ±8 | |||
| Non-Hispanic Black | 97 | 47 ± 10 | 49 ± 11 | 50 ± 10 | |||
| Other | 20 | 47 ±9 | 48 ±9 | 47 ±9 | |||
| Maternal Education | 0.51 | 0.54 | 0.26 | ||||
| High School or Less | 68 | 48 ±9 | 49 ± 10 | 50 ±9 | |||
| Tech School/Some College | 77 | 47 ±9 | 50 ± 11 | 50 ± 10 | |||
| Bachelor’s or More | 169 | 48 ±8 | 48 ±7 | 48 ±8 | |||
| Marital Status | 0.54 | 0.51 | 0.14 | ||||
| Not Married | 103 | 47 ± 10 | 49 ± 10 | 50 ±9 | |||
| Married | 211 | 48 ±8 | 49 ±8 | 49 ±8 | |||
| Maternal Age | 0.07 | 0.67 | 0.72 | ||||
| 18-25 Years | 70 | 47 ± 10 | 48 ± 11 | 50 ± 10 | |||
| 25-35 Years | 195 | 47 ±9 | 49 ±8 | 49 ±8 | |||
| >35 Years | 49 | 50 ±8 | 50 ±8 | 50 ±9 | |||
| Pre-Pregnancy BMI | 0.36 | 0.18 | 0.26 | ||||
| <25 | 166 | 47 ±9 | 48 ±9 | 49 ±8 | |||
| ≥25-30 | 85 | 49 ±8 | 48 ±7 | 49 ±8 | |||
| ≥30 | 63 | 47 ± 10 | 51 ± 11 | 51 ± 10 | |||
| Gestational Smoking | 0.48 | 0.52 | 0.56 | ||||
| Unexposed | 109 | 48 ±8 | 49 ±8 | 49 ±8 | |||
| Second Hand Smoke | 172 | 47 ±9 | 49 ± 10 | 49 ±9 | |||
| Active Smoking | 33 | 48 ± 10 | 50 ± 10 | 50 ±8 | |||
| Maternal Baseline BDI | 0.02 | 0.01 | 0.01 | ||||
| 0-14 | 257 | 47 ±8 | 48 ±8 | 48 ±8 | |||
| >14-<19 | 29 | 50 ± 10 | 51 ± 11 | 53 ±9 | |||
| ≥19 | 25 | 51 ±9 | 55 ± 10 | 55 ±9 | |||
| Gestational Alcohol Drinking | 0.27 | 0.23 | 0.28 | ||||
| Never or <1/Month | 269 | 47 ±9 | 49 ±9 | 49 ±9 | |||
| >1/Month | 19 | 48 ±8 | 51 ±7 | 51 ±7 | |||
| Binge | 26 | 50 ±8 | 50 ± 10 | 50 ±9 |
Note: SD, standard deviation; BMI, body mass index; BDI, Beck Depression Inventory.
When BASC-2 scores were available at more than one age, the oldest age was used.
P values were calculated using the Kruskal-Wallis test to compare the mean BASC-2 scores across the strata of covariates.
Using multiple informant models, we did not find evidence that the association of gestational or childhood phthalate concentrations with BASC-2 scores varied by visit. All of the heterogeneity p-values were >0.05, although the p-values for gestational ΣDEHP-externalizing problems and childhood MCPP-internalizing problems were 0.10 and 0.09, respectively (Supplemental Table S5 and Supplemental Figure S5). Thus, we focused on the geometric mean measurement-error-corrected gestational exposure and measurement-error-corrected childhood exposure intensity in the rest of our analyses.
In our main analyses, gestational urinary MCPP concentrations were positively associated with the three composite scores (internalizing problems: β=0.9, 95% confidence interval [CI]=−0.1, 1.9, P=0.07; externalizing problems: β=1.0, 95%CI=−0.1, 2.0, P=0.07; BSI: β=1.1, 95%CI=0.1, 2.1, P=0.03). Gestational urinary MBzP (β=0.8, 95%CI=−0.2, 1.9, P=0.13) and MiBP (β=0.7, 95%CI=−0.2, 1.6, P=0.14) concentrations were both positively associated with externalizing problem composite scores (Figure 1, Table 2, and Supplemental Table S6). The 95%CIs for most of these associations included the null. The associations between gestational phthalates and children’s BASC-2 scores did not differ by child sex (Table 2 and Supplemental Figure S6).
Figure 1.
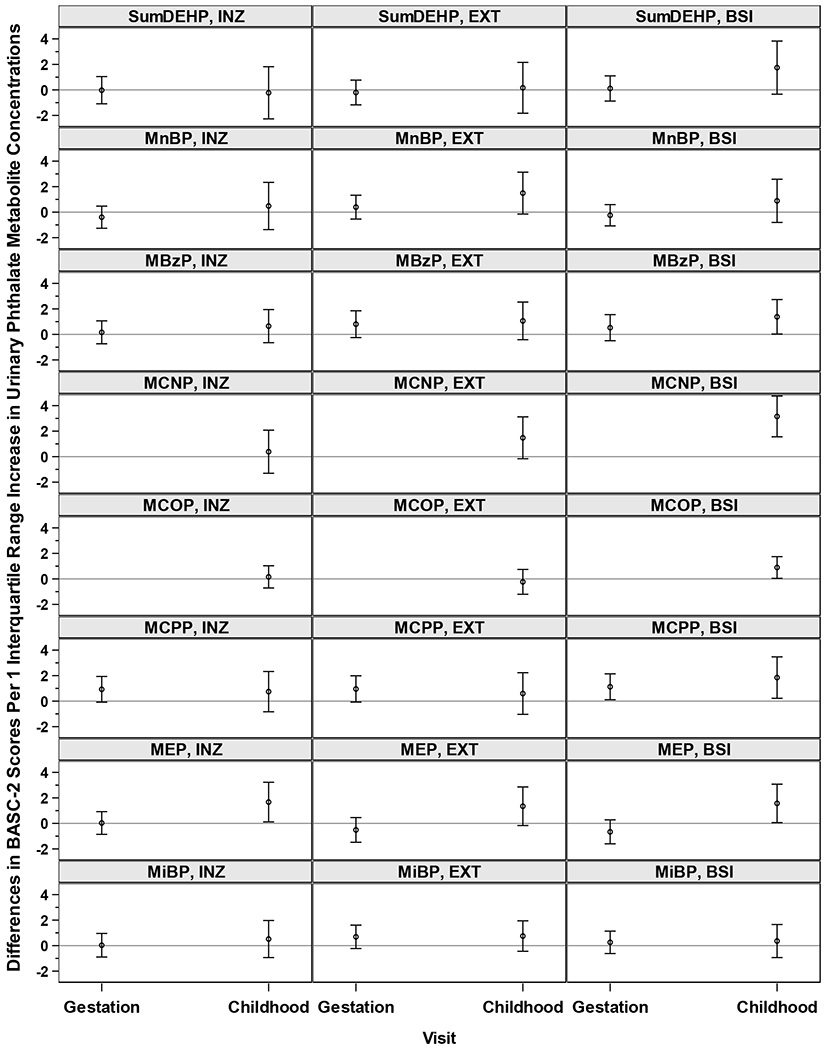
Adjusted Differences in BASC-2 Composite Scale Scores with 1-IQR Increase in Geometric Mean Measurement-Error-Corrected Log10-Transformed Gestational Creatinine-Standardized Urinary Phthalate Metabolite Concentrations or Measurement-Error-Corrected Exposure Intensity of Log10-Transformed Childhood Creatinine-Standardized Urinary Phthalate Metabolite Concentrations: The HOME Study.
Note: BASC-2, Behavioral Assessment System for Children-2; IQR, interquartile range; INZ, internalizing problems; EXT, externalizing problems; BSI, behavioral symptoms index; ΣDEHP, summary di(2-ethylhexyl) phthalate metabolite measure; MnBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
The sample size for estimating the associations between maternal phthalates and behavior is N=314, for the associations of childhood MiBP and MnBP with behavior is N=262, and for the associations between other childhood phthalates and behavior is N=312.
All estimates were adjusted for: maternal age at delivery, mother’s pre-pregnancy BMI, maternal gestational smoking (mean of log10-transformed serum cotinine concentrations during 16 and 26 weeks of pregnancy), mother’s Beck’s Depression Inventory score, gestational alcohol consumption (<1/month, >1/month, binge), maternal education (high school graduate or less, tech school or some college, college graduate or above), marital status (married, unmarried), and child’s sex (male/female), race (non-Hispanic White, non-Hispanic Black, other), and age at outcome assessment.
Table 2.
Adjusted Differences [95% Confidence Intervals (CI)] in BASC-2 Composite Scale Scores with 1-IQR Increase in Geometric Mean Measurement-Error-Corrected Log10-Transformed Gestational Creatinine-Standardized Urinary Phthalate Metabolite Concentrations or Measurement-Error-Corrected Exposure Intensity of Log10-Transformed Childhood Creatinine-Standardized Urinary Phthalate Metabolite Concentrations: The HOME Study.
| Phthalates & BASC-2 | Maternal Exposurea,b | Childhood Exposurea,b | ||||||
|---|---|---|---|---|---|---|---|---|
| Scales | All | Boys | Girls | Pint.c | All | Boys | Girls | Pint.c |
| ΣDEHP | ||||||||
| Internalizing Problems | 0.0 (−1.1, 1.0) | −0.6 (−2.1, 0.9) | 0.5 (−1.0, 1.9) | 0.77 | −0.2 (−2.3, 1.8) | 0.6 (−2.3, 3.5) | −0.3 (−3.2, 2.5) | 0.69 |
| Externalizing Problems | −0.2 (−1.2, 0.8) | −0.5 (−1.8, 0.9) | 0.1 (−1.2, 1.4) | 0.97 | 0.2 (−1.8, 2.2) | 0.7 (−2.2, 3.6) | 0.1 (−2.7, 2.8) | 0.05 |
| BSI | 0.1 (−0.9, 1.1) | −0.1 (−1.5, 1.4) | 0.3 (−1.0, 1.5) | 0.99 | 1.7 (−0.3, 3.8) | 3.3 (0.8, 5.9) | 1.2 (−1.9, 4.3) | 0.26 |
| MnBP | ||||||||
| Internalizing Problems | −0.4 (−1.3, 0.5) | −0.7 (−2.0, 0.5) | −0.2 (−1.5, 1.0) | 0.61 | 0.5 (−1.4, 2.3) | −0.8 (−3.2, 1.6) | 1.2 (−1.3, 3.6) | 0.11 |
| Externalizing Problems | 0.4 (−0.5, 1.3) | 0.4 (−0.9, 1.7) | 0.5 (−0.9, 1.8) | 0.95 | 1.5 (−0.1, 3.1) | 0.8 (−1.7, 3.3) | 1.7 (−0.4, 3.7) | 0.05 |
| BSI | −0.2 (−1.1, 0.6) | −0.4 (−1.5, 0.8) | −0.2 (−1.4, 1.0) | 0.89 | 0.9 (−0.8, 2.6) | 0.8 (−1.4, 3.1) | 0.7 (−1.6, 3.0) | 0.20 |
| MBzP | ||||||||
| Internalizing Problems | 0.2 (−0.7, 1.1) | 0.0 (−1.3, 1.2) | 0.5 (−1.0, 2.0) | 0.36 | 0.6 (−0.6, 1.9) | 0.4 (−1.4, 2.2) | 0.9 (−0.9, 2.7) | 0.34 |
| Externalizing Problems | 0.8 (−0.2, 1.9) | 0.8 (−0.5, 2.2) | 0.9 (−0.8, 2.6) | 0.60 | 1.1 (−0.4, 2.5) | −0.1 (−2.1, 1.9) | 1.8 (−0.4, 3.9) | 0.01 |
| BSI | 0.5 (−0.5, 1.6) | 0.4 (−0.8, 1.7) | 0.9 (−0.9, 2.6) | 0.58 | 1.4 (0.0, 2.7) | 1.3 (−0.5, 3.1) | 1.5 (−0.5, 3.5) | 0.19 |
| MCNP | ||||||||
| Internalizing Problems | - | - | - | 0.4 (−1.3, 2.1) | 1.7 (−0.5, 4.0) | −0.8 (−3.3, 1.7) | 0.90 | |
| Externalizing Problems | - | - | - | 1.5 (−0.2, 3.1) | 2.2 (−0.3, 4.8) | 0.6 (−1.5, 2.8) | 0.11 | |
| BSI | - | - | - | 3.2 (1.6, 4.8) | 4.1 (1.7, 6.5) | 2.6 (0.4, 4.8) | 0.37 | |
| MCOP | ||||||||
| Internalizing Problems | - | - | - | 0.2 (−0.7, 1.0) | 1.3 (0.3, 2.4) | −0.9 (−2.2, 0.5) | 0.08 | |
| Externalizing Problems | - | - | - | −0.2 (−1.2, 0.7) | 0.9 (−0.4, 2.2) | −1.5 (−2.7, −0.2) | 0.64 | |
| BSI | - | - | - | 0.9 (0.0, 1.7) | 1.5 (0.3, 2.7) | 0.4 (−0.8, 1.5) | 0.83 | |
| MCPP | ||||||||
| Internalizing Problems | 0.9 (−0.1, 1.9) | 0.7 (−0.8, 2.1) | 1.2 (−0.2, 2.7) | 0.96 | 0.7 (−0.8, 2.3) | 0.9 (−1.1,2.9) | 0.5 (−1.9, 3.0) | 0.90 |
| Externalizing Problems | 1.0 (−0.1,2.0) | 0.6 (−0.9, 2.0) | 1.5 (0.1, 3.0) | 0.78 | 0.6 (−1.0, 2.2) | 0.5 (−1.7, 2.7) | 0.4 (−1.9, 2.7) | 0.08 |
| BSI | 1.1 (0.1,2.1) | 1.2 (−0.3, 2.7) | 1.1 (−0.3, 2.4) | 0.58 | 1.8 (0.2, 3.5) | 2.4 (0.2, 4.6) | 1.3 (−1.0, 3.5) | 0.42 |
| MEP | ||||||||
| Internalizing Problems | 0.0 (−0.9, 0.9) | −0.1 (−1.4, 1.2) | 0.2 (−1.2, 1.5) | 0.41 | 1.7 (0.1, 3.2) | 0.7 (−1.4, 2.9) | 2.2 (0.2, 4.1) | 0.11 |
| Externalizing Problems | −0.5 (−1.5, 0.5) | −1.2 (−2.6, 0.1) | 0.0 (−1.3, 1.4) | 0.15 | 1.3 (−0.2, 2.9) | 0.3 (−1.9, 2.5) | 2.0 (0.1, 3.9) | 0.01 |
| BSI | −0.7 (−1.6, 0.3) | −1.1 (−2.5, 0.2) | −0.3 (−1.5, 1.0) | 0.36 | 1.6 (0.1, 3.1) | 1.1 (−1.0, 3.2) | 2.0 (−0.1, 4.0) | 0.10 |
| MiBP | ||||||||
| Internalizing Problems | 0.0 (−0.9, 1.0) | 0.0 (−1.4, 1.4) | 0.2 (−1.2, 1.6) | 0.40 | 0.5 (−0.9, 2.0) | −0.5 (−2.7, 1.6) | 1.0 (−0.9, 2.9) | 0.09 |
| Externalizing Problems | 0.7 (−0.2, 1.6) | 0.8 (−0.5, 2.2) | 0.6 (−0.7, 1.9) | 0.83 | 0.8 (−0.4, 1.9) | −0.5 (−2.3, 1.4) | 1.1 (−0.6, 2.7) | 0.02 |
| BSI | 0.3 (−0.6, 1.1) | 0.6 (−0.7, 1.8) | 0.0 (−1.3, 1.2) | 0.73 | 0.4 (−0.9, 1.7) | −0.3 (−2.3, 1.6) | 0.5 (−1.3, 2.3) | 0.13 |
Note: BASC-2, Behavior Assessment System for Children-2; IQR, interquartile range; Pint, P value for exposure*sex interaction; BSI, behavioral symptoms index; ΣDEHP, summary di(2-ethylhexyl) phthalate metabolite measure; MnBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
The sample size for estimating the associations between maternal phthalates and behavior is N=314, for the associations of childhood MiBP and MnBP with behavior is N=262, and for the associations between other childhood phthalates and behavior is N=312.
All estimates were adjusted for: maternal age at delivery, mother’s pre-pregnancy BMI, maternal gestational smoking (mean of log10-transformed serum cotinine concentrations during 16 and 26 weeks of pregnancy), mother’s Beck’s Depression Inventory score, gestational alcohol consumption (<1/month, >1/month, binge), maternal education (high school graduate or less, tech school or some college, college graduate or above), marital status (married, unmarried), and child’s sex (male/female), race (non-Hispanic White, non-Hispanic Black, other), and age at outcome assessment.
P values <0.05 indicate the interaction were significant.
Several childhood phthalate metabolites were associated with BASC-2 composite scores. Each 1-IQR increase in log10-transformed childhood urinary MEP concentrations were associated with 1.7 (95%CI=0.1, 3.2, P=0.03) higher scores on internalizing problem composite scale. Higher childhood urinary MnBP (β=1.5; 95%CI=−0.1, 3.1, P=0.07), MCNP (β=1.5; 95%CI=−0.2, 3.1, P=0.08), and MEP (β= 1.3; 95%CI=−0.2, 2.9, P=0.08) concentrations were associated with higher externalizing problem composite scores. Higher childhood urinary MBzP (β=1.4; 95%CI=0.0, 2.7, P=0.05), MCNP (β=3.2; 95%CI=1.6, 4.8, P<0.01), MCOP (β=0.9; 95%CI=0.0, 1.7, P=0.04), MCPP (β=1.8; 95%CI=0.2, 3.5, P=0.03), and MEP (β=1.6; 95%CI=0.1, 3.1, P=0.04) concentrations were associated with higher BSI composite scores (Figure 1, Table 2, and Supplemental Table S6). The 95%CIs for some of these associations included the null. For childhood phthalates, most of the associations were not different by child sex; however, we observed more pronounced positive associations between MEP concentrations and three composite scores in girls (internalizing problems: β=2.2; 95%CI=0.2, 4.1, P=0.03; externalizing problems: β=2.0; 95%CI=0.1, 3.9, P=0.04; BSI: β=2.0; 95%CI=−0.1, 4.0, P=0.06) compared to boys (internalizing problems: β=0.7; 95%CI=−1.4, 2.9, P=0.50; externalizing problems: β=0.3; 95%CI=−1.9, 2.5, P=0.81; BSI: β=1.1; 95%CI=−1.0, 3.2, P=0.32) (Table 2 and Supplemental Figure S6).
When examining the aggregate impact of all phthalate metabolites (exposure intensity through age 8 years) using WQS regression, an IQR increase in the weighted childhood phthalate index was positively associated with the three composite scale scores (internalizing problems: β= 1.5, 95%CI=−0.2, 3.1, P=0.08; externalizing problems: β=1.7, 95%CI=0.1, 3.5, P=0.048; BSI: β=1.7, 95%CI=0.2, 3.2, P=0.03). Compared to other metabolites, MCNP, MEP, and MBzP contributed largely to these associations, with the relative weights of 0.33, 0.28, and 0.15 for internalizing problems; 0.22, 0.15, and 0.36 for externalizing problems; and 0.28, 0.13, and 0.30 for BSI, respectively (Figures 2–4, Supplemental Table S7). MCNP, MEP, MBzP, ΣDEHP and MCPP (for internalizing problems); MCNP, MEP, MBzP, MnBP, MiBP, and ΣDEHP (for externalizing problems); and MCNP, MEP, MBzP and ΣDEHP (for BSI) had inclusion probabilities >0.125.
Figure 2.
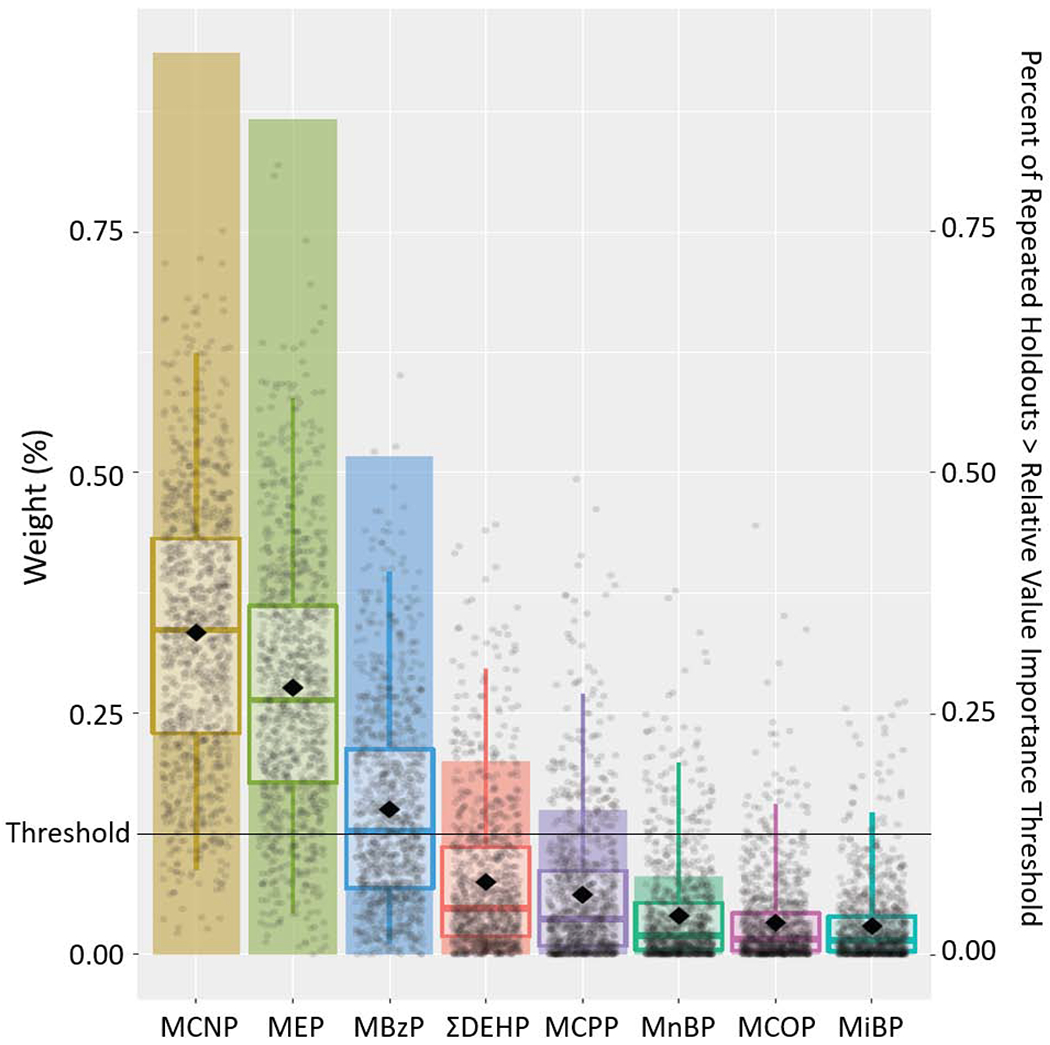
Results from Weighted Quantile Sum Regression Analysis with 1,000 Iterations on Childhood Exposure to Phthalate Mixture and Internalizing Problem Composite Scale: Relative Weights of Each Phthalate Metabolite and Percent of Repeated Holdouts Above Relative Variable Importance Threshold (N=228).
Note: Each individual dot represents weight for a given metabolite from a single repeated holdout. Box-and-whisker plots describe univariate statistics of these weights from 1,000 repeated holdouts. Bar height indicates the proportion of repeated holdouts where the weight had a value above the relative variable importance threshold (0.125, i.e., 1/8). DEHP, summary di(2-ethylhexyl) phthalate metabolite measure; MnBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
Adjusted for maternal age at delivery, mother’s pre-pregnancy BMI, maternal gestational smoking (mean of log10-transformed serum cotinine concentrations during 16 and 26 weeks of pregnancy), mother’s Beck’s Depression Inventory score, gestational alcohol consumption (<1/month, >1/month, binge), maternal education (high school graduate or less, tech school or some college, college graduate or above), marital status (married, unmarried), and child’s sex (male/female), race (non-Hispanic White, non-Hispanic Black, other), and age at outcome assessment.
Figure 4.
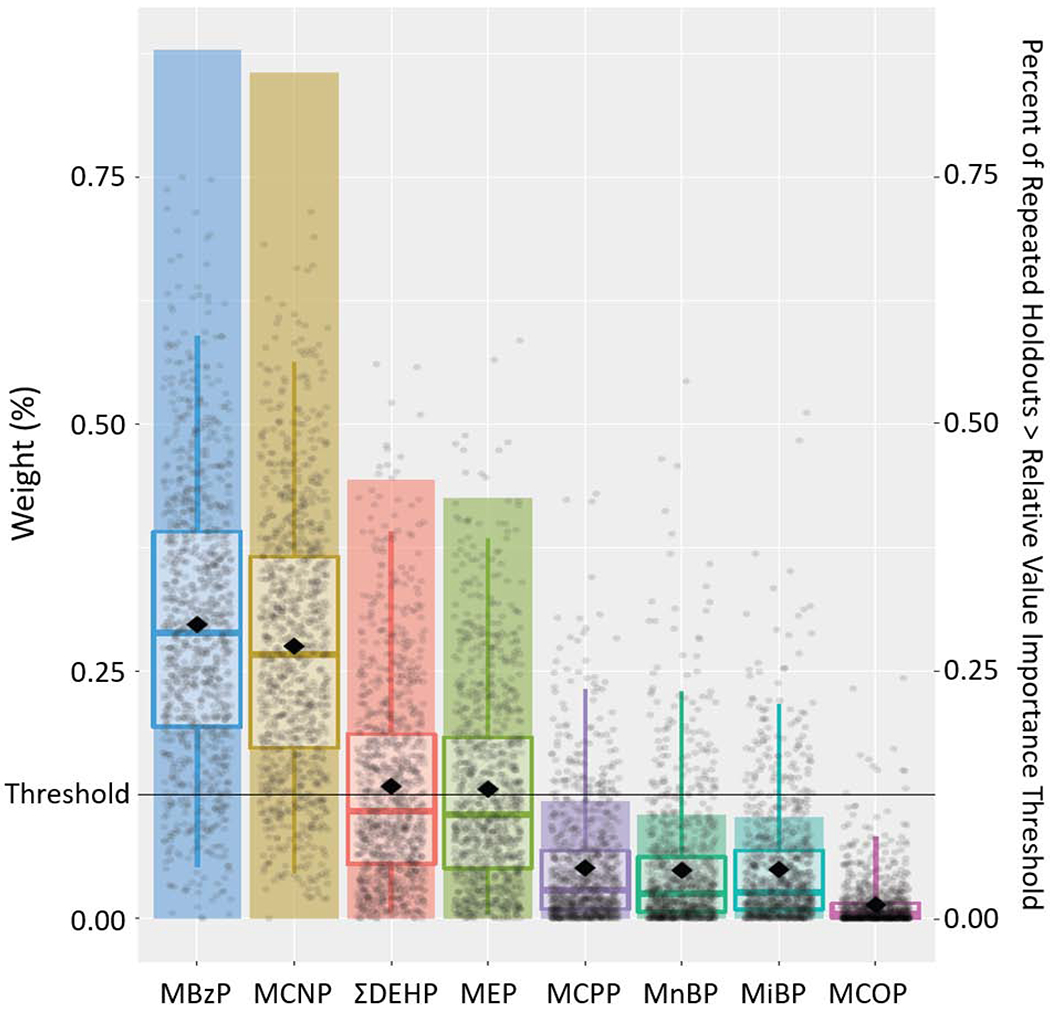
Results from Weighted Quantile Sum Regression Analysis with 1,000 Iterations on Childhood Exposure to Phthalate Mixture and Behavioral Symptoms Index Composite Scale: Relative Weights of Each Phthalate Metabolite and Percent of Repeated Holdouts Above Relative Variable Importance Threshold (N=228).
Note: Each individual dot represents weight for a given metabolite from a single repeated holdout. Box-and-whisker plots describe univariate statistics of these weights from 1,000 repeated holdouts. Bar height indicates the proportion of repeated holdouts where the weight had a value above the relative variable importance threshold (0.125, i.e., 1/8). DEHP, summary di(2-ethylhexyl) phthalate metabolite measure; MnBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
Adjusted for maternal age at delivery, mother’s pre-pregnancy BMI, maternal gestational smoking (mean of log10-transformed serum cotinine concentrations during 16 and 26 weeks of pregnancy), mother’s Beck’s Depression Inventory score, gestational alcohol consumption (<1/month, >1/month, binge), maternal education (high school graduate or less, tech school or some college, college graduate or above), marital status (married, unmarried), and child’s sex (male/female), race (non-Hispanic White, non-Hispanic Black, other), and age at outcome assessment.
When examining clinical subscales, childhood urinary concentrations of several phthalate metabolites were associated with somatization, depression, atypicality and withdrawal subscales (Supplemental Figure S7 and Supplemental Tables S8–S9). It is noteworthy that childhood urinary ΣDEHP (β=1.9; 95%CI=0.0, 3.8, P=0.05), MBzP (β=1.3; 95%CI=0.0, 2.7, P=0.06), MCNP (β=1.6; 95%CI=−0.1, 3.3, P=0.06), MCOP (β=1.0; 95%CI=0.0, 2.1, P=0.06), MCPP (β=2.0; 95%CI=0.3, 3.8, P=0.02), MEP (β=1.8; 95%CI=0.4, 3.2, P=0.01) and MiBP (β=1.3; 95%CI=−0.2, 2.7, P=0.10) concentrations were associated with somatization subscale scores. Gestational urinary phthalate metabolites were generally not associated with clinical subscales (Supplemental Figure S8).
In sensitivity analyses, when we mutually adjusted for gestational and childhood urinary phthalate metabolite concentrations, the results were not substantially different from the main analysis (Supplemental Figure S9). When we additionally adjusted for gestational urinary BPA and triclosan concentrations in our sex-specific prenatal models, and childhood triclosan concentrations in our sex-specific postnatal models, the results were not substantially different from the main analysis, except that the association between gestational phthalate metabolites and behavior in girls were slightly attenuated after adjusting for gestational BPA (results not shown). The results were not substantially altered after additionally adjusting for gestational or childhood blood lead concentrations, household income, pregnancy-induced hypertension, parity, caregiving environment, or maternal ADHD behavior (results not shown).
4. Discussion
Using data from a pregnancy and birth cohort, we examined the associations of repeated measures of urinary phthalate metabolite concentrations from 16 weeks of gestation to 8 years of age with longitudinal measures of caregiver-reported childhood problem behaviors from ages 2-8 years, accounting for urinary phthalate metabolite concentration measurement error. We did not observe strong evidence for periods of heightened susceptibility. Gestational urinary MCPP concentrations were positively associated with the three composite behavior scores examined. Higher childhood MEP concentrations were associated with higher internalizing and externalizing problem composite scores, while higher childhood urinary MBzP, MCNP, MCOP, MCPP, and MEP concentrations were associated with higher BSI composite scores. Finally, the mixture of childhood phthalates was positively associated with all three composites scores.
It is unclear how early-life phthalate exposure influence neurobehavior, but several potential mechanisms have been proposed (Akaike et al., 1991; Andrade et al., 2006; Boas et al., 2012; Breous et al., 2005; Howdeshell et al., 2008; Huang et al., 2007; Meeker et al., 2007; Miodovnik et al., 2014; Moog et al., 2017; Stein et al., 1991; Xu et al., 2007). Thyroid hormones play a critical role in early life brain development (Chan and Kilby, 2000; Hendrich et al., 1984; Porterfield and Hendrich, 1993). Exposure to several phthalates (e.g., DBP, DEHP, and butyl benzyl phthalate [BBzP, the parent chemical of MBzP, and to a lesser extent, MnBP]) have been shown to modulate thyroid function and reduce circulating thyroid hormone levels, which may adversely affect neurobehavior (Akaike et al., 1991; Breous et al., 2005; Meeker et al., 2007; Miodovnik et al., 2014; Moog et al., 2017; Poon et al., 1997; Stein et al., 1991; Sugiyama et al., 2005). Our group has previously found that gestational phthalate exposure was associated with cord blood thyroid hormone levels among HOME Study children (Romano et al., 2018). In addition, phthalates (e.g., DEHP) may alter lipid metabolism and lipid profiles of the brain (Xu et al., 2007; Xu et al., 2008). Lipids and their fatty acid substituents (e.g., docosahexaenoic acid and arachidonic acid) are critical for development of the central nervous system and fetal brain (Crawford et al., 1976; Neuringer et al., 1984). Altered lipid profiles could adversely influence neurodevelopment (Helland et al., 2003). Finally, certain phthalates (e.g., BBzP, DEHP, DnBP, and DiBP) are anti-androgenic and prior studies report concentration addition effects (Fisher et al., 2003; Foster et al., 2001; Hannas et al., 2011; Kavlock et al., 2002). Thus, exposure to these phthalates, as well as their mixture, in early life may inhibit testosterone production, which is critical for brain development, particularly sex-specific behaviors (Cohen-Bendahan et al., 2005; Dahl et al., 2018; Fisher et al., 2003; Foster et al., 2001; Hannas et al., 2011; Kavlock et al., 2002).
Previous rodent studies suggest that pre- or peri-natal phthalate exposure may impact certain offspring behaviors (Barakat et al., 2018; Hoshi and Ohtsuka, 2009; Kougias et al., 2018). Using a rat model of human prenatal phthalate exposure, a prior study found that perinatal exposure (from gestation through postnatal day 10) to a phthalate mixture was associated with decreased social play behavior in the offspring (Kougias et al., 2018). Another study found that low-dose DBP exposure during gestation decreased grooming behavior in the rat offspring when they were placed in a test cage, indicating low-dose DBP adversely influences emotional stability in a novel environment (Hoshi and Ohtsuka, 2009). Another rodent study found that gestational DEHP exposure increased anxiety behavior in offspring (Barakat et al., 2018).
We found that gestational MCPP was positively associated with internalizing and externalizing problem, and BSI composite scores in the present study, possibly driven by anxiety, aggression, and withdrawal subscale scores, respectively. Our findings are consistent with findings from the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study (Hyland et al., 2019). The CHAMACOS study found that gestational MCPP was positively associated with parent-reported BASC-2 scores assessed at ages 7-16 years, including internalizing and externalizing problem composite scores. They also found that gestational MCPP was associated with increased internalizing problem composite scores and anxiety subscale scores assessed via the self-report version of the BASC-2, which is consistent with our findings. Because MCPP is a metabolite of several phthalates and its concentrations are usually low compared to other phthalate metabolites, MCPP was not often examined in previous studies, which limited our ability to compare our findings with theirs. Our findings are, however, consistent with experimental animal studies. MCPP is a non-specific metabolite of several phthalates, including DBP, which was shown to be associated with decreased grooming behavior in a rodent study (Hoshi and Ohtsuka, 2009). DBP was also shown to disturb thyroid homeostasis and reduce gonadal hormones, which may subsequently influence neurobehavior (Breous et al., 2005; Fisher et al., 2003; Foster et al., 2001; Hannas et al., 2011; Kavlock et al., 2002; Meeker et al., 2007; Miodovnik et al., 2014; Poon et al., 1997; Sugiyama et al., 2005).
Previous studies showed mixed findings on gestational phthalate exposures and child behavior in terms of particular phthalate metabolites and distinct behavior profiles. For example, gestational MBzP was not associated with behavioral problems assessed by the Strength and Difficulties Questionnaires at age 7 years in a Spanish study, but it was associated with withdrawn and internalizing behaviors assessed by the Child Behavioral Checklist at age 3 years in an U.S. study (Gascon et al., 2015; Whyatt et al., 2012). A Polish study found that gestational MEP concentrations were associated with increased peer relationship problems at age 7 years, whereas the Spanish study reported that gestational MEP was associated with reduced inattention symptoms at 4 years (Gascon et al., 2015; Jankowska et al., 2019). In the present study, besides MCPP, gestational MBzP and MiBP were associated with externalizing problem composite scores. The discrepancies in the findings might be partly explained by the differences in the sociodemographic compositions of the study population, timing of phthalate measurements, age at behavioral assessment, and the instruments used. Most importantly, most previous studies only used one single spot urine to assess urinary phthalate metabolite concentrations, which is unlikely to represent the exposure pattern during entire gestational period given the short biological half-life of phthalates in the body. Therefore, previous findings are subject to exposure misclassification and biased results. Future studies should consider repeated measurements and correcting for measurement error when assessing phthalate metabolite concentrations.
Only three prospective cohorts have investigated the impact of childhood phthalate exposure on child behavior, and few studies have examined mixture effects of phthalate exposure (Huang et al., 2019; Jankowska et al., 2019; Ku et al., 2019). A Taiwanese study reported that childhood urinary MBzP concentrations at ages 2-8 years were associated with higher social problem scores, assessed by the Child Behavior Checklist, in 153 children ages 8-14 years (Huang et al., 2019). Another study based on the same cohort reported that urinary MBzP concentrations at age 2 years were associated with ADHD symptom traits, assessed by child activity level, distractibility, and low persistence (Ku et al., 2019). In our study, we found that childhood MBzP was associated with higher BSI composite scores, and increased depression, somatization, conduct problem, and atypicality subscale scores. The positive associations between MBzP and child behavior is consistent with experimental animal studies examining BBzP, the parent compound of MBzP, which has been shown to adversely affect neurobehavior by disturbing thyroid homeostasis and reducing gonadal hormones (Breous et al., 2005; Fisher et al., 2003; Foster et al., 2001; Hannas et al., 2011; Kavlock et al., 2002; Meeker et al., 2007; Miodovnik et al., 2014; Poon et al., 1997; Sugiyama et al., 2005). We also found that several childhood phthalate metabolites were associated with BSI composite scales, but null associations were observed in this Taiwanese study (Huang et al., 2019). The Polish Mother and Child Cohort reported that childhood urinary phthalate metabolite concentrations at age 2 years were not associated with child behavior at age 7 years, assessed using the Strengths and Difficulties Questionnaire (Jankowska et al., 2019). The inconsistencies in the findings might be explained by differences in sociodemographic compositions of the study population, age at behavior assessment, the instruments used, and whether exposure misclassification and mixture effects were accounted for.
Few studies have examined the impact of early life phthalate mixtures on child behavior. In our WQS analysis, childhood MEP, MCNP, and MBzP contributed largely to the positive associations of phthalate mixture with the three composite scores, which is consistent with our findings from single phthalate metabolite analysis where these same three metabolites were positively associated with the three composite scores. Given that WQS cannot currently accommodate repeated measurements, only the BASC-2 scores at age 8 years was used in the WQS analysis, whereas repeated measurements of BASC-2 scores at ages 2, 3, 4, 5, and 8 years were used in the single phthalate metabolite analysis, and thus may contribute to some slight discrepancies in the findings, even though the BASC-2 measurements were relatively stable during childhood. For gestational exposure, the associations between phthalate metabolites and behavior were not modified by child sex in the present study, which is consistent with most previous studies (Gascon et al., 2015; Huang et al., 2019; Kobrosly et al., 2014; Lien et al., 2015; Philippat et al., 2017). Only two previous studies reported modification by sex; however, the associations were not consistently stronger in boys (Engel et al., 2010; Whyatt et al., 2012). For childhood exposure, most phthalate-behavior associations were not modified by child sex in our study, although there were some exceptions. No effect measure modification by child sex was observed in the Taiwanese study that examined the impact of childhood phthalates on behavior (Huang et al., 2019). Admittedly, the moderate sample size in our study and most previous studies have limited the ability to detect effect measure modification by sex. Phthalates influence gonadal hormones that are critical for sex-specific neurodevelopment, and thus it is possible that the impact of phthalates on child behavior differ by sex (Cohen-Bendahan et al., 2005; Dahl et al., 2018; Doherty et al., 2017; Hannas et al., 2011). Future studies are needed to explore the sex modification and the underlying mechanisms.
There are some strengths and limitations to our study. First and foremost, we quantified urinary phthalate metabolite concentrations up to twice during gestation (16 and 26 weeks) and six times in childhood (annually at ages 1-5 years and age 8 years). Phthalates are rapidly metabolized, and therefore it is challenging to accurately assess phthalate exposure using a single urine sample. Moreover, there are changes in physiology, diet, activity, and personal care product use over the first few years of a child’s life, which may lead to changes in phthalate exposure (Adibi et al., 2008; Braun et al., 2012; Watkins et al., 2014). Thus, compared with most previous studies that only assessed phthalate metabolites in a single urine sample, our repeated collections allowed us to better capture the phthalate exposure during gestation, infancy, and childhood.
Relatedly, our study used regression calibration to account for phthalate metabolite measurement error. Urinary phthalate metabolite concentrations vary within individuals due to the relatively short biological half-life (<24 hours) and episodic nature of phthalate exposures (Koch and Angerer 2007; Koch et al., 2013; Watkins et al., 2014). None of the previous studies examining the impact of phthalate exposure on child behavior have accounted for measurement error, and are thus subject to exposure misclassification and potentially biased results. Using regression calibration, we accounted for measurement error by considering the means of both group- and individual-level phthalate metabolite concentrations, variation in the number of missing phthalate metabolite measurements, and age-associated changes in childhood phthalate metabolite concentrations. Further, given that phthalates are quickly metabolized in the body and phthalate exposure may change with age, we calculated childhood phthalate exposure intensity to better reflect the “average” exposure during childhood. Compared with traditional methods that take an arithmetic average of observed values from non-missing measurements at different ages, our methods could provide a more accurate estimate by accounting for variations in phthalate metabolite concentrations associated with age. Admittedly, we still cannot completely rule out the possibility of exposure misclassification. Future studies should consider collecting several urine samples within a short period of time (e.g., a week) to obtain a more accurate estimation of urinary phthalate metabolite concentrations.
Third, we investigated phthalate mixture exposure and child behavior using iterative WQS regression, which allowed us to fully quantify the uncertainty surrounding the aggregate impact of different phthalates on child behavior. Admittedly, WQS model does not consider actual exposure level differences between each chemical. Also, similar to most mixture approaches, WQS is statistical in nature and does not incorporate biological knowledge. Fourth, we used a valid and reliable tool, the BASC-2, to repeatedly assess child behavior during early and middle childhood. Because BASC-2 measurements were relatively stable during childhood, we used all BASC-2 measurements as the repeated outcome to increase our sample size and effect estimate precision. However, given that the behaviors problems may change with age, there is a possibility that some age-specific could be present. Fifth, our study has a modest sample size, and thus the multiple informant model has relatively low power to detect metabolite-time period interactions. Sixth, we collected a rich set of covariates using valid and reliable measures, which allowed us to adjust for a variety of potential confounders, including gestational and childhood urinary BPA and triclosan concentrations and blood lead concentrations, maternal depressive symptoms, and maternal ADHD behaviors. Still, there is always a possibility of residual confounding, such as other potential neurotoxicants or maternal/child behavior and lifestyle factors associated with phthalate exposure and neurobehavior. In addition, the timing of the urine collection varies from person to person. This may be of concern given the short biological half-lives of phthalates within human body. However, given that the timing of urine collection is independent of the outcome, it is unlikely that this could have biased the findings. Moreover, we used creatine-standardized phthalate metabolite concentrations as exposure instead of adjusting for creatinine in the multivariable models, which may potentially introduce bias (Barr et al., 2005). However, previous simulation studies indicate that the bias from this is relatively low and the 95% CI coverage is acceptable in most scenarios (O’Brien et al., 2016). Further, all pregnant woman subjects of the HOME Study were living in a home built before 1978, which may reduce the generalizability of our findings. Finally, we did not adjust for multiple comparisons since it is controversial and may undeservedly reduce statistical power (Rothman 1990). However, the consistent patterns of several phthalate metabolites associated with BSI composite scores, as well as gestational MCPP being associated with the three problem behavior composite scores suggests that these results may not be solely due to chance. The potential concern of false positive results necessitates replication of our findings.
In conclusion, gestational and childhood urinary concentrations of several phthalate metabolites (i.e., MBzP, MCNP, MCOP, MCPP, and MEP) may be related to higher behavioral problem scores among children in this cohort. Moreover, the mixture of childhood phthalate concentrations may be associated with more problem behaviors, with MCNP, MEP, and MBzP being the most important components of this mixture. Future epidemiologic studies investigating the health effect of early life phthalate exposure should consider accounting for measurement error and examining the mixture effects of multiple phthalates, and laboratory studies could investigate the underlying mechanisms of these associations.
Supplementary Material
Figure 3.
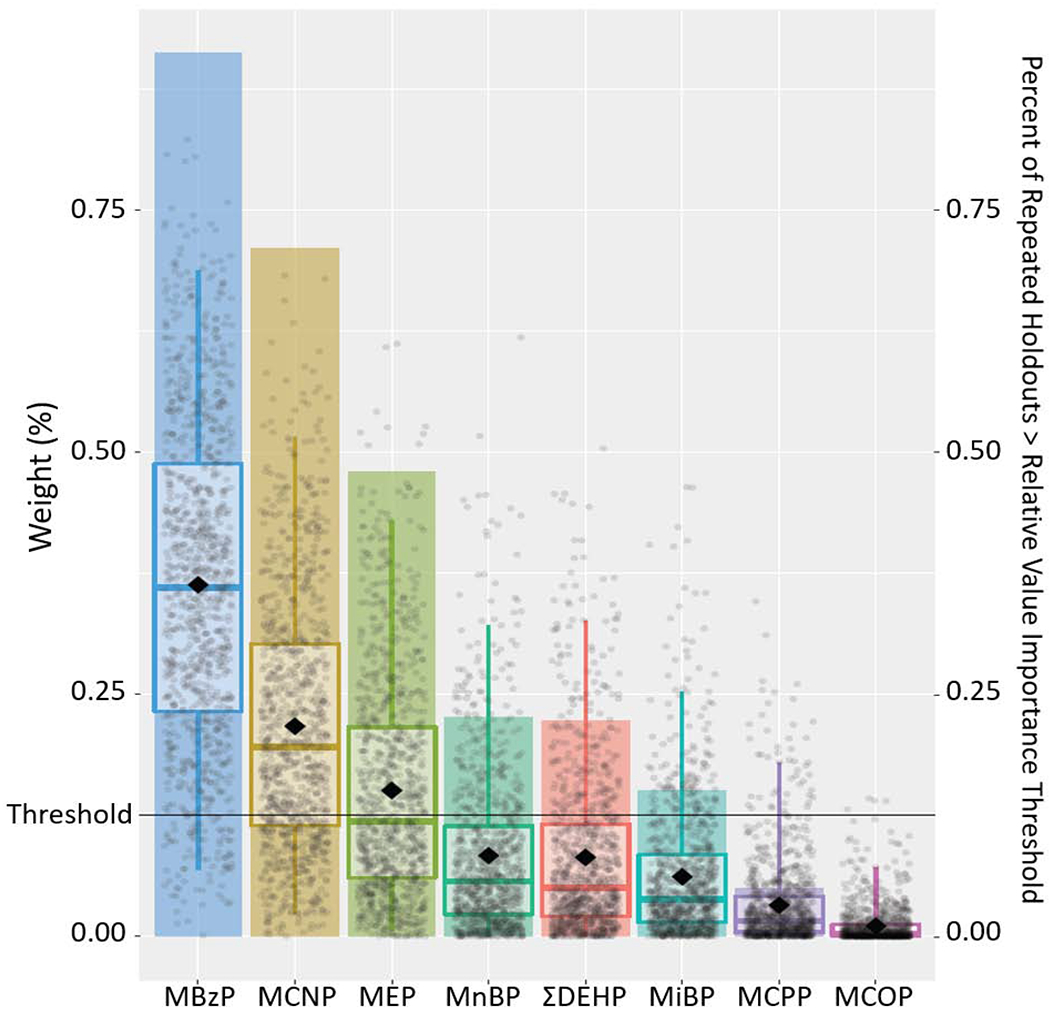
Results from Weighted Quantile Sum Regression Analysis with 1,000 Iterations on Childhood Exposure to Phthalate Mixture and Externalizing Problem Composite Scale: Relative Weights of Each Phthalate Metabolite and Percent of Repeated Holdouts Above Relative Variable Importance Threshold (N=228).
Note: Each individual dot represents weight for a given metabolite from a single repeated holdout. Box-and-whisker plots describe univariate statistics of these weights from 1,000 repeated holdouts. Bar height indicates the proportion of repeated holdouts where the weight had a value above the relative variable importance threshold (0.125, i.e., 1/8). DEHP, summary di(2-ethylhexyl) phthalate metabolite measure; MnBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate.
Adjusted for maternal age at delivery, mother’s pre-pregnancy BMI, maternal gestational smoking (mean of log10-transformed serum cotinine concentrations during 16 and 26 weeks of pregnancy), mother’s Beck’s Depression Inventory score, gestational alcohol consumption (<1/month, >1/month, binge), maternal education (high school graduate or less, tech school or some college, college graduate or above), marital status (married, unmarried), and child’s sex (male/female), race (non-Hispanic White, non-Hispanic Black, other), and age at outcome assessment.
Highlights.
Gestational and childhood phthalate exposures may be associated with behaviors
Childhood exposure to phthalate mixtures may be associated with behaviors
MCNP, MEP, and MBzP were the most important components of this mixture
Measurement error was accounted for in urinary phthalate metabolite concentrations
Acknowledgements:
We acknowledge the technical assistance of M. Silva, E. Samandar, J. Preau, and J. Tao (Centers for Disease Control and Prevention, Atlanta, Georgia) in measuring the urinary concentrations of phthalate metabolites.
Funding Source: This work was supported by National Institute of Environmental Health Sciences grants [grant numbers P01 ES011261, R01 ES014575, R01 ES024381, and R01 ES020349].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: JMB was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures and received an honoraria from Quest Diagnostic for serving on an expert panel related to endocrine disrupting chemicals. JMB’s institution was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water; these funds were not paid to JMB directly. The other authors declare they have no actual or potential competing financial interests.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, and Hauser R. 2008. “Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples.” Environ Health Perspect 116 (4):467–73. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike M, Kato N, Ohno H, and Kobayashi T. 1991. “Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism.” Neurotoxicol Teratol 13 (3):317–22. [DOI] [PubMed] [Google Scholar]
- Amrhein V, Greenland S, and McShane B. 2019. “Scientists rise up against statistical significance.” Nature 567 (7748):305–307. doi: 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- Andrade AJ, Grande SW, Talsness CE, Gericke C, Grote K, Golombiewski A, Sterner-Kock A, and Chahoud I. 2006. “A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats.” Toxicology 228 (1):85–97. doi: 10.1016/j.tox.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Barakat R, Lin PC, Park CJ, Best-Popescu C, Bakry HH, Abosalem ME, Abdelaleem NM, Flaws JA, and Ko C. 2018. “Prenatal Exposure to DEHP Induces Neuronal Degeneration and Neurobehavioral Abnormalities in Adult Male Mice.” Toxicol Sci 164 (2):439–452. doi: 10.1093/toxsci/kfy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, and Pirkle JL. 2005. “Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements.” Environ Health Perspect 113 (2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Guth D, Steer RA, and Ball R. 1997. “Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care.” Behav Res Ther 35 (8):785–91. [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, and Main KM. 2012. “Thyroid effects of endocrine disrupting chemicals.” Mol Cell Endocrinol 355 (2):240–8. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, and Sundell J. 2005. “Phthalates in indoor dust and their association with building characteristics.” Environ Health Perspect 113 (10):1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, and Hauser R. 2014. “Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic.” J Expo Sci Environ Epidemiol 24 (5):459–66. doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, and Lanphear BP. 2017. “Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study.” Int J Epidemiol 46 (1):24. doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Sathyanarayana S, and Hauser R. 2013. “Phthalate exposure and children’s health.” Curr Opin Pediatr 25 (2):247–54. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, and Hauser R. 2012. “Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy.” Environ Health Perspect 120 (5):739–45. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Wenzel A, and Loos U. 2005. “The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers.” Mol Cell Endocrinol 244 (1–2):75–8. doi: 10.1016/j.mce.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Buckley JP, Herring AH, Wolff MS, Calafat AM, and Engel SM. 2016. “Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children’s Environmental Health Study.” Environ Int 91:350–6. doi: 10.1016/j.envint.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM 2016. “Contemporary Issues in Exposure Assessment Using Biomonitoring.” Curr Epidemiol Rep 3 (2):145–153. doi: 10.1007/s40471-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell Bettye, and Bradley Robert. 2003. Home Inventory Administration Manual. Little Rock, AK: University of Arkansas at Little Rock. [Google Scholar]
- Carlstedt F, Jonsson BA, and Bornehag CG. 2013. “PVC flooring is related to human uptake of phthalates in infants.” Indoor Air 23 (1):32–9. doi: 10.1111/j.1600-0668.2012.00788.x. [DOI] [PubMed] [Google Scholar]
- Carroll R, Ruppert D, Stefanski L, and Crainiceanu C. 2006. Measurement Error in Nonlinear Models, A Modern Perspective, Second Edition Chapman and Hall/CRC. [Google Scholar]
- Chan S, and Kilby MD. 2000. “Thyroid hormone and central nervous system development.” J Endocrinol 165 (1):1–8. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, and Berenbaum SA. 2005. “Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings.” Neurosci Biobehav Rev 29 (2):353–84. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, and Sparrow E. 1999. Self-ratings of ADHD symptoms in adults I: factor structure and normative data. Journal of Attention Disorders. [Google Scholar]
- Crawford MA, Hassam AG, and Williams G. 1976. “Essential fatty acids and fetal brain growth.” Lancet 1 (7957):452–3. doi: 10.1016/s0140-6736(76)91476-8. [DOI] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, and Wheeler DC. 2015. “Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk.” Cancer Inform 14 (Suppl 2):159–71. doi: 10.4137/CIN.S17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, and Suleiman AB. 2018. “Importance of investing in adolescence from a developmental science perspective.” Nature 554 (7693):441–450. doi: 10.1038/nature25770. [DOI] [PubMed] [Google Scholar]
- Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, and Wolff MS. 2017. “Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children.” Environ Res 152:51–58. doi: 10.1016/j.envres.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, and Wolff MS. 2010. “Prenatal phthalate exposure is associated with childhood behavior and executive functioning.” Environ Health Perspect 118 (4):565–71. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SSM, Hoppin JA, Zeiner P, Knudsen GP, Reichborn-Kjennerud T, Herring AH, and Aase H. 2018. “Prenatal Phthalates, Maternal Thyroid Function, and Risk of Attention-Deficit Hyperactivity Disorder in the Norwegian Mother and Child Cohort.” Environ Health Perspect 126 (5):057004. doi: 10.1289/EHP2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, and Sharpe RM. 2003. “Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate.” Hum Reprod 18 (7):1383–94. [DOI] [PubMed] [Google Scholar]
- Foster PM, Mylchreest E, Gaido KW, and Sar M. 2001. “Effects of phthalate esters on the developing reproductive tract of male rats.” Hum Reprod Update 7 (3):231–5. [DOI] [PubMed] [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martinez D, Jdlvez J, Monfort N, Ventura R, Sunyer J, and Vrijheid M. 2015. “Prenatal exposure to phthalates and neuropsychological development during childhood.” Int J Hyg Environ Health 218 (6):550–8. doi: 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, and Gray LE. 2011. “Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate.” Toxicol Sci 123 (1):206–16. doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- Hauser R, and Calafat AM. 2005. “Phthalates and human health.” Occup Environ Med 62 (11):806–18. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Duty S, Godfrey-Bailey L, and Calafat AM. 2004. “Medications as a source of human exposure to phthalates.” Environ Health Perspect 112 (6):751–3. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, and Drevon CA. 2003. “Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age.” Pediatrics 111 (1):e39–44. [DOI] [PubMed] [Google Scholar]
- Hendrich CE, Jackson WJ, and Porterfield SP. 1984. “Behavioral testing of progenies of Tx (hypothyroid) and growth hormone-treated Tx rats: an animal model for mental retardation.” Neuroendocrinology 38 (6):429–37. doi: 10.1159/000123931. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, and Angerer J. 2007. “Phthalates: toxicology and exposure.” Int J Hyg Environ Health 210 (5):623–34. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hintze Jerry, and Nelson Ray. 2012. “Violin Plots: A Box Plot-Density Trace Synergism.” The American Statistician 52 (2): 181–184. [Google Scholar]
- Hornung Richard, and Reed Laurence. 1990. “Estimation of Average Concentration in the Presence of Nondetectable Values.” Applied Occupational and Environmental Hygiene 5 (1):46–51. [Google Scholar]
- Hoshi H, and Ohtsuka T. 2009. “Adult rats exposed to low-doses of di-n-butyl phthalate during gestation exhibit decreased grooming behavior.” Bull Environ Contam Toxicol 83 (1):62–6. doi: 10.1007/s00128-009-9729-1. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, and Gray LE. 2008. “A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner.” Toxicol Sci 105 (1): 153–65. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Huang HB, Kuo PH, Su PH, Sun CW, Chen WJ, and Wang SL. 2019. “Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study.” Environ Res 172:569–577. doi: 10.1016/j.envres.2019.02.029. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, and Lee CC. 2007. “Associations between urinary phthalate monoesters and thyroid hormones in pregnant women.” Hum Reprod 22 (10):2715–22. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Huen K, Harley K, Bradman A, Eskenazi B, and Holland N. 2010. “Longitudinal changes in PON1 enzymatic activities in Mexican-American mothers and children with different genotypes and haplotypes.” Toxicol Appl Pharmacol 244 (2):181–9. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland C, Mora AM, Kogut K, Calafat AM, Harley K, Deardorff J, Holland N, Eskenazi B, and Sagiv SK. 2019. “Prenatal Exposure to Phthalates and Neurodevelopment in the CHAMACOS Cohort.” Environ Health Perspect 127 (10): 107010. doi: 10.1289/EHP5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, and Braun JM. 2018. “Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children.” Environ Health Perspect 126 (5):057001. doi: 10.1289/EHP2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Yolton K, Lanphear BP, and Braun JM. 2019. “Early-life triclosan exposure and parent-reported behavior problems in 8-year-old children.” Environ Int 128:446–456. doi: 10.1016/j.envint.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska A, Polahska K, Hanke W, Wesolowska E, Ligocka D, Waszkowska M, Stahczak A, Tartaglione AM, Mirabella F, Chiarotti F, Gari M, and Calamandrei G. 2019. “Prenatal and early postnatal phthalate exposure and child neurodevelopment at age of 7 years - Polish Mother and Child Cohort.” Environ Res 177:108626. doi: 10.1016/j.envres.2019.108626. [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, and Meeker JD. 2015. “Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis.” Reprod Biol Endocrinol 13:4. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, and Zacharewski T. 2002. “NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate.” Reprod Toxicol 16 (5):489–527. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, and Swan SH. 2014. “Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age.” Environ Health Perspect 122 (5):521–8. doi: 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, and Angerer J. 2007. “Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP.” Int J Hyg Environ Health 210 (1):9–19. doi: 10.1016/j.ijheh.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Koch HM, Drexler H, and Angerer J. 2004. “Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP).” Int J Hyg Environ Health 207 (1):15–22. doi: 10.1078/1438-4639-00270. [DOI] [PubMed] [Google Scholar]
- Koch HM, Lorber M, Christensen KL, Palmke C, Koslitz S, and Bruning T. 2013. “Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns.” Int J Hyg Environ Health 216 (6):672–81. doi: 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Drexler H, and Angerer J. 2005. “Exposure of nursery school children and their parents and teachers to di-n-butylphthalate and butylbenzylphthalate.” Int Arch Occup Environ Health 78 (3):223–9. doi: 10.1007/s00420-004-0570-x. [DOI] [PubMed] [Google Scholar]
- Kougias DG, Cortes LR, Moody L, Rhoads S, Pan YX, and Juraska JM. 2018. “Effects of Perinatal Exposure to Phthalates and a High-Fat Diet on Maternal Behavior and Pup Development and Social Play.” Endocrinology 159 (2):1088–1105. doi: 10.1210/en.2017-03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku HY, Tsai TL, Wang PL, Su PH, Sun CW, Wang CJ, and Wang SL. 2019. “Prenatal and childhood phthalate exposure and attention deficit hyperactivity disorder traits in child temperament: A 12-year follow-up birth cohort study.” Sci Total Environ 699:134053. doi: 10.1016/j.scitotenv.2019.134053. [DOI] [PubMed] [Google Scholar]
- Larsen K 1972. “Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser.” Clin Chim Acta 38 (2):475–6. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, and Tellez-Rojo MM. 2013. “Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children.” Chemosphere 93 (10):2390–8. doi: 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, Chen WJ, and Wang SL. 2015. “Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study.” Environ Health Perspect 123 (1):95–100. doi: 10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, and Skaare JU. 2009. “Reproductive and developmental toxicity of phthalates.” J Toxicol Environ Health B Crit Rev 12 (4):225–49. doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, and Hauser R. 2007. “Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men.” Environ Health Perspect 115 (7):1029–34. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, and Swan SH. 2009. “Phthalates and other additives in plastics: human exposure and associated health outcomes.” Philos Trans R Soc Lond B Biol Sci 364 (1526):2097–113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Bellinger D, Minguez-Alarcon L, Romano ME, Ford JB, Williams PL, Calafat AM, Hauser R, and Braun JM. 2017. “Paternal and maternal preconception urinary phthalate metabolite concentrations and child behavior.” Environ Res 158:720–728. doi: 10.1016/j.envres.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatoya M, Itoh S, Yamazaki K, Araki A, Miyashita C, Tamura N, Yamamoto J, Onoda Y, Ogasawara K, Matsumura T, and Kishi R. 2018. “Prenatal exposure to bisphenol A and phthalates and behavioral problems in children at preschool age: the Hokkaido Study on Environment and Children’s Health.” Environ Health Prev Med 23 (1):43. doi: 10.1186/s12199-018-0732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, and Hauser R. 2014. “Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence.” Neurotoxicology 41:112–22. doi: 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, and Buss C. 2017. “Influence of maternal thyroid hormones during gestation on fetal brain development.” Neuroscience 342:68–100. doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose T, Knudsen LE, Hedegaard M, and Mortensen GK. 2007. “Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system.” Int J Toxicol 26 (3):221–9. doi: 10.1080/10915810701352721. [DOI] [PubMed] [Google Scholar]
- Neuringer M, Connor WE, Van Petten C, and Barstad L. 1984. “Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys.” J Clin Invest 73 (1):272–6. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, and Weinberg CR. 2016. “Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment.” Environ Health Perspect 124 (2):220–7. doi: 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R, and EDEN Mother-Child Study Group. 2017. “Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years.” Environ Health Perspect 125 (9):097014. doi: 10.1289/EHP1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, and Bates DM. 2000. “Mixed-Effects Models in S and S-PLUS.” Spinger; normal”> https://link.springer.com/content/pdf/10.1007/0-387-22747-4_1.pdf. [Google Scholar]
- Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, and Chu I. 1997. “Subchronic oral toxicity of di-n-octyl phthalate and di(2-Ethylhexyl) phthalate in the rat.” Food Chem Toxicol 35 (2):225–39. [DOI] [PubMed] [Google Scholar]
- Porterfield SP, and Hendrich CE. 1993. “The role of thyroid hormones in prenatal and neonatal neurological development-current perspectives.” Endocr Rev 14 (1):94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Nachman RM, and Cooper GS. 2020. “Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence.” Environ Int 137:105408. doi: 10.1016/j.envint.2019.105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, and Kamphaus RW. 2004. Behavior Assessment System for Children Manual. 2nd Bloomington, MN: Pearson. [Google Scholar]
- Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, Yolton K, Chen A, Lanphear BP, and Braun JM. 2018. “Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study.” Int J Hyg Environ Health 221 (4):623–631. doi: 10.1016/j.ijheh.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ 1990. “No adjustments are needed for multiple comparisons.” Epidemiology 1 (1):43–6. [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, and Brody JG. 2011. “Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention.” Environ Health Perspect 119 (7):914–20. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, and Tellez-Rojo MM. 2011. “Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants.” Environ Health Perspect 119 (3):409–15. doi: 10.1289/ehp.100245310.1289/ehp.1102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, Haaland W, Swan SH, and TIDES Team. 2017. “Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and Birth Outcomes.” J Clin Endocrinol Metab 102 (6):1870–1878. doi: 10.1210/jc.2016-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, and Mendola P. 2000. “Identifying critical windows of exposure for children’s health.” Environ Health Perspect 108 Suppl 3:451–5. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, and Calafat AM. 2004. “Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000.” Environ Health Perspect 112 (3):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AR, Lawrence WH, and Autian J. 1975. “Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats.” J Pharm Sci 64 (8):1347–50. [DOI] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, and Braun JM. 2017. “Early life bisphenol A exposure and neurobehavior at 8years of age: Identifying windows of heightened vulnerability.” Environ Int 107:258–265. doi: 10.1016/j.envint.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SA, Adams PM, Shanklin DR, Mihailoff GA, and Palnitkar MB. 1991. “Thyroid hormone control of brain and motor development: molecular, neuroanatomical, and behavioral studies.” Adv Exp Med Biol 299:47–105. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Shimada N, Miyoshi H, and Yamauchi K. 2005. “Detection of thyroid system-disrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis.” Toxicol Sci 88 (2):367–74. doi: 10.1093/toxsci/kfi330. [DOI] [PubMed] [Google Scholar]
- Sørensen LK 2006. “Determination of phthalates in milk and milk products by liquid chromatography/tandem mass spectrometry.” Rapid Commun Mass Spectrom 20 (7):1135–43. doi: 10.1002/rcm.2425. [DOI] [PubMed] [Google Scholar]
- Tanner EM, Bornehag CG, and Gennings C. 2019. “Repeated holdout validation for weighted quantile sum regression.” MethodsX 6:2855–2860. doi: 10.1016/j.mex.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan MJ, Polley EC, and Hubbard AE. 2007. “Super learner.” Stat Appl Genet Mol Biol 6: Article25. doi: 10.2202/1544-6115.1309. [DOI] [PubMed] [Google Scholar]
- Wassersteina RL, and Lazar NA 2016. “The ASA’s Statement on p-Values: Context, Process, and Purpose” The American Statistician 70 (2):129–133. [Google Scholar]
- Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, and Braun JM. 2014. “Variability and predictors of urinary concentrations of phthalate metabolites during early childhood.” Environ Sci Technol 48 (15):8881–90. doi: 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, Diaz D, Quinn J, Adibi J, Perera FP, and Factor-Litvak P. 2012. “Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age.” Environ Health Perspect 120 (2):290–5. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, and Angerer J. 2008. “Phthalates: metabolism and exposure.” Int J Androl 31 (2):131–8. doi: 10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, and Knipp GT. 2007. “Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure.” Arch Toxicol 81 (1):57–62. doi: 10.1007/s00204-006-0143-8. [DOI] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, and Knipp GT. 2008. “Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta.” Placenta 29 (11):962–9. doi: 10.1016/j.placenta.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


