Abstract
Loss of sensory function is a common consequence of neurological injury. Recent clinical and preclinical evidence indicates vagus nerve stimulation (VNS) paired with tactile rehabilitation, consisting of delivery of a variety of mechanical stimuli to the hyposensitive skin surface, yields substantial and long-lasting recovery of somatosensory function after median and ulnar nerve transection and repair. Here, we tested the hypothesis that a specific component of the tactile rehabilitation paired with VNS is necessary for recovery of somatosensory function. In a second experiment in a separate cohort, we investigated whether VNS paired with tactile rehabilitation could improve skilled forelimb motor function. Elements of the study design, including planned sample size, assessments, and statistical comparisons, were preregistered prior to beginning data collection (https://osf.io/3tm8u/). Animals received a peripheral nerve injury (PNI) causing chronic sensory loss. Eight weeks after injury, animals were given a VNS implant followed by six weeks of tactile rehabilitation sessions consisting of repeated application of one of two distinct mechanical stimuli, a filament or a paintbrush, to the previously denervated forepaw. VNS paired with either filament indentation or brushing of the paw significantly improved recovery of forelimb withdrawal thresholds after PNI compared to tactile rehabilitation without VNS. The effect size was twice as large when VNS was paired with brushing compared to VNS paired with point indentation. An independent replication in a second cohort confirmed that VNS paired with brush restored forelimb withdrawal thresholds to normal. These rats displayed significant improvements in performance on a skilled forelimb task compared to rats that did not receive VNS. These findings support the utility of pairing VNS with tactile rehabilitation to improve recovery of somatosensory and motor function after neurological injury. Additionally, this study demonstrates that the sensory characteristics of the rehabilitation paired with VNS determine the degree of recovery.
Keywords: vagal nerve stimulation, peripheral nerve injury, peripheral neuropathy, hypoesthesia, somatosensory function
1. Introduction
Loss of somatosensation accompanies many forms of neurological injury, including stroke and nerve damage [1–4]. Deficits in somatosensation, especially in the upper limb, strongly contribute to disability in patients with neurological damage [5–7]. At present, no interventional methods produce consistently effective restoration of somatosensory function. However, rehabilitation paradigms that emphasize sensory retraining may provide modest benefits [8–12].
Vagus nerve stimulation (VNS) has emerged as a novel strategy to enhance the efficacy of various rehabilitative paradigms [13]. VNS drives rapid, phasic activation of multiple neuromodulatory systems [14,15]. Co-activation of these neuromodulatory networks during training enhances plasticity in the motor and sensory circuits engaged by the rehabilitative paradigm [16–20]. A number of preclinical and clinical studies provide compelling evidence that VNS paired with motor rehabilitation yields significantly improved recovery of motor function after a range of neurological injuries, including chronic stroke [16,17,21–31]. Moreover, a recent proof-of-concept study in animals and a corresponding clinical case study demonstrate the utility of pairing VNS with sensory retraining [18,32]. Delivery of short bursts of VNS during tactile rehabilitation results in substantial, lasting improvements in somatosensory function.
Here, we sought to build on these promising initial findings and optimize the sensory retraining paradigm paired with VNS in a rat model of chronic sensory loss after neurological injury [33]. A number of studies demonstrate that the features of auditory or motor training paired with VNS dictate the specificity of VNS-dependent effects [34–38]. However, whether the nature of tactile input paired with stimulation would influence the somatosensory effects of VNS has not been evaluated. In a first experiment, we evaluated two mechanical stimuli with fundamentally different tactile features, a brush and a von Frey filament, paired with VNS on the degree of recovery of somatosensory function after nerve injury. In a second experiment, we sought to independently confirm these findings and determine if improved forelimb somatosensory function generalizes to gains in skilled motor function. These studies aim to identify whether the specifics of the stimuli presented during tactile therapy influence VNS-dependent enhancement of recovery in order to better inform the clinical implementation of VNS paired with sensory retraining therapy.
2. Methods
2.1. Experimental Design
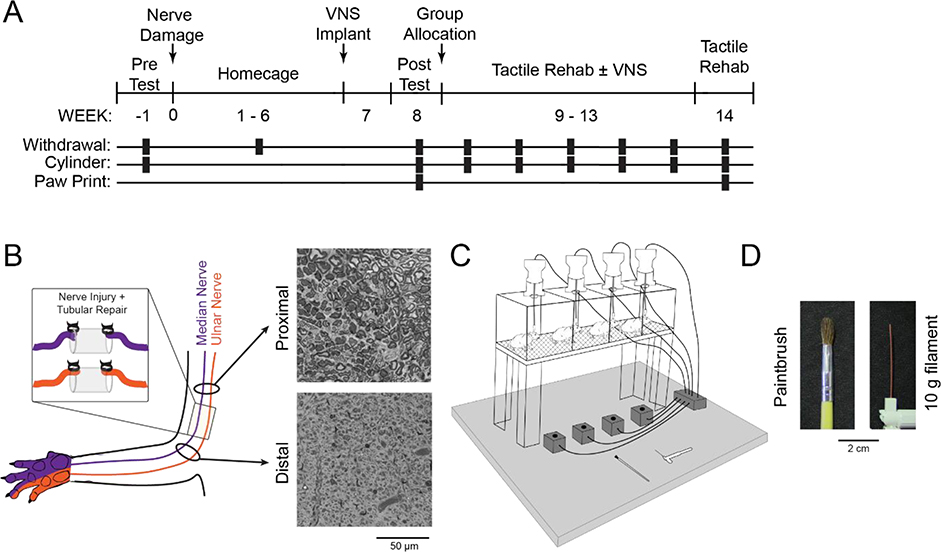
Elements of the design, including procedures, group sizes, outcome measures, statistical comparisons, and exclusion criteria were preregistered on Open Science Framework before data collection began (https://osf.io/3tm8u/). In a first experiment, we tested whether presentation of different mechanical stimuli during tactile rehabilitation would influence the degree of VNS-dependent recovery of somatosensory function after forelimb nerve injury. Before injury, all rats underwent baseline assessment of mechanosensory withdrawal thresholds using an automated aesthesiometer and forelimb use asymmetry using the cylinder task. All rats then underwent transection and tubular repair of the median and ulnar nerves in the right forearm and implantation of a stimulating cuff electrode on the left cervical vagus nerve. Beginning on week 8 post-injury, rats underwent baseline assessment of sensorimotor function and were dynamically allocated into three balanced groups based on mechanosensory withdrawal thresholds of the impaired forelimb. One group received tactile rehabilitation (Rehab, n = 4), comprised of 6 weeks of daily sessions in which 200 presentations of one of two distinct tactile stimuli, a paintbrush or a 10g filament, were applied to the ventral surface of the injured paw. The other two groups received equivalent tactile rehabilitation, but a 0.5 s train of VNS was paired with the delivery of each tactile stimulus (VNS+Brush, n = 9; VNS+Filament, n = 10). Mechanosensory withdrawal thresholds were measured weekly during therapy and one week after the cessation of therapy. Additional measures of forelimb sensorimotor function, including cylinder asymmetry and footprint analysis, were collected at multiple time points throughout the study (Fig. 1A).
Fig. 1. Experimental Design and Tactile Rehabilitation Paradigm.
(A) Timeline of experimental design. (B) Schematic showing nerve transection and repair. Representative images from proximal and distal cross-sections of the median nerve approximately 14 weeks after nerve transection and tubular repair are shown on the right. Reinnervation takes place, but the procedure results in chronic deficits in nerve architecture distal to the injury site. (C) Schematic of the tactile rehabilitation apparatus. Rats were placed in individual cages with a wire mesh floor. Either a paintbrush or 10 g filament were applied to the ventral surface of the right (injured) forepaw. A button press coincident with the delivery of the tactile stimuli initiated a 500 ms train of VNS in the appropriate groups. (D) Detailed view of the paintbrush and 10 g filament utilized during tactile rehabilitation.
In a second experiment, we tested whether VNS-dependent improvements resulting from tactile rehabilitation would generalize to recovery of motor function. A separate cohort of rats was trained on a skilled forelimb motor task and underwent nerve injury and VNS paired with tactile therapy as described in Experiment 1. Forelimb motor function was measured daily using the isometric force task [39], and mechanosensory withdrawal thresholds and forelimb asymmetry were assessed weekly.
Across both experiments, sixteen rats were excluded based on criteria defined in the study preregistration: mortality during surgery (n = 7), VNS device failure (n = 7), and autophagia (n = 2). All source data indexed across animals can be found in Supplementary Tables 1–7.
2.2. Subjects
Adult female Sprague Dawley rats (n = 52) weighing approximately 300g when they entered the study were obtained from Charles River Laboratories. The rats were housed in a 12:12 reversed light cycle environment, and behavioral training was performed during the dark cycle to increase daytime activity levels. All procedures performed in the study were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee (Protocols: 14–10 and 99–06).
2.3. Forelimb Nerve Injury
To generate chronic sensory loss in the forelimb, complete transection of both the median and ulnar nerves proximal to the elbow followed by tubular repair was performed as previously described [40]. Animals were deeply anesthetized with ketamine hydrochloride (50 mg/kg, i.p.), xylazine (20 mg/k, i.p.), and acepromazine (5 mg/kg, i.p.) and were given supplemental doses as needed to maintain anesthesia levels. A small incision proximal to the elbow of the right forelimb was made, and the median and ulnar nerves were carefully isolated and exposed. Both nerves were transected 1 cm proximal to the elbow. Immediately following transection, the proximal and distal stumps of each nerve were sutured 1 mm from the ends of a 8 mm saline-filled polyurethane tube (Micro-Renathane 0.095” I.D 0.066” O.D., Braintree Scientific, Inc., Braintree, MA), resulting in a 6 mm gap between nerve stumps. The skin incision was sutured and treated with antibiotic ointment. All animals were given enrofloxacin (10 mg/kg) immediately following surgery and sustained release buprenorphine (1.2 mg/kg) for 6 days following injury. Animals were placed in Elizabethan collars for approximately 1 week following injury to limit autophagia.
2.4. Vagus Nerve Stimulation Implantation Surgery
VNS implantation procedures were performed as described in previous studies [17,22,23,25–27,29,30,39]. Seven weeks after transection of the median and ulnar nerves, rats were anesthetized with ketamine hydrochloride (50 mg/kg, i.p.), xylazine (20 mg/kg, i.p.), and acepromazine (5 mg/kg, i.p.), and were placed in a stereotactic apparatus. An incision was made down the midline of the head to expose the skull. Bone screws were inserted into the skull at points surrounding the lamboid suture and over the cerebellum. A two-channel connector was mounted to the screws using acrylic. The rat was then removed from the stereotaxic apparatus and placed in a supine position. An incision was made on the left side of the neck and the overlying musculature was blunt dissected to isolate the vagus nerve. The nerve was placed into a bipolar stimulating cuff electrode, and the electrode leads were tunneled subcutaneously and connected with the two-channel skull-mounted connector. Incised skin was then sutured closed. All rats received enrofloxacin (s.c., 10 mg/kg) following surgery. To confirm cuff electrode functionality and proper placement, VNS-dependent activation of the Hering-Breuer reflex was assessed per standard procedures [41,42]. To do so, blood oxygenation saturation during trains of VNS (0.8 mA, 30 Hz, 100 μs pulse width, up to 5 s train duration) was monitored via pulse oximetry immediately after cuff implant. The cuff electrode was replaced if rats failed to demonstrate a reliable stimulation-dependent drop in oxygen saturation. Regardless of group assignment, all rats underwent implantation of the headmount and cuff electrode to ensure blinding.
2.5. Tactile Rehabilitation and Delivery of Vagus Nerve Stimulation
Tactile rehabilitation began nine weeks post-forelimb nerve injury and continued for 6 weeks. Sessions of tactile rehabilitation were performed once daily, four days per week, with each session lasting approximately 1.25 hours. During each session, up to 8 animals were placed in individual acrylic chambers (14 × 15 cm) with a mesh floor (Fig. 1C). Each session consisted of 200 touches to the ventral surface of the right (previously injured) forepaw with one of two mechanical stimuli (Fig. 1D and Supplemental Video 1): a 10g von Frey filament (North Coast Medical, Gilroy, CA) or a small paintbrush (Kiss Products, Port Washington, NY) as appropriate for the experimental group of the animal. Each tactile stimulus was applied for approximately 1 s by a blinded experimenter. The von Frey filament was applied perpendicularly to the surface of the paw and the digits with an upward force of 10 g. The paintbrush was applied across the paw and digits in varying directions and with an approximate upward force of 50 g.
In the appropriate groups, a train of VNS was triggered by a button press to coincide with delivery of each mechanical stimulus during tactile rehabilitation sessions. VNS parameters were equivalent to previous studies [16–20]. Each 0.5 s VNS train consisted of 0.8 mA 100 μsec biphasic pulses delivered at 30 Hz. No VNS was delivered during week 14 to assess effects lasting after the cessation of stimulation. Experimenters delivering tactile therapy were blinded to group assignment. All subjects in the study, regardless of group, were implanted with the same vagus nerve stimulation (VNS) device and headmount and were connected to a stimulator cable during therapy to ensure that they were indistinguishable in appearance. As a result, there were no visible differences between subjects to bias the experimenter administering the assessments.
2.6. Behavioral Testing
2.6.1. Mechanosensory Withdrawal Threshold Testing
Mechanosensory detection thresholds were assessed in all animals according to standard procedures [43]. Testing was performed in an acrylic chamber (19.5 × 9.6 cm) on a wire mesh floor. For each session, animals were allowed to acclimate to the behavioral chamber for 30 min before testing commenced. Mechanical withdrawal thresholds of the left and right forelimbs were tested using a dynamic plantar aesthesiometer (Cat. No. 37450, Ugo Basile, Switzerland). The actuator filament (0.5 mm diameter) was applied to the plantar surface of the forepaw, and a linearly increasing force was applied (20 s ramp time, 50 g maximal force). The force at which paw withdrawal occurred was captured for analysis. The left and right forelimbs were alternately tested with a minimum of 1 min between consecutive tests. Trials resulting in paw withdrawal due to spontaneous exploratory activity were excluded from analysis. Assessments were performed before injury (Week −1), before therapy (Week 8), and weekly during therapy (Weeks 9–14) by experimenters blinded to group.
2.6.2. Cylinder Forelimb Asymmetry Testing
Spontaneous use of the forelimbs during exploratory activity was measured using the cylinder forelimb asymmetry task, similar to previous descriptions [44]. Animals were placed in a transparent cylinder (20 cm diameter) and allowed to freely explore for two minutes. Video was be recorded from directly underneath the cylinder through a clear sheet of acrylic. The total number of both left and right forepaw contacts with the wall of the cylinder were recorded. An asymmetry index, describing the relative use of the injured forelimb, was calculated as [(right/(left + right)) x 100]. Assessments were performed before injury (Week −1), before therapy (Week 8), and weekly during therapy (Weeks 9–14) by experimenters blinded to group.
2.6.3. Pawprint Analysis
Pawprint analysis was performed using the stamp and paper method as previously described [45]. The forepaws of the animals were pressed into non-toxic ink, and the animals walked down a Plexiglas corridor (24 in x 4 in) with paper lining the floor. Each animal performed 1–3 trials to ensure three footprints from each paw could be analyzed. The paper was scanned and digitized, and three footprints from both the left and right paw were analyzed by a blinded experimenter using ImageJ software. Toe spread, defined as the distance between the center of the second and fifth digits, was measured and recorded. Assessments were performed at conclusion of therapy (Week 14) by experimenters blinded to group.
2.6.4. Isometric Pull Task
The isometric force task was used to measure volitional forelimb strength as previously described [16,24,25,27,39,40,46]. The behavioral training chamber consisted of an acrylic box (10 × 12 × 4.75 in) with a slot in the front right corner through which rats could access a handle manipulandum. Rats were trained to pull the handle, which was attached to a force transducer (Motor Pull Device and Motor Controller, Vulintus LLC, Sachse, TX). A trial was initiated when 10 g of force was exerted on the pull handle. If the peak pull force exceeded an adaptively scaled threshold within two seconds of trial initiation, a reward pellet was delivered (45 mg dustless precision pellet, BioServ, Frenchtown, NJ). The threshold was scaled adaptively based on the median peak force of the 10 preceding trials, with a fixed bounded minimum of 10 grams and maximum of 120 g based on previous studies.
Behavioral training sessions lasted 30 min and were conducted twice daily, five days per week, with the daily sessions separated by at least 2 hr. Prior to injury, rats were trained until they reached proficiency, defined as 10 consecutive sessions in which greater than 75% of trials exceeded 120 g. To reach proficiency, rats typically took 2–4 weeks of behavioral training. Eight weeks after injury, rats returned for behavioral testing and were dynamically allocated to balanced groups based on their post-injury performance. Rehabilitative training consisted of tactile rehabilitation daily for 4 days followed by 1 day of testing on the isometric pull task consisting of two 30-minute sessions. This weekly rehabilitation schedule persisted for 6 weeks. No rats received VNS during isometric force task training.
2.7. Statistical Analysis
All group sizes, outcome measures, and planned statistical comparisons were included in the study pre-registration prior to beginning data collection. Mechanical withdrawal thresholds, cylinder task right forelimb use, and isometric pull force were analyzed using a two-way repeated measures ANOVA, followed by post hoc Bonferroni-corrected unpaired t-tests where appropriate. Paired t-tests were used to compare measures within subjects from pre-injury to week 8 pre-therapy time points, where applicable. Statistical tests for each comparison are noted in the text. Figures depict mean ± standard error of the mean.
3. Results
Pairing VNS with tactile rehabilitation, consisting of repeated application of a variety of mechanical stimuli with different features including a brush, a filament, a stiff copper rod, and an air puff, significantly enhances recovery of forelimb somatosensory function in a model of chronic sensory loss caused by nerve injury [18]. Here, we varied the sensory characteristics of the rehabilitation paired with VNS and evaluated the influence on the degree of recovery using four behavioral assessments.
Rats underwent transection and tubular repair of the median and ulnar nerves in the right forelimb to produce chronic sensory loss despite reinnervation [16,18,33]. Eight weeks after nerve injury, the rats were allocated to groups to undergo tactile rehabilitation, consisting of 200 daily applications of a mechanical stimulus to the ventral surface of the previously denervated forepaw with or without VNS, as appropriate for each group (Fig. 1). Groups received either VNS paired with mechanical stimulation using a 10 g von Frey filament (VNS+Filament; n = 11), VNS paired with mechanical stimulation using a brush (VNS+Brush; n = 9), or equivalent mechanical stimulation with a brush or filament without VNS (Rehab only; n = 4). Therapy was delivered for 6 weeks, and forelimb withdrawal thresholds and sensorimotor function was assessed weekly.
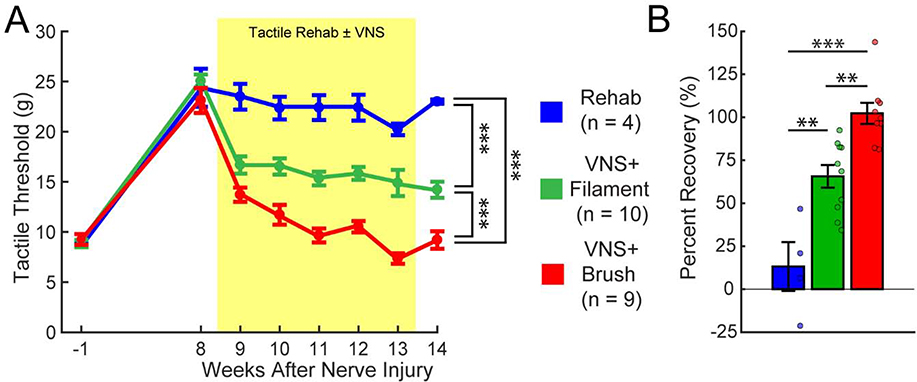
As expected, mechanosensory withdrawal thresholds in the previously injured forelimb were significantly elevated in all groups 8 weeks post-injury, indicative of chronic sensory loss (Fig. 2; PRE v. Wk 8; Paired t-test, t(22) = 17.55, p = 2.00 × 10−14). No differences in withdrawal thresholds were observed between groups prior to beginning therapy (One-way ANOVA across groups at week 8; F[2,22] = 0.29; p = 0.75).
Fig. 2. Distinct tactile experiences paired with VNS result in different recovery of somatosensory thresholds.
(A) Nerve damage results in chronic impairments in somatosensation in the forepaw, as indicated by a lasting increase in mechanical withdrawal thresholds. VNS paired with the either paintbrush (VNS+Brush) or filament (VNS+Filament) stimulation drives robust improvements in somatosensory thresholds compared to equivalent tactile rehabilitation without VNS (Rehab). However, VNS+Brush yields significantly greater restoration of tactile thresholds VNS+Filament, illustrating that specific features of rehabilitation impact VNS effects. The shaded region denotes when tactile therapy with or without VNS was delivered. Bonferroni corrected unpaired t-test across groups at week 14; *** denotes p < 0.00033. Error bars indicate mean ± SEM. (B) Percentage of recovery on tactile thresholds for each animal. VNS+Brush produced a virtually complete restoration of tactile threshold, whereas VNS+Filament produced significant, but incomplete, improvements in tactile threshold. Bonferroni corrected unpaired t-test across groups; ** denotes p < 0.0033, *** denotes p < 0.00033. Circles depict data from individual subjects. Error bars indicate mean ± SEM.
Comparison of withdrawal thresholds during therapy revealed a significant effect of group and time (Two-way repeated measures ANOVA, Effect of Group: F[1,21] = 231.14, p = 8.30 × 10−13; Effect of Time: F[6,126] = 6.56, p = 4.75 × 10−6; Interaction: F[6,126] = 0.43, p = 0.86). VNS paired with tactile rehabilitation consisting of either brush or filament stimulation significantly improved recovery compared to tactile stimulation without VNS (Fig. 2A, Week 14; Unpaired t-test; Rehab v. VNS+Filament, t(12) = 6.97; p = 1.50 × 10−5; Rehab v. VNS+Brush, t(11) = 13.80; p = 2.74 × 10−8). VNS paired with brush produced significantly more recovery of forelimb withdrawal thresholds compared to VNS paired with filament stimulation, indicating that the sensory stimuli delivered during rehabilitation with VNS influence functional outcomes (Fig. 2A, Week 14; Brush v. Filament; t(17) = 4.95; p = 1.23 × 10−4). Improvements in withdrawal thresholds were maintained on week 14 after the cessation of VNS (Fig. 2B; Unpaired t-test; Rehab v. VNS+Filament, t(12) = 3.85, p = 2.30 × 10−3; Rehab v. VNS+Brush, t(11) = 6.85, p = 2.75 × 10−5; VNS+Brush v. VNS+Filament, t(17) = 4.03, p = 8.72 × 10−4). As expected, no differences in sensory thresholds were observed in the uninjured forepaw at any time point (Two-way repeated measures ANOVA; Effect of Group: F[1,21] = 1.47, p = 0.24; Effect of Time: F[6,126] = 1.48, p = 0.21; Interaction: F[6,126] = 2.08, p = 0.06). These findings demonstrate that VNS paired with tactile rehabilitation produces substantial, stable improvements in somatosensory function in animals with chronic sensory loss and that the sensory features of the tactile rehabilitation paired with VNS influence the degree of recovery.
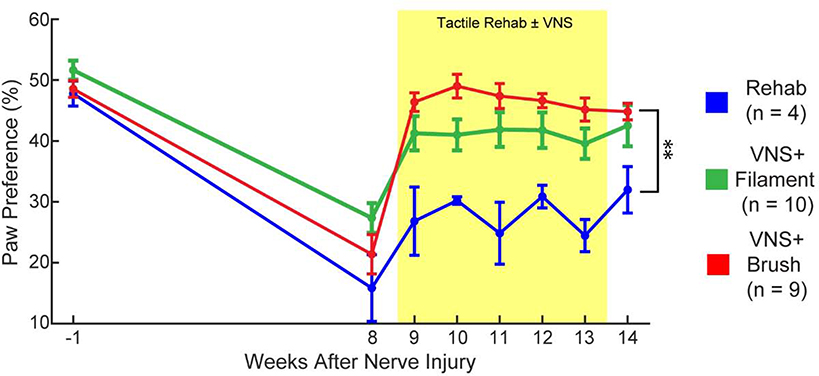
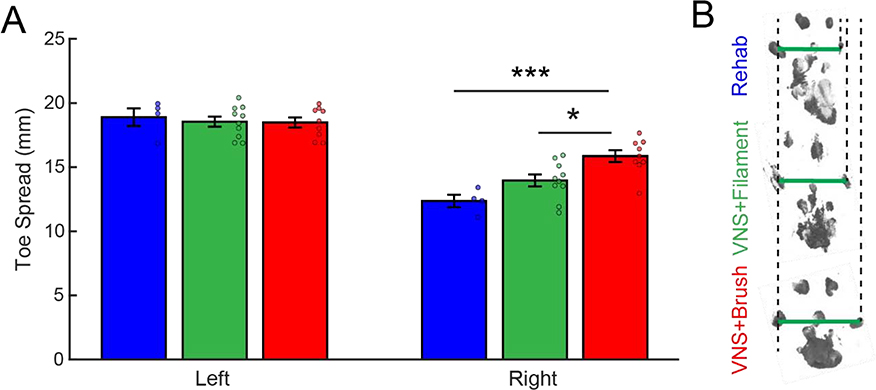
We next sought to determine whether the observed VNS-dependent improvements in somatosensory function would generalize to other measures of forelimb sensorimotor function. Spontaneous forelimb use, as measured with the cylinder task, revealed reduced reliance on the impaired limb, consistent with previous studies [16,18] (Fig. 3, PRE v. Wk 8; Paired t-test, t(22) = 12.20, p = 2.89 × 10−11). No differences were observed across groups before therapy (Fig. 3, Week 8; One-way ANOVA across groups at week 8; F[2,22] = 2.5; p = 0.11). Comparison of paw preference during therapy revealed a significant effect of group and time (Fig. 3, Two-way repeated measures ANOVA, Two-way repeated measures ANOVA, Effect of Group: F[1,21] = 26.87, p = 3.89 × 10−5; Effect of Time: F[6,126] = 2.45, p = 0.028; Interaction: F[6,126] = 0.72, p = 0.64). Matching the observed improvement in withdrawal thresholds, VNS paired with brush during tactile rehabilitation resulted in significantly greater use of the injured forelimb compared to tactile rehabilitation without VNS (Fig. 3, Week 14; Unpaired t-test, Rehab v. VNS+Brush, t(11) = 4.02, p = 2.00 × 10−3). Consistent with a lower degree of recovery of somatosensory function, VNS paired with filament during tactile rehabilitation did not result in significant improvements compared to tactile rehabilitation without VNS, although a trend was observed (Fig. 3, Week 14; Unpaired t-test, Rehab v. VNS+Filament, t(12) = 1.78, p = 0.10). Assessment of toe spread, a measure of weight bearing, revealed a similar distribution of VNS-dependent recovery. Again consistent with the degree of improvement in withdrawal thresholds, VNS paired with brush stimulation produced significantly greater improvements than VNS paired with filament stimulation and rehabilitation without VNS (Fig. 4A, Right Paw, One-way ANOVA; F[2,22] = 10.14; p = 9.10 × 10−4; post hoc unpaired t-test across groups; Rehab v. VNS+Filament, t(12) = 2.00, p = 0.06; Rehab v. VNS+Brush, t(11) = 4.58, p = 7.93 × 10−4; VNS+Brush v. VNS+Filament, t(17) = 2.90, p = 0.01). These findings indicate that the degree of VNS-dependent benefits in somatosensory recovery generalizes to other metrics of general forelimb function.
Fig. 3. VNS-dependent recovery generalizes to an untrained sensorimotor forelimb task.
Nerve damage and resultant sensory loss produces an overreliance on the use of the uninjured forelimb during exploration, demonstrated by a reduction in preference for the injured paw. VNS+Brush significantly increases use of the previously denervated forelimb compared to equivalent tactile rehabilitation without VNS (Rehab) after the completion of therapy. Unpaired t-tests across groups at week 14; ** denotes p < 0.0033. Error bars indicate mean ± SEM.
Fig. 4. VNS paired with brush improves toe spread during normal walking.
(A) Forelimb toe spread in the injured right forepaw was reduced compared to the intact left forepaw after nerve damage, consistent with sensorimotor dysfunction. VNS+Brush significantly increased toe spread compared to VNS+Filament or Rehab without VNS on week 14. (B) Representative examples of footprints collected from the injured right forepaw after the completion of tactile rehabilitation with or without VNS. Green lines illustrate the toe spread measurement, and dotted lines are shown for alignment. Bonferroni corrected unpaired t-tests across groups at week 14; * denotes p < 0.0167, *** denotes p < 0.00033. Circles depict data from individual subjects. Error bars indicate mean ± SEM.
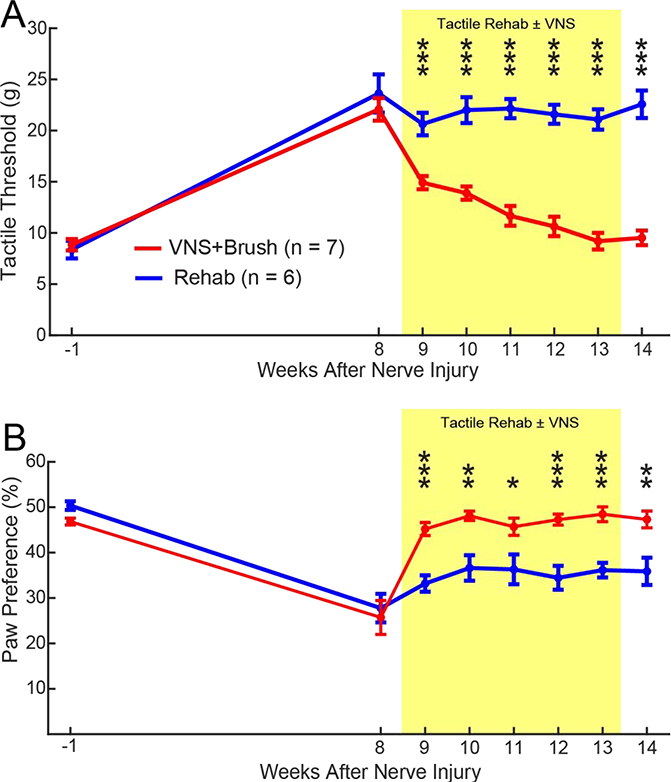
Somatosensory function and motor control are inherently integrated. Thus, we next sought to determine whether restoration in somatosensory function with VNS paired with tactile rehabilitation could produce improvements in skilled forelimb motor function. To do so, an independent cohort of animals underwent nerve injury, followed by tactile rehabilitation with brush stimulation with or without VNS. Confirming findings from the initial experiment, VNS paired with tactile rehabilitation significantly improved forelimb withdrawal thresholds compared to equivalent tactile rehabilitation without VNS (Fig. 5A, Two-way repeated measures ANOVA, Effect of Group: F[1,11] = 76.73, p = 2.73 × 10−6; Effect of Time: F[6,66] = 12.21, p = 3.47 × 10−9; Interaction: F[6,66] = 4.12, p = 1.42 × 10−3; post hoc Unpaired t-test on Week 14, Rehab v. VNS+Brush; t(11) = 8.96, p = 2.20 × 10−6). Spontaneous forelimb use was also significantly improved, providing additional corroboration of the initial experiment (Fig. 5B, Two-way repeated measures ANOVA, Effect of Group: F[1,11] = 66.04, p = 5.62 × 10−6; Effect of Time: F[6,66] = 2.52, p = 0.03; Interaction: F[6,66] = 0.31, p = 0.92; post hoc Unpaired t-test on Week 14, Rehab v. VNS+Brush; t(11) = 3.36, p = 6.41 × 10−3).
Fig. 5. Confirmation of VNS-dependent improvements in somatosensory function.
A separate cohort of rats confirms that VNS+Brush significantly improves tactile withdrawal thresholds (A) and sensorimotor function (B) compared to Rehab. Unpaired t-tests across groups at each week; * denotes p < 0.05, * denotes p < 0.01, *** denotes p < 0.001. Error bars indicate mean ± SEM.
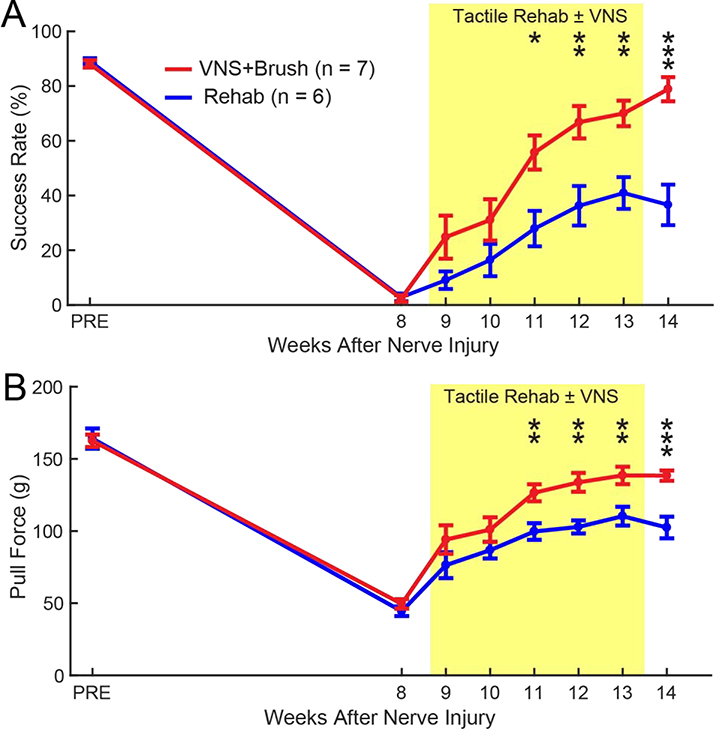
To evaluate skilled forelimb motor performance, all rats were trained to proficiency on the isometric pull task prior to nerve injury and assessed weekly during therapy [16,17,23,29,30,40,47–49]. As expected, nerve injury resulted in substantial impairments in skilled forelimb function [16,40] (Fig. 6A, PRE v. Wk 8; Paired t-test, t(12) = 68.43, p = 6.30 × 10−17). No differences were observed between groups prior to therapy (Fig. 6A, Wk 8, Unpaired t-test; Rehab v. VNS+Brush; t(11) = 0.26, p = 0.80). Evaluation of forelimb motor function during therapy revealed a significant effect of group and time (Fig. 6A, Two-way repeated measures ANOVA, Effect of Group: F[1,11] = 17.18, p = 1.63 × 10−3; Effect of Time: F[6,66] = 4.46, p = 7.54 × 10−4; Interaction: F[6,66] = 0.36, p = 0.90). VNS paired with tactile rehabilitation significantly improved recovery of forelimb motor performance compared to equivalent tactile rehabilitation without VNS (Fig. 6A, Week 14; Rehab v. VNS+Brush; Unpaired t-test, t(11) = 5.08, p = 3.54 × 10−4). Similar improvements in pull force were observed (Fig. 6B, Two-way repeated measures ANOVA, Effect of Group: F[1,11] = 15.34, p = 2.40 × 10−3; Effect of Time: F[6,66] = 1.92, p = 0.09; Interaction: F[6,66] = 1.21, p = 0.31; post hoc Unpaired t-test on Week 14, Rehab v. VNS+Brush; t(11) = 4.56, p = 8.20 × 10−4). These findings indicate that VNS pairing during tactile therapy produces associated improvements in motor function despite the fact that VNS was not explicitly paired with motor training.
Fig. 6. VNS paired with tactile rehabilitation improves skilled forelimb motor function.
(A) Success rate, defined as the percentage of pulls greater than 120 g on the isometric force task, was reduced following nerve injury consistent with impairment of skilled motor function. VNS+Brush significantly improved success rate compared tactile rehabilitation without VNS. (B) Similar effects were observed for pull force. The shaded region denotes when tactile therapy with or without VNS was delivered. Unpaired t-tests across groups at each week; at each time point; * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001. Error bars indicate mean ± SEM
4. Discussion
In the present study, we explored whether the sensory characteristics of the tactile rehabilitation paired with VNS influence the degree of recovery in a rat model of chronic sensory loss. Corroborating previous studies, we find that VNS paired with tactile rehabilitation consisting of applying either brush or filament stimulation to the previously denervated paw significantly improves restoration of somatosensory function compared to equivalent tactile rehabilitation without VNS [18,50]. Use of the brush to apply tactile stimulation during rehabilitation produced a greater degree of sensorimotor recovery than the filament, indicating that the features of the tactile rehabilitation regimen paired with VNS impact the efficacy of the therapy. Additionally, we find that VNS paired with tactile rehabilitation yields robust, generalized improvements in skilled motor function. Together, these findings support VNS paired with tactile rehabilitation as a novel strategy to enhance recovery of somatosensory and motor function after neurological injury and provide evidence to guide the selection of the features of the paired rehabilitative therapy.
Previous preclinical studies and a human case study indicate that pairing VNS with tactile rehabilitation improves recovery of somatosensory function following chronic sensory loss [18,50]. The tactile retraining paradigm employed in these studies was based on delivery of VNS coincident with application of tactile stimuli with a wide range of features to the reinnervated skin surface. VNS engages neuromodulatory networks to enhance plasticity, and this paradigm was premised on activating a variety of somatosensory receptors in combination with the pro-plasticity actions of VNS to promote reorganization of sensory networks in the central nervous system. A number of studies demonstrate that VNS-dependent plasticity is specific to features of the paired stimulus [16,17,27,35,51–57]. Thus, the features of tactile stimuli paired with stimulation may influence the effects of VNS on restoration of somatosensory function. Here, we explored tactile stimuli with fundamentally distinct features. The brush and filament stimuli applied differ in a variety of spatiotemporal features, including temporal pattern of activation, area of contact, and force of contact, and thus produce differential activation of exteroceptive somatosensory receptors. Pairing either tactile paradigm with VNS significantly enhanced recovery compared to equivalent tactile stimulation without VNS, consistent with previous studies using a combination of tactile stimuli [18]. Notably, VNS paired with tactile stimulation with the brush yielded significantly greater restoration of sensorimotor function on several measures compared to VNS paired with tactile stimulation with the filament. The restoration of somatosensory recovery in subjects that received VNS paired with brush stimulation is comparable to that observed in a previous study that used a combination of brush and filament stimulation [18]. Because the features of the individual stimuli used in the present study vary in a variety of dimensions, the results presented here cannot identify which features contribute to greater therapeutic efficacy when paired with VNS. However, these findings highlight the importance of the rehabilitation paradigm paired with VNS and suggest that clinical implementations should utilize a range of stimuli encompassing a variety of tactile features.
Motor and somatosensory function are highly integrated. As a result, improvements in somatosensory function driven by VNS paired with tactile rehabilitation may generalize to benefits in motor function. In the present study, we corroborated previous results that the benefits of VNS paired with tactile rehabilitation produce consequent improvements in measures of forelimb sensorimotor function [18]. Similar to withdrawal thresholds, pairing VNS with brush stimulation produced significantly greater improvements in other general sensorimotor measures, including spontaneous forelimb use and toe spread, compared to pairing VNS with filament stimulation. These findings provide further evidence that the selection of the tactile rehabilitation paradigm applied with VNS impacts the efficacy of the therapy.
Given that VNS paired with tactile rehabilitation produced generalized improvements in simple sensorimotor tasks, we evaluated whether VNS therapy would similarly facilitate recovery of skilled forelimb motor function. We elected to evaluate recovery of skilled motor function after delivery of VNS paired with brush stimulation, as this paradigm produced the greatest functional improvements. In an independent cohort of animals, we observed that VNS paired with brush stimulation significantly improved recovery of skilled forelimb motor performance compared to tactile rehabilitation without VNS. VNS paired with tactile rehabilitation resulted in an approximately 80% recovery of pre-injury motor performance and a virtually complete restoration of somatosensory recovery as measured by withdrawal thresholds. These findings indicate that VNS paired with tactile rehabilitation generalizes to improvements in skilled motor function. A previous study observed a similar generalization of sensory recovery when VNS was paired with motor rehabilitation [16]. In that study, pairing VNS with rehabilitative training on the skilled motor task produced a virtually complete restoration of motor function and an approximately 70% restoration of somatosensory function. Taken together, these data indicate that pairing VNS with somatosensory or motor rehabilitation produce partial generalization of recovery. As a result, clinical implementation in patients with somatosensory and motor dysfunction after neurological injury should incorporate both tactile and motor rehabilitative paradigms with VNS to ensure the greatest benefits.
VNS supports recovery by engaging neuromodulatory networks coincident with rehabilitation to promote synaptic plasticity in the rehabilitated circuits [16,17,30]. After injury, pairing VNS with motor rehabilitation drives synaptic reorganization in corticospinal projections to facilitate motor recovery [16,17,30]. Depletion of acetylcholine in the cortex or temporally uncoupling of VNS and rehabilitation prevents enhancement of plasticity and subsequently precludes recovery of motor function, indicating that VNS-directed synaptic plasticity underlies motor recovery. In the context of sensory improvements, pairing VNS with auditory stimuli drives reorganization in auditory circuits that is associated with a reduction in tinnitus [34,36,55,58–60]. Similar mechanisms likely underlie the VNS-dependent enhancement of somatosensory recovery observed here. Tactile rehabilitation produces activity in somatosensory circuits, and coincident VNS drives activation of neuromodulatory networks to facilitate synaptic plasticity. Future studies should explore the nature of VNS-dependent plasticity in somatosensory networks, such as changes in thresholds or receptive fields, to identify the neural mechanisms that subserve recovery [33].
This study was designed to provide direct translational evidence related to the development of VNS therapy to improve somatosensation, and several strengths and weakness merit consideration. Strengths include preregistration of experimental procedures and design, a confirmation of the main effect of VNS-dependent improvements in an independent cohort of animals, automated, quantitative, blinded behavioral assessments, and replication of parallel findings of VNS-dependent improvements in somatosensory function in a human case study. Additionally, the design of the study is predicated on the direct comparison of rehabilitative interventions, rather than comparison to an untreated control. The present results also need to be interpreted based on a number of limitations. First, while the study utilized several metrics of sensorimotor function, preclinical experiments lack the ability to capture the complexity of many aspects of somatosensation. Additionally, the coarse behavioral tools available limit assessment of fine characterization of sensory discrimination. Neurophysiological analysis of fine metrics of somatosensation, such as receptive field size, should be examined in subsequent studies. Second, the present study was performed exclusively in female rats. We elected to restrict evaluation to female rats because the tactile assessments and VNS parameters have been extensively validated in this sex [16,18,61,62,35,51–57]. While there is no evidence of a sex-specific effect of VNS on the nervous system in humans [21,31,63], future studies should confirm the present findings in male rats. Finally, the present study did not examine reinnervation. Although previous studies demonstrate that VNS does not influence any metrics of reinnervation after injury [16,18] and the presence of behavioral and neural responses to tactile stimulation indicate that reinnervation has occurred [33], we cannot explicitly rule out differences in reinnervation across groups in this study.
Recent clinical trials in chronic stroke patients demonstrates that VNS paired with physical rehabilitation represents a clinical viable strategy to restore motor function after neurological injury [13]. Here, we provide corroborating evidence to support the investigation of VNS paired with tactile rehabilitation as a novel strategy to restore somatosensory function in the context of chronic sensory loss [18,50]. Additionally, the data presented reinforce the need to identify the most effective rehabilitative paradigms in order to maximize the benefits of VNS-based therapies.
Supplementary Material
Highlights.
Chronic sensory loss is often associated with nerve injury
VNS paired with tactile rehabilitation drives recovery of somatosensory function
The tactile experience paired with VNS affects the degree of sensory recovery
VNS-dependent recovery of sensory function generalizes to other forelimb functions
Acknowledgments
We would like to thank Nikita Guntu, Forrest Smith, Mbinui Ghogomu, Eliezer Diaz, Monica Sheth, Yasir Mian Bilal, Wongani Kalengamaliro, Jimmy Tran, Ashleigh Abusomwan, Matt Buell, Khalil Abed Rabbo, and Nikki Simmons. This work was supported by The National Institutes of Health NIH R01 NS094384 (SAH) and R01 NS103803 (RLR).
Footnotes
Potential Conflicts of Interest: MPK has a financial interest in MicroTransponder, Inc., which is developing VNS for stroke. RLR is an owner of Teliatry, which is developing a VNS device. All other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Connell L, Lincoln N, Radford K, Somatosensory impairment after stroke: frequency of different deficits and their recovery, Clin. Rehabil. 22 (2008) 758–767. 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- [2].Carey L, Matyas T, Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting, J. Rehabil. Med. 43 (2011) 257–263. 10.2340/16501977-0662. [DOI] [PubMed] [Google Scholar]
- [3].Robinson LR, Traumatic injury to peripheral nerves, Muscle Nerve. 23 (2000) 863–873. [DOI] [PubMed] [Google Scholar]
- [4].Gilliatt RW, Wilson TG, ISCHAEMIC SENSORY LOSS IN PATIENTS WITH PERIPHERAL NERVE LESIONS, J. Neurol. Neurosurg. Psychiatry. 17 (1954) 104 10.1136/JNNP.17.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sullivan JE, Hedman LD, Sensory Dysfunction Following Stroke: Incidence, Significance, Examination, and Intervention, Top. Stroke Rehabil. 15 (2008) 200–217. 10.1310/tsr1503-200. [DOI] [PubMed] [Google Scholar]
- [6].Tyson SF, Hanley M, Chillala J, Selley AB, Tallis RC, Sensory Loss in Hospital-Admitted People With Stroke: Characteristics, Associated Factors, and Relationship With Function, Neurorehabil. Neural Repair. 22 (2008) 166–172. 10.1177/1545968307305523. [DOI] [PubMed] [Google Scholar]
- [7].Jaquet J, Shreuders T, Kalmijn S, Ruys ACJ, Coert H, Hovius SER, Median and Ulnar Nerve Injuries: Prognosis and Predictors for Clinical Outcome, J. Reconstr. Microsurg. 51 (2001) 687–692. 10.1055/s-2006-949697. [DOI] [Google Scholar]
- [8].Carey L, Macdonell R, Matyas TA, SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation, Neurorehabil. Neural Repair. 25 (2011) 304–313. 10.1177/1545968310397705. [DOI] [PubMed] [Google Scholar]
- [9].Schabrun S, Hillier S, Evidence for the retraining of sensation after stroke: a systematic review, Clin. Rehabil. 23 (2009) 27–39. 10.1177/0269215508098897. [DOI] [PubMed] [Google Scholar]
- [10].Doyle S, Bennett S, Fasoli SE, McKenna KT, Interventions for sensory impairment in the upper limb after stroke, Cochrane Database Syst. Rev. (2010). 10.1002/14651858.CD006331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Byl N, Roderick J, Mohamed O, Hanny M, Kotler J, Smith A, Tang M, Abrams G, Effectiveness of Sensory and Motor Rehabilitation of the Upper Limb Following the Principles of Neuroplasticity: Patients Stable Poststroke, Neurorehabil. Neural Repair. 17 (2003) 176–191. 10.1177/0888439003257137. [DOI] [PubMed] [Google Scholar]
- [12].Turville ML, Matyas TA, Blennerhassett JM, Carey LM, Initial severity of somatosensory impairment influences response to upper limb sensory retraining post-stroke, NeuroRehabilitation. 43 (2019) 413–423. 10.3233/NRE-182439. [DOI] [PubMed] [Google Scholar]
- [13].Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA, Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke, Front. Neurosci. 13 (2019). 10.3389/FNINS.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA, Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation, Exp. Neurol. 298 (2016) 21 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M, Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors, Neuroscience. 189 (2011) 207–214. [DOI] [PubMed] [Google Scholar]
- [16].Meyers EC, Kasliwal N, Solorzano BR, Lai E, Bendale G, Berry A, Ganzer PD, Romero-Ortega M, Rennaker RL, Kilgard MP, Hays SA, Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage, Nat. Commun. 10 (2019) 1–14. 10.1038/s41467-019-13695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, Adcock KS, James JT, Jeong HS, Becker A, Goldberg MP, Pruitt DT, Hays SA, Kilgard MP, Rennaker RL, Closed-loop Neuroprosthesis Restores Network Connectivity and Motor 2 Control after Spinal Cord Injury, Elife. (2018) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Darrow MJ, Mian TM, Torres M, Haider Z, Danaphongse T, Rennaker RL, Kilgard MP, Hays SA, Restoration of Somatosensory Function by Pairing Vagus Nerve Stimulation with Tactile Rehabilitation, Ann. Neurol. 87 (2020) 194–205. 10.1002/ana.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, Deshpande A, Stecker N, Pereira M, Kilgard M, Burress C, Pierce D, Rennaker R, Vanneste S, Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans., Sci. Rep. 7 (2017) 11960 10.1038/s41598-017-12178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Engineer ND, Møller AR, Kilgard MP, Directing Neural Plasticity to Understand and Treat Tinnitus, Hear. Res. (2012). [DOI] [PubMed] [Google Scholar]
- [21].Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, Tarver B, Engineer ND, Alexander Dickie D, Kline DK, Wigginton JG, Cramer SC, Dawson J, Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke, Stroke. 49 (2018) 2789–2792. 10.1161/STROKEAHA.118.022279. [DOI] [PubMed] [Google Scholar]
- [22].Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL, Hays SA, Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats., Neurorehabil. Neural Repair. 30 (2016) 676–84. 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, Kilgard MP, Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats, Neurobiol. Aging. 43 (2016) 111–118. 10.1016/j.neurobiolaging.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL, Kilgard MP, Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke, Neurobiol. Dis. 60 (2013). 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- [25].Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, Kilgard MP, Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke., Neurorehabil. Neural Repair. 28 (2014) 698–706. 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, Kilgard MP, Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage, Stroke. 45 (2014) 3097–100. 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, Kilgard MP, The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength, Neuroreport. 25(9) (2014) 676–682. 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pruitt DT, Danaphongse TT, Lutchman M, Patel N, Reddy P, Wang V, Parashar A, Rennaker RL, Kilgard MP, Hays SA, Optimizing dosing of vagus nerve stimulation for stroke recovery, Transl. Stroke Res. (n.d.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL, Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury, J. Neurotrauma. 33 (2016). 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, Kilgard MP, Hays SA, Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery, Stroke. 49 (2018) 710–717. 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N, Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired with Upper-Limb Rehabilitation after Ischemic Stroke, Stroke. 47 (2016) 143–150. https://doi.org/STROKEAHA.115.010477 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kilgard MP, Rennaker RL, Alexander J, Dawson J, Vagus Nerve Stimulation Improved Sensory Function in A Chronic Stroke Patient, NeuroRehabilitation. (n.d.). [DOI] [PubMed] [Google Scholar]
- [33].Hulsey DR, Mian TM, Darrow MJ, Hays SA, Quantitative assessment of cortical somatosensory digit representations after median and ulnar nerve injury in rats, Exp. Brain Res. 237 (2019) 2297–2304. 10.1007/s00221-019-05593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP, Reversing pathological neural activity using targeted plasticity, Nature. 470 (2011) 101–104. 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, II RLR, Kilgard MP, Rennaker RL, Kilgard MP, Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex, Cereb. Cortex. 22 (2011) 2365–2374. 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- [36].Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP, Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity, Brain Stimul. 8(3) (2015) 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Noble LJ, Meruva VB, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK, Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats, Brain Stimul. 12 (2019) 9–18. 10.1016/J.BRS.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP, Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex, Exp. Neurol. 233 (2011) 342–349. 10.1016/j.expneurol.2011.10.026. [DOI] [PubMed] [Google Scholar]
- [39].Hays SA, Khodaparast N, Sloan AM, Hulsey DR, Pantoja M, Ruiz AD, Kilgard MP, Rennaker RL, Rennaker RL, The isometric pull task: a novel automated method for quantifying forelimb force generation in rats, J. Neurosci. Methods. 212 (2012) 329–337. 10.1016/j.jneumeth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [40].Meyers EC, Granja R, Solorzano BR, Romero‐Ortega M, Kilgard MP, Rennaker RL, Hays SA, Median and ulnar nerve injuries reduce volitional forelimb strength in rats, Muscle Nerve. 56 (2017). 10.1002/mus.25590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rios M, Bucksot J, Rahebi K, Engineer C, Kilgard M, Hays S, Rios MU, Bucksot JE, Rahebi KC, Engineer CT, Kilgard MP, Hays SA, Protocol for Construction of Rat Nerve Stimulation Cuff Electrodes, Methods Protoc. 2 (2019) 19 10.3390/mps2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McAllen RM, Shafton AD, Bratton BO, Trevaks D, Furness JB, Calibration of thresholds for functional engagement of vagal A, B and C fiber groups in vivo, Bioelectron. Med. 1 (2018) 21–27. 10.2217/bem-2017-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shaikh S, Shortland P, Lauto A, Barton M, Morley JW, Mahns DA, Sensory perturbations using suture and sutureless repair of transected median nerve in rats, Somatosens. Mot. Res. 33 (2016) 20–28. 10.3109/08990220.2016.1142438. [DOI] [PubMed] [Google Scholar]
- [44].Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST, CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury, Neuropharmacology. 39 (2000) 777–787. [DOI] [PubMed] [Google Scholar]
- [45].Galtrey CM, Fawcett JW, Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb, J. Peripher. Nerv. Syst. 12 (2007) 11–27. [DOI] [PubMed] [Google Scholar]
- [46].Sloan AM, Fink MK, Rodriguez AJ, Lovitz AM, Khodaparast N, Rennaker RL, Hays SA, A within-animal comparison of skilled forelimb assessments in rats, PLoS One. 10 (2015). 10.1371/journal.pone.0141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pruitt D, Hays S, Schmid A, Choua C, Kim L, Trieu J, Kilgard MP, Rennaker RL, Controlled-cortical impact reduces volitional forelimb strength in rats, Brain Res. 1582 (2014) 91–98. 10.1016/j.brainres.2014.07.039. [DOI] [PubMed] [Google Scholar]
- [48].Darrow MJ, Torres M, Sosa MJ, Danaphongse TT, Haider Z, Rennaker RL, Kilgard MP, Hays SA, Vagus Nerve Stimulation Paired With Rehabilitative Training Enhances Motor Recovery After Bilateral Spinal Cord Injury to Cervical Forelimb Motor Pools., Neurorehabil. Neural Repair. 34 (2020) 200–209. 10.1177/1545968319895480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Becker AM, Meyers E, Sloan A, Rennaker R, Kilgard M, Goldberg MP, An automated task for the training and assessment of distal forelimb function in a mouse model of ischemic stroke, J. Neurosci. Methods. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kilgard MP, Rennaker RL, Alexander J, Dawson J, Vagus Nerve Stimulation Paired with Tactile Training Improved Sensory Function in a Chronic Stroke Patient, NeuroRehabilitation. 42 (2018) 159–165. [DOI] [PubMed] [Google Scholar]
- [51].Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, Hays SA, Vagus nerve stimulation intensity influences motor cortex plasticity., Brain Stimul. (2018). 10.1016/j.brs.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Buell EP, Loerwald KW, Engineer CT, Borland MS, Buell JM, Kelly CA, Khan II, Hays SA, Kilgard MP, Cortical map plasticity as a function of vagus nerve stimulation rate, Brain Stimul. (2018). 10.1016/j.brs.2018.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Loerwald KW, Buell EP, Borland MS, Rennaker RL, Hays SA, Kilgard MP, Varying Stimulation Parameters to Improve Cortical Plasticity Generated by VNS-tone Pairing, Neuroscience. 388 (2018) 239–247. 10.1016/J.NEUROSCIENCE.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Loerwald KW, Borland MS, Rennaker RL, Hays SA, Kilgard MP, The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation, Brain Stimul. (2017). 10.1016/j.brs.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Borland MS, Engineer CT, Vrana WA, Moreno NA, Engineer ND, Vanneste S, Sharma P, Pantalia MC, Lane MC, Rennaker RL, Kilgard MP, The Interval Between VNS-Tone Pairings Determines the Extent of Cortical Map Plasticity, Neuroscience. 369 (2018) 76–86. https://www.sciencedirect.com/science/article/pii/S0306452217307911 (accessed November 14, 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Buell EP, Borland MS, Loerwald KW, Chandler C, Hays SA, Engineer CT, Kilgard MP, Vagus Nerve Stimulation Rate and Duration Determine whether Sensory Pairing Produces Neural Plasticity, Neuroscience. 406 (2019) 290–299. 10.1016/J.NEUROSCIENCE.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Borland M, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Vanneste S, Kilgard MP, Engineer CT, Pairing vagus nerve stimulation with tones drives plasticity across the auditory pathway, J. Neurophysiol. (2019) jn.00832.2018. 10.1152/jn.00832.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kilgard MP, Harnessing plasticity to understand learning and treat disease, Trends Neurosci. 35 (2012) 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hays SA, Rennaker RL, Kilgard MP, Targeting plasticity with vagus nerve stimulation to treat neurological disease., Prog. Brain Res. 207 (2013) 275–99. 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Engineer ND, Møller AR, Kilgard MP, Directing neural plasticity to understand and treat tinnitus, Hear. Res. 295 (2013) 58–66. 10.1016/j.heares.2012.10.001. [DOI] [PubMed] [Google Scholar]
- [61].Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, Engineer CT, Kilgard MP, Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity, Brain Stimul. 9 (2016) 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, Hays SA, Vagus nerve stimulation intensity in fluences motor cortex plasticity, Brain Stimul. 12 (2019) 256–262. 10.1016/j.brs.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, Silverberg A, Rivera E, Geller E, Carlson C, Devinsky O, Doyle WK, Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: Long-term outcomes and predictors of response, Epilepsy Behav. 20 (2011) 57–63. 10.1016/J.YEBEH.2010.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.