Abstract
One of the most difficult challenges for risk assessment is evaluation of chemicals that predominately co-occur in mixtures like polycyclic aromatic hydrocarbons (PAHs). We previously developed a classification model in which systems biology data collected from mice short-term after chemical exposure accurately predict tumor outcome. The present study demonstrates translation of this approach into a human in vitro model in which chemical-specific bioactivity profiles from 3D human bronchial epithelial cells (HBEC) classify PAHs by carcinogenic potency. Gene expression profiles were analyzed from HBEC exposed to carcinogenic and non-carcinogenic PAHs and classification accuracies were identified for individual pathway-based gene sets. Posterior probabilities of best performing gene sets were combined via Bayesian integration resulting in a classifier with four gene sets, including aryl hydrocarbon receptor signaling, regulation of epithelial mesenchymal transition, regulation of angiogenesis, and cell cycle G2-M. In addition, transcriptional benchmark dose modeling of benzo[a]pyrene (BAP) showed that the most sensitive gene sets to BAP regulation were largely dissimilar from those that best classified PAH carcinogenicity challenging current assumptions that BAP carcinogenicity (and subsequent mode of action) is reflective of overall PAH carcinogenicity. These results illustrate utility of using systems toxicology approaches to analyze global gene expression towards carcinogenic hazard assessment.
1.0. Introduction
The environmental health science community recognizes PAHs as a re-emerging class of environmental pollutants due to their persistence and prominence in mixtures of concern (IARC 2010, IARC 2013, Jarvis, Dreij et al. 2014, IARC 2015, ATSDR 2017). PAHs are ubiquitous contaminants in the environment commonly generated by petrogenic and pyrogenic processes, including coal-tar pitch production and wood, and incomplete combustion of tobacco, and fossil fuels (Abdel-Shafy and Mansour 2016). They account for 3 of the top 10 chemicals of concern at priority pollutant sites and are considered among the most important carcinogens in air pollution (Ramesh, Archibong et al. 2011, ATSDR 2017). The World Health Organization’s International Agency for Research on Cancer (IARC) has designated several PAHs and mixtures, such as diesel exhaust, air pollution, coal and coke mixtures, as either Class 1 known or Class 2A/B probable/possible carcinogens to humans (IARC 2010, Benbrahim-Tallaa, Baan et al. 2012, IARC 2014, Rengarajan, Rajendran et al. 2015). Despite the fact that PAHs were the first class of chemicals identified as chemical carcinogens, little is known about the carcinogenic potential of many of the over 1500 polycyclic aromatic compounds or about potential mechanisms of carcinogenic action for this diverse class.
Current assessment of cancer risk for PAHs involves testing compounds in the 2-year rodent bioassay (EPA 2010). These studies are time and resource-intensive, and often lack reproducibility or concordance (Gottmann, Kramer et al. 2001). Furthermore, they require extrapolation of effects to humans, leading to further uncertainties regarding species-specific biology and chemical mode of action (MOA) (Gottmann, Kramer et al. 2001). Carcinogenic potential of PAHs has been historically evaluated for individual chemicals through intraperitoneal or dermal routes. However, PAH exposures occur as complex environmental mixtures through inhalation, oral, and dermal routes, which are difficult and cost-prohibitive to assess using traditional carcinogenicity assays. The primary method for estimating cancer risk of PAH mixtures is the relative potency factor (RPF) approach in which mixtures are evaluated based on a subset of individual component PAHs compared to benzo[a]pyrene (BAP) as a surrogate or reference (EPA 2010). However, we and others have found this approach inadequate for predicting carcinogenicity of mixtures and also for certain individual PAHs, particularly those that function through alternate pathways or exhibit greater promotional capacity compared to BaP (Courter, Luch et al. 2008, Siddens, Larkin et al. 2012, Tilton, Siddens et al. 2015, Chang, Siddens et al. 2019).
Previously we reported an approach for predicting potency of PAH chemicals and environmental PAH mixtures based on bioactivity profiles derived from global transcriptional analysis short-term post-exposure in a mouse skin cancer model (Tilton, Siddens et al. 2015). This classification approach overcomes limitations of using RPFs for complex mixtures since it does not require knowledge about individual components of mixtures nor does it assume a common mechanism of action for all PAHs. Instead, we found that chemical-specific signaling after exposure provides a unique signature or bioactivity profile for each PAH/mixture that is reflective of its MOA and can be used to discriminate carcinogenic potency. Similar genomic-based models have successfully been applied to individual chemicals after short-term exposure to identify modes of action for distinguishing carcinogens from non-carcinogens in rodent in vivo and human in vitro (Song, Song et al. 2012, Gusenleitner, Auerbach et al. 2014). Other studies that have modeled non-additive effects of polycyclic aromatic compounds in mixtures on hepatotoxicity with gene expression data report the strong correlation of gene response with other toxicity endpoints in vivo, including histopathology and gross physiology, showing the benefits of using gene expression to evaluate quantitative differences in toxicity (Kopec, Burgoon et al. 2010, Kopec, D’Souza et al. 2011).
In this study, we evaluated whether systems biology data collected from a human in vitro airway epithelial model can be used for accurate classification of carcinogenic potency of PAHs and PAH mixtures. Advanced cell culture and tissue engineering are increasingly recognized for their potential in mechanistic studies because the three-dimensional structure, metabolic and mitotic activity, multi-cellular communication and cell signaling better recapitulate in vivo response compared to cells grown in monolayer culture (Birgersdotter, Sandberg et al. 2005, McKim 2010, Kimlin, Kassis et al. 2013) Human bronchial epithelial cells cultured at the air-liquid interface were previously found to produce chemical-specific gene signatures after treatment with two carcinogenic PAHs, BAP and dibenzo[def,p]chrysene (DBC), that were consistent with gene changes observed in mouse and showed chemical-specific signaling related to oxidative stress and inflammation may drive different MOAs for these PAHs (Chang, Siddens et al. 2019). These differences in gene signaling and regulation may thus contribute to subsequent differences in carcinogenic potency observed in vivo (Tilton, Siddens et al. 2015). For this study, employing organotypic in vitro models allows for rapid generation of transcriptional biosignatures for analysis and comparison of PAH MOAs linked to adverse health outcomes.
2.0. Methods
2.1. Chemicals and reagents
Cell culture media and phosphate buffered saline (PBS) were provided by MatTek Corporation (Ashland, MA). Benzo[a]pyrene (BAP) CAS# 50–32-8 and dibenzo[def,p]chrysene (DBC) CAS# 189–64-0 were purchased from MRIGlobal (Kansas City, MO). Benz[a]anthracene (BAN) CAS# 56–55-3, phenanthrene (PHE) CAS# 85–01-8, and pyrene (PYR) CAS# 19–00-0 were acquired from Sigma-Aldrich dba. Millipore Sigma (St. Louis, MO.). Coal Tar Extract (CTE) (SRM 1597a) was purchased form the National Institute of Standards & Technology (Gaithersburg, MD.) DNase I, TRIzol® reagent, Superscript® III First Strand Synthesis System, qPCR primers, and Pierce™ LDH Cytotoxicity Assay Kit were from Thermo Fisher Scientific (Waltham, MA). 2X SsoAdvanced™ Universal SYBER®Green Supermix was purchased from BioRad Laboratories, Inc. (Hercules, CA.).
2.2. Tissue Culture and Chemical Exposures
For the 2D cell culture experiments, primary normal HBEC at passage 5 from Lonza Group (Basel, Switzerland) were expanded in PneumaCult™-Ex Plus Medium from Stemcell Technologies (Vancouver, Canada) and further subcultured onto black-walled, clear-bottom 96-well plates until confluent, then exposed to chemical treatments for 24 hours using 2% dimethyl sulfoxide (DMSO) in Dulbecco’s phosphate-buffered saline (DPBS) from Thermo Fisher Scientific (Waltham, MA).
For the differentiated 3D cell culture experiments, primary HBEC cultured on transwell inserts (EpiAirway™ 100, Mattek, Ashland, MA) were shipped overnight and received chilled on ice packs. Tissues were immediately transferred to 6-well plates each well containing 1 ml of assay medium and equilibrated for 24 hours at 37°C, 5% CO 2 followed by a change of fresh medium before any treatment regimens commenced. Tissues were prepared for treatment as follows. PBS (0.50 ml) was pipetted onto the apical surface of the inserts and the PBS was carefully removed along with mucus from the surface of the tissues to wash the cells prior to chemical exposure. The medium was replaced with 1 ml of fresh medium in the plate wells (basal side of the membrane). Single PAHs and coal tar were solubilized in acetone and applied (0.01 ml/insert) to the apical surface of tissues (n=4 per treatment) for up to 48 hrs, BAP (1–500 ug/ml), DBC (1–50 ug/ml), BAN (10–500 ug/ml), PYR (10–250 ug/ml), PHE (10–250 ug/ml) and CTE (250 −1500 ug/ml). Dosing was chosen based on relative potency in BAP equivalents as previously reported (Siddens, Larkin et al. 2012, Siddens, Bunde et al. 2015, Chang, Siddens et al. 2019). Basal media was transferred to clean, sterile tubes and stored at −80°C. At the end of each exposure regimen, lysis buffer was added to each insert, collected, and stored at −80°C until extraction using RNeasy extraction kit.
2.3. RNA Isolation and mRNA-Seq
Total RNA was isolated from HBEC (n=4) using RNeasy Mini Kit (Qiagen, Venlo, Netherlands) and was quantitated on a SYNERGY/HTX plate reader equipped with a Take3 module, then evaluated for quality with a Bioanalyzer 2100, Agilent (Santa Clara, CA). PAH dosing for sequencing was chosen based on BAP equivalents at concentrations similar to those used in prior animal studies (applied to mouse skin) (Siddens, Larkin et al. 2012, Siddens, Bunde et al. 2015, Chang, Siddens et al. 2019). Acceptable RNA quality was based on RIN ≥ 8.5. Isolated mRNA were sequenced on Illumina HiSeq 3000, which yielded >350 million reads per lane. Sequenced reads were first put through Cutadapt (version 1.8.1) to trim adapter sequences from the paired-end reads. The human genome assembly GRCh38.84 was indexed using Bowtie2-build (version 2.2.3) while the transcriptome was indexed using TopHat (version 2.1.1) (Trapnell, Pachter et al. 2009, Trapnell, Williams et al. 2010, Trapnell, Roberts et al. 2012). TopHat was used again to align the trimmed reads to indexed transcriptome and genome (Trapnell, Roberts et al. 2012). Featurecounts (from the Rsubread package v1.22.2) was used to summarize reads into count tables (Liao, Smyth et al. 2014). Differential expression was determined in DESeq2 (version 1.26.0) compared to vehicle control (Love, Huber et al. 2014). The differentially expressed gene lists (q<0.05) for each treatment were used for pathway enrichment analysis in MetaCore (GeneGO, Thomson Reuters). Statistical significance of over-connected interactions was calculated using a hypergeometric distribution, where the p value represents the probability of a particular mapping arising by chance for experimental data compared to the background (Nikolsky, Kirillov et al. 2009). Heatmaps were generated in MultiExperiment Viewer TM4 (Saeed, Sharov et al. 2003).
2.4. Posterior Probability Integration for Chemical Classification
Once significantly enriched pathways (p<0.05) were identified, they were further prioritized by differential significance across chemical exposure groups by calculating the standard deviations of the negative logarithm of the p values. The top 30 pathways with the greatest standard deviations were selected for chemical classification testing. PAH treatments were grouped based on RPF and BAP equivalence into four chemical classes (non-carcinogenic, low, moderate, and high). Non-carcinogenic PAHs were binned into Class 1, containing PYR and PHE. Lower potency carcinogenic treatments were assigned to Class 2, and the exposure groups were BAP10, BAP100, CTE, and BAN. Moderate-level carcinogenic potency was assigned to Class 3, with the highest dose of BAP (BAP500). High-level carcinogenic potency was assigned to Class 4, for DBC-exposed samples, which has been demonstrated in vivo to have up to 100-fold greater carcinogenic potency than BAP (Siddens, Larkin et al. 2012). Individual pathway gene sets were tested for classification performance in Visual Integration for Bayesian Evaluation (VIBE) v2.0 (Beagley, Stratton et al. 2010, Webb-Robertson, Metz et al. 2017), where probability matrices for significantly enriched pathways were calculated using Naïve Bayes statistical learning algorithm with 4-fold cross validation. Pathway gene sets with high-performing classification accuracies (0.70 and above) were prioritized for further testing in a Bayesian integration framework as described previously (Tilton, Siddens et al. 2015).
2.5. Transcriptional Benchmark Dose Modeling and Functional Classification
BMDExpress 2.0 (version 2.3) was used to perform benchmark dose (BMD) modeling analysis on transcriptomic data from 3D HBEC exposed to 10, 100, and 500 ug/uL BAP for 48 hours (Yang, Allen et al. 2007, Phillips, Svoboda et al. 2019). The imported RNAseq data set was analyzed using the EPA BMDS Models (parametric), and multiple models were used to fit gene expression dose-response data, including hill, power, linear, and polynomial. For each gene, the best fitting model was selected based on best fit and nested Chi-square test (cutoff of 0.05), as previously described (Labib, Williams et al. 2017, Phillips, Svoboda et al. 2019). A benchmark response (BMR) factor of 1.021 was selected to model a 5% response over background. Only genes with BMD values lower than the highest dose were included for the downstream Functional Classification Analysis using pre-defined biological process networks (Metacore). The median BMD of the selected process networks was used as the primary metric to evaluate overall pathway and process sensitivity to determine the most BAP-dose responsive and sensitive process networks.
2.6. Quantitative PCR
cDNA was synthesized using a Superscript® III First Strand Synthesis Supermix kit per manufacturer’s instructions. Reactions were diluted 1:5 with nuclease –free water and stored at −80°C until used for qPCR. A BioRad Laboratories, Inc. (Hercules, CA.) CFX96 Touch™ Real-Time PCR Detection System was used for running 20µl qPCR reactions to survey key gene targets. Each reaction contained 2µl cDNA template (10ng RNA), 150 nM of each primer, 10 µl 2X SsoAdvanced™ Universal SYBER®Green Supermix, and nuclease–free water. Primer sequences are as described in Chang et al. (2019) and reported in Supplemental File 1. The thermocycler was programmed for 1 cycle 95°C for 1 minute initial denaturing, 40 cycles 95°C for 15 sec denaturing, 60°C for 30 sec annealing/elongation, and a melt curve 65–95°C/0.5° per 5 sec for validating single product amplification. The relative expression differences among treatments were calculated using the ΔΔCt comparative method and normalized to the housekeeping gene peptidylprolyl isomerase A (PPIA). Genes significantly regulated by PAH treatment (p<0.05) were identified by one-way ANOVA with Tukey’s multiple testing correction.
2.7. Cell Viability
Lactate dehydrogenase (LDH) leakage was measured in media after treatment with PAHs (n=4) for 48 hrs using Pierce LDH Cytotoxicity Assay Kit (Thermo Scientific) following manufacturer instructions as previously described (Chang, Siddens et al. 2019). Briefly, basal medium samples (40µl) were aliquoted into the wells of a 96-well plate. LDH reaction reagent (40µl) was added to each sample and incubated at room temperature for 30 minutes while protected from light. Finally, 40µl of stop solution was added to each well and mixed. LDH activity was determined by subtracting absorbance at 680nm (background) from absorbance at 490nm (Synergy HTX plate reader, BioTek, Winooski, VT). A negative control of fresh cell culture medium, a positive control of medium from lysed cells, and vehicle only controls were included. Cytotoxicity was evaluated by one-way ANOVA with Dunnett’s multiple testing correction (p<0.05). The tested concentrations of PAHs had no effect on cell viability.
2.7. Oxidative Stress
For the reactive oxygen species detection assays, a 2’,7’–dichlorofluorescin diacetate (DCFDA) solution (from Sigma-Aldrich, St. Louis, MO) was prepared at 2% DMSO in HBSS, and pipetted onto the cells. Immediately, the plate was read by a SYNERGY/HTX plate reader (BioTek Instruments Inc., Winooski, VT) at 485/20 and 528/20 nm excitation and emission. A positive control of menadione and vehicle only controls were included. Chemical-induced fluorescence was expressed as relative fluorescence (RFU) normalized to vehicle control (RFU/vehicle), and the data were fit to a nonlinear regression with variable slope to model dose-response curves and calculate EC50s.
3.0. Results
3.1. Global gene expression analysis
In this study, PAH gene signatures were measured in 3D HBEC after 48 hour treatment using RNAseq. Raw and normalized sequencing files are available at NCBI Gene Expression Omnibus (GSE128471 and GSE156147). Of over 60,000 transcripts mapped and evaluated, there were 9,538 statistically significant transcripts (q < 0.05) identified as differentially regulated compared to vehicle control across all treatments. PAH treatment concentrations chosen for sequencing, which included a BAP dose-response and additional PAHs based on calculated BAP equivalents, did not result in any observed cytotoxicity compared to controls (data not shown). Overall, BAP 10ug/mL, 100ug/mL, and 500 ug/mL exposure resulted in 1222, 41, 6309 differentially regulated genes (DEGs), respectively, (q < 0.05); BAN 500ug/mL exposure resulted in 898 DEGs; CTE 1.5mg/mL exposure resulted in 1505 DEGs; DBC 10ug/mL exposure resulted in 3122 DEGs. Finally, the non-carcinogenic PAHs, PHE 100 ug/mL and PYR 500 ug/mL resulted in 1530 and 3 DEGs, respectively. There were no shared DEGs between all 8 treatment groups.
PAH treatments resulted in chemical-specific gene signatures as visualized by principal component analysis (PCA) (Fig 1A). Approximately 50% of the gene and sample variance across x and y axes are described in the first two components. Principal components 1 and 2 are shown in a 2D plot, with component 1 visualized across the x-axis (37.99% variance) and component 2 visualized across the y-axis (10.81%). In general, we observe marked separation between different PAH treatments, with the most separation observed in BAP 500ug/mL and DBC. There is slight overlap between BAN and CTE, and between BAP 100 ug/mL and PHE. Overall, treatment-specific response is observed, with PAH exposure groups clustering more closely with samples of the same chemical and dose, suggesting chemical-specific and dose-specific gene signatures.
Figure 1. Differential effects of PAHs in human bronchial epithelial cells. (A) Principal components analysis (PCA) of global gene expression of HBEC exposed to PAHs.
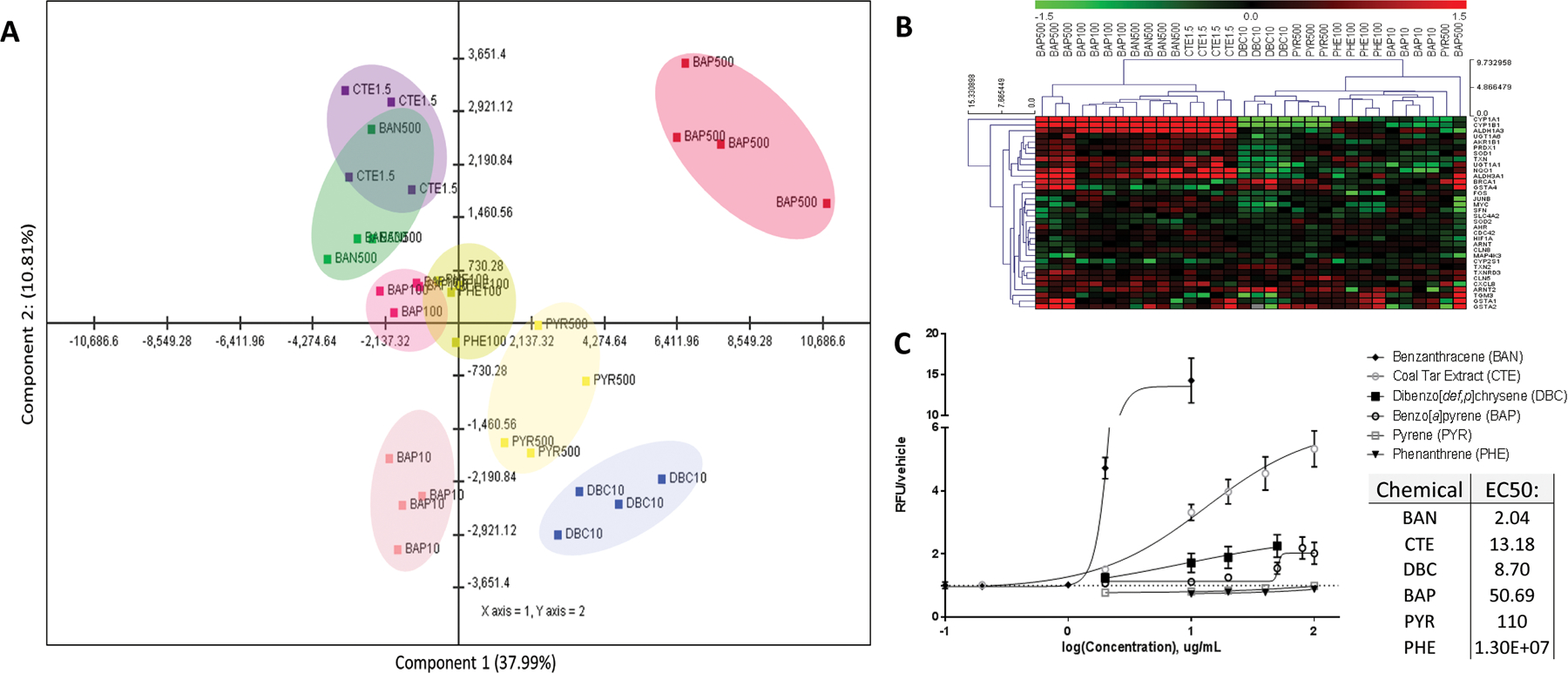
The horizontal axis represents PC 1 and the vertical axis represents PC 2. The captured variance of these first 2 components account for 47.22% of the total variance, and variance-correlation of gene expression resulted in variable clustering of PAHs. (B) Transcriptional regulation of genes by PAHs. Comparison of gene expression using RNASeq. Subsets of genes associated with xenobiotic metabolism, AhR signaling, oxidative stress, and cell cycle regulation were highlighted in a heatmap. Values are log2-fold change for all treatments compared with experimental control; red, green, and black represent upregulated, downregulated, and unchanged genes, respectively. (C) Intracellular ROS generation as measured by DCFDA. Chemical-induced fluorescence was expressed as relative fluorescence (RFU) normalized to vehicle control (RFU/vehicle), and the data were fit to a nonlinear regression with variable slope to model dose-response curves and calculate EC50s.
To compare PAH bioactivity at the gene level, subsets of genes associated with xenobiotic metabolism, aryl hydrocarbon receptor signaling, oxidative stress, and cell cycle regulation were highlighted in a heatmap (Fig 1B). Unsupervised hierarchical clustering was performed by Euclidean distance with average linkage clustering to generate gene and sample-level dendrograms. The heatmap illustrates unique patterns of expression across PAHs for some xenobiotic metabolism genes. CYP1A1, CYP1B1 and ALDH3A1 were upregulated by the two higher doses of BAP (100ug/mL and 500ug/mL), CTE, and BAN. However, DBC and PYR exposure resulted in decreased levels of these gene transcripts as confirmed by qPCR (Supplemental File 2). A similar pattern was observed for NQO1, SOD1, TXN, PRDX1, ALDH1A3, AKR1B1, and UGT1A1. For this subset of genes, DBC and the lowest sequenced dose of BAP (10ug/mL) clustered more closely with non-carcinogenic PAHs than the low and moderate potency carcinogens (BAN, CTE, BAP100ug/mL, BAP500ug/mL). Other antioxidant response genes (e.g. GSTA) followed a unique pattern of response that was also highly PAH-specific (Supplemental File 2). These data suggest that PAHs function through unique mechanisms and that typical PAH biomarkers (e.g. CYP1A1) are not predictive of carcinogenic potency.
3.2. PAH-related Reactive Oxygen Species Generation
Due to PAH-associated transcriptional differences in redox genes, the chemicals were further investigated for PAH-induced ROS generation using a high throughput DCFDA fluorescence assay. PAH-induced ROS were detected by fluorescent DCFDA signal in 2D cultured HBEC, and chemical-induced ROS were expressed as relative fluorescence units (RFUs) normalized to vehicle control (Fig. 1C). Based off of maximal responses, BAN exposure was the most effective at inducing intracellular ROS, followed by CTE, DBC, and BAP. PHE and PYR did not elicit ROS generation in 2D HBEC. The median effective concentration (EC50) values of these concentration-response curves were 2.04 ug/mL (9uM) for benz[a]anthracene, 8.70ug/mL (29 uM) for DBC, 13.18 ug/mL (78 uM) for CTE, and 50.69 ug/mL (225 uM) for BAP. When comparing EC50s between PAHs, the EC50 of BAP is notably higher than the EC50s of BAN and CTE, indicating that BAP may be a less potent intracellular ROS generator than BAN and CTE. In this assay, we observe that all chemical treatments with carcinogenic activity (BAP, BAN, CTE, DBC) had some level of ROS generation, while the non-carcinogenic PAHs (PYR, PHE) did not have any detectable ROS-generating activity. However, ROS generation potential in the 2D HBEC did not reflect chemical carcinogenic potency or RPF. For example, BAN is estimated to be five times less carcinogenic as BAP with an RPF of 0.2, yet demonstrates much greater potency in producing intracellular ROS, resulting in a much lower EC50 of 2.04 ug/mL (9uM). BAN surpassed all other PAHs tested in its ability to generate ROS, including the high molecular weight carcinogen DBC, which has a RPF estimate of 30–100.
3.3. Pathway-based classification of PAHs in 3D HBEC
Pathway selection for classification accuracy testing in Bayesian framework.
A primary goal of this study was to develop a predictive model using chemical-gene signatures following short-term exposure of a human organotypic culture model to non-carcinogenic and carcinogenic PAHs. With a limited dataset, we utilized an expert knowledge-driven approach to 1. Designate chemical classes based on carcinogenic potency informed by calculated RPFs and prior mouse in vivo studies (Siddens, Larkin et al. 2012, Siddens, Bunde et al. 2015), and 2. Develop a classification approach using in vitro data by testing and integrating prioritized pathway gene sets with high ability to correctly assign a sample into its designated class.
A pathway enrichment analysis was conducted to identify biological function associated with the 9,555 genes significantly regulated (q < 0.05) by the PAH exposure groups. Overall, the PAH exposure groups’ individual DEGs significantly enriched a total of 125 pathways. Overall, 92 pathways were significantly enriched by at least 2 treatments, indicating a wide range of biological coverage associated with PAH exposure. Pyrene did not significantly enrich any pathways. A key goal of this study is to identify pathways better suited for chemical carcinogenicity predictive classification, so statistical filtering was used to prioritize pathway gene sets to be tested for classification performance. Significantly enriched pathways were prioritized for classification testing based on differential significance (standard deviation of negative log p-value) to identify pathways that were most dissimilar among PAH exposure groups. The top 30 most differentially significant pathways were selected for further testing and visualized in a heatmap showing significance in yellow and white, and lack of significance in blue (Fig. 2). In addition, aryl hydrocarbon receptor signaling was included since it had previously been identified to be differentially regulated by PAHs (Fig. 1B, Supplemental File 2).
Figure 2. Pathway enrichment analysis of differentially regulated genes by PAH and PAH mixture exposure.
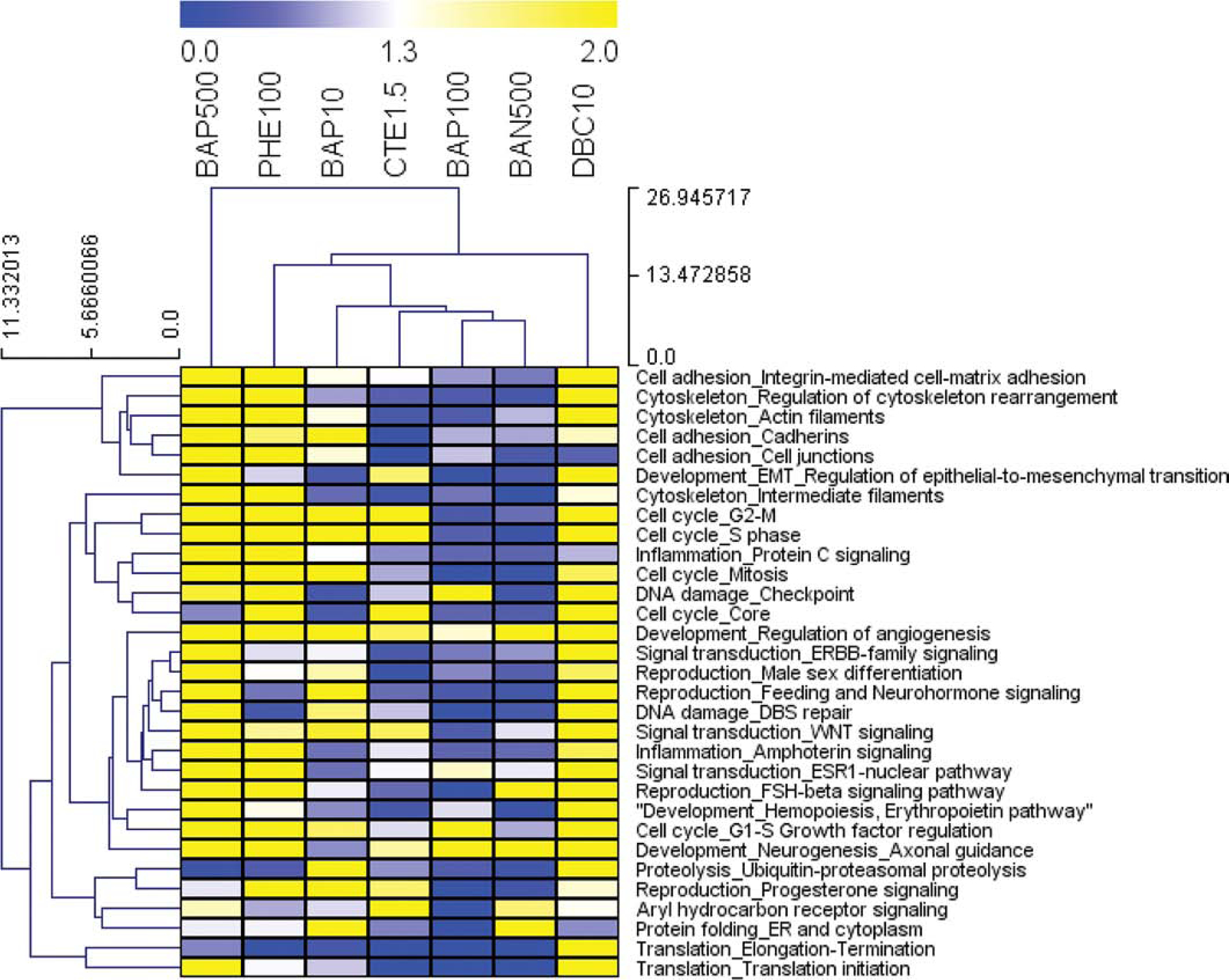
Functional enrichment analysis was performed in MetaCore (GeneGO, Thomson Reuters) based on mapping of the significant (p<0.05) genes in each treatment group onto built-in functional network processes. Heatmap visualizing the top 30 most differentially significant process networks (greatest std(−logpval) regulated by 7 PAH treatments (p<0.01 for at least one PAH group). Values are pval that have been transformed (−logpvalue) for pathway enrichment of treatments, where blue, white, and yellow represent −logpvalue = 0, 1.3 (p<0.05), and 2.0 (p<0.01), respectively.
Each gene set was tested individually for classification ability and quantified by a classification accuracy (CA). The resulting CAs for tested gene sets ranged 0.50 – 0.88. An example of a perfect classifier is shown in Fig. 3A1 in which the predicted (X-axis) and true class (y-axis) are identical resulting in 100% classification accuracy. Representative well-performing, poor performing and randomly generated gene sets are provided for comparison (Figs. 3A2–4). To generate the random gene set, 138 genes, which represents the average size of annotated gene sets, were randomly selected from the overall 9,555 DEG in the study. While a perfect classifier is returns a 1.00 or 100% CA, a random classifier is 0.50, or 50% CA. Some process, for example the translation elongation-termination gene set, did not classify chemicals any better than genes identified at random (Fig. 3A3). In contrast, other pathways containing gene sets yielding higher classification scores were able to successfully discriminate samples into chemical classes with higher probability (Fig. 3A2).
Figure 3. Classification of PAHs based gene sets.
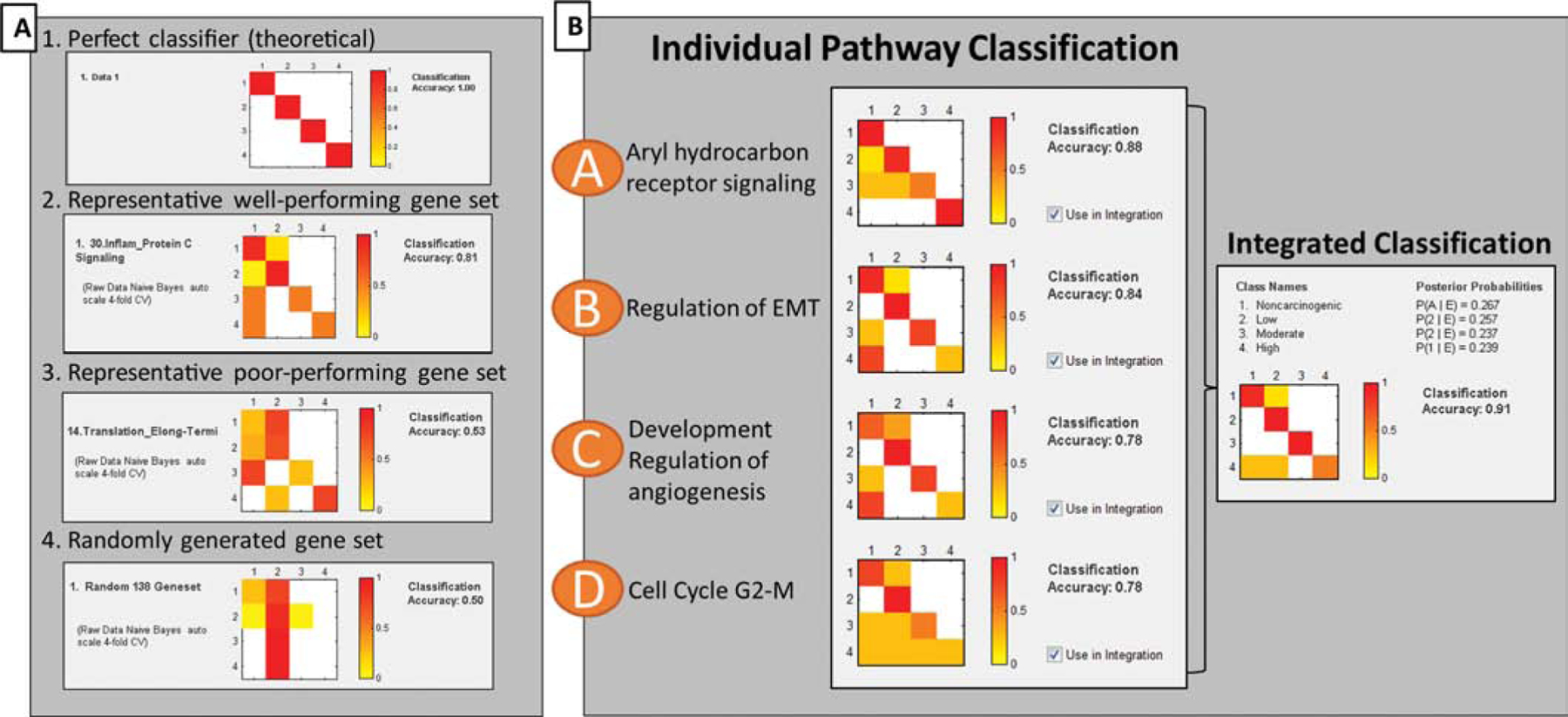
Classification accuracy (CA) heatmaps of gene-based classification performance, where classes 1–4 indicate carcinogenic potency (non-carcinogenic, low, moderate, high). Colors indicate proportion of samples classified into specific classes, where red represents most or all samples, and white represents zero samples. Therefore, a theoretical perfect classifier results in a diagonal line of red squares. (A) Probability matrices for gene sets were calculated using Naïve Bayes statistical learning algorithm with 4-fold cross validation. Here, the CA heatmaps demonstrate the possible range of classification performance, from theoretically perfect classifiers (1.00) to randomly generated gene sets that do not classify better than random (0.50). (B) Bayesian integration of top-performing pathway gene sets. Integrated CA (0.91) is indicated on the right, and exceeds the individual CA performance of individual pathway gene sets.
Figure 4. Benchmark dose (BMD) analysis of transcriptomic data from 3D HBEC exposed to Benzo[a]pyrene (BAP).
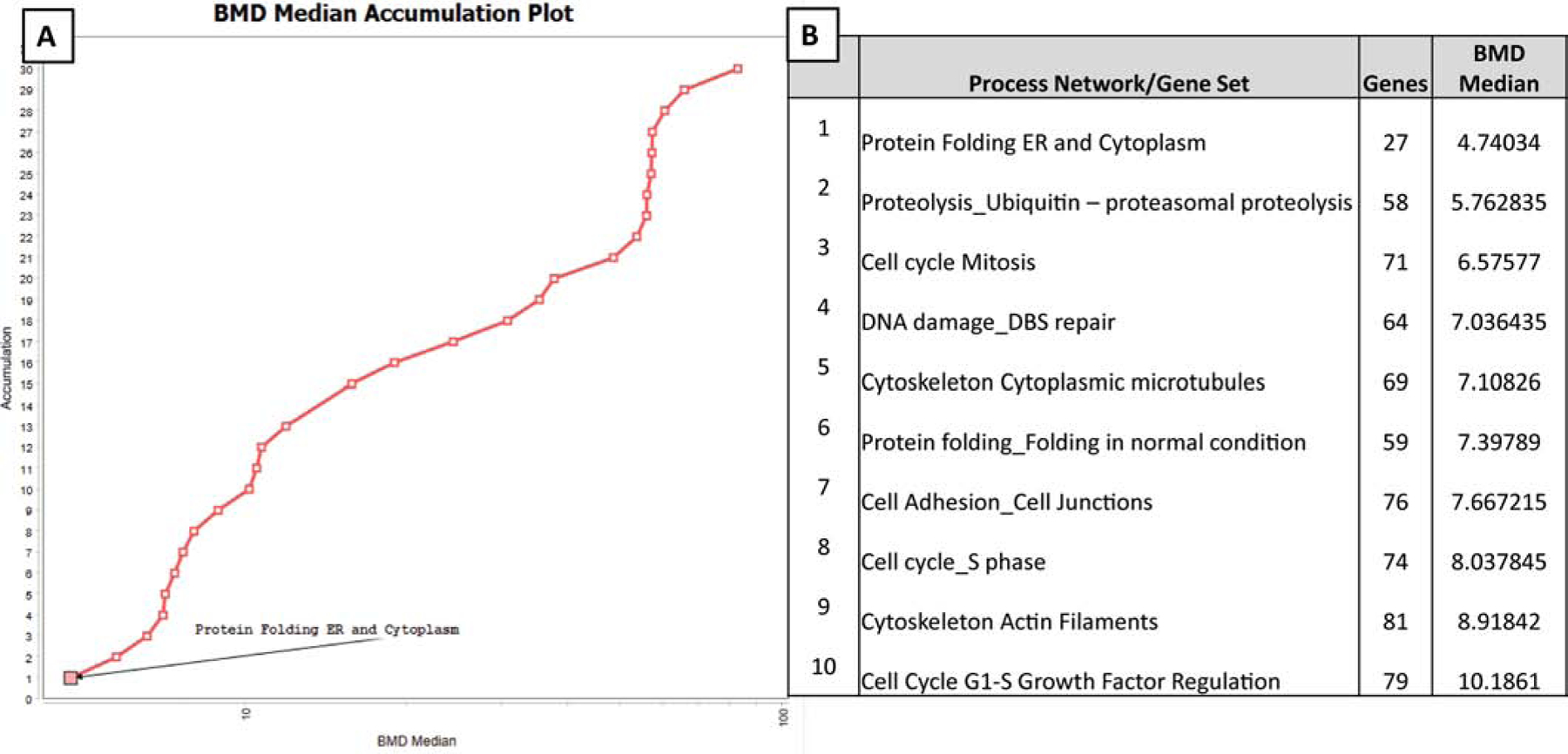
BMD values represent estimated median doses causing a 5% shift over the background rate of response associated with each gene set. (A) Distributions of modelled BMD estimates of BAP through a category analysis of the top 30 differentially significant pathway gene sets. The vertical axis represents the relative rank of the pathway gene sets, sorted by BMD median (ascending), while BMD median values are depicted across the horizontal axis. (B) Table of top ten most sensitive pathway gene sets to BAP gene regulation with number of genes modelled and BMD median. Genes from these pathways have the lowest BMD median values modelled, of the top 30 pathways.
Integration of multiple pathways for improved classification accuracy.
Based on prior data showing that integration across multiple pathways results in improved classification accuracy, candidate gene sets were identified and selected for Bayesian integration to identify a combination that improved CA performance (Tilton, Siddens et al. 2015). This integration approach was driven by knowledge that carcinogenic PAHs can act through different and possibly diverse modes of actions, and just like in vitro assay endpoints, there is unlikely to be one single pathway gene set that is predictive of different PAHs of many genotoxic and nongenotoxic MOAs. Testing combinations of pathway gene sets having 70% CA and higher yielded a resulting combination of 4 pathway gene sets with improved CA (Fig. 3B). A combination of four gene sets (aryl hydrocarbon receptor signaling, regulation of epithelial mesenchymal transition, regulation of angiogenesis, and cell cycle G2-M) resulted in the best CA score of 0.91, or 91% CA. Here, the CA performance of the integrated gene sets exceeded the CA performance of any of the individual gene sets (0.78–0.88). Overall, these results demonstrate that chemical biosignatures from in vitro studies can be used to inform classification based on in vivo outcomes and that integration across pathway-derived gene sets can improve overall accuracy.
3.5. Transcriptomic Dose-Response Modeling of BAP in 3D HBEC
To understand which genes and pathways are most sensitive to dose after treatment with BAP, transcriptomic dose-response modeling was performed with a three-point dose response of BAP-exposed HBEC. BMD analyses determines a chemical exposure concentration (ug/mL) at which a defined level of response occurs (5% standard deviation). The primary goal of conducting this analysis was to identify the biological functions most sensitive to BAP dose-response in the airway epithelium. In addition, we were interested in whether the gene sets that provided the highest CA across a group of PAHs overlapped with those that were highly sensitive to dose-response treatment by BAP. Pathway level BMD modeling was applied to the prioritized gene sets (Section 3.3) and the most sensitive gene set to BAP dose-response regulation in 3D HBEC was Protein folding in the endoplasmic reticulum (ER) and cytoplasm, which modelled 27 genes with the lowest BMD median (Fig. 4A). The top ten most sensitive pathways with lowest BMD medians are highlighted (Fig. 4B) and include processes associated with cell cycle (mitosis, S phase, and G1-S growth factor regulation); cytoskeleton (cytoplasmic microtubules and actin filaments), ubiquitin related proteolysis, and DNA damage double-strand repair. Notably, the top ten most sensitive gene sets to BAP dose-response regulation did not overlap with the pathways that performed best for PAH classification of carcinogenic potential. Overall, these data show that the bioactivity profiles most associated with PAH carcinogenesis are not consistent with those that are most sensitive to BAP suggesting that PAHs, as a class, can function through unique mechanisms when compared to BAP alone.
Due to the lack of overlap between the methods, the pathway-level BMD modeling for BAP was expanded to include all process network categories as described in Metacore (Thomson Reuters) beyond just those prioritized for classification. This grouping of process networks into broad biological categories allows for BMD to be calculated for each biological category ranking them in order of sensitivity to BAP dose-response. The top 3 most significantly enriched (lowest minimum p-value out of all 8 exposure groups) pathways per process network category were selected for custom upload into the BMD analysis and the resulting BMD medians were averaged (Supplemental File 3). Overall, the biological categories most sensitive to BAP dose-response gene regulation in HBEC were protein folding (avg BMD median = 4.74 ug/mL), DNA damage (avg BMD median = 6.58 ug/mL), cytoskeleton (8.92 ug/mL), cell adhesion (10.74 ug/mL), and cell cycle (11.06ug/mL). Comparatively, the least sensitive pathway categories to BAP gene regulation were proliferation (121.78 ug/mL) and autophagy (134.75 ug/mL).
4.0. Discussion
Previously, we developed an approach to use gene signatures generated from mouse skin short-term after chemical treatment to accurately predict cancer outcome (Tilton, Siddens et al. 2015). The present study demonstrates the successful translation of this approach into a human in vitro tissue model. We observed that gene biosignatures collected from 3D human bronchial epithelium exposed to a range of carcinogenic and non-carcinogenic PAHs can be used to accurately classify chemicals by potency. Gene expression profiles were analyzed to identify chemical-gene signatures at the pathway level. Gene sets related to several biological pathways were integrated and evaluated for classification performance to differentiate PAHs by carcinogenic class. Individual endpoints and biomarkers, such as intracellular ROS generation and CYP450 gene regulation, were insufficient predictors of carcinogenicity in this study. Benchmark dose modeling of BAP showed that the most sensitive pathway gene sets to BAP regulation were largely dissimilar from the pathways that best classified PAH carcinogenicity. These results illustrate the utility of using systems toxicology approaches to analyze global gene expression information towards carcinogenic hazard/risk assessment of PAHs. In addition to identification of gene sets, we also find evidence to challenge current assumptions that BAP carcinogenicity (and subsequent MOA) is reflective of overall PAH carcinogenicity.
4.1. Predicting carcinogenicity with individual biomarkers
Chemical carcinogenesis is a complex process that involves numerous biological processes and molecular targets during initiation, promotion, and progression. Thus, the complexity in mechanisms of carcinogenicity contributes to difficulty identifying singular endpoints or in vitro assays to predict carcinogenic hazard of chemicals (Tilton, Siddens et al. 2015, Labib, Williams et al. 2016, Chang, Siddens et al. 2019). While many carcinogenic PAHs have genotoxic modes of action and induce DNA damage through adduct formation (Ross, Nelson et al. 1995, Topinka, Schwarz et al. 1998, Baird and Mahadevan 2004), some carcinogenic PAHs also exhibit non-genotoxic modes of action that include estrogenic/antiestrogenic activity (Kummer, Maskova et al. 2008, Sievers, Shanle et al. 2013), dysregulation of cell proliferation (Vondráček, Kozubík et al. 2002, Plíšková, Vondráček et al. 2004), inhibition of gap-junctional intercellular communication (Upham, Masten et al. 1994, Bláha, Kapplová et al. 2002), and generation of ROS and oxidative stress (Penning, Burczynski et al. 1999, Park, Troxel et al. 2006, Hanzalova, Rossner et al. 2010, An, Yin et al. 2011).
Overall, we found that specific gene targets and endpoints in HBEC, such as CYP1A1 induction and ROS generation, were limited in their ability to predict degree of carcinogenic hazard. The diversity in MOAs for PAH toxicity may contribute to the difficulty in identifying single biomarkers and in vitro endpoints to evaluate carcinogenic hazard of chemicals with complex mechanisms. Other commonly reported biomarkers for carcinogenicity, such as DNA adduct formation, have also been observed to lack correlation with carcinogenesis (Tilton, Siddens et al. 2015). While specific biomarkers and endpoints may not adequately assess carcinogenic hazard, a growing body of research suggests utility in toxicogenomic biomarkers and gene sets in evaluating carcinogenic hazard and carcinogenic MOA. mRNA and miRNA gene expression profiles have been used successfully to discriminate among carcinogens in various experimental model systems (Song, Song et al. 2012, Gusenleitner, Auerbach et al. 2014). In this study, we did not observe a clear pattern of carcinogenicity driving the clustering and grouping of samples in PCA and hierarchical clustering from global gene expression data. For this reason, we focused our study towards identifying subsets of genes that are high-performing classifiers to degree of carcinogenic hazard.
4.2. Utilization of in vitro biosignatures to classify chemicals based on health outcomes
Toxicogenomics usage in carcinogenic hazard assessment has progressed considerably in the past two decades; numerous methods have been developed to successfully identify genes linked to specific carcinogenic mechanisms and to develop approaches to predict and classify chemicals. In earlier toxicogenomic studies, correlational analyses and hierarchical clustering were commonly employed methods to identify subsets of genes to describe mechanisms of chemical toxicity (Burczynski, McMillian et al. 2000). Similar approaches have been applied to identify patterns of gene expression that discriminate between genotoxic and non-genotoxic carcinogens (van Delft, van Agen et al. 2004, van Delft, van Agen et al. 2005, Magkoufopoulou, Claessen et al. 2012, Lee, Yum et al. 2013) or identify specific pathways that respond differently between genotoxic and non-genotoxic chemicals (Kim, Kwon et al. 2005, Watanabe, Suzuki et al. 2012). Over the years, studies have advanced towards modeling approaches in which gene expression profiling can robustly discriminate carcinogens based on clearly different MOAs (van Delft, van Agen et al. 2004, Mathijs, Brauers et al. 2009, Williams, Buick et al. 2015)
In our present study, we noted that PAH-specific signatures from global gene expression did not correlate with carcinogenic potency nor could be used to easily discriminate carcinogenic from non-carcinogenic PAHs. However, we hypothesized that global gene expression data could be organized into pathways or gene sets that may accurately classify treatments by potency. To address this challenge, we utilized a Bayesian posterior integration approach to assess classification accuracies for an integrated set of biological functions. These approaches have previously been applied to identify predictive biomarkers for chronic obstructive pulmonary disorder (COPD) (Wang, Webb-Robertson et al. 2013) and type I diabetes through integration of disparate data streams, including proteins, metabolites, and lipids (Webb-Robertson, Metz et al. 2017). We have also previously described application of a pathway-based classification model for predicting carcinogenic risk of PAHs in mouse skin in vivo in which integration across multiple gene sets improved overall accuracy compared to each gene set independently (Tilton, Siddens et al. 2015). A goal of the current study is to apply this approach to an organotypic human airway epithelial model to analyze, prioritize, and identify gene sets as classifiers and demonstrate feasibility of the classification framework in vitro. A benefit of the current approach is that the in vitro biosignatures are anchored to an established in vivo phenotype (cancer outcome and potency) through evaluation of their ability to predict cancer risk independent of whether the in vitro gene signatures would be identical to those measured in vivo. The resulting integrated CA of 91% indicates that when the gene sets for AhR signaling, regulation of epithelial-mesenchymal transition, regulation of angiogenesis, and cell cycle G2-M are combined in an integrated classification, the overall accuracy performance can improve (Fig. 3). Further, the best classifiers identified from human airway epithelium in vitro, while overlapping, are distinct from those previously identified in mouse skin epithelium in vivo, which may be due to the expanded number of gene sets or PAHs tested or due to model-specific responses. Similar genomic-based models have been applied to individual chemicals after short-term exposure in rats to identify modes of action for distinguishing carcinogens from non-carcinogens in multiple tissues showing that the prediction of carcinogenicity was tissue-dependent and was most effective with tissue-specific gene classifier identification (Gusenleitner, Auerbach et al. 2014).
These studies demonstrate the importance of diversifying toxicogenomics analysis approaches beyond traditional methods of global gene expression analyses and adopting specialized analysis approaches to appropriately filter and query data. As toxicogenomics continues to be adopted in predictive toxicology, the opportunities for novel analyses and data integration continue to expand. We and others have found success using prediction models and classification approaches towards transcriptomic approaches to discriminate chemicals by carcinogenic hazards (Magkoufopoulou, Claessen et al. 2012, Wang, Webb-Robertson et al. 2013, Tilton, Siddens et al. 2015). Eventually, we hope the toxicology community sufficiently develops criteria and guidelines for real-time prediction of carcinogenicity using -omics level data, including gene expression. However, more research is necessary to evaluate and validate biological pathway predictability in a range of human tissue models and chemical classes. Chemical carcinogenesis is a complicated process, affected by dose, timing, chemical mode(s) of action, and duration and frequency of exposure. Therefore, carcinogenic hazard prediction approaches using -omics technologies may be best applicable for a subset of carcinogens and exposure scenarios. Beyond carcinogenic hazard assessment, these approaches also have potential in further applications including disease prediction, biomarker development, drug safety, and mechanistic studies.
4.3. Dose-response modeling of BAP in human airway epithelium
Dose-response modeling of toxicogenomic data allows for quantitative assessment to estimate point of departure or threshold response for application to human health risk assessments (Farmahin, Williams et al. 2017, Guo and Mei 2018, Shao and Shapiro 2018, Mezencev and Auerbach 2020). In addition, it provides a better understanding of chemical MOA in different model systems. Through dose-response assessment of BAP in 3D HBEC, we observed that the most sensitive processes impacted by BAP in human airway epithelium included cell adhesion, cytoskeleton, DNA damage and protein folding/stress response. These data support prior studies that have reported these DNA damage and repair mechanisms and cytoskeleton processes as targets for PAH toxicity to the lung and airway epithelium in vivo (Marston, Pereira et al. 2001, Courter, Musafia-Jeknic et al. 2007, Courter, Luch et al. 2008, Siddens, Larkin et al. 2012, Banni, Sforzini et al. 2017). In particular, processes associated with barrier integrity were disrupted by exposure to BAP and PAHs in vitro in HepG2 and bronchial epithelium (McGarry, Charles et al. 2002, Oesterling, Toborek et al. 2008, Song, Kim et al. 2011, Chang, Siddens et al. 2019). PAH-induced disruption of the epithelial barrier and cell adhesion processes can lead to dysregulated inflammation and oxidative stress (Devalia, Bayram et al. 1997, Schamberger, Mise et al. 2014). Elevated oxidative stress and proinflammatory cytokines, in turn, disrupt tight junction function leading to increased airway inflammation (Coyne, Vanhook et al. 2002). Our BMD modelling results support in vitro and in vivo assay findings that cell adhesion, cytoskeleton, DNA damage, and stress response genes likely contributing modes of BAP toxicity and are among the most sensitive gene pathways to BAP-induced gene expression changes.
In addition, transcriptomic dose-response modeling allows for the direct comparison of gene sets most sensitive to BAP regulation to those identified as most predictive of PAH carcinogenicity in HBEC. Current EPA guidelines for PAH mixture carcinogenic risk assessment rely on comparing PAHs to BAP as a reference. A key assumption of this approach is that mechanisms of BAP carcinogenesis are representative of other carcinogenic PAHs However, there exist a wide range of known and proposed MOAs for PAHs suggesting that not all PAHs function through common mechanisms compared to BAP (Incardona, Day et al. 2006, Billiard, Meyer et al. 2007, Tilton, Siddens et al. 2015, Chang, Siddens et al. 2019). Our BMD modeling data support the hypothesis that PAHs can function through different mechanisms than BAP to contribute to carcinogenicity. In this study, the pathway gene sets most sensitive to BAP dose-response regulation were overall dissimilar to the high-performing gene set classifiers for PAH carcinogenicity. While the cell cycle category commonly found between the high-performing gene-set classifiers (cell cycle G2-M), and BAP (cell cycle S phase and G1-S regulation), the exact gene sets that performed best in classification did not rank among the top ten BAP-sensitive gene sets. In addition, BMD analysis identified stress response (protein folding and ubiquitin proteolysis) and DNA damage among the most sensitive pathways for BAP regulation. The transcriptional BMD median threshold values are well within range of in vivo derived points of departure for inhalation-specific cancer development (Thyssen, Althoff et al. 1981). While the goal of this study was not to derive points of departure for BAP, these results serve as an example of how toxicogenomics data can be modeled to support human health risk assessment.
5.0. Conclusion
Accurate carcinogenic assessment of PAHs and PAH mixtures has remained a challenge in toxicology and risk assessment. These studies show that chemical-gene biosignatures can be utilized to classify PAHs by carcinogenic hazard using in vitro models and inform potential mechanisms of action for PAHs in human bronchial epithelium. In addition, transcriptional BMD analysis show that processes most sensitive to BAP dose-response regulation are not necessarily the same as those that are most predictive of carcinogenic potential. These studies support the use of systems biology data collected from in vitro models for human health risk assessment. These studies support the use of systems biology data for use in human health risk assessments and to improve understanding of mechanisms associated with chemical toxicity and adverse health outcomes. Although the utilization of toxicogenomics in chemical carcinogenic hazard evaluation has developed significantly in the past decade, there have remained gaps in our understanding of PAH chemical and mixture effects in human systems. Overall, our findings support usage of organotypic human tissue cultures, implementation of toxicogenomics in cancer hazard evaluation, and a need for further studies to compare the applicability of BAP as a reference carcinogen for PAH mixture cancer risk assessment.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences Superfund Basic Research Program, National Institute of Health, P42 ES016465, by Public Health Service grant T32ES07060, and by a grant from the Center for Alternatives to Animal Testing, Johns Hopkins University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests
The authors declare that there are no conflicts of interest.
References
- Abdel-Shafy HI and Mansour MSM (2016). “A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation.” Egyptian Journal of Petroleum 25(1): 107–123. [Google Scholar]
- An J, Yin L, Shang Y, Zhong Y, Zhang X, Wu M, Yu Z, Sheng G, Fu J and Huang Y (2011). “The combined effects of BDE47 and BaP on oxidatively generated DNA damage in L02 cells and the possible molecular mechanism.” Mutat Res 721(2): 192–198. [DOI] [PubMed] [Google Scholar]
- ATSDR. (2017). “ATSDR. ATSDR’s Substance Priority List: Agency for Toxic Substances and Disease Registry.” Retrieved 11/18/2018, from https://www.atsdr.cdc.gov/spl/#2017spl.
- Baird WM and Mahadevan B (2004). “The uses of carcinogen-DNA adduct measurement in establishing mechanisms of mutagenesis and in chemoprevention.” Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 547(1): 1–4. [DOI] [PubMed] [Google Scholar]
- Banni M, Sforzini S, Arlt VM, Barranger A, Dallas LJ, Oliveri C, Aminot Y, Pacchioni B, Millino C, Lanfranchi G, Readman JW, Moore MN, Viarengo A and Jha AN (2017). “Assessing the impact of Benzo[a]pyrene on Marine Mussels: Application of a novel targeted low density microarray complementing classical biomarker responses.” PLoS One 12(6): e0178460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley N, Stratton KG and Webb-Robertson BJ (2010). “VIBE 2.0: visual integration for bayesian evaluation.” Bioinformatics 26(2): 280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Baan RA, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Guha N, Loomis D and Straif K (2012). “Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes.” The Lancet Oncology 13(7): 663–664. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV and Di Giulio RT (2007). “Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment.” Toxicological Sciences 105(1): 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgersdotter A, Sandberg R and Ernberg I (2005). “Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems.” Semin Cancer Biol 15(5): 405–412. [DOI] [PubMed] [Google Scholar]
- Bláha L, Kapplová P, Vondráček J, Upham B and Machala M (2002). “Inhibition of Gap-Junctional Intercellular Communication by Environmentally Occurring Polycyclic Aromatic Hydrocarbons.” Toxicological Sciences 65(1): 43–51. [DOI] [PubMed] [Google Scholar]
- Burczynski ME, McMillian M, Ciervo J, Li L, Parker JB, Dunn RT II, Hicken S, Farr S and Johnson MD (2000). “Toxicogenomics-Based Discrimination of Toxic Mechanism in HepG2 Human Hepatoma Cells.” Toxicological Sciences 58(2): 399–415. [DOI] [PubMed] [Google Scholar]
- Chang Y, Siddens LK, Heine LK, Sampson DA, Yu Z, Fischer KA, Löhr CV and Tilton SC (2019). “Comparative mechanisms of PAH toxicity by benzo[a]pyrene and dibenzo[def,p]chrysene in primary human bronchial epithelial cells cultured at air-liquid interface.” Toxicology and Applied Pharmacology 379: 114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courter LA, Luch A, Musafia-Jeknic T, Arlt VM, Fischer K, Bildfell R, Pereira C, Phillips DH, Poirier MC and Baird WM (2008). “The influence of diesel exhaust on polycyclic aromatic hydrocarbon-induced DNA damage, gene expression, and tumor initiation in Sencar mice in vivo.” Cancer Lett 265(1): 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courter LA, Musafia-Jeknic T, Fischer K, Bildfell R, Giovanini J, Pereira C and Baird WM (2007). “Urban dust particulate matter alters PAH-induced carcinogenesis by inhibition of CYP1A1 and CYP1B1.” Toxicol Sci 95(1): 63–73. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC and Johnson LG (2002). “Regulation of airway tight junctions by proinflammatory cytokines.” Mol Biol Cell 13(9): 3218–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalia JL, Bayram H, Rusznak C, Calderón M, Sapsford RJ, Abdelaziz MA, Wang J and Davies RJ (1997). “Mechanisms of pollution-induced airway disease: in vitro studies in the upper and lower airways.” Allergy 52(38 Suppl): 45–51; discussion 57–48. [DOI] [PubMed] [Google Scholar]
- EPA US (2010). “Development of a relative potency factor (RPF) approach for polycyclic aromatic hydrocarbon (PAH) mixtures.” EPA/635/R-08/012A, from http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=194584.
- Farmahin R, Williams A, Kuo B, Chepelev NL, Thomas RS, Barton-Maclaren TS, Curran IH, Nong A, Wade MG and Yauk CL (2017). “Recommended approaches in the application of toxicogenomics to derive points of departure for chemical risk assessment.” Arch Toxicol 91(5): 2045–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann E, Kramer S, Pfahringer B and Helma C (2001). “Data quality in predictive toxicology: reproducibility of rodent carcinogenicity experiments.” Environmental health perspectives 109(5): 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X and Mei N (2018). “Benchmark Dose Modeling of In Vitro Genotoxicity Data: a Reanalysis.” Toxicol Res 34(4): 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusenleitner D, Auerbach SS, Melia T, Gomez HF, Sherr DH and Monti S (2014). “Genomic models of short-term exposure accurately predict long-term chemical carcinogenicity and identify putative mechanisms of action.” PLoS One 9(7): e102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzalova K, Rossner P and Sram RJ (2010). “Oxidative damage induced by carcinogenic polycyclic aromatic hydrocarbons and organic extracts from urban air particulate matter.” Mutation Research/Genetic Toxicology and Environmental Mutagenesis 696(2): 114–121. [DOI] [PubMed] [Google Scholar]
- IARC (2010). “Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures.” IARC Monographs and the Evaluation of Carcinogenic Risks to Humans 92. [PMC free article] [PubMed] [Google Scholar]
- IARC (2013). “Diesel and Gasoline Engine Exhausts and Some Nitroarenes.” IARC Monographs and the Evaluation of Carcinogenic Risks to Humans 105. [Google Scholar]
- IARC (2014). “Diesel and gasoline engine exhausts and some nitrarenes.” IARC MONOGRAPHS ON THE EVALUATION OF CARCINOGENIC RISKS TO HUMANS 105. [PMC free article] [PubMed] [Google Scholar]
- IARC (2015). “Outdoor Air Pollution.” IARC Monographs and the Evaluation of Carcinogenic Risks to Humans 109. [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK and Scholz NL (2006). “Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism.” Toxicol Appl Pharmacol 217(3): 308–321. [DOI] [PubMed] [Google Scholar]
- Jarvis IW, Dreij K, Mattsson A, Jernstrom B and Stenius U (2014). “Interactions between polycyclic aromatic hydrocarbons in complex mixtures and implications for cancer risk assessment.” Toxicology 321: 27–39. [DOI] [PubMed] [Google Scholar]
- Kim J-Y, Kwon J, Kim JE, Koh WS, Chung M-K, Yoon S, Song CW and Lee M (2005). “Identification of potential biomarkers of genotoxicity and carcinogenicity in L5178Y mouse lymphoma cells by cDNA microarray analysis.” Environmental and Molecular Mutagenesis 45(1): 80–89. [DOI] [PubMed] [Google Scholar]
- Kimlin L, Kassis J and Virador V (2013). “3D in vitro tissue models and their potential for drug screening.” Expert Opin Drug Discov 8(12): 1455–1466. [DOI] [PubMed] [Google Scholar]
- Kopec AK, Burgoon LD, Ibrahim-Aibo D, Burg AR, Lee AW, Tashiro C, Potter D, Sharratt B, Harkema JR, Rowlands JC, Budinsky RA and Zacharewski TR (2010). “Automated dose-response analysis and comparative toxicogenomic evaluation of the hepatic effects elicited by TCDD, TCDF, and PCB126 in C57BL/6 mice.” Toxicol Sci 118(1): 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AK, D’Souza ML, Mets BD, Burgoon LD, Reese SE, Archer KJ, Potter D, Tashiro C, Sharratt B, Harkema JR and Zacharewski TR (2011). “Non-additive hepatic gene expression elicited by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153) co-treatment in C57BL/6 mice.” Toxicol Appl Pharmacol 256(2): 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer V, Maskova J, Zraly Z, Neca J, Simeckova P, Vondracek J and Machala M (2008). “Estrogenic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature Wistar rats.” Toxicol Lett 180(3): 212–221. [DOI] [PubMed] [Google Scholar]
- Labib S, Williams A, Guo CH, Leingartner K, Arlt VM, Schmeiser HH, Yauk CL, White PA and Halappanavar S (2016). “Comparative transcriptomic analyses to scrutinize the assumption that genotoxic PAHs exert effects via a common mode of action.” Arch Toxicol 90(10): 2461–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib S, Williams A, Kuo B, Yauk CL, White PA and Halappanavar S (2017). “A framework for the use of single-chemical transcriptomics data in predicting the hazards associated with complex mixtures of polycyclic aromatic hydrocarbons.” Archives of toxicology 91(7): 2599–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yum YN, Kim SC, Kim Y, Lim J, Lee WJ, Koo KH, Kim JH, Kim JE, Lee WS, Sohn S, Park SN, Park JH, Lee J and Kwon SW (2013). “Distinguishing between genotoxic and non-genotoxic hepatocarcinogens by gene expression profiling and bioinformatic pathway analysis.” Scientific Reports 3(1): 2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK and Shi W (2014). “featureCounts: an efficient general purpose program for assigning sequence reads to genomic features.” Bioinformatics 30(7): 923–930. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W and Anders S (2014). “Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2.” Genome Biol 15(12): 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magkoufopoulou C, Claessen SMH, Tsamou M, Jennen DGJ, Kleinjans JCS and van Delft JHM (2012). “A transcriptomics-based in vitro assay for predicting chemical genotoxicity in vivo.” Carcinogenesis 33(7): 1421–1429. [DOI] [PubMed] [Google Scholar]
- Marston CP, Pereira C, Ferguson J, Fischer K, Hedstrom O, Dashwood WM and Baird WM (2001). “Effect of a complex environmental mixture from coal tar containing polycyclic aromatic hydrocarbons (PAH) on the tumor initiation, PAH-DNA binding and metabolic activation of carcinogenic PAH in mouse epidermis.” Carcinogenesis 22(7): 1077–1086. [DOI] [PubMed] [Google Scholar]
- Mathijs K, Brauers KJJ, Jennen DGJ, Boorsma A, van Herwijnen MHM, Gottschalk RWH, Kleinjans JCS and van Delft JHM (2009). “Discrimination for Genotoxic and Nongenotoxic Carcinogens by Gene Expression Profiling in Primary Mouse Hepatocytes Improves with Exposure Time.” Toxicological Sciences 112(2): 374–384. [DOI] [PubMed] [Google Scholar]
- McGarry MA, Charles GD, Medrano T, Bubb MR, Grant MB, Campbell-Thompson M and Shiverick KT (2002). “Benzo(a)pyrene, but not 2,3,7,8-tetrachlorodibenzo-p-dioxin, alters cell adhesion proteins in human uterine RL95–2 cells.” Biochem Biophys Res Commun 294(1): 101–107. [DOI] [PubMed] [Google Scholar]
- McKim JM Jr. (2010). “Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance.” Comb Chem High Throughput Screen 13(2): 188–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezencev R and Auerbach SS (2020). “The sensitivity of transcriptomics BMD modeling to the methods used for microarray data normalization.” PLoS One 15(5): e0232955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolsky Y, Kirillov E, Zuev R, Rakhmatulin E and Nikolskaya T (2009). “Functional analysis of OMICs data and small molecule compounds in an integrated “knowledge-based” platform.” Methods Mol Biol 563: 177–196. [DOI] [PubMed] [Google Scholar]
- Oesterling E, Toborek M and Hennig B (2008). “Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway.” Toxicol Appl Pharmacol 232(2): 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, Troxel AB, Harvey RG and Penning TM (2006). “Polycyclic Aromatic Hydrocarbon (PAH) o-Quinones Produced by the Aldo-Keto-Reductases (AKRs) Generate Abasic Sites, Oxidized Pyrimidines, and 8-Oxo-dGuo via Reactive Oxygen Species.” Chemical Research in Toxicology 19(5): 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Hung C-F, McCoull KD, Palackal NT and Tsuruda LS (1999). “Dihydrodiol Dehydrogenases and Polycyclic Aromatic Hydrocarbon Activation: Generation of Reactive and Redox Active o-Quinones.” Chemical Research in Toxicology 12(1): 1–18. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Svoboda DL, Tandon A, Patel S, Sedykh A, Mav D, Kuo B, Yauk CL, Yang L, Thomas RS, Gift JS, Davis JA, Olszyk L, Merrick BA, Paules RS, Parham F, Saddler T, Shah RR and Auerbach SS (2019). “BMDExpress 2: enhanced transcriptomic dose-response analysis workflow.” Bioinformatics (Oxford, England) 35(10): 1780–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plíšková M, Vondráček J, Vojtěšek B, Kozubík A and Machala M (2004). “Deregulation of Cell Proliferation by Polycyclic Aromatic Hydrocarbons in Human Breast Carcinoma MCF-7 Cells Reflects Both Genotoxic and Nongenotoxic Events.” Toxicological Sciences 83(2): 246–256. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Archibong A, Hood DB, Guo Z and Loganathan BG (2011). Global environmental distribution and human health effects of polycyclic aromatic hydrocarbons. Boca Raton, FL, CRC Press. [Google Scholar]
- Rengarajan T, Rajendran P, Nandakumar N, Lokeshkumar B, Rajendran P and Nishigaki I (2015). “Exposure to polycyclic aromatic hydrocarbons with special focus on cancer.” Asian Pacific Journal of Tropical Biomedicine 5(3): 182–189. [Google Scholar]
- Ross JA, Nelson GB, Wilson KH, Rabinowitz JR, Galati A, Stoner GD, Nesnow S and Mass MJ (1995). “Adenomas induced by polycyclic aromatic hydrocarbons in strain A/J mouse lung correlate with time-integrated DNA adduct levels.” Cancer Res 55(5): 1039–1044. [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V and Quackenbush J (2003). “TM4: a free, open-source system for microarray data management and analysis.” Biotechniques 34(2): 374–378. [DOI] [PubMed] [Google Scholar]
- Schamberger AC, Mise N, Jia J, Genoyer E, Yildirim A, Meiners S and Eickelberg O (2014). “Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-β.” Am J Respir Cell Mol Biol 50(6): 1040–1052. [DOI] [PubMed] [Google Scholar]
- Shao K and Shapiro AJ (2018). “A Web-Based System for Bayesian Benchmark Dose Estimation.” Environ Health Perspect 126(1): 017002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddens LK, Bunde KL, Harper TA Jr., McQuistan TJ, Lohr CV, Bramer LM, Waters KM, Tilton SC, Krueger SK, Williams DE and Baird WM (2015). “Cytochrome P450 1b1 in polycyclic aromatic hydrocarbon (PAH)-induced skin carcinogenesis: Tumorigenicity of individual PAHs and coal-tar extract, DNA adduction and expression of select genes in the Cyp1b1 knockout mouse.” Toxicol Appl Pharmacol 287(2): 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddens LK, Larkin A, Krueger SK, Bradfield CA, Waters KM, Tilton SC, Pereira CB, Lohr CV, Arlt VM, Phillips DH, Williams DE and Baird WM (2012). “Polycyclic aromatic hydrocarbons as skin carcinogens: comparison of benzo[a]pyrene, dibenzo[def,p]chrysene and three environmental mixtures in the FVB/N mouse.” Toxicol Appl Pharmacol 264(3): 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers CK, Shanle EK, Bradfield CA and Xu W (2013). “Differential action of monohydroxylated polycyclic aromatic hydrocarbons with estrogen receptors α and β.” Toxicological sciences : an official journal of the Society of Toxicology 132(2): 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MK, Kim YJ, Song M, Choi HS, Park YK and Ryu JC (2011). “Polycyclic aromatic hydrocarbons induce migration in human hepatocellular carcinoma cells (HepG2) through reactive oxygen species-mediated p38 MAPK signal transduction.” Cancer Sci 102(9): 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MK, Song M, Choi HS, Kim YJ, Park YK and Ryu JC (2012). “Identification of molecular signatures predicting the carcinogenicity of polycyclic aromatic hydrocarbons (PAHs).” Toxicol Lett 212(1): 18–28. [DOI] [PubMed] [Google Scholar]
- Thyssen J, Althoff J, Kimmerle G and Mohr U (1981). “Inhalation studies with benzo[a]pyrene in Syrian golden hamsters.” J Natl Cancer Inst 66(3): 575–577. [PubMed] [Google Scholar]
- Tilton SC, Siddens LK, Krueger SK, Larkin AJ, Lohr CV, Williams DE, Baird WM and Waters KM (2015). “Mechanism-Based Classification of PAH Mixtures to Predict Carcinogenic Potential.” Toxicol Sci 146(1): 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topinka J, Schwarz LR, Kiefer F, Wiebel FJ, Gajdoš O, Vidová P, Dobiáš L, Fried M, Šrám RJ and Wolff T (1998). “DNA adduct formation in mammalian cell cultures by polycyclic aromatic hydrocarbons (PAH) and nitro-PAH in coke oven emission extract.” Mutation Research/Genetic Toxicology and Environmental Mutagenesis 419(1): 91–105. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L and Salzberg SL (2009). “TopHat: discovering splice junctions with RNA-Seq.” Bioinformatics 25(9): 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L (2012). “Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks.” Nat Protoc 7(3): 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter L (2010). “Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation.” Nat Biotechnol 28(5): 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Masten SJ, Lockwood BR and Trosko JE (1994). “Nongenotoxic effects of polycyclic aromatic hydrocarbons and their oxygenation by-products on the intercellular communication of rat liver epithelial cells.” Fundam Appl Toxicol 23(3): 470–475. [DOI] [PubMed] [Google Scholar]
- van Delft JHM, van Agen E, van Breda SGJ, Herwijnen MH, Staal YCM and Kleinjans JCS (2004). “Discrimination of genotoxic from non-genotoxic carcinogens by gene expression profiling.” Carcinogenesis 25(7): 1265–1276. [DOI] [PubMed] [Google Scholar]
- van Delft JHM, van Agen E, van Breda SGJ, Herwijnen MH, Staal YCM and Kleinjans JCS (2005). “Comparison of supervised clustering methods to discriminate genotoxic from non-genotoxic carcinogens by gene expression profiling.” Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 575(1): 17–33. [DOI] [PubMed] [Google Scholar]
- Vondráček J, Kozubík A and Machala M (2002). “Modulation of Estrogen Receptor-Dependent Reporter Construct Activation and G0/G1–S-Phase Transition by Polycyclic Aromatic Hydrocarbons in Human Breast Carcinoma MCF-7 Cells.” Toxicological Sciences 70(2): 193–201. [DOI] [PubMed] [Google Scholar]
- Wang J, Webb-Robertson BJ, Matzke MM, Varnum SM, Brown JN, Riensche RM, Adkins JN, Jacobs JM, Hoidal JR, Scholand MB, Pounds JG, Blackburn MR, Rodland KD and McDermott JE (2013). “A semiautomated framework for integrating expert knowledge into disease marker identification.” Dis Markers 35(5): 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Suzuki T, Natsume M, Nakajima M, Narumi K, Hamada S, Sakuma T, Koeda A, Oshida K, Miyamoto Y, Maeda A, Hirayama M, Sanada H, Honda H, Ohyama W, Okada E, Fujiishi Y, Sutou S, Tadakuma A, Ishikawa Y, Kido M, Minamiguchi R, Hanahara I and Furihata C (2012). “Discrimination of genotoxic and nongenotoxic hepatocarcinogens by statistical analysis based on gene expression profiling in the mouse liver as determined by quantitative real-time PCR.” Mutation Research/Genetic Toxicology and Environmental Mutagenesis 747(2): 164–175. [DOI] [PubMed] [Google Scholar]
- Webb-Robertson B-JM, Metz TO, Waters KM, Zhang Q and Rewers M (2017). Bayesian Posterior Integration for Classification of Mass Spectrometry Data Statistical Analysis of Proteomics, Metabolomics, and Lipidomics Data Using Mass Spectrometry. Datta S and Mertens BJA. Cham, Springer International Publishing: 203–211. [Google Scholar]
- Williams A, Buick JK, Moffat I, Swartz CD, Recio L, Hyduke DR, Li H-H, Fornace AJ, Aubrecht J and Yauk CL (2015). “A predictive toxicogenomics signature to classify genotoxic versus non-genotoxic chemicals in human TK6 cells.” Data in Brief 5: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Allen BC and Thomas RS (2007). “BMDExpress: a software tool for the benchmark dose analyses of genomic data.” BMC Genomics 8(1): 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


