Abstract
Objective.
Patients with dermatomyositis (DM) and polymyositis (PM) have reduced muscle endurance. The aim of this study was to streamline the Functional Index-2 (FI-2) by developing the Functional Index-3 (FI-3) and to evaluate its measurement properties, content and construct validity, intra- and inter-rater reliability.
Methods.
A data set of the previously performed and validated FI-2 (n=63) was analyzed for internal redundancy, floor and ceiling effects. The content of the FI-2 was revised into the FI-3. Construct validity and intra-rater reliability of FI-3 were tested on 43 DM and PM patients at two rheumatology centers. Inter-rater reliability was tested in 25 patients. The construct validity was compared with the Myositis Activities Profile (MAP), Health Assessment Questionnaire (HAQ), and Borg CR-10 using Spearman’s correlation coefficient.
Results.
Spearman’s correlation coefficients of 63 patients performing FI-3 revealed moderate to high correlations between shoulder flexion and hip flexion tasks and similar correlations with MAP and HAQ scores; there were lower correlations for neck flexion task. All FI-3 tasks had very low to moderate correlations with the Borg scale. Intra-class correlation coefficients (ICC) of FI-3 tasks for intra-rater reliability (n = 25) were moderate to good (range: 0.88-0.96). ICC of FI-3 tasks for inter-rater reliability (n = 17) were fair to good (range: 0.83-0.96).
Conclusion.
The FI-3 is an efficient and valid method for clinically assessing muscle endurance in DM and PM patients. FI-3’s construct validity is supported by the significant correlations between functional tasks and the MAP, HAQ, and Borg CR-10 scores.
Keywords: Functional index-2, Outcome Measures, Idiopathic Inflammatory myopathies, muscle endurance
The idiopathic inflammatory myopathies (IIM), dermatomyositis (DM) and polymyositis (PM), are inflammatory muscle diseases predominantly affecting the proximal skeletal muscles causing muscle weakness, exercise intolerance, and functional disability.1-3 Muscle inflammation leads to muscle damage, scarring, and chronic muscle atrophy with a lower proportion of type I muscle fibers responsible for muscle endurance relative to fast-twitch type II muscle fibers over time.4,5 The clinical course is usually characterized by periods of remission and relapse; hence, clinical assessments of muscle strength and function must have the sensitivity to discriminate changes in disease activity to guide treatment decisions.6-8
There are few valid and reliable tests available that measure muscle endurance as an assessment of disease activity and, by extension, physical function representative of the ability to perform activities of daily living (ADLs).6,9 The International Myositis Assessment and Clinical Studies Group (IMACS) recommends a 6 domain disease activity core set in clinical trials for the assessment of IIM patients involving physician and patient global disease activity, muscle strength testing by the manual muscle test (MMT), activity limitation by the Stanford Health Assessment Questionnaire (HAQ), muscle enzyme levels, and extra-skeletal involvement and physical function.10,11 The MMT is a widely used measure of muscle strength, but it does not reflect muscle endurance or correlate with the ability to perform ADLs.6,8,9 Indeed, patients with adult PM and DM are more limited in muscle endurance when assessed by the FI-2 compared to muscle strength assessed by the MMT8; the FI-2 also seems to reflect self-reported physical function.12
Tools to measure functional impairments and muscle endurance have been developed to clinically assess IIM patients. The Adult Myopathy Assessment Tool holds promise as a 13 item performance test designed to measure both physical function and endurance that requires no specialized equipment to perform but requires 20-30 minutes to complete, and it has been validated with strong intra-rater and inter-rater reliability scores.13 The Functional Index in myositis (FI) was the first outcome measure developed for assessing functional impairment in DM and PM patients by testing 14 repetitive tasks of selected muscle groups in both upper and lower limbs; this test was effective in discriminating patients from healthy individuals.14 However, the FI was time-consuming with observed floor and ceiling effects in patients with mild to moderate impairment.15 The ceiling effect occurs when patients of mild-to-moderate impairment cluster to the highest level of the measured outcome and therefore achieve the best score for the instrument. When a ceiling effect exists, any score beyond the upper limit cannot be measured; conversely, when an instrument has a floor effect, any score beyond the lowest limit cannot be measured.16 Hence, the Functional Index-2 (FI-2) was developed as a revision of the FI at the Karolinska University Hospital in Sweden and it has been partially validated in patients with adult patients with DM and PM.17 It involves testing7 repetitive tasks performed either bilaterally or unilaterally with a metronome to standardize movement pace. The FI-2 is useful without ceiling or floor effects and is well-tolerated by patients at varying stages of functional impairment. A prospective 7-week exercise study revealed the FI-2 to be sensitive to treatment outcomes with solid inter-and intra-rater reliability.18 The FI-2 demonstrates good to excellent inter-rater reliability (ICC 0.86-0.99) and good construct validity, but it requires a maximum of 33 minutes to complete and the concern for internal redundancy arises for some tasks such as shoulder abduction and step test.17,18 The goal of revising the FI-2 to the FI-3 was to shorten the administration time of a functional assessment tool and to derive a total score (summation of the individual tasks divided by 3) of the instrument to make it more useful to clinicians to follow patient progress.
Therefore, we revised the FI-2 into the Functional Index-3 (FI-3) as a clinical assessment tool to measure muscle endurance in patients with DM and PM for the purpose of incorporating into clinical practice and research trials in IIM. The objectives of our study were to validate the measurement properties of content and construct validity as well as intra- and interrater reliability of FI-3. Our prediction was that the FI-3 would correlate moderately to strongly to measures of physical function and perceived exertion. Our second hypothesis was that the FI-3 neck flexion would have the lowest correlation to the physical function measures as the neck muscles may not be directly involved in the daily activities included in the MAP and the HAQ.
MATERIALS AND METHODS
The study was approved by the Mayo Clinic institutional review and ethics board at Mayo Clinic Rochester, Minnesota (12-007485) and the local ethics committee at Karolinska Institutet in Stockholm, Sweden. All subjects gave informed written consent prior to participation.
Study design
This was a cross-sectional study of a cohort of patients with DM or PM at varying stages of disease (active or in remission). They were recruited from the rheumatology clinics at the Karolinska University Hospital and Mayo Clinic Rochester.
Inclusion and exclusion criteria
Inclusion criteria were patients aged 18 or over fulfilling Bohan and Peter criteria for DM or PM (at least 3 of 5 criteria needed for a probable diagnosis).1 Exclusion criteria were a diagnosis of inclusion body myositis, juvenile DM, severe pulmonary hypertension, acute fractures, or severe osteoporosis.
Patients
Three cohorts of patients were included in this study. Cohort 1 represented all patients performing the FI-2 during their early follow-up at the Karolinska University Hospital during 2006 (n=63) (site 1). Data were retrieved from the Swedish Myositis Register in 2010. Cohort 2 represented patients recruited at a second institution, Mayo Clinic Rochester, during 2010-2012 performing the FI-3 at site 2. Cohort 3 were patients performing FI-3 at site 1. These patients were seen on the same day as part of their scheduled follow-up visits.
Methods
The study was performed using the original FI-2 as the foundation for revision into the FI-3. The original FI-2 involves repetitive movements in 7 muscle groups to determine muscle endurance. It is made up of the following 7 tasks testing dynamic repetitive muscle function: shoulder flexion with 1 kg weight cuff on wrists, shoulder abduction, neck flexion/head lift, hip flexion, step test, heel lift, and toe lift. The patient performed many repetitions possible to a maximum of three minutes per task (60-120 repetitions) with a metronome (40 beats/minute generating 20 repetitions) to standardize the pace. A maximum of 3 minutes per task was allotted to reach the maximal number of repetitions. Patients did 5 learning repetitions to enhance performance. The entire test was performed in one sitting.
The study investigators met to scrutinize the goals of the tasks of the FI-2 a priori and then performed separate analysis of tasks for internal redundancy. In an effort to avoid bias, informal discussions among the authors of the FI-2 relating to the goals of the FI-2 occurred for advice on the development of the FI-3. Tasks that were felt to be essential to the functional assessment of DM/PM patients were the forward flexion, neck flexion and hip flexion tasks.19 The step test task was removed due to including non-essential elements of cardiovascular stress, balance and coordination. The heel lift and toe lift tasks were removed as they test distal muscle function which may not be limited in patients with PM and DM. The total score (summation of the individual tasks divided by 3) was developed for ease of use. Table 1 illustrates the differences between the FI-2 and the FI-3.
Table 1:
Characteristics of FI-2 and FI-3
| Characteristics | FI-2 | FI-3 |
|---|---|---|
| Total number of tasks | 7 | 3 |
| Performed unilaterally or bilaterally | yes | yes |
| Tasks involved | Shoulder flexion, Shoulder abduction. Neck flexion, Hip flexion, Step test, Heel lift, Toe lift |
Shoulder flexion, Neck flexion, Hip flexion |
| Pace standardized with metronome (40 or 80 beats per minute) | yes | yes |
| Time per task | 3 minutes | 3 minutes |
| Total time required to perform all tasks unilaterally/bilaterally | 21/33 minutes | 9/15 minutes |
| Repetitions per task | 60-120 | 60 |
| Total score derived | No | Yes |
For determining the FI-3 validation, study investigators at both sites participated in a 1-hour training session discussing the process of evaluating patients using the revised FI-2 and the scoring method prior to beginning the data collection (Appendices A,B in supplement). Scoring was based on the number of correctly performed repetitions with a score varying from 0-60. A score of 60 in a task reflects normal muscle endurance. The total score of the FI-3 was calculated on one side as right shoulder flexion, right hip flexion and neck flexion and divided by 3.
For assessing the inter-rater reliability, a total of 17 subjects performed the FI-3 twice at the same visit with a resting span of 30-60 minutes. The test was performed randomly led by assessor 1 and assessor 2 at each site. In the first session, subjects performed bilateral shoulder flexion (sequentially), neck/flexion/head lift, bilateral hip flexion (sequentially). The Borg CR-10 scale was filled out after each task. The Borg CR-10 is a category scale to rate perceived muscular exertion ranging from 0 = nothing at all to 10 = very strong.20 After completing first session, patients filled out the Myositis Activities Profile (MAP)21, 22 and the Health Assessments Questionnaire (HAQ)23,24.
The MAP is a myositis-specific questionnaire developed and validated in Sweden measuring limitations in daily life activities. Thirty-one items are scored on a 7-grade Likert scale from 1 to 7: 1 = no difficulty to perform and 7 = impossible to perform. The MAP has also has been validated in DM/PM patients in the United States.21,22 The HAQ is an arthritis-specific 20-item questionnaire assessing functional ability, and it is recommended for use also in myositis, including inflammatory myopathies following the effect of exercise interventions.23,24
For assessing the intra-rater reliability, a total of 29 subjects (Cohort 2) at site 2 and 14 patients (Cohort 3) at site 1 performed the FI-3 twice with a resting interval of 30-60 minutes by assessor 1 at site 2. At site 1 the FI-3 was performed twice within 4-7 days by the same assessor. After completing each task perceived exertion was again rated using the Borg CR-10 scale.
Statistical analysis
Descriptive data are presented using percentages, means, medians, standard deviations, and ranges with graphic depiction using box plots. Spearman’s correlation coefficient was used to assess internal redundancy (how all tasks correlate to each other) and internal consistency (how each task correlates to a sub-score of upper or lower extremities) of the FI-2. Correlation coefficients rs>0.90 were considered to indicate redundancy and rs<0.60 to indicate poor consistency. In analysis for construct validity, correlation coefficients of rs 0-0.25 were considered as no or very low, rs 0.25-0.49 as low, rs 0.50-0.69 as moderate, rs 0.70-0.89 as high and rs 0.90-10.00 as very high.25 Intra-class correlation coefficients (ICCs) were calculated for intra-rater and inter-rater reliability. ICCs < 0.75 indicate low to fair reliability and those >0.75 indicate good to excellent reliability. The reliability of the total score and error of measurement in all tasks were calculated. The level of significance was accepted as p < 0.05 for all comparisons. All data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Content validity
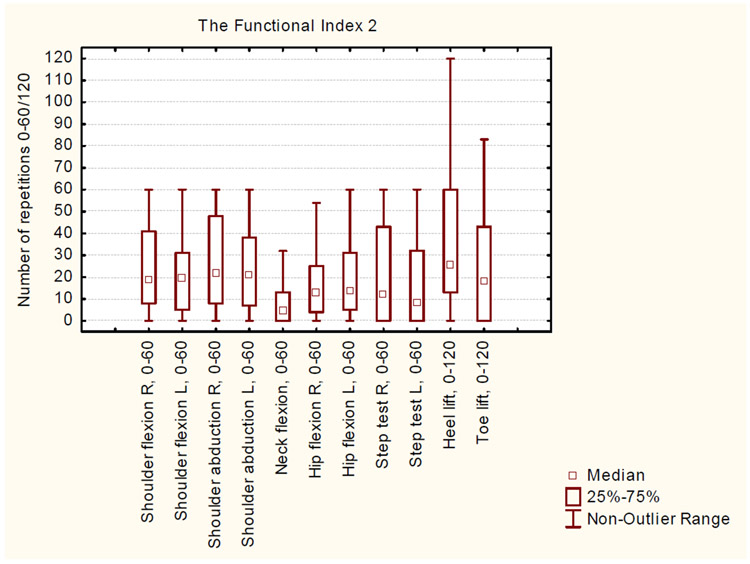
The FI-2 tasks were scrutinized and the range of FI-2 mean scores was 8.2 ± 10.8 to 40.2 ± 29.5. No ceiling effects (defined as median values of 20-50 percentile of total variation of values) were observed in any tasks. Correlation coefficients for analysis of internal redundancy for FI-2 tasks in the upper extremities varied between rs 0.89-0.94, with the highest correlations between shoulder flexion and shoulder abduction. The shoulder flexion task ranged between 0-60 with no tendency towards ceiling effect while the median shoulder abduction equaled the maximal number of repetitions 60; therefore, the shoulder abduction latter task was removed due to internal redundancy. Coefficients for analysis of tasks in the lower extremities varied between rs 0.63-0.84. The cut-off for exclusion due to poor internal redundancy was rs <0.60 (Figure 1).
Figure 1.
Descriptive data of 63 subjects who participated in the Functional Index 2 tests. Maximal FI-2 score is 60 for all tasks, except the heel lift and toe lift tasks (120 is maximal).
Clinical characteristics of patients in cohorts 2 and 3
Twenty-nine patients comprised cohort 2; 14 patients made up cohort 3. Table 2 describes the clinical characteristics of these patients. In cohort 2, the mean age was 57.9 (SD 11.2) years in cohort 2 and 66% were female. In cohort 3, the mean age was 60.2 (SD 13.5) years and 71% were female. In cohort 2, 52% had DM; 27% had PM; and 21% had anti-synthetase syndrome. Cohort 3 included 21% DM and 79% PM patients. The median creatine kinase level was 102 U/L (range: 43-850 U/L) in cohort 2 and 97 (range: 47-1470) U/L in cohort 3. The median myositis disease duration was 5 years in cohort 2 and 11.5 years in cohort 3. The median MAP score in session 1 was 2 (range: 1, 5). The median HAQ score in session 1 was 0.6 (range: 0.0, 2.0). Other than disease duration, the patient characteristics were similar in both cohorts, so the cohorts were combined for further analyses.
Table 2:
Demographic data of 29 patients in Cohort 2 and 14 patients in Cohort 3
| Clinical characteristics* | Cohort 2 (n=29) |
Cohort 3 (n= 14) |
|---|---|---|
| Age, years | 60 (31, 76) | 61.5 (43, 86) |
| Mean, SD | 57.9 (11.2) | 62.0 (13.5) |
| Myositis duration, years | 5 (1, 22) | 11.5 (5, 43) |
| Mean, SD | 6.1 (5.3) | 15.4 (10.9) |
| Creatine kinase (U/L) | 102 (43, 850) | 97 (47, 1470) |
| Sex, female | 19 (66%) | 10 (71%) |
| Diagnosis | ||
| PM | 6 (21%) | 11 (79%) |
| DM | 15 (52%) | 3 (21%) |
| Anti-synthetase syndrome | 8 (28%) | 0 (0%) |
| MAP score | 2 (1, 5) | 2 (1, 5) |
| HAQ score | 0.8 (0, 2) | 0.6 (0, 2) |
Construct validity
To measure construct validity, the correlations between FI-3 tasks (% maximum number of repetitions) and MAP scores (n=42), HAQ scores (n = 39), and Borg CR-10 scores (n=43) in session 1 were assessed (Table 3). The correlations between physical function and FI-3 total score were the following: MAP (−0.67, p < 0.001), HAQ (−0.72, p < 0.001). There were moderate to high correlations between shoulder flexion and hip flexion tasks and the MAP and the HAQ with lower correlations for the neck flexion task. All FI-3 tasks had very low to moderate correlations with the Borg scale.
Table 3.
Spearman correlations between MAP and HAQ scores and Borg CR-10 scores with FI-3 task scores (% of maximum number of repetitions) in session1 for 43 patients in cohorts 2 and 3.
| FI-3 task score | MAP score (n=42) | HAQ score (n=39) | Borg CR-10 scores (n=43) |
|||
|---|---|---|---|---|---|---|
| correlation | p-value | correlation | p-value | correlation | p-value | |
| Shoulder flexion right | −0.62 | <0.001 | −0.68 | <0.001 | −0.48 | 0.001 |
| Shoulder flexion left | −0.58 | <0.001 | −0.60 | <0.001 | −0.32 | 0.039 |
| Neck flexion | −0.38 | 0.011 | −0.43 | 0.006 | −0.30 | 0.049 |
| Hip flexion right | −0.70 | <0.001 | −0.76 | <0.001 | −0.21 | 0.16 |
| Hip flexion left | −0.61 | <0.001 | −0.68 | <0.001 | −0.10 | 0.52 |
| Total score | −0.67 | <0.001 | −0.72 | <0.001 | N/A | N/A |
Intra-rater reliability
Intra-rater reliability of the FI-3 tasks (% maximum number of repetitions) and Borg CR-10 scores was assessed in 25 subjects in cohorts 2 and 3 who were observed twice by the same rater (Table 4).
Table 4.
Intra-rater reliability of FI-3 task score (% of maximum number of repetitions) and Borg CR-10 scores for 25 patients in cohorts 2 and 3
| FI-3 tasks | Session 1 | Session2 | Difference | ICC (95% CI) | Measurement error |
|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | |||
| Shoulder flexion right | 100 (0, 100) | 100 (5, 100) | 0 (−28, 25) | 0.96 (0.92, 0.98) | 6.8 |
| Shoulder flexion left | 100 (8, 100) | 67 (7, 100) | 0 (−43, 11) | 0.92 (0.83, 0.97) | 9.3 |
| Neck flexion | 40 (0, 100) | 35 (0, 100) | 0 (−38, 20) | 0.94 (0.87, 0.97) | 9.0 |
| Hip flexion right | 50 (0, 100) | 33 (0, 100) | 0 (−57, 33) | 0.88 (0.74, 0.94) | 13.2 |
| Hip flexion left | 45 (0, 100) | 38 (0, 100) | 0 (−48, 40) | 0.93 (0.86, 0.97) | 10.0 |
| Total score | 69 (0, 100) | 62 (3, 100) | −1 (−32, 15) | 0.95 (0.89, 0.98) | 6.8 |
| Borg CR-10 scores | |||||
| Shoulder flexion right | 3 (0.5, 9) | 4 (0.5, 9) | 0 (−2, 4) | 0.76 (0.54, 0.89) | 1.0 |
| Shoulder flexion left | 4 (0.5, 9) | 4 (0.5, 9) | 0 (−2, 4) | 0.85 (0.68, 0.93) | 0.9 |
| Neck flexion | 5 (3, 10) | 5 (3, 9) | 0 (−4, 5) | 0.53 (0.17, 0.76) | 1.6 |
| Hip flexion right | 6 (3, 10) | 6 (0.5, 10) | 0 (−6, 2) | 0.72 (0.46, 0.86) | 1.2 |
| Hip flexion left | 6 (4, 10) | 7 (2, 10) | 0 (−6, 3) | 0.68 (0.38, 0.84) | 1.2 |
For FI-3, the ICC was 0.96 (95% confidence interval [CI]: 0.92, 0.98) for the right shoulder flexion task and 0.92 (95% CI: 0.83, 0.97) for the left shoulder flexion task. The ICC was 0.94 (95% CI: 0.87, 0.97) for the neck flexion task. The ICC was 0.88 (95% CI: 0.74, 0.94) for the right hip flexion task and 0.93 (95% CI: 0.86, 0.97) for the left hip flexion task. The measurement of error for each FI-3 task was calculated: right shoulder flexion (6.8); left shoulder flexion (9.3); neck flexion (9.0); right hip flexion (13.2); left hip flexion (10.0). The ICC for the total score was 0.95 (0.89-0.98).
Inter-rater reliability
To determine interrater reliability, 17 subjects in cohorts 2 and 3 were observed by 2 different assessors in session 1 and session 2. Table 5 shows the medians and ranges for each session and the ICC of the assessments. The measurement of error for each FI-3 task was calculated: right shoulder flexion (15.5); left shoulder flexion (13.9); neck flexion (14.1); right hip flexion (8.4); left hip flexion (7.4). The ICC for the total score was 0.93 (0.83, 0.98).
Table 5.
Inter-rater reliability of FI-3 task score (% of maximum number of repetitions) and Borg CR-10 scores for 17 patients in cohorts 2 and 3
| FI-3 tasks | Session 1 | Session2 | Difference | ICC (95% CI) | Measurement error |
|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | |||
| Shoulder flexion right | 83 (0, 100) | 72 (3, 100) | 0 (−73, 24) | 0.83 (0.59, 0.93) | 15.5 |
| Shoulder flexion left | 78 (8, 100) | 60 (7, 100) | 0 (−65, 9) | 0.83 (0.59, 0.94) | 13.9 |
| Neck flexion | 42 (0, 100) | 38 (0, 100) | 0 (−19, 55) | 0.85 (0.63, 0.94) | 14.1 |
| Hip flexion right | 32 (0, 100) | 33 (2, 100) | 0 (−40, 12) | 0.95 (0.88, 0.98) | 8.4 |
| Hip flexion left | 35 (0, 100) | 33 (0, 100) | 0 (−27, 14) | 0.96 (0.90, 0.99) | 7.4 |
| Total score | 51 (0, 100) | 47 (2, 100) | 0 (−28, 20) | 0.93 (0.83, 0.98) | 8.8 |
| Borg CR-10 scores | |||||
| Shoulder flexion right | 4 (0, 7) | 4 (0, 10) | 0 (−2, 4) | 0.78 (0.50, 0.92) | 1.2 |
| Shoulder flexion left | 4 (0, 9) | 4 (0, 10) | 0 (−1, 4) | 0.89 (0.74, 0.96) | 0.9 |
| Neck flexion | 5 (0, 9) | 6 (0, 10) | 1 (−1, 7) | 0.64 (0.24, 0.86) | 1.5 |
| Hip flexion right | 6 (0, 10) | 5 (0, 10) | 0 (−7, 2) | 0.75 (0.44, 0.90) | 1.4 |
| Hip flexion left | 5 (0, 10) | 5 (0, 10) | 0 (−4, 2) | 0.88 (0.69, 0.95) | 1.0 |
DISCUSSION
The FI-3 represents a streamlined version of the FI-2 in patients with DM and PM taking a maximum of 15 minutes with bilateral assessment and a maximum of 9 minutes with unilateral assessment. The FI-3 is a reliable and valid method for the functional assessment of DM/PM patients for muscle impairment of the major muscle groups in the neck, upper and lower extremities in patients at various stages of disease. The ICC scores for inter/intra-rater reliability for the FI-3 tasks were good to excellent; there were significant moderate to high associations with the MAP and HAQ scores to support construct validity. The neck flexion, bilateral shoulder flexion, and bilateral hip flexion tasks are in line with the common disease phenotype of bilateral proximal muscle involvement in DM/PM patients.2 All tasks of the FI-3 had good to excellent intra- and interrater reliability. As expected, the inter-rater ICC values were similar, but slightly lower than those for intra-rater reliability. This indicates that the FI-3 is easy to perform as it does not require more than one training session between assessors. There were no reported adverse effects from use of FI-3 in our patients. Furthermore, the FI-3 does not require specialized equipment or sophisticated training to conduct.
The FI-3 is to some extent similar to the Adult Myopathy Assessment Tool (AMAT)13; however, we anticipate that the advantages of the FI-3 over the AMAT is that it may be easier to use due to less tasks involved and therefore less training needed. Similar to the FI-3, the AMAT features functional and endurance tasks; yet, the original item development of the AMAT involved adults with DM/PM and inclusion body myositis and included the arm raise, modified push up, sit to stand, sit up, step up, supine to prone, supine to sit, head elevation, repeated heel rise, hip flexion, knee extension, modified push up, and arm raise. While all are important tasks to assess function and endurance, they are not specific for DM/PM patients but also include tasks for testing distal muscle strength and coordination that are relevant to patients with myopathies with distal muscle involvement. Moreover, we speculate that due to potential patient safety concerns, health care providers may be hesitant to administer certain tasks that require balance and coordination skills to patients with significant disabilities. Finally, the advantage of the FI-3 is that if time constraints exist, a single task can be measured to determine a baseline and then performance of that task may be followed over time for discrimination of change since each task has been validated separately.
Given the number of current tools, i.e. AMAT, FI-2 and FI-3, available to assess the functional status and endurance of myositis patients, there is a potential concern that there are many overlapping similarities. Hence, it could be challenging to decide which tool to use for clinical and/or research purposes. We believe that all the tools retain unique features that address different endurance tasks: the AMAT and FI-2 represent a comprehensive assessment of multiple muscle groups while incorporating balance and coordination skills, and the FI-3 offers a streamlined version of three major tasks with a derivation of a total score for ease of assessment of patient progress.
There were several potential limitations to this study. First, in session 2, the smaller sample size in the Mayo Clinic cohort may have affected the reliability scores. FI-3 task scores in site 2 cohort were more likely to decrease from session 1 to session 2; whereas, at site 1, the FI-3 scores were more likely to increase between the 2 sessions. This may be due to fatigue since the site 2 cohort performed the 2 sessions on the same day whereas the site 1 cohort performed the tasks on 2 separate days. There may not have been adequate time to rest between sessions (30-45 minutes, max). Second, there may be a ceiling effect (median values equal 20-50 percentile of the total variation of values) in the shoulder flexion task in patients at both sites. It was unexpected that both groups would perform so well in the shoulder flexion task. We expected that the most demanding tasks were hip flexion and neck flexion.19 Moreover, we observed that FI-3 tasks had low to moderate correlations to the Borg CR10 scale. The low correlations with the Borg index initially were surprising to us, but in reflection seem plausible since measures of perceived exertion are difficult to quantify given the subjectivity of patients self-rating their difficulties in performance of tasks, possibly related to motivational factors and attitudes. We speculate that patients may not have been motivated to perform as anticipated, or they perceived tasks to be more strenuous than expected. Some patients may experience a very fast depletion of muscle function, from one repetition to the next; in these cases, patients do not experience high muscle exertion even though they are unable to continue performing additional repetitions. This could also explain the low correlations between number of performed repetitions and the perceived exertion. Potentially, the construct validity of our study could be compromised with choosing the Borg scale to assess for correlations of tasks. However, we believe that the Borg scale will help the assessor to understand why the patient is unable to continue the test such as due to fatigue, pain or low motivation. A low perceived exertion warrants further questions to assess for limiting factors such as cardiovascular, pulmonary or musculoskeletal conditions that may lead to termination of testing. Finally, patients filled out the MAP and HAQ forms following completion of the FI-3; hence, their performances on the FI-3 may have influenced their self-assessments, including their MAP and HAQ scores.
In conclusion, the FI-3 is a valid and reliable tool to safely assess muscle endurance in patients with PM/DM. We anticipate the FI-3 can be incorporated into the routine functional assessment reflecting stamina and muscle endurance. We suggest to use the FI-3 tasks on the dominant side as it requires only 9 minutes to perform and scores can be calculated separately for each task or total score. The FI-3 can complement the FI-2 and the AMAT as well as the proposed IMACS core set of outcome measures.13,17
Supplementary Material
Acknowledgments
Grant(s) support: This project was supported by the small group division fund in rheumatology: CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), ALF Agreement between Karolinska Institutet and Region Stockholm, and Swedish Rheumatism Association. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement for all authors: We do not have any financial or non-financial potential conflicts of interest.
REFERENCES
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975; 292:344–7. [DOI] [PubMed] [Google Scholar]
- 2.Harris-Love MO, Shrader JA, Koziol D, Pahlajani N, Jain M, Smith M, et al. Distribution and severity of weakness among patients with polymyositis, dermatomyositis, and juvenile dermatomyositis. Rheumatology (Oxford) 2009; 48:134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiesinger GF, Quittan M, Nuhr M, Volc-Platzer B, Ebenbichler G, Zehetgruber M, et al. Aerobic capacity in adult dermatomyositis/polymyositis patients and healthy controls. Arch Phys Med Rehabil 2000; 81:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Dastmalchi M, Alexanderson H, Loell I, Ståhlberg M, Borg K, Lundberg IE, et al. Effect of physical training of the proportion of slow-twitch type I muscle fibers, a novel nonimmune-mediated mechanism for muscle impairment in polymyositis or dermatomyositis. Arthritis Rheum 2007; 57:1303–10. [DOI] [PubMed] [Google Scholar]
- 5.Loell I, Helmers SB, Dastmalchi M, Alexanderson H, Munters LA, Nennesmo I, et al. Higher proportion of fast-twitch (type II) muscle fibres in idiopathic inflammatory myopathies-evident in chronic but not in untreated newly diagnosed patients. Clin Physiol Funct Imaging 2011; 31:18–25. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Kiely PDW. Two simple, reliable and valid tests of proximal muscle function, and their application to the management of idiopathic inflammatory myopathies. Rheumatology 2006; 45:874–9. [DOI] [PubMed] [Google Scholar]
- 7.Miller FW. New approaches to the assessment and treatment of the idiopathic inflammatory myopathies. Ann Rheum Dis 2012; 71 Suppl 2:i82–5. [DOI] [PubMed] [Google Scholar]
- 8.Rider LG, Giannini EH, Harris-Love M, Joe G, Isenberg D, Pilkington C, et al. Defining clinical improvement in adult and juvenile myositis. J Rheumatol 2003; 30:603–17. [PubMed] [Google Scholar]
- 9.Harris-Love MO. Physical activity and disablement in the idiopathic inflammatory myopathies. Curr Opin Rheumatol 2003; 15:679–90. [DOI] [PubMed] [Google Scholar]
- 10.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980; 23:137–145. [DOI] [PubMed] [Google Scholar]
- 11.Miller FW, Rider LG, Chung YL, Cooper R, Danko K, Farewell V, et al. and the International Myositis Outcome Assessment Collaborative Study Group. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford) 2001; 40:1262–73. [DOI] [PubMed] [Google Scholar]
- 12.Alexanderson H, Regardt M, Ottoson C, Alemo Munters L, Dastmalchi M, Dani L, et al. Muscle strength and muscle endurance during the first year of treatment of polymyositis and dermatomyositis: a prospective study. J Rheumatol 2018; 45:4538–46. [DOI] [PubMed] [Google Scholar]
- 13.Harris-Love MO, Joe G, Davenport TE, Koziol D, Rose KA, Shrader JA, et al. Reliability of the adult myopathy assessment tool in individuals with myositis. Arthritis Care Res 2015; 67:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefson A, Romanus E, Carlsson J. A functional index in myositis. J Rheumatol 1996; 23:1380–4. [PubMed] [Google Scholar]
- 15.Alexanderson H, Stenström CH, Jenner G, Lundberg I. The safety of a resistive home exercise program in patients with recent onset active polymyositis or dermatomyositis. Scand J Rheumatol 2000; 29:295–301. [DOI] [PubMed] [Google Scholar]
- 16.Everitt BS and Skrondal A. The Cambridge Dictionary of Statistics. 4th edition. Cambridge, UK: Cambridge University Press; 2010;p84. [Google Scholar]
- 17.Alexanderson H, Broman L, Tollbäck A, Josefson A, Lundberg I, Stenström CH. Functional Index-2: Validity and reliability of a disease-specific measure of impairment in patients with polymyositis and dermatomyositis. Arthritis Rheum 2006; 55:114–22. [DOI] [PubMed] [Google Scholar]
- 18.Alexanderson H, Dastmalchi M, Esbjornsson-Liljedahl M, Opava CH, Lundberg IE. Benefits of intensive resistance training in patients with chronic polymyositis or dermatomyositis. Arthritis Rheum 2007; 57:768–77. [DOI] [PubMed] [Google Scholar]
- 19.Harris-Love MO, Shrader JA, Koziol D, Pahlajani N, Jain M, Smith M, et al. Distribution and severity of weakness among patients with polymyositis, dermatomyositis and juvenile dermatomyositis. Rheumatology (Oxford) 2009; 48:134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borg GA. Psychophysical bases of perceived exhaustion. Med Sci Sports Exerc 1982; 14:377–81. [PubMed] [Google Scholar]
- 21.Alexanderson H, Lundberg IE, Stenström CH. Development of the myositis activities profile-Validity and reliability of a self-administered questionnaire to assess activity limitations in patients with polymyositis/dermatomyositis. J Rheumatol 2002:29:2386–92. [PubMed] [Google Scholar]
- 22.Alexanderson H, Reed AM, Ytterberg SR. The Myositis Activities Profile-Initial Validation for Assessment of Polymyositis/Dermatomyositis in the USA. J Rheumatol 2012: 39(11):2134–41. [DOI] [PubMed] [Google Scholar]
- 23.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability, and pain scales. J Rheumatol 1982:789–93. [PubMed] [Google Scholar]
- 24.Ramey DR, Raynauld JP, Fries JF. The health assessment questionnaire 1992: status and review. Arthritis Care Res 1992:119–29. [DOI] [PubMed] [Google Scholar]
- 25.Munro BH. Statistical Methods for Health Care research. 3rd ed. Philadelphia: Lippincott; 1997; p235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.