Abstract
Zinc is an essential trace element. Deficiencies are frequently seen with gastrointestinal diseases, including chronic pancreatitis, nutritional deficiency, and reduced intestinal absorption. Additionally, reduced zinc levels have been linked to cellular changes associated with acute pancreatitis such as enhanced inflammation with increased macrophage activation and production of inflammatory cytokines such as IL-1β, impaired autophagy, and modulation of calcium homeostasis. Preliminary data suggest that zinc deficiency may lead to pancreatic injury in animal models. The purpose of this review is to explore the biologic effects of zinc deficiency that could impact pancreatic disease.
Keywords: Zinc, Cytokine, Pancreatitis, Malnutrition, inflammation, trace element
Introduction
Zinc deficiency can cause injury to a range of tissues; much of the research on its clinical impact and evolving role in disease states is recent. Zinc deficiency was first described as a clinical phenomenon in humans in 1963 after patients in Iran were characterized with a syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism [1]. In 1974 the Food and Drug Administration declared zinc an essential trace element [2]. Zinc homeostasis is tightly controlled in mammals, with minimal long-term storage in the body. However, zinc is vital in many processes at both the cellular and organ system levels, and even sub-clinical deficiencies can have negative effects. Accurately measuring zinc levels and assessing zinc therapy is a challenge in clinical studies because of fluctuations with age and technical issues related to measuring plasma and cellular levels. However, there is growing evidence suggesting that zinc deficiency may be common and may predispose individuals to or worsen a number of diseases including those involving the pancreas.
Zinc metabolism
Zinc is necessary for cellular and organ function. Within cells, zinc is present in the cytoplasm and stored in membrane-bound vesicles [3]. These cellular pools are particularly pronounced in muscle, erythrocytes, and bone, and have a slow turnover rate. Though the whole-body half-life of zinc is several weeks due release from cellular pools, plasma zinc levels can turn over within 30 min. Additionally, the volume of distribution of intravenously administered zinc is far greater than the volume of water in the human body, suggesting that zinc is concentrated in these cellular compartments [3]. General physiological functions of zinc are demonstrated in Fig. 1A.
Fig. 1. General physiological functions of zinc.
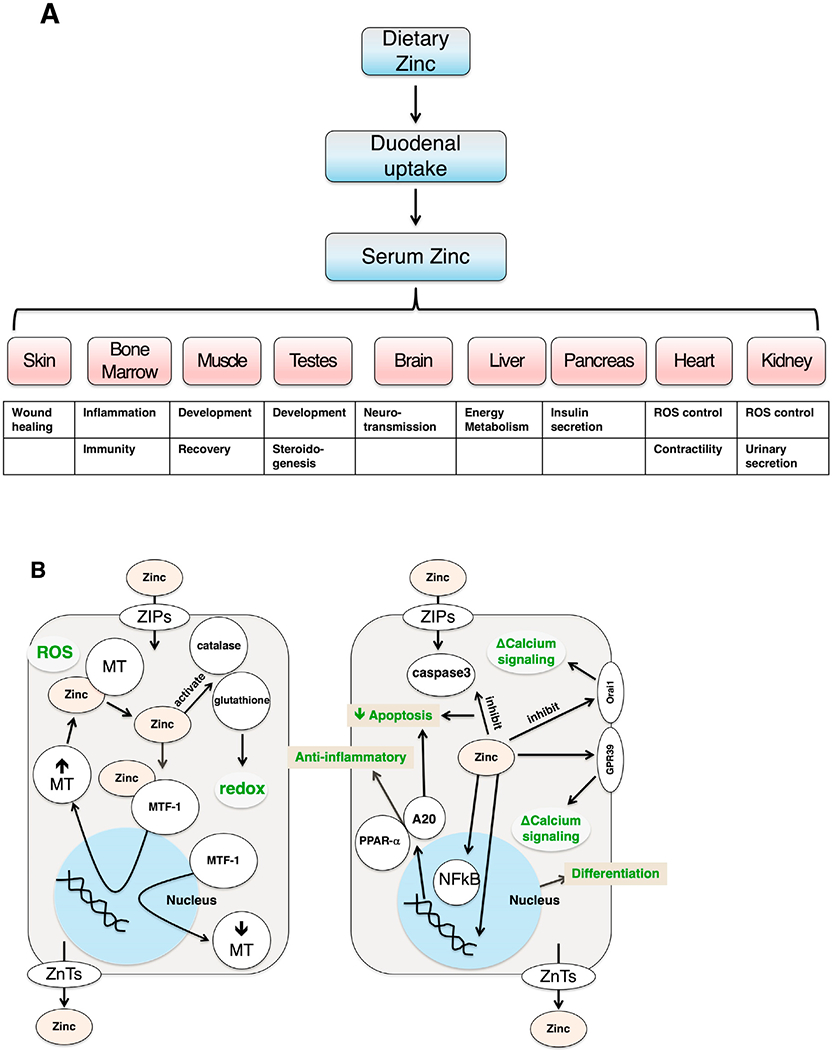
(A) within different tissues and (B) on a cellular level. ROS = reactive oxygen species; ZIPs = Zrt-Irt-like proteins; ZnTs = Zinc transporters; MT = metallothioneins; MTF-1 = metal response element binding transcription factor 1; Orai1 = calcium released-activated calcium modulator 1; GPR39 = G-protein coupled receptor 39; A20 = zinc finger protein; PPAR-α = peroxisome proliferator-activated receptor α
Two opposing families of transporter proteins largely regulate zinc homeostasis: ZIPs (Zrt-Irt-like Proteins, or the family of SLC39) and ZnTs (Zinc Transporters, or the family of SLC30). ZIPs transport zinc into the cytoplasm either from extracellular space or out of cytoplasmic vesicles. ZnTs facilitate transport in the opposite direction from ZIPs—they move zinc from the cytoplasm out of the cell or into membrane-bound vesicles [4,5]. There are over one hundred ZnTs and eighty-six ZIPs characterized, and they are found in prokaryotes and eukaryotes at all phylogenetic levels [4].
Though an extensive review of the specialized functions of each transporter is beyond the scope of this review, several of these transporters appear to be particularly important in the context of pancreatic disease . Zip5 is present on the basolateral membrane of intestinal enterocyte and pancreatic acinar cells. In Zip5 loss-of-function mice, the transporter has been shown to participate in zinc excretion in mice and may attenuate zinc-induced acute pancreatitis [6]. Increased Zip4 expression enhances cell proliferation and expression of downstream factors associated with pancreatic adenocarcinoma progression [7]. Zip8 downregulates IκB kinase activity and inhibits NF-κB activity, leading to an anti-inflammatory effect (see below) [8]. Thus, variations in the forms of zinc transporters can have a multitude of effects in both health and disease.
The dearth of zinc-excess-related pathologies suggests that zinc homeostasis is robust [9]. Given zinc’s established role in activating zinc finger proteins, which have central roles in mediating intracellular cell signaling and transcriptional regulation, its regulation is crucial to cell survival [10]. One set of key regulators are metallothioneins (MTs), which are cysteine-rich, zinc-binding proteins that sequester zinc. MTs are also electrophilic scavengers and cytoprotective. MTs exhibit two metal-cluster binding sites and can chelate up to seven zinc ions per MT molecule. Depending on their structure, MTs have a maximal half-life of 24 h and are therefore not considered long-term storage sites of zinc [11]. MTs interact with the metal response element binding transcription factor 1 (MTF-1) to regulate intracellular zinc homeostasis (Fig. 1B). Upon exposure to reactive oxygen species, MTs release zinc that is then chelated by MTF-1. Zinc binds to the zinc fingers in MTF-1, activating the complex, and inducing MT gene expression. Increased MT production then serves to bind excess zinc in the cytoplasm. Similarly, when MTF-1 releases zinc, MT gene expression is reduced [12]. In addition to the antioxidant effects of MTs, zinc serves many antioxidant and anti-inflammatory properties by activating antioxidant proteins like glutathione and catalase, stabilizing protein sulfhydryls against oxidation, participating in redox reactions with active metals, and decreasing NF-κB activation leading to increased expression of A20 and PPAR-α, zinc finger proteins associated with anti-inflammatory properties [8,13]. Therefore, zinc homeostasis involves a delicate balance of multiple proteins transport proteins; key mediators of its biologic action include metallothioneins and MTF-1, which provide both antioxidant and anti-inflammatory responses within cells.
Functional effects of zinc
Differentiation
Zinc levels can influence tissue development, renewal, and repair, including proliferation and apoptosis. Such responses have been shown in epithelial differentiation and proliferation, neuronal differentiation, and immunity [14–17]. Zinc is important in differentiation of myotubes, neurons, and epithelial cells [11,14]. Mechanistically, pre-adipocyte differentiation occurs with transport of MT-bound zinc into the nucleus, suggesting the importance of zinc-associated transcription factors.
Neurotransmission
In addition to the intracellular effects of zinc, it is concentrated in neurosecretory vesicles by ZnTs and functions intercellularly as a neurotransmitter. ZnT3 controls intra-vesicular zinc concentrations in presynaptic cells and gated Zn channels and ZIPs regulate zinc uptake in postsynaptic cells [11,18]. Zinc plays an inhibitory role on major post-synaptic channels including GABA-ergic channels [17,19]. A subset of glutamatergic receptors, including N-methyl-d-aspartic acid (NMDA) receptors, kainate receptors, and AMPA receptors are also zinc responsive [18]. NMDA receptors, for example, are noncompetitively inhibited by zinc at low micromolar concentrations [19]. The effects of zinc on NMDA receptors may be linked to the pathophysiology of depression [18].
Immunity
Zinc deficiency induces a persistent, but reversible, mixed immunological disorder with both immune-deficient and hyperinflammatory responses (Table 1, Fig. 2b) [20]. Zinc deficiency can cause changes in T cell proliferation and other defects in immunity. It can alter production of specific cytokines and their mRNAs by peripheral blood mononuclear cells [21]. For example, zinc deficiency causes IL-2 and interferon-gamma to decrease but IL-1β to increase in circulating mononuclear cells. This proinflammatory response is reversible with zinc repletion [21]. The immune decline associated with aging may be due in part to zinc deficiency in the elderly. Decreased zinc levels among elderly have been attributed to etiologies including decreased nutritional intake, reduced intestinal absorption secondary to medications and poor diet, and disease states such as diabetes [22].
Table 1.
Cytokine Responses to Zinc Deficiency that may be related to Acute Pancreatitis
| Cytokine | Role | Change in Zinc Deficiency | Net Effect of Zinc Deficient State | Change in Acute Pancreatitis |
|---|---|---|---|---|
| TNFα | Pro-inflammatory. With IL-1β, activates IL6/8. | Increased [20,68,69] | Increases injury via activation of IL6/8 and hyper-release of neutrophilic enzymes | Increased [50,70–72] |
| IL-1β | Pro-inflammatory. With TNF-a, activates IL6/8 | Increased [20,68,69,73,74] | Increase in sterile inflammation, via NLRP3; overexpression of IL-6, IL-8, increases effects of LPS | Little or no increase50,70–72,75 |
| IL-6 | Pro-inflammatory: lymphocyte-activating primary inducer of protein response to injury | Variable [20,68,69] | In heightened state, increased injury | Increased50,70–72,75 |
| IL-8 | Pro-inflammatory: targets neutrophil and causes release of enzymes | Variable [20,68] | Over-degranulation and overactivation of neutrophilic enzymes can cause injury themselves; decreased neutrophil and T cell migration | Increased [71,72] |
| IFNγ | Chemotactic for monocytes, activates B cells. Inhibits Th2, activates Th1 proliferation | Decreased [11,20,22,68] | Decreases Th1/Th2 ratio, reduces T-cell proliferation and differentiation, leading to immune deficiencies, reduces NK cell and monocyte activity and function | Increased72,75 |
| IL-10 | Anti-inflammatory | Unchanged [68] | Unchanged Th2 cytokines leads to net shift of Th1/Th2 ratio towards Th2 | Increased71,72,75 |
| IL-2 | Promotes T-cell differentiation; activates Th1 and NK cells | Decreased [11,20,22,68] | Decreases Th1/Th2 ratio, reduces T-cell proliferation and differentiation, leading to immune deficiencies, reduces NK cell and monocyte activity and function | Decreased [50,70,72] |
Fig. 2. Proposed effects of zinc-deficiency on pancreatitis responses.
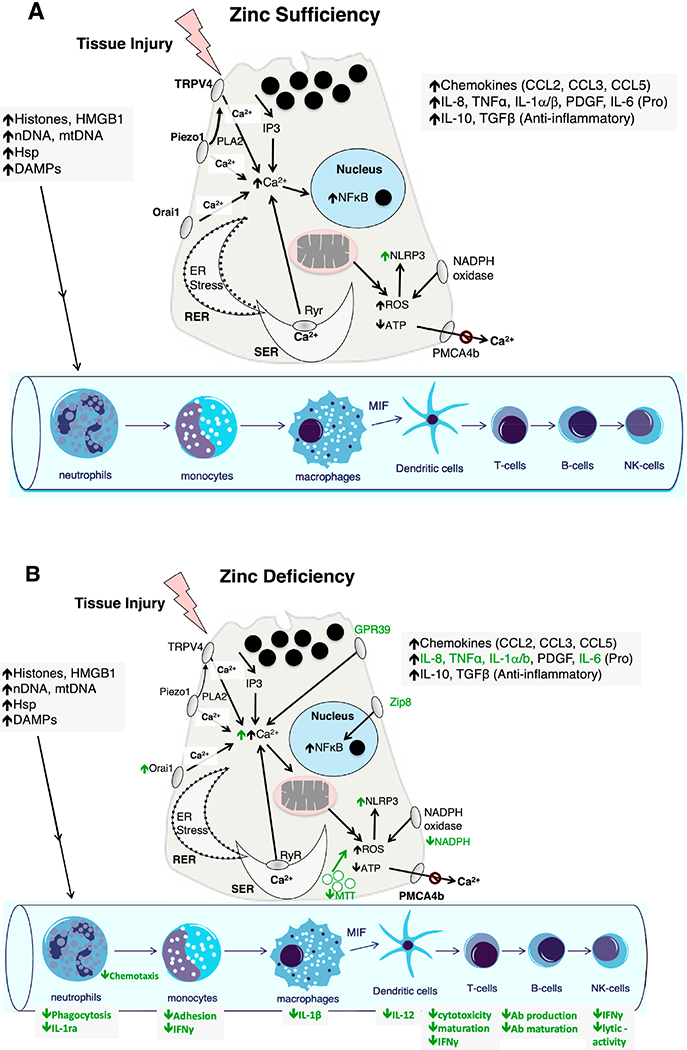
(A) Model of acute pancreatitis in with normal (Sufficiency) zinc levels; (B) Potential effects of zinc depletion (Deficiency) on acinar cell pancreatitis responses. HMGB1 = High mobility group box 1 protein; nDNA = normal DNA; mtDNA = mitochondrial DNA; Hsp = heat shock protein; Ab = antibody; DAMPs = damage-associated molecular patterns; TRPV4 = transient receptor potential cation channel subfamily V Member 4; PLA2 = phospholipase A2; Piezo1 = piezo type mechanosensitive ion channel component 1; Orai1 = calcium release-activated calcium modulator 1; RER = rough endoplasmic reticulum; SER = smooth endoplasmic reticulum; Ryr = ryanodine receptor; PMCA4b = plasma membrane calcium-transporting ATPase 4; NLRP3 = NLR family pyrin domain containing 3; Zip8 = Zrt-Irt-like protein 8; GPR39 = G-protein coupled receptor 39.
The overall number and function of immunological cells can be reduced in zinc deficiency (Fig. 2B). Thus, T cell numbers are reduced, lymphoid mass is reduced, and B cells are underdeveloped; this is proposed to be due to lack of activation of zinc finger transcription factors [14,21]. In addition, NK cell activity, antibody production, and phagocytosis are decreased [23]. Resolution of these effects, particularly in the elderly for whom zinc deficiency and compromised immunity are common, is often seen with zinc supplementation. Excess supplementation, however, can cause impaired immunity by suppressing levels of B cell [23].
Inflammation
A number of enhanced innate immune responses have been associated with zinc deficiency (Fig. 2B). Low zinc concentrations can lead to lysosomal dysfunction and activation of the NLRP3 inflammasome, leading to cell and tissue injury [24]. A20, a zinc-finger protein in the ovarian-tumor family of cysteine proteases, is involved with one of these responses, providing negative feedback inhibition on pro-inflammatory signaling. With zinc, A20 negatively regulates the NF-κB transcription factor, which normally induces both IL-1β and NLRP3 up-regulation. NF-κB in turn induces A20 gene transcription [25]. Zinc deficiency also leads to increased levels of the proinflammatory cytokines IL-6, IL-8, and TNF-α, and thus an imbalance between TH1 cytokines (including IL-2 and TNF-α) and TH2 cytokines (IL-6, IL-8, IL-10) [11].
Cell signaling
Zinc can activate a cell surface G-protein receptor, GPR39, and that in turn can modulate calcium signaling (Fig. 1B). The full-length form of GPR39, GPR39-1a, is expressed in intestinal organs including the stomach, intestine, and pancreas and also found in the spleen, lungs, liver, adipose tissue, and heart. Its truncated form, GPR39-1b (32kD), has a prematurely inserted stop codon and is also widely expressed, particularly in the stomach and intestine, and does not appear to be biologically active [26]. Constitutive activity in the full-length form, common for this family of GPRs, appears to occur through Gαq, primarily affecting inositol triphosphate turnover and cAMP-response element (CRE) mediated transcription, and through Gα12/13 it activates serum-response element (SRE) mediated transcription [27]. GPR39 has also been localized to pancreatic insulin-storing β-cells and exocrine duct cells [28]. GPR39 knockout mice have significantly decreased levels of insulin compared to control mice when fed a sucrose-rich diet [29]. Due to this connection with insulin, GPR39 has since become a target explored for its potential effects in diabetes.
ORAI1 is the most abundant store-operated calcium entry (SOCE) channel in pancreatic acinar cells. In settings of toxin-induced activation of ORAI1, cytosolic calcium levels are increased, contributing to the pathogenesis of acute pancreatitis. ORAI1 inhibitors are currently the target of drug development in treatment of patients with pancreatitis; one such drug is in early clinical trials [30]. Zinc has been found to decrease calcium cytoplasmic entry in Swiss murine fibroblasts, and genetic diseases associated with changes in calcium entry also have symptoms that overlap with signs of zinc deficiency [31]. ORAI1 channels are also highly expressed in tumors such as esophageal squamous cell carcinoma. Zinc inhibits ORAI1 and changes calcium concentrations in cancer cells [32]. Since disordered pancreatic acinar cell signaling is thought to initiate many forms of acute pancreatitis, zinc’s effects on intracellular calcium signaling may be important in considering its potential therapeutic benefit or role of zinc deficiency in predisposing to disease.
Cell death
Zinc also regulates apoptotic cell death [33]. Zinc indirectly suppresses signaling pathways leading to apoptosis by minimizing oxidative damage by stabilizing cellular membranes and proteins and potentially indirectly through effects on glutathione (Fig. 1B). Zinc may also act directly to inhibit caspase-3 cascade required for the pro-apoptotic cascade [33]. Additionally, the TNF inhibitor, A20, is recruited to TNF-receptor 1 signaling complex (Complex 1) through a zinc finger domain that protects cells from apoptosis through both linear (M1) ubiquitin network stabilization and deubiquitylase activity [34]. Free cellular zinc also protects against apoptotic injury while zinc chelation or dysfunction of zinc transporters can initiate apoptosis [11]. Experiments in Sertoli cells and cultured mouse hepatocytes show attenuation of ethanol-induced apoptosis with zinc supplementation [35]. This experiment has not been done in the pancreas, but it provides a strong case for zinc’s potential role in lessening injury in acute and chronic pancreatitis—conditions comorbid with heavy drinking. Additionally, zinc depletion may also lead to necrosis, particularly in cells for which activation is reduced [36]. The effect of zinc deficiency on the severity of acute pancreatitis has not been studied, but given the relationship between apoptosis and necrosis in the severity of acute pancreatitis, zinc deficiency may attenuate cell death pathways differently depending on the severity of acute pancreatitis.
Zinc deficiency and disease
Up to 17% of the global population may have zinc deficiency [37]. Zinc deficiency is largely attributed to inadequate dietary intake and increased phytate intake in some regions of the world [37]. The elderly and malnourished are particularly prone to zinc deficiency, and many zinc-related pathologies have higher prevalence among older patients [22].
In 1969, a report was published of a patient with growth retardation, hypogonadism, hypogammaglobulinemia, and chronic infection whose growth parameters improved with zinc supplementation [38]. This was the first report of zinc deficiency in the United States. Low circulating levels of the growth hormone insulin like growth factor 1 (IGF-1) has been associated with zinc deficiency, suggesting a mechanistic link between zinc deficiency and poor growth [39]. Since then, multiple studies have reported the essential role of zinc in humans and disease states aside from growth retardation.
Zinc deficiency is prototypically associated with acrodermatitis enteropathica, a disease due to mutations in the intestinal zinc transporter Zip4. Acrodermatitis enteropathica was discovered by in 1973 when a 2-year-old girl with severe dermatitis and diarrhea did not respond to dietary therapy but responded to oral zinc-sulfate [40]. Zinc supplementation is essentially curative for this otherwise lethal disease.
Zinc deficiency can cause selective organ dysfunction including diabetes mellitus, through pancreatic beta-cell destruction, impairment of intestinal permeability, and changing the gut microbiome [41–43]. Additionally, since zinc, through the ZnT8 transporter, plays a role in the synthesis, storage, and secretion of insulin, zinc deficiency increases the risk of type 2 diabetes. ZnT8 autoantibodies are also found in 60–80% of new onset type 1 diabetes [44]. Children with low exposure to zinc from drinking water have a higher risk of developing type 1 diabetes, and zinc deficiency may be associated with increased autoimmunity and cytokine-induced damage of islet cells [45,46].
Studies have also shown significant decreases in serum zinc concentrations in patients with depression, which appears to be related to this depletion’s effects on NMDA receptors [16]. Elevated corticosterone levels in zinc-deficient conditions have been theorized to inhibit glutamatergic transporter activity in astrocytes, thereby increasing glutamate concentrations in the synaptic cleft and increasing activation of post-synaptic NMDA neurons. About 50% of patients with depression show hyperactivation of the hypothalamic pituitary adrenal axis, which is implicated in increased corticosterone production under stressful conditions [18].
Zinc excess has been found to have biological effects, such as changes in gut microbiota, though few of these effects are known to be pathogenic. In one of these rare examples, increased zinc levels have been shown to reduce antibiotic effectiveness against C. difficile in zinc-supplemented mice [47]. M Microbiota dysbiosis and increased intestinal permeability has been associated with severe acute pancreatitis progression and salivary microbiota are altered in patients with chronic pancreatitis compared to healthy subjects; whether these changes are associated with zinc have not been established [48,49].Additionally, serum zinc levels are elevated in osteoarthritis. Zip8 expression is increased in osteoarthritis cartilage, leading to increased intracellular zinc concentration and activation of MFT-1, which leads to increase in MTs, ultimately leading to osteoarthritic damage to cartilage [8].
Zinc supplementation in disease treatment
The widest therapeutic use of zinc supplementation is in diarrheal disease. Evidence from several clinical trials has shown that zinc supplementation reduces both the prevalence and duration of diarrheal episodes among children in developing countries [50,67]. The World Health Organization (WHO) recommends routine zinc supplementation for 10–14 days in infants and children with diarrheal disease [51]. One recent systemic review of 33 studies concluded that zinc supplementation likely shortens the duration of diarrheal illness in children older than 6 months who are at risk for being zinc deficient [52]. Another analysis of randomized controlled trials examined zinc supplementation in the context of growth, general morbidity, and mortality and concluded that the apparent benefits of zinc supplementation are small but sufficient to advocate for zinc supplementation in individuals from 6 months to 12 years of age in areas where zinc deficiency is likely [53].
Zinc supplementation has also been used for the treatment of the common cold. Patients treated with zinc lozenges exhibit significantly shorter duration of cold symptoms, cough nasal discharge, and overall severity scores than controls with the common cold. There is no difference, however, in measured plasma pro-inflammatory cytokine levels in these patients [54,67].
Zinc is also used in the treatment of Wilson’s Disease, a genetic condition resulting in abnormal copper metabolism and copper deposition within the liver. Zinc therapy in Wilson’s Disease serves to block the intestinal absorption of copper through upregulation of intestinal MTs, leading to increased fecal copper loss and decreased accumulation of copper within tissue [55].
Zinc and the exocrine pancreas
Animal studies
The pathogenesis of acute pancreatitis is thought to most often begin with pathologic signaling in the pancreatic acinar cell (Table 1, Fig. 2A). This then modulates a series of inflammatory responses and cell death. A subset of the key acute pancreatitis mechanisms has been shown to be zinc-regulated (Fig. 2B). Studies in mice and rats have shown an association between zinc deficiency and worsened inflammation as well as a potential benefit of zinc supplementation in pancreatitis. In mice treated with injection of sodium taurocholate into the pancreatic duct to induce severe acute pancreatitis, animals that received zinc sulfate supplementation immediately following injection of bile acid had similar levels of erythrocyte glutathione peroxidase and erythrocyte superoxide dismutase (antioxidant enzymes) activity to controls, suggesting that zinc supplementation may protect against the oxidative stress caused by severe acute pancreatitis [56]. After intra-peritoneal cerulein-induced acute pancreatitis, pancreatic tissue concentrations of zinc, nickel, iron, and phosphorus are significantly decreased, suggesting that these elements may play a role in acute pancreatitis [57]. In a cecal puncture induced model of polymicrobial sepsis, zinc deficient mice with sepsis had a significantly higher mortality rate, more severe organ injury, and significantly higher plasma levels of pro-inflammatory cytokines IL-6 and IL-10 than controls, cytokines which play a role in the development of acute pancreatitis [58]. This model may have relevance to the inflammatory responses and organ failure observed in severe acute pancreatitis.
Human studies
The role of zinc in pancreatitis in humans remains unclear. In both acute and chronic pancreatitis, the literature is mixed as to whether zinc deficiency is associated worse disease. There is no significant difference in copper and zinc subunit concentrations contributing to the stability of the copper/zinc superoxide dismutase in patients with acute pancreatitis vs. controls, suggesting that zinc deficiency in acute pancreatitis may not be severe enough to affect antioxidant enzyme activity [59]. In patients with chronic pancreatitis, erythrocyte and serum zinc levels are reported to be significantly lower than controls [60,61]. Fecal elastase levels, a measure of exocrine pancreatic insufficiency (EPI), also positively correlates with erythrocyte zinc levels, suggesting a relationship between EPI and zinc deficiency [60]. Patients with EPI have a 50% reduction of zinc absorption, increased urinary zinc excretion, and reduced fecal zinc excretion compared to control patients, suggesting a possible compensatory mechanism of zinc retention [62]. In chronic pancreatitis patients, magnesium below 2.05 mg/dL; hemoglobin, albumin, prealbumin, retinol binding protein below lower limit of normal; and hemoglobin A1c above upper limit of normal were each associated with development of EPI; however, zinc levels have not been shown to be associated with development of EPI in chronic pancreatitis [63]. Given the confounding effects of aging on zinc levels and the lack of age-matched controls in many clinical studies, the data are currently too limited to ascertain whether zinc deficiency contributes to the phenotype of these diseases. However, since mechanistic studies from other systems that demonstrate effects on inflammatory responses and cell injury (Table 1 and Fig. 2) there are several potential roles for zinc in the context of acute and chronic pancreatitis.
Summary
Zinc has a range of effector mechanisms that may be implicated in the development of acute and chronic pancreatitis. It has an overall cytoprotective role in the context of its association with metal-binding proteins. Increased zinc levels are associated with upregulation of metallothioneins; the combination of zinc with metallothioneins provides antioxidant protection. Additionally, zinc activates other antioxidant proteins and decreases a range of inflammatory responses. Zinc can also activate the cell-surface receptor GPR39, which modulates calcium signaling and may affect insulin secretion. Since GPR39 is also found in many other tissues, its activation may affect other important cellular responses. This includes anti-inflammatory responses by macrophages [64].
The regulation of zinc in tissue and cellular compartments is also mediated by a broad array of zinc transporters, including ZIPs and ZnTs. Some of these zinc transporters have been implicated in pancreatic disease, including Zip5, which is associated with zinc excretion and is present on intestinal enterocytes and pancreatic acinar cells, and Zip8, which is associated with downregulation of NFkB [65].
Given zinc’s many roles and heterogeneous distribution across tissue and cellular compartments, its deficiency can cause a variety of responses. For example, whereas zinc plays an anti-inflammatory and cytoprotective role, zinc deficiency can enhance inflammatory responses and reduce tissue repair. Experimentally, the proinflammatory response associated with zinc deficiency is reversible with zinc repletion. Clinically, zinc supplementation improves the immunodeficiency often seen in elderly patients. These findings make it important to identify subjects who might benefit from zinc supplementation.
Known mechanisms of acute pancreatitis suggest that zinc deficiency could increase the risk of developing acute pancreatitis or increase the severity once disease has developed. The most common mechanism for initiating acute pancreatitis likely occurs with disruption in signaling in pancreatic acinar cells. Zinc’s association with Orai1 and GPR39 suggest a role for zinc in calcium disruption that could lead to acute pancreatitis. Since the severity of acute pancreatitis is mediated by inflammatory cells, zinc deficiency might also worsen disease by further stimulating the inflammatory response. Additionally, the relationship between apoptotic and non-apoptotic cell death in severity of acute pancreatitis may be affected by zinc’s role in apoptosis and non-apoptotic cell death.
Although human studies have shown variable results in relating zinc deficiency and acute pancreatitis, zinc deficiency is difficult to quantify. Zinc levels are also affected by a variety of factors including age, dietary intake, infection, stress, and inflammation. Additionally, zinc may be associated with prandial and postprandial fluctuations. The type of assay and measurement protocol can also affect zinc levels. For example, environmental zinc contamination, hemolysis from red blood cells, and timing from taking sample to separation of plasma have all been implicated in variable zinc measurements [66]. Future studies exploring zinc deficiency in the context of human disease must take these factors into account and be assessed under controlled conditions to understand its role in disease.
Finally, preliminary studies suggest that zinc deficiency is common in patients with chronic pancreatitis. While these findings need to be confirmed, these studies indicate a potential role of zinc in injury and lack of resolution of pancreatic disease. Further studies examining the role of zinc in chronic pancreatitis as well as other forms of pancreatic disease including recurrent acute pancreatitis, are therefore needed.
Acknowledgements
MW is supported by an NIH Fellowship (DK007017). MP was supported by a NIDDK T32 (DK007017). FSG is supported by a Veterans Administration Merit Award, DOD CA180514, DK54021 and PO1 DK098108. DY is supported by NIH U01 DK108306 and DoD, PR182623.
Abbreviations:
- ZIP
Zrt-Irt-like Proteins
- ZnTs
Zinc Transporters
- MT
metallothioneins
- MTF-1
metal response element binding transcription factor 1
- GPR
G-protein receptor
Footnotes
Declaration of competing interest
The authors have no financial disclosures or conflicts of interest for this review.
References
- [1].Prasad AS, Miale A Jr, Farid Z, Sandstead HH, Schulert AR. Clinical and experimental. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. J Lab Clin Med 1963;116(5):737–49. 1990. [PubMed] [Google Scholar]
- [2].Prasad AS. Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol 2014;28(4):357–63. [DOI] [PubMed] [Google Scholar]
- [3].Alpers DH, Young GP, Tran CD, et al. Drug-development concepts as guides for optimizing clinical trials of supplemental zinc for populations at risk of deficiency or diarrhea. Nutr Rev 2017;75(3):147–62. [DOI] [PubMed] [Google Scholar]
- [4].Eide DJ. The SLC39 family of metal ion transporters. Pflügers Archiv 2004;447(5):796–800. [DOI] [PubMed] [Google Scholar]
- [5].Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr 2004;24: 151–72. [DOI] [PubMed] [Google Scholar]
- [6].Geiser J, De Lisle RC, Andrews GK. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PLoS One 2013;8(11). e82149–e82149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li M, Zhang Y, Liu Z, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A 2007;104(47):18636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Takagishi T, Hara T, Fukada T. Recent advances in the role of SLC39A/ZIP zinc transporters in vivo. Int J Mol Sci 2017;18(12):2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vallee BL, Auld DS. Zinc metallochemistry in biochemistry. EXS 1995;73: 259–77. [DOI] [PubMed] [Google Scholar]
- [10].Kröncke K-D, Klotz L-O. Zinc fingers as biologic redox switches? Antioxidants Redox Signal 2009;11(5):1015–27. [DOI] [PubMed] [Google Scholar]
- [11].Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev 2015;14(4):277–85. [DOI] [PubMed] [Google Scholar]
- [12].Dong G, Chen H, Qi M, Dou Y, Wang Q. Balance between metallothionein and metal response element binding transcription factor 1 is mediated by zinc ions (review). Mol Med Rep 2015;11(3):1582–6. [DOI] [PubMed] [Google Scholar]
- [13].Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017;25(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol 2015;29:24–30. [DOI] [PubMed] [Google Scholar]
- [15].Rudolf E, Rudolf K. Low zinc environment induces stress signaling, senescence and mixed cell death modalities in colon cancer cells. Apoptosis 2015;20(12): 1651–65. [DOI] [PubMed] [Google Scholar]
- [16].Nowak G, Zinc future mono/adjunctive therapy for depression: mechanisms of antidepressant action. Pharmacol Rep 2015;67(3):659–62. [DOI] [PubMed] [Google Scholar]
- [17].Colvin RA, Fontaine CP, Laskowski M, Thomas D. Zn2+ transporters and Zn2+ homeostasis in neurons. Eur J Pharmacol 2003;479(1–3):171–85. [DOI] [PubMed] [Google Scholar]
- [18].Mlyniec K Zinc in the glutamatergic theory of depression. Curr Neuropharmacol 2015;13(4):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tóth K Zinc in neurotransmission. Annu Rev Nutr 2011;31:139–53. [DOI] [PubMed] [Google Scholar]
- [20].Bao B, Prasad AS, Beck FWJ, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab 2003;285(5):E1095–102. [DOI] [PubMed] [Google Scholar]
- [21].Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J Mol Sci 2017;18(10):2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing 2009;6 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr 2009;29:133–52. [DOI] [PubMed] [Google Scholar]
- [24].Jo E-K, Kim JK, Shin D-M, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016;13(2):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Evans PC, Ovaa H, Hamon M, et al. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem J 2004;378(Pt 3):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Depoortere I GI functions of GPR39: novel biology. Curr Opin Pharmacol 2012;12(6):647–52. [DOI] [PubMed] [Google Scholar]
- [27].Holst B, Egerod KL, Schild E, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 2007;148(1):13–20. [DOI] [PubMed] [Google Scholar]
- [28].Popovics P, Stewart AJ. GPR39: a Zn(2+)-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol Life Sci 2011;68(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tremblay F, Richard A-MT, Will S, et al. Disruption of G protein-coupled receptor 39 impairs insulin secretion in vivo. Endocrinology 2009;150(6): 2586–95. [DOI] [PubMed] [Google Scholar]
- [30].Wen L, Voronina S, Javed MA, et al. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology 2015;149(2):481–92. e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].O’Dell BL, Browning JD. Impaired calcium entry into cells is associated with pathological signs of zinc deficiency. Adv Nutr 2013;4(3):287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Choi S, Cui C, Luo Y, et al. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. Faseb J 2018;32(1):404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals 2001;14(3–4):315–30. [DOI] [PubMed] [Google Scholar]
- [34].Priem D, Devos M, Druwé S, et al. A20 protects cells from TNF-induced apoptosis through linear ubiquitin-dependent and -independent mechanisms. Cell Death Dis 2019;10(10). 692–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pourhassanali N, Roshan-Milani S, Kheradmand F, Motazakker M, Bagheri M, Saboory E. Zinc attenuates ethanol-induced Sertoli cell toxicity and apoptosis through caspase-3 mediated pathways. Reprod Toxicol 2016;61:97–103. [DOI] [PubMed] [Google Scholar]
- [36].Kolenko V, Uzzo RG, Bukowski R, et al. Dead or dying: necrosis versus apoptosis in caspase-deficient human renal cell carcinoma. Canc Res 1999;59(12):2838–42. [PubMed] [Google Scholar]
- [37].CDC CfDCaP. Micronutrient facts. https://www.cdc.gov/nutrition/micronutrient-malnutrition/micronutrients/index.html; 2019. [Google Scholar]
- [38].Caggiano V, Schnitzler R, Strauss W, et al. Zinc deficiency in a patient with retarded growth, hypogonadism, hypogammaglobulinemia and chronic infection. Am J Med Sci 1969;257(5):305–19. [DOI] [PubMed] [Google Scholar]
- [39].Cossack ZT. Decline in somatomedin-C (insulin-like growth factor-1) with experimentally induced zinc deficiency in human subjects. Clin Nutr 1991;10(5):284–91. [DOI] [PubMed] [Google Scholar]
- [40].Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc Roy Soc Med 1973;66(4):327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fukunaka A, Fujitani Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J Mol Sci 2018;19(2):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Reed S, Neuman H, Moscovich S, Glahn RP, Koren O, Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 2015;7(12):9768–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yang L, Wang C, Xiao F. Effects of different zinc nutritional status on iron metabolism in rats. Wei Sheng Yan Jiu 2013;42(4):647–51. [PubMed] [Google Scholar]
- [44].Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci 2014;71(17): 3281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Samuelsson U, Oikarinen S, Hyöty H, Ludvigsson J. Low zinc in drinking water is associated with the risk of type 1 diabetes in children. Pediatr Diabetes 2011;12(3 Pt 1):156–64. [DOI] [PubMed] [Google Scholar]
- [46].Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr 1998;17(2):109–15. [DOI] [PubMed] [Google Scholar]
- [47].Zackular JP, Moore JL, Jordan AT, et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med 2016;22(11): 1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tan C, Ling Z, Huang Y, et al. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas 2015;44(6):868–75. [DOI] [PubMed] [Google Scholar]
- [49].Habtezion A Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 2015;31(5):395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bhutta ZA, Black RE, Brown KH, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators’ Collaborative Group. J Pediatr 1999;135(6):689–97. [DOI] [PubMed] [Google Scholar]
- [51].Khan WU, Sellen DW. Zinc supplementation in the management of diarrhoea. https://www.who.int/elena/titles/bbc/zinc_diarrhoea/en/; 2011. [Google Scholar]
- [52].Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 2016;12(12). CD005436–CD005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mayo-Wilson E, Imdad A, Junior J, Dean S, Bhutta ZA. Preventive zinc supplementation for children, and the effect of additional iron: a systematic review and meta-analysis. BMJ Open 2014;4(6). e004647–e004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000;133(4):245–52. [DOI] [PubMed] [Google Scholar]
- [55].Roberts EA, Schilsky ML, American Association for Study of Liver D. Diagnosis and treatment of Wilson disease: an update. Hepatology 2008;47(6):2089–111. [DOI] [PubMed] [Google Scholar]
- [56].Tang Q-q, Su S-y, Fang M-y. Zinc supplement modulates oxidative stress and antioxidant values in rats with severe acute pancreatitis. Biol Trace Elem Res 2014;159(1—3):320–4. [DOI] [PubMed] [Google Scholar]
- [57].Kashiwagi M, Akimoto H, Goto J, Aoki T. Analysis of zinc and other elements in rat pancreas, with studies in acute pancreatitis. J Gastroenterol 1995;30(1):84–9. [DOI] [PubMed] [Google Scholar]
- [58].Knoell DL, Julian MW, Bao S, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 2009;37(4):1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Milnerowicz H, Bukowski R,Jabłonowska M, Ściskalska M, Milnerowicz U. The antioxidant profiles, lysosomal and membrane enzymes activity in patients with acute pancreatitis. Mediat Inflamm 2014;2014 376518–376518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Girish BN, Rajesh G, Vaidyanathan K, Balakrishnan V. Zinc status in chronic pancreatitis and its relationship with exocrine and endocrine insufficiency. JOP 2009;10(6):651–6. [PubMed] [Google Scholar]
- [61].Milnerowicz H, Jabłonowska M, Bizoń A. Change of zinc, copper, and metallothionein concentrations and the copper-zinc superoxide dismutase activity in patients with pancreatitis. Pancreas 2009;38(6):681–8. [DOI] [PubMed] [Google Scholar]
- [62].Dutta SK, Procaccino F, Aamodt R. Zinc metabolism in patients with exocrine pancreatic insufficiency. J Am Coll Nutr 1998;17(6):556–63. [DOI] [PubMed] [Google Scholar]
- [63].Lindkvist B, Domínguez-Muñoz JE, Luaces-Regueira M, Castiñeiras-Alvariño M, Nieto-Garcia L, Iglesias-Garcia J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology 2012;12(4):305–10. [DOI] [PubMed] [Google Scholar]
- [64].Muneoka S, Goto M, Kadoshima-Yamaoka K, Kamei R, Terakawa M, Tomimori Y. G protein-coupled receptor 39 plays an anti-inflammatory role by enhancing IL-10 production from macrophages under inflammatory conditions. Eur J Pharmacol 2018;834:240–5. [DOI] [PubMed] [Google Scholar]
- [65].Levaot N, Hershfinkel M. How cellular Zn(2+) signaling drives physiological functions. Cell Calcium 2018;75:53–63. [DOI] [PubMed] [Google Scholar]
- [66].Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr 2009;89(6):2040S–51S. [DOI] [PubMed] [Google Scholar]
- [67].Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 2009;12(6):646–52. [DOI] [PubMed] [Google Scholar]
- [68].Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients 2017;9(12):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Habtezion A, Algül H. Immune modulation in acute and chronic pancreatitis. Pancreapedia: The Exocrine Pancreas Knowledge Base; 2016. [Google Scholar]
- [70].Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Therapeut 2017;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Norman J The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg 1998;175(1):76–83. [DOI] [PubMed] [Google Scholar]
- [72].Kido T, Ishiwata K, Suka M, Yanagisawa H. Inflammatory response under zinc deficiency is exacerbated by dysfunction of the T helper type 2 lymphocyte-M2 macrophage pathway. Immunology 2019;156(4):356–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Summersgill H, England H, Lopez-Castejon G, et al. Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis 2014;5(1). e1040–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Szatmary P, Gukovsky I. The role of cytokines and inflammation in the genesis of experimental pancreatitis. Pancreapedia: Exocrine Pancreas Knowl Base 2016. 10.3998/panc.2016.29. [DOI] [Google Scholar]


