Abstract
Background:
There is a need to determine why prior concussion has been associated with adverse outcomes in some retired and active athletes. We examined whether serum inflammatory markers moderate the associations of prior concussion with hippocampal volumes and neurobehavioral functioning in active high school and collegiate athletes.
Methods:
Athletes (N=201) completed pre-season clinical testing and serum collection (C-reactive protein [CRP]; Interleukin-6 [IL]-6; IL-1 receptor antagonist [RA]) and in-season neuroimaging. Linear mixed-effects models examined associations of prior concussion with inflammatory markers, self-reported symptoms, neurocognitive function, and hippocampal volumes. Models examined whether inflammatory markers moderated associations of concussion history and hippocampal volume and/or clinical measures.
Results:
Concussion history was significantly associated with higher symptom severity, p=0.012, but not hippocampal volume or inflammatory markers (ps>0.05). A significant interaction of prior concussion and CRP was observed for hippocampal volume, p=0.006. Follow-up analyses showed that at high levels of CRP, athletes with two or more prior concussions had smaller hippocampal volume compared to athletes without prior concussion, p=0.008. There was a significant interaction between prior concussion and levels of IL-1RA on memory scores, p=0.044, i.e., at low levels of IL-1RA, athletes with two or more concussions had worse memory performance than those without prior concussion (p=0.014).
Conclusion:
Findings suggest that certain markers of systemic inflammation moderate the association between prior concussion and hippocampal volume and episodic memory performance. Current findings highlight potential markers for predicting at-risk individuals and identify therapeutic targets for mitigating the long-term adverse consequences of cumulative concussion.
Keywords: Concussion, inflammation, volumetrics, c-reactive protein
1. Introduction
Multiple studies have reported associations between concussion history, smaller hippocampal volumes, memory impairment, and a range of psychological and physical symptoms (Lepage et al., 2018; Misquitta et al., 2018; Strain et al., 2015). Emerging evidence suggests that the detrimental effects of prior concussion or cumulative exposure to repetitive head impacts (RHI) may also be present in younger athletes (Brett et al., 2019; Meier et al., 2016; Parivash et al., 2019; Singh et al., 2014). Concussion and RHI differ, in that concussion involves a manifestation of symptoms such as neurologic or cognitive dysfunction following a force to the head; whereas RHI involves head impacts that do not result in measurable acute symptoms or signs. Not all studies, however, have reported significant relationships between concussion history and smaller hippocampal volumes (Terry and Miller, 2017; Zivadinov et al., 2018) or greater symptom endorsement (Asken et al., 2017). Thus, there is a great need to identify moderating factors that could potentially explain why adverse outcomes are observed in subsets of athletes with prior concussion. Inflammation is implicated as one such factor.
Systemic markers of inflammation, such as C-reactive protein (CRP), have been linked with smaller hippocampal volumes, reduced memory function, and psychological and physical symptom endorsement in both diseased and healthy cohorts (Marsland et al., 2015; Parkin et al., 2019; Satizabal et al., 2012; Su et al., 2016). Markers of systemic inflammation (e.g., CRP and interleukin [IL]-6), as well as IL-1 receptor antagonist (IL-1RA), have been observed as also being elevated following acute concussion (Meier et al., 2020; Shetty et al., 2019). Inflammation is associated with potential risk-factors for poor outcome following brain injury or concussion, such as psychiatric disease (Iverson et al., 2017; Reus et al., 2015; Savitz and Harrison, 2018), and is associated with progressive diseases for which brain injury is thought to be a risk-factor (e.g., depression, dementia and multiple sclerosis) (Albrecht et al., 2019; Dendrou et al., 2015; Heppner et al., 2015; Montgomery et al., 2017; Reus et al., 2015). There is also evidence that chronic inflammation can change the reactive state of immune cells, sensitizing or “priming” the immune system to react more strongly to additional inflammatory triggers (Dilger and Johnson, 2008; Muccigrosso et al., 2016). Prior studies have reported a positive association between prior concussion and circulating concentrations of monocyte chemoattractant protein (MCP) 1 and MCP-2 among university athletes (Di Battista et al., 2016), as well between the number of prior concussions and plasma IL-6 levels in veterans (Guedes et al., 2020). Moreover, higher levels of neuroinflammation (assessed using PET) have been associated with greater exposure to contact sport participation among former professional American football players (Coughlin et al., 2017), providing additional indirect support that prior concussion can potentiate the inflammatory response to a subsequent concussion.
Despite this evidence linking inflammation, prior concussion, hippocampal volume, and neurobehavioral functioning, no studies to date have investigated the relationship between these factors in young, active athletes. Investigating such factors among young athletes is essential to better understand the association between concussive injury earlier in life and adverse long-term outcomes, as poor long-term outcomes identified in former athletes potentially reflect gradual processes that are initiated prior to the manifestation of clinical impairment and frank symptoms. Therefore, the aims of this study were twofold: 1) to determine whether prior concussion is associated with pre-season serum markers of inflammation, self-report and neurocognitive measures, and hippocampal volumes; and 2) to determine whether serum levels of inflammatory markers moderate associations between concussion history and hippocampal volume and/or clinical measures (i.e., interaction effect). Based on prior findings, we hypothesized that prior concussion would be associated with higher levels of serum inflammatory markers, more severe self-report symptoms, worse neurocognitive performance, and smaller hippocampal volumes (Brett et al., 2019; Parivash et al., 2019; Singh et al., 2014). Moreover, we hypothesized that the association of prior concussion with clinical measures and hippocampal volumes would be moderated by serum inflammatory-marker levels.
2. Materials and Methods
2.1. Participants
This work is a secondary analysis of data collected from high school and collegiate athletes enrolled as part of a prospective study of concussion between August 2015 and June 2018. Adult participants and parents of minor participants provided written informed consent. Minor participants provided written assent. This study was approved by the Medical College of Wisconsin Institutional Review Board (IRB) and the U.S. Department of Defense Human Research Protection Office (HRPO).
The parent study protocol (Project Head to Head II) is described in greater detail elsewhere (Chin et al., 2016; Klein et al., 2019; Meier et al., 2020). Briefly, athletes (i.e., American football players and non-contact sport athletes) participated in baseline visits (i.e., prior to their respective competitive sports season) that included a detailed clinical battery and collection of blood specimens, and also completed multiple in-season (i.e., during their competitive sports season) neuroimaging visits as part of the parent project as either acutely injured athletes or non-injured controls (median time from baseline to first MRI = 9.5 weeks, IQR =3-16). At the baseline visit, athletes were provided a standard definition of concussion when inquiring about previous injury history. The definition was based on that of the United States Department of Defense, which included “a blow to the head followed by a variety of symptoms that may include any of the following: headache, dizziness, loss of balance, blurred vision, seeing stars, feeling in a fog or slowed down, memory problems, poor concentration, nausea or throwing up. Getting knocked out or being unconscious does not always occur with a concussion” (Carney et al., 2014). For the parent study, exclusion criteria included: history or suspicion of conditions known to cause cognitive dysfunction (e.g., epilepsy, moderate to severe TBI, or other neurological disease), injury that would preclude participation in the study protocol, current psychotic disorder or narcotic use, any contraindication to study procedures. For the current study, additional exclusions included poor scan quality or processing errors (e.g., due to motion artifacts; see below), or missing data for primary variables of interest were excluded (Figure 1).
Figure 1: Study design.
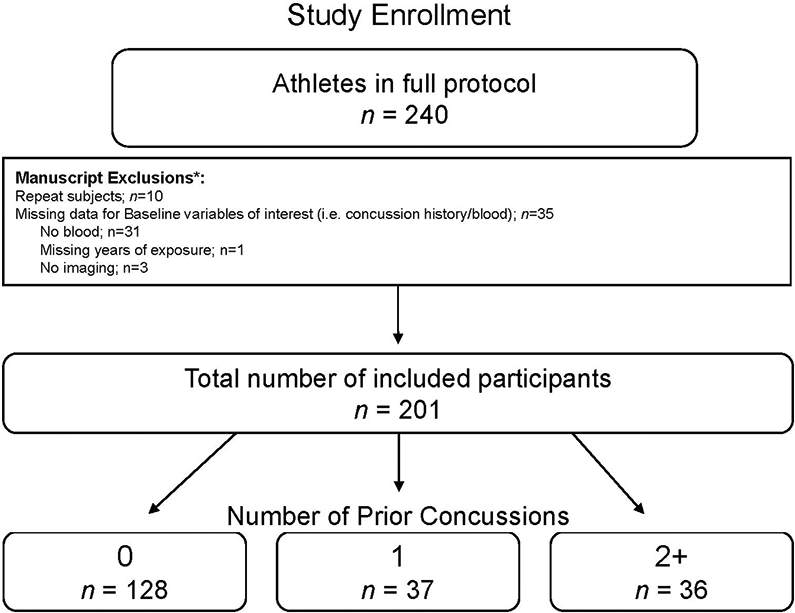
Shown is the consort diagram for the current study. *Indicates that participants could have multiple exclusion criteria. Repeat subjects refers to participants that completed the parent study in multiple subsequent years.
2.2. Clinical Battery
Demographic and health history information was collected at pre-season. Athletes self-reported the number of prior concussions (diagnosed and undiagnosed) and years of exposure to contact sport (e.g., football, soccer, etc.). The Wechsler Test of Adult Reading (WTAR) was collected to estimate pre-morbid intelligence. Based on prior published findings (Brett et al., 2019; Singh et al., 2014; Strain et al., 2015), a priori clinical outcomes of interest included select measures of clinical symptoms and neurocognition. Self-report symptom measures included the Sport Concussion Assessment Tool (SCAT) and Brief Symptom Inventory-18 Global Severity Index (BSI-18 GSI). Primary neurocognitive measures of interest included Memory (Verbal Memory and Visual Memory) and Speed (Visual Motor Speed and Reaction Time) composite scores previously established through a two-factor model (Schatz and Maerlender, 2013) of the Immediate Post-Concussion and Cognitive Testing (ImPACT) online neurocognitive test battery.
2.3. Imaging Protocol and Processing
High resolution T1-weighted images (1 mm x 1 mm x 1 mm) were acquired on 3 Tesla General Electric MR750 whole-body MR scanner using a 32-channel receiver coil array at the multiple in-season visits via a magnetization-prepared rapid gradient-echo sequence with the following parameters: FOV = 256 mm, acquisition matrix = 256, 160 slices, slice thickness = 1 mm, TR/TE/TI = 7.592/3.008/900 ms, flip angle = 8 degrees. Cortical surfaces were reconstructed and tissue segmentation was performed on the T1-weighted images using the following steps implemented in FreeSurfer v5.3: removal of non-brain tissue, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter structures, intensity normalization, tessellation of the gray/white matter boundary, automated topology correction, and surface deformation following the intensity gradients of the image in order to accurately place the boundaries between gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). The longitudinal stream was used to extract reliable volume estimates (Reuter et al., 2012). Visual inspection of each step of the processing stream was conducted.
2.4. Serum Biomarkers
Venous blood was collected using Red Top BD (East Rutherford, NJ) Vacutainer tubes, left to clot at room temperature for 30 minutes, and centrifuged at 1,500 relative centrifugal force for 15 minutes. Serum concentrations of IL-6, IL-1RA, and CRP were measured in duplicate, blinded to concussion status, using a Meso Scale Discovery QuickPlex SQ 120 instrument and MSD V-PLEX assays following manufacturer’s instructions. Samples that were lower/higher than level of detection or with coefficients of variation (CV) greater than 25% were excluded from analyses (n=1 for CRP; n=14 for IL-6).
2.5. Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics version 24 (Armonk, NY). Two generalized linear models (GLM) were fitted to determine the association of prior concussion with pre-season clinical measures and serum inflammatory markers (IL-6, IL-1RA, CRP), respectively. Age, years of contact sport exposure, and body mass index (BMI; for the inflammatory marker analysis only) were used as covariates in the analysis for self-report symptoms; race (coded as white or non-white) and WTAR scores (i.e., pre-morbid intelligence estimate) were included as additional covariates for models for the cognitive outcomes (i.e., ImPACT scores; Asken et al., 2019; Green et al., 2008; Houck et al., 2018). Concussion history was treated as a categorical variable, consisting of groups with 0, 1, and 2 or more prior concussions, based on the sample distribution (i.e., n=14, 7%, with 3 or more). Given the high propensity for positive skew, negative binomial models were fitted for self-report symptom measures (SCAT and BSI-GSI). Linear mixed effects models (LME) were used to determine the association of prior concussion with hippocampal volumes, using intracranial volume (ICV) in addition to the same covariates listed above. For volume analyses, participant was modeled as a random effect to account for repeating scanning over time. Moreover, group (current sport as American football or non-contact athlete, and whether they were injured at some point in the season) were including in the volumetric models to account for potential variance associated with acute injury. Sensitivity analyses in the current sample demonstrated no differences or changes across visit in hippocampal volume due to acute injury, consistent with prior reports showing that a single SRC does not cause measurable changes in volume in the acute and sub-acute time period (Espana et al., 2017).
To determine the interactive effects of prior concussion and pre-season inflammatory markers on clinical measures, GLMs were fit with each inflammatory marker (IL-6, IL-1RA, CRP, run separately) and the interaction of each inflammatory marker and prior concussion as well as the variables listed above. Similarly, LMEs were fitted for hippocampal volume analyses as above with the inclusion of the interaction of inflammatory marker and prior concussion. For ease of interpretation of significant interactive effects of prior concussion and serum inflammatory markers, post-hoc analyses examined the effects of prior concussion on outcomes at different levels of inflammatory markers (i.e., mean and ±1 SD). For all models, inflammatory markers were natural log-transformed and mean centered. Clinical measures of interest included SCAT symptom severity scores, BSI-18 GSI, and the Memory and Speed composite indices of ImPACT. Given our a priori hypotheses regarding prior concussion, an uncorrected alpha of 0.05 (two-tailed) was used for analyses.
Finally, secondary analyses were conducted to determine the interactive effects of contact sport exposure and inflammatory markers on outcomes of interest given prior reports of negative outcomes due to RHI without frank concussion (Stein et al., 2015; Tagge et al., 2018). Identical LME models were fit as above, with the additional interaction term of years of contact sport by inflammatory marker (IL-6, IL-1RA, CRP) included in the model. Due to their exploratory nature, an alpha of 0.01 (0.05/5 outcomes; two-tailed) was used for these secondary analyses.
3. Results
3.1. Participant Characteristics
A total number of 201 participants met the criteria for inclusion described above (Figure 1; Table 1). Prior concussions were reported as follows; 0 (n=128; 63.7%), 1 (n=37; 18.4%), and 2+ (n=36; 17.9%). On average, athletes reported 6.34 (SD=4.32) years of contact sport history.
Table 1.
Demographics and Sample Characteristics
| Demographic | M(SD) or N |
|---|---|
| N | 201 |
| Age | 18.32(1.67) |
| Race(N) | |
| White | 158 |
| Non-white | 41 |
| Not reported | 2 |
| Ethnicity (N) | |
| Non-Hispanic/Latino | 178 |
| Hispanic/Latino | 13 |
| Not reported | 10 |
| Education level | |
| College | 162 |
| High school | 39 |
| WTAR Standard Score | 100.38(12.98) |
| BMI | 28.12(5.66) |
| Years of Contact Sport Exposure | 6.34(4.32) |
| Number of Concussions (N) | |
| 0 | 128 |
| 1 | 37 |
| 2+ | 36 |
| Baseline Measures | M(SD) |
| SCAT Symp. Sev. Score | 2.79(5.05) |
| BSI-18 GSI | 3.24(4.82) |
| ImPACT Memory | 0.06(0.88) |
| ImPACT Speed | 0.00(0.87) |
| ln CRP (pg/mL) | 13.31(1.49) |
| ln IL-6 (pg/mL) | −0.67(0.71) |
| ln IL-1RA (pg/mL) | 5.56(0.52) |
M = mean, SD = standard deviation, N = number, NR= Not reported or unknown, WTAR= Wechsler Test of Adult Reading, BMI= Body Mass Index, SCAT Symp. Sev. = Sport Concussion Assessment Tool-3 symptom severity, BSI-GSI= Brief Symptom Inventory Global Severity Index, ImPACT = Immediate Post-Concussion Assessment and Cognitive Testing, ln = natural log transformed, C-reactive Protein (CRP), IL = interleukin, RA = receptor antagonist, pg/mL = picogram per milliliter.
3.2. Effect of prior concussion
Concussion history was significantly associated with SCAT symptom severity, p=0.012 (Table 2; Figure 2). Post-hoc testing showed that participants with 2 or more prior concussions reported significantly higher SCAT symptom scores compared to those with no prior concussions, p=0.008, and those reporting 1 prior concussion, p=0.007 (Table 2). There were no differences in SCAT scores between those who reported 0 or 1 prior concussion, p>0.05. There were no significant associations between prior concussion and general emotional distress (BSI-GSI), Speed or Memory Composite scores, all p>0.05; (Table 2).
Table 2:
Effects of prior concussion on variables of interest
| Clinical measures | Main Effect | Contrast | MD | Std. Error | 95 % CI | p |
|---|---|---|---|---|---|---|
| Symp. Sev. Score | χ2(2)=8.93, p=0.012 | 0 vs. 1 | 0.16 | 0.31 | −0.44, 0.77 | 0.60 |
| 0 vs. 2+ | −0.81 | 0.31 | −1.41, −0.12 | 0.01 | ||
| 1 vs. 2+ | −0.97 | 0.36 | −1.68, −0.26 | 0.01 | ||
| BSI-GSI | χ2(2)=5.95, p=0.051 | 0 vs. 1 | −0.03 | 0.29 | −0.60, 0.54 | 0.92 |
| 0 vs. 2+ | −0.69 | 0.30 | −1.27, −0.11 | 0.02 | ||
| 1 vs. 2+ | −0.66 | 0.34 | −1.33, 0.01 | 0.05 | ||
| ImPACT-Memory | χ2(2)=0.52, p=0.77 | 0 vs. 1 | −0.04 | 0.17 | −0.38, 0.29 | 0.79 |
| 0 vs. 2+ | 0.10 | 0.18 | −0.25, 0.44 | 0.58 | ||
| 1 vs. 2+ | 0.14 | 0.20 | −0.25, 0.54 | 0.48 | ||
| ImPACT-Speed | χ2(2)=0.50, p=0.78 | 0 vs. 1 | −0.01 | 0.16 | −0.32, 0.29 | 0.94 |
| 0 vs. 2+ | 0.10 | 0.16 | −0.21, 0.42 | 0.52 | ||
| 1 vs. 2+ | 0.12 | 0.18 | −0.25, 0.48 | 0.53 | ||
| Baseline Inflammatory markers |
||||||
| CRP | χ2(2)=0.11, p=0.94 | 0 vs. 1 | −0.09 | 0.29 | −0.67, 0.48 | 0.76 |
| 0 vs. 2+ | 0.01 | 0.30 | −0.58, 0.60 | 0.98 | ||
| 1 vs. 2+ | 0.10 | 0.35 | −0.58, 0.78 | 0.78 | ||
| IL-6 | χ2(2)=0.83, p=0.66 | 0 vs. 1 | 0.03 | 0.14 | −0.24, 0.31 | 0.82 |
| 0 vs. 2+ | −0.11 | 0.15 | −0.40, 0.18 | 0.44 | ||
| 1 vs. 2+ | −0.14 | 0.17 | −0.47, 0.19 | 0.39 | ||
| IL-1RA | χ2(2)=1.89, p=0.39 | 0 vs. 1 | −0.02 | 0.10 | −0.22, 0.18 | 0.86 |
| 0 vs. 2+ | −0.14 | 0.10 | −0.34, 0.06 | 0.19 | ||
| 1 vs. 2+ | −0.12 | 0.12 | −0.36, 0.11 | 0.31 | ||
| Subcortical Volume |
||||||
| Hippocampus | F(2, 192.01)=0.66, p=0.52 | 0 vs. 1 | 153.30 | 153.39 | −149.24, 455.85 | 0.32 |
| 0 vs. 2+ | 141.61 | 160.21 | −174.38, 457.60 | 0.38 | ||
| 1 vs. 2+ | −11.69 | 179.48 | −365.70, 342.32 | 0.95 |
Note: MD = estimated marginal mean difference, SE = standard error; CI = confidence interval, Symp. Sev. Score= SCAT-3 Symptom Severity Score, BSI-GSI = Brief Symptom Inventory Global Severity Index, C-reactive Protein (CRP), IL = interleukin, RA = receptor antagonist. Natural log-transformed values of inflammatory markers are presented (pg/mL). Symp. Sev. Score and BSI-GSI values obtained from the conducted negative binomial models with log link transformed are presented. Hippocampal volumes are presented in mm3. The lower and upper levels of quantification for markers in pg/mL are as follows: IL-1RA, 0.17-5.14 and 732-1,150; IL-6, 0.006-0.26 and 721-764; CRP, 1.11-4.62 and 202,000.
Figure 2: Effect of prior concussion on symptom severity.
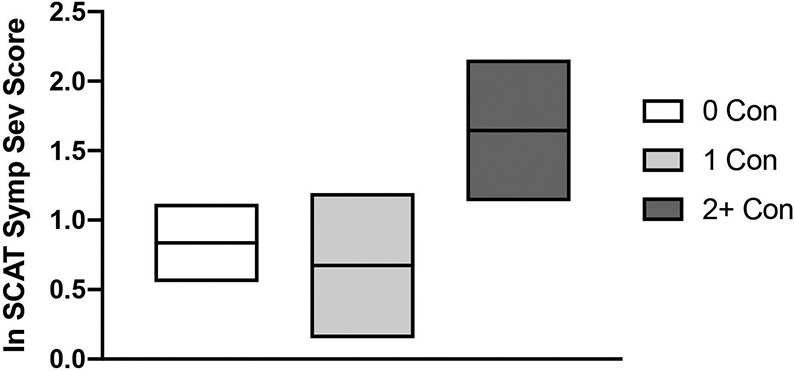
Shown are the mean (horizontal line) symptom severity scores with 95% confidence interval (top and bottom boundaries) for athletes with no prior concussion (0 Con), athletes with 1 prior concussion (1 Con) and athletes with 2 or more prior concussions (2+ Con). Log-transformed marginal means derived from the negative binomial model are displayed.
The relationship between prior concussion and hippocampal volume was not significant, p>0.05. Finally, concussion history was not significantly associated with any of the serum inflammatory markers, all p>0.05 (Table 2).
3.3. Moderating effects of inflammation - hippocampal volumes
A significant interaction of prior concussion and CRP was observed for hippocampal volume, p=0.006 (Table 3; Figure 3). Simple slope analysis showed that this effect was driven by an inverse association of prior concussion and hippocampal volume at high levels of CRP (i.e., 1 SD above the mean; Table 4). Specifically, at high levels of CRP athletes with 2+ prior concussions had significantly smaller hippocampal volumes compared to those with 0 prior concussions and a trend for smaller hippocampal volumes compared to those with 1 prior concussion. There were no significant interactions between prior concussion and either IL-6 or IL-1RA on hippocampal volume, all p>0.05. Significant main effects of serum blood markers of inflammation on hippocampal volume were not observed, all p>0.05.
Table 3:
Moderating effects of inflammatory markers
| Measure | Prior Con. by CRP | Prior Con. by IL-6 |
Prior Con. by IL-1RA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical measures | df | χ2 | p | df | χ2 | p | df | χ2 | p |
| Symp. Sev. | 2 | 0.68 | 0.71 | 2 | 5.50 | 0.06 | 2 | 2.11 | 0.35 |
| BSI-GSI | 2 | 3.35 | 0.19 | 2 | 4.26 | 0.12 | 2 | 4.15 | 0.12 |
| ImPACT-Memory | 2 | 4.15 | 0.13 | 2 | 3.71 | 0.16 | 2 | 6.23 | 0.044 |
| ImPACT-Speed | 2 | 0.77 | 0.68 | 2 | 1.47 | 0.48 | 2 | 0.68 | 0.71 |
| Subcortical Volume | df | F | p | df | F | p | df | F | p |
| Hippocampus | 2, 187.43 | 5.24 | 0.006 | 2, 174.79 | 1.07 | 0.34 | 2, 188.83 | 0.87 | 0.42 |
Prior Con = Prior Concussion, C-reactive Protein (CRP), IL = interleukin, RA = receptor antagonist, Symp. Sev. = SCAT-3 Symptom Severity Score, BSI-GSI= Brief Symptom Inventory Global Severity Index, ImPACT = Immediate Post-Concussion Assessment and Cognitive Testing.
Figure 3: Moderating effect of inflammatory markers on hippocampal volume.
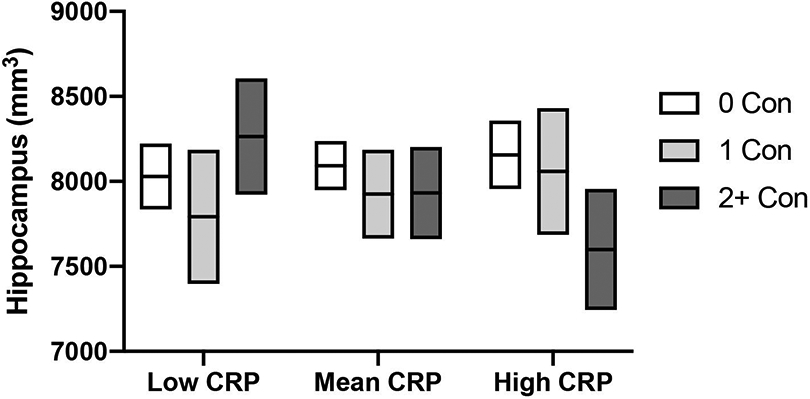
Mean (horizontal line) hippocampal volumes with 95% confidence interval (top and bottom boundaries) are shown for athletes with no prior concussion (0 Con), athletes with 1 prior concussion (1 Con) and athletes with 2 or more prior concussions (2+ Con) at low, mean, and high pre-season levels of c-reactive protein (CRP). Low and high levels were defined as values being 1 standard deviation below or above the mean values. Estimated marginal means based on corresponding models are shown.
Table 4.
Simple slope analyses for significant moderation effects
| Hippocampus and CRP | Test Statistic | p-value |
|---|---|---|
| Low CRP | F(2, 187.61)=1.72 | 0.18 |
| Mean CRP | F(2, 187.96)=0.86 | 0.43 |
| High CRP | F(2, 187.80)=3.63 | 0.028 |
| Contrast* | MD(SE), [95%CI] | |
| 0 vs 1 | 97.32(213.51), [−323.88, 518.52] | 0.65 |
| 0 vs 2+ | 556.19(248.34), [144.74, 967.65] | 0.008 |
| 1 vs 2+ | 458.87(208.53), [−31.02, 948.76] | 0.066 |
| Memory and IL-1RA | Test Statistic | p-value |
| Low IL-1RA | χ2 (2)=6.01 | 0.049 |
| Contrast* | MD(SE), [95%CI] | p-value |
| 0 vs 1 | 0.14(0.22), [−0.29, 0.57] | 0.52 |
| 0 vs 2+ | 0.57(0.23), [0.11, 1.02] | 0.014 |
| 1 vs 2+ | 0.42(0.28), [−0.12,0.97] | 0.12 |
| Mean IL-1RA | χ2 (2)=2.06 | 0.36 |
| High IL-1RA | χ2 (2)=0.63 | 0.73 |
Indicates within model contrasts performed for significant or trending simple slope effects.
MD = estimated marginal mean difference; SE = standard error; CI = confidence interval; CRP=c-reactive protein (CRP); IL-1RA = interleukin 1 receptor antagonist. Inflammatory markers are natural log-transformed for analyses (pg/mL). Subcortical volumes are presented in mm3.
3.4. Moderating effects of inflammation – clinical measures
There was no significant interaction between concussion history and serum blood markers of inflammation on self-reported symptom measures, all p>0.05 (Table 3). There was a significant interaction between prior concussion and levels of IL-1RA on the ImPACT Memory Composite, p=0.044 (Table 4; Figure 4). Simple slope analysis showed that this effect was driven by poorer memory performance in athletes with 2 or more concussions (versus no prior concussion) who also had low levels of IL-1RA. The interactions between pre-season serum inflammatory markers and prior concussion on the ImPACT Speed Composite were not significant, ps>0.05 (Table 3). Finally, for main effects within the interaction models, higher IL1-RA was significantly associated with higher BSI-GSI scores, χ2(1)= 3.98, p=0.046, and worse ImPACT Speed Composite scores, χ2(1)= 5.67, p=0.017, while higher CRP was also associated with worse ImPACT Speed Composite scores, χ2(1)= 6.50, p=0.011. Significant main effects of other inflammation markers on clinical outcomes were not observed.
Figure 4: Moderating effect of IL1-RA on memory scores.
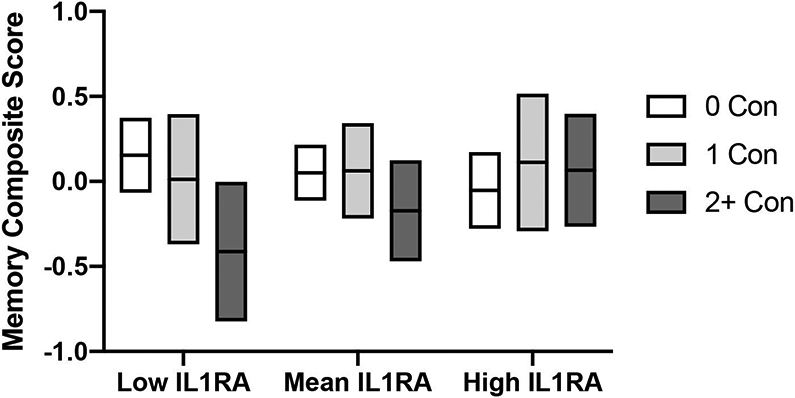
Mean (horizontal line) ImPACT memory composite scores with 95% confidence interval (top and bottom boundaries) are shown for athletes with no prior concussion (0 Con), athletes with 1 prior concussion (1 Con) and athletes with 2 or more prior concussions (2+ Con) at low, mean, and high pre-season levels of interleukin-1 receptor antagonist (IL-1RA). Low and high levels were defined as values being 1 standard deviation below or above the mean values. Estimated marginal means based on corresponding models are shown.
3.5. Secondary analyses: moderating effects of inflammatory markers on contact sport exposure
Exploratory analyses were conducted to determine whether inflammatory markers moderated the relationship between years of contact exposure and outcome variables (Table 5). For clinical outcome measures, there were no significant interactions between contact exposure and either CRP, IL-6, or IL-1RA (ps>0.10). Furthermore, the previous interaction between IL-1RA and prior concussion was no longer significant with the inclusion of the IL-1RA-by-exposure interaction (p=0.15), For hippocampal volume, there was a marginal interaction effect of years of contact sport exposure by IL-6, p=0.041; however, this was not significant following multiple comparison correction applied to these secondary analyses. There were no significant interactions between years of contact sport exposure and either CRP or IL-1RA on hippocampal volume, all p>0.05. Moreover, the interaction between CRP and prior concussion remained significant with the addition of the CRP-by-exposure interaction, as in the primary analyses (p=0.006).
Table 5:
Moderating effects of inflammatory markers on concussion and contact sport effects
| Measure | Prior Con. by CRP | Prior Con. by IL-6 |
Prior Con. by IL-1RA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical measures | df | χ2 | p | df | χ2 | p | df | χ2 | p |
| Symp. Sev. | 2 | 0.17 | 0.92 | 2 | 2.44 | 0.30 | 2 | 1.08 | 0.58 |
| BSI-GSI | 2 | 3.30 | 0.19 | 2 | 3.71 | 0.16 | 2 | 3.60 | 0.17 |
| ImPACT-Memory | 2 | 4.67 | 0.10 | 2 | 2.94 | 0.23 | 2 | 3.78 | 0.15 |
| ImPACT-Speed | 2 | 0.81 | 0.67 | 2 | 0.57 | 0.75 | 2 | 0.29 | 0.87 |
| Subcortical Volume | df | F | p | df | F | p | df | F | p |
| Hippocampus | 2, 186.49 |
5.35 | 0.006 | 2, 173.91 | 0.32 | 0.73 | 2, 187.91 | 1.10 | 0.34 |
| Measure | Contact Exposure by CRP |
Contact Exposure by IL-6 |
Contact Exposure by IL-1RA |
||||||
| Clinical measures | df | χ2 | p | df | χ2 | p | df | χ2 | p |
| Symp. Sev. | 1 | 1.27 | 0.26 | 1 | 0.26 | 0.61 | 1 | 0.40 | 0.53 |
| BSI-GSI | 1 | 1.10 | 0.30 | 1 | 0.04 | 0.84 | 1 | 0.03 | 0.86 |
| ImPACT-Memory | 1 | 0.74 | 0.39 | 1 | 0.04 | 0.85 | 1 | 0.27 | 0.60 |
| ImPACT-Speed | 1 | 0.69 | 0.41 | 1 | 0.48 | 0.49 | 1 | 0.16 | 0.69 |
| Subcortical Volume | df | F | p | df | F | p | df | F | p |
| Hippocampus | 1, 186.61 |
0.30 | 0.59 | 1, 173.91 | 4.23 | 0.041 | 1, 187.60 | 0.48 | 0.49 |
Prior Con = Prior Concussion, C-reactive Protein (CRP), IL = interleukin, RA = receptor antagonist, Symp. Sev. = SCAT-3 Symptom Severity Score, BSI-GSI= Brief Symptom Inventory Global Severity Index, ImPACT = Immediate Post-Concussion Assessment and Cognitive Testing.
4. Discussion
The current study investigated associations of prior concussion with self-reported symptoms, neurocognitive performance, and hippocampal volumes in active high school and collegiate athletes, as well as the potential moderating role of serum blood inflammatory markers on these associations. Results revealed that greater concussion history was associated with elevations in non-specific symptoms, but not neurocognitive performance, serum inflammatory marker levels, or hippocampal volumes. There was a significant interaction between serum levels of CRP and prior concussion on hippocampal volumes, such that more prior concussions were associated with smaller hippocampal volumes only in athletes with higher levels of CRP in serum. Further, serum levels of IL-1RA moderated the association between prior concussion and episodic memory performance, with poorer performance in athletes with 2 or more prior concussions only observed at low levels of IL-1RA. Each result is discussed below.
4.1. Moderating effects of inflammatory markers on concussion history
The current study provides the first evidence for the moderating influence of inflammation on the association between concussion history and differences in brain morphometry as well as episodic memory. Specifically, the association between prior concussion and hippocampal volume was only present in athletes with high CRP at pre-season. CRP is a stable pentameric protein that is general marker of inflammation (Kao et al., 2006). Higher peripheral levels of CRP are generally associated with poor health outcomes and have been associated with brain morphometry differences across the lifespan, particularly for the hippocampus (Bettcher et al., 2012; Lassale et al., 2019; Marsland et al., 2015; Satizabal et al., 2012). In line with this research, chronic inflammation has been shown to reduce neurogenesis in the hippocampus (Chesnokova et al., 2016). Moreover, there is evidence that chronic inflammation can result in immune system priming (Dilger and Johnson, 2008; Muccigrosso et al., 2016; Perry and Holmes, 2014). That is, immune cells become sensitized and respond more strongly upon subsequent exposure to inflammatory stimuli. In this context, athletes with higher peripheral levels of CRP – potentially due to a variety of factors such as diet, prior orthopedic injuries, prior head injuries, chronic stress or poor sleep – may be primed to have a more robust acute inflammatory response to concussive events that potentially affects hippocampal integrity (e.g., reduced neurogenesis or dendritic remodeling) (Norden et al., 2015). Additional work is needed to directly test this hypothesis and determine how inflammation potentially modifies the association between concussion history and smaller hippocampal volumes.
Interestingly, IL-1RA had a different moderating effect on memory performance compared to the results observed for hippocampal volume. Here, the association between prior concussion and worse memory performance was only observed in those with low pre-season serum levels of IL-1RA. IL-1RA is a competitive inhibitor of IL-1 activity, though it can also act as an acute phase protein (Palomo et al., 2015). Antagonism of IL-1 activity has been shown to be neuroprotective in preclinical TBI models, and IL-1 receptor antagonists are considered to be potential therapeutic treatments for severe brain injury, though much is still unknown regarding their effectiveness in humans (Helmy et al., 2014; Helmy et al., 2016; Jones et al., 2005; Sanderson et al., 1999; Sun et al., 2017). In one small randomized clinical trial, a modified human IL-1RA made recombinantly (anakinra) was administered to 10 patients following severe TBI and showed preliminary potential as a therapeutic candidate (Helmy et al., 2014). The degree to which these findings apply to cumulative more mild injury and chronic (i.e., not a response to a single more severe injury) requires future examination.
4.2. Hippocampal volume, memory, and inflammation
Within the current study, a moderating effect of CRP was observed on the association between concussion history and hippocampal volumes, but not memory performance. Conversely, there was a moderating effect of IL-1RA on the association between concussion history and memory performance, but not hippocampal volumes. Intuitively, one might expect analogous moderating effects of inflammatory markers on hippocampal volumes and memory performance given the prominent role of the hippocampus in memory storage and retrieval. It is important to note, however, that prior literature linking larger hippocampal volume with better memory performance in healthy, young adults is contradictory (Pohlack et al., 2014; Van Petten, 2004) It is therefore unsurprising that the effects of prior concussion on memory and hippocampal volume are not exactly concordant.
Similarly, we are unable to determine why different inflammatory markers moderated hippocampal volume and memory performance. One possibility is that IL-1RA has a more direct effect on memory performance, consistent with preclinical literature (Skelly et al., 2019), whereas CRP is more reflective of a general pro-inflammatory milieu that more directly impacts hippocampal volume, which may lead eventually lead to memory difficulties later in life (Marsland et al., 2015). It is noteworthy, however, that the IL1-RA by concussion history effect on memory was no longer significant when the interaction effect of IL1-RA by contact sport exposure was included in the model. In contrast, the CRP by concussion history interaction effect on hippocampal volume was unchanged, suggesting a more robust association. Prospective, longitudinal follow-up studies will help to better identify the exact mechanisms linking the current findings with adverse long-term effects that have been documented in retired athletes.
4.3. Concussion history, neurobehavioral functioning, hippocampal volumes, and inflammatory markers
The current study detected a significant association between prior concussion and elevated symptom report. Multiple studies have reported on similar associations between prior concussion history and elevations in pre-season symptom endorsement among active athletes (Brett et al., 2019; Iverson et al., 2015). Conversely, the current study did not observe a relationship between prior concussion history and neurocognitive (i.e., speed and memory), consistent with prior studies and meta-analyses (Alsalaheen et al., 2017; Singh et al., 2014). . The current findings demonstrate the need for prospective, longitudinal follow-up studies given the adverse long-term effects that have been documented in retired athletes.
Contrary to our hypothesis, there was no significant association between prior concussion and hippocampal volume. This finding, in a large sample of athletes, differs from previous studies in both retired and active athletes that have reported smaller hippocampal volumes associated with more contact sport exposure or repeated concussion (Adler et al., 2018; Lepage et al., 2018; Misquitta et al., 2018). Not all studies have reported this association, however (Strain et al., 2015; Terry and Miller, 2017; Zivadinov et al., 2018). Conflicting findings across studies may be due to a variety of factors, including study design, the extent to which the effects of years of participation or repetitive head impacts were accounted for, how prior concussion and contact sport exposure were operationalized and measured, differences in cohorts (including the range of concussion history among participants, age, etc.), or unmeasured moderating factors (e.g., levels of inflammation).
The current study also failed to find an association between concussion history and serum levels of inflammation at pre-season. Previous work has shown that concussion results in acute, transient increases in inflammatory markers, including IL-6, IL-1RA, and CRP, with recovery to normal or preinjury levels (Nitta et al., 2019; Parkin et al., 2019; Su et al., 2014). Additional work has demonstrated associations between inflammation and history of concussion or contact sport exposure. For example, one study in collegiate athletes found a positive association between prior concussion and plasma levels of MCP-1 and MCP-2 in female and male athletes, respectively (Di Battista et al., 2016), while a recent study documented an association between the number of prior concussions and plasma IL-6 levels in veterans (Guedes et al., 2020). Other studies have observed higher levels of neuroinflammation associated with greater exposure to contact sport participation among former professional football players via [11C]DPA-713 positron emission tomography (e.g., higher translocator protein signal across 8 of 12 regions examined compared to controls) (Coughlin et al., 2017) or at post-mortem (e.g., increased CCL11 protein levels, increased CD68 cell density, and enhanced microglia reactive morphology) (Cherry et al., 2017; Cherry et al., 2016). The seeming discrepancy between prior and current work could be due to selected inflammatory markers, differences in studied populations (e.g., varying degrees of concussion history), or the uncertain extent to which peripheral markers of inflammation reflect inflammation in CNS.
4.4. Limitations
This study was restricted to male athletes and it is uncertain the extent to which current results apply to women and non-football contact sport athletes. Years of participation and the number of prior concussions were based on self-report, which may under- or over-estimate true values (Wojtowicz et al., 2017). In addition, inflammation was measured in the periphery and it is unclear the extent to which peripheral markers of inflammation reflect neuroinflammation, though prior studies have reported blood-brain barrier dysfunction associated with concussion and contact sport exposure (i.e., participation of a football season) (Weissberg et al., 2014). Additionally, prior work has shown very high correlations between plasma and CSF CRP levels (~0.8-0.9) (Felger et al., 2018). Although the current study design was able to utilize multiple neuroimaging visits to increase the power of the analyses (i.e., up to 4 observations per individual), the clinical baseline measures were limited to a single visit, and these analyses may be underpowered for the contrasts involving multiple concussion history groups. There are also limitations in utilizing ImPACT to assess cognitive functioning, as this instrument has been validated mainly for detecting the acute effects of concussion (i.e., mainly within 48-72 hours of injury). Therefore, it may not be sensitive to potential subtle cognitive differences in non-impaired samples due to prior concussion. The parent study from which data for the current study were obtained had no requirements for fasting blood draws or time of day for blood collection, which is a limitation given the potential sensitivity of inflammatory markers to these factors. Finally, we are unable to account for the potential effects of other factors that could be associated with baseline measures (e.g., alcohol use) or identify confounding factors arising due to the time between baseline blood/clinical data and neuroimaging data collection (i.e., preseason versus in-season visits).
4.5. Conclusion
Current results support the hypothesis that systemic inflammation is one potential factor that moderates the association between history of concussion and hippocampal volume in young, active athletes. Longitudinal studies are needed to determine the extent to which these relationships in young, active athletes translate to long-term neurobehavioral deficits reported in some former contact sport athletes. If validated, the current findings highlight potential markers for predicting at-risk individuals and identify therapeutic targets for mitigating the long-term adverse consequences of cumulative concussion.
Highlights.
Inflammation, clinical measures, and hippocampal volume were assessed in athletes
We tested whether inflammation moderates associations with prior concussion
More concussions were associated with smaller hippocampi at higher levels of CRP
IL-1RA moderated the association between prior concussion and episodic memory
More concussions were associated with poorer memory at lower levels of IL-1RA.
Acknowledgements
The authors would like to thank Ashlee Taylor and Brenda Davis from University of Oklahoma Integrative Immunology Center for analysis of serum cytokines; Ashley LaRoche and Alexa Wild from the Department of Neurosurgery at the Medical College of Wisconsin for study coordination; and Dr. Aniko Szabo from the Department of Biostatistics at the Medical College of Wisconsin for statistical consultation; and the research and medical staff at each participating site. We are grateful for the participation of the student athletes without whom this research would not be possible.
Funding
Supported by the Department of Defense Broad Agency Announcement for Extramural Medical Research through award number W81XWH-14-1-0561, National Institute of Neurological Disorders and Stroke of the NIH under award number R21NS099789, and National Institute of Neurological Disorders and Stroke under award number R01NS102225. The National Institute of General Medical Sciences (P20GM121312) and the National Institute of Mental Health (R21MH113871) also provided support for this project. BLB acknowledges support from the National Institute of Neurological Disorders and Stroke under the National Institutes of Health under the award NO L301L30NS113158-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The REDCap electronic database used for this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. This research was completed in part with computational resources and technical support provided by the Research Computing Center at the Medical College of Wisconsin.
Footnotes
Competing Interests
Nothing to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, DelBello MP, Weber W, Williams M, Duran LRP, Fleck D, Boespflug E, Eliassen J, Strakowski SM, Divine J, 2018. MRI Evidence of Neuropathic Changes in Former College Football Players. Clin J Sport Med 28, 100–105. [DOI] [PubMed] [Google Scholar]
- Albrecht JS, Barbour L, Abariga SA, Rao V, Perfetto EM, 2019. Risk of Depression after Traumatic Brain Injury in a Large National Sample. J Neurotrauma 36, 300–307. [DOI] [PubMed] [Google Scholar]
- Alsalaheen B, Stockdale K, Pechumer D, Giessing A, He X, Broglio SP, 2017. Cumulative Effects of Concussion History on Baseline Computerized Neurocognitive Test Scores: Systematic Review and Meta-analysis. Sports Health 9, 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asken BM, Snyder AR, Clugston JR, Gaynor LS, Sullan MF, Bauer RM. Concussion-Like Symptom Reporting in Non-Concussed Collegiate Athletes Arch. Clin. Neuropsychol, 32 (8) (2017), pp. 963–971 [DOI] [PubMed] [Google Scholar]
- Asken BM, Houck ZM, Clugston JR, Larrabee GJ, Broglio SP, McCrea MA, McAllister TW, Bauer RM, Investigators CC, 2019. Word-reading ability as a "hold test" in cognitively normal young adults with history of concussion and repetitive head impact exposure: A CARE Consortium Study. Clin Neuropsychol, 1–18. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL, Kramer JH, 2012. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun 26, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BL, Huber DL, Wild A, Nelson LD, McCrea MA, 2019. Age of First Exposure to American Football and Behavioral, Cognitive, Psychological, and Physical Outcomes in High School and Collegiate Football Players. Sports Health, 1941738119849076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney N, Ghajar J, Jagoda A, Bedrick S, Davis-O'Reilly C, du Coudray H, Hack D, Helfand N, Huddleston A, Nettleton T, Riggio S, 2014. Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 75 Suppl 1, S3–15. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Stein TD, Tripodis Y, Alvarez VE, Huber BR, Au R, Kiernan PT, Daneshvar DH, Mez J, Solomon TM, Alosco ML, McKee AC, 2017. CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer's disease. PLoS One 12, e0185541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH, Mez J, Montenigro PH, Solomon TM, Alosco ML, Stern RA, McKee AC, Stein TD, 2016. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun 4, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Pechnick RN, Wawrowsky K, 2016. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun 58, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin EY, Nelson LD, Barr WB, McCrory P, McCrea MA, 2016. Reliability and Validity of the Sport Concussion Assessment Tool-3 (SCAT3) in High School and Collegiate Athletes. Am J Sports Med 44, 2276–2285. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Minn I, Bienko N, Ambinder EB, Xu X, Peters ME, Dougherty JW, Vranesic M, Koo SM, Ahn HH, Lee M, Cottrell C, Sair HI, Sawa A, Munro CA, Nowinski CJ, Dannals RF, Lyketsos CG, Kassiou M, Smith G, Caffo B, Mori S, Guilarte TR, Pomper MG, 2017. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol 74, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendrou CA, Fugger L, Friese MA, 2015. Immunopathology of multiple sclerosis. Nat Rev Immunol 15, 545–558. [DOI] [PubMed] [Google Scholar]
- Di Battista AP, Rhind SG, Richards D, Churchill N, Baker AJ, Hutchison MG, 2016. Altered Blood Biomarker Profiles in Athletes with a History of Repetitive Head Impacts. PLoS One 11, e0159929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW, 2008. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 84, 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana LY, Lee RM, Ling JM, Jeromin A, Mayer AR, Meier TB, 2017. Serial Assessment of Gray Matter Abnormalities after Sport-Related Concussion. J Neurotrauma 34, 3143–3152. [DOI] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le NA, Feinberg R, Tansey MG, Miller AH, 2018. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RE, Melo B, Christensen B, Ngo LA, Monette G, Bradbury C, 2008. Measuring premorbid IQ in traumatic brain injury: an examination of the validity of the Wechsler Test of Adult Reading (WTAR). J Clin Exp Neuropsychol 30, 163–172. [DOI] [PubMed] [Google Scholar]
- Guedes VA, Kenney K, Shahim P, Qu BX, Lai C, Devoto C, Walker WC, Nolen T, Diaz-Arrastia R, Gill JM, Investigators CMOS, 2020. Exosomal neurofilament light: A prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology 94, e2412–e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ, 2014. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab 34, 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Guilfoyle MR, Carpenter KLH, Pickard JD, Menon DK, Hutchinson PJ, 2016. Recombinant human interleukin-1 receptor antagonist promotes M1 microglia biased cytokines and chemokines following human traumatic brain injury. J Cereb Blood Flow Metab 36, 1434–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B, 2015. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
- Houck Z, Asken B, Clugston J, Perlstein W, Bauer R, 2018. Socioeconomic Status and Race Outperform Concussion History and Sport Participation in Predicting Collegiate Athlete Baseline Neurocognitive Scores. J Int Neuropsychol Soc 24, 1–10. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS, 2017. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med 51, 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Silverberg ND, Mannix R, Maxwell BA, Atkins JE, Zafonte R, Berkner PD, 2015. Factors Associated With Concussion-like Symptom Reporting in High School Athletes. JAMA Pediatr 169, 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Prior MJ, Burden-Teh E, Marsden CA, Morris PG, Murphy S, 2005. Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur J Neurosci 22, 72–78. [DOI] [PubMed] [Google Scholar]
- Kao PC, Shiesh SC, Wu TJ, 2006. Serum C-reactive protein as a marker for wellness assessment. Ann Clin Lab Sci 36, 163–169. [PubMed] [Google Scholar]
- Klein AP, Tetzlaff JE, Bonis JM, Nelson LD, Mayer AR, Huber DL, Harezlak J, Mathews VP, Ulmer JL, Sinson GP, Nencka AS, Koch KM, Wu YC, Saykin AJ, DiFiori JP, Giza CC, Goldman J, Guskiewicz KM, Mihalik JP, Duma SM, Rowson S, Brooks A, Broglio SP, McAllister T, McCrea MA, Meier TB, 2019. Prevalence of Potentially Clinically Significant Magnetic Resonance Imaging Findings in Athletes with and without Sport-Related Concussion. J Neurotrauma 36, 1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassale C, Batty GD, Steptoe A, Cadar D, Akbaraly TN, Kivimaki M, Zaninotto P, 2019. Association of 10-Year C-Reactive Protein Trajectories With Markers of Healthy Aging: Findings From the English Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 74, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage C, Muehlmann M, Tripodis Y, Hufschmidt J, Stamm J, Green K, Wrobel P, Schultz V, Weir I, Alosco ML, Baugh CM, Fritts NG, Martin BM, Chaisson C, Coleman MJ, Lin AP, Pasternak O, Makris N, Stern RA, Shenton ME, Koerte IK, 2018. Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB, 2015. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun 48, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Bellgowan PS, Bergamino M, Ling JM, Mayer AR, 2016. Thinner Cortex in Collegiate Football Players With, but not Without, a Self-Reported History of Concussion. J Neurotrauma 33, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Huber DL, Bohorquez-Montoya L, Nitta ME, Savitz J, Teague TK, Bazarian JJ, Hayes RL, Nelson LD, McCrea MA, 2020. A Prospective Study of Acute Blood-Based Biomarkers for Sport-Related Concussion. Ann Neurol 87, 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misquitta K, Dadar M, Tarazi A, Hussain MW, Alatwi MK, Ebraheem A, Multani N, Khodadadi M, Goswami R, Wennberg R, Tator C, Green R, Colella B, Davis KD, Mikulis D, Grinberg M, Sato C, Rogaeva E, Louis Collins D, Tartaglia MC, 2018. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. Neuroimage Clin 19, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S, Hiyoshi A, Burkill S, Alfredsson L, Bahmanyar S, Olsson T, 2017. Concussion in adolescence and risk of multiple sclerosis. Ann Neurol 82, 554–561. [DOI] [PubMed] [Google Scholar]
- Muccigrosso MM, Ford J, Benner B, Moussa D, Burnsides C, Fenn AM, Popovich PG, Lifshitz J, Walker FR, Eiferman DS, Godbout JP, 2016. Cognitive deficits develop 1month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain Behav Immun 54, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta ME, Savitz J, Nelson LD, Teague TK, Hoelzle JB, McCrea MA, Meier TB, 2019. Acute elevation of serum inflammatory markers predicts symptom recovery after concussion. Neurology 93, e497–e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, Godbout JP, 2015. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo J, Dietrich D, Martin P, Palmer G, Gabay C, 2015. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine 76, 25–37. [DOI] [PubMed] [Google Scholar]
- Parivash SN, Goubran M, Mills BD, Rezaii P, Thaler C, Wolman D, Bian W, Mitchell LA, Boldt B, Douglas D, Wilson EW, Choi J, Xie L, Yushkevich PA, DiGiacomo P, Wongsripuemtet J, Parekh M, Fiehler J, Do H, Lopez J, Rosenberg J, Camarillo D, Grant G, Wintermark M, Zeineh M, 2019. Longitudinal Changes in Hippocampal Subfield Volume Associated with Collegiate Football. J Neurotrauma 36, 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin GM, Clarke C, Takagi M, Hearps S, Babl FE, Davis GA, Anderson V, Ignjatovic V, 2019. Plasma Tumor Necrosis Factor Alpha Is a Predictor of Persisting Symptoms Post-Concussion in Children. J Neurotrauma 36, 1768–1775. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C, 2014. Microglial priming in neurodegenerative disease. Nat Rev Neurol 10, 217–224. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Meyer P, Cacciaglia R, Liebscher C, Ridder S, Flor H, 2014. Bigger is better! Hippocampal volume and declarative memory performance in healthy young men. Brain Struct Funct 219, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J, 2015. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300, 141–154. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B, 2012. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Saatman KE, Martin D, Miller G, McIntosh TK, 1999. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab 19, 1118–1125. [DOI] [PubMed] [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C, 2012. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 78, 720–727. [DOI] [PubMed] [Google Scholar]
- Savitz J, Harrison NA, 2018. Interoception and Inflammation in Psychiatric Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging 3, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P, Maerlender A, 2013. A two-factor theory for concussion assessment using ImPACT: memory and speed. Arch Clin Neuropsychol 28, 791–797. [DOI] [PubMed] [Google Scholar]
- Shetty T, Cogsil T, Dalal A, Kim E, Halvorsen K, Cummings K, Nguyen JT, 2019. High-Sensitivity C-Reactive Protein: Retrospective Study of Potential Blood Biomarker of Inflammation in Acute Mild Traumatic Brain Injury. J Head Trauma Rehabil 34, E28–E36. [DOI] [PubMed] [Google Scholar]
- Singh R, Meier TB, Kuplicki R, Savitz J, Mukai I, Cavanagh L, Allen T, Teague TK, Nerio C, Polanski D, Bellgowan PS, 2014. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 311, 1883–1888. [DOI] [PubMed] [Google Scholar]
- Skelly DT, Griffin EW, Murray CL, Harney S, O'Boyle C, Hennessy E, Dansereau MA, Nazmi A, Tortorelli L, Rawlins JN, Bannerman DM, Cunningham C, 2019. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry 24, 1533–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Alvarez VE, McKee AC, 2015. Concussion in Chronic Traumatic Encephalopathy. Curr Pain Headache Rep 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JF, Womack KB, Didehbani N, Spence JS, Conover H, Hart J Jr., Kraut MA, Cullum CM, 2015. Imaging Correlates of Memory and Concussion History in Retired National Football League Athletes. JAMA Neurol 72, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Faluyi YO, Hong YT, Fryer TD, Mak E, Gabel S, Hayes L, Soteriades S, Williams GB, Arnold R, Passamonti L, Rodriguez PV, Surendranathan A, Bevan-Jones RW, Coles J, Aigbirhio F, Rowe JB, O'Brien JT, 2016. Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. Br J Psychiatry 209, 525–526.27758838 [Google Scholar]
- Su SH, Xu W, Li M, Zhang L, Wu YF, Yu F, Hai J, 2014. Elevated C-reactive protein levels may be a predictor of persistent unfavourable symptoms in patients with mild traumatic brain injury: a preliminary study. Brain Behav Immun 38, 111–117. [DOI] [PubMed] [Google Scholar]
- Sun M, Brady RD, Wright DK, Kim HA, Zhang SR, Sobey CG, Johnstone MR, O'Brien TJ, Semple BD, McDonald SJ, Shultz SR, 2017. Treatment with an interleukin-1 receptor antagonist mitigates neuroinflammation and brain damage after polytrauma. Brain Behav Immun 66, 359–371. [DOI] [PubMed] [Google Scholar]
- Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, Wojnarowicz MW, Casey N, Lu H, Kokiko-Cochran ON, Saman S, Ericsson M, Onos KD, Veksler R, Senatorov VV Jr., Kondo A, Zhou XZ, Miry O, Vose LR, Gopaul KR, Upreti C, Nowinski CJ, Cantu RC, Alvarez VE, Hildebrandt AM, Franz ES, Konrad J, Hamilton JA, Hua N, Tripodis Y, Anderson AT, Howell GR, Kaufer D, Hall GF, Lu KP, Ransohoff RM, Cleveland RO, Kowall NW, Stein TD, Lamb BT, Huber BR, Moss WC, Friedman A, Stanton PK, McKee AC, Goldstein LE, 2018. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry DP, Miller LS, 2017. Repeated mild traumatic brain injuries is not associated with volumetric differences in former high school football players. Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- Van Petten C, 2004. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 42, 1394–1413. [DOI] [PubMed] [Google Scholar]
- Weissberg I, Veksler R, Kamintsky L, Saar-Ashkenazy R, Milikovsky DZ, Shelef I, Friedman A, 2014. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol 71,1453–1455. [DOI] [PubMed] [Google Scholar]
- Wojtowicz M, Iverson GL, Silverberg ND, Mannix R, Zafonte R, Maxwell B, Berkner PD, 2017. Consistency of Self-Reported Concussion History in Adolescent Athletes. J Neurotrauma 34, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Polak P, Schweser F, Bergsland N, Hagemeier J, Dwyer MG, Ramasamy DP, Baker JG, Leddy JJ, Wilier BS, 2018. Multimodal Imaging of Retired Professional Contact Sport Athletes Does Not Provide Evidence of Structural and Functional Brain Damage. J Head Trauma Rehabil 33, E24–E32. [DOI] [PMC free article] [PubMed] [Google Scholar]


