Abstract
Background
Opioid agonist therapy (OAT) models are generally provided without consideration of how pre-treatment characteristics may be associated with outcome. Therefore, we aimed to first characterize longitudinal trajectories of opioid use before initiating OAT. Then we explored the impact of OAT on opioid use across these pre-treatment trajectories.
Methods
Data were derived from three prospective cohort studies involving people who use drugs in Vancouver, Canada. Latent class growth analysis was applied to identify opioid use trajectories based on individual-level observations three years before starting OAT. Multivariable generalized linear mixed model was used to examine whether engaging in OAT was associated with lower risk of illicit opioid use among participants with different pre-treatment opioid use trajectories.
Results
464 participants were included in the study between September 2005 and November 2018. Two pre-treatment opioid use trajectories were identified: high frequency users (246, 53.0%) and gradually increasing frequency users (218, 47.0%). We observed different strengths of association between OAT engagement and illicit opioid use among high frequency users (adjusted odds ratio [AOR] = 0.36, 95% Confidence Interval [CI]: 0.20 – 0.63) and gradually increasing frequency users (AOR = 0.91, 95% CI: 0.53 – 1.56). Unstable housing, any stimulant use, experiencing violence, drug dealing, sex work involvement, and incarceration were independently and positively associated with ongoing illicit opioid use.
Conclusions
Distinct pre-treatment opioid use trajectories are likely to influence treatment outcomes. Research is required to determine if tailored strategies specific to people with different pre-treatment opioid use patterns have potential to improve outcomes of OAT.
Keywords: Opioid agonist therapy, Latent class growth analysis, Pre-treatment trajectories, Longitudinal studies
1. Introduction
It is estimated that globally, there were approximately 58 million people aged 15–64 years used opioids or used prescription opioids for non-medical purposes in 2018 (United Nations, 2020). Numerous studies have documented an extraordinary increase in opioid-related harms in recent years (Blanch et al., 2014; Degenhardt et al., 2014; Belzak and Halverson, 2018). Specifically, the number of opioid-related deaths has reached unprecedented levels in the United States and Canada. In the United States, overdose involving opioids resulted in 42249 deaths in 2016, with 40.4% due to the misuse of prescription opioids (Seth et al., 2018). In Canada, between January and June 2018, there were 2066 opioid-related deaths, with fentanyl or fentanyl analogues detected in 72.0% of cases (Government of Canada, 2018).
Opioid agonist therapy (OAT) is an evidence-based treatment approach whereby people with opioid use disorder can effectively reduce craving and prevent withdrawal symptoms by taking long-acting opioid drugs, such as buprenorphine-naloxone and methadone (Schuckit, 2016; British Columbia Centre on Substance Use, 2017). Besides effectively reducing opioid use, studies have shown that OAT can help reduce overdose and mortality (Degenhardt et al., 2011; Nolan et al., 2015), protect against HIV and HCV infection (MacArthur et al., 2012; Platt et al., 2018), improve health related quality of life (Krebs et al., 2016), decrease criminality (Krebs et al., 2014; Russolillo et al., 2018) and healthcare costs (Mohlman et al., 2016).
Despite the cumulative evidence for the effectiveness of OAT, studies have demonstrated considerable heterogeneity in terms of treatment outcomes. Particularly, people with opioid use disorder often experience multiple episodes of relapse and treatment readmission before achieving opioid abstinence (Hser et al., 2015). Aside from demographic variables, the characteristics of pre-treatment opioid use are amongst the factors that account for variations in treatment outcomes. A meta-analysis demonstrated that having a longer history of pre-treatment opioid use was positively associated with continued drug use after treatment (Brewer et al., 1998). Further, longer duration of regular opioid use at treatment enrollment was also found to be predictive for poorer treatment retention rate (Soyka et al., 2008; Hillhouse et al., 2013). In addition, several studies have shown that more frequent opioid use at treatment enrollment reduced the likelihood of achieving continuous opioid abstinence (Darke et al., 2005; Darke et al., 2007; Hillhouse et al., 2013). However, these studies focused on the characteristics of opioid use at treatment initiation, which cannot fully reflect the chronic relapsing nature of opioid use over time. Over the long term, some individuals tend to persist in their high frequency of opioid use, others show varying levels of opioid use, and yet others exhibit low or very sporadic opioid use histories (Hser et al., 2015). It is likely that people experience distinct trajectories of opioid use before treatment engagement, and these trajectories will further influence their treatment outcomes.
To our knowledge, no prior study has undertaken a detailed examination of longitudinal opioid use patterns prior to OAT despite high rates of OAT discontinuation (i.e., about 40% at 12 months) in different settings (O’Connor et al., 2020) and the association of discontinuation with dramatically increased mortality (Sordo et al., 2017). Given the growing interest in the development of treatment and continuing care strategies for people with opioid use disorder, research has shown that the effectiveness of the treatment could be improved by using flexible and adaptive algorithms, which take into account individual patient’s characteristics (McKay, 2009). Therefore, there is considerable clinical and scientific importance associated with understanding whether the benefit of OAT is conditional on pre-treatment opioid use trajectories. Therefore, in this study, we aimed to characterize the trajectories of opioid use prior to OAT initiation using data from three large, long-running community-recruited cohorts of people who use illicit drugs in a setting with a universal no-cost health care system and low-barrier access to OAT. Our secondary objective was to examine whether the impact of OAT engagement on the risk of opioid use were different among people with different pre-treatment opioid use patterns.
2. Methods
2.1. Study design and participants
Data were derived from three ongoing open prospective cohort studies of people who use drugs in Vancouver: the Vancouver Injection Drug Users Study (VIDUS), AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), and At-Risk Youth Study (ARYS). Detailed descriptions of these cohorts have been published elsewhere (Strathdee et al., 1998; Wood et al., 2009; Cheng et al., 2018). Briefly, recruitment for all cohorts uses extensive community-based methods, including self-referral and street outreach. To be eligible for VIDUS, participants had to be HIV-seronegative, at least 18 years of age, and report injecting an illicit drug in the month preceding enrollment. To be eligible for ACCESS, participants had to be HIV-seropositive, at least 18 years of age, and report using an illicit drug other than or in addition to cannabis in the month preceding enrollment. ARYS is a cohort study of street-involved youth. To be eligible, participants must be aged 14–26 years and also have used illegal drugs other than or in addition to cannabis in the past month at recruitment. Youth who were homeless or using services for homeless youth were considered street-involved in this study. For all cohorts, participants had to reside in the Greater Vancouver Regional District and provide written informed consent.
The baseline and follow-up procedures for these studies, including the questionnaires, are harmonized to allow for combined analyses of the cohorts. Specifically, at baseline and semi-annually thereafter, participants complete an interviewer-administered questionnaire. Nurses also examine participants and obtain blood samples for HIV and HCV serologic testing, and HIV disease monitoring, as appropriate. Participants receive a $40 (CAD) honorarium for each visit. These studies have been ethically approved by the University of British Columbia/Providence Health Care Research Ethics Board.
For the present analysis, participants were included if they were enrolled between September 2005 and November 2018. Because we were interested in understanding opioid use trajectories before engaging in OAT, the sample was restricted to opioid users who started OAT during study follow-ups and completed at least three study visits before starting OAT to allow for polynomial growth curve analysis.
2.2. Measures
Illicit opioid use was defined as self-reported injection or non-injection heroin or illicit prescription opioid use in the last six months. The frequency of illicit opioid use included “no use”, “less than monthly”, “at least monthly”, “at least weekly”, and “at least daily”. For primary analysis, the main outcome of interest was opioid use (yes vs. no) after OAT engagement. The primary exposure was a time-varying dichotomous variable of OAT engagement during the past six months (yes vs. no), including maintenance treatment using methadone or buprenorphine/naloxone. We also considered covariates that, based on a review of prior literature (Kerr et al., 2005; Socías et al., 2020), were hypothesized to potentially confound the relationship between OAT engagement and opioid use. We included self-reported baseline characteristics, including age (per year), sex (male vs. female), and ethnicity (white vs. others). Other time-varying sociodemographic factors included: being in a stable relationship, defined as being married, common law or having a regular partner (yes vs. no); employment, defined as having a regular job, temporary job or self-employed (yes vs. no); current housing status (unstable housing vs. stable housing). Unstable housing was defined as living in a single room occupancy hotel, shelter or other transitional housing, or living on the street. Substance use measures included: any stimulant use (i.e., cocaine, crack or crystal methamphetamine, yes vs. no); cannabis use (yes vs. no); at least daily alcohol use (yes vs. no); and benzodiazepine use (yes vs. no). Factors related to substance use treatment experience included: any OAT before study enrollment (yes vs. no); and any other addiction treatment or services except for OAT (yes vs. no). Other behavioural factors and social-structural exposures included: being attacked, assaulted, or suffered violence (yes vs. no); drug dealing (yes vs. no); sex work involvement (yes vs. no); and incarceration (yes vs. no). All behavioural variables referred to the period beginning six months before each study interview unless otherwise specified. We also included the calendar year of initiating OAT (per year) and study cohort designation (i.e., ACCESS vs. ARYS vs. VIDUS).
2.3. Statistical analysis
First, to investigate illicit opioid use trajectories before OAT initiation, latent class growth analysis (LCGA) was used on all the observations three years prior to OAT. LCGA is a semi-parametric, group-based analytical approach, aiming to discover meaningful distinctive subpopulations with homogeneous longitudinal trajectories within the larger heterogeneous population (Muthén and Muthén, 2000; Jung and Wickrama, 2008; Nagin, 2014).This methodology has been successfully applied in the field of substance use including alcohol use (Jackson et al., 2008; Witbrodt et al., 2012; Huh et al., 2013), tobacco smoking (Mathur et al., 2013; Klein et al., 2013), and drug use (Grella and Lovinger, 2011; Genberg et al., 2011; Mikolajczyk et al., 2014).
Illicit opioid use frequency was treated as a five-level ordinal variable (see Measures) and used to model opioid use trajectories before OAT initiation. To determine the optimal number of trajectories, we started with a single-class latent growth curve model and continued until a four-class model was fitted. Linear and quadratic parameters were fitted for the time trend for each trajectory group. Models were compared using Akaike information criterion (AIC) and Bayesian information criterion (BIC) (Jung and Wickrama, 2008; Van De Schoot et al., 2017). Lower absolute values for the information criteria suggest better model fit. Lo-Mendell-Rubin likelihood ratio test (LRT) was used to compare the likelihood of the model being tested with a model with one fewer class (Van De Schoot et al., 2017). Averaged posterior probability of group membership and entropy were used to evaluate classification quality. Furthermore, to ensure interpretability and usefulness of the latent classes, sample size per latent class and substantive importance of the trajectory groups were also taken into consideration.
Next, we summarized demographic characteristics, drug use behaviours, and social-structural exposures at OAT initiation stratified by pre-treatment opioid use trajectories. Finally, generalized linear mixed model (GLMM) with time-fixed and time-varying covariates was used to examine the association between OAT engagement and illicit opioid use. Observations from the first report of OAT engagement up to three years were included. To explore whether participants with different pre-treatment opioid use trajectories would benefit differently from OAT, an interaction term was tested between trajectory group membership and OAT engagement. All factors significant in the bivariable analyses at P < 0.10 were included in the final multivariable model. This model building approach has been utilized in previous research (Hadland et al., 2012; Hayden et al., 2014). As a secondary analysis, we examined the impact of OAT engagement on the probability of daily illicit opioid use, and further tested the interaction term between trajectory group membership and OAT engagement. The secondary analysis thus can complement the primary analysis by evaluating whether OAT helps reduce opioid use frequency across groups.
LCGA was conducted using the software Mplus version 8 (Muthén and Muthén, 2017) and all other analyses were performed using SAS 9.4 (SAS Institute, USA). All P values were two-sided.
3. Results
3.1. General characteristics
Between September 2005 and November 2018, a total of 3859 individuals were recruited. Of these, 2688 (69.7%) were not on OAT at baseline, among whom 741 (27.6%) subsequently initiated OAT during study follow-up. Two-hundred and seventy-seven participants were further excluded from the present study due to not completing at least three study visits before OAT. Compared to participants in the analytical sample (n = 464), the 277 excluded participants were younger at OAT engagement (median age: 34 vs. 36 years, P < 0.001), but there was no significant difference regarding sex (61.4% vs. 67.2% male, P = 0.105) and ethnicity (57.8% vs. 56.9% white, P = 0.691).
Among the 464 included participants, 312 (67.2%) were male, 264 (56.9%) self-reported white ethnicity, and the median age was 36 years (interquartile range [IQR]: 27–47). In the study visit that participants reported initiating OAT, 411 (88.6%) reported having received methadone as the medication, 47 (10.1%) reported having received buprenorphine/naloxone, and 6 (1.3%) reported having received both in the last 6 months. Compared to participants who initiated OAT with methadone, participants who reported having buprenorphine/naloxone were younger (median age: 27 vs. 37 years, P = 0.001), but there was no significant difference regarding sex (78.7% vs. 66.9% male, P = 0.100) and ethnicity (59.6% vs. 56.5% white, P = 0.682). Demographic characteristics, drug use behaviours, and social-structural exposures at opioid agonist therapy initiation are shown in Table 1.
Table 1.
Sociodemographic characteristics, substance use behaviours, treatment experience and other behavioural risk factors at opioid agonist therapy initiation stratified by pre-treatment opioid use trajectories among 464 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Characteristics | Total n=464 (%) | Gradually increasing frequency user n=218 (47.0%) | High frequency user n=246 (53.0%) | P-value |
|---|---|---|---|---|
| Opioid use a | ||||
| < monthly | 33 (7.1%) | 22 (10.1%) | 11 (4.5%) | <0.001 |
| ≥ monthly | 28 (6.0%) | 17 (7.8%) | 11 (4.5%) | |
| ≥ weekly | 87 (18.8%) | 46 (21.1%) | 41 (16.7%) | |
| ≥ daily | 246 (53.0%) | 90 (41.3%) | 156 (63.4%) | |
| Sociodemographic factors | ||||
| Age (years), median (interquartile range) | 36 (27 – 47) | 34 (26 – 48) | 37 (28 – 45) | 0.350 |
| Male | 312 (67.2%) | 156 (71.6%) | 156 (63.4%) | 0.062 |
| White ethnicity | 264 (56.9%) | 121 (55.5%) | 143 (58.1%) | 0.569 |
| Being in a stable relationship a | 135 (29.1%) | 65 (29.8%) | 70 (28.5%) | 0.747 |
| Employment status (regular/temporary job; self-employed) a | 98 (21.1%) | 49 (22.5%) | 49 (19.9%) | 0.500 |
| Unstable housing | 338 (72.8%) | 152 (69.7%) | 186 (75.6%) | 0.234 |
| Substance use a | ||||
| Any stimulant use | 377 (81.3%) | 179 (82.1%) | 198 (80.5%) | 0.581 |
| Cannabis use | 263 (56.7%) | 141 (64.7%) | 122 (49.6%) | 0.001 |
| Daily alcohol | 21 (4.5%) | 13 (6.0%) | 8 (3.3%) | 0.161 |
| Benzodiazepine use | 11 (2.4%) | 6 (2.8%) | 5 (2.0%) | 0.611 |
| Treatment experience | ||||
| Any opioid agonist therapy before study enrollment | 142 (30.6%) | 42 (19.3%) | 100 (40.7%) | <0.001 |
| Behavioural risk factors a | ||||
| Attacked, assaulted, or suffered violence | 79 (17.0%) | 42 (19.3%) | 37 (15.0%) | 0.217 |
| Drug dealing | 122 (26.3%) | 39 (17.9%) | 83 (33.7%) | <0.001 |
| Sex work involvement | 58 (12.5%) | 22 (10.1%) | 36 (14.6%) | 0.140 |
| Incarceration | 84 (18.1%) | 29 (13.3%) | 55 (22.4%) | 0.011 |
| Other factors | ||||
| Study cohort designation (ACCESS) | 115 (24.8%) | 62 (28.4%) | 53 (21.5%) | <0.001 |
| Study cohort designation (ARYS) | 135 (29.1%) | 83 (38.1%) | 52 (21.1%) | |
| Calendar year on opioid agonist therapy, median (interquartile range) | 2012 (2009 – 2015) | 2013 (2010 – 2016) | 2011 (2008 – 2015) | 0.004 |
ACCESS AIDS Care Cohort to evaluate Exposure to Survival Services; AYRS At-Risk Youth Study.
Denotes behaviours and events in the previous six months.
3.2. Identify opioid use trajectories before OAT engagement
As shown in Table 2, different model fit statistics were compared with an increasing number of trajectories. AIC and BIC continued to decrease but the reduction became relatively small when comparing models with two to three classes. LRT suggested that model was no longer improved with three classes (P = 0.594). Also, after taking into account the classification quality and interpretability, a two-class solution was chosen. The trajectories of daily opioid use are visualized in Figure 1. After assigning participants to each trajectory class based on their most likely latent class membership, we characterized the two classes as: high frequency users (246, 53.0%) and gradually increasing frequency users (218, 47.0%). The probability of daily illicit opioid use among high frequency users remained above 60.0% over the three years prior to OAT engagement. As shown in Figure 1, the probability of daily illicit opioid use for gradually increasing frequency users started low but then rapidly increased and reached above 40.0% at the time of OAT engagement.
Table 2.
Model comparison with an increasing number of trajectories.
| Number of Classes | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| AIC | 5307.7 | 4916.9 | 4888.5 | 4859.9 |
| BIC | 5332.6 | 4958.3 | 4946.5 | 4934.4 |
| LMR LRT P value | <0.001 | 0.594 | 0.199 | |
| Entropy | 0.704 | 0.634 | 0.642 | |
| Averaged posterior probability of group membership (range) | 0.91 – 0.92 | 0.79 – 0.89 | 0.69 – 0.87 | |
| Sample size per class (%, range) | 47.0 – 53.0 | 12.9 – 43.8 | 9.1 – 41.8 |
LMR LRT = Lo-Mendell-Rubin likelihood ratio test.
Figure 1.
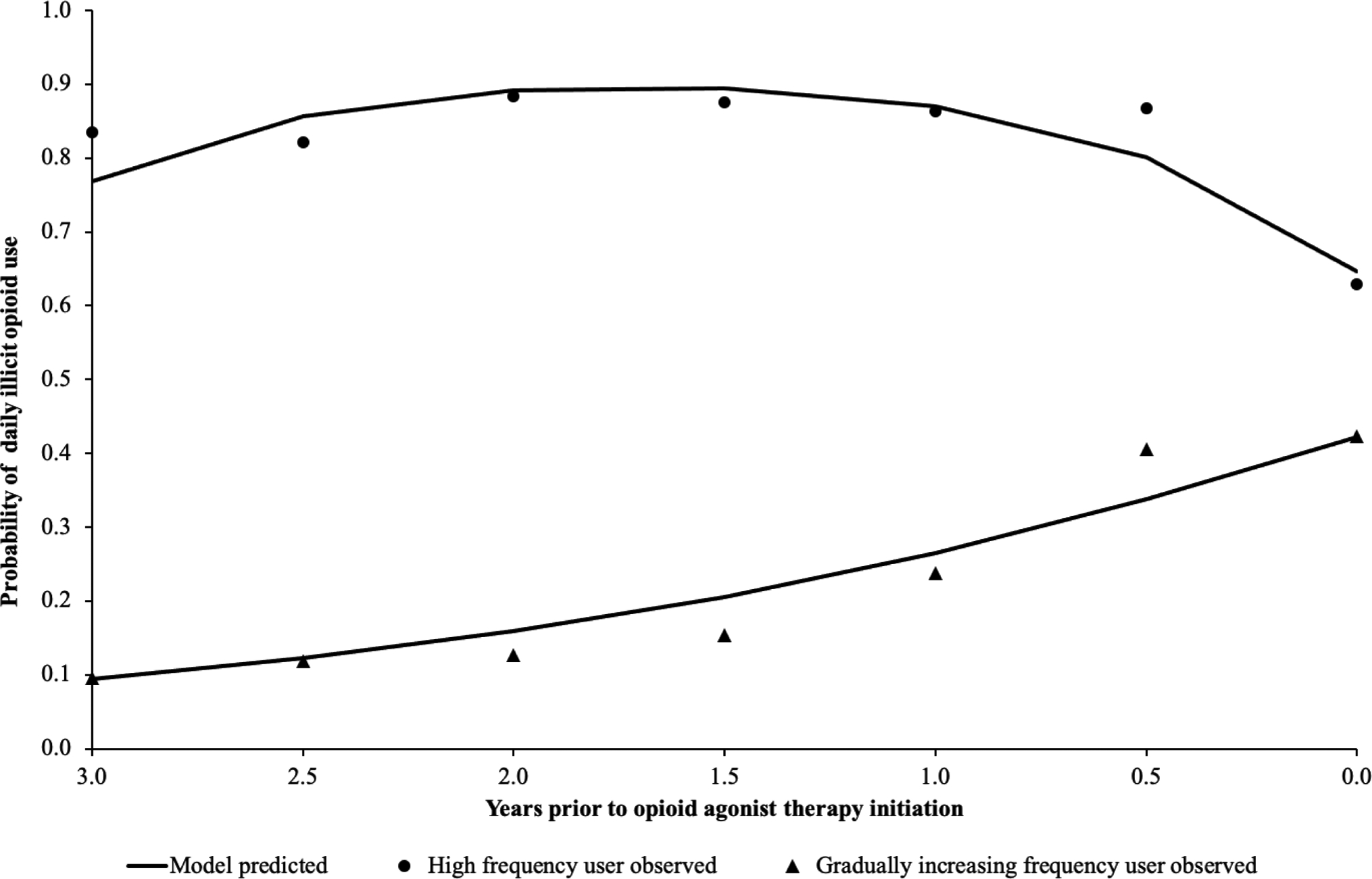
Illicit opioid use trajectory classes before engaging in opioid agonist treatment using latent class growth analysis among 464 people in Vancouver, British Columbia, Canada.
As shown in Table 1, for high frequency users, the median year of OAT initiation was 2011 (IQR: 2008 – 2015). Compared to high frequency users, most of the gradually increasing frequency users engaged in OAT in more recent years (median 2013, IQR: 2010 – 2016). Additionally, compared to gradually increasing frequency users, a significantly higher proportion of high frequency users had experienced any opioid agonist therapy before study enrollment, were more likely to be involved in drug dealing, and had been recently incarcerated. However, higher proportion of gradually increasing frequency users reported cannabis use.
3.3. Illicit opioid use after opioid agonist treatment among different trajectory group
In total, 177 (72.0%) high frequency users and 133 (61.0%) gradually increasing frequency users stopped illicit opioid use for at least six months by the end of the study period. After engaging in OAT, multiple episodes of cessation (i.e., each for at least six months) of and relapse to opioid use were commonly observed among the sample. Only 11 (4.5%) high frequency users and 18 (8.3%) gradually increasing frequency users stopped using opioid right after OAT engagement and remained opioid abstinence during the study period. Among these participants, the median time of opioid abstinence after engagement in OAT for high frequency users was 2.3 years (IQR: 1.0 – 2.8), which was not significantly different compared to the length among gradually increasing frequency users (median: 2.6 years, IQR: 0.5 – 3.0, P = 0.704).
The results of bivariable and multivariable GLMM analyses on any illicit opioid use are presented in Table 3. OAT engagement was negatively associated with the probability of illicit opioid use among high frequency users (adjusted odds ratio [AOR] = 0.36, 95% confidence interval [CI]: 0.20 – 0.63). However, we failed to observe a significant association between OAT engagement and illicit opioid use among gradually increasing frequency users (AOR = 0.91, 95% CI: 0.53 – 1.56), which represented a significantly weaker association compared to the association observed among high frequency users (interaction term P = 0.013).
Table 3.
Bivariable and multivariable generalized linear mixed models of sociodemographic characteristics, substance use behaviours, treatment experience and other behavioural risk factors associated with illicit opioid use among 464 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Characteristics | Bivariable Regression | Multivariable Regression | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P-value | Adjusted Odds Ratio (95% CI) | P-value | |
| Opioid agonist therapy, among: | ||||
| High frequency user | 0.57 (0.34, 0.95) | 0.031 | 0.36 (0.20, 0.63) | <0.001 |
| Gradually increasing frequency user | 1.35 (0.85, 2.14) | 0.203 | 0.91 (0.53, 1.56) | 0.735 |
| Gradually increasing frequency user vs. High frequency user, when: | ||||
| Not on opioid agonist therapy | 0.23 (0.11, 0.46) | <0.001 | 0.23 (0.11, 0.49) | <0.001 |
| On opioid agonist therapy | 0.54 (0.33, 0.88) | 0.014 | 0.59 (0.36, 0.99) | 0.047 |
| Sociodemographic factors | ||||
| Age, per year increase | 0.96 (0.94, 0.98) | <0.001 | 0.97 (0.94, 1.00) | 0.030 |
| Male | 1.27 (0.77, 2.08) | 0.349 | ||
| White ethnicity | 0.81 (0.50, 1.30) | 0.378 | ||
| Being in a stable relationship a | 1.00 (0.69, 1.45) | 0.983 | ||
| Employment status (regular/temporary job; self-employed) a | 1.15 (0.80, 1.66) | 0.448 | ||
| Unstable housing | 2.33 (1.66, 3.26) | <0.001 | 1.56 (1.09, 2.24) | 0.015 |
| Substance use a | ||||
| Any stimulant use | 5.16 (3.59, 7.41) | <0.001 | 3.69 (2.52, 5.39) | <0.001 |
| Cannabis use | 1.64 (1.18, 2.28) | 0.003 | 1.32 (0.93, 1.87) | 0.119 |
| Daily alcohol | 0.76 (0.39, 1.45) | 0.400 | ||
| Benzodiazepine use | 1.67 (0.71, 3.96) | 0.241 | ||
| Treatment experience | ||||
| Any opioid agonist therapy before study enrollment | 1.21 (0.73, 2.02) | 0.451 | ||
| Any other addiction treatment or services a | 0.50 (0.28, 0.91) | 0.023 | 0.47 (0.23, 0.97) | 0.040 |
| Behavioural risk factors a | ||||
| Attacked, assaulted, or suffered violence | 2.31 (1.48, 3.61) | <0.001 | 1.64 (1.02, 2.65) | 0.041 |
| Drug dealing | 5.20 (3.47, 7.77) | <0.001 | 3.30 (2.17, 5.02) | <0.001 |
| Sex work involvement | 5.23 (2.59, 10.54) | <0.001 | 2.75 (1.31, 5.78) | 0.008 |
| Incarceration | 3.22 (1.95, 5.33) | <0.001 | 2.24 (1.31, 3.83) | 0.003 |
| Other factors | ||||
| Study cohort designation (ACCESS) | 0.62 (0.35, 1.08) | 0.091 | 0.75 (0.43, 1.32) | 0.322 |
| Study cohort designation (ARYS) | 1.32 (0.74, 2.33) | 0.347 | 0.92 (0.42, 2.03) | 0.837 |
| Calendar year on opioid agonist therapy | 0.97 (0.91, 1.03) | 0.327 | ||
ACCESS AIDS Care Cohort to evaluate Exposure to Survival Services; AYRS At-Risk Youth Study; CI confidence interval.
Denotes behaviours and events in the previous six months.
As shown in Table 3, regardless whether or not they were engaged in OAT, gradually increasing frequency users had lower odds of illicit opioid use compared to high frequency users. Substance use and behavioural risk factors that were positively associated with the probability of illicit opioid use included unstable housing, any stimulant use, attacked, assaulted, or suffered violence, drug dealing, sex work involvement, and incarceration. Older age and engaging in any other addiction treatment services were associated with lower odds of illicit opioid use.
Results for the secondary analysis on daily illicit opioid use are summarized in Table 4. After adjusting for potential confounders, OAT engagement was negatively associated with daily illicit opioid use among both high frequency users (AOR = 0.25, 95% CI: 0.16 – 0.38) and gradually increasing frequency users (AOR = 0.41, 95% CI: 0.26 – 0.64). The interaction term between group membership and OAT exposure was no longer statistically significant (P = 0.111). Therefore, even though we failed to see an effect of OAT on reducing the risk of any opioid use among gradually increasing frequency users, the secondary analysis results indicated that involvement in OAT was associated with reduced frequency of illicit opioid use for both high frequency and gradually increasing frequency users.
Table 4.
Secondary analysis: bivariable and multivariable generalized linear mixed models of sociodemographic characteristics, substance use behaviours, treatment experience and other behavioural risk factors associated with daily illicit opioid use among 464 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Characteristics | Bivariable Regression | Multivariable Regression | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P-value | Adjusted Odds Ratio (95% CI) | P-value | |
| Opioid agonist therapy, among: | ||||
| High frequency user | 0.29 (0.19, 0.44) | <0.001 | 0.25 (0.16, 0.38) | <0.001 |
| Gradually increasing frequency user | 0.41 (0.27, 0.64) | <0.001 | 0.41 (0.26, 0.64) | <0.001 |
| Gradually increasing frequency user vs. High frequency user, when: | ||||
| Not on opioid agonist therapy | 0.25 (0.14, 0.46) | <0.001 | 0.21 (0.12, 0.40) | <0.001 |
| On opioid agonist therapy | 0.35 (0.23, 0.55) | <0.001 | 0.36 (0.23, 0.55) | <0.001 |
| Sociodemographic factors | ||||
| Age, per year increase | 0.96 (0.94, 0.97) | <0.001 | 0.96 (0.94, 0.97) | <0.001 |
| Male | 0.93 (0.59, 1.45) | 0.747 | ||
| White ethnicity | 0.68 (0.44, 1.03) | 0.069 | 0.73 (0.49, 1.09) | 0.120 |
| Being in a stable relationship a | 0.74 (0.53, 1.04) | 0.085 | 0.77 (0.54, 1.08) | 0.133 |
| Employment status (regular/temporary job; self-employed) a | 0.85 (0.61, 1.18) | 0.329 | ||
| Unstable housing | 2.72 (1.94, 3.79) | <0.001 | 2.02 (1.43, 2.86) | <0.001 |
| Substance use a | ||||
| Any stimulant use | 2.01 (1.44, 2.80) | <0.001 | 1.75 (1.23, 2.50) | 0.002 |
| Cannabis use | 0.88 (0.65, 1.18) | 0.386 | ||
| Daily alcohol | 1.35 (0.73, 2.50) | 0.332 | ||
| Benzodiazepine use | 1.70 (0.79, 3.67) | 0.178 | ||
| Treatment experience | ||||
| Any opioid agonist therapy before study enrollment | 1.11 (0.71, 1.75) | 0.640 | ||
| Any other addiction treatment or services a | 0.80 (0.46, 1.37) | 0.407 | ||
| Behavioural risk factors a | ||||
| Attacked, assaulted, or suffered violence | 2.07 (1.43, 3.00) | <0.001 | 1.61 (1.09, 2.37) | 0.016 |
| Drug dealing | 3.24 (2.37, 4.44) | <0.001 | 2.50 (1.80, 3.47) | <0.001 |
| Sex work involvement | 2.91 (1.72, 4.92) | <0.001 | 1.80 (1.05, 3.10) | 0.034 |
| Incarceration | 2.01 (1.35, 3.00) | <0.001 | 1.47 (0.97, 2.23) | 0.070 |
| Other factors | ||||
| Study cohort designation (ACCESS) | 0.80 (0.48, 1.32) | 0.377 | ||
| Study cohort designation (ARYS) | 1.29 (0.78, 2.12) | 0.326 | ||
| Calendar year on opioid agonist therapy | 1.06 (1.00, 1.12) | 0.051 | 1.09 (1.02, 1.15) | 0.007 |
ACCESS AIDS Care Cohort to evaluate Exposure to Survival Services; AYRS At-Risk Youth Study; CI confidence interval.
Denotes behaviours and events in the previous six months.
4. Discussion
This study sought to characterize pre-treatment illicit opioid use trajectories and examine how these trajectories affected treatment response in terms of reducing frequency of opioid use. Drawing on longitudinal data from three ongoing prospective cohort studies of people who use drugs in Vancouver, Canada, we identified two distinct opioid use trajectories over the three years prior to engaging in OAT. Participants with these different trajectories displayed different levels of treatment response, after adjustment for individual characteristics, drug use behaviours, and social-structural exposures. Differentiation of the pre-treatment characteristics and response to treatment may help clinicians in treatment planning and recommending more effective patient-centered interventions.
We found certain degree of heterogeneity in pre-treatment opioid use patterns. Specifically, over half of participants remained high frequency opioid users before engaging in treatment. Compared to high frequency users, most of the gradually increasing frequency users engaged in OAT in later years. This observation is likely reflective of the continuous effort to expand addiction care and treatment programming in British Columbia in recent years, including ensuring access to evidence-based and comprehensive addiction care, reducing prescriber restrictions and requirements (Canadian Research Initiative in Substance Misuse, 2016; Socías and Ahamad, 2016). Particularly in the fentanyl era, with the sharpest increase of overdose deaths since 2010 (National Institute on Drug Abuse, 2019), OAT has become more accessible to high-risk opioid users in an effort to reduce overdose deaths. Additionally, a higher proportion of gradually increasing frequency users reported cannabis use compared to high frequency users. Given the therapeutic effect of cannabis, it is possible that some people may substitute cannabis for opioids to treat pain or manage craving and withdrawal (Bergeria et al., 2020). On the other hand, high frequency users were more likely to be involved in drug dealing, which is consistent with previous finding that drug dealing is often associated with higher intensity addiction (Kerr et al., 2008). Similarly, we observed higher proportion of incarceration among high frequency users. Our finding supports previous research indicating that recent incarceration is associated negatively with drug use cessation, which might due to reduced access to mechanisms (e.g., addiction treatment, social support) that promote drug use cessation (DeBeck et al., 2009; Genberg et al., 2015).
In the primary and secondary analyses, OAT engagement was found to be strongly associated with lower risk of both illicit opioid use and daily illicit opioid use among high frequency users. Interestingly, in light of the observed beneficial effect of OAT in terms of reducing daily illicit opioid use among gradually increasing frequency users, we failed to find a significant reduction for the risk of any opioid use among this group of people. As shown in the trajectory plot, gradually increasing frequency users might represent those individuals who rapidly increased their frequency of opioid use before engaging in treatment. They had approximately a 40.0% chance of ongoing daily opioid use, and typically had experienced multiple episodes of cessation of and relapse to opioid use before OAT. This group had less opioid agonist therapy experience before study enrolment compared to high frequency users. It is possible that these individuals had more continuous exposure to the physiological, behavioural and social stressors that triggered opioid use, or experienced barriers accessing treatment. Research has shown that treatment outcomes could be improved by incorporating more integrated, comprehensive services, such as psychological counselling (including relapse prevention), behavioural therapy, pain management, and potentially more flexible and accessible models of substance use treatment (Amato et al., 2011; Volkow and McLellan, 2016; Schuckit, 2016; Hassan et al., 2017). Therefore, future research should be undertaken to explore whether such strategies are effective for individuals with different risk and pre-treatment profiles, particularly for the purpose of improving treatment adherence and reducing rates of relapse. Additionally, strategies that help expand treatment access and minimize barriers to treatment adherence are warranted (Socías and Ahamad, 2016).
In the study, when evaluating treatment outcomes, we accounted for the pre-treatment opioid use patterns over time by adjusting for the trajectory groups, which could not be achieved by only controlling for opioid use characteristics at baseline. Compared to high frequency users, gradually increasing frequency users had lower risk of illicit opioid use after OAT engagement. This result is consistent with previous findings that people with more severe history of opioid use are more likely to have poorer treatment outcomes and more protracted opioid use history (Brewer et al., 1998; Darke et al., 2005; Darke et al., 2007; Hillhouse et al., 2013). We have observed that more than 60% of the participants stopped illicit opioid use for at least six months after OAT engagement, however, only a very small number of participants achieved continuous opioid abstinence for an average of 2.5 years (i.e., 4.5% high frequency users and 8.3% gradually increasing frequency users). While there are variations in the definition of abstinence from opioid use, these observed rates of opioid abstinence are comparable to the findings in other study settings (Hser et al., 2015).
Stimulant use was found to be positively associated with any opioid use and daily opioid use. Several studies have found that stimulant use during opioid treatment often compromises opioid treatment (Williamson et al., 2006; Sullivan et al., 2010; Wang et al., 2017). Other risk behaviours including drug dealing, sex work involvement, and incarceration also increased the likelihood of illicit opioid use. Indeed past work has shown how engagement in alternative income generating activities is associated with high risk drug use and behaviour (DeBeck et al., 2007), and incarceration has also been associated with a reduced likelihood of ceasing injecting drug use (DeBeck et al., 2009). Accordingly, efforts should be made to provide alternatives to incarceration for non-violent substance users. Besides, acceptable low-threshold employment opportunities, which are easily accessible for active drug users and do not require abstinence from drug use should be explored (DeBeck et al., 2011). Examples for such opportunities include peer support positions (Kerr et al., 2006), jewellery making economic empowerment program (Sherman et al., 2006) and positions provided by organization that processes recyclable containers (Dale and Newman, 2010).
Some limitations in the current study require consideration. First, there could be errors of recall and social-desirability bias associated with using self-reported data, especially for socially stigmatized and criminalized behaviours (e.g., illicit substance use). Interviewers emphasized before each interview that anonymity and confidentiality were guaranteed. Further, self-reported data have been commonly used and found to be valid in studies involving people who use drugs (Darke, 1998; Langendam et al., 1999). Second, this study utilized a community-based sample recruited through snowball sampling, self-referral, and street outreach, which may limit the generalizability of the findings. Third, our study instrument did not allow for diagnosis of opioid use disorder based on DSM-5 criteria (American Psychiatric Association, 2013). Therefore, information about severity of opioid use disorder was not available. Fourth, although we considered various factors associated with treatment engagement and outcome, there is potential for unmeasured confounding, including confounding related to other substance use (e.g., hallucinogens), factors related to mental health status, interpersonal relationship, internal motivations, as well as factors due to combining three cohort samples for analyses. Fifth, research has shown that higher amount of opioid use is associated with greater risk of adverse events (Babu et al., 2019). However, the frequency of opioid use was considered in this study, and therefore pre-treatment opioid use patterns and the reduction in opioid use after OAT engagement could not reflect the variability in quantity of opioid use. Finally, the present analysis does not provide insight into long term treatment effects. Future studies are required in order to examine long term impact on sustained opioid cessation, opioid use relapse, and treatment readmission.
4.1. Conclusion
In summary, our study identified distinct illicit opioid use patterns prior to engagement in OAT and demonstrated that there are clear differences in treatment outcomes among those with different definable pre-treatment patterns of opioid use. Given the evidence regarding the benefits of OAT, our findings further support the implementation of OAT to reduce the frequency of opioid use. However, we also observed different levels of treatment response. This finding highlights the potential value of acquiring a better understanding of patients’ long-term opioid use patterns and the associated impacts of such patterns on OAT outcomes. Our findings further suggest a need for developing more comprehensive treatment strategies specific to people with different pre-treatment opioid use patterns in order to maximize the benefits of OAT.
Two opioid use trajectories were found before opioid agonist therapy (OAT) engagement.
Pre-treatment opioid use pattern was related to different level of response to OAT.
Tailored treatment strategies are needed to increase the benefits of OAT.
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
Role of Funding Sources
This work was supported by the US National Institutes on Drug Abuse at the US National Institutes of Health [U01-DA038886, U01-DA021525]. Huiru Dong is supported through a Canadian Institutes of Health Research (CIHR) Doctoral Award. Dr. Thomas Kerr is supported by a foundation grant from the CIHR [20R74326]. Dr. Evan Wood receives support through a Tier 1 Canada Research Chair in Addiction Medicine. Dr. Wood is also a consultant to a mental health wellness company called Numinus. Dr. Kanna Hayashi is supported by a CIHR New Investigator Award [MSH-141971], a Michael Smith Foundation for Health Research (MSFHR) Scholar Award, and the St. Paul’s Foundation. Dr. Nadia Fairbairn is supported by MSFHR Scholar Award and the St. Paul’s Foundation. Dr. Kora DeBeck is supported by a MSFHR/ St. Paul’s Hospital Foundation-Providence Health Care Career Scholar Award and a CIHR New Investigator Award. Dr. M-J Milloy is supported in part by the United States National Institutes of Health [U01-DA021525], a New Investigator Award from the CIHR, and a Scholar Award from the MSFHR. The University of British Columbia has received an arms’ length gift from NG Biomed, Ltd., a private firm seeking a license to produce cannabis, to support Dr. M-J Milloy. He is the Canopy Growth professor of cannabis science, a position at the University of British Columbia created by arms’ length gifts from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. Funding sources had no role in the design of this study; collection, analysis, and interpretation of the data; writing of the report; or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. M-J Milloy is supported in part by the United States National Institutes of Health [U01-DA021525], a New Investigator Award from the CIHR, and a Scholar Award from the MSFHR. The University of British Columbia has received an arms’ length gift from NG Biomed, Ltd., a private firm seeking a license to produce cannabis, to support Dr. M-J Milloy. He is the Canopy Growth professor of cannabis science, a position at the University of British Columbia created by arms’ length gifts from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. Dr. Evan Wood receives support through a Tier 1 Canada Research Chair in Addiction Medicine. Dr. Wood is also a consultant to a mental health wellness company called Numinus.
References
- Amato L, Minozzi S, Davoli M, Vecchi S, 2011. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. (9):CD005031. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Babu KM, Brent J, Juurlink DN, 2019. Prevention of opioid overdose. N Engl J Med. 380, 2246–2255. [DOI] [PubMed] [Google Scholar]
- Belzak L, Halverson J, 2018. Evidence synthesis - The opioid crisis in Canada: a national perspective. Health Promotion and Chronic Disease Prevention in Canada. 38, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeria CL, Huhn AS, Dunn KE, 2020. The impact of naturalistic cannabis use on self-reported opioid withdrawal. J Subst Abuse Treat. 108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanch B, Pearson SA, Haber PS, 2014. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol. 78, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB, 1998. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 93, 73–92. [PubMed] [Google Scholar]
- British Columbia Centre on Substance Use, 2017. A guideline for the clinical management of opioid use disorder. http://www.bccsu.ca/wp-content/uploads/2017/06/BC-OUD-Guidelines_June2017.pdf. Accessed December 23, 2018.
- Canadian Research Initiative in Substance Misuse, 2016. Moving towards improved access for evidence-based opioid addiction care in British Columbia. http://cfenet.ubc.ca/sites/default/files/uploads/news/releases/improved-access-opioid-addiction-care-bc-final-jun1.pdf. Accessed December 23, 2018.
- Cheng T, Small W, Dong H, Nosova E, Hayashi K, DeBeck K, 2018. An age-based analysis of nonmedical prescription opioid use among people who use illegal drugs in Vancouver, Canada. Subst Abuse Treat Prev Policy. 13, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A, Newman L, 2010. Social capital: a necessary and sufficient condition for sustainable community development?. Community Dev J. 45, 5–21. [Google Scholar]
- Darke S, Ross J, Mills KL, Williamson A, Havard A, Teesson M, 2007. Patterns of sustained heroin abstinence amongst long-term, dependent heroin users: 36 months findings from the Australian Treatment Outcome Study (ATOS). Addict Behav. 32, 1897–1906. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Teesson M, Ali R, Cooke R, Ritter A, Lynskey M, 2005. Factors associated with 12 months continuous heroin abstinence: findings from the Australian Treatment Outcome Study (ATOS). J Subst Abuse Treat. 28, 255–263. [DOI] [PubMed] [Google Scholar]
- Darke S, 1998. Self-report among injecting drug users: a review. Drug Alcohol Depend. 51, 253–263. [DOI] [PubMed] [Google Scholar]
- DeBeck K, Kerr T, Li K, Milloy MJ, Montaner J, Wood E, 2009. Incarceration and drug use patterns among a cohort of injection drug users. Addiction. 104, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeck K, Shannon K, Wood E, Li K, Montaner J, Kerr T, 2007. Income generating activities of people who inject drugs. Drug Alcohol Depend. 91, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeck K, Wood E, Qi J, Fu E, McArthur D, Montaner J, Kerr T, 2011. Interest in low-threshold employment among people who inject illicit drugs: implications for street disorder. Int J Drug Policy. 22, 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, Vos T, 2014. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction. 109, 1320–1333. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J, 2011. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 106, 32–51. [DOI] [PubMed] [Google Scholar]
- Genberg BL, Astemborski J, Vlahov D, Kirk GD, Mehta SH, 2015. Incarceration and injection drug use in Baltimore, Maryland. Addiction. 110, 1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, Mehta SH, 2011. Trajectories of injection drug use over 20 years (1988–2008) in Baltimore, Maryland. Am J Epidemiol. 173, 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Canada, 2018. Overview of national data on opioid-related harms and deaths. https://www.canada.ca/en/health-canada/services/substance-use/problematic-prescription-drug-use/opioids/data-surveillance-research/harms-deaths.html. Accessed December 17, 2018.
- Grella CE, Lovinger K, 2011. 30-year trajectories of heroin and other drug use among men and women sampled from methadone treatment in California. Drug Alcohol Depend. 118, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Marshall BD, Kerr T, Qi J, Montaner JS, Wood E, 2012. Suicide and history of childhood trauma among street youth. J Affect Disord. 136, 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden A, Hayashi K, Dong H, Milloy MJ, Montaner JS, Wood E, 2014. The impact of drug use patterns on mortality among polysubstance users in a Canadian setting: a prospective cohort study. BMC Public Health. 14:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AN, Howe AS, Samokhvalov AV, Le Foll B, George TP, 2017. Management of mood and anxiety disorders in patients receiving opioid agonist therapy: Review and meta-analysis. Am J Addict. 26, 551–563. [DOI] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, Ling W, 2013. Predictors of outcome after short-term stabilization with buprenorphine. J Subst Abuse Treat. 44, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y, Evans E, Grella C, Ling W, 2015. Long-Term Course of Opioid Addiction. Harv Rev Psychiatry. 23, 76–89. [DOI] [PubMed] [Google Scholar]
- Huh J, Huang Z, Liao Y, Pentz M, Chou CP, 2013. Transitional life events and trajectories of cigarette and alcohol use during emerging adulthood: latent class analysis and growth mixture modeling. J Stud Alcohol Drugs. 74, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Schulenberg JE, 2008. Conjoint developmental trajectories of young adult substance use. Alcohol Clin Exp Res. 32, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS, 2008. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2, 302–317. [Google Scholar]
- Kerr T, Marsh D, Li K, Montaner J, Wood E, 2005. Factors associated with methadone maintenance therapy use among a cohort of polysubstance using injection drug users in Vancouver. Drug Alcohol Depend. 80, 329–335. [DOI] [PubMed] [Google Scholar]
- Kerr T, Small W, Johnston C, Li K, Montaner JS, Wood E, 2008. Characteristics of injection drug users who participate in drug dealing: implications for drug policy. J Psychoactive Drugs. 40, 147–152. [DOI] [PubMed] [Google Scholar]
- Kerr T, Small W, Peeace W, Douglas D, Pierre A, Wood E, 2006. Harm reduction by a “user-run” organization: a case study of the Vancouver Area Network of Drug Users (VANDU). Int J Drug Policy. 17, 61–69. [Google Scholar]
- Klein EG, Bernat DH, Lenk KM, Forster JL, 2013. Nondaily smoking patterns in young adulthood. Addict Behav. 38, 2267–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E, Kerr T, Montaner J, Wood E, Nosyk B, 2014. Dynamics in the costs of criminality among opioid dependent individuals. Drug Alcohol Depend. 144, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E, Kerr T, Wood E, Nosyk B, 2016. Characterizing long-term health related quality of life trajectories of individuals with opioid use disorder. J Subst Abuse Treat. 67, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendam MW, Van Haastrecht HJ, Van Ameijden EJ, 1999. The validity of drug users’ self-reports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int J Epidemiol. 28, 514–520. [DOI] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Degenhardt L, Hickman M, 2012. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 345, e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur C, Erickson DJ, Stigler MH, Forster JL, Finnegan JR, 2013. Individual and neighborhood socioeconomic status effects on adolescent smoking: a multilevel cohort-sequential latent growth analysis. Am J Public Health. 103, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, 2009. Continuing care research: What we have learned and where we are going. J Subst Abuse Treat. 36, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk RT, Horn J, Prins M, Wiessing L, Kretzschmar M, 2014. Trajectories of injecting behavior in the Amsterdam Cohort Study among drug users. Drug Alcohol Depend. 144, 141–147. [DOI] [PubMed] [Google Scholar]
- Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C, 2016. Impact of medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abuse Treat. 67, 9–14. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén LK, 2000. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 24, 882–891. [PubMed] [Google Scholar]
- Muthén L.k., Muthén BO, 1998–2017. Mplus user’s guide. Eighth edition Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nagin DS, 2014. Group-based trajectory modeling: an overview. Ann Nutr Metab. 65, 205–210. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2019. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed January 31, 2019.
- Nolan S, Hayashi K, Milloy M, Kerr T, Dong H, Lima VD, Lappalainen L, Montaner J, Wood E, 2015. The impact of low-threshold methadone maintenance treatment on mortality in a Canadian setting. Drug Alcohol Depend. 156, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AM, Cousins G, Durand L, Barry J, Boland F, 2020. Retention of patients in opioid substitution treatment: A systematic review. PloS one. 15, e0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L, Minozzi S, Reed J, Vickerman P, 2018. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 113, 545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russolillo A, Moniruzzaman A, McCandless LC, Patterson M, Somers JM, 2018. Associations between methadone maintenance treatment and crime: a 17-year longitudinal cohort study of Canadian provincial offenders. Addiction. 113, 656–667. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, 2016. Treatment of opioid-use disorders. N Engl J Med. 375, 357–368. [DOI] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018. Overdose deaths involving opioids, cocaine, and psychostimulants — United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 67, 349–358. https://www.cdc.gov/mmwr/volumes/67/wr/mm6712a1.htm. Accessed December 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, German D, Cheng Y, Marks M, Bailey-Kloche M, 2006. The evaluation of the JEWEL project: an innovative economic enhancement and HIV prevention intervention study targeting drug using women involved in prostitution. AIDS care. 18, 1–11. [DOI] [PubMed] [Google Scholar]
- Socías ME, Ahamad K, 2016. An urgent call to increase access to evidence-based opioid agonist therapy for prescription opioid use disorders. CMAJ. 188, 1208–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socías ME, Dong H, Wood E, Brar R, Richardson L, Hayashi K, Kerr T, Milloy MJ, 2020. Trajectories of retention in opioid agonist therapy in a Canadian setting. Int J Drug Policy. 77, 102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H, 2008. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 11, 641–653. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, Schechter MT, Hogg RS, 1998. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 280, 547–549. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, O’Connor PG, Barry DT, Chawarski MC, Schottenfeld RS, Fiellin DA, 2010. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. Am J Addict. 19, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, 2020. World Drug Report 2020. https://wdr.unodc.org/wdr2020/field/WDR20_BOOKLET_1.pdf. Published June, 2020. Accessed August 2, 2020.
- Van De Schoot R, Sijbrandij M, Winter SD, Depaoli S, Vermunt JK, 2017. The GRoLTS-checklist: guidelines for reporting on latent trajectory studies. Structural Equation Modeling: A Multidisciplinary Journal. 24, 451–467. [Google Scholar]
- Volkow ND, McLellan AT, 2016. Opioid abuse in chronic pain--misconceptions and mitigation strategies. N Engl J Med. 374, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Wang L, Min JE, Krebs E, Evans E, Huang D, Liu L, Hser Y, Nosyk B, 2017. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy. 49, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teesson M, 2006. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend. 81, 293–300. [DOI] [PubMed] [Google Scholar]
- Witbrodt J, Kaskutas L, Bond J, Delucchi K, 2012. Does sponsorship improve outcomes above Alcoholics Anonymous attendance? A latent class growth curve analysis. Addiction. 107, 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, Harrigan PR, Montaner JS, 2009. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 338, b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]


