Abstract
Approach and avoidance represent two fundamental behavioral traits that develop early in life. Previous studies have examined the neural correlates of approach and avoidance traits in adults and adolescents. Here, using the data set of the Adolescent Brain Cognition Development project, we investigated the structural cerebral bases of behavioral activation system (BAS) and behavioral inhibition system (BIS) in children. We employed voxel-based morphometry to examine how gray matter volumes (GMV) related specifically to BAS and BIS traits in 11,542 children (5,491 girls, age 9 to 10 years) with 648 and 2,697 identified as monozygotic twins (MZ) and dizygotic twins/siblings (DZ), respectively. After accounting for the BIS score, higher BAS scores (residuals) were positively correlated with the GMV of the ventral striatum (VS), and the correlation was stronger in MZ than in DZ and unrelated children, with a heritability (h2) of 0.8463. Higher BAS scores were negatively correlated with the GMV of bilateral visual, lateral orbitofrontal, temporal, and inferior frontal cortex, as well as the precuneus. Higher BIS (after accounting for BAS) scores were negatively correlated with the GMVs of the ventral caudate and bilateral putamen/pallidum, hypothalamus, and right anterior insula, and the correlation was stronger in MZ than in DZ and unrelated children, with a heritability of 0.8848. A cluster in the VS showed positive and negative correlation with the BAS and BIS scores, respectively. These findings suggest shared and distinct cerebral volumetric bases of the BAS and BIS traits in children. Whereas both traits have a strong genetic basis, the BAS relative to BIS appears to be more amenable to environmental influences. These findings add to the literature of developmental neuroscience and may help identify genetic risk factors of externalizing and internalizing psychopathology.
Keywords: BAS, BIS, imaging, VBM, heritability, ABCD
1. Introduction
The development and formation of approach and avoidance traits in childhood is fundamental to individual differences in motivated behaviors and emotional experiences later in life. Major theories of personality and temperament have converged on the Behavioral Approach System (BAS) and the Behavioral Inhibition System (BIS) (Fowles, 1980; Gray, 1990; Elliot and Thrash, 2002). The BAS mediates responses to positive reinforcement to promote reward-seeking actions whereas the BIS responds to potentially aversive stimuli to inhibit behaviors that could lead to harmful outcomes. An over-active BAS has been associated with increased risks for impulse control, substance use, and attention-deficit/hyperactivity disorders (van den Berg et al., 2011; Becker et al., 2013; Park et al., 2013). Extreme BIS sensitivity, on the other hand, is linked to the development of anxiety disorders, depression, and psychosomatic illnesses (Vervoort et al., 2010; Sportel et al., 2011; Gudiño, 2013).
The neural bases of the BAS and BIS remain a topic of ongoing research. Functional imaging studies have employed a variety of paradigms to characterize the neural correlates of BAS and BIS traits (Reuter et al., 2004; Gray et al., 2005; Kennis et al., 2013; Fourie et al., 2014). For instance, individual BAS and BIS traits were each positively and negatively correlated with activation of the ventral striatum during receipt of reward in the monetary incentive delay task (Simon et al., 2010). Studies of adults have also suggested structural correlates of individual differences in BAS and BIS related traits. For instance, the BIS traits such as harm avoidance, behavioral inhibition, and punishment sensitivity showed a negative relationship with the gray matter volume (GMV) of the hippocampus (Yamasue et al., 2007), medial orbitofrontal cortex (Fuentes et al., 2012), and amygdala (Barros-Loscertales et al., 2006a), respectively. BAS traits, including novelty seeking, impulsivity, and reward sensitivity, were associated with higher GMV of the posterior cingulate areas (Gardini et al., 2009), and lower GMV of the dorsomedial prefrontal cortex (Muhlert and Lawrence, 2015) and superior frontal gyrus (Barros-Loscertales et al., 2006b), respectively. In studies of children and adolescents, a decline in reward sensitivity was noted in combination with a decrease in left nucleus accumbens GMV from the late teens to early twenties (Urosevic et al., 2012). Furthermore, females but not males experienced increases in sensitivity to threat, a change predicted by individual differences in the GMV of lateral orbitofrontal cortex at baseline. Together, the studies are consistent with the roles of a wide array of cortical and subcortical circuits in cognitive and emotional control and in the development of BAS and BIS traits (Ridderinkhof et al., 2004; Etkin et al., 2011; Kennis et al., 2013).
These previous findings demonstrate anatomical brain markers of BAS and BIS dispositions in adults and adolescents. On the other hand, studies focusing specifically on younger childhood, when BAS/BIS traits are in the formative stage, are sparse. Further, it is known that the imaging findings may also reflect the consequences of BAS/BIS traits, such as substance and alcohol use, in adolescents and adults (Krmpotich et al., 2013; Kim-Spoon et al., 2016; Yamamoto et al., 2017). Thus, it is important to investigate the neural correlates in children for whom the environmental influences are less prominent.
In the current study, we examined this issue using a large data set of children, 9 to 10 year old, collected of the National Institutes of Health’s Adolescent Brain Cognition Development (ABCD) project. We employed voxel-based morphometry (VBM) analysis to identify the structural correlates of BAS and BIS traits, as assessed by the Youth Behavioral Inhibition/Behavioral Approach System Scales (BIS/BAS), an version of BIS/BAS scale adapted for children (Pagliaccio et al., 2016). Previous studies have reported sex differences in the BAS and BIS traits as well as the neural bases of the sex differences (Li et al., 2014; Dragan et al., 2019). Thus, we examined the data both for boys and girls together and separately. Finally, the ABCD data comprised approximately 5.6% of monozygotic twins and 23.4% of dizygotic twins or siblings. We computed the heritability (h2) for BAS/BIS traits and the structural correlates identified from VBM, and examined to what extent these structural brain markers are determined by genetic and environmental factors.
2. Methods
2.1. Dataset
The ABCD study, funded by the NIH, is dedicated to the long-term investigation of brain development and child health in the United States (Bjork et al., 2017). The ABCD research consortium consists of a Coordinating Center (CC), a Data Analysis and Informatics Center (DAIC), and 21 research sites across the country. Children ages 9-10 years are invited to participate in the study with baseline and follow-up assessments over a period of 10 years. The ABCD CC, DAIC, and consortium workgroups have established standardized assessments of physical and mental health, neurocognition, substance use, culture and environment, as well as multimodal structural and functional imaging and bioassays protocols (https://abcdstudy.org/). Structural magnetic resonance imaging (MRI) data were acquired using optimized protocol for 3T machines (including Siemens Prisma, GE 750 and Philips) with voxel size of 1 mm isotropic (Casey et al., 2018).
We focused on a sample of 11,542 subjects (5,491 girls) obtained from the ABCD Project, Release 2. We only considered subjects for which raw structural images were available. The original cohort comprised 11,601 children; however, 59 subjects were not included in the current study because of questionable image quality and/or poor image segmentation (see below).
2.2. Assessments
The ABCD Youth BIS/BAS scales consisted of seven (#1-7) and thirteen (#8-20) items for BIS and BAS scores, respectively (Pagliaccio et al., 2016). One item (#5) of the BIS scale meant to be reverse-scored was excluded because that was the only “negative” question and children may not understand the question correctly amidst other items. This modified version shortens the BIS/BAS Reward Responsiveness subscales and includes the BAS “Fun” known to be a reliable predictor of substance misuse (Barch et al., 2018). The BAS signals a range of positive reinforcements and emotions and facilitates reward seeking behavior (Kambouropoulos and Staiger, 2001; Hamilton et al., 2012; Studer et al., 2016), and the BIS responds to salient stimuli associated with punishment by inhibiting ongoing behavior; i.e., to avoidance to mitigate harm (Gray, 1982).
2.3. Voxel-based morphometry (VBM)
Quality check of images was performed visually and quantitatively with the options “Display slices” and “Check sample homogeneity” in CAT12 toolbox (Gaser, 2016). A total of 29 subjects presented clearly faulty segmentation of brain tissues, and were removed from the group analyses. The faulty segmentation likely resulted from poor contrast or artifact of the structural images or abnormal brain shapes. Additionally, 30 subjects with a mean correlation < 0.70, suggesting higher variance of the grey matter densities, were also removed.
We implemented VBM to quantify the gray matter volume (GMV) of regions identified from high resolution T1-weighted images with the CAT12 toolbox (). VBM analysis identifies differences in the local composition of brain tissue, accounting for large-scale variation in gross anatomy and location. The analysis includes spatially normalizing individuals’ structural images to the same stereotactic space, segmenting the normalized images into distinct brain tissues, and smoothing the gray matter (GM) images. We used the raw images to avoid potential interference with the CAT12 preprocessing pipeline. T1-images were first co-registered to the MNI template space using a multiple-stage affine transformation during which the 12 parameters were estimated. Co-registration was performed with a coarse affine registration using mean square differences, followed by a fine affine registration using mutual information. Coefficients of the basis functions that minimize the residual squared difference (between individual image and the template) were estimated. Tissue probability maps constructed from 452 healthy subjects were used in affine transformation, and affine regularization was performed with the International Consortium for Brain Mapping (ICBM) template space. T1 images were then corrected for intensity bias field and a local means denoising filter and segmented into cerebrospinal fluid, gray, and white matter. Segmented and the initially registered tissue class maps were normalized using DARTEL (Ashburner, 2007), a fast diffeomorphic image registration algorithm of SPM. As a high-dimensional non-linear spatial normalization method, DARTEL generates mathematically consistent inverse spatial transformations. We used the standard DARTEL template in MNI space, constructed from 555 healthy subjects of the IXI-database (http://www.brain-development.org/), to drive the DARTEL normalization. Skull-stripping and final clean up (to remove remaining meninges and correct for volume effects in some regions) were performed with default parameters. Normalized GM maps were modulated to obtain the absolute volume of GM tissue corrected for individual brain sizes. Finally, the GM maps were smoothed by convolving with an isotropic Gaussian kernel (FWHM = 8mm).
In group analyses we performed a multiple regression of the GMVs of the whole brain against BAS and BIS scores, separately and with age (in months), intracranial volume and scanner manufacturer/model as a covariates, for the entire sample as well as for girls and boys separately. Because the BAS and BIS scores were highly correlated (see Results), we also performed whole-brain regression with the BAS and BIS residual scores – that is, with BAS residuals after regressing out the BIS score, and vice versa – again with age as a covariate, in order to identify volumetric correlates each specific to the BAS and BIS. All analyses were first evaluated with a voxel p < 0.05 corrected for family-wise error (FWE) of multiple comparisons, on the basis of Gaussian random field theory as implemented in the SPM. If there were no significant findings, we employed voxel p<0.001, uncorrected in combination with a cluster p<0.05 FWE-corrected to evaluate the multiple regressions. Clusters were overlaid on an unbiased pediatric standard MRI template obtained from 324 healthy children (Fonov et al., 2011). In this atlas, T1 weighted MRI data were warped into the MNI stereotaxic space using minctracc (Collins et al., 1994) by age-based subgroups using an iterative nonlinear coregistration algorithm (Fonov et al., 2009). Specifically, we used the asymmetric template MINC1 (10 to 14 years old; http://nist.mni.mcgill.ca/?p=974.)
In addition to the T maps, effect size maps were computed using tools available in CAT12, by approximating Cohen’s d (Cohen, 1988) from the t-statistics using the expression as employed in (Kleber et al., 2016). The effect sizes of two sample t-tests were computed according the equivalence given the sample sizes n1 and n2 of the two groups (Lakens, 2013). Custom computations were implemented in Matlab and verified with the equivalent effect size Hedge’s g calculated using the MES toolbox (https://github.com/hhentschke/measures-of-effect-size-toolbox) (Hentschke and Stuttgen, 2011).
2.4. Heritability of BAS and BIS traits and volumetric correlates
In the current sample of 11,542 children, 648 and 2,697 were identified as monozygotic twins (MZ) and dizygotic twins/siblings (DZ), respectively. We examined how MZ, DZ, and unrelated individuals may differ in the correlation of BAS/BIS residual scores and of volumetric correlates of BAS/BIS residual scores. To this end, we computed the Pearson correlation of BAS/BIS residual scores as well as the trait-related GMVs of MZ and DZ pairs. For unrelated individuals, we randomly sampled pair of subjects without replacement and computed the correlations. Confidence intervals were obtained by repeating the procedure 100 times. For each of these trait and volumetric measures, we performed slope tests (Zar, 1999) to examine differences in the correlations.
Further, for BAS and BIS residual scores as well as their volumetric correlates, we computed the heritability, shared environmental influence, and unique environmental influence (Visscher et al., 2006; Visscher et al., 2008), as follows:
where h2, c2, and e2 quantifies the fraction of phenotype variability attributed to genetic variation, common environmental influence, and unique environmental influence, respectively.
The data of identical or monozygotic twins (MZ) and fraternal or dizygotic twins (DZ) allowed us to examine the genetic and environmental influences on BAS/BIS traits, and their volumetric correlates. The univariate genetic or ACE model decomposes the observed variance into three categories: the additive genetic factors (A) known as heritability, the shared (common) environmental factors (C), and the unique (non-shared) environmental factors (E), in addition to the measurement error (Kohler et al., 2011). In the model, the correlation between the twin pair for the additive genetic variance is set to 1.0 and 0.5 for the MZ and DZ because 100% and 50% of their genes are shared, respectively. The correlation between the twin pair for the shared environmental variance is set to 1.0 for both the MZ and DZ twins because the common environment has the same influence on the twin pair. Finally, the correlation between the twin pair for the unique environmental variance is set to 0. The variance-covariance matrices with the MZ and DZ are used to estimate the contributions of the A, C, and E factors (Rijsdijk and Sham, 2002).
3. Results
3.1. BAS and BIS scores
Boys and girls were significantly different in BIS and BAS scores. Girls presented higher BIS score than boys (7.52 ± 3.66 vs. 7.05 ± 3.59; t = 6.93, Cohen’s d = 0.129, p= 4.33e-12, two-tailed two sample t-test). In contrast, girls demonstrated lower BAS score than boys (20.22 ± 6.85 vs. 21.35 ± 6.90; t = - 8.82, Cohen’s d = - 0.164, p= 1.36e-18). Additionally, BIS and BAS scores were significantly correlated across all groups (all: r= 0.363, p= 1.77e-307; girls: r= 0.368, p= 5.31e-173; boys: r= 0.354 and p= 3.29e-174, Pearson regression). This correlation may reflect shared intensity of BAS and BIS traits.
3.2. GMV correlates of BAS residual scores
Overall, brain regions with GMVs in correlation with BAS and BIS scores (Supplementary Figures S1 and S2) and those in correlation with BAS and BIS residual scores (Figure 1 and Figure 2) were similar. Thus, we report the results from the regressions with BAS/BIS residuals, which would reflect the GMV correlates specific to the BAS and BIS.
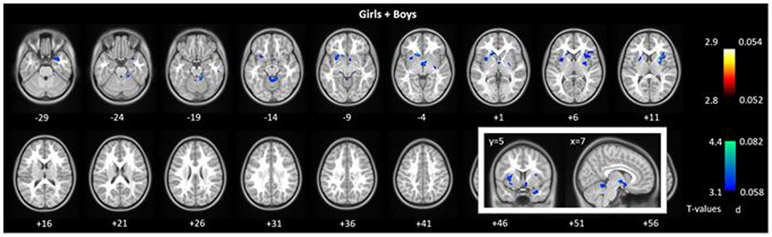
Figure 1.
GMV correlates of BAS residual scores for girls and boys combined. One-sample t-test, p<0.05, FWE corrected. Color bars show voxel T values and the corresponding Cohen’s d scores. Warm/cool colors: positive/negative correlations. The regression for the whole sample showed clusters both in positive and in negative correlation with BAS residual scores. The inset highlights the ventral striatal and cerebellar vermis clusters in coronal and sagittal sections. The results for girls’ and boys’ groups are shown in Supplementary Figure S3. The clusters are summarized in Supplementary Table S1.
Figure 2.
Structural correlates of BIS residual scores for girls and boys combined. One-sample t-test, p<0.001, uncorrected. Color bars show voxel T values and the corresponding Cohen’s d scores. Warm/cool colors: positive/negative correlations. The inset shows the ventral striatal cluster at the same coordinates in coronal and sagittal sections as in Figure 1. The results for girls’ and boys’ groups are shown in Supplementary Figure S4. The clusters are summarized in Supplementary Table S2.
Regions with GMVs that correlated positively with the BAS residuals were localized in a cluster in the ventral striatum (VS) extending to the subcallosal gyrus, another cluster in the cerebellar vermis, and two small clusters in bilateral parahippocampal gyri in boys and girls combined. The analyses of boys and girls alone did not show any brain regions with GMVs that correlated positively with the BAS residuals. For boys and girls combined, regions with GMVs that correlated negatively with the BAS residuals were identified in bilateral visual cortex, including the calcarine sulci and middle occipital gyrus, precuneus, bilateral lateral orbitofrontal cortex (OFC), inferior frontal cortex, middle/inferior temporal cortex and the right cerebellum. Boys and girls examined separately likewise showed some of these volumetric correlates but also involved brain regions that appeared distinct to each sex. In girls alone, bilateral amygdala and hippocampal gyri also showed GMVs in negative correlation with BAS residuals. In boys alone, the ventromedial prefrontal cortex (vmPFC) showed GMVs in negative correlation with BAS residuals. The clusters are summarized in Supplementary Table S1.
To examine potential sex differences, we used exclusive masking of the volumetric correlates. That is, for girls, we showed clusters by excluding the voxels observed for boys and vice versa. These “girl- and boy-specific” clusters are shown in pink and light green, respectively, in Supplementary Figure S3. Further, we combined these clusters into girl and boy specific ROI and extract the GMVs of these ROIs. We then performed a slope test (Zar, 1999) to examine sex differences in the linear regression of these GMVs against BAS residual scores. The results showed that girls and boys did not differ significantly in either regression (girl “specific” ROI: t=1.502, d = 0.028, p=0.133; boy “specific” ROI: t=1.501, d = 0.028, p=0.133). In addition to focusing on an ROI that combined all clusters, we performed the same analyses on bilateral amygdala and vmPFC – that each appeared to be girl and boy specific. The results of slope test similarly showed no significant sex differences (amygdala: t=0.739, d = 0.014, p=0.460; vmPFC: t=1.148, d = 0.021, p=0.251).
3.3. GMV correlates of BIS residual scores
For the GMV correlates of BIS residual scores, no voxels showed significant correlations at voxel p<0.05, FWE-corrected. Thus, we examined the results at voxel p<0.001 uncorrected, in combination with cluster p<0.05, FWE-corrected. In girls and boys combined, BIS residual scores were negatively correlated with the GMV of bilateral ventral caudate and putamen, hypothalamus, right anterior insula, and a cluster in the cerebellar vermis (Figure 2). Although girls and boys combined did not show voxels in positive correlation with the BIS residual scores, girls and boys examined separately showed a few clusters in correlation (Supplementary Figure S4). The clusters are summarized in Supplementary Table S2.
3.4. Shared correlates of the BAS and BIS
Notably, a cluster in the VS showed both a positive correlation with the BAS residuals (r = 0.05, p = 9.29e-07) and a negative correlation with the BIS residuals (r = −0.04, p = 3.29e-06), in girls and boys combined (insets, Figure 1 and 2). Another cluster in the cerebellar vermis also showed both a positive correlation with the BAS residuals (r = 0.04, p = 1.79e-05) and a negative correlation with the BIS residuals (r = - 0.04, p = 4.13e-06), in girls and boys combined. For both the VS and cerebellum clusters, the correlation of GMV with BAS vs. with BIS were significant in slope tests (VS: t= 6.40, p=1.54e-10; cerebellum: t=6.07, p= 1.26e-09) (Supplementary Figure S5).
3.5. Heritability of BAS/BIS residual scores and volumetric correlates
Of the 11,542 children, 648 and 2,697 were identified as monozygotic twins (MZ) and dizygotic twins/siblings (DZ), respectively. Thus, we performed an analysis to examine whether the BAS/BIS scores as well as the volumetric correlates are more significantly related in MZ, as compared to DZ and unrelated individuals (UR), and in DZ as compared to UR. To this end, we computed the correlation each for the BAS/BIS residual scores as well as the volumetric correlates for the MZ and DZ pairs. For the UR, pairs of children were randomly constructed by shuffling and splitting the sample into halves. This procedure was repeated 100 times, and mean regression lines were computed. For the correlations in MZ and DZ pairs, 95% confidence intervals were also calculated. We then performed slope tests, pair-wise, to examine the differences between MZ, DZ and UR.
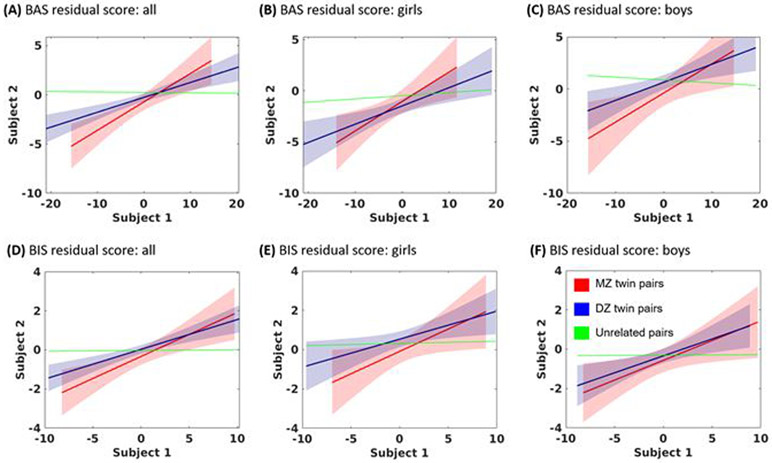
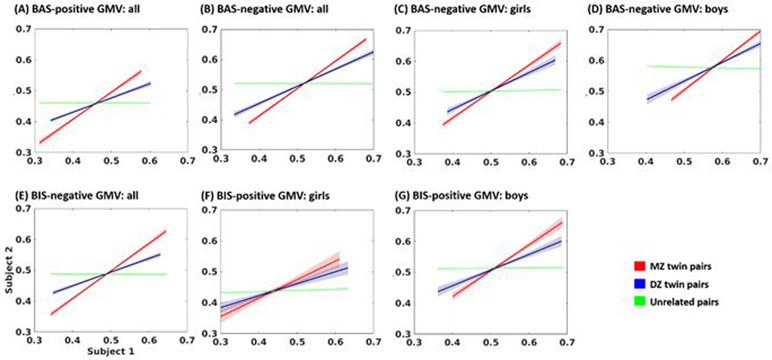
The results are shown in Figure 3 and Figure 4. Overall, for the BAS/BIS residual scores and the volumetric correlates, the correlations were stronger in MZ than in DZ and UR as well as in DZ than in UR. Supplementary Table S3 and S4 summarizes the statistics of slope tests.
Figure 3.
plots the correlation each of monozygotic twins (MZ, red), dizygotic twins and siblings (DZ, blue) and unrelated individuals (UR, green) of (A) BAS residual score for all; (B) BAS residual score for girls; (C) BAS residual scores for boys; (D) BIS residual score for all; (E) BIS residual score for girls; (F) BIS residual scores for boys. The results of slope test of these regressions are shown in Supplementary Table S3. Regression lines were built using pairs of scores for MZ and DZ. “Subject 1” and “Subject 2” represent MZ or DZ twin pairs. For UR, pairs of children were randomly constructed by shuffling and splitting the sample into halves. This procedure was repeated 100 times, and mean regression lines were computed. 95% confidence intervals were calculated and are represented in the plot by shaded areas for MZ and DZ.
Figure 4.
plots the correlation each of monozygotic twins (MZ), dizygotic twins and siblings (DZ) and unrelated individuals (UR) of (A) BAS residual-positive GMV correlates for all; (B) BAS residual-negative GMV correlates for all; (C) girls’ BAS residual-negative GMV correlates; (D) boys’ BAS residual-negative GMV correlates; and (E) BIS residual-negative GMV correlates for all; (F) girls’ BIS residual-positive GMV correlates; (G) boys’ BIS residual-positive GMV correlates. The results of slope test of these regressions are shown in Supplementary Table S4. Regression lines were built using pairs of average GMVs for MZ and DZ. “Subject 1” and “Subject 2” represent MZ or DZ twin pairs. For UR, pairs of children were randomly constructed by shuffling and splitting the sample into halves. This procedure was repeated 100 times, and mean regression lines were computed. 95% confidence intervals were calculated and are represented in the plot by shaded areas for MZ and DZ.
We computed the h2, c2 and e2 for the BAS residual scores, BIS residual scores, as well as the volumetric correlates of the BAS and BIS residual scores (Table 1). The results showed an h2 ~0.2 and e2 >0.7 for BAS residual scores, and an h2 ~0.15 and e2 >0.7 for BIS residual scores for the entire sample, suggesting that BAS and BIS residual scores were largely determined by unique environmental influences. In contrast, the GMV correlates ranged from 0.71 to 0.88 in h2 and 0.08 to 0.15 in e2 for the entire sample, suggesting that the volumetric markers were determined mostly by heritable factors. The shared volumetric correlate in the VS demonstrated an h2 of 0.8463. Further, the h2 (~0.88) of BIS GMV correlates was higher than the h2 (~0.71 to 0.76) of BAS GMV correlates in girls and boys combined, suggesting that the BAS may be more amenable to the influences of environmental factors, relative to the BIS.
Table 1.
Heritability of the BAS/BIS residual scores and the GMV correlates.
| h2 | c2 | e2 | |
|---|---|---|---|
| BAS residual score | |||
| all | 0.2335 | 0.0317 | 0.7348 |
| girls | 0.1724 | 0.0959 | 0.7317 |
| boys | 0.1665 | 0.0839 | 0.7496 |
| BIS residual score | |||
| all | 0.1612 | 0.0692 | 0.7697 |
| girls | 0.1481 | 0.0621 | 0.7899 |
| boys | 0.1273 | 0.0980 | 0.7747 |
| GMV | |||
| BAS residual +, all | 0.7612 | 0.0896 | 0.1492 |
| BAS residual −, all | 0.7128 | 0.2102 | 0.0770 |
| BAS residual −, girls | 0.6603 | 0.2713 | 0.0684 |
| BAS residual −, boys | 0.6033 | 0.3156 | 0.0811 |
| BIS residual −, all | 0.8848 | 0.0083 | 0.1070 |
| BIS residual +, girls | 0.5224 | 0.1170 | 0.3606 |
| BIS residual +, boys | 0.6948 | 0.1551 | 0.1501 |
In the interest of understanding how the h2 of the BAS/BIS volumetric correlates compared to those of other brain regions, we have computed the h2 for the whole-brain mask (h2 =0.8381) as well as for each one of the 116 masks available from the Automated Anatomical Labeling atlas (h2 = 0.7562 ± 0.0814, min=0.5137, max=0.9696). The BAS residual+, BAS residual- and BIS residual- GMV correlates each showed an h2 of 0.7612, 0.7128, and 0.8848, ranking at 53th, 23th, and 95th percentile, respectively. These results suggest that these GMV correlates of BIS but not BAS traits were more heritably than most brain regions as identified by the AAL atlas and that BIS correlates were more heritable than BAS correlates.
4. Discussion
The current results showed that the GMVs of a cluster in ventral striatum (VS) extending to the subcallosal gyrus were positively correlated with BAS scores in the entire cohort. BAS scores were negatively correlated with the GMVs in bilateral visual, lateral orbitofrontal, temporal, and inferior frontal cortex, as well as the precuneus. Although girls and boys appeared to demonstrate distinct volumetric markers of BAS when examined separately, the GMVs of these “sex-specific” regions did not show correlations with the BAS scores differently between girls and boys. In girls and boys combined, BIS residual scores were negatively correlated with the GMV of bilateral ventral caudate and putamen, hypothalamus, and right anterior insula. Importantly, the GMV of a cluster in the VS and in the cerebellar vermis showed both positive correlation with the BAS scores and negative correlation with the BIS scores in girls and boys combined, indicating a shared correlate of approach and avoidance traits. These volumetric correlates are heritable, suggesting an important role of genetics in determining approach and avoidance dispositions in children.
4.1. Shared BAS and BIS correlates in the ventral striatum (VS) and cerebellar vermis
The VS is best known as a hub of the reward circuit. Receiving dense dopaminergic projections from the midbrain, the VS responds to rewarding stimuli (Daniel and Pollmann, 2014; Van de Steen et al., 2020) and reward prediction error (Chase et al., 2015). The latter suggests that the VS may respond broadly to saliency and not just to reward (Hayes et al., 2014). Indeed, VS activation to rewarding stimuli depends on their saliency (Zink et al., 2004). A number of studies have also demonstrated VS response to salient but punishing stimuli, including exposure to fearful stimuli (de Haan et al., 2018), behaviorally relevant distractors (Zink et al., 2003) and noxious thermal stimulation (Becerra et al., 2001). VS response to reward or other salient stimuli varies with personality traits. For instance, individual differences in reward sensitivity were positively associated with VS response to reward receipt during reinforcement learning (Kim et al., 2015). VS responses to monetary wins and losses may depend on early-life experiences that shape behavioral dispositions (Luking et al., 2018). Together, the VS shows strong activations to rewarding stimuli, typically highly salient, as well as negative stimuli that are behaviorally salient. Studies are needed specifically to examine how individuals with different BAS and BIS traits may vary in VS responses to reward and punishment.
In addition to these functional studies, structural imaging has also demonstrated variation of the VS GMV in association with personality traits, but the results are less than consistent. For instance, behavioral impulsivity has been associated with smaller putamen (Cho et al., 2013), larger caudate (Tschernegg et al., 2015), and larger VS volumes in adolescents (Mackey et al., 2017), whereas another study failed to demonstrate an association between impulsivity and striatal GMV in adults (Caravaggio et al., 2018). For BIS traits, studies of adults likewise exhibited heterogeneity in findings. An earlier review revealed GMV of a number of brain regions, including the medial OFC and amygdala in healthy individuals scoring higher on negative emotionality traits, but did not implicate the VS (Mincic, 2015). Notably, these studies focused on adolescents or adults, comprised a small sample size, and involved different instruments in assessing impulsivity.
The current findings demonstrated VS volume as a shared correlates of BAS and BIS in children. Larger VS volume is associated with both higher BAS and lower BIS scores. Importantly, this neural marker is evident in children 9 to 10 years of age, suggesting that the variation in VS volume most likely reflect the predisposition to approach and avoidance behavior rather than reflect the consequences of these behaviors at this early stage of life. Indeed, the analysis of heritability showed that this volumetric trait is highly heritable with an h2 of 0.8463 in girls and boys combined (ranking at the 88th percentile, as compared to the 116 AAL regions), 0.6734 in girls and 0.6773 in boys. These findings in children do not mirror those in studies of adults, suggesting a significant role of environmental influences in shaping cerebral structures across the life span.
Although the cerebellum has received much less attention than the VS in the imaging literature, evidence has accumulated to implicate cerebellar processes in reward-related behavior (Wagner and Luo, 2020). In particular, recent studies have characterized cerebellar neuronal responses to prediction errors beyond cognitive motor control, suggesting a broader role of the cortical-cerebellar circuit to support motivated behavior (Hull, 2020). The extensive anatomical connections between the cerebellum and limbic circuits, including the hypothalamus, also suggested its importance in emotional processing and motivated behavior (Benagiano et al., 2018). On the other hand, although imaging studies have described cerebellar dysfunction in various clinical conditions involving externalizing and internalizing psychopathology (Moulton et al., 2014; Schutter, 2016), none to our knowledge have implicated the cerebellum in BAS or BIS traits in neurotypical populations. It is notable that the cerebellar cluster with GMV in positive and negative correlation each with the BAS and BIS trait was located in the anterior vermis, the “spinocerebellum” best known for its role in motor control. It remains to be seen how this cerebellar structure partake in reward-based behavioral control and how individual differences in BAS/BIS traits may be reflected in the cerebellar vermis during these behavioral contingencies.
4.2. Distinct volumetric correlates of the BAS and BIS
The BAS and BIS are also associated with distinct volumetric findings. The brain areas showing less GMVs in association with higher BAS scores include the visual cortex, bilateral lateral OFC (lOFC), bilateral but predominantly right inferior frontal cortex, and bilateral middle/inferior temporal cortex, in girls and boys combined. Higher BIS scores are specifically associated with lower GMVs of bilateral putamen, the hypothalamus, and right anterior insula. We will not attempt to offer post-hoc account of all of the associations. However, some of these findings can be considered with earlier functional imaging or lesion studies. For instance, the lOFC is known to process negatively valenced stimuli (Fujiwara et al., 2008; Rich and Wallis, 2014; Yan et al., 2016; Dugre et al., 2018). The putamen is a subcortical hub of the cognitive motor circuit. With extensive connections to motor, premotor, and supplementary motor cortex (Zhang et al., 2012), the putamen supports a wide range of cognitive and affective operations (Li, 2000; Hu and Li, 2012) and goal-directed actions (Ena et al., 2011; Schultz, 2016). Likewise, receiving heavy dopaminergic projections from the midbrain, the hypothalamus is implicated in a wide range of motivated behavior, including feeding, mating as well as “flight or fight” response (Zhang et al., 2018). Thus, lower lOFC and putamen/hypothalamus volume may reflect less efficient processing of negative information and a propensity to withdraw from goal-directed actions in individuals high on BAS and BIS traits, respectively.
These findings in children do not appear to mirror those reported in VBM studies of adults. For instance, combining brain imaging and a delay discounting task, a study demonstrated a positive correlation between the GMV of the right caudate head and a stronger tendency to discount future reward, a BAS correlate, in young adults (Tschernegg et al., 2015). A recent work reported a negative relationship between the striatal volume and reward sensitivity, as assessed by the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ), though only for male participants (Adrian-Ventura et al., 2019). Another VBM study of 400 healthy young adults showed that punishment sensitivity, as assessed with the SPSRQ, correlated positively with the GMV in the amygdala (Adrian-Ventura et al., 2019). An earlier review revealed lower GMV in the medial OFC (mOFC) and higher GMV in the left amygdala/anterior parahippocampal gyrus in healthy adults scoring high on negative emotionality traits (Mincic, 2015). Notably, although we did not observe variation of GMV in the mOFC and amygdala in link with BIS scores, girls and boys with higher BAS score demonstrated reduction of GMV in the amygdala and mOFC, respectively. The amygdala mOFC circuit is central to emotional regulation and likely amenable to the influences of many environmental factors (Diekhof et al., 2011; Hiser and Koenigs, 2018; Andrewes and Jenkins, 2019). Amygdalar functional connectivity varies significantly with age from early childhood to late adulthood (Xiao et al., 2018). Further, the amygdala interacts with subregions of the vmPFC in processing positive vs. negative emotions (Xiao et al., 2018; Yang et al., 2020). Thus, in order to meaningfully compare the findings across the life span, one would have to carefully consider not only age and sex of the study populations but also the instruments as well as anatomical or functional subareas of a brain region.
4.3. Sex differences
With a similar sample size and range of BAS and BIS scores, girls relative to boys showed higher BIS scores and, conversely, lower BAS scores. However, of the brain regions we identified and with consideration of multiple comparisons, none appear to show significant sex differences in the correlation with BAS or BIS scores, on the basis of slope tests. These findings suggest that girls and boys of 9 to 10 years old did not demonstrate significant sex-specific volumetric correlates of the BAS and BIS, despite differentiable subjective reports of behavioral tendency.
On the other hand, previous studies have associated BAS/BIS-related traits with specific brain correlates in either men or women only (Blankstein et al., 2009; Forsman et al., 2012; Hakamata et al., 2009; Yamasue et al., 2008). Higher GMV of the superior temporal gyrus was associated with novelty seeking in female but not male adults (Stam et al., 2019). As discussed earlier, a recent VBM study of healthy young adults showed a negative relationship between the striatal volume and reward sensitivity for male but not female participants (Adrian-Ventura et al., 2019). While the neural mechanisms underlying the development of such sex differences remain to be elucidated, girls and boys exhibit distinct trajectories in brain development during late childhood. From 7 to 17 years of age, boys as compared to girls showed faster dendritic pruning and greater white matter volume gains (De Bellis et al., 2001). Girls peaked in GMV 1-2 years earlier than boys (Lenroot et al., 2007). Further, anatomical connections may be optimized for within- and inter- hemispheric communications during development in boys and girls, respectively (Ingalhalikar et al., 2014). More studies clearly are warranted to understand the developmental neural processes of sex-specific cognitive and emotional characteristics (Cohn, 1991; Chaplin and Aldao, 2013).
Finally, some studies have suggested a stronger relationship between neurobiological correlates and personality traits in girls whereas environmental factors may contribute more significantly to personality in boys. For instance, BAS-related behaviors such as psychopathy and substance misuse have been shown to be modulated by environmental variables to a greater degree in boys than girls (Hicks et al., 2012; Schulte et al., 2009). In contrast, genetics plays a small but more influential role in aggression in girls relative to boys (Hudziak et al., 2003). Here, the heritability (h2) computed for BAS and BIS scores or the girls’ and boys’ volumetric correlates were very similar between girls and boys. It is possible that, as the children continue to grow, we may be able to see more differential influences of genetic and environmental factors of these personality and neural markers. Study of future releases of the ABCD data will help in elucidating these issues.
4.4. An overview of the heritability of neural correlates
The examination of the heritability of the BAS/BIS traits and their underlying neural substrates may enhance the understanding of individual differences in the BAS/BIS traits. In the combined sample, the h2 of the volumetric correlates of BIS and BAS traits ranged from 0.71 to 0.88, far higher than the trait BAS and BIS measures.
Further, based on the distribution of h2 (0.7562±0.0814) across the 116 AAL brain regions, the volumetric correlates of BIS ranked at 95th percentile, in contrast to 83th percentile of the whole intracranial volume. These findings together suggest high heritability, particularly for the BIS volumetric correlates, of these structural neural markers.
Previous studies showed that the BIS traits are weakly-to-highly heritable (19 to 62%) in the adolescents and moderately-to-highly heritable (38-62%) in the adults (Seroczynski et al., 1999; Takahashi et al., 2007; Zheng et al., 2019). In comparison, the heritability of the BIS traits in the children of the current study appears lower (16%), suggesting that the BIS trait may become more heritable across development from childhood to adulthood. This developmental trend of heritability has also been observed for cognitive abilities (Haworth et al., 2010; Briley and Tucker-Drob, 2013; Chen et al., 2020), and gene-environment interaction may contribute to the amplified genetic influences (Briley and Tucker-Drob, 2013; Tucker-Drob et al., 2013). Specifically, genetic factors may have positive influences on the environmental factors provided by parents, teachers, and peers which in turn leads to the longitudinal increase of the heritability. Relatively few studies have examined the heritability of the BAS traits, and earlier work showed that the BAS traits were less heritable (17-21%) compared to the BIS traits in adults (Takahashi et al., 2007). It remains to be seen whether the heritability of BAS traits would follow the same longitudinal patterns, increasing across the life span.
In the current study, the neural correlates of the BAS/BIS traits (h2neural ~ 71-88%) showed higher heritability than the clinical scores (h2traits ~ 16-23%). This finding does not appear to be consistent with those reported of fluid intelligence, working memory, and risk-taking in adults. Adults showed high heritability of both fluid intelligence scores (70-80%) (Brouwer et al., 2014) and the gray/white matter volumetric correlates of fluid intelligence (~80%) (Posthuma et al., 2002; Hulshoff Pol et al., 2006). Likewise, adults showed comparable heritability in working memory performance (44-57%), task-related brain responses (40-65%), and related gray matter density (48-62%) (Posthuma et al., 2003; Karlsgodt et al., 2010; Blokland et al., 2011). A more recent study demonstrated similar, moderate genetic influences for both risk-taking behavior (41-45%) and its functional correlates (29-47%) (Rao et al., 2018). It is not entirely clear what may account for higher h2neural than h2traits in the current study. Children may demonstrate less reliable responses in assessment with the clinical questionnaires. With the longitudinal clinical and imaging data from the ABCD project, investigators will have the opportunity to evaluate how these psychological and neural metrics evolve from childhood to young adulthood and whether the heritability of these metrics change accordingly.
4.5. Limitations, conclusions, and future research
A few limitations need to be considered for the current study. First, the brain undergoes extensive “maturation” during childhood, with substantial increases in GMV, white matter density, and myelination (Huttenlocher, 1979; Giedd et al., 1999; Gogtay et al., 2004). Studies have shown structural and functional brain changes through adolescence in relation to approach and avoidance traits (Fareri et al., 2008; Forbes et al., 2010; Urosevic et al., 2012; Urosevic et al., 2014; Berenbaum et al., 2015; Wierenga et al., 2018). Thus, the current findings from 9 and 10 years old may not generalize to the entire childhood. Studies of follow-up scans of the ABCD data will demonstrate how the BAS and BIS correlates evolve during adolescence. Second, we used an optimized adult tissue probability maps for the VBM analysis. Although the use of an age-specific template is recommended, the construction of customized DARTEL templates involves multiple issues, including the trade-off between more vs. less deformation (as conditioned to the warping algorithm parameters) and modeling demographic variables vs. averaging (Wilke et al., 2017). These issues are non-trivial. Further, the brain of children at 7-11 years is 95% the volume of the adults’ (Matsuzawa et al., 2001), and the patterns of uniform scaling are overall sustained during the final volumetric increment, between this age and adulthood, perhaps with exceptions to the cerebellum and the white matter (Caviness et al., 1996). Previous studies have also shown the feasibility of using adult templates in pediatric studies as young as 7 years of age (Burgund et al., 2002; Kang et al., 2003). Nonetheless, these methodological issues may need to be revisited by the imaging community. Third, cerebral GMV are influenced by early childhood experiences (e.g., Hanson et al., 2015). The ABCD project does include data on these important variables and research is needed to understand how BAS and BIS traits vary and potentially interact with these variables in determining brain development. Fourth, BAS and BIS represents very broad measures of approach and avoidance tendency; thus, clinical or behavioral assessments that target a specific dimension of these behavioral dispositions are needed to fully reveal the neural phenotypes of personality systems.
In conclusion, we identified shared and distinct cerebral volumetric markers of the BAS and BIS in children 9 to 10 years of age. The GMV of the ventral striatum is positively and negatively associated with the BAS and BIS scores, respectively, in girls and boys combined. These neural markers are highly heritable, showing a heritability (h2) of 0.71 to 0.88, far higher than the BAS or BIS scores (h2 < 0.23), suggesting their importance in advancing genetic research of behavioral dispositions in health and illness.
Supplementary Material
Acknowledgements
This study is supported by NIH grants DA023248, AA021449, DA044749, and DA045189. The NIH is otherwise not responsible for the conceptualization of the study, data collection and analysis, or in the decision in publishing the results.
Footnotes
Disclosures
We declare no conflicts of interest in the current study.
References
- Adrian-Ventura J, Costumero V, Parcet MA, Avila C (2019) Linking personality and brain anatomy: a structural MRI approach to Reinforcement Sensitivity Theory. Social cognitive and affective neuroscience 14:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrewes DG, Jenkins LM (2019) The Role of the Amygdala and the Ventromedial Prefrontal Cortex in Emotional Regulation: Implications for Post-traumatic Stress Disorder. Neuropsychology review 29:220–243. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D, Alia-Klein N, Potter AS, Paulus MP, Prouty D, Zucker RA, Sher KJ (2018) Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Developmental cognitive neuroscience 32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C (2006a) Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. Neuroimage 33:1011–1015. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C (2006b) Striatum gray matter reduction in males with an overactive behavioral activation system. The European journal of neuroscience 24:2071–2074. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D (2001) Reward circuitry activation by noxious thermal stimuli. Neuron 32:927–946. [DOI] [PubMed] [Google Scholar]
- Becker SP, Fite PJ, Garner AA, Greening L, Stoppelbein L, Luebbe AM (2013) Reward and punishment sensitivity are differentially associated with ADHD and sluggish cognitive tempo symptoms in children. Journal of Research in Personality 47:719–727. [Google Scholar]
- Benagiano V, Rizzi A, Lorusso L, Flace P, Saccia M, Cagiano R, Ribatti D, Roncali L, Ambrosi G (2018) The functional anatomy of the cerebrocerebellar circuit: A review and new concepts. J Comp Neurol 526:769–789. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM, Corley R (2015) The importance of puberty for adolescent development: conceptualization and measurement. Adv Child Dev Behav 48:53–92. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Straub LK, Provost RG, Neale MC (2017) The ABCD study of neurodevelopment: Identifying neurocircuit targets for prevention and treatment of adolescent substance abuse. Curr Treat Options Psychiatry 4:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland GA, McMahon KL, Thompson PM, Martin NG, de Zubicaray GI, Wright MJ (2011) Heritability of working memory brain activation. J Neurosci 31:10882–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM (2013) Explaining the increasing heritability of cognitive ability across development: a meta-analysis of longitudinal twin and adoption studies. Psychological science 24:1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer RM, Hedman AM, van Haren NE, Schnack HG, Brans RG, Smit DJ, Kahn RS, Boomsma DI, Hulshoff Pol HE (2014) Heritability of brain volume change and its relation to intelligence. Neuroimage 100:676–683. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL (2002) The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage 17:184–200. [DOI] [PubMed] [Google Scholar]
- Caravaggio F, Plaven-Sigray P, Matheson GJ, Plitman E, Chakravarty MM, Borg J, Graff-Guerrero A, Cervenka S (2018) Trait impulsivity is not related to post-commissural putamen volumes: A replication study in healthy men. PloS one 13:e0209584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ et al. (2018) The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental cognitive neuroscience 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS Jr., Kennedy DN, Richelme C, Rademacher J, Filipek PA (1996) The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cerebral cortex 6:726–736. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Aldao A (2013) Gender differences in emotion expression in children: a meta-analytic review. Psychol Bull 139:735–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Kumar P, Eickhoff SB, Dombrovski AY (2015) Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cogn Affect Behav Neurosci 15:435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen C, Wu T, Qiu B, Zhang W, Fan J (2020) Accessing the development and heritability of the capacity of cognitive control. Neuropsychologia 139:107361. [DOI] [PubMed] [Google Scholar]
- Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, Strafella AP (2013) Morphometric correlation of impulsivity in medial prefrontal cortex. Brain topography 26:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences. Hillsdale NJ: L. Erlbaum Associates. [Google Scholar]
- Cohn LD (1991) Sex differences in the course of personality development: a meta-analysis. Psychol Bull 109:252–266. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Daniel R, Pollmann S (2014) A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem 114:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM (2001) Sex differences in brain maturation during childhood and adolescence. Cerebral cortex 11:552–557. [DOI] [PubMed] [Google Scholar]
- de Haan MIC, van Well S, Visser RM, Scholte HS, van Wingen GA, Kindt M (2018) The influence of acoustic startle probes on fear learning in humans. Sci Rep 8:14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O (2011) Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 58:275–285. [DOI] [PubMed] [Google Scholar]
- Dragan WL, Jednorog K, Marchewka A (2019) Sex-Specific Relationship of Childhood Adversity With Gray Matter Volume and Temperament. Front Behav Neurosci 13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugre JR, Dumais A, Bitar N, Potvin S (2018) Loss anticipation and outcome during the Monetary Incentive Delay Task: a neuroimaging systematic review and meta-analysis. PeerJ 6:e4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot AJ, Thrash TM (2002) Approach-avoidance motivation in personality: approach and avoidance temperaments and goals. J Pers Soc Psychol 82:804–818. [DOI] [PubMed] [Google Scholar]
- Ena S, de Kerchove d’Exaerde A, Schiffmann SN (2011) Unraveling the differential functions and regulation of striatal neuron sub-populations in motor control, reward, and motivational processes. Front Behav Neurosci 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, Delgado MR (2008) Reward-related processing in the human brain: developmental considerations. Dev Psychopathol 20:1191–1211. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Brain Development Cooperative G (2011) Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL (2009) Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 47:S102. [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE (2010) Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry 49:162–172 e161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie MM, Thomas KG, Amodio DM, Warton CM, Meintjes EM (2014) Neural correlates of experienced moral emotion: an fMRI investigation of emotion in response to prejudice feedback. Soc Neurosci 9:203–218. [DOI] [PubMed] [Google Scholar]
- Fowles DC (1980) The three arousal model: implications of gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology 17:87–104. [DOI] [PubMed] [Google Scholar]
- Fuentes P, Barros-Loscertales A, Bustamante JC, Rosell P, Costumero V, Avila C (2012) Individual differences in the Behavioral Inhibition System are associated with orbitofrontal cortex and precuneus gray matter volume. Cogn Affect Behav Neurosci 12:491–498. [DOI] [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui K (2008) Personality-dependent dissociation of absolute and relative loss processing in orbitofrontal cortex. The European journal of neuroscience 27:1547–1552. [DOI] [PubMed] [Google Scholar]
- Gaser C (2016) CAT - a Computational Anatomy Toolbox for the Analysis of Structural MRI Data. In: Organization for Human Brain Mapping. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA (1982) The Neuropsychology of Anxiety - an Inquiry into the Functions of the Septo-Hippocampal System. Behav Brain Sci 5:469–484. [Google Scholar]
- Gray JA (1990) Brain Systems that Mediate both Emotion and Cognition. Cognition and Emotion 4:269–288. [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS (2005) Affective personality differences in neural processing efficiency confirmed using fMRI. Cognitive, Affective, & Behavioral Neuroscience 5:182–190. [DOI] [PubMed] [Google Scholar]
- Gudiño OG (2013) Behavioral inhibition and risk for posttraumatic stress symptoms in Latino children exposed to violence. J Abnorm Child Psychol 41:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Sinha R, Potenza MN (2012) Hazardous Drinking and Dimensions of Impulsivity, Behavioral Approach, and Inhibition in Adult Men and Women. Alcoholism-Clinical and Experimental Research 36:434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE (2015) Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol Psychiatry 78:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CM et al. (2010) The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular psychiatry 15:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Duncan NW, Xu J, Northoff G (2014) A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev 45:350–368. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Stuttgen MC (2011) Computation of measures of effect size for neuroscience data sets. The European journal of neuroscience 34:1887–1894. [DOI] [PubMed] [Google Scholar]
- Hiser J, Koenigs M (2018) The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry 83:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li CS (2012) Neural processes of preparatory control for stop signal inhibition. Human brain mapping 33:2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C (2020) Prediction signals in the cerebellum: beyond supervised motor learning. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, van Haren NE, Collins DL, Evans AC, Amunts K, Burgel U, Zilles K, de Geus E, Boomsma DI, Kahn RS (2006) Genetic contributions to human brain morphology and intelligence. J Neurosci 26:10235–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1979) Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain research 163:195–205. [DOI] [PubMed] [Google Scholar]
- Kambouropoulos N, Staiger PK (2001) The influence of sensitivity to reward on reactivity to alcohol-related cues. Addiction 96:1175–1185. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL (2003) Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage 19:16–28. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Kochunov P, Winkler AM, Laird AR, Almasy L, Duggirala R, Olvera RL, Fox PT, Blangero J, Glahn DC (2010) A multimodal assessment of the genetic control over working memory. J Neurosci 30:8197–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, Geuze E (2013) Neural correlates of personality: an integrative review. Neurosci Biobehav Rev 37:73–95. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Holmes C, Lee J, Chiu P, King-Casas B (2016) Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia 91:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Yoon H, Kim H, Hamann S (2015) Individual differences in sensitivity to reward and punishment and neural activity during reward and avoidance learning. Social cognitive and affective neuroscience 10:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber B, Veit R, Moll CV, Gaser C, Birbaumer N, Lotze M (2016) Voxel-based morphometry in opera singers: Increased gray-matter volume in right somatosensory and auditory cortices. Neuroimage 133:477–483. [DOI] [PubMed] [Google Scholar]
- Kohler HP, Behrman JR, Schnittker J (2011) Social science methods for twins data: integrating causality, endowments, and heritability. Biodemography Soc Biol 57:88–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotich TD, Tregellas JR, Thompson LL, Banich MT, Klenk AM, Tanabe JL (2013) Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug Alcohol Depend 129:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR (2000) Impairment of motor imagery in putamen lesions in humans. Neurosci Lett 287:13–16. [DOI] [PubMed] [Google Scholar]
- Li Y, Qiao L, Sun J, Wei D, Li W, Qiu J, Zhang Q, Shi H (2014) Gender-specific neuroanatomical basis of behavioral inhibition/approach systems (BIS/BAS) in a large sample of young adults: a voxel-based morphometric investigation. Behavioural brain research 274:400–408. [DOI] [PubMed] [Google Scholar]
- Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G (2018) Ventral Striatal Function Interacts With Positive and Negative Life Events to Predict Concurrent Youth Depressive Symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging 3:937–946. [DOI] [PubMed] [Google Scholar]
- Mackey S et al. (2017) Brain Regions Related to Impulsivity Mediate the Effects of Early Adversity on Antisocial Behavior. Biol Psychiatry 82:275–282. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T (2001) Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral cortex 11:335–342. [DOI] [PubMed] [Google Scholar]
- Mincic AM (2015) Neuroanatomical correlates of negative emotionality-related traits: A systematic review and meta-analysis. Neuropsychologia 77:97–118. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D (2014) The cerebellum and addiction: insights gained from neuroimaging research. Addict Biol 19:317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlert N, Lawrence AD (2015) Brain structure correlates of emotion-based rash impulsivity. Neuroimage 115:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luking KR, Anokhin AP, Gotlib IH, Hayden EP, Olino TM, Peng CZ, Hajcak G, Barch DM (2016) Revising the BIS/BAS Scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychol Assess 28:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Park YA, Lee HW, Jung HY, Lee J-Y, Choi J-S (2013) The effects of behavioral inhibition/approach system as predictors of Internet addiction in adolescents. Pers Indiv Differ 54:7–11. [Google Scholar]
- Posthuma D, De Geus EJC, Baaré WFC, Pol HEH, Kahn RS, Boomsma DI (2002) The association between brain volume and intelligence is of genetic origin. Nature Neuroscience 5:83–84. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baaré WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ (2003) Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Res 6:131–139. [DOI] [PubMed] [Google Scholar]
- Rao LL, Zhou Y, Zheng D, Yang LQ, Li S (2018) Genetic Contribution to Variation in Risk Taking: A Functional MRI Twin Study of the Balloon Analogue Risk Task. Psychological science 29:1679–1691. [DOI] [PubMed] [Google Scholar]
- Reuter M, Stark R, Hennig J, Walter B, Kirsch P, Schienle A, Vaitl D (2004) Personality and emotion: test of Gray’s personality theory by means of an fMRI study. Behav Neurosci 118:462–469. [DOI] [PubMed] [Google Scholar]
- Rich EL, Wallis JD (2014) Medial-lateral organization of the orbitofrontal cortex. Journal of cognitive neuroscience 26:1347–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS (2004) Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56:129–140. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC (2002) Analytic approaches to twin data using structural equation models. Brief Bioinform 3:119–133. [DOI] [PubMed] [Google Scholar]
- Schultz W (2016) Reward functions of the basal ganglia. J Neural Transm (Vienna) 123:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D (2016) A Cerebellar Framework for Predictive Coding and Homeostatic Regulation in Depressive Disorder. Cerebellum 15:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroczynski AD, Bergeman CS, Coccaro EF (1999) Etiology of the impulsivity/aggression relationship: genes or environment? Psychiatry research 86:41–57. [DOI] [PubMed] [Google Scholar]
- Sportel BE, Nauta MH, de Hullu E, de Jong PJ, Hartman CA (2011) Behavioral Inhibition and Attentional Control in Adolescents: Robust Relationships with Anxiety and Depression. J Child Fam Stud 20:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam D, Huang YA, Van den Stock J (2019) Non-overlapping and Inverse Associations Between the Sexes in Structural Brain-Trait Associations. Front Psychol 10:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer J, Baggio S, Dupuis M, Mohler-Kuo M, Daeppen JB, Gmel G (2016) Drinking Motives As Mediators of the Associations between Reinforcement Sensitivity and Alcohol Misuse and Problems. Frontiers in Psychology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Yamagata S, Kijima N, Shigemasu K, Ono Y, Ando J (2007) Continuity and change in behavioral inhibition and activation systems: A longitudinal behavioral genetic study. Pers Indiv Differ 43:1616–1625. [Google Scholar]
- Tschernegg M, Pletzer B, Schwartenbeck P, Ludersdorfer P, Hoffmann U, Kronbichler M (2015) Impulsivity relates to striatal gray matter volumes in humans: evidence from a delay discounting paradigm. Front Hum Neurosci 9:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA, Harden KP (2013) Genetic and Environmental Influences on Cognition Across Development and Context. Current directions in psychological science 22:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Lim K, Luciana M (2012) Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental psychology 48:1488–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Lim KO, Luciana M (2014) Pubertal status associations with reward and threat sensitivities and subcortical brain volumes during adolescence. Brain Cogn 89:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Steen F, Krebs RM, Colenbier N, Almgren H, Marinazzo D (2020) Effective connectivity modulations related to win and loss outcomes. Neuroimage 207:116369. [DOI] [PubMed] [Google Scholar]
- van den Berg L, Pieterse K, Malik JA, Luman M, Willems van Dijk K, Oosterlaan J, Delemarre-van de Waal HA (2011) Association between impulsivity, reward responsiveness and body mass index in children. Int J Obes (Lond) 35:1301–1307. [DOI] [PubMed] [Google Scholar]
- Vervoort L, Wolters LH, Hogendoorn SM, de Haan E, Boer F, Prins PJM (2010) Sensitivity of Gray’s Behavioral Inhibition System in clinically anxious and non-anxious children and adolescents. Pers Indiv Differ 48:629–633. [Google Scholar]
- Visscher PM, Hill WG, Wray NR (2008) Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet 9:255–266. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Medland SE, Ferreira MA, Morley KI, Zhu G, Cornes BK, Montgomery GW, Martin NG (2006) Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet 2:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Luo L (2020) Neocortex-Cerebellum Circuits for Cognitive Processing. Trends Neurosci 43:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, Schreuders E, Vd Kamp F, Peper JS, Tamnes CK, Crone EA (2018) Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology 91:105–114. [DOI] [PubMed] [Google Scholar]
- Wilke M, Altaye M, Holland SK, Consortium CA (2017) CerebroMatic: A Versatile Toolbox for Spline-Based MRI Template Creation. Front Comput Neurosci 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Zhang S, Lee LE, Chao HH, van Dyck C, Li CR (2018) Exploring Age-Related Changes in Resting State Functional Connectivity of the Amygdala: From Young to Middle Adulthood. Frontiers in aging neuroscience 10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Banich MT, Regner MF, Sakai JT, Tanabe J (2017) Behavioral approach and orbitofrontal cortical activity during decision-making in substance dependence. Drug Alcohol Depend 180:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato N, Kasai K (2007) Gender-Common and -Specific Neuroanatomical Basis of Human Anxiety-Related Personality Traits. Cerebral cortex 18:46–52. [DOI] [PubMed] [Google Scholar]
- Yan C, Su L, Wang Y, Xu T, Yin DZ, Fan MX, Deng CP, Hu Y, Wang ZX, Cheung EF, Lim KO, Chan RC (2016) Multivariate Neural Representations of Value during Reward Anticipation and Consummation in the Human Orbitofrontal Cortex. Sci Rep 6:29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Tsai SJ, Li CR (2020) Concurrent amygdalar and ventromedial prefrontal cortical responses during emotion processing: a meta-analysis of the effects of valence of emotion and passive exposure versus active regulation. Brain Struct Funct 225:345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH (1999) Biostatistical Analysis, Forth Edition. New Jersey: Prentice-Hall, Inc. [Google Scholar]
- Zhang S, Ide JS, Li CS (2012) Resting-state functional connectivity of the medial superior frontal cortex. Cerebral cortex 22:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang W, Zhornitsky S, Li CR (2018) Resting State Functional Connectivity of the Lateral and Medial Hypothalamus in Cocaine Dependence: An Exploratory Study. Front Psychiatry 9:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Chen J, Wang X, Zhou Y (2019) Genetic contribution to the phenotypic correlation between trait impulsivity and resting-state functional connectivity of the amygdala and its subregions. NeuroImage 201:115997. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS (2003) Human striatal response to salient nonrewarding stimuli. J Neurosci 23:8092–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS (2004) Human striatal responses to monetary reward depend on saliency. Neuron 42:509–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.