Abstract
Heart failure (HF) is a leading cause of morbidity. Strategies for preventing HF are paramount. Prevalent extra-coronary calcification is associated with HF risk but less is known about progression of mitral annular (MAC) and aortic valve calcification (AVC) and HF risk. Progression of valvular calcification (VC) [interval change of >0 units/yr] was assessed by 2 cardiac computed tomography scans over a median of 2.4 yrs. We used Cox regression to determine the risk of adjudicated HF and linear mixed effects models to determine 10-yr change in left ventricular (LV) parameters measured by cardiac magnetic resonance imaging associated with VC progression. We studied 5,591 MESA participants free of baseline cardiovascular disease. Mean ± SD age was 62±10 yrs; 53% women; 83% had no VC progression, 15% progressed at 1 site (AVC or MAC) and 3% at both sites. There were 251 incident HF over 15 yrs. After adjusting for cardiovascular risk factors, the Hazard Ratios (HR) (95% CI) of HF associated with VC progression at 1 and 2 sites were 1.62 (1.21–2.17) and 1.88 (1.14–3.09), respectively, compared with no progression (p-for-trend<0.001). HRs were higher for HFpEF [2.52 (1.63–3.90) and 2.49 (1.19–5.25)] but non-significant for HFrEF. Both AVC [1.61 (1.19–2.19)] and MAC [1.50 (1.09–2.07)] progression were associated with HF. VC was associated with worsening of some LV parameters over 10 yrs. In conclusion, VC progression was associated with increased risk of HF and change in LV function. Interventions targeted at reducing VC progression may also impact HF risk, particularly HFpEF.
Keywords: Calcification, Heart failure, aortic valvular calcification, mitral annular calcification
Heart failure (HF) is a complex clinical disease and shares many similar risk factors with atherosclerotic cardiovascular disease (ASCVD)1 (such increasing age, hypertension, diabetes, and physical inactivity) and sometimes results as a direct sequalae of clinical ASCVD.2, 3 It results from impairment in ventricular filling or cardiac ejection and can be classified as HF with preserved (HFpEF) or reduced (HFrEF) ejection fraction based on the left ventricular ejection fraction (LVEF). Compared to HFrEF, the pathophysiology of HFpEF is less well understood.4 Baseline coronary artery calcium (CAC) and extra coronary calcification have been shown to predict future coronary heart disease (CHD)5–10 and HF11 events. However, there is a paucity of knowledge on the effects of progression of valvular calcification (VC), possibly as a direct indicator of worsening subclinical atherosclerosis, on HF risk. We assessed the prospective association between progression of VC and incident HF and its subtypes, as well as indices of left ventricular (LV) structure and function, in a community-based cohort free from CHD and atrial fibrillation (AF) at baseline.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is an ethnically diverse cohort of 6,814 women and men aged 45–84 years old enrolled between July 2000 and August 2002 from Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA.12 The study design and methods are available at http://www.mesa-nhlbi.org and have been previously described.12 Participants were free of clinical cardiovascular disease and provided informed consent. Since inception, participants have been contacted every 9 to 12 months to assess clinical morbidity and mortality and have had five additional examinations: Exam 2 (September 2002-February 2004); Exam 3 (March 2004-September 2005); Exam 4 (September 2005-May 2007); Exam 5 (April 2010-December 2011); and Exam 6 (September 2016-June 2018). The institutional review board of each participating site approved the study.
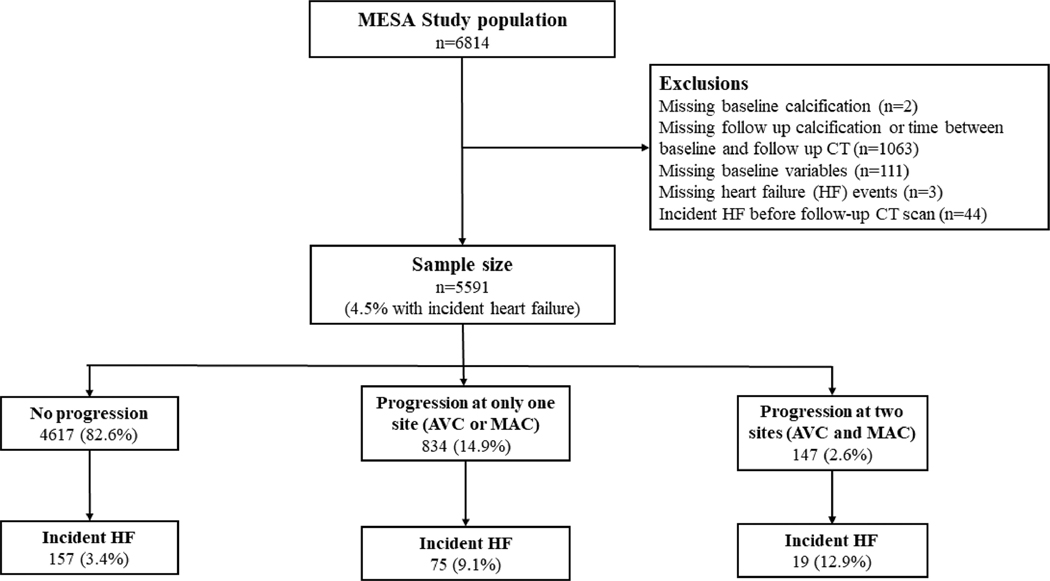
The current study includes 5,591 participants who were followed for HF events and had data on valvular calcium at both Exam 1 and Exam 2 or 3 (Figure 1). We excluded participants who had missing data on baseline calcification (n=2), missing data on follow-up valvular calcium or time between baseline and follow-up CT scans (n=1,063), missing covariates used in our main models (n=111), missing HF events (n=3) and participants who developed HF before the follow-up CT at Exam 2 or 3 (n=44).
Figure1.
Flow chart of study participants
Participants provided information on age, sex, race/ethnicity, education and smoking status during enrollment interview and questionnaire filling. The total metabolic equivalent of task (MET)-minutes/week of vigorous and moderate physical activity was derived from the Physical Activity Survey. Medication use was obtained from a medication inventory. Body mass index (BMI, kg/m2) was calculated. Systolic blood pressure (SBP) was calculated as the average of the last 2 of 3 measurements using a Dinamap automated blood pressure device. Total cholesterol and high-density lipoprotein cholesterol (HDL-C) was measured from blood obtained after a 12-hour fast. A participant was said to have diabetes if participant self-reported this, used diabetes medication or insulin, or had a fasting glucose ≥126 mg/dL. Estimated glomerular filtration rate (eGFR) was derived from the Chronic Kidney Disease Epidemiology Collaboration equation.13
Participants underwent an ECG-gated cardiac CT scanning at Exam 1, and at either Exam 2 or 3 (randomly assigned). Electron-beam CT was used at 3 centers and a 4-slice multi-detector row helical CT was used at the other 3 centers.14 Calcification was quantified using the Agatston scoring method.15 The scanning method, image reconstruction, and reading protocols have been previously reported.14 The equivalence across scanner types and inter-scanner reproducibility (kappa statistic of 0.94–0.96) have also been published.16, 17 The minimum, mean, median, and maximum time between baseline and follow-up CT scans in the 5,591 participants included in this study were 0.9, 2.4, 2.4, and 4.9 years, respectively.
The primary outcome of our analysis is incident HF which included both definite and probable HF. Participants were followed from Exam 2 or 3 (i.e. after the follow-up CT) till incident HF, non-HF related death, loss to follow-up, or December 31, 2016, whichever came first. Every 9–12 months participants or their next of kin were contacted for interim hospitalization. Medical records and death certifications were reviewed for HF diagnoses and adjudicated using standardized criteria by a MESA committee of physicians. Participants were diagnosed as having probable HF if diagnosis was defined by a physician or if participant was receiving HF treatment. An additional evidence of pulmonary edema/congestion on chest x-ray or reduced LV function by echocardiography or any ventriculography or any evidence of LV diastolic dysfunction was needed to make a definite diagnosis of HF. We further classified participants with data on LVEF as having HFpEF or HFrEF if LVEF was ≥ 50% or <50% respectively.
Ten-year change in cardiac magnetic resonance (CMR) measures of LV structure and function as ascertained at Exam 1 and Exam 5 were considered as secondary outcomes of our analysis; namely - left ventricular mass (LVM); left ventricular end diastolic volume (LVEDV); left ventricular end systolic volume (LVESV); stroke volume (SV); LVEF; and mass-to-volume ratio (LVM/LVEDV). We indexed LVM, LVEDV, LVESV to body surface area (BSA). This analysis was limited to 2,748 participants who had measures at both Exam 1 and 5 and were not missing any data as previously stated. The acquisition method of CMR images in MESA has been explained in detail in previous publications and was performed using 1.5 Tesla scanners.18
Statistical analysis was performed using Stata (version 15.1, College Station, TX, USA) and a p-value of <0.05 was considered to be statistically significant. The progression of VC was defined based on the number of left sided heart valves with an interval increase in calcification (Agatston units) between baseline and follow-up CT scans as 0 for no progression, 1 for progression at only 1 valve site (AVC or MAC), and 2 for progression at both sites (AVC and MAC). In another analysis we created binary variables - AVC progression compared to no AVC progression and similarly for MAC progression compared to no MAC progression. In this analyses, we compared participants with >0 Agatston units of change per year to those with ≤0 Agatston units of change per year between CT scans at each valve site.
Baseline characteristics were described by number of sites with progression of VC. The hazard ratios (HRs) and 95% confidence intervals (CI) for the associations between VC, AVC, and MAC progression with HF, HFpEF, and HFrEF were determined using Cox proportional hazard regression. Subgroup analysis by HF subtypes excluded participants with missing LVEF and those with the other HF subtype. Time to incident HF was the time from the second follow-up CT scan to HF event. Using the Schoenfeld residuals, we ensured that the proportional hazards assumption was not violated in unadjusted models.
The 10-year average adjusted change in CMR markers of LV structure and function was determined using linear mixed models with random intercepts and slopes.
The test for linear trend (p-for-trend) was derived by modeling the number of sites with progression of VC as a continuous variable. Analytical models were adjusted for several demographic, lifestyle, and ASCVD risk factors. Model 1 was adjusted for participant age, sex, race/ethnicity, MESA site (six centers), and CT scanner types. Analysis having the number of sites with progression of VC as the independent variable additional adjusted for the time between baseline and follow-up CT scan (years). Model 2 included Model 1 variables and educational status, BMI, smoking status, pack-years of smoking, and physical activity. Model 3 which was our main model included Model 2 variables and SBP, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, diabetes mellitus, and eGFR. A fourth model which additionally adjusted for baseline CAC (0, 1–99, 100–399, and ≥400) was used to determine if associations were independent of baseline CAC.
We tested for multiplicative interactions by age and sex. We performed several sensitivity analyses. First, we adjusted for incident CHD and AF as time varying covariates. Second, we excluded participants who developed incident CHD and AF prior to onset of HF. Third, we used competing risks model described by Fine and Gray19 for non-HF death to assess associations between progression of VC and HF. Fourth, we limited our assessment to participants with and without prevalent AVC or MAC at MESA exam 1 (i.e. Agatston score > 0). This was done to explore if the association between AVC or MAC progression and HF depended on the presence or absence of baseline AVC and MAC respectively.
Results
We studied 5591 MESA participants with mean (SD) age of 61.8 (10.2) years, 53% were women, and 40% White. At the time of baseline CT scan, 691 (12%) had prevalent AVC and 502 (9%) prevalent MAC. Eighty-three percent of participants had no progression of VC, 15% had progression of VC at only 1 site (AVC or MAC), and 3% at both sites (AVC and MAC) after a median of 2.4 years of follow-up. Table 1 shows the baseline characteristics of study participants stratified by number of sites with progression of VC. In summary, participants with progression of VC at both sites (AVC and MAC) tended to have a higher mean age, SBP, and median pack-years of cigarette smoking, and a lower mean eGFR, HDL-cholesterol, and median physical activity level. They also had a higher proportion of Whites, men, participants with diabetes, participants on anti-hypertensive and lipid-lowering medications, participants with CAC score of 100 to 399 and ≥400, and participants with interim CHD and AF compared to participants with no progression of VC.
Table 1.
Baseline characteristics of study participants; MESA
| Number of left valve sites with progression of calcium |
|||||
|---|---|---|---|---|---|
| Characteristics | Total | No progression | One site only (AVC or MAC) |
Both sites (AVC and MAC) |
p-for-trend |
| n=5591 | n=4617 | n=827 | n=147 | ||
| Age (years) | 61.8 ± 10.2 | 60.3 ± 9.8 | 68.6 ± 8.5 | 71.9 ± 7.1 | <0.001 |
| Women | 2939 (52.6%) | 2458 (53.2%) | 414 (50.1%) | 67 (45.6%) | 0.02 |
| White | 2215 (39.6%) | 1753 (38.0%) | 377 (45.6%) | 85 (57.8%) | <0.001 |
| Black | 1500 (26.8%) | 1286 (27.9%) | 189 (22.9%) | 25 (17.0%) | <0.001 |
| Hispanic | 1194 (21.4%) | 974 (21.1%) | 193 (23.3%) | 27 (18.4%) | 0.59 |
| Chinese | 682 (12.2%) | 604 (13.1%) | 68 (8.2%) | 10 (6.8%) | <0.001 |
| Education | |||||
| Less than high school | 922 (16.5%) | 715 (15.5%) | 184 (22.3%) | 23 (15.7%) | <0.001 |
| High school or vocational school | 2294 (41.0%) | 1890 (40.9%) | 341 (41.2%) | 63 (42.9%) | 0.68 |
| College, graduate or professional school | 2375 (42.5%) | 2012 (43.6%) | 302 (36.5%) | 61 (41.5%) | 0.002 |
| Body mass index (kg/m2) | 28.2 ± 5.4 | 28.1 ± 5.4 | 28.9 ± 5.4 | 28.8 ± 4.7 | <0.001 |
| Current smoker | 679 (12.1%) | 567 (12.3%) | 106 (12.8%) | 6 (4.1%) | 0.11 |
| Pack-years of smoking in eversmokers* | 16.0 (6.0 – 32.0) | 15.1 (5.8 – 30.0) | 19.0 (7.5 – 37.0) | 20.0 (6.0 – 43.5) | <0.001 |
| Physical activity (MET-minutes/week)* | 4140 (2055 – 7530) | 4238 (2100 – 7710) | 3818 (1713 – 6870) | 3360 (1710 – 6480) | <0.001 |
| Systolic blood pressure (mmHg) | 125.8 ± 21 | 124.4 ± 20.5 | 132.6 ± 22.6 | 133.1 ± 20.3 | <0.001 |
| Antihypertensive use | 1804 (32.3%) | 1365 (29.6%) | 363 (43.9%) | 76 (51.7%) | <0.001 |
| Lipid-lowering medication | 923 (16.5%) | 685 (14.8%) | 199 (24.1%) | 39 (26.5%) | <0.001 |
| Total cholesterol (mg/dL) | 194.2 ± 35.2 | 194.1 ± 35.2 | 195.4 ± 35.4 | 190.5 ± 32.1 | 0.98 |
| High density lipoprotein cholesterol (mg/dL) | 51 ± 14.7 | 51.2 ± 14.7 | 50.4 ± 14.9 | 49.3 ± 14.2 | 0.02 |
| Diabetes mellitus | 656 (11.7%) | 480 (10.4%) | 145 (17.5%) | 31 (21.1%) | <0.001 |
| Estimated glomerular filtration rate (ml/min per 1.73 m2) | 77.8 ± 15.9 | 79 ± 15.6 | 72.5 ± 16.2 | 69.8 ± 16.5 | <0.001 |
| Coronary artery calcium (Agatston units) | |||||
| 0 | 2875 (51.4%) | 2626 (56.9%) | 229 (27.7%) | 20 (13.6%) | <0.001 |
| 1 – 99 | 1463 (26.2%) | 1192 (25.8%) | 231 (27.9%) | 40 (27.2%) | 0.24 |
| 100 – 399 | 733 (13.1%) | 504 (10.9%) | 190 (23.0%) | 39 (26.5%) | <0.001 |
| ≥ 400 | 520 (9.3%) | 295 (6.4%) | 177 (21.4%) | 48 (32.7%) | <0.001 |
| Interim coronary heart disease and/ or atrial fibrillation | 1075 (19.2%) | 738 (16.0%) | 279 (33.7%) | 58 (39.5%) | <0.001 |
AVC= aortic valve calcium; MAC= mitral annular calcium
Data are presented as Mean ± Standard Deviation for continuous variables and frequency (percentage) for categorical variables unless otherwise specified.
Data presented as median (interquartile interval)
We identified a total of 251 participants (4.5%) with incident HF, including 103 with HFpEF and 124 with HFrEF, and 491 participants (8.9%) who died from non-HF related deaths during the 14.6 years of follow-up totaling 62,957 person-years. Twenty-four participants had missing data on LVEF at time of HF hospitalization. The unadjusted incidence rate (95% CI) of HF was 3.99 (3.52 – 4.51) per 1000 person-years in the whole cohort. In Table 2, in our main analytic model 3, we found an increased risk of incident HF and HFpEF but not HFrEF when comparing participants with no progression of VC to those with progression of at 1 and 2 sites respectively. Adjusting for baseline CAC attenuated results but remained statistically significant. We found no interactions by median age or sex for the association between the number of sites with progression of VC and HF (p>0.05). Adjusting for interim CHD and AF as time-varying covariates resulted in the attenuation of our results for the association between VC progression and HF but these remained statistically significant as with previous models (Table 2). However, excluding participants with interim CHD and AF (n=1075) resulted in non-significant associations in model 3 in a smaller sample size (Online Table 1). Results for competing risk analysis for non-HF related death showed an increased risk of HF with progression of VC with HR (95% CI) for 1 and 2 sites of 1.54 (1.12 – 2.12), and 1.84 (1.08 – 3.15) respectively (p-for-trend=0.002, results not shown).
Table2.
Incidence rates and Hazard ratios (95% confidence interval) of incident heart failure associated with number of sites with valvular calcification progression, MESA, 2000–2016
| No progression | One site only (AVC or MAC) | Two sites (AVC and MAC) | Total/p-for-trend | |
|---|---|---|---|---|
| Total Heart failure | ||||
| N (row %) | 4617 (82.6%) | 827 (14.8%) | 147 (2.6%) | 5591 |
| HF; n (%) | 157 (3.4%) | 75 (9.1%) | 19 (12.9%) | 251 (4.5) |
| Person-years | 53289 | 8302 | 1366 | 62957 |
| IR (95% CI)* | 2.95 (2.52 – 3.45) | 9.03 (7.20 – 11.33) | 13.91 (8.87 – 21.81) | 3.99 (3.52 – 4.51) |
| Model 1 | 1 (reference) | 1.92 (1.44 – 2.56) | 2.33 (1.42 – 3.83) | <0.001 |
| Model 2 | 1 (reference) | 1.73 (1.30 – 2.31) | 2.07 (1.26 – 3.40) | <0.001 |
| Model 3 | 1 (reference) | 1.62 (1.21 – 2.17) | 1.88 (1.14 – 3.09) | <0.001 |
| Model 4 | 1 (reference) | 1.49 (1.11 – 2.01) | 1.62 (0.98 – 2.68) | 0.005 |
| Heart failure with preserved ejection fraction | ||||
| N (row %) | 4514 (82.9%) | 792 (14.6%) | 137 (2.5%) | 5443 |
| HFpEF; n (%) | 54 (1.2%) | 40 (5.1%) | 9 (6.6%) | 103 (1.9) |
| Person-years | 52581 | 8070 | 1308 | 61959 |
| IR (95% CI)* | 1.03 (0.79 – 1.34) | 4.96 (3.64 – 6.76) | 6.88 (3.58 – 13.22) | 1.66 (1.37 – 2.02) |
| Model 1 | 1 (reference) | 2.98 (1.93 – 4.60) | 3.23 (1.54 – 6.76) | <0.001 |
| Model 2 | 1 (reference) | 2.63 (1.71 – 4.06) | 2.65 (1.26 – 5.59) | <0.001 |
| Model 3 | 1 (reference) | 2.52 (1.63 – 3.90) | 2.49 (1.19 – 5.25) | <0.001 |
| Model 4 | 1 (reference) | 2.44 (1.57 – 3.78) | 2.27 (1.07 – 4.82) | <0.001 |
| Heart failure with reduced ejection fraction | ||||
| N (row %) | 4549 (83.3%) | 780 (14.3%) | 135 (2.5%) | 5464 |
| HFrEF; n (%) | 89 (2.0%) | 28 (3.6%) | 7 (5.2%) | 124 (2.3) |
| Person-years | 52827 | 7989 | 1307 | 62122 |
| IR (95% CI)* | 1.68 (1.37 – 2.07) | 3.51 (2.42 – 5.08) | 5.36 (2.55 – 11.23) | 2.00 (1.67 – 2.38) |
| Model 1 | 1 (reference) | 1.33 (0.85 – 2.07) | 1.57 (0.71 – 3.48) | 0.12 |
| Model 2 | 1 (reference) | 1.22 (0.78 – 1.90) | 1.47 (0.66 – 3.26) | 0.24 |
| Model 3 | 1 (reference) | 1.07 (0.68 – 1.69) | 1.28 (0.57 – 2.86) | 0.55 |
| Model 4 | 1 (reference) | 0.95 (0.60 – 1.49) | 1.05 (0.47 – 2.35) | 0.95 |
| Total Heart failure adjusting from interim CHD and AF† | ||||
| Model 1 | 1 (reference) | 1.86 (1.40 – 2.48) | 2.23 (1.36 – 3.65) | <0.001 |
| Model 2 | 1 (reference) | 1.70 (1.28 – 2.27) | 2.01 (1.23 – 3.31) | <0.001 |
| Model 3 | 1 (reference) | 1.58 (1.18 – 2.11) | 1.86 (1.13 – 3.06) | <0.001 |
| Model 4 | 1 (reference) | 1.47 (1.09 – 1.97) | 1.62 (0.98 – 2.67) | 0.01 |
AF=atrial fibrillation; CHD=coronary heart disease; CI=confidence interval; HF=heart failure; HFpEF= heart failure with preserved ejection fraction; HFrEF= heart failure with reduced ejection fraction; IR=incidence rate
unadjusted and per 1,000 person-years
Models additional adjust for interim coronary heart disease and atrial fibrillation as time-varying covariates and use time updated age
Values in bold font are statistically significant, p<0.05
Model 1: age, race/ethnicity, sex, MESA site, CT scanner type, and time between baseline & follow-up CT
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, & physical activity
Model 3: Model 2 + systolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, diabetes status & eGFR
Model 4: Model 3 + baseline coronary calcium
Concerning the progression of calcification at each individual valve site, 561 participants (10%) had AVC progression and 560 (10%) had MAC progression. In Table 3, we found increased risk of HF and HFpEF but not HFrEF when comparing AVC progressors to non-progressors and MAC progressors to non-progressors. Adjusting for baseline CAC resulted in attenuated associations which remained mostly statistically significant for HF and HFpEF (Table 3). There were also no multiplicative interactions by median age or sex (p>0.05). Similarly, adjusting for interim CHD and AF as time-varying covariates resulted in the attenuation of results with the increased risk of HF persisting for AVC and MAC progressors (Table 3). Results for incident HF were weaker after excluding participants with interim CHD and AF and no longer significant in our main analytic model 3 (Online Table 2).
Table3.
Hazard ratios (95% confidence interval) of incident heart failure associated with progression of valvular calcification, MESA, 2000–2016
| Progressors vs non-progressors |
||
|---|---|---|
| Aortic valve calcium | Mitral annular calcium | |
| Heart failure, n (%)Progressors vs non-progressors | 59 (10.5) vs 192 (3.8) | 54 (9.6) vs 197 (3.9) |
| N Progressors vs non-progressors | 561 vs 5030 | 560 vs 5031 |
| Model 1 | 1.84 (1.36 – 49) | 1.80 (1.31 – 2.47) |
| Model 2 | 1.72 (1.27 – 2.32) | 1.60 (1.17 – 2.20) |
| Model 3 | 1.61 (1.19 – 2.19) | 1.50 (1.09 – 2.07) |
| Model 4 | 1.47 (1.08 – 2.01) | 1.37 (1.00 – 1.89) |
| HFpEF, n (%)Progressors vs non-progressors | 26 (4.9) vs 77 (1.6) | 32 (6.0) vs 71 (1.5) |
| N Progressors vs non-progressors | 528 vs 4915 | 538 vs 4905 |
| Model 1 | 2.06 (1.30 – 3.28) | 2.81 (1.80 – 4.38) |
| Model 2 | 1.93 (1.21 – 3.07) | 2.37 (1.52 – 3.71) |
| Model 3 | 1.84 (1.16 – 2.94) | 2.30 (1.47 – 3.59) |
| Model 4 | 1.75 (1.10 – 2.80) | 2.19 (1.40 – 3.43) |
| HFrEF, n (%)Progressors vs non-progressors | 25 (4.7) vs 99 (2.0) | 17 (3.3) vs 107 (2.2) |
| N Progressors vs non-progressors | 527 vs 4937 | 523 vs 4941 |
| Model 1 | 1.53 (0.97 – 2.41) | 1.14 (0.67 – 1.94) |
| Model 2 | 1.45 (0.92 – 2.29) | 1.04 (0.61 – 1.77) |
| Model 3 | 1.30 (0.82 – 2.05) | 0.94 (0.55 – 1.61) |
| Model 4 | 1.14 (0.72 – 1.82) | 0.84 (0.49 – 1.43) |
| Heart failure*, n (%)Progressors vs non-progressors | 59 (10.5) vs 192 (3.8) | 54 (9.6) vs 197 (3.9) |
| N Progressors vs non-progressors | 561 vs 5030 | 560 vs 5031 |
| Model 1 | 1.81 (1.34 – 2.45) | 1.74 (1.27 – 2.38) |
| Model 2 | 1.70 (1.26 – 2.30) | 1.58 (1.15 – 2.17) |
| Model 3 | 1.61 (1.19 – 2.18) | 1.47 (1.07 – 2.02) |
| Model 4 | 1.48 (1.09 – 2.01) | 1.35 (0.98 – 1.86) |
HFpEF= heart failure with preserved ejection fraction; HFrEF= heart failure with reduced ejection fraction
Values in bold font are statistically significant, p<0.05
Models additional adjust for interim coronary heart disease and atrial fibrillation as time-varying covariates and use time updated age
Model 1: age, race/ethnicity, sex, MESA site, & CT scanner type
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, & physical activity
Model 3: Model 2 + systolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, diabetes status & eGFR
Model 4: Model 3 + baseline coronary calcium
Additionally, stratifying by the presence or absence of baseline AVC or MAC resulted in no statistical difference in HF risk for participants with AVC or MAC progression compared to non-progressors in our main model (Online Table 3).
Analysis assessing associations with 10-year change in LV structure included 2748 participants who had data on CMR indices of LV structure and function at both MESA exams 1 and 5, spanning approximately 10 years. Of these, 2385 (86.8%) had no progression of VC, 311 (11.3%) with progression of VC at only 1 site (AVC or MAC), and 52 (1.9%) with progression at both sites. In Table 4, compared to participants with no progression of VC, participants with progression at only 1 site had a significant 10-year increase in Mass:Volume Ratio, LVM, and LVESV and a decrease in SV and LVEF. No significant associations were found in participants with progression of VC at both sites. Table 5 shows 10-year change in LV parameters by AVC and MAC progression. Participants with AVC progression had a significant 10-year increase in Mass:Volume ratio, and decrease in SV compared to participants with no AVC progression while participants with MAC progression had a significant 10-year change increase in LVESV and decrease in SV and LVEF.
Table 4.
Average adjusted 10-year change in left ventricular structure associated with number of valve sites with calcification progression: MESA.
| No progression | One site only (AVC or MAC) | Two sites (AVC and MAC) | p-for-trend | |
|---|---|---|---|---|
| N (row %) | 2385 (86.8%) | 311 (11.3%) | 52 (1.9%) | 2748 |
| Left ventricular mass-to-volume ratio | ||||
| Model 1 | Ref = 0 | 3.45 (0.79, 6.10) | 5.09 (−1.09, 11.28) | 0.004 |
| Model 2 | Ref = 0 | 3.45 (0.79, 6.11) | 5.08 (−1.11, 11.26) | 0.004 |
| Model 3 | Ref = 0 | 3.45 (0.79, 6.11) | 5.07 (−1.11, 11.26) | 0.004 |
| Model 4 | Ref = 0 | 3.45 (0.79, 6.11) | 5.07 (−1.11, 11.26) | 0.004 |
| Left ventricular mass | ||||
| Model 1 | Ref = 0 | 1.26 (0.05, 2.47) | 0.86 (−1.96, 3.68) | 0.06 |
| Model 2 | Ref = 0 | 1.26 (0.05, 2.47) | 0.85 (−1.97, 3.67) | 0.06 |
| Model 3 | Ref = 0 | 1.25 (0.04, 2.46) | 0.86 (−1.96, 3.68) | 0.06 |
| Model 4 | Ref = 0 | 1.25 (0.04, 2.46) | 0.86 (−1.96, 3.68) | 0.06 |
| Left ventricular end diastolic volume | ||||
| Model 1 | Ref = 0 | −0.13 (−1.63, 1.36) | −0.87 (−4.36, 2.61) | 0.66 |
| Model 2 | Ref = 0 | −0.13 (−1.63, 1.36) | −0.87 (−4.36, 2.61) | 0.66 |
| Model 3 | Ref = 0 | −0.13 (−1.63, 1.36) | −0.88 (−4.36, 2.61) | 0.66 |
| Model 4 | Ref = 0 | −0.13 (−1.63, 1.36) | −0.88 (−4.36, 2.61) | 0.66 |
| Left ventricular end systolic volume | ||||
| Model 1 | Ref = 0 | 1.35 (0.45, 2.25) | −0.40 (−2.50, 1.70) | 0.05 |
| Model 2 | Ref = 0 | 1.35 (0.45, 2.25) | −0.40 ( 2.50, 1.70) | 0.05 |
| Model 3 | Ref = 0 | 1.35 (0.45, 2.25) | −0.40 (−2.50, 1.70) | 0.05 |
| Model 4 | Ref = 0 | 1.35 (0.45, 2.25) | −0.40 (−2.50, 1.70) | 0.05 |
| Left ventricular stroke volume | ||||
| Model 1 | Ref = 0 | −3.66 (−5.77, −1.55) | −2.07 (−6.99, 2.85) | 0.002 |
| Model 2 | Ref = 0 | −3.67 (−5.78, −1.56) | −2.05 (−6.98, 2.87) | 0.002 |
| Model 3 | Ref = 0 | −3.68 (−5.79, −1.57) | −2.07 (−6.99, 2.85) | 0.002 |
| Model 4 | Ref = 0 | −3.68 (−5.79, −1.57) | −2.07 (−6.99, 2.85) | 0.002 |
| Left ventricular ejection fraction | ||||
| Model 1 | Ref = 0 | −1.78 (−2.72, −0.83) | 0.01 (−2.18, 2.21) | 0.01 |
| Model 2 | Ref = 0 | −1.78 (−2.72, −0.83) | 0.02 (−2.18, 2.21) | 0.01 |
| Model 3 | Ref = 0 | −1.78 (−2.72, −0.84) | 0.01 (−2.18, 2.21) | 0.01 |
| Model 4 | Ref = 0 | −1.78 (−2.72, −0.84) | 0.01 (−2.18, 2.21) | 0.01 |
Values in bold font are statistically significant, p<0.05
Model 1: age, race/ethnicity, sex, MESA site, CT scanner type, and time between baseline & follow-up CT
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, & physical activity
Model 3: Model 2 + systolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, diabetes status & eGFR
Model 4: Model 3 + baseline coronary calcium
Table5.
Average adjusted 10-year change in LV structure and function associated with AVC and MAC progression: MESA.
| Progressors vs non progressors | ||
|---|---|---|
| Aortic valve calcium | Mitral annular calcium | |
| N (row %) | 210 vs 2538 | 205 vs 2543 |
| Left ventricular mass-to-volume ratio | ||
| Model 1 | 4.47 (1.30, 7.63) | 2.81 (−0.39, 6.02) |
| Model 2 | 4.47 (1.30, 7.63) | 2.81 (−0.40, 6.02) |
| Model 3 | 4.47 (1.30, 7.63) | 2.81 (−0.40, 6.01) |
| Model 4 | 4.47 (1.30, 7.63) | 2.81 (−0.40, 6.01) |
| Left ventricular mass | ||
| Model 1 | 1.21 (−0.24, 2.65) | 0.95 (−0.51, 2.41) |
| Model 2 | 1.21 (−0.23, 2.65) | 0.95 (−0.51, 2.41) |
| Model 3 | 1.21 (−0.24, 2.65) | 0.95 (−0.51, 2.41) |
| Model 4 | 1.20 (−0.24, 2.65) | 0.95 (−0.51, 2.41) |
| Left ventricular end diastolic volume | ||
| Model 1 | −0.81 (−2.59, 0.97) | 0.20 (−1.60, 2.01) |
| Model 2 | −0.81 (−2.59, 0.97) | 0.20 (−1.60, 2.01) |
| Model 3 | −0.81 (−2.59, 0.97) | 0.20 (−1.60, 2.00) |
| Model 4 | −0.81 (−2.59, 0.97) | 0.20 (−1.60, 2.00) |
| Left ventricular end systolic volume | ||
| Model 1 | 0.26 (−0.81, 1.34) | 1.41 (0.32, 2.50) |
| Model 2 | 0.26 (−0.81, 1.34) | 1.41 (0.32, 2.50) |
| Model 3 | 0.26 (−0.81, 1.34) | 1.41 (0.32, 2.50) |
| Model 4 | 0.26 (−0.81, 1.34) | 1.41 (0.32, 2.50) |
| Left ventricular stroke volume | ||
| Model 1 | −2.97 (−5.48, −0.45) | −3.12 (−5.67, −0.56) |
| Model 2 | −2.95 (−5.46, −0.43) | −3.14 (−5.69, −0.59) |
| Model 3 | −2.95 (−5.47, −0.44) | −3.15 (−5.71, −0.60) |
| Model 4 | −2.95 (−5.47, −0.44) | −3.15 (−5.71, −0.60) |
| Left ventricular ejection fraction | ||
| Model 1 | −0.84 (−1.96, 0.29) | −1.62 (−2.75, −0.48) |
| Model 2 | −0.83 (−1.96, 0.29) | −1.62 (−2.75, −0.48) |
| Model 3 | −0.84 (−1.96, 0.29) | −1.62 (−2.76, −0.48) |
| Model 4 | −0.84 (−1.96, 0.29) | −1.62 (−2.76, −0.48) |
Values in bold font are statistically significant, p<0.05
Model 1: age, race/ethnicity, sex, MESA site, & CT scanner type
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, & physical activity
Model 3: Model 2 + systolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, diabetes status & eGFR
Model 4: Model 3 + baseline coronary calcium
Discussion
Baseline CAC and CAC progression have previously been shown to be associated with incident HF.11, 20 In this study, we now show that progression of VC at 1 or both sites after a median of 2.4 years is associated with an increased risk of HF in this multiethnic community-based cohort free from clinical cardiovascular disease at baseline independent of ASCVD risk factors. We also found stronger associations with the incidence of HFpEF, with a greater than two-fold risk of HFpEF with VC progression, and no significant associations with HFrEF. Similarly, AVC and MAC progression were associated with a significantly higher risk of HF and HFpEF. We also found that progression of VC at 1 site, AVC and MAC progression were all associated with various indices of LV structure and function except for LVEDV.
VC can be easily detected by echocardiography or CT imaging and it is possible that its progression serves as a subclinical marker of worsening atherosclerosis thereby predicting individuals at a higher risk of incident HF similar to that shown for CAC progression.20 Also, VC and HF share similar ASCVD risk factors. We however found that this increased risk of HF and HFpEF was independent of baseline ASCVD risk factors and CAC. This may suggest an added benefit of measuring VC in addition to CAC and other potential mechanisms other than shared risk factors. However, it remains unclear why the progression of VC was associated with an increased risk of overall HF and HFpEF but not HFrEF. VC and CAC are correlated (Spearman’s rho = 0.31 and 0.23 for baseline AVC and MAC in MESA respectively, p<0.001) and also share similar risk factors.21 CAC results in impairment of coronary endothelial function which may result in diastolic dysfunction.22 This and its correlation with VC may explain some but not all of the association between progression of VC and HF.
Among the various CMR indices of LV structure and function, we found progression of VC to be associated with reductions in both SV and LVEF and increase in Mass:Volume ratio, LVM, and LVESV after 10 years. Another MESA study showed CAC progression was associated with higher LVEDV and LVESV but not the other indices.20 This difference in findings may be as a result in difference in statistical analysis as the prior study only assessed LV function at 1 time point (MESA visit 5). These changes in LV structure and function may possibly explain some of the associations found with incident HF and HFpEF. However, associations of VC progression with LV parameters were only modest and likely do not fully explain the increased HFpEF risk. Additionally, it is possible that these changes, especially reductions in LVEF, were not clinically sufficient enough to increase the risk of HFrEF as participants enrolled in the MESA study were free from clinical ASCVD and HF at baseline. MAC progression however appeared to be better predictor of LVEF reduction and HFpEF compared to AVC but not overall HF.
Furthermore, in our supplemental analysis, we found that the associations between VC progression and overall HF risk to be independent of interim CHD and AF in our time-varying analysis suggesting that this associated was not mediated through CHD and AF. Excluding participants with interim CHD and AF led to attenuation of results, which may have resulted from reduced sample size and limited statistical power. This may explain why associations remained significant when adjusting for interim CHD and AF as time-varying covariates instead of excluding them. Studies assessing the associations between progression of VC and CHD may be necessary. In MESA, MAC progression was associated with increased AF risk23 and baseline extra coronary calcification predicted CHD risk.6–8
Our study has a number of strengths and limitations worthy of mention. To our knowledge, our study is the first to assess the associations between progression of VC, HF risk and indices of LV structure and function in a well-characterized multiethnic cohort of men and women. We were also able to show results for participants with HFpEF and HFrEF, although we had a few participants with missing LVEF at time of HF hospitalization. HF was ascertained from medical records and death certificates and may have been subject to misclassification. However, MESA used a standardized adjudication criteria for HF as described in the methods section which would have limited such misclassification. Categorizing our exposure assumes a homogenous effect within exposure groups which may not be accurate. It is possible that moving from a zero Agatston unit to a nonzero score may not confer the same risk as moving from a nonzero score to a higher nonzero score. We however attempted to stratify our analysis based on the presence of baseline VC and found no significant associations. Also, assessing continuous longitudinal changes in VC within a median of 2.4 years may not be long enough to provide clinically meaningful changes.
In sum, we found that the progression of VC was associated with increased risk of HF and HFpEF. Interventions targeted at reducing VC progression may also impact HF risk, particularly HFpEF.
Supplementary Material
Highlight.
Valve calcium progression and heart failure risk was assessed in a diverse cohort
Progression of valve calcification was associated with increased heart failure risk
Association seen for heart failure with preserved but not reduced ejection fraction
This was independent of interim coronary heart disease & atrial fibrillation events
Acknowledgements
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial support
This research was supported by R01 HL071739 and MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Drs. Michos and Zhao are additionally funded by the Blumenthal Scholars Award in Preventive Cardiology at Johns Hopkins University.
Conflicts of interest
Dr. Budoff receives grant support from General Electric. The other authors have no disclosures to make.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Trial registration
The MESA cohort design is registered at clinicaltrials.gov as follows: https://clinicaltrials.gov/ct2/show/NCT00005487.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Bui AL, Horwich TB and Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai Emily J and Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JGF, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O’Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV and Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail 2014;2:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ and Allison MA. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular EventsCoronary Artery Plaque and Cardiovascular EventsCoronary Artery Plaque and Cardiovascular Events. JAMA 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA and O’Brien KD. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging 2012;5:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, Wong ND, Blumenthal RS, Budoff MJ and Nasir K. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr 2015;9:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JAC, Detrano R, Blumenthal RS and Kronmal R. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2011;215:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Budoff MJ, Nasir K, Wong ND, Yeboah J, Al-Mallah MH, Shea S, Dardari ZA, Blumenthal RS, Blaha MJ and Cainzos-Achirica M. Thoracic aortic calcium, cardiovascular disease events, and all-cause mortality in asymptomatic individuals with zero coronary calcium: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2017;257:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas IC, McClelland RL, Michos ED, Allison MA, Forbang NI, Longstreth WT Jr., Post WS, Wong ND, Budoff MJ and Criqui MH. Density of calcium in the ascending thoracic aorta and risk of incident cardiovascular disease events. Atherosclerosis 2017;265:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leening MJG, Elias-Smale SE, Kavousi M, Felix JF, Deckers JW, Vliegenthart R, Oudkerk M, Hofman A, Steyerberg EW, Stricker BHC and Witteman JCM. Coronary Calcification and the Risk of Heart Failure in the Elderly: The Rotterdam Study. JACC: Cardiovascular Imaging 2012;5:874–880. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez RAV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS and Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD and Detrano RC. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M and Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 16.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, O’Brien KD and Carr JJ. Effect of Scanner Type on The Reproducibility of Extracoronary Measures of Calcification: The Multi-Ethnic Study of Atherosclerosis. Academic Radiology 2007;14:1043–1049. [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ and Kronmal R. Reproducibility of CT Measurements of Aortic Valve Calcification, Mitral Annulus Calcification, and Aortic Wall Calcification in the Multi-Ethnic Study of Atherosclerosis. Academic Radiology 2006;13:166–172. [DOI] [PubMed] [Google Scholar]
- 18.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JAC and Bluemke DA. Cardiovascular Function in Multi-Ethnic Study of Atherosclerosis: Normal Values by Age, Sex, and Ethnicity. American Journal of Roentgenology 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP and Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 20.Bakhshi H, Ambale-Venkatesh B, Yang X, Ostovaneh MR, Wu CO, Budoff M, Bahrami H, Wong ND, Bluemke DA and Lima JAC. Progression of Coronary Artery Calcium and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6:e005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekar C and Budoff M. Calcification of the heart: mechanisms and therapeutic avenues. Expert Rev Cardiovasc Ther 2018;16:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuragi S, Ichikawa K, Yamada K, Tanimoto M, Miki T, Otsuka H, Yamamoto K, Kawamoto K, Katayama Y, Tanakaya M and Ito H. An increase in the coronary calcification score is associated with an increased risk of heart failure in patients without a history of coronary artery disease. Journal of Cardiology 2016;67:358–364. [DOI] [PubMed] [Google Scholar]
- 23.O’Neal WT, Efird JT, Nazarian S, Alonso A, Michos ED, Szklo M, Heckbert SR and Soliman EZ. Mitral annular calcification progression and the risk of atrial fibrillation: results from MESA. Eur Heart J Cardiovasc Imaging 2018;19:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.