Abstract
Introduction
The present study aimed to evaluate the effect of a longer interval between the first and second stages of infected total knee arthroplasty (TKA) revision on the clinical and functional outcome.
Methods
This study included a total of 56 patients who underwent two-stage revision TKA with a dynamic spacer with a minimum of 2 years of follow-up. Patients were categorized into two groups according to time with the spacer: < 3 months (Group 1, 31 patients) or > 3 months (Group 2, 25 patients). Clinical outcome and quality of life were assessed by knee range of motion (ROM), Knee Society Score for Knee (KSS-K), Knee Society Score for Function (KSS-F) and Short Form 36 (SF-36).
Results
The mean follow-up period was 48 ± 19.1 months (range, 24–84 months). The KSS-K, KSS-F, and ROM values were significantly higher in Group 1 than in Group 2 (p < 0.05). The SF-36 scores for general health, physical function, and bodily pain were significantly higher in Group 1 (p < 0.05). Re-infection occurred in 10 patients (17.8%). Time with spacer was not associated with re-infection development (Group 1, n = 6, 19% vs. Group 2, n = 4, 16%; p > 0.05).
Conclusion
Increased duration with a spacer is associated with poorer clinical and functional outcomes as well as higher treatment costs in two-stage revision knee arthroplasty. Surgeons can attempt to reduce the time patients spend in a spacer to obtain better postoperative functional outcomes, as well as a better quality of life.
Level of Evidence
3.
Keywords: Two-stage revision arthroplasty, Total knee arthroplasty, Dynamic spacer, Time, Outcome, Cost, Periprosthetic infection
Introduction
Periprosthetic joint infection(PJI) is a serious complication of primary TKA and is associated with devastating consequences [1]. The incidence of PJI after TKA is 0.4–4% [2, 3], and infection is one of the most common indications for revision TKA [4, 5]. It is estimated that 1.5 million TKA’s per year will be performed in the USA by 2050 [6]. Therefore, it can be expected that the number of infections and related revisions will increase [1, 6].
Single- and two-stage revision are the main treatment options in chronic infected total knee arthroplasty (TKA). Two-stage revision arthroplasty is considered to be the optimal choice in the treatment of most chronic periprosthetic joint infection (PJI) cases [1, 7, 8].
Antibiotic-loaded dynamic spacers and static spacers are used as infection-eradicating strategies in two-stage revision, with reports suggesting that dynamic spacers are less likely to induce muscle atrophy, ligament shortening, or bone loss [9]. Therefore, dynamic spacers are reported to be associated with better functional outcomes than static spacers, which are indicated only in patients with severe bone loss or concomitant soft-tissue defects [10].
The presence of resistant microorganisms, such as Enterococcus, and immunosuppression is associated with longer intervals between the two stages of revision and thus longer time spent with spacers [11].
To the best of our knowledge, no study has evaluated the effect of time spent with a spacer on knee range of motion (ROM), functional outcomes, and quality of life of the patients. Neither has any study assessed the treatment costs associated with the increased duration with spacers.
The present study aimed to evaluate the effect of time spent with articulating spacer between the two stages of revision on knee ROM, functional outcome, quality of life, and treatment costs. We hypothesize that a shorter time interval with a spacer between two revision stages is associated with better knee ROM values, functional outcome scores, and quality of life, and lower treatment costs.
Materials and Methods
The study was approved by Erciyes University clinical investigations research ethics board (approval date and number: 06.06.2018–2018, 305). An informed consent was obtained from all patients. Patients who underwent revision TKA due to chronic periprosthetic knee infection between 2011 and 2017 were retrospectively evaluated. Patients with a minimum 2-year follow-up were included. Exclusion criteria were (1) treatment with static spacer, (2) single-stage revision, (3) insufficient regular follow-up data, (4) ipsilateral previous knee surgery, (5) ipsilateral neurologic impairment, and (6) inflammatory arthropathy.
A total of 74 patients who underwent two-stage revision TKA with dynamic antibiotic-loaded bone cement were recruited. After exclusions, 56 patients were included in this study.
The Musculoskeletal Infection Society (MSIS) criteria were used in the diagnosis of periprosthetic knee infection [3]. After detection of the infection, all implants were removed, radical bone and soft tissue debridement were performed, and an antibiotic-loaded dynamic spacer was inserted (Vancogenx-space knee, Tecres, IT). All patients received IV antibiotics for a minimum of 6 weeks according to culture results. The timing of the second stage operation was determined by serum infection markers (Complete blood count, sedimentation and C-reactive protein), clinical resolution of infection, negative joint aspiration, and intraoperative frozen section analysis in which the threshold value was 5 neutrophils in each high-power field [12]. Antibiotic loaded bone cement (40 g polymethylmethacrylate with 1 g gentamycin and 3 g vancomycin, 2 g meropenem, or 1 g gentamycin and 1 g clindamycin, according to suspected microorganism) was used to fix components in the second stage.
Patients who underwent second-stage surgery within 3 months of the first stage were categorized as Group 1, and those who received second-stage surgery after more than 3 months were categorized as Group 2. Baseline patient characteristics were compared. Preoperative and the last follow-up knee ROM measurements were used in the evaluation of clinical outcome. Knee Society Score—knee score (KSS-K), and KSS—function (KSS-F) were used in the evaluation of functional outcome [5]. Both scores are evaluated over 100 points. Higher score indicates better function. KSS-K evaluates pain, stability and ROM whereas KSS-F evaluates the walking distance, and the act of climbing and descending the stairs [13]. Postoperative health-related quality of life measurement was evaluated using the Short Form 36 (SF-36) patient-reported survey [14]. Responsible microorganisms obtained in the first stage were classified as resistant and non-resistant microorganisms according to their antibiotic resistance.
Costs of treatment were evaluated for each group. Costs of antibiotics, laboratory analysis, radiological analysis, hospital stay, and implants were calculated and compared between the two groups.
Statistical Analysis
Data are presented as mean ± standard deviation, median and interquartile range, frequency, or ratio. Distribution of variables was evaluated using Shapiro–Wilk test. Paired samples t test and Wilcoxon test were used for the analyses of quantitative dependent data. The independent samples t test and Mann–Whitney U test were used in the analyses of quantitative independent data. Chi-square and Fischer exact tests were used in the evaluation of qualitative independent data. Spearman correlation analysis was used in the correlation analysis. Kaplan–Meier survival analysis was performed to compare the infection-free survival time between two groups. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS for Windows, version 22 (IBM corp., Armonk, NY, USA).
Results
The mean follow-up period was 48.1 ± 19.1 months (range, 24–74 months). There was no significant difference between the two groups regarding patient characteristics (Table 1). Causative microorganisms were divided into two groups—resistant (methicillin-resistant Staphylococcus aureus, Enterococcus, Pseudomonas) and non-resistant (methicillin-sensitive Staphylococcus aureus, Coagulase-negative Staphylococci, Streptococcus agalactia). There was no significant difference in the distribution of organisms between the two groups (p > 0.05) (Table 2). The mean period between the first complaint related to PJI and the first stage surgery was 58.4 ± 12.3 days in Group 1 and 64.2 ± 19.7 days in Group 2 (p > 0.05). The mean CRP values before the second stage was 7.6 ± 3.4 mg/L in Group 1 and 7.9 ± 5.4 mg/L in Group 2 (p = 0.76).There was also no difference in terms of duration of IV antibiotics between two stages (Group 1: 7.1 ± 1.3 weeks, Group 2: 7.3 ± 1.6 weeks p = 0.87).
Table 1.
Patient characteristics
| Group 1 | Group 2 | p | ||
|---|---|---|---|---|
| (N = 31) | (N = 25) | |||
| Gender | Female | 17 (54.8%) | 18 (72.0%) | 0.192 |
| Male | 14 (45.2%) | 7 (28.0%) | ||
| Side | Right | 16 (51.6%) | 14 (56,0%) | 0.580 |
| Left | 15 (48.4%) | 11 (44.0%) | ||
| Age | 66.3 ± 8.6 | 67.8 ± 9.2 | 0.355 | |
| BMI | 29.2 ± 4.2 | 28.9 ± 4.8 | 0.624 | |
| Spacer time (Day) | 72.6 ± 8.8 | 166.7 ± 60.6 | 0.010 | |
| Follow-up time (Month) | 48.6 ± 19.1 | 47.1 ± 17.2 | 0.768 | |
| Comorbidity | Diabetes mellitus | 9 (%29) | 11 (%44) | 0.729 |
| Hypertension | 16 (%51.6) | 14 (%56) | 0.844 | |
| Malignancy | 2 (%6.4) | 1 (%4) | 0.803 | |
| Cerebrovascular disease | 1 (%3.2) | 2 (%8) | 0.820 | |
| Chronic renal failure | 2 (%6.4) | 3 (%12) | 0.625 | |
| Morbid obesity | 3 (%9.6) | 3 (%12) | 1.000 | |
| Chronic obstructive lung disease | 6 (%19.3) | 4 (%16) | 0.641 | |
| Coronary artery disease | 4 (%12.9) | 3 (%12) | 0.757 | |
Table 2.
Causing microorganism in both groups
| Microorganism | Group 1 n (%) | Group 2 n (%) | p value | Total n (%) |
|---|---|---|---|---|
| Coagulase-negative Staphylococcus | 10 (32.2%) | 8 (32%) | 0.94 | 18 (32.1%) |
| S.aureus | 4 (12.9%) | 3 (12%) | 0.88 | 7 (12.5%) |
| S. agalactia | 2 (6.4%) | 2 (8%) | 0.62 | 4 (7.1%) |
| Enterococcus | 2 (6.4%) | 1 (4%) | 0.42 | 3 (5.3%) |
| MRSA | 5 (16.1%) | 4 (16%) | 0.98 | 9 (16%) |
| Pseudomonas auriginosa | 2 (6.4%) | 1 (4%) | 0.42 | 3 (5.3%) |
| Mix organisms | 3 (9.6%) | 3 (12%) | 0.74 | 6 (10.7%) |
| Culture negative | 4 (12.9%) | 2 (8%) | 0.60 | 6 (10.7%) |
Group 1 had significantly better postoperative KSS-Knee and KSS-Function scores (p = 0.016 and p = 0.014, respectively) (Table 3). Further, general health, bodily pain, and physical function domains of the SF-36 score were significantly higher in Group 1 (p < 0.05) (Table 3). Spacer time was negatively correlated with KSS-Knee, KSS-Function and knee ROM (Figs. 1, 2 and 3) (p = 0.000; R = − 0.78, R = − 0.64, and R = − 0.75, respectively). The cost of treatment was significantly higher in Group 2 (Group 1 = 8734.80 ± 925.30 USD vs. Group 2 = 11157.60 ± 1325.40 USD, p = 0.035) (Table 4).
Table 3.
Pre- and postoperative ROM, KSS-K, KSS-F and SF-36 values in two groups
| Group 1 n = 31 (Mean ± SD) |
Group 2 n = 25 (Mean ± SD) |
p | |
|---|---|---|---|
| Preoperative ROM | 70 ± 13.1 | 72 ± 15.9 | 0.742 |
| Postoperative ROM | 111.4 ± 11.4 | 91.1 ± 16.8 | 0.012 |
| p | 0.035 | 0.040 | |
| Preoperative KSS-Knee | 42.7 ± 9.8 | 43.1 ± 12.7 | 0.654 |
| Postoperative KSS-Knee | 78.6 ± 10.7 | 58.1 ± 13.8 | 0.016 |
| p | 0.002 | 0.003 | |
| Preoperative KSS-F | 39.8 ± 10,3 | 38.7 ± 11.2 | 0.428 |
| Postoperative KSS-F | 76.8 ± 16.7 | 58.1 ± 19.1 | 0.014 |
| p | 0.001 | 0.026 | |
| Postoperative SF-36 | |||
| General health | 75.7 ± 16.1 | 60.9 ± 22.1 | 0.043 |
| Physical function | 41.8 ± 21.8 | 30.1 ± 20.7 | 0.038 |
| Bodily pain | 33.4 ± 16.8 | 23.0 ± 12.4 | 0.044 |
| Mental health | 81.3 ± 14.6 | 77.5 ± 21.4 | 0.724 |
| Role emotional | 84.2 ± 31.5 | 79.8 ± 39.9 | 0.950 |
| Role physical | 19.8 ± 32.4 | 17.3 ± 24.6 | 0.744 |
| Social function | 52.2 ± 35.5 | 48.7 ± ± 33.6 | 0.728 |
| Vitality | 70.5 ± 21.6 | 67.6 ± 21.1 | 0.854 |
ROM range of motion, KSS-K knee society score-knee score, KSS-F knee society score- function score
Fig. 1.
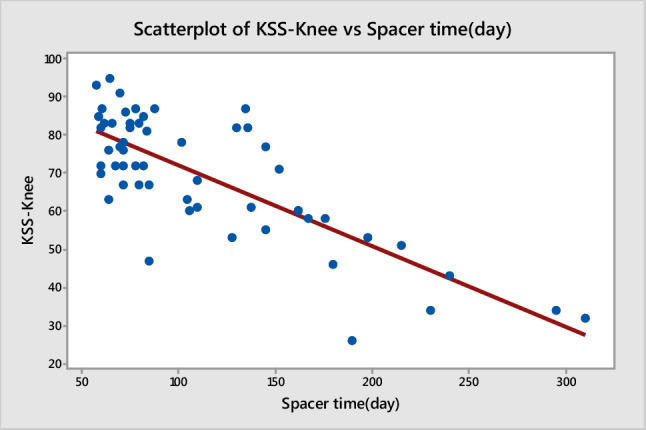
Correlation between spacer time and KSS-knee score
Fig. 2.
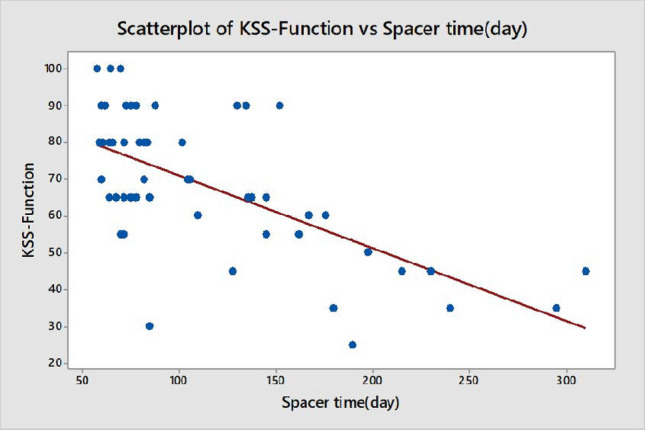
Correlation between spacer time and KSS-function score
Fig. 3.
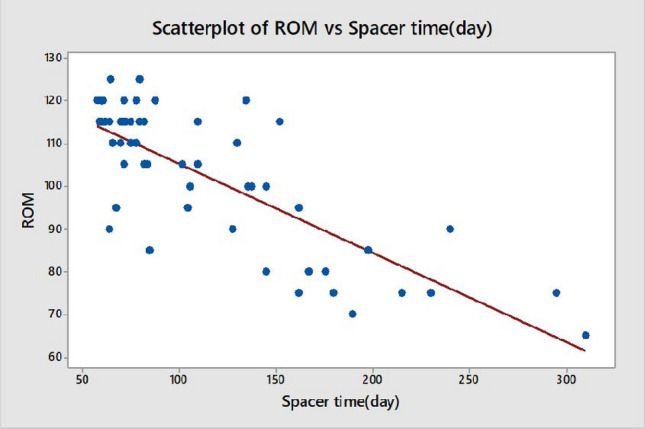
Correlation between spacer time and knee range of motion (ROM)
Table 4.
Comparison of the treatment costs between two groups
| Group 1 (Cost USD) Mean ± SD |
Group 2 (Cost USD) Mean ± SD |
p | |
|---|---|---|---|
| Antibiotics | 2110.5 ± 220.6 | 3902 ± 354.8 | 0.012 |
| Laboratory | 602.2 ± 92.6 | 828 ± 98.3 | 0.086 |
| Radiology | 96.0 ± 18.9 | 119.0 ± 23.4 | 0.144 |
| Prosthesis | 3908.5 ± 124.1 | 4020.0 ± 300.1 | 0.727 |
| Surgery | 998.2 ± 102.0 | 1160.6 ± 271.4 | 0.630 |
| Hospital stay | 1020.4 ± 114.2 | 2128.0 ± 194.8 | 0.011 |
| Total | 8734.8 ± 925.3 | 11,157. 6 ± 1325.4 | 0.035 |
Ten patients (17.8%) experienced reinfection during the follow-up period. There was no significant difference in reinfection rates between the two groups (Group 1, n = 6, 19% vs. Group 2, n = 4, 16%; p > 0.05). Infection-free survival times of each group was similar (Group 1:72.40 ± 4.90 vs. Group 2:71.28 ± 4.92 p = 0.920) (Fig. 4). Six patients with reinfections were treated with two-stage revision. Three patients underwent knee arthrodesis, and above-knee amputation was performed in one patient due to recalcitrant infection.
Fig. 4.
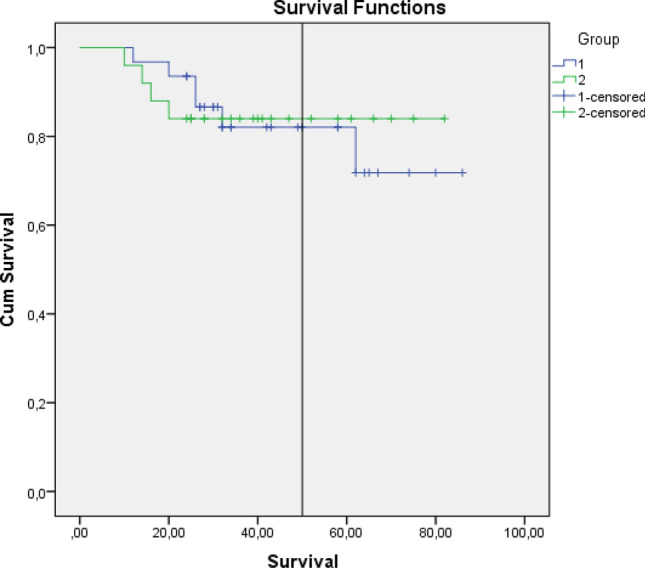
Comparison of infection-free survival times between two groups
In our study, 6 cases were culture negative (10.7%). They became culture positive in the cultures obtained from joint aspiration due to lack of clinical improvement during their follow-up. The most frequent microorganism was coagulase-negative staphylococci (4 out of 6 patients, 66.7%).
A sinus formation was detected in 9 patients in Group 1 and 6 patients in Group 2 (p > 0.05). One patient from each group received fascio-cutaneous antero-lateral thigh flap for soft tissue coverage, and full-thickness skin graft was performed in 2 patients due to skin necrosis.
In total, 16 (28.5%) patients experienced complications other than reinfection (Table 5). Hinged type revision prosthesis was used in patients with collateral ligament injuries (n = 8, 14.2%). Periprosthetic fractures (4 patients) were treated with long stem and plate. Patellar tendon reconstruction with allograft was performed in 4 patients due to traumatic patellar tendon rupture.
Table 5.
Distribution of non-infectious complications in both groups
| Complications | Total n (%) |
Group 1 n (%) |
Group 2 n (%) |
p |
|---|---|---|---|---|
| Patellar tendon rupture | 3 (5.3) | 1 (3.2) | 2 (8) | 0.56 |
| MCL rupture | 6 (10.7) | 3 (9.6) | 3 (12) | 0.86 |
| LCL rupture | 2 (3.5) | 1 (3.2) | 1 (4) | 0.82 |
| Periprosthetic fracture | 4 (7.1) | 3 (9.6) | 1 (4) | 0.64 |
| Total | 16 (28.5) | 8 (25.8) | 8 (32) | 0.75 |
Discussion
This is the first study which evaluates the effect of time interval between the stages on functional results after infeted TKA. Deciding proper timing for second stage surgery after infected TKA is challenging for orthopedic surgeons. Many predictors were used to decide to perform second surgery but the effect of the prolonged interval between stages on clinical and functional results remains unclear. Therefore, the most important finding of the present study was that patients spending less than 3 months with spacers had superior postoperative ROM value, KSS-K, KSS-F, and lower treatment cost. Moreover, general health, bodily pain, and physical function domains of the SF-36 survey scores were significantly higher in patients spending less than 3 months with spacers. In addition, time spent with spacer did not affect the reinfection rate and infection-free survival time.
Although dynamic knee spacers allow increased knee motion and partial weight bearing, the longer duration of use of these spacers has been associated with reduced improvement of functional outcomes [10]. In our study, we found significantly better clinical (knee ROM values) and functional (KSS values) outcomes in patients spending less than 3 months with dynamic spacers. It may be related to earlier full weight-bearing and rehabilitation.
There has been no clear data on the effect of time between the first and second stages on outcomes in infected TKA. Fu et al. [15] investigated the influence of the timing of the second stage in infected TKA and concluded that 12–16 weeks lead to more favorable results, when compared to a longer duration. However, they only evaluated reinfection development as the outcome and did not investigate functional outcomes [15]. Some authors suggested using spacer for a longer duration in the treatment of resistant microorganisms [16]. On the other hand, others suggested that increased time with spacers causes quadriceps shortening, muscle atrophy, and thickening of the soft tissues, which can contribute to poorer outcomes [17]. Cha et al. [18] evaluated prognostic factors after two staged revision, they divided the patients as reinfected and nonreinfected and there was no difference between two groups in terms of the interval between the first and second stages. In the present study, there was no significant difference in re-infection rates between the two groups (Group 1, 19% vs. Group 2, 16%). Moreover, knee ROM and functional outcome were better in patients with shorter durations of spacer use.
In their clinical study including 507 primary TKA patients, Lizaur‑Utrilla et al. reported the minimal clinically important difference (MCID) values for KSS-Knee and KSS-Function [19]. They found the MCID values for KSS-K and KSS-F to be 9 and 10, respectively. In the present study, the mean differences in KSS-K and KSS-F were 20.5 and 18.7, respectively. Accordingly, in patients spending less than 3 months with a spacer, the improvement in functional outcomes were found to be clinically meaningful. Since SF-36 scores could only be obtained postoperatively, we could not determine whether there was a significant difference compared to the preoperative period. However, we found significantly better general health, bodily pain, and physical function domain scores in patients spending less than 3 months with a spacer.
Recently, Faschingbauer et al. reported no difference in reinfection rates between resistant and non-resistant microorganism-caused PJI [20]. In the present study, 37.5% of the patients had resistant microorganisms and there was no significant difference in the reinfection rates between resistant and non-resistant microorganisms.
Reinfection after two-stage revision TKA is associated with high morbidity [21]. Petis et al. reported 17% reinfection rate after two-staged treatment of 245 patients [22]. When reinfection develops, more radical treatment options can be considered [21]. They suggested performing above the knee amputation in patients with good physical and mental conditions. In our study, reinfection developed in 10 patients (17.8%). Above the knee amputation and knee arthrodesis were performed in 1 patient and 3 patients, respectively.
Culture-negative PJI is another challenging situation in the treatment of PJI. It has been suggested that culture-negative PJI is related to high failure rates [23]. Tan et al. reported that 53.1% of the culture-negative cases become culture positive during the follow-up period. Of these, 38.5% were positive for methicillin-sensitive Staphylococcus aureus [23]. Muusa et al. recently reported a 33.3% culture-negative PJI rate [24]. In the current study, 10.7% of the patients were culture negative. They became culture positive in the cultures obtained from joint aspiration due to lack of clinical improvement during their follow-up. The most frequent microorganism was coagulase-negative staphylococci.
Periprosthetic knee infection represents a considerable financial burden on the healthcare system [25]. Kapadia et al. reported a fourfold higher mean total episode cost in patients with PJI [26]. Alp et al. reported that treatment costs are 2.8-fold higher in the case of infected TKA than in primary TKA [2]. In their systematic review, Fernandez-Fairen et al. showed that the cost for septic revision was between 2 and 4 times higher than that for primary surgery [27]. This study is the first attempt to specifically evaluate the cost related to time spending with articulating spacers. We found a 1.28-fold increase in the total treatment cost in patients spending more than 3 months using articulating spacers. This difference is related to the increased duration of IV antibiotics and longer hospital stay. Moreover, loss of labor is another concern but it was not included in the analysis.
In our study, total of 16 patients (28.5%) experienced noninfectious complications. The most frequent complication was MCL rupture (n = 6, 10.7%). Petis et al. [21] reported 5 (2%) extensor mechanism disruption, and 11 periprosthetic fracture (4%) after two staged revision following infected TKA, in another study Pelt et al. [28] retrospectively reviewed 58 patients who received Two-Stage Revision and they report 1 (2%) extensor mechanism injury and 2 (3.4%) periprosthetic fractures. In the current study, we had 3 (5.3%) extensor mechanism injuries and 4 (7.1%) periprosthetic fractures, which was consistent to the current literature.
The main limitation of the present study is the retrospective design and relatively small number of patients. We also could not determine the preoperative SF-36 values in the patients. Therefore, we could not make a preoperative and postoperative comparison of the quality of life between the patient groups. The follow-up period for reinfected patients was relatively short. The number of reinfections may increase in long-term follow-up. The duration of spacer usage is based on multiple factors, and many are not taken into consideration in this study. Future prospectively designed studies are needed to evaluate the effect of time spent using spacers on clinical and functional outcomes, as well as the quality of life and treatment costs.
Conclusions
Increased duration of spacer use between stages is associated with worse clinical and functional outcomes as well as treatment costs in 2-stage revision knee arthroplasty. Lesser duration of spacer use is not associated with reinfection development. Surgeons can try to reduce the time patients spend using a spacer, to obtain better postoperative clinical and functional outcomes, as well as better quality of life scores and lower treatment costs.
Author Contributions
FG: Data Collection and Statisical analysis. SO: Manuscipt writing. AM: Critical reviewing. AG: Data Collection.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Conflict of interest
Fatih Golgelioglu, Sinan Oguzkaya, Abdulhamit Misir and Ahmet Guney declare that they have no conflict of interest.
Ethical standard statement
Erciyes University Institutional Research Ethics Review Board approved the study protocol (Approval date/number: 06.06.2018/2018-305).
Informed consent
All patients signed informed constent form before be included into the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatih Golgelioglu, Email: fatihgolgelioglu@gmail.com.
Sinan Oguzkaya, Email: sinanoguzkaya@hotmail.com.
Abdulhamit Misir, Email: misirabdulhamitmd@gmail.com.
Ahmet Guney, Email: dr.aguney@gmail.com.
References
- 1.Wingert NC, Gotoff J, Parrilla E, Gotoff R, Hou L, Ghanem E. The ACS NSQIP risk calculator is a fair predictor of acute periprosthetic joint infection. Clinical Orthopaedics and Related Research. 2016;474(7):1643–1648. doi: 10.1007/s11999-016-4717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alp E, Cevahir F, Ersoy S, Guney A. Incidence and economic burden of prosthetic joint infections in a university hospital: a report from a middle-income country. J Infect Public Health. 2016;9(4):494–498. doi: 10.1016/j.jiph.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Vecchini E, Micheloni GM, Perusi F, Scaglia M, Maluta T, Lavini F, et al. Antibiotic-loaded spacer for two-stage revision of infected total knee arthroplasty. J Knee Surg. 2017;30(3):231–237. doi: 10.1055/s-0036-1584190. [DOI] [PubMed] [Google Scholar]
- 4.Koh CK, Zeng I, Ravi S, Zhu M, Vince KG, Young SW. Periprosthetic joint infection is the main cause of failure for modern knee arthroplasty: an analysis of 11,134 knees. Clinical Orthopaedics and Related Research. 2017;475(9):2194–2201. doi: 10.1007/s11999-017-5396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current epidemiology of revision total knee arthroplasty in the united states. J Arthroplast. 2017;32(9):2663–2668. doi: 10.1016/j.arth.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 6.Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States: an alternative projection model. Osteoarthr Cartil. 2017;25(11):1797–1803. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Warth LC, Hadley CJ, Grossman EL. Two-stage treatment for total knee arthroplasty infection utilizing an articulating prefabricated antibiotic spacer. J Arthroplast. 2020;35(3s):S57–s62. doi: 10.1016/j.arth.2019.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Pangaud C, Ollivier M, Argenson J-N. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019;4(8):495–502. doi: 10.1302/2058-5241.4.190003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guild GN, 3rd, Wu B, Scuderi GR. Articulating vs. Static antibiotic impregnated spacers in revision total knee arthroplasty for sepsis. A systematic review. J Arthroplast. 2014;29(3):558–563. doi: 10.1016/j.arth.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Mazzucchelli L, Rosso F, Marmotti A, Bonasia DE, Bruzzone M, Rossi R. The use of spacers (static and mobile) in infection knee arthroplasty. Curr Rev Musculoskelet Med. 2015;8(4):373–382. doi: 10.1007/s12178-015-9293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasouli MR, Tripathi MS, Kenyon R, Wetters N, Della Valle CJ, Parvizi J. Low rate of infection control in enterococcal periprosthetic joint infections. Clinical Orthopaedics and Related Research. 2012;470(10):2708–2716. doi: 10.1007/s11999-012-2374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Jt Surg Am. 1995;77(12):1807–1813. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Martimbianco AL, Calabrese FR, Iha LA, Petrilli M, Lira Neto O, Carneiro Filho M. Reliability of the American knee society score (AKSS) Acta Ortop Bras. 2012;20(1):34–38. doi: 10.1590/s1413-78522012000100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach CM, Nogler M, Steingruber IE, Ogon M, Wimmer C, Göbel G, et al. Scoring systems in total knee arthroplasty. Clinical Orthopaedics and Related Research. 2002;399:184–196. doi: 10.1097/00003086-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Fu J, Ni M, Li H, Li X, Chai W, Zhou Y, et al. The proper timing of second-stage revision in treating periprosthetic knee infection: reliable indicators and risk factors. J Orthop Surg Res. 2018;13(1):214. doi: 10.1186/s13018-018-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4(7):482–494. doi: 10.1302/2058-5241.4.180092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding H, Yao J, Chang W, Liu F. Comparison of the efficacy of static versus articular spacers in two-stage revision surgery for the treatment of infection following total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2017;12(1):151. doi: 10.1186/s13018-017-0644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha MS, Cho SH, Kim DH, Yoon HK, Cho HS, Lee DY, et al. Two-stage total knee arthroplasty for prosthetic joint infection. Knee Surg Relat Res. 2015;27(2):82–89. doi: 10.5792/ksrr.2015.27.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lizaur-Utrilla A, Gonzalez-Parreño S, Martinez-Mendez D, Miralles-Muñoz FA, Lopez-Prats FA. Minimal clinically important differences and substantial clinical benefits for knee society scores. Knee Surgery, Sports Traumatology, Arthroscopy. 2020;28(5):1473–1478. doi: 10.1007/s00167-019-05543-x. [DOI] [PubMed] [Google Scholar]
- 20.Faschingbauer M, Bieger R, Kappe T, Weiner C, Freitag T, Reichel H. Difficult to treat: are there organism-dependent differences and overall risk factors in success rates for two-stage knee revision? Archives of Orthopaedic and Trauma Surgery. 2020 doi: 10.1007/s00402-020-03335-4. [DOI] [PubMed] [Google Scholar]
- 21.Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD. Re-Infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS ONE. 2016;11(3):e0151537. doi: 10.1371/journal.pone.0151537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petis SM, Perry KI, Mabry TM, Hanssen AD, Berry DJ, Abdel MP. Two-stage exchange protocol for periprosthetic joint infection following total knee arthroplasty in 245 knees without prior treatment for infection. J Bone Jt Surg Am. 2019;101(3):239–249. doi: 10.2106/jbjs.18.00356. [DOI] [PubMed] [Google Scholar]
- 23.Tan TL, Kheir MM, Shohat N, Tan DD, Kheir M, Chen C, et al. Culture-negative periprosthetic joint infection: an update on what to expect. JB JS Open Access. 2018;3(3):e0060. doi: 10.2106/jbjs.Oa.17.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mussa M, Manciulli T, Corbella M, Mariani B, Cambieri P, Gipsz N, et al. Epidemiology and microbiology of prosthetic joint infections: a nine-year, single-center experience in Pavia Northern Italy. Musculoskelet Surg. 2020 doi: 10.1007/s12306-020-00638-y. [DOI] [PubMed] [Google Scholar]
- 25.Sousa A, Carvalho A, Pereira C, Reis E, Santos AC, Abreu M, et al. Economic impact of prosthetic joint infection - an evaluation within the portuguese national health system. J Bone Jt Infect. 2018;3(4):197–202. doi: 10.7150/jbji.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplast. 2014;29(5):929–932. doi: 10.1016/j.arth.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Fairen M, Torres A, Menzie A, Hernandez-Vaquero D, Fernandez-Carreira JM, Murcia-Mazon A, et al. Economical analysis on prophylaxis, diagnosis, and treatment of periprosthetic infections. Open Orthop J. 2013;7:227–242. doi: 10.2174/1874325001307010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelt CE, Grijalva R, Anderson L, Anderson MB, Erickson J, Peters CL. Two-stage revision TKA is associated with high complication and failure rates. Advances in Orthopedics. 2014;2014:659047. doi: 10.1155/2014/659047. [DOI] [PMC free article] [PubMed] [Google Scholar]


