Abstract
Rationale
In rodents, exposure to novel environments elicits initial anxiety-like behavior (neophobia) followed by intense exploration (neophilia) that gradually subsides as the environment becomes familiar. Thus, innate novelty-induced behaviors are useful indices of anxiety and motivation in animal models of psychiatric disease. Noradrenergic neurons are activated by novelty and implicated in exploratory and anxiety-like responses, but the role of norepinephrine (NE) in neophobia has not been clearly delineated.
Objective
We sought to define the role of central NE transmission in neophilic and neophobic behaviors.
Methods
We assessed dopamine β-hydroxylase knockout (Dbh −/−) mice lacking NE and their NE-competent (Dbh +/−) littermate controls in neophilic (novelty-induced locomotion; NIL) and neophobic (novelty-suppressed feeding; NSF) behavioral tests with subsequent quantification of brain-wide c-fos induction. We complimented the gene knockout approach with pharmacological interventions.
Results
Dbh −/− mice exhibited blunted locomotor responses in the NIL task and completely lacked neophobia in the NSF test. Neophobia was rescued in Dbh −/− mice by acute pharmacological restoration of central NE with the synthetic precursor L-3,4-dihydroxyphenylserine (DOPS), and attenuated in control mice by the inhibitory α2-adrenergic autoreceptor agonist guanfacine. Following either NSF or NIL, Dbh −/− mice demonstrated reduced c-fos in the anterior cingulate cortex, medial septum, ventral hippocampus, bed nucleus of the stria terminalis, and basolateral amygdala.
Conclusion
These findings indicate that central NE signaling is required for the expression of both neophilic and neophobic behaviors. Further, we describe a putative noradrenergic novelty network as a potential therapeutic target for treating anxiety and substance abuse disorders.
Keywords: norepinephrine, dopamine β-hydroxylase, neophobia, neophilia, novel environment, novelty suppressed feeding, guanfacine, c-fos, locus coeruleus, anxiety
Introduction
A novel environment may represent either an appetitive opportunity or a potential threat; the valence of the context is uncertain because it has never been encountered before (Kafkas and Montaldi 2018). In novel environments, it is adaptive for animals to exhibit initial anxiety-like behavior (neophobia) that gradually transitions to exploratory behavior (neophilia) once the environment has proven to be non-threatening (Barnett 1958; Dulawa and Hen 2005; Osorio-Gómez et al. 2018). Novelty-seeking and novelty-fearing personality traits correlate with substance abuse and anxiety disorders, respectively (Bardo et al. 1996; Dovey et al. 2008; Marcontell et al. 2003; Tulving et al. 1994; Weierich et al. 2010). In animal models of psychiatric disease, behavioral responses to novel environments are also strong predictors of drug-seeking and anxiety-like behavior (Dulawa 2009; Pawlak et al. 2008; Walker et al. 2009; Wingo et al. 2016).
Neophilic behavior can be assessed in mice with the novelty-induced locomotion (NIL) test, which measures exploration in a novel environment (Kabbaj et al. 2000; Walker et al. 2009). In the NIL test, high locomotor reactivity to novelty is interpreted as increased exploratory behavior, while low locomotor responses are interpreted as decreased exploratory behavior (Cubells et al. 2016; Schroeder et al. 2013; Stone et al. 1999). Neophobia can be assessed in mice with the novelty-suppressed feeding (NSF) test, a conflict-based model of anxiety-like behavior in which anxiety elicited by exposure to an unfamiliar environment suppresses feeding in hungry mice presented with food (Cryan and Sweeney 2011; Dulawa and Hen 2005). In the NSF test, longer latencies to begin feeding are interpreted as increases in neophobia.
Novel environments activate neural circuits involved in spatial memory (Takeuchi et al. 2016; Wagatsuma et al. 2018), attention (Aston-Jones et al. 2007; Gompf et al. 2010), motivation (Rinaldi et al. 2010; Struthers et al. 2005), arousal (Britton and Indyk 1990; Stone et al. 2005), and anxiety (Delini-Stula et al. 1984; File 2001; Sheth et al. 2008), which enables flexible responding to potential threats or rewards that may emerge under uncertain conditions (Aston-Jones et al. 1999; Kafkas and Montaldi 2018; Snyder et al. 2012). Although much is known about the biological mechanisms that drive conditioned responses to familiar contexts in animal models of anxiety and drug addiction (Koob and Simon 2009; Koob and Volkow 2016; Martin et al. 2009; Tovote et al. 2015), the basic neurochemistry and circuitry of the network that controls innate behavioral responses to novel environments remain largely unknown.
The locus coeruleus (LC) is the primary source of norepinephrine (NE) to the forebrain, projecting extensively within circuits that govern attention, arousal, emotion, and motivated behavior (Aston-Jones et al. 1999; Berridge et al. 1996; Sara and Bouret 2012; Uematsu et al. 2015). A longstanding theory is that the LC-NE system is sensitive to contextual novelty (Gompf et al. 2010; Sara et al. 1995), and it is well established that the firing rate of LC neurons increases in novel environments and declines as the context becomes familiar (Aston-Jones and Bloom 1981; Vankov et al. 1995). Besides the LC, the brainstem contains smaller, scattered populations of non-cerulean adrenergic cell groups such as A2 neurons in the nucleus of the solitary tract (NTS), which also send axonal projections to the forebrain to regulate affective and motivated behavior (Olson et al. 2006; Ricardo and Koh 1978; Rinaman 2011; Robertson et al. 2016).
Hyperactivity of the LC-NE system has been implicated in the pathophysiology of anxiety and substance abuse disorders (Aston-Jones et al. 1994; Brady 1994; Goddard et al. 2010; Tanaka et al. 2000; Weinshenker and Schroeder 2007; West et al. 2009), and drugs that suppress the activity of the LC or reduce NE signaling have therapeutic efficacy for patients with these conditions (Aston-Jones and Kalivas 2008; Boehnlein and Kinzie 2007; Fox et al. 2012). Experimental disruptions of the LC-NE system also suppress innate behavioral responses to novel environments and stimuli (Archer et al. 1981; Delini-Stula et al. 1984; Harro et al. 1995; Neophytou et al. 2001; Sara et al. 1995; Stone et al. 1999).
The LC-NE system innervates a constellation of cortical and limbic regions implicated in spatial memory and novelty detection, including the anterior cingulate cortex (ACC), medial septum/diagonal band (MS/DB) complex, and hippocampus (Chandler 2016; Guiard et al. 2008; Uematsu et al. 2015). LC-NE signaling within the basolateral amygdala (BLA) elicits anxiety-like behavior (Llorca-Torralba et al. 2019; McCall et al. 2017; Siuda et al. 2016), and NE transmission within the bed nucleus of the stria terminalis (BNST) is associated with stress responses, innate fear, and reward seeking (Avery et al. 2016; Lebow and Chen 2016; Weinshenker and Schroeder 2007). LC-NE inputs to the anterior insula (AI) and medial orbitofrontal cortex (MO) modulate decision making, emotion, and motivated behavior (Mather et al. 2016; Petrides 2007; Rojas et al. 2015; Sadacca et al. 2017). Thus, the LC is well poised to orchestrate both exploratory and anxious behavior in unfamiliar environments (Berridge and Dunn 1989; McCall et al. 2015; Snyder et al. 2012).
In the present study, we investigated the consequences of genetic or pharmacological disruption of central NE signaling on neophilic and neophobic behavior in the NIL and NSF tests using NE-deficient (dopamine β-hydroxylase knockout; Dbh −/−) mice and NE-sufficient (Dbh +/−) controls (Thomas et al. 1995). We also determined the effects of pharmacological restoration of central NE synthesis on neophobia in Dbh −/− mice and pharmacological reduction of NE transmission on neophobia in control mice (Mineur et al. 2015; Thomas et al. 1998). Finally, we measured how genetic NE deficiency alters c-fos induction in the LC and 15 of its target structures in the forebrain as a proxy measure of task-specific neuronal activity following NIL and NSF.
Methods
Subjects
Dbh −/− mice were maintained on a mixed 129/SvEv and C57BL/6J background, as described (Thomas et al. 1998; Thomas et al. 1995). Pregnant Dbh +/− dams were given drinking water supplemented with the β-adrenergic receptor (βAR) agonist isoproterenol and α1AR agonist phenylephrine (20 μg/ml each) + vitamin C (2 mg/ml) from E9.5-E14.5, and L-3,4-dihydroxyphenylserine (DOPS; 2 mg/ml + vitamin C 2 mg/ml) from E14.5-parturition to prevent developmental lethality associated with homozygous Dbh deficiency (Mitchell et al. 2008; Thomas et al. 1998; Thomas et al. 1995). Dbh −/− mice are easily identified by their ptosis phenotype, and genotypes were confirmed by PCR, as described (Mitchell et al. 2008; Thomas et al. 1995). Dbh +/− mice behavior and NE levels are indistinguishable from wild-type (Dbh +/+) mice and were used as controls (Bourdélat-Parks et al. 2005; Mitchell et al. 2006).
All experiments included adult mice (3–8 months old) of both sexes. Because sex differences were not reported in the literature nor observed in pilot experiments, approximately equal numbers of male and female mice of the same Dbh genotype were included in all experiments, and data from both sexes were pooled. All animal procedures and protocols were congruent with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Emory University Animal Care and Use Committee. Mice were maintained on a 12 h light/12 h dark cycle with access to food and water ad libitum. For all experiments, behavioral testing was conducted during the light cycle.
Drugs
The following drugs were used for behavioral pharmacology experiments: the α2AR agonist guanfacine hydrochloride (Sigma-Aldrich, St. Louis, MO), the peripheral aromatic acid decarboxylase inhibitor benserazide (Sigma-Aldrich), and the synthetic NE precursor l-3,4-dihydroxyphenylserine (DOPS; Lundbeck, Deerfield, IL). Guanfacine (0.3 mg/kg) was dissolved in sterile saline (0.9% NaCl) and injected i.p. at a volume of 10 ml/kg. Sterile saline vehicle was injected to control for any confounding effect of injection stress on behavior, and vehicle-treated animals were used for statistical comparison.
For the DOPS rescue experiment, DOPS was dissolved in distilled water with 2% HCl, 2% NaOH, and 2 mg/kg vitamin C, as described (Schank et al. 2008; Thomas et al. 1998). Dbh −/− mice were injected s.c. with DOPS (1 g/kg) + benserazide (250 mg/kg), then tested 5 h later once NE levels peaked (Rommelfanger et al. 2007; Thomas et al. 1998). Benserazide was included to prevent the conversion of DOPS to NE in the periphery, thus restricting restoration of NE to the brain (Murchison et al. 2004). The vehicle control for the DOPS experiment was also administered 5 h before testing.
Novelty-induced locomotion (NIL)
Individual mice were removed from their home cages and placed into large novel cages (10” × 18” × 10”) on locomotor activity testing racks (San Diego Instruments, San Diego, CA) (Schroeder et al. 2013; Weinshenker et al. 2002a). Novelty-induced ambulations (consecutive infrared beam breaks) were recorded for 1 h in 5 min bins. Testing occurred in a brightly lit room where the animals were housed. Test cages were covered with a lid and contained a thin layer of standard bedding substrate during NIL testing.
Novelty-suppressed feeding (NSF)
All chow was removed from the home cage of subject animals 24 h prior to behavioral testing. On the day of testing, mice were moved to the test room and allowed to habituate for at least 2 h before starting the test. For pharmacological experiments, mice were injected either 30 min (guanfacine) or 5 h (DOPS) prior to testing. Individual mice were removed from their home cages and placed into a large novel cage (10” ×18” × 10”) with a single pellet of standard mouse chow in the center. The latency for the mouse to feed (grasp and bite the food pellet) was recorded using a stopwatch; mice that did not feed within 10 min were assigned a feeding latency score of 600 sec (Dulawa and Hen 2005; Tillage et al. 2020). Testing occurred in a dark room under red light to reduce the potentially confounding effect of bright light on anxiety-like behavior, and isolate behavioral responses induced purely by the novel environment. Test cages were uncovered and contained a thin layer of standard bedding substrate.
Feeding in the home cage and food neophobia
Mice were singly housed in clean standard mouse cages without chow for 24 h prior to behavioral testing to allow for cage familiarization. On the day of testing, mice were moved to the test room and allowed to habituate for at least 2 h before starting the test. For home cage feeding experiments, the cage lid was removed, and a standard food pellet was introduced into the cage. For food neophobia experiments, the cage lid was removed, and a standard food pellet coated in cinnamon (2 g cinnamon/100 g chow; Penzeys Spices, Wauwatosa, WI) was introduced into the cage (Modlinska and Stryjek 2016; Modlinska et al. 2015). The latency to feed was recorded for both tests using a stopwatch; mice that did not feed within 10 min were assigned a feeding latency score of 600 sec. As with NSF, testing occurred in a dark room under red light.
c-fos immunohistochemistry (IHC)
Dbh −/− and control mice were exposed to NIL or NSF tasks, after which they were left undisturbed in the test cage for 90 min (from the start of the task). The mice were then euthanized with an overdose of sodium pentobarbital (Fatal Plus, 150 mg/kg, i.p.; Med-Vet International, Mettawa, IL) for transcardial perfusion with cold 4% paraformaldehyde in 0.01 M PBS. Another group of Dbh −/− and control mice was selected for comparisons of brainwide c-fos induction in animals that were naïve to the behavioral tasks and were immediately perfused after removal from the home cage.
After extraction, brains were post-fixed for 24 h in 4% paraformaldehyde at 4°C, and then transferred to cryoprotectant 30% sucrose/PBS solution for 72 h at 4°C. Brains were embedded in OCT medium (Tissue-Tek; Sakura, Torrance, CA) and serially sectioned by cryostat (Leica) into 40-μm coronal slices spanning the entire brain between the LC and orbitofrontal cortex. Brain sections were stored in 0.01 M PBS (0.02% sodium azide) at 4°C before IHC.
For IHC, brain sections were blocked for 1 h at room temperature in 5% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) diluted in 0.01 M PBS/0.1% Triton-X permeabilization buffer. Sections were then incubated for 48 h at 4°C in NGS blocking/permeabilization buffer, including primary antibodies raised against c-fos (rabbit anti-c-fos, Millipore, Danvers, MA, ABE457; 1:5000) and the NE transporter (NET; chicken anti-NET, #260006, Synaptic System, Goettingen, Germany; 1:3000). After washing in 0.01 M PBS, sections were incubated for 2 h in blocking/permeabilization buffer with goat anti-rabbit AlexaFluor 488 and goat anti-chicken AlexaFluor 568 (Invitrogen, Carlsbad, CA; 1:500). After washing, the sections were mounted onto Superfrost Plus slides and coverslipped with Fluoromount-G plus DAPI (Southern Biotech, Birmingham, AL).
Viral tracing of LC terminal fields in forebrain targets
Adult mice expressing Cre recombinase under the tyrosine hydroxylase promoter (TH-Cre; Jax #008601) were anesthetized with isoflurane (for induction and maintenance; Patterson Veterinary Supply, Devens, MA), placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and administered meloxicam (Patterson Veterinary Supply; 5 mg/kg, s.c.) for analgesia prior to the start of surgery. Mice received bilateral infusions of AAV10-EF1a-DIO-hChR2-eYFP (#20298, Addgene; Watertown, MA) into the LC (AP:−5.4 mm, ML: ±1.2 mm, DV, −4.0 mm) with a 5 μL Hamilton syringe (VWR, Radnor, PA) and stereotaxic injector pump (Stoelting, Wood Dale, IL). A total volume of 0.6 μL virus diluted 1:1 with sterile artificial cerebrospinal fluid (Harvard Apparatus, Holliston, MA) was infused per side at a rate of 0.15 μL/min. The infusion needle was left in place for 5 min after each infusion to allow for viral diffusion.
Mice were given at least 3 weeks to recover from surgery and allow full viral expression before they were euthanized and perfused, as described above. Brains were processed for IHC to visualize LC cell bodies and axon terminals in target regions using antibodies raised against TH (rabbit anti-TH, P40101–0, Pel-Freez, Rogers, AR; 1:1000) and YFP (chicken anti-GFP, ab13970, abcam, Cambridge, MA; 1:1000) All steps for IHC were identical to those described above except primary antibody incubations occurred over 24 h and primary antibodies were detected with goat anti-chicken 488 and goat anti-rabbit 568 (Invitrogen; 1:500).
Fluorescent imaging and c-fos quantification
Fluorescent micrographs of immunostained sections were acquired on a Leica DM6000B epifluorescent upright microscope at 10× magnification with uniform exposure parameters for c-fos quantification. For the viral tracing experiment, micrographs were acquired at 20× for visualization of LC cell bodies and axon terminals. NET and TH antibodies were used to define LC cell bodies and identify noradrenergic axon terminals in target regions.
For c-fos quantification, we selected atlas-matched sections from each animal at the level of the LC and 15 of its forebrain targets. A standardized region of interest was drawn for all images to delineate the borders of discrete structures in all animals. Image processing and analysis were performed using ImageJ software. The analysis pipeline included background subtraction, intensity thresholding (Otsu method), and automated cell counting within defined regions of interest, guided by automated size and shape criteria for c-fos+ cells (size: 50–100 pixel2, circularity: 0.6–1.0).
Statistical analysis
For NIL experiments, the effect of time on locomotion in Dbh −/− and Dbh +/− mice was compared using a two-way repeated measures ANOVA (genotype × time), with post hoc Bonferroni’s tests for multiple comparisons. The within-trial habituation curves for NIL were fit with a simple linear regression for statistical comparison between Dbh genotypes. For NSF experiments, the effect of test cage familiarity on latency to feed after food deprivation was compared by two-way ANOVA (genotype × test environment), with post hoc Sidak’s tests for multiple comparisons. Food neophobia between genotypes, NSF behavior between DOPS- and vehicle-treated Dbh −/− mice, and NSF behavior between guanfacine- and vehicle-treated Dbh +/− control mice were analyzed using unpaired t-tests.
For c-fos quantification, genotype differences were compared in the LC and 15 forebrain targets in naïve controls and after NIL or NSF. Comparisons were made within behavioral tasks and between genotypes by multiple t-tests using the Holm-Sidak correction for multiple comparisons. The threshold for adjusted significance was set at p < 0.05, and two-tailed variants of tests were used throughout. Analyses and graph design were performed using Prism v8 (GraphPad Software, San Diego, CA).
Results
Norepinephrine deficiency attenuates novelty-induced locomotion and promotes rapid habituation
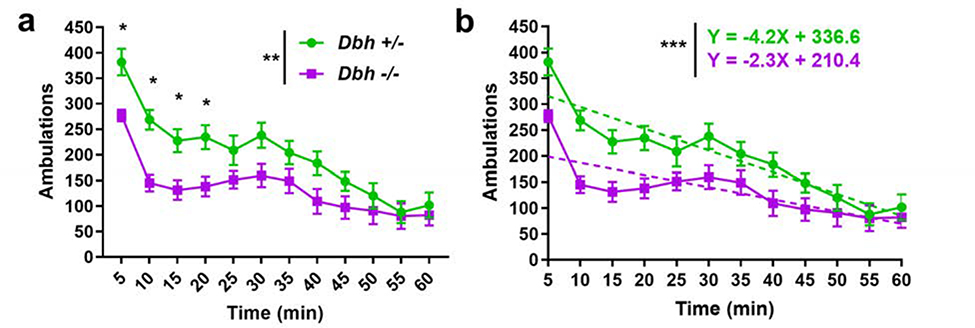
To determine the contribution of NE to neophilic behavior, a cohort of age- and sex-matched Dbh −/− and Dbh +/− control mice were compared in the NIL test, with locomotor activity measured in 5 min bins across 1 h. A two-way repeated measures ANOVA (genotype × time) showed a main effect of genotype (F(1,21) = 9.28, p < 0.01), time (F(11,231) = 30.16, p < 0.0001), and a time × genotype interaction (F(11,231) = 2.81, p = 0.02). Dbh −/− mice made fewer novelty-induced ambulations overall compared to control mice (1612±582 vs 2405±659), and post hoc analyses revealed that the Dbh −/− mice were less active than control mice at the beginning of the task during the 5 min (p = 0.01), 10 min (p < 0.01), 15 min (p = 0.03), and 20 min (p = 0.03) time bins (Fig. 1a).
Fig. 1.
Assessment of neophilia in Dbh −/− and Dbh +/− mice. a Novelty-induced locomotor behavior was attenuated in Dbh −/− mice (n = 11) compared to controls (n = 12). Mice of both Dbh genotypes demonstrated the most activity at the start of the task followed by habituation, with activity diminished over time. However, in the four earliest time bins of the test, Dbh −/− mice were less active than controls. b The change in novelty-induced locomotion over time for both Dbh genotypes was assessed using a simple linear regression analysis. The slope of the dashed fit lines of within-trial habituation to the novel environment indicates a blunted initial response to novelty in Dbh −/− mice (represented by the Y-intercept) and a flatter slope, suggesting more rapid habituation to the novel environment. Error bars ± SEM, ***p < 0.001, **p < 0.01, *p < 0.05.
Mice of both genotypes showed functional novelty detection, exhibiting the most activity in time bins at the beginning of the task and within-trial habituation to the novel environment. However, simple linear regression analysis of ambulations over time revealed that variation in locomotor activity was predicted more strongly by variation in time in the control mice (R2 = 0.43) than in Dbh −/− mice (R2 = 0.23). There was a significant difference in the slope of the fit lines for each Dbh genotype (F(1,272) = 11.22, p < 0.001). The flatter slope of the Dbh −/− fit line suggests more rapid locomotor habituation to the novel environment in NE-deficient mice (Fig. 1b).
Central norepinephrine is necessary and sufficient for neophobia in the novelty-suppressed feeding test
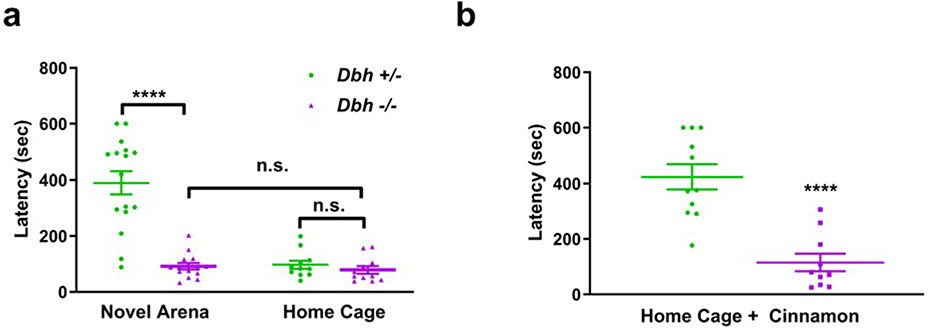
To assess whether NE is also required for neophobia, the behavior of Dbh −/− and control mice were compared in the NSF test. A two-way ANOVA (genotype × environment) showed a main effect of genotype (F(1,48) = 32.62, p < 0.0001), environment (F(1,48) = 30.38, p < 0.0001), and a genotype × environment interaction (F(1,48) = 25.39, p < 0.0001). Post hoc comparisons revealed that in the novel test environment, Dbh −/− mice ate much more rapidly than control mice (91.93±44.43 vs 389.49±162.81 s, p < 0.0001), but did not differ from control mice when assessed for feeding latency in the home cage (78.82±44.79 vs 92.45±47.43 sec, p > 0.05) (Fig. 2a). The amount of the food eaten over the course of 1 h following the NSF test also did not differ significantly between Dbh −/− mice and controls (0.37±0.05 vs 0.37±0.05 g, p > 0.05). Thus, the genotype differences in feeding latency are likely to be related to reduced neophobia rather than increased hunger. As expected, Dbh +/− mice ate significantly more rapidly in the home cage than they did in the novel cage (t(48) = 7.57, p < 0.0001). However, latency to feed did not differ between test environments in Dbh −/− mice (t(48) = 0.14, p > 0.05), suggesting NE deficient mice exhibit behavioral indifference to context familiarity when hunger is a motivating factor.
Fig. 2.
Assessment of novelty-suppressed feeding and food neophobia in Dbh −/− and Dbh +/− mice. a Compared to Dbh +/− controls (n = 16), Dbh −/− (n = 14) ate familiar mouse chow much more rapidly in a novel environment. Unlike controls, Dbh −/− mice did not have a significant difference in feeding latency between the novel test environment and familiar home cage. The latency to feed did not differ between Dbh +/− controls (n = 11) and Dbh −/− mice (n = 11) when tested in the home cage. b Compared to Dbh +/− controls (n = 11), Dbh −/− mice (n = 10) ate the novel-scented cinnamon chow more rapidly in a familiar environment, indicating diminished food neophobia. Error bars ± SEM, ****p < 0.0001, n.s. = not significant.
We also measured the effects of NE deficiency in a variation of the NSF test with novel-scented food presented in a familiar environment. To this end, we coated standard mouse chow pellets with cinnamon, a novel olfactory stimulus that is neither innately appetizing nor aversive to rodents (Modlinska and Stryjek 2016; Modlinska et al. 2015; Scholtysik 1980). Similar to the novel environment version of the test, Dbh −/− mice ate the novel-smelling pellet much more rapidly than control mice (115.3±99.7 vs 423.6±148.1 sec; t(19) = 5.54, p < 0.0001) (Fig. 2b).
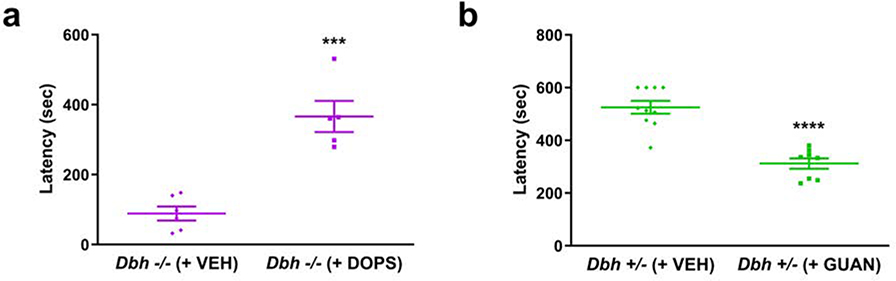
To evaluate whether replacing central NE in Dbh −/− mice would restore neophobic behavior in Dbh −/− mice, we compared knockouts treated with vehicle or the synthetic NE precursor DOPS in the NSF test. DOPS treatment significantly increased the latency to feed in Dbh −/− mice compared to vehicle treatment (366.4±99.3 vs 88.8±48.8 sec; t(9) = 6.07, p < 0.001), rescuing feeding latency to similar levels as control mice (Fig. 3a). To determine whether acutely disrupting NE transmission in normal mice confers resistance to neophobia, we also compared Dbh +/− mice treated with vehicle or guanfacine (0.3 mg/kg) in the NSF test. Guanfacine is an agonist of α2a inhibitory autoreceptors and inhibits NE release (Fox et al. 2012). Guanfacine produced an anxiolytic effect in Dbh +/− mice, significantly decreasing latency to feed in the novel environment (312.1±56.1 vs 525.3±76.4 sec; t(16) = 6.58, p < 0.0001) (Fig. 3b). Thus, neophobia can be restored in NE deficient mice by pharmacological rescue of brain NE synthesis, and attenuated in control mice by reducing NE release with guanfacine.
Fig. 3.
Pharmacological restoration and attenuation of neophobia. a Dbh −/− mice in which central NE was restored with DOPS (n = 5) before NSF testing demonstrate significantly longer latencies to feed than Dbh −/− mice treated with vehicle (VEH; n = 6). b Dbh +/− control mice in which central NE transmission was reduced with guanfacine (n = 8) ate more rapidly than control mice treated with VEH (n = 10) in the NSF test. Error bars ± SEM, ****p < 0.0001, ***p < 0.001.
LC neurons project to forebrain regions implicated in responses to novelty
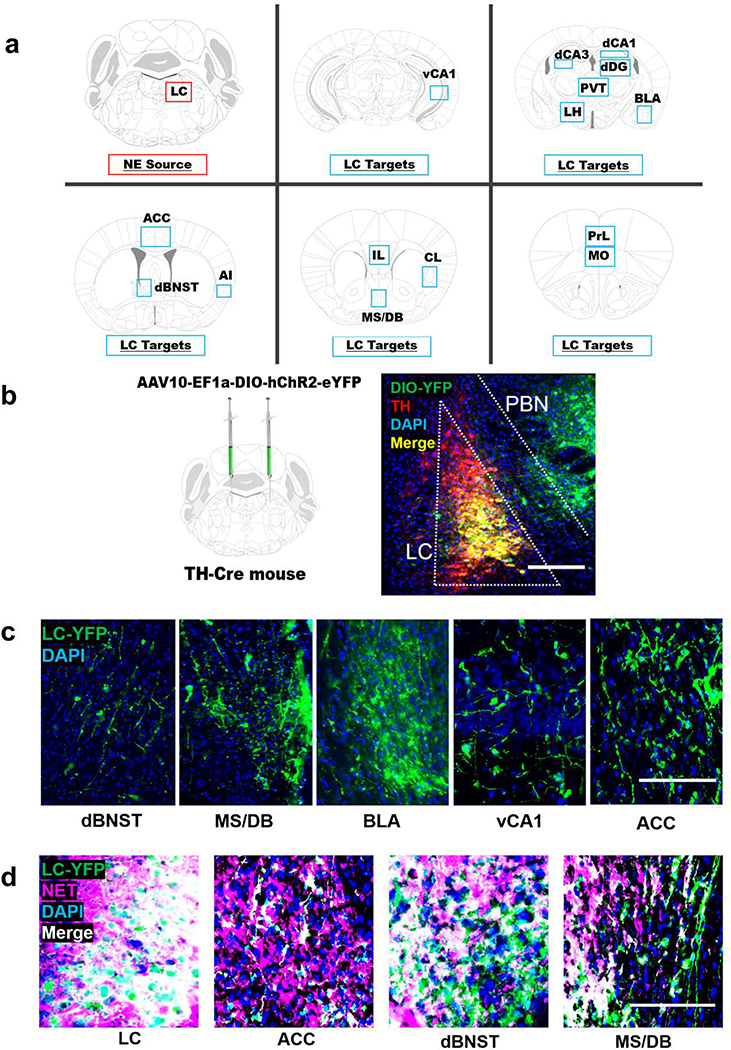
To determine whether LC neurons project to forebrain region implicated in novelty responses, we bilaterally injected a viral vector encoding Cre-dependent ChR2-eYFP into the LC of TH-Cre mice to label noradrenergic LC neurons and their axon terminals (Fig. 4a,b). Brainstem cell body expression of eYFP was histologically confirmed for all cases and was largely restricted to TH+ neurons in the LC, although moderate expression was also observed in the neighboring parabrachial nucleus (PBN) (Fig. 4b). TH-Cre activity in the parabrachial nucleus has been described previously (Lindeberg et al. 2004; Savitt et al. 2005), and was observed in neurons that were not immunoreactive for TH (Fig. 4b).
Fig. 4.
Viral tracing of locus coeruleus (LC) fibers in forebrain regions implicated in novelty. a Anatomical location of the LC and 15 target regions in the forebrain: medial orbitofrontal cortex (MO), prelimbic (PrL) and infralimbic cortices (IL), anterior cingulate cortex (ACC), agranular insula (AI), claustrum (CL), medial septum/diagonal band (MS/DB), dorsal bed nucleus of the stria terminalis (dBNST), basolateral amygdala (BLA), dorsal dentate gyrus (dDG), dorsal hippocampal subfields CA1 (dCA1) and CA3 (dCA3), ventral hippocampus subfield CA1 (vCA1), lateral hypothalamus (LH), and the paraventricular nucleus of the thalamus (PVT). b TH-Cre mice received bilateral intra-LC infusions of a Cre-dependent viral vector encoding ChR2-eYFP, which becomes concentrated in axons and axon terminals and can be used as a tool for projection mapping. ChR2-DIO-YFP expression (green) substantially overlaps with immunostaining for TH (red) in the LC and is largely restricted to TH+ LC neurons, with some expression in TH- cells of the neighboring parabrachial nucleus (PNB). c Representative micrographs of YFP+ terminals from the LC in the dorsal bed nucleus of the stria terminals (dBNST), medial septum/diagonal band complex (MS/DB), basolateral amygdala (BLA), the CA1 subfield of the ventral hippocampus (vCA1), and the anterior cingulate cortex (ACC). d All YFP+ cell bodies in the LC and most YFP+ terminals in the ACC, dBNST, and MS/DB overlap with immunostaining for the norepinephrine transporter (NET), indicating that these axon terminals originate from LC neurons rather than the PBN. The scale bar denotes 100 μM in all micrographs.
We next examined 15 forebrain regions previously implicated in novelty responses and reported to receive noradrenergic innervation from the LC (Robertson et al. 2016; Schwarz and Luo 2015; Uematsu et al. 2015; Zaborszky 1989), including the medial orbitofrontal cortex (mOFC), prelimbic (PrL) and infralimbic cortices (IL), anterior cingulate cortex (ACC), agranular insula (AI), claustrum (CL), medial septum/diagonal band (MS/DB), dorsal bed nucleus of the stria terminalis (dBNST), basolateral amygdala (BLA), dorsal dentate gyrus (dDG), dorsal hippocampal subfields CA1 (dCA1) and CA3 (dCA3), ventral hippocampus subfield CA1 (vCA1), lateral hypothalamus (LH), and the paraventricular nucleus of the thalamus (PVT) (Fig. 4a). All forebrain regions analyzed contained eYFP+ fibers, demonstrating direct inputs from the LC to novelty-sensitive forebrain targets (Fig. 4c). Because some YFP expression was observed in the PBN, which sends axonal projections to some of the same forebrain targets as the LC (Chiang et al. 2019), we confirmed that YFP+ terminals in target regions were noradrenergic (NET+) (Fig. 4d).
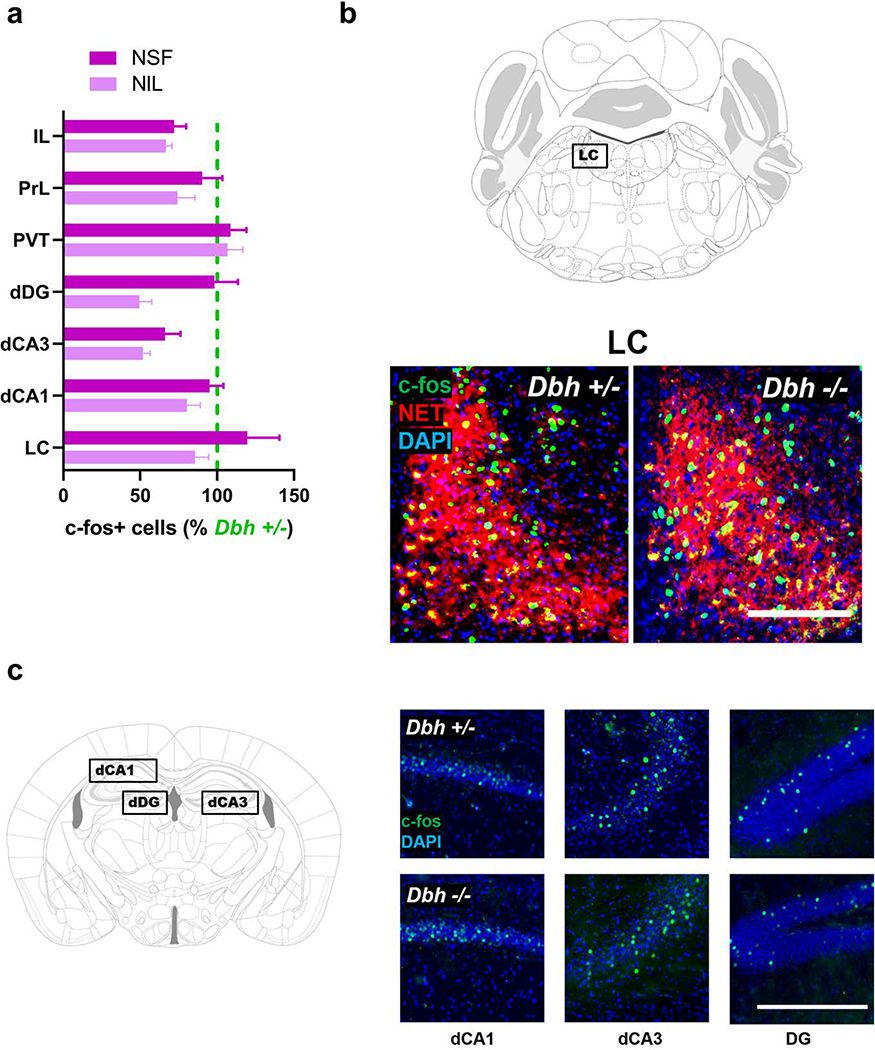
Norepinephrine deficiency is associated with blunted c-fos induction in several forebrain targets of the locus coeruleus following novelty exposure
To identify potential NE-sensitive novelty circuits from among the identified forebrain LC targets, Dbh −/− and Dbh +/− control mice were euthanized for quantification of c-fos+ cells in the LC and 15 of its forebrain targets (Fig. 4a) under naïve conditions and following NIL or NSF. c-fos expression was negligible in all regions examined in naïve mice, and there were no significant differences in c-fos+ cells between Dbh genotypes (p > 0.05 for all regions examined) (Lustberg et al. 2020) (Fig. S1a). Control and Dbh −/− mice exposed to a novel environment demonstrated marked increases in c-fos induction in the LC and many of its forebrain targets (Fig. S1b) (Lustberg et al. 2020).
Exposure to the novel environment in either NSF or NIL tests resulted in intense c-fos induction in the LC regardless of Dbh genotype (t(9) = 0.69, p > 0.05 for NIL; t(6) = 0.81, p > 0.05 for NSF) (Fig. 5a,b), suggesting that NE deficiency does not impair LC activation in response to spatial novelty. There were also no genotype differences observed for the PVT, PrL, IL, dCA1, dCA3, and dDG (p > 0.05 for all comparisons) (Fig. 5c).
Fig. 5.
Assessing the effects of NE deficiency on neuronal activity in the locus coeruleus (LC) and forebrain target regions following exposure to a novel environment. a Quantification of c-fos+ neurons in Dbh −/− mice in the LC and forebrain targets after NSF (darker purple) and NIL (lighter purple), expressed as a percentage of the average number of c-fos+ cells in Dbh +/− controls for each behavioral test and region. There were no significant differences between genotypes in the LC, dorsal hippocampal subfields CA1 (dCA1), CA3 (dCA3), and dentate gyrus (dDG), prelimbic cortex (PrL), infralimbic cortex (IL), or the paraventricular nucleus of the thalamus (PVT). b Representative micrographs showing similar levels of c-fos (green) induction between Dbh genotypes after NSF in NET+ (red) LC neurons. Nuclei are counterstained with DAPI (blue). c Dbh genotype does not affect the number of c-fos+ cells observed following NSF in dorsal hippocampal subregions. Error bars denote group mean ± SEM. The scale bar denotes 100 μM in all micrographs.
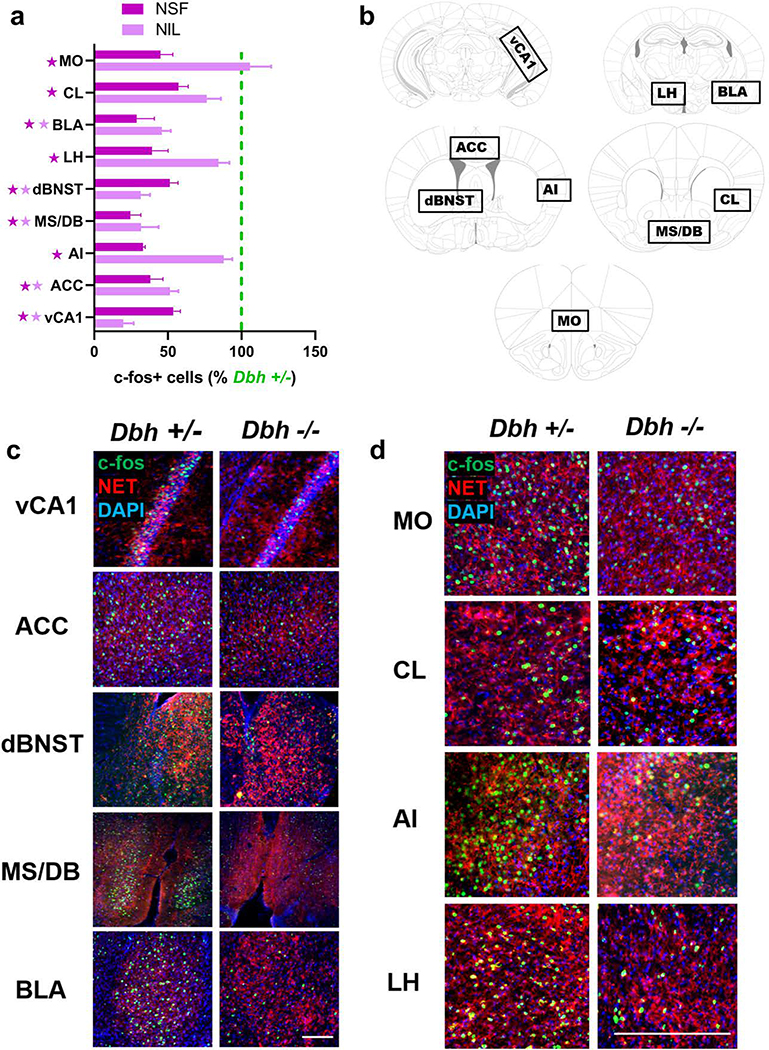
By contrast, profound genotype differences in c-fos induction emerged in several other forebrain targets of the LC during novelty exposure (Fig. 6a,b). Following either NIL or NSF, Dbh −/− mice had significantly fewer c-fos+ cells than control mice in vCA1 (t(9) = 5.02, p < 0.01 for NIL; t(9) = 5.13, p < 0.01 for NSF), ACC (t(9) = 4.86, p = 0.01 for NIL; t(9) = 5.20, p < 0.01 for NSF), dBNST (t(8) = 9.12, p < 0.001 for NIL; t(9) = 4.24, p = 0.02 for NSF), MS/DB (t(8) = 5.25, p < 0.01 for NIL; t(8) = 6.63, p < 0.01 for NSF), and BLA (t(8) = 5.92, p < 0.01 for NIL; t(9) = 5.63, p < 0.01 for NSF) (Fig. 6a,c). After NSF, but not after NIL, NE-deficient mice also had fewer c-fos+ cells than controls in MO (t(9) = 5.63, p < 0.01), CL (t(9) = 4.38, p = 0.02), AI (t(9) = 3.69, p = 0.04), and LH (t(9) = 5.69, p < 0.01) (Fig. 6a,d).
Fig. 6.
Visualization of NET+ noradrenergic fibers and c-fos induction in target regions after novelty-suppressed feeding (NSF). a c-fos induction in Dbh −/− mice in the LC and forebrain targets after NSF (darker purple) and NIL (lighter purple), expressed as a percentage of the average number of c-fos+ cells in Dbh +/− controls for each behavioral test and region. Dark purple stars indicate p < 0.05 for NSF, light purple stars indicate p < 0.05 for NIL. b Anatomical location of brain regions that were hypoactive in Dbh −/− mice after either NSF or NIL, including ventral hippocampus subfield CA1 (vCA1), anterior cingulate cortex (ACC), dorsal bed nucleus of the stria terminalis (dBNST), medial septum/diagonal band (MS/DB), basolateral amygdala (BLA), medial orbitofrontal cortex (MO), claustrum (CL), agranular insula (AI), and lateral hypothalamus (LH). c Representative micrographs showing immunostaining for NET (red) and c-fos (green) between Dbh genotypes after NSF. Nuclei are counterstained with DAPI (blue). Following either NSF or NIL, Dbh −/− mice showed fewer c-fos+ cells than controls in vCA1, ACC, dBNST, MS/DB, and BLA. d Following the NSF test, but not following NIL, Dbh −/− mice showed fewer c-fos+ cells than controls in MO, CL, AI, and LH. Error bars denote group mean ± SEM. The scale bar denotes 100 μM in all micrographs.
Discussion
Norepinephrine is required for sustained locomotor activation in novel environments
In this study, we first compared neophilic behavior in NE-deficient and NE-competent littermate control mice in the NIL test. We found that over the course of 1 h, NE-deficient mice exhibited reduced locomotor activity and more rapid habituation in the novel test environment compared to control mice. These findings are congruent with previous reports from our lab and suggest that novelty may have reduced incentive value or intrinsic salience in NE-deficient animals (Cubells et al. 2016; Porter-Stransky et al. 2019; Weinshenker et al. 2002a).
Dbh −/− mice show a modest deficit in motor learning in the rotarod test but exhibit normal locomotor activity in the open field test and home cage compared to littermate controls, and they can readily acquire new spatial memories (Marino et al. 2005; Thomas and Palmiter 1997). Importantly, novel environments elicit an initial increase in locomotion in Dbh −/− mice that habituates over time, suggesting that spatial memory mechanisms that distinguish novel and familiar environments remain intact in the absence of NE. Previous work has also demonstrated functional single-trial learning of a novel environment in Dbh −/− mice; total locomotion during the first exposure to the novel environment was significantly greater than total locomotion during re-exposure to the same environment 24 h later in both Dbh −/− and control mice (Weinshenker et al. 2002a).
It is interesting to note that this particular type of “one-shot” contextual learning was recently shown to depend on LC transmission of dopamine (DA) to the dorsal hippocampus (Kempadoo et al. 2016; Takeuchi et al. 2016; Wagatsuma et al. 2018). Because DBH catalyzes the conversion of DA to NE, Dbh −/− mice make DA instead of NE in LC neurons, and thus LC-DA transmission would theoretically be undisturbed (or even augmented) in Dbh −/− mice (BourdélatParks et al. 2005; Schank et al. 2006; Weinshenker et al. 2002b).
Norepinephrine is necessary and sufficient for novelty-suppressed feeding
The present study also investigated the effect of NE deficiency on neophobia in the NSF test. NE-deficient mice demonstrated a total absence of neophobia in the novel test environment that could be rescued to control levels by restoring central NE synthesis with DOPS. In control mice, reducing NE release with guanfacine produced rapid anxiolytic effects in the NSF test. Together, these findings demonstrate that NE is both necessary and sufficient for species-typical neophobia in NSF. Importantly, feeding latencies and food consumption in NE-deficient mice did not differ from controls in the familiar home cage environment, ruling out the possibility that NE-deficient mice are simply hungrier after food deprivation than NE-competent mice (Cryan and Sweeney 2011; Dulawa and Hen 2005).
The profound lack of anxiety displayed by the Dbh −/− mice in the NSF test contrasts sharply with the seemingly “normal” phenotype of these mice in canonical tests of anxiety, including the elevated plus and zero mazes (EPM/EZM), light/dark box (LDB), and open field test (OFT) (Lustberg et al. 2020; Marino et al. 2005; Schank et al. 2008). These conflict-based models of anxiety-like behavior are widely used in rodent studies, but they rely on the underlying assumption that mice are motivated to explore novel environments (open arms of EPM/EZM, light compartment of LDB, center of OFT). The conflict in these canonical tasks is between innate fear (open arms, light, center of field) and the drive to explore the novel environment (Cryan and Sweeney 2011; Kalueff et al. 2007).
Given that Dbh −/− mice exhibit blunted exploratory behavior in novel environments, it is possible that any anxiolytic phenotype would be occluded by a lack of neophilia. In other words, the behavioral measures of anxiolysis in these canonical anxiety tasks is exploratory behavior, which in NE-deficient mice is confounded by attenuated neophilia. In NSF, the conflict occurs between the fear of the novel environment and the motivation to eat. The behavioral measure of anxiolysis in NSF is latency to feed; thus, curiosity and neophilia are not a factor in this task.
The fact that a robust anxiolytic phenotype emerged in Dbh −/− mice during NSF but not in more commonly employed tests of anxiety-like behavior should serve as a reminder that any behavioral model has underlying assumptions and associated confounds (Cryan and Sweeney 2011; Kalueff et al. 2007). We urge researchers investigating anxiety-like behavior to include NSF in the battery of behavioral testing in order to account for potentially confounding effects of neophilia in more canonical anxiety tests, which all fundamentally measure exploration.
NE-deficient mice show reduced c-fos induction in select targets of the LC following NIL and NSF
Following exposure to the novel environment in the NIL and NSF tests, robust c-fos induction was observed in both NE-deficient and control LC. This finding suggests that LC activation in response to novelty does not depend on NE signaling and may be induced by glutamatergic inputs to the LC, or an excitatory neuropeptide transmitter such as orexin or corticotropin-releasing factor (CRF) (Gompf et al. 2010; Soya et al. 2017; Valentino et al. 1993).
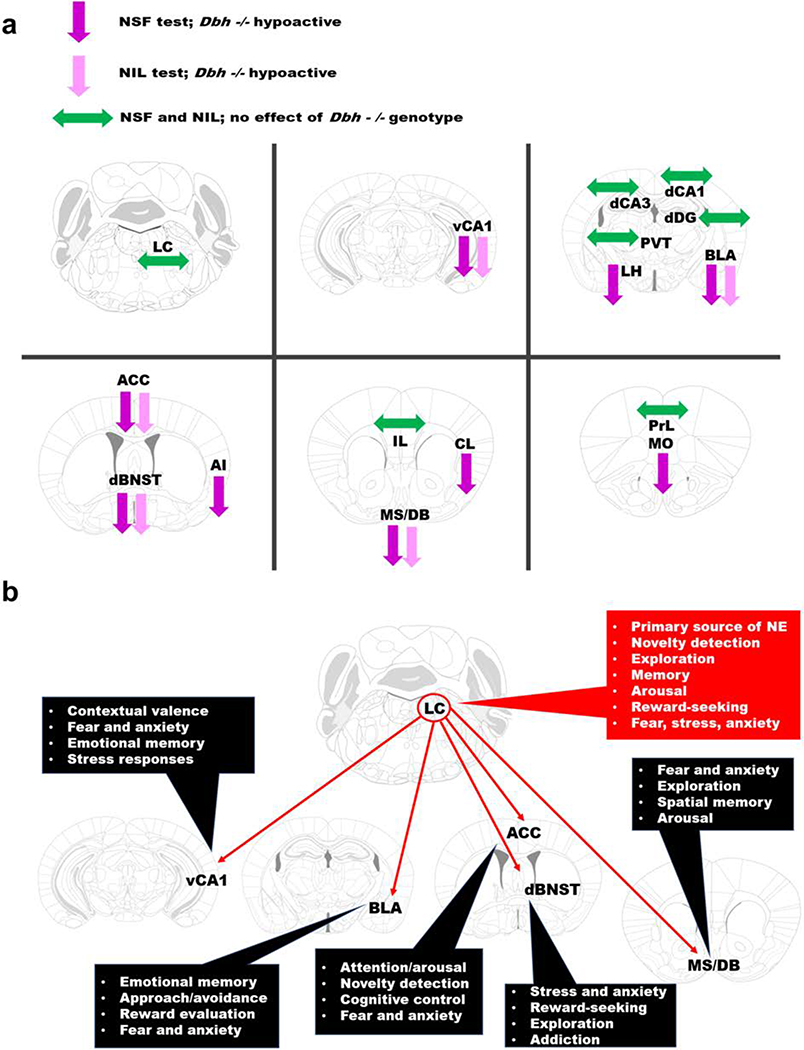
Despite demonstrating similar levels of LC activation compared to controls after NIL or NSF, Dbh −/− mice had fewer c-fos+ cells in several forebrain targets of the LC, including vCA1, ACC, dBNST, MS/DB, and BLA. Because these regions were hypoactive in the absence of NE following novelty exposure in both tests (Fig. 7a), we propose that they comprise the nodes of a noradrenergic novelty network (NNN) that drives both neophilic and neophobic behavior (Fig. 7b). With outputs extending throughout the prefrontal cortex, striatum, hypothalamus, midbrain, and brainstem, the NNN is well positioned to coordinate affective and motor responses to unfamiliar environments (Lebow and Chen 2016; Li and Wang 2018; Padilla-Coreano et al. 2016; Stachniak et al. 2014; Tovote et al. 2015).
Fig. 7.
The noradrenergic novelty network (NNN). a Summary of effects of Dbh genotype on c-fos induction in the LC and forebrain targets following NSF (darker purple) or NIL (lighter purple). Downward arrows denote regional hypoactivity in Dbh −/− mice and sideways green arrows denote no significant genotype differences following either test. Abbreviations: locus coeruleus (LC), medial orbitofrontal cortex (MO), prelimbic (PrL) and infralimbic cortices (IL), anterior cingulate cortex (ACC), agranular insula (AI), claustrum (CL), medial septum/diagonal band (MS/DB), dorsal bed nucleus of the stria terminalis (dBNST), basolateral amygdala (BLA), dorsal dentate gyrus (dDG), dorsal hippocampal subfields CA1 (dCA1) and CA3 (dCA3), ventral hippocampus subfield CA1 (vCA1), lateral hypothalamus (LH), and the paraventricular nucleus of the thalamus (PVT). b LC-NE inputs to vCA1, ACC, dBNST, MS/DB, and BLA may support diverse and flexible behavioral responses in novel environments. The outputs of the various nodes of the NNN could “prime” either fear-related or exploratory-related circuits in unfamiliar contexts, thus controlling both neophobic and neophilic behavior. Dysregulation of the NNN could be a common feature in certain psychiatric conditions, including anxiety and substance abuse disorders.
Recently, a population of spatially- and emotionally-tuned cells in vCA1 were shown to fire preferentially in anxiogenic environments (Jimenez et al. 2018). These “anxiety cells” are analogous to place cells in dCA1 which support spatial learning and memory, and exert their anxiogenic behavioral effects through excitatory glutamatergic projections to LH (Jimenez et al. 2018). The ventral hippocampus has been implicated in emotional learning and neophobia (Bannerman et al. 2002; Bannerman et al. 2004; Fanselow and Dong 2010; Padilla-Coreano et al. 2016; Santarelli et al. 2003), but the effects of manipulating LC-NE signaling within vCA1 on neophobia have not been described.
Reciprocal connections between the LC and the ACC support vigilance in novel environments, and destruction of LC terminals in the ACC suppresses electrophysiological measures of arousal associated with exposure to contextual novelty (Gompf et al. 2010). Recently, our group reported hypoactivity in the ACC of NE-deficient mice following cage change stress or exposure to a novel environment containing marbles (Lustberg et al. 2020). Unlike control mice, NE-deficient mice demonstrated virtually no stress-induced repetitive behaviors following cage change or novelty exposure, suggesting that NE engagement of the ACC may be necessary for typical affective responses to contextual change (Egner 2011).
The dBNST is a region with a well-established role in innate fear, stress responses, and anxiety-like behavior (Avery et al. 2016; Lebow and Chen 2016). CRF-containing neurons in the dBNST are activated by NE and engage the hypothalamic-pituitary-adrenal axis to synchronize endocrine and behavioral responses to stress (Dabrowska et al. 2016; Egli et al. 2005; Vranjkovic et al. 2017). Activation of this region is also implicated in stress-induced reinstatement of drug-seeking behavior (Mantsch et al. 2016), an animal model of relapse, as well as threat-detection and stimulus evaluation under conditions of uncertainty (Lebow and Chen 2016).
Cholinergic neurons in the MS/DB of the basal forebrain are densely innervated by the LC (Bergado et al. 2007; Schwarz and Luo 2015; Schwarz et al. 2015), and control both exploratory and anxious behavior in novel environments (Bannerman et al. 2004; Carpenter et al. 2017; Myhrer 1989; Zhang et al. 2017). These cholinergic projection neurons likely modulate exploratory and avoidant behavior in novel environments via projections to the hippocampus and medial prefrontal cortex (Jiang et al. 2018; Tereshchenko et al. 2008). Although LC inputs to the MS/DB have not been well characterized, pharmacological studies suggest that the NE increases excitability of cholinergic neurons in this region (Berridge and Espana 2000; Berridge et al. 1996).
The BLA is critical for fear learning and anxiety-like behavior, but is also implicated in novelty responses (Balderston et al. 2011; Jhang et al. 2018). LC-NE signaling within the BLA elicits anxiety-like behavior in canonical anxiety tests as well measures of social anxiety (Llorca-Torralba et al. 2019; McCall et al. 2017; Siuda et al. 2016), but the role of the LC-NE → BLA circuit in neophobia has not been described.
NE-deficient mice show diminished c-fos induction in select targets of the LC following NSF
After NSF, but not after NIL, NE-deficient mice had fewer c-fos+ cells than controls in MO, AI, CL, and LH. The MO is a behavioral control center at the anterior pole of the brain that is bidirectionally connected to the LC (Aston-Jones and Cohen 2005; Rolls and Grabenhorst 2008; Sadacca et al. 2017). MO neurons participate in the evaluation of risk and reward, decision making, and executive control of emotional responses (Petrides 2007; Woon et al. 2019). The AI is a cortical region involved in processing gustatory and interoceptive information (Naqvi and Bechara 2010; Uddin et al. 2017), and it is implicated in panic and anxiety disorders (Gehrlach et al. 2019; Klumpp et al. 2012; Poletti et al. 2015; Wittmann et al. 2014). NE signaling within the AI mediates food neophobia and is derived from the LC as well as non-cerulean brainstem groups (Robertson et al. 2016; Rojas et al. 2015). The CL is located between the AI and striatum, has connectivity with virtually all cortical regions, and is involved in contextual memory, novelty detection, allocation of attention, and action selection (Brown et al. 2017; Crick and Koch 2005; Qadir et al. 2018).
The LH is comprised of a heterogenous population of neurons that are also bidirectionally connected to the LC and participate in feeding, anxiety, stress responses, and motivated behavior (Bonnavion et al. 2016; Tanaka et al. 2000). One possible reason that NE-deficient mice demonstrated hypoactivity in these regions after NSF but not after NIL may be the relative demands and parameters of these two tests. For instance, NSF requires the animals to be food-deprived before testing so that the mice are motivated to eat in the test. On test day, NSF also requires the animals to make a behavioral “choice” between avoidance and approach to the food pellet in the novel environment. Thus, NE transmission in NSF may modulate gustatory reward-evaluation and action selection circuits in MO, AI, and CL, as well as motivational and emotional circuits within the LH in the context of the NSF test. The NIL test does not require food deprivation or decision making, potentially masking genotype differences in NE-dependent c-fos induction in these regions.
NE-deficient mice show similar c-fos induction to controls in dorsal hippocampus and paraventricular thalamus after following NIL and NSF
By definition, novelty detection requires engagement of memory systems (Kafkas and Montaldi 2018). In order to determine that an environment is novel, it must be compared with existing representations of previously encountered environments that are stored in memory. Despite their striking absence of neophilia and neophobia, NE-deficient mice retain the ability to recognize an environment as novel or familiar (Weinshenker et al. 2002a). The dissociation between novelty detection and novelty responding in the NE-deficient animals suggests at least partially non-overlapping neural substrates for these operations (Bannerman et al. 2002; Harro et al. 1995; Kafkas and Montaldi 2014; Tereshchenko et al. 2008; Wingo et al. 2016).
Studies in rodents suggest that the hippocampus is functionally segregated along the dorsoventral axis; the dorsal hippocampus is required for spatial memory and navigation, while the ventral hippocampus is required for emotional memory and anxiety (Bannerman et al. 2002; Fanselow and Dong 2010). Although NE-deficient mice had reduced emotional responses and diminished neuronal activity in vCA1 after novelty exposure, they showed no impairment in the detection of spatial novelty and displayed neuronal activity similar to controls in dCA1, dCA3, and dDG after novelty exposure. Dbh −/− mice also had similar levels of activity compared to controls in the PVT, a midline thalamic structure that integrates complex contextual signals from hypothalamus and brainstem and which becomes highly activated under conditions of uncertainty (Choi and McNally 2017; Kirouac 2015). As mentioned above, LC-DA transmission is theoretically intact in NE-deficient mice, and recent studies have shown that LC-DA transmission excites neurons within the dorsal hippocampus and PVT (Beas et al. 2018; Wagatsuma et al. 2018).
Limitations and future directions
A limitation of our study is that we cannot infer a causal role for LC-NE transmission to any of the identified forebrain regions in the expression of NIL and NSF behaviors. Other non-cerulean adrenergic cell groups in the brainstem, such as A2 neurons in the NTS, organize behaviors related to stress, reward, appetite, and affect via forebrain projections (Rinaman 2011). In addition to providing noradrenergic inputs to the LC itself, LC and A2 axon terminal fields overlap in prefrontal cortex (including PrL and IL), thalamus (including PVT), hypothalamus (including LH), and extended amygdala (including dBNST) (Ricardo and Koh 1978; van der Kooy et al. 1984). However, to our knowledge, the LC is the sole source of NE to the ACC, MS/DB, BLA, and hippocampus (including vCA1). The hypoactivity of these four nodes of the NNN, which receive NE exclusively from LC projections, following exposure to novelty suggests that the absence of LC-derived NE transmission at one or more these regions may underlie blunted affective responses to novelty in Dbh −/− mice.
Another important consideration when interpreting these findings is that the LC is not a homogenous nucleus (Schwarz and Luo 2015; Uematsu et al. 2015), and NE transmission from distinct sets of LC cells can play functionally opposite roles on behavior (Buffalari and Grace 2007; Chandler et al. 2014). Moreover, elegant intersectional genetic techniques have led to even finer parcellations of adrenergic cell groups and interrogations of their functions (Robertson et al. 2013; Robertson et al. 2016). A recent and surprising example of the functional diversity of the central NE system is the finding that chemogenetic activation of NE neurons with a shared genetic history of Hoxb1 actually reduced stress responses and anxiety-like behavior (Chen et al. 2019). These Hoxb1 NE neurons are concentrated in the A2 nucleus and in the subcoeruleus regions below the LC, and send noradrenergic projections to the LC, hypothalamus, and extended amygdala (Chen et al. 2019). Thus, additional experiments using optogenetic and chemogenetic tools to bidirectionally manipulate distinct forebrain targets of the LC are necessary to functionally dissect the NNN and understand how NE organizes affective responses to novelty.
Although we have clearly demonstrated a critical role for central NE transmission in neophobia, the neurochemistry underlying innate anxiety is complex and likely to involve multiple neuromodulatory systems. Intriguingly, serotonin (5-HT) deficient mice (Tph2 −/−) also exhibit dramatic reductions in neophobia in the NSF test (Angoa-Pérez et al. 2012; Mosienko et al. 2012). Moreover, these 5-HT deficient mice display increased aggressive and compulsive behaviors, which are absent in NE deficient mice (Angoa-Pérez et al. 2012; Lustberg et al. 2020; Marino et al. 2005; Mosienko et al. 2012). Indeed, the phenotypes of Tph2 −/− and Dbh −/− mice are perfectly opposed with the notable exception of reduced neophobia in both mutants, suggesting that behavioral expression of neophobia may require both NE and 5-HT (Blier and El Mansari 2007).
Conclusions
In summary, these findings support an expanded role of central NE transmission in the expression of neophilic and neophobic behaviors. Guanfacine and related drugs have been used both on- and off-label for the treatment of anxiety and substance abuse disorders (Fox et al. 2012; Hoehn-Saric et al. 1981), but this is the first study to demonstrate anxiolytic effects of guanfacine in the NSF test. We propose that guanfacine should be investigated for rapid anxiolytic effects in patients with context-specific anxiety symptoms, such as agoraphobia (Marazziti et al. 2012; Wittmann et al. 2014).
Supplementary Material
Acknowledgments
We thank Lundbeck for providing the DOPS. This work was supported by the National Institutes of Health (AG061175, NS102306, and DA038453 to DW; GM8602–22 to DL; MH116622 to RPT).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Angoa-Pérez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM, Rosenberg DR, Thomas DM, Kuhn DM. (2012). Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. Journal of neurochemistry 121:974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T, Ogren S-O, Ross SB. (1981). N-2-chloroethyl-N-ethyl-2-bromobenzylamine hydrochloride (DSP4), a new selective noradrenaline neurotoxin, and taste neophobia in the rat. Physiological Psychology 9:197–202 [Google Scholar]
- Aston-Jones G, Bloom F. (1981). Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. Journal of Neuroscience 1:887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28:403–450 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Iba M, Clayton E, Rajkowski J, Cohen J. (2007). The locus coeruleus and regulation of behavioral flexibility and attention: Clinical implications. Brain norepinephrine: Neurobiology:196–235 [Google Scholar]
- Aston-Jones G, Kalivas PW. (2008). Brain norepinephrine rediscovered in addiction research. Biological psychiatry 63:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. (1999). Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry 46:1309–1320 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Valentino RJ, Van Bockstaele EJ, Meyerson AT. (1994). Locus coeruleus, stress, and PTSD: neurobiological and clinical parallels. [Google Scholar]
- Avery S, Clauss J, Blackford J. (2016). The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Schultz DH, Helmstetter FJ. (2011). The human amygdala plays a stimulus specific role in the detection of novelty. Neuroimage 55:1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D, Deacon R, Offen S, Friswell J, Grubb M, Rawlins J. (2002). Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral neuroscience 116:884. [DOI] [PubMed] [Google Scholar]
- Bannerman D, Matthews P, Deacon R, Rawlins J. (2004). Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behavioral neuroscience 118:1033. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. (1996). Psychobiology of novelty seeking and drug seeking behavior. Behavioural brain research 77:23–43 [DOI] [PubMed] [Google Scholar]
- Barnett SA. (1958). Experiments on ‘neophobia’in wild and laboratory rats. British journal of psychology 49:195–201 [DOI] [PubMed] [Google Scholar]
- Beas BS, Wright BJ, Skirzewski M, Leng Y, Hyun JH, Koita O, Ringelberg N, Kwon H-B, Buonanno A, Penzo MA. (2018). The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nature neuroscience 21:963–973 doi: 10.1038/s41593-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado JA, Frey S, López J, Almaguer-Melian W, Frey JU. (2007). Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiology of Learning and Memory 88:331–341 doi: 10.1016/j.nlm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Berridge C, Espana R. (2000). Synergistic sedative effects of noradrenergic α1-and β-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience 99:495–505 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Bolen SJ, Manley MS, Foote SL. (1996). Modulation of forebrain electroencephalographic activity in halothane-anesthetized rat via actions of noradrenergic β-receptors within the medial septal region. Journal of Neuroscience 16:7010–7020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Dunn AJJJoN. (1989). Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. 9:3513–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, El Mansari M. (2007). The importance of serotonin and noradrenaline in anxiety. International journal of psychiatry in clinical practice 11:16–23 [DOI] [PubMed] [Google Scholar]
- Boehnlein JK, Kinzie JDJJoPP. (2007). Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. 13:72–78 [DOI] [PubMed] [Google Scholar]
- Bonnavion P, Mickelsen LE, Fujita A, Lecea L, Jackson AC. (2016). Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. The Journal of physiology 594:6443–6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdélat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. (2005). Effects of dopamine β-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology 183:72–80 [DOI] [PubMed] [Google Scholar]
- Brady LSJBrb. (1994). Stress, antidepressant drugs, and the locus coeruleus. 35:545–556 [DOI] [PubMed] [Google Scholar]
- Britton DR, Indyk E. (1990). Central effects of corticotropin releasing factor (CRF): evidence for similar interactions with environmental novelty and with caffeine. Psychopharmacology 101:366–370 [DOI] [PubMed] [Google Scholar]
- Brown SP, Mathur BN, Olsen SR, Luppi P-H, Bickford ME, Citri A. (2017). New Breakthroughs in Understanding the Role of Functional Interactions between the Neocortex and the Claustrum. The Journal of neuroscience : the official journal of the Society for Neuroscience 37:10877–10881 doi: 10.1523/JNEUROSCI.1837-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. (2007). Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of α−2 and β receptor activation. Journal of Neuroscience 27:12358–12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F, Burgess N, Barry C. (2017). Modulating medial septal cholinergic activity reduces medial entorhinal theta frequency without affecting speed or grid coding. Scientific reports 7:14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Gao W-J, Waterhouse BD. (2014). Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proceedings of the National Academy of Sciences 111:6816–6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJJBr. (2016). Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations. 1641:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-W, Das M, Oyarzabal EA, Cheng Q, Plummer NW, Smith KG, Jones GK, Malawsky D, Yakel JL, Shih Y-YI, Jensen P. (2019). Genetic identification of a population of noradrenergic neurons implicated in attenuation of stress-related responses. Molecular Psychiatry 24:710–725 doi: 10.1038/s41380-018-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. (2019). Parabrachial Complex: A Hub for Pain and Aversion. The Journal of Neuroscience 39:8225 doi: 10.1523/JNEUROSCI.1162-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EA, McNally GP. (2017). Paraventricular thalamus balances danger and reward. Journal of Neuroscience 37:3018–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FC, Koch C. (2005). What is the function of the claustrum? Philosophical transactions of the Royal Society of London. Series B, Biological sciences 360:1271–1279 doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. (2011). The age of anxiety: role of animal models of anxiolytic action in drug discovery. British journal of pharmacology 164:1129–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Schroeder JP, Barrie ES, Manvich DF, Sadee W, Berg T, Mercer K, Stowe TA, Liles LC, Squires KE, Mezher A, Curtin P, Perdomo DL, Szot P, Weinshenker D. (2016). Human Bacterial Artificial Chromosome (BAC) Transgenesis Fully Rescues Noradrenergic Function in Dopamine β-Hydroxylase Knockout Mice. PLOS ONE 11:e0154864 doi: 10.1371/journal.pone.0154864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG. (2016). Targeting Corticotropin-Releasing Factor Projections from the Oval Nucleus of the Bed Nucleus of the Stria Terminalis Using Cell-Type Specific Neuronal Tracing Studies in Mouse and Rat Brain. Journal of neuroendocrinology 28:10.1111/jne.12442 doi: 10.1111/jne.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delini-Stula A, Mogilnicka E, Hunn C, Dooley D. (1984). Novelty-oriented behavior in the rat after selective damage of locus coeruleus projections by DSP-4, a new noradrenergic neurotoxin. Pharmacology Biochemistry Behavior 20:613–618 [DOI] [PubMed] [Google Scholar]
- Dovey TM, Staples PA, Gibson EL, Halford JCG. (2008). Food neophobia and ‘picky/fussy’eating in children: a review. Appetite 50:181–193 [DOI] [PubMed] [Google Scholar]
- Dulawa SC (2009) Novelty-induced hypophagia In: Mood and Anxiety Related Phenotypes in Mice. Springer, pp 247–259 [Google Scholar]
- Dulawa SC, Hen R. (2005). Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neuroscience Biobehavioral Reviews 29:771–783 [DOI] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. (2005). Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30:657. [DOI] [PubMed] [Google Scholar]
- Egner T (2011). Surprise! A unifying model of dorsal anterior cingulate function? Nature Neuroscience 14:1219. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19 doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. (2001). Factors controlling measures of anxiety and responses to novelty in the mouse. Behavioural brain research 125:151–157 [DOI] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. (2012). Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. Journal of psychopharmacology 26:958–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrlach DA, Dolensek N, Klein AS, Roy Chowdhury R, Matthys A, Junghänel M, Gaitanos TN, Podgornik A, Black TD, Reddy Vaka N, Conzelmann K-K, Gogolla N. (2019). Aversive state processing in the posterior insular cortex. Nature neuroscience 22:1424–1437 doi: 10.1038/s41593-019-0469-1. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. (2010). Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depression and anxiety 27:339–350 [DOI] [PubMed] [Google Scholar]
- Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J. (2010). Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. Journal of Neuroscience 30:14543–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier PJMp. (2008). Crosstalk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus coeruleus, and dorsal hippocampus. [DOI] [PubMed]
- Harro J, Oreland L, Vasar E, Bradwejn JJEN. (1995). Impaired exploratory behaviour after DSP-4 treatment in rats: implications for the increased anxiety after noradrenergic denervation. 5:447–455 [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Merchant AF, Keyser ML. (1981). Effects of clonidine on anxiety disorders. Archives of General Psychiatry 38:1278–1282 [DOI] [PubMed] [Google Scholar]
- Jhang J, Lee H, Kang MS, Lee H-S, Park H, Han J-H. (2018). Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response. Nature communications 9:2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y-Y, Zhang Y, Cui S, Liu F-Y, Yi M, Wan Y. (2018). Cholinergic neurons in medial septum maintain anxiety-like behaviors induced by chronic inflammatory pain. Neuroscience Letters 671:7–12 doi: 10.1016/j.neulet.2018.01.041. [DOI] [PubMed] [Google Scholar]
- Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L. (2018). Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97:670–683. e676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. (2000). Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. Journal of Neuroscience 20:6983–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. (2014). Two separate, but interacting, neural systems for familiarity and novelty detection: A dual-route mechanism. Hippocampus 24:516–527 [DOI] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. (2018). How do memory systems detect and respond to novelty? Neuroscience Letters 680:60–68 doi: 10.1016/j.neulet.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A, Wheaton M, Murphy D. (2007). What’s wrong with my mouse model?: Advances and strategies in animal modeling of anxiety and depression. Behavioural brain research 179:1–18 [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. (2016). Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proceedings of the National Academy of Sciences 113:14835–14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ. (2015). Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neuroscience & Biobehavioral Reviews 56:315–329 doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. (2012). Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology 89:273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Simon EJ. (2009). The Neurobiology of Addiction: Where We Have Been and Where We Are Going. Journal of drug issues 39:115–132 doi: 10.1177/002204260903900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2016). Neurobiology of addiction: a neurocircuitry analysis. The lancet. Psychiatry 3:760–773 doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A. (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry 21:450–463 doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang L. (2018). Modulation of Innate Defensive Responses by Locus Coeruleus-Superior Colliculus Circuit. Journal of experimental neuroscience 12:1179069518792035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Söderström S, Ebendal T. (2004). Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40:67–73 doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Llorca-Torralba M, Suárez-Pereira I, Bravo L, Camarena-Delgado C, Garcia-Partida JA, Mico JA, Berrocoso E. (2019). Chemogenetic silencing of the locus coeruleus–basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biological Psychiatry 85:1021–1035 [DOI] [PubMed] [Google Scholar]
- Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. (2020). Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. (2016). Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41:335–356 doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Carlini M, Dell’Osso L. (2012). Treatment strategies of obsessive-compulsive disorder and panic disorder/agoraphobia. Current topics in medicinal chemistry 12:238–253 [DOI] [PubMed] [Google Scholar]
- Marcontell DK, Laster AE, Johnson J. (2003). Cognitive-behavioral treatment of food neophobia in adults. Journal of anxiety disorders 17:243–251 [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdélat-Parks BN, Liles LC, Weinshenker D. (2005). Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behavioural brain research 161:197–203 [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. (2009). The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. The Psychiatric clinics of North America 32:549–575 doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. (2016). Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. The Behavioral and brain sciences 39:e200–e200 doi: 10.1017/S0140525X15000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. (2015). CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87:605–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR. (2017). Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 6:e18247 doi: 10.7554/eLife.18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Bentham MP, Zhou W-L, Plantenga ME, McKee SA, Picciotto MRJP. (2015). Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. 232:3539–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D. (2006). The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biological psychiatry 60:1046–1052 [DOI] [PubMed] [Google Scholar]
- Mitchell HA, Bogenpohl JW, Liles LC, Epstein MP, Bozyczko-Coyne D, Williams M, Weinshenker D. (2008). Behavioral responses of dopamine β-hydroxylase knockout mice to modafinil suggest a dual noradrenergic–dopaminergic mechanism of action. Pharmacology Biochemistry Behavior 91:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlinska K, Stryjek R. (2016). Food Neophobia in Wild Rats (Rattus norvegicus) Inhabiting a Changeable Environment-A Field Study. PloS one 11:e0156741–e0156741 doi: 10.1371/journal.pone.0156741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlinska K, Stryjek R, Pisula W. (2015). Food neophobia in wild and laboratory rats (multi-strain comparison). Behavioural processes 113:41–50 [DOI] [PubMed] [Google Scholar]
- Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. (2012). Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Translational psychiatry 2:e122–e122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA. (2004). A distinct role for norepinephrine in memory retrieval. Cell 117:131–143 [DOI] [PubMed] [Google Scholar]
- Myhrer T (1989). Exploratory behavior and reaction to novelty in rats: effects of medial and lateral septal lesions. Behavioral neuroscience 103:1226. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function 214:435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou SI, Aspley S, Butler S, Beckett S, Marsden CAJPin-p, psychiatry b. (2001). Effects of lesioning noradrenergic neurones in the locus coeruleus on conditioned and unconditioned aversive behaviour in the rat. 25:1307–1321 [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. (2006). Role of Noradrenergic Signaling by the Nucleus Tractus Solitarius in Mediating Opiate Reward. Science 311:1017 doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Osorio-Gómez D, Guzmán-Ramos K, Bermúdez-Rattoni F (2018) Neurobiology of neophobia and its attenuation In: Food Neophobia. Elsevier, pp 111–128 [Google Scholar]
- Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA. (2016). Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron 89:857–866 doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak CR, Ho Y-J, Schwarting RKW. (2008). Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neuroscience & Biobehavioral Reviews 32:1544–1568 [DOI] [PubMed] [Google Scholar]
- Petrides M (2007). The orbitofrontal cortex: novelty, deviation from expectation, and memory. Annals of the New York Academy of Sciences 1121:33–53 [DOI] [PubMed] [Google Scholar]
- Poletti S, Radaelli D, Cucchi M, Ricci L, Vai B, Smeraldi E, Benedetti F. (2015). Neural correlates of anxiety sensitivity in panic disorder: a functional magnetic resonance imaging study. Psychiatry Research: Neuroimaging 233:95–101 [DOI] [PubMed] [Google Scholar]
- Porter-Stransky KA, Centanni SW, Karne SL, Odil LM, Fekir S, Wong JC, Jerome C, Mitchell HA, Escayg A, Pedersen NP. (2019). Noradrenergic Transmission at Alpha1-Adrenergic Receptors in the Ventral Periaqueductal Gray Modulates Arousal. Biological Psychiatry 85:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir H, Krimmel SR, Mu C, Poulopoulos A, Seminowicz DA, Mathur BN. (2018). Structural connectivity of the anterior cingulate cortex, claustrum, and the anterior insula of the mouse. Frontiers in neuroanatomy 12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. (1978). Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain research 153:1–26 [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Romeo S, Agustin-Pavon C, Oliverio A, Mele A. (2010). Distinct patterns of Fos immunoreactivity in striatum and hippocampus induced by different kinds of novelty in mice. Neurobiology of learning & memory 94:373–381 [DOI] [PubMed] [Google Scholar]
- Rinaman L (2011). Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. American journal of physiology. Regulatory, integrative and comparative physiology 300:R222–R235 doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. (2013). Developmental origins of central norepinephrine neuron diversity. Nature Neuroscience 16:1016–1023 doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, Jensen P. (2016). Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain research 1641:234–244 doi: 10.1016/j.brainres.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas S, Diaz-Galarce R, Jerez-Baraona JM, Quintana-Donoso D, Moraga-Amaro R, Stehberg J. (2015). The insula modulates arousal-induced reluctance to try novel tastes through adrenergic transmission in the rat. Frontiers in behavioral neuroscience 9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. (2008). The orbitofrontal cortex and beyond: from affect to decision-making. Progress in neurobiology 86:216–244 [DOI] [PubMed] [Google Scholar]
- Rommelfanger K, Edwards G, Freeman K, Liles L, Miller G, Weinshenker D. (2007). Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proceedings of the National Academy of Sciences 104:13804–13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Wikenheiser AM, Schoenbaum G. (2017). Toward a theoretical role for tonic norepinephrine in the orbitofrontal cortex in facilitating flexible learning. Neuroscience 345:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809 [DOI] [PubMed] [Google Scholar]
- Sara Susan J, Bouret S. (2012). Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 76:130–141 doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Dyon-Laurent C, Hervé A. (1995). Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Cognitive Brain Research 2:181–187 [DOI] [PubMed] [Google Scholar]
- Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM. (2005). Bcl-x is required for proper development of the mouse substantia nigra. Journal of Neuroscience 25:6721–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. (2008). Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biological psychiatry 63:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. (2006). Dopamine β-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology 31:2221. [DOI] [PubMed] [Google Scholar]
- Scholtysik G (1980). Pharmacology of guanfacine. British journal of clinical pharmacology 10:21S–24S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. (2013). The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Luo L. (2015). Organization of the locus coeruleus-norepinephrine system. Current Biology 25:R1051–R1056 [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. (2015). Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524:88–92 doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth A, Berretta S, Lange N, Eichenbaum H. (2008). The amygdala modulates neuronal activation in the hippocampus in response to spatial novelty. Hippocampus 18:169–181 [DOI] [PubMed] [Google Scholar]
- Siuda ER, Al-Hasani R, McCall JG, Bhatti DL, Bruchas MR. (2016). Chemogenetic and Optogenetic Activation of Gαs Signaling in the Basolateral Amygdala Induces Acute and Social Anxiety-Like States. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41:2011–2023 doi: 10.1038/npp.2015.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang W-W, Han R, McFadden K, Valentino RJ. (2012). Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, Sakimura K, Sakurai T. (2017). Orexin modulates behavioral fear expression through the locus coeruleus. Nature communications 8:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. (2014). Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→ midbrain pathway for feeding behavior. Neuron 82:797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D. (1999). Brain alpha 1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience 94:1245–1252 [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Ahsan MR, Quartermain D. (2005). α 1-Adrenergic and α 2-adrenergic balance in the dorsal pons and gross behavioral activity of mice in a novel environment. Psychopharmacology 183:127. [DOI] [PubMed] [Google Scholar]
- Struthers WM, DuPriest A, Runyan J. (2005). Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex. Experimental brain research 167:136–140 [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW. (2016). Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H. (2000). Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. European journal of pharmacology 405:397–406 [DOI] [PubMed] [Google Scholar]
- Tereshchenko Y, Brandewiede J, Schachner M, Irintchev A, Morellini F. (2008). Novelty-induced behavioral traits correlate with numbers of brainstem noradrenergic neurons and septal cholinergic neurons in C57BL/6 J mice. Behavioural brain research 191:280–284 [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. (1998). Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine β-hydroxylase. Journal of neurochemistry 70:2468–2476 [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. (1995). Noradrenaline is essential for mouse fetal development. Nature 374:643. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. (1997). Disruption of the dopamine Β-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behavioral neuroscience 111:579. [DOI] [PubMed] [Google Scholar]
- Tillage RP, Sciolino NR, Plummer NW, Lustberg D, Liles LC, Hsiang M, Powell JM, Smith KG, Jensen P, Weinshenker D. (2020). Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Structure Function:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience 16:317. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S. (1994). Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. (2017). Structure and function of the human insula. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society 34:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Johansen JP. (2015). Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learning & memory (Cold Spring Harbor, N.Y.) 22:444–451 doi: 10.1101/lm.037283.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME. (1993). The Locus Coeruleus as a Site for Integrating Corticotropin-Releasing Factor and Noradrenergic Mediation of Stress Responses a. Annals of the New York Academy of Sciences 697:173–188 [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. (1984). The organization of projections from the cortes, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. Journal of Comparative Neurology 224:1–24 [DOI] [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ. (1995). Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. European Journal of Neuroscience 7:1180–1187 [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Pina M, Kash TL, Winder DG. (2017). The bed nucleus of the stria terminalis in drug-associated behavior and affect: a circuit-based perspective. Neuropharmacology 122:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagatsuma A, Okuyama T, Sun C, Smith LM, Abe K, Tonegawa S. (2018). Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proceedings of the National Academy of Sciences 115:E310–E316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM. (2009). Novelty-induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacology Biochemistry and Behavior 91:398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. (2010). Novelty as a dimension in the affective brain. Neuroimage 49:2871–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. (2002a). Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proceedings of the National Academy of Sciences 99:13873–13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. (2007). There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, White SS, Javors MA, Palmiter RD, Szot P. (2002b). Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Research 946:239–246 doi: 10.1016/S0006-8993(02)02889-5. [DOI] [PubMed] [Google Scholar]
- West CHK, Ritchie JC, Boss-Williams KA, Weiss JM. (2009). Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. The international journal of neuropsychopharmacology 12:627–641 doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo T, Nesil T, Choi J-S, Li MD. (2016). Novelty seeking and drug addiction in humans and animals: from behavior to molecules. Journal of Neuroimmune Pharmacology 11:456–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann A, Schlagenhauf F, Guhn A, Lueken U, Gaehlsdorf C, Stoy M, Bermpohl F, Fydrich T, Pfleiderer B, Bruhn H. (2014). Anticipating agoraphobic situations: the neural correlates of panic disorder with agoraphobia. Psychological medicine 44:2385–2396 [DOI] [PubMed] [Google Scholar]
- Woon EP, Sequeira MK, Barbee BR, Gourley SL. (2019). Involvement of the rodent prelimbic and medial orbitofrontal cortices in goal-directed action: A brief review. Journal of Neuroscience Research n/a doi: 10.1002/jnr.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L (1989). Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. EXS 57:12–32 doi: 10.1007/978-3-0348-9138-7_2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang Y-Y, Shao S, Zhang C, Liu F-Y, Wan Y, Yi M. (2017). Inhibiting medial septal cholinergic neurons with DREADD alleviated anxiety-like behaviors in mice. Neuroscience Letters 638:139–144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.