Abstract
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) have a strong clinical, genetic and pathological overlap. This review focuses on the current understanding of structural, functional and molecular neuroimaging signatures of genetic FTD and ALS. We overview quantitative neuroimaging studies on the most common genes associated with FTD (MAPT, GRN), ALS (SOD1), and both (C9orf72), and summarize visual observations of images reported in the rarer genes (CHMP2B, TARDBP, FUS, OPTN, VCP, UBQLN2, SQSTM1, TREM2, CHCHD10, TBK1).
Keywords: frontotemporal dementia, amyotrophic lateral sclerosis, motor neuron disease, presymptomatic, genetics, neuroimaging
1. INTRODUCTION
Frontotemporal dementia (FTD) is a clinically heterogeneous group of neurodegenerative diseases characterized by early prominent changes in behavior and/or language accompanied by focal atrophy in frontal and temporal cortices. In contrast, amyotrophic lateral sclerosis (ALS) involves progressive degeneration of both upper and lower motor neurons, leading to progressive muscle weakness, and paralysis. Despite these distinctions in clinical presentation, the clinical, genetic, and pathological overlap between FTD and ALS has been well established, framing these diseases as part of a continuum. Clinically, 15% of patients with FTD develop motor neuron disease, and conversely, most patients with ALS develop increasing cognitive and behavioral deficits as their disease progresses (Burrell et al., 2011; Crockford et al., 2018; Lomen-Hoerth et al., 2002). Certain genetic variants such as the C9orf72 expansion and mutations in TBK1, VCP, and TARDBP are known to cause FTD, ALS, or both, whereas other genetic mutations are predominantly associated either with FTD or ALS (Fig. 1; Nguyen et al., 2018).
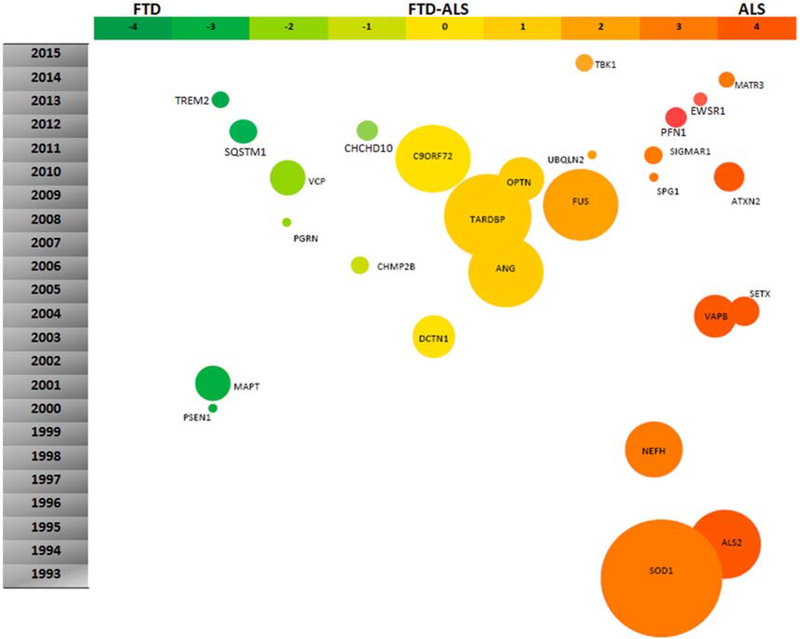
Fig. 1.
Genes whose mutations are associated with FTD-ALS spectrum disorders. Reprinted from Kumar et al. (2016) with permission from Elsevier.
Because mutation-specific therapies are currently undergoing human clinical trials for genetic FTD and ALS, determining the neuroanatomical regions and selective cell populations that are targeted in each genetic mutation is critical for understanding disease trajectories. While the earliest imaging studies characterized patients by clinical syndrome, genotypic stratification has revealed mutation-specific vulnerabilities in neuroanatomical regions targeted in genetic FTD (Chen and Kantarci, 2020; Greaves and Rohrer, 2019) and ALS (Dharmadasa et al., 2018) that appear distinct from the sporadic forms of these diseases. For example, in FTD due to the C9orf72 expansion, the characteristic frontotemporal atrophy may be milder or even absent and the atrophy pattern includes parietal (Cash et al., 2018; Irwin et al., 2013; Sha et al., 2012; Whitwell et al., 2012) and occipital (Sha et al., 2012; Whitwell et al., 2012) cortices. Patient-level findings in C9orf72 expansion carriers suggest even greater heterogeneity with little or no atrophy in some patients with mild, slowly progressive, or even advanced dementia (Boeve et al., 2012; Khan et al., 2012).
Studying the genetic forms of FTD and ALS has also enabled the characterization of the earliest symptomatic and even presymptomatic phases in mutation carriers, with neuroimaging changes preceding symptom onset by years. Most studies have focused on identifying regional atrophy patterns as measured by brain volumetrics in structural T1-weighted magnetic resonance imaging (MRI) (Dopper et al., 2014; Olm et al., 2018; Panman et al., 2019; Rohrer et al., 2015). Microstructural changes (e.g. myelin thickness, axon density, cell swelling) in the white matter of presymptomatic carriers have been measured with diffusion MRI (Bertrand et al., 2018; Floeter et al., 2018; Olm et al., 2018; Panman et al., 2019). Functional MRI, which indirectly measures neural activity by assessing hemodynamics, has revealed presymptomatic abnormalities in resting-state or task-free network connectivity (Dopper et al., 2014; Lee et al., 2017; Menke et al., 2016). Metabolic and molecular imaging provide information about physiological changes such as abnormalities in cellular glucose metabolism measured by [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) or perfusion weighted MRI (Dopper et al., 2016; Jacova et al., 2013; Mutsaerts et al., 2019), and in the concentrations of specific metabolites measured by proton magnetic resonance spectroscopy (1H MRS) (Carew et al., 2011a; Chen et al., 2019d). While none of these imaging modalities enable identification of the specific neuropathological diagnoses in FTD or ALS, they provide useful data on alterations in brain function and structure. Here, we review neuroimaging studies of genetic mutations that cause 1) FTD, 2) ALS, and 3) FTD and/or ALS. First, we briefly examine neuroimaging studies in sporadic FTD and ALS as a backdrop to a more in-depth review of neuroimaging literature in patients with genetic forms of these diseases.
2. SPORADIC FTD AND ALS
2.1. FTD
FTD serves as an umbrella term for three clinical subtypes, which include behavioral variant FTD (bvFTD), and the semantic (svPPA) and nonfluent variants (nfvPPA) of primary progressive aphasia (Gorno-Tempini et al., 2011; Neary et al., 1998; Rascovsky et al., 2011). While these three syndromes converge on frontotemporal atrophy (Gorno-Tempini et al., 2004; Rosen et al., 2002), each clinical syndrome targets a distinct network of regions whose dysfunction is associated with changes in social and emotional cognition and cognitive and motor function (Fig. 2; Seeley et al., 2009). Focal neurodegeneration can be asymmetric: in bvFTD, the right hemisphere often shows greater atrophy than the left, while the PPAs typically feature left hemispheric atrophy, corresponding to the higher frequency of left-sided language dominance in the population (Schroeter et al., 2007). With disease progression, atrophy spreads next to homologous regions in the contralateral hemisphere (Kumfor et al., 2016; Rohrer et al., 2009c, 2012).
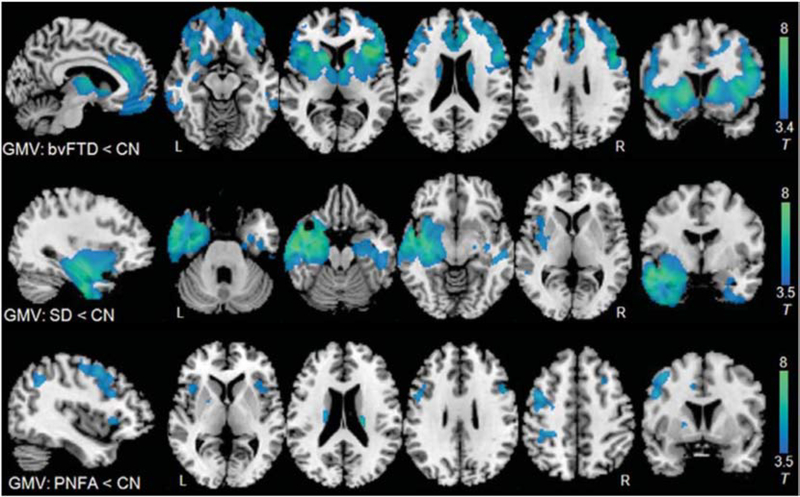
Fig. 2.
Comparison of grey matter atrophy patterns in three FTD syndromes. Patients with bvFTD, svPPA, nfvPPA were compared to healthy age-matched control subjects using voxel-based morphometry. Maps are thresholded at p < 0.001 uncorrected and superimposed on a study-specific template. BvFTD, behavioral variant frontotemporal dementia; SD, semantic dementia, also known as semantic variant PPA; PNFA, progressive nonfluent aphasia, also known as nonfluent variant PPA; GMV, grey-matter volume. Adapted from Zhang et al. (2013).
2.1.1. bvFTD
BvFTD presents with early changes in personality or behavior, with absent or mild cognitive symptoms in early stages of the disease. Clinical criteria include a wide variety of neuropsychiatric behaviors, including disinhibited behavior, profound apathy, loss of empathy, repetitive or compulsive behavior, and hyperorality or rigid food preferences (Neary et al., 1998; Rascovsky et al., 2011). In bvFTD, neurodegeneration starts in the pregenual anterior cingulate and the frontoinsular cortex (Kim et al., 2012; Seeley et al., 2007a, 2008), with orbitofrontal cortex, striatum, amygdala, thalamus, and brainstem also targeted later in the disease (Broe et al., 2003). Unlike Alzheimer’s disease, the parietal lobes are typically spared. Axonal degeneration, likely associated with gray matter neuron loss, often arises in the anterior corpus callosum, cingulum bundle and uncinate fasciculus (Agosta et al., 2012; Elahi et al., 2017; Lam et al., 2014; Mahoney et al., 2015, 2014; Whitwell et al., 2010; Zhang et al., 2013).
Studies show that specific types of behavioral symptoms in bvFTD are related to atrophy in distinct neuroanatomical regions. Disinhibited behavior has been associated with atrophy in right orbitofrontal cortex, while apathy is related to atrophy in anterior cinguate and medial prefrontal cortex (Rosen et al., 2005, 2002). Loss of empathy is associated with atrophy in several key hubs, including the right anterior temporal lobe, right anterior cingulate, anterior insula, and ventral striatum (Rankin et al., 2006). Simple stereotyped motor behavior relates to striatal atrophy, while more complex compulsions correlate with orbitofrontal, temporal lobe, and caudate atrophy (Josephs et al., 2008; Perry et al., 2012; Rosen et al., 2005; Rosso et al., 2001). Eating behaviors, which include increased carbohydrate consumption, eating of inedible objects, or rigid or ritualistic food preferences or schedules, are linked to degeneration of orbitofrontal cortex, right temporal lobe, right insula, striatum, and hypothalamus (Henry et al., 2014; Piguet et al., 2011; Whitwell et al., 2007b; Woolley et al., 2007).
Neurodegenerative syndromes target specific networks of distributed regions that are identifiable in healthy individuals, as measured by task-free fMRI intrinsic connectivity network (ICN) analysis (Greicius et al., 2004; Seeley et al., 2009). The key hubs of neurodegeneration in bvFTD form the salience network, proposed to evaluate the importance of emotionally significant stimuli (Seeley et al., 2007b). BvFTD typically is associated with disrupted connectivity within the salience network (Day et al., 2013; Filippi et al., 2013; Zhou et al., 2010), though some studies report hyperconnectivity or connectivity similar to controls (Farb et al., 2013; Hafkemeijer et al., 2015; Rytty et al., 2013). Intriguingly, the default mode network, targeted in Alzheimer’s disease, shows decreased but also increased connectivity in bvFTD, proposed to underlie enhanced visuospatial abilities and emerging visual artistic creativity in some patients (Zhou et al., 2010).
[18F]FDG-PET shows glucose hypometabolism within orbitofrontal, dorsomedial and dorsolateral prefrontal regions, anterior temporal pole and basal ganglia, which may be detectable in early stages before patients meet clinical criteria for probable bvFTD (Ber et al., 2006; Morbelli et al., 2016; Varma et al., 2002). Studies have reported that [18F]FDG-PET may distinguish patients with bvFTD from other patients with dementia (Buhour et al., 2017a; Diehl-Schmid et al., 2007; Morbelli et al., 2016; Tosun et al., 2016; Verfaillie et al., 2015; Vijverberg et al., 2016), yet frontal hypometabolism has also been reported in psychiatric disorders and Alzheimer’s disease, which confounds diagnostic accuracy (Vijverberg et al., 2016). Tau PET tracers, such as [18F]flortaucipir, show limited sensitivity and specificity in FTD (Tsai et al., 2019).
2.1.2. svPPA
SvPPA is a progressive language disorder characterized by loss of word and object meaning and surface dyslexia, yet fluent, grammatically correct speech (Gorno-Tempini et al., 2011; Hodges and Patterson, 1996; Snowden et al., 1989). The anterior temporal lobe, typically more atrophied on the left compared with the right, shows prominent focal degeneration, sometimes with “knife-edge” gyri in patients with severe atrophy (Gorno-Tempini et al., 2004; Rogalski et al., 2011). DTI studies consistently show disruption in anterior and inferior temporal white matter tracts, including the inferior longitudinal fasciculi and uncinate fasciculi (Agosta et al., 2015, 2012; Lam et al., 2014; Mahoney et al., 2013; Schwindt et al., 2013; Tu et al., 2015; Zhang et al., 2013).
Task-free fMRI studies in healthy individuals show that the anterior temporal lobe has strong intrinsic connectivity to the anterior cingulate, orbitofrontal cortex, frontoinsula, striatum and thalamus (Guo et al., 2013; Seeley et al., 2009). Patients with svPPA also show disrupted connectivity within this anterior temporal network, also known as the semantic appraisal network, which processes semantic stimuli across various modalities (Agosta et al., 2014b; Guo et al., 2013).
Mirroring gray matter atrophy patterns, [18F]FDG-PET in svPPA typically features asymmetrical bilateral temporal hypometabolism (Cerami et al., 2017).
2.1.3. nfvPPA
In contrast to svPPA, the hallmarks of nfvPPA include effortful speech production and agrammatism with preservation of object knowledge and single-word comprehension (GornoTempini et al., 2011; Grossman et al., 1996; Hodges and Patterson, 1996; Snowden et al., 1989). The left posterior frontoinsula and the left inferior frontal gyrus usually show the most profound atrophy (Gorno-Tempini et al., 2011, 2004). Left orbitofrontal and intrafrontal tracts, the superior longitudinal fasciculus and frontostriatal pathways are involved (Agosta et al., 2015, 2012; Lam et al., 2014; Mahoney et al., 2013; Mandelli et al., 2014; Schwindt et al., 2013; Zhang et al., 2013).
In healthy individuals, the inferior frontal gyrus is intrinsically connected to frontal operculum, middle frontal gyrus, primary and supplementary motor cortices, and inferior parietal lobule, a network of regions critical for language and motor speech fluency (Battistella et al., 2020; Seeley et al., 2009), and patients with nfvPPA show atrophy and reduced connectivity with these regions. [18F]FDG-PET shows hypometabolism reported in left inferior frontal gyrus, dorsolateral frontal cortex, anterior cingulate and insula (Cerami et al., 2017; Mesulam, 2003).
2.2. ALS
ALS is a progressive motor neuron disease that causes degeneration in upper and lower motor neurons. Clinical heterogeneity exists with respect to the site of onset, the degree of upper and lower motor neuron involvement, the rate of progression, and cognitive and behavioral symptoms (Strong et al., 2017). Patients with ALS have clinical overlap with FTD. Most patients develop some degree of behavioral and cognitive impairment with disease progression (Crockford et al., 2018; Lomen-Hoerth et al., 2002), with 15% of patients meeting clinical criteria for FTD (Ringholz et al., 2005).
Clinically, MRI has been used in ALS to exclude mimics of motor neuron disease, such as nerve root compression and neoplastic, vascular, and demyelinating diseases of the brain and spinal cord. Typically, conventional MRI shows mild or absent gray matter atrophy (Bede and Hardiman, 2018; Grieve et al., 2016; Walhout et al., 2015b). Upon visual assessment of the corticospinal tract or precentral gyrus, hypointensities on T2-weighted, fluid-attenuated inversion recovery or proton density sequences are variably noted, yet not adequately sensitive or specific to confirm a diagnosis of ALS (Filippi et al., 2010).
Quantitative MRI approaches such as voxel-based morphometry show that patients with ALS have atrophy in the precentral gyrus and inferior frontal cortex (Grosskreutz et al., 2006; Mezzapesa et al., 2013; Shen et al., 2016) with subcortical regions such as the striatum, thalamus and the cerebellum also targeted (Bede et al., 2013c; Bede and Hardiman, 2018; Menke et al., 2014; Westeneng et al., 2015). Cervical and upper thoracic spinal cord atrophy is associated with clinical severity and disease duration (El Mendili et al., 2014; Valsasina et al., 2006) and progressive longitudinal changes are detectable (Agosta et al., 2009; El Mendili et al., 2014). Those patients with bulbar versus limb onset show the expected corresponding atrophy within primary motor cortex (Bede et al., 2013a). Patients with ALS and FTD harbor more widespread frontotemporal atrophy (Lillo et al., 2012; Masuda et al., 2016; Omer et al., 2017).
Reflecting the disruption to upper motor neuron axons in ALS, diffusion MRI studies consistently revealed microstructural changes along the corticospinal tracts and in mid and posterior corpus callosum (Fig. 3; Bede and Hardiman, 2018; Broad et al., 2019; Müller et al., 2016; van der Graaff et al., 2011). Patients with primary lateral sclerosis, which involves upper motor neurons but not lower motor neurons, have more pronounced white matter disruption compared to patients with ALS (Agosta et al., 2014a). Studies of patients with lower motor neuron predominant presentations may also show extensive white matter disruption, suggesting that DTI may be capturing subclinical upper motor neuron disease in these patients (Müller et al., 2018). Patients with ALS who also feature behavioral or cognitive symptoms, including patients with frank bvFTD, additionally show white matter involvement in frontotemporal tracts (Agosta et al., 2016; Lillo et al., 2012; Omer et al., 2017; Spinelli et al., 2016; Trojsi et al., 2013).
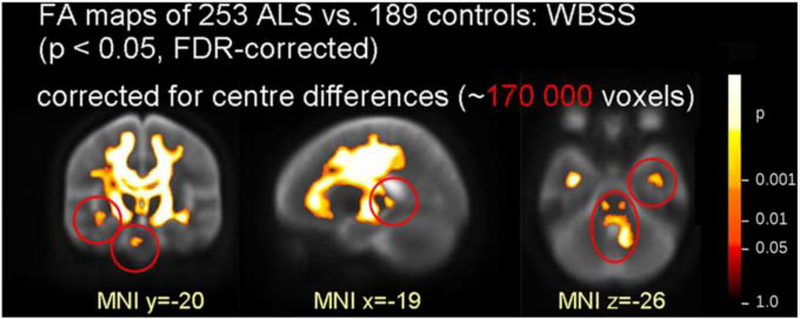
Fig. 3.
White-matter involvement in sporadic ALS revealed by a multi-site DTI study. FA decreases were found along the corticospinal tracts, frontal lobe, brainstem and hippocampi. Red circles indicate deficits only significant with correction for site differences. ALS, amyotrophic lateral sclerosis; WBSS, whole-brain-based spatial statistics; FA, fractional anisotropy; FDR, false discovery rate. Adapted from Müller et al. (2016) with permission from BMJ Publishing Group Ltd.
In task-free fMRI, the most consistent intrinsic connectivity network alteration is decreased sensorimotor network connectivity (Agosta et al., 2013; Douaud et al., 2011; Tedeschi et al., 2012; Trojsi et al., 2015). Most whole-brain investigations have additionally discovered connectivity decreases or increases between motor and non-motor regions and within extra-motor networks such as salience, default mode, and frontoparietal networks (Agosta et al., 2013; Menke et al., 2018; Mohammadi et al., 2009; Qiu et al., 2019; Tedeschi et al., 2012; Trojsi et al., 2015). Similary, task-based fMRI studies have highlighted additional recruitment of non-motor brain regions during motor tasks in patients with ALS compared to controls (Abidi et al., 2019; Konrad et al., 2006; Poujois et al., 2013; Schoenfeld et al., 2005; Stanton et al., 2007). This functional reorganization is commonly interpreted to result from both degenerative and compensatory mechanisms.
Extra-motor involvement in ALS has been long suggested by PET studies, which show hypometabolism in non-motor cortex (Dalakas et al., 1987; Hatazawa et al., 1988; Kew et al., 1993). More recently, larger [18F]FDG PET studies have converged on hypometabolism within the premotor and frontal cortices (Buhour et al., 2017b; Cistaro et al., 2012; Pagani et al., 2014; Van Laere et al., 2014). Several studies have also observed increased [18F]FDG uptake, particularly in the brainstem (Cistaro et al., 2012; Pagani et al., 2014; Van Laere et al., 2014) and cervical spinal cord (Marini et al., 2016; Yamashita et al., 2017) of ALS patients, hypothesized to be due to glial cells surrounding degenerating neurons. Increased uptake of PET tracers that are associated with microglial activation and oxidative stress and decreased binding of GABAergic, serotonergic, and dopaminergic ligands has also been reported (Fu et al., 2017; Ikawa et al., 2015; Lloyd et al., 2000; Turner, 2005; Turner et al., 2005).
1H MRS studies have revealed decreased NAA (an estimate of neuronal density) and, less consistently, low concentrations of myoinositol, glutamate, glutamine, and GABA along the corticospinal tract, posterior limb of the internal capsule, periventricular white matter (Atassi et al., 2017; Cheong et al., 2017; Pyra et al., 2010; Westeneng et al., 2017). NAA and myoinositol levels also correlate with measures of clinical severity in cervical spinal cord (Carew et al., 2011b; Ikeda et al., 2013) and prefrontal cortex (Hanstock et al., 2020).
Despite these advances, neuroimaging features alone are not able to diagnose bvFTD, PPA or ALS and instead are supportive in rendering clinical diagnoses. For bvFTD, frontal and/or anterior temporal atrophy on MRI or CT and/or hypoperfusion on PET and SPECT in these regions are supportive criteria in concert with symptoms, neuropsychological testing and functional assessment (Rascovsky et al., 2011). Similarly, diagnostic criteria for PPA also incorporate imaging features. Predominant left posterior fronto-insular atrophy on MRI or predominant left posterior fronto-insular hypoperfusion or hypometabolism on SPECT or PET supports the diagnosis of nfvPPA (Gorno-Tempini et al., 2011). Predominant anterior temporal lobe atrophy (MRI) and/or hypoperfusion (SPECT/PET) support a diagnosis of svPPA. For ALS, neuroimaging studies are currently used only to exclude other conditions that may cause upper or lower neuron signs that mimic ALS (Brooks et al., 2000).
3. NEUROIMAGING STUDIES OF GENETIC MUTATIONS FOR FTD AND ALS
3.1. Genetic mutations for FTD and ALS: a brief overview
In 1994, autosomal dominant inheritance was first identified in a family with FTD and parkinsonism which was linked to chromosome 17q21.2 (Lynch et al., 1994) and the causative genetic mutation was identified as the microtubule protein-associated tau (MAPT) (Clark et al., 1998; Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998), which plays a role in microtubule stabilization and assembly. In 2006, the second autosomal dominant mutation for FTD was identified as GRN, a mere 6.2 MB away from MAPT (Baker et al., 2006). GRN encodes for progranulin, a protein that is ubiquitously expressed and involved in wound repair, inflammation and lysosomal function (Kao et al., 2017; Petkau and Leavitt, 2014).
For ALS, the first autosomal dominant mutation was discovered in a cytosolic, Cu/Zn-binding superoxide dismutase (SOD1). Although the clinical overlap between FTD and ALS syndromes had been noted as early as the 1980s, the discovery of rare genetic mutations such as CHMP2B (Gydesen et al., 2002), TARDBP (Sreedharan et al., 2008), FUS (Kwiatkowski et al., 2009; Sreedharan et al., 2008), and VCP (Johnson et al., 2010) that cause either FTD, ALS or both syndromes united these two syndromes based on their underlying pathobiology. In 2011, a hexanucleotide expansion in the C9orf72 gene was discovered as the most common cause of familial and FTD and ALS (DeJesus-Hernandez et al., 2011; Renton et al., 2011). For ALS, the C9orf72 expansion and SOD1 mutations account for the majority of familial ALS in Caucasian populations, causing 30 to 40 percent and 15 to 20 percent of cases, respectively (Renton et al., 2014). Since the discovery of the C9orf72 expansion, other rare mutations that cause either FTD and/or ALS have been identified, including SQSTM1 (Fecto, 2011), CHCHD10 (Bannwarth et al., 2014), and TBK1 (Cirulli et al., 2015; Freischmidt et al., 2015).
3.2. Genetic FTD
3.2.1. MAPT
MAPT mutations typically cause bvFTD with or without parkinsonism. Some patients with parkinsonism meet criteria for progressive supranuclear palsy (PSP) or corticobasal syndrome (CBS) (Ghetti et al., 2011). Less commonly, MAPT mutations have been identified in patients with primary progressive aphasia (PPA) (Munoz et al., 2007; Rohrer et al., 2009b) and, rarely, ALS (Karch et al., 2018; Origone et al., 2018). Over 50 pathogenetic mutations in MAPT have been described (Greaves and Rohrer, 2019), yet the rarity of MAPT mutations in the population and clinical heterogeneity found even within families carrying the same MAPT mutation (Janssen et al., 2002; van Herpen et al., 2003; Van Swieten et al., 1999) has made it challenging to ascertain genotype-phenotype correlations. Nevertheless, mutations that do not affect the splicing of exon 10 are typically associated with the bvFTD phenotype, while mutations that affect exon 10 splicing and increase the ratio of four repeat (4R) tau to three repeat (3R) tau most frequently cause bvFTD with PSP or parkinsonism (Delisle et al., 1999; Iijima et al., 1999; Skoglund et al., 2008).
Structural neuroimaging
Due to the rarity of MAPT mutations, most neuroimaging-studies have examined MAPT-FTD cohorts with a mixture of mutation subtypes. MAPT-related neuroimaging studies are summarized in Supplementary Table S1. In general, MAPT-FTD features frontotemporal atrophy similar to sporadic disease, yet atrophy is typically relatively symmetric and most prominent within anterior and mesial temporal lobes, while involvement of orbitofrontal, lateral prefrontal, and parietal regions is less consistent (Fig. 4; Beck et al., 2008; Boeve, 2005; Cash et al., 2018; Deters et al., 2014; Fumagalli et al., 2018; Olney et al., 2020; Rohrer et al., 2010, 2011a; Whitwell et al., 2009a, 2009b, 2012). A distinctive feature of MAPT-FTD is pronounced mesial temporal lobe atrophy (Deters et al., 2014; Olney et al., 2020; Rohrer et al., 2010; Whitwell et al., 2009a), associated with correspondingly greater memory impairment than seen in sporadic bvFTD (Rascovsky et al., 2011; Rohrer and Warren, 2011; Ygland et al., 2018). Longitudinal studies suggest that atrophy progresses symmetrically within the regions atrophied at baseline, namely the anteromedial temporal lobes and orbitofrontal cortex (Rohrer et al., 2010; Whitwell et al., 2015). Different MAPT mutation subtypes may be associated with distinct atrophy patterns (Ghetti et al., 2015; Whitwell et al., 2009a), yet it remains unknown whether MAPT mutations that share pathophysiological mechanisms selectively target specific neuroanatomical regions.
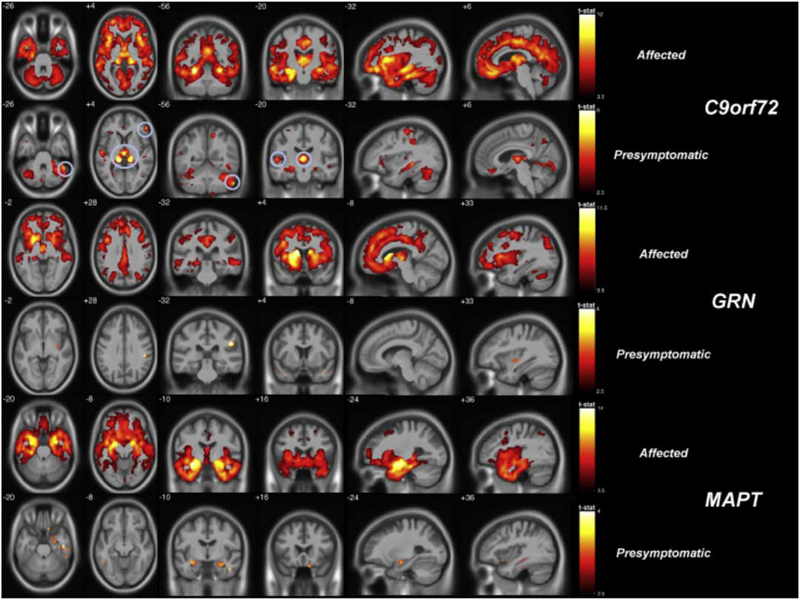
Fig. 4.
Gray matter deficits in FTD mutation carriers measured by voxel-based morphometry. In both affected (symptomatic) and presymptomatic stages of disease, carriers of the C9orf72 expansion, MAPT and GRN mutations each exhibited distinct atrophy patterns with overlap in frontotemporal cortex and insula. Both C9orf72 and GRN feature parieto-occipital atrophy in addition to frontotemporal atrophy, while MAPT targets the mesial temporal lobe. In the presymptomatic C9orf72 group, circles indicate significantly low gray matter volume in the thalamus. Maps illustrate brain regions with reduced grey matter probability in affected (p < 0.05 FWE-corrected) and presymptomatic (p < 0.001 uncorrected) carriers compared to noncarriers. FTD, frontotemporal dementia; FWE, familywise error rate. Reprinted from Cash et al. (2018).
Early studies with smaller cohorts suggested that presymptomatic MAPT mutation carriers may lack gray matter volume differences (Dopper et al., 2014; Whitwell et al., 2011). In contrast, studies with larger cohorts have shown that presymptomatic carriers have subtle gray matter deficits (Cash et al., 2018; Domínguez-Vivero et al., 2020; Fumagalli et al., 2018; Panman et al., 2019; Rohrer et al., 2015) in temporal lobe, cingulate and lingual cortices (Domínguez-Vivero et al., 2020) as well as hippocampi and amygdala (Rohrer et al., 2015). These mixed results across studies could be attributable to differences in cohort size or subject heterogeneity with respect to actual time from symptom onset. Yet, even in presymptomatic carriers with no gray matter deficits at baseline, greater longitudinal temporal lobe (Chen et al., 2019b) and hippocampal (Panman et al., 2019) grey matter volume decline compared to noncarriers has been reported. In 5 presymptomatic carriers converting to bvFTD, declines in frontal and temporal volume appeared around 2 years before symptom onset (Jiskoot et al., 2019). These studies suggest that the characteristic mesial temporal atrophy of MAPT mutations may also be detectable in presymptomatic carriers, but further studies are needed to determine this relationship.
Cross-sectional and longitudinal DTI studies have revealed that in MAPT-FTD the most prominent white matter deficits appear within entorhinal white matter, limbic tracts, and frontotemporal tracts such as the left uncinate fasciculus (Chen et al., 2019a; Jiskoot et al., 2018; Mahoney et al., 2015, 2014). Similarly, reduced white matter integrity arises in presymptomatic carriers principally within frontotemporal tracts such as the uncinate fasciculus and parahippocampal cingulum bundle (Chen et al., 2019a; Dopper et al., 2014; Jiskoot et al., 2018; Panman et al., 2019; Rohrer et al., 2015). White matter deficits are estimated to appear as early as 20–30 years before estimated onset and may predate low gray matter volume change during the presymptomatic phase (Dopper et al., 2014; Greaves and Rohrer, 2019; Jiskoot et al., 2018).
Functional and molecular neuroimaging
Studies have probed the question of whether brain function as measured by task-free fMRI, [18F]FDG-PET, and 1H MRS may be more sensitive to early-stage or preclinical deficits. One task-free fMRI connectivity study of 9 presymptomatic MAPT mutation carriers showed no network connectivity alterations (Dopper et al., 2014), while another study of 8 carriers showed regions with increased and decreased default mode network connectivity compared to noncarriers (Whitwell et al., 2011). Both of these studies had small cohorts of MAPT carriers which may account for disparate results. Compared to healthy controls, patients with MAPT-FTD have reduced [18F]FDG uptake (Deters et al., 2014) or hypoperfusion (Seelaar et al., 2011) in frontal, temporal, and parietal lobes. Presymptomatic metabolic changes have been elusive with studies reporting either negative findings (Dopper et al., 2016; Mutsaerts et al., 2019) or hypometabolism in a medial temporal ROI (Deters et al., 2014). The disparate results may suggest that hypometabolism is not a prominent early imaging finding across all carriers or MAPT mutational variants, but hypometabolism has been found in two presymptomatic carriers known to have converted soon after imaging (Arvanitakis et al., 2007; Dopper et al., 2016).
Tau PET tracers such as [18F]AV 1451 and [11C]PBB3 have been less promising in FTD, since off-target binding has led to false-positive tau positivity in subjects who are unlikely to harbor tau neuropathology (Tsai et al., 2019; Wang and Edison, 2019). [18F]AV-1451, however, has shown strong binding to neurofibrillary tangles matching the pattern of paired helical filament (PHF) immunochemistry in a subset of MAPT mutations such as V337M and R406W (Jones et al., 2018; Smith et al., 2016; Tsai et al., 2019). Other PET studies have reported high levels of microglial activation in MAPT-FTD postmortem (Lant et al., 2014) and dopaminergic dysfunction and glial activation in presymptomatic carriers (Bevan-Jones et al., 2019; Miyoshi et al., 2010; Wu et al., 2018). In 1H MRS, patients with MAPT-FTD show abnormalities in the posterior cingulate and medial frontal cortex, such as decreased NAA/Cr and increased mI/Cr ratios in line with neuronal dysfunction and inflammation, respectively (Chen et al., 2019c; Kantarci et al., 2010), with abnormalities in medial frontal regions observed even presymptomatically (Chen et al., 2019d). In contrast, the posterior cingulate has increases in mI/Cr but normal NAA/Cr, which may reflect a period of reactive astrocytosis in MAPT-related degeneration (Chen et al., 2019d; Kantarci et al., 2010).
3.2.2. GRN
Mutations in the GRN gene cause missense and premature termination codons in GRN mRNA that are degraded by nonsense-mediated decay, which results in a haploinsufficiency of progranulin protein (Baker et al., 2006; Cruts et al., 2006), most commonly leading to bvFTD and less frequently PPA, CBS or an Alzheimer’s-like amnestic syndrome (Le Ber et al., 2008). FTD-ALS has been reported infrequently in association with GRN mutations, and pure ALS is rare (Chen-Plotkin et al., 2011; Yu et al., 2010). Interestingly, GRN mutation carriers show the most clinical heterogeneity among the common FTD mutations, despite the fact that the 79 GRN mutations identified to date all cause a haploinsuffiency of progranulin which has been hypothesized to be the underlying cause of neurodegeneration. GRN mutation carriers also show incomplete disease penetrance, with 90% of carriers becoming symptomatic by age 70 (Cruts et al., 2006). TDP-43 type A neuropathology is most commonly found at autopsy (Beck et al., 2008; Mackenzie et al., 2006), yet the mechanisms by which progranulin deficiency lead to TDP pathology remain unknown.
Structural neuroimaging
GRN-related neuroimaging findings are summarized in Supplementary Table S2. Patients with FTD due to GRN mutations may have markedly asymmetric cortical atrophy that involves frontotemporal but also parietal cortices (Fig. 4; Beck et al., 2008; Borroni et al., 2012; Bozzali et al., 2013; Cash et al., 2018; Fumagalli et al., 2018; Le Ber et al., 2008; Olm et al., 2018; Premi et al., 2016; Rohrer et al., 2010; Whitwell et al., 2015, 2012, 2009b, 2007a). Longitudinal studies suggest that atrophy in most brain regions is faster in FTD due to GRN mutations than atrophy in MAPT mutations and the C9orf72 expansion (Whitwell et al., 2015). After symptom onset, atrophy accelerates particularly in the temporal cortex (Chen et al., 2020) and grows more asymmetrical in the later stages of disease (Rohrer et al., 2010)
Many studies using voxel-based morphometry or cortical thickness measurements in presymptomatic GRN mutation carriers have found no detectable abnormalities in gray matter (Borroni et al., 2012, 2008; Fumagalli et al., 2018; Jacova et al., 2013; Lee et al., 2019; Moreno et al., 2013; Panman et al., 2019; Popuri et al., 2018). Other studies of presymptomatic carriers have identified low cortical grey matter volume (Cash et al., 2018; Cruchaga et al., 2009; Dopper et al., 2014; Olm et al., 2018; Olney et al., 2020; Rohrer et al., 2015), thickness (Pievani et al., 2014), and morphological abnormalities (Gazzina et al., 2018). Three longitudinal studies in presymptomatic carriers show converging evidence of volumetric loss in frontal cortex, though in contrast to studies of symptomatic carriers, insular, temporal and parietal involvement is less consistent (Caroppo et al., 2015b; Chen et al., 2020; Olm et al., 2018). In general, the studies that report low gray matter volume analyzed larger subject cohorts, suggesting that gray matter deficits may be detectable, yet subtle during the presymptomatic stage. Overall, the literature suggests that presymptomatic carriers may have targeted regions of gray matter deficits, but when present, these are mild. The mixed results across these studies may be attributable to heterogeneity across study cohorts with respect to subjects’ age of future symptom onset and clinical syndrome or targeted neuroanatomy.
White matter hyperintensities are uncommon in FTD, yet several studies have reported white matter hyperintensities in some patients with GRN-FTD (Ameur et al., 2016; Caroppo et al., 2014; Le Ber et al., 2008; Paternicò et al., 2016; Sudre et al., 2017). Recent studies have revealed an association between longitudinal accumulation of white matter hyperintensities and atrophy and executive deficits in patients with GRN-FTD (Sudre et al., 2019) and also found white matter hyperintensities in presymptomatic carriers (Benussi et al., 2019; Sudre et al., 2019). A report of a patient with GRN-FTD with severe white matter hyperintensities showed that white matter disease on MRI scans (in vivo and cadaveric) corresponded to promient microglial activation and microglial dystrophy at autopsy, but only mild axonal loss and minimal vascular pathology, supporting the notion that white matter hyperintensities in GRN-FTD are not due to small vessel cerebrovascular disease (Woollacott et al., 2018).
DTI studies show that white matter tracts such as the inferior longitudinal and uncinate fasciculi, anterior corpus callosum, and the long intrahemispheric association tracts have reduced integrity in GRN-FTD (Bozzali et al., 2013; Premi et al., 2016; Rohrer et al., 2010). Across various cross-sectional DTI studies, presymptomatic GRN mutation carriers also show diminished white matter integrity within tracts affected during the symptomatic phase. These tracts include the superior longitudinal and uncinate fasciculi, the corticospinal tract, and anterior corpus callosum (Borroni et al., 2008; Dopper et al., 2014; Jiskoot et al., 2018; Olm et al., 2018; Pievani et al., 2014). Differences in white matter integrity have been proposed to develop 10 years before symptom onset (Jiskoot et al., 2018). Longitudinal DTI studies in presymptomatic carriers have been mixed, either showing no change (Panman et al., 2019) or a greater annualized FA reduction in the right superior longitudinal fasciculus and frontal corpus callosum (Olm et al., 2018). One possible explanation is that differences across studies could be attributable to subject heterogeneity, and subjects either farther from or closer to symptom onset could be driving the overall group result in each study.
Functional and molecular neuroimaging
The first studies that identified task-free fMRI connectivity alterations in presymptomatic FTD mutation carriers explored salience and default mode networks, which show prominent alterations in patients with sporadic FTD. For the salience network, a region of reduced connectivity was found in the midcingulate cortex in 9 presymptomatic GRN mutation carriers (Borroni et al., 2012), while another study of 28 presymptomatic GRN mutation carriers revealed connectivity disruption of an anterior midcingulate seed region and parietal regions including precuneus, posterior cingulate, and lateral parietal cortex (Dopper et al., 2014). Neither of these studies showed default mode network differences in presymptomatic carriers compared to controls. A study of 5 carriers showed no differences within functional networks (Pievani et al., 2014), suggesting that the differences across studies are due to different sample sizes, methodological differences, and/or subject heterogeneity. Recently, Lee et al. (2019) showed that presymptomatic carriers have hyperconnectivity within the four networks that correspond to the most common clinical syndromes reported during the symptomatic stage of GRN, which included salience network for bvFTD, nfvPPA and CBS networks, and the default mode network for AD. For each four networks, hyperconnectivity converged in the thalamus, paralleling the finding in GRN −/− mice that the thalamus is a key region implicated in GRN pathobiology (Lui et al., 2016).
In addition to these studies examining seed-based and ICA-derived connectivity network alterations, presymptomatic carriers and patients with FTD due to GRN mutations have shown alterations in measures of local connectivity (Premi et al., 2014). Another study compared the ability of various imaging modalities such as gray and white matter volume loss, ICA-derived connectivity networks (salience, frontoparietal, dorsal attentional, executive, and default mode), and local connectivity measures to classify GRN-FTD and presymptomatic GRN versus controls (Premi et al., 2016). Overall, reduced gray matter volume was able to best distinguish patients with GRN-FTD from controls, while decreases in local fMRI connectivity (fractional amplitude of low frequency fluctuations) in frontoparietal cortex and increases in local connectivity in prefrontal areas most accurately distinguished presymptomatic carriers from controls. Early involvement of the prefrontal cortex in GRN related disease is also supported by task-based fMRI studies (Alexander et al., 2018; Cruchaga et al., 2009).
Metabolic imaging by [18F]FDG-PET and arterial spin labeling (ASL) MRI have revealed frontotemporal hypometabolism and hypoperfusion in GRN mutation carriers, even in presymptomatic carriers that show no significant differences in brain volume. Patients with GRN-FTD have [18F]FDG-PET hypometabolism in frontotemporal and parietal cortices, corresponding to regional atrophy patterns seen in structural imaging (Cruchaga et al., 2009; Huey et al., 2006). Similar to symptomatic carriers, group studies of presymptomatic carriers show discrete regions of hypometabolism appear in right anterior cingulate, insula and orbitofrontal cortex (Jacova et al., 2013), left lateral temporal lobe (Caroppo et al., 2015b), and frontal, parietal, and hippocampal regions (Le Ber et al., 2008). Longitudinally, more pronounced hypometabolism has been found within regions of lateral temporal and frontal cortex (Caroppo et al., 2015b). For ASL, presymptomatic GRN mutation carriers have shown hypoperfusion cross-sectionally in frontoparietal cortex, and longitudinally in frontal, temporal, parietal, and subcortical regions (Dopper et al., 2016). Across studies, metabolic changes are estimated to emerge 7–25 years before symptom onset (Alexander et al., 2019; Caroppo et al., 2015b; Jacova et al., 2013).
GRN-FTD is typically associated with TDP-43 type A pathology. While there is no molecular imaging available for TDP to date, Alzheimer’s co-pathology has been described in GRN mutation cariers (Perry et al., 2013). Consistent with this report, GRN-FTD shows amyloid beta accumulation as measured by [11C]PiB-PET more frequently than seen in sporadic disease and MAPT and C9orf72 expansion carriers (Tan et al., 2017).
The hexanucleotide expansion in C9orf72 is the most common cause of genetic FTD and ALS and neuroimaging studies are discussed in section 3.4.1 below.
3.3. Genetic ALS
3.3.1. SOD1
To date, more than 180 mutations in superoxide dismutase (SOD1) have been identified. Superoxide dismutase is an abundant enzyme ubiquitously expressed in the body and its main function is to bind copper and zinc to eliminate toxic superoxide radicals that cause oxidative stress. While it remains unknown how SOD1 mutations lead to ALS, the mutations cause a toxic gain of function (Rothstein, 2009). The neuropathological hallmark of SOD1-ALS is the deposition of misfolded SOD1 protein inclusions in motor neurons (Saberi et al., 2015), and curiously, the TDP-43 pathology that is characteristic of sporadic ALS is absent (Mackenzie et al., 2007). Co-occurrence of FTD in SOD1-ALS is rare (Millecamps et al., 2012) but cognitive impairment, principally executive dysfunction, is seen in SOD1-ALS as in sporadic ALS (Agosta et al., 2018). SOD1 variants show phenotypic heterogeneity. For example, D90A carriers have a markedly long disease duration ranging between 14–20 years in contrast to the 2–5 years typical of sporadic ALS (Andersen et al., 1996; Weber et al., 2000), while the A4V mutation is typically associated with death within a year of symptom onset (Aggarwal and Nicholson, 2005; Cudkowicz et al., 1997).
SOD1-related neuroimaging studies are summarized in Supplementary Table S3. As in genetic FTD, most neuroimaging studies have investigated different SOD1 mutations in a combined cohort, although some have focused exclusively on D90A homozygous recessive carriers. The earliest structural imaging studies focused on D90A SOD1 mutation carriers with ALS and compared them to sporadic ALS. While both D90A SOD1 and sporadic ALS have atrophy in motor and premotor cortex, D90A SOD1-ALS showed more prominent atrophy in anteromedial frontal cortex (Turner et al., 2007a). Patients with D90A SOD1-ALS show a lesser degree of corticospinal tract deficits than sporadic patients with similar disease severity (Blain et al., 2011; Stanton et al., 2009) as measured by DTI fractional anisotropy. PET imaging of GABAergic [11C]flumazenil (Turner et al., 2005) and serotonergic [11C]WAY100635 (Turner et al., 2007b) ligands have shown that both sporadic and D90A SOD1-ALS patients have reduced binding compared to controls. Interestingly, sporadic ALS had reduced binding of GABA in premotor and motor cortex and posterior motor association areas, while D90A SOD1-ALS was associated with lower binding in the left frontotemporal junction and anterior cingulate.
A multimodal imaging study with a larger cohort of 20 patients with SOD1-ALS, most of whom carried the L144F mutation, also found that SOD1-ALS showed distinct patterns of corticospinal tract and sensorimotor network functional connectivity compared with sporadic ALS despite no group differences on manual muscle testing (Agosta et al., 2018). Both sporadic ALS and SOD1-ALS showed decreased corticospinal tract white matter integrity compared to controls, but corticospinal tract integrity was more preserved in SOD1-ALS compared to sporadic ALS. Another DTI study, which included a wider range of SOD1 mutations, did not find significant FA differences in the corticospinal tract and frontal and prefrontal tracts in SOD1-ALS patients compared to controls (Müller et al., 2020). Additionally, while sporadic ALS has been shown to be associated with alterations in sensorimotor network connectivity, Agosta et al. (2018) found that patients with SOD1-ALS had sensorimotor network connectivity similar to controls, but cervical cord atrophy greater than seen in sporadic disease. Overall, these DTI and task-free fMRI studies suggest that SOD1-ALS shows relatively spared motor networks and corticospinal tract integrity compared to sporadic disease, but with greater cervical cord atrophy which may be a distinguishing characteristic of SOD1-ALS. The relative sparing of motor networks may reflect the milder motor cortex and corticospinal tract involvement reported in certain patients with SOD1-ALS at autopsy when compared to sporadic ALS (Cudkowicz et al., 1998; Ince et al., 1998).
During the presymptomatic phase, SOD1 carriers do not typically show structural brain deficits (Menke et al., 2016; Vucic et al., 2010), but one DTI study showed reduced integrity in the posterior limb of the internal capsule (Ng et al., 2008). Consistent with the notion that SOD1-ALS targets the cervical cord, presymptomatic SOD1 carriers show in 1H MRS reduced NAA/Cho and NAA/mIns ratios in the cervical spinal cord relative to healthy controls (Carew et al., 2011a). Echoing the finding that SOD1-ALS spares cortical motor networks, an fMRI functional connectivity study found no sensorimotor network alterations in presymptomatic SOD1 carriers, although they showed increased precuneus-cingulate-middle frontal network connectivity as did the sporadic ALS patients in their study (Menke et al., 2016).
3.4. Mutations that cause either FTD or ALS, or both
The C9orf72 expansion is the most common known genetic cause of FTD and ALS in people with Northern European ancestry. CHMP2B, TARDBP, FUS, VCP, SQSTM1, TBK1, CHCHD10, are all rare genes that may cause FTD or ALS.
3.4.1. C9orf72
Abnormal G4C2 hexanucleotide repeats in either the promoter region or the first intron of chromosome 9 open reading frame 72 (C9orf72) usually manifest as bvFTD, ALS, or both (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Less commonly, C9orf72 expansion carriers may have clinical phenotypes such as an Alzheimer’s disease-like syndrome, a Huntington’s disease-like syndrome, progressive muscular atrophy, or corticobasal or ataxic syndromes (Anor et al., 2015; Lesage et al., 2013; Lindquist et al., 2013; Liu et al., 2014). Although the function of the C9orf72 protein remains unclear, three proposed pathophysiological mechanisms include haploinsufficiency of C9orf72 protein, toxic gain-of-function due to the accumulation of aberrant RNA foci (DeJesus-Hernandez et al., 2011) and dipeptide repeat proteins both derived from the repeat expansion (Mori et al., 2013). Diverse neuropathological features have been described, but in addition to RNA foci and the dipeptide repeat proteins, TDP-43 type B pathology is most common at autopsy (Mackenzie et al., 2013; Murray et al., 2011; Snowden et al., 2012).
The age of onset in C9orf72-related FTD and ALS ranges widely between 30 to 90 years (Murphy et al., 2017). Similar to sporadic FTD, C9orf72-FTD has an average disease duration of 14 years (Kaivorinne et al., 2013), but with a subset of cases progressing considerably slower (Devenney et al., 2014; Gómez-Tortosa et al., 2014; Khan et al., 2012; Llamas-Velasco et al., 2018). C9orf72-ALS tends to progress more rapidly than sporadic and most other genetic ALS subtypes (Cooper-Knock et al., 2012; DeJesus-Hernandez et al., 2011; Millecamps et al., 2012). In FTD, the expansion is associated with an increased risk of psychosis, hallucinations, and parkinsonism (Cooper-Knock et al., 2012; Snowden et al., 2012). ALS patients with the C9orf72 expansion have a three times higher frequency of cognitive impairment compared to those with sporadic ALS (Byrne et al., 2012; Smith et al., 2013). Factors such as the size of the repeat expansion, environmental influences, and disease-modifying genes have been speculative and are not clearly established (Chi et al., 2016).
Structural neuroimaging
Neuroimaging studies related to the C9orf72 expansion are summarized in Supplementary Table S4. Similar to sporadic bvFTD, patients with bvFTD due to the C9orf72 expansion have frontotemporal and insular atrophy that is often accompanied by parietal, occipital, thalamic, and cerebellar atrophy (Fig. 4; Boeve et al., 2012; Boxer et al., 2011; Cash et al., 2018; Irwin et al., 2013; Lee et al., 2014; Mahoney et al., 2012a; McMillan et al., 2015; Sha et al., 2012; Whitwell et al., 2012). Cortical atrophy is generally symmetric, and longitudinal changes are most apparent in core regions affected at baseline, such as frontotemporal cortex, thalamus and cerebellum (Boeve et al., 2012; Floeter et al., 2016; Le Blanc et al., 2020; Mahoney et al., 2012b; Whitwell et al., 2015). Individual patients have notable heterogeneity, at times exhibiting minimal atrophy (Boeve et al., 2012; Devenney et al., 2015, 2014; Solje et al., 2015) or a more posterior pattern with relatively spared frontal or temporal lobes (Boeve et al., 2012; Boxer et al., 2011; Sha et al., 2012). C9orf72 expansion carriers also show heterogeneity at the individual subject level with respect to which brain regions are most prominently targeted. For example, a recent data-driven modelling study suggested that C9orf72-FTD features two distinct subtypes of atrophy patterns, either predominantly frontotemporal atrophy or predominantly subcortical atrophy (Young et al., 2018).
C9orf72 expansion carriers with ALS or MND typically exhibit relatively symmetric volume loss and cortical thinning. C9orf72-ALS appears clinically similar to sporadic ALS, yet gray matter volume loss is pronounced in expansion carriers, particularly in frontotemporal and parieto-occipital cortex (Agosta et al., 2017; Bede et al., 2013b; Byrne et al., 2012; Floeter et al., 2016; van der Burgh et al., 2020; Westeneng et al., 2016) and in subcortical structures such as the thalamus, cerebellum, and hippocampi (Agosta et al., 2017; Bede et al., 2013c; Schönecker et al., 2018; van der Burgh et al., 2020; Westeneng et al., 2016). These cortical deficits correlate with the cognitive impairment in C9orf72-ALS (Bede et al., 2013b; Floeter et al., 2016). In parallel, frontotemporal and insular changes have been found to be associated with cognitive and behavioral impairments in sporadic ALS (Agosta et al., 2017, 2016; Christidi et al., 2018; Consonni et al., 2019; Tsujimoto et al., 2011; Westeneng et al., 2016). In addition to these cortical and subcortical volume differences, C9orf72-ALS do not have extensive cervical cord thinning compared to sporadic ALS (van der Burgh et al., 2019).
Patients with C9orf72 with both FTD and ALS show a lesser degree of cortical atrophy compared with sporadic FTD-ALS (Omer et al., 2017) and a distinct pattern of subcortical atrophy mainly in thalamic nuclei connected to motor and sensory cortical areas (Bede et al., 2018). One hypothesis is that abnormalities in subcortical brain structures such as the thalamus and cerebellum may be a more robust imaging signature of the C9orf72 expansion than cortical atrophy patterns (Mahoney et al., 2012b, 2012a). Low gray matter volume is detectable in presymptomatic C9orf72 expansion carriers, generally within regions atrophied in C9orf72-bvFTD and C9orf72-ALS (Bertrand et al., 2018; Cash et al., 2018; De Vocht et al., 2020; Lee et al., 2017; Panman et al., 2019; Papma et al., 2017; Popuri et al., 2018; Rohrer et al., 2015; Walhout et al., 2015a). In particular, atrophy of the posterior thalamus correlates with disease severity in C9orf72-FTD and -ALS (Agosta et al., 2017; McMillan et al., 2015; Schönecker et al., 2018) and low gray matter in this region is apparent even in young presymptomatic carriers (Bertrand et al., 2018; Cash et al., 2018; Lee et al., 2017; Olney et al., 2020; Papma et al., 2017; Rohrer et al., 2015). Recent investigations focused on the involvement of individual thalamic nuclei in FTD–ALS have characterized pulvinar nucleus atrophy as unique to C9orf72 expansion carriers with FTD (Bocchetta et al., 2020) but other thalamic nuclei have been associated with the C9orf72 expansion with motor neuron disease (Chipika et al., 2020).
DTI studies reveal that white matter tracts are also disrupted in C9orf72 expansion carriers. Studies of patients with C9orf72-bvFTD have revealed disruption of frontotemporal association tracts and corpus callosum similar to the white matter tracts affected in sporadic bvFTD (Mahoney et al., 2015, 2014). Both C9orf72-bvFTD and sporadic bvFTD show the greatest longitudinal decline in the paracallosal cingulum bundle (Mahoney et al., 2015). Patients with C9orf72-ALS/MND and varying degrees of cognitive impairment show motor tract involvement similar to sporadic ALS, but also systematic involvement of frontotemporal tracts such as the uncinate fasciculus, superior longitudinal fasciculus, and inferior longitudinal fasciculus (Agosta et al., 2017; Bede et al., 2013b; Floeter et al., 2018; Müller et al., 2020; Omer et al., 2017; van der Burgh et al., 2019; Westeneng et al., 2016). Though gray matter atrophy patterns overlap in C9orf72-FTD and -ALS, white matter tract involvement may better reflect each phenotype than do volumetric deficits (Bertrand et al., 2018; Floeter et al., 2018; Querin et al., 2019). For example, a longitudinal DTI study on a cohort of C9orf72 expansion carriers with different clinical diagnoses found that changes in cognitive-behavioral and motor symptom severity correlated with progressive deficits along the frontotemporal and corticospinal tracts, respectively, over a 6 month interval (Floeter et al., 2018).
In presymptomatic C9orf72 expansion carriers, white matter deficits commonly appear in tracts connecting the frontal lobe, the thalamic radiation, and tracts associated with motor functioning (Bertrand et al., 2018; Jiskoot et al., 2018; Lee et al., 2017; Panman et al., 2019; Papma et al., 2017; Wen et al., 2018). Cross-sectionally, both gray and white matter deficits in presymptomatic carriers emerge early, up to 30 years before symptom onset (Jiskoot et al., 2018; Lee et al., 2017), and are present even in presymptomatic carriers younger than 40 years old (Bertrand et al., 2018; Le Blanc et al., 2020; Lee et al., 2017). These regions of low gray matter volume and reduced white matter integrity are generally congruent with regions of gray matter atrophy found during the symptomatic phase. In the cervical spinal cord, only presymptomatic carriers older than 40 years showed atrophy at each vertebral level, and corticospinal tract FA reductions were detectable at an 18 month follow up (Querin et al., 2019). Consistent with cross-sectional studies, a recent study of 137 carriers that included longitudinal data for a subset of subjects estimated that the thickness of the medial frontal and parietal cortex and scattered lateral frontal, parietal, and temporal regions begins to decline during the early thirties with no acceleration around the estimated age of symptom onset (Le Blanc et al., 2020). Longitudinal changes in grey matter volume (Floeter et al., 2016; Panman et al., 2019) and white matter integrity (Panman et al., 2019) have been elusive in presymptomatic cohorts thus far. Taken together, these studies point to gray and white matter deficits at an early age during the presymptomatic phase, suggesting that these abnormalities may represent neurodevelopmental differences in C9orf72 expansion carriers (Bertrand et al., 2018; Lee et al., 2017).
Functional and molecular imaging
Although patients with C9orf72-bvFTD have a distinct, yet overlapping atrophy pattern compared to those with sporadic bvFTD, both converge on salience and sensorimotor network connectivity disruption, suggesting that ICN maps rather than atrophy patterns may better represent the clinical syndrome (Lee et al., 2014). Salience network connectivity disruption correlated with left medial pulvinar thalamic atrophy, suggesting that the medial pulvinar degeneration may contribute to the bvFTD syndrome in C9orf72 by disrupting salience network connectivity. In contrast to sporadic bvFTD, which shows both default mode network connectivity increases and decreases, C9orf72-bvFTD shows default mode network connectivity similar to controls (Lee et al., 2014). A study comparing 7 C9orf72-bvFTD to those with sporadic disease described pronounced anti-correlation between thalamic nodes of the salience network and the default mode network and also found connectivity increases within the right dorsal attention network (Rytty et al., 2014). A comparison between 19 C9orf72-MND and 24 sporadic MND with comparable cognitive deficits revealed that C9orf72-MND have sensorimotor network decreases but enhanced visual network connectivity (Agosta et al., 2017). During the presymptomatic stage, robust intrinsic connectivity network disruption is detectable in the salience, sensorimotor and default mode networks and to the medial pulvinar, and these anomalies are apparent as early as the third decade of life (Lee et al., 2017).
Metabolic imaging in patients with C9orf72-FTD typically reveals hypoperfusion and hypometabolism in the frontal and/or temporal lobes and limbic structures, in keeping with the clinical phenotype (Boeve et al., 2012; Castelnovo et al., 2019; Diehl-Schmid et al., 2019). Likely due to individual subject heterogeneity, a notable subset of patients with C9orf72-bvFTD lack hypometabolism in frontotemporal cortex or show at least a comparable degree of hypometabolism in the parietal lobe, thalamus, or cerebellum (Boeve et al., 2012; Devenney et al., 2014; Diehl-Schmid et al., 2019; Khan et al., 2012; Solje et al., 2015). Compared to sporadic bvFTD, C9orf72-bvFTD show pronounced focal hypometabolism in bilateral thalami (Diehl-Schmid et al., 2019). In C9orf72-ALS, metabolic signatures are more variable (Castelnovo et al., 2019). Group studies in patients with ALS suggest, however, that the expansion is associated with pronounced [18F]FDG-PET hypometabolism in thalamus and posterior cingulate (Cistaro et al., 2014; Van Laere et al., 2014; Verschueren et al., 2013) and hypermetabolism in midbrain and occipital and inferior temporal cortices (Cistaro et al., 2014). Metabolic abnormalities also occur in presymptomatic C9orf72 expansion carriers (De Vocht et al., 2020; Mutsaerts et al., 2019; Westeneng et al., 2017). Mutsaerts et al. (2019) estimated that insular, temporal, and parietal hypoperfusion emerges early, at least 12 years from estimated symptom onset. De Vocht et al. (2020) identified [18F]FDG-PET hypometabolism in the insular cortices, central opercular cortex, and thalami in 82% of presymptomatic participants studied, whereas abnormally low gray matter volume and elevated neurofilament light levels (a marker of axonal injury) were less frequently observed (62% and 19%, respectively).
3.5. Rare genetic mutations that cause either FTD or ALS
In recent years, genome-wide association studies have revealed a range of causal and risk variants for FTD and ALS. While rare genes such as CHMP2B and TREM2 have been associated with FTD and OPTN has been associated with ALS, other genes including TARDBP, DCTN1, FUS, VCP, UBQLN2, SQSTM1, CHCHD10, TBK1, CCNF, are associated with a heterogeneous array of disorders in the FTD-ALS spectrum. Below we review imaging findings for these rarer genetic FTD or ALS variants, including studies reporting visual assessment of structural MRI brain or [18F]FDG-PET scans in addition to quantitative neuroimaging studies.
3.5.1. CHMP2B
Charged multivesicular body protein 2B (CHMP2B) is expressed in multiple human tissues, including frontal and temporal lobes. CHMP2B protein is a component of the endosomal secretory complex required for transport III, and in cell culture, mutations in CHMP2B disrupt the protein’s localization and results in the formation of dysmorphic organelles of the late endosomal pathway (Skibinski et al., 2005). The pathology associated with CHMP2B mutations is poorly understood, with the FTLD UPS aggregates either representing an unknown disease-specific protein or a more general defect in endosomal trafficking and lysosomal protein degradation (Neumann and Mackenzie, 2019). CHMP2B mutations comprise less than 1% of FTD mutations, and have been described in large Danish and Belgian families (Gydesen et al., 2002; van der Zee et al., 2008), but are rare in other cohorts. Most CHMP2B mutation carriers develop bvFTD, although FTD-ALS and CBS and PSP syndromes have been reported (Isaacs et al., 2011). Dyscalculia, limb apraxia and dynamic aphasia have also been described (Gydesen et al., 2002).
Patients with bvFTD due to truncating CHMP2B mutations generally show atrophy and reduced cerebral blood flow in frontotemporal and sometimes parietal regions (Gydesen et al., 2002; Isaacs et al., 2011; Lindquist et al., 2008). In contrast to sporadic FTD, FTD due to CHMP2B mutations additionally involves parietal and posterior central regions, hypothesized to correspond to dyscalculia and limb apraxia, also unusual features of sporadic FTD (Gydesen et al., 2002; Isaacs et al., 2011). Both FTD and ALS have also been associated with missense CHMP2B mutations (Isaacs et al., 2011). Autopsy studies reveal that truncating and missense CHMP2B mutations have pathologically distinct features (Neumann and Mackenzie, 2019), but the associated imaging signatures of each type of mutation have not been systematically compared.
Group comparisons between presymptomatic carriers of truncating CHMP2B mutations and non-carrier family members have revealed focal atrophy in inferotemporal, superior frontal, and insular cortex (Eskildsen et al., 2009; Rohrer et al., 2009a). Presymptomatic CHMP2B carriers also show significantly decreased cerebral blood flow in occipital and parietal regions (Lunau et al., 2012).
3.5.2. TARDBP
TAR DNA-binding protein (TARDBP) is a nuclear protein involved with RNA processing and metabolism (Xia et al., 2016). During stress conditions, hyperphosphorylated, ubiquitinated and cleaved TDP-43 deposits aggregate in the cytoplasm. In addition to TDP pathology, other neuropathologies such as tau, amyloid and alpha-synuclein have been found in TARDBP mutation carriers (Gelpi et al., 2014; Kovacs et al., 2009; Moreno et al., 2015). TARDBP mutations account for 3% of familial ALS, but rarely cause dementia or parkinsonism (Lattante et al., 2013b), and account for less than 1% of patients with FTD (Caroppo et al., 2016). When TARDBP mutation carriers develop dementia, bvFTD is the most frequent syndrome, though both nonfluent and semantic PPA have been reported (Caroppo et al., 2016; Floris et al., 2015), and less than half develop motor neuron disease (Caroppo et al., 2016). Among the FTD syndromes, svPPA is usually sporadic and not genetic, yet patients with TARDP mutations develop svPPA at a higher rate compared to patients with the C9orf72 expansion and MAPT and GRN mutations (Caroppo et al., 2016).
Case series describe that patients with TARDBP-FTD have atrophy in frontotemporal and less commonly in parietal cortex, hippocampus, and amygdala (Caroppo et al., 2016; Cheng et al., 2016; Floris et al., 2015; Synofzik et al., 2014). Correspondingly, hypoperfusion and hypometabolism has been observed in frontotemporal cortex and more rarely in parietal cortex and the caudate (Benajiba et al., 2009; Borroni et al., 2009; Caroppo et al., 2016; Cheng et al., 2016; Floris et al., 2015; Synofzik et al., 2014). As expected, those with the svPPA syndrome typically develop left-lateralized anterior temporal and sometimes frontal gray matter loss, hypoperfusion and hypometabolism (Benajiba et al., 2009; Caroppo et al., 2016; Cheng et al., 2016; Floris et al., 2015; Gelpi et al., 2014; González-Sánchez et al., 2018).
Most case reports on pure TARDBP-MND or -ALS have found no apparent imaging abnormalities upon visual inspection of brain or cervical cord MRI (Agosta et al., 2020; Cheng et al., 2016; Chiò et al., 2010; Del Bo et al., 2009), but individual imaging reports have described abnormalities such as frontotemporal hypometabolism in SPECT and [18F]FDG-PET (Borghero et al., 2011) and temporal atrophy (Del Bo et al., 2009). In TARDBP-ALS with concomitant FTD or behavioral deficits, cortical atrophy and metabolic changes appear common within frontal and/or temporal lobes and less often involve parietal and anterior cingulate cortex and the head of the caudate (Cheng et al., 2016; Chiò et al., 2010).
3.5.3. FUS
FUS encodes the fused in sarcoma protein, an RNA-binding protein involved in cell proliferation, DNA repair, transcription regulation, and RNA splicing and transport (Deleon and Miller, 2018). ALS patients with FUS mutations show aggregated FUS protein in the cytoplasm. Interestingly, no patient with FTLD with FUS neuropathology has yet been identified to carry a FUS mutation (Neumann and Mackenzie, 2019).
Studies in patients with FTD or FTD-ALS with FUS mutations have reported frontal, temporal and parietal atrophy and hypometabolism (Akiyama et al., 2016; Blair et al., 2010; Broustal et al., 2010; Huey et al., 2012). Individual case reports on FUS-ALS generally describe normal brain imaging (Blair et al., 2010; Chiò et al., 2009; Rademakers et al., 2010; Zhou et al., 2020), although other reports have described instances of individual patients with cortical and cerebellar atrophy (Yan et al., 2010), nonspecific scattered white matter changes (Wongworawat et al., 2020), and decreases in cerebral blood flow in the right striatum, thalamus, and frontotemporal lobe (Tateishi et al., 2010).
3.5.4. OPTN
The OPTN gene encodes optineurin, a multifunctional protein involved in protein degradation via autophagy. OPTN mutations are associated with ALS, primary open-angle glaucoma (Rezaie, 2002), and Paget’s disease of bone (Albagha et al., 2010). OPTN mutations have been reported in 1–2 % of familial ALS and up to 3.5 % of sporadic cases (Belzil et al., 2011; Del Bo et al., 2011). While OPTN mutations are rarely found in patients with FTD, they are not considered causative of FTD (Pottier et al., 2015). Imaging reports on patients with OPTN-ALS have reported atrophy in frontal, temporal and motor cortices (Feng et al., 2019; Ito et al., 2011; Kamada et al., 2014; Ueno et al., 2011) as well as brainstem and cerebellum (Ueno et al., 2011).
3.5.5. VCP
The valosin-containing protein (VCP) gene is involved with various cellular activities, and pathological mutations are hypothesized to disrupt its role in protein degradation (Watts et al., 2004; Weihl et al., 2009). VCP mutations are associated with a disease characterized by inclusion body myopathy, Paget’s disease of bone, early onset FTD (IBMPFD) (Taylor, 2015; Watts et al., 2004) and other clinical syndromes including ALS (Johnson et al., 2010). The FTD phenotype develops in about one third of VCP mutation carriers, whereas ALS is rarer (Abramzon et al., 2012; Al-Obeidi et al., 2018; Johnson et al., 2010; Kimonis and Watts, 2005; Koppers et al., 2012).
Imaging reports on VCP-FTD range from having no apparent atrophy to focal frontotemporal or diffuse cortical and subcortical atrophy (Djamshidian et al., 2009; Kimonis et al., 2008; Kovach et al., 2001; Saracino et al., 2018; van der Zee et al., 2009). Rare reports also describe hippocampal or parietal atrophy (Fanganiello et al., 2011; Kim et al., 2011; Rohrer et al., 2011b). [18F]FDG-PET typically shows predominantly frontotemporal hypometabolism with parietal and cerebellar involvement in patients with VCP-FTD (Kim et al., 2011; Rohrer et al., 2011b; van der Zee et al., 2009; Viassolo et al., 2008). Compared to sporadic svPPA, patients with VCP-svPPA have more extensive frontal (Krause et al., 2007) or posterolateral temporal and parietal cortical atrophy (Kim et al., 2011), and curiously, the anterior temporal lobe is relatively spared. VCP-FTD and VCP-ALS have been suggested to share a focal atrophy in the temporal lobes and hippocampi (Hirano et al., 2017, 2015).
3.5.6. UBQLN2
The ubiquilin-2 (UBQLN2) gene encodes for a protein involved in proteasomal degradation (Deng et al., 2011; Hjerpe et al., 2016). UBQLN2 mutations have been associated with juvenile and adult onset ALS (Daoud et al., 2012; Deng et al., 2011; Gellera et al., 2013) and more rarely with FTD, FTD-ALS, and PSP (Synofzik et al., 2012; Vengoechea et al., 2013). Imaging reports in two UBQLN2-related FTD cases described bilateral frontotemporal atrophy and hypometabolism (Leger et al., 2017; Synofzik et al., 2012), whereas findings in UBQLN2-ALS with cognitive impairment have ranged from a lack of apparent atrophy (Kotan et al., 2016) to diffuse atrophy patterns in a family with heterogeneous clinical presentations (Fahed et al., 2014).
3.5.7. SQSTM1
The sequestome 1 (SQSTM1) gene encodes for p62, a multi-functional protein that targets specific cargoes for autophagy (Rea et al., 2014). SQSTM1 mutations are associated with Paget’s disease of bone (Goode and Layfield, 2010), FTD (Rubino et al., 2012) and ALS (Fecto, 2011; Yilmaz et al., 2020). In FTD and ALS cohorts, the mutation frequency of SQSTM1 is estimated to be 1–3% and SQSTM1 mutations are associated with TDP-43 neuropathology (Fecto, 2011; Le Ber, 2013; Rubino et al., 2012; van der Zee et al., 2014).
Imaging findings of individual patients with SQSTM1-bvFTD suggest atrophy and hypometabolism predominantly in frontotemporal and cingulate cortices, putamen, and basal ganglia, often more pronounced in one hemisphere (Kovacs et al., 2016; Le Ber, 2013; Rubino et al., 2012; Sun et al., 2018). Compared to sporadic FTD, ten patients with SQSTM1-FTD showed pronounced atrophy in inferior and medial orbitofrontal cortex, anterior insula, and precentral gyri (Luis et al., 2016). Brain imaging signatures in SQSTM1-ALS have not yet been systematically investigated.
3.5.8. TREM2
TREM2 (triggering receptor expressed on myeloid cells 2) encodes a receptor exclusively expressed in immune cells, and is thought to interfere with anti-inflammatory function and the removal of apoptotic tissue (Giraldo et al., 2013). TREM2 mutations were first detected in Nasu-Hakola disease, which manifests as recurrent bone fractures and early-onset dementia (Paloneva et al., 2002). Since then, TREM2 variants have been identified to cause familial FTD and to increase risk of AD and FTD without bone involvement (Borroni et al., 2014; Giraldo et al., 2013; Guerreiro et al., 2013; Lattante et al., 2013a; Le Ber et al., 2014). Parkinsonism is common in TREM2 mutation carriers, but genetic association studies do not support an association with ALS (Rayaprolu et al., 2013). Characteristic neuroimaging features in Nasu-Hakola disease include atrophy and white matter abnormalities most prominent in frontal cortices along with calcifications of the basal ganglia (Klünemann et al., 2005). Mild hypoperfusion in basal ganglia has also been reported in two preclinical TREM2 carriers (Montalbetti et al., 2005). TREM2 carriers with a pure FTD diagnosis exhibit frontal and/or temporal atrophy with parietal and hippocampal involvement along with extensive white matter lesions and reduced thickness of the corpus callosum (Giraldo et al., 2013; Guerreiro et al., 2013; Le Ber et al., 2014).
3.5.9. CHCHD10
CHCHD10 encodes for coiled-coil-helix-coiled-coil-helix domain containing protein 10, a mitochondrial protein located in the intermembrane space and enriched at cristae junctions (Bannwarth et al., 2014). Mutations in CHCHD10 cause structural abnormalities of mitochondria leading to fragmentation and suggest a role for mitochondrial dysfunction in FTD-ALS. The first report of a family with a CHCHD10 mutation revealed clinical heterogeneity within the family and included features such as motor neuron disease, cerebellar ataxia, frontal cognitive and behavioral changes, parkinsonism, and mitochondrial myopathy (Bannwarth et al., 2014). Subsequent reports have described other clinical phenotypes including pure ALS and pure FTD, mitochondrial myopathy, and spinal motor neuronopathy (Ajroud-Driss et al., 2015; Dols-Icardo et al., 2015; Jiao et al., 2016; Penttilä et al., 2015; Zhang et al., 2015). Based on mutation frequencies in ethnically matched FTD and ALS cohorts, CHCHD10 mutations appear to be more frequently associated with FTD phenotypes than with pure ALS (Chaussenot et al., 2014; Jiao et al., 2016; Marroquin et al., 2016; Teyssou et al., 2016; Wong et al., 2015). Disease progression in CHCHD10-related FTD and MND/ALS can be markedly slow, lasting up to 40 years (Bannwarth et al., 2014; Müller et al., 2014; Zhang et al., 2015).
Neuroimaging in patients with CHCHD10 related FTD and ALS with cognitive impairment generally shows atrophy or [18F]FDG-PET hypometabolism in frontal regions, and insular, temporal, parietal and cerebellar involvement have also been described (Bannwarth et al., 2014; Chaussenot et al., 2014; Dols-Icardo et al., 2015; Zhang et al., 2015). Structural MRI scans of CHCHD10 mutation carriers with a pure MND/ALS phenotype, however, have not shown apparent abnormalities upon visual inspection (Bannwarth et al., 2014; Müller et al., 2014; Ronchi et al., 2015).
3.5.10. TBK1
The tumor necrosis factor receptor-associated factor NF-κB activator (TANK)-binding kinase 1 (TBK1) gene encodes for a protein that is active in autophagy and inflammatory signaling (Weidberg and Elazar, 2011). Clinically, TBK1 loss-of-function mutations manifest as bvFTD, PPA, and ALS and are associated with TDP-43 type B pathology (Freischmidt et al., 2015; Gijselinck et al., 2015). Movement disorders such as PSP and cerebellar syndromes have also been reported (Wilke et al., 2018). It is estimated that over half of carriers develop a pure ALS phenotype and less than fifth develop FTD or a mixed phenotype, yet cognitive impairment is among the most common initial symptoms and found in up to half of TBK1 carriers (Freischmidt et al., 2015; Yu et al., 2019). In addition to bvFTD-ALS, patients with TBK1 may develop svPPA-ALS and nfvPPA-ALS (Caroppo et al., 2015a).
Imaging reports of TBK1-FTD show asymmetrical atrophy and hypometabolism in frontal and/or temporal lobes, reflecting the clinical phenotype (Caroppo et al., 2015a; Hirsch-Reinshagen et al., 2019; Koriath et al., 2017; Lamb et al., 2019; Pottier et al., 2015; Van Mossevelde et al., 2016; Yu et al., 2019). In the majority of TBK1-FTD patients, hypometabolism extends to parietal regions (Hirsch-Reinshagen et al., 2019; Koriath et al., 2017; Schönecker et al., 2016; Van Mossevelde et al., 2016), though parietal symptoms are not common. The few reports with imaging on pure ALS and FTD-ALS due to TBK1 mutations have described variable vulnerable brain regions, but involvement of temporal cortex, hippocampi, and cerebellum appear common across various reports (Jiao et al., 2018; McCombe et al., 2018; Tohnai et al., 2018; Van Mossevelde et al., 2016).
4. DISCUSSION
Literature overview
Taken together, neuroimaging studies of genetic FTD and ALS suggest that each genetic mutation shows a distinct pattern of targeted neuroanatomy that overlaps with the focal degeneration seen in the sporadic forms of these diseases. Because clinical trials for disease-modifying treatments have failed in symptomatic patients thus far, an ongoing hypothesis in the field of neurodegenerative diseases is that treatments may only be effective prior to profound neurodegeneration and may need to be administered during the presymptomatic phase. Numerous studies spanning various imaging modalities have revealed abnormalities in presymptomatic genetic mutation carriers for FTD and ALS, which generally converge within anatomical regions affected during the symptomatic stage. These studies suggest that abnormalities in functional connectivity networks, brain metabolism, gray matter volume and white matter integrity are detectable prior to the onset of profound neurodegeneration.
Clinical application of neuroimaging findings
As mentioned in the sections describing sporadic FTD and ALS, the clinical diagnostic criteria for bvFTD and PPA incorporate neuroimaging findings such as atrophy patterns on structural MRI or regions of hypometabolism on PET as supportive criteria. For ALS, neuroimaging is currently used only to exclude other conditions that may mimic ALS. In our opinion, the literature supports the idea that certain imaging features that may increase the clinician’s suspicion of genetic FTD or ALS. Although the ultimate confirmation of genetic FTD or ALS is made by genetic testing and not with neuroimaging, the following neuroimaging features may raise the index of suspicion for a genetic etiology, perhaps even in people without a known family history. For FTD or ALS due to the C9orf72 expansion, structural and functional MRI studies suggest that subcortical structures, the thalamus in particular, are more likely to be involved compared to sporadic disease. Patients with bvFTD due to MAPT mutations often have early memory impairment with corresponding focal mesial temporal involvement (atrophy or hypometabolism), both of which are not typical in sporadic bvFTD, GRN or C9orf72. Parietal lobe involvement has been reported in C9orf72, GRN and TBK1 carriers, while sporadic bvFTD typically spares the parietal lobes. Patients with FTD due to GRN or TBK1 mutations have been reported to have prominently asymmetric atrophy, most evident in the late stages of disease. Compared to sporadic ALS, more widespread frontotemporal involvement emerges in C9orf72-ALS, even in the absence of cognitive or behavioral symptoms, and many SOD1 variants are associated with more prominent cervical spinal cord atrophy with relative sparing of cortical motor networks.
Limitations in the current literature and future directions
Thus far, many neuroimaging studies of genetic FTD and ALS have however been performed in relatively small cohorts in group analyses using cross-sectional data. In the past decade, international consortia for genetic FTD and ALS that collect harmonized multisite data have addressed this need for larger longitudinal cohorts. These longitudinal studies have begun building neuroimaging biomarker trajectories for different genetic mutations. For example, research on both genetic FTD and ALS suggests that patients with different mutations show differences in the order of involvement and rate of decline of specific brain structures (Jiskoot et al., 2019; Müller et al., 2020; Rohrer et al., 2015; van der Burgh et al., 2020; Whitwell et al., 2012; Young et al., 2018). Cross-sectional fMRI connectivity studies suggest that network connectivity may change dynamically throughout the lifespan in carriers of GRN (Borroni et al., 2012; Lee et al., 2019) or SOD1 (Menke et al., 2016) mutations, or the C9orf72 expansion (Agosta et al., 2017; Lee et al., 2017).
While these studies have been groundbreaking, group analyses are more vulnerable to individual subject heterogeneity and have limited ability to determine trajectories in individual patients. Assessing longitudinal trajectories at the individual-subject level will be necessary for developing neuroimaging as a biomarker for disease detection and monitoring.
Supplementary Material
Supplementary Table S1. Summary of neuroimaging findings related to MAPT mutations
Supplementary Table S2. Summary of neuroimaging findings related to GRN mutations
Supplementary Table S3. Summary of neuroimaging findings related to SOD1 mutations
Supplementary Table S4. Summary of neuroimaging findings related to C9orf72 expansions
5. ACKNOWLEDGEMENTS
This work was supported by NIH AG058233, Tau Consortium, Bluefield Project to Cure FTD, and the Päivikki and Sakari Sohlberg foundation. The authors alone are responsible for the content and writing of the paper.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abidi M, de Marco G, Couillandre A, Feron M, Mseddi E, Termoz N, Querin G, Pradat P, Bede P, 2019. Adaptive functional reorganisation in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. Eur J Neurol. doi: 10.1111/ene.14042 [DOI] [PubMed] [Google Scholar]
- Abramzon Y, Johnson JO, Scholz SW, Taylor JP, Brunetti M, Calvo A, Mandrioli J, Benatar M, Mora G, Restagno G, Chiò A, Traynor BJ, 2012. Valosin-containing protein (VCP) mutations in sporadic amyotrophic lateral sclerosis. Neurobiol Aging 33, 2231.e1–2231.e6. doi: 10.1016/j.neurobiolaging.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A, Nicholson G, 2005. Age-dependent penetrance of three different superoxide dismutase 1 (SOD1) mutations. Int J Neurosci 115, 1119–1130. doi: 10.1080/00207450590914392 [DOI] [PubMed] [Google Scholar]
- Agosta F, Canu E, Valsasina P, Riva N, Prelle A, Comi G, Filippi M, 2013. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol Aging 34, 419–427. doi: 10.1016/j.neurobiolaging.2012.04.015 [DOI] [PubMed] [Google Scholar]
- Agosta F, Ferraro PM, Riva N, Spinelli EG, Chiò A, Canu E, Valsasina P, Lunetta C, Iannaccone S, Copetti M, Prudente E, Comi G, Falini A, Filippi M, 2016. Structural brain correlates of cognitive and behavioral impairment in MND. Hum Brain Mapp 37, 1614–1626. doi: 10.1002/hbm.23124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Ferraro PM, Riva N, Spinelli EG, Domi T, Carrera P, Copetti M, Falzone Y, Ferrari M, Lunetta C, Comi G, Falini A, Quattrini A, Filippi M, 2017. Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol Aging 57, 206–219. doi: 10.1016/j.neurobiolaging.2017.05.024 [DOI] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Magnani G, Marcone A, Martinelli D, Antonietta Volontè M, Riva N, Iannaccone S, Ferraro PM, Caso F, Chiò A, Comi G, Falini A, Filippi M, 2015. MRI signatures of the frontotemporal lobar degeneration continuum. Hum Brain Mapp 36, 2602–2614. doi: 10.1002/hbm.22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Riva N, Chiò A, Messina S, Iannaccone S, Calvo A, Silani V, Copetti M, Falini A, Comi G, Filippi M, 2014a. Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp 35, 1710–1722. doi: 10.1002/hbm.22286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Valsasina P, Canu E, Meani A, Marcone A, Magnani G, Falini A, Comi G, Filippi M, 2014b. Disrupted brain connectome in semantic variant of primary progressive aphasia. Neurobiol Aging 35, 2646–2655. doi: 10.1016/j.neurobiolaging.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Agosta F, Rocca MA, Valsasina P, Sala S, Caputo D, Perini M, Salvi F, Prelle A, Filippi M, 2009. A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 80, 53–55. doi: 10.1136/jnnp.2008.154252 [DOI] [PubMed] [Google Scholar]
- Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, Copetti M, Caso F, Cerami C, Comi G, Cappa SF, Falini A, Filippi M, 2012. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex 22, 2705–2714. doi: 10.1093/cercor/bhr288 [DOI] [PubMed] [Google Scholar]
- Agosta F, Spinelli E, Ghirelli A, Riva N, Magnani G, Caso F, Caroppo P, Prioni S, Tremolizzo L, Appollonio I, Silani V, Carrera P, Filippi M, 2020. Deep grey matter and hippocampal involvement in genetic cases of frontotemporal lobar degeneration (1508). Neurology 94, 1508. [Google Scholar]
- Agosta F, Spinelli EG, Marjanovic IV, Stevic Z, Pagani E, Valsasina P, Salak-Djokic B, Jankovic M, Lavrnic D, Kostic VS, Filippi M, 2018. Unraveling ALS due to SOD1 mutation through the combination of brain and cervical cord MRI. Neurology 90, e707–e716. doi: 10.1212/WNL.0000000000005002 [DOI] [PubMed] [Google Scholar]
- Ajroud-Driss S, Fecto F, Ajroud K, Lalani I, Calvo SE, Mootha VK, Deng H-X, Siddique N, Tahmoush AJ, Heiman-Patterson TD, Siddique T, 2015. Mutation in the novel nuclear-encoded mitochondrial protein CHCHD10 in a family with autosomal dominant mitochondrial myopathy. Neurogenetics 16, 1–9. doi: 10.1007/s10048-014-0421-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Warita H, Kato M, Nishiyama A, Izumi R, Ikeda C, Kamada M, Suzuki N, Aoki M, 2016. Genotype-phenotype relationships in familial amyotrophic lateral sclerosis with FUS/TLS mutations in Japan: FUS/TLS linked FALS in Japan. Muscle Nerve 54, 398–404. doi: 10.1002/mus.25061 [DOI] [PubMed] [Google Scholar]
- Albagha OME, Visconti MR, Alonso N, Langston AL, Cundy T, Dargie R, Dunlop MG, Fraser WD, Hooper MJ, Isaia G, Nicholson GC, del Pino Montes J, Gonzalez-Sarmiento R, di Stefano M, Tenesa A, Walsh JP, Ralston SH, 2010. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat Genet 42, 520–524. doi: 10.1038/ng.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Pisner D, Jacova C, 2019. Predementia brain changes in progranulin mutation: a systematic review of neuroimaging evidence. Dement Geriatr Cogn Disord 47, 1–18. doi: 10.1159/000494968 [DOI] [PubMed] [Google Scholar]
- Alexander C, Zeithamova D, Hsiung G-YR, Mackenzie IR, Jacova C, 2018. Decreased prefrontal activation during matrix reasoning in predementia progranulin mutation carriers. J Alzheimers Dis 62, 583–589. doi: 10.3233/JAD-170716 [DOI] [PubMed] [Google Scholar]
- Al-Obeidi E, Al-Tahan S, Surampalli A, Goyal N, Wang AK, Hermann A, Omizo M, Smith C, Mozaffar T, Kimonis V, 2018. Genotype-phenotype study in patients with valosin-containing protein mutations associated with multisystem proteinopathy. Clin Genet 93, 119–125. doi: 10.1111/cge.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameur F, Colliot O, Caroppo P, Ströer S, Dormont D, Brice A, Azuar C, Dubois B, Le Ber I, Bertrand A, 2016. White matter lesions in FTLD: distinct phenotypes characterize GRN and C9ORF72 mutations. Neurol Genet 2, e47. doi: 10.1212/NXG.0000000000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PM, Forsgren L, Binzer M, Nilsson P, Ala-Hurula V, Keranen ML, Bergmark L, Saarinen A, Haltia T, Tarvainen I, Kinnunen E, Udd B, Marklund SL, 1996. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain 119 ( Pt 4), 1153–1172. doi: 10.1093/brain/119.4.1153 [DOI] [PubMed] [Google Scholar]
- Anor CJ, Xi Z, Zhang M, Moreno D, Sato C, Rogaeva E, Tartaglia MC, 2015. Mutation analysis of C9orf72 in patients with corticobasal syndrome. Neurobiol Aging 36, 2905.e1–2905.e5. doi: 10.1016/j.neurobiolaging.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Witte RJ, Dickson DW, Tsuboi Y, Uitti RJ, Slowinski J, Hutton ML, Lin S-C, Boeve BF, Cheshire WP, Pooley RA, Liss JM, Caviness JN, Strongosky AJ, Wszolek ZK, 2007. Clinical-pathologic study of biomarkers in FTDP-17 (PPND family with N279K tau mutation). Parkinsonism Relat Disord 13, 230–239. doi: 10.1016/j.parkreldis.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Atassi N, Xu M, Triantafyllou C, Keil B, Lawson R, Cernasov P, Ratti E, Long CJ, Paganoni S, Murphy A, Salibi N, Seethamraju R, Rosen B, Ratai E-M, 2017. Ultra high-field (7tesla) magnetic resonance spectroscopy in amyotrophic lateral sclerosis. PLoS One 12, e0177680. doi: 10.1371/journal.pone.0177680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M, 2006. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919. doi: 10.1038/nature05016 [DOI] [PubMed] [Google Scholar]
- Bannwarth S, Ait-El-Mkadem S, Chaussenot A, Genin EC, Lacas-Gervais S, Fragaki K, Berg-Alonso L, Kageyama Y, Serre V, Moore DG, Verschueren A, Rouzier C, Le Ber I, Augé G, Cochaud C, Lespinasse F, N’Guyen K, de Septenville A, Brice A, Yu-Wai-Man P, Sesaki H, Pouget J, Paquis-Flucklinger V, 2014. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137, 2329–2345. doi: 10.1093/brain/awu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Borghesani V, Henry M, Shwe W, Lauricella M, Miller Z, Deleon J, Miller BL, Dronkers N, Brambati SM, Seeley WW, Mandelli ML, Gorno-Tempini ML, 2020. Task-free functional language networks: reproducibility and clinical application. J Neurosci 40, 1311–1320. doi: 10.1523/JNEUROSCI.1485-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, Warrington EK, Stevens J, Revesz T, Holton J, Al-Sarraj S, King A, Scahill R, Warren JD, Fox NC, Rossor MN, Collinge J, Mead S, 2008. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 131, 706–720. doi: 10.1093/brain/awm320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N, Hampel H, Gallagher L, Lynch C, Fagan AJ, Pender N, Hardiman O, 2013a. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84, 766–773. doi: 10.1136/jnnp-2012-302674 [DOI] [PubMed] [Google Scholar]
- Bede P, Bokde ALW, Byrne S, Elamin M, McLaughlin RL, Kenna K, Fagan AJ, Pender N, Bradley DG, Hardiman O, 2013b. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81, 361–369. doi: 10.1212/WNL.0b013e31829c5eee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P, Elamin M, Byrne S, McLaughlin RL, Kenna K, Vajda A, Pender N, Bradley DG, Hardiman O, 2013c. Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology 81, 2107–2115. doi: 10.1212/01.wnl.0000437313.80913.2c [DOI] [PubMed] [Google Scholar]
- Bede P, Hardiman O, 2018. Longitudinal structural changes in ALS: a three time-point imaging study of white and gray matter degeneration. Amyotroph Lateral Scler Frontotemporal Degener 19, 232–241. doi: 10.1080/21678421.2017.1407795 [DOI] [PubMed] [Google Scholar]
- Bede P, Omer T, Finegan E, Chipika RH, Iyer PM, Doherty MA, Vajda A, Pender N, McLaughlin RL, Hutchinson S, Hardiman O, 2018. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging Behav 12, 1696–1707. doi: 10.1007/s11682-018-9837-9 [DOI] [PubMed] [Google Scholar]
- Belzil VV, Daoud H, Desjarlais A, Bouchard J-P, Dupré N, Camu W, Dion PA, Rouleau GA, 2011. Analysis of OPTN as a causative gene for amyotrophic lateral sclerosis. Neurobiol Aging 32, 555.e13–555.e14. doi: 10.1016/j.neurobiolaging.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, Legallic S, Salachas F, Hannequin D, Decousus M, Lacomblez L, Guedj E, Golfier V, Camu W, Dubois B, Campion D, Meininger V, Brice A, French Clinical and Genetic Research Network on Frontotemporal Lobar Degeneration/Frontotemporal Lobar Degeneration with Motoneuron Disease, 2009. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol 65, 470–473. doi: 10.1002/ana.21612 [DOI] [PubMed] [Google Scholar]
- Benussi A, Gazzina S, Premi E, Cosseddu M, Archetti S, Dell’Era V, Cantoni V, Cotelli MS, Alberici A, Micheli A, Benussi L, Ghidoni R, Padovani A, Borroni B, 2019. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol Aging 76, 133–140. doi: 10.1016/j.neurobiolaging.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Ber IL, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, Decousus M, Hannequin D, Vera P, Lacomblez L, Camuzat A, Didic M, Puel M, Lotterie JA, Golfier V, Bernard A-M, Vercelletto M, Magne C, Sellal F, Namer I, Michel BF, Pasquier J, Salachas F, Bochet J, Brice A, Habert MO, Dubois B, 2006. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain 129, 3051–3065. doi: 10.1093/brain/awl288 [DOI] [PubMed] [Google Scholar]
- Bertrand A, Wen J, Rinaldi D, Houot M, Sayah S, Camuzat A, Fournier C, Fontanella S, Routier A, Couratier P, Pasquier F, Habert M-O, Hannequin D, Martinaud O, Caroppo P, Levy R, Dubois B, Brice A, Durrleman S, Colliot O, Le Ber I, for the Predict to Prevent Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis (PREV-DEMALS) Study Group, 2018. Early cognitive, structural, and microstructural changes in presymptomatic C9orf72 carriers younger than 40 years. JAMA Neurol 75, 236. doi: 10.1001/jamaneurol.2017.4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan-Jones WR, Cope TE, Jones PS, Passamonti L, Hong YT, Fryer T, Arnold R, Coles JP, Aigbirhio FI, O’Brien JT, Rowe JB, 2019. In vivo evidence for presymptomatic neuroinflammation in a MAPT mutation carrier. Ann Clin Transl Neurol 6, 373–378. doi: 10.1002/acn3.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain CRV, Brunton S, Williams VC, Leemans A, Turner MR, Andersen PM, Catani M, Stanton BR, Ganesalingham J, Jones DK, Williams SCR, Leigh PN, Simmons A, 2011. Differential corticospinal tract degeneration in homozygous “D90A” SOD-1 ALS and sporadic ALS. J Neurol Neurosurg Psychiatry 82, 843–849. doi: 10.1136/jnnp.2010.236018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, Blumbergs PC, Vucic S, Kiernan MC, Nicholson GA, 2010. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry 81, 639–645. doi: 10.1136/jnnp.2009.194399 [DOI] [PubMed] [Google Scholar]
- Bocchetta M, Iglesias JE, Neason M, Cash DM, Warren JD, Rohrer JD, 2020. Thalamic nuclei in frontotemporal dementia: Mediodorsal nucleus involvement is universal but pulvinar atrophy is unique to C9orf72. Hum Brain Mapp 41, 1006–1016. doi: 10.1002/hbm.24856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, 2005. Longitudinal characterization of two siblings with frontotemporal dementia and parkinsonism linked to chromosome 17 associated with the S305N tau mutation. Brain 128, 752–772. doi: 10.1093/brain/awh356 [DOI] [PubMed] [Google Scholar]
- Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME, Dickson DW, Josephs KA, Rush BK, Machulda MM, Fields JA, Ferman TJ, Baker M, Rutherford NJ, Adamson J, Wszolek ZK, Adeli A, Savica R, Boot B, Kuntz KM, Gavrilova R, Reeves A, Whitwell J, Kantarci K, Jack CR, Parisi JE, Lucas JA, Petersen RC, Rademakers R, 2012. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135, 765–783. doi: 10.1093/brain/aws004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghero G, Floris G, Cannas A, Marrosu MG, Murru MR, Costantino E, Parish LD, Pugliatti M, Ticca A, Traynor BJ, Calvo A, Cammarosano S, Moglia C, Cistaro A, Brunetti M, Restagno G, Chiò A, 2011. A patient carrying a homozygous p.A382T TARDBP missense mutation shows a syndrome including ALS, extrapyramidal symptoms, and FTD. Neurobiol Aging 32, 2327.e1–2327.e5. doi: 10.1016/j.neurobiolaging.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Cercignani M, Premi E, Serra L, Cerini C, Cosseddu M, Pettenati C, Turla M, Archetti S, Gasparotti R, Caltagirone C, Padovani A, Bozzali M, 2012. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol Aging 33, 2506–2520. doi: 10.1016/j.neurobiolaging.2011.10.031 [DOI] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C, Gasparotti R, Di Luca M, Perani D, Padovani A, 2008. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res 11, 585–595. doi: 10.1089/rej.2007.0623 [DOI] [PubMed] [Google Scholar]
- Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, Papetti A, Stuani C, Di Luca M, Gennarelli M, Padovani A, 2009. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat 30, E974–E983. doi: 10.1002/humu.21100 [DOI] [PubMed] [Google Scholar]
- Borroni B, Ferrari F, Galimberti D, Nacmias B, Barone C, Bagnoli S, Fenoglio C, Piaceri I, Archetti S, Bonvicini C, Gennarelli M, Turla M, Scarpini E, Sorbi S, Padovani A, 2014. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol Aging 35, 934.e7–-934.e10.. doi: 10.1016/j.neurobiolaging.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, Feldman H, Hsiung G-YR, Rutherford N, Laluz V, Whitwell J, Foti D, McDade E, Molano J, Karydas A, Wojtas A, Goldman J, Mirsky J, Sengdy P, DeArmond S, Miller BL, Rademakers R, 2011. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry 82, 196–203. doi: 10.1136/jnnp.2009.204081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Battistoni V, Premi E, Alberici A, Giulietti G, Archetti S, Turla M, Gasparotti R, Cercignani M, Padovani A, Borroni B, 2013. Structural brain signature of FTLD driven by Granulin mutation. J Alzheimers Dis 33, 483–494. doi: 10.3233/JAD-2012-121273 [DOI] [PubMed] [Google Scholar]
- Broad RJ, Gabel MC, Dowell NG, Schwartzman DJ, Seth AK, Zhang H, Alexander DC, Cercignani M, Leigh PN, 2019. Neurite orientation and dispersion density imaging (NODDI) detects cortical and corticospinal tract degeneration in ALS. J Neurol Neurosurg Psychiatry 90, 404–411. doi: 10.1136/jnnp-2018-318830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM, 2003. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 60, 1005–1011. doi: 10.1212/01.WNL.0000052685.09194.39 [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL, 2000. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1, 293–299. doi: 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- Broustal O, Camuzat A, Guillot-Noel L, Guy N, Millecamps S, Deffond D, Lacomblez L, Golfier V, Hannequin D, Salachas F, Camu W, Didic M, Dubois B, Meininger V, Le Ber I, Brice A, French clinical and genetic research network on FTD/FTD-MND., 2010. FUS mutations in frontotemporal lobar degeneration with amyotrophic lateral sclerosis. J Alzheimers Dis 22, 765–769. [PubMed] [Google Scholar]
- Buhour M-S, Doidy F, Laisney M, Pitel AL, de La Sayette V, Viader F, Eustache F, Desgranges B, 2017a. Pathophysiology of the behavioral variant of frontotemporal lobar degeneration: a study combining MRI and FDG-PET. Brain Imaging Behav 11, 240–252. doi: 10.1007/s11682-016-9521-x [DOI] [PubMed] [Google Scholar]
- Buhour M-S, Doidy F, Mondou A, Pélerin A, Carluer L, Eustache F, Viader F, Desgranges B, 2017b. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res 7. doi: 10.1186/s13550-017-0267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell JR, Kiernan MC, Vucic S, Hodges JR, 2011. Motor Neuron dysfunction in frontotemporal dementia. Brain 134, 2582–2594. doi: 10.1093/brain/awr195 [DOI] [PubMed] [Google Scholar]
- Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, McLaughlin RL, Iyer PM, O’Brien C, Phukan J, Wynne B, Bokde AL, Bradley DG, Pender N, Al-Chalabi A, Hardiman O, 2012. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol 11, 232–240. doi: 10.1016/S1474-4422(12)70014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JD, Nair G, Andersen PM, Wuu J, Gronka S, Hu X, Benatar M, 2011a. Presymptomatic spinal cord neurometabolic findings in SOD1-positive people at risk for familial ALS. Neurology 77, 1370–1375. doi: 10.1212/WNL.0b013e318231526a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JD, Nair G, Pineda-Alonso N, Usher S, Hu X, Benatar M, 2011b. Magnetic resonance spectroscopy of the cervical cord in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12, 185–191. doi: 10.3109/17482968.2010.515223 [DOI] [PubMed] [Google Scholar]
- Caroppo P, Camuzat A, De Septenville A, Couratier P, Lacomblez L, Auriacombe S, Flabeau O, Jornéa L, Blanc F, Sellal F, Cretin B, Meininger V, Fleury M-C, Couarch P, Dubois B, Brice A, Le Ber I, 2015a. Semantic and nonfluent aphasic variants, secondarily associated with amyotrophic lateral sclerosis, are predominant frontotemporal lobar degeneration phenotypes in TBK1 carriers. Alzheimers Dement (Amst) 1, 481–486. doi: 10.1016/j.dadm.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P, Camuzat A, Guillot-Noel L, Thomas-Antérion C, Couratier P, Wong TH, Teichmann M, Golfier V, Auriacombe S, Belliard S, Laurent B, Lattante S, Millecamps S, Clot F, Dubois B, van Swieten JC, Brice A, Le Ber I, 2016. Defining the spectrum of frontotemporal dementias associated with TARDBP mutations. Neurol Genet 2, e80. doi: 10.1212/NXG.0000000000000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P, Durrleman S, Funkiewiez A, Perlbarg V, Hahn V, Bertin H, Gaubert M, Routier A, Hannequin D, Deramecourt V, Pasquier F, Rivaud-Pechoux S, Vercelletto M, Edouart G, Valabregue R, Lejeune P, Didic M, Corvol J-C, Benali H, Lehericy S, Dubois B, Colliot O, Brice A, Le Ber I, 2015b. Lateral temporal lobe: an early imaging marker of the presymptomatic GRN disease? J Alzheimers Dis 47, 751–759. doi: 10.3233/JAD-150270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P, Le Ber I, Camuzat A, Clot F, Naccache L, Lamari F, De Septenville A, Bertrand A, Belliard S, Hannequin D, Colliot O, Brice A, 2014. Extensive white matter involvement in patients with frontotemporal lobar degeneration: think progranulin. JAMA Neurol 71, 1562. doi: 10.1001/jamaneurol.2014.1316 [DOI] [PubMed] [Google Scholar]
- Cash DM, Bocchetta M, Thomas DL, Dick KM, van Swieten JC, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe JB, Graff C, Tagliavini F, Frisoni GB, Laforce R, Finger E, de Mendonça A, Sorbi S, Rossor MN, Ourselin S, Rohrer JD, Andersson C, Archetti S, Arighi A, Benussi L, Black S, Cosseddu M, Fallström M, Ferreira C, Fenoglio C, Fox N, Freedman M, Fumagalli G, Gazzina S, Ghidoni R, Grisoli M, Jelic V, Jiskoot L, Keren R, Lombardi G, Maruta C, Mead S, Meeter L, van Minkelen R, Nacmias B, Öijerstedt L, Padovani A, Panman J, Pievani M, Polito C, Premi E, Prioni S, Rademakers R, Redaelli V, Rogaeva E, Rossi G, Rossor M, Scarpini E, Tang-Wai D, Tartaglia C, Thonberg H, Tiraboschi P, Verdelho A, Warren J, 2018. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol Aging 62, 191–196. doi: 10.1016/j.neurobiolaging.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnovo V, Caminiti SP, Riva N, Magnani G, Silani V, Perani D, 2019. Heterogeneous brain FDG-PET metabolic patterns in patients with C9orf72 mutation. Neurol Sci 40, 515–521. doi: 10.1007/s10072-018-3685-7 [DOI] [PubMed] [Google Scholar]
- Cerami C, Dodich A, Greco L, Iannaccone S, Magnani G, Marcone A, Pelagallo E, Santangelo R, Cappa SF, Perani D, 2017. The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J Alzheimers Dis 55, 183–197. doi: 10.3233/JAD-160682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussenot A, Le Ber I, Ait-El-Mkadem S, Camuzat A, de Septenville A, Bannwarth S, Genin EC, Serre V, Augé G, Brice A, Pouget J, Paquis-Flucklinger V, 2014. Screening of CHCHD10 in a French cohort confirms the involvement of this gene in frontotemporal dementia with amyotrophic lateral sclerosis patients. Neurobiol Aging 35, 2884.e1–2884.e4. doi: 10.1016/j.neurobiolaging.2014.07.022 [DOI] [PubMed] [Google Scholar]
- Chen Q, Boeve BF, Schwarz CG, Reid R, Tosakulwong N, Lesnick TG, Bove J, Brannelly P, Brushaber D, Coppola G, Dheel C, Dickerson BC, Dickinson S, Faber K, Fields J, Fong J, Foroud T, Forsberg L, Gavrilova RH, Gearhart D, Ghoshal N, Goldman J, Graff-Radford J, Graff-Radford NR, Grossman M, Haley D, Heuer HW, Hsiung G-YR, Huey E, Irwin DJ, Jack CR, Jones DT, Jones L, Karydas AM, Knopman DS, Kornak J, Kramer J, Kremers W, Kukull WA, Lapid M, Lucente D, Lungu C, Mackenzie IRA, Manoochehri M, McGinnis S, Miller BL, Pearlman R, Petrucelli L, Potter M, Rademakers R, Ramos EM, Rankin KP, Rascovsky K, Sengdy P, Shaw L, Syrjanen J, Tatton N, Taylor J, Toga AW, Trojanowski J, Weintraub S, Wong B, Boxer AL, Rosen H, Wszolek Z, Kantarci K, 2019a. Tracking white matter degeneration in asymptomatic and symptomatic MAPT mutation carriers. Neurobiol Aging 83, 54–62. doi: 10.1016/j.neurobiolaging.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Boeve BF, Senjem M, Tosakulwong N, Lesnick T, Brushaber D, Dheel C, Fields J, Forsberg L, Gavrilova R, Gearhart D, Graff-Radford J, Graff-Radford N, Jack CR, Jones D, Knopman D, Kremers WK, Lapid M, Rademakers R, Ramos EM, Syrjanen J, Boxer AL, Rosen H, Wszolek ZK, Kantarci K, 2020. Trajectory of lobar atrophy in asymptomatic and symptomatic GRN mutation carriers: a longitudinal MRI study. Neurobiol Aging 88, 42–50. doi: 10.1016/j.neurobiolaging.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Boeve BF, Senjem M, Tosakulwong N, Lesnick TG, Brushaber D, Dheel C, Fields J, Forsberg L, Gavrilova R, Gearhart D, Graff-Radford J, Graff-Radford NR, Jack CR, Jones DT, Knopman DS, Kremers WK, Lapid M, Rademakers R, Syrjanen J, Boxer AL, Rosen H, Wszolek ZK, Kantarci K, 2019b. Rates of lobar atrophy in asymptomatic MAPT mutation carriers. Alzheimers Dement (N Y) 5, 338–346. doi: 10.1016/j.trci.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Boeve BF, Tosakulwong N, Lesnick T, Brushaber D, Dheel C, Fields J, Forsberg L, Gavrilova R, Gearhart D, Haley D, Gunter JL, Graff-Radford J, Jones D, Knopman D, Graff-Radford N, Kraft R, Lapid M, Rademakers R, Syrjanen J, Wszolek ZK, Rosen H, Boxer AL, Kantarci K, 2019c. Frontal lobe 1H MR spectroscopy in asymptomatic and symptomatic MAPT mutation carriers. Neurology 93, e758–e765. doi: 10.1212/WNL.0000000000007961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Boeve BF, Tosakulwong N, Lesnick T, Brushaber D, Dheel C, Fields J, Forsberg L, Gavrilova R, Gearhart D, Haley D, Gunter JL, Graff-Radford J, Jones D, Knopman D, Graff-Radford N, Kraft R, Lapid M, Rademakers R, Wszolek ZK, Rosen H, Boxer AL, Kantarci K, 2019d. Brain MR spectroscopy changes precede frontotemporal lobar degeneration phenoconversion in Mapt mutation carriers. J Neuroimaging 29, 624–629. doi: 10.1111/jon.12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kantarci K, 2020. Imaging Biomarkers for neurodegeneration in presymptomatic familial frontotemporal lobar degeneration. Front Neurol 11. doi: 10.3389/fneur.2020.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YW, Lee MJ, Chen TF, Cheng TW, Lai YM, Hua MS, Chiu MJ, 2016. A single nucleotide TDP-43 mutation within a Taiwanese family: a multifaceted demon. Amyotroph Lateral Scler Frontotemporal Degener 17, 292–294. doi: 10.3109/21678421.2015.1111905 [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Martinez-Lage M, Sleiman PMA, Hu W, Greene R, Wood EM, Bing S, Grossman M, Schellenberg GD, Hatanpaa KJ, Weiner MF, White CL, Brooks WS, Halliday GM, Kril JJ, Gearing M, Beach TG, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Pickering-Brown SM, Snowden J, van Swieten JC, Heutink P, Seelaar H, Murrell JR, Ghetti B, Spina S, Grafman J, Kaye JA, Woltjer RL, Mesulam M, Bigio E, Lladó A, Miller BL, Alzualde A, Moreno F, Rohrer JD, Mackenzie IRA, Feldman HH, Hamilton RL, Cruts M, Engelborghs S, De Deyn PP, Van Broeckhoven C, Bird TD, Cairns NJ, Goate A, Frosch MP, Riederer PF, Bogdanovic N, Lee VMY, Trojanowski JQ, Van Deerlin VM, 2011. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch Neurol 68, 488. doi: 10.1001/archneurol.2011.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong I, Marjańska M, Deelchand DK, Eberly LE, Walk D, Öz G, 2017. Ultra-high field proton MR spectroscopy in early-stage amyotrophic lateral sclerosis. Neurochem Res 42, 1833–1844. doi: 10.1007/s11064-017-2248-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S, Jiang T, Tan L, Yu J-T, 2016. Distinct neurological disorders with C9orf72 mutations: genetics, pathogenesis, and therapy. Neurosci Biobehav Rev 66, 127–142. doi: 10.1016/j.neubiorev.2016.03.033 [DOI] [PubMed] [Google Scholar]
- Chiò A, Calvo A, Moglia C, Restagno G, Ossola I, Brunetti M, Montuschi A, Cistaro A, Ticca A, Traynor BJ, Schymick JC, Mutani R, Marrosu MG, Murru MR, Borghero G, 2010. Amyotrophic lateral sclerosis–frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations. Arch Neurol 67, 1002–1009. doi: 10.1001/archneurol.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A, Restagno G, Brunetti M, Ossola I, Calvo A, Mora G, Sabatelli M, Monsurrò MR, Battistini S, Mandrioli J, Salvi F, Spataro R, Schymick J, Traynor BJ, La Bella V, 2009. Two Italian kindreds with familial amyotrophic lateral sclerosis due to FUS mutation. Neurobiol Aging 30, 1272–1275. doi: 10.1016/j.neurobiolaging.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipika RH, Finegan E, Li Hi Shing S, McKenna MC, Christidi F, Chang KM, Doherty MA, Hengeveld JC, Vajda A, Pender N, Hutchinson S, Donaghy C, McLaughlin RL, Hardiman O, Bede P, 2020. “Switchboard” malfunction in motor neuron diseases: Selective pathology of thalamic nuclei in amyotrophic lateral sclerosis and primary lateral sclerosis. NeuroImage Clin 27, 102300. doi: 10.1016/j.nicl.2020.102300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christidi F, Karavasilis E, Riederer F, Zalonis I, Ferentinos P, Velonakis G, Xirou S, Rentzos M, Argiropoulos G, Zouvelou V, Zambelis T, Athanasakos A, Toulas P, Vadikolias K, Efstathopoulos E, Kollias S, Karandreas N, Kelekis N, Evdokimidis I, 2018. Gray matter and white matter changes in non-demented amyotrophic lateral sclerosis patients with or without cognitive impairment: A combined voxel-based morphometry and tract-based spatial statistics whole-brain analysis. Brain Imaging Behav 12, 547–563. doi: 10.1007/s11682-017-9722-y [DOI] [PubMed] [Google Scholar]
- Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu Y-F, Wang Q, Krueger BJ, Ren Z, Keebler J, Han Y, Levy SE, Boone BE, Wimbish JR, Waite LL, Jones AL, Carulli JP, Day-Williams AG, Staropoli JF, Xin WW, Chesi A, Raphael AR, McKenna-Yasek D, Cady J, Vianney de Jong JMB, Kenna KP, Smith BN, Topp S, Miller J, Gkazi A, FALS Sequencing Consortium, Al-Chalabi A, van den Berg LH, Veldink J, Silani V, Ticozzi N, Shaw CE, Baloh RH, Appel S, Simpson E, Lagier-Tourenne C, Pulst SM, Gibson S, Trojanowski JQ, Elman L, McCluskey L, Grossman M, Shneider NA, Chung WK, Ravits JM, Glass JD, Sims KB, Van Deerlin VM, Maniatis T, Hayes SD, Ordureau A, Swarup S, Landers J, Baas F, Allen AS, Bedlack RS, Harper JW, Gitler AD, Rouleau GA, Brown R, Harms MB, Cooper GM, Harris T, Myers RM, Goldstein DB, 2015. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347, 1436–1441. doi: 10.1126/science.aaa3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistaro A, Pagani M, Montuschi A, Calvo A, Moglia C, Canosa A, Restagno G, Brunetti M, Traynor BJ, Nobili F, Carrara G, Fania P, Lopiano L, Valentini MC, Chiò A, 2014. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging 41, 844–852. doi: 10.1007/s00259-013-2667-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistaro A, Valentini MC, Chiò A, Nobili F, Calvo A, Moglia C, Montuschi A, Morbelli S, Salmaso D, Fania P, Carrara G, Pagani M, 2012. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging 39, 251–259. doi: 10.1007/s00259-011-1979-6 [DOI] [PubMed] [Google Scholar]
- Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, Li D, Payami H, Awert F, Markopoulou K, Andreadis A, D’Souza I, Lee VM, Reed L, Trojanowski JQ, Zhukareva V, Bird T, Schellenberg G, Wilhelmsen KC, 1998. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci U S A 95, 13103–13107. doi: 10.1073/pnas.95.22.13103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni M, Cappa SF, Dalla Bella E, Contarino VE, Lauria G, 2019. Cortical correlates of behavioural change in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 90, 380–386. doi: 10.1136/jnnp-2018-318619 [DOI] [PubMed] [Google Scholar]
- Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S, Martindale J, Hartley J, Walsh T, Gelsthorpe C, Baxter L, Forster G, Fox M, Bury J, Mok K, McDermott CJ, Traynor BJ, Kirby J, Wharton SB, Ince PG, Hardy J, Shaw PJ, 2012. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 135, 751–764. doi: 10.1093/brain/awr365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Newton J, Lonergan K, Chiwera T, Booth T, Chandran S, Colville S, Heverin M, Mays I, Pal S, Pender N, Pinto-Grau M, Radakovic R, Shaw CE, Stephenson L, Swingler R, Vajda A, Al-Chalabi A, Hardiman O, Abrahams S, 2018. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 91, e1370–e1380. doi: 10.1212/WNL.0000000000006317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Fernández-Seara MA, Seijo-Martínez M, Samaranch L, Lorenzo E, Hinrichs A, Irigoyen J, Maestro C, Prieto E, Martí-Climent JM, Arbizu J, Pastor MA, Pastor P, 2009. Cortical atrophy and language network reorganization associated with a novel progranulin mutation. Cereb Cortex 19, 1751–1760. doi: 10.1093/cercor/bhn202 [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin J-J, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C, 2006. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924. doi: 10.1038/nature05017 [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, McKenna-Yasek D, Chen C, Hedley-Whyte ET, Brown RH, 1998. Limited corticospinal tract involvement in amyotrophic lateral sclerosis subjects with the A4V mutation in the copper/zinc superoxide dismutase gene. Ann Neurol 43, 703–710. doi: 10.1002/ana.410430604 [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, Schoenfeld DA, Hosler BA, Horvitz HR, Brown RH, 1997. Epidemiology of mutations in superoxide dismutase in amyotrophic lateal sclerosis. Ann Neurol 41, 210–221. doi: 10.1002/ana.410410212 [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Hatazawa J, Brooks RA, Di Chiro G, 1987. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann Neurol 22, 580–586. doi: 10.1002/ana.410220504 [DOI] [PubMed] [Google Scholar]
- Daoud H, Suhail H, Szuto A, Camu W, Salachas F, Meininger V, Bouchard J-P, Dupré N, Dion PA, Rouleau GA, 2012. UBQLN2 mutations are rare in French and French–Canadian amyotrophic lateral sclerosis. Neurobiol Aging 33, 2230.e1–2230.e5. doi: 10.1016/j.neurobiolaging.2012.03.015 [DOI] [PubMed] [Google Scholar]
- Day GS, Farb NAS, Tang-Wai DF, Masellis M, Black SE, Freedman M, Pollock BG, Chow TW, 2013. Salience network resting-state activity: prediction of frontotemporal dementia progression. JAMA Neurol 70, 1249–1253. doi: 10.1001/jamaneurol.2013.3258 [DOI] [PubMed] [Google Scholar]
- De Vocht J, Blommaert J, Devrome M, Radwan A, Van Weehaeghe D, De Schaepdryver M, Ceccarini J, Rezaei A, Schramm G, van Aalst J, Chiò A, Pagani M, Stam D, Van Esch H, Lamaire N, Verhaegen M, Mertens N, Poesen K, van den Berg LH, van Es MA, Vandenberghe R, Vandenbulcke M, Van den Stock J, Koole M, Dupont P, Van Laere K, Van Damme P, 2020. Use of multimodal imaging and clinical biomarkers in presymptomatic carriers of C9orf72 repeat expansion. JAMA Neurol. doi: 10.1001/jamaneurol.2020.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung G-YR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R, 2011. Expanded GGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256. doi: 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bo R, Ghezzi S, Corti S, Pandolfo M, Ranieri M, Santoro D, Ghione I, Prelle A, Orsetti V, Mancuso M, Sorarù G, Briani C, Angelini C, Siciliano G, Bresolin N, Comi GP, 2009. TARDBP (TDP-43) sequence analysis in patients with familial and sporadic ALS: identification of two novel mutations. Eur J Neurol 16, 727–732. doi: 10.1111/j.1468-1331.2009.02574.x [DOI] [PubMed] [Google Scholar]
- Del Bo R, Tiloca C, Pensato V, Corrado L, Ratti A, Ticozzi N, Corti S, Castellotti B, Mazzini L, Soraru G, Cereda C, D’Alfonso S, Gellera C, Comi GP, Silani V, The SLAGEN Consortium, 2011. Novel optineurin mutations in patients with familial and sporadic amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 82, 1239–1243. doi: 10.1136/jnnp.2011.242313 [DOI] [PubMed] [Google Scholar]
- Deleon J, Miller BL, 2018. Frontotemporal dementia, in: Handbook of Clinical Neurology. Elsevier, pp. 409–430. doi: 10.1016/B978-0-444-64076-5.00027-2 [DOI] [PubMed] [Google Scholar]
- Delisle MB, Murrell JR, Richardson R, Trofatter JA, Rascol O, Soulages X, Mohr M, Calvas P, Ghetti B, 1999. A mutation at codon 279 (N279K) in exon 10 of the Tau gene causes a tauopathy with dementia and supranuclear palsy. Acta Neuropathol. 98, 62–77. [DOI] [PubMed] [Google Scholar]
- Deng H-X, Chen W, Hong S-T, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T, 2011. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477, 211–215. doi: 10.1038/nature10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deters KD, Risacher SL, Farlow MR, Unverzagt FW, Kareken DA, Hutchins GD, Yoder KK, Murrell JR, Spina S, Epperson F, Gao S, Saykin AJ, Ghetti B, 2014. Cerebral hypometabolism and grey matter density in MAPT intron 10 +3 mutation carriers. Am J Neurodegener Dis 3, 103–114. [PMC free article] [PubMed] [Google Scholar]
- Devenney E, Foxe D, Dobson-Stone C, Kwok JB, Kiernan MC, Hodges JR, 2015. Clinical heterogeneity of the C9orf72 genetic mutation in frontotemporal dementia. Neurocase 21, 535–541. doi: 10.1080/13554794.2014.951058 [DOI] [PubMed] [Google Scholar]
- Devenney E, Hornberger M, Irish M, Mioshi E, Burrell J, Tan R, Kiernan MC, Hodges JR, 2014. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol 71, 331. doi: 10.1001/jamaneurol.2013.6002 [DOI] [PubMed] [Google Scholar]
- Dharmadasa T, Huynh W, Tsugawa J, Shimatani Y, Ma Y, Kiernan MC, 2018. Implications of structural and functional brain changes in amyotrophic lateral sclerosis. Expert Rev Neurother 18, 407–419. doi: 10.1080/14737175.2018.1464912 [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Goldhardt O, Förstl H, Yakushew I, Otto M, Anderl-Straub S, Beer A, Ludolph AC, Landwehrmeyer GB, Levin J, Danek A, Fliessbach K, Spottke A, Fassbender K, Lyros E, Prudlo J, Krause BJ, Volk A, Edbauer D, Schroeter ML, Drzezga A, Kornhuber J, Lauer M, Grimmer T, Lauer M, Grimmer T, 2019. FDG-PET underscores the key role of the thalamus in frontotemporal lobar degeneration caused by C9ORF72 mutations. Transl Psychiatry 9. doi: 10.1038/s41398-019-0381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Förstl H, Schwaiger M, Kurz A, 2007. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging 28, 42–50. doi: 10.1016/j.neurobiolaging.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Djamshidian A, Schaefer J, Haubenberger D, Stogmann E, Zimprich F, Auff E, Zimprich A, 2009. A novel mutation in the VCP gene (G157R) in a german family with inclusion-body myopathy with paget disease of bone and frontotemporal dementia. Muscle Nerve 39, 389–391. doi: 10.1002/mus.21225 [DOI] [PubMed] [Google Scholar]
- Dols-Icardo O, Nebot I, Gorostidi A, Ortega-Cubero S, Hernández I, Rojas-García R, García-Redondo A, Povedano M, Lladó A, Álvarez V, Sánchez-Juan P, Pardo J, Jericó I, Vázquez-Costa J, Sevilla T, Cardona F, Indakoechea B, Moreno F, Fernández-Torrón R, Muñoz-Llahuna L, Moreno-Grau S, Rosende-Roca M, Vela Á, Muñoz-Blanco JL, Combarros O, Coto E, Alcolea D, Fortea J, Lleó A, Sánchez-Valle R, Esteban-Pérez J, Ruiz A, Pastor P, López De Munain A, Pérez-Tur J, Clarimón J, on behalf of the Dementia Genetics Spanish Consortium (DEGESCO), 2015. Analysis of the CHCHD10 gene in patients with frontotemporal dementia and amyotrophic lateral sclerosis from Spain. Brain 138, e400–e400. doi: 10.1093/brain/awv175 [DOI] [PubMed] [Google Scholar]
- Domínguez-Vivero C, Wu L, Lee S, Manoochehri M, Cines S, Brickman AM, Rizvi B, Chesebro A, Gazes Y, Fallon E, Lynch T, Heidebrink JL, Paulson H, Goldman JS, Huey E, Cosentino S, 2020. Structural brain changes in pre-clinical FTD MAPT mutation carriers. J Alzheimers Dis 1–12. doi: 10.3233/JAD-190820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopper EGP, Chalos V, Ghariq E, den Heijer T, Hafkemeijer A, Jiskoot LC, de Koning I, Seelaar H, van Minkelen R, van Osch MJP, Rombouts SARB, van Swieten JC, 2016. Cerebral blood flow in presymptomatic MAPT and GRN mutation carriers: A longitudinal arterial spin labeling study. NeuroImage Clin 12, 460–465. doi: 10.1016/j.nicl.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopper EGP, Rombouts SARB, Jiskoot LC, den Heijer T, de Graaf JRA, de Koning I, Hammerschlag AR, Seelaar H, Seeley WW, Veer IM, van Buchem MA, Rizzu P, van Swieten JC, 2014. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology 83, e19–e26. doi: 10.1212/WNL.0000000000000583 [DOI] [PubMed] [Google Scholar]
- Douaud G, Filippini N, Knight S, Talbot K, Turner MR, 2011. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain 134, 3470–3479. doi: 10.1093/brain/awr279 [DOI] [PubMed] [Google Scholar]
- El Mendili M-M, Cohen-Adad J, Pelegrini-Issac M, Rossignol S, Morizot-Koutlidis R, Marchand-Pauvert V, Iglesias C, Sangari S, Katz R, Lehericy S, Benali H, Pradat P-F, 2014. Multi-parametric spinal cord MRI as potential progression marker in amyotrophic lateral sclerosis. PLoS One 9, e95516. doi: 10.1371/journal.pone.0095516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi FM, Marx G, Cobigo Y, Staffaroni AM, Kornak J, Tosun D, Boxer AL, Kramer JH, Miller BL, Rosen HJ, 2017. Longitudinal white matter change in frontotemporal dementia subtypes and sporadic late onset Alzheimer’s disease. NeuroImage Clin 16, 595–603. doi: 10.1016/j.nicl.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen SF, Østergaard LR, Rodell AB, Østergaard L, Nielsen JE, Isaacs AM, Johannsen P, 2009. Cortical volumes and atrophy rates in FTD-3 CHMP2B mutation carriers and related non-carriers. NeuroImage 45, 713–721. doi: 10.1016/j.neuroimage.2008.12.024 [DOI] [PubMed] [Google Scholar]
- Fahed AC, McDonough B, Gouvion CM, Newell KL, Dure LS, Bebin M, Bick AG, Seidman JG, Harter DH, Seidman CE, 2014. UBQLN2 mutation causing heterogeneous X-linked dominant neurodegeneration. Ann Neurol 75, 793–798. doi: 10.1002/ana.24164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanganiello RD, Kimonis VE, Côrte CC, Nitrini R, Passos-Bueno MR, 2011. A Brazilian family with hereditary inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Braz J Med Biol Res 44, 374–380. doi: 10.1590/S0100-879X2011007500028 [DOI] [PubMed] [Google Scholar]
- Farb NAS, Grady CL, Strother S, Tang-Wai DF, Masellis M, Black S, Freedman M, Pollock BG, Campbell KL, Hasher L, Chow TW, 2013. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex 49, 1856–1873. doi: 10.1016/j.cortex.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Fecto F, 2011. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol 68, 1440. doi: 10.1001/archneurol.2011.250 [DOI] [PubMed] [Google Scholar]
- Feng Shu-Man, Che C, Feng Shu-Yan, Liu C, Li L, Li Y, Huang H, Zou Z, 2019. Novel mutation in optineurin causing aggressive ALS+/−frontotemporal dementia. Ann Clin Transl Neurol 6, 2377–2383. doi: 10.1002/acn3.50928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Agosta F, Abrahams S, Fazekas F, Grosskreutz J, Kalra S, Kassubek J, Silani V, Turner MR, Masdeu JC, 2010. EFNS guidelines on the use of neuroimaging in the management of motor neuron diseases. Eur J Neurol 17, 526–e20. doi: 10.1111/j.1468-1331.2010.02951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Agosta F, Scola E, Canu E, Magnani G, Marcone A, Valsasina P, Caso F, Copetti M, Comi G, Cappa SF, Falini A, 2013. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex 49, 2389–2401. doi: 10.1016/j.cortex.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Floeter MK, Bageac D, Danielian LE, Braun LE, Traynor BJ, Kwan JY, 2016. Longitudinal imaging in C9orf72 mutation carriers: relationship to phenotype. NeuroImage Clin 12, 1035–1043. doi: 10.1016/j.nicl.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Danielian LE, Braun LE, Wu T, 2018. Longitudinal diffusion imaging across the C9orf72 clinical spectrum. J Neurol Neurosurg Psychiatry 89, 53–60. doi: 10.1136/jnnp-2017-316799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris G, Borghero G, Cannas A, Di Stefano F, Murru MR, Corongiu D, Cuccu S, Tranquilli S, Cherchi MV, Serra A, Loi G, Marrosu MG, Chiò A, Marrosu F, 2015. Clinical phenotypes and radiological findings in frontotemporal dementia related to TARDBP mutations. J Neurol 262, 375–384. doi: 10.1007/s00415-014-7575-5 [DOI] [PubMed] [Google Scholar]
- Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Müller K, Marroquin N, Nordin F, Hübers A, Weydt P, Pinto S, Press R, Millecamps S, Molko N, Bernard E, Desnuelle C, Soriani M-H, Dorst J, Graf E, Nordström U, Feiler MS, Putz S, Boeckers TM, Meyer T, Winkler AS, Winkelman J, de Carvalho M, Thal DR, Otto M, Brännström T, Volk AE, Kursula P, Danzer KM, Lichtner P, Dikic I, Meitinger T, Ludolph AC, Strom TM, Andersen PM, Weishaupt JH, 2015. Haploinsufficiency of TBK1 causes familial ALS and frontotemporal dementia. Nat Neurosci 18, 631–636. doi: 10.1038/nn.4000 [DOI] [PubMed] [Google Scholar]
- Fu X, Zhu W, Guo Z, Shu G, Cui F, Yang F, Zhang Y, Ren Y, Zhang Xiaojun, Zhang Xiaolan, Chen Z, Ling L, Huang X, Zhang J, 2017. 18 F-fallypride PET-CT of dopamine D2/D3 receptors in patients with sporadic amyotrophic lateral sclerosis. J Neurol Sci 377, 79–84. doi: 10.1016/j.jns.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Fumagalli GG, Basilico P, Arighi A, Bocchetta M, Dick KM, Cash DM, Harding S, Mercurio M, Fenoglio C, Pietroboni AM, Ghezzi L, van Swieten J, Borroni B, de Mendonça A, Masellis M, Tartaglia MC, Rowe JB, Graff C, Tagliavini F, Frisoni GB, Laforce R, Finger E, Sorbi S, Scarpini E, Rohrer JD, Galimberti D, Andersson C, Archetti S, Benussi L, Binetti G, Black S, Cosseddu M, Fallström M, Ferreira C, Fox NC, Freedman M, Gazzina S, Ghidoni R, Grisoli M, Jelic V, Jiskoot L, Keren R, Lombardi G, Maruta C, Mead S, Meeter L, van Minkelen R, Nacmias B, Öijerstedt L, Ourselin S, Padovani A, Panman J, Pievani M, Polito C, Premi E, Prioni S, Rademakers R, Redaelli V, Rogaeva E, Rossi G, Rossor MN, Tang-Wai D, Thomas DL, Thonberg H, Tiraboschi P, Verdelho A, Warren JD, on behalf of the Genetic FTD Initiative (GENFI), 2018. Distinct patterns of brain atrophy in Genetic Frontotemporal Dementia Initiative (GENFI) cohort revealed by visual rating scales. Alzheimers Res Ther 10, 46. doi: 10.1186/s13195-018-0376-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzina S, Benussi A, Premi E, Paternicò D, Cristillo V, Dell’Era V, Cosseddu M, Archetti S, Alberici A, Gasparotti R, Padovani A, Borroni B, 2018. Neuroanatomical correlates of transcranial magnetic stimulation in presymptomatic granulin mutation carriers. Brain Topogr 31, 488–497. doi: 10.1007/s10548-017-0612-9 [DOI] [PubMed] [Google Scholar]
- Gellera C, Tiloca C, Del Bo R, Corrado L, Pensato V, Agostini J, Cereda C, Ratti A, Castellotti B, Corti S, Bagarotti A, Cagnin A, Milani P, Gabelli C, Riboldi G, Mazzini L, Sorarù G, D’Alfonso S, Taroni F, Comi GP, Ticozzi N, Silani V, Consortium TS, 2013. Ubiquilin 2 mutations in Italian patients with amyotrophic lateral sclerosis and frontotemporal dementia. J Neurol Neurosurg Psychiatry 84, 183–187. doi: 10.1136/jnnp-2012-303433 [DOI] [PubMed] [Google Scholar]
- Gelpi E, van der Zee J, Turon Estrada A, Van Broeckhoven C, Sanchez-Valle R, 2014. TARDBP mutation p.Ile383Val associated with semantic dementia and complex proteinopathy. Neuropathol Appl Neurobiol 40, 225–230. doi: 10.1111/nan.12063 [DOI] [PubMed] [Google Scholar]
- Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M, 2015. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene ( MAPT ) mutations: a chameleon for neuropathology and neuroimaging: MAPT mutations and FTD. Neuropathol Appl Neurobiol 41, 24–46. doi: 10.1111/nan.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti B, Wszolek ZK, Boeve BF, Spina S, Goedert M, 2011. Frontotemporal Dementia and Parkinsonism Linked to Chromosome 17, in: Dickson DW, Weller RO (Eds.), Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Wiley-Blackwell, Oxford, UK, pp. 110–134. doi: 10.1002/9781444341256.ch14 [DOI] [Google Scholar]
- Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Philtjens S, Heeman B, Engelborghs S, Vandenbulcke M, De Baets G, Bäumer V, Cuijt I, Van den Broeck M, Peeters K, Mattheijssens M, Rousseau F, Vandenberghe R, De Jonghe P, Cras P, De Deyn PP, Martin J-J, Cruts M, Van Broeckhoven C, 2015. Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort. Neurology 85, 2116–2125. doi: 10.1212/WNL.0000000000002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, Muñoz C, Ramirez-Restrepo M, Gaiteri C, Myers AJ, Caselli RJ, Kosik KS, Reiman EM, Huentelman MJ, 2013. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol Aging 34, 2077.e11–2077.e18. doi: 10.1016/j.neurobiolaging.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Tortosa E, Serrano S, de Toledo M, Pérez-Pérez J, Sainz MJ, 2014. Familial benign frontotemporal deterioration with C9ORF72 hexanucleotide expansion. Alzheimers Dement 10, S284–S289. doi: 10.1016/j.jalz.2013.09.013 [DOI] [PubMed] [Google Scholar]
- González-Sánchez M, Puertas-Martín V, Esteban-Pérez J, García-Redondo A, Borrego-Hernández D, Méndez-Guerrero A, Llamas-Velasco S, Herrero-San Martín A, Cordero-Vázquez P, Herrero-Manso MC, Pérez-Martínez DA, Villarejo-Galende A, 2018. TARDBP mutation associated with semantic variant primary progressive aphasia, case report and review of the literature. Neurocase 24, 301–305. doi: 10.1080/13554794.2019.1581225 [DOI] [PubMed] [Google Scholar]
- Goode A, Layfield R, 2010. Recent advances in understanding the molecular basis of Paget disease of bone. J Clin Pathol 63, 199–203. doi: 10.1136/jcp.2009.064428 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL, 2004. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55, 335–346. doi: 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M, 2011. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves CV, Rohrer JD, 2019. An update on genetic frontotemporal dementia. J Neurol 266, 2075–2086. doi: 10.1007/s00415-019-09363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V, 2004. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 101, 4637–4642. doi: 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Menon P, Korgaonkar MS, Gomes L, Foster S, Kiernan MC, Vucic S, 2016. Potential structural and functional biomarkers of upper motor neuron dysfunction in ALS. Amyotroph Lateral Scler Frontotemporal Degener 17, 85–92. doi: 10.3109/21678421.2015.1074707 [DOI] [PubMed] [Google Scholar]
- Grosskreutz J, Kaufmann J, Frädrich J, Dengler R, Heinze H-J, Peschel T, 2006. Widespread sensorimotor and frontal cortical atrophy in Amyotrophic Lateral Sclerosis. BMC Neurol 6, 17. doi: 10.1186/1471-2377-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding X-S, Alavi A, Reivich M, 1996. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci 8, 135–154. doi: 10.1162/jocn.1996.8.2.135 [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Lohmann E, Brás JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J, 2013. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol 70, 78–84. doi: 10.1001/jamaneurol.2013.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Gorno-Tempini ML, Gesierich B, Henry M, Trujillo A, Shany-Ur T, Jovicich J, Robinson SD, Kramer JH, Rankin KP, Miller BL, Seeley WW, 2013. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain 136, 2979–2991. doi: 10.1093/brain/awt222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gydesen S, Brown JM, Brun A, Chakrabarti L, Gade A, Johannsen P, Rossor M, Thusgaard T, Grove A, Yancopoulou D, Spillantini MG, Fisher EMC, Collinge J, Sorensen SA, 2002. Chromosome 3 linked frontotemporal dementia (FTD-3). Neurology 59, 1585–1594. doi: 10.1212/01.WNL.0000034763.54161.1F [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A, Möller C, Dopper EGP, Jiskoot LC, Schouten TM, van Swieten JC, van der Flier WM, Vrenken H, Pijnenburg YAL, Barkhof F, Scheltens P, van der Grond J, Rombouts SARB, 2015. Resting state functional connectivity differences between behavioral variant frontotemporal dementia and Alzheimer’s disease. Front Hum Neurosci 9, 474. doi: 10.3389/fnhum.2015.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstock C, Sun K, Choi C, Eurich D, Camicioli R, Johnston W, Kalra S, 2020. Spectroscopic markers of neurodegeneration in the mesial prefrontal cortex predict survival in ALS. Amyotroph Lateral Scler Frontotemporal Degener 1–6. doi: 10.1080/21678421.2020.1727926 [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Brooks RA, Dalakas MC, Mansi L, Chiro GD, 1988. Cortical motor-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J Comput Assist Tomogr 12, 630–636. doi: 10.1097/00004728-198807000-00019 [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, von Hippel C, 2014. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia 56, 53–62. doi: 10.1016/j.neuropsychologia.2013.12.024 [DOI] [PubMed] [Google Scholar]
- Hirano M, Nakamura Y, Saigoh K, Sakamoto H, Ueno S, Isono C, Mitsui Y, Kusunoki S, 2015. VCP gene analyses in Japanese patients with sporadic amyotrophic lateral sclerosis identify a new mutation. Neurobiol Aging 36, 1604.e1–1604.e6. doi: 10.1016/j.neurobiolaging.2014.10.012 [DOI] [PubMed] [Google Scholar]
- Hirano M, Yamagishi Y, Yanagimoto S, Saigoh K, Nakamura Y, Kusunoki S, 2017. Time course of radiological imaging and variable interindividual symptoms in amyotrophic lateral sclerosis and frontotemporal dementia associated with p.Arg487His mutation in the VCP gene. Eur Neurol 78, 78–83. doi: 10.1159/000478906 [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Alfaify OA, Hsiung G-YR, Pottier C, Baker M, Perkerson RB, Rademakers R, Briemberg H, Foti DJ, Mackenzie IR, 2019. Clinicopathologic correlations in a family with a TBK1 mutation presenting as primary progressive aphasia and primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 20, 568–575. doi: 10.1080/21678421.2019.1632347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerpe R, Bett JS, Keuss MJ, Solovyova A, McWilliams TG, Johnson C, Sahu I, Varghese J, Wood N, Wightman M, Osborne G, Bates GP, Glickman MH, Trost M, Knebel A, Marchesi F, Kurz T, 2016. UBQLN2 mediates autophagy-independent protein aggregate clearance by the proteasome. Cell 166, 935–949. doi: 10.1016/j.cell.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, 1996. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc 2, 511–524. doi: 10.1017/S1355617700001685 [DOI] [PubMed] [Google Scholar]
- Huey ED, Ferrari R, Moreno JH, Jensen C, Morris CM, Potocnik F, Kalaria RN, Tierney M, Wassermann EM, Hardy J, Grafman J, Momeni P, 2012. FUS and TDP43 genetic variability in FTD and CBS. Neurobiol Aging 33, 1016.e9–1016.e17. doi: 10.1016/j.neurobiolaging.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Grafman J, Wassermann EM, Pietrini P, Tierney MC, Ghetti B, Spina S, Baker M, Hutton M, Elder JW, Berger SL, Heflin KA, Hardy J, Momeni P, 2006. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol 60, 374–380. doi: 10.1002/ana.20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P, 1998. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705. doi: 10.1038/31508 [DOI] [PubMed] [Google Scholar]
- Iijima M, Tabira T, Poorkaj P, Schellenberg G, Trojanowski J, Lee V, Schmidt M, Takahashi K, Nabika T, Matsumoto T, Yamashita Y, Yoshioka S, Ishino H, 1999. A distinct familial presenile dementia with a novel missense mutation in the tau gene. Neuroreport 10, 497–501. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Okazawa H, Tsujikawa T, Matsunaga A, Yamamura O, Mori T, Hamano T, Kiyono Y, Nakamoto Y, Yoneda M, 2015. Increased oxidative stress is related to disease severity in the ALS motor cortex: A PET study. Neurology 84, 2033–2039. doi: 10.1212/WNL.0000000000001588 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Murata K, Kawase Y, Kawabe K, Kano O, Yoshii Y, Takazawa T, Hirayama T, Iwasaki Y, 2013. Relationship between cervical cord 1 H-magnetic resonance spectroscopy and clinoco-electromyographic profile in amyotrophic lateral sclerosis. Muscle Nerve 47, 61–67. doi: 10.1002/mus.23467 [DOI] [PubMed] [Google Scholar]
- Ince PG, Tomkins J, Slade JY, Thatcher NM, Shaw PJ, 1998. Amyotrophic lateral sclerosis associated with genetic abnormalities in the gene encoding Cu/Zn superoxide dismutase: molecular pathology of five new cases, and comparison with previous reports and 73 sporadic cases of ALS. J Neuropath Exp Neur 57, 895–904. doi: 10.1097/00005072-199810000-00002 [DOI] [PubMed] [Google Scholar]
- Irwin DJ, McMillan CT, Brettschneider J, Libon DJ, Powers J, Rascovsky K, Toledo JB, Boller A, Bekisz J, Chandrasekaran K, Wood EM, Shaw LM, Woo JH, Cook PA, Wolk DA, Arnold SE, Van Deerlin VM, McCluskey LF, Elman L, Lee VM-Y, Trojanowski JQ, Grossman M, 2013. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 84, 163–169. doi: 10.1136/jnnp-2012-303507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs AM, Johannsen P, Holm I, Nielsen E, J., Consortium, Fr., 2011. Frontotemporal dementia caused by CHMP2B mutations. Curr Alzheimer Res 8, 246–251. doi: 10.2174/156720511795563764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Nakamura M, Komure O, Ayaki T, Wate R, Maruyama H, Nakamura Y, Fujita K, Kaneko S, Okamoto Y, Ihara M, Konishi T, Ogasawara K, Hirano A, Kusaka H, Kaji R, Takahashi R, Kawakami H, 2011. Clinicopathologic study on an ALS family with a heterozygous E478G optineurin mutation. Acta Neuropathol 122, 223–229. doi: 10.1007/s00401-011-0842-y [DOI] [PubMed] [Google Scholar]
- Jacova C, Hsiung G-YR, Tawankanjanachot I, Dinelle K, McCormick S, Gonzalez M, Lee H, Sengdy P, Bouchard-Kerr P, Baker M, Rademakers R, Sossi V, Stoessl AJ, Feldman HH, Mackenzie IR, 2013. Anterior brain glucose hypometabolism predates dementia in progranulin mutation carriers. Neurology 81, 1322–1331. doi: 10.1212/WNL.0b013e3182a8237e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen JC, Warrington EK, Morris HR, Lantos P, Brown J, Revesz T, Wood N, Khan MN, Cipolotti L, Fox NC, Rossor MN, 2002. Clinical features of frontotemporal dementia due to the intronic tau 10 +16 mutation. Neurology 58, 1161–1168. doi: 10.1212/WNL.58.8.1161 [DOI] [PubMed] [Google Scholar]
- Jiao B, Sun Q, Yuan Z, Wang J, Zhou L, Yan X, Tang B, Shen L, 2018. Rare TBK1 variants in patients with frontotemporal dementia and amyotrophic lateral sclerosis in a Chinese cohort. Transl Neurodegener 7, 31. doi: 10.1186/s40035-018-0136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao B, Xiao T, Hou L, Gu X, Zhou Y, Zhou L, Tang B, Xu J, Shen L, 2016. High prevalence of CHCHD10 mutation in patients with frontotemporal dementia from China. Brain 139, e21–e21. doi: 10.1093/brain/awv367 [DOI] [PubMed] [Google Scholar]
- Jiskoot LC, Bocchetta M, Nicholas JM, Cash DM, Thomas D, Modat M, Ourselin S, Rombouts SARB, Dopper EGP, Meeter LH, Panman JL, van Minkelen R, van der Ende EL, Donker Kaat L, Pijnenburg YAL, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe J, Graff C, Tagliavini F, Frisoni GB, Laforce R, Finger E, de Mendonça A, Sorbi S, on behalf of the Genetic Frontotemporal dementia Initiative (GENFI), Papma JM, van Swieten JC, Rohrer JD, 2018. Presymptomatic white matter integrity loss in familial frontotemporal dementia in the GENFI cohort: a cross-sectional diffusion tensor imaging study. Ann Clin Transl Neurol 5, 1025–1036. doi: 10.1002/acn3.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiskoot LC, Panman JL, Meeter LH, Dopper EGP, Donker Kaat L, Franzen S, van der Ende EL, van Minkelen R, Rombouts SARB, Papma JM, van Swieten JC, 2019. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain 142, 193–208. doi: 10.1093/brain/awy288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, Ding J, McCluskey L, Martinez-Lage M, Falcone D, Hernandez DG, Arepalli S, Chong S, Schymick JC, Rothstein J, Landi F, Wang Y-D, Calvo A, Mora G, Sabatelli M, Monsurrò MR, Battistini S, Salvi F, Spataro R, Sola P, Borghero G, Galassi G, Scholz SW, Taylor JP, Restagno G, Chiò A, Traynor BJ, 2010. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68, 857–864. doi: 10.1016/j.neuron.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Knopman DS, Graff-Radford J, Syrjanen JA, Senjem ML, Schwarz CG, Dheel C, Wszolek Z, Rademakers R, Kantarci K, Petersen RC, Jack CR, Lowe VJ, Boeve BF, 2018. In vivo 18 F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology 90, e947–e954. doi: 10.1212/WNL.0000000000005117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Jack CR, 2008. Anatomic correlates of stereotypies in frontotemporal lobar degeneration. Neurobiol Aging 29, 1859–1863. doi: 10.1016/j.neurobiolaging.2007.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaivorinne A-L, Bode MK, Paavola L, Tuominen H, Kallio M, Renton AE, Traynor BJ, Moilanen V, Remes AM, 2013. Clinical characteristics of C9ORF72-linked frontotemporal lobar degeneration. Dement Geriatr Cogn Disord Extra 3, 251–262. doi: 10.1159/000351859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada M, Izumi Y, Ayaki T, Nakamura M, Kagawa S, Kudo E, Sako W, Maruyama H, Nishida Y, Kawakami H, Ito H, Kaji R, 2014. Clinicopathologic features of autosomal recessive amyotrophic lateral sclerosis associated with optineurin mutation: Autosomal recessive OPTN-ALS. Neuropathology 34, 64–70. doi: 10.1111/neup.12051 [DOI] [PubMed] [Google Scholar]
- Kantarci K, Boeve BF, Wszolek ZK, Rademakers R, Whitwell JL, Baker MC, Senjem ML, Samikoglu AR, Knopman DS, Petersen RC, Jack CR, 2010. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology 75, 771–778. doi: 10.1212/WNL.0b013e3181f073c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AW, McKay A, Singh PP, Brunet A, Huang EJ, 2017. Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci 18, 325–333. doi: 10.1038/nrn.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Wen N, Fan CC, Yokoyama JS, Kouri N, Ross OA, Höglinger G, Müller U, Ferrari R, Hardy J, Schellenberg GD, Sleiman PM, Momeni P, Hess CP, Miller BL, Sharma M, Van Deerlin V, Smeland OB, Andreassen OA, Dale AM, Desikan RS, for the International Frontotemporal Dementia (FTD)–Genomics Consortium, International Collaboration for Frontotemporal Dementia, Progressive Supranuclear Palsy (PSP) Genetics Consortium, and International Parkinson’s Disease Genomics Consortium, 2018. Selective genetic overlap between amyotrophic lateral sclerosis and diseases of the frontotemporal dementia spectrum. JAMA Neurol 75, 860–875. doi: 10.1001/jamaneurol.2018.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JJM, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, Frackowiak RSJ, Brooks DJ, 1993. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis: A neuropsychological and positron emission tomography study. Brain 116, 1399–1423. doi: 10.1093/brain/116.6.1399 [DOI] [PubMed] [Google Scholar]
- Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, Karydas AM, Wu T, Ketelle RS, Baker MC, Hernandez M-D, Coppola G, Geschwind DH, Rademakers R, Lee SE, Rosen HJ, Rabinovici GD, Seeley WW, Rankin KP, Boxer AL, Miller BL, 2012. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J Neurol Neurosurg Psychiatry 83, 358–364. doi: 10.1136/jnnp-2011-301883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E-J, Park Y-E, Kim D-S, Ahn B-Y, Kim H-S, Chang YH, Kim S-J, Kim H-J, Lee H-W, Seeley WW, Kim S, 2011. Inclusion body myopathy with Paget disease of bone and frontotemporal dementia linked to VCP p.Arg155Cys in a Korean family. Arch Neurol 68, 787–796. doi: 10.1001/archneurol.2010.376 [DOI] [PubMed] [Google Scholar]
- Kim E-J, Sidhu M, Gaus SE, Huang EJ, Hof PR, Miller BL, DeArmond SJ, Seeley WW, 2012. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex 22, 251–259. doi: 10.1093/cercor/bhr004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis VE, Watts GDJ, 2005. Autosomal dominant inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Alzheimer Dis Assoc Disord 19, S44–S47. doi: 10.1097/01.wad.0000183081.76820.5a [DOI] [PubMed] [Google Scholar]
- Kimonis Virginia.E., Mehta SG, Fulchiero EC, Thomasova D, Pasquali M, Boycott K, Neilan EG, Kartashov A, Forman MS, Tucker S, Kimonis K, Mumm S, Whyte MP, Smith CD, Watts GDJ, 2008. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet A 146A, 745–757. doi: 10.1002/ajmg.a.31862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klünemann HH, Ridha BH, Magy L, Wherrett JR, Hemelsoet DM, Keen RW, De Bleecker JL, Rossor MN, Marienhagen J, Klein HE, Peltonen L, Paloneva J, 2005. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology 64, 1502–1507. doi: 10.1212/01.WNL.0000160304.00003.CA [DOI] [PubMed] [Google Scholar]
- Konrad C, Jansen A, Henningsen H, Sommer J, Turski PA, Brooks BR, Knecht S, 2006. Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172, 361–369. doi: 10.1007/s00221-006-0352-7 [DOI] [PubMed] [Google Scholar]
- Koppers M, van Blitterswijk MM, Vlam L, Rowicka PA, van Vught PWJ, Groen EJN, Spliet WGM, Engelen-Lee J, Schelhaas HJ, de Visser M, van der Kooi AJ, van der Pol W-L, Pasterkamp RJ, Veldink JH, van den Berg LH, 2012. VCP mutations in familial and sporadic amyotrophic lateral sclerosis. Neurobiol Aging 33, 837.e7–837.e13. doi: 10.1016/j.neurobiolaging.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Koriath CAM, Bocchetta M, Brotherhood E, Woollacott IOC, Norsworthy P, Simón-Sánchez J, Blauwendraat C, Dick KM, Gordon E, Harding SR, Fox NC, Crutch S, Warren JD, Revesz T, Lashley T, Mead S, Rohrer JD, 2017. The clinical, neuroanatomical, and neuropathologic phenotype of TBK1-associated frontotemporal dementia: a longitudinal case report. Alzheimers Dement (Amst) 6, 75–81. doi: 10.1016/j.dadm.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotan D, Iskender C, Ozoguz Erimis A, Basak AN, 2016. A Turkish Family with a Familial ALS-positive UBQLN2-S340I Mutation. Arch Neuropsychiatr 53, 283–285. doi: 10.5152/npa.2016.12371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach MJ, Waggoner B, Leal SM, Gelber D, Khardori R, Levenstien MA, Shanks CA, Gregg G, Al-Lozi MT, Miller T, Rakowicz W, Lopate G, Florence J, Glosser G, Simmons Z, Morris JC, Whyte MP, Pestronk A, Kimonis VE, 2001. Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab 74, 458–475. doi: 10.1006/mgme.2001.3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, Budka H, Ghetti B, Spina S, 2009. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 24, 1842–1847. doi: 10.1002/mds.22697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, van der Zee J, Hort J, Kristoferitsch W, Leitha T, Höftberger R, Ströbel T, Van Broeckhoven C, Matej R, 2016. Clinicopathological description of two cases with SQSTM1 gene mutation associated with frontotemporal dementia: clinicopathology of SQSTM1 gene mutations. Neuropathology 36, 27–38. doi: 10.1111/neup.12233 [DOI] [PubMed] [Google Scholar]
- Krause S, Göhringer T, Walter MC, Schoser BGH, Reilich P, Linn J, Pöpperl GE, Frölich L, Hentschel F, Lochmüller H, Danek A, 2007. Brain imaging and neuropsychology in late-onset dementia due to a novel mutation (R93C) of valosin-containing protein. Clin Neuropathol 26, 232–240. doi: 10.5414/NPP26232 [DOI] [PubMed] [Google Scholar]
- Kumar V, Islam A, Hassan Md.I., Ahmad F, 2016. Delineating the relationship between amyotrophic lateral sclerosis and frontotemporal dementia: Sequence and structure-based predictions. Biochim Biophys Acta 1862, 1742–1754. doi: 10.1016/j.bbadis.2016.06.011 [DOI] [PubMed] [Google Scholar]
- Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, Piguet O, 2016. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain 139, 986–998. doi: 10.1093/brain/awv387 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH, 2009. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208. doi: 10.1126/science.1166066 [DOI] [PubMed] [Google Scholar]
- Lam BYK, Halliday GM, Irish M, Hodges JR, Piguet O, 2014. Longitudinal white matter changes in frontotemporal dementia subtypes. Hum Brain Mapp 35, 3547–3557. doi: 10.1002/hbm.22420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R, Rohrer JD, Real R, Lubbe SJ, Waite AJ, Blake DJ, Walters RJ, Lashley T, Revesz T, Holton JL, Morris HR, 2019. A novel TBK1 mutation in a family with diverse frontotemporal dementia spectrum disorders. Cold Spring Harb Mol Case Stud 5, a003913. doi: 10.1101/mcs.a003913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lant SB, Robinson AC, Thompson JC, Rollinson S, Pickering-Brown S, Snowden JS, Davidson YS, Gerhard A, Mann DMA, 2014. Patterns of microglial cell activation in frontotemporal lobar degeneration: Microglia and frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 40, 686–696. doi: 10.1111/nan.12092 [DOI] [PubMed] [Google Scholar]
- Lattante S, Le Ber I, Camuzat A, Dayan S, Godard C, Van Bortel I, De Septenville A, Ciura S, Brice A, Kabashi E, 2013a. TREM2 mutations are rare in a French cohort of patients with frontotemporal dementia. Neurobiol Aging 34, 2443.e1–2443.e2. doi: 10.1016/j.neurobiolaging.2013.04.030 [DOI] [PubMed] [Google Scholar]
- Lattante S, Rouleau GA, Kabashi E, 2013b. TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum Mutat 34, 812–826. doi: 10.1002/humu.22319 [DOI] [PubMed] [Google Scholar]
- Le Ber I, 2013. SQSTM1 mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol 70, 1403–1410. doi: 10.1001/jamaneurol.2013.3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, Puel M, Ghanim M, Lacomblez L, Mikol J, Deramecourt V, Lejeune P, de la Sayette V, Belliard S, Vercelletto M, Meyrignac C, Van Broeckhoven C, Lambert J-C, Verpillat P, Campion D, Habert M-O, Dubois B, Brice A, 2008. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 131, 732–746. doi: 10.1093/brain/awn012 [DOI] [PubMed] [Google Scholar]
- Le Ber I, De Septenville A, Guerreiro R, Bras J, Camuzat A, Caroppo P, Lattante S, Couarch P, Kabashi E, Bouya-Ahmed K, Dubois B, Brice A, 2014. Homozygous TREM2 mutation in a family with atypical frontotemporal dementia. Neurobiol Aging 35, 2419.e23–2419.e25. doi: 10.1016/j.neurobiolaging.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc G, Jetté Pomerleau V, McCarthy J, Borroni B, van Swieten J, Galimberti D, Sanchez-Valle R, LaForce R, Moreno F, Synofzik M, Graff C, Masellis M, Tartaglia MC, Rowe JB, Vandenberghe R, Finger E, Tagliavini F, de Mendonça A, Santana I, Butler C, Gerhard A, Danek A, Levin J, Otto M, Frisoni G, Sorbi S, Rohrer JD, Ducharme S, GENetic Frontotemporal dementia Initiative (GENFI), 2020. Faster cortical thinning and surface area loss in presymptomatic and symptomatic C9orf72 repeat expansion adult carriers. Ann Neurol. doi: 10.1002/ana.25748 [DOI] [PubMed] [Google Scholar]
- Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, Takada LT, Karydas AM, Block NR, Coppola G, Pribadi M, Geschwind DH, Rademakers R, Fong JC, Weiner MW, Boxer AL, Kramer JH, Rosen HJ, Miller BL, Seeley WW, 2014. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 137, 3047–3060. doi: 10.1093/brain/awu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Sias AC, Kosik EL, Flagan TM, Deng J, Chu SA, Brown JA, Vidovszky AA, Ramos EM, Gorno-Tempini ML, Karydas AM, Coppola G, Geschwind DH, Rademakers R, Boeve BF, Boxer AL, Rosen HJ, Miller BL, Seeley WW, 2019. Thalamo-cortical network hyperconnectivity in preclinical progranulin mutation carriers. NeuroImage Clin 22, 101751. doi: 10.1016/j.nicl.2019.101751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Sias AC, Mandelli ML, Brown JA, Brown AB, Khazenzon AM, Vidovszky AA, Zanto TP, Karydas AM, Pribadi M, Dokuru D, Coppola G, Geschwind DH, Rademakers R, Gorno-Tempini ML, Rosen HJ, Miller BL, Seeley WW, 2017. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. NeuroImage Clin 14, 286–297. doi: 10.1016/j.nicl.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger GC, Banks SJ, Leverenz JB, Bekris LM, 2017. Behavioral variant FTD caused by UBQLN2 P525S mutation, without evidence of motor neuron disease. Alzheimers Dement 13, P1492. doi: 10.1016/j.jalz.2017.07.579 [DOI] [Google Scholar]
- Lesage S, Le Ber I, Condroyer C, Broussolle E, Gabelle A, Thobois S, Pasquier F, Mondon K, Dion PA, Rochefort D, Rouleau GA, Dürr A, Brice A, for the French Parkinson’s Disease Genetics (PDG) Study Group, 2013. C9orf72 repeat expansions are a rare genetic cause of parkinsonism. Brain 136, 385–391. doi: 10.1093/brain/aws357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M, 2012. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One 7, e43993. doi: 10.1371/journal.pone.0043993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Duno M, Batbayli M, Puschmann A, Braendgaard H, Mardosiene S, Svenstrup K, Pinborg L, Vestergaard K, Hjermind L, Stokholm J, Andersen B, Johannsen P, Nielsen J, 2013. Corticobasal and ataxia syndromes widen the spectrum of C9ORF72 hexanucleotide expansion disease. Clin Genet 83, 279–283. doi: 10.1111/j.1399-0004.2012.01903.x [DOI] [PubMed] [Google Scholar]
- Lindquist SG, Braendgaard H, Svenstrup K, Isaacs AM, Nielsen JE, on behalf of the FReJA Consortium, 2008. Frontotemporal dementia linked to chromosome 3 (FTD-3) - current concepts and the detection of a previously unknown branch of the Danish FTD-3 family: frontotemporal dementia linked to chromosome 3. Eur J Neurol 15, 667–670. doi: 10.1111/j.1468-1331.2008.02144.x [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu J-T, Zong Y, Zhou J, Tan L, 2014. C9ORF72 mutations in neurodegenerative diseases. Mol Neurobiol 49, 386–398. doi: 10.1007/s12035-013-8528-1 [DOI] [PubMed] [Google Scholar]
- Llamas-Velasco S, García-Redondo A, Herrero-San Martín A, Puertas Martín V, González-Sánchez M, Pérez-Martínez DA, Villarejo-Galende A, 2018. Slowly progressive behavioral frontotemporal dementia with C9orf72 mutation. Case report and review of the literature. Neurocase 24, 68–71. doi: 10.1080/13554794.2018.1428353 [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Richardson MP, Brooks DJ, Al-Chalabi A, Leigh PN, 2000. Extramotor involvement in ALS: PET studies with the GABAA ligand [11C]flumazenil. Brain 123, 2289–2296. doi: 10.1093/brain/123.11.2289 [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B, 2002. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59, 1077–1079. doi: 10.1212/WNL.59.7.1077 [DOI] [PubMed] [Google Scholar]
- Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang H-Y, Shang Y, Oldham MC, Martens LH, Gao F, Coppola G, Sloan SA, Hsieh CL, Kim CC, Bigio EH, Weintraub S, Mesulam M-M, Rademakers R, Mackenzie IR, Seeley WW, Karydas A, Miller BL, Borroni B, Ghidoni R, Farese RV, Paz JT, Barres BA, Huang EJ, 2016. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935. doi: 10.1016/j.cell.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis E, Ortiz A, Eudave L, Ortega-Cubero S, Borroni B, van der Zee J, Gazzina S, Caroppo P, Rubino E, D’Agata F, Le Ber I, Santana I, Cunha G, Almeida MR, Boutoleau-Bretonnière C, Hannequin D, Wallon D, Rainero I, Galimberti D, Van Broeckhoven C, Pastor MA, Pastor P, 2016. Neuroimaging correlates of frontotemporal dementia associated with SQSTM1 mutations. J Alzheimers Dis 53, 303–313. doi: 10.3233/JAD-160006 [DOI] [PubMed] [Google Scholar]
- Lunau L, Mouridsen K, Rodell A, Østergaard L, Nielsen JE, Isaacs A, Johannsen P, The FReJA Consortium, 2012. Presymptomatic cerebral blood flow changes in CHMP2B mutation carriers of familial frontotemporal dementia (FTD-3), measured with MRI. BMJ Open 2, e000368. doi: 10.1136/bmjopen-2011-000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T, Sano M, Marder KS, Bell KL, Foster NL, Defendini RF, Sima AA, Keohane C, Nygaard TG, Fahn S, 1994. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology 44, 1878–1884. doi: 10.1212/wnl.44.10.1878 [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Arzberger T, Kremmer E, Troost D, Lorenzl S, Mori K, Weng S-M, Haass C, Kretzschmar HA, Edbauer D, Neumann M, 2013. Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta Neuropathol 126, 859–879. doi: 10.1007/s00401-013-1181-y [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Baker M, Pickering-Brown S, Hsiung G-YR, Lindholm C, Dwosh E, Gass J, Cannon A, Rademakers R, Hutton M, Feldman HH, 2006. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129, 3081–3090. doi: 10.1093/brain/awl271 [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM-Y, Trojanowski JQ, 2007. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61, 427–434. doi: 10.1002/ana.21147 [DOI] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, Rossor MN, Hardy J, Collinge J, Revesz T, Mead S, Warren JD, 2012a. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 135, 736–750. doi: 10.1093/brain/awr361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Downey LE, Ridgway GR, Beck J, Clegg S, Blair M, Finnegan S, Leung KK, Yeatman T, Golden H, Mead S, Rohrer JD, Fox NC, Warren JD, 2012b. Longitudinal neuroimaging and neuropsychological profiles of frontotemporal dementia with C9ORF72 expansions. Alzheimers Res Ther 4, 41. doi: 10.1186/alzrt144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Malone IB, Ridgway GR, Buckley AH, Downey LE, Golden HL, Ryan NS, Ourselin S, Schott JM, Rossor MN, Fox NC, Warren JD, 2013. White matter tract signatures of the progressive aphasias. Neurobiol Aging 34, 1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Ridgway GR, Malone IB, Downey LE, Beck J, Kinnunen KM, Schmitz N, Golden HL, Rohrer JD, Schott JM, Rossor MN, Ourselin S, Mead S, Fox NC, Warren JD, 2014. Profiles of white matter tract pathology in frontotemporal dementia. Hum Brain Mapp 35, 4163–4179. doi: 10.1002/hbm.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Simpson IJA, Nicholas JM, Fletcher PD, Downey LE, Golden HL, Clark CN, Schmitz N, Rohrer JD, Schott JM, Zhang H, Ourselin S, Warren JD, Fox NC, 2015. Longitudinal diffusion tensor imaging in frontotemporal dementia. Ann Neurol 77, 33–46. doi: 10.1002/ana.24296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, Amirbekian B, Dronkers N, Miller BL, Henry RG, Gorno-Tempini ML, 2014. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci 34, 9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini C, Cistaro A, Campi C, Calvo A, Caponnetto C, Nobili FM, Fania P, Beltrametti MC, Moglia C, Novi G, Buschiazzo A, Perasso A, Canosa A, Scialò C, Pomposelli E, Massone AM, Bagnara MC, Cammarosano S, Bruzzi P, Morbelli S, Sambuceti G, Mancardi G, Piana M, Chiò A, 2016. A PET/CT approach to spinal cord metabolism in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging 43, 2061–2071. doi: 10.1007/s00259-016-3440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin N, Stranz S, Müller K, Wieland T, Ruf WP, Brockmann SJ, Danzer KM, Borck G, Hübers A, Weydt P, Meitinger T, Strom T-M, Rosenbohm A, Ludolph AC, Weishaupt JH, 2016. Screening for CHCHD10 mutations in a large cohort of sporadic ALS patients: no evidence for pathogenicity of the p.P34S variant. Brain 139, e8–e8. doi: 10.1093/brain/awv218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Senda J, Watanabe H, Epifanio B, Tanaka Y, Imai K, Riku Y, Li Y, Nakamura R, Ito M, Ishigaki S, Atsuta N, Koike H, Katsuno M, Hattori N, Naganawa S, Sobue G, 2016. Involvement of the caudate nucleus head and its networks in sporadic amyotrophic lateral sclerosis-frontotemporal dementia continuum. Amyotroph Lateral Scler Frontotemporal Degener 17, 571–579. doi: 10.1080/21678421.2016.1211151 [DOI] [PubMed] [Google Scholar]
- McCombe PA, Ngo ST, Guo CC, Fazlollahi A, Bollmann S, Wang L, Hu X, Barth M, Salvado O, Davis M, Ceslis A, Robinson G, Henderson RD, Steyn FJ, 2018. Patient with ALS with a novel TBK1 mutation, widespread brain involvement, behaviour changes and metabolic dysfunction. J Neurol Neurosurg Psychiatry 90, 952–954. doi: 10.1136/jnnp-2018-318823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Russ J, Wood EM, Irwin DJ, Grossman M, McCluskey L, Elman L, Van Deerlin V, Lee EB, 2015. C9orf72 promoter hypermethylation is neuroprotective: Neuroimaging and neuropathologic evidence. Neurology 84, 1622–1630. doi: 10.1212/WNL.0000000000001495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke RAL, Körner S, Filippini N, Douaud G, Knight S, Talbot K, Turner MR, 2014. Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain 137, 2546–2555. doi: 10.1093/brain/awu162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke RAL, Proudfoot M, Talbot K, Turner MR, 2018. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. NeuroImage Clin 17, 953–961. doi: 10.1016/j.nicl.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke RAL, Proudfoot M, Wuu J, Andersen PM, Talbot K, Benatar M, Turner MR, 2016. Increased functional connectivity common to symptomatic amyotrophic lateral sclerosis and those at genetic risk. J Neurol Neurosurg Psychiatry 87, 580–588. doi: 10.1136/jnnp-2015-311945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, 2003. Primary progressive aphasia — a language-based dementia. N Engl J Med 349, 1535–1542. doi: 10.1056/NEJMra022435 [DOI] [PubMed] [Google Scholar]
- Mezzapesa DM, D’Errico E, Tortelli R, Distaso E, Cortese R, Tursi M, Federico F, Zoccolella S, Logroscino G, Dicuonzo F, Simone IL, 2013. Cortical thinning and clinical heterogeneity in amyotrophic lateral sclerosis. PLoS One 8, e80748. doi: 10.1371/journal.pone.0080748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps S, Boillée S, Le Ber I, Seilhean D, Teyssou E, Giraudeau M, Moigneu C, Vandenberghe N, Danel-Brunaud V, Corcia P, Pradat P-F, Le Forestier N, Lacomblez L, Bruneteau G, Camu W, Brice A, Cazeneuve C, LeGuern E, Meininger V, Salachas F, 2012. Phenotype difference between ALS patients with expanded repeats in C9ORF72 and patients with mutations in other ALS-related genes. J Med Genet 49, 258–263. doi: 10.1136/jmedgenet-2011-100699 [DOI] [PubMed] [Google Scholar]
- Miyoshi M, Shinotoh H, Wszolek ZK, Strongosky AJ, Shimada H, Arakawa R, Higuchi M, Ikoma Y, Yasuno F, Fukushi K, Irie T, Ito H, Suhara T, 2010. In vivo detection of neuropathologic changes in presymptomatic MAPT mutation carriers: a PET and MRI study. Parkinsonism Relat Disord 16, 404–408. doi: 10.1016/j.parkreldis.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Münte TF, 2009. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp Neurol 217, 147–153. doi: 10.1016/j.expneurol.2009.01.025 [DOI] [PubMed] [Google Scholar]
- Montalbetti L, Ratti MT, Greco B, Aprile C, Moglia A, Soragna D, 2005. Neuropsychological tests and functional nuclear neuroimaging provide evidence of subclinical impairment in Nasu-Hakola disease heterozygotes. Funct Neurol 20, 71–75. [PubMed] [Google Scholar]
- Morbelli S, Ferrara M, Fiz F, Dessi B, Arnaldi D, Picco A, Bossert I, Buschiazzo A, Accardo J, Picori L, Girtler N, Mandich P, Pagani M, Sambuceti G, Nobili F, 2016. Mapping brain morphological and functional conversion patterns in predementia late-onset bvFTD. Eur J Nucl Med Mol I 43, 1337–1347. doi: 10.1007/s00259-016-3335-3 [DOI] [PubMed] [Google Scholar]
- Moreno F, Rabinovici GD, Karydas A, Miller Z, Hsu SC, Legati A, Fong J, Schonhaut D, Esselmann H, Watson C, Stephens ML, Kramer J, Wiltfang J, Seeley WW, Miller BL, Coppola G, Grinberg LT, 2015. A novel mutation P112H in the TARDBP gene associated with frontotemporal lobar degeneration without motor neuron disease and abundant neuritic amyloid plaques. Acta Neuropathol Commun 3, 19. doi: 10.1186/s40478-015-0190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno F, Sala-Llonch R, Barandiaran M, Sánchez-Valle R, Estanga A, Bartrés-Faz D, Sistiaga A, Alzualde A, Fernández E, Martí Massó JF, López de Munain A, Indakoetxea B, 2013. Distinctive age-related temporal cortical thinning in asymptomatic granulin gene mutation carriers. Neurobiol Aging 34, 1462–1468. doi: 10.1016/j.neurobiolaging.2012.11.005 [DOI] [PubMed] [Google Scholar]
- Mori K, Weng S-M, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, Haass C, Edbauer D, 2013. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339, 1335–1338. doi: 10.1126/science.1232927 [DOI] [PubMed] [Google Scholar]
- Müller H-P, Agosta F, Riva N, Spinelli EG, Comi G, Ludolph AC, Filippi M, Kassubek J, 2018. Fast progressive lower motor neuron disease is an ALS variant: A two-centre tract of interest-based MRI data analysis. NeuroImage Clin 17, 145–152. doi: 10.1016/j.nicl.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H-P, Del Tredici K, Lulé D, Müller K, Weishaupt JH, Ludolph AC, Kassubek J, 2020. In vivo histopathological staging in C9orf72-associated ALS: A tract of interest DTI study. NeuroImage Clin 27, 102298. doi: 10.1016/j.nicl.2020.102298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H-P, Turner MR, Grosskreutz J, Abrahams S, Bede P, Govind V, Prudlo J, Ludolph AC, Filippi M, Kassubek J, 2016. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 87, 570–579. doi: 10.1136/jnnp-2015-311952 [DOI] [PubMed] [Google Scholar]
- Müller K, Andersen PM, Hübers A, Marroquin N, Volk AE, Danzer KM, Meitinger T, Ludolph AC, Strom TM, Weishaupt JH, 2014. Two novel mutations in conserved codons indicate that CHCHD10 is a gene associated with motor neuron disease. Brain 137, e309–e309. doi: 10.1093/brain/awu227 [DOI] [PubMed] [Google Scholar]
- Munoz DG, Ros R, Fatas M, Bermejo F, de Yebenes JG, 2007. Progressive nonfluent aphasia associated with a new mutation V363I in tau gene. Am J Alzheimers Dis Other Demen 22, 294–299. doi: 10.1177/1533317507302320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NA, Arthur KC, Tienari PJ, Houlden H, Chiò A, Traynor BJ, 2017. Age-related penetrance of the C9orf72 repeat expansion. Sci Rep 7, 2116. doi: 10.1038/s41598-017-02364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, Rademakers R, Dickson DW, 2011. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol 122, 673–690. doi: 10.1007/s00401-011-0907-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts HJMM, Mirza SS, Petr J, Thomas DL, Cash DM, Bocchetta M, de Vita E, Metcalfe AWS, Shirzadi Z, Robertson AD, Tartaglia MC, Mitchell SB, Black SE, Freedman M, Tang-Wai D, Keren R, Rogaeva E, van Swieten J, Laforce R, Tagliavini F, Borroni B, Galimberti D, Rowe JB, Graff C, Frisoni GB, Finger E, Sorbi S, de Mendonça A, Rohrer JD, MacIntosh BJ, Masellis M, GENetic Frontotemporal dementia Initiative (GENFI), Andersson, C., Archetti S, Arighi A, Benussi L, Binetti G, Cosseddu M, Dick KM, Fallström M, Ferreira C, Fenoglio C, Fox NC, Fumagalli G, Gazzina S, Ghidoni R, Grisoli M, Jelic V, Jiskoot L, Lombardi G, Maruta C, Mead S, Meeter L, van Minkelen R, Nacmias B, Öijerstedt L, Ourselin S, Padovani A, Panman J, Pievani M, Polito C, Premi E, Prioni S, Rademakers R, Redaelli V, Rossi G, Rossor MN, Scarpini E, Thonberg H, Tiraboschi P, Verdelho A, Warren JD, 2019. Cerebral perfusion changes in presymptomatic genetic frontotemporal dementia: a GENFI study. Brain 142, 1108–1120. doi: 10.1093/brain/awz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF, 1998. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. doi: 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Neumann M, Mackenzie IRA, 2019. Review: neuropathology of non-tau frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 45, 19–40. doi: 10.1111/nan.12526 [DOI] [PubMed] [Google Scholar]
- Ng M-C, Ho JT, Ho S-L, Lee R, Li G, Cheng T-S, Song Y-Q, Ho PW-L, Fong GC-Y, Mak W, Chan K-H, Li LS-W, Luk KD-K, Hu Y, Ramsden DB, Leong LL-Y, 2008. Abnormal diffusion tensor in nonsymptomatic familial amyotrophic lateral sclerosis with a causative superoxide dismutase 1 mutation. J Magn Reson Imaging 27, 8–13. doi: 10.1002/jmri.21217 [DOI] [PubMed] [Google Scholar]
- Nguyen HP, Van Broeckhoven C, van der Zee J, 2018. ALS genes in the genomic era and their implications for FTD. Trends Genet 34, 404–423. doi: 10.1016/j.tig.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Olm CA, McMillan CT, Irwin DJ, Van Deerlin VM, Cook PA, Gee JC, Grossman M, 2018. Longitudinal structural gray matter and white matter MRI changes in presymptomatic progranulin mutation carriers. NeuroImage Clin 19, 497–506. doi: 10.1016/j.nicl.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney NT, Ong E, Goh S-YM, Bajorek L, Dever R, Staffaroni AM, Cobigo Y, Bock M, Chiang K, Ljubenkov P, Kornak J, Heuer HW, Wang P, Rascovsky K, Wolf A, Appleby B, Bove J, Bordelon Y, Brannelly P, Brushaber D, Caso C, Coppola G, Dickerson BC, Dickinson S, Domoto-Reilly K, Faber K, Ferrall J, Fields J, Fishman A, Fong J, Foroud T, Forsberg LK, Gearhart DJ, Ghazanfari B, Ghoshal N, Goldman J, Graff-Radford J, Graff-Radford NR, Grant I, Grossman M, Haley D, Hsiung G, Huey ED, Irwin DJ, Jones DT, Kantarci K, Karydas AM, Kaufer D, Kerwin D, Knopman DS, Kramer JH, Kraft R, Kremers W, Kukull W, Lapid MI, Litvan I, Mackenzie IR, Maldonado M, Manoochehri M, McGinnis SM, McKinley EC, Mendez MF, Miller BL, Onyike C, Pantelyat A, Pearlman R, Petrucelli L, Potter M, Rademakers R, Ramos EM, Rankin KP, Roberson ED, Rogalski E, Sengdy P, Shaw LM, Syrjanen J, Tartaglia MC, Tatton N, Taylor J, Toga A, Trojanowski JQ, Weintraub S, Wong B, Wszolek Z, Boxer AL, Boeve BF, Rosen HJ, 2020. Clinical and volumetric changes with increasing functional impairment in familial frontotemporal lobar degeneration. Alzheimers Dement 16, 49–59. doi: 10.1016/j.jalz.2019.08.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer T, Finegan E, Hutchinson S, Doherty M, Vajda A, McLaughlin RL, Pender N, Hardiman O, Bede P, 2017. Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener 18, 611–623. doi: 10.1080/21678421.2017.1332077 [DOI] [PubMed] [Google Scholar]
- Origone P, Geroldi A, Lamp M, Sanguineri F, Caponnetto C, Cabona C, Gotta F, Trevisan L, Bellone E, Manganelli F, Devigili G, Mandich P, 2018. Role of MAPT in pure motor neuron disease: report of a recurrent mutation in Italian patients. Neurodegener Dis 18, 310–314. doi: 10.1159/000497820 [DOI] [PubMed] [Google Scholar]
- Pagani M, Chio A, Valentini MC, Oberg J, Nobili F, Calvo A, Moglia C, Bertuzzo D, Morbelli S, De Carli F, Fania P, Cistaro A, 2014. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 83, 1067–1074. doi: 10.1212/WNL.0000000000000792 [DOI] [PubMed] [Google Scholar]
- Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjærg L, Konttinen Y, Peltonen L, 2002. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71, 656–662. doi: 10.1086/342259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panman JL, Jiskoot LC, Bouts MJRJ, Meeter LHH, van der Ende EL, Poos JM, Feis RA, Kievit AJA, van Minkelen R, Dopper EGP, Rombouts SARB, van Swieten JC, Papma JM, 2019. Gray and white matter changes in presymptomatic genetic frontotemporal dementia: a longitudinal MRI study. Neurobiol Aging 76, 115–124. doi: 10.1016/j.neurobiolaging.2018.12.017 [DOI] [PubMed] [Google Scholar]
- Papma JM, Jiskoot LC, Panman JL, Dopper EG, den Heijer T, Donker Kaat L, Pijnenburg YAL, Meeter LH, van Minkelen R, Rombouts SARB, van Swieten JC, 2017. Cognition and gray and white matter characteristics of presymptomatic C9orf72 repeat expansion. Neurology 89, 1256–1264. doi: 10.1212/WNL.0000000000004393 [DOI] [PubMed] [Google Scholar]
- Paternicò D, Premi E, Gazzina S, Cosseddu M, Alberici A, Archetti S, Cotelli MS, Micheli A, Turla M, Gasparotti R, Padovani A, Borroni B, 2016. White matter hyperintensities characterize monogenic frontotemporal dementia with granulin mutations. Neurobiol Aging 38, 176–180. doi: 10.1016/j.neurobiolaging.2015.11.011 [DOI] [PubMed] [Google Scholar]
- Penttilä S, Jokela M, Bouquin H, Saukkonen AM, Toivanen J, Udd B, 2015. Late onset spinal motor neuronopathy is caused by mutation in CHCHD10. Ann Neurol 77, 163–172. doi: 10.1002/ana.24319 [DOI] [PubMed] [Google Scholar]
- Perry DC, Lehmann M, Yokoyama JS, Karydas A, Lee JJ, Coppola G, Grinberg LT, Geschwind D, Seeley WW, Miller BL, Rosen H, Rabinovici G, 2013. Progranulin mutations as risk factors for Alzheimer disease. JAMA Neurol 70, 774–778. doi: 10.1001/2013.jamaneurol.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Whitwell JL, Boeve BF, Pankratz VS, Knopman DS, Petersen RC, Jack CR, Josephs KA, 2012. Voxel-based morphometry in patients with obsessivecompulsive behaviors in behavioral variant frontotemporal dementia: Compulsions in FTD. Eur J Neurol 19, 911–917. doi: 10.1111/j.1468-1331.2011.03656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau TL, Leavitt BR, 2014. Progranulin in neurodegenerative disease. Trends Neurosci 37, 388–398. doi: 10.1016/j.tins.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Pievani M, Paternicò D, Benussi L, Binetti G, Orlandini A, Cobelli M, Magnaldi S, Ghidoni R, Frisoni GB, 2014. Pattern of structural and functional brain abnormalities in asymptomatic granulin mutation carriers. Alzheimers Dement 10, S354–S363.e1. doi: 10.1016/j.jalz.2013.09.009 [DOI] [PubMed] [Google Scholar]
- Piguet O, Petersén Å, Yin Ka Lam B, Gabery S, Murphy K, Hodges JR, Halliday GM, 2011. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann Neurol 69, 312–319. doi: 10.1002/ana.22244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD, 1998. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43, 815–825. doi: 10.1002/ana.410430617 [DOI] [PubMed] [Google Scholar]
- Popuri K, Dowds E, Beg MF, Balachandar R, Bhalla M, Jacova C, Buller A, Slack P, Sengdy P, Rademakers R, Wittenberg D, Feldman HH, Mackenzie IR, Hsiung G-YR, 2018. Gray matter changes in asymptomatic C9orf72 and GRN mutation carriers. NeuroImage Clin 18, 591–598. doi: 10.1016/j.nicl.2018.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C, Bieniek KF, Finch N, van de Vorst M, Baker M, Perkersen R, Brown P, Ravenscroft T, van Blitterswijk M, Nicholson AM, DeTure M, Knopman DS, Josephs KA, Parisi JE, Petersen RC, Boylan KB, Boeve BF, Graff-Radford NR, Veltman JA, Gilissen C, Murray ME, Dickson DW, Rademakers R, 2015. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol 130, 77–92. doi: 10.1007/s00401-015-1436-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poujois A, Schneider FC, Faillenot I, Camdessanché J-P, Vandenberghe N, Thomas-Antérion C, Antoine JC, 2013. Brain plasticity in the motor network is correlated with disease progression in amyotrophic lateral sclerosis: functional cerebral reorganization, disease progression, and ALS. Hum Brain Mapp 34, 2391–2401. doi: 10.1002/hbm.22070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premi E, Cauda F, Costa T, Diano M, Gazzina S, Gualeni V, Alberici A, Archetti S, Magoni M, Gasparotti R, Padovani A, Borroni B, 2016. Looking for neuroimaging markers in frontotemporal lobar degeneration clinical trials: a multi-voxel pattern analysis study in granulin disease. J Alzheimers Dis 51, 249–262. doi: 10.3233/JAD-150340 [DOI] [PubMed] [Google Scholar]
- Premi E, Cauda F, Gasparotti R, Diano M, Silvana Archetti, Padovani A, Borroni B, 2014. Multimodal fMRI resting-state functional connectivity in granulin mutations: the case of fronto-parietal dementia. PLoS One 9, e106500. doi: 10.1371/journal.pone.0106500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyra T, Hui B, Hanstock C, Concha L, Wong JCT, Beaulieu C, Johnston W, Kalra S, 2010. Combined structural and neurochemical evaluation of the corticospinal tract in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11, 157–165. doi: 10.3109/17482960902756473 [DOI] [PubMed] [Google Scholar]
- Qiu T, Zhang Y, Tang X, Liu X, Wang Y, Zhou C, Luo C, Zhang J, 2019. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: a multimodal MRI analysis. Hum Brain Mapp 40, 3464–3474. doi: 10.1002/hbm.24609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querin G, Bede P, El Mendili MM, Li M, Pélégrini-Issac M, Rinaldi D, Catala M, Saracino D, Salachas F, Camuzat A, Marchand-Pauvert V, Cohen-Adad J, Colliot O, Le Ber I, Pradat P, for The Predict to Prevent Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis (PREV DEMALS) Study Group, 2019. Presymptomatic spinal cord pathology in c9orf72 mutation carriers: a longitudinal neuroimaging study. Ann Neurol. doi: 10.1002/ana.25520 [DOI] [PubMed] [Google Scholar]
- Rademakers R, Stewart H, Dejesus-Hernandez M, Krieger C, Graff-Radford N, Fabros M, Briemberg H, Cashman N, Eisen A, Mackenzie IRA, 2010. Fus gene mutations in familial and sporadic amyotrophic lateral sclerosis. Muscle Nerve 42, 170–176. doi: 10.1002/mus.21665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL, 2006. Structural anatomy of empathy in neurodegenerative disease. Brain 129, 2945–2956. doi: 10.1093/brain/awl254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini M-L, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL, 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, Josephs KA, Knopman DS, White CL, Caselli R, Mackenzie IR, Miller BL, Boczarska-Jedynak M, Opala G, Krygowska-Wajs A, Barcikowska M, Younkin SG, Petersen RC, Ertekin-Taner N, Uitti RJ, Meschia JF, Boylan KB, Boeve BF, Graff-Radford NR, Wszolek ZK, Dickson DW, Rademakers R, Ross OA, 2013. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener 8, 19. doi: 10.1186/1750-1326-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Majcher V, Searle MS, Layfield R, 2014. SQSTM1 mutations – bridging Paget disease of bone and ALS/FTLD. Exp Cell Res 325, 27–37. doi: 10.1016/j.yexcr.2014.01.020 [DOI] [PubMed] [Google Scholar]
- Renton AE, Chiò A, Traynor BJ, 2014. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17, 17–23. doi: 10.1038/nn.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita V-M, Kaivorinne A-L, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ, 2011. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268. doi: 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, 2002. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295, 1077–1079. doi: 10.1126/science.1066901 [DOI] [PubMed] [Google Scholar]
- Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE, 2005. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65, 586–590. doi: 10.1212/01.wnl.0000172911.39167.b6 [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam M-M, 2011. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 76, 1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ahsan RL, Isaacs AM, Nielsen JE, Ostergaard L, Scahill R, Warren JD, Rossor MN, Fox NC, Johannsen P, 2009a. Presymptomatic generalized brain atrophy in frontotemporal dementia caused by CHMP2B mutation. Dement Geriatr Cogn Disord 27, 182–186. doi: 10.1159/000200466 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Clarkson MJ, Kittus R, Rossor MN, Ourselin S, Warren JD, Fox NC, 2012. Rates of hemispheric and lobar atrophy in the language variants of frontotemporal lobar degeneration. J Alzheimers Dis 30, 407–411. doi: 10.3233/JAD-2012-111556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Guerreiro R, Vandrovcova J, Uphill J, Reiman D, Beck J, Isaacs AM, Authier A, Ferrari R, Fox NC, Mackenzie IRA, Warren JD, de Silva R, Holton J, Revesz T, Hardy J, Mead S, Rossor MN, 2009b. The heritability and genetics of frontotemporal lobar degeneration. Neurology 73, 1451–1456. doi: 10.1212/WNL.0b013e3181bf997a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, de Silva R, Warrington E, Troakes C, Al-Sarraj S, King A, Borroni B, Clarkson MJ, Ourselin S, Holton JL, Fox NC, Revesz T, Rossor MN, Warren JD, 2011a. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134, 2565–2581. doi: 10.1093/brain/awr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, van Minkelen R, Rombouts SA, Cardoso MJ, Clegg S, Espak M, Mead S, Thomas DL, De Vita E, Masellis M, Black SE, Freedman M, Keren R, MacIntosh BJ, Rogaeva E, Tang-Wai D, Tartaglia MC, Laforce R, Tagliavini F, Tiraboschi P, Redaelli V, Prioni S, Grisoli M, Borroni B, Padovani A, Galimberti D, Scarpini E, Arighi A, Fumagalli G, Rowe JB, Coyle-Gilchrist I, Graff C, Fallström M, Jelic V, Ståhlbom AK, Andersson C, Thonberg H, Lilius L, Frisoni GB, Binetti G, Pievani M, Bocchetta M, Benussi L, Ghidoni R, Finger E, Sorbi S, Nacmias B, Lombardi G, Polito C, Warren JD, Ourselin S, Fox NC, Rossor MN, 2015. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 14, 253–262. doi: 10.1016/S1474-4422(14)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, Rossor MN, Warren JD, 2010. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage 53, 1070–1076. doi: 10.1016/j.neuroimage.2009.12.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, 2011. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol 24, 542–549. doi: 10.1097/WCO.0b013e32834cd442 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Ourselin S, Fox NC, 2009c. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 72, 1562–1569. doi: 10.1212/WNL.0b013e3181a4124e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Reiman D, Uphill J, Beck J, Collinge J, Rossor MN, Isaacs AM, Mead S, 2011b. A novel exon 2 I27V VCP variant is associated with dissimilar clinical syndromes. J Neurol 258, 1494–1496. doi: 10.1007/s00415-011-5966-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi D, Riboldi G, Del Bo R, Ticozzi N, Scarlato M, Galimberti D, Corti S, Silani V, Bresolin N, Comi GP, 2015. CHCHD10 mutations in Italian patients with sporadic amyotrophic lateral sclerosis. Brain 138, e372–e372. doi: 10.1093/brain/awu384 [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL, 2005. Neuroanatomical correlates of behavioural disorders in dementia. Brain 128, 2612–2625. doi: 10.1093/brain/awh628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno–Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL, 2002. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208. doi: 10.1212/WNL.58.2.198 [DOI] [PubMed] [Google Scholar]
- Rosso SM, Roks G, Stevens M, de Koning I, Tanghe HLJ, Kamphorst W, Ravid R, Niermeijer MF, van Swieten JC, 2001. Complex compulsive behaviour in the temporal variant of frontotemporal dementia. J Neurol 248, 965–970. doi: 10.1007/s004150170049 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, 2009. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol 65, S3–S9. doi: 10.1002/ana.21543 [DOI] [PubMed] [Google Scholar]
- Rubino E, Rainero I, Chiò A, Rogaeva E, Galimberti D, Fenoglio P, Grinberg Y, Isaia G, Calvo A, Gentile S, Bruni AC, St. George-Hyslop PH, Scarpini E, Gallone S, Pinessi L, For the TODEM Study Group, 2012. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology 79, 1556–1562. doi: 10.1212/WNL.0b013e31826e25df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytty R, Nikkinen J, Paavola L, Abou Elseoud A, Moilanen V, Visuri A, Tervonen O, Renton AE, Traynor BJ, Kiviniemi V, Remes AM, 2013. GroupICA dual regression analysis of resting state networks in a behavioral variant of frontotemporal dementia. Front Hum Neurosci 7, 461. doi: 10.3389/fnhum.2013.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytty R, Nikkinen J, Suhonen N, Moilanen V, Renton AE, Traynor BJ, Tervonen O, Kiviniemi V, Remes AM, 2014. Functional MRI in patients with the C9ORF72 expansion associate frontotemporal dementia. Mol Biol 03. doi: 10.4172/2168-9547.1000117 [DOI] [Google Scholar]
- Saberi S, Stauffer JE, Schulte DJ, Ravits J, 2015. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin 33, 855–876. doi: 10.1016/j.ncl.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracino D, Clot F, Camuzat A, Anquetil V, Hannequin D, Guyant-Maréchal L, Didic M, Guillot-Noël L, Rinaldi D, Latouche M, Forlani S, Ghassab Y, Coppola C, Di Iorio G, David I, Le Guern E, Brice A, Le Ber I, 2018. Novel VCP mutations expand the mutational spectrum of frontotemporal dementia. Neurobiol Aging 72, 187.e11–187.e14. doi: 10.1016/j.neurobiolaging.2018.06.037 [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Tempelmann C, Gaul C, Kühnel GR, Düzel E, Hopf J-M, Feistner H, Zierz S, Heinze H-J, Vielhaber S, 2005. Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252, 944–952. doi: 10.1007/s00415-005-0787-y [DOI] [PubMed] [Google Scholar]
- Schönecker S, Brendel M, van der Zee J, van Broeckhoven C, Rominger A, Danek A, Levin J, 2016. Ein Geschwisterpaar mit frontotemporaler Lobärdegeneration und amyotropher Lateralsklerose und einer neuen Mutation im TBK1-Gen (Thr462Lysfs). Fortschr Neurol Psychiatr 84, 494–498. doi: 10.1055/s-0042-110653 [DOI] [PubMed] [Google Scholar]
- Schönecker S, Neuhofer C, Otto M, Ludolph A, Kassubek J, Landwehrmeyer B, Anderl-Straub S, Semler E, Diehl-Schmid J, Prix C, Vollmar C, Fortea J, Deutsches FTLD-Konsortium, Huppertz H-J, Arzberger T, Edbauer D, Feddersen B, Dieterich M, Schroeter ML, Volk AE, Fließbach K, Schneider A, Kornhuber J, Maler M, Prudlo J, Jahn H, Boeckh-Behrens T, Danek A, Klopstock T, Levin J, 2018. Atrophy in the thalamus but not cerebellum is specific for C9orf72 FTD and ALS patients – an atlas-based volumetric MRI study. Front Aging Neurosci 10, 45. doi: 10.3389/fnagi.2018.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, Yves von Cramon D, 2007. Towards a nosology for frontotemporal lobar degenerations—A meta-analysis involving 267 subjects. NeuroImage 36, 497–510. doi: 10.1016/j.neuroimage.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Schwindt GC, Graham NL, Rochon E, Tang-Wai DF, Lobaugh NJ, Chow TW, Black SE, 2013. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum Brain Mapp 34, 973–984. doi: 10.1002/hbm.21484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H, Papma JM, Garraux G, de Koning I, Reijs AE, Valkema R, Rozemuller AJM, Salmon E, van Swieten JC, 2011. Brain perfusion patterns in familial frontotemporal lobar degeneration. Neurology 77, 384–392. doi: 10.1212/WNL.0b013e3182270456 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Allman JM, Carlin DA, Crawford RK, Macedo MN, Greicius MD, DeArmond SJ, Miller BL, 2007a. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord 21, S50–S57. doi: 10.1097/WAD.0b013e31815c0f14 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML, 2008. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 65. doi: 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD, 2009. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52. doi: 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007b. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, Khan B, Karydas A, Baker MC, DeJesus-Hernandez M, Pribadi M, Coppola G, Geschwind DH, Rademakers R, Lee SE, Seeley W, Miller BL, Boxer AL, 2012. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology 79, 1002–1011. doi: 10.1212/WNL.0b013e318268452e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Cui L, Fang J, Cui B, Li D, Tai H, 2016. Voxel-wise meta-analysis of gray matter changes in amyotrophic lateral sclerosis. Front Aging Neurosci 8, 64. doi: 10.3389/fnagi.2016.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sørensen SA, Gydesen S, Fisher EM, Collinge J, 2005. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37, 806–808. doi: 10.1038/ng1609 [DOI] [PubMed] [Google Scholar]
- Skoglund L, Viitanen M, Kalimo H, Lannfelt L, Jonhagen ME, Ingelsson M, Glaser A, Herva R, 2008. The tau S305S mutation causes frontotemporal dementia with parkinsonism. Eur J Neurol 15, 156–161. doi: 10.1111/j.1468-1331.2007.02017.x [DOI] [PubMed] [Google Scholar]
- Smith BN, Newhouse S, Shatunov A, Vance C, Topp S, Johnson L, Miller J, Lee Y, Troakes C, Scott KM, Jones A, Gray I, Wright J, Hortobágyi T, Al-Sarraj S, Rogelj B, Powell J, Lupton M, Lovestone S, Sapp PC, Weber M, Nestor PJ, Schelhaas HJ, Asbroek A.A. ten, Silani V, Gellera C, Taroni F, Ticozzi N, Van den Berg L, Veldink J, Van Damme P, Robberecht W, Shaw PJ, Kirby J, Pall H, Morrison KE, Morris A, de Belleroche J, Vianney de Jong JMB, Baas F, Andersen PM, Landers J, Brown RH, Weale ME, Al-Chalabi A, Shaw CE, 2013. The C9ORF72 expansion mutation is a common cause of ALS+/−FTD in Europe and has a single founder. Eur J Hum Genet 21, 102–108. doi: 10.1038/ejhg.2012.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Puschmann A, Schöll M, Ohlsson T, van Swieten J, Honer M, Englund E, Hansson O, 2016. 18 F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain 139, 2372–2379. doi: 10.1093/brain/aww163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D, 1989. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 2, 167–182. [Google Scholar]
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AMT, Jones M, Gerhard A, Davidson YS, Robinson A, Gibbons L, Hu Q, DuPlessis D, Neary D, Mann DMA, Pickering-Brown SM, 2012. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 135, 693–708. doi: 10.1093/brain/awr355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solje E, Aaltokallio H, Koivumaa-Honkanen H, Suhonen NM, Moilanen V, Kiviharju A, Traynor B, Tienari PJ, Hartikainen P, Remes AM, 2015. The phenotype of the C9ORF72 expansion carriers according to revised criteria for bvFTD. PLoS One 10, e0131817. doi: 10.1371/journal.pone.0131817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B, 1998. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 95, 7737–7741. doi: 10.1073/pnas.95.13.7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli EG, Agosta F, Ferraro PM, Riva N, Lunetta C, Falzone YM, Comi G, Falini A, Filippi M, 2016. Brain MR imaging in patients with lower motor neuronpredominant disease. Radiology 280, 545–556. doi: 10.1148/radiol.2016151846 [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE, 2008. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672. doi: 10.1126/science.1154584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton BR, Shinhmar D, Turner MR, Williams VC, Williams SCR, Blain CRV, Giampietro VP, Catani M, Leigh PN, Andersen PM, Simmons A, 2009. Diffusion tensor imaging in sporadic and familial (D90A SOD1) forms of amyotrophic lateral sclerosis. Arch Neurol 66. doi: 10.1001/archneurol.2008.527 [DOI] [PubMed] [Google Scholar]
- Stanton BR, Williams VC, Leigh PN, Williams SCR, Blain CRV, Jarosz JM, Simmons A, 2007. Altered cortical activation during a motor task in ALS: evidence for involvement of central pathways. J Neurol 254, 1260–1267. doi: 10.1007/s00415-006-0513-4 [DOI] [PubMed] [Google Scholar]
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, Mioshi E, Roberts-South A, Benatar M, HortobáGyi T, Rosenfeld J, Silani V, Ince PG, Turner MR, 2017. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18, 153–174. doi: 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Bocchetta M, Cash D, Thomas DL, Woollacott I, Dick KM, van Swieten J, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe JB, Graff C, Tagliavini F, Frisoni G, Laforce R, Finger E, de Mendonça A, Sorbi S, Ourselin S, Cardoso MJ, Rohrer JD, Andersson C, Archetti S, Arighi A, Benussi L, Binetti G, Black S, Cosseddu M, Fallström M, Ferreira C, Fenoglio C, Fox NC, Freedman M, Fumagalli G, Gazzina S, Ghidoni R, Grisoli M, Jelic V, Jiskoot L, Keren R, Lombardi G, Maruta C, Mead S, Meeter L, van Minkelen R, Nacmias B, Öijerstedt L, Padovani A, Panman J, Pievani M, Polito C, Premi E, Prioni S, Rademakers R, Redaelli V, Rogaeva E, Rossi G, Rossor MN, Scarpini E, Tang-Wai D, Thonberg H, Tiraboschi P, Verdelho A, Warren JD, 2017. White matter hyperintensities are seen only in GRN mutation carriers in the GENFI cohort. NeuroImage Clin 15, 171–180. doi: 10.1016/j.nicl.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Bocchetta M, Heller C, Convery R, Neason M, Moore KM, Cash DM, Thomas DL, Woollacott IOC, Foiani M, Heslegrave A, Shafei R, Greaves C, van Swieten J, Moreno F, Sanchez-Valle R, Borroni B, Laforce R, Masellis M, Tartaglia MC, Graff C, Galimberti D, Rowe JB, Finger E, Synofzik M, Vandenberghe R, de Mendonça A, Tagliavini F, Santana I, Ducharme S, Butler C, Gerhard A, Levin J, Danek A, Frisoni GB, Sorbi S, Otto M, Zetterberg H, Ourselin S, Cardoso MJ, Rohrer JD, 2019. White matter hyperintensities in progranulin-associated frontotemporal dementia: A longitudinal GENFI study. NeuroImage Clin 24, 102077. doi: 10.1016/j.nicl.2019.102077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Rong Z, Li W, Zheng H, Xiao S, Li X, 2018. Identification of a novel hemizygous SQSTM1 nonsense mutation in atypical behavioral variant frontotemporal dementia. Front Aging Neurosci 10, 26. doi: 10.3389/fnagi.2018.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M, Born C, Rominger A, Lummel N, Schöls L, Biskup S, Schüle C, Grasshoff U, Klopstock T, Adamczyk C, 2014. Targeted high-throughput sequencing identifies a TARDBP mutation as a cause of early-onset FTD without motor neuron disease. Neurobiol Aging 35, 1212.e1–1212.e5. doi: 10.1016/j.neurobiolaging.2013.10.092 [DOI] [PubMed] [Google Scholar]
- Synofzik M, Maetzler W, Grehl T, Prudlo J, vom Hagen JM, Haack T, Rebassoo P, Munz M, Schöls L, Biskup S, 2012. Screening in ALS and FTD patients reveals 3 novel UBQLN2 mutations outside the PXX domain and a pure FTD phenotype. Neurobiol Aging 33, 2949.e13–2949.e17. doi: 10.1016/j.neurobiolaging.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Tan RH, Kril JJ, Yang Y, Tom N, Hodges JR, Villemagne VL, Rowe CC, Leyton CE, Kwok JBJ, Ittner LM, Halliday GM, 2017. Assessment of amyloid β in pathologically confirmed frontotemporal dementia syndromes. Alzheimers Dement (Amst) 9, 10–20. doi: 10.1016/j.dadm.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi T, Hokonohara T, Yamasaki R, Miura S, Kikuchi H, Iwaki A, Tashiro H, Furuya H, Nagara Y, Ohyagi Y, Nukina N, Iwaki T, Fukumaki Y, Kira J, 2010. Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol 119, 355–364. doi: 10.1007/s00401-009-0621-1 [DOI] [PubMed] [Google Scholar]
- Taylor JP, 2015. Multisystem proteinopathy: intersecting genetics in muscle, bone, and brain degeneration. Neurology 85, 658–660. doi: 10.1212/WNL.0000000000001862 [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Trojsi F, Tessitore A, Corbo D, Sagnelli A, Paccone A, D’Ambrosio A, Piccirillo G, Cirillo M, Cirillo S, Monsurrò MR, Esposito F, 2012. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol Aging 33, 886–898. doi: 10.1016/j.neurobiolaging.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Teyssou E, Chartier L, Albert M, Bouscary A, Antoine J-C, Camdessanché J-P, Rotolo F, Couratier P, Salachas F, Seilhean D, Millecamps S, 2016. Genetic analysis of CHCHD10 in French familial amyotrophic lateral sclerosis patients. Neurobiol Aging 42, 218.e1–218.e3. doi: 10.1016/j.neurobiolaging.2016.03.022 [DOI] [PubMed] [Google Scholar]
- Tohnai G, Nakamura R, Sone J, Nakatochi M, Yokoi D, Katsuno M, Watanabe Hazuki, Watanabe Hirohisa, Ito M, Li Y, Izumi Y, Morita M, Taniguchi A, Kano O, Oda M, Kuwabara S, Abe K, Aiba I, Okamoto K, Mizoguchi K, Hasegawa K, Aoki M, Hattori N, Onodera O, Naruse H, Mitsui J, Takahashi Y, Goto J, Ishiura H, Morishita S, Yoshimura J, Doi K, Tsuji S, Nakashima K, Kaji R, Atsuta N, Sobue G, 2018. Frequency and characteristics of the TBK1 gene variants in Japanese patients with sporadic amyotrophic lateral sclerosis. Neurobiol Aging 64, 158.e15–158.e19. doi: 10.1016/j.neurobiolaging.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Rabinovici GD, Ayakta N, Miller BL, Jagust W, Kramer J, Weiner MM, Rosen HJ, 2016. Diagnostic utility of ASL-MRI and FDG-PET in the behavioral variant of FTD and AD. Ann Clin Transl Neurol 3, 740–751. doi: 10.1002/acn3.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojsi F, Corbo D, Caiazzo G, Piccirillo G, Monsurrò MR, Cirillo S, Esposito F, Tedeschi G, 2013. Motor and extramotor neurodegeneration in amyotrophic lateral sclerosis: A 3T high angular resolution diffusion imaging (HARDI) study. Amyotroph Lateral Scler Frontotemporal Degener 14, 553–561. doi: 10.3109/21678421.2013.785569 [DOI] [PubMed] [Google Scholar]
- Trojsi F, Esposito F, de Stefano M, Buonanno D, Conforti FL, Corbo D, Piccirillo G, Cirillo M, Monsurrò MR, Montella P, Tedeschi G, 2015. Functional overlap and divergence between ALS and bvFTD. Neurobiol Aging 36, 413–423. doi: 10.1016/j.neurobiolaging.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Tsai RM, Bejanin A, Lesman-Segev O, LaJoie R, Visani A, Bourakova V, O’Neil JP, Janabi M, Baker S, Lee SE, Perry DC, Bajorek L, Karydas A, Spina S, Grinberg LT, Seeley WW, Ramos EM, Coppola G, Gorno-Tempini ML, Miller BL, Rosen HJ, Jagust W, Boxer AL, Rabinovici GD, 2019. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther 11, 13. doi: 10.1186/s13195-019-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M, Senda J, Ishihara T, Niimi Y, Kawai Y, Atsuta N, Watanabe H, Tanaka F, Naganawa S, Sobue G, 2011. Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. J Neurol Sci 307, 34–40. doi: 10.1016/j.jns.2011.05.025 [DOI] [PubMed] [Google Scholar]
- Tu S, Leyton CE, Hodges JR, Piguet O, Hornberger M, 2015. Divergent longitudinal propagation of white matter degradation in logopenic and semantic variants of primary progressive aphasia. J Alzheimers Dis 49, 853–861. doi: 10.3233/JAD-150626 [DOI] [PubMed] [Google Scholar]
- Turner MR, 2005. [11C]-WAY100635 PET demonstrates marked 5-HT1A receptor changes in sporadic ALS. Brain 128, 896–905. doi: 10.1093/brain/awh428 [DOI] [PubMed] [Google Scholar]
- Turner MR, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, Leigh PN, 2005. Distinct cerebral lesions in sporadic and “D90A” SOD1 ALS: studies with [11C]flumazenil PET. Brain 128, 1323–1329. doi: 10.1093/brain/awh509 [DOI] [PubMed] [Google Scholar]
- Turner MR, Hammers A, Allsop J, Al-Chalabi A, Shaw CE, Brooks DJ, Nigel Leigh P, Andersen PM, 2007a. Volumetric cortical loss in sporadic and familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler 8, 343–347. doi: 10.1080/17482960701538734 [DOI] [PubMed] [Google Scholar]
- Turner MR, Rabiner EA, Al-Chalabi A, Shaw CE, Brooks DJ, Leigh PN, Andersen PM, 2007b. Cortical 5-HT1A receptor binding in patients with homozygous D90A SOD1 vs sporadic ALS. Neurology 68, 1233–1235. doi: 10.1212/01.wnl.0000259083.31837.64 [DOI] [PubMed] [Google Scholar]
- Ueno H, Kobatake K, Matsumoto M, Morino H, Maruyama H, Kawakami H, 2011. Severe brain atrophy after long-term survival seen in siblings with familial amyotrophic lateral sclerosis and a mutation in the optineurin gene: a case series. J Med Case Rep 5, 573. doi: 10.1186/1752-1947-5-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsasina P, Agosta F, Benedetti B, Caputo D, Perini M, Salvi F, Prelle A, Filippi M, 2006. Diffusion anisotropy of the cervical cord is strictly associated with disability in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 78, 480–484. doi: 10.1136/jnnp.2006.100032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burgh HK, Westeneng H-J, Meier JM, van Es MA, Veldink JH, Hendrikse J, van den Heuvel MP, van den Berg LH, 2019. Cross-sectional and longitudinal assessment of the upper cervical spinal cord in motor neuron disease. NeuroImage: Clinical 24, 101984. doi: 10.1016/j.nicl.2019.101984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burgh HK, Westeneng H-J, Walhout R, van Veenhuijzen K, Tan HHG, Meier JM, Bakker LA, Hendrikse J, van Es MA, Veldink JH, van den Heuvel MP, van den Berg LH, 2020. Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology 94, e2592–e2604. doi: 10.1212/WNL.0000000000009498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff MM, Sage CA, Caan MWA, Akkerman EM, Lavini C, Majoie CB, Nederveen AJ, Zwinderman AH, Vos F, Brugman F, van den Berg LH, de Rijk MC, van Doorn PA, Van Hecke W, Peeters RR, Robberecht W, Sunaert S, de Visser M, 2011. Upper and extra-motoneuron involvement in early motoneuron disease: a diffusion tensor imaging study. Brain 134, 1211–1228. doi: 10.1093/brain/awr016 [DOI] [PubMed] [Google Scholar]
- van der Zee J, Pirici D, Van Langenhove T, Engelborghs S, Vandenberghe R, Hoffmann M, Pusswald G, Van den Broeck M, Peeters K, Mattheijssens M, Martin J-J, De Deyn PP, Cruts M, Haubenberger D, Kumar-Singh S, Zimprich A, Van Broeckhoven C, 2009. Clinical heterogeneity in 3 unrelated families linked to VCP p.Arg159His. Neurology 73, 626–632. doi: 10.1212/WNL.0b013e3181b389d9 [DOI] [PubMed] [Google Scholar]
- van der Zee J, Urwin H, Engelborghs S, Bruyland M, Vandenberghe R, Dermaut B, De Pooter T, Peeters K, Santens P, De Deyn PP, Fisher EM, Collinge J, Isaacs AM, Van Broeckhoven C, 2008. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum Mol Genet 17, 313–322. doi: 10.1093/hmg/ddm309 [DOI] [PubMed] [Google Scholar]
- van der Zee J, Van Langenhove T, Kovacs GG, Dillen L, Deschamps W, Engelborghs S, Matěj R, Vandenbulcke M, Sieben A, Dermaut B, Smets K, Van Damme P, Merlin C, Laureys A, Van Den Broeck M, Mattheijssens M, Peeters K, Benussi L, Binetti G, Ghidoni R, Borroni B, Padovani A, Archetti S, Pastor P, Razquin C, Ortega-Cubero S, Hernández I, Boada M, Ruiz A, de Mendonça A, Miltenberger-Miltényi G, do Couto FS, Sorbi S, Nacmias B, Bagnoli S, Graff C, Chiang H-H, Thonberg H, Perneczky R, Diehl-Schmid J, Alexopoulos P, Frisoni GB, Bonvicini C, Synofzik M, Maetzler W, vom Hagen JM, Schöls L, Haack TB, Strom TM, Prokisch H, Dols-Icardo O, Clarimón J, Lleó A, Santana I, Almeida MR, Santiago B, Heneka MT, Jessen F, Ramirez A, Sanchez-Valle R, Llado A, Gelpi E, Sarafov S, Tournev I, Jordanova A, Parobkova E, Fabrizi GM, Testi S, Salmon E, Ströbel T, Santens P, Robberecht W, De Jonghe P, Martin J-J, Cras P, Vandenberghe R, De Deyn PP, Cruts M, Sleegers K, Van Broeckhoven C, 2014. Rare mutations in SQSTM1 modify susceptibility to frontotemporal lobar degeneration. Acta Neuropathol 128, 397–410. doi: 10.1007/s00401-014-1298-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herpen E, Rosso SM, Serverijnen L-A, Yoshida H, Breedveld G, Van De Graaf R, Kamphorst W, Ravid R, Willemsen R, Dooijes D, Majoor-Krakauer D, Kros JM, Crowther RA, Goedert M, Heutink P, Van Swieten JC, 2003. Variable phenotypic expression and extensive tau pathology in two families with the noveltau mutation L315R. Ann Neurol 54, 573–581. doi: 10.1002/ana.10721 [DOI] [PubMed] [Google Scholar]
- Van Laere K, Vanhee A, Verschueren J, De Coster L, Driesen A, Dupont P, Robberecht W, Van Damme P, 2014. Value of 18fluorodeoxyglucose–positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol 71, 553. doi: 10.1001/jamaneurol.2014.62 [DOI] [PubMed] [Google Scholar]
- Van Mossevelde S, van der Zee J, Gijselinck I, Engelborghs S, Sieben A, Van Langenhove T, De Bleecker J, Baets J, Vandenbulcke M, Van Laere K, Ceyssens S, Van den Broeck M, Peeters K, Mattheijssens M, Cras P, Vandenberghe R, De Jonghe P, Martin J-J, De Deyn PP, Cruts M, Van Broeckhoven C, 2016. Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort. Brain 139, 452–467. doi: 10.1093/brain/awv358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Swieten JC, Stevens M, Rosso SM, Rizzu P, Joosse M, De Koning I, Kamphorst W, Ravid R, Spillantini MG, Niermeijer MF, Heutink P, 1999. Phenotypic variation in hereditary frontotemporal dementia with tau mutations. Ann Neurol 46, 617–626. doi: [DOI] [PubMed] [Google Scholar]
- Varma AR, Adams W, Lloyd JJ, Carson KJ, Snowden JS, Testa HJ, Jackson A, Neary D, 2002. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand 105, 261–269. doi: 10.1034/j.1600-0404.2002.1o148.x [DOI] [PubMed] [Google Scholar]
- Vengoechea J, David MP, Yaghi SR, Carpenter L, Rudnicki SA, 2013. Clinical variability and female penetrance in X-linked familial FTD/ALS caused by a P506S mutation in UBQLN2. Amyotroph Lateral Scler Frontotemporal Degener 14, 615–619. doi: 10.3109/21678421.2013.824001 [DOI] [PubMed] [Google Scholar]
- Verfaillie SCJ, Adriaanse SM, Binnewijzend MAA, Benedictus MR, Ossenkoppele R, Wattjes MP, Pijnenburg YAL, van der Flier WM, Lammertsma AA, Kuijer JPA, Boellaard R, Scheltens P, van Berckel BNM, Barkhof F, 2015. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: two sides of the same coin? Eur Radiol 25, 3050–3059. doi: 10.1007/s00330-015-3696-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschueren J, Vanhee A, De Coster L, Van Damme P, Van Laere K, 2013. Impact of the C9Orf72 expansion on brain glucose metabolism in ALS patients. J Nucl Med 54, 155–155. [Google Scholar]
- Viassolo V, Previtali S, Schiatti E, Magnani G, Minetti C, Zara F, Grasso M, Dagnà-Bricarelli F, Di Maria E, 2008. Inclusion body myopathy, Paget’s disease of the bone and frontotemporal dementia: recurrence of the VCP R155H mutation in an Italian family and implications for genetic counselling. Clin Genet 74, 54–60. doi: 10.1111/j.1399-0004.2008.00984.x [DOI] [PubMed] [Google Scholar]
- Vijverberg EGB, Wattjes MP, Dols A, Krudop WA, Möller C, Peters A, Kerssens CJ, Gossink F, Prins ND, Stek ML, Scheltens P, van Berckel BNM, Barkhof F, Pijnenburg YAL, 2016. Diagnostic accuracy of MRI and additional [18F]FDG-PET for behavioral variant frontotemporal dementia in patients with late onset behavioral changes. J Alzheimers Dis 53, 1287–1297. doi: 10.3233/JAD-160285 [DOI] [PubMed] [Google Scholar]
- Vucic S, Winhammar JMC, Rowe DB, Kiernan MC, 2010. Corticomotoneuronal function in asymptomatic SOD-1 mutation carriers. Clin Neurophysiol 121, 1781–1785. doi: 10.1016/j.clinph.2010.02.164 [DOI] [PubMed] [Google Scholar]
- Walhout R, Schmidt R, Westeneng H-J, Verstraete E, Seelen M, van Rheenen W, de Reus MA, van Es MA, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH, 2015a. Brain morphologic changes in asymptomatic C9orf72 repeat expansion carriers. Neurology 85, 1780–1788. doi: 10.1212/WNL.0000000000002135 [DOI] [PubMed] [Google Scholar]
- Walhout R, Westeneng H-J, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH, 2015b. Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86, 288–294. doi: 10.1136/jnnp-2013-306839 [DOI] [PubMed] [Google Scholar]
- Wang YT, Edison P, 2019. Tau imaging in neurodegenerative diseases using positron emission tomography. Curr Neurol Neurosci Rep 19, 45. doi: 10.1007/s11910-019-0962-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts GDJ, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE, 2004. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36, 377–381. doi: 10.1038/ng1332 [DOI] [PubMed] [Google Scholar]
- Weber M, Eisen A, Stewart HG, Andersen PM, 2000. Preserved slow conducting corticomotoneuronal projections in amyotrophic lateral sclerosis with autosomal recessive D90A CuZn-superoxide dismutase mutation. Brain 123 ( Pt 7), 1505–1515. doi: 10.1093/brain/123.7.1505 [DOI] [PubMed] [Google Scholar]
- Weidberg H, Elazar Z, 2011. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci Signal 4, p e39. doi: 10.1126/scisignal.2002355 [DOI] [PubMed] [Google Scholar]
- Wen J, Zhang H, Alexander DC, Durrleman S, Routier A, Rinaldi D, Houot M, Couratier P, Hannequin D, Pasquier F, Zhang J, Colliot O, Le Ber I, Bertrand A, 2018. Neurite density is reduced in the presymptomatic phase of C9orf72 disease. J Neurol Neurosurg Psychiatry 90, 387–394. doi: 10.1136/jnnp-2018-318994 [DOI] [PubMed] [Google Scholar]
- Westeneng HJ, Verstraete E, Walhout R, Schmidt R, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH, 2015. Subcortical structures in amyotrophic lateral sclerosis. Neurobiol Aging 36, 1075–1082. doi: 10.1016/j.neurobiolaging.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Westeneng HJ, Walhout R, Straathof M, Schmidt R, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH, 2016. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry 87, 1354–1360. doi: 10.1136/jnnp-2016-313959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westeneng HJ, Wismans C, Nitert A, Walhout R, Lujiten P, Wijnen J, van den Berg LH, 2017. Metabolic differences between asymptomatic C9orf72 carriers and non-carriers assessed by brain 7T MRSI. Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, USA Abstract 0028. [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Petersen RC, Josephs KA, Jack CR, 2010. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 74, 1279–1287. doi: 10.1212/WNL.0b013e3181d9edde [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Boeve BF, Weigand SD, Senjem ML, Gunter JL, Baker MC, DeJesus-Hernandez M, Knopman DS, Wszolek ZK, Petersen RC, Rademakers R, Jack CR, Josephs KA, 2015. Brain atrophy over time in genetic and sporadic frontotemporal dementia: a study of 198 serial magnetic resonance images. Eur J Neurol 22, 745–752. doi: 10.1111/ene.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Baker M, Rademakers R, Adamson J, Boeve BF, Knopman DS, Parisi JF, Petersen RC, Dickson DW, Hutton ML, Josephs KA, 2007a. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol 64, 371. doi: 10.1001/archneur.64.3.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Senjem ML, Baker M, Ivnik RJ, Knopman DS, Wszolek ZK, Petersen RC, Rademakers R, Josephs KA, 2009a. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology 73, 1058–1065. doi: 10.1212/WNL.0b013e3181b9c8b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Senjem ML, Baker M, Rademakers R, Ivnik RJ, Knopman DS, Wszolek ZK, Petersen RC, Josephs KA, 2009b. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 72, 813–820. doi: 10.1212/01.wnl.0000343851.46573.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Avula R, Tosakulwong N, Weigand SD, Senjem ML, Vemuri P, Jones DT, Gunter JL, Baker M, Wszolek ZK, Knopman DS, Rademakers R, Petersen RC, Boeve BF, Jack CR, 2011. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology 77, 866–874. doi: 10.1212/WNL.0b013e31822c61f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, Warren JD, 2007b. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. NeuroImage 35, 207–213. doi: 10.1016/j.neuroimage.2006.12.006 [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, Rutherford NJ, Baker M, Knopman DS, Wszolek ZK, Parisi JE, Dickson DW, Petersen RC, Rademakers R, Jack CR, Josephs KA, 2012. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 135, 794–806. doi: 10.1093/brain/aws001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C, Baets J, De Bleecker JL, Deconinck T, Biskup S, Hayer SN, Züchner S, Schüle R, De Jonghe P, Synofzik M, 2018. Beyond ALS and FTD: the phenotypic spectrum of TBK1 mutations includes PSP-like and cerebellar phenotypes. Neurobiol Aging 62, 244.e9–244.e13. doi: 10.1016/j.neurobiolaging.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Wong CH, Topp S, Gkazi AS, Troakes C, Miller JW, de Majo M, Kirby J, Shaw PJ, Morrison KE, de Belleroche J, Vance CA, Al-Chalabi A, Al-Sarraj S, Shaw CE, Smith BN, 2015. The CHCHD10 P34S variant is not associated with ALS in a UK cohort of familial and sporadic patients. Neurobiol Aging 36, 2908.e17–2908.e18. doi: 10.1016/j.neurobiolaging.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Wongworawat YC, Liu YA, Raghavan R, White CL, Dietz R, Zuppan C, Rosenfeld J, 2020. Aggressive FUS-mutant motor neuron disease without profound spinal cord pathology. J Neuropath Exp Neur 79, 365–369. doi: 10.1093/jnen/nlaa011 [DOI] [PubMed] [Google Scholar]
- Woollacott IOC, Bocchetta M, Sudre CH, Ridha BH, Strand C, Courtney R, Ourselin S, Cardoso MJ, Warren JD, Rossor MN, Revesz T, Fox NC, Holton JL, Lashley T, Rohrer JD, 2018. Pathological correlates of white matter hyperintensities in a case of progranulin mutation associated frontotemporal dementia. Neurocase 24, 166–174. doi: 10.1080/13554794.2018.1506039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini M-L, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL, 2007. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 69, 1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23 [DOI] [PubMed] [Google Scholar]
- Wu L, Liu J, Feng X, Dong J, Qin W, Liu Y, Wang J, Lu J, Chen K, Wang Y, Jia J, 2018. 11C-CFT-PET in presymptomatic FTDP-17: a potential biomarker predicting onset. J Alzheimers Dis 61, 613–618. doi: 10.3233/JAD-170561 [DOI] [PubMed] [Google Scholar]
- Xia Q, Wang G, Wang H, Hu Q, Ying Z, 2016. Folliculin, a tumor suppressor associated with Birt–Hogg–Dubé (BHD) syndrome, is a novel modifier of TDP-43 cytoplasmic translocation and aggregation. Hum Mol Genet 25, 83–96. doi: 10.1093/hmg/ddv450 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Hatakeyama T, Sato K, Fukui Y, Hishikawa N, Ohta Y, Nishiyama Y, Kawai N, Tamiya T, Abe K, 2017. Flow-metabolism uncoupling in the cervical spinal cord of ALS patients. Neurol Sci 38, 659–665. doi: 10.1007/s10072-017-2823-y [DOI] [PubMed] [Google Scholar]
- Yan J, Deng H-X, Siddique N, Fecto F, Chen W, Yang Y, Liu E, Donkervoort S, Zheng JG, Shi Y, Ahmeti KB, Brooks B, Engel WK, Siddique T, 2010. Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia. Neurology 75, 807–814. doi: 10.1212/WNL.0b013e3181f07e0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ygland E, van Westen D, Englund E, Rademakers R, Wszolek ZK, Nilsson K, Nilsson C, Landqvist Waldö M, Alafuzoff I, Hansson O, Gustafson L, Puschmann A, 2018. Slowly progressive dementia caused by MAPT R406W mutations: longitudinal report on a new kindred and systematic review. Alzheimers Res Ther 10, 2. doi: 10.1186/s13195-017-0330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz R, Müller K, Brenner D, Volk AE, Borck G, Hermann A, Meitinger T, Strom TM, Danzer KM, Ludolph AC, Andersen PM, Weishaupt JH, Weyen U, Hermann A, Regensburger M, Winkler J, Linker R, Winner B, Hagenacker T, Koch JC, Lingor P, Göricke B, Zierz S, Jordan B, Baum P, Wolf J, Winkler A, Young P, Bogdahn U, Prudlo J, Kassubek J, 2020. SQSTM1/p62 variants in 486 patients with familial ALS from Germany and Sweden. Neurobiol Aging 87, 139.e9–139.e15. doi: 10.1016/j.neurobiolaging.2019.10.018 [DOI] [PubMed] [Google Scholar]
- Young AL, Marinescu RV, Oxtoby NP, Bocchetta M, Yong K, Firth NC, Cash DM, Thomas DL, Dick KM, Cardoso J, van Swieten J, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe JB, Graff C, Tagliavini F, Frisoni GB, Laforce R, Finger E, de Mendonça A, Sorbi S, Warren JD, Crutch S, Fox NC, Ourselin S, Schott JM, Rohrer JD, Alexander DC, Andersson C, Archetti S, Arighi A, Benussi L, Binetti G, Black S, Cosseddu M, Fallström M, Ferreira C, Fenoglio C, Freedman M, Fumagalli GG, Gazzina S, Ghidoni R, Grisoli M, Jelic V, Jiskoot L, Keren R, Lombardi G, Maruta C, Meeter L, Mead S, van Minkelen R, Nacmias B, Öijerstedt L, Padovani A, Panman J, Pievani M, Polito C, Premi E, Prioni S, Rademakers R, Redaelli V, Rogaeva E, Rossi G, Rossor M, Scarpini E, Tang-Wai D, Thonberg H, Tiraboschi P, Verdelho A, Weiner MW, Aisen P, Petersen R, Jack CR, Jagust W, Trojanowki JQ, Toga AW, Beckett L, Green RC, Saykin AJ, Morris J, Shaw LM, Khachaturian Z, Sorensen G, Kuller L, Raichle M, Paul S, Davies P, Fillit H, Hefti F, Holtzman D, Mesulam MM, Potter W, Snyder P, Schwartz A, Montine T, Thomas RG, Donohue M, Walter S, Gessert D, Sather T, Jiminez G, Harvey D, Bernstein M, Thompson P, Schuff N, Borowski B, Gunter J, Senjem M, Vemuri P, Jones D, Kantarci K, Ward C, Koeppe RA, Foster N, Reiman EM, Chen K, Mathis C, Landau S, Cairns NJ, Householder E, Taylor-Reinwald L, Lee V, Korecka M, Figurski M, Crawford K, Neu S, Foroud TM, Potkin S, Shen L, Faber K, Kim S, Nho K, Thal L, Buckholtz N, Albert Marylyn, Frank R, Hsiao J, Kaye J, Quinn J, Lind B, Carter R, Dolen S, Schneider LS, Pawluczyk S, Beccera M, Teodoro L, Spann BM, Brewer J, Vanderswag H, Fleisher A, Heidebrink JL, Lord JL, Mason SS, Albers CS, Knopman D, Johnson Kris, Doody RS, Villanueva-Meyer J, Chowdhury M, Rountree S, Dang M, Stern Y, Honig LS, Bell KL, Ances B, Carroll M, Leon S, Mintun MA, Schneider S, Oliver A, Marson D, Griffith R, Clark D, Geldmacher D, Brockington J, Roberson E, Grossman H, Mitsis E, de Toledo-Morrell L, Shah RC, Duara R, Varon D, Greig MT, Roberts P, Albert Marilyn, Onyike C, D’Agostino D, Kielb S, Galvin JE, Cerbone B, Michel CA, Rusinek H, de Leon MJ, Glodzik L, De Santi S, Doraiswamy PM, Petrella JR, Wong TZ, Arnold SE, Karlawish JH, Wolk D, Smith CD, Jicha G, Hardy P, Sinha P, Oates E, Conrad G, Lopez OL, Oakley M, Simpson DM, Porsteinsson AP, Goldstein BS, Martin K, Makino KM, Ismail MS, Brand C, Mulnard RA, Thai G, Mc-Adams-Ortiz C, Womack K, Mathews D, Quiceno M, Diaz-Arrastia R, King R, Weiner M, Martin-Cook K, DeVous M, Levey AI, Lah JJ, Cellar JS, Burns JM, Anderson HS, Swerdlow RH, Apostolova L, Tingus K, Woo E, Silverman DH, Lu PH, Bartzokis G, Graff-Radford NR, Parfitt F, Kendall T, Johnson H, Farlow MR, Hake AM, Matthews BR, Herring S, Hunt C, van Dyck CH, Carson RE, MacAvoy MG, Chertkow H, Bergman H, Hosein C, Stefanovic B, Caldwell C, Hsiung G-YR, Feldman H, Mudge B, Assaly M, Kertesz A, Rogers J, Bernick C, Munic D, Kerwin D, Mesulam M-M, Lipowski K, Wu C-K, Johnson N, Sadowsky C, Martinez W, Villena T, Turner RS, Johnson Kathleen, Reynolds B, Sperling RA, Johnson KA, Marshall G, Frey M, Lane B, Rosen A, Tinklenberg J, Sabbagh MN, Belden CM, Jacobson SA, Sirrel SA, Kowall N, Killiany R, Budson AE, Norbash A, Johnson PL, Allard J, Lerner A, Ogrocki P, Hudson L, Fletcher E, The Genetic FTD Initiative (GENFI), The Alzheimer’s Disease Neuroimaging Initiative (ADNI), 2018. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun 9, 1–16. doi: 10.1038/s41467-018-05892-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-E, Bird TD, Bekris LM, Montine TJ, Leverenz JB, Steinbart E, Galloway NM, Feldman H, Woltjer R, Miller CA, Wood EM, Grossman M, McCluskey L, Clark CM, Neumann M, Danek A, Galasko DR, Arnold SE, Chen-Plotkin A, Karydas A, Miller BL, Trojanowski JQ, Lee VM-Y, Schellenberg GD, Van Deerlin VM, 2010. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch Neurol 67, 161–170. doi: 10.1001/archneurol.2009.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Yu W, Luo S, Yang Y, Liu F, Zhang Y, Chen Y, Sun Y, Wu J, 2019. Association of the TBK1 mutation p.Ile334Thr with frontotemporal dementia and literature review. Mol Genet Genom Med 7, e547. doi: 10.1002/mgg3.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xi Z, Zinman L, Bruni AC, Maletta RG, Curcio SAM, Rainero I, Rubino E, Pinessi L, Nacmias B, Sorbi S, Galimberti D, Lang AE, Fox S, Surace EI, Ghani M, Guo J, Sato C, Moreno D, Liang Y, Keith J, Traynor BJ, St George-Hyslop P, Rogaeva E, 2015. Mutation analysis of CHCHD10 in different neurodegenerative diseases. Brain 138, e380–e380. doi: 10.1093/brain/awv082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tartaglia MC, Schuff N, Chiang GC, Ching C, Rosen HJ, Gorno-Tempini ML, Miller BL, Weiner MW, 2013. MRI signatures of brain macrostructural atrophy and microstructural degradation in frontotemporal lobar degeneration subtypes. J Alzheimers Dis 33, 431–444. doi: 10.3233/JAD-2012-121156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang H, Cai Y, Wen H, Wang L, Zhu M, Chen Y, Yu Y, Lu X, Zhou M, Fang P, Li X, Hong D, 2020. FUS P525L mutation causing amyotrophic lateral sclerosis and movement disorders. Brain Behav 10, e01625. doi: 10.1002/brb3.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW, 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367. doi: 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Summary of neuroimaging findings related to MAPT mutations
Supplementary Table S2. Summary of neuroimaging findings related to GRN mutations
Supplementary Table S3. Summary of neuroimaging findings related to SOD1 mutations
Supplementary Table S4. Summary of neuroimaging findings related to C9orf72 expansions