Abstract
Background
Ethylene is a gaseous plant hormone that acts as a requisite role in many aspects of the plant life cycle, and it is also a regulator of plant responses to abiotic and biotic stresses. In this study, we attempt to provide comprehensive information through analyses of existing data using bioinformatics tools to compare the identified ethylene biosynthesis genes between Arabidopsis (as dicotyledonous) and rice (as monocotyledonous).
Results
The results exposed that the Arabidopsis proteins of the ethylene biosynthesis pathway had more potential glycosylation sites than rice, and 1-aminocyclopropane-1-carboxylate oxidase proteins were less phosphorylated than 1-aminocyclopropane-1-carboxylate synthase and S-adenosylmethionine proteins. According to the gene expression patterns, S-adenosylmethionine genes were more involved in the rice-ripening stage while in Arabidopsis, ACS2, and 1-aminocyclopropane-1-carboxylate oxidase genes were contributed to seed maturity. Furthermore, the result of miRNA targeting the transcript sequences showed that ath-miR843 and osa-miR1858 play a key role to regulate the post-transcription modification of S-adenosylmethionine genes in Arabidopsis and rice, respectively. The discovered cis- motifs in the promoter site of all the ethylene biosynthesis genes of A. thaliana genes were engaged to light-induced response in the cotyledon and root genes, sulfur-responsive element, dehydration, cell cycle phase-independent activation, and salicylic acid. The ACS4 protein prediction demonstrated strong protein-protein interaction in Arabidopsis, as well as, SAM2, Os04T0578000, Os01T0192900, and Os03T0727600 predicted strong protein-protein interactions in rice.
Conclusion
In the current study, the complex between miRNAs with transcript sequences of ethylene biosynthesis genes in A. thaliana and O. sativa were identified, which could be helpful to understand the gene expression regulation after the transcription process. The binding sites of common transcription factors such as MYB, WRKY, and ABRE that control target genes in abiotic and biotic stresses were generally distributed in promoter sites of ethylene biosynthesis genes of A. thaliana. This was the first time to wide explore the ethylene biosynthesis pathway using bioinformatics tools that markedly showed the capability of the in silico study to integrate existing data and knowledge and furnish novel insights into the understanding of underlying ethylene biosynthesis pathway genes that will be helpful for more dissection.
Keywords: Cis-acting elements, miRNAs, Post-transcriptions modifications, Ligand binding site, Pathway study
Background
The gas ethylene has been known as a signaling molecule, which regulates stress responses and various developmental processes in plants [1, 2], such as a boost of fruit ripening, petal and leaf abscission, flower senescence, incitement of root initiation, and prevention of seedling elongation [3]. Besides, ethylene is produced in response to environmental stresses [3], consisting of wounding [4], flooding [5], bacteria, viruses, fungi, nematodes, and insects [6]. Ethylene has been known as a regulated hormone under stress conditions [7], and several studied ecotypes on stress-responsive genes revealed various basal expression levels [8]. Increment the production of ethylene works as a signaling mechanism with intense physiological outcomes [1, 9, 10]. Ethylene is synthesized from methionine through its transformation to S-adenosylmethionine that it is converted via the enzyme 1-aminocyclopropane-1-carboxylate synthase into methylthioadenosine and 1-aminocyclopropane-1-carboxylic acid (ACC) as the precursor of ethylene [11]. 1-aminocyclopropane-1-carboxylic acid (ACC) is oxidized to HCN, CO2, and C2H4 by ACC oxidase (ACO) [12]. Besides, ACC could be turned from transformation to ethylene by forming the conjugate N-malonyl-ACC [13]. Bleecker et al [11] the 1-aminocyclopropane-1-carboxylate synthase (ACS) activity is regulated at the transcriptional and post-transcriptional levels [1, 9]. Owing to the influence of ethylene on senescence and ripening, vast vegetables, fruit, and flowers are lost. Therefore, as a reversible manner, the endeavor has been done to delay or prevent fruit ripening. The activity of ACC synthase has been illustrated with antisense RNA experiments in the role of the rate-limiting phase in ethylene synthesis [14].
The natural diversity of ethylene production suggests that plants by fine-tuning biosynthetic of ethylene and signaling pathways can adapt to different environments. Observation of some stress-responsive genes revealed that this adaptation could be associated to modify the expression of ACS genes via epigenetic modifications [8, 15]. Moreover, it has been demonstrated that ethylene influence the transcription and translation of many genes which are related to ripening [16], in tomato, at least eight ACS genes have been recognized [17]. The Arabidopsis genome consists of 12 putative ACS-like genes, further, from the ACS genes, ACS3 was identified as pseudogene by a short sequence, besides, ACS12 and ACS10 encode an aminotransferase sans the catalytic activity of ACS [18]. The nine remaining ACS genes encode an ACS proteins group which could be categorized into 3 types, according to the absence or presence of putative phosphorylation sites at the proteins C-terminal extension [19, 20]. Type-1 ACS proteins consist of an almost lengthy C-terminal domain that shares the target sites and extremely conserved sequences for a calcium-dependent protein kinase (CDPK) and mitogen-activated protein kinase (MAPK) [21–24], while, type-2 have only the anticipated CDPK phosphorylation site. Nonetheless, type-2 ACS proteins consist of an exclusive regulatory motif named a target of ethylene overproducer (ETO1) (TOE) that overlaps by the CDPK target site. Besides, TOE motif mediates interaction by ETO1 E3 ligase, and its two paralogs, ETO1-Like (EOL1 and EOL2), also it is needed for type-2 ACS degradation [24–28]; type-3 ACS contains only a short expansion of amino acids in the C-terminal domain, and no target sites for a MAPK and CDPK [19, 24]. Another plant that was selected for this study was rice as monocotyledonous, rice is the main staple cereal that feeds almost half of the world’s population. Owing to the enhancing worldwide demand of the growing population, approximately, 50% enhance in production of rice will be needed [10]. Rice has the shortest genome of the main cereals and wealthy genetic diversity. Moreover, the sequence of rice whole-genome furnishes the basis to identify the homologous genes for other crops [29, 30]. Its sustainability and productivity are crucially threatened via several biotic and abiotic stresses such as submergence, drought, salinity, and chilling, but ethylene plays an initial role in adopting plants under stress conditions [20, 31]. In deep water rice, it has been demonstrated that OsACO1 is involved in the internode elongation, also, submergence enhances the ACO enzyme activity and levels of OsACO1 mRNA [32, 33]. The expression of OsACO3 and OsACO2 genes in etiolated rice seedlings was also revealed to be diversely controlled via auxin and ethylene [34].
Ethylene is the main hormone, which controls many physiological pathways. Ethylene has been suggested to be more potent versus necrotrophic pathogens (like B. cinerea) than against biotrophic pathogens. Ethylene insensitive mutants etr1, ein3, and ein2, display increased susceptibility to B. cinerea [35, 36]. Also, plants that overexpress transcription factors associated with the jasmonic acid and ethylene pathways expose an enhanced resistance to different necrotrophs [37–39]. In Arabidopsis, overexpression of AP2C1 that encodes a Thr or Ser protein type 2C phosphatase decreased production of ethylene and compromises resistance to the necrotrophic pathogen B. cinerea [40]. On the other hand, the ACSs could be adjusted via putative endogenous signal receptors like phytohormones and intracellular accumulation of secondary metabolites, such as calcium [3]. Moreover, usage of ACC or ethylene could enhance plant salinity tolerance, mainly by increasing the expression of reactive oxygen species scavengers [41–43]. The expression of ACO genes from various species is also associated with the ethylene biosynthesis rate, as well as ACS, and the transcript levels of multiple ACO genes are regulated under stress conditions [44, 45]. There are some ethylene response factors (ERFs) gene family, across the environmental stress-responsive genes, the mRNA levels of various ERF are controlled via several molecules produced and hormones in various stress conditions [46]. Ethylene plays a biphasic role, inhibiting and stimulating growth dependent upon the species, developmental stages of organs or tissue, and environmental conditions [47, 48]. Ethylene prevents hypocotyl elongation by the switch on the transcription factors ethylene response factor 1 (ERF1) [49–51] and waved-dampened 5 (WDL5) in Arabidopsis [52] in the low light severity or dark. Transcription factor hypocotyl 5 (HY5) also gets involved in this action that is degraded via the E3 ligase constitutive photomorphogenic 1 (COP1) [53]. Adjustment of the ACS transcript levels seems to be a critical mechanism to control the alteration of plant ethylene production. Nonetheless, recent studies put forward that posttranslational modifications, like ubiquitination and phosphorylation, provide as a momentous mechanism to adjust the stability of the ACS proteins that will be led to regulate the levels of ethylene in plants [24, 54, 55].
Considering the riches of the genome sequence information of rice and Arabidopsis which is supplying a valuable resource to study and dissect ethylene biosynthesis genes in monocotyledons (rice) and dicotyledons (Arabidopsis). The genes that are responsible for the biosynthesis of ethylene in Arabidopsis and rice were retrieved delicately. Regarding the importance of the post transcription and translation modifications, the study of the phosphorylation, glycosylation, and miRNA target ethylene biosynthesis genes will be useful. Besides, the cis-regulatory elements in promoter regions of ethylene biosynthesis genes will give a better understanding of the regulation of these genes expression. Moreover, the perception of cis-acting regulatory elements can help to change gene expression patterns through plant genetic engineering approaches to avoid biotic and abiotic stress damages. The present study was the first study to provide comprehensive information and a wide analysis of ethylene biosynthesis genes by available bioinformatics tools for dissection of promoter regions, mRNA, and protein sequences of ethylene biosynthesis genes of two important model plants including Arabidopsis and rice.
Methods
Retrieve the ethylene genes and sequence analysis
The involved genes identification for the pathway of ethylene biosynthesis in Arabidopsis and rice were performed using the Plantcyc (https://www.plantcyc.org/). The sequences of transcript and polypeptide of all involving genes in ethylene biosynthesis of Arabidopsis thaliana from the Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/) and Oryza sativa from Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu/index.shtml) were retrieved, respectively [56].
Biochemical characteristics
Prediction of biochemical traits such as molecular weight (MW), isoelectric point (pI), aliphatic index, instability index, and grand average of hydropathy (GRAVY) was done by polypeptide sequences of ethylene biosynthesis genes and ProtParam tool of Expasy database [57] (https://web.expasy.org/protparam/). The subcellular location of proteins was predicted using Plant-mPLoc (https://www.csbio.sjtu.edu.cn/bioinf/plant-multi) for both Arabidopsis thaliana and Oryza sativa.
Evolutionary analysis
The full length of the amino acid sequence of all predicted SAM, ACS, and ACO proteins of rice and Arabidopsis were used to align using ClustalX. The phylogenetic tree was constructed using the neighbor-joining method of clustal omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
3D protein structure prediction and domain analysis
Three-dimensional (3D) protein structure and ligand-binding site of SAM, ACS, and ACO genes were predicted using the homology modeling of SWISS-MODEL [58]. Also, protein sequences of studied genes were analyzed using the MOTIF Search program https://www.genome.jp/tools/motif/ for finding the conserved motifs and domains.
Gene expression analysis and identification of miRNA targets
Microarray expression of intended genes in Arabidopsis thaliana and Oryza sativa under biotic and abiotic stresses and hormones treatment were obtained from the Genevistigator database [59]. The Affymetrix rice genome array (2836 samples) and Affymetrix Arabidopsis ATH1 genome array (10615 samples) were selected to study the expression patterns of ethylene biosynthesis genes in rice and Arabidopsis, respectively. The psRNATarget server (http://plantgrn.noble.org/psRNATarget/) applied to find existing miRNAs of Arabidopsis thaliana and Oryza sativa at 3.5 expectation level by searching all the transcript sequences of desired genes in Arabidopsis and rice [60].
Prediction of putative Cis-elements
To identify the probable cis-regulatory elements, the promoter sequences (1500 bp upstream of transcription start site) of ethylene biosynthesis pathway genes in Arabidopsis and rice were perused by plantpan2 database (http://plantpan2.itps.ncku.edu.tw/index.html) [61].
Prediction the glycosylation and phosphorylation sites
The NetNGlyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc/) was utilized to determine potential N-glycosylation sites [62]. The predictable phosphorylation sites were identified by NetPhos 3.1 server (http://www.cbs.dtu.dk/services/NetPhos/) [63].
Results
Biochemical characteristics SAM, ACS, and ACO genes in Arabidopsis thaliana and Oryza sativa
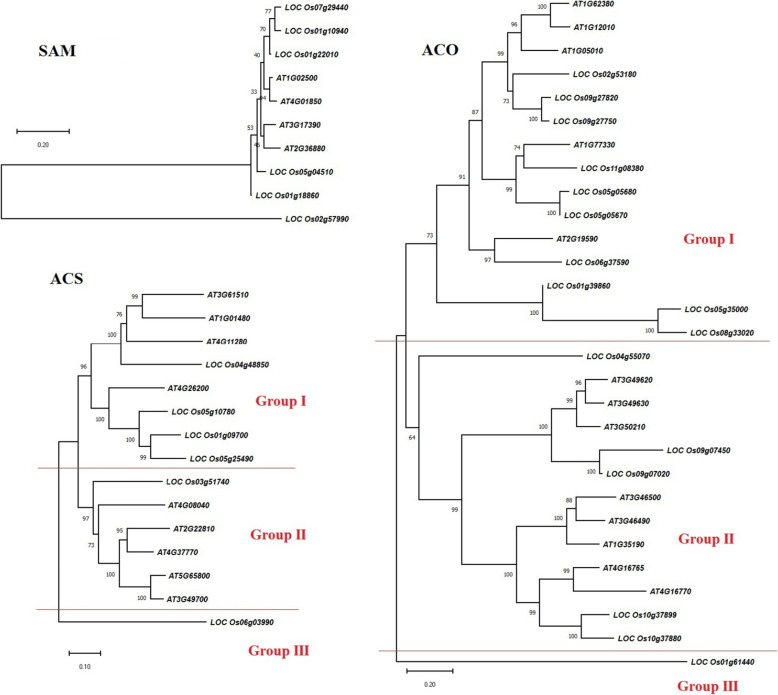
The genes which are involved in the pathway of biosynthesis of ethylene in Arabidopsis and rice were detected by the Plantcyc database. According to the ethylene biosynthesis pathway, 26 and 28 engaging enzymes were predicted in A. thaliana and O. sativa, respectively (Fig. 1). Besides, from 26 identified genes 4, 9, and 13 were identified as methionine adenosyltransferase (SAM), aminocyclopropane-1-carboxylate synthase (ACS), and aminocyclopropane-1-carboxylate oxidase (ACO), respectively in A. thaliana, as well as, number of 6, 6, and 16 predicted genes were involved in SAM, ACS, and ACO, respectively, in O. sativa. The number of ACO engaged genes was more than the genes which were involved in SAM and ACS (Fig. 1, Table 1).
Fig. 1.
Table 1.
The properties of ethylene biosynthesis genes in Arabidopsis thaliana and Oryza sativa
| Locus ID | Gene name | Length (aa) | MW (kDa) | pl | Instability index | Aliphatic index | GRAVY | Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| AT2G36880 | SAM3 | 390 | 42.50 | 5.76 | Stable | 86.26 | −0.209 | Chloroplast |
| AT4G01850 | SAM2 | 393 | 43.26 | 5.67 | Stable | 83.74 | −0.236 | Chloroplast |
| AT3G17390 | SAM4 | 393 | 42.80 | 5.51 | Stable | 79.34 | −0.353 | Chloroplast |
| AT1G02500 | SAM1 | 393 | 43.16 | 5.51 | Stable | 85.04 | −0.255 | Chloroplast |
| LOC_Os01g22010 | SAM | 394 | 42.90 | 5.68 | Stable | 81.14 | −0.264 | Chloroplast |
| LOC_Os01g18860 | SAM | 396 | 43.31 | 5.22 | Stable | 82.7 | −0.274 | Chloroplast |
| LOC_Os05g04510 | SAM | 396 | 43.22 | 5.74 | Stable | 82.45 | −0.289 | Chloroplast |
| LOC_Os02g57990 | SAM | 317 | 34.27 | 5.98 | Stable | 96.53 | 0.036 | Golgi apparatus |
| LOC_Os07g29440 | SAM | 164 | 17.80 | 4.77 | Unstable | 75.49 | −0.268 | Cytoplasm |
| LOC_Os01g10940 | SAM | 164 | 17.85 | 4.78 | Stable | 75.49 | −0.291 | Cytoplasm |
| AT3G61510 | ACS1 | 488 | 55.00 | 7.18 | Unstable | 80.33 | −0.306 | Chloroplast |
| AT1G01480 | ACS2 | 496 | 55.53 | 7.2 | Stable | 83.52 | −0.205 | Chloroplast |
| AT2G22810 | ACS4 | 474 | 53.80 | 8.5 | Stable | 87.46 | −0.201 | Cytoplasm |
| AT5G65800 | ACS5 | 470 | 53.31 | 7.55 | Unstable | 83.71 | −0.351 | Cytoplasm |
| AT4G37770 | ACS8 | 496 | 53.37 | 8 | Stable | 81.72 | −0.339 | Cytoplasm |
| AT4G26200 | ACS7 | 447 | 50.67 | 5.94 | Unstable | 82.75 | −0.386 | Chloroplast |
| AT4G11280 | ACS6 | 495 | 55.52 | 6.23 | Unstable | 83.31 | −0.337 | Chloroplast |
| AT4G08040 | ACS11 | 460 | 51.80 | 6.34 | Stable | 80.77 | −0.271 | Cytoplasm |
| AT3G49700 | ACS9 | 470 | 53.17 | 6.73 | Stable | 80.28 | −0.36 | Cytoplasm |
| LOC_Os05g10780 | ACS | 437 | 47.73 | 6.1 | Unstable | 84.92 | −0.111 | Chloroplast |
| LOC_Os01g09700 | ACS | 510 | 55.44 | 7.16 | Unstable | 85.98 | −0.077 | Chloroplast |
| LOC_Os05g25490 | ACS | 496 | 53.51 | 5.66 | Stable | 85.65 | −0.077 | Chloroplast |
| LOC_Os06g03990 | ACS | 542 | 59.48 | 8.99 | Unstable | 87.51 | −0.148 | Chloroplast |
| LOC_Os03g51740 | ACS | 487 | 53.14 | 8.49 | Unstable | 85.17 | −0.115 | Cytoplasm |
| LOC_Os04g48850 | ACS | 483 | 54.34 | 6.83 | Unstable | 80.6 | −0.259 | Chloroplast |
| AT3G46500 | ACO | 251 | 28.48 | 6.38 | Stable | 85.66 | −0.31 | Cytoplasm |
| AT3G49620 | ACO | 357 | 40.70 | 6.22 | Stable | 80 | −0.366 | Cytoplasm |
| AT1G35190 | ACO | 329 | 37.64 | 5.47 | Stable | 89.75 | −0.226 | Cytoplasm |
| AT3G49630 | ACO | 332 | 37.46 | 5.65 | Stable | 79.12 | −0.409 | Cytoplasm |
| AT4G16765 | ACO | 247 | 27.75 | 5.33 | Stable | 89.22 | −0.219 | Cytoplasm |
| AT3G50210 | ACO | 332 | 37.22 | 5.16 | Stable | 89.15 | −0.152 | Cytoplasm |
| AT4G16770 | ACO | 258 | 29.08 | 5.66 | Unstable | 84.25 | −0.266 | Cytoplasm |
| AT3G46490 | ACO | 330 | 37.61 | 5.75 | Stable | 78.22 | −0.381 | Cytoplasm |
| AT1G77330 | ACO5 | 307 | 34.95 | 5.05 | Stable | 77.42 | −0.441 | Cytoplasm |
| AT2G19590 | ACO1 | 310 | 35.20 | 6.17 | Stable | 76.84 | −0.574 | Cytoplasm |
| AT1G62380 | ACO2 | 320 | 36.18 | 4.98 | Stable | 74.55 | −0.487 | Cytoplasm |
| AT1G12010 | ACO | 320 | 36.53 | 5.09 | Stable | 78.56 | −0.498 | Cytoplasm |
| AT1G05010 | ACO4 | 323 | 36.68 | 5.24 | Stable | 81.97 | −0.43 | Cytoplasm |
| LOC_Os10g37899 | ACO | 544 | 59.36 | 6.66 | Unstable | 79.15 | −0.363 | Chloroplast, Cytoplasm |
| LOC_Os04g55070 | ACO | 326 | 35.80 | 5.34 | Unstable | 83.22 | −0.247 | Cytoplasm |
| LOC_Os05g35000 | ACO | 222 | 24.68 | 11.18 | Unstable | 70.45 | −0.662 | Chloroplast |
| LOC_Os09g07450 | ACO | 202 | 22.93 | 5.45 | Stable | 93.66 | −0.311 | Cytoplasm |
| LOC_Os08g33020 | ACO | 286 | 31.16 | 11 | Unstable | 73.11 | −0.698 | Chloroplast, Nucleus |
| LOC_Os01g61440 | ACO | 394 | 40.50 | 4.96 | Unstable | 80.61 | −0.09 | Cytoplasm |
| LOC_Os09g27820 | ACO | 322 | 36.44 | 4.99 | Unstable | 82.7 | −0.352 | Cytoplasm |
| LOC_Os02g53180 | ACO | 344 | 38.29 | 6.81 | Unstable | 78.63 | −0.368 | Cytoplasm |
| LOC_Os05g05680 | ACO | 308 | 34.58 | 5.11 | Stable | 71.59 | −0.452 | Cytoplasm |
| LOC_Os06g37590 | ACO | 293 | 33.62 | 5.88 | Unstable | 80.82 | −0.423 | Cytoplasm |
| LOC_Os01g39860 | ACO | 312 | 34.03 | 5.21 | Stable | 81.7 | −0.206 | Cytoplasm |
| LOC_Os09g27750 | ACO | 322 | 36.34 | 5.2 | Unstable | 84.25 | −0.315 | Cytoplasm |
| LOC_Os10g37880 | ACO | 308 | 34.24 | 5.35 | Stable | 83.64 | −0.25 | Cytoplasm |
| LOC_Os11g08380 | ACO | 309 | 34.75 | 4.93 | Unstable | 79.61 | −0.403 | Cytoplasm |
| LOC_Os09g07020 | ACO | 435 | 48.94 | 7.1 | Stable | 77.49 | −0.315 | Cytoplasm |
| LOC_Os05g05670 | ACO | 157 | 17.53 | 5.07 | Stable | 73.95 | −0.522 | Cytoplasm |
The total number of amino acids in studied genes ranged from 251–490 aa that AT3G46500 was the smallest protein involved in ACO and AT4G37770 was the largest predicted protein at ACS in Arabidopsis (Table 1). Also, the length of amino acids varied from 157 to 544 aa in rice that LOC_Os05g05670 was the smallest protein, and LOC_Os10g37899 was the largest protein both engaged with ACO. Furthermore, the high length of proteins was contributed to the ACS in both Arabidopsis and rice (Table 1). The GRAVY values of A. thaliana were varied between −0.152 (AT3G50210) a − 0574 (AT2G19590), besides, the GRAVY range was from −0.077 to 0.036 in O. sativa (Table 1).
Molecular weight (MW) of proteins in A. thaliana varied between 27.75 and 55.53 kDa while in O. sativa, they ranged between 17.53 and 59.48 kDa. Isoelectric points (pI) of proteins in A. thaliana ranged from 5.16 (AT3G50210) to 8.5 (AT2G22810) while in O. sativa, they varied from 4.77 (LOC_Os07g29440) to 11.18 (LOC_Os05g35000). Most of the predicted proteins in A. thaliana were stable except the proteins involved in ACS; however, in rice, the larger part of the proteins that contribute to the ACS and ACO was unstable (Table 1). The range of the aliphatic index was from 74.55 (AT1G62380) to 89.75 (AT1G35190) in A. thaliana, further, the aliphatic index was varied in O. sativa between 70.45 (LOC_Os05g35000) and 96.53 (LOC_Os02g57990). The lowest and highest aliphatic indices presented in ACO and SAM rice predicted proteins, respectively (Table 1). The predicted localization of the proteins was diverse and included the chloroplast, Golgi apparatus, cytoplasm, and nucleus (Table 1). The majority of SAM proteins were localized to the chloroplast in both A. thaliana and O. sativa except LOC_Os02g57990 (Golgi apparatus), LOC_Os07g29440, and LOC_Os01g10940 (cytoplasm) in O. sativa (Table 1). The ACS predicted proteins were localized in the chloroplast and cytoplasm. Besides, most of the ACO proteins were associated with the cytoplasm in both A. thaliana and O. sativa; however, the LOC_Os10g37899 and LOC_Os08g33020 were located in the chloroplast as well as cytoplasm and nucleus, respectively (Table 1). The results revealed that genes involved in ethylene biosynthesis from rice are more varied than these genes from Arabidopsis.
Phylogenetic relationship
To investigate the evolutionary relationships among involved genes of the ethylene biosynthesis pathway, we constructed the phylogenetic tree by the rooted neighbor-joining method using the amino acid sequences from Arabidopsis, and rice (Fig. 2). According to the phylogenic tree, the LOC_Os2g57990 that was predicted to have Golgi apparatus localization had more genetic distance than other rice-SAM genes. Also, two rice-SAM proteins (LOC_Os07g29440 and LOC_Os01g10940) which were predicted to cytoplasm localization had high similarity based on amino acid sequences (Table 1, Fig. 2). According to the evolutionary relationships among SAM proteins, it seems that rice SAM genes had more variation than Arabidopsis SAM genes (Fig. 2). ACS proteins were clustered into three groups that 8 of 15 ACSs were located in the first group. Interestingly, a predicted ACS protein of rice (LOC_Os06g03990) had a high distance with others (Fig. 2). In the first group, AT3G61510, AT1G01480, AT4G11280 (ACS6), and LOC_Os04g8850 had more distance than other ACS proteins from rice and Arabidopsis and it was worth noting that these proteins were predicted to locate in the chloroplast (Table 1, Fig. 2). Also, the evolutionary relationships of ACO proteins revealed that they could be clustered into three groups based on the similarity of amino acid sequences. In this way, the first group contained 15 ACOs while 13 ACOs were clustered into second. The LOC_Os01g61440 had more genetic distance than other the studied ACOs. All Arabidopsis ACO proteins were predicted to have cytoplasm localization, but rice ACO proteins were different in terms of protein localization. Also, phylogenetic analysis between ACO proteins showed that rice ACO proteins had high variation than Arabidopsis ACO proteins (Table 1, Fig. 2).
Fig. 2.
Phylogenetic analysis of methionine adenosyltransferase (SAM), aminocyclopropane-1-carboxylate synthase (ACS), and aminocyclopropane-1-carboxylate oxidase (ACO) enzymes of Arabidopsis, tomato and rice based on amino acid sequences using the neighbor-joining method of clustal omega (https://www.ebi.ac.uk/Tools/msa/clustalo/)
Protein structure and domain analysis
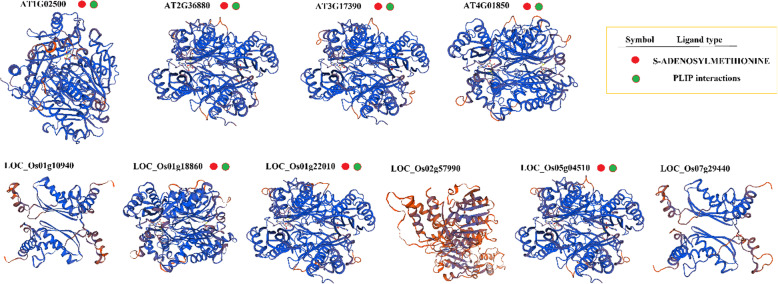
In this study, the 3D protein structure of all SAM, ACS, and ACO genes and their ligand-binding site was predicted based on the homology model using the SWISS-MODEL database for predicting the protein-protein interactions (Figs. 3, 4, 5). The ligand-sites for S-adenosylmethionin and protein-ligand interaction profiler (PLIP) were observed in all Arabidopsis-SAM proteins and three rice-SAM proteins (LOC_Os01g18860, LOC_Os01g22010, and LOC_Os0504510) (Fig. 3). The ligand site of MES (2-(N-Morpholino)-ethanesulfonic acid) was observed in all predicted-ACS proteins except AT1G01480 (Fig. 4). Also, the ligand-binding site of PLP (Pyridoxal-5- Phosphate) was found in the structure of ACO proteins. However, the binding sites of AAD ((2-Aminooxy-Ethyl)-[5-(6-Amino-Purin-9-YL)-3, 4-Dihydroxy-Tetrahydro-Furan-2-Ylmethyl]-Methyl-Sulfonium) and 2-Amino-4-(2-Amino-Ethoxy)-Butyric acid were observed only in Arabidopsis-ACS proteins (Fig. 5). For most ACO proteins, the ligand-binding site was not predicted; however, the ion-binding sites (Fe, zinc, and nickel ion) were observed in some ACO proteins (Fig. 5). According to the 3D structure and ligand type, AT2G19590 was most similar to LOC_Os09g27750 and LOC_Os09g27820, and also, AT3G46500 was similar to LOC_Os10g37899 (Fig. 5).
Fig. 3.
The predicted 3D model structure and ligand type of methionine adenosyltransferase (SAM) proteins in Arabidopsis and rice using SWISS-MODEL [58]
Fig. 4.
The predicted 3D model structure and ligand type of aminocyclopropane-1-carboxylate synthase (ACS) proteins in Arabidopsis and rice using SWISS-MODEL [58]
Fig. 5.
The predicted 3D model structure and ligand type of aminocyclopropane-1-carboxylate oxidase (ACO) proteins in Arabidopsis and rice using SWISS-MODEL [58]
The motif analysis for SAM, ACS, and ACO proteins was carried out using the MOTIF Search program (https://www.genome.jp/tools/motif/), separately (Fig. 6). According to the result of motif analysis, location and order of 3 motifs in SAM proteins were similar except for LOC_Os02g57990, LOC_Os07g29440, and LOC_Os01g10940. Moreover, motifs with different lengths and locations observed in LOC_Os02g57990, that the LOC_Os2g57990 was predicted to have Golgi apparatus localization had more genetic distance than other rice-SAM genes (Table 1, Fig. 2, 6). Aminotron_1_2 motif was detected in all of the studied ACS proteins in Arabidopsis and rice, which are almost located in the same position. Besides, Beta_elim_lase was identified in LOC_O05g25490, LOC_Os01g09700, At04g08040, At04g26200, and At01g01480 (Fig. 6). Two motifs were detected in most of the ACO proteins with identical order in Arabidopsis and rice; however, in some ACO proteins such as At02g19590 and LOC_Os05g05670, proteins had two additional different motifs with various length and locations. The At02g19590 and LOC_Os05g05670 proteins with 310 aa and 157 aa length, respectively, were predicted as stable proteins and localized in the cytoplasm (Table 1, Fig. 6).
Fig. 6.
Motif and domain analysis of methionine adenosyltransferase (SAM), aminocyclopropane-1-carboxylate synthase (ACS), and aminocyclopropane-1-carboxylate oxidase (ACO) proteins in Arabidopsis and rice using the MOTIF Search program (https://www.genome.jp/tools/motif/)
Gene expression: Anatomy, development stages, biotic, and abiotic stresses and hormones treatment
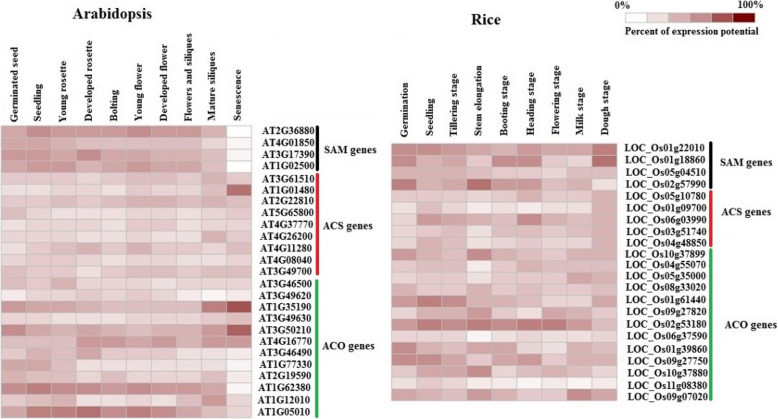
The expression patterns of SAM, ACS, and ACO genes were evaluated in different tissues, organs, growth and development stages, as well as, under biotic and abiotic stresses and hormones treatment in Arabidopsis and rice via microarray data analysis available online using the Genevestigator database (Figs. 7, 8, 9). SAM genes including SAM1, SAM2, METK3, and METK4 high expressed in the primary cell, seedling, inflorescence, shoot and root in Arabidopsis, whereas, LOC_Os01g22010 and LOC_Os05g04510 showed a high level of expression in studied tissue and organs of rice, also, LOC_Os01g18860 and LOC_Os02g57990 expressed in medium level (Fig. 7). In Arabidopsis, ACS1, ACS2, ACS4, ACS5, ACS8, ACS7, ACS6, ACS11, and ACS9 showed exclusive expressions in different tissues and organs. Most of the ACS genes showed medium expressions in studied tissue and organs except ACS5 that expressed in low level in all studied tissues and organs, and ACS6 showed a high level of expression in the primary cell, shoot, and root (Fig. 7). In rice, LOC_Os06g03990 displayed a high level of expression in studied tissues and organs, whereas, LOC_Os05g10780, LOC_Os03g51740, LOC_Os04g48850, and LOC_Os01g09700 showed a medium level of expression in all studied tissues and organs except LOC_Os01g09700 had a low level of expression in the inflorescence (Fig. 7). Most of the ACO genes expressed in medium level, DIN11 and At03g46500 showed the lowest level of expression in inflorescence, as well as, At3g46500 had a low expression level in shoot among the studied ACO genes in Arabidopsis. Besides, At01g77330, ACO1, At03g50210, and At01g35190 expressed at a high level at the shoot, also At01g77330 had a high level of expression in the primary cell and seedling. Moreover, At03g50210 demonstrated a high level of expression in the primary cell and inflorescence (Fig. 7). Nine studied ACO showed various expression levels in rice, according to the obtained results LOC_Os10g37899, and LOC_Os02g53180 revealed the highest level of expression, whereas, LOC_O04g55070 and LOC_O05g35000 showed low expression levels in studied tissue and organs of rice (Fig. 7). It could be concluded that almost all SAM, ACS, and ACO expressed in studied tissue and organs, but at different levels. The results of SAM, ACS, and ACO genes expression were investigated in different growth and development stages (Fig. 8). The Arabidopsis-SAM genes are mostly expressed in the germination stage, while two rice-SAM genes (LOC_Os01g18860 and LOC_Os01g22010) are highly expressed in the ripening stage. Furthermore, Arabidopsis-ACS2 more induced than other ACS genes that showed high expression in the seeds (Fig. 8). Among ACO genes, At01g35190, and At03g50210 were more up-regulated than others and they had high expression in the seeds. Regarding the gene expression patterns, SAM genes were more involved in the rice-ripening stage, while in Arabidopsis, ACS and ACO genes were contributed in maturity (Fig. 8).
Fig. 7.
Expression level of ethylene biosynthesis genes in various tissues and organs of rice and Arabidopsis. The expression data were obtained from Affymetrix Arabidopsis ATH1 genome array using the Genevistigator database [59]
Fig. 8.
Expression patterns of ethylene biosynthesis genes at different development stages in rice and Arabidopsis. The expression data were obtained from Affymetrix Arabidopsis ATH1 genome array using the Genevistigator database [59]
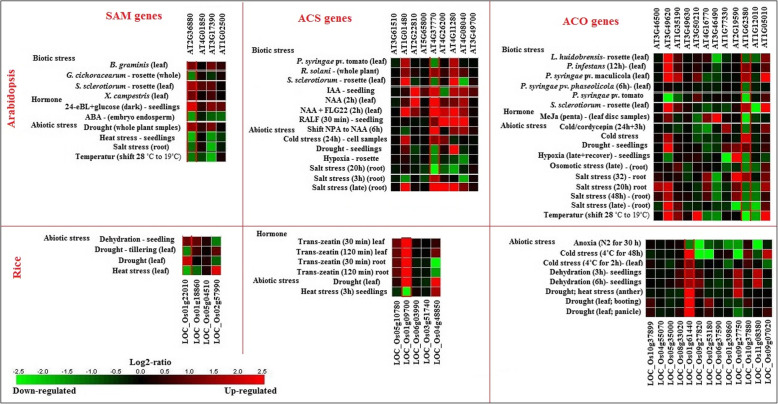
Fig. 9.
Differential expression of ethylene biosynthesis genes in rice and Arabidobsis under different biotic and abiotic stresses and hormones treatment. The expression data were obtained from Affymetrix Arabidopsis ATH1 genome array using the Genevistigator database [59]
The expression of SAM, ACS, and ACO genes was studied under biotic and abiotic stresses and hormones treatment in Arabidopsis and rice through the existence of microarray data (Fig. 9). The At02g36880 (SAM gene) showed high differential expression under stress conditions in Arabidopsis. The At02g36880 gene is up-regulated in 24-CBL+ glucose (dark) in hormone treatment at the seedling stage, but it down-regulated under heat (seedling), salt (root), and temperature, as well as, G. cichoracearum (biotic stress). In rice, the LOC_Os01g22010 gene up-regulated under drought (leaf) and dehydration, on the contrary, it down-regulated in the drought (tillering) and heat stresses, interestingly LOC_Os02g57990 displayed vice-versa pattern in studied conditions (Fig. 9). Considering to filtration of ACS genes in Arabidopsis, At04g37770 demonstrated various expressions under different stress conditions, where this gene is up-regulated under IAA (seedling), NAA, NAA + FLG22, RALF and shift NPA to NAA, while it down-regulated in some abiotic stresses. Besides, the gene expression profile of some ACS genes illustrated that LOC_Os01g09700 gene is especially up-regulated at the different time courses of trans-zeatin treatment and drought stress condition, but it showed down-regulation under heat stress (Fig. 9). Regarding the expression pattern of ACO genes in Arabidopsis, the AT03g49620 and At01g62380 genes showed up-regulation and down-regulation in most of the studied conditions, respectively. While, LOC_Os01g61440 up-regulated under abiotic stresses consisting of the cold, dehydration, drought, and heat condition in rice (Fig. 9).
It is worth noting that some of the studied genes with similar expression patterns under particular stress had a more close evolutionary relationship with each other and were categorized in the same groups. For instance, some ACS genes including At04g37770, At04g26200, and At04g11280 genes are up-regulated under salt stress (late, root) which belonged to group I in phylogeny analysis, also LOC_Os01g09700 and LOC_Os04g48850 showed a high level of expression under drought conditions that categorized in group II (Figs. 2, 9). Moreover, among ACO genes At01g62380 and Ato1g12010 down-regulated under temperature (28 °C to 19 °C), and LOC_ Os09g27820, LOC_ Os02g531810 and LOC_ Os09g27750 down-regulated under cold stress (4 °C for 48 h) that clustered in group III regarded to phylogeny analysis (Figs. 2, 9). It seems that similar expression patterns of these genes in exclusive stresses were associated with the alike cis-elements underlying the promoter region of these genes. It reveals that the transcript of these genes adjusted with the identified transcription factors in the same conditions. Thus, gene expression study under various conditions showed environmental signals and stresses influence on the regulation of ethylene biosynthesis pathway, the achieved results could help to figure out how the underlying pathway gene networks were organized and adjusted in various tissues, organs, developmental stages, and stress conditions.
Prediction the miRNA targets
In the present study, the sites of microRNAs (miRNA) were predicted using published miRNA sequences of psRNATarget server for Arabidopsis and rice (Table 2). The result of miRNA targeting the transcript sequences of SAM, ACS, and ACO genes revealed that SAM1 (AT1G02500) from Arabidopsis was targeted by ath-miR843 while osa-miR1858 targeted two rice-SAMs (LOC-Os01g22010 and LOC-Os05g04510) transcripts. Two rice-ACOs and one Arabidopsis-ACO contained the link-sites of ath-miR3933, osa-miR5809, and osa-miR531, respectively. All microRNAs inhibition involved the transcript cleavage. In our study, the complex between published miRNAs with transcript sequences of ethylene biosynthesis genes in A. thaliana and O. sativa were identified that would be helpful to understand the regulation the gene expression after the transcription process.
Table 2.
Putative miRNAs targeted the transcripts of ethylene biosynthesis genes
| miRNA ID | Target ID | miRNA sequence (3-5) | Target position | Expectation | Inhibition |
|---|---|---|---|---|---|
| ath-miR843 | AT1G02500 (SAM1) | AGGUUACUUCGAGCUGGAUUU | 1333–1353 | 3 | Cleavage |
| ath-miR843 | AT2G22810 (ACS4) | AGGUUACUUCGAGCUGGAUUU | 675–694 | 2.5 | Cleavage |
| ath-miR159a | AT2G22810 (ACS4) | AUCUCGAGGGAAGUUAGGUUU | 437–457 | 3 | Cleavage |
| ath-miR159a | AT4G37770 (ACS8) | UCCUCGAGGGAAGUUAGGUUU | 458–478 | 1.5 | Cleavage |
| ath-miR3933 | AT1G77330 (ACO5) | GGCUCAGCAGUAAAACGAAGA | 739–759 | 2.5 | Cleavage |
| osa-miR1858 | LOC_Os01g22010 (SAM) | CGGGGUGAGGCAGGAGGAGAG | 567–587 | 3 | Cleavage |
| osa-miR1858 | LOC_Os05g04510 (SAM) | CGGGGUGAGGCAGGAGGAGAG | 570–590 | 3 | Cleavage |
| osa-miR5809 | LOC_Os05g05680 (ACO) | CGACACCAGCGGCCGCUGCU | 802–821 | 3 | Cleavage |
| osa-miR531a | LOC_Os11g08380 (ACO) | UACCGCCGUGCGUCGGGGCCGCUC | 900–923 | 3 | Cleavage |
| osa-miR531b | LOC_Os11g08380 (ACO) | GCCGUGCGUCGGGGCCGCUC | 904–923 | 3 | Cleavage |
Cis-regulatory elements in promoter site
Gene expression is broadly adjusted in the transcription phase, where the interactions amongst cis-regulatory elements and transcription factors in the promoter region of the genes which perform a crucial role. In other words, the cis-regulatory elements (CREs) as non-coding DNA are mainly located in upstream of genes, which are determined via transcription factors that control the gene expression in various conditions. Analyses of the promoter region of the induced genes led to the discovering of cis-acting elements, also the ethylene-responsive element-binding protein (EREBP) family that interacts with ethylene response factors (ERFs) and DNA [3]. Transcription factors related to the ERF family have been demonstrated to be engaged in several developmental processes [64–66], abiotic [67, 68], and biotic [69] stress responses. The upstream of studied genes (promoter site) was screened to identify the key cis-elements that regulate the gene expression under different conditions (Table 3). The AAACAAA sequence named anaero1 consensus was observed in the most promoter sites of ethylene biosynthesis genes of A. thaliana and O. sativa. The anaero1 consensus is one of the motifs found in the promoters of anaerobic genes involved in the fermentative pathway [76]. The binding sites of common transcription factors such as MYB, WRKY, and ABRE that control target genes in abiotic and biotic stresses were generally distributed in promoter sites of ethylene biosynthesis genes of A. thaliana. SORLIP1AT, SURECOREATSULTR11, ABRELATERD1, MYBCOREATCYCB1, and LS7ATPR1, discovered in promoter site of all the ethylene biosynthesis genes of A. thaliana genes that these cis-motifs are engaged to response light-induced cotyledon and root genes [70], sulfur-responsive element [71], dehydration [72], cell cycle phase-independent activation and salicylic acid [73], respectively. Besides, the binding sites of some key cis-regulatory elements such as BIHD1OS, CGACGOSAMY3 and GARE2OSREP1 that involved in disease resistance [78], sugar starvation [79] and gibberellin-responsive element (GARE) [81], respectively, were commonly distributed in promoter sites of ethylene biosynthesis genes of O. sativa.
Table 3.
List of key cis-regulatory elements of promoter site of ethylene biosynthesis genes
| Identifier | Sequence | Cover % | Annotation | |
|---|---|---|---|---|
| Arabidopsis | Rice | |||
| SORLIP1AT | GCCAC | 100 | – | Over-represented in light-induced cotyledon and root common genes and root-specific genes [70] |
| SURECOREATSULTR11 | GAGAC | 100 | – | Sulfur-responsive element [71] |
| ABRELATERD1 | ACGTG | 100 | – | Responsive to dehydration [72] |
| MYBCOREATCYCB1 | AACGG | 100 | – | Involved in cell cycle phase-independent activation |
| WBBOXPCWRKY1 | TTTGACY | 100 | – | W box; WRKY |
| LS7ATPR1 | ACGTCATAGA | 100 | – | A positive salicylic acid-inducible element [73] |
| XYLAT | ACAAAGAA | 96.2 | – | Involved in secondary xylem development and wood formation [74] |
| CCA1ATLHCB1 | AAMAATCT | 92.3 | – | Involved in the phytochrome regulation [75] |
| ANAERO1CONSENSUS | AAACAAA | 96.2 | 96.2 | Involved in the fermentative pathway [76] |
| SITEIOSPCNA | CCAGGTGG | – | 100 | Resemble G-box; May contribute in part to transcriptional activation [77] |
| BIHD1OS | TGTCA | – | 100 | Involved in disease resistance [78] |
| CGACGOSAMY3 | CGACG | – | 100 | Involved in sugar starvation [79] |
| E2F1OSPCNA | GCGGGAAA | – | 100 | Involved in actively dividing cells and tissue [80] |
| GARE2OSREP1 | TAACGTA | – | 100 | Gibberellin-responsive element (GARE) [81] |
Potential phosphorylation and glycosylation sites
Phosphorylation and glycosylation are the prevalent post-translational modification of proteins which could modify object site and activity of protein [82]. The potential phosphorylation sites of studied proteins were predicted based on the presence of serine, threonine, and tyrosine amino acids (Fig. 10). Phosphorylation is catalyzed by kinases that transmit a phosphoryl group commonly from ATP, but also from ADP to the hydroxyl group of particular Ser, Tyr, or Thr residues in their target proteins. Nevertheless, also His and both Asp and His in plant two-component signaling can be phosphorylated [83–86]. The result illustrated that the LOC_Os05g05670 (as an ACO protein) had the minimum phosphorylation sites while the highest phosphorylation number (53 sites) predicted in LOC_Os06g03990 (as an ACS protein). The predicted phosphorylation sites in SAM, ACS and ACO proteins of Arabidopsis ranged from 19 (AT3G49630 as an ACO protein) to 48 (AT1G01480 as an ACS protein). According to our findings, the ACO proteins were less phosphorylated than ACS and SAM proteins. This is likely that phosphorylation of ACS adjusts ethylene production was supported through the study that mutation of the C-terminal extension of ACS5 in Arabidopsis persuades the eto2-1 mutant to overproduce. The predicted-glycosylation sites within amino acid sequences of SAM, ACS, and ACO proteins were presented in table 4. All Arabidopsis-SAMs showed similar glycosylation patterns while the glycosylation patterns were very different in rice-SAMs and 50% of them were not predicted any glycosylation site. ACS proteins showed the highest glycosylation sites whereas AT2G22810 (ACS4) had four predicted-glycosylation sites (as hyperglycosylated protein). The rice-ACO proteins showed the minimum predicted-glycosylation sites that 75% had no glycosylation site. Also, 38% of Arabidopsis-ACO proteins had no potential glycosylation site.
Fig. 10.
The predicted sites of phosphorylation in amino sequences of methionine adenosyltransferase (SAM), aminocyclopropane-1-carboxylate synthase (ACS), and aminocyclopropane-1-carboxylate oxidase (ACO) proteins in Arabidopsis and rice using NetPhos 3.1 server (http://www.cbs.dtu.dk/services/NetPhos/) [63]
Table 4.
The predicted N-glycosylation sites in amino sequences of methionine adenosyltransferase (SAM), aminocyclopropane-1-carboxylate synthase (ACS), and aminocyclopropane-1-carboxylate oxidase (ACO) proteins in Arabidopsis and rice using NetNGlyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc/) [62]
| Arabidopsis thaliana | Oryza sativa | |||
|---|---|---|---|---|
| Locus ID | Glycosylation site | Locus ID | Glycosylation site | |
| SAM proteins | AT2G36880 | 2 (158, 233) | LOC_Os01g22010 | 2 (160, 235) |
| AT4G01850 | 2 (158, 233) | LOC_Os01g18860 | 1 (236) | |
| AT3G17390 | 2 (158, 233) | LOC_Os05g04510 | 2 (161, 236) | |
| AT1G02500 | 2 (158, 233) | LOC_Os02g57990 | 0 | |
| LOC_Os07g29440 | 0 | |||
| LOC_Os01g10940 | 0 | |||
| ACS proteins | AT3G61510 | 1 (131) | LOC_Os05g10780 | 1 (189) |
| AT1G01480 | 2 (132, 207) | LOC_Os01g09700 | 2 (264, 496) | |
| AT2G22810 | 4 (124, 199, 447, 456) | LOC_Os05g25490 | 1 (217) | |
| AT5G65800 | 2 (124, 199) | LOC_Os06g03990 | 2 (140, 282) | |
| AT4G37770 | 2 (124, 199) | LOC_Os03g51740 | 2 (46, 211) | |
| AT4G26200 | 2 (50, 214) | LOC_Os04g48850 | 2 (21, 204) | |
| AT4G11280 | 3 (27, 210, 229) | |||
| AT4G08040 | 3 (34, 122, 197) | |||
| AT3G49700 | 2 (124, 199) | |||
| ACO proteins | AT3G46500 | 0 | LOC_Os10g37899 | 0 |
| AT3G49620 | 1 (124) | LOC_Os04g55070 | 1 (307) | |
| AT1G35190 | 2 (3, 23) | LOC_Os05g35000 | 0 | |
| AT3G49630 | 1 (99) | LOC_Os09g07450 | 1 (98) | |
| AT4G16765 | 0 | LOC_Os08g33020 | 0 | |
| AT3G50210 | 1 (98) | LOC_Os01g61440 | 0 | |
| AT4G16770 | 1 (194) | LOC_Os09g27820 | 0 | |
| AT3G46490 | 1 (3) | LOC_Os02g53180 | 0 | |
| AT1G77330 | 0 | LOC_Os05g05680 | 0 | |
| AT2G19590 | 1 (110) | LOC_Os06g37590 | 0 | |
| AT1G62380 | 0 | LOC_Os01g39860 | 0 | |
| AT1G12010 | 0 | LOC_Os09g27750 | 0 | |
| AT1G05010 | 1 (99) | LOC_Os10g37880 | 1 (178) | |
| LOC_Os11g08380 | 0 | |||
| LOC_Os09g07020 | 3 (65, 70, 205) | |||
| LOC_Os05g05670 | 0 | |||
Discussion
Ethylene is one of the simplest well-characterized plant hormones and ethylene biosynthesis including three simple steps, beginning from the amino acid methionine in both dicot and monocot plants [13]. Firstly, methionine is transformed to S-adenosyl methionine (SAM), which is afterward converted to 1-aminocyclopropane-1-carboxylic acid (ACC) via ACC synthases (ACS). Eventually, ACC is converted to ethylene through ACC oxidases (ACO) [87]. In this study, 26 and 28 engaging genes involving in the ethylene biosynthesis pathway, were predicted in A. thaliana and O. sativa, respectively. The selected SAM, ACS, and ACO genes in Arabidopsis and rice were variable in physicochemical properties including protein length, GRAVY value, aliphatic index, molecular weight, isoelectric points (pI), and instability index. The GRAVY values in involved-ethylene biosynthesis genes of Arabidopsis were more varied than rice. The GRAVY value associated with the solubility of proteins, and it could calculate the sum of hydropathy values [88, 89]. According to the predicted GRAVY value, ACS enzymes of Arabidopsis are more hydrophilic than ACS enzymes of rice. Besides, the ACOs of rice showed high variation based on physicochemical properties. Besides, the lowest and highest aliphatic indices observed in ACO and SAM rice predicted proteins, respectively. The aliphatic index is an important factor for the thermostability of proteins [90]. Research showed that Arabidopsis 14-3-3 protein exploits as positively adjusting the ethylene biosynthesis via increasing the ACS protein stability by the interaction with ACS proteins [55]. The proteins by high aliphatic index may have a greater half-life and they could be engaged in high reaction temperature [91].
Regarding the appearance and advancement of the genomic era, progressively, more genome sequences are released, which pave the way for evolutionary and comprehensive studies of any gene family from various species [82]. Our result based on studied predicted proteins indicated that involved-proteins in the ethylene biosynthesis pathway of rice had high variation than Arabidopsis. Lee and Yoon [20] indicated that the similarity of structure and the conserved regulatory motif discovered in both ACS proteins from these two plant species rice and Arabidopsis indicate the being of an evolutionally conserved mechanism, which underlies the ethylene biosynthesis regulation in rice and Arabidopsis. Also, the different ligand sites were observed in the predicted-3D structure of ACO and ACS proteins. Illuminating the biochemical and biological roles of proteins to determine their interacting partners, could be time-consuming and hardly implement by in vivo and/or in vitro approaches, besides most of the recently sequenced proteins will have unclear functions and structures as well. Although, computational approaches for predicting protein–ligand binding sites suggest an alternating practical solution. Therefore, it is momentous to discover these key sites to understand the protein function [92–94]. MES (2-(N-Morpholino)-ethanesulfonic acid) binding site was observed in all predicted-ACS proteins except AT1G01480, while the binding sites of AAD ((2-Aminooxy-Ethyl)-[5-(6-Amino-Purin-9-YL)-3,4-Dihydroxy-Tetrahydro-Furan-2-Ylmethyl]-Methyl-Sulfonium) and 2-Amino-4-(2-Amino-Ethoxy)-Butyric acid just observed in Arabidopsis-ACS proteins. The structure of the ligand-binding site can influence the protein function, protein evolution, and protein-protein interaction [95].
Ethylene plays a main role in the senescence and fruit ripening initiation, also boosts the transcription and translation of responsive genes engaged to fruit softening, cell-wall metabolism, and membrane metabolism, via switching on the ethylene signaling transduction [89, 96, 97]. The results of gene expression demonstrated that SAM, ACS, and ACO genes were differentially induced in plant development stages and they had different expression patterns in monocots and dicotyledonous in response to stresses. ACS2 gene of Arabidopsis is more induced than other ACS genes showing high expression in seeds. In Arabidopsis, ACS transcripts have been illustrated in etiolated seedlings, roots, stems, leaves, siliques, and flowers [18, 98, 99]. Each of the multigene family is differentially expressed for the time of auxin treatment, wounding, and ripening [100]. For example, LE-ACS4, and LE-ACS2 genes are expressed at the ripening time in tomato [101], persuaded in mature green fruits after treatment by exogenous ethylene [101, 102] and over induced upon pericarp tissues wounding [103]. Some ACS genes including At04g37770, At04g26200, and At04g11280 genes were up-regulated under salt stress. Lelièvre et al. indicated that expression of the ACC synthase gene is controlled through ethylene only during/ after chilling treatment, but the expression of the ACC oxidase gene could be regulated separately through either ethylene or chilling [104]. As already noted, in Arabidopsis, various abiotic stresses often enhance ethylene biosynthesis by enhancing the transcription of distinct subsets of ACS genes. Transcript levels of the ACS6 gene elevate in response to ozone [105]. ACS2, ACS9, ACS6, and ACS7 are induced during hypoxia [106], but the expression of all the ACS genes decreased under anaerobic conditions in Arabidopsis [98]. Nevertheless, the transcript levels of separate subsets of the ACS genes enhance in response to osmotic stress, drought, high temperatures conditions, and after wounding [98, 99]. Gene expression is broadly adjusted in the transcription phase, where the interactions amongst cis-regulatory elements and transcription factors in the promoter region of the genes which perform a crucial role. The binding sites of important transcription factors including ABRE, MYB, and WRKY that regulate target genes under stresses were generally distributed in promoter sites of ethylene biosynthesis genes of A. thaliana. Considering the regulatory role of these elements could distinguish much of plant stress response by these elements existence [46, 107, 108]. Also, different cis motifs including sulfur-responsive element, dehydration, and hormone (salicylic acid, gibberellin, and abscisic acid) responsive elements were observed in upstream of SAM, ACO, and ACS genes. Cis-acting elements are particular binding sites for proteins that engaged in the initiation and regulation of transcription, which is suppressing or activating the gene transcription in response to altering growth conditions and different environmental stress [109]. Our results indicated that the most SAM and ACO genes were down-regulated in response to abiotic stresses that various factors such as type of cis-regulatory elements may affect the expression patterns. Collectively, the current study revealed that involved genes in the ethylene biosynthesis pathway play key roles, not only in regulating development stages such as the ripening stage but also in regulating the response to abiotic and biotic stresses tolerance.
The result of miRNA targeting the transcript sequences of SAM, ACS, and ACO genes showed that ath-miR843 and osa-miR1858 play a key role to regulate the post-transcription modification of SAM genes in Arabidopsis and rice, respectively. The ath-miR843 involves in response to low-oxygen (hypoxia) stress [110], and osa-miR1858 is one of the mirRNA that is related to rice grains development [111]. Also, the target site of ath-miR159a was found in the transcript sequence of AT2G22810 and AT4G37770 as ACS genes. MIR159a is a key microRNA that targets mRNAs coding of MYB proteins that bind to the regulative site of floral meristem identity gene LEAFY [112], also ath-miR159a involved in hypoxia stress [110]. The prediction result of the post-translation modification showed that ACS proteins were more phosphorylated and glycosylated. Phosphorylation and glycosylation are the prevalent post-translational modification of proteins which could modify object site and activity of protein [82]. Phosphorylation, as one of the most plentiful post-translational modifications, plays the main role in plant metabolism and signal transduction via modifying protein interactions, protein activities, or subcellular location [24, 86, 113–115]. Regarding evidence, it seems that the biosynthesis of ethylene is adjusted by phosphorylation events that probably affect the ACS protein turnover. Working on the usage of phosphatase inhibitors and kinase in tomato tissues and suspension cell cultures demonstrated that phosphorylation influence the activity and/or turnover of ACS [116]. Thus, it seems that ACS phosphorylation preserves the protein from the destruction that in turn may lead ACS to accumulate and ACS activity to enhance, considering for the burst of ethylene production via ripening fruit [117], noteworthy, LeACS2 protein of tomato has been discovered to be phosphorylated in response to wounding [117]. The glycosylation could make alterations to the stability of the protein [118] and protein’s molecular weight [119]. To sum up, it seems the ethylene biosynthesis proteins from Arabidopsis were more glycosylated than rice’s proteins. Some studies highlight the possibility of posttranslational regulation of ACS [115, 118].
Conclusion
Nowadays, computational analysis plays a substantial role in plant science. Appropriate computational approaches coupled with suitable databases are fundamental for detecting, organizing, integrating data information content furnishing novel insights into the involved genes in important pathways and biological systems as well. Ethylene is a gaseous hormone that controls various physiological pathways. In this study, the involved genes in ethylene biosynthesis were evaluated using available bioinformatics tools in Arabidopsis and rice. Results revealed that involved-enzymes in ethylene biosynthesis had more variation based on physic-chemical characters and patterns of gene expression, protein structure, post-translation modification, and type of cis-regulatory elements. The genes in the ethylene biosynthesis pathway of rice had high variation than Arabidopsis indicated that probably SAM, ACS, and ACO genes of dicots such as Arabidopsis are derived from monocot such as rice. All SAM, ACS, and ACO genes are expressed in studied tissue and organs, but at different levels. SAM genes are more involved in the rice-ripening stage, while in Arabidopsis, ACS and ACO genes are contributed in maturity. Also, the SAM, ACS, and ACO genes expression of rice in different tissue and organs demonstrated more variation in comparison with the Arabidopsis genes. Regarding the post-translation modification result, the ACO proteins were less phosphorylated than ACS, and SAM proteins, and it seems the ethylene biosynthesis proteins from Arabidopsis were more glycosylated than rice’s proteins that can affect the protein activity, or subcellular location. Overall, the current study described that involved genes in the ethylene biosynthesis pathway play the key roles in controlling the response to abiotic and biotic stresses tolerance that various factors such as PPIs, type of cis-regulatory elements, and post-transcription/translation modifications could affect their expression. Our study was the first in silico and review study which widely assessed SAM, ACS, and ACO genes that are involved in ethylene biosynthesis and it provided an expanded landscape of computational analysis for further dissection and functional characterization of SAM, ACS, and ACO genes.
Acknowledgements
Not applicable.
Abbreviations
- PGRs
-
Plant growth regulators
miRNA
Micro-RNA
SAM
S-adenosylmethionine
ACC
1-Aminocyclopropane-1-carboxylate
ACO
ACC oxidase
CDPK
Calcium-dependent protein kinase
MAPK
Mitogen-activated protein kinase
ERF
Ethylene response factor
TFs
Transcription factors
CAREs
Cis-acting regulatory elements
MW
Molecular weight
pI
Isoelectric point
GRAVY
Grand average of hydropathy
PPI
Protein-protein interaction
Authors’ contributions
PH designed and managed the work. MA, SH, and PH analyzed and wrote the original draft. All authors read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
The datasets and raw data are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mostafa Ahmadizadeh, Email: ahmadizadeh.mostafa@gmail.com.
Jen-Tsung Chen, Email: jentsung@nuk.edu.tw.
Soosan Hasanzadeh, Email: sousan.hasanzadeh@gmail.com.
Sunny Ahmar, Email: sunnyahmar@webmail.hzau.edu.cn.
Parviz Heidari, Email: heidarip@shahroodut.ac.ir.
References
- 1.Argueso CT, Hansen M, Kieber JJ. Regulation of ethylene biosynthesis. J Plant Growth Regul. 2007;26:92–105. doi: 10.1007/s00344-007-0013-5. [DOI] [Google Scholar]
- 2.Tsuchisaka A, Yu G, Jin H, et al. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics. 2009;183:979–1003. doi: 10.1534/genetics.109.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arraes FBM, Beneventi MA, Lisei de Sa ME, et al. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015;15:213. doi: 10.1186/s12870-015-0597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell PJ, Calvert C, Atzorn R, et al (1996) Ethylene as a signal mediating the wound response of tomato plants. Science (80- ) 274:1914–1917. 10.1126/science.274.5294.1914 [DOI] [PubMed]
- 5.Grichko VP, Glick BR (2001) Ethylene and flooding stress in plants. Plant Physiol Biochem 39:1–9. 10.1016/S0981-9428(00)01213-4
- 6.Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology. Academic Press
- 7.Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller M, Song Q, Shi X, et al. Natural variation in timing of stress-responsive gene expression predicts heterosis in intraspecific hybrids of Arabidopsis. Nat Commun. 2015;6:7453. doi: 10.1038/ncomms8453. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Yin C-C, Zhao H, Ma B, et al. Diverse roles of ethylene in regulating agronomic traits in rice. Front Plant Sci. 2017;8:1676. doi: 10.3389/fpls.2017.01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleecker AB, Kende H. Ethylene: A gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Dong JG, Fernandez-Maculet JC, Yang SF. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci. 1992;89:9789–9793. doi: 10.1073/pnas.89.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 14.Jakubowicz M. Structure, catalytic activity and evolutionary relationships of 1-aminocyclopropane-1-carboxylate synthase, the key enzyme of ethylene synthesis in higher plants. Acta Biochim Pol. 2002;49:757–774. doi: 10.18388/abp.2002_3784. [DOI] [PubMed] [Google Scholar]
- 15.Song Q, Ando A, Xu D, et al. Diurnal down-regulation of ethylene biosynthesis mediates biomass heterosis. Proc Natl Acad Sci. 2018;115:5606–5611. doi: 10.1073/pnas.1722068115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 17.Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- 18.Yamagami T, Tsuchisaka A, Yamada K, et al. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem. 2003;278:49102–49112. doi: 10.1074/jbc.M308297200. [DOI] [PubMed] [Google Scholar]
- 19.Chae HS, Kieber JJ. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005;10:291–296. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee HY, Yoon GM. Regulation of ethylene biosynthesis by phytohormones in etiolated rice (Oryza sativa L.) seedlings. Mol Cell. 2018;41:311–319. doi: 10.14348/molcells.2018.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastià CH, Hardin SC, Clouse SD, et al. Identification of a new motif for CDPK phosphorylation in vitro that suggests ACC synthase may be a CDPK substrate. Arch Biochem Biophys. 2004;428:81–91. doi: 10.1016/j.abb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Kim CY, Liu Y, Thorne ET, et al. Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell. 2003;15:2707–2718. doi: 10.1105/tpc.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon GM. New insights into the protein turnover regulation in ethylene biosynthesis. Mol Cell. 2015;38:597–603. doi: 10.14348/molcells.2015.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell. 2003;15:545–559. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christians MJ, Gingerich DJ, Hansen M, et al. The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 2009;57:332–345. doi: 10.1111/j.1365-313X.2008.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang KL-C, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–950. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida H, Nagata M, Saito K, et al. Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biol. 2005;5:14. doi: 10.1186/1471-2229-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki T, Matsumoto T, Antonio B, Nagamura Y. From mapping to sequencing, post-sequencing and beyond. Plant Cell Physiol. 2005;46:3–13. doi: 10.1093/pcp/pci503. [DOI] [PubMed] [Google Scholar]
- 30.Du H, Yu Y, Ma Y, et al. Sequencing and de novo assembly of a near complete indica rice genome. Nat Commun. 2017;8:15324. doi: 10.1038/ncomms15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahi C, Singh A, Kumar K, et al. Salt stress response in rice: genetics, molecular biology, and comparative genomics. Funct Integr Genomics. 2006;6:263–284. doi: 10.1007/s10142-006-0032-5. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto M, Baba-kasai A, Kiyota S, et al. ACO1, a gene for aminocyclopropane-1-carboxylate oxidase: effects on internode elongation at the heading stage in rice. Plant Cell Environ. 2010;33:805–815. doi: 10.1111/j.1365-3040.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- 33.Mekhedov SL, Kende H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 1996;37:531–537. doi: 10.1093/oxfordjournals.pcp.a028976. [DOI] [PubMed] [Google Scholar]
- 34.Chae HS, Cho YG, Park MY, et al. Hormonal cross-talk between auxin and ethylene differentially regulates the expression of two members of the 1-aminocyclopropane-1-carboxylate oxidase gene family in rice (Oryza sativa L.) Plant Cell Physiol. 2000;41:354–362. doi: 10.1093/pcp/41.3.354. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari S, Vairo D, Ausubel FM, et al. Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell. 2003;15:93–106. doi: 10.1105/tpc.005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomma BPHJ, Eggermont K, Tierens KFM-J, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pré M, Atallah M, Champion A, et al. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 2008;147:1347–1357. doi: 10.1104/pp.108.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berrocal-Lobo M, Molina A, Solano R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 2002;29:23–32. doi: 10.1046/j.1365-313x.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- 39.Berrocal-Lobo M, Molina A. Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Mol Plant-Microbe Interact. 2004;17:763–770. doi: 10.1094/MPMI.2004.17.7.763. [DOI] [PubMed] [Google Scholar]
- 40.Schweighofer A, Kazanaviciute V, Scheikl E, et al. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell. 2007;19:2213–2224. doi: 10.1105/tpc.106.049585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao W-H, Liu J, He X-J, et al. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007;143:707–719. doi: 10.1104/pp.106.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng J, Li Z, Wen X, et al. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet. 2014;10:e1004664. doi: 10.1371/journal.pgen.1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao J-J, Chen H-W, Ma B, et al. The role of ethylene in plants under salinity stress. Front Plant Sci. 2015;6:1059. doi: 10.3389/fpls.2015.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linkies A, Leubner-Metzger G. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep. 2012;31:253–270. doi: 10.1007/s00299-011-1180-1. [DOI] [PubMed] [Google Scholar]
- 45.Matilla AJ, Matilla-Vázquez MA. Involvement of ethylene in seed physiology. Plant Sci. 2008;175:87–97. doi: 10.1016/j.plantsci.2008.01.014. [DOI] [Google Scholar]
- 46.Pegoraro C, Farias D da R, Mertz LM, et al. Ethylene response factors gene regulation and expression profiles under different stresses in rice. Theor Exp Plant Physiol. 2013;25:261–274. doi: 10.1590/S2197-00252013000400004. [DOI] [Google Scholar]
- 47.Yu Y, Wang J, Zhang Z, et al. Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genet. 2013;9:e1004025. doi: 10.1371/journal.pgen.1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori Y, Nagai K, Furukawa S, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 49.Zhong S, Shi H, Xue C, et al. A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol. 2012;22:1530–1535. doi: 10.1016/j.cub.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi H, Shen X, Liu R, et al. The red light receptor phytochrome B directly enhances substrate-E3 ligase interactions to attenuate ethylene responses. Dev Cell. 2016;39:597–610. doi: 10.1016/j.devcel.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi H, Liu R, Xue C, et al. Seedlings transduce the depth and mechanical pressure of covering soil using COP1 and ethylene to regulate EBF1/EBF2 for soil emergence. Curr Biol. 2016;26:139–149. doi: 10.1016/j.cub.2015.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J, Ma Q, Mao T. Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiol. 2015;169:325–337. doi: 10.1104/pp.15.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- 54.Harpaz-Saad S, Yoon GM, Mattoo AK, Kieber JJ. Annual Plant Reviews Volume 44. Oxford, UK: Wiley-Blackwell; 2012. The formation of ACC and competition between polyamines and ethylene for SAM; pp. 53–81. [Google Scholar]
- 55.Yoon GM, Kieber JJ. 14-3-3 regulates 1-aminocyclopropane-1-carboxylate synthase protein turnover in Arabidopsis. Plant Cell. 2013;25:1016–1028. doi: 10.1105/tpc.113.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawahara Y, de la Bastide M, Hamilton JP, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gasteiger E, Hoogland C, Gattiker A, et al. The Proteomics Protocols Handbook. Totowa, NJ: Humana Press; 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [Google Scholar]
- 58.Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hruz T, Laule O, Szabo G, et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinforma. 2008;2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai X, Zhuang Z, Zhao PX. psRNATarget: a plant small RNA target analysis server (2017 release) Nucleic Acids Res. 2018;46:W49–W54. doi: 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chow C-N, Zheng H-Q, Wu N-Y, et al. PlantPAN 2.0: an update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 2016;44:D1154–D1160. doi: 10.1093/nar/gkv1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta R, Brunak S (2002) Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput:310–322 [PubMed]
- 63.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 64.Elliott RC, Betzner AS, Huttner E, et al. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutilier K, Offringa R, Sharma VK, et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vahala J, Felten J, Love J, et al. A genome-wide screen for ethylene-induced ethylene response factors (ERFs) in hybrid aspen stem identifies ERF genes that modify stem growth and wood properties. New Phytol. 2013;200:511–522. doi: 10.1111/nph.12386. [DOI] [PubMed] [Google Scholar]
- 67.Cheng M-C, Liao P-M, Kuo W-W, Lin T-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013;162:1566–1582. doi: 10.1104/pp.113.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu K, Xu X, Fukao T, et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto S, Suzuki K, Shinshi H. Elicitor-responsive, ethylene-independent activation of GCC box-mediated transcription that is regulated by both protein phosphorylation and dephosphorylation in cultured tobacco cells. Plant J. 1999;20:571–579. doi: 10.1046/j.1365-313X.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- 70.Jiao Y, Ma L, Strickland E, Deng XW. Conservation and divergence of light-regulated genome expression patterns during seedling development in rice and Arabidopsis. Plant Cell. 2005;17:3239–3256. doi: 10.1105/tpc.105.035840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, et al. Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J. 2005;42:305–314. doi: 10.1111/j.1365-313X.2005.02363.x. [DOI] [PubMed] [Google Scholar]
- 72.Simpson SD, Nakashima K, Narusaka Y, et al. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003;33:259–270. doi: 10.1046/j.1365-313X.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 73.Johnson C, Boden E, Arias J. Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell. 2003;15:1846–1858. doi: 10.1105/tpc.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ko J-H, Beers EP, Han K-H. Global comparative transcriptome analysis identifies gene network regulating secondary xylem development in Arabidopsis thaliana. Mol Gen Genomics. 2006;276:517–531. doi: 10.1007/s00438-006-0157-1. [DOI] [PubMed] [Google Scholar]
- 75.Wang ZY, Kenigsbuch D, Sun L, et al. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohanty B, Krishnan SPT, Swarup S, Bajic VB. Detection and preliminary analysis of motifs in promoters of anaerobically induced genes of different plant species. Ann Bot. 2005;96:669–681. doi: 10.1093/aob/mci219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kosugi S, Suzuka I, Ohashi Y. Two of three promoter elements identified in a rice gene for proliferating cell nuclear antigen are essential for meristematic tissue-specific expression. Plant J. 1995;7:877–886. doi: 10.1046/j.1365-313X.1995.07060877.x. [DOI] [PubMed] [Google Scholar]
- 78.Luo H, Song F, Goodman RM, Zheng Z. Up-regulation of OsBIHD1, a rice gene encoding BELL homeodomain transcriptional factor, in disease resistance responses. Plant Biol. 2005;7:459–468. doi: 10.1055/s-2005-865851. [DOI] [PubMed] [Google Scholar]
- 79.Hwang YS, Karrer EE, Thomas BR, et al. Three cis-elements required for rice alpha-amylase Amy3D expression during sugar starvation. Plant Mol Biol. 1998;36:331–341. doi: 10.1023/A:1005956104636. [DOI] [PubMed] [Google Scholar]
- 80.Kosugi S, Ohashi Y. E2F sites that can interact with E2F proteins cloned from rice are required for meristematic tissue-specific expression of rice and tobacco proliferating cell nuclear antigen promoters. Plant J. 2002;29:45–59. doi: 10.1046/j.1365-313x.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 81.Sutoh K, Yamauchi D. Two cis-acting elements necessary and sufficient for gibberellin-upregulated proteinase expression in rice seeds. Plant J. 2003;34:635–645. doi: 10.1046/j.1365-313X.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- 82.Heidari P, Ahmadizadeh M, Izanlo F, Nussbaumer T (2019) In silico study of the CESA and CSL gene family in Arabidopsis thaliana and Oryza sativa: Focus on post-translation modifications. Plant Gene 19. 10.1016/j.plgene.2019.100189
- 83.Bigeard J, Rayapuram N, Pflieger D, Hirt H. Phosphorylation-dependent regulation of plant chromatin and chromatin-associated proteins. Proteomics. 2014;14:2127–2140. doi: 10.1002/pmic.201400073. [DOI] [PubMed] [Google Scholar]
- 84.Heyl A, Brault M, Frugier F, et al. Nomenclature for members of the two-component signaling pathway of plants. Plant Physiol. 2013;161:1063–1065. doi: 10.1104/pp.112.213207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP. Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J Exp Bot. 2013;64:445–458. doi: 10.1093/jxb/ers354. [DOI] [PubMed] [Google Scholar]
- 86.Friso G, van Wijk KJ (2015) Update: Post-translational protein modifications in plant metabolism. Plant Physiol 01378(2015) 10.1104/pp.15.01378 [DOI] [PMC free article] [PubMed]
- 87.Bidonde S, Ferrer MA, Zegzouti H, et al. Expression and characterization of three tomato 1-aminocyclopropane-1-carboxylate oxidase cDNAs in yeast. Eur J Biochem. 1998;253:20–26. doi: 10.1046/j.1432-1327.1998.2530020.x. [DOI] [PubMed] [Google Scholar]
- 88.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 89.Heidari P, Mazloomi F, Nussbaumer T, Barcaccia G (2020) Insights into the SAM synthetase gene family and its roles in tomato seedlings under abiotic stresses and hormone treatments. Plants 9. 10.3390/plants9050586 [DOI] [PMC free article] [PubMed]
- 90.Ikai A. Thermostability and aliphatic index of globular proteins. J Biochem. 1980;88:1895–1898. [PubMed] [Google Scholar]
- 91.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 92.Roche DB, Brackenridge DA, McGuffin LJ. Proteins and their interacting partners: An introduction to protein-ligand binding site prediction methods. Int J Mol Sci. 2015;16:29829–29842. doi: 10.3390/ijms161226202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dukka BKC. Structure-based methods for computational protein functional site prediction. Comput Struct Biotechnol J. 2013;8:e201308005. doi: 10.5936/csbj.201308005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ezzat A, Kwoh CK. Comparison of structure-based tools for the prediction of ligand binding site residues in apo-structures. In: Procedia Computer Science. Elsevier B.V; 2012. pp. 115–126. [Google Scholar]
- 95.Abrusán G, Marsh JA. Ligand binding site structure influences the evolution of protein complex function and topology. Cell Rep. 2018;22:3265–3276. doi: 10.1016/j.celrep.2018.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alonso JM, Stepanova AN (2004) The ethylene signaling pathway. Science (80- ) 306:1513–1515. 10.1126/science.1104812 [DOI] [PubMed]
- 97.Liu C, Lü R, Li J, et al. Characterization and expression profiles of MaACS and MaACO genes from mulberry (Morus alba L.) J Zhejiang Univ B. 2014;15:611–623. doi: 10.1631/jzus.B1300320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang NN, Shih M-C, Li N. The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot. 2005;56:909–920. doi: 10.1093/jxb/eri083. [DOI] [PubMed] [Google Scholar]
- 100.Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- 101.Olson DC, White JA, Edelman L, et al. Differential expression of two genes for 1-aminocyclopropane-1-carboxylate synthase in tomato fruits. Proc Natl Acad Sci U S A. 1991;88:5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lincoln JE, Campbell AD, Oetiker J, et al. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum). Expression in Escherichia coli, structural characterization, expression characteristics, and phylogenetic analysis. J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- 103.Rottmann WH, Peter GF, Oeller PW, et al (1991) 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol 222:937–961. 10.1016/0022-2836(91)90587-V [DOI] [PubMed]
- 104.Lelièvre JM, Tichit L, Dao P, et al. Effects of chilling on the expression of ethylene biosynthetic genes in Passe-Crassane pear (Pyrus communis L.) fruits. Plant Mol Biol. 1997;33:847–855. doi: 10.1023/A:1005750324531. [DOI] [PubMed] [Google Scholar]
- 105.Arteca JM, Arteca RN. A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (ACS6) in mature Arabidopsis leaves. Plant Mol Biol. 1999;39:209–219. doi: 10.1023/A:1006177902093. [DOI] [PubMed] [Google Scholar]
- 106.Peng H-P, Lin T-Y, Wang N-N, Shih M-C. Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant Mol Biol. 2005;58:15–25. doi: 10.1007/s11103-005-3573-4. [DOI] [PubMed] [Google Scholar]
- 107.Ahmadizadeh M, Heidari P. Bioinformatics study of transcription factors involved in cold stress. Biharean Biol. 2014;8:83–86. [Google Scholar]
- 108.Rezaee S, Ahmadizadeh M, Heidari P. Genome-wide characterization, expression profiling, and post- transcriptional study of GASA gene family. Gene Reports. 2020;20:100795. doi: 10.1016/j.genrep.2020.100795. [DOI] [Google Scholar]
- 109.Ibraheem O, Botha CEJ, Bradley G. In silico analysis of cis-acting regulatory elements in 5′ regulatory regions of sucrose transporter gene families in rice (Oryza sativa Japonica) and Arabidopsis thaliana. Comput Biol Chem. 2010;34:268–283. doi: 10.1016/j.compbiolchem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Moldovan D, Spriggs A, Yang J, et al. Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J Exp Bot. 2010;61:165–177. doi: 10.1093/jxb/erp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu Q-H, Spriggs A, Matthew L, et al. A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res. 2008;18:1456–1465. doi: 10.1101/gr.075572.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rhoades MW, Reinhart BJ, Lim LP, et al (2002) Prediction of plant microRNA targets. Cell 110:513–520. 10.1016/s0092-8674(02)00863-2 [DOI] [PubMed]
- 113.van Wijk KJ, Friso G, Walther D, Schulze WX. Meta-analysis of Arabidopsis thaliana phospho-proteomics data reveals compartmentalization of phosphorylation motifs. Plant Cell. 2014;26:2367–2389. doi: 10.1105/tpc.114.125815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schönberg A, Baginsky S. Signal integration by chloroplast phosphorylation networks: an update. Front Plant Sci. 2012;3:256. doi: 10.3389/fpls.2012.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silva-Sanchez C, Li H, Chen S. Recent advances and challenges in plant phosphoproteomics. Proteomics. 2015;15:1127–1141. doi: 10.1002/pmic.201400410. [DOI] [PubMed] [Google Scholar]
- 116.Kamiyoshihara Y, Iwata M, Fukaya T, et al (2010) Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J 64:no-no. 10.1111/j.1365-313X.2010.04316.x [DOI] [PubMed]
- 117.Tatsuki M, Mori H. Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J Biol Chem. 2001;276:28051–28057. doi: 10.1074/jbc.M101543200. [DOI] [PubMed] [Google Scholar]
- 118.Solá RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009;98:1223–1245. doi: 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hamilton SR, Gerngross TU. Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr Opin Biotechnol. 2007;18:387–392. doi: 10.1016/j.copbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and raw data are available from the corresponding author on reasonable request.