Abstract
Background
During the last century, total hip arthroplasties have become more popular. They have had a huge impact on the quality of life, pain, range of motion, social interaction, and psychological well-being. A number of studies have emphasized the importance of using templates to choose the appropriate implant size when planning the surgery. Our aim is to use MediCad® software to analyze the ability of the digital template system MediCad® to predict the size of the implant needed in total hip arthroplasties.
Materials and Methods
An arthroplasty preoperative plan was created according to the MediCad® software guidelines, on anteroposterior hip X-ray by one junior resident, one senior resident, and three experienced hip surgeons.
Results
The median size accuracy was 0.7 (range: 0.27–0.87) for the cup, 0.73 (range: 0.36–0.83) for the stem, and 0.28 (range: −0.14–0.69) for the neck. Interobserver reliability was good (kappa > 0.4) and stronger when measuring the stem than when doing so with the cup. Conclusion: Digital preoperative total hip arthroplasty planning is a good method for predicting component size, restoring hip anatomy (vertical offset and horizontal offset), with good interobserver reliability.
Keywords: Digital templating, Hip arthroplasty, Total hip arthroplasty, Total hip arthroplasty preoperative planning
Introduction
Total hip arthroplasties (THAs) are one of the greatest medical innovations of the 20th century [1], due to their impact upon the quality of life, pain, range of motion, social interaction, and psychological well-being [2]. Good long term results encourage performing total hip replacements at earlier ages to achieve an active lifestyle after the procedure with the greatest possible implant survival. Obtaining excellent radiological results has hence become imperative. Therefore, a good preoperative plan of implants’ size and position should be done. Furthermore, using these templates, the risks associated with potential complications such as periprosthetic fractures, the inappropriate difference in leg length, and dislocation are decreased, leading to higher implant survival rates and less implant wear.
During the past two decades, digital image acquisition and reviewing have become widespread, and with it, digital templating is becoming more and more popular. These digital templates are 15%–20% [3] magnified, and according to Conn et al., they are only accurate in 65% of the cases [4].
Most studies consider their final choice of component size the gold standard with which to compare the prediction. However, only Gamble et al. have analyzed their final components on radiographs to check they were the appropriate size [5]. Strøm and Reikerås evaluated their surgical outcome based on leg length equality [6]. In our study, we did not only analyze the postoperative radiographs but also made objective measurements to determine that the implant positioning was correct.
Despite the consensus in the literature on the usefulness of this software, the preoperative plan should not only consider the use of templates but also a study of individual biomechanical and anatomical factors that are key during the surgery. However, the later is not easy to assess and digital planning software is not able to fully evaluate them. This is where the surgeon’s experience in preoperative planning is important and could make a difference when choosing the appropriate implant size.
Our aim is to use MediCad® software to analyze the ability of this digital template system to predict the size of the implant needed in THA. In addition, interobserver variability of the data obtained was also assessed, taking into account that the different observers have variable levels of experience in this kind of surgery.
Materials and Methods
This is a prospective and descriptive study conducted between January and December 2015. Our inclusion criteria were patients who underwent primary uncemented THA, excluding those where a trabecular metal supplement or bone graft was planned. To obtain a uniform sample and minimize bias, we excluded patients who underwent cemented, partial, or resurfacing bilateral hip arthroplasty or hip revision surgery from our study. Moreover, like Shaarani et al., we consider that when a radiograph with an adequate femur rotation was impossible to obtain because of a fixed flexion deformity of the hip, it is not possible to make an accurate estimation [7]. To solve this problem in these cases, we have planned the components’ size using the contralateral healthy side. Furthermore, to confirm that the final components were correct in size and orientation, a postoperative analysis of anteroposterior (AP) radiograph was performed by two members of the team [5, 8, 9]. The patients that did not have an adequate postoperative cup positioning at 10°–15° anteversion [8] and 35°–45° inclination [9] were excluded. We considered the position to be important because we could not ensure that the final components were appropriate in size if they lacked optimal orientation. This ensures that we can consider our final components as the gold standard.
All the patients undergoing primary uncemented THA were selected and included in the study. Preoperative planning was performed in all these patients. However, when in postoperative radiographs either the size or orientation was considered not optimal, the patients were excluded.
Surgical Procedure
All surgeries were performed by the same surgical team using the same surgical technique and a modified Watson Jones approach in supine position under general anesthesia. Antibiotic prophylaxis with cefazolin 2 g was used. A second postoperative dose was given 8 h after the procedure. In all cases, an uncemented total hip prosthesis press-fit Allofit cup® (Zimmer Inc.®, Warsaw, IN, USA) and CLS Spottorno Stem® (Zimmer Ltd.®, Germany) was implanted. In 34 (87.2%) cases, a cup size bigger than 50 with a 36 mm diameter head was implanted. In five (12.8%) cases, a cup size 50 or smaller was implanted in combination with a 28 mm diameter head.
Intraoperative anatomical criteria such as the surrounding soft-tissue tension, leg length discrepancy, intraoperative implant stability, and dislocation maneuvers were all taken into account to make a decision regarding the final component size.
X-ray Preoperative Study
The preoperative study included an AP hip X-ray following the recommendations published by Campbell [10], both feet in approximately 15º of internal rotation to allow better visualization of the femoral neck by reducing the normal femoral anteversion. A correct biometric analysis can only be possible if the pelvis has been placed symmetrically, the longitudinal axis of the femur is parallel to the X-ray detector and the patella is at rest. A correct X-ray should show the 10 references noted by Blumetritt in his mechanical loading model of the hip joint [11, 12]. Note that the MediCad® software requires a 25 mm diameter radio-opaque ball as a reference that must be placed in the inner area of the thigh, as close as possible to the femoral head to adjust the degree of magnification.
Templating
Before surgery, the surgical team including one junior resident, one senior resident, and three experienced hip surgeons performed the preoperative planning independently. All observers were blinded to each other’s results.
The arthroplasty plan was devised according to the MediCad® software guidelines, following these sequential steps: [13].
The femoral head center of rotation was specified by marking three points in its circumference.
Offset was measured from the teardrop medial to the acetabulum to the femoral longitudinal axes [14].
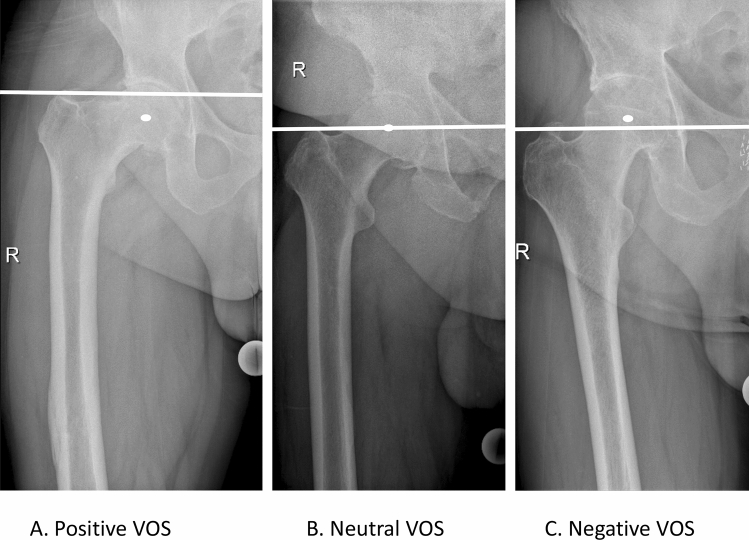
Vertical offset (VOS) was analyzed as positive, negative, or neutral according to the position of the greater trochanter relative to the femoral head’s center of rotation (Fig. 1).
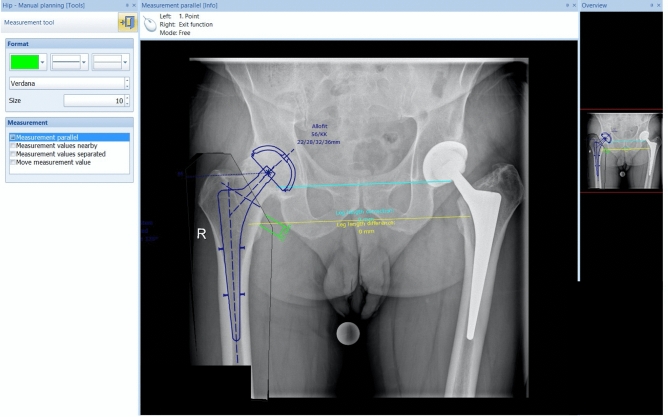
Thereafter, cup, stem, and neck size were determined using the program’s templates (Fig. 2).
Fig. 1.
MediCad® Software: Vertical offset: a Positive vertical offset: The greater trochanter is above the femoral head’s center of rotation. b Neutral vertical offset: The greater trochanter is aligned with the femoral head’s center of rotation. c Negative vertical offset: The greater trochanter is below the femoral head’s center of rotation
Fig. 2.
MediCad® Software: Choosing the stem, neck and cup size
Postoperative X-ray Study
After the surgery, VOS and horizontal offset were measured on the AP hip X-ray view. To ensure that the sizes selected during surgery were optimal and could, therefore, be used as our gold standard, cup size and positioning were analyzed under the assumptions that proper cup implantation should have an anteversion of 10°–15° [8] and an inclination of 35°–45° [9] and be placed 5 mm above the inferior teardrop, < 5 mm from Kohler’s line [5]. Appropriate stem size was defined as an adequate canal fill with cortical contact in the metaphyseal–diaphyseal junction and < 5° of varus or valgus [5].
Statistical Methods
The agreement was analyzed as weighted kappa. It was measured for each component (stem, neck, and head) and surgeon. Full agreement was weighted as 1 and one size difference was weighted as 0.8, differences equal or over two sizes were given a weight of 0. The results were categorized as an excellent agreement when kappa values exceeded 0.75, good agreement when kappa values were between 0.4 and 0.75 or poor agreement when kappa values were < 0.4 [15].
Statistical analysis was performed with STATA version 9.0. and Microsoft® Excel version 15.28. The graphical representation of agreement was done with the agreement chart of Bandingwala [16] on RStudio version 0.99.484 (RStudio, Boston, Massachusetts, USA).
Results
Of the 60 patients who underwent THA from January to December 2015, 39 met the inclusion criteria. Our sample included 24 (61.5%) men and 15 (38.5%) women, with a mean age of 65 (standard deviation [SD]: 9). Fourteen (35.9%) surgeries were performed on the left side and 25 (64.1%) were right.
Two patients were excluded from the analysis because their prosthesis components were considered inappropriate due to size or positioning, following exclusion criteria.
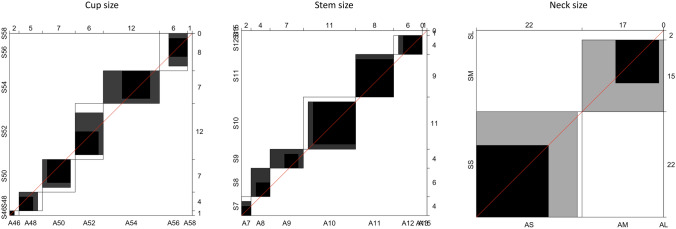
The agreement analyses results are summarized in Table 1. The agreement Bandingwala chart for our best observer is represented in Fig. 3.
Table 1.
Agreement values
| Observer | Cup | Stem | Neck |
|---|---|---|---|
| C1 | 0.7 | 0.57 | 0.51 |
| C2 | 0.7 | 0.83 | 0.28 |
| C3 | 0.87 | 0.77 | 0.69 |
| R1 | 0.58 | 0.73 | 0.13 |
| R2 | 0.27 | 0.36 | − 0.14 |
Fig. 3.
Our best observer’s agreement Bandingwala chart
The Cup
The median cup size accuracy was 0.7 (range: 0.27–0.87). The most experienced consultant made the most accurate predictions, with a kappa value of 0.87 (excellent agreement). The senior resident made the least accurate prediction with a kappa value of 0.27 (poor agreement).
The Stem
The median stem size accuracy was 0.73 (range: 0.36–0.83). The consultant with intermediate experience made the most accurate predictions, with a kappa value of 0.83 (excellent agreement). The senior resident made the least accurate prediction with a kappa value of 0.36 (poor agreement).
The Head Neck
The median neck size accuracy was 0.28 (range: 0.14–0.69). The most experienced consultant made the most accurate prediction in the range of good, with a kappa value of 0.69 (good agreement). The senior resident made the least accurate prediction with a kappa value of −0.14 (poor agreement).
Interobserver Reliability
Interobserver reliability results are summarized in Table 2. There was a good agreement between the consultants. This agreement was stronger when the consultants had more experience. Moreover, there was also a good agreement between the consultants and the junior resident. However, the senior resident was not as accurate, and therefore, displayed a “poor agreement” with the consultants and was on the verge of the “good agreement” range with the junior resident.
Table 2.
Interobserver reliability
| Observer | C1 | C2 | C3 | R1 |
|---|---|---|---|---|
| Stem | ||||
| C2 | 0.42 | |||
| C3 | 0.54 | 0.61 | ||
| R1 | 0.51 | 0.73 | 0.67 | |
| R2 | 0.23 | 0.43 | 0.45 | 0.55 |
| Cup | ||||
| C2 | 0.56 | |||
| C3 | 0.66 | 0.74 | ||
| R1 | 0.51 | 0.54 | 0.49 | |
| R2 | 0.44 | 0.39 | 0.41 | 0.45 |
| Neck | ||||
| C2 | 0.05 | |||
| C3 | 0.39 | 0.12 | ||
| R1 | 0.06 | 0.25 | 0.09 | |
| R2 | − 0.06 | 0.06 | − 0.03 | 0.13 |
Furthermore, the agreement was stronger when measuring the stem than when doing so with the cup. The agreement parameters regarding the neck size were chaotic. There was a very poor agreement amongst all the observers.
Horizontal Offset and Vertical Offset Discrepancy
Preoperative and postoperative horizontal offset was maintained constant with a mean difference between preoperative and postoperative offset of 0.57 mm (SD: 16 mm IC 95%). We maintained the same VOS in 20 (54%) of our patients. In 12 (32%) of our patients, the VOS changed positive to neutral or neutral to negative. In five (14%), the VOS changed from neutral to positive or negative to neutral (Fig. 1).
Discussion
Our study aims to test the reliability of digital templating in predicting component size in THA surgery. It also intends to assess interobserver reliability between five observers of increasing experience.
Preoperative size prediction is important because it shortens surgical time [17, 18], ensures implants are available (identifying rare sizes) and minimizes costs [19]. Preoperative planning also increases surgical precision [17, 20], aiding in femoral offset restoration, leg length symmetry, and alignment optimization [21–31]. Finally, it reduces the risks of implant loosening, bone stock loss periprosthetic fractures, and instability [21, 32, 33].
Our results suggest that preoperative planning using Medicad® is accurate in experienced hands. Choosing the appropriate component size in THA reduces the risk of disrupting hip anatomy and minimizes the risk of prosthesis instability that could potentially contribute to increased survival of the prosthesis and improvement of functional results. Therefore, we consider preoperative clinical and radiological planning an essential step for the success of this procedure, since it allows the surgeon to anticipate any possible difficulties that might arise during the process.
THA is one of the most successful and cost-effective procedures in orthopedics [34, 35]. Digital planning minimized the costs and space associated with inventory and archives. It has already been established in the literature that both digital and analogical methods provide accurate predictions [5]. Taking into account that, hardcopy X-rays are hardly used and need physical storage space, it seems more appropriate to start using digital methods. Despite their similarities, some authors have found that the digital method tends to overestimate the cup size and underestimate the stem [36]. We have found that using our digital templating system, we tend to overestimate rather than underestimate both the cup and stem size. To minimize this overestimation, a metallic ball is placed in the inner thigh as close as possible to the femoral head [37]. This is one of the main differences with other software. Other programs use a disk on the medial aspect of the thigh [5] or two metallic markers on the lateral aspect [13]. This marker will influence the distances measured therefore a sphere is considered more accurate because no matter the angle of incidence of the X-ray, the diameter (the length used for calibration) will always be the same, whereas for a disk or 2 markers the length can change depending on how angulated the X-ray tube is when taking the radiographs. Moreover, it seems it is better to place it as near to the femoral head as possible [5]. We consider that placing the marker in the inner thigh as close as possible to the femoral head is a better option because it minimizes the magnification error.
Most authors [3, 37] have not tested their gold standard (component size implanted during surgery) that could lead to bias assessments. We consider this an important part of our study because intraoperative decisions are not always correct. Adequate choice of component size and position can be analyzed with various methods such as the ones used by us or the one used by Shaarani et al. in his postoperative radiographic analyses [7].
Our results are similar to those previously reported in the literature [3, 7, 36–38]. They show similar agreement values to those studies that have used both analog and digital methods [36] (Table 3). We agree with the fact that there are stronger agreements between surgeons with more surgical experience [39]. Likewise, we consider that the level of experience with the planning software seems to influence the results. This is how we explain that our junior resident provided more accurate predictions than the senior resident. This could be due to the ability in identifying anatomical landmarks or the practice using the software. Although our senior resident has a wide experience using analog templates, our junior resident has more experience using the digital templates in the planning software. We have not been able to identify or quantify the elements conditioning this learning curve and therefore cannot analyze to what degree it influences the observer’s final decision. This should be furthered analyzed in future studies.
Table 3.
Results reported in the literature
| Planning method | Author | Exact cup | Cup ± 1 size | Exact stem | Stem ± 1 size |
|---|---|---|---|---|---|
| Analog (%) | Unnanuntana et al. | 42.2 | – | 68.8 | 98.2 |
| The et al. | 67 | – | 56 | – | |
| Suh et al. | 58 | 100 | 79 | 100 | |
| Iorio et al. | – | 78 | – | 77 | |
| Digital (%) | Kumar et al. | 56 | 91 | 62 | 78 |
| Gamble et al. | 38 | 80 | 35 | 85 | |
| Steinberg | 50 | 88 | 47 | 87 | |
| Bertz et al. | 60 | 94 | 64 | 95 | |
| Gallart et al. | 49.1 | 65.5 | 43.6 | 46.1 | |
| Iorio et al. | 60 | 74 | |||
| Sharaani et al. | 75 | 80 | |||
| Efe et al. | 36 | 82.3 |
One of the drawbacks of using these softwares is that they can be pricey. For this reason, some surgeons have already started using other more accessible, flexible software such as Photoshop, which is also less expensive [40]. However, this takes time because the plastic templates have to be scanned and stored as PNG (.png) and the radiographs saved as JPEG (.jpg). Furthermore, the scale calibration can be tricky.
The software has some limitations. In patients with advanced degenerative arthritis or patients with hip fractures who tend to have the leg externally rotated [7], it is not possible to obtain a correct X-ray for a good preoperative plan using Medicad®. This could affect our ability to predict the correct stem size. Sometimes, we can minimize this problem using the contralateral side as a size reference. Moreover, Dong et al. have demonstrated that the accuracy of templating for THA can be improved in radiographs with limb rotation and osteoporotic changes [41]. This can be done by adjusting the stem in one or two sizes according to the cortices width and the thickness of the lesser trochanter, and changing the cup in one or two sizes depending on the degree of sclerosis and presence of osteophytes.
We have a very limited ability to predict the neck size. This could be explained because the software does not consider the effect of soft-tissue tension on the hip arthroplasty components. Intraoperative soft-tissue tension, leg length discrepancy, and dislocation maneuvers are especially important when determining neck final size.
In our statistical analyses, kappa values provided a more reliable method of analyzing categorical data (size). Other studies use interclass coefficients [19, 20], but these should only be employed to analyze continuous data.
Our research is not exempt from limitations. The sample size is small though it is equivalent to those mentioned in the literature. However, our inclusion criteria are strict to make the sample homogeneous, increasing the value of our conclusion. Furthermore, although we analyze the AP and axial X-ray, the program only uses an AP X-ray image to make the prediction, meaning that this could lead to oversizing the stem in a patient that has a wider femur canal in the coronal plane than in the sagittal plane. Moreover, there is a possible bias because the three experienced surgeons were the same ones predicting the sizes using the templating software. We have tried to minimize this bias by including the resident’s predictions in the study and including surgeries, not all performed by the same surgeon.
Conclusion
Digital preoperative THA planning is a good method for predicting component size, with good interobserver reliability. Therefore, we recommend this method in preoperative THA planning.
Financial Support and Sponsorship
Nil
Compliance with Ethical Standards
Conflict of Interest
There are no conflicts of interest.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study informed consent is not required.
Patient Declaration Statement
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American college of sports medicine position stand Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medical Science Sports Exercise. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Bourne R, Rorabeck C, Feeny D, Wong C, Tugwell P, et al. The effect of elective total hip replacement on health-related quality of life. Journal Bone Joint surgery American Volume. 1993;75:1619–1626. doi: 10.2106/00004623-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Gallart X, Daccach JJ, Fernández-Valencia JA, García S, Bori G, Rios J, et al. Study of the consistency of a system for preoperative planning digital in total arthroplasty of the hip. Revista Española Cirugía Ortopédica Traumatología. 2012;56:471–477. doi: 10.1016/j.recot.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Conn KS, Clarke MT, Hallett JP. A simple guide to determine the magnification of radiographs and to improve the accuracy of preoperative templating. J Bone Joint surgery British Volume. 2002;84:269–272. doi: 10.1302/0301-620x.84b2.12599. [DOI] [PubMed] [Google Scholar]
- 5.Gamble P, de Beer J, Petruccelli D, Winemaker M. The accuracy of digital templating in uncemented total hip arthroplasty. Journal Arthroplasty. 2010;25:529–532. doi: 10.1016/j.arth.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Strøm NJ, Reikerås O. Templating in uncemented THA. On accuracy and postoperative leg length discrepancy. Journal Orthopaedic. 2018;15:146–150. doi: 10.1016/j.jor.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaarani SR, McHugh G, Collins DA. Accuracy of digital preoperative templating in 100 consecutive uncemented total hip arthroplasties: a single surgeon series. Journal Arthroplasty. 2013;28:331–337. doi: 10.1016/j.arth.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Widmer KH. A simplified method to determine acetabular cup anteversion from plain radiographs. Journal Arthroplasty. 2004;19:387–390. doi: 10.1016/j.arth.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. Journal Arthroplasty. 1999;14:509–513. doi: 10.1016/s0883-5403(99)90110-9. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Seminars Roentgenol. 2005;40:290–319. doi: 10.1053/j.ro.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Blumentritt S. Biomechanical construction principles of the human hip joint in frontal plane. Gegenbaurs morphologisches. 1988;134:221–240. [PubMed] [Google Scholar]
- 12.Eschweiler J, Fieten L, Dell’Anna J, Kabir K, Gravius S, Tingart M, et al. Application and evaluation of biomechanical models and scores for the planning of total hip arthroplasty. Proc Inst Mech Eng H. 2012;226:955–967. doi: 10.1177/0954411912445261. [DOI] [PubMed] [Google Scholar]
- 13.Bono JV. Digital templating in total hip arthroplasty. Journal Bone Joint Surgery American. 2004;86:118–122. doi: 10.2106/00004623-200412002-00016. [DOI] [PubMed] [Google Scholar]
- 14.Lecerf G, Fessy MH, Philippot R, Massin P, Giraud F, Flecher X, et al. Femoral offset: anatomical concept, definition, assessment, implications for preoperative templating and hip arthroplasty. Orthopaedics Traumatology Surgery Research. 2009;95:210–219. doi: 10.1016/j.otsr.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Horneij E, Holmströms E, Hemborg B, Isberg P-E, Ekdahl C. Interrater reliability and between-days repeatability of light physical performance test. Advances Physiotherapy. 2002;4:146–160. [Google Scholar]
- 16.Bangdiwala SI, Shankar V. The agreement chart. BMC Medical Research Methodology. 2013;13:97. doi: 10.1186/1471-2288-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackley HR, Howell GE, Rorabeck CH. Planning and management of the difficult primary hip replacement: preoperative planning and technical considerations. Instructional Course Lectures. 2000;49:3–11. [PubMed] [Google Scholar]
- 18.Schiffers N, Schkommodau E, Portheine F, Radermacher K, Staudte HW. Planning and performance of orthopedic surgery with the help of individual templates. orthopaedica. 2000;29:636–640. doi: 10.1007/s001320050504. [DOI] [PubMed] [Google Scholar]
- 19.Kosashvili Y, Shasha N, Olschewski E, Safir O, White L, Gross A, et al. Digital versus conventional templating techniques in preoperative planning for total hip arthroplasty. Canadian Journal Surgery. 2009;52:6–11. [PMC free article] [PubMed] [Google Scholar]
- 20.Knight JL, Atwater RD. Preoperative planning for total hip arthroplasty. Quantitating its utility and precision. Journal Arthroplasty. 1992;7(1):403–409. doi: 10.1016/s0883-5403(07)80031-3. [DOI] [PubMed] [Google Scholar]
- 21.Haddad FS, Masri BA, Garbuz DS, Duncan CP. The prevention of periprosthetic fractures in total hip and knee arthroplasty. Orthopedic Clinics of North America. 1999;30:191–207. doi: 10.1016/s0030-5898(05)70074-2. [DOI] [PubMed] [Google Scholar]
- 22.Cech O, Fassbender M, Kirschner P, Rozkydal Z. Preoperative planning and surgical technic in achieving stability and leg length equality in total hip joint arthroplasty. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca. 2002;69:362–368. [PubMed] [Google Scholar]
- 23.Eggli S, Pisan M, Müller ME. The value of preoperative planning for total hip arthroplasty. Journal of Bone and Joint Surgery. British Volume. 1998;80:382–390. doi: 10.1302/0301-620x.80b3.7764. [DOI] [PubMed] [Google Scholar]
- 24.Michalíková M, Bednarčíková L, Petrík M, Živčák J, RašI R. The digital pre-operative planning of total hip arthroplasty. Acta Polytechnica Hungarica. 2010;7:137–152. [Google Scholar]
- 25.Goldstein WM, Gordon A, Branson JJ. Leg length inequality in total hip arthroplasty. Orthopedics. 2005;28:s1037–s1040. doi: 10.3928/0147-7447-20050902-06. [DOI] [PubMed] [Google Scholar]
- 26.Goodman SB, Huene DS, Imrie S. Preoperative templating for the equalization of leg lengths in total hip arthroplasty. Contemporary Orthopaedics. 1992;24:703–710. [PubMed] [Google Scholar]
- 27.Lindgren JU, Rysavy J. Restoration of femoral offset during hip replacement. A radiographic cadaver study. Acta Orthopaedica Scandinavica. 1992;63:407–410. doi: 10.3109/17453679209154755. [DOI] [PubMed] [Google Scholar]
- 28.Rubash HE, Parvataneni HK. The pants too short, the leg too long: leg length inequality after THA. Orthopedics. 2007;30:764–765. doi: 10.3928/01477447-20070901-30. [DOI] [PubMed] [Google Scholar]
- 29.Schmalzried TP. Preoperative templating and biomechanics in total hip arthroplasty. Orthopedics. 2005;28:s849–s851. doi: 10.3928/0147-7447-20050802-09. [DOI] [PubMed] [Google Scholar]
- 30.Suh KT, Cheon SJ, Kim DW. Comparison of preoperative templating with postoperative assessment in cementless total hip arthroplasty. Acta Orthopaedica Scandinavica. 2004;75:40–44. doi: 10.1080/00016470410001708070. [DOI] [PubMed] [Google Scholar]
- 31.Crooijmans HJ, Laumen AM, van Pul C, van Mourik JB. A new digital preoperative planning method for total hip arthroplasties. Clinical Orthopaedics and Related Research. 2009;467:909–916. doi: 10.1007/s11999-008-0486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller ME. Lessons of 30 years of total hip arthroplasty. Clinical Orthopaedics and Related Research. 1992;274:12–21. [PubMed] [Google Scholar]
- 33.Haddad FS, Masri BA, Garbuz DS, Duncan CP. Femoral bone loss in total hip arthroplasty: classification and preoperative planning. Instructional Course Lectures. 2000;49:83–96. [PubMed] [Google Scholar]
- 34.Jenkins PJ, Clement ND, Hamilton DF, Gaston P, Patton JT, Howie CR, et al. Predicting the cost-effectiveness of total hip and knee replacement: A health economic analysis. Bone Joint Journal. 2013;95:115–121. doi: 10.1302/0301-620X.95B1.29835. [DOI] [PubMed] [Google Scholar]
- 35.Daigle ME, Weinstein AM, Katz JN, Losina E. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Practice & Research Clinical Rheumatology. 2012;26:649–658. doi: 10.1016/j.berh.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iorio R, Siegel J, Specht LM, Tilzey JF, Hartman A, Healy WL, et al. A comparison of acetate vs. digital templating for preoperative planning of total hip arthroplasty: Is digital templating accurate and safe. Journal Arthroplasty. 2009;24:175–179. doi: 10.1016/j.arth.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Bertz A, Indrekvam K, Ahmed M, Englund E, Sayed-Noor AS. Validity and reliability of preoperative templating in total hip arthroplasty using a digital templating system. Skeletal Radiology. 2012;41:1245–1249. doi: 10.1007/s00256-012-1431-4. [DOI] [PubMed] [Google Scholar]
- 38.Efe T, El Zayat BF, Heyse TJ, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J, et al. Precision of preoperative digital templating in total hip arthroplasty. Acta Orthopaedica Belgica. 2011;77:616–621. [PubMed] [Google Scholar]
- 39.Schmidutz F, Steinbrück A, Wanke-Jellinek L, Pietschmann M, Jansson V, Fottner A, et al. The accuracy of digital templating: a comparison of short-stem total hip arthroplasty and conventional total hip arthroplasty. International Orthopaedics. 2012;36:1767–1772. doi: 10.1007/s00264-012-1532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Si HB, Zeng Y, Cao F, Pei FX, Shen B. Accuracy of a simple digital templating in primary uncemented total hip arthroplasty. Chinese Medical Sciences Journal. 2015;30:150–155. doi: 10.1016/s1001-9294(15)30039-0. [DOI] [PubMed] [Google Scholar]
- 41.Dong N, Yang C, Li SQ, Gao YH, Liu JG, Qi X. A novel digital templating methodology for arthroplasty: experience from patients with osteonecrosis of the femoral head. Hip International. 2017;27:82–86. doi: 10.5301/hipint.5000427. [DOI] [PubMed] [Google Scholar]