Abstract
Double hit lymphomas (DHL) and double expresser lymphomas (DEL) are subsets of diffuse large B cell lymphomas (DLBCL) which are being increasingly recognised as cause of treatment failure. This emphasizes the need for their separation from other DLBCL cases in order to prognosticate and administer more aggressive treatment to this set of patients. The present study was conducted with the aim to identify the DHL/DEL patients and study their distinctive clinicopathological profile and overall survival. This retrospective analysis involved 172 cases of DLBCL sub-classified on the basis of cell of origin. Immunohistochemical (IHC) analysis for MYC, BCL2, BCL6, MUM1 and CD10 was performed. Rearrangement studies were performed using break apart Fluorescent in situ hybridization. Overall survival (OS) was also evaluated. Distinctive clinical and pathological features of DHL and DEL were identified. Rearrangement study by FISH revealed seven cases of DHL (MYC + BCL2 &/or BCL6 rearrangement). Also, 20 patients (11.6%) showed a concurrent expression of BCL2 and MYC oncoproteins (DEL) on IHC. Most (6/7) DHL patients were double expressors also. The DHL patients demonstrated a significant association with female gender, high serum LDH levels (> 750 U/L) and GCB phenotype. DEL patients contrarily predominated amongst males, had intermediate LDH levels (251–500 U/L) and non GCB phenotype. The OS of the patients was 63.8% at 4 years. The OS of the DLBCL, DEL and DHL patients was 71.9%, 46.9%, and 0%, respectively at 4 years (p value 0.010). In case of DEL subtype, factors such as age < 60 years (66.7%), male sex (60.8%), nodal localization (52.5%), early disease stage (84.6%), low IPI score (60%), absence of B symptoms (50%), LDH < 250 U/L (80%) and GCB phenotype (53.3%) were associated with better OS. Further, the OS of DHL cases was 0% at 4 years. Double hit and double expresser lymphomas have poor prognostic outcomes and should be separated from DLBCL. All DELs should be tested for DHLs and especially those with immunoblastic morphology. DHL and DEL subtypes delineate the subtypes with inferior OS and reinstate the need for aggressive interventions.
Electronic supplementary material
The online version of this article (10.1007/s12288-019-01248-w) contains supplementary material, which is available to authorized users.
Keywords: Double hit lymphoma, Double expresser lymphoma, Diffuse large B cell lymphoma, C-MYC, BCL2, BCL6
Introduction
Current therapeutic decisions on the treatment of lymphomas are based upon the histological classification. Diffuse large B cell lymphomas (DLBCL) account for nearly 30% of all lymphomas with diverse behaviour and therapeutic outcomes [1–3]. Gene expression profiling (GEP) of nearly 20,000 genes has identified various subtypes of DLBCL namely Germinal Centre ‘B’ Cell (GCB) type, Activated ‘B’ Cell (ABC) type and Mediastinal ‘B’ Cell type which also show differences in their pathogenetic mechanisms [4–6]. This Cell of Origin (COO) based classification has emphatically separated the survival in favour of GCB type. Yet, around 20% patients of GCB type recur within the first year of therapy while 50% of cases of ABC type have good long term survival [6, 7]. The COO classification of DLBCL therefore is inadequate to prognosticate effectively.
Further research into DLBCL has helped define the occult categories within DLBCL based upon MYC oncogene rearrangement either alone or in combination with BCL2 &/or BCL6 and are referred to as single hit, double hit or triple hit lymphomas (SHL/DHL/THL) [8–10]. These subtypes have been identified in 2–12% of DLBCL patients with poor response to standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) therapy [9–13]. An advanced stage of the disease, high International Prognostic Index (IPI), markedly raised lactic acid dehydrogenase (LDH), bone marrow involvement and early disease progression have also been associated with DHL [9–17]. Researchers attempting to separate these cases by immunohistochemistry (IHC) as surrogates [18, 19] serendipitously found a new group of ‘Double Expresser’ lymphomas (DEL) that showed concurrent expression of MYC and BCL2 oncoprotein but were not necessarily rearranged at the genetic level to always qualify as DHL [19–22].
The present study was conducted with the aim to identify the DHL/DEL patients amongst the DLBCL cases so as to separate these subsets on the basis of their distinctive clinicopathological profile and overall survival (OS) with the ultimate goal of enriching the cohort for genetic rearrangement testing for the identification of DHL patients (testing for all cases of DLBCL will not be cost effective or readily available).
Materials and Methods
The present study is a retrospective analysis involving 172 cases of DLBCL diagnosed between January 2014 and December 2015 at a tertiary cancer care centre. These cases were retrieved from the archives and re-examined for confirmation of diagnosis of DLBCL.
Initially, 200 cases of DLBCL were selected for the study, out of which, 28 cases of core biopsies were found to be inadequate for complete workup and were excluded leaving a total of 172 evaluable cases. For 120 (67.9%) cases, core biopsy was the available tissue while 52 cases (30.3%) had excision biopsy. The cases were then further sub-classified on the basis of cell of origin (COO) into GCB and Non-GCB type using Hans algorithm [23]. In addition, all the cases were further immunostained for MYC and BCL2 to identify the DEL subtype. Immunostaining for Ki-67 was also performed in all the cases. IHC was performed on Ventana Benchmark XT, Tucson, Arizona, USA employing heat induced epitope retrieval and ultra view labelling system.
For immunohistochemical analysis, the percentage of positive cells and their mean intensity of staining was recorded. The panel of markers is outlined in Supplementary Table 1. Threshold percentage for assessing positivity was moderate to strong nuclear staining in ≥ 40% of the cells for MYC, strong nuclear staining in ≥ 30% of the cells for BCL6 & MUM1, strong cytoplasmic staining in ≥ 70% of the cells for BCL2 and strong membranous staining in ≥ 30% of the cells for CD10 respectively [19–22, 24]. Any intensity of nuclear staining with Ki-67 antibody was considered positive and contributed to Ki-67 labelling index [25].
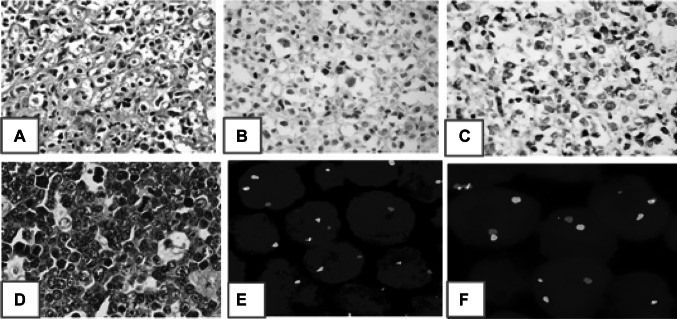
The rearrangement studies were performed using break apart Fluorescent in situ hybridization (FISH) with standard probes procured from ZytoVision. The panel of FISH probes is given in Supplementary Table 2. The test was performed on 4-micron formalin fixed and paraffin embedded tissue sections following standardized protocol which included pre-treatment (dewax/proteolysis), denaturation, probe application and hybridization, application of DAPI/antifade solution and analysis of slides using Leica fluorescent microscope (DM6000B). At least 100 contiguous non-overlapping nuclei were assessed and percentage of positive nuclei was computed. Cases with break apart signals in greater than/equal to 15% of nuclei (laboratory determined cut off based upon presence of split signals in reactive lymph nodes) were considered positive for presence of rearrangement. Figure 1 shows a representative staining of DEL on IHC and DHL by FISH.
Fig. 1.
Representative images of a case of double expresser (a) H&E stained section, (b) CMYC positivity (> 40% nuclear staining) on IHC (c), BCL2 positivity (> 70% cytoplasmic staining) on IHC (c); d–f a case of double hit lymphoma (d) H&E section which showed BCLU morphology, (e) C-MYC rearrangement on FISH using break apart probe seen as separate red and green signals, (f) BCL6 rearrangement on FISH using break apart probe seen as separate red and green signals
SPSS version 23 for Windows (SPSS Inc, Chicago IL, USA) was used for statistical analysis. Pearson χ2 or Fisher’s Exact Test, whichever appropriate, was used for categorical variables. OS analysis was performed using the Kaplan–Meier method [26]. OS was calculated as the time from the date of initial diagnosis to the date of death or the date of last follow-up. Log Rank test was used to compare the difference in survival among the groups. A two sided p value < 0.05 was considered as significant. This study was approved by the Institutional Review Board of the Institute (IRB No. RGCIRC/IRB/59/2016) and was performed in accordance with the principles of the Declaration of Helsinki.
Results
A total of 172 patients with a diagnosis of DLBCL were included in the study of which 57.6% were males. The mean age of the patients was 56 years. As per the classification based on COO, 49.4% patients were GCB type. The demographic, clinical and pathological profile of the patients is presented in Table 1. Majority of the patients presented with stage III–IV disease (60.5%), extranodal site (57%), IPI score 0–2 (50.6%), presence of B symptoms (52.9%), LDH levels 251–500 U/L (39%), centroblastic morphology (86.1%), ABC phenotype (50.6%) and rituximab based regime (87.2%).
Table 1.
Demographic, clinical and pathological profile of 172 patients with diffuse large B-cell lymphoma
| Characteristics | N (%) |
|---|---|
| Age (years) | |
| < 60/≥ 60 | 86 (50)/86 (50) |
| Sex | |
| Female/male | 73 (42.4)/99 (57.6) |
| Stage | |
| I–II/III–IV | 68 (39.5)/104 (60.5) |
| Site | |
| Extranodal/nodal | 98 (57)/74 (43) |
| IPI score | |
| Low (0–2)/high(3–5) | 87 (50.6)/85 (49.4) |
| B symptoms | |
| Present/absent | 91 (52.9)/81 (47.1) |
| LDH (U/L) | |
| < 250/251–500/501–750/> 750 | 57 (33.1)/67 (39)/23 (13.4)/25 (14.5) |
| Morphology | |
| Centroblastic/immunoblastic/anaplastic/BCLU | 148 (86.1)/12 (7)/6 (3.5)/6 (3.5) |
| Phenotype | |
| GCB/non GCB | 85 (49.4)/87 (50.6) |
| Treatment regime | |
| Rituximab based/non rituximab based/no treatment | 150 (87.2)/7 (4.1)/15 (8.7) |
N number, IPI International Prognostic Index, LDH lactic acid dehydrogenase, BCLU B cell lymphoma unclassifiable, GCB germinal centre type
Table 2 shows the comparison of demographic, clinical and pathological parameters of DLBCL, DHL and DEL cases. Seven cases of DHL were identified on rearrangement study (MYC + BCL2-4/7; MYC + BCL6-2/7; MYC + BCL2 + BCL6-1/7). The DHL patients were more commonly associated with age group ≥ 60 years (57.1%), female sex (85.7%), stage III–IV disease (100%), extranodal site (71.4%), IPI score 3–5 (100%), presence of B symptoms (100%), LDH levels > 750 U/L (100%) and GCB phenotype (100%). The Ki-index of the DHL cases ranged from 50 to 95% (mean 80 ± 15.5). The mean index was 69.8 ± 15.4 in the DLBCL cases (range 30–99).
Table 2.
Comparison of demographic, clinical and pathological parameters of DLBCL, DHL and DEL cases
| Characteristics | DLBCL | DHL | DEL | p value* | p value** | p value*** |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| N | 145 | 7 | 20 | – | – | – |
| Age (years) | ||||||
| < 60 | 75 (51.7) | 3 (42.9) | 8 (40) | 1.000 | 0.341 | 1.000 |
| ≥ 60 | 70 (48.3) | 4 (57.1) | 12 (60) | |||
| Sex | ||||||
| Male | 86 (59.3) | 1 (14.3) | 12 (60) | 0.042 | 1.000 | 0.041 |
| Female | 59 (40.7) | 6 (85.7) | 8 (40) | |||
| Stage | ||||||
| I–II | 61 (42.1) | 0 (0) | 7 (35) | 0.042 | 1.000 | 0.025 |
| III–IV | 84 (57.9) | 7 (100) | 13 (65) | |||
| Site | ||||||
| Extranodal | 83 (57.2) | 5 (71.4) | 10 (50) | 0.699 | 0.633 | 0.408 |
| Nodal | 62 (42.8) | 2 (28.6) | 10 (50) | |||
| IPI score | ||||||
| Low (0–2) | 79 (54.5) | 0 (0) | 8 (40) | 0.005 | 0.242 | 0.015 |
| High (3–5) | 66 (45.5) | 7 (100) | 12 (60) | |||
| B symptoms | ||||||
| Present | 72 (49.7) | 7 (100) | 12 (60) | 0.014 | 0.477 | 0.068 |
| Absent | 73 (50.3) | 0 (0) | 8 (40) | |||
| LDH (U/L) | ||||||
| < 250 | 52 (35.9) | 0 (0) | 5 (25) | < 0.0001 | 0.182 | 0.004 |
| 251–500 | 61 (42.1) | 0 (0) | 6 (30) | |||
| 501–750 | 18 (12.4) | 0 (0) | 5 (25) | |||
| > 750 | 14 (9.7) | 7 (100) | 4 (20) | |||
| Morphology | ||||||
| Centroblastic | 131 (90.3) | 5 (71.4) | 12 (60) | 0.121 | < 0.0001 | 0.908 |
| Immunoblastic | 6 (4.1) | 1 (14.3) | 5 (25) | |||
| Anaplastic | 5 (3.5) | 0 (0) | 1 (5) | |||
| BCLU | 3 (2.1) | 1 (14.3) | 2 (10) | |||
| Phenotype | ||||||
| GCB | 72 (49.7) | 7 (100) | 6 (30) | 0.014 | 0.099 | 0.001 |
| Non GCB | 73 (50.3) | 0 (0) | 14 (70) | |||
| Treatment regime | ||||||
| Rituximab based | 126 (86.9) | 7 (100) | 17 (85) | 1.000 | 0.023 | 0.545 |
| Non Rituximab based | 4 (2.8) | 0 (0) | 3 (15) | |||
| No treatment | 15 (10.3) | 0 (0) | 0 (0) |
The figures in bold indicate significant associations
N, number; LDH, lactic acid dehydrogenase; IPI, International Prognostic Index; BCLU, B cell lymphoma unclassifiable; GCB, germinal centre type; DLBCL, diffuse large B-cell lymphoma; DHL, double hit lymphomas; DEL, double expresser lymphomas; p value*, DLBCL versus DHL; p value**, DLBCL versus DEL; p value***, DHL versus DEL
Twenty patients (11.6%) of DEL with a concurrent expression of BCL2 and MYC oncoproteins (DEL) on IHC were identified. Among these, 14 (70%) patients were of non GCB phenotype whereas, 6 (30%) patients were of GCB phenotype. DEL patients showed a predominance of characteristics such as age group ≥ 60 years (60%), male sex (60%), stage III–IV disease (65%), IPI score 3–5 (60%), presence of B symptoms (60%), LDH levels 251–500 U/L (30%) and non GCB phenotype (70%). None of the features significantly associated with DHL was repeated in DEL (Table 2). The Ki-index of the DEL cases ranged from 50 to 100% (mean 81.7 ± 13.7). Immunoblastic morphology over represented significantly (25%) in DEL.
Six of seven DHL patients were double expressors as well. One case of DHL which was not DEL showed 0% and 75% immunostaining for MYC and BCL2 oncoproteins, respectively with a Ki-index of 90%.
The OS of the patients was 63.8% at 4 years. Figure 2 profiles the OS of the DLBCL, DEL and DHL patients which was 71.9%, 46.9%, and 0%, respectively at 4 years (p value 0.010). In case of DEL cases, factors including age < 60 years (66.7%), male sex (60.8%), nodal site of disease (52.5%), early disease stage (84.6%), low IPI score (60%), absence of B symptoms (50%), LDH < 250 U/L (80%) and GCB phenotype (53.3%) were associated with better OS. However, variations were noted in the case of DHL patients and all the 7 patients with a diagnosis of DHL had died at 4 years and hence the OS was 0% with respect to the various factors.
Fig. 2.
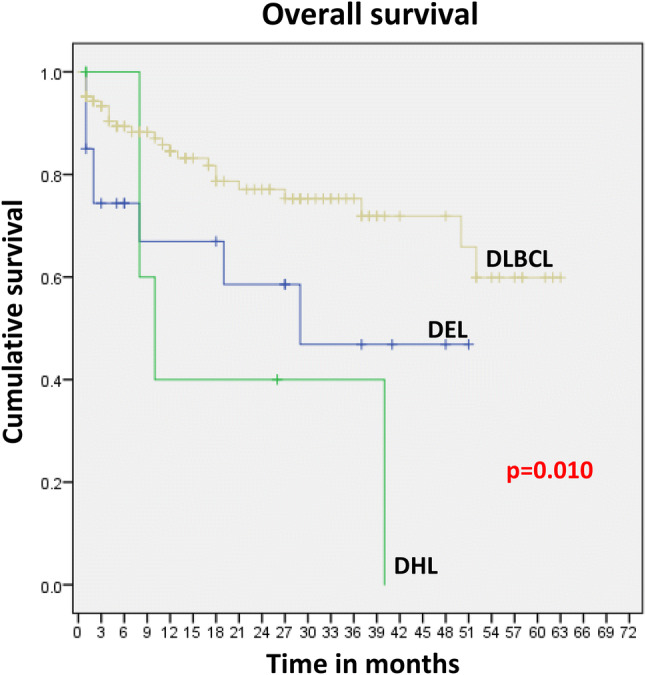
Overall survival of DLBCL, DHL and DEL cases. Comparison of OS between DLBCL versus DHL, p value 0.010; DLBCL versus DEL, p value 0.036; DEL versus DHL, p value 0.546. DLBCL diffuse large B-cell lymphoma, DHL double hit lymphomas, DEL double expresser lymphomas
Incidentally, two cases were seropositive for HIV, of which, one patient had stage I DLBCL while the other had stage III DLBCL with plasmablastic differentiation. None of these two cases were DEL or DHL. Leukemic phase was seen in four cases of DLBCL of which one was a case of DEL.
Discussion
DLBCL is considered a heterogenous and an aggressive lymphoma with varied clinical outcome [9, 15]. Attempt to prognostically categorise these lymphomas according to COO led to the identification of GCB and non GCB phenotype. In our study, majority of the patients were non GCB type (50.6%) which is in agreement with the study by Ayurek et al. [27]. However, our results are dissimilar when compared to the study by Johnson et al. [18] and Hu et al. [21] who reported a higher percentage of GCB cases in their cohort (76% and 66%, respectively).
The incidence of DHL in our study was 4%, which is comparable to the study by Scott et al. [11], Barrans et al. [28], Visco et al. [29]. Six of seven DHL patients were females which is in stark contrast to most studies which state that DHL cases are more common in males [17, 30]. All cases of DHL had stage III/IV disease and high LDH levels. Most cases had extranodal disease. Bone marrow and CNS involvement was seen in 66.6% and 33.3% cases, respectively. The clinicopathological parameters were similar to that reported in the previous studies [2, 4, 9–20, 28–33]. The authors recommend that these unique clinical features can be used to select the patients of DLBCL for further testing for molecular cytogenetics for rearrangement.
DEL are defined as lymphomas having concurrent expression of MYC and BCL2 oncoprotein. It was initially thought that DEL are same as DHL, however, subsequent studies revealed that DEL are not equivalent to DHL, even though 80–90% of the DHL are also DEL. Apart from gene rearrangement (which defines DHL cases), gene can be amplified or mutated which result in increased oncoprotein expression (which define DEL cases) [9, 22, 24]. In our study, there were 20 (11.6%) cases of DEL. The incidence is slightly lower than stated in the literature (19–34%) [22, 24, 34]. Interestingly, 6 out of 7 DHL patients were also DEL. Since DEL concentrates DHL cases, all cases of DEL should undergo FISH testing for assignment as DHL. Moreover, such separation is also necessary as DEL cases which were not DHL fared better than the former. This observation is a reaffirmation of the studies by Hu et al. and Green et al. [20, 22, 24].
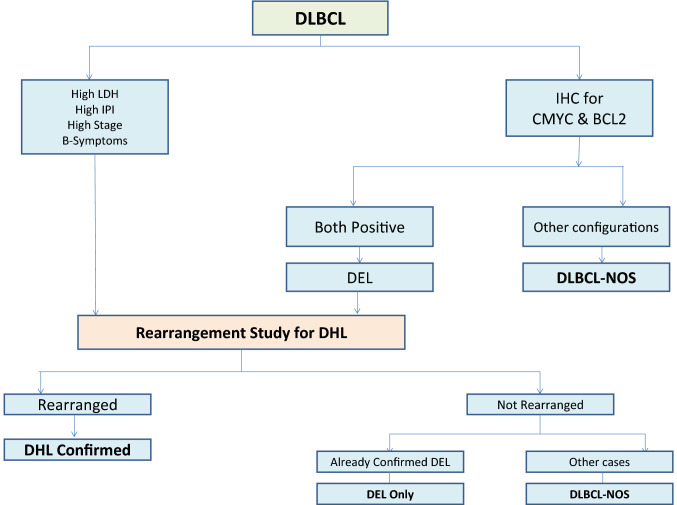
In our study, the Ki-index of DHL ranged from 50 to 95%, whereas that of DEL cases ranged from 50 to 100%. This observation brings forth the fact that Ki-index cannot be relied upon to sift out these subsets of lymphomas from the DLBCL cases in contrast to the commonly held belief that DHL and DEL are highly proliferative lymphomas with Ki67 labelling exceeding 95% [9, 35, 36]. Our observation, however, resonates with the study by Mationg‐Kalaw which also highlighted the lack of value of Ki67 in enriching the DLBCL cases for genetic rearrangement studies [37]. We propose a schema (Fig. 3) for the assignment of DEL and DHL subtypes based upon our findings with a view to optimize the use of resources.
Fig. 3.
Proposed algorithm for the testing of DHL/DEL patients. DLBCL diffuse large B-cell lymphoma, DHL double hit lymphomas, DEL double expresser lymphomas
The OS of the entire cohort was 63.8% at 4 years and was 71.9%, 46.9%, and 0% at 4 years for DLBCL, DEL and DHL respectively. In DEL cases, factors like age < 60 years, male sex, nodal site of disease, early disease stage, low IPI score, absence of B symptoms, LDH < 250 U/L and GCB phenotype were associated with better OS. However, all 7 DHL patients, irrespective of the afore mentioned factors that tempered the outcome in DEL died by the end of 4 years of follow up. The statistical correlations observed in terms of OS among the different groups points towards the fact that the specific subtypes of DLBCL patients considered along with clinico-pathological factors determines the survival. The 4-year OS in patients with DEL was 56% (95% CI 40–69%). In a study by Herrera et al., the 4 year OS in patients with DHL was inferior to that of patients without DHL (25% vs. 66%; p < .001). Patients with DHL had decreased OS (25%; 95% CI 5–54%) compared with patients with DEL but not DHL (OS 61%, 95% CI 45–74%), and patients with neither DEL nor DHL (OS 70%, 95% CI 55–80%; P = .002) [38].
The strength of the present study is the comprehensive IHC and molecular diagnostic work up that allowed definitive diagnosis of DHL/DEL patients and study their distinctive clinicopathological profile and OS. These findings will help delineate a smaller cohort of patients that shall undergo genetic rearrangement testing to select patients with DHL/DEL. The limitations of the study include the fact that some previously stored biopsies were inadequate for performing the IHC and rearrangement studies, thus compromising on the sample size. Multivariate analysis was not performed and the COO was identified by surrogate IHC with its inherent discordance with gene expression profiling in 10–15% of the cases.
In conclusion, the double hit and double expresser lymphomas have poor prognostic outcomes. There are significant differences between DLBCL and DHL in stage at presentation, IPI, LDH levels, B symptoms, phenotype, and morphology and these clinicopathological parameters can enrich the subset of population for testing for MYC, BCL2 and BCL6 rearrangements using FISH. Such distinctive features were however not observed in the DEL group. All patients of DLBCL therefore must undergo additional MYC and BCL2 IHC to identify DEL. DELs however, concentrate DHLs to the extent of 85% and hence once identified, all DELs should be tested for DHLs. DHL and DEL subtypes delineate the subtypes with inferior OS and reinstate the need for aggressive interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to the technical staff of Department of Laboratory Services, Rajiv Gandhi Cancer Institute & Research Centre, New Delhi for their enormous help in performing immunohistochemistry and FISH experiments.
Funding
The financial support was provided by Rajiv Gandhi Cancer Institute and Research Centre.
Compliance with Ethical Standards
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Tilly H, Silva MG, Vitolo U, Jack A, Meignan M, Walewski J. Clinical practice guidelines diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol. 2015;26(Supplement 5):bv116–bv125. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 4.Nowakowski GS, Czuczman MS (2015) Management of DLBCL based on molecular profile ABC, GCB, and double-hit diffuse large B-cell lymphoma: does subtype make a difference in therapy selection? Am Soc Clin Oncol Educ Book 449–457 [DOI] [PubMed]
- 5.Lenz G, Wright GW, Emre NCT, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105(36):13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havranek O, Xu J, Stefan K, Wang Z, Becker L, Comer JM, et al. Tonic B-cell receptor signaling in diffuse large B-cell lymphoma. Blood. 2017;130(8):995–1006. doi: 10.1182/blood-2016-10-747303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer PN, Fu K, Greiner TC, Smith LM, Delabe J, Gascowyne RD, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse largeB-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2):200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal A, Younes A, Phoenix CA, Kettering S, York N. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: double hit and triple hit lymphomas anddouble expressing lymphoma. Blood Rev. 2017;31(2):37–42. doi: 10.1016/j.blre.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow SH. Diagnosis of “double hit” diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology. 2014;2014(1):90–99. doi: 10.1182/asheducation-2014.1.90. [DOI] [PubMed] [Google Scholar]
- 10.Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, Kluin PM. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 11.Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, et al. High grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060–2064. doi: 10.1182/blood-2017-12-820605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merron B, Davies A. Double hit lymphoma: how do we define it and how do we treat it? Best Pract Res Clin Haematol. 2018;31(3):233–240. doi: 10.1016/j.beha.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Landsburg DJ, Falkiewicz MK, Maly J, Blum KA, Howlett C, Feldman T, et al. Outcomes of patients with double-hit lymphoma who achieve first complete remission. J Clin Oncol. 2017;35(20):2260–2267. doi: 10.1200/JCO.2017.72.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedberg JW. How I treat “Double Hit” lymphoma. Blood. 2017;130(5):590–596. doi: 10.1182/blood-2017-04-737320. [DOI] [PubMed] [Google Scholar]
- 16.Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120(24):3884–3895. doi: 10.1002/cncr.28899. [DOI] [PubMed] [Google Scholar]
- 17.Snuderl M, Kolman OK, Chen YB, Hsu JJ, Ackerman AM, Dal Cin P, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34:327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson NA, Slack GW, Savage KJ, Conners JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens DM, Sweetenham JW. Clinical controversies of double-hit lymphoma. Am J Hematol Oncol. 2015;2(4):10–16. [Google Scholar]
- 20.Smith SM. Aggressive B-cell lymphoma: the double-hit and double-expresser phenotypes. Clin Adv Hematol Oncol. 2017;15(1):40–42. [PubMed] [Google Scholar]
- 21.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Clin Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 23.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 24.Molina TJ, Briere J, Copie-Bergman C, et al. Overexpression of MYC, BCL2, MYC/BCL2, IGM, and non-germinal centre B cell-like immunophenotype predicts a worse progression-free sur- vival and overall survival in a series of 670 de novo diffuse large B-cell lymphomas: S Lysa Study. Hematol Oncol Clin North Am. 2013;31(suppl 1):151–200. [Google Scholar]
- 25.Broyde A, Boycov O, Strenov Y, Okon E, Shpilberg O, Bairey O. Role and prognostic significance of the Ki-67 index in non-Hodgkin’s lymphoma. Am J Hematol. 2009;84(6):338–343. doi: 10.1002/ajh.21406. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Non Parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 27.Akyurek N, Uner A, Benekli M, Barista I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-celllymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2012;118(17):4173–4183. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 28.Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 29.Visco C, Tzankov A, Xu-monette ZY, Miranda RN, Tai YC, Li Y, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98(2):255–263. doi: 10.3324/haematol.2012.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry AM, Crockett D, Dave BJ, Althof P, Smith LM, Chan WC, et al. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma: study of 39 cases. Br J Haematol. 2013;162(1):40–49. doi: 10.1111/bjh.12343. [DOI] [PubMed] [Google Scholar]
- 31.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32. doi: 10.1182/blood-2014-05-577189. [DOI] [PubMed] [Google Scholar]
- 32.Sesques P, Johnson NA. Review Article Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280–288. doi: 10.1182/blood-2016-02-636316. [DOI] [PubMed] [Google Scholar]
- 33.Landsburg DJ, Petrich AM, Abramson JS, et al. Impact of oncogene rearrangement patterns on outcomes in patients with double-hit non Hodgkin lymphoma. Cancer. 2016;122:559–564. doi: 10.1002/cncr.29781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. 2015;16(15):e555–e567. doi: 10.1016/S1470-2045(15)00005-4. [DOI] [PubMed] [Google Scholar]
- 35.Mation-Kalaw E, Tan LHC, Tay K, et al. Does the proliferation fraction help identify mature B cell lymphomas with double- and triple-hit translocations? Histopathology. 2012;61:1214–1218. doi: 10.1111/j.1365-2559.2012.04351.x. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira CC, Maciel-guerra H, Kucko L, Hirama EJ, Brilhante AD, Quevedo FC, et al. Double-hit lymphomas: clinical, morphological, immunohistochemical and cytogenetic study in a series of Brazilian patients with high-grade non-Hodgkin lymphoma. Diagn Pathol. 2017;12(1):3. doi: 10.1186/s13000-016-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mationg-Kalaw E, Tan LH, Tay K, Lim ST, Tang T, Lee YY, Tan SY. Does the proliferation fraction help identify mature B cell lymphomas with double-and triple-hit translocations? Histopathology. 2012;61(6):1214–1218. doi: 10.1111/j.1365-2559.2012.04351.x. [DOI] [PubMed] [Google Scholar]
- 38.Herrera AF, Mei M, Low L, et al. Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J Clin Oncol. 2017;35(1):24–31. doi: 10.1200/JCO.2016.68.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.