Abstract
Marginal zone lymphomas (MZLs) are rare and indolent subtypes of non-Hodgkin lymphomas, and their clinical behaviours are heterogeneous. The aim of this study was to evaluate the clinical and prognostic characteristics of MZL. In this multicentre retrospective study, we analyzed demographical, clinical and prognostic features of 64 MZL patients. The median age was 54.0 and 78.1% of the patients had extra-nodal disease at presentation. Most of the patients were treated with chemotherapy. The 5 years and 10 years overall survival (OS) rates were 74.5% and 62.1%, respectively. The analysis of factors associated with OS showed that ECOG performance score was an important prognostic factor, with 133.0 months (95% CI 49.3–216.5) versus 18.0 months (95% CI 12.1–23.7) for ECOG 0–1 and 2–3, respectively (p = 0.011). Prognosis of MZL is favorable and ECOG performance score was found associated with OS. Further detailed studies with large patient numbers are needed to clarify the clinical features and treatment management of MZLs.
Keywords: Marginal zone lymphoma, MALT lymphoma, Chemotherapy, Radiotherapy, Prognosis
Introduction
Marginal zone lymphoma (MZL) is a rare subtype of non-Hodgkin lymphomas (NHLs), accounting for 3–9% of all NHL cases [1]. It arises from post-germinal center marginal zone B cells. According to World Health Organization classification of lymphoid neoplasms, there are subtypes of MZL; extranodal MZL of mucosa associated lymphoid tissue (MALT lymphoma), nodal MZL and splenic MZL. All subtypes of MZL share morphologic and immunophenotypical similarities; however molecular and clinical characteristics differ for each entity [2]. Primary splenic and nodal MZLs are rare, each accounting for approximately less than 2% of the NHLs. The extranodal MZLs of MALT type represent around 7–8% of the NHL, including both the common gastrointestinal and the less usual non-gastrointestinal localizations [3].
MALT lymphoma develops in the lymphoid tissue of the mucosa or tissue that lines body organs as well as in body cavities including the gastrointestinal tract, lungs, eyes, skin, salivary glands, and breast. MZL has usually a slow-growing, indolent clinical course as opposite to other lymphoma types and median survival usually exceeds a decade [4]. However, they can sometimes transform into an aggressive type of NHL like diffuse large B cell lymphoma and it is the most important poor prognostic factor of MZL [5].
The aim of this study was to evaluate the clinical and prognostic characteristics of MZL.
Materials and Methods
The study was conducted as a multicentre retrospective study. It was approved by the Institutional Review Board and conducted according to Helsinki Declaration and good clinical practice.
Patients with a histopathologically confirmed diagnosis of Marginal Zone Lymphoma (MZL) were evaluated retrospectively. The final pathological diagnoses of the patients were made on the basis of morphological (having small to medium sized lymphocytes surrounding a follicule and having a plasmacytic differentiation), immunohistochemical (CD20+, CD79+, CD5−, CD10−, CD23−) and enzyme linked immunosorbent assay (surface IgM+ and IgD−) characteristics, which are useful in the pathological differential diagnosis of MZL from other hematological malignancies. Patients who were ≥ 18 years old were included. We excluded the patients who had a diagnosis of lymphoma including 2 and more histopathological subtype, history of other malignancies or inadequate medical records. The age, gender, sociodemographic data and comorbidities were recorded. In addition, the primary location of the disease, laboratory work up at the initial evaluation (haemoglobin, leucocyte and platelet count, lactate dehydrogenase, calcium levels), treatment modalities and disease characteristics after recurrence were documented. The staging of the primary disease was recorded according to Lugano modification of Ann Arbor staging system.
Baseline characteristics of the patient group were described using proportions for dichotomous and categorical variables. The effects of clinical parameters on mDFS and mOS were investigated by using the log ranks test. The Kaplan–Meier survival estimates were calculated. A separate log ranks test was used to identify the independent effect of parameters on survival. All analyses were performed using SPSS 17.0 for Windows (IBM Corp., Armonk, NY). p value of less than 0.05 was considered as statistically significant.
Results
Sixty-four patients, who had a diagnosis of MZL between 1995 and 2016 were evaluated. The basely characteristics of the study population were summarized in Table 1. The median age was 54.0 years (range 24–84 years) and 60.9% of the patients were male. Twenty-five (39.1%) of the 64 patients had at least one comorbid disease and most of the patients had 0–1 ECOG performance scores. Extra-nodal disease was present in 78.1% of the patients and majority (64.1%) of the patients had stage I-II disease at presentation. While 82.8% of patients had chemotherapy, only 1 patient was followed without any treatment modality. The analysis of regimens used showed that the most commonly used combination was CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) in 46.8% of the patients. The other regimens were as follows: R-CVP (cyclophosphamide, vincristine sulfate, and prednisone) in 17.0%, R-CHOP in 12.7%, CVP in 6.3% and monotherapy with Rituximab in 14.8% of the chemo-treated patients.
Table 1.
Characteristics of patients, disease and treatment
| Properties | N (%) |
|---|---|
| Age, years, median (range) | 54.0 (24–84) |
| Male | 39 (60.9) |
| Female | 25 (39.1) |
| Smoking history | |
| Active smoker | 14 (21.9) |
| Ex-smoker | 8 (12.5) |
| Non-smoker | 42 (65.6) |
| Comorbidity present | 25 (39.1) |
| Performance score (ECOG) | |
| 0–1 | 55 (85.9) |
| 2–3 | 9 (14.1) |
| B symptoms present | 11 (17.2) |
| Tumor location | |
| Nodal | 14 (21.9) |
| Extra-nodal | 50 (78.1) |
| Extranodal-gastric | 27 (42.1) |
| Bone marrow involvement | 8 (12.5) |
| Stage | |
| I | 21 (32.8) |
| II | 20 (31.2) |
| III | 12 (18.8) |
| IV | 11 (17.2) |
| Treatment modalities | |
| Follow-up | 1 (1.6) |
| Chemotherapy | 47 (73.4) |
| Chemoradiotherapy | 6 (9.4) |
| Radiotherapy | 7 (10.9) |
| Other | 3 (4.7) |
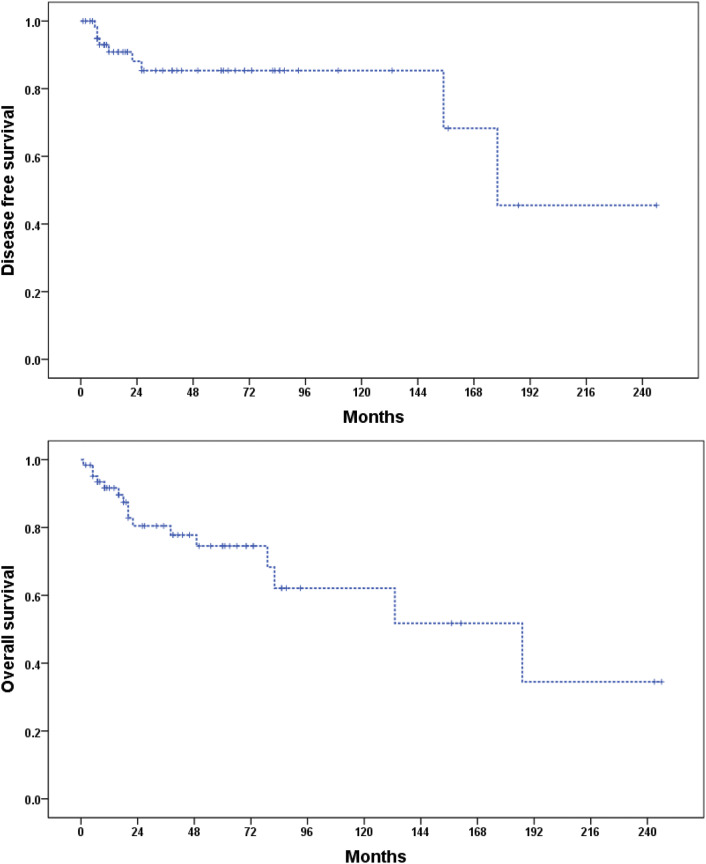
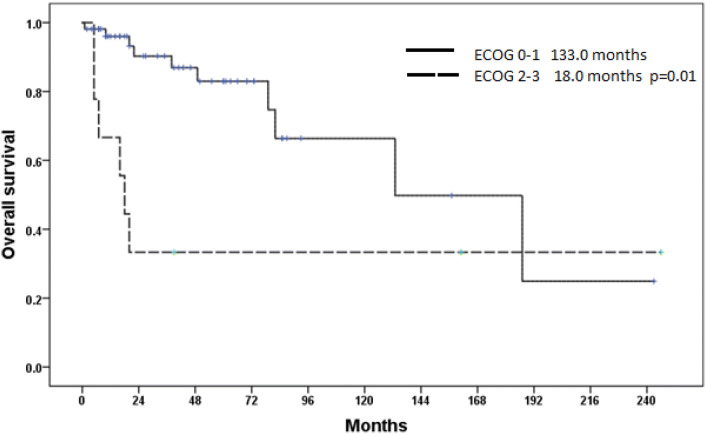
During follow-up, 12.5% (8/64) of the patients had recurrence and all the recurrences were detected in the locoregional lymph nodes of the primary disease. The median disease free survival (mDFS) was 178 months (95% CI 143.1–218.8) and 5 year DFS rate was 85.3%. The median overall survival (mOS) was 187 months (65.0–308.9 months). The 5 year and 10 year OS rates were 74.5% and 62.1%, respectively. Figure 1 shows DFS and OS curves of the patients. The analysis of factors associated with mOS showed that ECOG performance score was an important prognostic factor, with 133.0 months (95% CI 49.3–216.5) versus 18.0 months (95% CI 12.1–23.7) for ECOG 0–1 and 2–3, respectively (p = 0.011). Figure 2 shows OS curves of the patients with ECOG 0–1 and ECOG 2–3.
Fig. 1.
Disease free survival and overall survival curves of the patients
Fig. 2.
Overall survival curves of the patients with ECOG 0–1 and ECOG 2–3
Discussion
Marginal zone lymphoma is a rare form of NHL. In a large patient population study, it was shown that its incidence was higher in females than in males [4]. With respect to its subtypes, the median age at diagnosis may vary from 50 to 69 years [6]. Among its subtypes, the most common type of MZL is MALT lymphoma, which causes approximately two-thirds of all MZL cases per year. In overall, more than 50% of MZL patients have early stage disease at presentation. MALT lymphoma patients are mostly presented in stage I disease, but nodal and splenic MZL patients are mostly presented in stage III or IV disease [4]. In this study, median age and distribution of stages at diagnosis of included patients and the percentage of MALT lymphoma cases were similar with the literature. But in contrast to above-mentioned study, we found a male predominance. The MALT lymphoma has been reported most commonly in stomach. But, several non-gastric sites, such as, conjunctiva, larynx, salivary gland, thyroid, skin, liver, prostate, lung, breast and kidney have been involved [7]. In consistent with literature, gastric origin was the most common source of MALT lymphoma in our study.
Bone marrow involvement can be detected in splenic MZL, nodal MZL and MALT lymphoma with the ratios up to 95%, 44% and 20%, respectively [8–10]. On the other hand, different rates were reported for the presence of B symptoms in MZL patients, ranging between 8.3% and 24% [11, 12]. Systemic B symptoms may be observed in 27% of patients with splenic MZL, 8% of patients with nodal MZL and 5% of patients with MALT lymphoma [11]. In our study, bone marrow involvement was detected in 12.5% of cases. The low percentage in our study can be explained by the absence of splenic MZL patients and the predominance of MALT lymphoma patients in the study. However, B symptoms were present in 17.2% of patients and this result was consistent with the literature.
Treatment approaches may differ depending on the stage and subtype of MZL. Helicobacter pylori (HP) eradication is recommended to all gastric MALT lymphoma patients, regardless of stage. For persistent localized disease, radiotherapy (RT) or rituximab (if RT is contraindicated) should be added to the HP eradication treatment. In general, RT is the preferred treatment option for localized MZL. For systemic disease, systemic chemotherapy and/or immunotherapy, rituximab, observation may be administered. The different aspect of our study is, only 13 (20.3%) of 64 MZL patients had RT. When compared to current treatment strategies, our cohort have been mostly treated with chemotherapy. This approach could be explained by the broad range of the timeline in which the patients have been treated. In addition, the problems in approaching to a radiation oncology facility could be an another reason. Ortega et al. retrospectively evaluated the impact of upfront chemotherapy and showed an 80% failure free survival and 100% overall survival. There were no relapses in the upfront chemotherapy arm [13]. Currently, the suggested systemic treatment option for MZL is Rituximab based regimens providing 75% response rate [14].
The prognosis of MALT lymphoma is better than splenic and nodal MZL. In addition, the gastrointestinal and pulmonary origins have worse prognosis when compared to ocular, cutaneous, and endocrine sites [4]. We couldn’t assess the impact of origin due to the limited number of patients in our study. When compared to literature data, the DFS and OS were similar with the previous studies [4]. The MZL has generally an indolent course with a 5-year progression free survival (PFS) and OS rates of 74% and 92%, respectively [15]. We found a 5-year DFS and OS rates of 85.3% and 74.5%, respectively. Table 2 shows a summary of MZLs from the English language literature. The age, elevated lactate dehydrogenase (LDH) and high Follicular Lymphoma International Prognostic Index (FLIPI) scores were associated with poor PFS and OS [16]. In the study, the only statistically significant factor for prognosis was performance score. Having ECOG performance scores of 2–3 was associated with worse OS in comparison to having ECOG 0–1, with mOS times of 18.0 versus 133.0 months, respectively (p = 0.011).
Table 2.
A summary of Marginal Zone Lymphomas from the English language literature
| Studies | Number of patients | MZL subtype (%) | Median age (range) | Gender (%) | Stage I–II; III–IV (%) | B symptoms (%) | BM involvement (%) | Overall survival | Used treatment modality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mazloom et al. [11] | 275 |
Splenic (13) Nodal (10) MALT (77) |
64 (N/S) 56 (N/S) 59 (N/S) |
F (65) F (63) F (53) |
0; 100 41; 59 58; 42 |
27 8 5 |
N/S N/S N/S |
5-year 93% 5-year 89% 5-year 87% |
RT (34.9) SysT (45.8) Obs (6.5) Sx (29) Other (9.8) |
| Kang et al. [17] | 40 |
Splenic (0) Nodal (30) MALT (70) |
56 (29–77) | M (60) | 0; 100 | 17 | 20 | 3-year 95% | SysT (100) |
| Olszewski et al. [4] | 15,908 |
Splenic (8.2) Nodal (29.7) MALT (62.1) |
68 (56–77) | F (54.4) | 57.4; 33.6 | 11.3 | N/S |
5-year 71.5% 10-year 53.3% |
RT (20.8) SysT (N/S) Sx (N/S, 51% for Splenic MZLs) |
| Heilgeist et al. [18] | 144 |
Splenic (11) Nodal (22) MALT (67) |
62 (29–88) | M (53) | 42.3; 57.7 | 6 | N/S |
5-year 82% 5-year 89% 5-year 92% |
RT (24) SysT (43.5) Obs (12) Sx (19.4) Other (10.1) |
| Oh et al. [19] | 45 |
Splenic (0) Nodal (33) MALT (67) |
54 (33–77) | M (71) | 0; 100 | 16 | 24 | 3-year 90% | SysT (100) |
| Present study | 64 |
Splenic (0) Nodal (21.9) MALT (78.1) |
54 (24–84) | M (60.9) | 64.1; 35.9 | 17.2 | 12.5 | 5-year 74.5% |
RT (20.3) SysT (82.8) Obs (1.6) Other (4.7) |
BM bone marrow, F female, M male, MALT mucosa-associated lymphoid tissue, MZL marginal zone lymphoma, N/S not specified, Obs observation, Other antibiotics etc., RT radiotherapy, Sx surgery, SysT systemic treatment (chemotherapy ± immunotherapy)
The study had some inevitable limitations. It was a retrospective analysis and there were insufficient numbers of patients and data for detailed analysis for factors associated with DFS and OS. In addition, we couldn’t analyze the impact of different types of MZL.
In conclusion, MZL has an indolent course and the prognosis is favorable. Moreover, some patients may live for many years without progression, even though they did not receive any treatment for their MZL. Further detailed studies with large patient numbers are needed and may help to clarify the clinical features and treatment management of MZLs.
Authors Contributions
GT, AA and ÖT wrote the manuscript. All authors contributed substantially to the conception, acquisition, analysis, and interpretation of the data for the work and approved the final approval of the version to be published.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest or grant support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salama ME, Lossos IS, Warnke RA, Natkunam Y. Immunoarchitectural patterns in nodal marginal zone B-cell lymphoma: a study of 51 cases. Am J Clin Pathol. 2009;132(1):39–49. doi: 10.1309/AJCPZQ1GXBBNG8OG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87. [PubMed] [Google Scholar]
- 4.Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2013;119(3):629–638. doi: 10.1002/cncr.27773. [DOI] [PubMed] [Google Scholar]
- 5.Casulo C, Friedberg J. Transformation of marginal zone lymphoma (and association with other lymphomas) Best Pract Res Clin Haematol. 2017;30(1–2):131–138. doi: 10.1016/j.beha.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Sriskandarajah P, Dearden CE. Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract Res Clin Haematol. 2017;30(1–2):84–91. doi: 10.1016/j.beha.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Vannata B, Stathis A, Zucca E. Management of the marginal zone lymphomas. Cancer Treat Res. 2015;165:227–249. doi: 10.1007/978-3-319-13150-4_9. [DOI] [PubMed] [Google Scholar]
- 8.Arcaini L, Paulli M, Boveri E, Vallisa D, Bernuzzi P, Orlandi E, et al. Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles. Cancer. 2004;100(1):107–115. doi: 10.1002/cncr.11893. [DOI] [PubMed] [Google Scholar]
- 9.Thieblemont C. Clinical presentation and management of marginal zone lymphomas. Hematol Am Soc Hematol Educ Program. 2005;2005:307–313. doi: 10.1182/asheducation-2005.1.307. [DOI] [PubMed] [Google Scholar]
- 10.Thieblemont C, Felman P, Berger F, Dumontet C, Arnaud P, Hequet O, et al. Treatment of splenic marginal zone B-cell lymphoma: an analysis of 81 patients. Clin Lymphoma. 2002;3(1):41–47. doi: 10.3816/CLM.2002.n.010. [DOI] [PubMed] [Google Scholar]
- 11.Mazloom A, Medeiros LJ, McLaughlin PW, Reed V, Cabanillas FF, Fayad LE, et al. Marginal zone lymphomas: factors that affect the final outcome. Cancer. 2010;116(18):4291–4298. doi: 10.1002/cncr.25325. [DOI] [PubMed] [Google Scholar]
- 12.Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224–2232. doi: 10.1182/blood-2016-10-747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega JL, Cabanillas F, Rivera N, Tirado-Gomez M, Hallman D, Pardo WI, et al. Results of upfront therapy for marginal zone lymphoma. Clin Lymphoma Myeloma Leuk. 2017;17(12):879–883. doi: 10.1016/j.clml.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Conconi A, Martinelli G, Thieblemont C, Ferreri AJ, Devizzi L, Peccatori F, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102(8):2741–2745. doi: 10.1182/blood-2002-11-3496. [DOI] [PubMed] [Google Scholar]
- 15.Ayyappan S, William BM. Marginal zone lymphoma: clinicopathologic variations and approaches to therapy. Curr Oncol Rep. 2018;20(4):33. doi: 10.1007/s11912-018-0687-9. [DOI] [PubMed] [Google Scholar]
- 16.Starr AG, Caimi PF, Fu P, Massoud MR, Meyerson H, Hsi ED, et al. Dual institution experience of extranodal marginal zone lymphoma reveals excellent long-term outcomes. Br J Haematol. 2016;173(3):404–412. doi: 10.1111/bjh.13975. [DOI] [PubMed] [Google Scholar]
- 17.Kang HJ, Kim WS, Kim SJ, Lee JJ, Yang DH, Kim JS, et al. Phase II trial of rituximab plus CVP combination chemotherapy for advanced stage marginal zone lymphoma as a first-line therapy: consortium for improving survival of lymphoma (CISL) study. Ann Hematol. 2012;91(4):543–551. doi: 10.1007/s00277-011-1337-6. [DOI] [PubMed] [Google Scholar]
- 18.Heilgeist A, McClanahan F, Ho AD, Witzens-Harig M. Prognostic value of the follicular lymphoma international prognostic index score in marginal zone lymphoma: an analysis of clinical presentation and outcome in 144 patients. Cancer. 2013;119(1):99–106. doi: 10.1002/cncr.27704. [DOI] [PubMed] [Google Scholar]
- 19.Oh SY, Kim WS, Kim JS, Kim SJ, Yoon DH, Yang DH, et al. Phase II study of R-CVP followed by rituximab maintenance therapy for patients with advanced marginal zone lymphoma: consortium for improving survival of lymphoma (CISL) study. Cancer Commun (Lond) 2019;39(1):58. doi: 10.1186/s40880-019-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]