Abstract
Functional near-infrared spectroscopy (fNIRS) is a relatively new imaging modality in the functional neuroimaging research arena. The fNIRS modality non-invasively investigates the change of blood oxygenation level in the human brain utilizing the transillumination technique. In the last two decades, the interest in this modality is gradually evolving for its real-time monitoring, relatively low-cost, radiation-less environment, portability, patient-friendliness, etc. Including brain-computer interface and functional neuroimaging research, this technique has some important application of clinical perspectives such as Alzheimer’s disease, schizophrenia, dyslexia, Parkinson’s disease, childhood disorders, post-neurosurgery dysfunction, attention, functional connectivity, and many more can be diagnosed as well as in some form of assistive modality in clinical approaches. Regarding the issue, this review article presents the current scopes of fNIRS in medical assistance, clinical decision making, and future perspectives. This article also covers a short history of fNIRS, fundamental theories, and significant outcomes reported by a number of scholarly articles. Since this review article is hopefully the first one that comprehensively explores the potential scopes of the fNIRS in a clinical perspective, we hope it will be helpful for the researchers, physicians, practitioners, current students of the functional neuroimaging field, and the related personnel for their further studies and applications.
Keywords: Functional near-infrared spectroscopy (fNIRS), Anesthetic depth monitoring, Behavioral disorder, Neurological disorder, Assistive modality
Introduction
Frans Jöbsis, a pioneer in biomedical optics, showed in 1977 that brain tissue transparency is relatively high in the near-infrared (NIR) range (700–900 nm) of the optical spectrum which allows us to record the average hemoglobin-oxyhemoglobin equilibrium in continuous time non-invasively using infrared transillumination spectroscopy [1]. After several demonstrations and different types of tests on laboratory animals, in 1985, this technique was first used to study cerebral oxygenation on sick newborn babies [2]. The first prototype of the near-infrared spectroscopy (NIRS) instrument was deployed by Marco Ferrari, in 1980. He started utilizing the prototype NIRS instruments for measuring the changes in brain oxygenation in human adults [3, 4]. In 1984, David Delpy started developing several NIRS instruments. The first quantitative measurement of numerous hemodynamic oxygenation parameters in sick newborn children was published. The tests recorded increases in oxygenated (HbO2) concentrations, deoxygenated (dHb) concentration, total hemoglobin levels (HbT), cerebral blood flow (CBV), and blood circulation (CBF) in the brain [5].
NIRO-1000 was the first commercial single-channel continuous wave clinical instrument by Hamamatsu Photonics K.K. (Hamamatsu City, Japan), built in 1989 after Cope and Delpy introduced four-wavelength system just 1 year before [6]. After that, different companies have developed their own NIRS prototypes in different time periods and different purposes.
Functional near-infrared spectroscopy (fNIRS) provides the relative change of blood oxygenation level in the superficial layer of the brain tissue. Functional magnetic resonance imaging (fMRI) also provides the same result [7] and this modality provides more accurate results than the fNIRS. The main drawback of the fMRI is its temporal resolution. With the poor temporal resolution, fMRI is very sensitive with motion as well as this is a costly and bulky imaging modality. On this contrary, fNIRS becomes popular because it provides real-time monitoring (with moderate temporal and fine spatial resolution), relatively low cost, radiation-less environment, portability, very fewer motion sensitivities, patient-friendliness feature, etc.
The main fields of fNIRS are related to neurology, psychiatry, psychology, education, cognitive neuroscience, and many more. These include a brain-computer interface (BCI), Alzheimer’s disease, Parkinson’s disease, post-neurosurgery dysfunction, anxiety disorders, childhood disorders, attention, functional connectivity, neuroergonomics, and so on. The fNIRS is developing rapidly over these years and the researchers of the diverse fields are engaging to better understand the functionality of the human brain. On the other hand, due to being a new neuroimaging modality, the different researchers of the arena of Biomedical Engineering are becoming interested in the medical applications of the fNIRS. Although there is a number of medical applications of the fNIRS, so far our knowledge, there is no review article that explores most of the medical applications of the fNIRS. Therefore, it could be an interesting scope to present a review article based on the medical applications of the fNIRS so that the researchers can focus on the future perspectives of this modality and its usages in the clinical applications.
The main contribution of this paper is to present how the fNIRS technology can assist medical and clinical decision making in different kinds of diseases. This review paper studied more than 200 papers of the applications of fNIRS and scrutinized some selected articles that are directly related to the medical or clinical applications of the fNIRS. Significant outcomes of the articles have been reported in this work in a narrative aspect. In addition, the study environment, the significance of the outcomes, limitations, future perspectives, etc. are also discussed in this review article.
This paper is organized as follows: the fundamental working principle of the fNIRS modality is described in “The Fundamentals of fNIRS Modality”. The clinical applications of fNIRS till now are presented in “fNIRS in Clinical Practices”. The drawback of this modality in clinical practice is described in the section titled as “Limitation of This Modality”. This review work has been concluded with its future perspectives in “Conclusions”.
The Fundamentals of fNIRS Modality
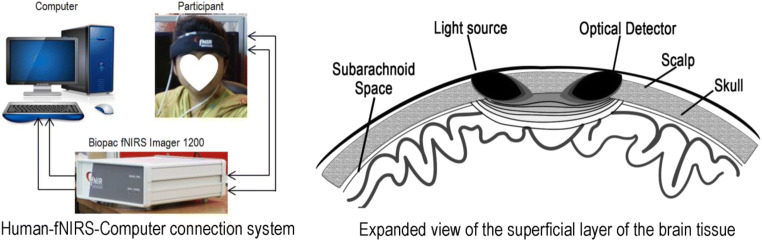
Functional near-infrared spectroscopy or fNIRS is a non-invasive optical functional imaging system. fNIRS uses the utility of near-infrared optical window (700–900 nm) which is almost transparent to skin, bone, and brain tissue [1]. The chromophores (HbO2 and dHb) existed in the blood absorbs NIR, and most importantly, the absorption coefficients of HbO2 and dHb are different. Therefore, the scattered-back light after absorption can provide information about the amount of the chromophores in that region which can be evaluated by modified Beer-Lambert law (mBLL). The blood circulation and the brain functionality are related to each other by the neuro-vascular coupling which is also known as the hemodynamic response.
When any region of our brain becomes activated, the neurons of that region need energy. This energy is produced from glucose metabolization, and to metabolize glucose, there needs oxygen. This oxygen is transported to that region by blood by combining with hemoglobin as HbO2. Therefore, the increasing amount of HbO2 or decreased amount of Hb provides the activation information, indirectly. This blood-dependent activation is also known as the BOLD (blood-oxygenation-level-dependent) response. Through the method of neurovascular coupling, neuronal activities are linked to related variations in regional cerebral blood flow (rCBF). In addition, this is also the core idea of fMRI and that is why the fNIRS and fMRI provide similar results in functional brain imaging [7].
In the hardware section of fNIR devices, the infrared light emitter diode and detectors are generally placed 3–4 cm apart as Fig. 1. While infrared light penetrates the cerebrum, it traces a banana-shaped path (shown in Fig. 1) through emitter to the detector. A series of sources and detectors are oriented in a defined manner in an optode band. Since the NIR light absorbs by HbO2 and dHb while traveling from emitter to detector, the intensity of the NIR light is decreased. From the difference between the intensity emitting and scattered back NIR light, the relative change of the oxygen concentration is assessed. The detailed procedure of measuring the oxygen saturation through fNIRS technology is worth reading the paper in [8, 9]. Since the absorption coefficients of HbO2 and Hb are different, the detected intensity contains two variables. To meet the challenge, simultaneously, the lights of two different wavelengths are supplied from the emitter. The increased amount of HbO2 concentration reports the activation of a specific region of the brain. Since oxygen is transported by the hemoglobin, with the increment of HbO2, there occurs decrement in the concentration of Hb. Therefore, fNIRS signals of HbO2 are negatively correlated with dHb.
Fig. 1.
fNIRS modality-based BOLD signal monitoring from the prefrontal cortex
fNIRS in Clinical Practices
The fNIRS modality is being used in many areas of researches and applications. Since this article scopes to pay attention only to its clinical applications and future perspective, we divided the application areas of the fNIRS modality into some major subjects. There are several applications of the fNIRS technology that have been reported in one or two articles. Such applications are excluded in the area of major clinical applications of the fNIRS. We paid our attention to some of the clinical applications that contributed significantly to this field by different approaches. The concerning major applications are described by the following subsections.
Monitoring the Anesthetic Depth
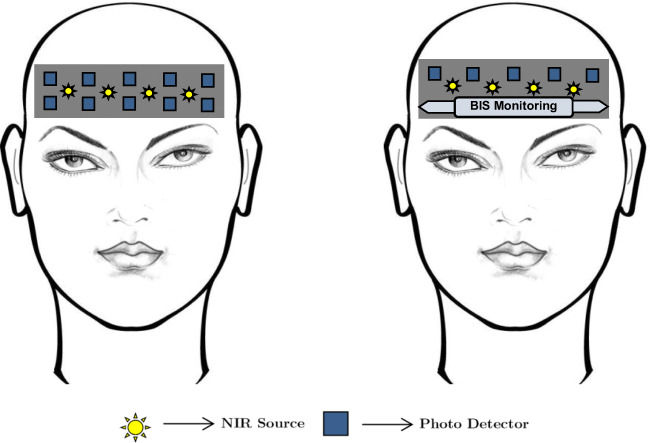
One of the remarkable applications of fNIRS as a clinical tool is anesthetic depth monitoring. From 2009 to 2017, a wide range of investigations had been conducted by several research groups to correlate the anesthetic depth to neurovascular coupling. A complete anesthetic delivery monitoring system requires different continuous measures such as peripheral oxygenation, circulation, ventilation, and temperature [10]; those aid to assure the safety of anesthesia procedures. As an anesthetic monitoring tool to evaluate the effect of general anesthesia, a combined method (Bispectral Index monitor (BIS) [11]) includes the investigations of electroencephalographic (EEG) signals, facial electromyography (EMG), and auditory evoked potential (AEP). A combined arrangement of fNIRS and BIS is shown in Fig. 2. Although this method provides the patient’s anesthetized condition, it is not good enough to predict the level of anesthetic depth for proper guidance of anesthetic delivery [12, 13]. Aforesaid complaint creates scope to develop a new method that can facilitate the optimal anesthetic delivery to enhance patient safety.
Fig. 2.
Anesthetic monitoring by the fNIR sensor alone (left figure). The combination of fNIRS and BIS system for anesthetic condition monitoring is given in the right figure. In the combination system, only the upper channels of the fNIRS system are considered [11]
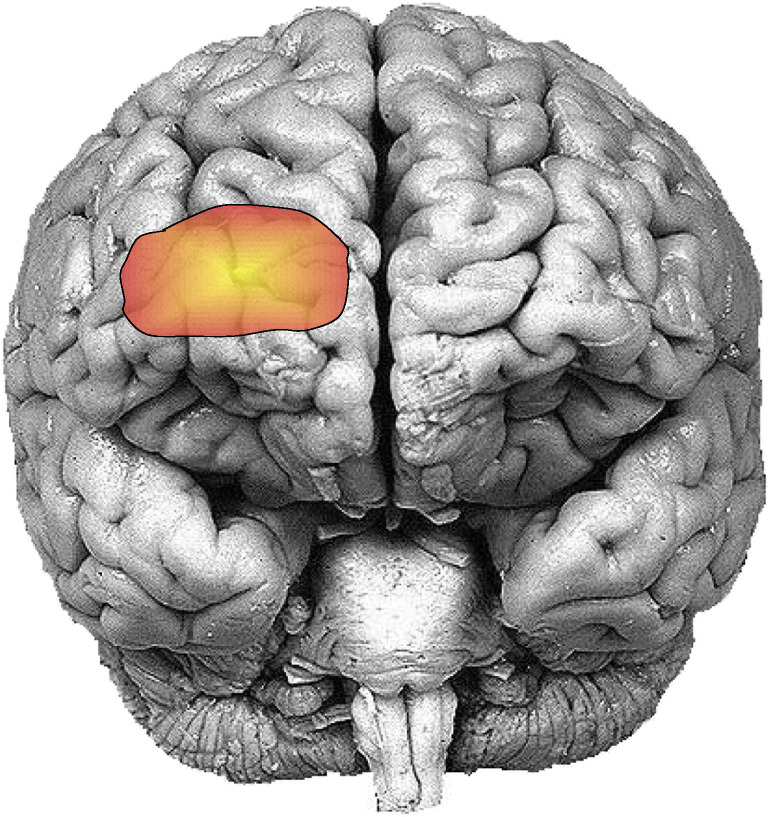
The research works [14, 15] introduced for the first time the correlation between the anesthetic depth and the neurovascular coupling in the human body. Therefore, a new dimension was added to neuroscience finding the pathway to assess the quantitative measurement of anesthetic depth. The anesthesia delivery process can alter brain hemodynamics which affects the regional cerebral blood flow (rCBF) as well as a neurovascular coupling in the cortical regions exclusively the prefrontal cortex (PFC) [16–19]. It was reported in [19] that the most activated region regarding the emergence of consciousness is the dorsomedial superficial layer of the prefrontal cortex as given in Fig. 3. The modality, fNIRS, is capable to evaluate the neural activities associated with hemodynamics through brain-energy metabolism research [20–22]. This preliminary research work established the foundation to demonstrate fNIRS as a clinical tool for real-time monitoring of the effect of anesthesia or sedation on cerebral oxygenation. With a variety of research work, it has been proved that fNIRS can provide the measurements of cerebral oxygenation that can discriminate among anesthetic states [23–26], concentrations of the same anesthetic (such as 5% versus 8% sevoflurane) [19, 24, 27], and different anesthetics [24, 26].
Fig. 3.
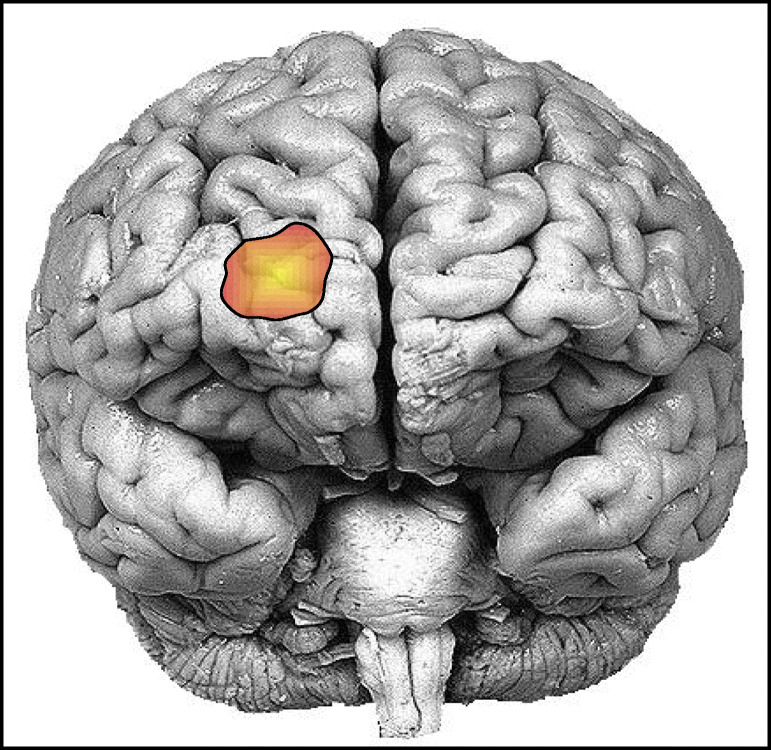
The position of the prefrontal cortex that shows the most significant activation regarding the emergence of consciousness during the anesthetic period. This area is equivalent to the channel number 11 of the 16 channel optodes of the fNIRS devices [19]
In anesthetic depth monitoring period, one of the major important observations is the changes in hemoglobin concentration (HbX: HbO2 and dHb) during the transition from the deeply anesthetized state (maintenance) to light anesthetized state (emergence) which was excluded as the possible confounders in the previous work described in [27]. A beautiful research work [28] investigated this problem and found that significant reductions took place in the global mean of HbO2 and HbT while the subjects experienced a transition from maintenance to emergence. This work proposed two postulates to describe the hemodynamics as (i) there is less space to be occupied as the vasodilatory effect wears off and (ii) the molecules move faster through the area of interest. Besides, this research work showed a high accuracy classification (94.7%) performance based on the corresponding signal features (standard deviation, range, minimum dHb, and HbO2) to discriminate the transition from maintenance to emergency state. Eventually, this work [28] found the fNIRS findings as biomarkers to predict the real-time state of the emergence due to sevoflurane-based anesthesia based on the measurement of the changes in CMR (cerebral metabolic rate), CBF, and CBV during the emergence.
Usage in Alzheimer’s Disease
AD is a neurodegenerative disease that worsens over time. It is termed as a clinical state of reducing utility in several cognitive areas, like memory, thinking, visuospatial abilities, decision, executive function, praxis, and language. fNIRS may be used for detecting disease-specific variations in the brain whichever can be of therapeutic measure or diagnostic parameters.
In the study of Hock et al., 38 individuals (as a guideline of the National Institute of Neurological and Communicative Disorders and Stroke criteria, 19 stable elderly people and 19 patients with a likely moderate AD [29]) were examined. This work claimed that in the case of the healthy participants, there occurred a rise in HbO2 and HbT concentration and a small decline in dHb concentration. On the other hand, most patients with AD exhibited a drop in HbO2 and HbT concentration throughout the verbal fluency test (VFT) which is a psychological test in which participants produce as many words as possible from a category in a given time. This occurrence is more prominent in the parietal cortex than in the frontal cortex.
A newer study by Fallgatter et al. [30] studied 10 AD victims and 10 well participants as control. During VFT, the AD victims lost mostly the left hemispheric stimulations noticed in controls. An overall decrease in HbO2 concentration related to ordinary aging despite the lack of hemispheric asymmetries indicated by the previous fNIRS studies [31–33]. So, a feasible characteristic for AD might be this discovery with a reduction of asymmetry during VFT. An initial possible study of fNIRS done by Fladby et al. [34] showed a reduced reaction to an olfactory stimulus in the temporal cortex in 13 subjects with mild cognitive impairment (MCI), very mild AD, or subjective memory complaints. According to the route for olfactory stimulus through the entorhinal cortex, the response can be diminished during the primary phase of AD. As a result, for the detection of temporal lobe dysfunction in the AD, fNIRS might be used as a promising method.
fNIRS showed upcoming feasible clinical practice for perceiving the effects of medications like cholinesterase inhibitor. Multichannel NIRS was used by Herrmann et al. to explore 16 patients with AD and 16 healthy persons throughout the enactment of VFT [35]. They found less increment of HbO2 concentration in the dorsolateral prefrontal cortex in patients with AD. These outcomes could be useful future applications related to therapeutic studies, for a prior work showing that the cholinesterase inhibitor AD patients were categorized by bilateral lower dorsolateral frontal perfusion [36]. This finding might be helpful to quantify the importance of functional brain activation for the anticipated result of the forthcoming cholinesterase inhibitor AD patients.
The study was led by Arai et al. [37] on 32 healthy controls, 15 patients with the AD, and 15 individuals spotted with MCI. Based on the activation index computation and the heft of variations in the HbO2 concentration in each zone during the activation task, they established that the activation index was considerably lesser in the bilateral parietal and frontal areas in the AD group, while the concentration change of the HbO2 in the MCI group was meaningfully inferior only in the right parietal area. This study found that in case of the average value of A-index (an A-index is a mathematical procedure that constantly calculates the t value from the consecutive groups with a fixed time interval) of the control group, in the frontal and bilateral parietal areas, the AD group was substantially lower while in the parietal right area, the MCI group had a noticeably lower average A-index value. The representative outcomes of each group are shown by the waveforms in Fig. 4. The results of the right parietal data seemed interesting for the clinical evaluation. If the cutoff point could be considered as 0.071 for the A-index to differentiate the AD or MCI patients from the controls set at an A-Index value of 0.071, the sensitivity for AD and for MCI was 71% and 40%, respectively, along with 94% specificity. Therefore, fNIRS has a future perspective to be an initial screening device for AD patients in the community health care system.
Fig. 4.
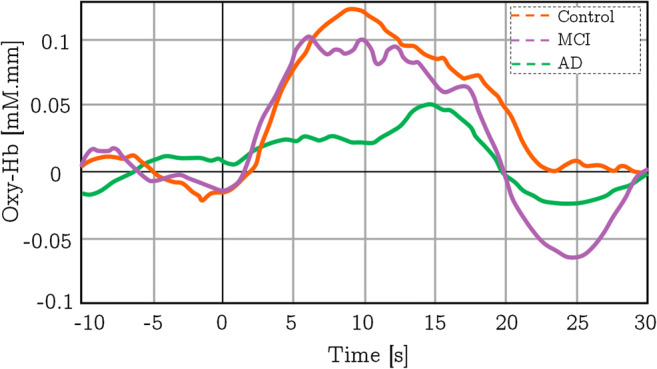
The overall average changes in the concentration of HbO2 of different groups during the verbal fluency test in the right parietal area of the brain. This figure is redrawn from the information found in [37]
Zeller et al. inspected the visuospatial deficits between 13 subjects with an assumed mild AD applying the Benton line orientation task [38]. The Benton line orientation task is a broadly practiced neuropsychological test gauging visuospatial judgment. Fascinatingly, they discovered no variance in task performance between patients and controls. However, fNIRS revealed activation deficiency in the parietal lobe in patients with AD. This suggests that fNIRS could feasibly be a quick AD detection technique.
Application in Schizophrenia
Schizophrenia is a condition that is categorized by positive and negative symptoms and cognitive dysfunction with enduring social deficits. An assessment was done by Watanabe et al. in cerebral hemoglobin oxygen saturation in the left frontal area with 62 schizophrenia patients and 31 healthy subjects in the course of a VFT and letter-number span test (LN) measured by fNIRS [39]. Also, previous studies suggest that activities of the frontopolar prefrontal cortex in recurrent schizophrenia and their resultant biomarkers are linked to their operations. Watanabe and Kato identified that HbO2 and dHb variations were reduced in LFT between healthy controls and schizophrenia patients. This study also demonstrated improved activities and related HbO2 improvements relative to studies in patients given medication with traditional antipsychotic drugs. The corresponding changes in HbO2 concentration due to VFT and LN for the control state are given in Fig. 5. The patients with schizophrenia revealed a minor peak in HbO2 concentration and a decrease in dHb concentration during VFT than controls. Alternatively, change in the concentration of HbO2 is increased during LN analogous to controls, but the relative concentration of dHb is decreased slightly from controls in schizophrenia patients. The contrast among patients who receive atypical antipsychotics and those receiving standard antipsychotics in the variations in HbO2 and dHb concentration between VFT and LN is also reported by this analysis. This further research revealed that during VFT and LN test, patients treated with atypical antipsychotics exhibited a greater rise in HbO2 concentration and a comparable reduction in dHb concentration compared with that patient were medicated with typical antipsychotics. The corresponding results are given in Fig. 6. This outcome proposes that typical antipsychotics may weaken task reaction and brain activity.
Fig. 5.
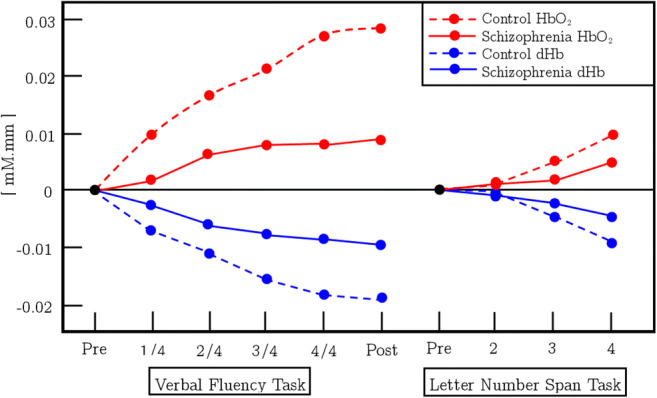
This figure represents the tasks (VFT and LN) and averaged change in [HbO2] and [dHb]
Fig. 6.
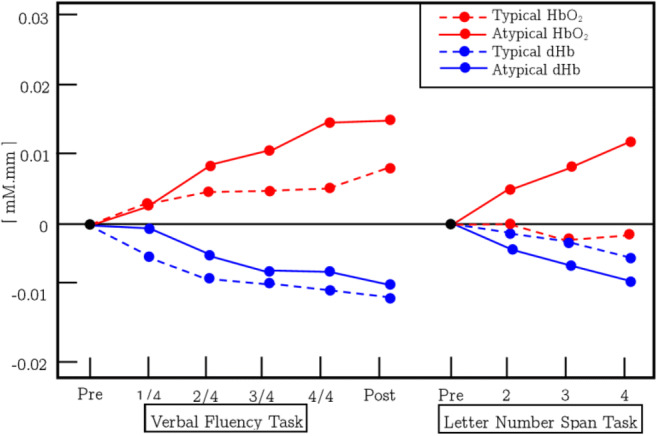
This figure represents the tasks (VFT and LN) and related averaged change in the concentration of HbO2 and dHb of the patients who were medicated by atypical antipsychotics and compared with the control group
Furthermore, we get that some more works [40–42] utilized fNIRS to measure non-invasive hemodynamic response on the surface of the scalp for schizophrenia as well as depression. As previous fNIRS investigations have represented that patients with schizophrenia have reduced activity and distinguishing signal patterns in the prefrontal cortex throughout the LN of the VFT, and part of these results have been permitted as one of the Advanced Medical Technologies as a support for the differential diagnosis of depressive symptoms by the Ministry of Health, Labor, and Welfare of Japan in 2009 [43–45] between victims with bipolar disorder [45, 46], major depressive illness [42, 45–50], and schizophrenia [45, 50, 51]. Such approval is prominent in the field of psychiatry.
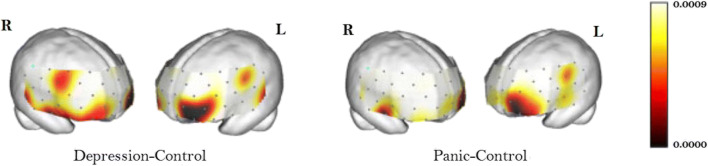
In [47], the authors considered the panic disordered and major depressive disordered patients for their functional change in HbX during word fluency tasks (WFT). They found that there were no significant differences in performances (results are given in Fig. 7) between the three groups (panic disorder group, major depressive disorder group, and healthy controls). To produce as many words as possible from an initial syllable of /a/, /ka/, or /sa/, participants were advised. In addition, every 20 s during the 60-s task, the three original syllables switched. As a task performance indicator, the right number of words produced during WFT has been evaluated.
Fig. 7.
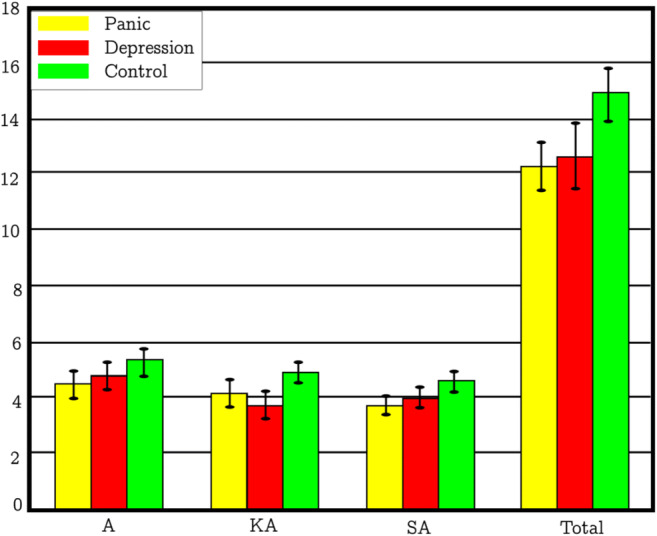
The number of appropriate answers for panic disorder, major depressive disorder, and stable controls on the Word Fluency Test (WFT) during fNIRS
From the functional change in HbX, this work found that the concentration of HbO2 was increased during WFT in the bilateral frontal areas in subjects with panic disorder and was diminished in the case of healthy control subjects. The corresponding functional brain images are given in Fig. 8. This kind of panic disorder hypofrontality is similar to results from some earlier works [10, 11, 14]. The panic and key anxiety condition classes display significant bilateral hypofrontality variations and the likelihood of laterality discrepancies. The left medial inferior frontal lobe can be deemed the most important functional area of the group.
Fig. 8.
Significance diagram of the category of panic disorders (right panel) and the major depressive disorders (left panel) compared with stable controls. The color bars on the right display gradient colors as a measure of significance
In another study, the results of a schizophrenic brain function during LFT have been confirmed by the Quaresuma et al., although no major improvements have been observed in the visual-spatial working memory task [52]. Ikezawa et al. showed major hemoglobin improvement on PFC through the usage of two-channel fNIRS in Tower of Hanoi (TOH), LFT, CFT, Sternberg’s, and Stroop’s task and found that brain functions were substantially different in schizophrenia and stable control patients during LFT and TOH [53].
fNIRS in Addiction Treatment
Increasing knowledge of the role of the prefrontal cortex on addiction indicates that the modality of fNIRS neuroimaging is a promising technique to predict recurrence [54–57]. fNIRS provides a different neuroimaging modality which is safe, patient-friendly, and affordable. The fNIRS could be easily employed in a bar setting, in the clinical office, for delivering an objective measure of predictive or analytic efficacy.
In a study, Bunce et al. [58] used fNIRS to assess the hypothesis made by Wilson et al. [59], i.e., that continuing alcoholic subjects without any intent to avoid drunkenness will indicate a rise in activation signal for 90–180-day care patients and casual drinkers in the dorsolateral prefrontal cortex (DLPFC)/orbitofrontal cortex (OFC). Researchers also measured the reactions of the participants to natural incentive stimuli, which projected a decreased reaction to incentives between active drinkers relative to rehab patients and social drinkers. Four adult alcoholics pursuing no counselling (one female), six alcoholics still in recovery (two females), and four responsible recreational drinkers (two females) have been recorded totalling 14 right-handed non-smokers in three classes. A cued response task was requested from participants. The findings revealed that active drinkers have seen a rise in alcohol activation across the right middle/inferior frontal gyrus which was anticipated compared with casual drinkers and recovery participants.
Bunce et al. obtained similar findings in patients undergoing opiate dependency using fNIRS imaging. In the initial study [60], patients who recently undergone opiate detoxification have been found to have decreased activity in the right lateral prefrontal cortex compared with patients under intensive residential monitoring between 60 and 90 days.
Such experiments have provided clear evidence that fNIRS can provide an unbiased index of preference for repetition. Clinicians should use fNIRS in order to improve better care utilizing an unbiased biomarker and to offer effective diagnosis by customized medicine.
Dyslexic Patient Identification
Dyslexia is a common learning difficulty that can cause reading, writing, and spelling. People with dyslexia find it hard to recognize the different sounds that complete words and relate these to letters. It is a disorder present at birth and cannot be prevented or cured. This research work [61] shows that reading is a complex task and a complex neural network is involved in different brain regions.
The dual-route model (DRM) of reading is a proposal for describing the processing of reading. According to the model, the information processing pathway has two routes. One is the orthographic route which is more active in finding semantic context, identifying the appropriate spoken representation of a word, and identifying the whole pattern representing the written word. The other one is the phonological route which is activated during combining the grapheme with the phoneme and providing a phonological representation of the written word [62]. However, one of the root causes of dyslexia was the latter route [63].
Lexical decision task (LDT) is a method for investigating the DRM [61, 64, 65]. In 2014, three reading classes, 12-year-old children, young adult dyslexic readers, and young adult typical readers, were studied for the comparative left frontal lobe activation during the LDT depending on the Shatila’s 1-min test. All participants were right-handed, regular, or corrected-to-average vision with both eyes and natural hearing tested. Neither individual reported persistent medication usage. The children displayed less activity under the word rule, and the dyslexic readers reported less activity even relative to normal readers under the pseudo-word scheme. All typical children and young adults were classified as typical readers for reading, and the standard Hebrew reading test was used by all young dyslexic readers. [66]. Although it is more difficult for unpointed Hebrew scripts to decode compared with pointed scripts [67], the decoding complexity of a single word, such as LDT, may not be high.
The results provide evidence that fNIRS can be used for upper left frontal lobe involvement in LDT through the DRM and the phonological deficit theory of dyslexia can be examined.
Contributions of fNIRS in ADHD Study
Attention-deficit/hyperactivity disorder is a neurological disorder that can be present from childhood. But, ADHD may pick up from adolescence and adulthood. It is a disorder that makes it difficult for a person to concentrate on and impulsive behavior control. Some warning signs for ADHD are inattention, impulsivity, non-stop talking, hyperactivity, etc.
Though there is no cure for ADHD yet, current ADHD treatments may help lower symptoms and improve impulsive functioning. Some treatments include medication, education or training, therapy, or a combination of treatments. Currently, neurofeedback is frequently used in the treatment of attention-deficit/hyperactivity disorder (ADHD) which is a trouble-some and frequent disorder that strikes adults approximately 2.5–5% globally [68, 69]. NF is a comparatively new evolving technique for behavioral therapy. In this protocol, patients learn to control their specific brain activity with the help of different feedbacks such as visual, audio, or combined with real continuous-time monitoring.
fNIRS is used to feedback blood-oxygenation level-dependent response which reveals the cortical activation latent to the brain region in a new NF protocol for ADHD [70–72]. fNIRS provides some ideal precedence in NF for ADHD patients such as low sensitivity to motion artifacts, easy and fast setup, portable, has a good spatial and temporal resolution as well as it measures cortical hemodynamic response [73–75], though functional connectivity is not clear in successful trials and failed trials in neurofeedback protocol yet [76, 77].
In a recent study by Hudak et al. [78], no significant changes were found between trials and the rest in functional connectivity analysis but differences were found in success and failure. But, the researchers claimed that they cannot confirm without a healthy control group in their study. They analyzed six different ROIs: the bilateral dorsolateral prefrontal cortex (dlPFC), bilateral inferior frontal gyrus (IFG), and bilateral parietal area. They also suggested that functional connectivity-based neurofeedback designs would be a great path to mark ADHD. Multimodal neuroimaging can be a favorable approach to investigate electrophysiological and neurological parameters that also aid fNIRS. Dolu et al. [79] conducted a study on 18 children with ADHD and 18 gender-matched healthy controls to see the effects of methylphenidate in ADHD. The focus was on oxy-Hb signals and the ROI was right PFC as it is a potential biomarker in the effects of methylphenidate for ADHD [80, 81]. The ROI of the possibly most activated region has been registered on the brain MRI image in Fig. 9. It was shown in the work [79] that fNIRS/EEG-based modality is clinically advantageous for the treatment effects of methylphenidate in children with ADHD.
Fig. 9.
The most affected ROI on the prefrontal cortex due to the effects of methylphenidate during the auditory oddball paradigm test by the ADHD children
Stroke Rehabilitation
Functional electrical stimulation (FES), which employs an electrical signal to stimulate motor neurons in particular muscle tissue, is one technique for rehabilitation [82]. FES should be implemented throughout self-regulated brain activation, to activate neuroplastic reorganization and to start learning control of the affected limbs. An optical BCI may be used to monitor the brain condition. fNIRS was used for examining the correlations of real imagery of movement. fNIRS-BCI for controlling FES and evaluating the brain function of healthy participants while self-regulated FES feedback and passive FES are being built. The optode setup has been selected to include a primary motor cortex in the frontal lobe that controls the upper extremity and the premotor cortex. The research study carried out by 10 healthy subjects allowed them either to visualize their right hand extending fingers or relaxation. The time series analysis of oxygenated and deoxygenated hemoglobin demonstrated a variation in brain activity in the motor imagery when participants checked the FES compared with passively induced ones. This research demonstrates that FES alongside fNIRS could be used as a method for neuroplastic re-organization and motor therapy in a BCI environment for stroke victims.
In particular, the evaluation of vasomotor reactivity in occlusive carotid artery disease was endorsed by fNIRS as a substitute or supplementary technique. The findings suggest that the sensitivity is similar and/or less than adequate to implement the methodology widely in this field [83, 84]. However, in all phases of the cerebrovascular disorder, the mixture of transcranial Doppler (TCD) and fNIRS has been reported. The growing use of fNIRS imaging devices that provides chapped topographical data might support a cumulative flow-velocity assessment in large vessels (TCD) and the fNIRS index. The delineation of tissue is far beyond the scope of the technique in significant parts of cortical and subcortical tissue. Continuous monitoring of cerebrovascular variables could be considered the most promising ground for fNIRS in clinical neurology mostly during the under-acute stage after diagnosis and initial treatment. A systemic decline in arterial, ABP, and partial oxygen saturation reveals that the cerebral oxygen decrease as monitored by the fNIRS has continuously been observed overnight in nine patients with an anterior sub-acute cerebral artery stroke [85]. A scarcity of the specification of the fNIRS variables and the physiological assumptions underlying these results is the viability of long-term assessment of the main flaw. The effects of sleep-related respiratory disorders in underlying stroke patients were evaluated in another study [86]. Two frontal samples have been used to minimize extra-cranial contamination in a multi-distance method. In 11 subjects, successful overnight recordings were taken in answer to obstructive apneas with profound cerebral deoxygenation. In 24 subjects with variable-level stenosis cases, fNIRS measured relative oxygen saturation for bilateral front areas and middle cerebral arteries (MCAs) [87]. The most important area, for the clinical neurologist, might be the surveillance of sub-acute stroke and cerebrovascular disorders in the overview. The methods could easily be incorporated into the surveillance and therapeutic context that an increasing number of individuals in specific stroke units can expect.
fNIRS is used to identify vivid prefrontal regions of the diseased brain functionally. Significant trends in response patterns are being reported in patients who have an ischemic stroke and variable degrees of carotid artery stenosis [88, 89]. Revascularization has also been shown to normalize the response [90]. Although these findings indicate that the reactivity of the vascular system has largely improved, another study illustrates the dynamic relationship between a transformed neurovascular connection and pathologic brain function. A significant difference may also result in changes in cortico-subcortical loops in cortical oxygen consumption. This was shown in Parkinson’s disease patients who received deep brain stimulation (DBS) and serious tremor (n = 1) [91, 92]. Specific reaction patterns based on the location for the stimulus were observed by oxygenation of the front lobe (bilateral probes). While the clinical setting pertinence can be minimal, detecting functional cortical alterations in this population induced by DBS may be a valuable use of fNIRS.
fNIRS can be used to resolve changes in neurovascular connectivity in the suffering brain and the cycles of brain activity during recovery. A transformed cortical activation pattern with improved premotor recruitment was shown in the post-stroke rehabilitation field [93]. Also, the lateralization level of the fNIRS reaction in the premotor cortex was changed for a 2-month rehabilitation [94]. In mild and severely paretic stroke patients’ therapeutic interventions had been researched trying to make gait is one of the most highly researched areas of fNIRS neurorehabilitation [95, 96]. The fNIRS could also be delicate to more cognitive locomotive aspects [97]. In the reiteration of the studies previously covering [98, 99], it is suggested that the fNIRS will include two specific features of functional reorientation. One, adding specific prefrontal regions for activities that do not necessitate intense executive regulation of balanced tests, and another one is a brain lateralization map for tasks like motor and language arbitrarily. The creation of neurofeedback devices and brain-computer (BCI) interfaces is another area that has the potential for neurorehabilitation. Neurofeedback fNIRS or the usage of fNIRS as another way to identify brain activity trends has been used frequently in recent approaches [100, 101]. Current methods have portability, the key to truly exploit the functionality of fNIRS. Many organizations have developed wearable devices, such as strategies for simultaneous EEG acquisition, successfully [102, 103]. So, the feasibility of the preciseness of fNIRS could be much higher in stroke rehabilitation.
Autism
Visual facial processing weaknesses of individuals with autism spectrum disorder (ASD) may be caused by unusual brain structure and operation. Studies evaluating the asymmetric function of the brain among ASD people suggested that the lateralization of facial processing in neuro-typical (NT) individuals is less likely to be lateralized in ASD. The researchers initially tried this hypothesis using fNIRS by evaluating trends of lateralized cognitive function in facial treatment areas of the homologous temporal-occipital while observing the face of an ASD and NT group. As anticipated, as opposed to the NT participants, the ASD group demonstrated reduced human-side right hemisphere asymmetry. Judging by recent cognitive findings that robots can make it more possible for ASD to interact with human counterparts, researchers have also monitored responses to faces of robots to establish whether these stimulation trends were lateralized in each group. All groups demonstrated identical asymmetry trends for robotic faces in the exploratory study. The study suggests that human faces in ASD have lowered asymmetry as well as provide an initial basis for future tests on how the clinically useful use of conclusively different social stimuli in these populations can be [104].
Autism is a neurodevelopmental illness with symptoms of behavior and different structural and operational brain damage. Earlier neuroimaging experiments investigated the disparities in brain function among people with and without ASD. Researchers have managed to research the variations between people on the autism spectrum in specific brain characteristics. Their primary purpose was to investigate the variations in the growth of the functional network capability and rates of autistic behavior, and also the connection within functional network capability and age, in young children with ASD. A recent study involved 46 children with ASD and assessed their parents’ autistic behaviors. The network performance was found from the HbO2-, dHb-, and HbT-based functional networks. The analysis revealed that in young kids with autism in the dHb- and HbT-based networks, network effectiveness decreased with age, and in the HbO2-based network children with a comparatively high level of autistic actions. Findings indicate that independent brain development differs in young ASD children, offering insights into autism psychopathology [105].
Initially, by implementing fNIRS, cognitive development researchers could have a better insight into brain functionality and organization, particularly once they are fully conscious or hyperactive, in babies and young people. New research on fNIRS usefulness in new-born populations has shown, however, that fNIRS is indeed an effective solution in the research of infants who may be more probable to have an older sibling and to evolve ASD than new-borns without the family background of developmental disorders and also have a large likelihood of developing ASD [106–110]. Based on important physiological information given by infants, initial symptoms of ASD brain structural and functional characteristics may be clarified in studies of infants [111, 112]. Second, fNIRS has shown its suitability for the analysis of ASD [113, 114]. It is difficult to engage in an fMRI trial for those with a chronic condition as some of the participants cannot regulate the hyperkinetic activity or tolerate the confined space and the high noise. The significantly larger visible room and the soothing ring of fNIRS enable patients to encounter more natural conditions throughout experiments compared with the restricted, claustrophobic room used in fMRI studies. The fNIRS is also decent for examining the linguistic perspective that is crucial to sufficient interpersonal interactions and frequently deteriorated in ASD due to lower noise. Nevertheless, this should be remembered that by utilizing fNIRS, there are still some drawbacks. Yet together, fNIRS is a successful method to investigate ASD neurodevelopment from an early phase and overlooks its pitfalls in work on brain functions for individuals with ASD [115, 116].
Depression
In major depressive disorder (MDD), decreased oxygenation shifts in the prefrontal cortex were documented during cognitive tasks. Nonetheless, in individuals with MDD, prefrontal asymmetry was somewhat commonly investigated throughout mental exercises and their connection to suicidal thoughts. Researchers examined prefrontal asymmetry and its moderating effect in patients with MDD during mental performance between depression severity and the suicidal thoughts [117]. Forty-two patients with MDD and 64 healthy controls (HCs) were evaluated with fNIRS verbal fluency task (VFT), stop-task, and two-back task for alterations in HbO2 and dHb. The self-report questionnaires evaluated depression, anxiety, as well as suicide thoughts. For MDD patients, comparatively lower-left HbO2 shifts were reported contrary to HCs during VFT but not during strop or two-back activities. Moreover, the impact on suicide idea of depressive magnitude during VFT has been mitigated by prefrontal asymmetry and is associated substantially and strongly with suicide ideations in MDD patients. More left HbO2 shifts have been linked with higher ideation to suicide. The results demonstrate that prefrontal asymmetry, fNIRS-measured, is a possible biomarker for MDD and suicidal risk management in MDD patients.
Usually, patients with MDD have an omnipresent depressed mood or anhedonia, along with many cognitive and physical conditions. In another research, the random hemodynamic behavior of the PFC was examined by fNIRS where 21 MDD patients took part in an 8-min rest state measurement [118]. Their findings found that patients with MDD exhibited substantially reducing interhemispheric association with PFC and impaired cortical organizations with PFC, by the study of the interhemispheric association and the PFC correlation charts. This research indicates that the neurological symptoms of depression can be tested accurately and comfortably.
Depressed people often experience psychologically passive facial gestures. It remains uncertain what is neurobiologically dependent on this impact. In consideration of the emotional and neutral facial expressions with the assistance of fNIRS, researchers analyzed the variations in PFC activity in stressed individuals vs. healthy controls (HC). The task of emotional intensity ratings in experiment 1, 33 depressed people, and 20 HC assessed the intensity of face facial reactions. Experiment 2 performed a separate group of subjects (18 persons distressed and 16 HC) during their PFC activation. Both studies have shown stressed people to perceive optimistic, but not joyful and anxious facial emotional signals more gradually and less specifically. Experiment 2 demonstrated a decreased performance of supportive facial emotions with a lower right stimulation of PFC in anxious persons, but not HC. In relation, the right PFC stimulation for the recognition of joyful facial expressions is lower contrary to HC for depressed people. For anxious people, the perception of sympathetic facial gestures is compromised. Higher PFC stimulation during neutral facial development coincides with greater disability. The perception of positive facial gestures is equivalent to unhappy people and HC, but retired patients have slightly fewer correct PFC stimulation. These results altogether indicate that deviant processing of the correct PFC might influence the capacities of depressed individuals to differentiate in rational and emotional stimuli in the context [119].
Emotional phrases that inspire left cortex activity in depressed patients have been discovered from earlier studies. This result was replicated primarily through a data received and the brain region linked with the levels of depression was evaluated using an emotional stop task. The fNIRS records HbO2 and dHb of 14 patients with MDD and 22 normal controls in the brain. In the left frontal cortex, the sensitivity of hyperactivated HbO2 to undesirable conditions was detected; yet, there was no distinction between patients with distress and safe sensitivity controls. This outcome is consistent with previous findings. There was a negative correlation with depression intensity between an evoked pulse correlated with the left upper frontal cortex and desirable stimuli. Their current fNIRS work offers a possible indication of the position of the symptoms of depression throughout the top left front. Future research will test and expand existing results to an effective etiology of depression [120].
The central neuroimaging predictor for unipolar (UNI) and bipolar (BI) disorder is modified pre-frontal brain function, for example, hypofrontality, through executive activities like work memory. In a memory task that includes different mechanisms and materials with fNIRS over the pre-front cortex, researchers examined first-line UNI (n = 16) and BI (n = 14) patients. The variations to HbO2 and dHb, relative to patient populations, in stable controls (n = 15), display an improvement in ventrolateral, dorsolateral prefrontal, and superior frontal cortex function similar to the conditions to regulation for artifacts and the spatial visual cognitive function. By comparison, in both functioning memory settings, all patient types displayed decreased brain function. The findings demonstrated unspecific deficiencies and could not differentiate between unipolar and bipolar disorder, based on processing memory mechanisms or elements. fNIRS can, therefore, be considered a legitimate instrument for measuring prefrontal cortex functions that is simple to use, low cost, and fast [121].
Epilepsy
The diagnosis and treatment of epilepsy are clinical, as they normally are not observed by the healthcare professionals in the history of chronic seizures [122]. fNIRS research analyzed (i) cortical oxygenation problems during the seizing phase, (ii) concentration localization, and (iii) whether functional imaging would help to delineate the functionally vivid brain areas in advance of surgery. In fNIRS studies, the oxygenation reactivity to epileptic activity had been researched most frequently. Recent studies revealed that complicated partial seizures cause significant increases in HbT in the frontal lobe [123], but deoxygenation over the frontal lobe ipsilateral to the target has been documented in two subjects with temporal lobe epilepsy, and in three specific patients including the baby, separate peri-ictal findings have been recorded [124].
An electroconvulsive therapy (ECT) study also supports the motif of deoxygenation in reaction to generalized epileptic activity. ECT is being used in pharmacologically resistant depression and was investigated with fNIRS as a framework for widespread human seizures [125]. fNIRS and TCD were followed in 90 subjects induced seizures. Oxygenation decreased, and the number of cytochrome-oxidase decreased, whereas TCD demonstrated an increase in the flow rate as an effect of the electrocution. The development of fNIRS imaging devices, in particular, has turned epileptic focusing into yet another excellently studied implementation. The concurrent use of fNIRS and intracranial EEG was used to evaluate 29 patients with pharmacologically refractory epilepsy [126]. The affected hemisphere as described by intracranial EEG was recognized by fNIRS in 28; yet, only 20 patients were shown to have proper lateralization. Concurrent scalp-EEG and fNIRS studies were carried out in a variety of patients with non-lesional drug-resistant frontal lobe epilepsy, the subject of which had been identified by separate intracranial recordings [127]. fNIRS has illustrated a segment of subclinical ictal action, missed by scalp-EEG scanning. fNIRS also indicated that stimulation should be expanded rapidly in opposite areas of the hemisphere.
Fluctuations in oxygenation will model general epileptic outflow in subcortical and cortical areas [128]. New research identified oxygenation reductions of up to 15 min before seizure start around the frontal lobe [129] in patients with temporal lobe epilepsy. Such improvements have been made to the supposed target ipsilaterally. The pathophysiological basis of such a substantial time delay remains unclear as to the application of a 2-channel fNIRS system, which selectively samples an area away from the suspected focus. Even so, the research highlights the durable monitoring possibility of fNIRS and the possibility of detecting anticipatory seizure signals. The previous research comparing written word production with a drawing activity for motor stimulation demonstrates that 11 healthy subjects were managed to be reliable lateralization. In six patients who received an assessment of the presurgical lateralization, a test based on the unilateral angiographic use of the fast anesthetic amobarbital was correlated with the Wada test [130]. The results were repeated in a later analysis utilizing the same role in eight participants, but the association between Wada and fNIRS had been decreased in 16 patients [131].
The strong connection between the fNIRS assessment and both the Wada test and fMRI test results was seen in 8 patients and 3 control subjects utilizing orally verbal fluency (control: nonsense-syllable repetition). This could be an excellent added measure of language prevalence and an alternate method in patients without Wada, PET, or fMRI imaging [132, 133]. Besides, an equally non-invasive TCD evaluation is viable [134]. An amazing case study on a 10-year-old student initially evaluated oxygenation responses to seizure and then tested language lateralization in a later word generation task [135], highlighting that perhaps the fNIRS might be used in various facets of the disorder assessment. Yet, another significant problem would be how to evaluate continuous fNIRS information when distinct unexpected events are to be found. A notable article evaluating various techniques using simulated data and examine data on one patient was used explicitly resolved to this question [136]. Signal-to-noise ratio (SNR) is, of instance, an important determinant of the identification susceptibility. The question if the alterations in migraine activity correlate to the underlying photosensitive epilepsy has also been contentiously discussing [137].
Migraine
The effect of migraines mostly on the vascular systems had been shown [138]. Migraine’s latter pathophysiological trait reflects the rare illness of the migraine stroke [139] and the controversial increased risk of migraine stroke [140]. Research conducted in fNIRS has highlighted (i) vasoreactivity alteration, (ii) vascular reaction to cortical depression (CSD), and (iii) certain therapeutic or vascular prevention facets of patients with migraine. A relaxation procedure has been tested for six people undergoing with migraine with aura [141] that is one of the very first trials of vasomotor reactions. Such patients displayed a common trend of the initial decline and a steep increase in dHb over around 20 s of respiration. Similarly, another study demonstrates an oxygenation attenuation of 30 migraine patients with an aura contrast to age-matched controls, using breath-holding as more of a vasoreactivity test [142]. The fNIRS-TCD configuration allows the cerebral vascular reaction alterations to be assigned to a better degree, but no chronic hemodynamic shifts control was employed. Twelve patients with migraine without aura have confirmed a far more remarkable result concerning lateralized alterations in vasoreactivity [143]. Throughout the right hemisphere, a tiny increment in HbT preferentially demonstrates a head-down rotating operation compared with age-related controls within those patients.
Important earlier studies with fNIRS in a rat model explored the vascular reaction and observational key changes of CSD [144]. Because auras have a very short period (~ 30 min), the study of natural auras in humans is not easy, but fNIRS is quite well suited for providing further evidence on this extremely fascinating scientific phenomenon. The study on 8 random auras patients explains a decline in fNIRS oxygenation in tissue and a decline in the TCD flow rate [145]. Such results concur with the non-ischemic character of CSD and their hemisphere lateralization contrary to the syndromes endorses the cerebral origins of the fNIRS findings. In a very large cohort (n = 88) of fNIRS patients, a patent foramen ovale (PFO) has been tested [146]. When contrasting the fNIRS protocol with a normal comparison strengthened TCD method, the result reveals a rather high diagnostic reliability (84%).
Another recent study explicitly focuses on patients at risk of a stroke. In contrast to age-balanced monitoring, ten patients were tested interictally and displayed a longer period interval between electrocardiogram R-wave and the NIRS signal [147]. One small-scale fNIRS article discusses oxygenation alterations mostly during a migraine attack in reaction to relieving pain. Four symptomatic participants undergo subcutaneously sumatriptan and four controls received saline sham injection [148]. The researchers reported a concurrent reduction in HbO2, measured by fNIRS and skin blood circulation, measured by Doppler flowmetry in patients who received sumatriptan migraines. As fNIRS and skin blood flow changes have been strongly correlated, both techniques could quite reinforce radical changes in hemodynamic parameters than specifically in the brain.
The concept of a modified neurovascular reaction and prophylactic actions in migraineurs were integrated into an interesting experiment. Based on a predetermined protocol for visually stimulated treatment [149], 20 patients were subjected to diverse visual stimuli. The vascular reaction was comparable but with variations in period (mainstream of HbO2), in contrast to age-based controls. In much the same population, a second study utilized color lenses independently adjusted to reduce the visual discomfort of migraines. The use of such lenses has caused the patient group to normalize its reaction. The research demonstrates how well the preventive effects of fNIRS concerning fundamental pathophysiology might well be measured outside clear clinical aims.
Limitations of This Modality
Oddly, the flexibility of the technique can be yet another reason for this evident difference. This ranges from the straightest measurement of oxygenated, deoxygenated, and total hemoglobin, to the less accurate cytochrome oxidase redox changes [150], to many notations that give oxygenation measures including regional saturation of oxygen and tissue oxygenation [151, 152]. The plenty of variables may be scientifically valuable, but significantly reduces consistency between study groups and is therefore not best suited for clinical use. The fact which half of the brain cortex may be questioned in grown-ups at best impedes the incorporation of fNIRS in clinical neurology. Mesial, insular, and even cortex cannot be attained in deep sulci and every subcortical or infratentorial area of the brain. The application and critical assessment of the brain throughout cardiac or carotid artery surgery seem to be most sophisticated in the case of brain monitoring [153–156]. Within this field, fNIRS systems concentrated primarily on the entire approach to correlate in cerebral oxygenation with any postoperative neurological impairment in a relatively small region of the cerebral cortex. Conversely, the treatment of neurological differing disease causing the central nervous system (CNS) normally seeks to evaluate a somewhat limited lesion or dysfunction detection, which can then distinguish among underlying illness. Evidentially, fNIRS may only examine the brain surface and thus the crucial first phase until the immediate operation cannot accurately distinguish between ischemia and hemorrhage. However, it can provide appropriate additional data to evaluate the altered vascular reaction trends in the proximity of the disordered brain tissue and provide a better understanding of the durable reorganization of DBS in fNIRS, which does not provide adequate spatial resolution for succinct preoperative modeling of cortical functions. The use of fNIRS may even be restricted in the “hyper-acute” diagnostic phase.
Conclusions
The study has found from the current literatures so far that the fNIRS modality contributes some significant applications in clinical aspects. One thing should be noted that fNIRS does not contribute directly for diagnosis; rather, it is an assistive device that reads the functional activities of the brain and indirectly informs abnormality of the brain functionality. For example, in clinical practices, the neurosurgeons or neurologists utilize the feature of this modality in anesthetic depth monitoring to be confirmed that the patient is in a deep sedating state. In some other mentioned applications like Alzheimer’s, schizophrenia, dyslexia, addiction, ADHD, epilepsy, depression, etc., fNIRS are used as a predictive modality that discriminates the functional activity of the hemodynamics with some behavioral test. Since the researches on fNIRS-based study have started the journey a few years ago, different forms of new clinical applications with strong validity of fNIRS-based study are surely coming shortly.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Md. Asadur Rahman, Email: bmeasadur@gmail.com.
Abu Bakar Siddik, Email: yeashasiddik@gmail.com.
Tarun Kanti Ghosh, Email: tarunbge@gmail.com.
Farzana Khanam, Email: farzanabme@just.edu.bd.
Mohiuddin Ahmad, Email: ahmad@eee.kuet.ac.bd.
References
- 1.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 2.Brazy JE, Lewis DV, Mitnick MH, Jöbsis vander Vliet FF. Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics. 1985;75:217–225. [PubMed] [Google Scholar]
- 3.Ferrari M, Giannini I, Carpi A, Fasella P, Fieschi C, Zanette E: Noninvasive, infrared monitoring of tissue oxygenation and circulatory parameters. XII World Congress of Angiology, Athens, September 7–12, abs. 663, 1980
- 4.Ferrari M, Giannini I, Sideri G, Zanette E. Continuous non invasive monitoring of human brain by near infrared spectroscopy. Adv Exp Med Biol. 1985;191:873–882. doi: 10.1007/978-1-4684-3291-6_88. [DOI] [PubMed] [Google Scholar]
- 5.Wolf M, Naulaers G, van Bel F, Kleiser S, Greisen G: A review of near-infrared spectroscopy for term and preterm newborns. J Near Infrared Spectrosc. 20, 2012. 10.1255/jnirs.972
- 6.Cope M, Delpy DT. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput. 1988;26:289–294. doi: 10.1007/BF02447083. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54(4):2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman MA and Ahmad M: Identifying appropriate feature to distinguish between resting and active condition from FNIRS, 3rd International Conference on Signal Processing and Integrated Networks (SPIN), Noida, pp. 671-675, 2016. 10.1109/SPIN.2016.7566781
- 9.Rahman MA and Ahmad M: Movement related events classification from functional near infrared spectroscopic signal, Int. Conf. on Computer and Information Technology (ICCIT), Dhaka, Bangladesh, 18-20 December, 2016, Dhaka, Bangladesh. 10.1109/ICCITECHN.2016.7860196
- 10.American Society of Anesthesiologists (ASA): Standards for basic anesthetic monitoring, ASA house of delegates. 1986
- 11.Medical Advisory Secretariat: Bispectral index monitor: an evidence based analysis. Ontario Health Technology Assessment Series vol. 4, no. 9, 2004 [PMC free article] [PubMed]
- 12.Gajraj RJ, Doi M, Mantzaridis H, Kenny GN. Analysis of the EEG bispectrum, auditory evoked potentials and the EEG power spectrum during repeated transitions from consciousness to unconsciousness. Br J Anaesth. 1998;80(1):46–52. doi: 10.1093/bja/80.1.46. [DOI] [PubMed] [Google Scholar]
- 13.Von Delius S, Thies P, Rieder T, Wagenpfeil S, Herberich E, Karagianni A, Frimberger E, Meining A, Ludwig L, Ebert MP, Schulte-Frohlinde E, Neu B, Prinz C, Schimid RM, Huber W. Auditory evoked potentials compared with bispectral index for monitoring of midazolam and propofol sedation during colonoscopy. Am J Gastroenterol. 2009;104(2):318–25. doi: 10.1038/ajg.2008.73. [DOI] [PubMed] [Google Scholar]
- 14.Ou W, Nissilä I, Radhakrishnan H, Boas DA, Hämäläinen MS, Franceschini MA. Study of neurovascular coupling in humans via simultaneous magnetoencephalography and diffuse optical imaging acquisition. Neuroimage. 2009;46:624–32. doi: 10.1016/j.neuroimage.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosengarten B, Kaps M. A simultaneous EEG and transcranial Doppler technique to investigate the neurovascular coupling in the human visual cortex. Cerebrovasc Dis. 2010;29:211–6. doi: 10.1159/000267840. [DOI] [PubMed] [Google Scholar]
- 16.Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. J Cereb Blood Flow Metab. 2012;32(7):1233–47. doi: 10.1038/jcbfm.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John ER, Prichep LS. The anesthetic cascade: a theory of how anesthesia suppresses consciousness. Anesthesiology. 2005;102(2):447. doi: 10.1097/00000542-200502000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Heinke W, Fiebach CJ, Schwarzbauer C, Meyer M, Olthoff D, Alter K. Sequential effects of propofol on functional brain activation induced by auditory language processing: an event related functional magnetic resonance imaging study. British J Anaesth. 2004;92(5):641–50. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- 19.Leon-Dominguez U, Izzetoglu M, Leon-Carrion J, Solís-Marcos KL, Garcia-Torrado FJ, Forastero-Rodríguez A, Mellado-Miras P, Villegas-Duque D, Lopez-Romero J, Izzetoglu K. Molecular concentration of deoxyHb in human prefrontal cortex predicts the emergence and suppression of consciousness. Neuroimage. 2014;85:616–25. doi: 10.1016/j.neuroimage.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Magistretti PJ: Cellular bases of functional brain imaging: insights from neuron-glia metabolic coupling, Brain Res, vol. 886, no. 1-2, pp. 108–112, 2000. 10.1016/S0006-8993(00)02945-0 [DOI] [PubMed]
- 21.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans Royal Soc B Biol Sci. 1999;354(1387):1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajapakse JC, Kruggel F, Maisog JM, von Cramon DY. Modeling hemodynamic response for analysis of functional MRI time-series. Hum Brain Mapp. 1998;6(4):283–300. doi: 10.1002/(sici)1097-0193(1998)6:4<283::aid-hbm7>3.0.co;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleck T, Schubert S, Ewert P, Stiller B, Nagdyman N, Berger F. Propofol effect on cerebral oxygenation in children with congenital heart disease. Pediatr Cardiol. 2015;36(3):543–549. doi: 10.1007/s00246-014-1047-7. [DOI] [PubMed] [Google Scholar]
- 24.Fassoulaki A, Kaliontzi H, Petropoulos G, Tsaroucha A. The effect of desflurane and sevoflurane on cerebral oximetry under steady-state conditions. Anesth Analg. 2006;102(6):1830–1835. doi: 10.1213/01.ane.0000205739.37190.14. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki K, Nomoto Y, Ishiwata M, Yokota T, Ogawa R. Vital capacity induction with 8% sevoflurane and N2O causes cerebral hyperemia. J Anesth. 2003;17(1):3–7. doi: 10.1007/s005400300001. [DOI] [PubMed] [Google Scholar]
- 26.Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554–558. doi: 10.1097/00000539-199903000-00017. [DOI] [PubMed] [Google Scholar]
- 27.A. Curtin, K. Izzetoglu, J. Reynolds et al., Functional near-infrared spectroscopy for the measurement of propofol effects in conscious sedation during outpatient elective colonoscopy, NeuroImage, vol. 85, pp. 626–636, 2014. 10.1016/j.neuroimage.2013.07.009. [DOI] [PubMed]
- 28.Hernandez-Meza G, Izzetoglu M, Osbakken M, Green M, Abubakar H, Izzetoglu K. Investigation of optical neuro-monitoring technique for detection of maintenance and emergence states during general anesthesia. J Clin Monit Comput. 2018;32(1):147–163. doi: 10.1007/s10877-017-9998-x. [DOI] [PubMed] [Google Scholar]
- 29.Hock C, Villringer K, Müller-Spahn F, Hofmann M, Schuh-Hofer S, Heekeren H, Wenzel R, Dirnagl U, Villringer A. Near infrared spectroscopy in the diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 1996;777:22–9. doi: 10.1111/j.1749-6632.1996.tb34397.x. [DOI] [PubMed] [Google Scholar]
- 30.Fallgatter AJ, Roesler M, Sitzmann L, Heidrich A, Mueller TJ, Strik WK. Loss of functional hemispheric asymmetry in Alzheimer's dementia assessed with near-infrared spectroscopy. Brain Res Cogn Brain Res. 1997;6(1):67–72. doi: 10.1016/S0926-6410(97)00016-5. [DOI] [PubMed] [Google Scholar]
- 31.Hock C, Müller-Spahn F, Schuh-Hofer S, Hofmann M, Dirnagl U, Villringer A. Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near-infrared spectroscopy study. J Cereb Blood Flow Metab. 1995;15(6):1103–8. doi: 10.1038/jcbfm.1995.137. [DOI] [PubMed] [Google Scholar]
- 32.Hoshi Y, Tamura M. Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man. Neurosci Lett. 1993;150:5e8. doi: 10.1016/0304-3940(93)90094-2. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939e44. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 34.Fladby T, Bryhn G, Halvorsen O, Rosé I, Wahlund M, Wiig P, et al. Olfactory response in the temporal cortex of the elderly measured with near-infrared spectroscopy: a preliminary feasibility study. J Cereb Blood Flow Metab. 2004;24:677e80. doi: 10.1097/01.WCB.0000119966.74298.5C. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann MJ, Langer JB, Jacob C, Ehlis AC, Fallgatter AJ. Reduced prefrontal oxygenation in Alzheimer disease during verbal fluency tasks. Am J Geriatr Psychiatry. 2008;16:125e35. doi: 10.1097/JGP.0b013e3180cc1fbc. [DOI] [PubMed] [Google Scholar]
- 36.Mega MS, Dinov ID, Lee L, O’Connor SM, Masterman DM, Wilen B, et al. Orbital and dorsolateral frontal perfusion defect associated with behavioral response to cholinesterase inhibitor therapy in Alzheimer’s disease. J Neuropsychiatr Clin Neurosci. 2000;12:209e18. doi: 10.1176/jnp.12.2.209. [DOI] [PubMed] [Google Scholar]
- 37.Arai H, Takano M, Miyakawa K, Ota T, Takahashi T, Asaka H, et al. A quantitative near-infrared spectroscopy study: a decrease in cerebral hemoglobin oxygenation in Alzheimer’s disease and mild cognitive impairment. Brain Cogn. 2006;61:189e94. doi: 10.1016/j.bandc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Zeller JB, Herrmann MJ, Ehlis AC, Polak T, Fallgatter AJ. Altered parietal brain oxygenation in Alzheimer’s disease as assessed with near-infrared spectroscopy. Am J Geriatr Psychiatry. 2010;18:433e41. doi: 10.1097/JGP.0b013e3181c65821. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe A, Kato T. Cerebrovascular response to cognitive tasks in patients with schizophrenia measured by near-infrared spectroscopy. Schizophr Bull. 2004;30:435–44. doi: 10.1093/oxfordjournals.schbul.a007090. [DOI] [PubMed] [Google Scholar]
- 40.Koike S, Takizawa R, Nishimura Y, Takano Y, Takayanagi Y, Kinou M, et al. Different hemodynamic response patterns in the prefrontal cortical sub-regions according to the clinical stages of psychosis. Schizophr Res. 2011;132:54–61. doi: 10.1016/j.schres.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Takizawa R, Kasai K, Kawakubo Y, Marumo K, Kawasaki S, Yamasue H, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multichannel near-infrared spectroscopy study. Schizophr Res. 2008;99:250–62. doi: 10.1016/j.schres.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Suto T, Fukuda M, Ito M, Uehara T, Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. BiolPsychiatry. 2004;55:501–11. doi: 10.1016/j.biopsych.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, et al.: Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage, 2013. 10.1016/j.neuroimage.2013.05.126 [DOI] [PubMed]
- 44.Fukuda M, Mikuni M. Clinical application of near-infrared spectroscopy (NIRS) in psychiatry: the advanced medical technology for differential diagnosis of depressive state. Seishin Shinkeigaku Zasshi. 2012;114:801–6. [PubMed] [Google Scholar]
- 45.Kameyama M, Fukuda M, Yamagishi Y, Sato T, Uehara T, Ito M, et al. Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. Neuroimage. 2006;29:172–84. doi: 10.1016/j.neuroimage.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Noda T, Yoshida S, Matsuda T, Okamoto N, Sakamoto K, Koseki S, et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatry Res. 2012;46:905–12. doi: 10.1016/j.jpsychires.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Ohta H, Yamagata B, Tomioka H, Takahashi T, Yano M, Nakagome K, et al. Hypofrontality in panic disorder and major depressive disorder assessed by multi-channel near-infrared spectroscopy. Depress Anxiety. 2008;25:1053–9. doi: 10.1002/da.20463. [DOI] [PubMed] [Google Scholar]
- 48.Pu S, Matsumura H, Yamada T, Ikezawa S, Mitani H, Adachi A, et al. Reduced frontopolar activation during verbal fluency task associated with poor social functioning in late-onset major depression: multi-channel near-infrared spectroscopy study. Psychiatry Clin Neurosci. 2008;62:728–37. doi: 10.1111/j.1440-1819.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- 49.Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Mitani H, et al. The relationship between the prefrontal activation during a verbal fluency task and stress-coping style in major depressive disorder: a near-infrared spectroscopy study. J Psychiatry Res. 2012;46:1427–34. doi: 10.1016/j.jpsychires.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Takizawa R, Kasai K, Kawakubo Y, Marumo K, Kawasaki S, Yamasue H, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. 2008;99:250–62. doi: 10.1016/j.schres.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 52.Quaresima V, Giosue P, Roncone R, Casacchia M, Ferrari M. Prefrontal cortex dysfunction during cognitive tests evidenced by functional near-infrared spectroscopy. Psychiatry Res. 2009;171:252–7. doi: 10.1016/j.pscychresns.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Ikezawa K, Iwase M, Ishii R, Azechi M, Canuet L, Ohi K, et al. Impaired regional hemodynamic response in schizophrenia during multiple prefrontal activation tasks: a two-channel near-infrared spectroscopy study. Schizophr Res. 2009;108:93–103. doi: 10.1016/j.schres.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Janes AC, Pizzagalli DA, Richardt S. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grüsser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 56.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 57.Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacol. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 58.Bunce SC, Bixler EO, Harris J, Meyer RE. A clinical laboratory model of allostasis of the brain reward system diurnal cortisol and sleep in recently detoxified opioid dependent patients, normal control subjects & patients drug free for 60-90 days. Neuropsychopharmacol. 2012;38:S444–S445. doi: 10.1007/978-3-642-39454-6_26. [DOI] [Google Scholar]
- 59.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bunce SC, Izzetoglu K, Izzetoglu M, Ayaz H, Pourrezaei K, Onaral B. Treatment status predicts differential prefrontal cortical responses to alcohol and natural reinforcer cues among alcohol dependent individuals. In: Zhang H, Hussain A, Liu D, Wang Z, editors. BICS 2012. LNCS. Heidelberg: Springer; 2012. pp. 183–191. [Google Scholar]
- 61.Hofmann J, Herrmann MJ, Martin DI, Obrig H, Conrad M, Kuchinke L, Jacobs AM, Fallgatter AJ. Differential activation of frontal and parietal regions during visual word recognition: an optical topography study. NeuroImage. 2008;40(3):1340–1349. doi: 10.1016/j.neuroimage.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 62.Coltheart M, Rastle K, Perry C, Langdon R, Ziegter J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- 63.Snowling MJ. Phonological processing and developmental dyslexia. J Res Read. 1995;18(2):132–138. doi: 10.1111/j.1467-9817.1995.tb00079.x. [DOI] [Google Scholar]
- 64.Sela I, Horowitzkraus T, Izzetoglu M, Shewokis P, Izzetoglu K, Onaral B, Breznitz Z. Brain activity of young and adult Hebrew speakers during Lexical Decision Task: FNIR application to language. In: Schmorrow D, Fidopiastis C, editors. Foundations of augmented cognition: Directing the future of adaptive systems. Berlin: Springer; 2011. pp. 231–239. [Google Scholar]
- 65.Bergmann J, Wimmer H. A dual-route perspective on poor reading in a regular orthography: evidence from phonological and orthographic lexical decisions. Cogn Neuropsychol. 2008;25(5):653–676. doi: 10.1080/02643290802221404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MATAL: Diagnosis of learning disabilities and attention disorders. The National Institute for Testing and Evaluations (NITE). Jerusalem, Israel, 2007
- 67.Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychol Bull. 2005;131(1):3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
- 68.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 69.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/ajp.2006.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blume F, Hudak J, Dresler T, Ehlis A-C, KuÈhnhausen J, Renner TJ, et al. NIRS-based neurofeedback training in a virtual reality classroom for children with attention-deficit/hyperactivity disorder: study protocol for a randomized controlled trial. Trials. 2017;18:41. doi: 10.1186/s13063-016-1769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marx A-M, Ehlis A-C, Furdea A, Holtmann M, Banaschewski T, Brandeis D, et al. Near-infrared spectroscopy (NIRS) neurofeedback as a treatment for children with attention deficit hyperactivity disorder (ADHD): a pilot study. Front Hum Neurosci. 2015;8:1–13. doi: 10.3389/fnhum.2014.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayer K, Wyckoff SN, Fallgatter AJ, Ehlis A-C, Strehl U. Neurofeedback as a nonpharmacological treatment for adults with attention-deficit/hyperactivity disorder (ADHD): study protocol for a randomized controlled trial. Trials. 2015;16:174. doi: 10.1186/s13063-015-0683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehlis A-C, Schneider S, Dresler T, Fallgatter AJ: Application of functional near-infrared spectroscopy in psychiatry. Neuroimage. Elsevier Inc. 85 Pt 1: 478-88, 2014. 10.1016/j.neuroimage.2013.03.067 [DOI] [PubMed]
- 74.Mehta RK, Parasuraman R, Mckinley A. Neuroscience A. Neuroergonomics. 2013;7:1–10. doi: 10.3389/fnhum.2013.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–731. doi: 10.1006/nimg.2002.1227. [DOI] [PubMed] [Google Scholar]
- 76.Radua J, Stoica T, Scheinost D, Pittenger C, Hampson M. Neural correlates of success and failure signals during neurofeedback learning. Neuroscience. 2018;378:11–21. doi: 10.1016/j.neuroscience.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruzelier JH. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci Biobehav Rev. 2014;44:124–41. doi: 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 78.Hudak J, Rosenbaum D, Barth B, Fallgatter AJ, Ehlis A-C. Functionally disconnected: a look at how study design influences neurofeedback data and mechanisms in attention-deficit/hyperactivity disorder. PLoS One. 2018;13(8):e0200931. doi: 10.1371/journal.pone.0200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dolu Nazan, Altınkaynak Miray, Güven Ayşegül, Özmen Sevgi, Demirci Esra, İzzetoğlu Meltem & Pektaş Ferhat: Effects of methylphenidate treatment in children with ADHD: a multimodal EEG/fNIRS approach. Psychiatry Clin Psychopharmacol, 2018. 10.1080/24750573.2018.1542779
- 80.Monden Y, Dan H, Nagashima M, et al. Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Clin Neurophysiol. 2012;123:1147–1157. doi: 10.1016/j.clinph.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Monden Y, Dan H, Nagashima M, et al. Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: An fNIRS study. Neuroimage Clin. 2012;1(1):131–140. doi: 10.1016/j.nicl.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lynch CL. Functional Electrical Stimulation. IEEE Control Syst Mag. 2008;28(2):40–50. doi: 10.1109/MCS.2007.914689. [DOI] [Google Scholar]
- 83.Palazzo P, Tibuzzi F, Pasqualetti P, Altamura C, Silvestrini M, Passarelli F, Rossini PM, Vernieri F. Is there a role of near-infrared spectroscopy in predicting the outcome of patients with carotid artery occlusion? J Neurol Sci. 2010;292:36–39. doi: 10.1016/j.jns.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 84.Vernieri F, Tibuzzi F, Pasqualetti P, Rosato N, Passarelli F, Rossini PM, Silvestrini M. Transcranial Doppler and near-infrared spectroscopy can evaluate the hemodynamic effect of carotid artery occlusion. Stroke. 2004;35:64–70. doi: 10.1161/01.STR.0000106486.26626.E2. [DOI] [PubMed] [Google Scholar]
- 85.Aries MJ, Coumou AD, Elting JW, van der Harst JJ, Kremer BP, Vroomen PC. Near infrared spectroscopy for the detection of desaturations in vulnerable ischemic brain tissue: a pilot study at the stroke unit bedside. Stroke. 2012;43:1134–1136. doi: 10.1161/STROKEAHA.111.636894. [DOI] [PubMed] [Google Scholar]
- 86.Pizza F, Biallas M, Kallweit U, Wolf M, Bassetti CL. Cerebral hemodynamic changes in stroke during sleep-disordered breathing. Stroke. 2012;43:1951–1953. doi: 10.1161/STROKEAHA.112.656298. [DOI] [PubMed] [Google Scholar]
- 87.Vasdekis SN, Tsivgoulis G, Athanasiadis D, Andrikopoulou A, Voumvourakis K, Lazaris AM, Stamboulis E. Cerebrovascular reactivity assessment in patients with carotid artery disease: a combined TCD and NIRS study. J Neuroimaging. 2012;22:261–265. doi: 10.1111/j.1552-6569.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 88.Murata Y, Sakatani K, Katayama Y, Fukaya C. Increase in focal concentration of deoxyhaemoglobin during neuronal activity in cerebral ischaemic patients. J Neurol Neurosurg Psychiatry. 2002;73:182–184. doi: 10.1136/jnnp.73.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murata Y, Sakatani K, Hoshino T, Fujiwara N, Kano T, Nakamura S, Katayama Y. Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and BOLD contrast functional MRI in stroke patients. Stroke. 2006;37:2514–2520. doi: 10.1161/01.STR.0000239698.50656.3b. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura S, Sakatani K, Kano T, Hoshino T, Fujiwara N, Murata Y, Katayama Y. Effects of revascularisation on evoked cerebral blood oxygenation responses in stroke patients. Adv Exp Med Biol. 2010;662:525–530. doi: 10.1007/978-1-4419-1241-1_76. [DOI] [PubMed] [Google Scholar]
- 91.Murata Y, Katayama Y, Oshima H, Kawamata T, Yamamoto T, Sakatani K, Suzuki S. Changes in cerebral blood oxygenation induced by deep brain stimulation: study by near-infrared spectroscopy (NIRS) Keio J Med. 2000;49(Suppl. 1):A61–A63. [PubMed] [Google Scholar]
- 92.Sakatani K, Katayama Y, Yamamoto T, Suzuki S. Changes in cerebral blood oxygenation of the frontal lobe induced by direct electrical stimulation of thalamus and globus pallidus: a near infrared spectroscopy study. J Neurol Neurosurg Psychiatry. 1999;67:769–773. doi: 10.1136/jnnp.67.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? Neuroimage. 2007;37:1338–1345. doi: 10.1016/j.neuroimage.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 94.Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34:2866–2870. doi: 10.1161/01.STR.0000100166.81077.8A. [DOI] [PubMed] [Google Scholar]
- 95.Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, Kubota K. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52:188–194. doi: 10.1002/ana.10274. [DOI] [PubMed] [Google Scholar]
- 96.Miyai I, Suzuki M, Hatakenaka M, Kubota K. Effect of body weight support on cortical activation during gait in patients with stroke. Exp Brain Res. 2006;169:85–91. doi: 10.1007/s00221-005-0123-x. [DOI] [PubMed] [Google Scholar]
- 97.Lin PY, Chen JJ, Lin SI: The cortical control of cycling exercise in stroke patients: an fNIRS study. Hum Brain Mapp, 2012. 10.1002/hbm.22072 [DOI] [PMC free article] [PubMed]
- 98.Arenth PM, Ricker JH, Schultheis MT. Applications of functional near-infrared spectroscopy (fNIRS) to neurorehabilitation of cognitive disabilities. Clin Neuropsychol. 2007;21:38–57. doi: 10.1080/13854040600878785. [DOI] [PubMed] [Google Scholar]
- 99.Obrig H, Steinbrink J. Non-invasive optical imaging of stroke. Philos Transact A Math Phys Eng Sci. 2011;369:4470–4494. doi: 10.1098/rsta.2011.0252. [DOI] [PubMed] [Google Scholar]
- 100.Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, Okibayashi M, Danjo N, Ishikawa A, Inoue Y, Kubota K. Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLoS One. 2012;7:e32234. doi: 10.1371/journal.pone.0032234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fazli S, Mehnert J, Steinbrink J, Curio G, Villringer A, Muller KR, Blankertz B. Enhanced performance by a hybrid NIRS–EEG brain computer interface. Neuroimage. 2012;59:519–529. doi: 10.1016/j.neuroimage.2011.07.084. [DOI] [PubMed] [Google Scholar]
- 102.Atsumori H, Kiguchi M, Obata A, Sato H, Katura T, Funane T, Maki A. Development of wearable optical topography system for mapping the prefrontal cortex activation. Rev Sci Instrum. 2009;80:043704. doi: 10.1063/1.3115207. [DOI] [PubMed] [Google Scholar]
- 103.Lareau E, Lesage F, Pouliot P, Nguyen D, le Lan J, Sawan M. Multichannel wearable system dedicated for simultaneous electroencephalography near-infrared spectroscopy real-time data acquisitions. J Biomed Opt. 2011;16:096014. doi: 10.1117/1.3625575. [DOI] [PubMed] [Google Scholar]
- 104.Jung CE, Strother L, Feil-Seifer DJ, Hutsler JJ. Atypical asymmetry for processing human and robot faces in autism revealed by fNIRS. PLoS One. 2016;11(7):1–13. doi: 10.1371/journal.pone.0158804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Yu D. Variations of the functional brain network efficiency in a young clinical sample within the autism spectrum: a fNIRS investigation. Front Physiol. 2018;9:67. doi: 10.3389/fphys.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siddiqui MF, Lloyd-Fox S, Kaynezhad P, Tachtsidis I, Johnson MH, Elwell CE. Non-invasive measurement of a metabolic marker of infant brain function. Sci Rep. 2017;7:1330. doi: 10.1038/s41598-017-01394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gervain J. Near-infrared spectroscopy: recent advances in infant speech perception and language acquisition research. Front Psychol. 2014;5:1–2. doi: 10.3389/fpsyg.2014.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gervain J, Mehler J, Werker JF, Nelson CA, Csibra G, Lloyd-Fox S, Shukla M, Aslin RN: Near-infrared spectroscopy: a report from the McDonnell infant methodology consortium. Dev Cogn Neurosci, 2011 10.1016/j.dcn.2010.07.004 [DOI] [PMC free article] [PubMed]
- 109.Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Wilcox T, Biondi M. fNIRS in the developmental sciences. Wiley Interdiscip Rev Cogn Sci. 2015;6:263–283. doi: 10.1002/wcs.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bölte S, Marschik PB, Falck-Ytter T, Charman T, Roeyers H, Elsabbagh M. Infants at risk for autism: a European perspective on current status, challenges and opportunities. Eur Child Adolesc Psychiatry. 2013;22:341–348. doi: 10.1007/s00787-012-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ernst LH, Schneider S, Ehlis AC, Fallgatter AJ. Functional near infrared spectroscopy in psychiatry: a critical review. J Near Infrared Spectrosc. 2012;20:93–105. doi: 10.1255/jnirs.970. [DOI] [Google Scholar]
- 114.Iwanaga R, Tanaka G, Nakane H, Honda S, Imamura A, Ozawa H. Usefulness of near-infrared spectroscopy to detect brain dysfunction in children with autism spectrum disorder when inferring the mental state of others. Psychiatry Clin Neurosci. 2013;67:203–209. doi: 10.1111/pcn.12052. [DOI] [PubMed] [Google Scholar]
- 115.Zhu H, Fan Y, Guo H, Huang D, He S. Reduced interhemispheric functional connectivity of children with autism spectrum disorder: evidence from functional near infrared spectroscopy studies. Biomed Opt Express. 2014;5:1262. doi: 10.1364/BOE.5.001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wijeakumar S, Spencer JP, Bohache K, Boas DA, Magnotta VA. Validating a new methodology for optical probe design and image registration in fNIRS studies. NeuroImage. 2015;106:86–100. doi: 10.1016/j.neuroimage.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baik SY, Kim JY, Choi J, et al. Prefrontal asymmetry during cognitive tasks and its relationship with suicide ideation in major depressive disorder: an fNIRS study. Diagnostics (Basel) 2019;9(4):193. doi: 10.3390/diagnostics9040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Jiangxue, Zhu Huilin, Li, Xinge, Peng, Hongjun, Xu Jie, Cai Tingting & He Sailing: Spontaneous hemodynamic activity in prefrontal cortex of depression patients assessed with functional near-infrared spectroscopy, 2015
- 119.Anna M, Theodore JH, Erin R, Holly AS, Mary LP. The role of the right prefrontal cortex in recognition of facial emotional expressions in depressed individuals: fNIRS study. J Affect Disord. 2019;258:151–158. doi: 10.1016/j.jad.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishizawa Y, Kanazawa T, Kawabata Y, Matsubara T, Maruyama S, Kawano M, Kinoshita S, Koh J, Matsuo K, Yoneda H. fNIRS assessment during an emotional stroop task among patients with depression: replication and extension. Psychiatry Investig. 2019;16(1):80–86. doi: 10.30773/pi.2018.11.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schecklmann M, Dresler T, Beck S, et al. Reduced prefrontal oxygenation during object and spatial visual working memory in unipolar and bipolar depression. Psychiatry Res. 2011;194(3):378–384. doi: 10.1016/j.pscychresns.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 122.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 123.Villringer A, Planck J, Stodieck S, Botzel K, Schleinkofer L, Dirnagl U. Noninvasive assessment of cerebral hemodynamics and tissue oxygenation during activation of brain cell function in human adults using near infrared spectroscopy. Adv Exp Med Biol. 1994;345:559–565. doi: 10.1007/978-1-4615-2468-7_74. [DOI] [PubMed] [Google Scholar]
- 124.Adelson PD, Nemoto E, Scheuer M, Painter M, Morgan J, Yonas H. Noninvasive continuous monitoring of cerebral oxygenation periictally using near-infrared spectroscopy: a preliminary report. Epilepsia. 1999;40:1484–1489. doi: 10.1111/j.1528-1157.1999.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 125.Saito S, Yoshikawa D, Nishihara F, Morita T, Kitani Y, Amaya T, Fujita T. The cerebral hemodynamic response to electrically induced seizures in man. Brain Res. 1995;673:93–100. doi: 10.1016/0006-8993(94)01408-A. [DOI] [PubMed] [Google Scholar]
- 126.Watanabe E, Nagahori Y, Mayanagi Y. Focus diagnosis of epilepsy using near-infrared spectroscopy. Epilepsia. 2002;43(Suppl. 9):50–55. doi: 10.1046/j.1528-1157.43.s.9.12.x. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen DK, Tremblay J, Pouliot P, Vannasing P, Florea O, Carmant L, Lepore F, Sawan M, Lesage F, Lassonde M: Noninvasive continuous functional near-infrared spectroscopy combined with electroencephalography recording of frontal lobe seizures. Epilepsia, 2012b. 10.1111/epi.12011 [DOI] [PubMed]
- 128.Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008;39:1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 129.Slone E, Westwood E, Dhaliwal H, Federico P, Dunn JF. Near-infrared spectroscopy shows preictal haemodynamic changes in temporal lobe epilepsy. Epileptic Disord. 2012;14:371–378. doi: 10.1684/epd.2012.0535. [DOI] [PubMed] [Google Scholar]
- 130.Watanabe E, Maki A, Kawaguchi F, Takashiro K, Yamashita Y, Koizumi H, Mayanagi Y. Non-invasive assessment of language dominance with near-infrared spectroscopic mapping. Neurosci Lett. 1998;256:49–52. doi: 10.1016/S0304-3940(98)00754-X. [DOI] [PubMed] [Google Scholar]
- 131.Watson NF, Dodrill C, Farrell D, Holmes MD, Miller JW. Determination of language dominance with near-infrared spectroscopy: comparison with the intracarotid amobarbital procedure. Seizure. 2004;13:399–402. doi: 10.1016/j.seizure.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 132.Gallagher A, Bastien D, Pelletier I, Vannasing P, Legatt AD, Moshe SL, Jehle R, Carmant L, Lepore F, Beland R, Lassonde M. A noninvasive, presurgical expressive and receptive language investigation in a 9-year-old epileptic boy using near-infrared spectroscopy. Epilepsy Behav. 2008;12:340–346. doi: 10.1016/j.yebeh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 133.Gallagher A, Beland R, Lassonde M. The contribution of functional near-infrared spectroscopy (fNIRS) to the presurgical assessment of language function in children. Brain Lang. 2012;121:124–129. doi: 10.1016/j.bandl.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 134.Knecht S, Deppe M, Ebner A, Henningsen H, Huber T, Jokeit H, Ringelstein EB. Noninvasive determination of language lateralization by functional transcranial Doppler sonography: a comparison with the Wada test. Stroke. 1998;29:82–86. doi: 10.1161/01.STR.29.1.82. [DOI] [PubMed] [Google Scholar]
- 135.Gallagher A, Lassonde M, Bastien D, Vannasing P, Lesage F, Grova C, Bouthillier A, Carmant L, Lepore F, Beland R, Nguyen DK. Non-invasive presurgical investigation of a 10 year-old epileptic boy using simultaneous EEG NIRS. Seizure. 2008;17:576–582. doi: 10.1016/j.seizure.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 136.Machado A, Lina JM, Tremblay J, Lassonde M, Nguyen DK, Lesage F, Grova C. Detection of hemodynamic responses to epileptic activity using simultaneous electroencephalography (EEG) / near infrared spectroscopy (NIRS) acquisitions. Neuroimage. 2011;56:114–125. doi: 10.1016/j.neuroimage.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 137.Chen WT, Wang SJ, Fuh JL, Lin CP, Ko YC, Lin YY. Persistent ictal-like visual cortical excitability in chronic migraine. Pain. 2011;152:254–258. doi: 10.1016/j.pain.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 138.Tietjen GE. Migraine as a systemic vasculopathy. Cephalalgia. 2009;29:987–996. doi: 10.1111/j.1468-2982.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 139.Laurell K, Artto V, Bendtsen L, Hagen K, Kallela M, Meyer EL, Putaala J, Tronvik E, Zwart JA, Linde M. Migrainous infarction: a Nordic multicenter study. Eur J Neurol. 2011;18:1220–1226. doi: 10.1111/j.1468-1331.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- 140.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. 2005;128:2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 141.Akin A, Bilensoy D, Emir UE, Gulsoy M, Candansayar S, Bolay H. Cerebrovascular dynamics in patients with migraine: near-infrared spectroscopy study. Neurosci Lett. 2006;400:86–91. doi: 10.1016/j.neulet.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 142.Liboni W, Molinari F, Allais G, Mana O, Negri E, Grippi G, Benedetto C, D'Andrea G, Bussone G. Why do we need NIRS in migraine? Neurol. Sci. 2007;28(Suppl. 2):S222–S224. doi: 10.1007/s10072-007-0782-4. [DOI] [PubMed] [Google Scholar]
- 143.Shinoura N, Yamada R. Decreased vasoreactivity to right cerebral hemisphere pressure in migraine without aura: a near-infrared spectroscopy study. Clin Neurophysiol. 2005;116:1280–1285. doi: 10.1016/j.clinph.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 144.Wolf T, Lindauer U, Obrig H, Dreier J, Back T, Villringer A, Dirnagl U. Systemic nitric oxide synthase inhibition does not affect brain oxygenation during cortical spreading depression in rats: a noninvasive near-infrared spectroscopy and laser-Doppler flowmetry study. J Cereb Blood Flow Metab. 1996;16:1100–1107. doi: 10.1097/00004647-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 145.Viola S, Viola P, Litterio P, Buongarzone MP, Fiorelli L. Pathophysiology of migraine attack with prolonged aura revealed by transcranial Doppler and near infrared spectroscopy. Neurol Sci. 2010;31(Suppl. 1):S165–S166. doi: 10.1007/s10072-010-0318-1. [DOI] [PubMed] [Google Scholar]
- 146.Liboni W, Molinari F, Allais GB, Mana O, Negri E, D'Andrea G, Bussone G, Benedetto C. Patent foramen ovale detected by near-infrared spectroscopy in patients suffering from migraine with aura. Neurol Sci. 2008;29(Suppl. 1):S182–S185. doi: 10.1007/s10072-008-0920-7. [DOI] [PubMed] [Google Scholar]
- 147.Viola S, Viola P, Litterio P, Buongarzone MP, Fiorelli L. Stroke risk and migraine: near-infrared spectroscopy study. Neurol Sci. 2012;33(Suppl. 1):S173–S175. doi: 10.1007/s10072-012-1077-y. [DOI] [PubMed] [Google Scholar]
- 148.Watanabe Y, Tanaka H, Dan I, Sakurai K, Kimoto K, Takashima R, Hirata K. Monitoring cortical hemodynamic changes after sumatriptan injection during migraine attack by near-infrared spectroscopy. Neurosci Res. 2011;69:60–66. doi: 10.1016/j.neures.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 149.Griffin M, Prior D, Cooper CE, Wilkins AJ, Elwell CE. Near infrared spectroscopy as a noninvasive assessment of cortical abnormality in migraine? Adv Exp Med Biol. 2006;578:203–208. doi: 10.1007/0-387-29540-2_33. [DOI] [PubMed] [Google Scholar]
- 150.Tisdall MM, Tachtsidis I, Leung TS, Elwell CE, Smith M. Near-infrared spectroscopic quantification of changes in the concentration of oxidized cytochrome c oxidase in the healthy human brain during hypoxemia. J Biomed Opt. 2007;12:024002. doi: 10.1117/1.2718541. [DOI] [PubMed] [Google Scholar]
- 151.Al-Rawi PG, Kirkpatrick PJ. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke. 2006;37:2720–2725. doi: 10.1161/01.STR.0000244807.99073.ae. [DOI] [PubMed] [Google Scholar]
- 152.Pocivalnik M, Pichler G, Zotter H, Tax N, Muller W, Urlesberger B. Regional tissue oxygen saturation: comparability and reproducibility of different devices. J Biomed Opt. 2011;16:057004. doi: 10.1117/1.3575647. [DOI] [PubMed] [Google Scholar]
- 153.Pennekamp CW, Bots ML, Kappelle LJ, Moll FL, de Borst GJ. The value of near-infrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention. A review. Eur J Vasc Endovasc Surg. 2009;38:539–545. doi: 10.1016/j.ejvs.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 154.Vohra HA, Modi A, Ohri SK. Does use of intra-operative cerebral regional oxygen saturation monitoring during cardiac surgery lead to improved clinical outcomes? Interact Cardiovasc Thorac Surg. 2009;9:318–322. doi: 10.1510/icvts.2009.206367. [DOI] [PubMed] [Google Scholar]
- 155.Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW: Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg, 2012 10.1213/ANE.0b013e318277a255 [DOI] [PMC free article] [PubMed]
- 156.Smith M. Shedding light on the adult brain: a review of the clinical applications of near-infrared spectroscopy. Philos Transact A Math Phys Eng Sci. 2011;369:4452–4469. doi: 10.1098/rsta.2011.0242. [DOI] [PubMed] [Google Scholar]