Abstract
Purpose
Analyzing effectiveness and cost-effectiveness of voriconazole versus fluconazole prophylaxis in hematopoietic stem cell transplantation (HSCT).
Methods
The research included 70 patients; 34 undergoing allogeneic HSCT and 36 undergoing autologous stem cell transplantation (ASCT), alternated to receive either voriconazole or fluconazole prophylaxis for 180 days on a 1:1 basis. Patients were monitored for occurrence of invasive fungal infections (IFI), IFI-related death (IRD) and total death events. Cost-effectiveness of both agents in both groups was also assessed.
Results
Antifungal prophylactic drug had no impact on incidence of IFI and IRD in both allogeneic HSCT and ASCT (P = .452 and P = 1.000; P = .457 and P = .146 respectively). An insignificant difference occurred among patients receiving voriconazole or fluconazole regarding overall survival (OS) and fungal infection-free survival (FFS) in both groups (P = .705 and P = .879; P = .713 and P = .681 respectively). Regarding cost-effectiveness, voriconazole dominated fluconazole regarding prevention of IFI and IRD but was less costly/less effective regarding prevention of total death events and gaining life years in the allogeneic HSCT setting. In the ASCT setting, voriconazole was not cost-effective regarding avoidance of IFI and IRD and was dominated by fluconazole regarding avoidance of total death events and gaining life years.
Conclusions
Voriconazole does not differ from fluconazole regarding its efficacy in prevention of IFI and IRD and does not improve OS and FFS in both allogeneic HSCT and ASCT settings. Voriconazole is cost-effective regarding protection from IFI and IRD in allogeneic HSCT but not cost-effective in ASCT.
Electronic supplementary material
The online version of this article (10.1007/s12288-020-01259-y) contains supplementary material, which is available to authorized users.
Keywords: Hematopoietic stem cell transplantation, Antifungal prophylaxis, Invasive fungal infection, Invasive fungal infection-related death, Cost-effectiveness
Introduction
The incidence of invasive fungal infections (IFI) has been rising during the last decades because of the increasing number of patients at risk and the widespread use of transplantation in clinical practice [1]. IFI have become a major determinent of morbidity and mortality in hematopoietic stem cell transplantation (HSCT) recipients [2]. The most prevalent IFI in HSCT patients is invasive aspergillosis (IA) (incidence = 11–14% at 1 year) [3]. Although newer drugs may have nowadays improved outcome, death from IA after allogeneic HSCT is still high (67–87%) [4].
As IFIs are difficult to diagnose and treat early, concerns were focused on prophylaxis [5]. However, the most suitable antifungal prophylactic drug is still controversial and identification of more efficient agent with acceptable toxicity profile is needed. Invasive candidiasis is effectively avoided following engraftment by use of fluconazole, but it lacks activity against Aspergillus [6]. Itraconazole which is a broad-spectrum azole with activity against filamentous fungi has shown activity against Aspergillus. Nevertheless, itraconazole tablets have variable bioavailability and the suspension is poorly tolerated which in turn restricted its administration in antifungal prophylaxis [7]. Posaconazole which is a second-generation triazole has effectiveness against moulds and has shown effectiveness in allogeneic HSCT as a prophylactic agent [8]. Voriconazole, another second-generation broad-spectrum triazole, also has shown effect against yeasts and moulds but not zygomycetes [9]. However, itraconazole, posaconazole and voriconazole are stronger inhibitors of the hepatic cytochrome P450 3A4 (CYP 3A4) than fluconazole and may widely interact with many drugs used in HSCT [10].
As the economic burden of IFI is high, the cost-effectiveness of prophylactic agents should be evaluated to determine which one is optimum choice for restricted healthcare resources [11]. Nevertheless, due to the wide differences in patient features, underlying diseases, hospital policies and research methods as well as the rarity of head to head comparative studies, it is hard to determine the economic previlages of any prophylactic drug [11]. Hence, we compared, head to head, two antifungal drugs available in oral and parenteral forms and with high bioavailability (> 90%) when administered orally (voriconazole and fluconazole) to evaluate their efficacy in preventing IFI and IFI-related death (IRD) in HSCT recipients and also to assess their cost-effectiveness.
Materials and Methods
Study Design
This research was an open-label prospective one and involved 70 subjects performing HSCT at our transplant center during the period 1/2016–12/2017; 34 undergoing allogeneic HSCT and 36 undergoing autologous stem cell transplantation (ASCT). Patients in each transplantation group were alternated to receive either voriconazole (loading dose = 400 mg BID PO or 6 mg/kg BID IV for two doses ensued by maintenance dose = 200 mg BID PO or 4 mg/kg BID IV) or fluconazole (dose = 400 mg once daily PO or IV) prophylaxis on a one to one basis and in consecutive order for 180 days [2]. Parenteral forms were used only if severe gastrointestinal mucositis interfering with oral administration developed. Patients younger than 16 years, those with previous history of IFI, those with moderate to severe hepatic compromise and those with psychological disorders were excluded from the study. Comorbidities were graded according to the hematopoietic cell transplantation comorbidity index (HCT-CI) [12]. Patients receiving grafts from matched related donors administered ciclosporin and methotrexate for prevention of graft versus host disease (GVHD) whereas those receiving grafts from haploidentical donors administered post-transplant cyclophosphamide and ciclosporin.
Monitoring for Occurrence of IFI and Its Treatment
The patients have been followed up for occurrence of IFI, IRD and total death events during the first 180 days following HSCT which is the time needed for quantitative T cell recovery post-HSCT and for completion of immunosuppressive medication withdrawal in allogeneic HSCT setting [13]. IFI that occurred following day 180 were excluded from the analysis. European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) Consensus Group revised definitions published in 2008 were used for defining the diagnosis of IFI clinically and by laboratory and radiological investigations [14]. In brief, possible IFI included only those cases with clinical evidence consistent with IFI with absence of laboratory and radiological mycological support. Probable IFI needed the cooccurrence of clinical criteria and laboratory and/or radiological mycological support. Diagnosis of proven IFI needed proof of IFI by finding fungal elements in involved tissue. Antifungal prophylaxis was replaced by antifungal treatment in case of occurrence of fungal infection necessitating treatment and prophylaxis was resumed by the same prophylactic drug after cure from IFI. Antifungal treatment consisted of amphotericin B in case of possible IFI, amphotericin B in case of probable IFI other than probable IA, parenteral voriconazole in case of probable IA, amphotericin B in case of proven mucormycosis (MM), parenteral voriconazole in case of proven IA and antifungal agent based on results of fungal culture and sensitivity in case of proven MM and IA infections resistant to amphotericin B and voriconazole respectively. A clear definition of IRD is lacking and, hence, it was defined in our study as death in patients with probable or proven IFI which can not be attributed to other HSCT complications e.g. bacterial infections, cytomegalovirus (CMV), GVHD, disease relapse, organ toxicity, … etc. [15].
Estimation Of Cost-Effectiveness
Voriconazole was considered the dominant prophylactic treatment when it lead to favorable effects while reducing costs. When voriconazole lead to unfavorable effects while increasing costs, it was considered to be dominated by fluconazole. When both costs and effects were elevated, the incremental cost-effectiveness ratio (ICER) was assessed by the formula:
where Cv is the mean cost in voriconazole arm, Cf is the mean cost in fluconazole arm, Ev is the effectiveness of voriconazole and Ef is the effectiveness of fluconazole. Accordingly, when the incremental costs per additional unit of effect for voriconazole were below a willingness-to-pay threshold (determined by our center at the Egyptian gross domestic product (GDP) per capita in year 2016 which was 62,558 Egyptian Pounds (EGP) obtained from the website of the Central Agency for Public Mobilization and Statistics of Egypt), it was defined as cost-effective [16]. On the other hand, when they were above the threshold, it was not considered cost-effective. The cost included the cost of prophylaxis till day 180 post-transplant, the cost of preemptive fungal infection treatment, the cost of management of prophylactic drug side effects including use of alternative agent cost, the cost of IFI management and the cost of late hospitalization because of IFI. The cost did not involve the cost of managing the underlying disease or the cost of managing other HSCT complications. The prices of drugs, investigations and hospitalization were obtained from the price list of year 2016/2017 of our center. Effectiveness was defined as efficacy of either drug in preventing IFI, IRD and total death events and in gainng life years.
End Points and Statistical Methods
Overall survival (OS) was defined as the time interval between stem cell transfusion and death whatever the cause. Fungal infection-free survival (FFS) was defined as time interval between stem cell transfusion and occurrence of IFI or death whatever the cause whichever came first. Life years gained (LYG) were calculated by measuring the area between the survival curves using Kaplan Meier method. Descriptive statistical analysis of patient characteristics was performed (mean, standard deviation, range, number and percentage). Comparisons between groups have been performed using unpaired t test and Chi-square test for continuous variables and for categorical variables respectively. Survival probabilities were analyzed by the Kaplan Meier method and survival curves were compared by the log-rank test. Statistical signifcance was defined at the 0.05 level. All P values were 2-sided. Standard computer program SPSS for Windows, version 20.0 (SPSS Inc, USA), was used for data entry and statistical analysis.
Results
Patient Characteristics
Patient characteristics are illustrated in Table 1. Patients were well balanced regarding their characteristics.
Table 1.
Comparison of patient characteristics in voriconazole and fluconazole prophylactic arms in allogeneic HSCT and ASCT settings
| Variable | Allogeneic HSCT | ASCT | ||||
|---|---|---|---|---|---|---|
| Voriconazole (N = 17) | Fluconazole (N = 17) | P | Voriconazole (N = 18) | Fluconazole (N = 18) | P | |
| Mean age ± SD, years | 26.9 ± 6.2 | 30.2 ± 12 | 0.321 | 45.5 ± 12.5 | 40.9 ± 14.1 | 0.313 |
| Sex | ||||||
| Male | 13 (76.5%) | 8 (47.1%) | 0.078 | 10 (55.6%) | 6 (33.3%) | 0.180 |
| Female | 4 (23.5%) | 9 (52.9%) | 8 (44.4%) | 12 (66.7%) | ||
| HCT-CI | ||||||
| 0 | 13 (76.5%) | 14 (82.4%) | 0.427 | 14 (77.8%) | 16 (88.9%) | 0.567 |
| 1 | 4 (23.5%) | 2 (11.8%) | 3 (16.7%) | 1 (5.6%) | ||
| 2 | 0 (0%) | 1 (5.9%) | 1 (5.6%) | 1 (5.6%) | ||
| Diagnosis | ||||||
| AL | 12 (70.6%) | 7 (41.2%) | 0.371 | 0 (0%) | 0 (0%) | 0.317 |
| BM failure | 2 (11.8%) | 5 (29.4%) | 0 (0%) | 0 (0%) | ||
| MPN/MDS | 2 (11.8%) | 3 (17.6%) | 0 (0%) | 0 (0%) | ||
| R/R lymphoma | 1 (5.9%) | 2 (11.8%) | 8 (44.4%) | 11 (61.1%) | ||
| MM/PCL | 0 (0%) | 0 (0%) | 10 (55.6%) | 7 (38.9%) | ||
| TBI-based conditioning | ||||||
| +ve | 5 (29.4%) | 2 (11.8%) | 0.203 | 0 (0%) | 0 (0%) | * |
| −ve | 12 (70.6%) | 15 (88.2%) | 18 (100%) | 18 (100%) | ||
| RIC | ||||||
| +ve | 5 (29.4%) | 4 (23.5%) | 0.697 | 0 (0%) | 0 (0%) | * |
| −ve | 12 (70.6%) | 13 (76.5%) | 18 (100%) | 18 (100%) | ||
| ATG use | ||||||
| +ve | 5 (29.4%) | 2 (11.8%) | 0.203 | 0 (0%) | 0 (0%) | * |
| −ve | 12 (70.6%) | 15 (88.2%) | 18 (100%) | 18 (100%) | ||
| Steroid use | ||||||
| +ve | 9 (52.9%) | 8 (47.1%) | 0.732 | 7 (38.9%) | 4 (22.2%) | 0.278 |
| −ve | 8 (47.1%) | 9 (52.9%) | 11 (61.1%) | 14 (77.8%) | ||
| Donor | ||||||
| ASCT | 0 (0%) | 0 (0%) | 0.070 | 18 (100%) | 18 (100%) | * |
| MRD | 14 (82.4%) | 17 (100%) | 0 (0%) | 0 (0%) | ||
| Haploidentical | 3 (17.6%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Graft failure | ||||||
| +ve | 4 (23.5%) | 3 (17.6%) | 0.671 | 0 (0%) | 2 (11.1%) | 0.146 |
| −ve | 13 (76.5%) | 14 (82.4%) | 18 (100%) | 16 (88.9%) | ||
| Grade II–IV GVHD | ||||||
| +ve | 4 (23.5%) | 6 (35.3%) | 0.452 | 0 (0%) | 0 (0%) | * |
| −ve | 13 (76.5%) | 11 (64.7%) | 18 (100%) | 18 (100%) | ||
HSCT hematopoietic stem-cell transplantation, ASCT autologous stem cell transplantation, N number, SD standard deviation, HCT-CI hematopoietic cell transplantation comorbidity index, AL acute leukemia, BM bone marrow, MPN myeloproliferative neoplasm, MDS myelodysplastic syndrome, R/R relapsed/refractory, MM multiple myeloma, PCL plasma cell leukemia, TBI total body irradiation, RIC reduced intensity conditioning, ATG antithymocyte globulin, MRD matched related donor, GVHD graft versus host disease
*No statistics were computed because all cases were censored
Comparison of Antifungal Prophylactic Agents Regarding IFI and IRD Events
IFI occurred in 10 patients (29.4%) in the allogeneic HSCT group (3 possible, 4 probable, 3 proven) and in 10 patients (27.8%) in the ASCT group (4 possible, 6 probable). There was no difference between antifungal prophylactic agents used regarding IFI events, need for preemptive antifungal treatment, incidence of side effects necessitating change of prophylactic antifungal agent, need for late hospitalization because of IFI, IRD events, types of probable/proven IFI, sites of probable/proven IFI and mean number of hospitalization days whether in the allogeneic HSCT or ASCT groups (Table 2). Also, IFI and IRD events did not differ between voriconazole and fluconazole arms in allogeneic HSCT and ASCT patients when categorized according to age, gender, HCT-CI, diagnosis, total body irradiation-containing conditioning regimen, antithymocyte globulin administration, steroid administration, type of donor, graft failure occurrence and grade II–IV GVHD occurrence (as shown in Online Resource 1). In the allogeneic HSCT setting, mortality in voriconazole arm was due to bacterial septicemia (number (N) = 2), fungal pneumonia (N = 2), GVHD (N = 1), disease recurrence (N = 1) and CMV encephalitis (N = 1); whereas in fluconazole arm it was due to bacterial septicemia (N = 2), MM (N = 2), GVHD (N = 1) and sinusoidal obstruction syndrome (N = 1). In the ASCT setting, causes of death were bacterial septicemia (N = 2), disease relapse (N = 1) and cardiac arrythmia (N = 1) in the voriconazole arm; whereas in fluconazole arm, mortality was due to fungal pneumonia (N = 2) and bacterial septicemia (N = 1). Side effects necessitating change of prophylactic antifungal agent were visual hallucinations (N = 1) in the voriconazole group and hepatotoxicity (N = 3) in the fluconazole group; all were confined to ASCT recipients.
Table 2.
Comparison of voriconazole and fluconazole prophylaxis in allogeneic HSCT and ASCT settings
| Variable | Antifungal prophylaxis | |||
|---|---|---|---|---|
| N | Voriconazole | Fluconazole | P | |
| IFI in Allogeneic HSCT | ||||
| +ve | 10 | 4 (40%) | 6 (60%) | 0.452 |
| −ve | 4 | 13 (54.2%) | 11 (45.8%) | |
| IFI in ASCT | ||||
| +ve | 10 | 4 (40%) | 6 (60%) | 0.457 |
| −ve | 26 | 14 (53.8%) | 12 (46.2%) | |
| Need for preemptive antifungal treatment in allogeneic HSCT | ||||
| +ve | 15 | 7 (46.7%) | 8 (53.3%) | 0.730 |
| −ve | 19 | 10 (52.6%) | 9 (47.4%) | |
| Need for preemptive antifungal treatment in ASCT | ||||
| +ve | 10 | 5 (50%) | 5 (50%) | 1.000 |
| −ve | 26 | 13 (50%) | 13 (50%) | |
| Replacement of antifungal agent by another one because of side effects in ASCT | ||||
| +ve | 4 | 1 (25%) | 3 (75%) | 0.289 |
| −ve | 32 | 17 (53.1%) | 15 (46.9%) | |
| IFI requiring late hospitalization in allogeneic HSCT | ||||
| +ve | 4 | 1 (25%) | 3 (75%) | 0.287 |
| −ve | 30 | 16 (53.3%) | 14 (46.7%) | |
| IFI requiring late hospitalization in ASCT | ||||
| +ve | 2 | 0 (0%) | 2 (100%) | 0.146 |
| −ve | 34 | 18 (52.9%) | 16 (47.1%) | |
| IRD in allogeneic HSCT | ||||
| +ve | 4 | 2 (50%) | 2 (50%) | 1.000 |
| −ve | 30 | 15 (50%) | 15 (50%) | |
| IRD in ASCT | ||||
| +ve | 2 | 0 (0%) | 2 (100%) | 0.146 |
| −ve | 34 | 18 (52.9%) | 16 (47.1%) | |
| Type of probable/proven IFI in allogeneic HSCT | ||||
| −ve | 27 | 15 (55.6%) | 12 (44.4%) | 0.282 |
| MM | 2 | 0 (0%) | 2 (100%) | |
| IA | 5 | 2 (40%) | 3 (60%) | |
| IC | 0 | 0 (0%) | 0 (0%) | |
| Type of probable/proven IFI in ASCT | ||||
| −ve | 30 | 15 (50%) | 15 (50%) | 1.000 |
| MM | 0 | 0 (0%) | 0 (0%) | |
| IA | 6 | 3 (50%) | 3 (50%) | |
| IC | 0 | 0 (0%) | 0 (0%) | |
| Sites of probable/proven IFI in allogeneic HSCT | ||||
| −ve | 27 | 15 (55.6%) | 12 (44.4%) | 0.282 |
| PNS | 2 | 0 (0%) | 2 (100%) | |
| Pulmonary | 5 | 2 (40%) | 3 (60%) | |
| Sites of probable/proven IFI in ASCT | ||||
| −ve | 30 | 15 (50%) | 15 (50%) | 1.000 |
| Pulmonary | 6 | 3 (50%) | 3 (50%) | |
| Mean duration of hospitalization in allogeneic HSCT ± SD, days (range) | 36.2 ± 16.5 (21–87) | 36.9 ± 19.8 (17–81) | 0.903 | |
| Mean duration of hospitalization in ASCT ± SD, days (range) | 27.3 ± 11.2 (16–53) | 31.3 ± 27.4 (12–109) | 0.570 | |
IFI invasive fungal infection, HSCT hematopoietic stem cell transplantation, ASCT autologous stem cell transplantation, IRD IFI-related death, MM mucormycosis, IA invasive aspergillosis, IC invasive candidiasis, PNS paranasal sinuses, SD standard deviation, N number
Impact Of Antifungal Prophylactic Agent Used On Transplant Outcome
Non-significant difference occurred among patients receiving voriconazole and those receiving fluconazole as regard OS in the allogeneic HSCT group (OS = 58.8% vs. 64.7%; mean survival = 140.3 days vs. 142.4 days respectively; P = 0.705) (Fig. 1a). Furthermore, non-significant difference occurred among patients receiving voriconazole and those receiving fluconazole regarding OS in the ASCT group (OS = 77.8% vs. 83.3%; mean survival = 160.3 days vs. 164.9 days respectively; P = 0.713) (Fig. 1b). Non-significant difference occurred among patients receiving voriconazole and those receiving fluconazole regarding FFS in the allogeneic HSCT group (FFS = 52.9% vs. 52.9%; mean survival = 137.2 days vs. 123 days respectively; P = 0.879) (Fig. 2a). Additionally, non-significant difference occurred among patients administering voriconazole and those administering fluconazole regarding FFS in the ASCT group (FFS = 55.6% vs. 61.1; mean survival = 128.4 days vs. 132.7 days respectively; P = 0.681) (Fig. 2b).
Fig. 1.
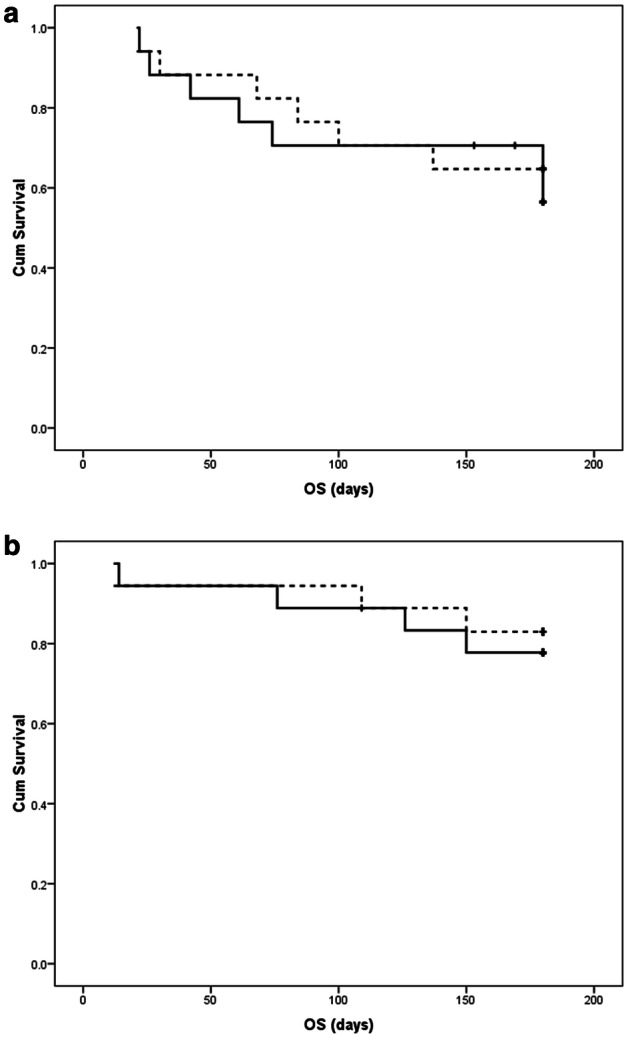
Comparison of overall survival (OS) of patients receiving voriconazole (continuous line) and fluconazole (dashed line) prophylaxis using Kaplan Meier curves in the setting of: a allogeneic stem cell transplantation; b autologous stem cell transplantation
Fig. 2.
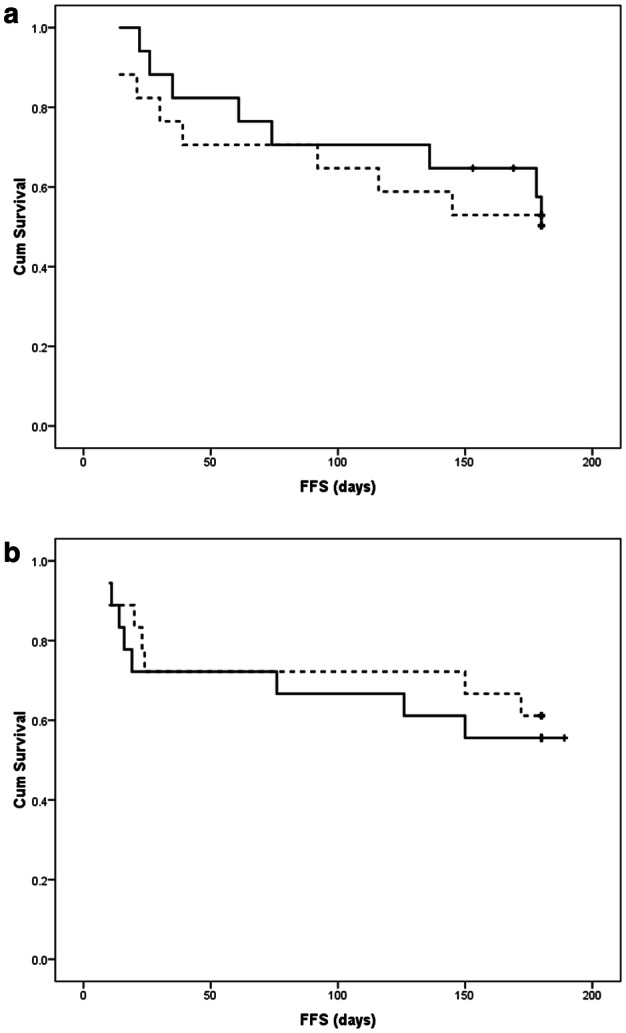
Comparison of fungal infection-free survival (FFS) of patients receiving voriconazole (continuous line) and fluconazole (dashed line) prophylaxis using Kaplan Meier curves in the setting of: a allogeneic stem cell transplantation; b autologous stem cell transplantation
Cost-Effectiveness of Voriconazole Versus Fluconazole
In the allogeneic HSCT group, voriconazole use total cost was lower than fluconazole use total cost. As regard effectiveness, voriconazole was more effective regarding avoidance of IFI and equally effective regarding avoidance of IRD but less effective regarding avoidance of total death events and gaining life years. Hence, voriconazole was dominant on fluconazole and cost-effective regarding prevention of IFI and IRD in allogeneic HSCT (Table 3). On the other hand, voriconazole use total cost was higher than fluconazole use total cost and although voriconazole was more effective regarding avoidance of IFI and IRD in the setting of ASCT, the calculated ICER for IFI avoided (− 112,505 EGP) and ICER for IRD avoided (− 112,505 EGP) in the ASCT setting were higher than the willingness to pay threshold (62,558 EGP). Also, voriconazole was dominated by fluconazole regarding avoidance of total death events and gaining life years in the setting of ASCT (Table 3). Therefore, voriconazole was not cost-effective in ASCT setting.
Table 3.
Cost-effectiveness of voriconazole and fluconazole in allogeneic HSCT and ASCT
| HSCT group | Allogeneic HSCT | ASCT | ||||
|---|---|---|---|---|---|---|
| Prophylactic antifungal agent | Voriconazole | Fluconazole | Difference | Voriconazole | Fluconazole | Difference |
| Price of drug, EGP | ||||||
| Tablet | 195 (200 mg) | 35 (200 mg) | 195 (200 mg) | 35 (200 mg) | ||
| Vial | 700 (200 mg) | 60 (100 mg) | 700 (200 mg) | 60 (100 mg) | ||
| Mean prophylaxis cost, EGP (range) | 50,012 (5070–70,200) | 28,560 (1920–43,200) | 21,452 | 56,247 (2730–70,200) | 32,467 (1920–43,200) | 23,780 |
| Mean preemptive antifungal treatment cost, EGP (range) | 6914 (2500–20,900) | 12,214 (3120–36,150) | − 5300 | 5280 (3000–9900) | 7356 (4170–14,040) | − 2076 |
| Mean side effect management cost, EGP (range) | 0 | 0 | 0 | 31,060* | 12,367 (7000–19,600) | 18,693 |
| Mean IFI monitoring cost, EGP (range) | 1129 (900–3800) | 1518 (900–4700) | − 389 | 1117 (900–2300) | 1194 (900–2800) | − 77 |
| Mean IFI management cost, EGP (range) | 25,275 (1750–78,950) | 53,844 (16,100–170,015) | − 28,569 | 15,788 (4750–35,100) | 30,787 (1920–61,510) | − 14,999 |
| Mean hospitalization cost, EGP (range) | 9325 (3300–18,000) | 8025 (3000–13,500) | 1300 | 0 | 12,833 (1000–19,500) | − 12,833 |
| Total cost, EGP | 92,655 | 104,161 | − 11,506 | 109,492 | 97,004 | 12,488 |
| IFI events | 0.235 | 0.353 | − 0.118 | 0.222 | 0.333 | − 0.111 |
| ICER (EGP/IFI avoided) | Dominant | − 112,505 | Not cost-effective | |||
| IRD events | 0.118 | 0.118 | 0 | 0 | 0.111 | − 0.111 |
| ICER (EGP/IRD avoided) | Lower cost/equal effectiveness | − 112,505 | Not cost-effective | |||
| Total death events | 0.412 | 0.353 | 0.059 | 0.222 | 0.167 | 0.055 |
| ICER (EGP/total death avoided) | Lower cost/lower effectiveness | Dominated | ||||
| LYG | 0.384 | 0.390 | − 0.006 | 0.439 | 0.452 | − 0.013 |
| ICER (EGP/LYG) | Lower cost/lower effectiveness | Dominated | ||||
HSCT hematopoietic stem cell transplantation, ASCT autologous stem cell transplantation, EGP Egyptian pounds, IFI invasive fungal infection, ICER incremental cost-effectiveness ratio, IRD IFI-related death, LYG life years gained
*No range as it is a single value
Discussion
Transplanters frequently meet the hard situation of choosing the most suitable and effective agent for antifungal prophylaxis in HSCT patients [17]. Fluconazole was the first drug used orally and parenterally for IFI prevention [18]. Fluconazole has been evaluated in many randomized controlled trials [19]. Fluconazole at the prophylactic dose (400 mg) is vulnerable to little CYP-mediated metabolism and it inhibits CYP 3A4 weakly and hence does not seem to interact with calcineurin inhibitors used for prevention and treatment of GVHD [20]. Although fluconazole is effective against most Candida strains, some are constituvely resistant (e.g. Candida glabrata and Candida kruzei) [21]. On the other hand, the broad spectrum of voriconazole makes it under consideration as a prophylactic drug specially in HSCT [9, 22]. However, the idea that voriconazole is regarded the first line treatment for IA may pose an issue due to the hazard of overgrowth of resistant strains in patients failing prophylaxis and may be even responsible for the increase in zycomycosis incidence observed at some transplant centers [23]. Other advantages that favor the use of voriconazole as a prophylactic agent are its good penetration into the lungs, its availability in oral and parenteral forms and its high tolerability by patients [23]. On the other hand, its administration is associated with some issues, e.g. visual hallucinations, prolongation of QT-interval, hepatic toxicity, risk of squamous cell carcinoma and melanoma (because of the photosensitivity induced by this drug), some drug–drug interactions and some variability regarding its pharmacokinetics [23].
In this study, we compared voriconazole and fluconazole regarding their effectiveness and cost-effectiveness in prevention of IFI and IRD in HSCT recipients. Regarding allogeneic HSCT group, both agents did not differ regarding their efficacy in preventing IFI and IRD as well as their FFS and OS rates in both allogeneic and ASCT groups. In this respect, we agree with a randomized double-blind trial involving 35 centers comparing voriconazole and fluconazole prophylaxis used for 100 days in myeloablative allogeneic HSCT [24]. In spite of a trend towards lower incidence of Aspergillus infections in voriconazole arm in the other study, there were non-significant differences in IFI, FFS and OS rates between voriconazole and fluconazole [24]. Similarly, a recent Singaporean meta-analysis showed that voriconazole was more efficient than fluconazole in reducing IFIs and IRD events among HSCT recipients although the difference was non-significant [25]. Moreover, a Chinese multicenter prospective observational study found that the rate of IFI did not differ significantly among patients administering voriconazole and those administering fluconazole whether as primary or secondary prophylaxis in the setting of allogeneic HSCT [26]. Conversely, a Canadian meta-analysis of randomized clinical trials analyzing oral antifungal prophylaxis in allogeneic HSCT reported that voriconazole was more efficient in prevention of proven and probable IFI at 6 months post-transplant in comparison to fluconazole [17]. However, the differences regarding this outcome did not reach statistical significance [17]. This study did not include patients with possible IFI which can be the reason for this difference.
Regarding cost-effectiveness, voriconazole was dominant on fluconazole regarding prevention of IFI and was less costly with equal effectiveness regarding prevention of IRD in the setting of allogeneic HSCT making it a favorable choice. We disagree with Mauskopf et al. who concluded that voriconazole was dominated by fluconazole regarding avoidance of IFI [27]. Such conclusion can be explained by the exclusion of side effect management costs, IFI-monitoring costs and hospitalization costs in the other study [27]. Another study compared the cost-effectiveness of itraconazole, fluconazole, voriconazole and posaconazole and reported that voriconazole was not cost-effective regarding avoidance of IFI [25]. This study was not confined to HSCT recipients and included myeloid malignancy patients receiving chemotherapy which may be the reason for this difference. A Mexican study reported that voriconazole was cost-effective in comparison to fluconazole in allogeneic HSCT recipients when referring to a willingness-to-pay threshold of ~ 1 GDP per capita per life year gained in contrast to our study and that although voriconazole was more costly than fluconazole, breakthrough IFI and in turn prospective resource consumption were lower [28]. This difference can be attributed to the higher number of total death events and fewer number of life years gained in the voriconazole arm in our study which in turn can be explained by the inclusion of haploidentical transplants conditioned by reduced intensity regimens which are complicated by increased non-relapse mortality rates [29].
Regarding ASCT group, voriconazole was not cost-effective regarding prevention of IFI and IRD. The calculated ICERs exceeded the Egyptian GDP per capita in year 2016 (62,558 EGP) [16]. Despite similar immune reconstitution process following allogeneic HSCT and ASCT, GVHD and immunosuppressive drug administration in the setting of allogeneic HSCT affect the initial steps of immune reconstitution [30]. Therefore, the types of antifungal prophylactic agent may have significant influence in term of their effectiveness in avoiding IFI and IRD in allogeneic HSCT, whereas in ASCT the cost of the drug will be the major determinant of its choice. Moreover, voriconazole was dominated by fluconazole regarding its cost-effectiveness in avoidance of total death events and in gaining life years making it an unfavorable option in ASCT. To the best of our knowledge, no other study has assessed the cost effectiveness of voriconazole and fluconazole as prophylactic anti-fungal agents in the ASCT setting.
This study holds significance because it involved a head to head comparison of two prophylactic agents in a one to one basis. Also, it is the first study comparing cost-effectiveness of antifungal prophylactic agents in ASCT. Our study has some limitations which are the single center nature of the study and the lack of comparison with other antifungal agents, e.g. posaconazole, itraconazole, amphotericin B, micafungin, … etc. We conclude that voriconazole did not differ from fluconazole regarding its efficacy in prevention of IFI and IRD and that its use did not have impact on OS or FFS in both allogeneic HSCT and ASCT. In addition, voriconazole seems to be a good choice for money in the setting of allogeneic HSCT despite it did not result in better avoidance of total death events and gaining of additional life years. On the other hand, voriconazole is not cost-effective and is even dominated by fluconazole in the setting of ASCT. Hence, we recommend using voriconazole prophylaxis in the allogeneic HSCT setting and using fluconazole prophylaxis in the ASCT setting. However, a comparison between the two drugs on a larger scale of patients should be carried out to decide whether these recommendations should be considered the standard of care in HSCT or not. The availability of generic voriconazole may provide a favorable influence on the cost-effectiveness of voriconazole in the ASCT setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AE-G, YA, MK and HF. The first draft of the manuscript was written by AE-G and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Human and Animal Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethical committee of Faculty of Medicine, Ain Shams University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
It was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amro Mohamed Sedky El-Ghammaz, Email: amro.sedky@yahoo.com.
Maha El-Zimaity, Email: mahazimaity@gmail.com.
Amal Mostafa Elafifi, Email: amalelafifi_24@hotmail.com.
Essam Abdelwahed, Email: abdelwahed.essam@yahoo.com.
Mohamed Mahmoud Moussa, Email: moussanz@yahoo.com.
Yasmin Ahmed Aboelmagd, Email: yasminabouelmagd@hotmail.com.
Mohamed Gamal Kotob, Email: mhmdkotop@gmail.com.
Hebatullah Magdy Fares, Email: Hebatullahfares@gmail.com.
References
- 1.Warnock DW. Trends in the epidemiology of invasive fungal infections. Nihon Ishinkin Gakkai Zasshi. 2007;48(1):1–12. doi: 10.3314/jjmm.48.1. [DOI] [PubMed] [Google Scholar]
- 2.Kontoyiannis DP. Antifungal prophylaxis in hematopoietic stem cell transplant recipients: the unfinished tale of imperfect success. Bone Marrow Transpl. 2011;46(2):165–173. doi: 10.1038/bmt.2010.256. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47(8):1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks DI, Pagliuca A, Kibbler CC, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic hematopoietic stem-cell transplantation. Br J Haematol. 2011;155(3):318–327. doi: 10.1111/j.1365-2141.2011.08838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–833. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 6.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis. 1993;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 7.Simon A, Besuden M, Vezmar S, et al. Itraconazole prophylaxis in pediatric cancer patients receiving conventional chemotherapy or autologous stem cell transplants. Support Care Cancer. 2007;15:213–220. doi: 10.1007/s00520-006-0125-7. [DOI] [PubMed] [Google Scholar]
- 8.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 9.Cecil JA, Wenzel RP. Voriconazole: a broad-spectrum triazole for the treatment of invasive fungal infections. Exp Rev Hematol. 2009;2:237–254. doi: 10.1586/ehm.09.13. [DOI] [PubMed] [Google Scholar]
- 10.Brüggemann RJM, Alffenaar JC, Blijlevens NMA, et al. Pharmacokinetic drug interactions of azoles. Curr Fungal Infect Rep. 2008;2:20–27. doi: 10.1007/s12281-008-0004-4. [DOI] [Google Scholar]
- 11.Pechlivanoglou P, De Vries R, Daenen SM, Postma MJ. Cost benefit and cost effectiveness of antifungal prophylaxis in immunocompromised patients treated for haematological malignancies: reviewing the available evidence. Pharmacoeconomics. 2011;29(9):737–751. doi: 10.2165/11588370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Storb R, et al. Heamatopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talekar MK, Olson T. Immune reconstitution after hematopoietic stem cell transplantation. In: Brown VI, editor. Hematopoietic stem cell transplantation for the pediatric hematologist/oncologist. Cham: Springer; 2018. pp. 371–383. [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drgona L, Khachatryan A, Stephens J, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on preemptive and emperical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014;33(1):7–21. doi: 10.1007/s10096-013-1944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egyptian gross Domestic Product (2016). https://www.capmas.gov.eg/HomePage.aspx. Accessed 15 Sept 2018
- 17.Bow EJ, Vanness DJ, Slavin M, et al. Systematic review and mixed treatment comparison meta-analysis of randomized clinical trials of primary oral antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. BMC Infect Dis. 2015;15:128. doi: 10.1186/s12879-015-0855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagano L, Caira M. The role of primary antifungal prophylaxis in patients with haematological malignancies. Clin Microbiol Infect. 2014;20(Suppl. 6):19–26. doi: 10.1111/1469-0691.12464. [DOI] [PubMed] [Google Scholar]
- 19.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 20.Cronin S, Chandrasekar PH. Safety of triazole antifungal drugs in patients with cancer. J Antimicrob Chemother. 2010;65:410–416. doi: 10.1093/jac/dkp464. [DOI] [PubMed] [Google Scholar]
- 21.Meunier F. Candidiasis. Eur J Clin Microbiol Infect Dis. 1989;8:438–447. doi: 10.1007/BF01964058. [DOI] [PubMed] [Google Scholar]
- 22.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J. 2002;21:240–248. doi: 10.1097/00006454-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Hicheri Y, Cook G, Cordonnier C. Antifungal prophylaxis in haematology patients: the role of voriconazole. Clin Microbiol Infect. 2012;18(Suppl. 2):1–15. doi: 10.1111/j.1469-0691.2012.03772.x. [DOI] [PubMed] [Google Scholar]
- 24.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao YJ, Khoo AL, Tan G, et al. Network meta-analysis and pharmacoeconomic evaluation of fluconazole, itraconazole, posaconazole, and voriconazole in invasive fungal infection prophylaxis. Antimicrob Agents Chemother. 2016;60:376–386. doi: 10.1128/AAC.01985-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao L, Sun Y, Meng F, et al. Antifungal prophylaxis of patients undergoing allogenetic hematopoietic stem cell transplantation in China: a multicenter prospective observational study. J Hematol Oncol. 2016;9:97. doi: 10.1186/s13045-016-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauskopf J, Chirila C, Graham J, et al. Cost-effectiveness analysis of voriconazole compared with fluconazole for prevention of invasive fungal infection in patients receiving allogeneic hematopoietic cell transplants. Am J Health Syst Pharm. 2013;70(17):1518–1527. doi: 10.2146/ajhp120599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morfín-Otero R, Alvarado-Ibarra M, Rodriguez-Noriega E, et al. Cost-effectiveness analysis of voriconazole, fluconazole, and amphotericin B for invasive fungal infections following allogeneic hematopoietic stem cell transplantation in Mexico. Clin Outcomes Res. 2018;10:511–520. doi: 10.2147/CEOR.S157642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang B, Yu R, Cai L, et al. Haploidentical versus matched donor stem cell transplantation for patients with hematological malignancies: a systemic review and meta-analysis. Bone Marrow Transpl. 2019;54(1):99–122. doi: 10.1038/s41409-018-0239-9. [DOI] [PubMed] [Google Scholar]
- 30.Guillaume T, Rubinstein DB, Symann M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood. 1998;92(5):1471–1490. doi: 10.1182/blood.V92.5.1471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


