Abstract
Introduction:
Cancer-related fatigue and muscle wasting have received significant attention over the last few decades with the goal of establishing interventions that can improve cancer patient life quality and survival. Increased physical activity (PA) has shown to reduce cancer associated fatigue and has been proposed as a promising therapeutic to attenuate cancer induced wasting. However, significant gaps remain in our understanding of how PA impacts the compositional and functional changes that initiate muscle wasting. The purpose of the current study was to determine the effect of wheel exercise on body composition and functional indices of cancer cachexia prior to the development of significant wasting.
Methods:
Thirteen-week old male ApcMin/+ (MIN) and C57BL/6 (B6) mice were given free wheel access (W) or a locked wheel (Sed) for 5 weeks.
Results:
Wheel activity was reduced in the MIN compared to B6; however, wheel access increased Complex II expression in isolated skeletal muscle mitochondria regardless of genotype. Wheel access had no effect on tumor burden or plasma IL-6 in the MIN. MIN-W increased body weight and lean mass compared to MIN-Sed, and there was a direct correlation between wheel distance and lean mass change. MIN-W increased grip strength and treadmill time to fatigue compared to MIN-Sed. Within MIN-W mice, skeletal muscle fatigability was only improved in high runners (>60min/day).
Conclusion:
Our results suggest there were therapeutic benefits of increased activity related to body composition, behavior, and whole body function that were not dependent on exercise duration; however, there was an exercise threshold needed to improve skeletal muscle fatigability in tumor-bearing mice. Interestingly, wheel access was able to improve compositional and functional outcomes without mitigating tumor number or size.
Keywords: Cancer, Exercise, Skeletal Muscle, Muscle Wasting, Fatigue
INTRODUCTION
Reduced volitional activity and increased skeletal muscle fatigue have been reported in cancer patients and preclinical tumor models, often preceding or independent of body weight and muscle mass changes (1–3). Decreased physical activity (PA) results in increased oxidative stress, reduced mitochondrial content, and decreased protein synthesis, which can all negatively impact muscle mass, strength, and fatigue resistance (4, 5). In contrast, exercise increased skeletal muscle oxidative capacity which was associated with improved muscle fatigue resistance in both healthy and diseased conditions (6–9). There is unequivocal evidence that PA has systemic wide effects that can benefit an individual’s overall health, which includes skeletal muscle alterations, that impact systemic metabolism and body mass maintenance (4). Identifying whether fatigue and disuse are causal or a consequence of muscle wasting with cancer has eluded investigators likely due to the complexity of the syndrome. Moreover, several proposed therapeutics have failed in clinical trials due to their inability to improve muscle function (10).
Fatigue and weakness are among the most commonly reported consequences of cancer and cancer treatments, which contributes to reduced perceived life quality and poor treatment outcomes (11, 12). Cancer-related fatigue (CRF) has been defined as patient perceived tiredness, which is not associated with activity status and persists with rest (13). The subjectivity of CRF’s definition lacks mechanistic insight; however, the underlying cause has been hypothesized to include hypothalamic-pituitary-anterior pituitary (HPA) axis dysregulation, elevated proinflammatory cytokines, and skeletal muscle mass loss (13). Skeletal muscle fatigue, specifically, has been classically defined as the inability to maintain a given force output over time and is dependent on the muscle’s contractile phenotype and capacity to generate ATP through glycolytic and oxidative processes (14). Skeletal muscle fatigability is highly sensitive to muscle use. Decreased use has been shown to induce muscle atrophy, reduce muscle oxidative capacity, and increase muscle fatigability which creates a feedback loop that can contribute to the initiation and/or potentiation of cancer-induced muscle mass loss (1, 15, 16). While our understanding of the drivers regulating muscle mass loss has dramatically improved, the role of PA in the etiology of fatigue and body compositional changes with cancer remains largely unknown.
The ApcMin/+ (MIN) is an established pre-clinical model of cancer-cachexia characterized by the development of intestinal polyps and a slow progressive wasting phenotype (17). MIN mice have a nonsense germline mutation in the Adenomatous polyposis coli (Apc) gene which predisposes mice to multiple intestinal neoplasia (min) resulting in intestinal polyp development. MIN mice begin to develop polyps at 4 weeks of age which continues until 12-14 weeks at which time polyp number ceases to increase but polyp size can progress (18). Reduced voluntary wheel activity and reduced oxidative metabolism are present ~14-15 weeks of age, preceding body weight loss which is initiated around 16-18 weeks of age (16). We have previously identified exercise’s ability to improve muscle mass, reduce polyp number, and reduce the systemic inflammatory environment in the MIN when exercise was initiated at 4 weeks of age, prior to the completion of polyp development (19, 20). While the cachectic phenotype and muscle’s functional characteristics have been previously characterized in the MIN mouse (1, 16, 17), it is still unclear if voluntary exercise, after polyp-development, is sufficient to attenuate skeletal muscle mass loss, fatigability, and weakness.
Exercise has been shown to spare muscle mass loss during cancer progression through improvements to muscle proteostasis and/or oxidative capacity in several preclinical cancer models (21, 22); however, the effects of exercise on whole body and skeletal muscle function in tumor-bearing mice has not been investigated. Therefore, the purpose of the current study was to determine the effect of wheel exercise on body composition and functional indices of cachexia prior to the development of significant wasting in tumor-bearing mice. A secondary aim of the study was to determine if there was a relationship between wheel activity and body compositional and functional changes independent of weight loss in male MIN mice. We hypothesized that wheel exercise would improve skeletal muscle specific fatigue and would be directly related to wheel distance and duration. Additionally, we also hypothesized that MIN mice given wheel access would have improved fatigue resistance associated with greater muscle mass and better whole body function. To test this hypothesis, male C57BL/6 (B6) and MIN mice were given either free access to wheels (W) or a locked wheel (Sed) for 5 weeks. As previously mentioned, MIN polyp development is complete by 12-14 weeks at which point the polyps continue to grow in size, not number (18). To control for exercise’s effects on polyp development, wheel access was initiated between 13-14 weeks, after MIN polyp development. We have previously shown that the tibialis anterior (TA), which is primarily type II myofibers, is susceptible to cancer and inflammation-induced skeletal muscle dysfunction and a preferential loss of type II fibers has been postulated with early cachexia (1, 23). Additionally, the TA is especially sensitive to wheel exercise despite being a dorsiflexor (24). Based on these notions, we limited our skeletal muscle function analysis to the TA.
METHODS
Animals
Male C57BL/6 (B6) and ApcMin/+ (MIN) mice were originally purchased from Jackson Laboratories and bred at the University of South Carolina’s Animal Resources Facility. All animals were group housed and kept on a 12:12-h light-dark cycle. Body weights were measured weekly, and animals were monitored for signs of distress. Animals were given food and water ad libitum throughout the duration of the study. All animals were fasted 5 hours prior to tissue collection. Mice were anesthetized with a ketamine-xylazine-acepromazine cocktail, and hindlimb muscles and select organs were carefully dissected and snap frozen in liquid nitrogen and stored at −80⁰C. All animal experiments were approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
MIN mice share the C57BL/6 background but have a nonsense germline mutation in the Adenomatous polyposis coli (Apc) gene which predisposes mice to multiple intestinal neoplasia (min) resulting in intestinal polyp development. MIN mice begin to develop polyps at 4 weeks of age at develop between 60-100 polyps by 14 weeks of age which is maintained throughout their survival (18). The MIN is an established preclinical model of familial adenomatous polyposis (FAP) and our lab and others have extensively characterized its cachectic phenotype. The 50% survival rate of the MIN is ~17-18 weeks of age (Jackson Laboratories) and therefore experiments were conducted in ~13-18 week old C57BL/6 and MIN mice.
Voluntary Wheel Running
Voluntary wheel running was used as an exercise treatment as well as a marker of volitional activity and was performed as previously described (16). At 13 weeks of age, B6 and MIN mice were individually housed in cages with 9.5-in.-diameter stainless steel wheels (MiniMitter, Bend, OR) for 5 weeks. B6 and MIN mice were randomized into 2 additional groups with either a fixed wheel (B6-Sed, n=10; MIN-Sed, n=10) or free wheel access (B6-W, n=9; MIN-W, n=10). Wheel activity was monitored and recorded daily from 13 to 18 weeks of age. Wheel activity was measured by bicycle computers (Specialized, Morgan Hill, CA) with magnetic sensors which calculated average speed, distance, time, and maximum speed by sensing 1 full rotation of the wheel in either direction while using the program wheel circumference.
Intestinal Polyp Quantification
Intestinal segments were excised, cleaned with PBS, cut into 5 equal segments, and stored in 10% neutral formalin until tumor count analysis. Intestinal polyps were analyzed after a deionized water rinse and 0.1% methylene blue staining. Total polyp counts were performed using dissecting micro-scope (model SMZ168, Motic, Xiamen, China) by an investigator blinded to the treatment groups as previously described (17). Polyps were categorized as >2mm, 1-2 mm, and <1mm in all segments.
Treadmill run to fatigue.
Mice were run on a treadmill to examine time to exhaustion as a measure of whole body fatigue. Three days before the fatigue test, mice were acclimated to the treadmill by running at a 5% grade for a total of 20 min, with 5 m/min incremental increases in speed starting at 5 m/min and finishing at 20 m/min. After acclimation, the mice were run on a treadmill until complete exhaustion, determined by 2 min of resistance to hand prodding. The fatigue test consisted of 5 min at 5, 10, and 15 m/min and 30 min at 20 m/min, and then increased to the final speed of 25 m/min until the fatigue was determined.
Grip strength
Combined hindlimb and forelimb rodent grip strength was measured with the Grip Tester (Columbus Instruments, Columbus, OH). Mice were placed with all four limbs on a metal grid mounted at a 45° angle connected to a force transducer. Mice were pulled by the tail until they let go of the grid with all 4 paws. Each mouse went through a series of two sets of five repetitions of force measurements, with a 2- to 3-min rest period between each set. The highest and the lowest scores from each set of five repetitions were removed and the remainders averaged to provide a mean force measurement for each mouse.
RotoRod
Fall latency using the RotoRod (Columbus Instruments) was used to crudely measure neuromuscular performance. The protocol consisted of a ramping protocol from 0 to 25 rpm over a period of 90 seconds. The protocol continued at 25 rpm from 90 to 120 seconds. Each mouse performed the protocol three times, with each trial separated by a 1- to 2-min rest period. The average time of the three tests was recorded for each mouse.
Cage activity monitoring
To assess volitional activity mice were placed in activity monitor cages (Opto-M3 Activity Meter, Columbus Instruments). Activity was measured for 3 days and the average number of beams crossed in an X–Y plane as well as the Z plane was recorded. Food consumption was also recorded during this time while the mice were single-housed.
Analysis of Muscle Function
At ~19 weeks of age, mice were anesthetized with 2% isoflurane inhalation and kept anesthetized at 1.5 % isoflurane for ~1hr throughout the duration of the procedure (~1 hour). Muscle function analysis of the tibialis anterior (TA) in situ, which maintains the host nerve and blood supply, has been previously described (25). Briefly, the distal tendon of the left TA was isolated and tied to a force transducer (Aurora Scientific, Ontario, Canada) using 5-0 silk sutures. The mouse was placed on the apparatus maintained at 37ºC throughout the entirety of the procedure. The sciatic nerve was exposed proximal to the knee and maintained using warmed mineral oil. The sciatic nerve was then subjected to a single stimulus to determine optimal length (Lo). Once Lo was obtained, a force-frequency curve was generated, and maximal tetanic force was determined. Specific tension was determined using TA muscle CSA calculated by muscle mass/(Lf x 1.06), where Lf represents fiber length determined by multiplying Lo by 0.6, the predetermined TA muscle length to fiber length ratio, and 1.06 represents the skeletal muscle density. After a 5-minute rest the TA was subjected to an intermittent fatigue protocol consisting of 0.5 second submaximal stimulation (50Hz) every second for 5 minutes. Immediately, after submaximal fatigue the TA was subjected to an intermittent fatigue protocol consisting of 0.5 second maximal stimulation (200Hz) every second for 5 minutes. The fatiguing properties of skeletal muscle were determined by the relative change in force (% of initial contraction) throughout the duration of the fatiguing contraction. Absolute muscle fatigue was measured as the reduction in maximal force (% of max force) following either the submaximal or maximal fatiguing contraction. After the completion of the fatigue protocol the muscle was rested for 5 minutes at Lo and then maximally stimulated (200hz) to determine fatigue recovery.
Skeletal Muscle Enriched Mitochondrial Fraction
Mitochondrial isolation from the right quadriceps muscle was performed following a previously described protocol (26). Briefly, freshly dissected quadriceps muscles were minced and digested for 30 min in isolation buffer 1 (2 M Tris·HCl, 1 M KCl, and 0.5 M EDTA/Tris) containing 0.05% trypsin solution. The digested solution was spun at 200 g for 3 min, and the pellet was resuspended and homogenized using a Teflon pestle. The homogenate was spun at 700 g for 10 min and the supernatant was collected and spun at 8,000 g for another 10 min. The supernatant was discarded, and the pellet was resuspended in 5 ml of isolation buffer 1 and spun again at 8,000 g for 10 min. The resulting pellet was gently resuspended in 50 μL of isolation buffer 2 (0.1 M EGTA/Tris and 1 M Tris·HCl) and contained the isolated mitochondrial suspension. The Bradford method was used to determine total mitochondria protein concentration in the purified mitochondrial fraction.
Myosin Heavy Chain isoform expression
The relative distribution of myosin heavy chain (MyHC) isoforms was determined according to the method of Toth et. al with slight modifications (27). Briefly, the tibialis anterior (TA) was homogenized in KCl buffer (300mM KCl, 150mM KH2PO4, 10mM NaP2O7, 200mM DTT, 200mM MgCl2, proteinase inhibitor cocktail) and protein concentration was determined using the standard Bradford method. Aliquoted homogenates were diluted to 40ng/ul in sample prep buffer (2%SDS, 62.5mM Tris, 10% glycerol, 0.001% bromophenol blue) and heated at 90ºC for 5 minutes. 200ng of protein was loaded to a 4% bis-acrylamide stacking gel and separated by SDS-PAGE (37.5% glycerol (w/v), 7% 50:1 bis-acrylamide, 200mM Tris/HCL, 100mM Glycine, 0.4% SDS, 0.08% APS) at 70V for 16hrs followed by 200V for 4hrs at 4ºC. The gel was then subjected to silver stain according to the manufacturer’s instruction (Bio-rad Laboratories) and MyHC isoforms were stained and quantified. Relative isoform expression was calculated by taking the percent expression of a single isoform band relative to all 3 quantified isoform bands.
Western blotting
Western blot analysis was performed as previously described (26). Briefly, isolated mitochondrial fraction homogenates were fractionated on SDS-polyacrylamide gels and transferred to PVDF membrane. After the membranes were blocked in 5% non-fat milk, antibodies Total OXPHOS Cocktail (Cat #: ab110413; Abcam, Cambridge, United Kingdom), cytochrome c (Cat #:11940S; Cell Signaling Technology), and VDAC (Cat #:4661S; Cell Signaling Technology) were incubated at dilutions of 1:5000 overnight at 4°C in 1% TBST milk. Anti-rabbit IgG-conjugated secondary antibodies (Cell Signaling Technology) were incubated with the membranes at 1:5000 dilutions for 1 h in 1% TBST milk. Enhanced chemiluminescence developed by autoradiography was used to visualize the antibody–antigen interactions. Blots were analyzed by measuring the integrated optical density (IOD) of each band with ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
Values are presented as means ± standard error of the mean (SEM). Unpaired student t-tests were performed to compare body composition changes, whole body functional changes, and skeletal muscle fatiguability in MIN-Sed and MIN-W mice. To determine a dose effect of wheel, MIN-W mice were divided into High (>60 minutes/day of wheel running; n=5) and Low (<60 minutes/day of wheel; n=5). Unpaired student t-tests were performed to compare skeletal muscle fatiguability between MIN-W high and low runners. Two-way ANOVA’s (genotype x wheel access) were used to compare animal characteristics, body composition, behavior and function characteristics, muscle contractile properties, and protein expression between all groups. Two-way repeated measures ANOVA (genotype x time) was used to compare wheel distance, time, average speed, and maximum speed on wheel. Post hoc analyses were performed with student Newman-Keuls methods. A Bartlett’s test was used to determine significantly different standard deviations (p<0.05). If a significant difference was observed between group standard deviations, a non-parametric Kruskal-Wallis one-way ANOVA was used. A Pearson correlation was used to determine correlations between cachexia indices and skeletal muscle functional properties in MIN mice. Significance was set at p≤0.05.
RESULTS
Animal Characteristics
Between 12 and 13 weeks of age, B6 and MIN mice were randomized to either a fixed or free access wheel for 5 weeks (Figure 1A). There was a main effect of MIN to have increased total polyp number, large polyp number, and plasma IL-6 compared to B6 (Table 1). There was a main effect of MIN to have increased spleen weight by 424% compared to B6 (p<0.0001). We have previously examined body compositional changes in MIN mice, characterized by decreased lean mass during cachexia progression (17, 28). We extended these results to identify if wheel exercise could attenuate the body compositional changes that occur in the MIN. There was a main effect of MIN to have reduced body weight by 4% (p=0.04) and 6% (p=0.002) at 12 and 18 weeks of age, respectively (Table 1). There was an 8% (p<0.0001), 7% (p<0.0001), and 8% (p=0.006) increase in body weight between 12 and 18 weeks within B6-Sed, B6-W, and MIN-W, respectively; however, MIN-Sed did not increase body weight over time (Table 1). Percent body weight loss was also measured using the loss from peak body weight to body weight at sacrifice, and MIN-Sed and MIN-W mice had a mean percent body weight loss of 4.8 ± 2.2% (Peak: 24.7 ± 0.3; Final: 23.6 ± 0.6) and 3.2 ± 1.4% (Peak: 25.1 ± 0.5; Final: 24.4 ± 0.7), respectively, classifying the MINs as weight stable or pre-cachectic (<5% BW loss). B6-Sed and B6-W mice had a mean percent body weight loss of 1.0 ± 0.3% (Peak: 25.8 ± 0.4; Final: 25.6 ± 0.5) and 0.9 ± 0.4% (Peak: 25.5 ± 0.3; Final: 25.3 ± 0.2), respectively. There was a main effect of MIN to have reduced lean mass by 4% (p=0.03) at 18 weeks of age (Table 1). There was an 8% (p<0.0001), 9% (p<0.0001), and 9% (p=0.01) increase in lean mass between 12 and 18 weeks within B6-Sed, B6-W, and MIN-W, respectively; however, MIN-Sed did not increase lean mass over time (Table 1). There were no significant changes to fat mass (Table 1).
Figure 1.
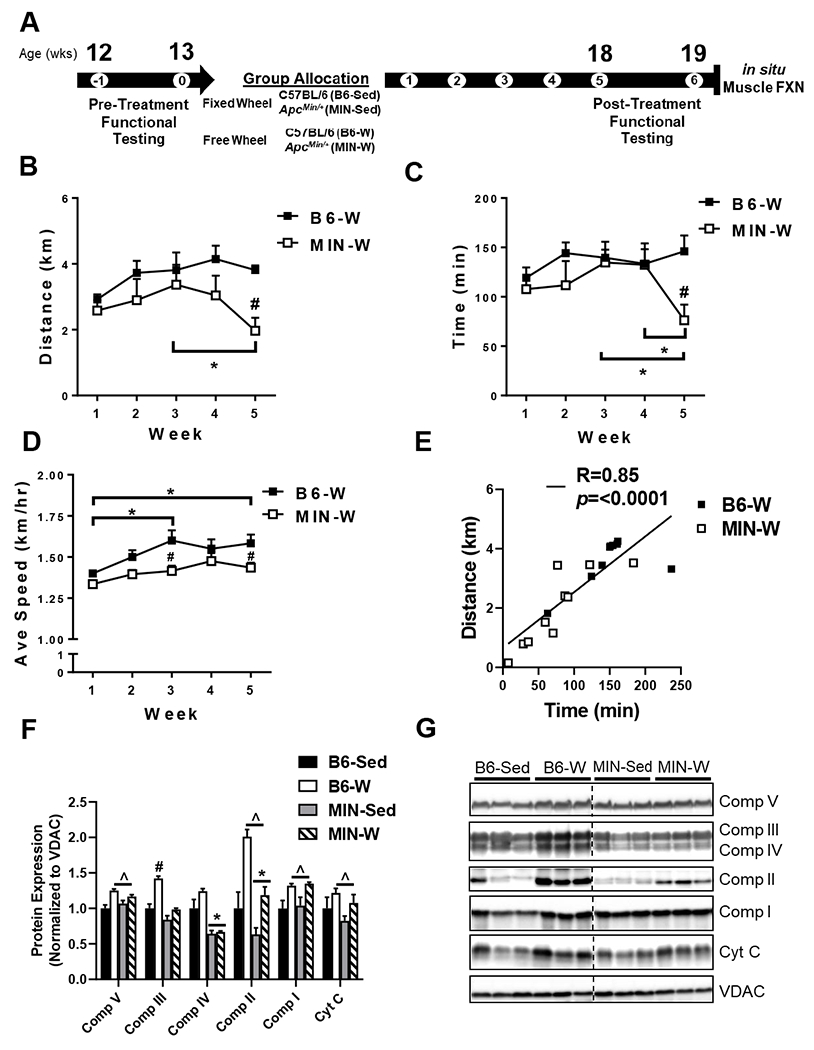
Experimental design and treatment validation. A) Experimental design and procedure timeline. Pre-treatment measurements were performed at 12 weeks (wks) of age. Mice were then singled house with either a fixed or free wheel for 5 weeks. Post-treatment measurements were performed at 18 wks of age. Several properties of skeletal muscle function were assessed at ~19 wks of age in all mice. B) Average daily distance travelled on the wheel shown as the average kilometers (km) per day for each week. C) Average daily time spent on the wheel each day for mice given free wheel access shown as the average minutes per day for each week. D) Average speed on the wheel each day for mice given free wheel access shown as kilometers per hour (km/hr) per day for each week. E) Pearson’s correlation (R) between distance (km/day) and time (min) during week 5 in B6-W and MIN-W. F) Protein expression of key mitochondrial proteins, complexes I-V and cytochrome c in mitochondrial isolated from the quadriceps muscle. Expression was normalized to VDAC loading control and shown relative to B6-C. G) representative images of mitochondrial protein expression. Dotted line indicates where cropped; all samples were run and analyzed together. C57BL/6 (B6). ApcMin/+ (MIN). Sedentary controls (Sed; B6-Sed n=10, MIN-Sed n=10). Free wheel access (W; B6-W n=9, MIN-W n=10). Two-way ANOVA. *Significant main effect of genotype. *Significant main effect of treatment. @Significant main effect of time. #Significant difference between MIN-Sed and B6-W. %Significantly different from MIN-High. Significance was set a p≤0.05.
Table 1.
Animal Characteristics
| B6 |
MIN |
|||
|---|---|---|---|---|
| Control |
Wheel Access |
Control |
Wheel Access |
|
| n | 10 | 9 | 10 | 10 |
| Disease Progression | ||||
| Age (wk) | 19.6 ± 0.2 | 19.8 ± 0.1 | 19.2 ± 0.4 | 19.4 ± 0.2 |
| Polyp number (#) | 0.0 ± 0.0 | 0.0 ± 0.0 | 91.1 ± 7.6* | 90.5 ± 4.3* |
| Large Polyps (#) | 0.0 ± 0.0 | 0.0 ± 0.0 | 71.9 ± 7.8* | 65.9 ± 5.0* |
| Spleen (mg) | 71 ± 3 | 68 ± 5 | 313 ± 34* | 279 ± 21* |
| Plasma IL-6 (pg/mL) | 0.0 ± 0.0 | 0.0 ± 0.0 | 27.9 ± 10.8* | 31.7 ± 8.7* |
| Body Composition | ||||
| Body Weight (g) | ||||
| 12 wk | 23.6 ± 0.3 | 23.8 ± 0.2 | 23.3 ± 0.5* | 22.6 ± 0.4* |
| 18 wk | 25.5 ± 0.5$ | 25.4 ± 0.2$ | 23.6 ± 0.5* | 24.4 ± 0.7*$ |
| Δ | 1.96 ± 0.20 | 1.58 ± 0.19 | 0.41 ± 0.71 | 1.84 ± 0.52 |
| Lean Mass (g) | ||||
| 12 wk | 17.9 ± 0.3 | 17.9 ± 0.1 | 17.9 ± 0.4 | 17.6 ± 0.3 |
| 18 wk | 19.3 ± 0.3$ | 19.5 ± 0.1$ | 18.0 ± 0.4* | 19.1 ± 0.5*$ |
| Δ | 1.43 ± 0.08 | 1.56 ± 0.11^ | 0.12 ± 0.41* | 1.47 ± 0.47*^ |
| Fat Mass (g) | ||||
| 12 wk | 2.7 ± 0.1 | 2.6 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 |
| 18 wk | 2.8 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.2 | 2.5 ± 0.1 |
| Δ | 0.14 ± 0.14 | −0.18 ± 0.11 | 0.02 ± 0.25 | 0.15 ± 0.13 |
Values are means ± SEM. Age in weeks (wk). Intestinal polyp number observed in segments 1-5. Intestinal polyps that were ≥2mm were considered large polyps. Spleen weight given in milligrams (mg). Plasma levels of interleukin-6 (IL-6) in picograms per milliliter (pg/mL). Plasma IL-6 and intestinal polyps were not detectable in all B6 mice. Body weights, lean mass, and fat mass given in grams (g) measured by Dual-Energy X-Ray Analysis (DEXA) at 12 and 18 weeks of age. Delta (Δ) between 12 and 18 weeks. C57BL/6 (B6; sedentary n=10, wheel n=9). ApcMin/+ (MIN; sedentary n=10, wheel n=10). Two-way ANOVA.
Main effect of genotype.
Main effect of treatment.
Repeated measures, effect of time. Significance was set a p≤0.05
Wheel Running
There was a 39% (p=0.004) decrease in total wheel distance at week 5 in MIN-W compared to B6-W (Table 2, Figure 1B); however, there was no observed difference in weeks 1-4 (Figure 1B). Average daily wheel distance within the MIN-W was decreased 42% (p=0.03) at week 5 compared to week 3, while there were no differences within the B6-W (Figure 1B). There was a 23% (p=0.05) decrease in total wheel time and a 49% (p=0.004) decrease in week 5 wheel time in the MIN-W compared to B6-W (Figure 1C). Average time on the wheels within the MIN-W was decreased 43% (p=0.02) and 42% (p=0.03) at week 5, compared to weeks 3 and 4, respectively, while there were no differences within the B6-W (Figure 1C). There was an 8% (p=0.002) decrease in total average speed and a 9% (p=0.02) decrease in week 5 average speed in MIN-W compare to B6-W (Table 2). Average speed within the B6-W was increased 14% (p=0.007) and 13% (p=0.02) at week 3 and 5, compared to week 1, respectively, while there were no differences within the MIN-W (Figure 1D).There was an 8% (p=0.02) and 9% (p=0.002) decrease in the average speed at weeks 3 and 5, respectively, in the MIN-W compared to B6-W (Figure 1D). There was a 10% (p=0.035) decrease in maximal speed during week 5 in the MIN-W compared to B6-W (Table 2). There was a strong direct relationship between distance run and time on the wheel demonstrating that the mice that ran the shortest amount of time also ran the shortest distance (Figure 1E). In isolated mitochondria, there was a main effect of wheel activity to increase protein expression of mitochondrial complex V by 17% (p=0.0001), II by 96% (p=0.0005), and I by 31% (p=0.0013) (Figure 1F). There was a main effect of wheel to have increased cytochrome c expression by 26% (p=0.045). There was a main effect of MIN to have decreased mitochondrial complex IV by 16% (p<0.0001) and II by 40% (p=0.01) (Figure 1F). There were no differences between MIN-W high and low runners in any of the measured mitochondrial proteins (data not shown).
Table 2.
Wheel Activity
| B6-W | MIN-W | Body Weight | Lean Mass | Time to Fatigue | Grip Strength | |
|---|---|---|---|---|---|---|
| Distance on Wheel (km) | Correlation to MIN-W Distance on Wheel (R) | |||||
| Total | 127.4 ± 7.8 | 103.4 ± 15.3 | 0.32 | 0.43 | 0.12 | −0.20 |
| Week 1 | 20.4 ± 1.1 | 18.1 ± 2.2 | 0.00 | 0.09 | −0.17 | −0.25 |
| Week 5 | 25.1 ± 1.8 | 15.2 ± 2.6* | 0.25 | 0.75# | 0.25 | 0.10 |
| Time on Wheel (min/day) | Correlation to MIN-W Time on Wheel (R) | |||||
| Total | 147.0 ± 7.6 | 112.6 ± 14.7* | 0.30 | 0.37 | 0.10 | −0.14 |
| Week 1 | 119.1 ± 11.1 | 107.7 ± 12.1 | −0.24 | −0.17 | −0.19 | −0.29 |
| Week 5 | 149.0 ± 15.0 | 76.2 ± 15.9* | 0.44 | 0.48 | 0.06 | 0.04 |
| Average Speed (km/hr) | Correlation to MIN-W Average Speed (R) | |||||
| Total | 1.53 ± 0.02 | 1.41 ± 0.03* | 0.12 | 0.07 | 0.30 | −0.52 |
| Week 1 | 1.40 ± 0.19 | 1.34 ± 0.02 | −0.25 | −0.30 | −0.27 | −0.44 |
| Week 5 | 1.58 ± 0.05 | 1.44 ± 0.03* | 0.41 | 0.28 | 0.41 | 0.00 |
| Maximal Speed (km/hr) | Correlation to MIN-W Maximal Speed (R) | |||||
| Total | 2.31 ± 0.12 | 2.17 ± 0.11 | 0.03 | 0.37 | 0.38 | −0.37 |
| Week 1 | 2.10 ± 0.19 | 2.25 ± 0.26 | 0.13 | −0.28 | −0.18 | −0.02 |
| Week 5 | 2.20 ± 0.06 | 1.96 ± 0.09* | 0.74# | 0.31 | 0.28 | 0.02 |
Values are means ± SEM and Pearson’s correlation (R). C57BL/6 (B6). ApcMin/+ (MIN). Wheel access (W; B6-W n=9, MIN-W n=10). Correlations were between each wheel measurement and the percent change between 12 and 18 weeks on compositional and functional outcomes in MIN-W. Two-way ANOVA.
Main effect of genotype.
Main effect of treatment.
Repeated measures, effect of time.
Significant correlation. Significance was set a p≤0.05
Animal behavior and whole body functional capacity
The MIN-W percent body weight change between week 12 and 18 was increased 8% (p=0.05) compared to the MIN-Sed (Figure 2A). There was a positive relationship between body weight change and maximal wheel speed at week 5 in MIN-W (Table 2). The MIN-W percent lean mass change between week 12 and 18 was increased 8% (p<0.05) compared to the MIN-Sed (Figure 2B). There was a significant positive relationship between lean mass change and week 5 total distance in MIN-W (Table 2).
Figure 2.
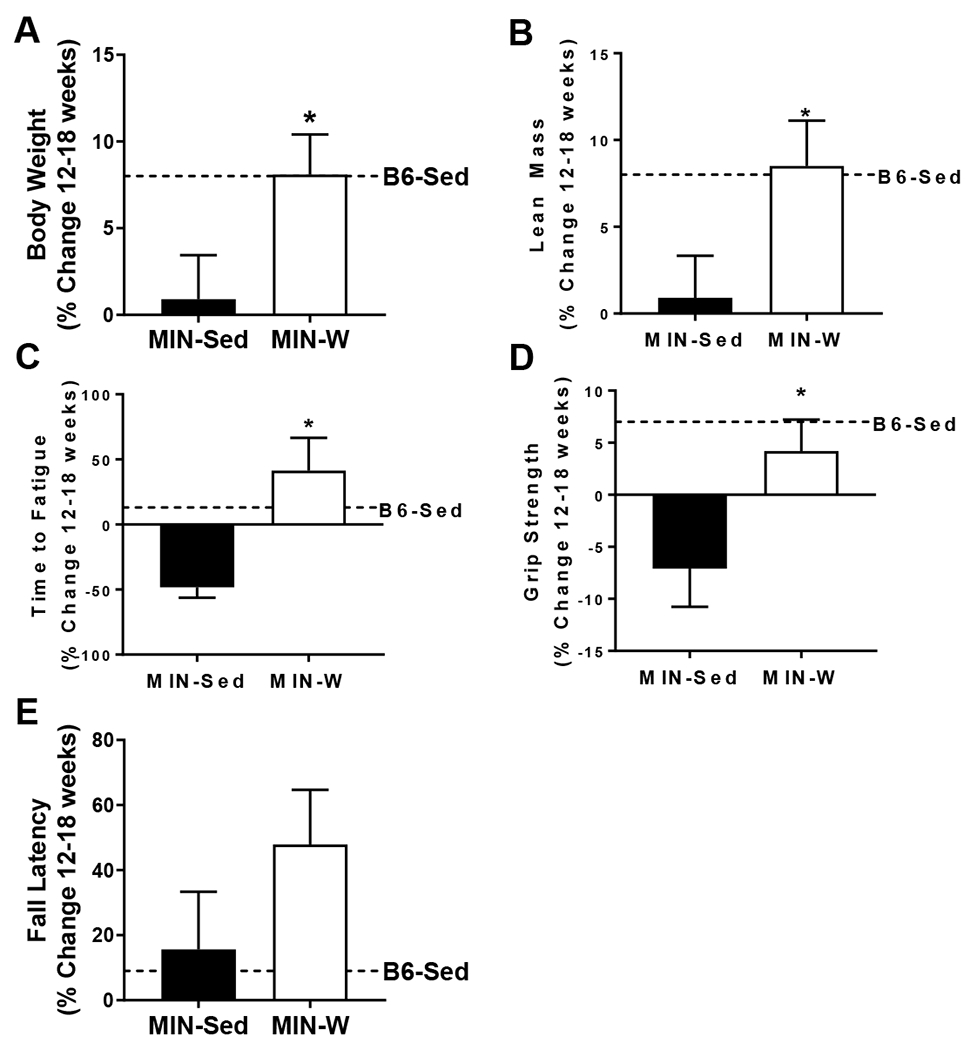
Body composition and whole body function changes in ApcMin/+ mice given free wheel access. A) Percent body weight change after 5 weeks of wheel access. B) Percent lean mass change after 5 weeks of wheel access measured by DEXA. C) Percent fat mass change after 5 weeks of wheel access measured by DEXA. D) Percent grip strength change after 5 weeks of wheel access. E) Percent time to fatigue change after 5 weeks of wheel access. F) Percent fall latency change after 5 weeks of wheel access. C57BL/6 (B6). ApcMin/+ (MIN). Sedentary controls (Sed; B6-Sed n=10, MIN-Sed n=10). Free wheel access (W; MIN-W n=10). Student’s t-test. *Significantly different from MIN-Sed. Significance was set a p≤0.05.
There were no differences in daily food intake between all groups at 12 weeks of age. There was an 8% (p=0.035) and 16% (p=0.037) increase in food intake between week 12 and 18 in the B6-Sed and B6-W, respectively (Table 3). There was a main effect of MIN to have reduced food intake by 8% (p=0.003) at 18 weeks (Table 3). There was no effect of wheel on food intake (Table 3). There were no differences in cage activity between all groups at 12 weeks. There was a main effect of MIN to have reduced cage activity by 52% (p<0.0001) compared to B6 at 18 weeks of age (Table 3). Both MIN-W and MIN-Sed had significantly reduced cage activity by 43% (p=0.01) and 40% (p=0.01) between week 12 and 18, respectively, while both B6-Sed and B6-W both increased cage activity by 35% (p=0.03) and 52% (p=0.004) between week 12 and 18, respectively (Table 3).
Table 3.
Behavioral and Functional Characteristics
| B6 |
MIN |
|||
|---|---|---|---|---|
| Sedentary |
Wheel |
Sedentary |
Wheel |
|
| Behavior | ||||
| Food Intake (g) | ||||
| 12 wk | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.2 | 3.7 ± 0.1 |
| 18 wk | 4.0 ± 0.1$ | 4.3 ± 0.2$ | 3.7 ± 0.2* | 3.9 ± 0.1* |
| Δ | 0.33 ± 0.13 | 0.54 ± 0.22 | −0.16 ± 0.33* | 0.18 ± 0.13* |
| Cage Activity (x1000 counts) | ||||
| 12 wk | 19.5 ± 1.3 | 19.6 ± 1.4 | 22.0 ± 2.7 | 23.4 ± 2.8 |
| 18 wk | 26.4 ± 2.0$ | 29.8 ± 2.6$ | 12.6 ± 3.4*$ | 14.1 ± 2.0*$ |
| Δ | 6.9 ± 2.7 | 10.2 ± 2.6 | −9.4 ± 2.9* | −9.4 ± 3.0* |
| Whole-body Function | ||||
| Grip Strength (mN/g) | ||||
| 12 wk | 106.2 ± 0.9 | 103.4 ± 2.5 | 108.4 ± 2.9 | 103.5 ± 2.1 |
| 18 wk | 113.1 ± 3.8 | 112.7 ± 1.1$ | 100.0 ± 2.0* | 107.4 ± 2.0* |
| Δ | 6.9 ± 3.9 | 9.3 ± 3.0^ | −8.4 ± 4.0* | 3.9 ± 3.0*^ |
| Fall Latency (sec) | ||||
| 12 wk | 61.5 ± 4.6 | 62.1 ± 5.9 | 61.3 ± 4.4 | 56.4 ± 8.4 |
| 18 wk | 76.7 ± 5.7 | 68.1 ± 5.2 | 69.0 ± 9.7 | 72.2 ± 3.4 |
| Δ | 15.2 ± 5.1 | 6.1 ± 6.2 | 7.8 ± 9.6 | 15.8 ± 7.2 |
| Time to Fatigue (min) | ||||
| 12 wk | 77.9 ± 4.1 | 79.7 ± 6.0 | 78.8 ± 6.7 | 74.2 ± 6.1 |
| 18 wk | 86.6 ± 3.6 | 150.3 ± 11.7$^ | 40.8 ± 6.9$* | 99.9 ± 15.2*^ |
| Δ | 8.7 ± 4.1 | 70.7 ± 13.7^ | −38 ± 7.3 | 25.7 ± 15.2*^ |
| Skeletal Muscle Function | ||||
| Twitch Properties | ||||
| 1/2 RT (ms) | 7.3 ± 0.2 | 7.5 ± 0.1 | 9.4 ± 0.8* | 10.3 ± 1.2* |
| TPT (ms) | 15.1 ± 0.2 | 14.8 ± 0.2 | 16.2 ± 0.4* | 17.6 ± 1.3* |
| Force Production | ||||
| Po (mN) | 1544 ± 42 | 1647 ± 36 | 1300 ± 82* | 1356 ± 87* |
| sPo (kN/m2) | 285 ± 9 | 309 ± 8 | 271 ± 12* | 279 ± 9* |
| Contraction Rates | ||||
| + dP/dtmax (N/s) | 24.0 ± 1.0 | 23.2 ± 1.0 | 20.6 ± 1.9 | 20.9 ± 1.7 |
| − dP/dtmax (N/s) | 17.5 ± 1.3 | 19.7 ± 1.0 | 12.5 ± 1.3* | 13.1 ± 1.6* |
Values are means ± SEM. Average daily food intake measured over 3 days in grams (g). Average daily cage activity measured over 3 days in beams crossed (counts). Relative grip strength given in milliNewtons force /grams of body weight (mN/g). Time on the rotorod (fall latency) given in seconds (sec). Time to fatigue during a graded exercise treadmill test given in minutes (min). All behavior and whole-body function outcomes were measured at 12 and 18 weeks (wk). Delta (Δ) between 12 and 18 weeks. Properties of skeletal muscle function were measured at ~18 wks prior to sacrifice. 1/2 Relaxation time (RT) and time to peak twitch (TPT) given in milliseconds (ms) measured during an isolated twitch contraction. Absolute (Po) and specific (sPo) maximal force production given in milliNewtons (mN) and kiloNewtons/meters2 (kN/m2), respectively, measured at 200 hz. Rate of contraction (+dP/dtmax) and rate of relaxation (−dP/dtmax) given in newtons/second (N/s) measured at 200 hz. C57BL/6 (B6; sedentary n=10, wheel n=9). ApcMin/+ (MIN; sedentary n=10, wheel n=10). Two-way ANOVA.
Main effect of genotype.
Main effect of treatment.
Repeated measures, effect of time. Significance was set a p≤0.05
There was a main effect of MIN to have reduced time to fatigue by 41% (p=0.0002) and a main effect of wheel to have increased time to fatigue by 96% (p<0.0001) at 18 weeks of age (Table 3). There was an increase in time to fatigue by 88% (p=0.0009) within the B6-W between week 12 and 18 (Table 3). There was a decrease in time to fatigue by 48% (p=0.0006) in the MIN-Sed between week 12 and 18 (Table 3). There was a 186% (p=0.003) increase in the percent change in time to fatigue in MIN-W compared to MIN-C (Figure 2C). There were no relationships between wheel speed, duration, or distance and time to fatigue change in the MIN-W (Table 1). There was a main effect of MIN to have reduced grip strength by 8% at 18 weeks of age (p=0.002; Table 3). There was an increase in grip strength by 9% (p=0.01) in the B6-W between week 12 and 18 (Table 3). There was a 159% (p=0.03) increase in the percent change in grip strength in the MIN-W compared to MIN-Sed (Figure 2D). There were no relationships between wheel speed, duration, or distance and grip strength change in the MIN-W (Table 1). There was no difference in fall latency between all groups throughout the study (Table 3, Figure 2E).
Skeletal Muscle Contractile Properties
Skeletal muscle function has been classically characterized by the muscle’s twitch properties, force production capabilities, and intrinsic fatigability (29). There was a main effect of MIN to have reduced TA weight by 8% (p=0.001), but there was no effect of wheel (B6-Sed 49.0 ± 0.9 mg; B6-W 48.4 ± 0.8 mg; MIN-Sed 42.0 ± 2.0 mg; MIN-W 44.5 ± 1.9 mg). Similar to what has been previously reported in the MIN mouse (1), there was a main effect of MIN to have increased ½ RT and TPT by 33% (p=0.003) and 14% (p=0.008), respectively, indicating a slower contractile phenotype (Table 3). There was a main effect of MIN to have a reduced rate of relaxation (−dP/dtmax) from peak tetanic force by 31% (p=0.0002); however, rate of peak contraction (dP/dtmax) was not changed (Table 3). There was a main effect of MIN to have reduced absolute tetanic force by 17% (p=0.0002) (Po; Table 3). There was a main effect of MIN to have reduced maximal specific force by 8% (p=0.01) (sPo; Table 3).
Skeletal Muscle Fatigability
To measure the effects of wheel exercise on skeletal muscle specific fatigability, the sciatic nerve was stimulated 1 contraction/second for 5 minutes at 50Hz to elicit submaximal contractions followed by 1 contraction/second for 5 minutes at 200Hz to elicit maximal contractions. The relative (% of initial) force-time tracing throughout both contraction periods is shown in Figure 3A. There was no difference in relative maximal force production (% of max force) following 300 submaximal contractions between MIN-Sed and MIN-W (Figure 3C); however, there was a significant increase by 44% (p=0.046) in the MIN-High compared to MIN-Low runners (Figure 3D). Similarly, there was no difference in relative maximal force production (% of max force) following 600 contractions (300 submaximal and 300 maximal) between MIN-Sed and MIN-W (Figure 3E); however, there was a significant increase by 36% (p=0.023) in the MIN-High compared to MIN-Low runners (Figure 3F). In order to measure recovery from fatigue, the TA was rested at Lo for 5 minutes and then measured for maximal force production. There was a 27% (p=0.05) increase in force recovery with the MIN-W compared to MIN-Sed (Figure 3G). Interestingly, there was a significant increase by 18% (p=0.020) in the MIN-High compared to MIN-Low (Figure 3H).
Figure 3.
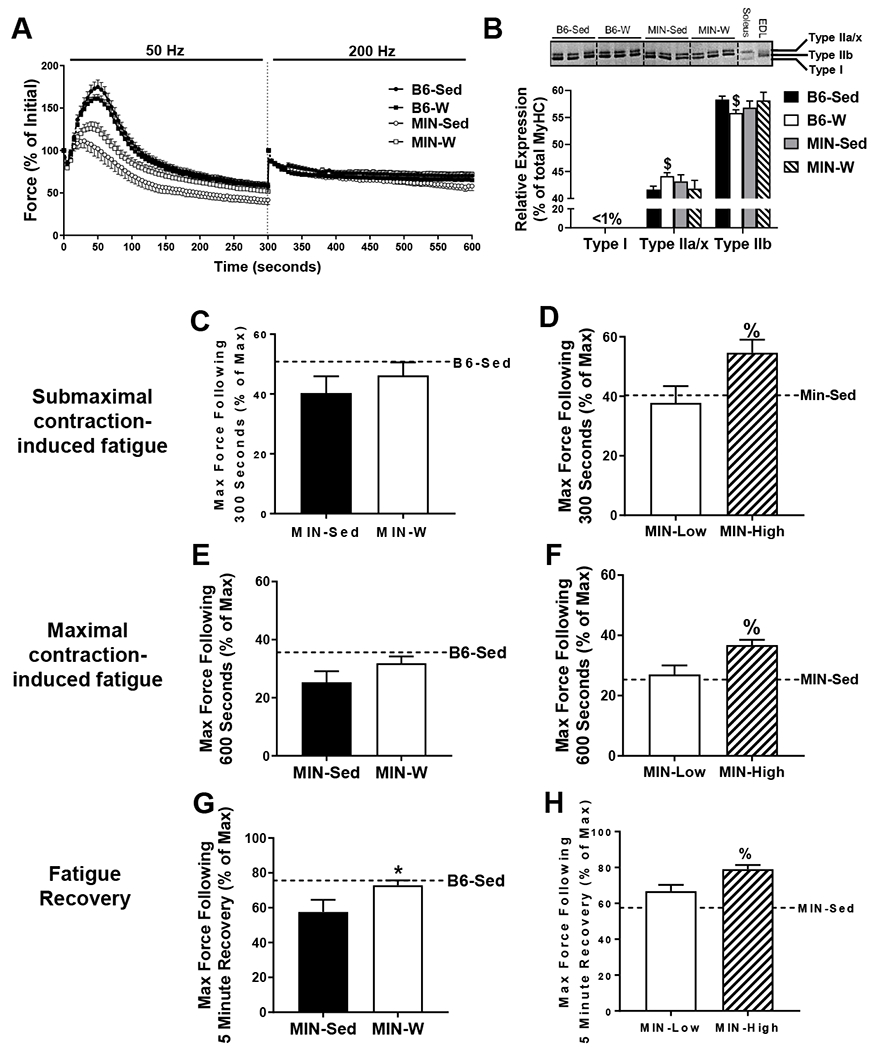
The effect of wheel on skeletal muscle fatigability in the ApcMin/+ mouse. A) Force-time tracing of the intermittent skeletal muscle fatigue protocol. Measurements shown as a percent of the first submaximal contraction and then the first maximal contraction. B) Myosin heavy chain (MyHC) isoform expression in all mice. C) Maximal force production following 300 submaximal contractions relative to Po in all MIN mice. D) Maximal force production following 300 submaximal contractions relative to Po in all MIN-W mice stratified by MIN-High (>60minutes/day) and MIN-Low (<60minutes/day) runners. E) Maximal force production following 300 submaximal contractions and 300 maximal contractions (600 total) relative to Po in all MIN mice. F) Maximal force production following 300 submaximal contractions and 300 maximal contractions (600 total) relative to Po in all MIN-W mice stratified by MIN-High (>60minutes/day) and MIN-Low (<60minutes/day) runners. G) Maximal force production following a 5 minute rest after the completion of the fatigue protocol relative to Po in all MIN mice. H) Maximal force production following 5 minute rest after the completion of the fatigue protocol relative to Po in all MIN-W mice stratified by MIN-High (>60minutes/day) and MIN-Low (<60minutes/day) runners. C57BL/6 (B6). ApcMin/+ (MIN). Sedentary controls (Sed; B6-Sed n=10, MIN-Sed n=10). Free wheel access (W; B6-W n=9, MIN-W n=10). MIN-High runners (>60minutes/day; n=5) and MIN-Low runners (<60minutes/day; n=5). Student’s t-test. *Significantly different than control. %Significantly different from MIN-High runners. $Significantly different from B6-C (preplanned t-test). Significance was set a p≤0.05.
Myosin heavy chain isoform expression
In addition to the metabolic properties of skeletal muscle (Figure 1F), the MyHC isoform expression is classically sensitive to activity and contributes to the contractile and fatiguing properties of skeletal muscle (29). Therefore, we examined the relative MyHC fiber type abundance in the TA of each group (Figure 2B). There was a 6.7% (p=0.005; preplanned t-test) increase in Type IIa/x and decrease in Type IIb in the B6-W compared to B6-Sed (Figure 3B) confirming previous reports that the TA has been previously shown to be sensitive to 4 weeks of wheel activity (24). There were no observed differences between MIN-C or MIN-WA and any group. Additionally, there was no difference in relative MyHC isoform expression between the MIN-W high and low runners (Type IIa/x MIN-Low:41 ± 1% vs MIN-High 43 ± 3%; p=0.65)
DISCUSSION
The current study sought to better understand if wheel exercise could improve body composition as well as whole body and skeletal muscle function during the early stages of cachexia progression. We have previously demonstrated reduced volitional activity and increased whole body and skeletal muscle fatigability in both weight stable and cachectic male MINs (1). While inactivity has established deleterious effects on muscle mass and function, return to activity can reverse these effects in healthy individuals (5, 6, 8); however, it still remains unclear if increasing volitional activity can attenuate skeletal muscle fatigue that is associated with wasting. Herein, we show that regardless of tumor presence and elevated circulating IL-6, there were apparent therapeutic benefits of increased activity related to body composition, behavior, and whole body function, that were not dependent on exercise duration. There was an exercise threshold needed to improve skeletal muscle fatigability in tumor-bearing mice. Furthermore, all of these benefits occurred without mitigating tumor number or size.
Regimented exercise and increased daily physical activity are commonly prescribed to cancer patients with the goal of improving physical function and quality of life (8), however our understanding of how PA can improve muscle’s functional capacity during cachexia progression is still developing. Furthermore, our understanding of the progression of central and musculoskeletal fatigue during cancer remains extremely limited. While a cause-effect relationship between central and peripheral fatigue is generally accepted, their mechanistic link and causal sequence with aging and chronic disease is not well understood (30). The current study demonstrated that a low dose of exercise was sufficient to improve treadmill fatigue test running time as well as relative grip strength. Interestingly, wheel access was unable to improve central fatigue, demonstrated by no difference in cage activity between sedentary and wheel groups. While our results are limited regarding central fatigue, our study suggests that wheel access can improve several aspects of physical function without necessarily improving central drive. One potential hypothesis for the inability of wheel access to increase volitional activity is elevated systemic inflammation and tumor-burden which were not changed by exercise (31). Chronic inflammation negatively impacts central fatigue since several inflammatory cytokines, namely TNF-α, IL-6, and IFNγ, have been shown to cross the blood brain barrier and disrupt the hypothalamic-pituitary-adrenal (HPA) axis (32). Therefor we speculate that circulating IL-6 may still be disrupting the HPA axis control of central drive. Whether activity altered neural inflammation of tumor-bearing mice was not determined, however this question serves as an intriguing inquiry for further research.
Decreased volitional activity has previously been considered to be consequence of muscle mass loss (15). However, several studies have provided evidence that reduced activity levels and increased sedentary behavior occurs prior to, or without, significant weight loss (6, 8, 16). Our lab has previously reported that reductions in voluntary wheel speed and distance precede body weight loss in male MIN mice (16), further supporting decreased activity as a contributor of cachexia initiation. We have extended these findings by showing that a reduction in cage activity, wheel distance, and time spent running on the wheel can occur without significant body weight loss. Interestingly, we have previously shown an exercise modality specific effect (wheel versus treadmill) on polyp number when initiated at 4 weeks in the MIN; however, both treadmill and wheel exercise reduced spleen weight and plasma IL-6 (19). To account for exercise effects on tumor-burden, the current study initiated wheel access at ~13 weeks of age, after polyp development in the MIN plateaus. We report that wheel activity did not affect tumor number, plasma IL-6, or spleen weight when started at this advanced age. While MIN-Sed mice maintained body weight and lean mass from 12-18 weeks, MIN-W mice, regardless of exercise duration, were able to increase lean mass and body weight over time similar to healthy controls. Based on the study design, our MIN-Sed animals could not be classified as cachectic, but rather weight stable or pre-cachectic (15). Therefore, we are limited in our ability to rule out stunted growth rather than cachexia initiation; however, we have previously shown that MIN mice will reach their peak body weight ~16 weeks of age followed by a slow progressive loss of body weight (16). While our results show no differences in lean mass or body weight between 12 and 18 weeks, MIN-Sed mice continued to increase body weight to ~16 weeks and lost ~4% BW by 18 weeks. This strongly suggests that the MIN mice were initiating cachexia rather than only having stunted growth. The beneficial effects of exercise on tumor metastasis, development, and growth have been studied extensively; however, our results demonstrate that wheel access was able to increase lean body mass and body weight without affecting tumor number. Interestingly, our lab recently demonstrated that the cancer environment disrupts diurnal PA fluxes associated with aberrant mTORC1 (mechanistic target of rapamycin complex 1) signaling which is a key regulator of muscle mass and is sensitive to changes in muscle use (31). While low levels of physical activity have been strongly linked to the incidence of colon, breast, kidney, and digestive cancers (6), our results and others suggest that maintaining physical activity levels following diagnosis is important in maintaining cancer-patient muscle mass and function independent of tumor growth (33).
While the regulators of skeletal muscle mass with cachexia have received significant attention, less is known about how skeletal muscle function is affected by cachexia. Disrupted skeletal muscle function have been reported (1, 3, 25); however, these studies were limited in mechanistic insight and have provided equivocal findings likely due to the muscle measured, tumor model used, and degree of weight loss at the time of measurement. Additionally, a disconnect between muscle mass and function has contributed to unsuccessful treatment outcomes by targeting mass without accounting for functional changes (10). Skeletal muscle function analysis has often been relegated to the simple assessments of skeletal muscle strength without appropriately testing skeletal muscle fatigability. Improved muscle strength without improved muscle fatigue resistance has limited translation to cancer patients, since CRF affects the majority of cancer patients (13). We provide evidence of a relationship between low activity levels, skeletal muscle fatigue, and fatigue recovery. While hindlimb muscle mass and skeletal muscle force were not changed with wheel activity, we have previously shown that repeated eccentric muscle contractions, a model of resistance exercise, could attenuate the loss of muscle CSA in the male MIN mouse (22). Further research is justified to examine if increased activity and resistance exercise can improve overall functional quality of cancer patients through attenuating the deleterious effects of the tumor environment on muscle mass, strength, and fatigability.
There are numerous factors that contribute to the fatiguing properties of skeletal muscle; however, intrinsic to the muscle, the metabolic properties and MyHC fiber type abundance emerge as key regulators. The murine TA contains primarily Type II muscle fibers (Type I: <1%, Type IIa/x: ~40%; Type IIb: ~60%); however, remains sensitive to wheel activity (24). Interestingly, preferential loss of type II fibers with early cachexia has been postulated (34); however, we did not observe a loss of fiber type relative abundance with early cachexia in the MIN. There has previously been reports of myofiber atrophy of the Type II fibers and a relative increase in Type IIb in the EDL of C26 mice; however preferential atrophy of Type IIa and Type IIb with no significant changes to Type I fibers in the Soleus of these same mice (3). Together, it appears that the loss of myofibrillar size and MyHC abundance with cachexia is not apparent in skeletal muscle without a significant portion of Type I fibers (e.g. TA, EDL). It is important to note that human skeletal muscle is much more heterogenous compared to murine muscle, and the fiber type sensitivity in the TA and EDL is difficult to translate to cachectic cancer patients. To this end, Toth et al. showed reduced muscle cross sectional area (CSA) only of Type I and Type IIa in cancer patients with no significant changes in Type IIa/x fiber size; however, they show an increase in relative Type IIa/x muscle fiber abundance (35). Interestingly, while mitochondrial proteins were sensitive to wheel activity in both the B6 and MIN, we were unable to observe an effect of wheel activity on the relative fiber type abundance in the MIN. We are limited in our ability to suggest changes in myosin light chain expression; however, changes in the potentiation observed within the first 100 contractions of the skeletal muscle fatigue protocol highlight a need for additional work investigating the changes to myosin light chain expression with cachexia progression.
Emerging evidence suggests that metabolic dysfunction is a key driver of cancer-induced muscle mass and function loss (34). Disrupted oxidative metabolism and mitochondrial dysfunction have been demonstrated in both pre-cachectic and cachectic muscle which may directly disrupt the functional quality of skeletal muscle (36, 37). Our lab has previously shown that voluntary wheel running is reduced prior to weight loss in male MIN mice associated with anemia and decreased muscle citrate synthase (CS) activity (16). Regular treadmill exercise and wheel access initiated at 4 weeks of age increased CS activity compared to sedentary controls; however, only treadmill training was able to reduce total polyp number. Both exercise types reduced spleen weight and plasma IL-6 (19). The current study provides evidence that wheel exercise was able to improve mitochondrial complex II, called succinate dehydrogenase (SDH), in both healthy and tumor-bearing mice. Increased SDH is a hallmark of skeletal muscle’s response to exercise training (38) and the loss of SDH activity with cachexia progression has been established (22, 39). Mitochondria are especially susceptible to inflammation-induced wasting (37) and our lab has recently demonstrated that elevated IL-6 alone is sufficient to increase skeletal muscle fatigability (23) and disrupt mitochondrial quality control (40). Exercise has been shown to mitigate chronic inflammation, improve mitochondria quality control, and increase mitochondrial content (9); however, our results extend these findings to suggest that exercise can increase the relative expression of several mitochondrial proteins within isolated mitochondria and improve fatigue resistance without necessarily reducing circulating IL-6. While treadmill exercise training has been shown to be protective against IL-6-induced cachexia in the MIN mouse, and was associated with improved triglyceride and glucose metabolism, further work is needed to understand how exercise can impact skeletal muscle oxidative metabolism prior to significant wasting (20).
The loss of functional independence and the burden of CRF plagues most cancer patients and contributes substantially to reduced life quality and survival. Therefore, a better understanding of these musculoskeletal alteration and functional deficits should improve the efficacy of future therapeutics. The current study identified deficits in skeletal muscle mass, strength, and fatigue resistance before the initiation of body weight loss. Additionally, these changes occurred concomitant with reduced volitional activity and decreased mitochondrial content. Whether there is an exercise threshold needed for cancer patients is an active area of inquiry by the American College of Sports Medicine and requires additional work. Our results highlight an importance to looking beyond cellular signing and muscle fiber-type abundance to the functional capability as the exercise threshold to observe differences in metabolic biochemical signaling versus function changes may be distinct. We provide further evidence supporting the therapeutic benefits of increased activity on lean mass, whole body function, and skeletal muscle fatigability. While investigation into the combination and comparison of exercise modalities with cancer patients is needed, our results in conjunction with others suggest that increasing volitional activity has the potential to improve cancer patient life quality and survival.
ACKNOWLEDGEMENTS
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and the results of the present study were not endorsed by ACSM.
The research described in this report was supported by R01CA-121249 (National Cancer Institute) to J. A. Carson, and SPARC graduate research grant from the Vice President of Research at the University of South Carolina to B. N. VanderVeen.
Funding: This work was supported by National Institutes of Health Grants R01CA-121249 (National Cancer Institute) to JAC and SPARC graduate research grant from the Vice President of Research at the University of South Carolina to BNV.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
REFERENCES
- 1.VanderVeen BN, Hardee JP, Fix DK, Carson JA. Skeletal muscle function during the progression of cancer cachexia in the male Apc(Min/+) mouse. J Appl Physiol (1985). 2018;124(3):684–95. Epub 2017/11/11. doi: 10.1152/japplphysiol.00897.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79(9-10):1479–86. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun. 2013;435(3):488–92. doi: 10.1016/j.bbrc.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R337–44. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bogdanis GC. Effects of physical activity and inactivity on muscle fatigue. Front Physiol. 2012;3:142 Epub 2012/05/26. doi: 10.3389/fphys.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic review. Physiother Can. 2010;62(1):25–34. Epub 2011/01/05. doi: 10.3138/physio.62.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol. 2012;2(4):2775–809. Epub 2013/05/31. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.al-Majid S, McCarthy DO. Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs. 2001;2(3):186–97. doi: 10.1177/109980040100200304. [DOI] [PubMed] [Google Scholar]
- 9.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40(3):159–64. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramage MI, Skipworth RJE. The relationship between muscle mass and function in cancer cachexia: smoke and mirrors? Curr Opin Support Palliat Care. 2018. Epub 2018/08/24. doi: 10.1097/SPC.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 11.Chang VT, Hwang SS, Feuerman M, Kasimis BS. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer. 2000;88(5):1175–83. Epub 2000/03/04. [DOI] [PubMed] [Google Scholar]
- 12.Vigano A, Dorgan M, Buckingham J, Bruera E, Suarez-Almazor ME. Survival prediction in terminal cancer patients: a systematic review of the medical literature. Palliat Med. 2000;14(5):363–74. [DOI] [PubMed] [Google Scholar]
- 13.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–39. Epub 2015/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74(1):49–94. Epub 1994/01/01. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Baltgalvis KA, Berger FG, Pena MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol (1985). 2010;109(4):1155–61. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 18.Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. 2011;1812(12):1601–6. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol (1985). 2005;98(6):2219–25. Epub 2005/05/17. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 20.Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, et al. The effect of exercise on IL-6-induced cachexia in the Apc ( Min/+) mouse. J Cachexia Sarcopenia Muscle. 2012;3(2):117–37. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Sugimoto K, Fujimoto T, Xie K, Takahashi T, Akasaka H, et al. Preventive effects of low-intensity exercise on cancer cachexia-induced muscle atrophy. FASEB J. 2019:fj201802430R Epub 2019/03/28. doi: 10.1096/fj.201802430R. [DOI] [PubMed] [Google Scholar]
- 22.Hardee JP, Mangum JE, Gao S, Sato S, Hetzler KL, Puppa MJ, et al. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol (1985). 2016;120(1):29–37. doi: 10.1152/japplphysiol.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderVeen BN, Fix DK, Montalvo RN, Counts BR, Smuder AJ, Murphy EA, et al. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin-6. Exp Physiol. 2019;104(3):385–97. Epub 2018/12/24. doi: 10.1113/EP087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol (1985). 2001;90(5):1900–8. Epub 2001/04/12. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 25.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Dis Model Mech. 2012;5(4):533–45. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fix DK, Hardee JP, Gao S, VanderVeen BN, Velazquez KT, Carson JA. The Role of gp130 in Basal and Exercise Trained Skeletal Muscle Mitochondrial Quality Control. J Appl Physiol (1985). 2018. Epub 2018/02/02. doi: 10.1152/japplphysiol.01063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth MJ, Matthews DE, Ades PA, Tischler MD, Van Buren P, Previs M, et al. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol Endocrinol Metab. 2005;288(4):E685–92. Epub 2004/11/25. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 28.Narsale AA, Puppa MJ, Hardee JP, VanderVeen BN, Enos RT, Murphy EA, et al. Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice. Oncotarget. 2016;7(37):59482–502. Epub 2016/07/28. doi: 10.18632/oncotarget.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52(1):129–97. [DOI] [PubMed] [Google Scholar]
- 30.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89. Epub 2001/10/03. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 31.Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23(8):2461–78. Epub 2015/05/16. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaVoy EC, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev. 2016;22:82–93. Epub 2016/02/09. [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–95. Epub 2005/07/21. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 34.Carson JA, Hardee JP, VanderVeen BN. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. 2016;54:53–67. Epub 2015/11/26. doi: 10.1016/j.semcdb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toth MJ, Callahan DM, Miller MS, Tourville TW, Hackett SB, Couch ME, et al. Skeletal muscle fiber size and fiber type distribution in human cancer: Effects of weight loss and relationship to physical function. Clin Nutr. 2016. doi: 10.1016/j.clnu.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2017. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderVeen BN, Fix DK, Carson JA. Disrupted Skeletal Muscle Mitochondrial Dynamics, Mitophagy, and Biogenesis during Cancer Cachexia: A Role for Inflammation. Oxid Med Cell Longev. 2017;2017:3292087 Epub 2017/08/09. doi: 10.1155/2017/3292087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnard RJ, Edgerton VR, Peter JB. Effect of exercise on skeletal muscle. I. Biochemical and histochemical properties. J Appl Physiol. 1970;28(6):762–6. Epub 1970/06/01. doi: 10.1152/jappl.1970.28.6.762. [DOI] [PubMed] [Google Scholar]
- 39.White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R201–11. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fix DK, VanderVeen BN, Counts BR, Carson JA. Regulation of Skeletal Muscle DRP-1 and FIS-1 Protein Expression by IL-6 Signaling. Oxid Med Cell Longev. 2019;2019:8908457 Epub 2019/03/29. doi: 10.1155/2019/8908457. [DOI] [PMC free article] [PubMed] [Google Scholar]


