Abstract
Background:
The role of omega-3 fatty acid in multiple sclerosis (MS) susceptibility is unclear.
Objective:
To determine whether fish/seafood intake or genetic factors that regulate omega-3 fatty acids levels are associated with MS risk.
Methods:
We examined the association of fish and shrimp consumption, 13 tag single nucleotide polymorphisms (SNPs) in FADS1, FADS2 and ELOV2 with risk of MS in 1153 individuals from the MS Sunshine Study, a case-control study of incident MS or clinically isolated syndrome (CIS), recruited from Kaiser Permanente Southern California.
Results:
Consuming fish/seafood at least once a week or at least once a month with regular fish oil use was associated with 44% reduced odds of MS/CIS (adjusted OR=0.56; 95%CI=0.41–0.76; p=0.0002) compared with consuming fish/seafood less than once a month and no fish oil supplementation. Two FADS2 SNPs (rs174611, rs174618) were independently associated with a lower risk of MS (adjusted ORs 0.74, 0.79, p=0.0056, 0.0090, respectively). Association of FADS2 SNPs with MS risk were confirmed in an independent dataset.
Conclusions:
These findings suggest that omega-3 fatty acid intake may be an important modifiable risk factor for MS. This is consistent with the other known health benefits of fish consumption and complementary genetic studies supporting a key role for omega-3 regulation.
Keywords: multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that often leads to diffuse neurodegeneration. While the cause is unknown, the rising prevalence of MS has led to increased interest in identifying modifiable risk factors including diet.
Fish or other seafood consumption is particularly interesting because it is the main determinant of circulating and tissue levels of omega-3 polyunsaturated fatty acids (omega-3 PUFAs). Omega-3 PUFAs modulate inflammation and cognition and are strongly associated with the risk of cardiovascular disease. Recently, single nucleotide polymorphisms (SNPs) in a cluster of genes that encode fatty acid biosynthesis enzymes have been directly linked to circulating and tissue levels of PUFAs1, accounting for up to 28% of the variability2. These SNPs have been used as objective surrogate measures to strengthen the causal association between omega-3 PUFAs, neurocognitive development, cardiovascular disease and other conditions3.
Whether higher fish or omega-3 consumption reduces the risk of MS is unclear. Few studies have addressed this question, employing heterogeneous methods and producing mixed results. These studies reported either a protective effect4–7, protective effect in a select subgroup only8 or no association9, 10. None of these studies examined fatty acid biosynthesis genes.
The primary objective of the analyses presented herein was to determine whether omega-3 PUFAs may be associated with a lower risk of MS by augmenting data on self-reported fish/seafood consumption and fish oil supplement use, with information on SNPs in fatty acid biosynthesis genes.
METHODS
Primary study population
Kaiser Permanente Southern California (KPSC) is a large health maintenance organization with over 4 million members representative of the general population in Southern California11. KPSC uses an integrated electronic health record (EHR) system which includes all inpatient and outpatient encounters, diagnostic tests, diagnoses and medications, and some demographic and behavioural characteristics.
Participants in the MS Sunshine Study were identified and recruited from the KPSC membership between December 2011 and December 2014 as previously described12. Briefly, potential incident cases were identified via the EHR; diagnosis of MS or CIS was confirmed by an MS Specialist (ALG). At least one control participant from the KPSC population, matched to the case on race/ethnicity, birthdate (within 2 years), sex and KPSC facility (a surrogate measure for socioeconomic status) was identified from the EHR and recruited. The controls were assigned the same index date as their matched case (symptom onset date). Detailed participation rates have been previously described12. Data were collected from the EHR, structured in-person interview and blood draw.
Standard Protocol Approvals, Registrations, and Patient Consents.
The study protocol was approved by the KPSC institutional review board. Written informed consent was obtained from all participants.
Data Collection
Covariates obtained from the in-person interview included fish/seafood consumption, fish oil or cod liver oil supplement use in the 12 months prior to symptom onset/index date and lifetime smoking history. Fish intake was assessed through 2 questions: 1) “How often did you eat 3 to 4 ounces of fresh fish (not including shrimp), mussels or raw oysters?”; and 2) “How often did you eat 3 to 4 ounces of shrimp or canned or dried fish?” with 8 categorical response options (appendix). Fish and cod liver oil supplements were obtained as follows: “did you use any of the following dietary or vitamin supplements on a regular basis at least 3 times a week for 1 month or more?” Response options yes (recorded type/brand, dose, frequency, start and stop dates), no or don’t know.
Self-identified race/ethnicity was obtained from the interview. White, non-Hispanics were classified as white; any black race regardless of ethnicity was classified as black; and those who identified themselves as white and Hispanic were classified as Hispanics.
Genotyping
DNA samples were genotyped for HLA-DRB1*15:01 status via tag SNP (rs3135388). Genetic ancestry was determined with the software STRUCTURE Version 2·3·113 using a genome-wide set of 67547 linkage disequilibrium pruned loci selected by PLINK 1·0714 as previously described15. SNPs in fatty acid desaturase 1 and 2 (FADS1, FADS2) and elongation of very long-chain fatty acids 2 (ELOV2) region were genotyped, followed by imputation using standard methods (appendix).
FADS1, FADS2 and ELOV2 SNP selection
We selected 2 SNPs a priori, s174548 on FADS1 and rs3734398 on ELVOL2, based on a large meta-analysis of genetic variants that modulate fatty acid levels and cardiovascular risk and are stable across racial/ethnic groups1, 16.
FADS2 SNP selection was more challenging as there is little consistency in the literature on precisely which SNPs on FADS2 are associated with disease status3. There are 95 imputed SNPs in FADS2 with MAF no less than 10%, and 81 of them have missing values less than 10%. A total of 16 tag SNPs capturing common variation across FADS2 were studied. Six of 16 tag SNPs (rs968567, rs99780, rs174570, rs174575, rs2727271, and rs498793) were selected because they have been associated with modulating EPA levels following fish oil supplementation17. Exploratory analyses of the remaining 10 tag SNPs (rs93923, rs2851682, rs174592, rs174593, rs174611, rs174618, rs639394, rs34013632, rs174622, and rs11539526) were also conducted18.
Statistical analysis
The genotyping data were available on 1159 (97.6%) of the 1187 participants who had completed the study protocol by 6/2/2015. Six participants were excluded due to missing data. The final analysis cohort included 1153 participants: 252 Blacks, 386 Hispanics, and 515 whites.
Multivariate logistic regression models were used to evaluate the associations of fish/seafood intake and the additive effect of each copy of the minor allele (effect allele) in fatty acid biosynthesis SNPs with MS. The models were adjusted for age at index date, sex, HLA-DRB1*15:01 status19, genetic ancestry and smoking (ever/never). Significance threshold was set at p<0.05.
Fish/seafood/fish oil consumption was classified a priori as low if subjects reported never or rare (<1 time/month) seafood consumption and no fish oil supplement use. High and medium consumption was based on a combination of fish/seafood consumption and fish oil (omega-3) supplement use. First, we defined high and medium fish/seafood consumption based on the distribution in controls as follows: high was defined as consuming fish/seafood at least once a week and medium intake was defined as 1–3 times per month. To account for PUFA intake through supplements, subjects who reported medium fish/seafood intake but also regularly (≥3 times/week) used fish oil supplements were classified as high intake, and those who reported no or rare (<1/month) fish/seafood intake but regularly used fish oil supplements were classified as medium intake. We were unable to examine cod liver oil supplement use as fewer than 10 participants reported regular use. To determine whether the effect of fish/seafood/fish oil consumption varied by race/ethnicity we tested for multiplicative interaction. No significant interaction was detected; thus, all models were adjusted for genetic ancestry.
For those fatty acid biosynthesis SNPs that met the threshold for independent association with MS (FADS2: rs174611 and rs174618), we then tested whether these associations were independent of fish/seafood/fish oil consumption. Tests for multiplicative or additive interactions were also performed (appendix).
Sensitivity analyses included restricting the analysis to fresh fish/mussels/raw oyster consumption only (no shrimp, canned or dried fish or supplement use); additional adjustment for socioeconomic status; and adjustment for deseasonalized serum 25-hydroxyvitamin D levels as previously described12.
Chi-square and Fisher Exact test was used to compare the frequencies of binary variables, Wilcoxon rank sum test was used to compare the medians of continuous variables between cases and controls. All analyses were conducted using SAS software v9.3 (Cary, NC).
Replication Dataset
To determine whether the tag SNPs in FADS2 that showed a nominally significant independent association with MS risk in our cohort were chance findings, the association of these SNPs (rs174611, rs174618, rs174622) with MS were tested in a large, independent dataset comparing white cases recruited from the Pediatric MS Network (n=486) and a large population of white controls recruited largely from KP Northern California (n=1,362), as previously described20, 21 (appendix).
RESULTS
Table 1 shows fish intake and selected clinical and demographic characteristics in cases and controls. Low fish intake was common in both cases and controls. Approximately 25% of participants rarely consumed any seafood and did not use fish oil supplements in the 12 months prior to symptom onset. Over 40% of participants reported rare fresh fish consumption. Cases were more likely to carry at least one HLA-DRB1*15:01 risk allele or to have smoked than controls. The median time from diagnosis to interview among cases was 10.3 months (interquartile range 7.1–15.3 months).
Table 1:
Selected Characteristics and Fish intake at Multiple Sclerosis Symptom Onset/Index Date
| Case (n=552) | Control (n=601) | Total (n=1153) | P | |
|---|---|---|---|---|
| Age, yrs, median (Q1,Q3) | 36 (27, 45) | 36 (27, 46) | 36 (27, 46) | 0.8554 |
| Disease duration, yrs, median (Q1, Q3) | 1.7 (0.9,3.2) | |||
| Female sex, n (%) | 392 (71.0) | 427 (71.0) | 819 (71.0) | 0.9899 |
| Race/Ethnicity, n (%) | 0.9707 | |||
| Black | 119 (21.6) | 133 (22.1) | 252 (21.9) | |
| Hispanic | 186 (33.7) | 200 (33.3) | 386 (33.5) | |
| White | 247 (44.7) | 268 (44.6) | 515 (44.7) | |
| Ever smoker, n (%) | 188 (34.1) | 161 (26.8) | 349 (30.3) | 0.0073 |
| HLA-DRB1*15:01*, n (%) | <0.0001 | |||
| GG | 370 (67.0) | 509 (84.7) | 879 (76.2) | |
| AG | 161 (29.2) | 86 (14.3) | 247 (21.4) | |
| AA | 21 (3.8) | 6 (1.0) | 27 (2.3) | |
| Fresh fish/mussels/oysters**, n (%) | 0.0044 | |||
| Never or less than once a month | 282 (51.1) | 249 (41.4) | 531 (46.1) | |
| 1–3 per month | 151 (27.4) | 194 (32.3) | 345 (29.9) | |
| once a week or more | 118 (21.4) | 157 (26.1) | 275 (23.9) | |
| Canned fish or shrimp**, n (%) | 0.2614 | |||
| Never or less than once a month | 237 (42.9) | 230 (38.3) | 467 (40.5) | |
| 1–3 per month | 208 (37.7) | 246 (40.9) | 454 (39.4) | |
| once a week or more | 106 (19.2) | 125 (20.8) | 231 (20.0) | |
| Fish oil supplement use**, n (%) | 0.0121 | |||
| yes | 66 (12.0) | 103 (17.1) | 169 (14.7) | |
| Fish/seafood/fish oil intake**, n (%) | 0.0013 | |||
| low | 163 (29.5) | 131 (21.8) | 294 (25.5) | |
| medium | 209 (37.9) | 219 (36.4) | 428 (37.1) | |
| high | 180 (32.6) | 251 (41.8) | 431 (37.4) | |
Abbreviations: yrs=years; Q1=first quartile; Q3=third quartile;
tag SNP rs3135388
in the 12 months prior to symptom onset/index date; 1 case could not recall fresh or canned fish intake; 1 control - fresh fish intake, and 2 controls could not recall fish oil supplement use
Fish/seafood/fish oil consumption and MS
Cases reported significantly less fresh fish (p=0.004) and less fish oil supplement intake (p=0.01) in the 12 months prior to symptom onset/index date, but similar consumption of canned fish/shrimp (p=0.26) than controls. When these sources of seafood and fish oil supplements are considered in combination, cases reported significantly less fish/seafood/fish oil consumption compared to controls (p=0.001, Table 1). Most subjects in the high or medium intake category were classified as such by fish/seafood consumption (86% and 92%, respectively) rather than supplement use.
Higher consumption of fish/seafood /fish oil was associated with a reduced risk of MS/CIS in a dose-dependent fashion even after adjusting for age, sex, smoking, HLA-DRB1*15:01 and genetic ancestry (Figure 1). Accounting for 25OHD levels or socioeconomic status showed similar results (appendix). Analyses examining fresh fish/mussels/oysters intake only showed a similar association with reduced MS risk but an attenuated dose effect (medium intake, adjusted OR=0.70, 95% CI 0.53–0.93; high intake, OR=0.68, 95% CI 0.50–0.92, low intake reference group, p(trend)= 0.011).
Figure 1. Fish/Seafood/Fish Oil Consumption and Multiple Sclerosis.
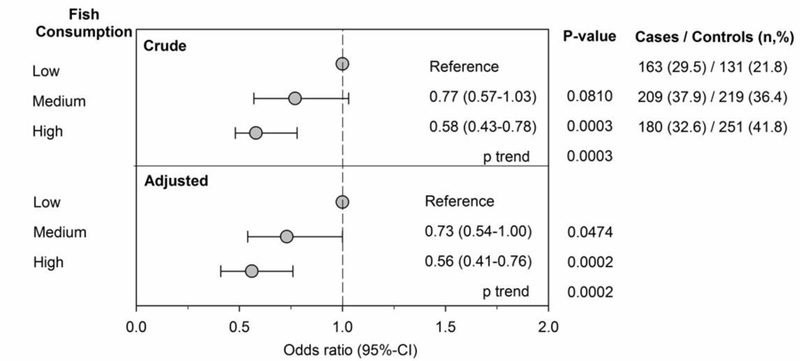
Depicted are the crude (upper panel) and adjusted (lower panel) odds ratios (OR) and 95% confidence intervals (95% CI) of the association between the consumption of fish/seafood/fish oil supplements in the 12 months prior to symptom onset (cases) or index date (controls) and multiple sclerosis/clinically isolated syndrome. The number (n) and percent (%) of cases and matched controls in each analysis are listed in the right-hand column. Fish/seafood/fish oil consumption was classified as high (≥1 per week fish/seafood, or 1–3/month + fish oil), medium (1–3/month fish/seafood + no fish oil, or fish oil only) or low (never or ≤ 1/month fish/seafood and no fish oil). OR are adjusted for age, smoking, genetic ancestry and HLADRB1*15:01. Higher fish/seafood/fish oil consumption was associated with a significantly lower risk of MS/CIS in a dose-response manner in both crude and adjusted analyses (p=0.0003 and 0.0002 for trend, respectively).
Polymorphisms in FADS1, FADS2, ELOV2 and MS
We found no statistically significant association between MS risk in crude or adjusted analyses and SNPs in FADS1, ELOVL2 or the 6 SNPs on FADS2 selected based on published literature (Table 2). Of the 10 exploratory tag SNPs on FADS2, carrying each one copy of the minor allele (C) in two (rs174611, rs174618) tag SNPs were significantly associated with reduced MS risk compared to the major allele (T, Table 2). The independent association of carrying the minor allele in these two FADS2 tag SNPs (rs174611, rs174618) and fish/seafood/fish oil intake with a reduced risk of MS remained significant in mutually adjusted models (Table 3). No significant additive or multiplicative interactions were detected between these 2 SNPs and fish/seafood/fish oil intake on MS risk (appendix).
Table 2.
Association of tag SNPs on fatty acid biosynthesis genes with multiple sclerosis risk
| MS Sunshine Study | Replication Dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude* | Adjusted OR (95% CI)*** | Adjusted OR (95% CI)**** | |||||||||
| Gene | SNP | major/minor allele | p value | OR | 95% CI | p value | OR | 95% CI | p value | ||
| FADS1 | rs174548** | C/G | 0.13 | 0.88 | 0.73 | 1.05 | 0.15 | nt | |||
| ELOVL2 | rs3734398** | T/C | 0.41 | 0.96 | 0.80 | 1.14 | 0.63 | nt | |||
| FADS2 | rs968567** | C/T | 0.21 | 0.82 | 0.63 | 1.07 | 0.15 | nt | |||
| rs99780** | C/T | 0.15 | 0.89 | 0.74 | 1.06 | 0.18 | nt | ||||
| rs174570** | C/T | 0.39 | 0.96 | 0.76 | 1.21 | 0.71 | nt | ||||
| rs174575** | C/G | 0.91 | 1.01 | 0.83 | 1.22 | 0.95 | nt | ||||
| rs2727271** | A/T | 0.20 | 0.89 | 0.69 | 1.14 | 0.35 | nt | ||||
| rs498793** | C/T | 0.96 | 1.00 | 0.85 | 1.19 | 0.96 | nt | ||||
| rs93923 | C/T | 0.85 | 0.99 | 0.80 | 1.24 | 0.96 | nt | ||||
| rs2851682 | A/G | 0.0269 | 0.77 | 0.59 | 1.00 | 0.05 | nt | ||||
| rs174592 | A/G | 0.79 | 1.00 | 0.83 | 1.19 | 0.96 | nt | ||||
| rs174593 | T/C | 0.71 | 1.04 | 0.86 | 1.26 | 0.71 | nt | ||||
| rs174611 | T/C | 0.0063 | 0.74 | 0.60 | 0.92 | 0.0056 | 0.83 | 0.70 | 0.98 | 0.0287 | |
| rs174618 | T/C | 0.0162 | 0.79 | 0.66 | 0.94 | 0.0090 | 0.86 | 0.74 | 1.01 | 0.0651 | |
| rs639394 | A/G | 0.59 | 0.95 | 0.69 | 1.31 | 0.76 | nt | ||||
| rs34013632 | GT/G | 0.55 | 0.93 | 0.71 | 1.22 | 0.61 | nt | ||||
| rs174622 | G/A | 0.0288 | 0.77 | 0.62 | 0.96 | 0.0206 | 0.95 | 0.78 | 1.17 | 0.6587 | |
| rs11539526 | C/T | 0.33 | 0.87 | 0.67 | 1.13 | 0.29 | nt | ||||
additive genetic model, the OR measures the change in odds of having MS associated with carrying each one copy of the minor allele compared to the major allele
a priori tag SNPs selected from published literature
adjusted for age at index date, sex, HLA-DRB1*15:01 status, genetic ancestry, and smoking
adjusted for age at index date, sex, HLA-DRB1*15:01 status and genetic ancestry
Abbreviations: fatty acid desaturase 1 and 2 (FADS1, FADS2), elongation of very long-chain fatty acids 2 (ELOV2), SNP single nucleotide polymorphisms, OR=odds ratio, CI=confidence interval, nt=not tested
Table 3.
Independent associations of high fish/seafood/fish oil intake and FADS2 tag SNPs with MS risk
| FADS2 SNP | Adjusted OR (95% CI)* | |||
|---|---|---|---|---|
| OR | LCL | UCL | p value | |
| rs174611 (effect allele, C) | 0.75 | 0.60 | 0.92 | 0.007 |
|
fish/seafood/fish oil intake** medium |
0.74 | 0.55 | 1.01 | 0.058 |
| high | 0.56 | 0.41 | 0.77 | 0.0003 |
| rs174618 (effect allele, C) | 0.80 | 0.67 | 0.96 | 0.014 |
|
fish/seafood/fish oil intake** medium |
0.74 | 0.54 | 1.00 | 0.051 |
| high | 0.57 | 0.41 | 0.77 | 0.0004 |
odds ratios additionally adjusted for age, sex, smoking, genetic ancestry and HLADRB1*15:01
reference group is low intake group abbreviations: OR=odds ratio, LCL=lower confidence limit, UCL=upper confidence limit, FADS2=fatty acid desaturase 2, SNP=single nucleotide polymorphism
In the replication dataset, the direction and magnitude of effect for FADS2 SNPs rs174611 and rs174618 were consistent with our initial findings. Although rs174618 did not reach statistical significance, 2 SNPs within the same LD block did (rs11407273 adjusted OR=0.82, 95%CI=0.69–0.96, P=0.01293; rs35622765 adjusted OR=0.82, 95%CI=0.70–0.96, P=0.01664). The third tag SNP (rs174622) with nominal significance (P<0.05) in our initial analyses showed no association (Table 2).
DISCUSSION
We found that consuming fish/seafood at least once a week or at least once a month in addition to regular fish oil supplements, was associated with 44% lower odds of MS compared to those who rarely or never consume fish/seafood/fish oil. In addition, common polymorphisms in the fatty acid biosynthesis gene, FADS2, were also associated with MS risk. Taken together with previous studies, these findings suggest that omega-3 PUFAs may play an important role in reducing MS risk.
Omega-3 PUFAs are central in maintaining health, including visual and cognitive development and cardiovascular health. They are present in a wide array of tissues with broad functions including the active component of phospholipid cell membranes and substrate for molecular signalling pathways. Omega-3 PUFAs have been shown to be neuroprotective during aging22 and suppress MS-related inflammation through multiple mechanisms23, 24 in animal models. This provides at least two biologically plausible mechanisms whereby higher omega-3 PUFA intake and biosynthesis could protect against development of MS.
Tissue and circulating levels of omega-3 PUFAs are chiefly dependent on direct dietary intake from fish or shellfish or intake of their precursors from plants, which then require conversion to physiologically active omega-3 PUFAs. High fish intake is strongly associated with a lower risk of cardiovascular disease25 and metabolic syndrome26 and consuming fish rich in omega-3 PUFAs at least 2 times a week is part of the American Heart Association’s heart healthy diet recommendations27.
Over the past decade, a cluster of genes has been identified that also contribute to the regulation of PUFA levels in the body1. These genes, fatty acid desaturase 1 and 2 (FADS1, FADS2) and elongation of very long-chain fatty acids 2 (ELOV2), regulate desaturation and elongation of plant-derived PUFA precursors to physiologically active omega-3 PUFAs. Scientists have utilized large GWAS studies of SNPs in these genes as a more objective method of examining the role of omega-3 PUFAs in complex diseases. Perhaps not surprisingly, polymorphisms in these genes have been associated with neurodevelopment, cognition, cardiovascular disease, metabolic syndrome and inflammation3. Whether they play a role in MS susceptibility has until now not been studied.
The few published studies of MS and omega-3/fish consumption4–10 employed very heterogeneous methods which makes it difficult to draw clear conclusions. These methodological variations include: 1) definition of the exposure, ranging from any fish8, 10, fatty or non-fatty fish5, cod liver oil6 or total omega-3 intake7, 9; 2) timing of the exposure, ranging from childhood6, adolescence6, adulthood prior to diagnosis8, 9, 12 months prior to symptom onset7, after diagnosis10 or overlapping before and after diagnosis5; 3) ascertainment of the exposure, ranging from validated full food frequency8, 9 or fatty acid intake questionnaires7 to unspecified10 or unvalidated instruments5, 6; and 4) overall study design ranging from prevalent case/control6, 10, incident case/control5, 7, 8, to prospective cohort study9 recruited from population-based5–8, 10 or selected sources9. Our study design is most like the recent Australian population-based incident case-control study7, although we did not use a complete food frequency questionnaire to assess diet as they did. None of these studies also examined PUFA biosynthesis genes.
Most studies reported a protective association as we did between their definition of fish or omega-3 PUFA intake, particularly in the 127 or 24 months5 prior to symptom onset. One study reported a protective effect of fish consumption only in females8, and another reported no association between total omega-3 PUFA intake and MS risk9. Whether the discrepancy of these findings with ours is due to differences in definition of the exposure, change in the MS diagnostic criteria, cohort effect, or selection bias9 is unclear.
A small number of clinical trials investigated the hypothesis that omega-3 PUFA supplementation improves prognosis in MS28–30; however, the results are inconclusive, at least partly due to limitations in study design. To our knowledge, no clinical trials have examined omega-3 PUFA supplementation and MS risk.
While some authors concluded that the protective association with fish and MS is due to vitamin D (vitD)5, 6, we hypothesized that omega-3 PUFAs also play a key role. Many fish and shellfish are rich in omega-3 PUFAs yet most are only a minor source of vitD which is mainly derived from sun exposure. In addition, the vitD content of seafood varies widely even among oily fish that typically contain the highest amounts. For instance, the vitD content of the most popular seafoods consumed in California range from only 3 IU per 3oz serving of shrimp, to 68 (canned tuna), 193 (fresh tuna) and as high as 375 IU (fresh salmon) per 3oz serving of these oily fish. The recommended daily intake of vitD is at least 400IU. In contrast, the omega-3 PUFA content of shrimp, canned tuna, fresh tuna and fresh salmon is 0.5g, 0.94g, 1.2g and 3.3g respectively with a recommended adequate daily intake of 1.1–1.6 grams31. Furthermore, when we restricted the analysis to vitD-rich seafood, we no longer detected a dose-response relationship with MS risk as when we included the major sources of seafood-based omega-3 PUFAs. This, taken together with the association of SNPs in the omega-3 PUFA biosynthesis genes and MS risk, supports an important role for omega-3 PUFAs in MS risk.
Some scientists have suggested that high fish consumption may be particularly important in maintaining health in people with genetic adaptations to a high fish diet32 (i.e. ‘lazy’ PUFA biosynthesis genotypes), but evidence for interaction between genotype and fish intake on adverse health outcomes to date is very weak33. It appears a high fish diet is good for everyone regardless of genotype, which is consistent with our findings. Like other investigators3, we were interested in examining the SNP associations in FADS and ELOV2 with disease as surrogate for tissue and circulating omega-3 PUFAs to improve our understanding of whether omega-3s are protective as opposed to our results being due to residual confounding34.
While we favour a protective association of higher omega-3 intake from fish/seafood/fish oil and MS risk, the main limitation of this study is that we measured only certain foods. Therefore, we cannot exclude a substitution effect- that those who rarely consume fish/seafood consume more foods that increase the risk of MS. In particular, we did not measure meat consumption, an important source of omega-6 PUFAs and saturated fats. This is important because FADS1 and FADS2 are also the main enzymes that convert omega-6 precursors to physiologically active omega-6 PUFAs. Ecological studies and a few case-control studies have suggested that a diet high in saturated fat and meats may increase this risk of MS35–37, although other, methodologically more rigorous studies showed no association between total omega-6 PUFA or meat intake and MS risk7, 9. We also did not measure plant-based sources of omega-3 PUFAs or circulating or tissue levels of PUFAs which would be ideal. Future studies should address these limitations.
A further limitation of our study is that we did not use an established food frequency questionnaire or otherwise validated questionnaire to capture fish or omega-3 intake. Our questions did not separate shrimp and canned fish, or distinguish between oily and non-oily fish. Fresh oily fish and canned fish (which is likely to be oily fish) are higher in omega-3 PUFA than shrimp or fresh, non-oily fish. In addition, we did not assess participants’ ability to judge a 3–4 ounce serving. Thus, the cut-points chosen in our study for defining high and medium fish/seafood/fish oil intake should be interpreted cautiously. While our findings support a dose-effect, precisely how much omega-3 consumption may be protective in MS needs to be addressed in future studies with more rigorous measures.
Because MS is an uncommon disease, the appropriate study type is the case-control design and exposures are typically recalled. Nevertheless, dietary intake is typically recalled, regardless of the study design, over some prior period; measured intake at a single timepoint may not be relevant to risk for a disease such as MS. Furthermore, recall bias in dietary recall is unlikely to be differential as diet is not commonly considered a risk factor for MS. Selection bias of controls is another potential limitation although the FADS and ELOV2 SNP distributions are consistent with expected rates from multi-ethnic population-based studies1. We inquired only about the timeframe 12-months prior to symptom onset; thus, we cannot address whether diet during childhood or adolescence influences MS risk. We also cannot exclude the possibility of false-positive or negative associations of SNPs in FADS2 and FADS1 or true interaction between fish/seafood/fish oil intake and MS risk given our sample size.
Strengths of this study include that it is a population-based multi-ethnic study of incident MS cases, all recruited from same geographic area over a relatively short period of time, thus minimizing the potential for selection bias, confounding by geography, influence of marked changes in MS diagnostic criteria or a birth cohort effect. Replication of genetic findings in an independent dataset is also a strength. Our approach to simultaneously examine fish, other seafood, supplement use, and fatty acid biosynthesis genes is novel. That the genetic and fish/seafood intake findings are consistent strengthens a causal interpretation34.
Taken together with the existing literature, these analyses provide more evidence that a diet rich in fish/seafood has health benefits. In addition to promoting improved cardiovascular health, a high-fish/seafood diet may also reduce the risk of developing MS. It is concerning that ~25% of our study population rarely consumed fish/seafood and do not take fish oil. Future studies to replicate our findings and determine whether these are mediated by the anti-inflammatory, metabolic and/or neuroprotective actions of PUFAs are needed.
Supplementary Material
ACKNOWLEDGEMENTS
Conflicts of interest:
Annette Langer-Gould was site principal investigator for one industry-sponsored phase 3 clinical trial (Biogen Idec). She has received grant support and awards from the National Institutes of Health, the Patient Centered Outcomes Research Institute, and the National MS Society. She currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review.
Emmanuelle Waubant is site principal investigator for industry-sponsored phase 3 and 4 trials (Roche, Novartis). She receives grant support from the National MS Society, the Patient Centered Outcomes Research Institute and Race to Erase MS.
Robyn Lucas receives grant support from Cancer Australia and the National Health and Medical Research Council of Australia.
Lucinda Black, Jessica Smith, Jun Wu, Edlin Gonzales, Corinna Koebnick and Anny Xiang report no disclosures.
Study funding: This research was supported by NIH (NINDS 1R01NS075308 Langer-Gould; NIH/NIEHS R01 ES017080 Barcellos; NINDS R01NS071463 Waubant)
Role of the sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Lemaitre RN, Tanaka T, Tang W, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet 2011; 7: e1002193 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaeffer L, Gohlke H, Muller M, et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet 2006; 15: 1745–1756. 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 3.Lattka E, Illig T, Heinrich J, et al. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin Nutr 2010; 29: 277–287. 10.1016/j.clnu.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Kampman MT, Wilsgaard T and Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 2007; 254: 471–477. 10.1007/s00415-006-0395-5. [DOI] [PubMed] [Google Scholar]
- 5.Baarnhielm M, Olsson T and Alfredsson L. Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult Scler 2014; 20: 726–732. 10.1177/1352458513509508. [DOI] [PubMed] [Google Scholar]
- 6.Cortese M, Riise T, Bjornevik K, et al. Timing of use of cod liver oil, a vitamin D source, and multiple sclerosis risk: The EnvIMS study. Mult Scler 2015; 21: 1856–1864. 10.1177/1352458515578770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoare S, Lithander F, van der Mei I, et al. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: Results from the Ausimmune Study. Mult Scler 2016; 22: 884–892. 10.1177/1352458515604380. [DOI] [PubMed] [Google Scholar]
- 8.Ghadirian P, Jain M, Ducic S, et al. Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol 1998; 27: 845–852. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SM, Willett WC, Hernan MA, et al. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am J Epidemiol 2000; 152: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 10.Berr C, Puel J, Clanet M, et al. Risk factors in multiple sclerosis: a population-based case-control study in Hautes-Pyrenees, France. Acta Neurol Scand 1989; 80: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koebnick C., Langer Gould A, Gould MK, et al. Do the sociodemographic characteristics of members of a large, integrated health care system represent the population of interest? Permanente Journal 2012; 16: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer-Gould A, Lucas R, Xiang AH, et al. MS Sunshine Study: Sun Exposure But Not Vitamin D Is Associated with Multiple Sclerosis Risk in Blacks and Hispanics. Nutrients 2018; 10 10.3390/nu10030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard JK, Stephens M and Donnelly P. Inference of population structure using multilocus genotype data. Genetics 2000; 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 2007; 81: 559–575. 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langer-Gould A, Wu J, Lucas R, et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: A multiethnic study. Neurology 2017; 89: 1330–1337. 10.1212/WNL.0000000000004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CE, Follis JL, Nettleton JA, et al. Dietary fatty acids modulate associations between genetic variants and circulating fatty acids in plasma and erythrocyte membranes: Meta-analysis of nine studies in the CHARGE consortium. Mol Nutr Food Res 2015; 59: 1373–1383. 10.1002/mnfr.201400734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meldrum SJ, Li Y, Zhang G, et al. Can polymorphisms in the fatty acid desaturase (FADS) gene cluster alter the effects of fish oil supplementation on plasma and erythrocyte fatty acid profiles? An exploratory study. Eur J Nutr 2017. 10.1007/s00394-017-1529-5. [DOI] [PubMed]
- 18.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265. 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 19.Waubant E, Mowry EM, Krupp L, et al. Antibody response to common viruses and human leukocyte antigen-DRB1 in pediatric multiple sclerosis. Mult Scler 2013; 19: 891–895. 10.1177/1352458512469693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianfrancesco MA, Stridh P, Rhead B, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology 2017; 88: 1623–1629. 10.1212/WNL.0000000000003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianfrancesco MA, Stridh P, Shao X, et al. Genetic risk factors for pediatric-onset multiple sclerosis. Mult Scler 2017: 1352458517733551 10.1177/1352458517733551. [DOI] [PMC free article] [PubMed]
- 22.Freitas HR, Ferreira GDC, Trevenzoli IH, et al. Fatty Acids, Antioxidants and Physical Activity in Brain Aging. Nutrients 2017; 9 10.3390/nu9111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim W and Lee H. Advances in nutritional research on regulatory T-cells. Nutrients 2013; 5: 4305–4315. 10.3390/nu5114305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen MJ, Fan YY, Monk JM, et al. n-3 PUFAs reduce T-helper 17 cell differentiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4(+) T cells. J Nutr 2014; 144: 1306–1313. 10.3945/jn.114.194407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saravanan P, Davidson NC, Schmidt EB, et al. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010; 376: 540–550. 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 26.Vessby B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol 2003; 14: 15–19. 10.1097/01.mol.0000052859.26236.5f. [DOI] [PubMed] [Google Scholar]
- 27.AHA. Fish and Omega-3 Fatty Acids, http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/HealthyDietGoals/Fish-and-Omega-3-Fatty-Acids_UCM_303248_Article.jsp#.WmAg0K6nGHs (accessed January 18 2018).
- 28.Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG, et al. Efficacy of fish oil on serum of TNF alpha , IL-1 beta , and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid Med Cell Longev 2013; 2013: 709493 10.1155/2013/709493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torkildsen O, Wergeland S, Bakke S, et al. omega-3 fatty acid treatment in multiple sclerosis (OFAMS Study): a randomized, double-blind, placebo-controlled trial. Arch Neurol 2012; 69: 1044–1051. 10.1001/archneurol.2012.283. [DOI] [PubMed] [Google Scholar]
- 30.Bates D, Cartlidge NE, French JM, et al. A double-blind controlled trial of long chain n-3 polyunsaturated fatty acids in the treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry 1989; 52: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIoH. Omega-3 Fatty Acids: Fact Sheet for Health Professionals, https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/#h2 (accessed January 19 2018).
- 32.Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 2015; 349: 1343–1347. 10.1126/science.aab2319. [DOI] [PubMed] [Google Scholar]
- 33.Minihane AM. Impact of Genotype on EPA and DHA Status and Responsiveness to Increased Intakes. Nutrients 2016; 8: 123 10.3390/nu8030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponsonby AL and Dwyer T. Statistics: Biomedicine must look beyond P values. Nature 2014; 507: 169 10.1038/507169b. [DOI] [PubMed] [Google Scholar]
- 35.Swank RL. Multiple sclerosis; a correlation of its incidence with dietary fat. Am J Med Sci 1950; 220: 421–430. [PubMed] [Google Scholar]
- 36.Jahromi SR, Toghae M, Jahromi MJ, et al. Dietary pattern and risk of multiple sclerosis. Iran J Neurol 2012; 11: 47–53. [PMC free article] [PubMed] [Google Scholar]
- 37.Zorzon M, Zivadinov R, Nasuelli D, et al. Risk factors of multiple sclerosis: a case-control study. Neurol Sci 2003; 24: 242–247. 10.1007/s10072-003-0147-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


