Abstract
Objective
There is little evidence to guide patient selection for subdural hemorrhage (SDH) evacuation. This study was designed to assess the benefit of surgical evacuation of SDH, identify predictors of functional outcome, and create a bedside score to guide the clinical management of SDH.
Methods
A cohort of 331 patients presenting to a single center from 2010 to 2014 with a principal diagnosis of subdural hemorrhage was identified. Clinical and radiographic information were extracted from the medical record. Outcomes of interest were (1) the occurrence of surgical evacuation of SDH, and (2) an unfavorable 90-day functional status represented by a modified Rankin score (mRS) ≥ 3. Propensity score matching and adjustment techniques were employed to assess the benefit of surgery accounting for confounding by indication. Multivariable logistic regression models predicting follow-up functional outcome were generated and bootstrapped separately among those with acute SDH and those with either subacute or chronic SDH. Clinical scores were created using model coefficients.
Results
In this cohort [65% male, mean age 67 years], 47% underwent surgery. Age, focal neurologic deficit, SDH thickness > 10 mm, midline shift > 5mm, and SDH acuity predicted undergoing surgery. Propensity score matching analysis demonstrated that operated patients overall were less likely to have unfavorable 90-day mRS outcome (OR 0.35, 95% C.I. 0.15–0.82). Among patients with acute SDH, age, female sex, pre-admission mRS, focal neurologic deficit, and neuropsychiatric symptoms predicted 90-day functional outcome (c-statistic 0.89, optimism-corrected c-statistic 0.87) and were incorporated into an acute SDH score (range 1—10). Patients with SDH score > 4 were significantly more likely to have an unfavorable outcome if treated medically versus surgically; there was no difference in 90-day functional status by treatment strategy among patients with SDH score ≤ 4. No difference in outcome was seen by surgical status across the spectrum of chronic SDH scores.
Conclusions
Surgical evacuation of subdural hematomas overall is associated with favorable outcome. Patient selection for evacuation is enhanced by the application of the acute SDH score. Future studies are necessary to validate the SDH score in an external cohort.
Keywords: Intracranial hemorrhages, Hematoma, Subdural, Epidemiologic methods, Outcome
Introduction
Acute subdural hematoma (SDH) is a common neurological emergency. Epidemiological data are limited but prior studies report a frequency of 11%—20% in patients with head injury.1,2 Chronic SDH also occur frequently, with an incidence of 1.7–20.6 per 100,000 individuals per year.3 SDH can manifest with headache, psychiatric manifestations, cognitive impairment, seizures, and focal neurologic deficits.4 Inpatient costs are high5,6, and mortality rates range from 55% to 80%.3,7
SDH management is based largely on institutional/personal experience and anecdotal evidence. The Surgical Management of Traumatic Brain Injury Author Group recommends surgical evacuation for acute SDH with thickness > 10 mm or midline shift > 5 mm, regardless of Glasgow Coma Scale (GCS) score at presentation.8 These guidelines were based on small studies that did not account for baseline clinical and demographic characteristics, and confounders such as “do not resuscitate/do not intubate” (DNR/DNI) code status that could affect the decision to operate or the eventual clinical outcome. There are no guidelines for subacute or chronic SDH management. In the absence of robust evidence-based guidelines, decision-making in the emergency department becomes challenging. The aims of our study were to assess the benefit of surgery, identify independent predictors of poor outcome, and create a bedside score to guide surgical decision-making.
Methods
This retrospective cohort study was designed in accordance with STROBE recommendations and approved by our hospital’s Human Research Committee.
Patients
Using the Massachusetts General Hospital’s Research Patient Database Repository, we identified 827 consecutive patients presenting to our hospital from September 2010 to September 2014 with International Classification of Dis- eases-9 discharge code 432.1 (SDH). Data abstraction was completed for 400 randomly-selected adult patients with a first occurrence of SDH. Patients with pre-SDH modified Rankin (mRS) score 4 or 5 (n = 20), DNR/DNI code status (n = 22), and missing 90-day mRS scores (n = 27) were excluded, yielding 331 patients with complete data.
SDH management
In our hospital, SDH patients are initially evaluated in the emergency department. SDH is identified by non-contrast head CT. Anti-thrombotics are held and any coagulopathy is reversed as clinically indicated. Traumatic SDH patients receive seizure prophylaxis for 7 days. Witnessed seizures are treated with anti-epileptics. Patients with suspected seizures or unexplained altered sensorium are monitored with electroencephalogram. Steroids are not usually administered. GCS is recorded on admission and daily thereafter. Patients are monitored every 4 X and more often if warranted. Decisions regarding surgery are made collaboratively by neurosurgeons and neurologists, based on SDH location, size, mass effect, midline shift, acuity, patient age and co-morbidities, the degree of neurological deficits, and patient preference including prior DNR/DNI status. Intracranial pressure monitoring is performed if clinically warranted. Inpatient rehabilitation therapy is routinely offered. Follow-up visits are typically scheduled around 3 months.
Clinical and imaging data
Chart review was performed by neurology co-investigators (R.S., E.R, M. P.) to extract the following variables: demographics, baseline mRS score, medical co-morbidities, pre-admission antithrombotic usage, admission GCS, presence of delirium, focal neurologic deficits (ani- socoria, aphasia, neglect, visual field defect, weakness, numbness), and clinical/electrographic seizure. The premorbid Charlson Index score9 was computed since it incorporates factors important for management decisions such as age, dementia, prior stroke, cancer and others, and is a validated outcome predictor.9 Head CT and brain MRI data, collected from clinical radiology reports, included SDH chronicity (acute, subacute, chronic), laterality (left, right, both), location (convexity, tentorium, both), diameter >10 mm (yes/no), and midline shift > 5 (mm). Patients were coded as having surgery if they underwent SDH evacuation by burr hole, mini-craniotomy, full craniotomy or craniectomy during the admission. Follow-up clinical and physical therapy notes were reviewed to capture mRS scores. A 90-day mRS score ≥ 3 was an unfavorable outcome.
Statistical analysis
All analyses were performed using SAS 9.4 (Cary, NC). Unpaired t-test and χ2 test were used as appropriate for the univariate analysis. A value of p < 0.05 for 2-sided hypothesis tests was considered statistically significant.
Predictors of surgery
To account for factors that influence surgical evacuation (and in turn clinical outcome), we first performed univariate and multivariable stepwise logistic regression analyses to identify predictors of surgery. To address biases that might drive surgical decision-making, we used the predicted probabilities derived from covariates in the multivariable logistic regression model to create a ‘propensity to undergo surgery’ score for each patient.
Predictors of outcome
Two propensity-based methods were used to determine if surgical evacuation is associated with an unfavorable outcome compared with conservative management. Logistic regression was performed without and with adjustment for the propensity to undergo surgery score. Additionally, patients in the surgical and nonsurgical groups were matched 1:1 on their individual propensity scores using a published SAS macro10 where the threshold for matching was defined by the formula: absolute difference of 0.2* standard deviation of the logit of the propensity score. Fifty-seven matched pairs identified were included in a separate logistic regression model with 90-day mRS ≥ 3 as the outcome variable.
Next, univariate and multivariable stepwise logistic regression analysis were performed to identify predictors of unfavorable outcome separately in patients with acute SDH and those with either subacute or chronic SDH. Variables were included in multivariable logistic regression models in the acute and subacute/chronic groups by stepwise selection if significant in univariate analysis (p < 0.05). Model discrimination was assessed by the c-statistic. The stability of the base model in each acuity cohort was tested by bootstrapping to obtain an optimism-corrected c-statistic, an internal validation method that allows estimation of statistics by repeated sampling of the data without assumption of sample distribution.11,12
SDH score
Separate acute and subacute/chronic SDH scores were created using the regression coefficients of the significant outcome predictors in each model. Cutoff points were demarcated by the endpoint of the tertiles of each SDH score. The percentage of patients with unfavorable outcomes in the surgical and non-surgical groups was compared in patients with SDH scores below the level of each cutoff by χ2 tests for each acuity group. For both groups, the number need to treat (NNT) was computed in patients above the SDH score cutoff from the difference in percentage of patients with poor outcome in the surgical versus non-surgical groups (1/absolute risk reduction).
As a means of comparison, we applied the current guidelines for acute SDH surgical evacuation to our acute SDH cohort (midline shift > 5 mm or diameter > 10mm at presentation).8 We calculated the percentage of patients meeting current guideline criteria at presentation for surgical evacuation. Within the strata of patients who did and did not meet guideline criteria for surgery, we compared the percentage of patients with favorable outcome among those managed surgically versus medically.
Results
Table 1 shows 153 patients who underwent surgical evacuation and 178 patients managed conservatively. There were no differences in the age, gender, race, comorbidities, pre-admission mRS, or presentation GCS. Operated patients had significantly more focal neurologic deficits, a greater frequency of convexity location of SDH, and larger SDH dimensions. Operated patients more often had imaging findings suggesting subacute or chronic SDH. The median time from discharge to follow-up was 78 days (IQR 53–113) in the surgical cohort and 71 days (IQR 47–103) in the non-surgical cohort. There was no significant difference in the proportions of patients with follow-up mRS scores 0–2 versus mRS 3–6 (p = 0.26) by treatment status.
Table 1.
Univariate analysis: surgical versus non-surgical management.
| Variables | Full cohort (N = 331) | No evacuation (N = 178) | Surgical evacuation (N = 153) | P value |
|---|---|---|---|---|
| Age (years) | 67.3 (16.8) | 67.4(17.9) | 67.2 (15.6) | 0.89 |
| Male | 215 (64.5) | 113 (63.5) | 102 (66.7) | 0.55 |
| Race | 0.75 | |||
| White | 275 (87.9) | 149 (87.7) | 126 (88.1) | |
| Black or African-American | 9 (2.9) | 4 (2.4) | 5 (3.5) | |
| Other | 29 (9.3) | 17 (10.0) | 12 (8.4) | |
| Charlson comorbidity index | 3.8 (2.9) | 3.7 (2.8) | 4.1 (2.9) | 0.23 |
| Baseline mRS | 0.59 | |||
| 0 | 120 (36.3) | 68 (38.2) | 52 (34.0) | |
| 1 | 113 (34.1) | 55 (30.9) | 58 (37.9) | |
| 2 | 55 (16.6) | 30 (16.9) | 25 (16.3) | |
| 3 | 43 (13.0) | 25 (14.0) | 18 (11.8) | |
| Anticoagulant use | 58 (17.5) | 36 (20.2) | 22 (14.5) | 0.17 |
| GCS at presentation | 12.6 (4.3) | 13.1 (3.9) | 12.2 (4.7) | 0.06 |
| Neuropsychiatric symptoms | 80 (24.2) | 38 (21.4) | 42 (27.5) | 0.20 |
| Seizures | 38 (11.5) | 22 (12.4) | 16 (10.5) | 0.59 |
| Focal neurologic deficit | 118 (35.7%) | 29 (16.3%) | 89 (58.2%) | < 0.001 |
| Laterality | 0.28 | |||
| Left | 109 (32.9) | 52 (29.2) | 57 (37.3) | |
| Right | 122 (36.9) | 68 (38.2) | 54 (35.3) | |
| Both | 100 (30.2) | 58 (32.6) | 42 (27.5) | |
| Location | < 0.001 | |||
| Convexity only | 238 (71.9) | 117(65.7) | 121 (79.1) | |
| Tentorium only | 28 (8.5) | 27 (15.2) | 1 (0.7) | |
| Both | 65 (19.6) | 34(19.1) | 31 (20.3) | |
| Diameter > 10 mm | 201 (60.7) | 75 (42.1) | 126 (82.4) | < 0.001 |
| Midline shift > 5 mm | 119 (36.0) | 17 (9.6) | 102 (66.7) | < 0.001 |
| Hematoma acuity | < 0.001 | |||
| Acute | 128 (38.7) | 90 (50.9) | 37 (24.2) | |
| Mixed | 27 (8.2) | 14 (7.9) | 13 (8.5) | |
| Chronic | 176 (53.2) | 61 (41.8) | 73 (62.4) | |
| SDH recurrence | 43 (13.9) | 23 (13.9) | 20(13.6) | 0.93 |
| 90-day mRS score | ||||
| 0–2 | 101 (30.5) | 119(66.9%) | 111 (72.6%) | 0.26 |
| 3–6 | 230 (69.5) | 59 (33.2%) | 42 (27.5%) | |
Abbreviations: mRS: modified Rankin Scale; GCS: Glasgow Coma Scale; SDH: subdural hematoma. The values represent mean (standard deviation) or counts (percentages).
Table 2 displays the results of the stepwise regression analysis for independent predictors of surgical evacuation. Variables in this model included all the statistically significant (p < 0.05) variables from Table 1. Age, focal neurologic deficit, SDH thickness > 10mm, midline shift > 5mm, and SDH acuity (reference: acute) were independent predictors of surgical evacuation. The ‘propensity to undergo surgery’ score for each patient was created using the predicted probabilities of surgical evacuation based on the 5 variables shown in Table 2. The logistic regression model was then adjusted by propensity score, after which the variables no longer remained significant predictors of surgical evacuation (Table 2, right-most column), suggesting that inclusion of the ‘propensity for surgery’ score in regression models balanced the distribution of variables across the surgical and non-surgical cohorts and allowed more appropriate analysis of surgery’s effects on clinical outcome.
Table 2.
Logistic regression: predictors of surgical evacuation.
| Predictors of surgery | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) adjusted for propensity score | P-value |
|---|---|---|---|---|
| Age | 0.97 | 0.007 | 0.98 | 0.208 |
| (0.95, 0.99) | (0.95, 1.01) | |||
| Focal neurologic deficit | 4.89 | < 0.001 | 2.98 | 0.266 |
| (2.57, 9.30) | (0.44, 20.47) | |||
| Diameter > 10 mm | 4.18 | < 0.001 | 2.84 | 0.190 |
| (2.05, 8.54) | (0.60, 13.58) | |||
| Midline shift > 5 mm | 10.82 | < 0.001 | 4.81 | 0.292 |
| (5.55,21.11) | (0.26, 89.26) | |||
| Subdural acuity | 3.30 | 0.001 | 1.54 | 0.241 |
| (Reference: acute) | (1.63,6.70) | (0.75, 3.19) | ||
Note: The left column shows odds ratios and 95% confidence intervals (C.I.) for covariables that proved statistically significant and the admission Glasgow coma scale (GCS) score; these variables were used to create the propensity score for surgical evacuation. The right column shows results after adjustment for the propensity score.
The effect of surgical evacuation on follow-up outcome was assessed before and after adjustment for the ‘propensity to undergo surgery’ score. There was no significant association between surgical evacuation and unfavorable outcome (OR 0.76, 95% C.I. 0.48–1.22); however, after adjustment for the propensity score, patients who underwent surgical evacuation were significantly less likely to have an unfavorable outcome (OR 0.38, 95% C.I. 0.19–0.76). Propensity score matching yielded 57 1:1 matched pairs; within these matched pairs, there was no significant difference in the 5 model variables among patients who did or did not undergo surgery. Among the matched pairs, patients who underwent surgical evacuation versus conservative management were again significantly less likely to have an unfavorable outcome at follow-up (OR 0.35, 95% C.I. 0.15–0.82).
Table 3 compares patients with favorable (N = 230) and unfavorable (N = 101) outcomes among patients with acute (N =128) and subacute/chronic SDH (N = 203). In the acute SDH group, patients with unfavorable outcome were older, less likely male, had higher Charlson comorbidity index scores, had higher pre-admission mRS scores, were more likely to use an anti-coagulant, and demonstrated neuropsychiatric symptoms, focal neurologic deficit, and midline shift > 5mm. Among patients with subacute or chronic SDH, patients with unfavorable outcome were older, had higher Charlson comorbidity index scores, had higher pre-admission mRS scores, and were more likely to have neuropsychiatric symptoms and a focal neurologic deficit.
Table 3.
Univariate analysis: 90-day modified Rankin Scale Score.
| Variable | Full acute cohort (N = 128) |
Acute |
Full subacute/chronic cohort (N=203) |
Subacute or
chronic |
||||
|---|---|---|---|---|---|---|---|---|
| 90-Day mRS score 0–2 (N=92) | 90-Day mRS score ≥ 3 (N=36) | P-value | 90-Day mRS score 0–2 (N=138) | 90-Day mRS score ≥ 3 (N=65) | P-value | |||
| Age (years) | 62.3 (18.3) | 58.6 (17.9) | 71.8(15.7) | 0.0002 | 70.5 (15.1) | 67.2(15.0) | 77.5 (12.7) | <0.0001 |
| Male | 85 (66.4) | 66 (71.7) | 19 (52.8) | 0.0411 | 130(64.0) | 91 (65.9) | 39 (60.0) | 0.4105 |
| Race | 0.0796 | 1.0000 | ||||||
| White | 101 (84.9) | 68 (80) | 33 (97.1) | 174 (89.7) | 119(89.5) | 55 (90.2) | ||
| Black or African-American | 3 (2.5) | 3 (3.5) | 0 (0.0) | 6(3.1) | 4 (3.0) | 2(3.3) | ||
| Other | 15 (12.6) | 14 (16.5) | 1 (2.9) | 14 (7.2) | 10(7.5) | 4 (6.6) | ||
| Charlson Comorbidity Index | 3.6 (3.4) | 3.0 (3.2) | 5.4 (3.3) | 0.0001 | 4.0 (2.5) | 3.3 (2.1) | 5.4 (2.7) | <0.0001 |
| Baseline mRS | ||||||||
| 0 | 56 (43.8) | 46 (50.0) | 10 (27.8) | <0.0001 | 64(31.5) | 53 (38.4) | 11 (16.9) | <0.0001 |
| 1 | 40(31.3) | 34 (37.0) | 6 (16.7) | 73 (36.0) | 66 (47.8) | 7 (10.8) | ||
| 2 | 22(17.2) | 12(13.0) | 10 (27.8) | 33 (16.3) | 18(13.0) | 15 (23.1) | ||
| 3 | 10(7.8) | 0 (0.0) | 10 (27.8) | 33 (16.3) | 1 (0.7) | 32 (49.2) | ||
| Anticoagulant Use | 27 (21.1) | 15 (16.3) | 12 (33.3) | 0.0337 | 31 (15.4) | 23 (16.8) | 8 (12.3) | 0.4092 |
| GCS at presentation | 11.3 (5.1) | 11.6 (5.2) | 10.6 (4.8) | 0.3326 | 13.5 (3.5) | 13.7 (3.3) | 12.9 (3.8) | 0.1236 |
| Neuropsychiatric symptoms | 32 (25.0) | 17 (18.5) | 15 (41.7) | 0.0064 | 48 (23.7) | 23 (16.7) | 25 (38.5) | 0.0007 |
| Seizures | 19(14.8) | 13 (14.1) | 6 (16.7) | 0.7167 | 19 (9.4) | 16(11.6) | 3 (4.6) | 0.1112 |
| Focal neurologic deficit | 40(31.3) | 20 (21.7) | 20 (55.6) | 0.0002 | 78 (38.4) | 46 (33.3) | 32 (49.2) | 0.0298 |
| Laterality | 0.2381 | 0.2588 | ||||||
| Left | 47 (36.7) | 35 (38.0) | 12 (33.3) | 62 (30.5) | 39 (28.3) | 23 (35.4) | ||
| Right | 50(39.1) | 32 (34.8) | 18 (50.0) | 72 (35.5) | 47 (34.1) | 25 (38.5) | ||
| Both | 31 (24.2) | 25 (27.2) | 6 (16.7) | 69 (34.0) | 52 (37.7) | 17 (26.2) | ||
| Location | 0.7037 | 0.6210 | ||||||
| Convexity | 71 (55.5) | 53 (57.6) | 18 (50.0) | 167 (82.3) | 115 (83.3) | 52 (80.0) | ||
| Tentorium | 27 (21.1) | 19 (20.7) | 8 (22.2) | 1 (0.5) | 1 (0.72) | 0 (0.0) | ||
| Both | 30(23.4) | 20 (21.7) | 10 (27.8) | 35 (17.2) | 22(15.9) | 13 (20.0) | ||
| Diameter > 10 mm | 49 (38.3) | 35 (38.0) | 14 (38.9) | 0.9295 | 152 (74.9) | 49 (75.4) | 103 (74.6) | 0.9089 |
| Midline shift > 5 mm | 34 (26.6) | 20 (21.7) | 14 (38.9) | 0.0482 | 85 (41.9) | 59 (42.8) | 26 (40.0) | 0.7106 |
| SDH evacuation | 37 (28.9) | 28 (30.4) | 9 (25.0) | 0.5420 | 116(57.1) | 83 (60.1) | 33 (50.8) | 0.2079 |
| SDH Recurrence | 9 (7.8) | 3 (3.5) | 6 (21.4) | 0.0063 | 34(17.3) | 15 (10.9) | 19 (32.2) | 0.0003 |
Abbreviations: mRS: modified Rankin Scale; GCS: Glasgow Coma Scale; SDH: subdural hematoma.
Having shown by propensity score adjustment and matched analyses that surgical evacuation is a major factor driving outcome, our next aim was to determine which patients benefited most from surgical evacuation. Therefore, multivariate logistic regression analysis with stepwise selection was performed using statistically significant variables from the univariate analysis (Table 3) to predict unfavorable outcome. Predictors of unfavorable outcome in the acute SDH group included age > 65 (OR 4.25, 95% C.I. 1.40–12.89), female sex (OR 4.25, 95% C.I. 1.37–11.73), pre-admission mRS (OR 3.29, 95% C.I. 1.83–5.93), neuropsychiatric symptoms on presentation (OR 6.51, 95% C.I. 1.98–21.44), and focal neurologic deficit on presentation (OR 5.74, 95% C.I. 1.95–16.95) (Table 4a). This model had a c-statistic or area under the curve of 0.889. The model was internally validated by bootstrapping in 200 replicate samples using the 5 variables to yield an optimism-corrected c-statistic of 0.874, suggesting excellent robustness. In the subacute/chronic SDH group, age > 65 (OR 3.06, 95% C.I. 1.28–7.31), pre-admission mRS (OR 3.62, 95% C.I. 2.43–5.40), and neuropsychiatric symptoms (OR 2.50, 95% C.I. 1.09–5.76) predicted unfavorable outcome (Table 4b). The associated c-statistic was 0.837 and optimism-corrected c-statistic was 0.836.
Table 4.
Logistic regression: predictors of unfavorable outcome.
| Table 4a. Acute subdural hematoma | |||||
| Covariates | Odds ratio(95% C.I.) | Coefficient | P-value | C-statistic | Optimism-corrected C-statistic |
| Admission Age > 65 | 4.25 (1.40, 12.89) | 1.4472 | 0.0105 | 0.889 | 0.874 |
| Female sex | 4.01 (1.37, 11.73) | 0.6945 | 0.0111 | ||
| Pre-admission mRS | 3.29(1.83,5.93) | 1.1910 | <0.0001 | ||
| Neuropsychiatric features | 6.51 (1.98,21.44) | 1.8738 | 0.0021 | ||
| Focal neurologic deficit | 5.74(1.95, 16.95) | 1.7477 | 0.0016 | ||
| Table 4b. Subacute and chronic subdural hematoma | |||||
| Covariates | Odds ratio (95% C.I.) | Coefficient | P-value | C-statistic | Optimism-corrected C-statistic |
| Age > 65 | 3.06 (1.28,7.31) | 1.1175 | 0.0120 | 0.837 | 0.836 |
| Pre-admission mRS | 3.62 (2.43, 5.40) | 1.2867 | < 0.0001 | ||
| Neuropsychiatric symptoms | 2.50(1.09,5.76) | 0.9163 | 0.0315 | ||
Abbreviation: mRS: modified Rankin scale.
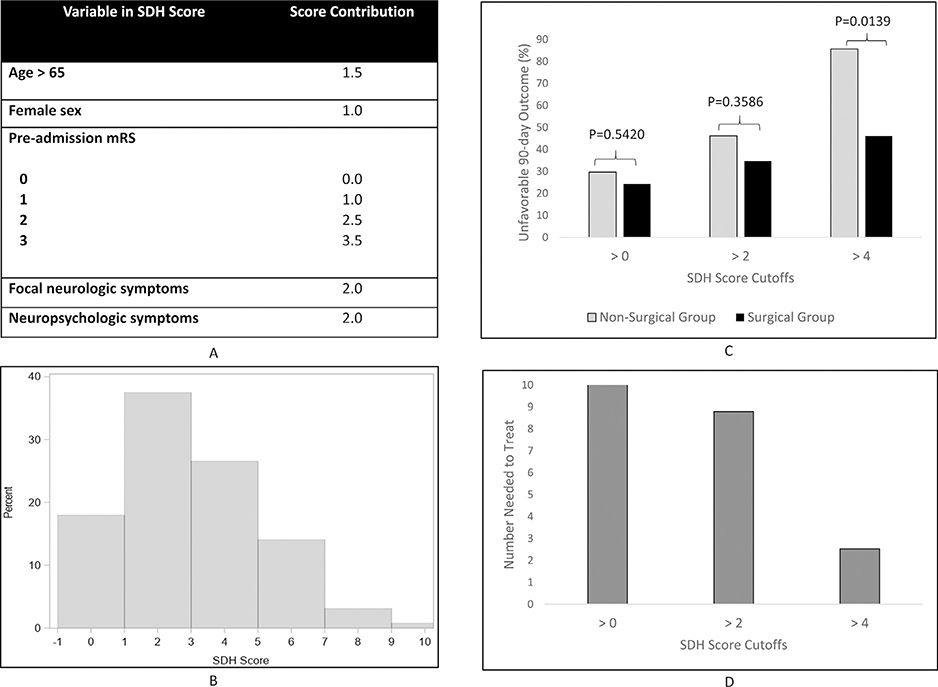
An SDH score was created for the acute and the subacute/chronic SDH groups using parameter coefficients of the base model derived from the full cohort (Figs. 1 and 2).
Fig. 1.
The SDH Score in patients with acute SDH. (A) Components of the acute subdural hematoma (SDH) score; (B) Distribution of patients across the SDH score; (C) Percentage of patients with favorable outcomes at follow-up stratified by treatment (gray: non-surgical, black: surgical) below each SDH score cutoff; (D) Number needed to treat in patients with SDH scores below each cutoff.
Fig. 2.
The SDH Score in patients with subacute or chronic SDH. (A) Components of the subacute/chronic subdural hematoma (SDH) score; (B) Distribution of patients across the SDH score; (C) Percentage of patients with favorable outcomes at follow-up stratified by treatment (gray: non-surgical, black: surgical) below each SDH score cutoff; (D) Number needed to treat in patients with SDH scores below each cutoff.
Each regression coefficient contribution was rounded to the nearest 0.5. The acute SDH score ranged from 1 to 10 (Fig. 1B). The subacute/chronic SDH score ranged from 0 to 6 (Fig. 2B). The SDH score for each group was divided into tertiles. The acute SDH tertiles were demarcated with the score cutoff boundaries of 0, 2 and 4 and the subacute/ chronic SDH tertiles were delineated by scores 0,2 and 3.
In the acute SDH analysis depicted in Fig. 1C, at cutoff > 4 (27% of patients), nearly 46% of operated patients had an unfavorable outcome at follow-up compared to 86% among those medically managed (p = .014). Below a cutoff of 4 (73% of patients), 13% had an unfavorable outcome in the surgical group versus 13% of those managed conservatively (p = 1.000). There was no statistically significant difference in the percentage of patients with unfavorable outcome in surgically versus medically managed patients with SDH scores > than the thresholds of 0 and 2. The NNT was 3 in patients with SDH scores > 4 (Fig. 1D). Among subacute/chronic SDH patients, there was no significant difference in outcome by surgical status at the cutoffs of the tertile boundaries (Fig. 2C).
Finally, we assessed the outcomes associated with each treatment as recommended by the current guideline in the acute SDH group. (Table 5) Among the 48% of patients who would be recommended for surgery based on current guidelines, 53% underwent evacuation and 48% were managed conservatively. There was no significant difference in the percentage of patients with unfavorable outcomes in those who underwent surgery versus medical management (25% versus 35%, p = .42) among those who meet current guidelines for surgery.
Table 5.
Functional outcomes among patients with acute SDH stratified by SDH score and current guidelines.
| Patient groups | Surgery= 1 | Surgery=0 | Unfavorable outcome (Surgery= 1) | Unfavorable outcome (Surgery=0) | P-value |
|---|---|---|---|---|---|
| SDH score cutoff = 4 | |||||
| SDH Score ≤ 4 (N=94; 73.4%) | 24 (25.5) | 70 (74.5) | 3 (12.5) | 9 (12.9) | 1.000 |
| SDH Score > 4 (N=34; 26.6%) | 13 (38.2) | 21 (61.8) | 6 (46.2) | 18 (85.7) | .014 |
| Current guidelines | |||||
| Do not meet current guidelines (N=67; 52.3%) | 5 (7.5) | 62 (92.5) | 1 (20.0) | 17 (27.4) | 1.000 |
| Meet current guidelines (N=61; 47.7%) | 32 (52.5) | 29 (47.5) | 8 (25.0) | 10 (34.5) | .417 |
Note: Data presented as N, %
Discussion
The management of SDH is controversial in the absence of evidence-based guidelines and randomized controlled trials. Numerous prior studies have identified predictors of outcome, such as age, co-morbidities and SDH evacuation,13–25 however these studies are limited by factors such as small sample size, heterogeneity in management, variable outcome measures, and most importantly, confounders that influence surgical decision-making and hence clinical outcome. In this relatively large single-center study, we investigated the association between SDH evacuation and outcome while controlling for potential confounding by indication. Age, presence of focal neurologic deficits, SDH thickness > 10mm, midline shift > 5mm, and SDH acuity were independent predictors of surgical evacuation. Propensity-matched analysis demonstrated that patients who underwent surgical evacuation were nearly three times less likely to have an unfavorable outcome at follow-up compared to those treated with medical management. To better understand whether surgery confers benefit over conservative management uniformly or in specific groups of patients, we created SDH scores for acute and subacute/chronic SDH patients and compared outcomes in operated and non-operated patients for each score category. Among acute SDH patients, the probability of a favorable outcome was similar at lower SDH score cutoffs; however, for scores above 4, we found a significant benefit of surgery. There was no differential benefit of surgery across the spectrum of SDH score among patients with subacute/chronic SDH. Patients with acute SDH recommended for surgery by the SDH score threshold had significantly better functional outcomes than those managed medically; in contrast, there was no difference in outcome among those who met criteria for surgery per current guidelines and ultimately were managed surgically or medically. Furthermore, 44% fewer acute SDH patients were delegated for surgery by the SDH score compared to the guidelines with no compromise in functional outcome, suggesting superior patient selection. This simple bedside tool may be useful for surgical decision-making and has potential to harmonize clinical practice.
While our overall goal was to identify independent predictors of poor outcome, the initial analysis focused on predictors of surgical evacuation since these factors could independently influence outcome. For example, sicker individuals have greater propensity for poor outcome; surgeons may thus be less likely to operate on sicker individuals. Thus, outcome may not be attributable to surgery alone. As shown in Table 2, greater clinical and imaging severity (e.g. focal deficits, greater midline shift) predicted surgical evacuation, whereas older age predicted conservative management. The surgical predictors identified in our study are broader than those suggested by guidelines. For example, the presence of any focal neurological deficit predicted surgery, and we identified SDH acuity as another independent predictor of surgery. Neurosurgeons may be more inclined to operate if a patient is symptomatic, regardless of GCS. A more chronic and liquefied hematoma may be technically easier to evacuate by a less aggressive method such as a Burr hole rather than craniotomy.
We then identified predictors of follow-up functional status. Surgery remained a strong predictor of outcome after adjustment for the ‘propensity to undergo surgery’ score and in the propensity-matched analysis. Patient outcomes were predicated on baseline variables (age and pre-admission mRS) as well as clinical data at the time of presentation. Having a focal neurologic deficit and neuropsychiatric symptoms independently predicted follow-up function, again suggesting that more subtle symptoms may have long-term ramifications on a patient’s ability to maintain independence. Our results are consistent with prior studies13–15,26–32 showing that factors such as age, baseline status, and surgical evacuation are associated with outcome. The novelty of our analysis lies in the adjustment for propensity for surgery, even after which surgery remained highly beneficial, and the unveiling of other clinical prognostic markers.
Although our results demonstrate that surgical evacuation overall is beneficial, it is clinically meaningful to understand the magnitude of benefit for each individual to decide who should be offered surgery. To that end, we developed separate SDH scores for patients with acute SDH and those with subacute/chronic SDH. Segmentation of each SDH score at endpoints of its tertiles allowed direct comparison of patients treated surgically or conservatively in each quintile. The SDH score is intuitive, easily applicable at the bedside, and threshold scores provide direction for management. In the acute group, the benefit of surgery was incremental for higher SDH scores, with significantly less unfavorable outcomes noted among surgically versus medically managed patients with SDH score > 4 (Fig. 1) with an NNT of 3. These findings suggest that acute patients with higher SDH scores, nearly 27% of acute SDH patients, may be at risk of worse functional status if managed conservatively. Conversely, there does not appear to be any benefit of surgery in those with lower scores. In contrast, there was no difference in follow-up functional status by actual treatment in patients who met criteria from the current guidelines for surgical evacuation. Patient selection by the acute SDH score appears superior to triaging by current guidelines. The SDH score identifies a smaller and more defined group of patients with potential to benefit from surgery, within which patients who are managed surgically rather than medically have better functional outcomes. Thus, the acute SDH score may provide a better method for identifying patients for surgery and prevent unnecessary SDH evacuations in patients who will have a favorable outcome even if managed medically. There was no similar benefit of surgery across SDH scores in subacute and chronic SDH patients, suggesting limited utility of surgical evacuation of SDH in this group.
Our study has limitations. Despite propensity score adjustment, there are likely unmeasured confounders such as heterogeneity of neurosurgeons involved that were not addressed. These unmeasured confounders may have introduced selection bias for surgical intervention. Our results were internally validated but still require external validation. The retrospective study design may be considered a limitation; however, prospective randomized controlled trials pose ethical limitations. Our results, if validated, may provide the foundation for future studies: while conservative and surgical management appears appropriate for low and high scores respectively, the surgical benefit for intermediate scores may be worth investigating in randomized trials. Our study results may not be generalizable since this was a single-center study performed at an academic, tertiary-care hospital. We did not investigate the association between outcome and the timing of intervention, surgical technique, and SDH recurrences. One prior study found an association with anticoagulant use16; indeed, this was significant in our univariate analysis but not after adjusting for other factors. Although our sample size compares favorably with prior studies, the number of patients at the extremes of the SDH score was small. Further studies are needed to validate score performance and assess its utility over the current practice guideline.
Conclusion
Our novel SDH score shows high discriminatory ability to predict functional outcome in patients undergoing surgery versus conservative treatment of an acute SDH. Patients with acute SDH and SDH scores > 4 had greater likelihood of favorable outcome with surgical evacuation. This simple bedside score utilizing clinical information readily available on admission, promises to be a valuable tool for joint decision-making by neurologists and neurosurgeons, patients, and their families about the expected neurologic outcome with and without SDH evacuation.
Study sponsorship/funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
A. Singhal is supported by grants from the NIH (U10 NS086729; 1U01NS095869; 1R01NS105875; 1R01DC012584), and the Dana Foundation. R. Sharma is an NIH StrokeNet Fellow (NIH U10 NS086729). Dr. Rocha’s visiting scholarship at Massachusetts General Hospital and Harvard Medical School, Boston, was sponsored by the Capes Foundation, Ministry of Education, Brazil (grant no. 88,881.133101/2016–01).
Disclosures
Dr. Sharma reports the following disclosure: research support, NIH grant U10 NS086729.
Dr. Rocha reports the following disclosures: research support, Capes Foundation, Ministry of Education, Brazil.
Dr. Pasi reports no disclosures.
Dr. Lee reports no disclosures.
Dr. Patel reports the following disclosures: Consultant, Medtronic PLC; Penumbra, Inc.; Microvention, Inc.
Dr. Singhal reports the following disclosures: Consulting, Omniox; honoraria, Medlink, UptoDate; stock and/ or stock options, Biogen, Zafgen; research support, NIH grants U10 NS086729, U01NS095869, R01NS105875 and R01DC012584, the Dana Foundation, and Boehringer- Ingelheim, Inc.
References
- 1.Servadei F Prognostic factors in severely head injured adult patients with acute subdural haematoma’s. Acta Neurochir Wien 1997;139:279–285. [DOI] [PubMed] [Google Scholar]
- 2.Servadei F, Nasi MT, Giuliani G, et al. CT prognostic factors in acute subdural haematomas: the value of the ‘worst’ CT scan. Br J Neurosurg 2000;14:110–116. [DOI] [PubMed] [Google Scholar]
- 3.Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg 2016;11:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir Wien 1988;90:111–116. [DOI] [PubMed] [Google Scholar]
- 5.Kalanithi P, Schubert RD, Lad SP, Harris OA, Boakye M. Hospital costs, incidence, and inhospital mortality rates of traumatic subdural hematoma in the United States. J Neurosurg 2011;115:1013–1018. [DOI] [PubMed] [Google Scholar]
- 6.Frontera JA, de los Reyes K, Gordon E, et al. Trend in outcome and financial impact of subdural hemorrhage. Neu- rocrit Care 2011;14:260–266. [DOI] [PubMed] [Google Scholar]
- 7.Rosenorn J, Gjerris F. Long-term follow-up review of patients with acute and subacute subdural hematomas. J Neurosurg 1978;48:345–349. [DOI] [PubMed] [Google Scholar]
- 8.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery 2006;58:S16–S24. discussion Si-iv. [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 10.Computerized Matching of Cases to Controls.
- 11.Efron BTR. An Introduction to the Bootstrap. 1st ed. Boca Raton: Chapman and Hall; 1993. [Google Scholar]
- 12.Miao YCI, Kirby K, Boscardin WEstimating Harrell’s Optimism on Predictive Indices Using Bootstrap Samples 2013.
- 13.Weimer JM, Gordon E, Frontera JA. Predictors of functional outcome after subdural hematoma: a prospective study. Neurocrit Care 2017;26:70–79. [DOI] [PubMed] [Google Scholar]
- 14.Won SY, Dubinski D, Brawanski N, et al. Significant increase in acute subdural hematoma in octo- and nonagenarians: surgical treatment, functional outcome, and predictors in this patient cohort. Neurosurg Focus 2017;43:E10. [DOI] [PubMed] [Google Scholar]
- 15.Katsigiannis S, Hamisch C, Krischek B, et al. Independent predictors for functional outcome after drainage of chronic subdural hematoma identified using a logistic regression model. J Neurosurg Sci 2017. [DOI] [PubMed] [Google Scholar]
- 16.Leroy HA, Aboukais R, Reyns N, et al. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci 2015;22:1895–1900. [DOI] [PubMed] [Google Scholar]
- 17.Atsumi H, Sorimachi T, Honda Y, Sunaga A, Matsumae M. Effects of Pre-Existing Comorbidities on Outcomes in Patients with Chronic Subdural Hematoma. World Neu- rosurg 2019;122:e924–ee32. [DOI] [PubMed] [Google Scholar]
- 18.Lavrador JP, Teixeira JC, Oliveira E, Simao D, Santos MM, Simas N. Acute subdural hematoma evacuation: predictive factors of outcome. Asian J Neurosurg 2018;13:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneck MJ, Maheswaran M, Leurgans S. Predictors of outcomes after nontraumatic subdural hematoma. J Stroke Cerebrovasc Dis 2004;13:192–195. [DOI] [PubMed] [Google Scholar]
- 20.Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Correlation between CT scan findings and outcome. Acta Neurochir Wien 1996;138:185–191. [DOI] [PubMed] [Google Scholar]
- 21.Wilberger JE Jr., Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg 1991;74:212–218. [DOI] [PubMed] [Google Scholar]
- 22.Howard MA 3rd, Gross AS, Dacey RG Jr., Winn HR. Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg 1989;71:858–863. [DOI] [PubMed] [Google Scholar]
- 23.Sawauchi S, Murakami S, Ogawa T, Abe T. [Acute subdural hematoma associated with diffuse brain injury: analysis of 526 cases in Japan neurotrauma data bank]. No Shinkei Geka 2007;35:43–51. [PubMed] [Google Scholar]
- 24.Cagetti B, Cossu M, Pau A, Rivano C, Viale G. The outcome from acute subdural and epidural intracranial haematomas in very elderly patients. Br J Neurosurg 1992;6:227–231. [DOI] [PubMed] [Google Scholar]
- 25.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg 2005;107:223–229. [DOI] [PubMed] [Google Scholar]
- 26.Hussain R, Afzal M, Joshi S, et al. Factors affecting the survival following surgical treatment of chronic subdural haematoma: Single institutional experience. J Clin Neurosci 2017;44:75–79. [DOI] [PubMed] [Google Scholar]
- 27.Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg 2013;119:1296–1301. [DOI] [PubMed] [Google Scholar]
- 28.Leitgeb J, Mauritz W, Brazinova A, et al. Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg 2012;117:324–333. [DOI] [PubMed] [Google Scholar]
- 29.Kotwica Z, Brzezinski J. Acute subdural haematoma in adults: an analysis of outcome in comatose patients. Acta Neurochir Wien 1993;121:95–99. [DOI] [PubMed] [Google Scholar]
- 30.Koc RK, Akdemir H, Oktem IS, Meral M, Menku A. Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev 1997;20:239–244. [DOI] [PubMed] [Google Scholar]
- 31.Zumkeller M, Behrmann R, Heissler HE, Dietz H. Computed tomographic criteria and survival rate for patients with acute subdural hematoma. Neurosurgery 1996;39:708–712. discussion 12–3. [DOI] [PubMed] [Google Scholar]
- 32.Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol 1993;40:22–25. [DOI] [PubMed] [Google Scholar]