Abstract
Introduction
Dry eye disease (DED) prevalence is estimated at 9.3% of the US adult population, although diagnosed rate is much lower. This study examined real-world incidence rates (IR) and prevalence rates (PR) of DED in adults using continuous positive airway pressure (CPAP) or nasal mask therapy (NMT) devices to treat sleep apnea.
Methods
Using IBM MarketScan Commercial and Medicare Supplemental claims databases, this study identified adults with ≥1 claim of CPAP or other NMT device between January 1, 2014 and June 30, 2018, ≥1 diagnosis of sleep apnea during a 12-month pre-index period, and continuous benefit enrollment ≥12 pre- and post-index date. The date of the first CPAP or NMT device claim was considered the index date. Descriptive analyses included PR, IR, and IR per 100-person years (100PY) for the overall population and subgroups including age, sex, and baseline comorbidities.
Results
The 1-, 2-, and 3-year PR of DED was 6.2%, 10.0%, and 13.0%, while the IR of DED was 4.0%, 7.3%, and 10.3%, respectively. Females had a higher IR of DED compared to males: 5.8%, 10.8%, and 15.1% vs 3.0%, 5.4%, and 7.9%, respectively. DED increased with age with a 1-, 2-, and 3-year PR for patients aged 18–24 years of 2.2%, 3.4%, and 5.0% vs 17.6%, 25.8%, and 32.1% in patients aged ≥75, respectively. Overall, IR per 100PY of DED was 3.68, higher for females than males (5.51 vs 2.73). PR and IR of DED were high among patients with comorbid inflammatory or metabolic conditions.
Conclusion
The PR and IR of DED in CPAP or NMT users were higher than the reported prevalence of DED in the general population. CPAP/NMT users who were female, older, or had comorbid inflammatory or metabolic conditions may experience a higher incidence and prevalence of DED.
Keywords: dry eye disease, obstructive sleep apnea, prevalence rate, incidence rate, CPAP, nasal mask therapy device
Introduction
Dry eye disease (DED) is a chronic, multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film leading to tear film instability, hyperosmolarity, and ocular surface inflammation and damage.1 In the US, 6.8% of the population (approximately 16.4 million adults) are diagnosed with DED, and an additional 2.5% are estimated to have dry eye disease without a clinical diagnosis.2 Prevalence of DED is two times higher in females compared to males and increases more than threefold for adults aged ≥50 years compared to adults aged 18–49 years.2 Sleep apnea affects approximately 13–14% of men and 5–6% of women in the US;3 the 5-year incidence of sleep apnea in adults is approximately 16% for mild to moderate disease and 7.5% for moderate to severe disease.4 Moreover, the prevalence of sleep apnea has increased substantially in the US from 14% to 55% depending on age, sex, and severity of sleep apnea when comparing data from 1988–1994 to 2007–2010—representing an increase in millions of patients diagnosed with sleep apnea.3
The definition of sleep apnea has evolved, although a general consensus involves partial to complete upper-airway collapse and decreased airflow causing oxygen desaturation and/or arousal from sleep.5 According to the American Academy of Sleep Medicine guidelines, the recommended treatment for sleep apnea involves continuous positive airway pressure (CPAP) or other nasal mask therapy (NMT) devices.6 The benefits to CPAP use in patients with sleep apnea are considerable, with patients experiencing significant improvements in quality of life measures such as daily functioning, emotional functioning, and social interactions, as well as decreased daytime fatigue after 6 months of treatment with CPAP.7 Compliance to CPAP therapy is high for patients with sleep apnea with approximately 80% of patients accepting CPAP therapy after initial titration.8
Patients using CPAP or NMT devices to treat sleep apnea may develop secondary ocular disorders, with approximately 50% of patients with sleep apnea exhibiting abnormal eye findings.9 Twenty-one percent of CPAP users experience mask edge leakage, which may result in increased ocular irritation, abnormal tear film, lid laxity, and floppy eyelid syndrome.10 Patients with sleep apnea and floppy eyelid syndrome often present with symptoms such as foreign body sensation, redness, eyelid swelling, and excessive tearing in response to underlying dryness—leading to an increased risk of DED.11 Moreover, the severity of dry eye symptoms is directly associated with apnea-hypopnea index values, a scale used to determine the severity of sleep apnea based on the number of apneic episodes experienced in one hour of sleep.12,13 Thereby, as the severity of sleep apnea increases, ocular surface inflammation, hypoxemia, and mechanical tissue stress on the eye increase as well.13 The growing prevalence of ocular manifestations in patients with sleep apnea reveals a significant need to raise awareness and establish collaboration between sleep physicians and ophthalmic practitioners.10 This study aimed to examine the real-world prevalence and incidence of dry eye disease in patients using CPAP or NMT devices to treat sleep apnea.
Materials and Methods
Data Source
This study was a retrospective, real-world analysis utilizing data from the IBM MarketScan® Commercial and Medicare Supplemental Databases, which contains inpatient, outpatient, and outpatient prescription-drug data for several million adults and children in the US, covered under a variety of fee-for-service and capitated health plans including exclusive provider organizations, preferred provider organizations, point of service plans, indemnity plans, and health maintenance organizations as well as Medicare supplemental insurance. More than 100 large employers and health insurance plans contributed data to MarketScan. All database records are de-identified and fully compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996.
Patient Population
This study identified adult patients with ≥1 claim of CPAP or NMT device between January 1, 2014 and June 30, 2018. Patients included in the study had ≥1 claim of an inpatient or outpatient diagnosis of sleep apnea in the 12 months prior to the device claim and continuous enrollment with medical and pharmacy benefits for at least 12 months before and after the index date. The index date was defined as the date of the first device claim. Diagnosis of sleep apnea was defined by ICD-9 and ICD-10 diagnosis codes. DED was defined using ICD-9 and ICD-10 diagnosis and current procedural terminology (CPT) codes, while CPAP and NMT devices were identified via the healthcare common procedure coding system and CPT codes. There were no specifications or requirements for types of CPAP or NMT devices used; therefore, a variety of masks were included in this study. The study period ranged from January 1, 2013 to June 30, 2018. Patients were followed from the index date to the earliest of the following: inpatient death, end of continuous enrollment, or end of the study period (June 30, 2018).
Clinical and demographic characteristics for patients were assessed in the pre-index period or on the index date and included eye-related conditions; major autoimmune disorders, ocular surgery—which may be a risk factor for DED; concomitant medications such as antidepressants, anxiolytics, antihypertensives, and antihistamines; and the Deyo-Charlson Comorbidity Index (DCCI)—an aggregate measure of comorbidity expressed as a numeric score based on the presence of select diagnoses for various conditions, each with a specific weight ranging from 1 to 6.14
Outcome Measures
This analysis estimated the cumulative prevalence rate and incidence rate of DED over 12-, 24-, and 36-month follow-up periods after the index date and incidence rate per 100 person-years (100PY) for the overall population and selected subgroups. The prevalence rate of DED among users of CPAP or NMT devices was defined as the number of patients with ≥1 inpatient or outpatient claim with a diagnosis of DED over the 12-, 24-, and 36-month follow-up periods divided by the total number of CPAP or NMT users. The incidence of DED was examined among the subset of patients with no DED during the pre-index period. The incidence rate was defined as the number of patients with ≥1 inpatient or outpatient claim with a diagnosis of DED over the 12-, 24-, or 36-month follow-up period divided by the total number of patients with evidence of CPAP or other NMT device use and with at least 12, 24, and 36 months of follow-up, respectively. The incidence rate per 100PY was calculated as the number of patients with DED diagnosis in the variable-length follow-up period divided by the total number of years from the index date to the first DED diagnosis for patients with DED plus the total number of years of follow-up for patients without DED multiplied by 365 and 100. The number of days from the index date to the first diagnosis of DED was reported.
Statistical Analyses
Results were summarized using descriptive statistics for all patients, and subgroup analyses were reported by age, sex, a combination of age and sex, and 5 selected comorbidities. Continuous measures were presented as mean, standard deviation (SD), and median. Categorical measures were presented as counts and percentages. Time from the first use of CPAP or other NMT devices to the first diagnosis of DED was further evaluated with Kaplan–Meier curves.
Results
Patient Demographics and Clinical Characteristics
At baseline, among all patients meeting study criteria (n = 350,420), the mean (SD) age was 53.1 (11.8) years with 63.3% male. Among all patients without a diagnosis of DED prior to their first CPAP or NMT device claim (n = 330,926), the mean (SD) age was 52.7 (11.7) years, and 64.5% were male (Table 1). The mean DCCI (SD) for both populations was 0.9 (1.5).
Table 1.
Baseline Demographics and Clinical Characteristics
| Prevalence Population | Incidence Population | |||||
|---|---|---|---|---|---|---|
| With DED n = 21,810 |
Without DED n = 328,610 | Overall N = 350,420 |
With DED n = 13,176 |
Without DED n = 317,750 |
Overall N = 330,926 |
|
| Sex, male | 9403 (43.1) | 212,279 (64.6) | 221,682 (63.3) | 6296 (47.8) | 207,013 (65.1) | 213,309 (64.5) |
| Age, median (range), years | 60 (18–97) | 53 (18–103) | 54 (18–103) | 59 (18–97) | 53 (18–103) | 53 (18–103) |
| Age (years) | ||||||
| 18–24 | 89 (0.4) | 3941 (1.2) | 4030 (1.2) | 65 (0.5) | 3877 (1.2) | 3942 (1.2) |
| 25–34 | 355 (1.6) | 17,273 (5.3) | 17,628 (5.0) | 265 (2.0) | 17,023 (5.4) | 17,288 (5.2) |
| 35–44 | 1556 (7.1) | 56,504 (17.2) | 58,060 (16.6) | 1096 (8.3) | 55,526 (17.5) | 56,622 (17.1) |
| 45–54 | 4529 (20.8) | 100,512 (30.6) | 105,041 (30.0) | 2969 (22.5) | 97,981 (30.8) | 100,950 (30.5) |
| 55–64 | 8522 (39.1) | 110,605 (33.7) | 119,127 (34.0) | 5077 (38.5) | 106,504 (33.5) | 111,581 (33.7) |
| 65–74 | 4157 (19.1) | 27,609 (8.4) | 31,766 (9.1) | 2313 (17.6) | 25,786 (8.1) | 28,099 (8.5) |
| 75+ | 2602 (11.9) | 12,166 (3.7) | 14,768 (4.2) | 1391 (10.6) | 11,053 (3.5) | 12,444 (3.8) |
| Payer | ||||||
| Commercial | 14,814 (67.9) | 286,579 (87.2) | 301,393 (86.0) | 9337 (70.9) | 278,749 (87.7) | 288,086 (87.1) |
| Medicare | 6996 (32.1) | 42,031 (12.8) | 49,027 (14.0) | 3839 (29.1) | 39,001 (12.3) | 42,840 (12.9) |
| Index year | ||||||
| 2014 | 7319 (33.6) | 104,821 (31.9) | 112,140 (32.0) | 4553 (34.6) | 101,407 (31.9) | 105,960 (32.0) |
| 2015 | 6629 (30.4) | 99,348 (30.2) | 105,977 (30.2) | 4039 (30.7) | 95,806 (30.2) | 99,845 (30.2) |
| 2016 | 5542 (25.4) | 86,023 (26.2) | 91,565 (26.1) | 3264 (24.8) | 83,199 (26.2) | 86,463 (26.1) |
| 2017 | 2320 (10.6) | 38,418 (11.7) | 40,738 (11.6) | 1320 (10.0) | 37,338 (11.8) | 38,658 (11.7) |
| Comorbid conditions | ||||||
| Diabetes, mild to moderate | 5952 (27.3) | 69,985 (21.3) | 75,937 (21.7) | 3630 (27.6) | 67,036 (21.1) | 70,666 (21.4) |
| COPD | 5552 (25.5) | 59,080 (18.0) | 64,632 (18.4) | 3208 (24.3) | 56,435 (17.8) | 59,643 (18.0) |
| Rheumatoid arthritis | 1035 (4.7) | 4968 (1.5) | 6003 (1.7) | 458 (3.5) | 4593 (1.4) | 5051 (1.5) |
| Psoriasis | 421 (1.9) | 4846 (1.5) | 5267 (1.5) | 241 (1.8) | 4617 (1.5) | 4858 (1.5) |
| IBS | 264 (1.2) | 2776 (0.8) | 3040 (0.9) | 150 (1.1) | 2655 (0.8) | 2805 (0.8) |
| Concomitant medications | ||||||
| Antihypertensives | 14,619 (67.0) | 191,136 (58.2) | 205,755 (58.7) | 8719 (66.2) | 184,025 (57.9) | 192,744 (58.2) |
| Antidepressants | 9116 (41.8) | 106,762 (32.5) | 115,878 (33.1) | 5283 (40.1) | 102,423 (32.2) | 107,706 (32.5) |
| Anxiolytics | 9073 (41.6) | 102,555 (31.2) | 111,628 (31.9) | 5241 (39.8) | 98,315 (30.9) | 103,556 (31.3) |
| Topical ophthalmic preservatives† | 5459 (25.0) | 29,101 (8.9) | 34,560 (9.9) | 2359 (17.9) | 25,378 (8.0) | 27,737 (8.4) |
| Antihistamines | 2056 (9.4) | 19,385 (5.9) | 21,441 (6.1) | 1141 (8.7) | 18,490 (5.8) | 19,631 (5.9) |
| Hormone replacement therapy | 1604 (7.4) | 10,065 (3.1) | 11,669 (3.3) | 822 (6.2) | 9422 (3.0) | 10,244 (3.1) |
| Parkinson’s disease treatment | 1078 (4.9) | 9301 (2.8) | 10,379 (3.0) | 615 (4.7) | 8834 (2.8) | 9449 (2.9) |
Notes: Data shown as n (%) unless otherwise indicated. P-values for all data shown are statistically significant for patients with DED vs without DED (P <0.001). †Preservatives include antibiotic, antifungal, anti-inflammatory, miotic, vasoconstrictor, antiallergenic, and antiviral ophthalmic agents.
Abbreviations: COPD, chronic obstructive pulmonary disease; DED, dry eye disease; IBS, irritable bowel syndrome.
Among all study patients, patients with DED were statistically significantly older (mean age 59.9 years vs 52.7 years; P <0.001) and were predominantly female (56.9% vs 43.1; P <0.001) compared to patients without DED. Patients without prior DED who developed DED after CPAP or NMT use were generally older than patients without a DED diagnosis (mean age 58.9 years vs 52.5 years) and were slightly more likely to be female (52.2%). Among all patients, a significantly greater percentage of patients with DED vs patients without DED had inflammatory or metabolic comorbid diseases such as diabetes (27.3% vs 21.3%; P <0.001) and chronic obstructive pulmonary disease (COPD; 25.5% vs 18.0%; P <0.001). Additionally, the percentage of patients with DED who received antidepressants, anxiolytics, and antihypertensives was statistically significantly greater compared with patients without DED (P <0.001 for all).
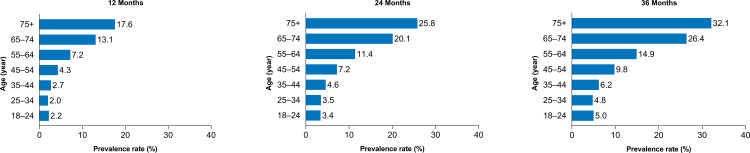
Prevalence of DED at 12, 24, and 36 Months
The overall prevalence of DED in CPAP or other NMT device users at 12, 24, and 36 months was 6.2%, 10.0%, and 13.0%, respectively. The prevalence also increased with age; the 12-, 24-, and 36-month prevalence in patients aged 18–24 years vs patients aged ≥75 years was 2.2%, 3.4%, and 5.0% vs 17.6%, 25.8%, and 32.1%, respectively (Figure 1). Females had an increased prevalence of DED compared to males, which also increased over time (12-, 24-, and 36-month prevalence for females was 9.6%, 15.2%, and 19.5% vs 4.2%, 7.0%, and 9.6%, for males, respectively). Furthermore, the prevalence of DED increased over time in patients with comorbidities. The prevalence in patients with psoriasis, irritable bowel syndrome (IBS), COPD, rheumatoid arthritis (RA), and diabetes was 17.2%, 8.7%, 8.6%, 8.0%, and 7.8% at 12 months vs 29.6%, 17.0%, 18.0%, 15.7%, and 16.0% at 36 months, respectively.
Figure 1.
Prevalence of dry eye disease by age and time.
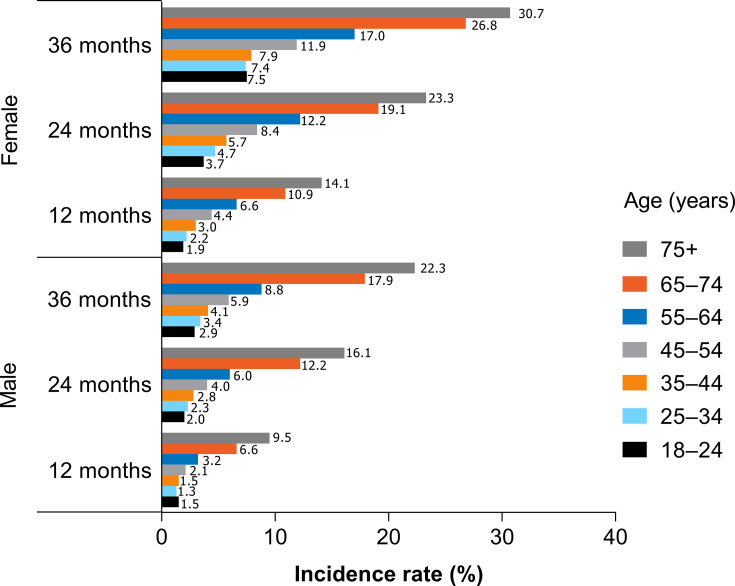
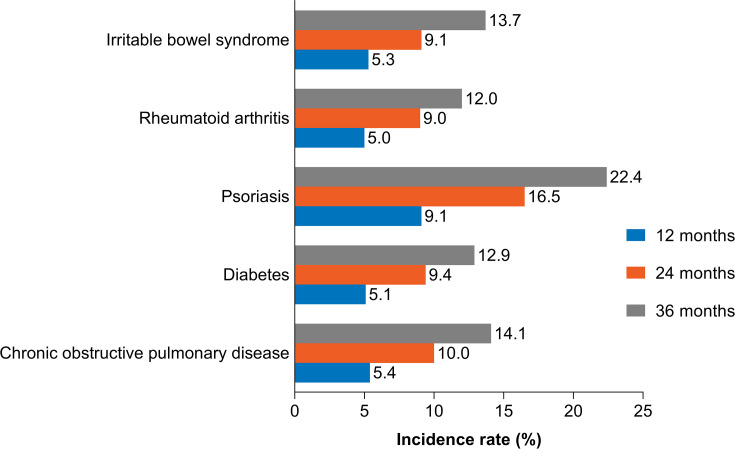
Incidence of DED at 12, 24, and 36 Months
The overall incidence of DED in patients using CPAP or other NMT devices at 12-, 24-, and 36-months was 4.0%, 7.3%, and 10.3%, respectively. As observed with prevalence, the incidence of DED increased with age; the 12-, 24-, and 36-month incidence in patients aged 18–24 years vs patients aged ≥75 years was 1.6%, 2.6%, and 4.3% vs 11.2%, 18.7%, and 25.4%, respectively (Figure 2). Incidence in female patients was also higher than in males at 12, 24, and 36 months of follow-up (5.8%, 10.8%, 15.1% vs 3.0%, 5.4%, 7.9%, respectively;Figure 2). The incidence in patients with comorbidities increased as the length of time patients used CPAP or other NMT devices increased. The incidence of DED in patients with psoriasis, COPD, IBS, diabetes, and RA was 9.1%, 5.4%, 5.3%, 5.1%, and 5.0% at 12 months vs 22.4%, 14.1%, 13.7%, 12.9%, and 12.0% at 36 months, respectively (Figure 3).
Figure 2.
Incidence by age and sex.
Figure 3.
Incidence of dry eye disease by comorbidity.
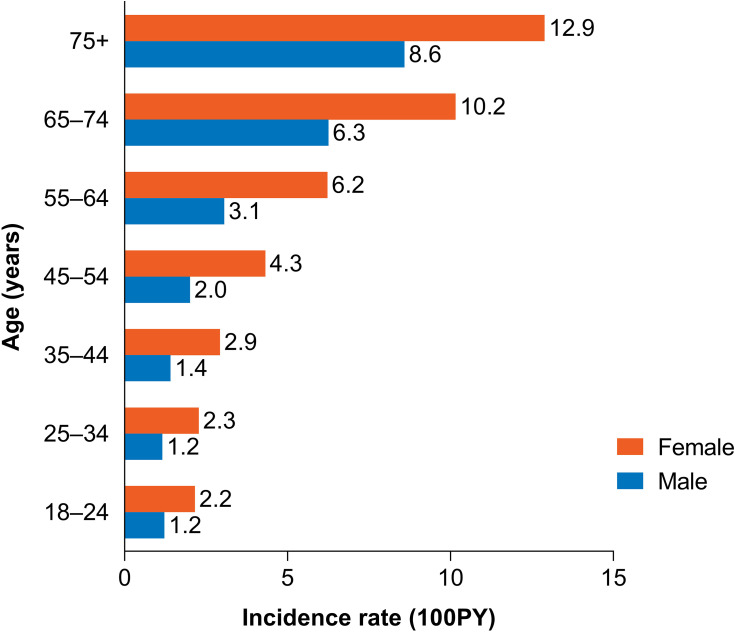
Incidence Rates of DED per 100 Person-Years
The overall incidence of DED in patients using CPAP or other NMT devices was 3.68 per 100PY. Females had a higher incidence of DED compared to males per 100PY (5.51 vs 2.73, respectively), and patients aged ≥75 years had a considerably greater incidence per 100PY than those aged 18–24 years (10.11 vs 1.52, respectively; Figure 4). Of the 5 most common comorbid conditions, the incidence rate per 100PY for psoriasis, COPD, IBS, diabetes, and RA was 8.31, 4.99, 4.83, 4.77, and 4.43, respectively.
Figure 4.
Incidence rate per 100 person-years by sex and age.
Abbreviation: 100PY, 100 person-years.
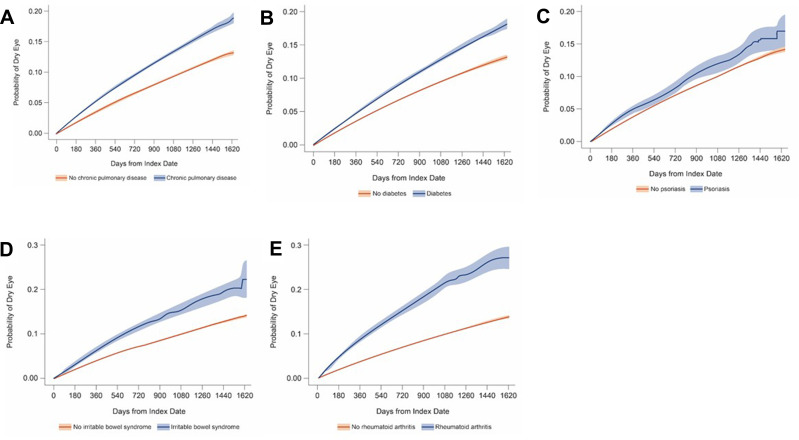
Probability of Dry Eye Disease Based on Kaplan–Meier Curves
The 12-, 24-, and 36-month probability (95% confidence interval) of DED in CPAP or other NMT device users were 4.0% (3.9–4.1%), 7.3% (7.2–7.4%), and 10.3% (10.1–10.4%), respectively. Similar to the trend of prevalence and incidence rates of DED, the probability of DED increased with age and was greater in females vs males (Table 2). Additionally, the probability of DED in patients who had inflammatory or metabolic comorbidity was greater than patients without these comorbidities (Figure 5).
Table 2.
Probability of Dry Eye by Sex and Age
| 12 Months (95% CI) | 24 Months (95% CI) | 36 Months (95% CI) | |
|---|---|---|---|
| Sex | |||
| Female | 5.9 (5.7–6.0) | 10.7 (10.5–10.9) | 14.9 (14.7–15.2) |
| Male | 3.0 (2.9–3.0) | 5.4 (5.3–5.5) | 7.7 (7.6–7.9) |
| Age (years) | |||
| 18–24 | 1.7 (1.3–2.1) | 2.8 (2.3–3.4) | 4.7 (3.7–5.6) |
| 25–34 | 1.5 (1.4–1.7) | 2.9 (2.6–3.1) | 4.2 (3.8–4.6) |
| 35–44 | 1.9 (1.8–2.1) | 3.7 (3.5–3.9) | 5.4 (5.2–5.6) |
| 45–54 | 2.9 (2.8–3.1) | 5.5 (5.4–5.7) | 8.0 (7.8–8.2) |
| 55–64 | 4.6 (4.4–4.7) | 8.3 (8.1–8.5) | 11.8 (11.6–12.0) |
| 65–74 | 8.2 (7.9–8.6) | 14.5 (14.0–14.9) | 19.8 (19.2–20.4) |
| ≥75 | 11.2 (10.6–11.7) | 18.7 (18.0–19.5) | 25.1 (24.2–26.0) |
Note: Data shown as %, unless otherwise indicated.
Abbreviation: CI, confidence interval.
Figure 5.
Probability of dry eye with (A) chronic obstructive pulmonary disease, (B) diabetes, (C) psoriasis, (D) irritable bowel syndrome, (E) rheumatoid arthritis.
Discussion
This study demonstrated an increased prevalence and incidence rate of DED for patients using CPAP or other NMT devices to treat sleep apnea compared with the general population. Moreover, we found that females and patients of older age had higher prevalence and incidence rates of DED compared with males and young adults, respectively. These findings support earlier publications reporting the prevalence and incidence of DED in the general population. In a recent study using data from the US Department of Defense Military Health System, the overall prevalence of DED was 2–3 times higher in females vs males across all ages—0.3% vs 0.1% (aged 2–17 years), 3.1% vs 1.3% (aged 18–39 years), 8.5% vs 2.8% (aged 40–49 years), and 15.9% vs 7.0% (≥50 years), respectively.15 Additionally, the annual prevalence of DED tripled over 7 years from 2005 to 2012.15 Although grouping by age varied between this study and our analysis, we found patients with at least 36 months of CPAP or NMT use had much greater prevalence rates of DED than adults in the general US population (13.0% vs 6.7%).15 Similar to prevalence, the annual incidence of DED in the general US population increased over time from 2008 to 2012 and ranged between 0.6% and 0.9%.15 In comparison, the overall incidence rate we found in patients utilizing CPAP or other NMT devices for at least 36 months was 10.3%.
Comorbid diseases also factored as a driver in the increase in prevalence and incidence of DED in patients using CPAP or NMT devices. Specifically, our analysis demonstrated patients with inflammatory and metabolic conditions had the highest rates of DED. After 36 months of follow-up, patients with psoriasis had the highest prevalence of DED (29.6%), followed by COPD (18.0%), IBS (17.0%), diabetes (16.0%), and RA (15.7%). These rates were higher than the overall prevalence rate in patients using CPAP or NMT devices at 36 months follow-up (13.0%). Incidence of DED at 36 months follow-up mirrored the prevalence rates with the highest incidence reported in patients with psoriasis, then COPD, IBS, diabetes, and RA. DED has been associated with various comorbid diseases such as hypertension, type II diabetes, hypothyroidism, depression, and autoimmune disease including RA, Sjögren’s syndrome, psoriasis, ulcerative colitis, and Crohn’s disease.16 Comorbid conditions often found in patients with sleep apnea include hypertension, obesity, COPD, cardiovascular disease, and type II diabetes.17 Accordingly, the comorbid conditions often linked to DED and sleep apnea overlapped with the 5 most common comorbid conditions observed in patients in this study. Therefore, patients with sleep apnea and comorbid inflammatory, metabolic, or autoimmune conditions may be at a heightened risk of developing DED.
Our analysis also revealed sequential rises in the prevalence and incidence of DED, as the length of time patients used CPAP or NMT devices increased. Over the study period, the prevalence rate of DED increased from 6.2% at the initial 12-month follow-up to 10.0% at the 24-month follow-up to 13.0% at the final 36-month follow-up. Patients with higher apnea-hypopnea index scores often have increased inflammation of the ocular surface and mechanical stress on the tissues of the eye due to CPAP or NMT devices—leading to a vicious cycle of damage to the surface of the eye.13 In addition, patients with moderate to severe sleep apnea are more likely to require CPAP or other NMT device treatment for longer periods than those with the more mild disease; thereby, those patients with the highest CPAP/NMT use may also be the most at risk for developing ocular disorders and DED. Moreover, in a study evaluating CPAP compliance in patients with sleep apnea, increased age and female sex were found to be significantly correlated with greater CPAP compliance and use.18 This association may also contribute to the results observed in the current analysis where patients of the female sex, increased age, and those who had increased use of CPAP or other NMT devices had increased prevalence and incidence of DED.
The findings of this study should be interpreted considering some limitations, including those inherent in any retrospective healthcare claims-based analysis. Diagnoses and outcomes were identified through administrative claims data, which are subject to data coding limitations and data entry error. There may be a potential for misclassification of diagnoses or study outcomes. These findings are also limited only to patients with commercial health or Medicare supplemental coverage and may not be generalizable to individuals with other insurance coverage or without health insurance coverage. Consequently, this population of CPAP and NMT users may differ from the general population of patients in the US with DED. Claims data cannot identify with certainty whether a case is truly an incident or represents an individual who may not have obtained care for a prolonged period. To reduce this uncertainty, we required continuous enrollment with no prior CPAP/NMT use and no evidence of DED in 12-months prior to first CPAP or NMT use. Administrative claims lack information on race/ethnicity or health behaviors that might provide further insight into epidemiology of DED. Lastly, this database analysis did not record patient compliance to their CPAP/NMT device, which may underestimate the incidence of DED in this population. Further research into the prevalence and incidence of DED in patients using CPAP or NMT devices is necessary to expand our understanding of the association between sleep apnea and DED in patients with other insurances and with information on health behaviors or other risk factors.
Conclusions
Prevalence and incidence of DED were higher in patients utilizing CPAP or other NMT devices compared to the general population, and prevalence and incidence rates increased as the length of time patients used a CPAP or NMT device increased. Furthermore, CPAP or NMT users who were female, older, or had comorbid inflammatory or metabolic conditions experienced a greater prevalence and incidence of DED. The rising prevalence and incidence of DED in patients with sleep apnea reveal a need for sleep physicians and ophthalmic practitioners to collaborate to provide comprehensive treatment plans for their patients.
Acknowledgments
This study was sponsored and funded by Sun Pharmaceutical Industries, Inc. We thank Dr. Yang Zhao for feedback and contributions to this study. Writing and editorial support for manuscript preparation were provided by Zehra Gundogan, VMD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc. All authors met the International Council of Medical Journal Editors criteria and received neither honoraria nor payment for authorship. Portions of this manuscript were presented at the 2020 Association for Research in Vision in Ophthalmology annual meeting as a poster presentation with interim findings. The poster’s abstract was published in Investigative Ophthalmology and Visual Science, June 2020 (https://iovs.arvojournals.org/article.aspx?articleid=2766373).
Disclosure
CM has received consultant fees from Aerie Pharmaceuticals; Alcon; Bausch & Lomb; Bruder Healthcare; Checked Up; EyePoint; EyeVance; Johnson & Johnson; Kala Pharmaceuticals; Lacriscience; Lenstex; Lumenis; Novartis; Ocular Science; Ocular Therapeutix; Olympic; Quidel; Sun Pharmaceutical Industries, Inc.; TearLab; TissueTech; and Ziedel; and personal fees from Progressive Tech Training, Strathspey Crown, and Veterinarian Recommended Solutions. XS, and AS-N are employees of IBM Watson Health, which received funding from Sun Pharmaceutical Industries, Inc., to conduct this study. IC was an employee of IBM Watson Health at the time of the study. AO is an employee of Sun Pharmaceutical Industries, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi: 10.1016/j.jtos.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 2.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289(17):2230–2237. doi: 10.1001/jama.289.17.2230 [DOI] [PubMed] [Google Scholar]
- 5.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142(3):187–197. doi: 10.7326/0003-4819-142-3-200502010-00010 [DOI] [PubMed] [Google Scholar]
- 6.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335–343. doi: 10.5664/jcsm.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas CI, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012;16(2):563–569. doi: 10.1007/s11325-011-0543-8 [DOI] [PubMed] [Google Scholar]
- 8.Pepin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124–1129. doi: 10.1164/ajrccm.160.4.9802027 [DOI] [PubMed] [Google Scholar]
- 9.Mojon DS, Goldblum D, Fleischhauer J, et al. Eyelid, conjunctival, and corneal findings in sleep apnea syndrome. Ophthalmology. 1999;106(6):1182–1185. doi: 10.1016/S0161-6420(99)90256-7 [DOI] [PubMed] [Google Scholar]
- 10.Kadyan A, Asghar J, Dowson L, Sandramouli S. Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye (Lond). 2010;24(5):843–850. doi: 10.1038/eye.2009.212 [DOI] [PubMed] [Google Scholar]
- 11.Santos M, Hofmann RJ. Ocular manifestations of obstructive sleep apnea. J Clin Sleep Med. 2017;13(11):1345–1348. doi: 10.5664/jcsm.6812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. doi: 10.1093/sleep/32.2.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acar M, Firat H, Acar U, Ardic S. Ocular surface assessment in patients with obstructive sleep apnea–hypopnea syndrome. Sleep Breath. 2013;17(2):583–588. doi: 10.1007/s11325-012-0724-0 [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 15.Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age united states health care system. Am J Ophthalmol. 2019;202:47–54. doi: 10.1016/j.ajo.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 16.Dana R, Bradley JL, Guerin A, Pivneva I, Evans AM, Stillman IÖ. Comorbidities and prescribed medications in patients with or without dry eye disease: a population-based study. Am J Ophthalmol. 2019;198:181–192. doi: 10.1016/j.ajo.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 17.Huang QR, Qin Z, Zhang S, Chow CM. Clinical patterns of obstructive sleep apnea and its comorbid conditions: a data mining approach. J Clin Sleep Med. 2008;4(6):543–550. doi: 10.5664/jcsm.27348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430–435. doi: 10.1378/chest.121.2.430 [DOI] [PubMed] [Google Scholar]