Abstract
Objective
Surgical site infection (SSI) is one of the leading causes of hospital-acquired infection among hospitalized patients. It causes significant health problems and results in an extended length of hospital stay, increased cost, and increased patient morbidity and mortality. To prevent the development of SSI, surgical antibiotic prophylaxis (SAP) administration before surgery is an evidence-based practice. Therefore, this study aimed to assess the prevalence of SSIs and surgical antibiotic prophylaxis practice, and identifying the gap in practicing prophylactic surgical antibiotic use.
Methods
A retrospective cross-sectional study design was conducted on randomly selected 281 participants who fulfilled the inclusion criteria. Appropriateness of surgical antibiotic prophylaxis was assessed by clinical pharmacists based on the standard treatment guideline. Descriptive and multivariate logistic regression analyses were performed in SPSS version 25. Statistical significance was set at p <0.05.
Results
The overall prevalence of SSI was 19.6% (95% CI: 19–20.2). Majority of surgical patients (88.6%) got surgical antibiotic prophylaxis. Ceftriaxone and metronidazole (45.4%), and ceftriaxone (33.3%) were the most frequently used prophylactic antibiotics. Presence of comorbidity (AOR=9.18, 95% CI: 5.17–17.9, p<0.001), contaminated (AOR=6.01, 95% CI: 1.77–16.8, p=0.019) and dirty (AOR=7.20, 95% CI: 1.23–12.1, p=0.029) wound classes, devoid of prophylactic antibiotics (AOR=6.63, 95% CI: 0.89–19.3, p=0.006), the timing of prophylactic antibiotic administration between 1 hour and 2 hours before incision (AOR=8.2, 95% CI: 4.34–18.1, p=0.001), and 48 hours duration of surgical antimicrobial prophylaxis (AOR=7.20, 95% CI: 1.23–28.17, p=0.027) were significantly associated with the development of SSIs.
Conclusion
The prevalence of SSI was relatively high despite most surgical patients were given prophylactic antibiotics. The presence of comorbidity, contaminated and dirty wound classes, devoid of prophylactic antibiotics, administering prophylactic antibiotics between 1 hour and 2 hours before incision, and 48 hours duration of surgical antibiotic prophylaxis were significantly associated with SSIs.
Keywords: surgical site infection, surgical antibiotic prophylaxis, Finote Selam General Hospital, Ethiopia
Introduction
Hospital-acquired infections remain a major clinical problem that caused significant morbidity and mortality, and increased healthcare cost.1 In developing countries, it is estimated that about 10% of hospitalized patients acquired hospital-acquired infections. Most of them are SSIs that accounted for 5.6% of surgical admitted patients.2 SSI is an infection occurring at or near surgical incision within 30 days of operation or after one year in case of an implant and affecting either the incision or the deep tissue in parts of the body where the surgery took place.3 Patients who developed SSI were more likely to have an outpatient and emergency department visits, frequent radiological service use, high rate of readmission, and home health aide service.4 In addition, SSI was a significant predictor of patient morbidity and mortality,5 associated with an extended duration of hospital stay,6 and resulted in an increased healthcare cost.7 Further, antimicrobial resistance in SSI was another challenge in the healthcare system.8,9
In low- and middle-income countries, more than one in ten surgical patients developed SSI. Even the risk of SSI is three to five times more susceptible to SSI in low- and middle-income countries than in high-income countries.10,11 In Africa, SSIs were the leading hospital-acquired infections, and the cumulative incidence varies from 2.5% to 30.9% as reported in a systematic review.12 In Ethiopia, although there was limited information, 10.9–75% of surgical patients developed SSI at different teaching hospitals.13–16 Furthermore, a meta-analysis reported that the pooled prevalence of SSI in Ethiopia was 12.3%.17
Surgical antibiotic prophylaxis (SAP) administration before surgery is an evidence-based practice to prevent the development of SSI.18 The literature recommends that antibiotics should be administered at least 30 minutes, but not more than 60 minutes, before incision.19 However, the nature of the pathogen, pharmacokinetics and pharmacodynamic profile of antibiotics, correct timing, dose, and route of administration are significantly related to the prevention of SSI and appropriate prophylaxis antibiotic use.1 According to the World Health Organization (WHO), the maximum duration of post-operative prophylaxis administration should be within 24 hours of the incision.20 Furthermore, antibiotic prophylaxis is usually indicated in contaminated wounds, penetrating wounds, abdominal trauma, compound fractures and wounds with devitalized tissue, which has a higher risk of infection.21 Despite this recommendation, evidence showed that SAP was often inappropriately reported by different studies.1,22,23 Inappropriate choice, timing, and the SAP duration were the commonly reported irrational use of prophylactic antibiotics.1,22,24 30–50% of surgical patients prescribed prophylactic antibiotics, and of which, 30–90% was inappropriate.25,26 Due to these irrational prophylactic antibiotic uses, increased medical care costs, prolonged hospitalization, superinfection, resistance, and adverse drug reaction were the frequently reported adverse effects.18,22
Although several studies have been conducted on SSIs, with significant variations in different regions, there was a paucity of data in the Northwest part of Ethiopia. Besides, these previous studies showed that antibiotics use associated problems were highly prevalent in Ethiopia.27 Therefore, this study aimed to assess surgical antibiotic prophylaxis practice and prevalence of SSI among surgically operated patients at Finote Selam General Hospital (FSGH), which will help promote SSI control and rational antibiotic prophylaxis utilization.
Methods
Study Design and Setting
A retrospective cross-sectional study design was conducted at FSGH located 387 km from Addis Ababa, Northwest Ethiopia. It is one of the biggest general hospitals in Northwest Ethiopia and provides specialized services in five major departments: Pediatrics, Surgery, Gynecology, Outpatient Department, and Internal Medicine. FSGH provides services to people residing in Finote-Selam town and its surroundings. The data were collected from 1st June 2020 to 31st July 2020.
Study Population
All patients admitted from 1st June 2019 to 31st May 2020 for elective or emergency surgical procedures were eligible. The study sample size was determined using a single population proportion formula by considering proportions of SSI rate was 23.5%28 with 5% marginal error and a confidence level of 95% as follows:
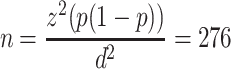 |
where
p = the proportion of favorable outcome
n= sample size
Z = is standard normal distribution usually set as 1.96 (which corresponds to 95% confidence level)
d = marginal error
Therefore, with a 5% contingency for incomplete medical records, the final sample size was 290 surgical patients.
Eligibility Criteria
All medical records of surgical patients admitted from 1st June 2019 to 31st May 2020 for elective or emergency surgical procedures with complete medical records were included in the study. Nonetheless, medical records that did not have complete information, such as list of surgical antibiotics prophylaxis, were excluded from the study.
Study Variables
The outcome variable for this study was SSI, while age, types of surgery, co-morbidity status, duration of surgery in hours, wound class, prophylactic antibiotic use, timing of prophylactic antibiotic administration, and duration of surgical antimicrobial prophylaxis administration were the main independent variables of the study.
Sampling Technique and Sampling Procedure
A simple random sampling technique was used among patients who underwent surgery from 1st June 2019 to 31st May 2020. The list of patients who underwent surgery was obtained from the record department of the hospital. Each eligible patient was given specific identification number and written in a piece of paper. Then, this paper was folded and placed in a basket. After meticulous mixing, the data collectors randomly chose study participants using the lottery method until the estimated sample size was attained. Finally, all the randomly selected medical records were assessed by trained data collector.
Data Collection Tool and Procedure
A structured data abstraction format was prepared considering the pretest result, which was conducted in 5% of the sample size at Debre Tabor General Hospital, to ensure the validity of data collection instrument. It contained socio-demographic characteristics, clinically related information, and prophylactic antibiotic regimens. Proper training was given for the four clinical pharmacists involved in the data collection. Data were retrospectively collected from the patients’ medical record. Patients’ medical record was reviewed from the time of patient admission to discharge. Appropriateness of surgical antibiotic prophylaxis was assessed by clinical pharmacists based on the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Surgical Infection Society, and the Society for Healthcare Epidemiology of America,29 Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery29 and World Health Organization SAP guidelines.30
Data Analysis
The collected data were cleaned, entered, and analyzed using Statistical Package for the Social Sciences (SPSS) version 25.0 software. Descriptive statistics (frequency, mean, and standard deviation) were employed. In addition, bivariate logistic regression analysis was conducted to investigate risk factors of SSIs. All variables with p<0.20 in the bivariate analysis were considered during multivariate logistic regression. Adjusted Odds Ratio (COR/AOR) with its p-value and confidence interval (95%) were reported in each logistic regression analysis. P-value < 0.05 considered as statistically significant.
Operational Definition of Terms
Surgical Antimicrobial Prophylaxis
Any antibiotics given before surgery to prevent surgical site infections.
Appropriate Surgical Antibiotics Prophylaxis
Refers to the use right antibiotics to the right indication at the right dose, frequency, route, and duration of antibiotics during the surgical procedure as per the WHO guideline.
Results
Socio-Demographic and Clinical Characteristics
In this study, 281 patients were assessed for SSI and appropriateness of surgical antibiotic prophylaxis use. As depicted in Table 1, 55.2% (155) were males, and almost half of them (52.3%) lived in urban areas. Forty-six percent of patients were in the age range of 21–40 years.
Table 1.
Socio-Demographic and Clinical Characteristics of the Study Participants (N = 281)
| Variables | Categories | N (%) |
|---|---|---|
| Sex | Male | 155 (55.2) |
| Female | 126 (44.8) | |
| Age | ≤20 | 28(10) |
| 21–40 | 131 (46.6) | |
| 41–60 | 87 (31.0) | |
| ≥61 | 35 (12.5) | |
| Residence | Urban | 147 (52.3) |
| Rural | 134 (47.7) | |
| Presence of comorbid conditions | Yes | 72 (25.6) |
| Types of surgery | Elective | 150 (53.4) |
| Emergency | 131 (46.6) | |
| Types of surgical procedures conducted | General surgery | 110 (39.1) |
| GI surgery | 74 (26.3) | |
| Gynecologic surgery | 41 (14.6) | |
| Urological surgery | 33 (11.7) | |
| Orthopedic surgery | 14 (5.0) | |
| Other surgery | 9 (3.2) | |
| Wound class | Clean | 107 (38.1) |
| Clean-contaminated | 74 (26.3) | |
| Contaminated | 66 (23.5) | |
| Dirty | 34 (12.5) | |
| Preoperative duration of hospitalization | ≤1 day | 208 (74.0) |
| >1 day | 73 (26.0) | |
| Postoperative duration of hospitalization | 1 day | 9 (3.2) |
| 2 days | 113 (40.2) | |
| 3 days | 128 (45.6) | |
| 4 days | 31(11) | |
| Duration of surgery (in hours) | <1 | 85 (30.2) |
| 1–2 | 115 (40.9) | |
| >23 | 61 (21.7) | |
| >34 | 20 (7.1) |
Note: Other surgery: neck surgery and appendectomy.
Abbreviation: GI, gastrointestinal.
Regarding clinical characteristics of study participants, general surgery (39.1%) and gastrointestinal surgery (26.3%) were the frequent surgical procedures in the study setting. More than half of these surgical procedures (53.4%) were elective. In addition, clean contaminated and contaminated wounds constituted 26.3% and 23.5% of surgical procedures, respectively. The predominant proportion of surgical procedures (62.6%) was taken between 1 hour and 3 hours of incision. Further, 26.0% of patients stayed in the hospital for more than one day before surgical procedure, while 56.6% of patients stayed in the hospital for more than two days after surgical procedure (Table 1).
Prevalence of Surgical Site Infections
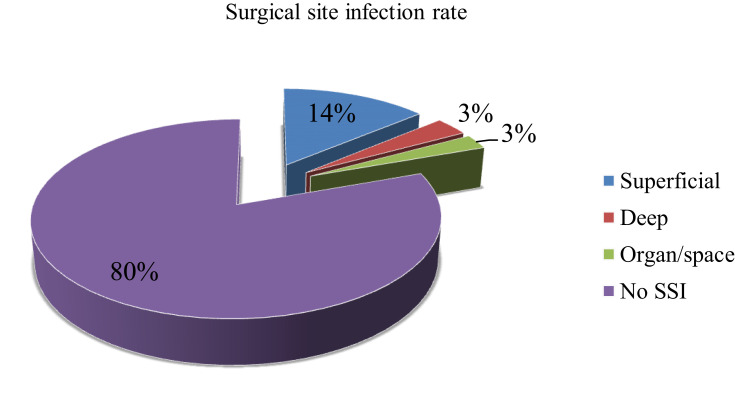
The present study showed that 19.6% (95% CI: 18–21.1) of patients developed SSIs. Of the developed SSI, 14.5%, 3% and 3% involved superficial, deep, and organ structures, respectively (Figure 1).
Figure 1.
Type of surgical site infection.
Surgical antibiotic prophylaxis was administered for more than three-fourth of patients (88.6%). 4.4%11 of prophylactic antibiotics were per-oral medications. In addition, 40.6% of antibiotic prophylaxis was given before 30 minutes of incision, while 82.8% of them were given between 30 minutes to 1 hour before incision. Of the prescribed antibiotics, 7.2% were inappropriate and 5.4% of these antibiotics’ dosage was inappropriate. Further, 43.8% of the prophylactic antibiotics were administered once (Table 2). Ceftriaxone (45.4%), and ceftriaxone and metronidazole (33.3%) were the most frequently used prophylactic antibiotics (Table 3).
Table 2.
Characteristics of Surgical Antimicrobial Prophylaxis Administration
| Variables | Frequency (%) | |
|---|---|---|
| Antibiotic surgical prophylaxis use | Yes | 249 (88.6) |
| Timing of antibiotic prophylaxis Administration |
30 minutes before surgery | 100 (40.6) |
| 30 minutes to 1 hour before incision | 108 (42.2) | |
| 1–2 hours before incision | 31 (13.2) | |
| Not known | 10 (4.0) | |
| Duration of SAP administration | Single dose | 109 (43.8) |
| 24 hours | 92 (36.9) | |
| 48 hours | 39 (15.7) | |
| 72 hours | 9 (3.6) | |
| Indication of SAP | Indicated and administered | 235 (94.4) |
| Administered but not indicated | 14 (5.4) | |
| Choice of antibiotics prophylaxis | Appropriate | 218 (92.8) |
| Inappropriate | 17 (7.2) | |
| Dosage appropriateness | Appropriate | 235 (94.4) |
| Inappropriate | 14 (5.4) | |
| Route | Intravenous | 203 (81.5) |
| Intravenous and oral | 35 (14.1) | |
| Oral | 11 (4.4) |
Abbreviation: SAP, surgical antibiotic prophylaxis.
Table 3.
Pattern of Prophylactic Antibiotic Use in Surgical Patients
| Prophylaxis Drug | Frequency (%) |
|---|---|
| Ceftriaxone | 113 (45.4) |
| Ceftriaxone + metronidazole | 83 (33.3) |
| Cloxacillin | 32 (12.9) |
| Ceftriaxone + gentamicin + metronidazole | 15 (6.0) |
| Ciprofloxacillin | 6 (2.4) |
Note: + Plus.
Factors Associated with Surgical Site Infections
Univariate logistic regression analysis showed that age, types of surgery, co-morbidity status, duration of surgery in hours, wound class, prophylactic antibiotic use, timing of prophylactic antibiotic administration, and duration of surgical antimicrobial prophylaxis administration were selected for final logistic regression analysis (p < 0.2).
At the end, multivariate regression analysis revealed that presence of comorbidity (p < 0.001), contaminated (p = 0.019) and dirty (p = 0.029) wound classes, devoid of prophylactic antibiotics (p = 0.006), the timing of prophylactic antibiotic between 1 hour and 2 hours before incision (p = 0.001), and 48 hours duration of surgical antimicrobial prophylaxis (p = 0.027) were significant predictors of SSI (Table 4).
Table 4.
Factors Associated with Surgical Site Infections Occurrence Among Surgical Patients
| Variables | Categories | Status of SSI | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| No (N, %) | Yes (N, %) | COR (95% CI) | AOR (95% CI) | p-value | ||
| Sex | Male | 128(82.6) | 27(17.4) | 1 | 1 | |
| Female | 98(77.8) | 28 (22.2) | 1.35(0.75–2.44) | 7.23(0.27–13.6) | 0.801 | |
| Age (in years) | ≤20 | 25(89.3) | 3(10.7) | 1 | 1 | |
| 21–40 | 114 (87.0) | 17(13.0) | 1.24(0.34–4.57) | 0.71(0.09–5.63) | 0.749 | |
| 41–60 | 63(72.4) | 24(27.6) | 3.18(0.88–11.49)* | 6.63(0.89–19.3) | 0.065 | |
| ≥61 | 24(68.6) | 11(31.4) | 3.82(0.95–15.4)* | 0.33(0.19–5.81) | 0.450 | |
| Types of surgery | Elective | 131(87.1) | 19(12.9) | 1 | 1 | |
| Emergency | 95(72.5) | 36(27.5) | 2.61(1.41–4.83)* | 2.82(0.47–16.8) | 0.254 | |
| Comorbidity status | Yes | 38(52.8) | 34(47.2) | 8.01 (4.20–15.28)* | 9.18(5.17–17.9) | <0.001 |
| No | 188(90) | 21(10) | 1 | 1 | ||
| Duration of surgery in hours | <1 | 75(88.2) | 10(11.8) | 1 | 1 | |
| 1–2 | 95(82.6) | 20(17.4) | 1.58(0.7–3.58) | 0.85(0.12–6.10) | 0.870 | |
| >23 | 48(78.7) | 13(21.3) | 2.03(0.83–5.0)* | 0.52(0.033–8.15) | 0.640 | |
| >34 | 8(40) | 12 (60) | 11.25(3.7–34.2)* | 0.38(0.012–12.9) | 0.803 | |
| Wound class | Clean | 92(86) | 15(14) | 1 | 1 | |
| Clean-contaminated | 63(85.1) | 11(14.9) | 1.07(0.46–2.48) | 1.94(0.145–15.8) | 0.617 | |
| Contaminated | 49(74.2) | 17(25.8) | 2.13(0.98–4.62)* | 6.01(1.77–16.8) | 0.019 | |
| Dirty | 22 (64.7) | 12 (35.3) | 3.35(1.37–8.15)* | 7.20(1.23–12.1) | 0.029 | |
| Prophylactic antibiotics | Received | 210(84.3) | 39(15.7) | 1 | 1 | |
| Not received | 16(50) | 16(50) | 5.39(2.49–11.66)* | 6.63(1.89–19.3) | 0.006 | |
| Timing of prophylaxis administration |
30 minutes before surgery | 88(88) | 12(12) | 1 | 1 | |
| 30 minutes to 1 hour before incision | 98(90.7) | 10(9.3) | 0.8(0.32–1.97) | 0.56(0.1–3.04) | 0.499 | |
| 1 to 2 hours before incision | 14(45.2) | 17(54.8) | 10.2(3.93–26.62)* | 8.2(4.34–18.1) | 0.001 | |
| Not known | 5 (50) | 5 (50) | 7.82(1.95–31.37)* | 10.33(0.89–22.1) | 0.062 | |
| Duration of SAP administration | Single dose | 85(81) | 24(19) | 1 | 1 | |
| 24 hours | 86(93.5) | 6(6.5) | 0.26(0.1–0.68)* | 0.30(0.64–1.45) | 0.136 | |
| 48 hours | 29(74.4) | 10(25.6) | 1.22(0.52–2.86)* | 7.20(1.23–28.17) | 0.027 | |
| 72 hours | 4 (44.4) | 5 (55.6) | 9.43(1.71–51.93) | 5.89(0.43–12.28) | 0.834 | |
Note: *p<0.2.
Abbreviations: AOR, adjusted odds ratio; COR, crude odds ratio; SAP, surgical antimicrobial prophylaxis.
Discussion
In this study, antibiotics were used preoperatively and postoperatively, and the incidence of SSIs was assessed in FSGH, Ethiopia. Socio-demographic and clinical characteristics, antibiotics utilization practice in surgery, SAP, and factors associated with SSIs were studied. SSIs are the most common cause of hospital-acquired infections that result in considerable morbidity and mortality, extended duration of hospital stay, increased cost, and high rate of antimicrobial resistance and pose an additional burden to the healthcare system.2,5,6
In the present study, 19.6% (95% CI: 19–20.2) of patients developed SSIs, which is consistent with other previous study conducted by Tekie et al (18%),31 Sievert et al (19.1%),32 Nobandegani et al (20%),33 and Halawi et al (20.6%).34 The finding is higher than studies conducted by Lubega et al (16.4%),35 Fisha et al (9.9%),36 and Alamrew et al (11.1%).25 The higher prevalence of SSI in our setting could be due to the lack of adequate infection control systems, poor practices, and inappropriate use of antibiotics. Likewise, the prevalence of SSI was higher than studies conducted by Mukagendaneza et al (10.9%)37 and Ghali et al (8.6%).38 This variation may be because of the different healthcare services, type of and level of healthcare professionals, sanitation status, and the number of patients seeking medical care.
Nonetheless, the prevalence was lower than studies conducted by Misganaw et al (23.4%)28 and Mezemir et al (24.6%).39 In addition, it was lower than the recent systematic review performed in Ethiopian literature (25.22%, 95% CI: 17.30–3.14%).40 This disparity could probably be due to the higher prevalence of prophylactic surgical antibiotic use (88.6%) in our setting.
The present study showed that most surgical procedures (71.2%) were undertaken within 1–2 hours. Further, a higher number of surgical procedures were clean (38.1%) surgeries, which is lower as compared to Aragaw et al study,41 where 54% of surgical wounds were clean and 80% of patients received preoperative prophylaxis.
In our study, 14.7% SSIs were superficial, followed by deep (3%) infections, which is different from Mezemir et al,39 study that reported 40.7% and 37.4% were deep and organ spaced infections, respectively. In addition, a higher percentage of surgical procedures were general (39.1%). On the other hand, Legesse et al,15 and Awoke et al,42 found that head and neck procedures (29.5%) and abdominal surgery (42.9%) were the leading surgical procedures, respectively. Further, most of the procedures were elective (53.4%), which is lower than the study conducted by Awoke et al (62.8%).42 The different patterns of surgical cases and the type of surgery performed in two hospitals may be responsible for this variation.
Regarding prophylactic antibiotic use, 88.6% of surgical patients got surgical antibiotic prophylaxis, which is greater than Alamrew et al (72.1%)25 and lower than Lijaemiro et al (92.0%)43 study. This difference may be due to the variation of cases, urgency, and healthcare professionals involved. One hundred (40.6%) of surgical antibiotic prophylaxis was given 30 minutes before incision in line with the recommended guideline. This is comparable to a study conducted by Alamrew et al25 and Lee et al.44 According to American Family Physicians recommendations, prophylactic antibiotics should be initiated within 1 hour before surgical incision.45 This supports our study finding as most of our study participants (208, 83.5%) received SAP within 1 hour before surgical incision.
Of the prescribed antibiotics, 7.2% of them were inappropriate, while 5.4% used inappropriate dosages. Besides, a single antibiotic was used more than half of the surgical patients (60.7%), and ceftriaxone (45.4%) was the most frequently administered antibiotic. The finding is in contrast to Misganaw et al28 and Alamrew et al25 studies, which reported that a combination of ceftriaxone and metronidazole (47.46%) was the frequently used antibiotics. Such variation may be because of the different types of surgical procedures conducted among these studies and variation in the drug’s accessibility. Accordingly, appropriate antibiotic selection, time of administration, and dosage form should be advocated to reduce inappropriate antibiotic use and development of SSIs.
Our study identified a few risk factors that contributed to SSIs. Patients with comorbidity were nine times more risk to develop SSI than patients without comorbidity. This is in line with Misganaw et al study.28 The presence of comorbidity may expose patients to infection due to lowered immunity. Besides, contaminated and dirty wound classes were six and seven times more susceptible to SSIs than clean wound classes. This finding is in line with Weldu et al,46 Mezemir et al,39 and Misganaw et al28 studies. These types of wound classes are suitable for the colonization and multiplication of different pathogens. Patients who did not get prophylactic antibiotics were seven (6.6) times more likely to develop SSI than patients who had prophylactic antibiotics, which is in line with Misganaw et al28 and Alamrew et al25 studies. Administering prophylactic antibiotic prevent colonization and spread of microbes. Prophylactic antibiotic administration between 1 hour and 2 hours before the incision was eight times more likely to have SSIs than patients who got these antibiotics before 30 minutes of surgery. Our finding is in line with Legesse et al15 and Misganaw et al28 studies. Prophylactic antibiotic administration within 30 minutes of incision reduces the risk of SSIs. Guidelines recommended that SAP should be administered 30 minutes before incision. The present study also showed that the duration of surgical antibiotic prophylaxis with 48 hours was seven times more likely to develop SSI than patients with a single dose, which is in line with Misganaw et al study.28
Our study’s strength involves a sufficient number of study participants and comprehensive data collection to generalize to the source population, while the main limitation is the retrospective nature of the study.
Conclusion
Our study found that most of the surgical procedures were general and gastrointestinal. Of these procedures, half of them were either contaminated or dirty wounds. Besides, despite more than three-fourth of surgical patients got surgical antibiotic prophylaxis, one in five of these patients developed SSI. Superficial infections were common SSIs. In addition, more than half of the prescribed prophylactic antibiotics were given after the recommended time limit (30 minutes before the incision). Further, the presence of comorbidity, contaminated and dirty wound class, devoid of prophylactic antibiotics, administering prophylactic antibiotics between 1 hour and 2 hours before incision, and 48 hours duration of surgical antibiotic prophylaxis were significantly associated with SSIs. Therefore, healthcare professionals should consider the above risk factors while they are undergoing surgical procedures. They should also adhere to the recommended treatment guideline to prescribe appropriate prophylactic surgical antibiotics.
Acknowledgments
We would like to acknowledge FSGH medical ward staffs and data abstractors for their valuable contribution towards this project.
Funding Statement
There is no funding to report.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; FSGH, Finote Selam General Hospital; SAP, surgical antibiotic prophylaxis; SPSS, Statistical Package for the Social Sciences; SSI, surgical site infection; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Ethical Review Board of Debre Tabor University (Approval number: DTU/RE/1/189/12), and subsequent permission was obtained from the Medical Record Department of FSGH. The Ethics Committee and the Hospital waived the informed written consent from the study participants due to the retrospective nature of this study. Patients’ personal information and clinical information were recorded by ensuring patient confidentiality without recording their names and address. The methods were carried out following the relevant guidelines and regulations of Debre Tabor University. Our study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable
Author Contributions
All authors made a significant contribution to the work reported, in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Alemkere G. Antibiotic usage in surgical prophylaxis: A prospective observational study in the surgical ward of Nekemte referral hospital. PLoS One. 2018;13(9):e0203523. doi: 10.1371/journal.pone.0203523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. doi: 10.1016/S0140-6736(10)61458-4 [DOI] [PubMed] [Google Scholar]
- 3.Smith MA, Dahlen NR, Bruemmer A, Davis S, Heishman C. Clinical practice guideline surgical site infection prevention. Orthopaedic Nursing. 2013;32(5):242–248. doi: 10.1097/NOR.0b013e3182a39c6b [DOI] [PubMed] [Google Scholar]
- 4.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196. doi: 10.3201/eid0902.020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astagneau P, Rioux C, Golliot F, Brücker G. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hospital Infection. 2001;48(4):267–274. doi: 10.1053/jhin.2001.1003 [DOI] [PubMed] [Google Scholar]
- 6.Mahmoud NN, Turpin RS, Yang G, Saunders WB. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect. 2009;10(6):539–544. doi: 10.1089/sur.2009.006 [DOI] [PubMed] [Google Scholar]
- 7.Monahan M, Jowett S, Pinkney T, et al. Surgical site infection and costs in low- and middle-income countries: A systematic review of the economic burden. PLoS One. 2020;15(6):e0232960. doi: 10.1371/journal.pone.0232960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmanov AG, Dyndar OA, Vdovychenko YP, et al. Surgical site infections and antimicrobial resistance in Kyiv City Hospitals, Ukraine. Wiad Lek. 2019;72(5 cz 1):760–764. [PubMed] [Google Scholar]
- 9.Zahran WA, Zein-Eldeen AA, Hamam SS, Sabal MSE. Surgical site infections: problem of multidrug-resistant bacteria. Menoufia Medical J. 2017;30(4):1005. doi: 10.4103/mmj.mmj_119_17 [DOI] [Google Scholar]
- 10.WHO. Stop infections after surgery; what is the problem? 2016. Available from: https://www.who.int/gpsc/ssi-infographic.pdf?ua=1. Accessed August21, 2020.
- 11.Curcio D, Cane A, Fernández F, Correa J. Surgical site infection in elective clean and clean-contaminated surgeries in developing countries. Int J Infectious Diseases. 2019;80:34–45. doi: 10.1016/j.ijid.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 12.Nejad SB, Allegranzi B, Syed SB, Ellis B, Pittet D. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011;89:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulu W, Kibru G, Beyene G, Damtie H. Associated risk factors for postoperative nosocomial infections among patients admitted at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Clin Med Res. 2013;2(6):140–147. doi: 10.11648/j.cmr.20130206.15 [DOI] [Google Scholar]
- 14.Mengesha RE, Kasa BG-S, Saravanan M, Berhe DF, Wasihun AG. Aerobic bacteria in post surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res Notes. 2014;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laloto TL, Gemeda DH, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis. 2017;17(1):119. doi: 10.1186/s12879-016-2167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gedefaw G, Asires A, Shiferaw S, Addisu D. Factors associated with surgical site infection among women undergoing obstetrics surgery at Felegehiwot referral hospital, Bahir Dar, Northwest Ethiopia: a retrospective cross-sectional study. Safety in Health. 2018;4(1):14. doi: 10.1186/s40886-018-0081-1 [DOI] [Google Scholar]
- 17.Shiferaw WS, Aynalem YA, Akalu TY, Petrucka PM. Surgical site infection and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Surg. 2020;20:1–15. doi: 10.1186/s12893-020-00764-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59–74. doi: 10.1016/j.jamcollsurg.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 19.Crader MF, Varacallo M. Preoperative antibiotic prophylaxis. StatPearls. 2020. [PubMed] [Google Scholar]
- 20.WHO Guidelines Development Group, Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288–e303. doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 21.Organization WH. Prevention and management of wound infection. 2010. [Google Scholar]
- 22.Ayele Y, Taye H. Antibiotic utilization pattern for surgical site infection prophylaxis at Dil Chora Referral Hospital Surgical Ward, Dire Dawa, Eastern Ethiopia. BMC Res Notes. 2018;11(1):537. doi: 10.1186/s13104-018-3629-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoglu S, Aslan S, Akalin S, Bosnak V. Audit of quality of perioperative antimicrobial prophylaxis. Pharmacy World Sci. 2009;31(1):14–17. doi: 10.1007/s11096-008-9259-7 [DOI] [PubMed] [Google Scholar]
- 24.Nabovati E, Vakili-Arki H, Taherzadeh Z, Hasibian MR, Abu-Hanna A, Eslami S. Drug-drug interactions in inpatient and outpatient settings in Iran: a systematic review of the literature. DARU J Pharm Sci. 2014;22(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alamrew K, Tadesse TA, Abiye AA, Shibeshi W. Surgical antimicrobial prophylaxis and incidence of surgical site infections at ethiopian tertiary-care teaching hospital. Infectious Diseases. 2019;12:1178633719892267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzal Khan A, Mirshad P, MohAMMed rAfiuddin rAshed GB. A study on the usage pattern of antimicrobial agents for the prevention of surgical site infections (SSIs) in a tertiary care teaching hospital. JCDR. 2013;7(4):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tefera GM, Feyisa BB, Kebede TM. Antimicrobial use–related problems and their costs in surgery ward of Jimma University Medical Center: prospective observational study. PLoS One. 2019;14(5):e0216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misganaw D, Linger B, Abesha A. Surgical antibiotic prophylaxis use and surgical site infection pattern in Dessie Referral Hospital, Dessie, Northeast of Ethiopia. Biomed Res Int. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14(1):73–156. doi: 10.1089/sur.2013.9999 [DOI] [PubMed] [Google Scholar]
- 30.Organization WH. Implementation Manual to Support the Prevention of Surgical Site Infections at the Facility Level: Turning Recommendations into Practice: Interim Version. World Health Organization; 2018. [Google Scholar]
- 31.Tekie K Surgical wound infection in Tikur Anbessa hospital with special emphasis on Pseudomonas aeruginosa. Unpublished MSc thesis in medical microbiology. Addis Ababa University, Medical Faculty; Ethiopia; 2008. Available from: http://etd aau edu et/bitstream/handle/123456789/5772/Kassaye% 20Tekie pdf. Accessed January31, 2014. [Google Scholar]
- 32.Sievert DM, Ricks P, Edwards JR, et al. National Healthcare Safety Network (NHSN) Team and Participating NHSN facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. [DOI] [PubMed] [Google Scholar]
- 33.Nobandegani Zinat M, Doulatabad Shahla N, Masoumeh R, Ardeshir A. Surgical site infection incidence after a clean-contaminated surgery in Yasuj Shahid Beheshti hospital, Iran. Investigación y Educación En Enfermería. 2011;29(3):435–441. [Google Scholar]
- 34.Halawi E, Assefa T, Hussen S. Pattern of antibiotics use, incidence and predictors of surgical site infections in a tertiary care teaching hospital. BMC Res Notes. 2018;11(1):538. doi: 10.1186/s13104-018-3643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubega A, Joel B, Justina Lucy N. Incidence and etiology of surgical site infections among emergency postoperative patients in mbarara regional referral hospital, South Western Uganda. Surgery Res Practice. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisha K, Azage M, Mulat G, Tamirat KS. The prevalence and root causes of surgical site infections in public versus private hospitals in Ethiopia: a retrospective observational cohort study. Patient Saf Surg. 2019;13(1):26. doi: 10.1186/s13037-019-0206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukagendaneza MJ, Munyaneza E, Muhawenayo E, et al. Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg. 2019;13(1):10. doi: 10.1186/s13037-019-0190-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghali H, Ben Rejeb M, Chahed C, et al. Incidence and risk factors of surgical site infection in general surgery department of a Tunisian tertiary teaching hospital: A prospective observational study. Canadian J Infection Control. 2018;33:1. [Google Scholar]
- 39.Mezemir R, Seid A, Gishu T, Demas T, Gize A. Prevalence and root causes of surgical site infections at an academic trauma and burn center in Ethiopia: a cross-sectional study. Patient Saf Surg. 2020;14(1):3. doi: 10.1186/s13037-019-0229-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birhanu Y, Endalamaw A. Surgical site infection and pathogens in Ethiopia: a systematic review and meta-analysis. Patient Saf Surg. 2020;14(1):1–8. doi: 10.1186/s13037-020-00232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argaw NA, Shumbash KZ, Asfaw AA, Hawaze S. Assessment of surgical antimicrobial prophylaxis in orthopaedics and traumatology surgical unit of a tertiary care teaching hospital in Addis Ababa. BMC Res Notes. 2017;10(1):1–8. doi: 10.1186/s13104-017-2475-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awoke N, Arba A, Girma A. Magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia. PLoS One. 2019;14(12):e0226140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lijaemiro H, Berhe Lemlem S, Tesfaye Deressa J. Incidence of surgical site infection and factors associated among cesarean deliveries in selected government Hospitals in Addis Ababa, Ethiopia, 2019. Obstet Gynecol Int. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JS, Lee HH, Song KY, Park CH, Jeon HM. The feasibility of short term prophylactic antibiotics in gastric cancer surgery. J Gastric Cancer. 2010;10(4):206–211. doi: 10.5230/jgc.2010.10.4.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salkind AR, Rao KC. Antibiotic prophylaxis to prevent surgical site infections. Am Fam Physician. 2011;83(5):585–590. [PubMed] [Google Scholar]
- 46.Weldu MG, Berhane H, Berhe N, et al. Magnitude and determinant factors of surgical site infection in Suhul hospital Tigrai, northern Ethiopia: a cross-sectional study. Surg Infect. 2018;19(7):684–690. doi: 10.1089/sur.2017.312 [DOI] [PubMed] [Google Scholar]