Introduction
Immune checkpoint inhibitors (ICIs) are being increasingly used for the treatment of certain advanced cancers. Each drug targets a specific signaling pathway to reduce inhibition of the antitumor immune response and thus activate the body’s immune cells against cancer cells. Nivolumab, most often used in treatment of melanoma, renal cell cancer, and non–small cell lung cancer, is a human immunoglobulin G4 monoclonal antibody that selectively inhibits the programmed cell death-1 (PD-1) receptor.1 Cardiotoxicity from ICIs is thought to result from the loss of T-cell regulation and subsequent dysregulated autoimmune response to cardiac antigens.2 This can manifest clinically as myocarditis, pericarditis, Takotsubo cardiomyopathy, arrhythmias, and vasculitis.3
Key Teaching Points.
-
•
Cardiac conduction abnormalities and ventricular arrhythmias are possible complications of immune checkpoint inhibitor (ICI)–associated myocarditis.
-
•
Corticosteroid therapy has been shown to reverse cardiotoxicity due to systolic dysfunction in up to two-thirds of cases of ICI myocarditis. Reversibility of conduction abnormalities has not been well described, owing to the rarity of this toxicity.
-
•
Permanent pacing for heart block with ICI-associated myocarditis may be necessary. Current guidelines support permanent pacing in patients with any symptomatic atrioventricular block attributable to a known reversible cause that does not resolve despite treatment of this cause.
Case report
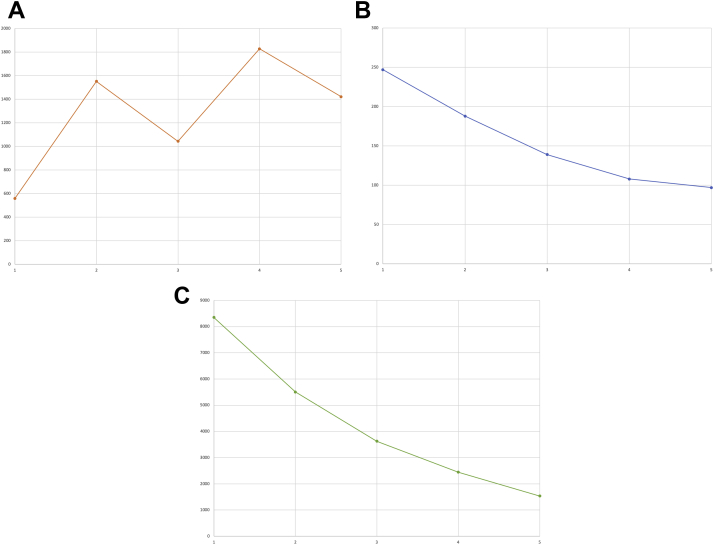
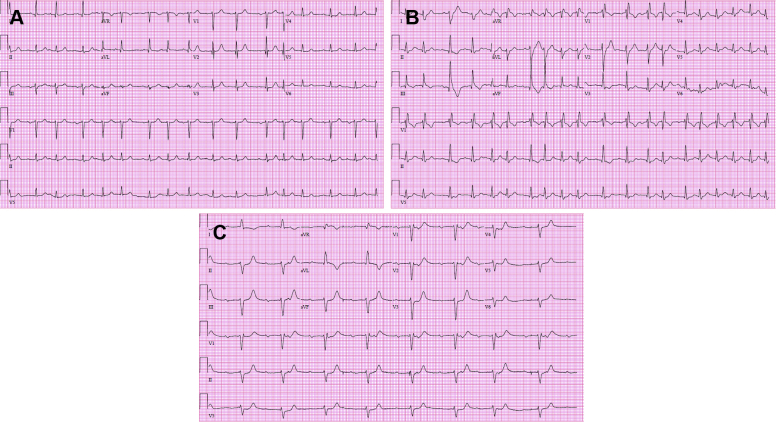
An 88-year-old man presented with generalized weakness and inability to transfer out of bed. The patient had been diagnosed with pT4b invasive melanoma of the scalp 3 months prior. He had opted for systemic treatment instead of surgery and radiation therapy and had received his first dose of nivolumab (480 mg intravenous [IV]) 22 days prior. Initial examination was unremarkable, without evidence of poor tissue perfusion or volume overload. Fifth-generation troponin T peaked at 1828 ng/L (normal <22), creatine kinase MB isoenzyme was at 247 ng/mL (<4.8), and total creatine phosphokinase was 8350 U/L (<175) (Figure 1A–C). Initial electrocardiogram (ECG) demonstrated normal sinus rhythm without conduction abnormalities (Figure 2A). Echocardiogram showed preserved systolic function and no significant valvular disease (Supplemental Video). With his symptoms, significant cardiac biomarker elevations, and recent nivolumab infusion, ICI-associated myositis and myocarditis was suspected clinically. He was started on a burst of prednisone 40 mg daily and further nivolumab infusions were held. Subsequent daily ECGs showed progression from normal sinus rhythm with premature atrial contractions and without conduction disease (hospital day 1) to new bifascicular block (hospital day 4), then complete heart block (hospital day 5) (Figure 2A–C). On hospital day 5, intermittent complete heart block was demonstrated on telemetry, and a temporary transvenous pacemaker was placed. Oral prednisone was escalated to IV methylprednisolone 125 mg daily on hospital days 5–6, which was then increased to 1 g IV methylprednisolone daily thereafter. Corticosteroid dosing and intervals were determined by the patient’s oncology consultants. The patient also received 1 dose of infliximab 5 mg/kg on hospital day 9. Mycophenolate was also considered but was ultimately not given owing to a previous adverse reaction of hives. The need for permanent pacemaker placement was considered. Given lack of improvement with high-dose corticosteroid therapy and infliximab, he ultimately received a dual-chamber pacemaker on hospital day 10. Following his pacemaker placement, the patient continued to decline clinically and with the involvement of his family, he chose not to be resuscitated in the event of a cardiopulmonary arrest. On hospital day 15 he went into ventricular fibrillation followed by asystole, and then died. An autopsy was offered but was declined by his surviving family.
Figure 1.
A: Fifth-generation troponin T trend (x-axis = days, y-axis = ng/L). B: Creatine kinase MB isoenzyme trend (x-axis = days, y-axis = ng/mL). C: Creatine phosphokinase trend (x-axis = days, y-axis = U/L).
Figure 2.
A: Electrocardiogram (ECG) on hospital day 1 showing sinus rhythm with premature atrial contractions. B: ECG on hospital day 4 showing sinus rhythm with new bifascicular block and increased atrial ectopy. C: ECG on hospital day 5 showing complete heart block.
Discussion
The authors have presented a case of ICI-associated myocarditis in a patient with invasive melanoma on nivolumab. The patient developed progressive conduction disease and ultimately complete heart block, followed by ventricular fibrillation leading to sudden cardiac death—a remarkable clinical course in just over 2 weeks’ time. The short duration over which these complications occurred makes this a uniquely challenging and noteworthy case to discuss.
The true prevalence of myocarditis from ICI is not known. A range from <0.06% to 2.4% has been reported from preclinical trials, case series, registries, and randomized clinical trials.3,4 Data from 1 multicenter registry found the median time to onset of ICI-associated myocarditis was 34 days (interquartile range 21–75 days) from the first drug infusion.5 In that study, patients with myocarditis were more likely to have received combination ICI as compared to monotherapy.
Electrical complications associated with ICI-associated myocarditis may include atrial fibrillation, supraventricular tachycardia, progressive heart block, and lethal ventricular arrhythmias. The prevalence of these complications following ICI therapy is also not well understood. One observational study reported findings of conduction disease and ventricular arrhythmias in 17% and 27% of patients with ICI-associated myocarditis, respectively.4 Only 3 previously published case reports of ICI-associated complete heart block have been reported.6,7 In all of these 3 previously described cases of complete heart block, patients had received combination therapy with anti-PD-1 and anti–cytotoxic T lymphocyte–associated protein 4 ICIs, compared to ICI monotherapy with only anti-PD-1 (nivolumab) in this case report.
The American Society for Clinical Oncology practice guidelines recommend hospital admission, withholding of further ICI therapy, and early initiation of corticosteroid therapy for moderate-to-severe cases of suspected ICI-associated myocarditis.3 One to 2 mg/kg of prednisone or methylprednisolone is recommended for moderate (grade 2 or 3) cases, while the addition of mycophenolate, antithymocyte globulin, or infliximab may be considered for severe (grade 4) cases. Corticosteroid therapy has been shown to reverse cardiotoxicity due to systolic dysfunction in up to two-thirds of cases.4 Registry data suggest that use of higher doses of corticosteroids is associated with lower rates of peak serum troponin levels and major adverse cardiac events.5 The reversibility of conduction abnormalities has not been well described, owing to the rarity of this toxicity.
The patient characterized in this case report presented with symptoms compatible with some element of heart failure, although his initial echocardiogram was normal. This may not be an uncommon finding; Mahmood and colleagues5 found that more than half of patients with ICI-associated myocarditis in a multicenter registry had a normal left ventricular ejection fraction (LVEF) on initial assessment. Additional cardiac imaging was not felt to be indicated at that early juncture of his clinical presentation; however, it was suspected that this patient may have evolved with left ventricle dysfunction over time; no guidelines exist to aid in determining the needed frequency of LVEF re-evaluation. Cardiac magnetic resonance imaging was considered as his condition deteriorated but was not pursued, as it probably would not have changed management. In hindsight, a focused echocardiogram evaluating LVEF after conduction disease had progressed may have been prognostic. The patient then progressed to complete heart block in the setting of ICI-associated myocarditis and did not improve after 10 days of high-dose corticosteroids and 1 dose of infliximab. This presented a management dilemma with regard to the indication for and timing of permanent pacemaker placement. Reddy and colleagues7 have reported on the only known case of complete heart block due to ICI-associated myocarditis that reversed with treatment. This was, however, months later in follow-up, after receiving high-dose corticosteroid therapy, mycophenolate mofetil, a transvenous pacer, and later a permanent pacemaker. The 2018 ACC guidelines8 give a class IC recommendation for permanent pacing in patients with symptomatic atrioventricular block attributable to a known reversible cause that does not resolve despite treatment of this cause. Given the paucity of data on reversibility of heart block in this setting, the guidelines seem to support the clinicians’ decision to place a permanent pacemaker. Our patient also developed ventricular arrhythmias as a likely toxicity. Up until the moment of sudden cardiac death, however, the patient had not demonstrated significant ventricular ectopy on telemetry. Ventricular arrhythmias are common in all types of myocarditis, particularly if the LVEF is depressed.9 Owing to the apparent high rates of ventricular arrhythmias reported in the literature, the authors suggest that placement of an implantable cardioverter-defibrillator (ICD) should be considered if permanent pacing is felt to be indicated. Additionally, in those who are not implanted with ICD therapy, a wearable defibrillator may be considered, particularly in patients at high risk of life-threatening ventricular arrhythmias.10 The life expectancy of the patient must also be considered in these decisions, as the mortality rate of ICI-induced myocarditis can be up to 50%.3 To help guide treatment to avoid sudden cardiac death in patients with ICI-related myocarditis, future studies should assess the risk of ventricular arrhythmias and sudden death in this population.
Conclusion
ICI-associated myocarditis is rare, and cardiac conduction abnormalities and arrhythmia complications are even rarer. To our knowledge this is the first case of complete heart block to occur as a toxicity from monotherapy with a single ICI. Reversibility of heart block is unknown, and permanent pacemaker placement may be necessary. Placement of an ICD lead for prevention of sudden cardiac death may be considered in select patients.
Footnotes
Conflict of Interest: Dr Hsu has received honoraria from Medtronic, Abbott, Boston Scientific, Biotronik, Janssen, Bristol-Myers Squibb, Zoll Medical, Altathera Pharmaceuticals, and Biosense-Webster; has received research grants from Biosense-Webster and Biotronik; and has equity interest in Acutus Medical and Vektor Medical. All other authors have no disclosures to report.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.07.015.
Appendix. Supplementary data
Transthoracic echocardiogram; Apical four chamber view
References
- 1.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti–pd-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mir H., Alhussein M., Alrashidi S. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol. 2018;34:1059–1068. doi: 10.1016/j.cjca.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Ball S., Ghosh R.K., Wongsaengsak S. Cardiovascular toxicities of immune checkpoint inhibitors. J Am Coll Cardiol. 2019;74:1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 4.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy N., Moudgil R., Lopez-Mattei J.C. Progressive and reversible conduction disease with checkpoint inhibitors. Can J Cardiol. 2017;33:1335.e13–1335.e15. doi: 10.1016/j.cjca.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay. J Am Coll Cardiol. 2019;74:e51–e156. doi: 10.1016/j.jacc.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P.S., Bordachar P., Ellenbogen K.A. Indications and use of the wearable cardiac defibrillator. Eur Heart J. 2017;38:258–267. doi: 10.1093/eurheartj/ehw353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiogram; Apical four chamber view