Introduction
The nodofascicular (NF) and nodoventricular (NV) pathways originate from the atrioventricular node and connect to the right bundle branch (RBB) or ventricular muscle. They cause orthodromic reciprocating tachycardia (ORT) and antidromic reciprocating tachycardia1, 2, 3 or act as a bystander in other supraventricular tachycardia (SVT) cases. The diagnosis of SVT with these pathways is mainly established by ventricular pacing maneuvers during tachycardia.4,5 Here, we present a case of a short RP′ SVT (SVT-1) with coexisting long RP′ SVT (SVT-2). SVT-1 was suspected to be typical atrioventricular nodal reentrant tachycardia (AVNRT) because of its earliest atrial activation in the His region and the long postpacing interval (PPI) after ventricular overdrive pacing (VOP). The existence of a bystander NF/NV pathway was suspected because His-refractory ventricular premature contractions (VPCs) reset the SVTs. However, the definitive diagnosis of these SVTs was very difficult. Finally, based on the findings during VPCs with varying prematurity, as well as VOP, SVT-1 and SVT-2 were diagnosed as typical and atypical AVNRT with a bystander NV pathway, respectively. This case report demonstrated the usefulness of the findings during early VPCs in diagnosing cases with NF/NV pathways.
Key Teaching Points.
-
•
The conductibility of the nodofascicular/nodoventricular (NF/NV) pathways during diagnostic ventricular pacing maneuvers depends on their prematurity.
-
•
The responses to earlier ventricular premature contractions and ventricular overdrive pacing are useful in diagnosing supraventricular tachycardia with NF/NV pathways.
-
•
The NF/NV pathway can reset 2 different atrioventricular nodal reentrant tachycardias (AVNRTs) by His-refractory ventricular stimuli without changing the atrial sequences of each AVNRT.
Case report
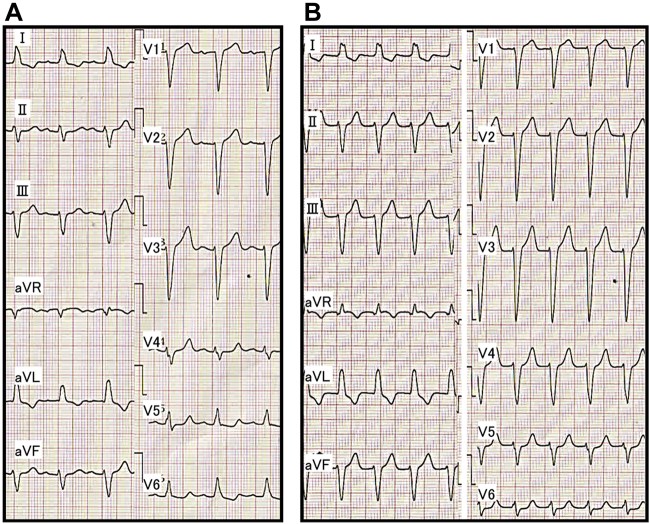
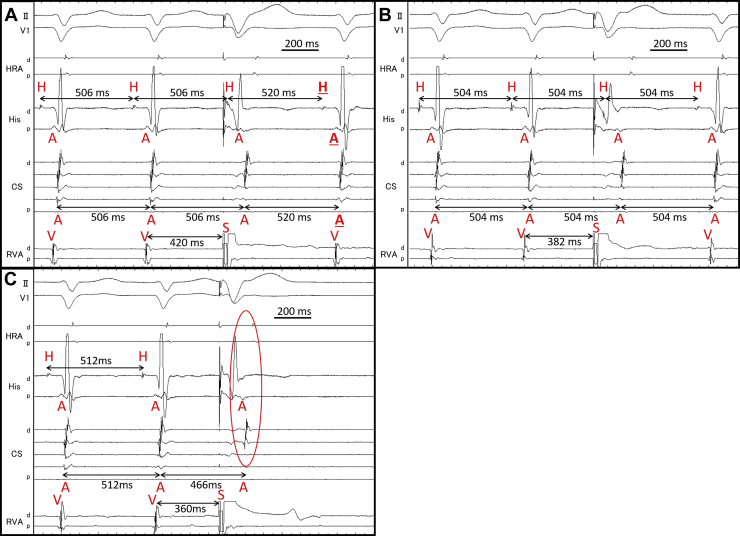
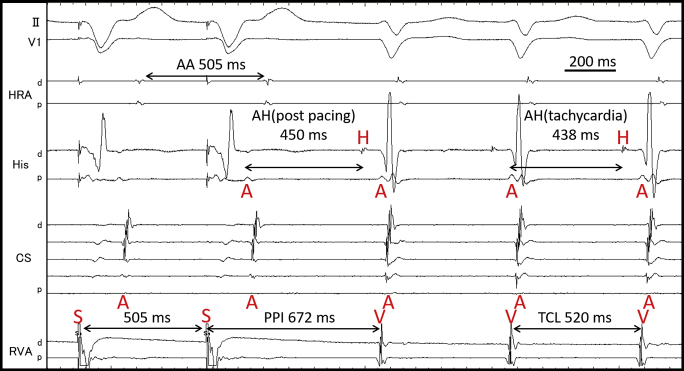
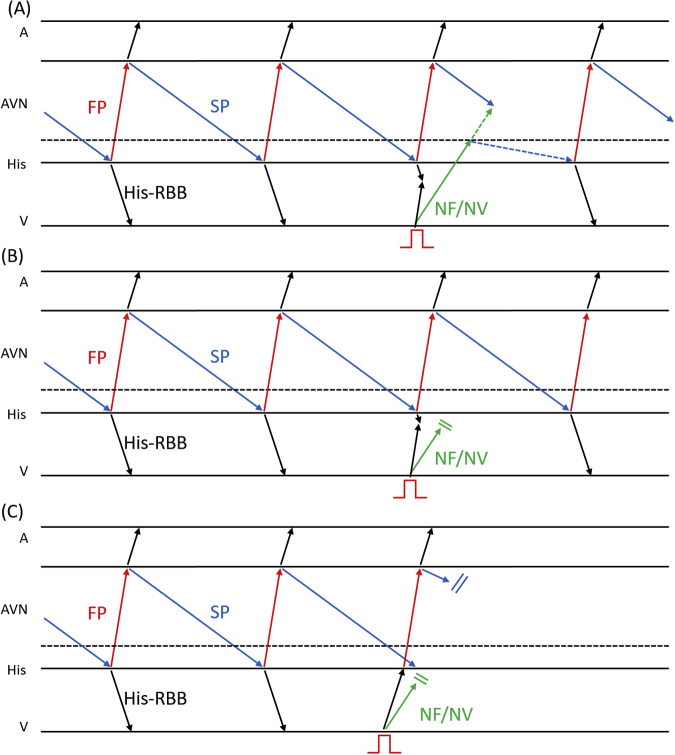
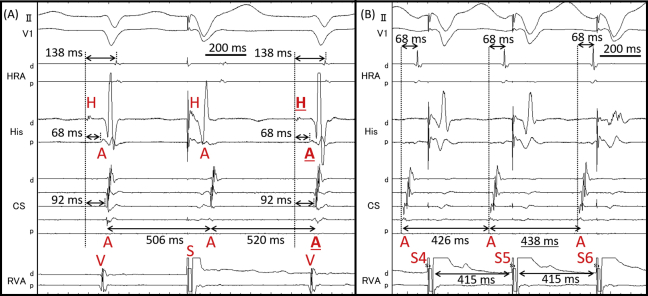
A 75-year-old woman was referred to our hospital because of drug-resistant SVT attacks. She also had left bundle branch block (Figure 1A) that was observed during clinical SVT (Figure 1B). Coronary computed tomography and echocardiography revealed no structural heart diseases. We proceeded with an electrophysiological examination of the patient. Baseline A-H interval was 92 ms, and the H-V interval was 64 ms. Programmed single atrial extrastimuli showed decremental conduction without a jump-up phenomenon. SVT-1 was induced by the programmed single ventricular extrastimulus without retrograde jump-up phenomenon. SVT-1 was a short RP′ tachycardia, and its earliest atrial activation site (EAAS) was the His region, wherein the H-A-V sequence was observed. We delivered VPCs and VOP from the right ventricular apex (RVA). However, the results were conflicting. The His-refractory VPC reset the tachycardia with delay (Figure 2A) of the subsequent His and atrial electrograms without influencing the atrial electrograms immediately after stimulation. When increasing the prematurity of His-refractory VPCs, the resetting phenomenon disappeared (Figure 2B), and the VPC earlier than His terminated SVT-1 with atrial advancement immediately after the stimulation (Figure 2C). The VOP from RVA showed V-A-V response with a long corrected PPI minus tachycardia cycle length (cPPI-TCL) of 140 ms (Supplementary Figure 1).6 ORT with a normal accessory pathway (AP) and atrial tachycardia were excluded because of the H-A-V sequence of the His region and the V-A-V response after VOP.7 These results indicated that SVT-1 was a typical AVNRT with a bystander NF/NV pathway. NF/NV ORT was unlikely because “earlier” His-refractory VPC neither reset nor terminated SVT-1 (Figure 2B), indicating that the connection of the NF/NV pathway toward the tachycardia circuit was blocked and it was not necessary for the continuation of SVT-1. The speculated ladder diagrams of Figure 2A–C are shown in Supplementary Figure 2A–C. The long cPPI-TCL after VOP, which was probably caused by the entrainment via the conduction axis, not the NF/NV pathway, also favored the diagnosis of AVNRT.
Figure 1.
A: Electrocardiogram during sinus rhythm (after the supraventricular tachycardia [SVT] termination). B: Electrocardiogram during clinical SVT.
Figure 2.
A: His-refractory ventricular premature contraction (VPC) reset supraventricular tachycardia (SVT)-1 with the delay of subsequent His and next atrium (underlined H and A). B: The reset phenomenon disappeared by “earlier” His-refractory VPC. C: SVT-1 was terminated by VPC earlier than His with atrial advancement (circle). A = atrium; d = distal; H = His; p = proximal; S = stimulation; V = ventricle.
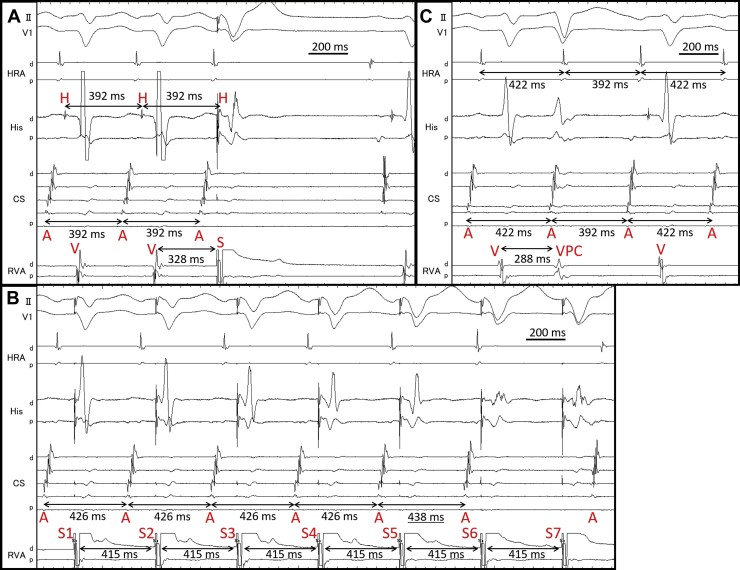
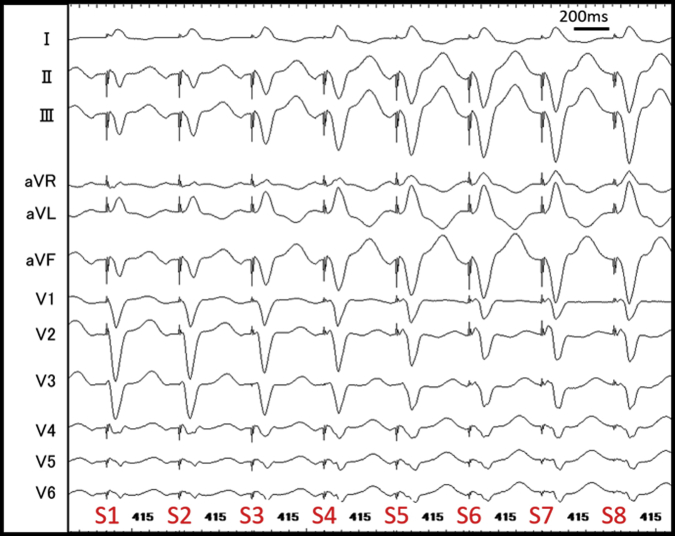
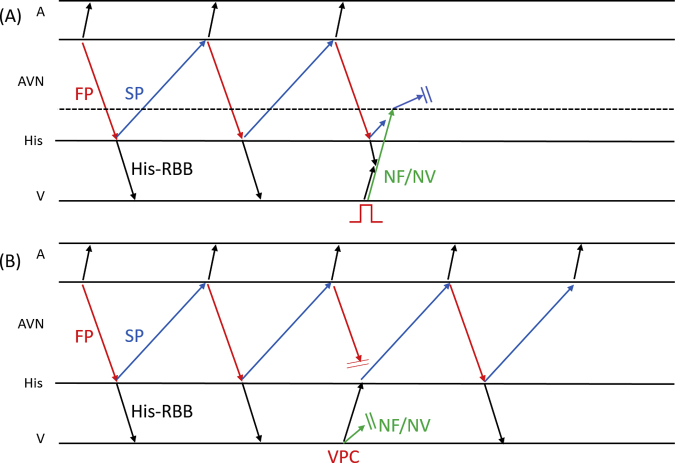
After initiation of low-dose isoproterenol infusion (10–30 μg/h), a programmed single atrial extrastimulus from the coronary sinus induced long RP′ SVT (SVT-2) without a jump-up phenomenon. The EAAS of SVT-2 was the proximal coronary sinus. The His-refractory VPCs terminated SVT-2 without capturing the atrium (Figure 3A). SVT-2 was also repeatedly terminated during VOP; however, its atrial electrogram was reset without changing its sequence during the QRS fusion period, which also favored the presence of AP (Figure 3B, Supplementary Figure 3). The electroanatomical mapping of SVT-2 revealed that the EAAS of SVT-2 was the rightward slow pathway (SP) region. At that point, SVT-2 was speculated to be an atypical AVNRT with a bystander NF/NV pathway or NF/NV ORT. We diagnosed SVT-2 to be an atypical AVNRT with a bystander NF/NV pathway because very early VPC unintentionally induced by RVA catheter contact did not terminate SVT-2; however, it advanced SVT-2 without changing the atrial sequence (Figure 3C). According to the findings during SVT-1, we speculated that His-refractory VPCs captured the SP via the NF/NV pathway and reset SVT-2. The conduction block in the SP resulted in tachycardia termination, probably owing to the decremental property of the SP. Considering that even His-refractory VPCs resulted in tachycardia termination without atrial capture, the atrial advancement by this very early VPC could have been caused not by the NF/NV pathway but by the retrograde capture of SP via the RBB-His conduction. As observed in SVT-1 (Figure 2B and C), the conduction via the NF/NV pathway was speculated to be blocked at that point. The speculated ladder diagram corresponding to Figure 3A and C is shown in Supplementary Figure 4. Although a simple ladder diagram of fast-slow AVNRT is shown in this figure, the same theory can be applied if SP is utilized for retrograde atrial activation regardless of the subtype of atypical AVNRT (fast-slow or slow-slow variant) and the existence of a lower common pathway. We proceeded with SP ablation, and no SVT was inducible after that. No symptomatic recurrence was observed during the 6-month follow-up.
Figure 3.
A: Supraventricular tachycardia (SVT)-2 was terminated without atrial capture by His-refractory ventricular premature contraction (VPC). B: SVT-2 was reset during the QRS fusion period (by fifth ventricular stimulus). Twelve-lead electrocardiogram during this period is shown in Supplementary Figure 3. C: Very early VPC advanced SVT-2 without termination. A = atrium; d = distal; H = His; p = proximal; S = stimulation; V = ventricle.
Discussion
A characteristic finding of concealed NF/NV pathways is the tachycardia reset by His-refractory VPC.4,5 However, finding them and diagnosing whether they are components of the tachycardia circuit (NF/NV ORT) or mere bystanders is difficult. For example, para-Hisian pacing, which is useful in detecting ordinary APs, is not useful in detecting NF/NV pathways owing to its decremental conduction properties. Ventricular pacing maneuvers such as measurement of PPI after VOP, manifest entrainment, and cycle length prolongation during bundle branch block, were reported to be useful in diagnosing the involvement of NF/NV pathways in the tachycardia circuit.4,5
This case demonstrated 2 types of SVTs, which were reset by the His-refractory VPCs. We diagnosed SVT-1 (speculated to be clinical SVT) to be a typical AVNRT with a bystander NF/NV pathway based on the results of the VPC scan pacing from RVA. SVT-1 was reset without affecting the atrial electrogram immediately after stimulation. This type of reset phenomenon was also observed in previous reports on the NF/NV pathway.8,9 It is noteworthy that SVT-1 in this case demonstrated the reset with delay. We speculated that the decremental conduction of the NF/NV pathway and SP offset the prematurity of the VPC. By increasing the prematurity of His-refractory VPCs, this reset phenomenon was diminished, and finally, the VPC earlier than His terminated SVT-1 with the advancement of the atrium immediately after the stimulus. This phenomenon is explained in Supplementary Figure 2B and C. The important point was that the His-refractory VPC earlier than that which could reset SVT-1 neither reset nor terminated SVT-1 (Figure 2B, Supplementary Figure 2B). This could be attributed to the loss of the orthodromic activation of the slow pathway via the NF/NV pathway. This finding excluded NF/NV ORT and was consistent with the decremental property of the NF/NV pathway. Long cPPI-TCL after VOP also favored the diagnosis of typical AVNRT. During VOP, conduction via the NF/NV pathway was probably blocked, and entrainment pacing was achieved via the conduction axis; therefore, cPPI-TCL was indicative of AVNRT.
We used these theories to diagnose SVT-2. SVT-2 was also reset by His-refractory VPCs. Considering the findings during SVT-1, this phenomenon was probably due to the orthodromic capture of the SP via the NF/NV pathway. Although this finding alone could not determine whether SVT-2 was atypical AVNRT with a bystander NF/NV pathway or NF/NV ORT, the tachycardia reset with the advancement by very early VPC followed by the continuation of the tachycardia (Figure 3C) strongly supported the diagnosis of a bystander NF/NV pathway. These findings are especially important in this case because the previously reported maneuvers (entrainment pacing by VOP and inducing transient RBB block)4,5 to differentiate whether the NF/NV pathway was a circuit component or a mere bystander in long RP′ tachycardia could not be performed.
We would like to highlight other important findings in this case. First, to the best of our knowledge, this is the first case report on an NF/NV pathway that can reset 2 different AVNRTs by His-refractory ventricular stimuli (in SVT-2, atrial capture during fusion period during VOP) without changing the atrial sequences of each AVNRT (Supplementary Figure 5). This finding demonstrates the connection of the NF/NV pathway to the slow pathway, which was captured in an orthodromic manner in each type of AVNRT by His-refractory ventricular stimuli. This phenomenon cannot occur if the AP is an ordinary A-V AP.
Second, we also investigated whether the concealed AP was the NF or NV pathway. The NF and NV pathways are different in their manner of insertion into the ventricle; the NF pathway inserts into the RBB, whereas the NV pathway inserts into the myocardium of the ventricular septum directly. Therefore, ventricular overdrive pacing during NV-ORT can demonstrate constant and progressive fusion because the ventricular muscle is a component of the tachycardia circuit.10 In SVT-2, the tachycardia was reset with delay by VOP during the QRS fusion period (Figure 3B, Supplementary Figure 3); it meant that ventricular activation waves collided in the ventricular myocardium when the tachycardia was reset. This phenomenon is consistent with the characteristics of the NV pathway, as demonstrated above.
Conclusion
This case report demonstrated the conduction property of the NF/NV pathway during ventricular pacing maneuvers and the usefulness of the findings during early VPCs. These theories can help to differentiate NF/NV ORT from a bystander NF/NV pathway when other diagnostic maneuvers fail.
Acknowledgments
We greatly appreciate the assistance of our hospital’s medical engineers.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflicts to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.06.016.
Appendix. Supplementary data
Supplementary Figure 1.
Entrainment pacing of SVT-1 by VOP from RVA. Corrected PPI was calculated to be 140 ms ([672 -520] – [450-438] = 140). A, atrium; d, distal; H, His; p, proximal; PPI, post pacing interval; S, stimulation; TCL, tachycardia cycle length; V, ventricle
Supplementary Figure 2.
(A) The speculated ladder diagrams corresponds to Figure 2A. The Blue dotted line indicates the conduction delay within the slow pathway. (B) corresponds to Figure 2B and (C) corresponds to Figure 2C. Dotted horizontal lines indicate the connection site of NF/NV pathway to the SP (also in Supplementary Figure 4). AVN, atrioventricular node; RBB, right bundle branch; FP, fast pathway; NF/NV, nodofascicular/nodoventricular pathway; SP, slow pathway
Supplementary Figure 3.
12-lead electrocardiogram during the QRS fusion period of VOP for SVT-2. S, stimulation
Supplementary Figure 4.
(A) The speculated ladder diagram corresponds to Figure 3A. (B) corresponds to Figure 3C. AVN, atrioventricular node; RBB, right bundle branch; FP, fast pathway; NF/NV, nodofascicular/nodoventricular pathway; SP, slow pathway; VPC, ventricular premature contraction
Supplementary Figure 5.
The comparison of atrial sequences before and after the reset of tachycardias. (A) SVT-1 reset by His-refractory VPC. (B) SVT-2 reset during the fusion period of VOP. A, atrium; H, His; S, stimulation; V, ventricle
References
- 1.Mahaim I., Benatt A. Nouvelles recherches sur les connexions superieures de la branche gauche du faisceau de His-Tarawa avec cloison interventriculaire. Cardiologia. 1938;1:61. [Google Scholar]
- 2.Gallagher J.J., Smith W.M., Kasell J.H., Benson D.W., Jr., Sterba R., Grant A.O. Role of Mahaim fibers in cardiac arrhythmias in man. Circulation. 1981;64:176–189. doi: 10.1161/01.cir.64.1.176. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M., Campos J., Marcus F.I., Papouin G., Clémenty J. Involvement of a nodofascicular connection in supraventricular tachycardia with VA dissociation. J Cardiovasc Electrophysiol. 1994;5:854–862. doi: 10.1111/j.1540-8167.1994.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 4.Ho R.T., Frisch D.R., Pavri B.B., Levi S.A., Greenspon A.J. Electrophysiological features differentiating the atypical atrioventricular node–dependent long RP supraventricular tachycardias. Circ Arrhythm Electrophysiol. 2013;6:597–605. doi: 10.1161/CIRCEP.113.000187. [DOI] [PubMed] [Google Scholar]
- 5.Soares Correa F., Lokhandwala Y., Sánchez-Quintana D. Unusual variants of pre-excitation: From anatomy to ablation: Part III—Clinical presentation, electrophysiologic characteristics, when and how to ablate nodoventricular, nodofascicular, fasciculoventricular pathways, along with considerations of permanent junctional reciprocating tachycardia. J Cardiovasc Electrophysiol. 2019;30:3097–3115. doi: 10.1111/jce.14247. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Torrecilla E., Arenal A., Atienza F. First postpacing interval after tachycardia entrainment with correction for atrioventricular node delay: a simple maneuver for differential diagnosis of atrioventricular nodal reentrant tachycardias versus orthodromic reciprocating tachycardias. Heart Rhythm. 2006;3:674–679. doi: 10.1016/j.hrthm.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Knight B.P., Zivin A., Souza J. A technique for the rapid diagnosis of atrial tachycardia in the electrophysiology laboratory. J Am Coll Cardiol. 1999;33:775–781. doi: 10.1016/s0735-1097(98)00614-7. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama M., Uetake S., Miyauchi Y., Shimizu W. An uncommon response to a ventricular extrastimulus during a short RP supraventricular tachycardia: What is the mechanism? J Cardiovasc Electrophysiol. 2018;29:634–637. doi: 10.1111/jce.13423. [DOI] [PubMed] [Google Scholar]
- 9.Wakamatsu Y., Nagashima K., Watanabe R. Novel V-V-A response after right ventricular entrainment pacing for narrow QRS tachycardia: What is the mechanism? J Cardiovasc Electrophysiol. 2019;30:2528–2530. doi: 10.1111/jce.14131. [DOI] [PubMed] [Google Scholar]
- 10.Han F.T., Riles E.M., Badhwar N., Scheinman M.M. Clinical features and sites of ablation for patients with incessant supraventricular tachycardia from concealed nodofascicular and nodoventricular tachycardias. JACC Clin Electrophysiol. 2017;3:1547–1556. doi: 10.1016/j.jacep.2017.07.015. [DOI] [PubMed] [Google Scholar]