Introduction
Sarcoidosis is an autoimmune disorder in which granulomatous inflammation leads to multiorgan dysfunction. Cardiac involvement in particular is associated with substantial morbidity and mortality.1 Potential cardiac manifestations include atrioventricular block, ventricular tachycardia (VT), heart failure, and sudden death. Steroids are recommended for pulmonary sarcoidosis and may be effective for cardiac involvement as well. For instance, steroid therapy is reported to be associated with improvement in atrioventricular block and left ventricular (LV) function.2,3 Based on this sort of observational data, the Heart Rhythm Society recommends immunosuppression for active cardiac sarcoidosis.4
Key Teaching Points.
-
•
Cardiac sarcoidosis is a disease in which granulomatous inflammation in the heart leads to cardiac dysfunction. Cardiac sarcoidosis most commonly manifests with left ventricular dysfunction, heart block, and/or atrial or ventricular arrhythmias.
-
•
Ventricular tachycardia (VT) is a particularly devastating complication of cardiac sarcoidosis and is associated with increased morbidity and mortality. Despite optimal treatment, patients often have recurrence of VT and are at increased risk of sudden cardiac death.
-
•
VT in cardiac sarcoidosis may occur as a result of either inflammation or scar. Scar-mediated VT is generally caused by re-entry around sites of scar, whereas inflammatory VT is generally a result of increased automaticity.
-
•
VT in cardiac sarcoidosis may be treated with immunosuppression, antiarrhythmic drugs, or catheter ablation. Immunosuppression seems to be most useful for patients with automaticity-mediated VT secondary to inflammation.
-
•
In our case series, high-dose intravenous (IV) glucocorticoids were an effective treatment for malignant ventricular arrhythmias in cardiac sarcoidosis patients with active myocardial inflammation. The use of high-dose IV glucocorticoids has previously been validated in other systemic rheumatologic diseases, including rheumatoid arthritis, lupus, and organ transplant rejection.
VT may occur in up to 17% of patients with cardiac sarcoidosis and is associated with increased mortality.3,5 VT may occur owing to active inflammation or scar, or by both mechanisms.6 Currently, the Heart Rhythm Society considers immunosuppression to be a reasonable treatment for ventricular arrhythmias due to the proarrhythmic effects of myocardial inflammation.4 Improvement in inflammation on myocardial positron emission tomography (PET) is associated with fewer VT episodes.7 However, despite some evidence of benefit with conventional steroid treatment, rates of failure and breakthrough VT are unacceptably high.8, 9, 10 One explanation is that steroids may be effective for treating inflammatory but not scar-mediated VT.6 Notably, patients in these studies generally received between 30 and 60 mg prednisone daily, which conforms with available guidelines.4 Antiarrhythmic drugs and catheter ablation are also commonly used to treat VT in cardiac sarcoidosis.
The use of high-dose intravenous (IV) glucocorticoids has been studied in other autoimmune diseases such as lupus erythematosus, rheumatoid arthritis, and solid organ rejection.11,12 High-dose IV glucocorticoids have a more rapid onset of action and greater lymphocyte inhibition compared with lower dosages.13 Currently, it is unknown whether high-dose IV glucocorticoids are effective in treating VT due to cardiac sarcoidosis.3
In our case series, we describe 3 patients with cardiac sarcoidosis at a single academic center who presented with VT storm with evidence of active myocarditis who were successfully treated with high-dose IV glucocorticoids (Table 1).
Table 1.
Clinical courses for each patient
| Hospitalization number | Presentation | Active inflammation (yes/no/unknown) | Methylprednisolone dose | Concomitant therapies | Disease-free interval | |
|---|---|---|---|---|---|---|
| Patient 1 | 1 | VT storm | Yes | 500 mg × 3 | IV amiodarone Mexiletine 300 mg TID Prednisone 60 mg daily Adalimumab |
15 months |
| 2 | PVC/NSVT | Yes | 1000 mg × 3 | Methotrexate 12.5 mg weekly Mexiletine 300 mg TID Adalimumab |
1 month | |
| 3 | PVC/NSVT | Unknown | 1000 mg × 3 | Methotrexate Mexiletine 300 mg TID Adalimumab |
>2 months | |
| Patient 2 | 1 | VT storm | Yes | 500 mg × 1 | IV amiodarone | 6 months |
| 2 | VT storm | Yes | 1000 mg × 1 500 mg × 1 |
IV amiodarone Methotrexate 15 mg weekly |
1 month | |
| Patient 3 | 1 | VT storm | Yes | 500 mg × 3 | IV amiodarone | >16 months |
IV = intravenous; NSVT = nonsustained ventricular tachycardia; PVC = premature ventricular complexes; TID = 3 times per day; VT = ventricular tachycardia.
Case report
Patient 1
A 56-year-old man with a history of Crohn disease on adalimumab and biopsy-confirmed pulmonary sarcoidosis with probable cardiac involvement presented after receiving multiple shocks from his implantable cardioverter-defibrillator (ICD) (Table 1). His history was also notable for complete heart block and systolic heart failure status post cardiac resynchronization device implantation (CRT-D).
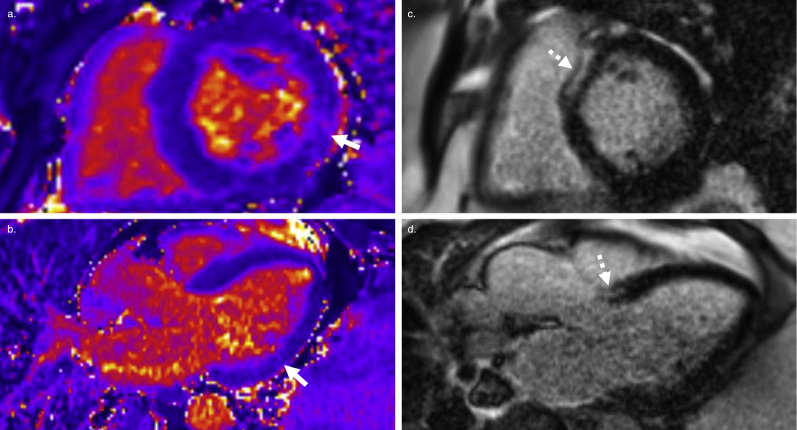
Four months prior to this admission for ICD shocks, he was admitted for palpitations. A device interrogation at that time revealed VT, which had terminated successfully with antitachycardia pacing (ATP). He was treated with 40 mg daily prednisone and sotalol. He continued to experience symptomatic, multifocal premature ventricular contractions (PVCs) and a PET demonstrated avid fluorodeoxyglucose (FDG) uptake (Figure 1c/d); his sotalol was subsequently switched to amiodarone 200 mg 3 times daily and his prednisone was increased to 60 mg daily.
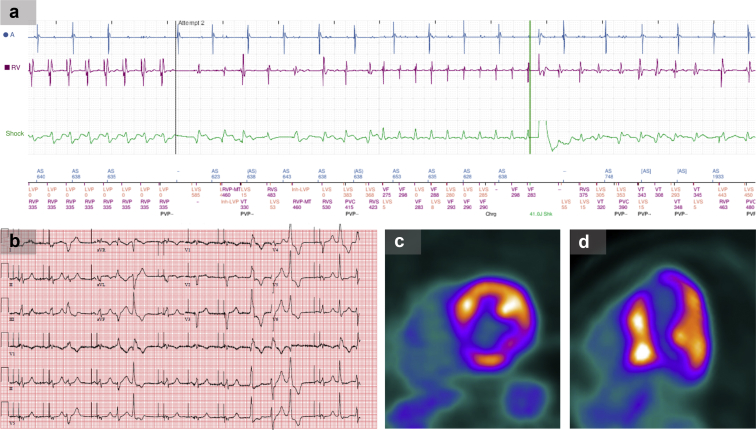
Figure 1.
Findings for patient 1. a: Electromyography from implantable cardioverter-defibrillator (ICD) interrogation showing antitachycardia pacing, which transiently suppresses ventricular tachycardia (VT), followed by VT recurrence and ICD shock. b: Electrocardiogram demonstrating multifocal premature ventricular contractions. c,d: Positron emission tomography scan demonstrating avid fluorodeoxyglucose uptake consistent with active inflammation.
Despite these interventions, the patient developed palpitations while walking his dog, followed by 2 ICD shocks. While in the emergency room, he received a third ICD shock for witnessed VT. His electrocardiogram revealed sinus rhythm with frequent multifocal PVCs (Figure 1b). ICD interrogation demonstrated 17 episodes of sustained VT with elevated PVC burden (29%). On 3 occasions he had received multiple rounds of failed ATP for VT followed by an appropriate ICD shock (Figure 1a).
He was started on amiodarone infusion and mexiletine 300 mg 3 times daily but 24 hours later continued to have episodes of sustained VT (Table 1). Given the team’s concern for inflammatory-mediated VT despite oral prednisone, he was treated with high-dose, 500 mg/day IV methylprednisolone for 3 days. Within 24 hours of the first IV dose, his VT resolved, with no further episodes during his hospitalization. A repeat PET scan demonstrated an improvement in FDG uptake. He was discharged on oral prednisone 60 mg daily and transitioned to methotrexate as an outpatient. A follow-up device interrogation revealed improved PVC burden (7%) without ICD therapies.
The patient initially did well but was readmitted 15 months later after an attempted steroid wean with nonsustained VT (NSVT) and multifocal PVCs resulting in decreased CRT-D pacing. A repeat PET showed an interval increase in FDG uptake compared to his prior study. He was treated with 1000 mg IV methylprednisolone daily for 3 days with improvement in NSVT and PVC burden. He was rehospitalized a third time 1 month later with recurrent multifocal PVCs and heart failure, again responding favorably to 3 daily doses of 1000 mg IV methylprednisolone. His VT was eventually controlled with the addition of infliximab. Follow-up device interrogations demonstrated appropriate CRT-D pacing without further ICD therapies.
Patient 2
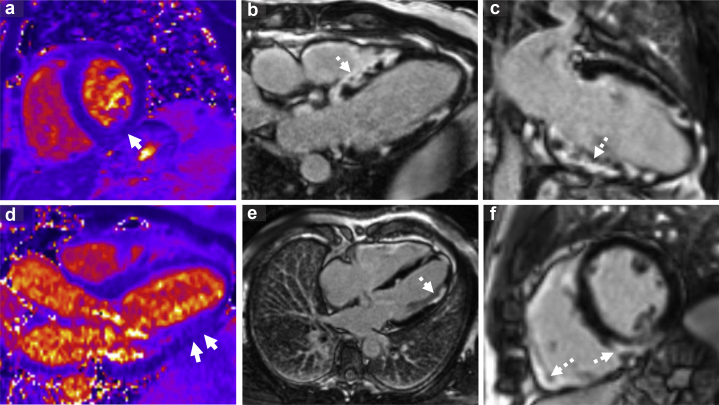
A 65-year-old man with a history of pulmonary and cardiac sarcoid presented with palpitations and was found to be in monomorphic VT (Table 1). Several days prior to admission, he had presented similarly with VT. At that time, a left heart catheterization was performed, which was normal. A cardiac magnetic resonance (CMR) demonstrated mild systolic dysfunction (LV ejection fraction = 44%) with striking scar in the septal, inferior, and lateral walls with inflammation on T2 mapping consistent with cardiac sarcoidosis (Figure 2). His VT terminated without intervention. Unfortunately, he was discharged prior to completing further workup.
Figure 2.
Cardiac magnetic resonance for patient 2. a,b: T2 maps demonstrating increased inflammation/edema in the inferolateral wall (solid arrow), Panel b demonstrates septal scar. c–f: Late gadolinium enhancement imaging demonstrating striking, patchy epicardial, midmyocardial, and transmural scar in the septum and inferior and lateral walls (dashed arrows). Panel c demonstrates predominantly inferior wall fibrosis. Panel e demonstrates lateral wall and panel f demonstrates fibrosis in both the septum and right ventricular free wall.
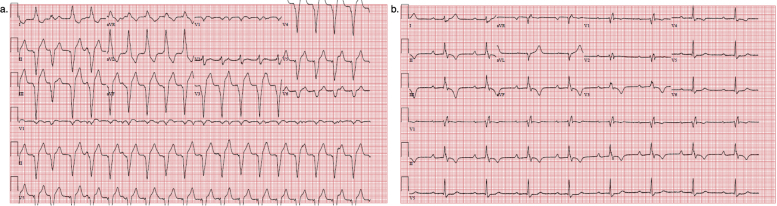
Two days later, he was readmitted with palpitations and was again found to be in monomorphic VT (Supplemental Figure 1). He converted to sinus rhythm after receiving IV amiodarone but continued to have prolonged NSVT episodes. Given his ongoing ventricular arrhythmias and active inflammation on CMR, he was treated with 1 dose of 500 mg IV methylprednisolone. His NSVT improved, with only 1 additional episode during his admission. He underwent ICD implantation and was discharged on 60 mg daily prednisone and amiodarone. On outpatient follow-up, his ejection fraction had improved on echocardiography to 60%–65% and device interrogation revealed no PVCs. He was transitioned to methotrexate as an outpatient.
Seven months later after an attempted steroid wean, he presented with palpitations and was again in slow VT. No ICD therapies had been delivered, as the VT was below the detection rate. He received IV amiodarone but had breakthrough VT and NSVT. He was treated with a single dose of 1000 mg IV methylprednisolone followed by 500 mg the next day. Subsequently, the patient’s NSVT resolved. Prior to discharge, a PET scan showed avid FDG uptake in the lateral and septal walls, consistent with active inflammation and scar in the basal septum. He was discharged on prednisone 40 mg daily with a prolonged taper.
One month later, he developed recurrent monomorphic VT, which was treated successfully with ATP. He was started on infliximab. A subsequent PET demonstrated reduced inflammation. However, despite treatment with infliximab, methotrexate, dofetilide, and mexiletine, the patient continued to have ATP-responsive VT and had a decline in systolic function. Ultimately, his VT was controlled after he underwent VT ablation and CRT-D upgrade. Subsequent ICD interrogations did not reveal any further therapies.
Patient 3
A 52-year-old man with a biopsy-proven pulmonary sarcoidosis and paroxysmal atrial fibrillation presented with several days of shortness of breath and fatigue (Table 1). Four months prior to this presentation, he had undergone atrial fibrillation ablation. He was later placed on amiodarone after developing recurrent atrial arrhythmias 2 weeks prior to his presentation with VT.
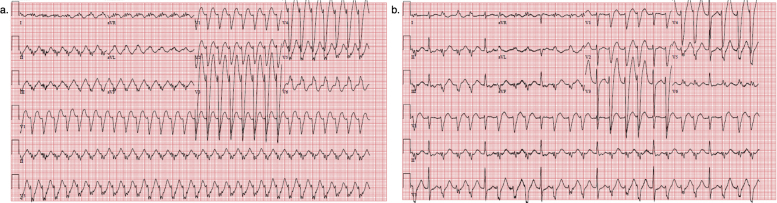
On arrival, he was found to be in monomorphic VT (Supplemental Figure 2). Serum angiotensin-converting enzyme was elevated but additional lab work was unremarkable. He converted to sinus rhythm after treatment with IV amiodarone but had multifocal PVCs and breakthrough VT. A CMR was performed, which showed severe LV systolic dysfunction (LV ejection fraction = 28%), patchy midmyocardial fibrosis in the septum, and increased inflammation on T2 mapping consistent with cardiac sarcoidosis (Supplemental Figure 3). A myocardial PET demonstrated active inflammation in the inferolateral wall.
As his VT storm was unresponsive to intravenous amiodarone, the patient was treated with 500 mg IV methylprednisolone daily for 3 days, which resulted in improved ectopy without further VT. He underwent ICD implantation and was discharged on 60 mg daily prednisone and oral amiodarone. He was ultimately transitioned to methotrexate as an outpatient and to date has had no recurrence of ventricular arrhythmias.
Discussion
We describe 3 patients with cardiac sarcoidosis presenting with either VT storm or significant NSVT that responded to high-dose IV glucocorticoids. In each case, ventricular arrhythmias were refractory to antiarrhythmic drugs and/or oral steroids prior to receiving high-dose glucocorticoids (Table 1). Each patient also had evidence of active inflammation by PET or CMR. Patients were given 500–1000 mg IV methylprednisolone for 1–3 days under the supervision of a heart failure cardiologist. All 3 patients had significant improvement in their arrhythmia burden within 24–48 hours of therapy. Additionally, no patients experienced serious infectious complications, likely owing to the short duration of high-dose IV glucocorticoids received.14 To our knowledge, this is the first case series of high-dose IV glucocorticoid use in patients with cardiac sarcoidosis presenting with ventricular arrhythmias. The results of this case series are informative for the management of VT in this population.
Although not well studied, there is at least rationale for the use of high-dose IV glucocorticoids for inflammatory-mediated VT in cardiac sarcoidosis. Previous studies have demonstrated glucocorticoids to be effective in the treatment of pulmonary and cardiac sarcoidosis.3,15 Additionally, high-dose IV glucocorticoids seem to be effective for acute manifestations of other autoimmune disorders.11,12
In cardiac sarcoidosis, ventricular arrhythmias may occur owing to either scar or active sarcomyocarditis.6 Scar-mediated VT generally results from re-entry, whereas VT due to sarcomyocarditis is caused by triggered activity or enhanced automaticity. In patient 1, the failure of ATP together with the response to high-dose glucocorticoids suggests inflammatory VT. Additionally, all 3 patients had multifocal PVCs and evidence of inflammation on imaging. The presence of inflammation may explain why our patients responded favorably to high-dose IV glucocorticoids. It is important to note that patient 2—who had the highest scar burden on CMR—also had the most malignant course and ultimately required VT ablation. Immunosuppression is theorized to help with inflammatory-mediated but not scar-mediated VT.6 Patients with predominantly scar-mediated VT may respond better to antiarrhythmic drug therapy or catheter ablation.6
It is further notable that VT recurred in all but 1 patient, highlighting the tendency for relapses with this disease. Patients in whom VT recurred ultimately required increased immunosuppression or VT ablation. Thus, high-dose IV glucocorticoids should not be seen as definitive therapy for inflammatory VT. Rather, they should be viewed as a bridge to additional interventions such as increased immunosuppression or catheter ablation. This parallels the role of high-dose IV glucocorticoids in other rheumatologic illnesses in which patients are often transitioned to steroid-sparing immunosuppressive agents long term.
A limitation of our case series is its small sample size and relatively short follow-up in 2 patients. Further studies assessing high-dose IV glucocorticoids for ventricular arrhythmias in cardiac sarcoidosis are needed. Another limitation of our data is the simultaneous use of antiarrhythmic and immunosuppressive drugs in our patients. However, this is representative of a real-world cohort where patients often receive a combination of therapies acutely for VT. Additionally, both patient 1 and patient 3 responded to high-dose IV glucocorticoids despite experiencing breakthrough VT after several weeks of amiodarone therapy. Thus, the concomitant use of antiarrhythmic drugs should not completely discredit our findings.
Conclusions
In conclusion, in our case series, high-dose IV glucocorticoids were effective in treating VT storm or symptomatic NSVT in patients with cardiac sarcoidosis and active inflammation. Future prospective studies are warranted to validate our findings.
Footnotes
None of the authors involved in this case have relevant conflicts of interest to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data associated with this article can be found in the online version at https://10.1016/j.hrcr.2020.06.028.
Appendix. Supplementary data
Supplementary Figure 1.
Electrocardiograms (EKG) for patient 2. Panel a: initial presenting EKG demonstrating monomorphic VT. Panel b: EKG after conversion to sinus rhythm demonstrating sinus rhythm with right bundle branch block and left posterior fascicular block. There are also anterolateral and inferior t-wave inversions present. (VT=ventricular tachycardia)
Supplementary Figure 2.
Electrocardiograms (EKG) for patient 3. Panel a: initial presenting EKG demonstrating monomorphic VT. Panel b: EKG after conversion to sinus rhythm demonstrating frequent triplets of non-sustained VT of identical morphology to his VT strip. (VT=ventricular tachycardia)
Supplementary Figure 3.
Cardiac Magnetic Resonance (CMR) Imaging for Patient 3. Demonstrates CMR findings consistent with cardiac sarcoidosis for patient 3. In panel A-B, T2 maps demonstrate increased T2 relaxation time in the inferolateral wall (solid arrow) consistent with inflammation/edema in this region. Panel C-D: late gadolinium enhancement (LGE) imaging demonstrating prominent mid-myocardial fibrosis in the basal anteroseptum (dashed arrow).
References
- 1.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 2.Sadek M.M., Yung D., Birnie D.H., Beanlands R.S., Nery P.B. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–1041. doi: 10.1016/j.cjca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Chiu C.Z., Nakatani S., Zhang G. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 4.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Yazaki Y., Isobe M., Hiroe M. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 6.Yalagudri S., Zin Thu N., Devidutta S. Tailored approach for management of ventricular tachycardia in cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2017;28:893–902. doi: 10.1111/jce.13228. [DOI] [PubMed] [Google Scholar]
- 7.Muser D., Santangeli P., Castro S.A. Prognostic role of serial quantitative evaluation of. Eur J Nucl Med Mol Imaging. 2018;45:1394–1404. doi: 10.1007/s00259-018-4001-8. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y., Morimoto S., Uemura A., Hiramitsu S., Ito T., Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–137. [PubMed] [Google Scholar]
- 9.Winters S.L., Cohen M., Greenberg S. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol. 1991;18:937–943. doi: 10.1016/0735-1097(91)90750-4. [DOI] [PubMed] [Google Scholar]
- 10.Furushima H., Chinushi M., Sugiura H., Kasai H., Washizuka T., Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004;27:217–222. doi: 10.1002/clc.4960270409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mussche M.M., Ringoir S.M., Lameire N.N. High intravenous doses of methylprednisolone for acute cadaveric renal allograft rejection. Nephron. 1976;16:287–291. doi: 10.1159/000180613. [DOI] [PubMed] [Google Scholar]
- 12.Ponticelli C., Tarantino A., Pioltelli P., Invernizzi F. High-dose methylprednisolone pulses in active lupus nephritis. Lancet. 1977;1:1063. doi: 10.1016/s0140-6736(77)91307-1. [DOI] [PubMed] [Google Scholar]
- 13.Badsha H., Edwards C.J. Intravenous pulses of methylprednisolone for systemic lupus erythematosus. Semin Arthritis Rheum. 2003;32:370–377. doi: 10.1053/sarh.2002.50003. [DOI] [PubMed] [Google Scholar]
- 14.Badsha H., Kong K.O., Lian T.Y., Chan S.P., Edwards C.J., Chng H.H. Low-dose pulse methylprednisolone for systemic lupus erythematosus flares is efficacious and has a decreased risk of infectious complications. Lupus. 2002;11:508–513. doi: 10.1191/0961203302lu243oa. [DOI] [PubMed] [Google Scholar]
- 15.Padala S.K., Peaslee S., Sidhu M.S., Steckman D.A., Judson M.A. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol. 2017;227:565–570. doi: 10.1016/j.ijcard.2016.10.101. [DOI] [PubMed] [Google Scholar]