Enterococcus faecalis, long implicated in serious systemic infections and failure of root canal treatment, is a persistent inhabitant of oral periapical lesions. Dendritic cells (DCs) and other innate immune cells patrol the oral mucosa for infecting microbes. Dendritic cells are efficient at capturing microbes when immature, whereupon they can transform into potent antigen-presenting cells upon full maturation. Autophagy, a sophisticated intracellular process first described for elimination of damaged organelles, regulates DC maturation and other important immune functions of DCs.
KEYWORDS: antigen presentation, autophagy, bone marrow-derived stem cells, dendritic cells, Enterococcus faecalis, infection, inflammatory cytokines
ABSTRACT
Enterococcus faecalis, long implicated in serious systemic infections and failure of root canal treatment, is a persistent inhabitant of oral periapical lesions. Dendritic cells (DCs) and other innate immune cells patrol the oral mucosa for infecting microbes. Dendritic cells are efficient at capturing microbes when immature, whereupon they can transform into potent antigen-presenting cells upon full maturation. Autophagy, a sophisticated intracellular process first described for elimination of damaged organelles, regulates DC maturation and other important immune functions of DCs. The present study examined how E. faecalis influences the differentiation of murine bone marrow-derived stem cells (BMSCs) into functional DCs in the presence of the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Although the viability and differentiation of DCs were not affected by E. faecalis, expression of the autophagy-related proteins ATG7, Beclin1, and LC3bI/II were significantly suppressed in an mTOR-dependent manner. Ultrastructurally, E. faecalis was identified in single-membrane vacuoles, some of which were in the process of binary fission. Bacterium-containing autophagosomes were absent within the cytoplasm. Accessory molecules (major histocompatibility complex class II [MHC-II], CD80, and CD86) and anti-inflammatory cytokine (transforming growth factor β1 [TGF-β1]) were suppressed in E. faecalis-induced DCs, while IL-1β, tumor necrosis factor alpha (TNF-α), and IL-12 levels were upregulated. When pulsed with ovalbumin (OVA), the E. faecalis-induced DCs showed reduction in CD4+ OVA-specific OT-II T cell proliferation. It is concluded that E. faecalis promotes the differentiation of bone marrow stem cells into CD11c-positive DCs with aberrant immune functions while retaining the capability of proinflammatory cytokine induction.
INTRODUCTION
Enterococcus faecalis is a Gram-positive, facultative anaerobic bacterium that is found in the intestines of healthy individuals, the oral cavity, and vaginal tract. Upon entering the bloodstream (i.e., bacteremia), E. faecalis can cause serious complications that include abdominal and pelvic infections, urinary tract infections, septicemia, wound infections, endocarditis, or enterococcal meningitis (1).
Enterococcus faecalis plays a critical role in the failure of root-treated teeth and is frequently identified from the canal space of teeth with posttreatment secondary infections (2). Previous studies showed that E. faecalis was found in 4% to 40% of primary endodontic infections, with a much higher frequency in persistent periradicular lesions. Other studies reported a 24% to 77% prevalence of E. faecalis in root-treated teeth with periapical infections (3).
Enterococcus faecalis has received much attention because of its reported resistance to antibiotics and ability to survive under harsh conditions. In the starvation phase, E. faecalis can remain in a viable state for extended periods of time and become resistant to UV light irradiation, heat, sodium hypochlorite, hydrogen peroxide, ethanol, and acid (4). One of the most critical characteristics of E. faecalis is its ability to form a biofilm that enhances resistance to destruction by increasing resistance to phagocytosis, antibodies, and antimicrobial agents (5).
The effect of E. faecalis on the innate immune response was reported previously. The bacterium inhibits apoptosis of macrophages and enhances their survival (6). This feature was found to be independent of the bacterial strain (7). Another study showed that E. faecalis causes immune suppression of macrophages and alters the host immune response to facilitate infection with other pathogens (8). In high doses, E. faecalis delays wound healing and infiltration of immune cells and suppresses the expression of inflammatory cytokines in a mouse model (9). To date, the interactions of E. faecalis with dendritic cells (DCs) and their progenitors and the effects of such interactions on the body’s immune response are not clear.
Dendritic cells play a vital role in the immune system. They function as “bridges” between innate and adaptive immune responses. DC precursors, circulating monocytes derived from bone marrow cells, are recruited rapidly from the bloodstream to the infected site (10, 11). Monocytes differentiate into two major cell types, DCs and macrophages, depending local growth factors and the inflammatory mediators present. In the presence of murine granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), monocytes differentiate to immature DCs that capture microbes and other antigens, which are routed into their intracellular compartments for degradation through the process of autophagy (12). Disruption of autophagy in DCs is a critical survival tactic and virulence factor of the “keystone” oral pathogen Porphyromonas gingivalis (13, 14). Dendritic cells infiltrate periapical infections and are involved in the T cell response (15). Downstream of autophagy in DCs are key proteins that are required to fight infection (16) and for supporting antigen-specific T cell proliferation, including expression of costimulatory molecules and inflammatory cytokines. Efficient antigen presentation by DCs is highly dependent on their active cellular autophagic machinery (17).
The objective of the present study was to determine how E. faecalis influences the ability of bone marrow-derived stem cells (BMSCs) to differentiate into DCs. The present work has important medical significance, because E. faecalis has great potential to spread virulence and antibiotic resistance genes via horizontal gene transfer and is an important cause of nosocomial infections (18). The bacterium is also a frequent pathogen is dental diseases (periodontitis, peri-implantitis, and secondary endodontic infections). Integration of foodborne enterococci into oral biofilms has been identified in vivo (19). The impact of the present work is the novel discovery that while viability and differentiation of DCs are not influenced by E. faecalis, the bacterium dysregulates their ability to function as antigen-presenting cells via a mechanism involving mammalian target of rapamycin (mTOR)-dependent inhibition of key autophagy-related proteins. Surprisingly, these immune-aberrant DCs still retain their ability to produce proinflammatory cytokines.
RESULTS
DC lineage marker expression and viability are unaffected and autophagy-related proteins are suppressed in E. faecalis-induced DCs.
Our preliminary studies established that E. faecalis at a multiplicity of infection (MOI) of 1 for 8 days had no significant influence on GM-CSF/IL-4-induced differentiation of bone marrow-derived stem cells into bona fide CD11c+ DCs relative to that of controls (P = 0.4256) (see Fig. S1A and B in the supplemental material). There was no difference in the viability of the DCs relative to that of control DCs, as examined by annexin V and 7-amino-actinomycin D (7-AAD) for apoptosis and necrosis, respectively (P = 0.1367) (Fig. S1C and D). Therefore, an MOI of 1 was used in subsequent experiments.
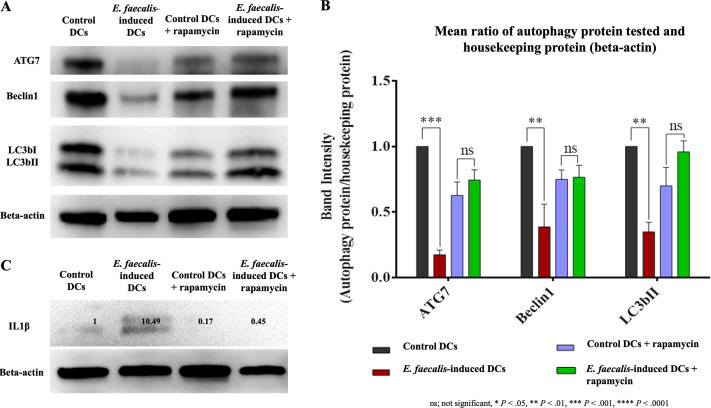
Activation of autophagy, an important antimicrobial mechanism linked to antigen-presenting capability (17), was examined in E. faecalis-induced DCs relative to that in control groups. The expression levels of ATG7 (P = 0.0011), Beclin1 (P = 0.0023), and LC3bII (P = 0.0059) were significantly downregulated in E. faecalis-induced DCs (Fig. 1A and B). Augmenting autophagy by the mTOR inhibitor rapamycin restored autophagy activity in E. faecalis-induced DCs. Interestingly, IL-1β was expressed in E. faecalis-induced DCs (Fig. 1C) but not in the control DCs or in the groups treated with rapamycin.
FIG 1.
Suppression of autophagy-related proteins in E. faecalis-induced DCs. (A) Western blot of ATG7, Beclin1, and LC3bI/II expressed by E. faecalis-induced DCs relative to expression in control DCs and E. faecalis-induced DCs treated with mTOR inhibitor rapamycin; β-actin was used as loading control. (B) Bar graphs show the band intensities of autophagy proteins normalized to β-actin. (C) Western blot showing the expression of IL-1β in E. faecalis-induced DCs that is not evident in the other groups. The experiment was repeated in triplicates three times.
Uptake and intracellular localization of E. faecalis.
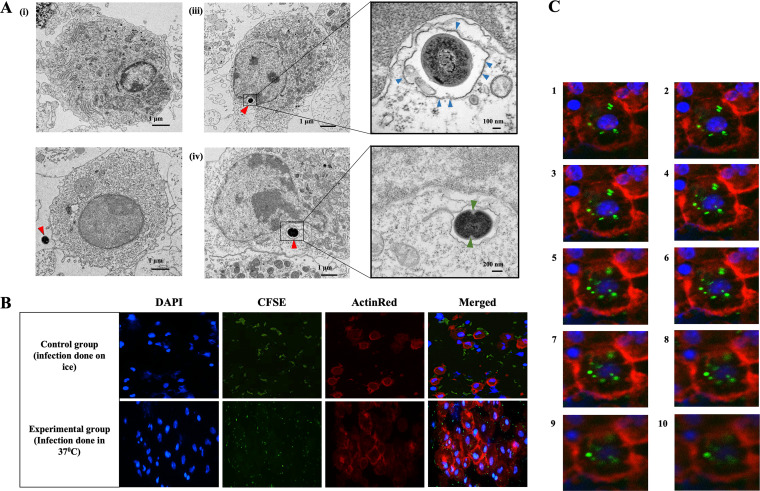
The E. faecalis-induced DCs were examined by transmission electron microscopy (TEM) for intracellular bacteria and presence/absence within double-membrane autophagosomes, as identified previously in DCs (13). Although no autophagosomes were detected, bacteria were located within single-membrane vacuoles in the vicinity of the nucleus (Fig. 2A). Some of the engulfed bacteria were in the process of binary fission.
FIG 2.
Ultrastructural evidence of bacterial phagocytosis by differentiating and control DCs. (A) TEM images of immature control DC (i) and immature DC with extracellular E. faecalis (red arrow) (iii). (iii, left) E. faecalis engulfed during DC differentiation and found within the cytoplasm. (iii, right) Magnified image of E. faecalis enclosed in a vacuole and surrounded by a single membrane (blue arrows) (iv, left) Intracellular E. faecalis undergoing binary fission. (iv, right) Magnified image showing cell wall invagination (green arrows) that is consistent with cell division. (B) Immunofluorescence staining images showing uptake of CFSE (green)-labeled E. faecalis by control DCs (differentiated in the absence of E. faecalis) (Top) Control DCs incubated on ice, shows E. faecalis outside DCs. (Bottom) DCs at 37°C internalize E. faecalis. (C) Z-stack images, through the DCs, confirming the presence of E. faecalis within the DCs. The experiment was repeated in triplicates three times.
The uptake of E. faecalis by DCs was confirmed by confocal microscopy imaging of carboxyfluorescein succinimidyl ester (CFSE)-labeled E. faecalis cocultured with DCs. Results showed that CFSE-labeled bacteria (green) were internalized by DCs (red); however, these bacteria were aggregated on the outer surface when infection was performed on ice (Fig. 2B). To confirm the presence of bacteria inside the cells and to exclude any superimposition, Z-stack images were recorded and inspected. The analysis of Z-stack images at 1-μm-depth intervals confirmed efficient internalization by the DCs, with many bacteria in the same focal plane as the DC cell nuclei (Fig. 2C).
Aberrant maturation of E. faecalis-induced DCs.
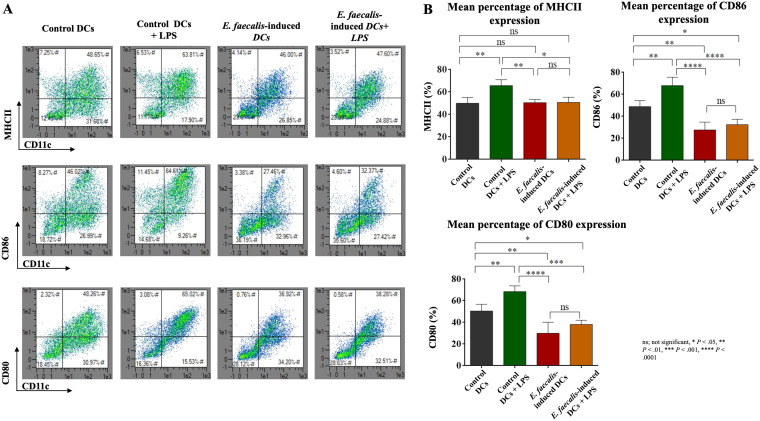
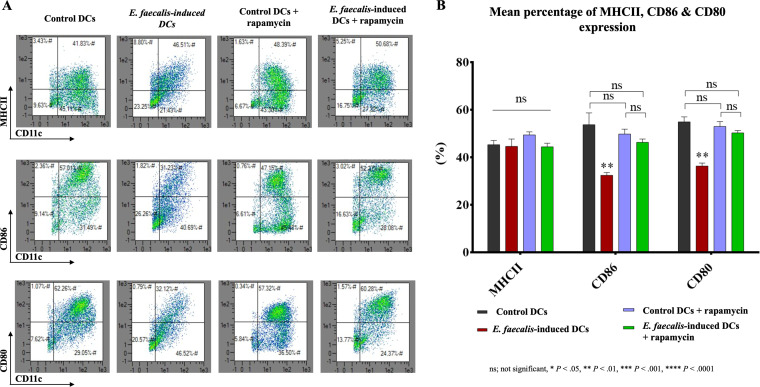
Robust maturation is required for DCs to promote antigen presentation to T cells. Accordingly, the ability of E. faecalis-induced DCs to upregulate accessory molecules major histocompatibility complex class II (MHCII), CD86, and CD80 in response to lipopolysaccharide (LPS) was examined. All three accessory molecules were upregulated in the control DCs. In contrast, E. faecalis-induced DCs did not demonstrate a robust maturation response to LPS (Fig. 3A and B). Treatment of E. faecalis-induced DCs with rapamycin increased expression of CD86 and CD80 but not MHCII (Fig. 4A and B), which is suggestive of the contribution of impaired autophagy to the maturation deficit. For comparison, control DCs cocultured with E. faecalis after differentiation were capable of undergoing maturation (see Fig. S2A and B).
FIG 3.
Aberrant maturation function of E. faecalis-induced DCs. (A) Scattergrams from flow cytometry of percentage expression of MHCII, CD86, and CD80 by E. faecalis-induced CD11c+ DCs relative to that in the control groups. Upper right quadrants show percent double-positive CD11c+ MHCII+ (top), CD11c+ CD86+ (middle), and CD11c+ CD80+ (bottom) DCs. (B) Bar graphs showing the mean percentages of double-positive CD11c+ MHCII+ (top left), CD11c+ CD86+ (top right), and CD11c+ CD80+ (bottom) cells in the four groups. The experiment was repeated in triplicates three times.
FIG 4.
Rapamycin treatment of E. faecalis-induced DCs upregulated the expression of costimulatory molecules. (A) Scattergrams from flow cytometry of percentage expression of MHCII, CD86, and CD80 by E. faecalis-induced CD11c+ DCs that were treated with mTORC1 inhibitor rapamycin relative to that in the control groups shown. Upper right quadrants show percent double-positive CD11c+ MHCII+ (top), CD11c+ CD86+ (middle), and CD11c+ CD80+ (bottom) DCs. (B) A bar graph showing the mean percentages of double-positive CD11c+ MHCII+, CD11c+ CD86+, and CD11c+ CD80+ cells in the four groups. The experiment was repeated in triplicates three times.
Potentiated proinflammatory cytokine response of E. faecalis-induced DCs.
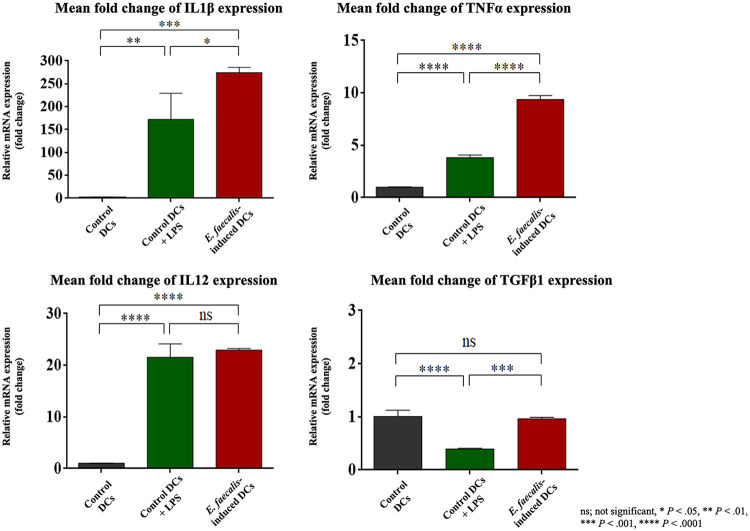
Proinflammatory cytokines, including IL-1β, tumor necrosis factor alpha (TNF-α), and IL-12, serve as the second signal required for DCs to promote a robust T cell response, while transforming growth factor β1 (TGF-β1) is considered anti-inflammatory. Because the IL-1β protein levels were upregulated in E. faecalis-induced DCs (Fig. 1C), the proinflammatory cytokine mRNA responses in E. faecalis-induced DCs were further examined. There were significant increases in mRNA expression for IL-1β (P = 0.0002), TNF-α (P < 0.0001), and IL-12 (P < 0.0001) and a significant decrease in anti-inflammatory TGF-β1(P < 0.0001) relative to that in control immature DCs. The proinflammatory response of E. faecalis-induced DCs was greater than that induced by LPS, although a reduction in TGF-β1 was not observed (Fig. 5). Interestingly, E. faecalis cells that were cocultured with fully differentiated DCs were less inflammatory, as evidenced by the lower levels of cytokines relative to those induced by LPS (Fig. S2C).
FIG 5.
Proinflammatory cytokine response potentiated in E. faecalis-induced DCs. Levels of mRNA expression of IL-1β, TNF-α, IL-12, TGF-β1 by RT-PCR, normalized relative to GAPDH, in control DCs, LPS-stimulated control DCs, and E. faecalis-induced DCs. The experiment was repeated three times in triplicates.
Ability of E. faecalis-induced DCs to stimulate OVA-specific T cell proliferation.
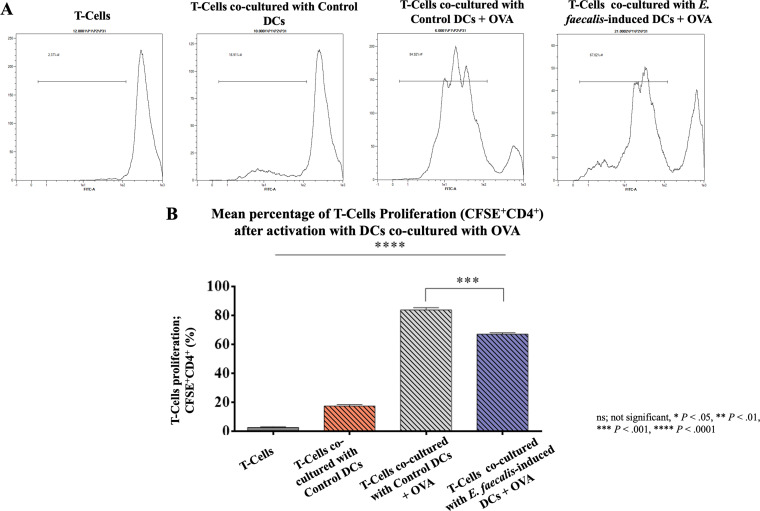
The aberrant maturation identified in E. faecalis-induced DCs, combined with an exuberant inflammatory cytokine response, called into question whether these aberrant DCs would be capable of antigen presentation. We therefore examined the ability of E. faecalis-induced ovalbumin (OVA)-DCs to stimulate proliferation of OVA-specific T cells. In the absence of DCs, there was an apparent reduction in T cell proliferation, while control DCs induced ∼20% proliferation and control DCs pulsed with OVA induced ∼80% proliferation of T cells. In contrast to control DCs plus OVA, the proliferation was lower by ∼20% in the E. faecalis-induced DCs plus OVA group in comparison to that in the positive control (P < 0.001) (Fig. 6A and B).
FIG 6.
Immunosuppressive effect of E. faecalis-induced DCs on OVA-specific T cell proliferation. (A) Histograms showing the peaks of CFSE+ CD4+ T cells after coculture with control DCs, with control DCs plus OVA, or E. faecalis-induced DCs plus OVA. Proliferation indicated by peaks shifted to the left. (B) Mean percentage proliferation of CD4+ T cells, ± standard errors (SEs). Results are representative of three separate experiments performed in triplicates.
DISCUSSION
This is the first study to examine the influence of E. faecalis on GM-CSF/IL-4-induced differentiation of primary-isolated BMSCs into DCs. From an immunobiological perspective, the present results show that differentiated DCs vary extensively depending on when the DCs are exposed to E. faecalis. Monocytes derived from bone marrow cells which differentiate into DCs in the presence of GM-CSF/IL-4 are recruited to the tissues in response to invading pathogens (10, 11). Early exposure during differentiation leads to aberrant DCs with compromised ability to undergo maturation and induce antigen-directed T cell proliferation, despite retention of an intact proinflammatory cytokine response. The aforementioned events do not occur when fully differentiated DCs are exposed to E. faecalis. Although other innate immune cells such as macrophages or B lymphocytes also serve as antigen-presenting cells, we focused on DCs because of their key role in active patrol of the oral mucosa for microbes (20–24) and relation of DCs to the severity of periapical lesions after infected teeth undergo necrosis (15). Dendritic cells are highly migratory and are the only antigen-presenting cells capable of activating naive T cells (25). This is due to the high responsiveness of DCs to microbes and other maturation signals via upregulation of key signals for antigen presentation such as accessory molecules, inflammatory cytokines, and the Ag-MHCII complex (26). Previous studies showed that aberrant DCs have impairments in the pathogen recognition capability and T cell responses, with increases in infection susceptibility and inflammation progression (27–29).
Enterococcus faecalis, while considered a normal commensal of the human gastrointestinal tract, can cause severe medical conditions when displaced into the bloodstream and in an immunocompromised host (1). From the dental perspective, E. faecalis is detected profusely in failed root-treated teeth, especially those with persistent apical periodontitis (2). This microbe resists eradication during disinfection of the instrumented canal space with antimicrobial agents (30, 31).
It is presently unclear how E. faecalis evades immune clearance in periapical tissues. Previous studies indicate that E. faecalis is capable of surviving in macrophages (7, 8) through evasion of autophagy and enclosure in single-membrane vacuoles but not double-membraned autophagosomes (32). Here, we used a low-grade infection model to decipher this process in DCs and their progenitors. Results derived from the ultrastructural part of the present study are in line with macrophage studies, showing intracellular E. faecalis in single-membrane vacuoles undergoing binary fission (Fig. 1C). Autophagy regulates DC maturation and phenotypic changes, and activation of autophagy in DCs upregulates the expression of CD86 and enhances antigen delivery to the MHCII complex for T cell activation (17, 33). Application of the autophagy activator rapamycin to pre-DCs before infection with E. faecalis restored the immune functions of E. faecalis-induced DCs, where the expression of MHCII, CD86, and CD80 as well as the expression of autophagy proteins was restored as in the control groups. These findings signify the importance of autophagy activation to resist the suppressive effects of the bacteria on DCs and the potential of rapamycin treatments. Mycobacterium tuberculosis has also been reported to infect DCs and evade autophagy (34). The periodontal pathogen P. gingivalis infects DCs through a specific receptor ligand mechanism involving C-type lectins and Toll-like receptor 2 on DCs (35, 36). This activates a unique antiapoptotic and antiautophagy signaling pathway (24) that promotes evasion of autophagy (13). We presently know very little about the specific mechanisms in E. faecalis-induced DCs.
Interestingly, fully differentiated DCs responded robustly to E. faecalis, in the expression of MHCII, CD86, and CD80 and in increased antigen presentation but not in cytokine response. Dendritic cells exposed to E. faecalis during differentiation responded with robust proinflammatory cytokine gene expressions, including those for IL-1β, TNF-α, and IL-12. Both IL-1β and TNF-α are associated with tissue damage and bone resorption in chronic apical periodontitis (37). Interleukin-1β is considered a contributing factor in the development of periapical granulomas in primary teeth (38). Both IL-1β and TNF-α are critical inflammatory cytokines in the progression of periodontal disease. These proinflammatory cytokines stimulate periodontal tissue destruction and the release of tissue-degrading enzymes. The blockage of IL-1β is considered a therapeutic alternative for the treatment of periodontitis (39). The proinflammatory IL-12 has been shown to suppress Th2 cytokines and facilitate progression of periapical infections (40).
Within the limitations of the present in vitro study, it may be concluded that E. faecalis has distinct influences on DCs and their precursors, depending on when exposure occurs during the differentiation process. Early exposure to E. faecalis promotes aberrant inflammatory DCs that are immunocompromised and unable to promote a robust T cell proliferative response. Further study of the role of autophagy in this duality by using rapamycin and specific gene inhibitors of autophagy pathways are needed as well as in vivo studies.
MATERIALS AND METHODS
Animals.
C57BL/6 mice (8 to 12 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). All the procedures were approved by the Institutional Animal Care and Use Committee at Augusta University (protocol number 2013-0586).
Isolation and culture of mouse BMSCs and DCs.
Murine stem cells were collected from the bone marrow of femurs and tibia of mice that were euthanized by CO2 asphyxiation, as previously described (41). Briefly, the cell suspension was collected and centrifuged twice at 1,100 rpm for 10 min in complete medium (RPMI 1640 and 10% fetal bovine serum [FBS] supplemented with 1% penicillin-streptomycin and 100 μl/ml β-mercaptoethanol). The supernatant was then discarded, and the pellet was suspended in ACK lysis buffer (Invitrogen, Thermo Fisher Scientific, Waltham, MA) to eliminate erythrocytes. The number of cells was counted, and cells were plated in tissue culture dishes at 1 × 106 cells/ml of complete medium, with the addition of 20 ng/ml GM-CSF and IL-4 (Peprotech, Rocky Hill, NJ). The cells were incubated at 37°C and 5% CO2 for 8 days with the addition of complete medium on day 3 with fresh growth factors. On day 6, the cells were collected and centrifuged twice. The supernatant was discarded, and the cells were counted and plated again with fresh new medium and growth factors.
Preparation of E. faecalis.
Enterococcus faecalis (ATCC 29212; American Type Culture Collection, Manassas, VA) was incubated in sterilized brain heart infusion medium (Bacto brain heart infusion; Becton, Dickinson, Franklin Lakes, NJ) at 37°C and 5% CO2 in an aerobic chamber for 24 h. The optical density of the bacterium-containing medium was adjusted to 1 at 600 nm, corresponding to 108 cells/ml, using a UV-visible spectrophotometer (BioMate 3S; Thermo Fisher Scientific) (42).
Coculture of E. faecalis with pre-DCs during differentiation and with fully differentiated DCs.
Stem cells cultured in GM-CSF/IL-4 (referred to as pre-DCs) were cocultured with E. faecalis (3 × 106) at a multiplicity of infection (MOI) of 1. Bacterial coculturing was performed at day 0 immediately after cell plating to ensure exposure during differentiation. A second exposure was applied on day 6 with the same MOI (referred to as E. faecalis-induced DCs). As a positive control for level of maturation, day-6 GM-CSF/IL-4-induced immature DCs were incubated with 100 ng/ml lipopolysaccharides (LPS) from Escherichia coli (MilliporeSigma, Burlington, MA) (43). In another group, LPS was added to E. faecalis-induced DCs on day 6. In addition, E. faecalis at an MOI of 1 was added to immature DCs on day 6 to analyze the effect of infection on the already differentiated immature DCs.
Rapamycin treatment of immature and E. faecalis-induced DCs.
Rapamycin, which activates autophagy by inhibiting the mTOR pathway (44), was added on day 0 to stem cells for 1 h prior to the addition of E. faecalis and to the control group. On day 6, the control group and the E. faecalis-induced DCs were treated with another dose of rapamycin to ensure its presence during downstream DC functions, including maturation. The DCs were analyzed by flow cytometry.
Bacterial uptake by pre-DCs and DCs.
The interactions of E. faecalis with pre-DCs and fully differentiated DCs were examined by transmission electron microscopy (TEM) and fluorescence microscopy. For TEM, E. faecalis-induced DC pellets were fixed in 4% paraformaldehyde and 2% glutaraldehyde, postfixed in 2% osmium tetroxide, stained en bloc with 2% uranyl acetate, dehydrated with a graded ethanol series, and embedded in Araldite-Epon resin. Thin sections were cut, collected on copper grids, and stained with uranyl acetate and lead citrate. Stained grids were observed in a JEM-1230 TEM (JEOL USA Inc., Peabody, MA) at 110 kV and imaged with an UltraScan 4000 charge-coupled-device (CCD) camera (Gatan Inc., Pleasanton, CA).
For fluorescence microscopy, E. faecalis were prelabeled with 10 μM carboxyfluorescein succinimidyl ester (CFSE; eBioscience, San Diego, CA) (45) and left for 1 h at 37°C in a shaker to allow the dye to diffuse into the bacteria. The bacteria were washed with phosphate-buffered saline (PBS) to remove excess dye. Fully differentiated DCs on day 6 were cocultured with immature DCs (3 × 106) and infected with CFSE-stained E. faecalis at an MOI of 1. The cells were incubated at 37°C and 5% CO2 for 6 h. Another group of immature DCs was infected with CFSE-stained E. faecalis at an MOI of 1. Control cells were incubated on ice for 6 h (46). The DCs were transferred to microscope slides using a Cytospin 4 cytocentrifuge (Thermo Fisher Scientific). The cells were fixed with 4% formaldehyde and permeabilized with 0.1% Triton X-100 Surfact-Amps detergent solution (Thermo Fisher Scientific) in PBS with no calcium or magnesium. The cells were counterstained with ActinRed-555 ReadyProbes reagent (Thermo Fisher Scientific), and the nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (ab228549; Abcam, Burlingame, CA) and visualized using an inverted confocal microscope (LSM 780; Carl Zeiss AG, Oberkochen, Germany).
Antigen presentation.
To study the antigen-presenting ability of E. faecalis-induced DCs for CD4+ T cells, the spleens of OT-II transgenic mice were dissected, and T cells were isolated to 95% purity using negative selection with a mouse T cell enrichment kit (MagniSort; Thermo fisher Scientific) and then stained with CFSE as previously described (47). The E. faecalis-induced DCs and control DCs were incubated with OVA peptide (O1641; MilliporeSigma). Both DC types were then washed and cocultured with CD4+ T cells at a ratio of 1:10 DCs to T cells in complete medium. After incubation for 5 days at 37°C and 5% CO2, T cell proliferation, based on the percentage loss of the CFSE label in proliferative T cells, was evaluated by flow cytometry.
Antibodies and flow cytometry.
Cells were analyzed using a MACSQuant Analyzer flow cytometer, and data were analyzed using MACSQuantify software (Miltenyi Biotec Inc., Auburn, CA). Fluorophore-conjugated antibodies were added to the cells on ice for 30 min, after which, the cells were washed and resuspended in staining buffer (Thermo Fisher Scientific) for data acquisition. Flow cytometry antibodies used were anti-mouse CD11c monoclonal antibody (N418; eBioscience), anti-MHC class II antibodies (mouse clone M5/114.15.2, VioGreen; Miltenyi Biotec, Bergisch Gladbach, Germany), anti-mouse CD86 monoclonal antibody (GL1; eBioscience), and anti-mouse CD80 monoclonal antibody (1G10; Thermo Fisher Scientific).
Apoptosis/necrosis.
Flow cytometry was used to study the effect of E. faecalis on apoptosis/necrosis of cells by using an annexin V fluorescein isothiocyanate (FITC) kit (apoptosis) and 7-AAD (7-amino-actinomycin D) (necrosis) (eBioscience). The data were analyzed using MACSQuantify software.
Inflammatory gene expression.
Gene expression was analyzed with reverse transcriptase PCR (RT-PCR). An RNeasy minikit (Qiagen, Germantown, MD) was used to isolate total RNA from pre-DCs and DCs. The purity and concentration of the isolated RNAs were examined using the NanoDrop 1000 UV-visible (UV-Vis) spectrophotometer (Thermo Fisher Scientific). The RNA was reverse transcribed to cDNA using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific). Quantitative real-time PCR was performed using TaqMan gene expression primers specific for the IL-1β (Mm00434228_m1), TNF-α (Mm00443258_m1), TGF-β1 (Mm01178820_m1), and IL-12 (Mm01288989) genes in a StepOnePlus real-time PCR system (all from Thermo Fisher Scientific). Delta-delta threshold cycle (CT) calculations were used for gene expression, normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and plotted as relative fold changes.
Autophagy protein expression.
The E. faecalis-induced DCs and control immature DCs were analyzed for expression of autophagy proteins ATG7, Beclin1, and LC3bI/II by Western blotting. Briefly, radioimmunoprecipitation assay (RIPA) buffer with the addition of a protease/phosphatase inhibitor cocktail was used for protein extraction from the DCs. A Mini-PROTEAN TGX Precast protein gel (Bio-Rad Laboratories, Hercules, CA) was used to separate proteins at a concentration of 10 μg. The separated proteins were transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat dry milk and incubated overnight at 4°C with 1:1,000 dilutions of primary antibodies. The membrane was washed thrice in Tris-buffered saline (TBS) and incubated with horseradish peroxidase-conjugated secondary antibodies. Imaging was performed using the ChemiDoc MP imaging system (Bio-Rad).
Statistical analysis.
The data were statistically analyzed for the validity of their normality (Shapiro-Wilk test) and homoscedasticity (modified Levene test) assumptions prior to the use of parametric statistical methods. Multiple group comparisons were analyzed using one-way analysis of variance. Post hoc pairwise comparisons were conducted using the Tukey test. An unpaired t test was used for the comparison of 2 groups. Statistical significance was preset at an α level of 0.05. All experiments were conducted at least three times.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this work was provided by a grant from the Carlos and Marguerite Mason Trust Foundation and the Augusta University Office of Vice President of Research intramural grants program.
We declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Mohamed Mohamed Elashiry contributed to conception, design, data acquisition and interpretation, and analysis and drafted and critically revised the manuscript. Mahmoud Elashiry contributed to design and data acquisition and interpretation and critically revised the manuscript. Rana Zeitoun, Ranya Elsayed, and Fucong Tian contributed to data acquisition and critically revised the manuscript. Shehab Eldin Saber and Salma Hasan Elashry contributed to data interpretation and critically revised the manuscript. Franklin R. Tay contributed to data interpretation and drafted and critically revised the manuscript. Christopher W. Cutler contributed to conception, design, and data interpretation and drafted and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, Kupelian A, Rohn JL. 2013. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS One 8:e83637. doi: 10.1371/journal.pone.0083637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. 2006. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod 32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 3.Rôças IN, Siqueira JF Jr, Santos KR. 2004. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod 30:315–320. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hollenbeck BL, Rice LB. 2012. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence 3:421–433. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Distel JW, Hatton JF, Gillespie MJ. 2002. Biofilm formation in medicated root canals. J Endod 28:689–693. doi: 10.1097/00004770-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect Immun 67:2160–2165. doi: 10.1128/IAI.67.5.2160-2165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou J, Shankar N. 2014. Enterococcus faecalis infection activates phosphatidylinositol 3-kinase signaling to block apoptotic cell death in macrophages. Infect Immun 82:5132–5142. doi: 10.1128/IAI.02426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tien BYQ, Goh HMS, Chong KKL, Bhaduri-Tagore S, Holec S, Dress R, Ginhoux F, Ingersoll MA, Williams RBH, Kline KA. 2017. Enterococcus faecalis promotes innate immune suppression and polymicrobial catheter-associated urinary tract infection. Infect Immun 85:e00378-17. doi: 10.1128/IAI.00378-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong KKL, Tay WH, Janela B, Yong AMH, Liew TH, Madden L, Keogh D, Barkham TMS, Ginhoux F, Becker DL, Kline KA. 2017. Enterococcus faecalis modulates immune activation and slows healing during wound infection. J Infect Dis 216:1644–1654. doi: 10.1093/infdis/jix541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, Pamer EG. 2011. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong H, Pamer EG. 2015. Monocytes and infection: modulator, messenger and effector. Immunobiology 220:210–214. doi: 10.1016/j.imbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glick D, Barth S, Macleod KF. 2010. Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, Waller JL, Genco CA, Slocum C, Manning M, Schoenlein PV, Cutler CW. 2015. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog 10:e1004647. doi: 10.1371/journal.ppat.1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meghil MM, Tawfik OK, Elashiry M, Rajendran M, Arce RM, Fulton DJ, Schoenlein PV, Cutler CW. 2019. disruption of immune homeostasis in human dendritic cells via regulation of autophagy and apoptosis by Porphyromonas gingivalis. Front Immunol 10:2286. doi: 10.3389/fimmu.2019.02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueiredo JAP, Machado AM, Oliveira VPD, Hartmann R, Waltrick SBG, Borba MG, Brew MC, Araujo Estrela CR, Böttcher DE. 2018. Dendritic cells and their relation to apical peridontitis. Braz Oral Res 32(Suppl 1):e71. doi: 10.1590/1807-3107BOR-2018.vol32.0071. [DOI] [PubMed] [Google Scholar]
- 16.Mellman I. 2013. Dendritic cells: master regulators of the immune response. Cancer Immunol Res 1:145–149. doi: 10.1158/2326-6066.CIR-13-0102. [DOI] [PubMed] [Google Scholar]
- 17.Ghislat G, Lawrence T. 2018. Autophagy in dendritic cells. Cell Mol Immunol 15:944–952. doi: 10.1038/cmi.2018.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Tyne D, Gilmore MS. 2014. Friend turned foe: evolution of enterococcal virulence and antibiotic resistance. Annu Rev Microbiol 68:337–356. doi: 10.1146/annurev-micro-091213-113003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson AC, Jonas D, Huber I, Karygianni L, Wölber J, Hellwig E, Arweiler N, Vach K, Wittmer A, Al-Ahmad A. 2015. Enterococcus faecalis from food, clinical specimens, and oral sites: prevalence of virulence factors in association with biofilm formation. Front Microbiol 6:1534. doi: 10.3389/fmicb.2015.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jotwani R, Palucka AK, Al-Quotub M, Nouri-Shirazi M, Kim J, Bell D, Banchereau J, Cutler CW. 2001. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol 167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jotwani R, Cutler CW. 2003. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J Dent Res 82:736–741. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- 22.Jotwani R, Cutler CW. 2004. Fimbriated Porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect Immun 72:1725–1732. doi: 10.1128/iai.72.3.1725-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, Bear A, Genco CA, Brown DL, Cutler CW. 2012. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol 189:3178–3187. doi: 10.4049/jimmunol.1201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arjunan P, Meghil MM, Pi W, Xu J, Lang L, El-Awady A, Sullivan W, Rajendran M, Rabelo MS, Wang T, Tawfik OK, Kunde-Ramamoorthy G, Singh N, Muthusamy T, Susin C, Teng Y, Arce RM, Cutler CW. 2018. Oral pathobiont activates anti-apoptotic pathway, promoting both immune suppression and oncogenic cell proliferation. Sci Rep 8:16607. doi: 10.1038/s41598-018-35126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellman I, Steinman RM. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 26.Volcheck GW. 2008. Overview of the human immune response, p 1–39. Clinical allergy. Springer, New York, NY. [Google Scholar]
- 27.Agrawal A, Agrawal S, Gupta S. 2017. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front Immunol 8:896. doi: 10.3389/fimmu.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding D, Mehta H, McCune WJ, Kaplan MJ. 2006. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol 177:5878–5889. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 29.Monrad SU, Rea K, Thacker S, Kaplan MJ. 2008. Myeloid dendritic cells display downregulation of C-type lectin receptors and aberrant lectin uptake in systemic lupus erythematosus. Arthritis Res Ther 10:R114. doi: 10.1186/ar2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portenier I, Waltimo TMT, Haapasalo M. 2003. Enterococcus faecalis – the root canal survivor and ‘star’ in post-treatment disease. Endod Topics 6:135–159. doi: 10.1111/j.1601-1546.2003.00040.x. [DOI] [Google Scholar]
- 31.Vatkar N, Hegde V, Sathe S. 2016. Vitality of Enterococcus faecalis inside dentinal tubules after five root canal disinfection methods. J Conserv Dent 19:445–449. doi: 10.4103/0972-0707.190019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou J, Shankar N. 2016. The opportunistic pathogen Enterococcus faecalis resists phagosome acidification and autophagy to promote intracellular survival in macrophages. Cell Microbiol 18:831–843. doi: 10.1111/cmi.12556. [DOI] [PubMed] [Google Scholar]
- 33.Morris S, Swanson MS, Lieberman A, Reed M, Yue Z, Lindell DM, Lukacs NW. 2011. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J Immunol 187:3953–3961. doi: 10.4049/jimmunol.1100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saini NK, Baena A, Ng TW, Venkataswamy MM, Kennedy SC, Kunnath-Velayudhan S, Carreno LJ, Xu J, Chan J, Larsen MH, Jacobs WR Jr, Porcelli SA. 2016. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat Microbiol 1:16133. doi: 10.1038/nmicrobiol.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeituni AE, Jotwani R, Carrion J, Cutler CW. 2009. Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. J Immunol 183:5694–5704. doi: 10.4049/jimmunol.0901030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeituni AE, McCaig W, Scisci E, Thanassi DG, Cutler CW. 2010. The native 67-kilodalton minor fimbria of Porphyromonas gingivalis is a novel glycoprotein with DC-SIGN-targeting motifs. J Bacteriol 192:4103–4110. doi: 10.1128/JB.00275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomes BPFA, Herrera DR. 2018. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz Oral Res 32(Suppl 1):e69. doi: 10.1590/1807-3107bor-2018.vol32.0069. [DOI] [PubMed] [Google Scholar]
- 38.Yang NY, Zhou Y, Zhao HY, Liu XY, Sun Z, Shang JJ. 2018. Increased interleukin 1α and interleukin 1β expression is involved in the progression of periapical lesions in primary teeth. BMC Oral Health 18:124. doi: 10.1186/s12903-018-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng R, Wu Z, Li M, Shao M, Hu T. 2020. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int J Oral Sci 12:2. doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Queiroz-Junior CM, Silva MJB, Corrêa JD, Madeira MFM, Garlet TP, Garlet GP, Cunha FQ, Teixeira MM, Silva TA. 2010. A controversial role for IL-12 in immune response and bone resorption at apical periodontal sites. Clin Dev Immunol 2010:327417. doi: 10.1155/2010/327417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Li J, Wu K, Azhati B, Rexiati M. 2016. Culture and Identification of mouse bone marrow-derived dendritic cells and their capability to induce T lymphocyte proliferation. Med Sci Monit 22:244–250. doi: 10.12659/msm.896951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Layton G, Wu WI, Selvaganapathy PR, Friedman S, Kishen A. 2015. Fluid dynamics and biofilm removal generated by syringe-delivered and 2 ultrasonic-assisted irrigation methods: a novel experimental approach. J Endod 41:884–889. doi: 10.1016/j.joen.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. 1999. Early events in dendritic cell maturation induced by LPS. Microbes Infect 1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi S, Sreenivasmurthy SG, Liu J, Wang Z, Tong BC, Song J, Lu J, Cheung KH, Li M. 2019. Balancing mTOR signaling and autophagy in the treatment of Parkinson's disease. Int J Mol Sci 20:728. doi: 10.3390/ijms20030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuominen-Gustafsson H, Penttinen M, Hytönen J, Viljanen MK. 2006. Use of CFSE staining of borreliae in studies on the interaction between borreliae and human neutrophils. BMC Microbiol 6:92. doi: 10.1186/1471-2180-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedwell DB. 1999. Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol 30:101–111. doi: 10.1111/j.1574-6941.1999.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 47.Elsayed R, Kurago Z, Cutler CW, Arce RM, Gerber J, Celis E, Sultan H, Elashiry M, Meghil M, Sun C, Auersvald CM, Awad ME, Zeitoun R, Elsayed R, Eldin M, Elshikh M, Isales C, Elsalanty ME. 2020. Role of dendritic cell-mediated immune response in oral homeostasis: a new mechanism of osteonecrosis of the jaw. FASEB J 34:2595–2608. doi: 10.1096/fj.201901819RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.