Recent efforts to develop an enterotoxigenic Escherichia coli (ETEC) vaccine have focused on the antigenically conserved tip adhesins of colonization factors. We showed previously that intranasal immunization with dsc19CfaE, a soluble variant of the in cis donor strand-complemented tip adhesin of a colonization factor of the class 5 family (CFA/I) fimbria, is highly immunogenic and protects against oral challenge with CFA/I-positive (CFA/I+) ETEC strain H10407 in the Aotus nancymaae nonhuman primate.
KEYWORDS: enterotoxigenic Escherichia coli, ETEC, CfaE, vaccine, mice, H10407, nonhuman primates, chimera LTB, chimera, guinea pigs
ABSTRACT
Recent efforts to develop an enterotoxigenic Escherichia coli (ETEC) vaccine have focused on the antigenically conserved tip adhesins of colonization factors. We showed previously that intranasal immunization with dsc19CfaE, a soluble variant of the in cis donor strand-complemented tip adhesin of a colonization factor of the class 5 family (CFA/I) fimbria, is highly immunogenic and protects against oral challenge with CFA/I-positive (CFA/I+) ETEC strain H10407 in the Aotus nancymaae nonhuman primate. We also reported a cholera toxin (CT)-like chimera (called dsc19CfaE-CTA2/CTB) in which the CTA1 domain of CT was replaced by dsc19CfaE that was strongly immunogenic when administered intranasally or orogastrically in mice. Here, we evaluate the immunogenicity and protective efficacy (PE) of a refined and more stable chimera comprised of a pentameric B subunit of ETEC heat-labile toxin (LTB) in lieu of the CTB pentamer and a donor strand truncation (dsc14) of CfaE. The refined chimera, dsc14CfaE-sCTA2/LTB, was highly immunogenic in mice when administered intranasally or intradermally, eliciting serum and fecal antibody responses against CfaE and LTB, as well as strong hemagglutination inhibition titers, a surrogate for neutralization of intestinal adhesion mediated by CfaE. Moreover, the chimera was safe and highly immunogenic when administered intradermally to guinea pigs. In A. nancymaae, intradermal (i.d.) immunization with chimera plus single-mutant heat-labile toxin [LT(R192G)] elicited strong serum anti-CfaE and anti-LTB antibody responses and conferred significant reduction of diarrhea compared to phosphate-buffered saline (PBS) controls (PE = 84.1%; P < 0.02). These data support the further evaluation of dsc14CfaE-sCTA2/LTB as an ETEC vaccine in humans.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is one of the leading causes of secretory bacterial diarrheal disease worldwide in travelers, deployed U.S. military personnel, and children living in low- to middle-income countries (1–7). Multiple virulence factors, including colonization factors (CFs) and the heat-labile (LT) and heat-stable (ST) enterotoxins, expressed by ETEC strains add complexity to the pathogenesis and serve as potential targets for vaccine development efforts. However, the heterogeneity of CFs produced by pathogenic ETEC isolates has further complicated efforts to develop a broadly protective vaccine to prevent ETEC diarrheal disease.
Our ETEC vaccine strategy is focused on eliciting antibodies against CFs expressed by clinically prevalent ETEC strains in order to inhibit ETEC attachment to the small intestine and prevent onset of disease. CFs of the class 5 family (CFA/I, CS17, and CS2, among others) contain a minor and a major subunit, which form the adhesin tip and stalk of the fimbrial structures, respectively. Among the class 5 family members, the minor subunits of the CFs display greater sequence conservation than the major subunits (8). This suggests that the incorporation of a subset of key CF subunits, in combination with an LT-based component, into a multivalent ETEC vaccine platform may provide sufficient protection against the highly diverse serotypes observed in disease-prone regions (8, 9). We previously developed dsc19CfaE, a soluble and highly stable in cis donor strand-complemented form of the CFA/I fimbrial tip adhesin (10), and demonstrated it to be strongly immunogenic when administered intranasally (i.n.) or transcutaneously to BALB/c mice and i.n. to Aotus nancymaae nonhuman primates (NHPs) (11–13). Immunization of dams with dsc19CfaE passively protected their pups in the neonatal mouse challenge model (14), and immunization with dsc19CfaE conferred active protection in the CFA/I-positive (CFA/I+) ETEC strain H10407 A. nancymaae challenge model (13). Further, bovine colostrum-derived anti-CfaE IgG antibodies orally administered to human volunteers protected against the challenge with the CFA/I+ ETEC strain H10407 (5).
We previously demonstrated that anti-adhesin immune responses were enhanced by coadministration of the soluble adhesin antigen (dsc19CfaE) with the B subunits of LT or cholera toxin (CT) as adjuvant (12, 13). LT and CT are related protein toxins, each composed of a single-polypeptide A subunit (called LTA or CTA, respectively) and a homopentameric ring-shaped B subunit (called LTB or CTB, respectively). The amino-terminal domain, or A1 domain, of CTA/LTA harbors the latent ADP-ribosylating activity, while the carboxyl-terminal A2 domain extends into the core of the pentameric B subunit ring, which has five ganglioside GM1-binding sites. The heterohexameric holotoxin is assembled and stabilized by noncovalent interactions.
To exploit the ability of CT to bind to GM1 receptors on enterocytes and direct antigens to the intestinal surface, chimeras were created in which the A1 domain of CT was replaced with TcpA or TcpF, which can function as colonization factors for Vibrio cholerae. The resulting proteins were evaluated for immunogenicity in adult mice and protective efficacy (PE) in the infant mouse model of cholera (15, 16). Similarly, we have shown that a chimera with the ETEC adhesin dsc19CfaE replacing the A1 domain of CT was highly immunogenic in mice, giving rise to systemic anti-CfaE immune responses that were comparable or superior to those elicited by an admixture of the two separate components (17). Although CT is antigenically related to LT, for the purposes of developing an ETEC vaccine, we wanted to ensure an anti-LT specific response by incorporating the B subunit of LT in the chimera. Herein, we describe the development of a stable, refined chimera in which dsc14CfaE is genetically fused to a shortened CTA2 chain of CT (designated sCTA) and coexpressed with LTB, resulting in the assembly of the dsc14CfaE-sCTA2/LTB chimera. Furthermore, we assessed the immunogenicity of dsc14CfaE-sCTA2/LTB in mice, its safety and immunogenicity in guinea pigs, and its protection against diarrhea in A. nancymaae NHPs challenged orogastrically with the CFA/I+ ETEC strain H10407 after immunization by the clinically suitable intradermal (i.d.) route.
RESULTS
Characterization of chimera.
During design and expression of an initial CfaE-LTB chimeric protein, we observed that the dsc19CfaE-CTA2 fusion underwent proteolytic cleavage between the dsc19CfaE and CTA2 components during storage and handling of the protein, causing release of dsc19CfaE from the chimera (data not shown). We subsequently found that shortening the donor strand, used to stabilize CfaE in the fusion polypeptide, from 19 to 14 amino acids and truncating the CTA2 domain at its N terminus (sCTA2) conferred increased resistance to proteolysis for the resulting chimera, dsc14CfaE-sCTA2/LTB. A comparison of the former dsc19CfaE-CTA2/LTB and the refined dsc14CfaE-sCTA2/LTB chimeras indicated that, for the most part, both elicited comparable immune responses, except for hemagglutination-inhibiting (presumed to reflect adherence-neutralizing) antibodies, which were higher with dsc14CfaE-sCTA2/LTB (Fig. S1 in the supplemental material). SDS-PAGE separation of a heated sample of the refined chimera showed two components (Fig. 1A, lane 1): the dsc14CfaE-sCTA2 protein (43 kDa) and monomeric LTB (12 kDa), which reacted with CfaE antisera (Fig. 1B, lane 1) and LTB antisera (Fig. 1C, lane 1), respectively. Without the addition of reducing agents, the dsc14CfaE-sCTA2 fusion (Fig. 1A to C, lane 3) migrates faster than its reduced form (Fig. 1A to C, lane 1), which indicates the presence of one or more of three possible disulfide bonds within the dsc14CfaE domain. In the absence of heat treatment prior to loading the sample onto the SDS-PAGE gel, two additional protein species were observed (Fig. 1A to C, lane 2). One is the fully assembled chimera (∼103 kDa), which was reactive to both LTB and CfaE antisera. The second species comigrated with the dsc14CfaE-sCTA2 fusion band and was reactive with LTB antisera. The species is likely the pentameric form of LTB (∼59 kDa). Thus, without heat treatment, a portion of the LTB remains assembled as either the free pentamer or as part of the intact heterohexameric chimera complex even in the presence of SDS, resulting in an observable reduction in the monomeric LTB band on the gel (∼12 kDa). Analysis of the chimera by high-performance liquid chromatography size exclusion chromatography (HPLC-SEC) showed three peaks. The primary peak was the intact chimera, and the two minor peaks were free dsc14CfaE-sCTA2 and LTB, which likely formed from the spontaneous disassociation of the chimera complex. Peak identities were confirmed by SDS-PAGE (Fig. S2). The dsc14CfaE-sCTA2/LTB-refined chimera is hereafter referred to as chimera.
FIG 1.
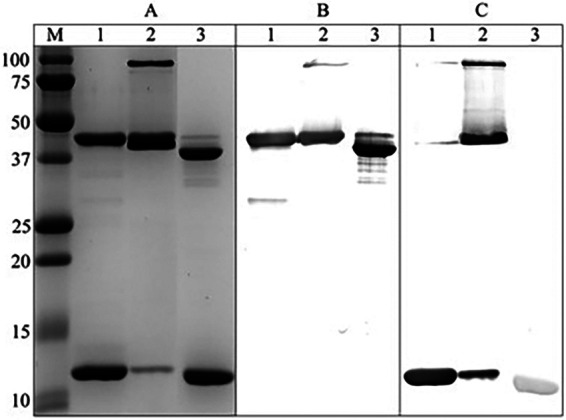
Characterization of chimera. (A) dsc14CfaE-sCTA2/LTB chimera was separated by 15% SDS-PAGE and stained with Coomassie blue. (B) Immunodetection with CfaE antisera. (C) Immunodetection with LTB antisera. Lanes: M, molecular weight marker (Bio-Rad Precision Plus Protein Standards); 1, heat treated, reduced; 2, unheated, reduced; 3, heat treated, not reduced. Original images of SDS-PAGE gels and blots used to assemble this figure are shown in Fig. S3 in the supplemental material.
Chimera binds to GM1 and bovine erythrocytes.
The functionalities of the LTB and CfaE components of the chimera were confirmed by ganglioside GM1-binding enzyme-linked immunosorbent assays (ELISAs) and by mannose-resistant hemagglutination assays, respectively. When increasing amounts of chimera were added to ELISA wells coated with GM1, washed, incubated with CfaE antisera, and developed with horseradish peroxidase-conjugated anti-IgG, a dose-dependent increase in optical density at 450 nm (OD450) was observed (Fig. 2). As expected, the positive control CTB also bound to GM1, but the negative-control dsc19CfaE did not. Further, when either the chimera or the positive control dsc19CfaE was absorbed to polystyrene beads, they agglutinated bovine erythrocytes in the presence of mannose, but the negative-control LT did not (Fig. S2). Together, these results demonstrate that the chimera retains the functional activities of its individual components, CfaE and LTB.
FIG 2.
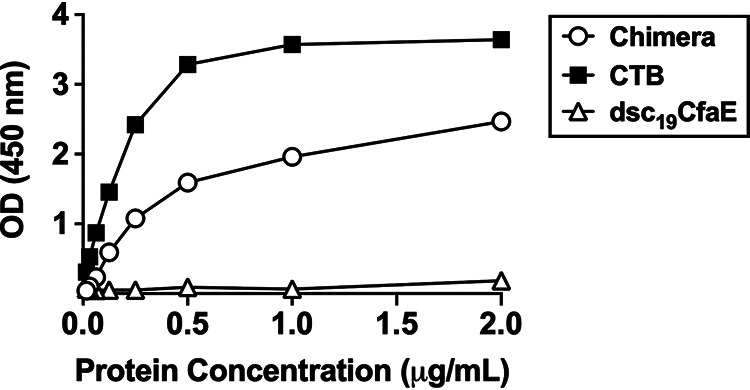
Binding of chimera (dsc14CfaE-sCTA2/LTB), CTB, and dsc19CfaE to GM1. GM1 binding activity of chimera and dsc19CfaE (negative control) was detected using CfaE antisera, while CTB (positive control) binding activity was detected using CTB antisera (see Materials and Methods for details).
Chimera is immunogenic in mice by multiple routes.
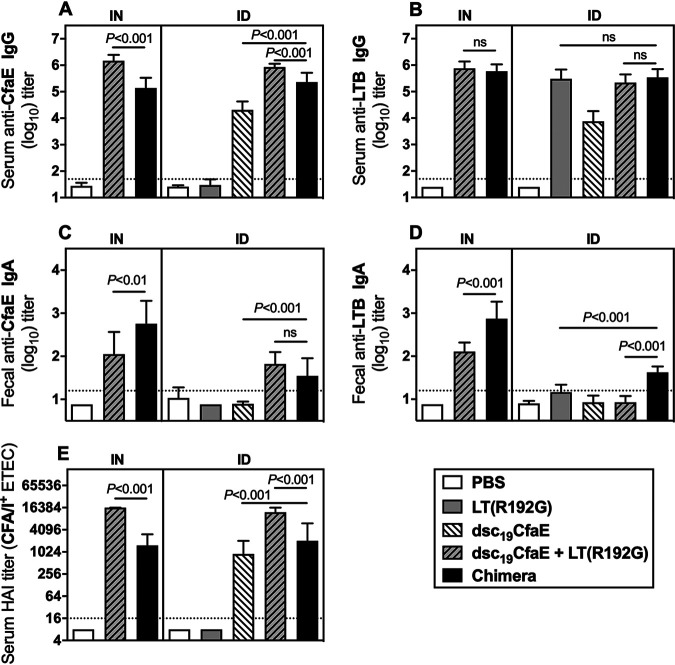
Serum samples collected from mice immunized with chimera, dsc19CfaE, or dsc19CfaE plus single-mutant heat-labile toxin [LT(R192G)] were evaluated for anti-CfaE and anti-LTB antibody responses. Antibody responses measured 2 weeks after the third dose (day 42) are shown in Fig. 3. Mice immunized with chimera, as well as with dsc19CfaE or dsc19CfaE with LT(R192G), by either i.n. or i.d. routes, had anti-CfaE and anti-LTB IgG titers significantly greater than the corresponding phosphate-buffered saline (PBS) control groups (P < 0.001) (Fig. 3A and B). While dsc19CfaE plus LT(R192G) elicited higher anti-CfaE IgG antibody levels than chimera by both routes (P < 0.001), comparable anti-LTB IgG levels were observed between those two groups. We also observed modest anti-LTB IgG antibody titers (mean ± standard deviation [SD], 3.9 ± 0.38) in the animals immunized i.d. with dsc19CfaE only, which was likely the result of minute contamination with LT(R192G) during dose preparation. In fact, we recently demonstrated that a dose as low as 0.01 μg of LT(R192G) is able to elicit an immune response (18). Mice given chimera i.d. had significantly greater anti-CfaE IgG titers than those given dsc19CfaE alone (P < 0.001; Fig. 3A).
FIG 3.
Serum and fecal antibody responses in mice immunized i.n. or i.d. with chimera. Mice (10/group) were immunized i.d. on days 0, 14, and 28 with 21 μg chimera, 8 μg dsc19CfaE with or without 0.1 μg LT(R192G), 0.1 μg LT(R192G), or PBS. Three additional groups of mice were immunized i.n. with 21 μg chimera, 8 μg dsc19CfaE plus 0.1 μg LT(R192G), or PBS. (A) Serum anti-CfaE IgG antibody response on day 42; (B) serum anti-LTB IgG antibody response on day 42; (C) fecal anti-CfaE IgA antibody response on day 33; (D) fecal anti-LTB IgA antibody response on day 33. ELISA results (panels A to D) are presented as mean log10 titers ± SD. (E) Functional antibody titers measured by the HAI assay (median ± interquartile ranges). Horizontal dotted lines denote the limit of detection for the assays. Statistical significance was determined by using a one-way ANOVA and Tukey’s post hoc test. ns, not significant.
Significant fecal anti-CfaE IgA antibody titers were observed in mice given chimera or dsc19CfaE plus LT(R192G), either by the i.n. or i.d. routes, compared to the corresponding PBS control groups (all P < 0.001; Fig. 3C). While fecal anti-CfaE IgA antibody titers were increased in the chimera group compared to dsc19CfaE plus LT(R192G) group when administered i.n. (P < 0.01), comparable titers were observed when the same formulations were administered i.d. Fecal anti-LTB IgA responses significantly higher than those in the PBS group were seen in the groups given chimera i.n. or i.d. (both P < 0.001) and in groups given dsc19CfaE plus LT(R192G) by the i.n. route (P < 0.001) or LT(R192G) by the i.d. route (P < 0.01) (Fig. 3D). By both routes, groups given chimera had significantly greater anti-LTB fecal antibody responses than those given the dsc19CfaE with LT(R192G) (both P < 0.001; Fig. 3D).
All groups given chimera or dsc19CfaE [alone or with LT(R192G)] showed functional neutralization titers, as judged by hemagglutination inhibition (HAI) assays, significantly higher than those in the PBS group (all P < 0.001). Immunization with dsc19CfaE with LT(R192G) elicited higher HAI titers than chimera by both routes (both P < 0.001) (Fig. 3E).
Chimera is safe and immunogenic in guinea pigs by the i.d. route.
Intradermal administration with chimera with or without LT(R192G) in Hartley guinea pigs was well tolerated, and all animals survived to termination with no adverse clinical signs. Induration at the site of injection was more evident when chimera was administered in combination with LT(R192G) than when administered alone, but resolved over time (Fig. S3). A similar profile was observed with the administration of dsc19CfaE plus LT(R192G). The chimera was highly immunogenic whether administered alone or with LT(R192G) (Fig. 4). The anti-CfaE IgG response and HAI titers rose earlier, by day 21, in groups given chimera [with or without LT(R192G)] than in groups given dsc19CfaE with or without LT(R192G), despite having only 5 μg of dsc14CfaE in the chimera versus 25 μg of dsc19CfaE (Fig. 4A and B). For both anti-CfaE IgG and HAI parameters, addition of LT(R192G) adjuvant tended to enhance responses on study days 42 and 63. While anti-LTB IgG antibody responses were observed after vaccination with dsc19CfaE with LT(R192G) as well as chimera [with or without LT(R192G)], the highest titers were observed in the groups that received the LT(R192G) adjuvant (Fig. 4C).
FIG 4.
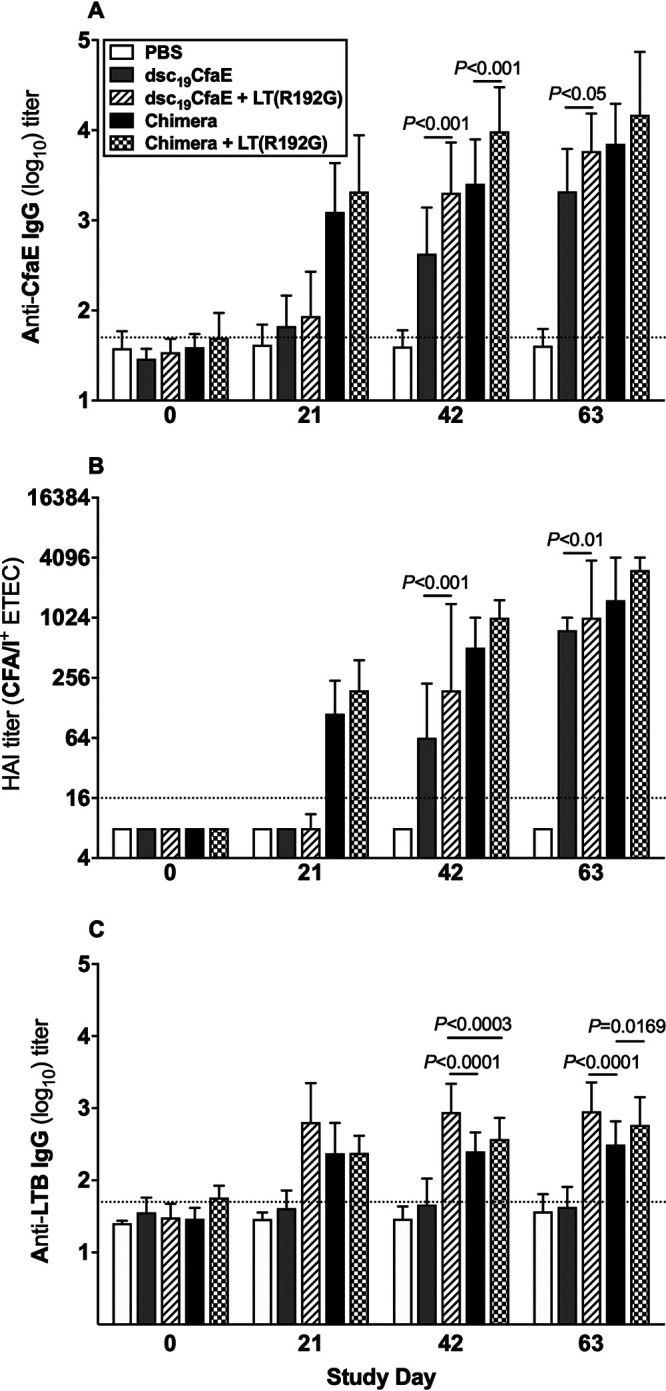
Serological immune responses in guinea pigs immunized i.d. with chimera. Animals (24/group) were immunized i.d. on days 0, 21, 42, and 63 with 13 μg chimera with or without 0.1 μg LT(R192G), 25 μg dsc19CfaE with or without 0.1 μg LT(R192G), or PBS. (A) Anti-CfaE IgG antibody responses (mean ± SD); (B) functional antibody titers measured by the HAI assay (median ± interquartile ranges); (C) anti-LTB IgG antibody response (mean ± SD). Horizontal dotted lines denote the limit of detection of the assays. Statistical significance was determined by using one-way ANOVA and Tukey’s post hoc test.
Chimera is immunogenic and protective in A. nancymaae.
We used the A. nancymaae NHP challenge model (13) to test whether immunization with chimera by the i.d. route would elicit anti-adhesin immune responses and protect against challenge with the CFA/I+ ETEC strain H10407. Intradermal immunization with the chimera alone induced little to no induration at any time point (Fig. S4). Animals dosed with chimera with LT(R192G) exhibited induration, which peaked after the second dose, decreased in size with each subsequent dose, and tended to self-resolve over time.
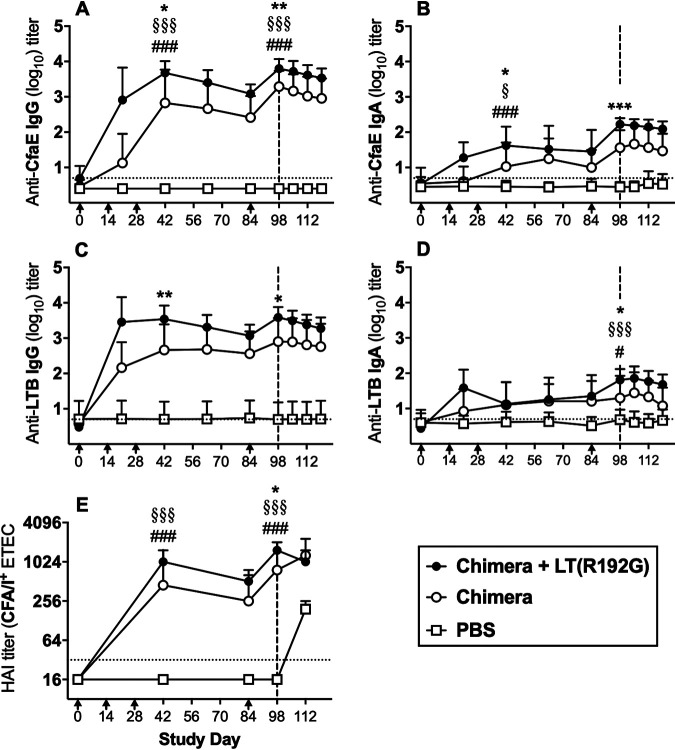
Immunization with chimera with or without LT(R192G) led to significant anti-CfaE and anti-LTB immune responses (Fig. 5). Serum anti-CfaE IgG immune responses in test groups peaked two weeks following the third immunization (day 42) and again after the boost on day 84 (i.e., day 98; Fig. 5A), and thus, analyses focused on these two time points. Groups that received chimera with or without LT(R192G) developed anti-CfaE IgG immune responses significantly higher than those in the PBS group on both days 42 and 98 (all P < 0.001). Co-administration of chimera with LT(R192G) led to significantly higher anti-CfaE responses on days 42 and 98 than the chimera-only group (P < 0.05 and P < 0.01, respectively; Fig. 5A). On days 42 and 98, anti-CfaE IgA responses in groups receiving chimera or chimera with or without LT(R192G) were significantly different from each other (P < 0.05 and P < 0.001, respectively; Fig. 5B).
FIG 5.
Serological immune responses in A. nancymaae. Animals (9 to 10/group) were immunized i.d. with 129 μg chimera with or without 1 μg LT(R192G) or PBS on days 0, 14, 28, and 84 (arrows below x axis) and challenged on day 98 with 4.95 × 1011 CFU of ETEC strain H10407 (vertical dashed line). (A) Anti-CfaE IgG antibody titers; (B) anti-CfaE IgA antibody titers; (C) anti-LTB IgG antibody titers; (D) anti-LTB IgA antibody titers. ELISA results (panels A to D) are shown as mean log10 titers ± SD. (E) Hemagglutination inhibition titers with CFA/I+ ETEC strain H10407 (median ± interquartile ranges). Horizontal dotted lines denote the limit of detection for each assay (see Materials and Methods). Statistical comparisons were performed by one-way ANOVA and Tukey’s post hoc test on data collected from day 42 and 98 sera. #, P < 0.05, and ###, P < 0.001 for comparisons between chimera and PBS groups; §, P < 0.05, and §§§, P < 0.001 for comparisons between chimera with LT(R192G) and PBS groups; *, P < 0.05; **, P < 0.01; and ***, P < 0.001 for comparisons between chimera and chimera with LT(R192G) groups.
Strong anti-LTB IgG antibody responses were elicited in animals in both test groups but were significantly stronger in those given chimera plus LT(R192G) than in those without LT(R192G) (day 42, P < 0.01; day 98, P < 0.05; Fig. 4C). Anti-LTB IgA antibody responses in both test groups were significantly greater than those in PBS controls on day 98 [chimera, P < 0.05; chimera with LT(R192G), P < 0.001]. On day 98, the responses in each test group were significantly different from each other (P < 0.05; Fig. 5D).
As with the mouse and guinea pig studies, A. nancymaae serum samples were assessed for the presence of fimbrial adhesion neutralizing antibodies using the HAI assay. Serum HAI responses seen in groups receiving chimera [with or without LT(R192G)] were significantly higher than those in PBS controls on both days 42 and 98 (all P < 0.001; Fig. 5E). Responses in test groups were significantly different from each other on day 98 (P < 0.05; Fig. 5E).
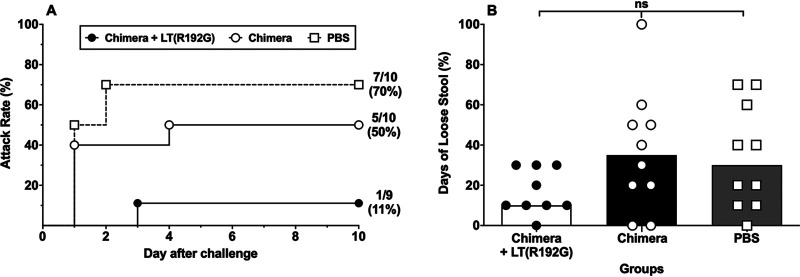
Following challenge with ETEC strain H10407, on day 98, fecal shedding of the bacteria was observed in all animals, regardless of treatment group, beginning 1 day after challenge (Table S1). Animals immunized with the chimera alone or with LT(R192G) adjuvant had lower diarrhea rates (50% and 11%, respectively) than PBS-immunized animals (70%) (Fig. 6A). This translates into PE of 28.6% for chimera alone (P = 0.65) and 84.1% for chimera with LT(R192G) (P = 0.02) (Table 1). The proportion of days of loose stools was similar among all groups (Fig. 6B).
FIG 6.
Disease assessment in A. nancymaae after challenge with CFA/I+ ETEC. A. nancymaae NHPs (9 to 10/group) were immunized i.d. on days 0, 14, 28, and 84 with 129 μg chimera with or without 0.1 μg LT(R192G) or PBS and challenged on day 98 with 4.95 × 1011 CFU of CFA/I+ ETEC strain H10407. (A) Diarrheal attack rate (day 0 on the x axis refers to the day of challenge, which corresponds to study day 98). (B) Percent of days with loose stools after challenge (data from individual animals are shown as well as the median [bar] for the group).
TABLE 1.
Protective efficacy of vaccines in A. nancymaae
| Vaccine | No. with diarrhea/total (%) | Protective efficacy (%) | P valuea |
|---|---|---|---|
| Chimera | 5/10 (50) | 28.6 | 0.65 |
| Chimera with LT(R192G) | 1/9 (11) | 84.1 | 0.02 |
| PBS | 7/10 (70) |
Fisher’s exact test, two-tailed, comparing frequency of diarrhea in test groups to PBS group.
DISCUSSION
The high degree of antigenic heterogeneity of ETEC strains requires a broadly protective multivalent vaccine. Our focus has been in identifying the minimum critical number of components necessary to optimize coverage, as well as the most efficacious formulation and route of administration. Here, we evaluated the safety, immunogenicity, and efficacy of a chimeric protein combining the prototypical class 5a ETEC adhesin, CfaE, and a pentamer of the B subunit of the heat-labile toxin, LTB, a strategy that can reduce the number of proteins of an ETEC multivalent vaccine. Although i.n. vaccination is able to generate mucosal responses, it is not a viable route for an LT-adjuvanted ETEC vaccine due to the associated risk of Bell’s palsy (19, 20). Hence, we investigated whether i.d. administration could be used as an alternative and effective route of immunization. Administration of vaccines through the skin has several advantages, such as less pain, small volumes, and dose sparing. In addition, skin vaccination seems to be an attractive approach to promote mucosal immunity (21–23). We found that chimera, administered i.n. or i.d. to mice, elicits strong serum anti-adhesin (i.e., anti-CfaE) IgG and fecal anti-adhesin IgA antibody responses, as well as strong serum anti-LTB IgG and fecal anti-LTB IgA antibody responses. Importantly, immunization with chimera by both routes leads to the generation of high levels of functional antibodies, as judged by HAI assays, an in vitro proxy parameter for intestinal adhesion inhibition. Previous studies have demonstrated that, while dsc19CfaE is highly immunogenic when administered via different immunization routes, higher anti-adhesin and functional antibody levels were elicited with the coadministration of LT- or CT-based adjuvants (12, 13, 18). In addition to adjuvant properties, those proteins also elicit anti-toxin responses that are important for protection, particularly against LT-producing (i.e., LT plus ST and LT-only) ETEC strains (9, 24–27). However, the toxicity associated with the native holotoxins by i.n. and orogastric administration, resulting in Bell’s palsy and diarrhea, respectively, precludes their use as vaccine components via those routes. Incorporating only the LTB subunit into a chimera plus an adhesin antigen not only allows the generation of anti-toxin antibodies (24) but also eliminates enterotoxic effects. Furthermore, since a transcutaneous vaccine with only LT failed to protect travelers against ETEC diarrhea, evaluation of vaccine candidates that include colonization factors is warranted (28).
Given the encouraging results seen with i.d. immunization in mice, we investigated the chimera’s safety profile in guinea pigs. The species was chosen for the toxicology study because it has historically been used to investigate vaccines administered through the skin (29, 30) and because guinea pigs have a well-defined epidermis compared to other small animals (31). No systemic adverse reactions were observed in the group receiving chimera only, and local reactogenicity at the injection site was minimal, transient, and self-limited. Moreover, immunization with chimera or chimera plus LT(R192G) elicited an earlier onset of anti-CfaE and HAI responses than the dsc19CfaE plus LT(R192G), suggesting that fewer doses of chimera might be needed to reach similar responses.
We proceeded to investigate chimera’s immunogenicity and protective efficacy when administered i.d. to A. nancymaae NHP. The animals responded with a strong anti-adhesin antibody response that was enhanced by the coadministration of LT(R192G) adjuvant. Importantly, NHPs immunized i.d. with chimera plus LT(R192G) were significantly protected against the challenge with CFA/I+ ETEC strain H10407, similar to previous results seen with dsc19CfaE with LT(R192G) administered by the i.n. route (13).
The experiments described here have some limitations. While the mucosal immune response in the murine model was investigated, we were not able to do so in the A. nancymaae model, as fecal antibody assays were not developed at the time of this study. However, the primary aim of the NHP model was to investigate whether or not i.d. immunization with chimera could protect against the challenge with ETEC. Interestingly, only the administration of chimera with LT(R192G) adjuvant afforded significant protection. While we speculate that a larger dose of the chimera antigen could possibly elicit comparable protection in NHP in the absence of the adjuvant, our data suggest that LT-derived adjuvants, such as LT(R192G), are able to enhance mucosal responses even if administered by nonmucosal routes. The final ETEC multivalent vaccine formulation will most likely require an adjuvant, and while the reactogenicity of the LT(R192G) is acceptable, as seen here and elsewhere (18), and its adjuvanticity has successfully been evaluated in clinical trials by the transcutaneous (https://www.clinicaltrials.gov identifier NCT01382095) and i.d. routes (https://www.clinicaltrials.gov identifier NCT01644565) (M. Maciel, Jr., unpublished data), it is likely that future ETEC vaccine formulations would benefit from a less reactogenic LT toxoid. The recently developed LT(R192G/L211A) adjuvant (double-mutant LT) (32) incorporates a second mutation that reduces reactogenicity, while preserving its adjuvanticity, when administered i.d. (18) and, as such, is a promising alternative. The lack of an adjuvant-only group in the current NHP study precludes the assessment of the protection conferred by the anti-toxin-only response. However, that question has been recently investigated in a study in which A. nancymaae were immunized i.d. with LT(R192G/L211A) and challenged with CS6+ ETEC strain B7A. No significant protection was seen after the challenge in that group (PE 37.5%; P = 0.32 compared to PBS controls), in spite of an anti-toxin response comparable to the group that received adhesin plus LT(R192G/L211A), which did show significant protection (S. J. Savarino, unpublished data). Alternatively, other adjuvants such as Toll-like receptor 4 (TLR-4) agonists can also be used to deliver ETEC vaccine candidates (33). In that case, a single chimeric protein such the one described here can provide both antigens.
Our results support the notion that the immunogenicity of both antigens in the chimera, i.e., CfaE and LTB, is preserved, and both antigens can be delivered as a single macromolecular protein complex without loss of critical epitopes. Of note, while the immunogenicity of the chimera is comparable to that of dsc19CfaE plus LT(R192G) by the i.d. route in mice, we recently demonstrated that the chimera is a superior antigen for sublingual delivery, a mucosal route (34), likely because of its ability to bind to the GM1 receptor. Proteins fused with CTA2 and coexpressed with CTB often retain the ability to assemble into chimeras (15–17, 35), where only CTA2, and not CTA1, is necessary to bind to the CTB subunit and form a GM1-binding complex (35). Although we have not directly investigated the role of the GM1 receptor binding for i.d. immunization with chimera, Yamaoka and Imamura have elegantly demonstrated that the full adjuvant effect of the cholera toxin in the skin is dependent upon both GM1 binding and ADP-ribosyltransferase activity (36). The absence of induration after i.d. injection with chimera is likely due to the lack of ADP-ribosyltransferase activity and possibly explains why the use of an adjuvant with both activities, such as LT(R192G), was necessary to achieve protection of A. nancymaae against challenge at the doses of chimera used for immunization in our experiments.
The present study supports several important conclusions. First, we demonstrated that immunization with chimera is not only able to elicit systemic and mucosal immune responses against ETEC antigens but also generated functional antibody responses. Second and importantly, immunization with chimera coadministered with LT(R192G) adjuvant conferred protection against the challenge with CFA/I+ ETEC strain H10407. Finally, the i.d. route of vaccination was safe and effective at promoting immune responses that protected against diarrhea. The development of a broadly protective subunit ETEC vaccine will need to expand these evaluations to other key adhesin antigens to determine the wider suitability of the i.d. immunization.
MATERIALS AND METHODS
Construction of expression vectors and clones.
(i) Expression clone for dsc14CfaE-sCTA2/LTB chimera. The dsc14*cfaE gene encoding the 338 residue CfaE genetically fused at its C terminus with a 4-codon linker turn (encoding Asp-Asn-Lys-Gln), and the first 14 codons of the mature CfaB major subunit was PCR amplified from pET24-dsc19cfaE[his]6 (10) and joined with sequences encoding a two-codon linker (encoding Ser-Ile) and the last 30 residues (LDEYQ to IKDEL) of the cholera toxin A2 domain (called sCTA2), resulting in the sequence coding for the dsc14CfaE-sCTA2 fusion protein (17). The fusion protein also has a pelB leader derived from the vector pET22b(+) (Novagen, EMD Biosciences, Inc.) that directs its secretion into the periplasm, and the processed mature fusion protein has Met1 in place of Ala1 in native CfaE. A separate gene codes for a variant form of the 103-residue mature B subunit of heat-labile enterotoxin (LTB) protein with two substitutions (R13H and N94H) corresponding to histidine residues in the cholera toxin B subunit (CTB) that allow it to bind to metal-chelate resins. The LTB gene also contains an LT-IIb B leader sequence to allow for secretion to the periplasm after expression (37). Both genes are controlled by plasmid-encoded AraC and expressed under separate pBAD arabinose-inducible promoters derived from the Salmonella enterica serovar Typhimurium ara operon vector pAR3 (38). This expression plasmid, p1115K1(07), contains a pUC19-derived origin of replication and the kanamycin resistance gene aph (aminoglycoside 3′-O-phosphotransferase) from Tn5 via the vector pK18 (39). For expression of the protein, Escherichia coli BL21 Veggie competent cells (Novagen) were transformed with p1115K1(07), yielding the chimera expression strain BL21(p1115K1(07).
(ii) Expression clone for LTB. The LT-IIb B subunit leader with the LTB gene carried by p1115K1(07) was cloned into pK19 (37) to make an LTB-only clone, p0104K1(06). As described above, BL21 Veggie competent cells (Novagen) were transformed with this plasmid, yielding the LTB expression strain BL21[p0104K1(06)].
Expression and purification of recombinant proteins.
(i) Recombinant dsc14CfaE-sCTA2/LTB chimera. E. coli BL21 cells (Novagen, Madison, WI) containing p1115K1(07) were used to inoculate a baffled 1-liter Fernbach flask containing growth media (APS superbroth [BD Difco] and 50 μg/ml kanamycin sulfate). The flasks were grown at 37°C with 200 rpm agitation to an approximate optical density at 600 nm (OD600) of 1 and used to seed 10 liters of growth medium in a 10-liter New Brunswick fermentation system (BioFlo 3000 or 310; Eppendorf North America, Hauppauge, NY). The 10-liter culture was grown at 37°C to an OD600 between 10 and 20 using 10-liter/min airflow and a 200- to 800-rpm agitation cascade designed to maintain dissolved oxygen above 30%. Upon reaching the target OD600, the temperature was lowered to 25°C, and expression was induced with a continuous feed (0.7 ml/min) of a glucose (12.5%) and arabinose mixture (10%) over a period of 11 h. The cell culture was harvested 12 h postinduction and lysed by microfluidization using a model M-110Y microfluidizer (Microfluidics Corporation, Westwood, MA). Cell debris was removed by centrifugation at 4°C, and the soluble protein fraction was applied to a Talon Superflow metal affinity column (Clontech Laboratories, Mountain View, CA). The chimera was eluted using a stepwise imidazole gradient. Eluted fractions were pooled and applied to an SP Sepharose cation exchange column (GE Healthcare), and protein was eluted with a stepwise salt (NaCl) gradient. Fractions containing purified chimera were pooled, concentrated, and sterilized by 0.22 μM filtration. The dsc14CfaE-sCTA2/LTB chimera used in the guinea pig study was manufactured under good manufacturing practices (GMP) at the Walter Reed Institute of Research Pilot BioProduction Facility (WRAIR-PBF, Silver Spring, MD).
(ii) Recombinant dsc19CfaE. Recombinant dsc19CfaE[His]6 (referred throughout as dsc19CfaE), used for immunization in the murine and guinea pig studies and ELISAs for all studies, was produced and purified using previously described expression clone and production methods (10). The recombinant dsc19CfaE used in the guinea pig studies was manufactured under current good manufacturing practices (cGMP) at the WRAIR-PBF.
Recombinant LTB containing the R13H and N94H mutations was expressed in E. coli expression strain BL21[p0104K1(06)]. The expression strain was grown in a 10-liter culture of APS Superbroth containing 50 μg/ml kanamycin using a New Brunswick BioFlo 3000 fermentation system at 25°C and 850 rpm agitation. After reaching an OD600 of approximately 6, the temperature was increased to 37°C, and expression was induced with 0.2% arabinose. The culture was harvested by centrifugation at 4°C after 3 h of induction. The recombinant LTB protein was then purified as described for the chimera.
SDS-PAGE and Western blot analysis of chimera.
The chimera was analyzed by SDS-PAGE and Western blotting as described previously (40). For Western blotting, membranes were incubated with rabbit polyclonal antisera against either recombinant LTB or dsc19CfaE.
Binding of the chimera to ganglioside GM1 detected by ELISA.
The ability of the chimera, CTB (LIST Biological Laboratories), or dsc19CfaE to bind to GM1 was determined by ELISA as described previously (35). Samples were analyzed in triplicate.
Polystyrene bead adsorption and MRHA.
Fifty micrograms of chimera, dsc19CfaE, or recombinant LTB purified protein preparations were adsorbed to 3-μm polystyrene beads and subsequently assayed for mannose-resistant hemagglutination (MRHA) with bovine erythrocytes as previously described (10).
HPLC-SEC.
Fifty microliters of the chimera were applied to a TSK G2000 size exclusion column at a flow rate of 1.0 ml/min using phosphate-buffered saline, pH 7.4, as the mobile phase. Eluting protein peaks were monitored by UV light absorbance at 280 nm.
Vaccine proteins and adjuvant and ELISA antigens.
The research-grade chimera (mouse studies) and cGMP chimera (WRAIR PBF, lot 1648; guinea pig and NHP studies) were both 99% pure (endotoxin <12 and <7 endotoxin units [EU]/mg, respectively). The dsc19CfaE protein used for mouse studies and ELISA reagent was produced as described previously (10) and was ≥92% pure and low in endotoxin content (≤158 EU/mg). The dsc19CfaE protein used in the guinea pig study was manufactured under cGMP at the WRAIR-PBF (lot 1587; purity of 99%; endotoxin content, 17 EU/mg). LTB was purified and released by the biochemistry laboratory at Naval Medical Research Center (NMRC) (purity of 100%; endotoxin content of 177.5 EU/mg). Single-mutant heat-labile toxin [LT(R192G)] was prepared under cGMP at the WRAIR-BPF (lot 0816; endotoxin content, 24 EU/mg).
Mouse immunization and sample collection.
The mouse studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the Naval Medical Research Center (protocol no. D05-09). Female BALB/c mice, aged 6 to 8 weeks, were purchased from Jackson Laboratory (Bar Harbor, ME). Groups of 10 mice were immunized i.n. with 21 μg chimera, 8 μg dsc19CfaE with 1.5 μg LT(R192G), or PBS. For evaluation of the i.d. route, animals (10/group) received 21 μg chimera, 8 μg dsc19CfaE with or without 0.1 μg LT(R192G), 0.1 μg LT(R192G), or PBS. For both routes, immunizations were performed on days 0, 14, and 28. The dose of dsc19CfaE was molar matched to the approximate amount present in 21 μg of chimera. For i.n. immunization, mice were anesthetized with isoflurane (Halocarbon Products Corporation, River Edge, NJ), and 20 μl vaccine preparation was delivered via pipette into the external nares (10 μl per nare). For i.d. immunization, mice were restrained, and the dorsal surface of each animal was shaved using an electric clipper 1 day prior to immunization. Injections were delivered in 20 μl/dose using a 31G needle fitted to a 1-ml syringe, inserted almost parallel to the skin. After delivery, the syringe was removed quickly; a bleb at the site of injection indicated a proper intradermal delivery of the formulation. Each of three doses was administered in a different quadrant on the animal’s dorsum (18). Blood was sampled by tail bleeding at baseline (day −1) and on days 13 and 27 and by cardiac puncture on day 42. Serum was separated from clotted blood samples and stored at −20˚C until use. Fecal samples were collected on day 33, incubated on ice in protease inhibitor cocktail (Sigma-Aldrich Corp., St. Louis, MO), and homogenized, and supernatants were stored at −80˚C until use.
Guinea pig immunization and sample collection.
The good laboratory practice (GLP) guinea pig study protocol was approved by AVANZA Laboratory IACUC (Gaithersburg, MD). We purchased 120 Hartley guinea pigs, 60 per sex, ranging in weight from 409 to 677 g, from Elm Hill Breeding Labs, Inc. (North Chelmsford, MA). Groups of 24 guinea pigs were immunized with 13 μg chimera with or without 0.1 μg LT(R192G), 25 μg dsc19CfaE with or without 0.1 μg LT(R192G), or PBS by the i.d. route on days 0, 21, 42, and 63. On each animal, four dose sites were shaved and marked 1 day prior to immunization. Animals were dosed on day 0 (site 1), 21 (site 2), 42 (site 3), and 63 (site 4) by i.d. injection in a 100-μl/dose volume. Dose sites were evaluated for induration prior to dosing on day 0 and then 1, 2, 3, 7, 10, and 14 days after dosing, then weekly after that if signs of reactogenicity had not resolved. To measure induration at the dose sites, medial-to-lateral (width) and caudal-to-cranial (length) measurements were taken and results calculated as the average (in millimeters). Other parameters collected for assessment of safety included edema, erythema, weight, body temperature, gross pathology, and weight of several organs (brain, heart, liver, spleen, lungs, ovary, kidneys, testes, ovaries, and uterus), plasma fibrinogen, albumin-to-globulin ratio, blood chemistry, and hematology (data not shown). Blood samples for immunology were collected via retro-orbital puncture while animals were anesthetized with isoflurane prior to dose 1 and on days 21, 42, 63, and then via cardiac puncture on day 84. Whole blood was processed for serum and used to detect anti-CfaE IgG antibodies by ELISA and functional antiadhesive antibodies by hemagglutination inhibition assay.
Aotus nancymaae immunization, challenge, and sample collection.
The study was approved by the IACUC at the U.S. Naval Medical Research Unit No. 6 (NAMRU-6, Lima, Peru) and the Bureau of Medicine (BUMED) (IACUC protocol no. 11-02). Twenty-nine New World monkey Aotus nancymaae NHPs were purchased from the Instituto Veterinario de Investigaciones Tropicales y de Altura (IVITA), Universidad Nacional Mayor de San Marcos (Lima, Peru). The animals had not been previously used in an ETEC study and were excluded if prescreening revealed a reciprocal anti-CFA/I IgG titer >250. Animals were housed in the NAMRU-6 Primate Facility (Lima, Peru), fed a standard monkey diet supplemented with fruit, and provided water ad libitum. Animals were caged individually for 4 days (starting on day 94) prior to and during the challenge period, but pair housed at all other times during the immunization period. Nine male and 20 female A. nancymaae ranging in age from 25.2 to 45.8 months and in weight from 710 to 1,010 g at study onset were used. Weights, temperatures, complete blood counts, and blood chemistry were monitored by the veterinary staff throughout the experiment. Animals (n = 9 to 10 per group) were anesthetized by intramuscular injection of ketamine hydrochloride (10 mg/kg) and immunized i.d. in the abdomen with a volume of 100 μl containing 129 μg of chimera, 129 μg of chimera with 0.1 μg LT(R192G), or PBS on study days 0, 14, 28, and 84. Skin reactogenicity to the vaccine was measured 1, 2, 3, 7, and 14 days after each dose at the site of injection by averaging the length and width of induration. Blood was collected from the femoral vein on study days 0, 21, 42, 63, 84, 98, 105, 112, and 119. Animals were orogastrically challenged on day 98 with CFA/I+ ETEC strain H10407 (41) as previously described (13), observed for 10 days for symptoms of illness, and then treated with enrofloxacin (5 mg/kg) administered intramuscularly once daily for 5 days. Bacterial count in the inoculum preparation was retrospectively quantified and equaled 4.95 × 1011 CFU.
Disease assessment.
Following challenge, monkeys were observed twice daily for 10 days for the development of diarrhea, and stool was graded on a scale from 1 to 5 as previously described (13). A diarrhea episode was defined as a period that begins with two or more consecutive days of grade 3 or higher stool consistency and ends the day prior to two or more consecutive days of grade 2 or lower stool consistency. Animals meeting the case definition of diarrhea prior to challenge were excluded from analysis. Percent of total loose stool days was calculated as the number of days a stool of grade 3 or higher was observed divided by the total number of observation days times 100. Fecal excretion of challenge bacteria was monitored daily for 10 days as previously described (13). A period of fecal shedding was defined as the isolation of ETEC strain H10407 from stool collected after challenge that begins (onset) as early as day 1 after challenge and ends (duration) on the last day up to day 10 that bacteria were shed in the stool.
All studies were reviewed and approved by the NMRC Institutional Animal Care and Use Committee and performed in compliance with all applicable federal regulations governing the protection of animals in research.
Quantification of antigen-specific IgG and IgA antibodies.
Serum and fecal samples were tested by ELISA for the presence of antibodies against immunizing antigens. Serum anti-CfaE IgG and IgA and murine fecal anti-CfaE IgA were performed with plates coated with dsc19CfaE at 2 μg/ml. For anti-LTB assays, plates were initially coated with GM1 (Sigma-Aldrich) diluted to 0.5 μg/ml in PBS, blocked with 5% nonfat milk (Sigma-Aldrich) in 0.05% Tween 20 (Sigma-Aldrich) in PBS, and coated with LTB at 0.5 μg/ml. After a blocking step, samples were serially diluted 3-fold beginning with dilutions of 1:50 (murine and guinea pig serum samples), 1:15 (murine fecal samples), and 1:5 (A. nancymaae serum samples). Antigen-specific murine IgG and IgA antibodies were detected with goat anti-mouse IgG horseradish peroxidase (HRP) (Jackson ImmunoResearch Laboratories, West Grove, PA) and goat anti-mouse IgA HRP (KPL, Milford, MA), respectively, and diluted 1:1,000 (serum) or 1:2,000 (fecal). Binding of IgG antibodies in guinea pig assays was detected with goat anti-guinea pig IgG-HRP conjugate (KPL) diluted 1:1,000. Quantification of IgG and IgA antibodies in A. nancymaae assays was performed with rabbit anti-A. nancymaae IgG-HRP conjugate (lot number 043063739; Lampire Biological Laboratories, Pipersville, PA) and rabbit anti-A. nancymaae IgA-HRP conjugate (lot number 043063740; Lampire Biological Laboratories), respectively, both diluted 1:4,000. Plates were developed with orthophenylenediamine (Sigma-Aldrich), 1-Step Ultra TMB (Thermo Fisher Scientific, Waltham, MA), or 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase substrate (KPL) and absorbance measured at optical density at 450 nm. Endpoint titers were assigned as the interpolated dilutions of the samples, giving an absorbance value of 0.4 OD units above background, and the antibody titer ascribed to each sample represented the geometric mean of duplicate determinations. Samples with undetectable titers, i.e., reciprocal endpoint titer <50 (mouse and guinea pig sera), <15 (mouse fecal extracts), and <5 (A. nancymaae serum), were assigned values of 25, 7.5, and 2.5, respectively, for computational purposes.
Hemagglutination inhibition assay.
Serum samples were tested in 12-well ceramic plates (Thermo Fisher; A. nancymaae assays) or 96-well plates (Falcon Microtest U-bottom tissue culture-treated plates; BD, Franklin Lakes, NJ; mouse and guinea pig assays) as follows. Each day, the minimum hemagglutination titer (MHT) was determined, defined as the reciprocal of the highest dilution of bacteria that agglutinated bovine erythrocytes (Lampire Biological Laboratories). CFA/I+ ETEC strain H10407 was grown overnight (O/N) on CFA agar plates, harvested, and resuspended in 0.5% d-mannose (Sigma-Aldrich) in PBS (PBS-M) to a final solution of OD650 0.4 ± 0.02 or 0.2 ± 0.02 for 12- or 96-well plates, respectively. Twenty-five microliters of this suspension were added to the wells followed by 2-fold serial dilution with PBS-M. Twenty-five microliters of 3% (12-well plates) or 1.5% (96-well plates) erythrocytes in PBS-M were added to each dilution, followed by 25 μl of PBS-M for a total of 75 μl per well. Twelve-well plates were agitated on ice for 20 min at 100 rpm, and 96-well plates were agitated at 500 rpm for 30 min at 4°C. The highest dilution of bacteria to give visible agglutination was taken as the MHT for the day. The HAI assay was performed with a bacterial suspension one (12-well plates) or two dilutions (96-well plates) more concentrated than the MHT. For HAI assays, 25 μl of 2-fold serial dilutions of serum in PBS-M, beginning with 1:16 or 1:8 for 12- or 96-well plates, respectively, was added to wells with an equal volume of bacterial suspension, forming final serum dilutions beginning with 1:32 or 1:16 for 12- or 96-well plates, respectively. Plates were agitated for 30 min at room temperature (RT), and then 25 μl of 3% (12-well plates) or 1.5% (96-well plates) erythrocytes was added to each well. Ceramic plates were agitated on ice for 20 min, and 96-well plates were agitated at 500 rpm for 30 min at 4°C. The presence or absence of agglutination was recorded immediately after incubation. Each sample was tested in duplicate, and the HAI titer was defined as the average of the reciprocal of the highest serum dilution that completely inhibited agglutination. Serum samples that did not agglutinate at the lowest dilutions tested were assigned reciprocal titers of 16 and 8 for the 12-well and 96-well plate assays, respectively, for computational purposes.
Statistical analyses.
ELISA and HAI titers were log10 transformed for statistical analysis. Serum and fecal titers in mice, 2 weeks after the third dose (days 42 and 33, respectively) and in monkeys, 2 weeks after the third dose (day 42) and 2 weeks after the fourth dose (day 98), were compared between groups using a one-way analysis of variance (ANOVA), and a Tukey’s post hoc test was used for pairwise comparisons. Intradermal and i.n. data in the mouse studies were analyzed separately. ELISA and HAI data from the guinea pig study were analyzed using separate ANOVAs and Tukey’s post hoc tests for each time point. For the A. nancymaae study, the proportion of animals experiencing diarrhea in both test groups was compared to a PBS control group with a Fisher’s exact test. The proportion of days with loose stool was compared between groups using a Kruskal-Wallis test followed by Dunn’s multiple-comparison tests. Frequency analyses were not adjusted for multiple comparisons. All tests were interpreted in a two-tailed fashion, and P values of <0.05 were considered significant. GraphPad Prism version 6.07 for Windows (GraphPad Software, San Diego, CA) was used for all statistical analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Matthew Reader, William Hulsey, Glomil Corbin, Diana Zhang, Gladys Nuñez, and Nereyda Espinosa for technical assistance.
This research was supported by the National Institutes of Health grant U01 AI070638 (to S.J.S.), the U.S. Army Military Infectious Diseases Research Program Work Unit Number A0307 (to S.J.S.), the Henry M. Jackson Foundation for the Advancement of Military Medicine (to S.J.S.), and the National Institutes of Health grant R01 AI031940 (to R.K.H.). Funding for the GLP guinea pig experiment performed at Avanza Laboratories (Gaithersburg, MD, USA) was provided by a Defense Health Program grant to M.M., Jr., and S.J.S. (JON BB607).
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government.
C.M.W., S.A.S., R.C.M., M.J.G., M.G.P., and S.J.S. served as military service members over the course of this work. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that Copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. government as part of that person's official duties.
S.J.S., R.K.H., and M.G.J. have received income from royalties on licensure of a U.S. patent (US 9,107,866 B2) for an adhesin-enterotoxin chimera based immunogenic composition against enterotoxigenic Escherichia coli. All other authors declare that there are no financial, institutional, or other relationships that might lead to bias or a conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hosangadi D, Smith PG, Giersing BK. 2019. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine 37:7372–7380. doi: 10.1016/j.vaccine.2017.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Riddle MS, Sanders JW, Putnam SD, Tribble DR. 2006. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg 74:891–900. doi: 10.4269/ajtmh.2006.74.891. [DOI] [PubMed] [Google Scholar]
- 4.Troeger C, Colombara DV, Rao PC, Khalil IA, Brown A, Brewer TG, Guerrant RL, Houpt ER, Kotloff KL, Misra K, Petri WA Jr, Platts-Mills J, Riddle MS, Swartz SJ, Forouzanfar MH, Reiner RC Jr, Hay SI, Mokdad AH. 2018. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health 6:e255–e269. doi: 10.1016/S2214-109X(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarino SJ, McKenzie R, Tribble DR, Porter CK, O'Dowd A, Cantrell JA, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, Grahek SL, Brinkley C, Crabb JH, Bourgeois AL. 2017. Prophylactic efficacy of hyperimmune bovine colostral anti-adhesin antibodies against enterotoxigenic Escherichia coli diarrhea: a randomized, double-blind, placebo-controlled, phase 1 trial. J Infect Dis 216:7–13. doi: 10.1093/infdis/jix144. [DOI] [PubMed] [Google Scholar]
- 6.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. IAI 75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah ED, Riddle MS, Chang C, Pimentel M. 2012. Estimating the contribution of acute gastroenteritis to the overall prevalence of irritable bowel syndrome. J Neurogastroenterol Motil 18:200–204. doi: 10.5056/jnm.2012.18.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anantha RP, McVeigh AL, Lee LH, Agnew MK, Cassels FJ, Scott DA, Whittam TS, Savarino SJ. 2004. Evolutionary and functional relationships of colonization factor antigen I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect Immun 72:7190–7201. doi: 10.1128/IAI.72.12.7190-7201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 10.Poole ST, McVeigh AL, Anantha RP, Lee LH, Akay YM, Pontzer EA, Scott DA, Bullitt E, Savarino SJ. 2007. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63:1372–1384. doi: 10.1111/j.1365-2958.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- 11.Rollenhagen JE, Woods CM, O'Dowd A, Poole ST, Tian JH, Guebre-Xabier M, Ellingsworth L, Prouty MG, Glenn G, Savarino SJ. 2019. Evaluation of transcutaneous immunization as a delivery route for an enterotoxigenic E. coli adhesin-based vaccine with CfaE, the colonization factor antigen 1 (CFA/I) tip adhesin. Vaccine 37:6134–6138. doi: 10.1016/j.vaccine.2019.08.057. [DOI] [PubMed] [Google Scholar]
- 12.Sincock SA, Hall ER, Woods CM, O'Dowd A, Poole ST, McVeigh AL, Nunez G, Espinoza N, Miller M, Savarino SJ. 2016. Immunogenicity of a prototype enterotoxigenic Escherichia coli adhesin vaccine in mice and nonhuman primates. Vaccine 34:284–291. doi: 10.1016/j.vaccine.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Rollenhagen JE, Jones F, Hall E, Maves R, Nunez G, Espinoza N, O'Dowd A, Prouty MG, Savarino SJ. 2018. Establishment, validation and application of a New World Primate model of ETEC disease for vaccine development. Infect Immun 87. doi: 10.1128/IAI.00634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luiz WB, Rodrigues JF, Crabb JH, Savarino SJ, Ferreira LC. 2015. Maternal vaccination with a fimbrial tip adhesin and passive protection of neonatal mice against lethal human enterotoxigenic Escherichia coli challenge. Infect Immun 83:4555–4564. doi: 10.1128/IAI.00858-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price GA, Holmes RK. 2014. Immunizing adult female mice with a TcpA-A2-CTB chimera provides a high level of protection for their pups in the infant mouse model of cholera. PLoS Negl Trop Dis 8:e3356. doi: 10.1371/journal.pntd.0003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price GA, Holmes RK. 2012. Evaluation of TcpF-A2-CTB chimera and evidence of additive protective efficacy of immunizing with TcpF and CTB in the suckling mouse model of cholera. PLoS One 7:e42434. doi: 10.1371/journal.pone.0042434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobling MG, Poole ST, Rasulova-Lewis F, O'Dowd A, McVeigh AL, Balakrishnan A, Sincock SA, Prouty MG, Holmes RK, Savarino SJ. 2020. Biochemical and immunological characterization of an ETEC CFA/I adhesin cholera toxin B subunit chimera. PLoS One 15:e0230138. doi: 10.1371/journal.pone.0230138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maciel M Jr, Smith M, Poole ST, Laird RM, Rollenhagen JE, Kaminski RW, Wenzel H, Bourgeois AL, Savarino SJ. 2019. Evaluation of the reactogenicity, adjuvanticity and antigenicity of LT(R192G) and LT(R192G/L211A) by intradermal immunization in mice. PLoS One 14:e0224073. doi: 10.1371/journal.pone.0224073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. 2009. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med 350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 21.Combadiere B, Liard C. 2011. Transcutaneous and intradermal vaccination. Hum Vaccin 7:811–827. doi: 10.4161/hv.7.8.16274. [DOI] [PubMed] [Google Scholar]
- 22.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. 2004. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest 113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belyakov IM, Ahlers JD. 2009. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 24.Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, Huda N, Khan MU, Kay BA, Khan MR. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis 158:372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- 25.Glenn GM, Francis DH, Danielsen EM. 2009. Toxin-mediated effects on the innate mucosal defenses: implications for enteric vaccines. Infect Immun 77:5206–5215. doi: 10.1128/IAI.00712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrens RH, Cramer JP, Jelinek T, Shaw H, von Sonnenburg F, Wilbraham D, Weinke T, Bell DJ, Asturias E, Pauwells HL, Maxwell R, Paredes-Paredes M, Glenn GM, Dewasthaly S, Stablein DM, Jiang ZD, Dupont HL. 2014. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers' diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis 14:197–204. doi: 10.1016/S1473-3099(13)70297-4. [DOI] [PubMed] [Google Scholar]
- 27.Mansour A, Shaheen HI, Amine M, Hassan K, Sanders JW, Riddle MS, Armstrong AW, Svennerholm AM, Sebeny PJ, Klena JD, Young SY, Frenck RW. 2014. Diarrhea burden due to natural infection with enterotoxigenic Escherichia coli in a birth cohort in a rural Egyptian community. J Clin Microbiol 52:2595–2603. doi: 10.1128/JCM.00215-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riddle MS, Savarino SJ. 2014. Moving beyond a heat-labile enterotoxin-based vaccine against enterotoxigenic Escherichia coli. Lancet Infect Dis 14:174–175. doi: 10.1016/S1473-3099(13)70355-4. [DOI] [PubMed] [Google Scholar]
- 29.Veeraraghavan N. 1959. Improvement of the antigenicity of antirabies vaccine by pooling checked by post-challenge vaccination of guinea-pigs. Bull World Health Organ 20:121–131. [PMC free article] [PubMed] [Google Scholar]
- 30.Anonymous. 1955. Experimental studies of vaccination, allergy, and immunity in tuberculosis. II. Effect of varying the dose of BCG. Bull World Health Organ 12:31–45. [PMC free article] [PubMed] [Google Scholar]
- 31.Schultheis K, Schaefer H, Yung BS, Oh J, Muthumani K, Humeau L, Broderick KE, Smith TR. 2017. Characterization of guinea pig T cell responses elicited after EP-assisted delivery of DNA vaccines to the skin. Vaccine 35:61–70. doi: 10.1016/j.vaccine.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements JD, Norton EB. 2018. The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere 3:e00215-18. doi: 10.1128/mSphere.00215-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang H, Poncet D, Seydoux E, Rintala ND, Maciel M Jr, Ruiz S, Orr MT. 2019. The TLR4 agonist adjuvant SLA-SE promotes functional mucosal antibodies against a parenterally delivered ETEC vaccine. NPJ Vaccines 4:19. doi: 10.1038/s41541-019-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maciel M Jr, Bauer D, Baudier RL, Bitoun J, Clements JD, Poole ST, Smith MA, Kaminski RW, Savarino SJ, Norton EB. 2019. Intradermal or sublingual delivery and heat-labile enterotoxin proteins shape immunologic responses to a CFA/I fimbria-derived subunit antigen vaccine against enterotoxigenic Escherichia coli. Infect Immun 87:e00460-19. doi: 10.1128/IAI.00460-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jobling MG, Holmes RK. 1992. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun 60:4915–4924. doi: 10.1128/IAI.60.11.4915-4924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaoka J, Imamura S. 1998. Analysis of mechanisms of epidermal proliferation induced by intracutaneous injection of cholera toxin by the use of site-specifically mutated cholera toxins. J Dermatol Sci 16:182–190. doi: 10.1016/s0923-1811(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 37.Jobling MG, Palmer LM, Erbe JL, Holmes RK. 1997. Construction and characterization of versatile cloning vectors for efficient delivery of native foreign proteins to the periplasm of Escherichia coli. Plasmid 38:158–173. doi: 10.1006/plas.1997.1309. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Perez J, Marquez G, Barbero JL, Gutierrez J. 1994. Increasing the efficiency of protein export in Escherichia coli. Biotechnology (N Y) 12:178–180. doi: 10.1038/nbt0294-178. [DOI] [PubMed] [Google Scholar]
- 39.Pridmore RD. 1987. New and versatile cloning vectors with kanamycin-resistance marker. Gene 56:309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- 40.Jobling MG, Holmes RK. 1993. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun 61:1168. doi: 10.1128/IAI.61.3.1168-.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans DG, Silver RP, Evans DJ Jr, Chase DG, Gorbach SL. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun 12:656–667. doi: 10.1128/IAI.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.