Enucleated cells or cytoplasts (cells whose nucleus is removed in vitro) represent an unexplored biological model for intracellular infection studies due to the abrupt interruption of nuclear processing and new RNA synthesis by the host cell in response to pathogen entry. Using enucleated fibroblasts hosting the protozoan parasite Leishmania amazonensis, we demonstrate that parasite multiplication and biogenesis of large parasitophorous vacuoles in which parasites multiply are independent of the host cell nucleus.
KEYWORDS: RNA sequencing, cytoplast, fibroblasts, leishmania
ABSTRACT
Enucleated cells or cytoplasts (cells whose nucleus is removed in vitro) represent an unexplored biological model for intracellular infection studies due to the abrupt interruption of nuclear processing and new RNA synthesis by the host cell in response to pathogen entry. Using enucleated fibroblasts hosting the protozoan parasite Leishmania amazonensis, we demonstrate that parasite multiplication and biogenesis of large parasitophorous vacuoles in which parasites multiply are independent of the host cell nucleus. Dual RNA sequencing of both host cytoplast and intracellular parasite transcripts identified host transcripts that are more preserved or degraded upon interaction with parasites and also parasite genes that are differentially expressed when hosted by nucleated or enucleated cells. Cytoplasts are suitable host cells, which persist in culture for more than 72 h and display functional enrichment of transcripts related to mitochondrial functions and mRNA translation. Crosstalk between nucleated host de novo gene expression in response to intracellular parasitism and the parasite gene expression to counteract or benefit from these host responses induces a parasite transcriptional profile favoring parasite multiplication and aerobic respiration, and a host-parasite transcriptional landscape enriched in host cell metabolic functions related to NAD, fatty acid, and glycolytic metabolism. Conversely, interruption of host nucleus-parasite cross talk by infection of enucleated cells generates a host-parasite transcriptional landscape in which cytoplast transcripts are enriched in phagolysosome-related pathway, prosurvival, and SerpinB-mediated immunomodulation. In addition, predictive in silico analyses indicated that parasite transcript products secreted within cytoplasts interact with host transcript products conserving the host V-ATPase proton translocation function and glutamine/proline metabolism. The collective evidence indicates parasite-mediated control of host cell transcripts half-life that is beneficial to parasite intracellular multiplication and escape from host immune responses. These findings will contribute to improved drug targeting and serve as database for L. amazonensis-host cell interactions.
INTRODUCTION
Mass enucleation was developed in the 1970s. The process involves the centrifugation of cell monolayers in the presence of cytochalasins that disorganize the actin cytoskeleton. This allows the extrusion of the nuclei, which are denser than the cytoplasm, covered by plasma membrane (1–4). The enucleated cells, called cytoplasts, preserve all the cytoplasmic organelles (5–7). After enucleation of cultured epithelial cells or fibroblasts, the ultrastructure, distribution of the major organelles, and several cellular functions are preserved (1, 6–16).
Considering the maintenance of several cellular functions despite the absence of the nucleus, cytoplasts are a promising model for studying the role of cell nucleus (and especially of the gene expression modulation) in infections by intracellular pathogens. The main challenge to the use of cytoplasts in these studies is to ensure their survival to guarantee the observation of the parasite intracellular phenotype. Decay of cytoplasts is probably associated with a halt in mRNA production, leading to progressive reduction of protein synthesis, although proteins for cells continue to be synthesized 10 h after enucleation (17–19).
The maintenance of cytoplasts for several hours in vitro allows intracellular infection assays in enucleated cells infected by pathogens sheltered in pathogen-containing vacuoles, such as Toxoplasma gondii (20, 21), Chlamydia trachomatis (22), and C. psittaci (23). These assays can also involve bacteria that have lodged and multiplied in the cytosol following their exit from the vacuoles, such as Rickettsia prowazekii (24) and S. flexneri (25), or that remain in the vacuoles, such as Mycobacterium smegmatis (26).
Shigella flexneri and Mycobacterium species bacteria and Toxoplasma gondii and Trypanosoma cruzi protozoa survive inside cytoplasts by using and subverting a limited and regressive number of enucleated host factors (25–27). In studies using cytoplasts obtained by mass enucleation of L cells, clone 929 (a mouse tumor fibroblast line), or L929 fibroblasts, Trypanosoma cruzi trypomastigotes were observed within enucleated cells after 18 h of infection. In host L929 cytoplasts or L929 nucleated cells, the parasites multiplied and differentiated (27).
How do intracellular parasites establish and grow within cells without nuclei? The association between parasite and host is a system with emergent properties. Their relationship involves new phenotypic characteristics expressed by both only in the context of their interaction. The durable interaction between parasite and host depends on these emergent properties, which arise from the mutual influence or interference of both organisms on their genome expression (28). The removal of the host cell nucleus excludes from this complex system the modulation of host genes by intracellular parasites, allowing for identification of complete and robust interaction networks between host transcripts established prior to infection that are minimally required for parasite multiplication. The infected cytoplasts are therefore a valuable, but unexplored, model in the study of the host-parasite relationship, since cytoplasts are unable to synthesize new RNAs and respond to intracellular infection only at the posttranscriptional level.
RNA sequencing (RNA-seq) has been successfully used to study infections by different pathogens. This approach allows the construction of complex transcription analysis networks. Here, we used RNA-seq to investigate the host cytoplast and intracellular parasite transcriptome. The analysis, termed dual RNA-seq (29–31), took into account the interaction of the two organisms to identify the minimum transcripts of the host that are necessary for the multiplication of the parasite and to clarify differences in parasite gene expression due to interactions in cytoplasts. The intracellular pathogen model, Leishmania (Leishmania) amazonensis, is the causative agent of localized and diffuse cutaneous leishmaniasis. The latter is the chronic form of the disease and does not respond to the conventional therapy (32, 33). L. amazonensis is an intracellular parasite adapted to a durable relationship with host cells, including fibroblasts (34, 35). With this model, we investigated the hypothesis that the parasite affects the half-life of specific mRNA that is beneficial for parasitism. Enucleated cells infected by L. amazonensis provide a suitable model in which a restricted and self-limiting number of transcripts and host proteins are sufficient for intracellular parasitism. Transcriptomics was used to map and identify the transcripts present in the cytoplasts that were maintained during L. amazonensis intracellular infection (see workflow in Fig. S1 in the supplemental material).
RESULTS
Mass-enucleated fibroblasts (cytoplasts) survive in culture for more than 48 h and preserve mitochondrion structure and mitochondrial and ribosomal transcripts.
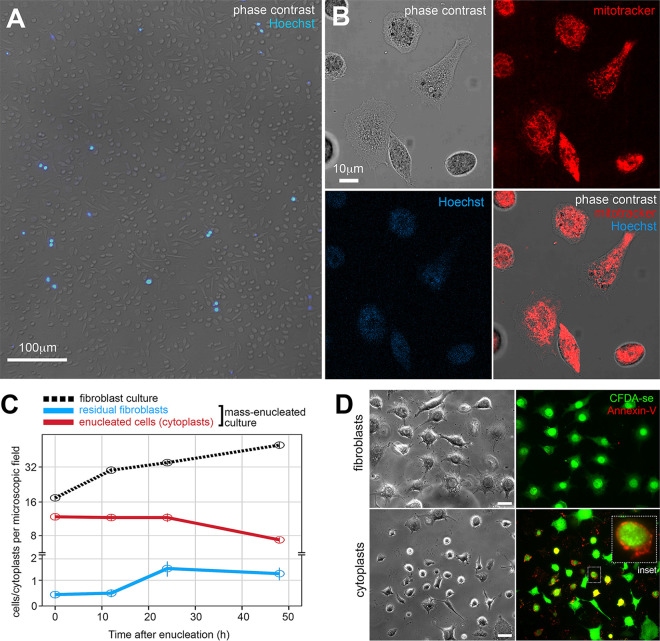
To optimize the generation of enucleated cells in bulk and to increase the yield of extracted RNA, we performed the enucleation of cells adherent to glass slides (2, 25, 27). We obtained samples with a satisfactory level of cytoplast purity corresponding to approximately 90% of the cytoplasts generated after mass enucleation (Fig. 1A to C), which conserved vital organelles, including mitochondria (Fig. 1B). The residual nucleated fibroblasts in the enucleated cell cultures displayed limited multiplication 48 h after enucleation (Fig. 1C).
FIG 1.
Cytoplasts generated from L929 fibroblasts by enucleation. (A) Epifluorescence microscopy of viable cells from enucleation cell culture. Enucleated cells (cytoplasts) and nucleated cells (postenucleation residual fibroblasts) were distinguished using the Hoechst 33342 nuclear fluorescent probe. Scale bar, 100 μm. (B) Confocal microscopy of cytoplasts stained for mitochondria using the MitoTracker fluorescent probe. MitoTracker staining is shown in red, and Hoechst 33342 staining is shown in blue (absent in the cytoplasts). Scale bar, 10 μm. (C) Number of cells and cytoplasts counted per microscopic field throughout 48 h of in vitro cultivation after enucleation. Multiplication of fibroblasts in nonenucleated cultures is indicated by the dotted black line and residual fibroblasts by the blue line. The stability in enucleated cultures is shown. The example shown is representative from three independent experiments. (D) Epifluorescence microscopy of cultures of live fibroblasts (upper panel) and cytoplasts (bottom panel) 96 h postenucleation, following staining with the cell viability dye carboxyfluorescein diacetate succinimidyl ester (green) and annexin V (red) to identify apoptotic cells (CFDA-se+Annexin V+). The inset shows an apoptotic, enucleated cell. Scale bar, 20 μm.
All the cytoplasm organelles are preserved in cytoplasts (5–7). Only long-term cultivation of cytoplasts (from 96 h postenucleation) leads to programmed cell death, not necrosis, in a fraction of enucleated cells (Fig. 1D). These results allowed us to establish 48 h postinfection as the time for host-parasite transcriptomic profiling in nucleated and enucleated cells.
Transcriptomics was done by selecting cytoplast preparations in which more than 90% of the cells were enucleated at 48 h postenucleation, as confirmed by evaluation of quality indexes evaluation of extracted RNA (RIN, A260/A280 ratio; Table 1). Since RNA degradation is inherent to cell enucleation, RNA integrity is close to the minimum RIN that is usually required for sequencing. The RNA content of cytoplasts comprised only 0.36% (0.102 pg/cytoplast) of the total RNA of noninfected fibroblasts and 0.5% (0.173 pg/cytoplast) of the total RNA of Leishmania-infected fibroblasts. We therefore opted for a coverage of over 100 million reads for uninfected cytoplasts and 200 million reads for those infected to accurately detect host versus parasite transcripts among transcripts in the degradation process.
TABLE 1.
Purity, integrity, and number of sequencing reads for RNA obtained from nucleated cells and cytoplasts harboring or not L. amazonensis after 48 h of in vitro culturea
| Sample | Organism(s) | A260/A280 ratio | RIN | No. of reads (in millions) | Notes |
|---|---|---|---|---|---|
| L929 fibroblasts | M. musculus | 2.21 | 10 | 101 | |
| M. musculus | 2.10 | 9.1 | 129 | ||
| M. musculus | 2.09 | 9.7 | 194 | ||
| L929 fibroblasts hosting L. amazonensis | M. musculus, L. amazonensis | 2.12 | 9.4 | 275 | Around 40% cells hosting parasites |
| M. musculus, L. amazonensis | 2.09 | 9.3 | 292 | Around 40% cells hosting parasites | |
| M. musculus, L. amazonensis | 2.12 | 9.2 | 305 | Around 40% cells hosting parasites | |
| L929 cytoplasts | M. musculus | 2.05 | 7.8 | 174 | >90% of enucleated cells |
| M. musculus | 2.39 | 7.8 | 182 | >90% of enucleated cells | |
| L929 cytoplasts hosting L. amazonensis | M. musculus, L. amazonensis | 2.72 | 7.6 | 317 | >90% of enucleated cells, 50% cytoplasts hosting parasites |
| M. musculus, L. amazonensis | 2.19 | 7.9 | 232 | Around 90% of enucleated cells, 70% cytoplasts hosting parasites | |
| M. musculus, L. amazonensis | 2.48 | 7.6 | 212 | Around 90% of enucleated cells, 70% cytoplasts hosting parasites |
Evaluations were performed on an Agilent Bioanalyzer 2100 instrument. Notes indicate the efficiency of enucleation (the percentage of cytoplasts among the population of enucleated fibroblast cultures) and the percentage of infected cells/cytoplasts.
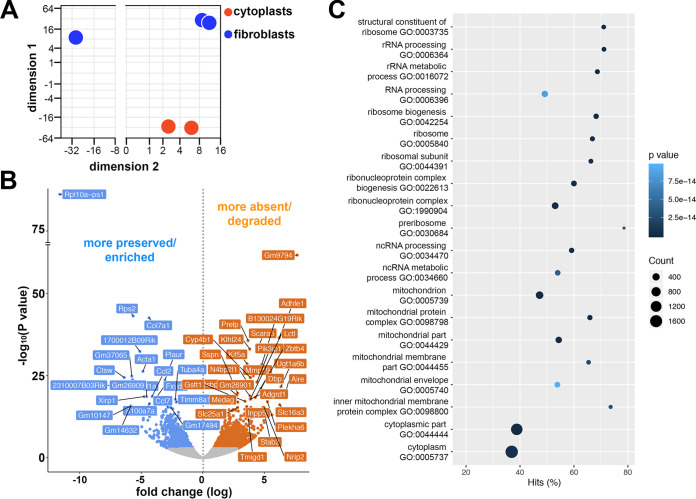
The analysis of transcripts that were differentially present in cytoplasts compared to their nucleated counterpart revealed significant differences in the transcript profiles of these populations after 48 h culture (Fig. 2A; see Table S1 in the supplemental material). The comparison between the transcripts of the cells with or without nuclei demonstrated that 2,556 transcripts were more preserved/enriched (blue) and 3,436 transcripts were less prevalent or more degraded (orange) in the cytoplasts compared to the nucleated cells (adjusted P < 0.1; Fig. 2B). Transcripts enriched in cytoplasts were subjected to gene ontology (GO) analysis of functional clusters to demonstrate functions that were preferentially preserved after enucleation (Fig. 2C). As expected, due to the absence of nuclei, cytoplasts preferentially preserved transcripts related to extranuclear cellular components, such as mitochondria and ribosomes, which were capable of maintaining the survival of these cells. The enrichment of mitochondrial transcripts indicates the potential energy source of cytoplasts, which would consequently also support the intracellular parasitism of L. amazonensis. Transcripts of translation and RNA processing machineries were present and enriched in cytoplasts, allowing for the synthesis of proteins in the absence of the cell nucleus.
FIG 2.
Transcriptomic profile and functional enrichment analysis comparison of enucleated cells (cytoplasts) and nucleated cells (fibroblasts). (A) Principal component analysis (PCA) of M. musculus transcripts in noninfected fibroblasts (blue) and noninfected cytoplasts (red). Each point represents a biological replicate, i.e., independent experiments. (B) Volcano plots in a pairwise format showing the –log10 (P value) and fold change (log2) of transcripts detected in a pairwise comparison between fibroblasts and cytoplasts. Transcripts with a negative fold change signal (in blue, adjusted P < 0.1) correspond to transcripts that are more preserved/enriched in cytoplasts or less prevalent in fibroblasts. Transcripts with a positive fold change signal (in orange, adjusted P < 0.1) correspond to transcripts that are more frequently detected in fibroblasts or are more absent/degraded in cytoplasts. Transcripts with lowest adjusted P values are identified by their gene names. (C) Top-ranking biological pathways by GO enrichment. GO functional enrichment analysis performed by GOseq showing the top GO terms enriched in the list of transcripts that are more preserved/enriched in cytoplasts, and the percentage of differentially detected transcripts fitting each category (Hits%). Coordinates are represented by dots whose size and color indicate the number of differentially detected transcripts fitting the GO category, and whose P value is determined by a colorimetric scale of P values (darker for higher and lighter for lower P values).
Cytoplasts support L. amazonensis intracellular multiplication in large parasitophorous vacuoles.
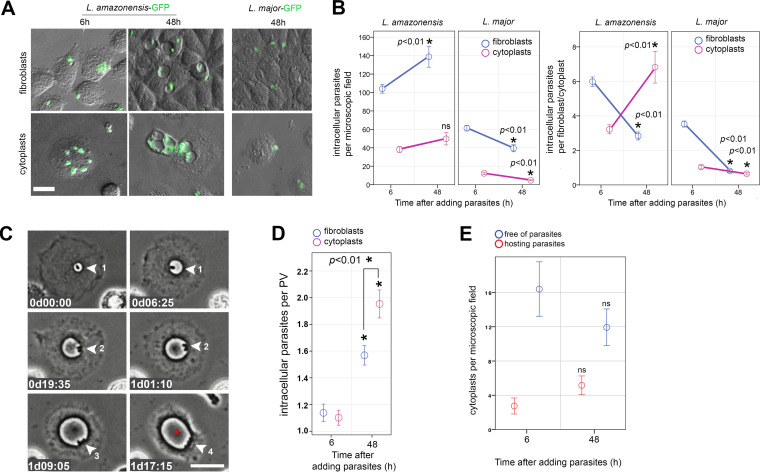
Intracellular infection of cytoplasts appeared to be Leishmania-specific since cytoplasts supported L. amazonensis parasite multiplication for up to 48 h at 34°C. In contrast, parasitism of Leishmania major was not sustained in cytoplasts (Fig. 3A and B). We observed an increase in L. amazonensis per cytoplasts compared to the lack of L. major multiplication in the enucleated cells. The decrease in parasites/fibroblasts in L. amazonensis group (Fig. 3B, right graph) is not due to L. amazonensis inability to multiply in fibroblasts but due to multiplication of fibroblasts themselves, which split intracellular parasites among daughter cells interfering in the comparative analysis with nondividing cytoplasts. To better assess parasite multiplication, we count parasites per parasitophorous vacuoles (PVs), structures that stably harbor Leishmania parasites throughout their intracellular life cycle and which do not split between mother-daughter cells during cell division (36). Cytoplasts supported the biogenesis of large PVs characteristic of macrophage infection by this parasite species (35, 36), in which parasites efficiently multiply up to two parasite-replication cycles (Fig. 3C and D; see also Movie S1 in the supplemental material). The number of parasites per PV increases not only in cytoplasts but also in fibroblasts (Fig. 3D), indicating that parasites multiply within PVs formed in both cytoplasts and fibroblasts, with a slightly but statistically significant increase in the enucleated host cell. This measure excludes the influence of cell multiplication from the quantitative analysis. The number of noninfected or L. amazonensis-infected cytoplasts remained the same throughout in vitro infection assays, indicating their viability in culture for up to 48 h postinfection and also discarding the influence of cytoplast decay in our quantifications (Fig. 3E).
FIG 3.
Cytoplasts support L. amazonensis amastigote multiplication in enlarging parasitophorous vacuoles. (A) Fibroblast (upper) and cytoplast (bottom) cultures after interaction with green fluorescent protein-expressing L. amazonensis or L. major for 6 and 48 h postinteraction. The phase-contrast images are merged with the green fluorescent signal of the parasites. In contrast to L. major, L. amazonensis amastigotes are internalized by cytoplasts, within which they develop large parasitophorous vacuoles (PVs) that are visible by phase-contrast imaging. Scale bar, 10 μm. (B) Quantification of the number of internalized parasites per microscopic field (left graph) or parasites per fibroblast/cytoplast (right graph) after 6 or 48 h of host cell-parasite interaction using cytoplasts or fibroblasts and L. amazonensis or L. major lesion-derived amastigotes. A Student t test was used to compare 6- and 48-h time points under each condition. The asterisk indicates P < 0.01 (n = 3 independent experiments). (C) Live imaging of infected cytoplasts showing the multiplication of one intracellular parasite (amastigote form, round, no apparent flagellum) into four parasites (arrowheads and numbers representing parasites) in an enlarging PV (red asterisk). Image acquisition began 2 h after cytoplast-parasite interaction and is displayed as “day-hours:minutes” (dhh:mm). Scale bar, 20 μm. (D) Quantification of the number of internalized parasites per PV in fibroblasts (blue) or cytoplasts (magenta) showing mean multiplication of parasites from approximately one to two amastigotes per PV. A Student t test was used to compare 6- and 48-h time points or comparing parasite number per PV in fibroblasts and cytoplasts at 48 h postinfection. The asterisk indicates P < 0.01 (n = 3 independent experiments). (E) Number of cytoplasts hosting (red) or not hosting (blue) parasites counted per microscopic field in the same infected cytoplasts preparation at 6 and 48 h after cytoplast-parasite interaction. A Student t test was used to compare 6- and 48-h time points under each condition. ns, nonsignificant (P > 0.05; n = 3 independent experiments).
L. major parasites are also sheltered in PVs, although these PVs fission as parasite divides, hindering the use of parasite/PV quantifications to assess L. major multiplication. Nevertheless, L. major parasites are less internalized by fibroblasts and cytoplasts and are unable to multiply in both models (Fig. 3A and B).
Therefore, in the L. amazonensis model infection, the translation of messengers prior to enucleation is sufficient for the multiplication of the parasites in cytoplasts and also for the biogenesis of their parasitophorous vacuoles.
Intracellular infection extends the half-life of host cytoplast transcripts involved in phagolysosome biogenesis and proteolysis inhibition.
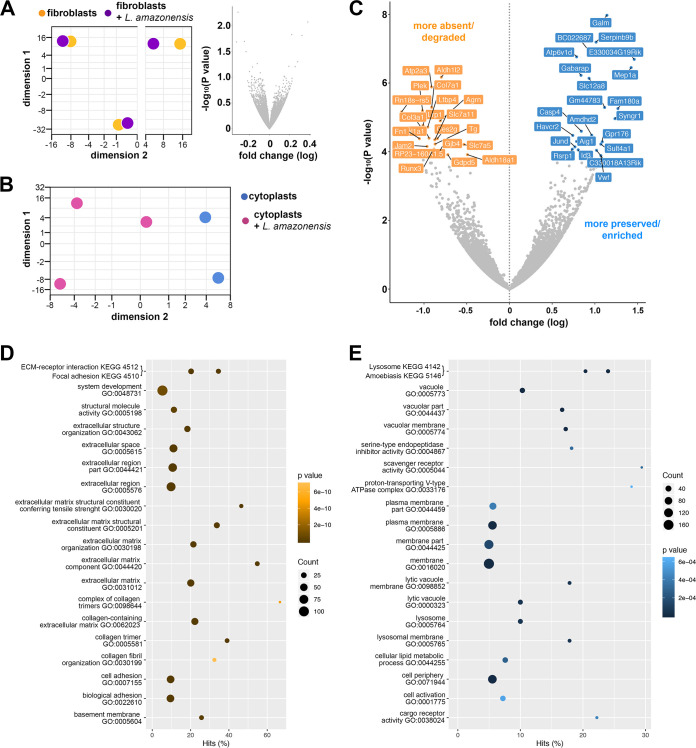
Examination of nucleated cells harboring or not harboring L. amazonensis could not determine with statistical accuracy which transcripts were more or less expressed in fibroblasts after 48 h of intracellular infection (adjusted P > 0.1; Fig. 4A; see also Table S2). The data suggested that Leishmania parasites circumvented the host fibroblast cell response, favoring the intracellular persistence and chronicity of infection of the parasites, as previously demonstrated (37). Only 40% of fibroblasts hosted parasites, which could have also influenced the statistical power concerning the detection of differentially expressed genes.
FIG 4.
Transcriptomic profile and functional enrichment comparison of infected cytoplasts and noninfected cytoplasts. (A) PCA of M. musculus transcripts found in noninfected fibroblasts (yellow) and infected fibroblasts (fibroblasts plus L. amazonensis, purple; each point represents a biological replicate, i.e., independent experiments) and volcano plot in a pairwise format showing the –log10 (P value) and fold change (log2) of transcripts detected in a pairwise comparison between these two groups. No statistically significant difference in differential gene expression was detected. (B) PCA of M. musculus transcripts found in noninfected cytoplasts (blue) and infected cytoplasts (cytoplasts plus L. amazonensis, magenta). Each point represents a biological replicate, i.e., independent experiments. (C) Volcano plot in a pairwise format showing –log10 (P value) and fold change (log2) of transcripts detected in a pairwise comparison between infected cytoplasts and noninfected cytoplasts. Transcripts with negative fold change signal (in orange, adjusted P < 0.1) correspond to those that are absent/degraded more by intracellular infection or present more in noninfected cytoplasts. Transcripts with a positive fold change signal (in blue, adjusted P < 0.1) correspond to transcripts that are more preserved/enriched by intracellular infection. Transcripts with adjusted P < 0.1 are identified by their gene names. (D and E) Top-ranking biological pathways by GO enrichment. GO functional enrichment analysis performed using GOseq showing the top GO terms enriched in the list of cytoplasts transcripts that are more absent/degraded (D) or more preserved/enriched by intracellular infection (E). Graphs show the ranked top GO terms and the percentage of differentially detected transcripts that fit in each category (Hits%). Coordinates are represented by dots whose size and color indicate the number of differentially detected transcripts fitting the GO category and whose P value is determined by a colorimetric scale (darker for higher and lighter for lower P values).
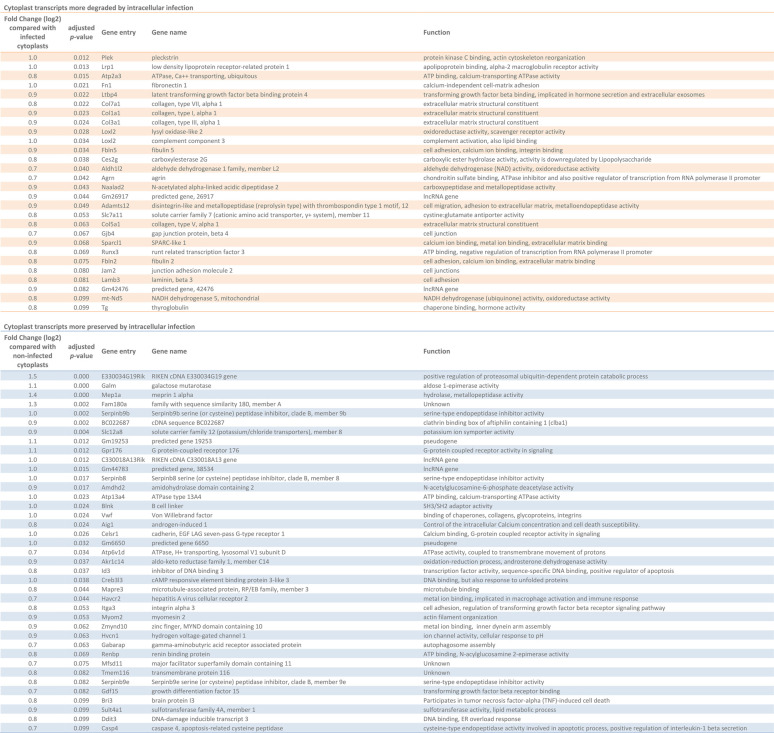
However, unlike nucleated cells, cytoplasts harboring parasites displayed a set of differentially preserved transcripts compared to noninfected cytoplasts (Fig. 4B and C, Table 2; see also Table S3). Thirty-nine of the transcripts were more preserved, and 28 were less represented transcripts (considered here as being more degraded) in parasite-hosting cytoplasts compared to uninfected cytoplasts (adjusted P < 0.1) (Table 2).
TABLE 2.
List of cytoplast transcripts that were more degraded or more preserved after 48 h of L. amazonensis infectiona
Cytoplast transcripts that were more degraded (in the upper, orange portion of the table) or more preserved (in the lower, blue portion of the table) are shown. The fold change difference compared to either infected or noninfected cytoplasts, the adjusted P value of differential gene expression analysis, the gene name, and the function as described in the databases are also presented.
The overall analysis of the biological processes regulated by the products of more preserved or more degraded host transcripts in infected cytoplasts was conducted for both transcript groups using GO analysis (Fig. 4D and E). Transcripts whose encoding molecules were involved in biological pathways, including extracellular matrix, cell adhesion, and epithelial growth, and concerning structural cellular functions of fibroblasts, were depleted in infected cytoplasts (Fig. 4D). Although not detected in GO analysis, there is also a decrease in transcripts whose products are involved in catalytic activity and cellular immune response against L. amazonensis, such as the complement system component C3 (38, 39) and Runx3 (Runt-related transcription factor 3), and transcription factor involved in regulation of type 1 T helper (Th1) cells response, such as the expression of gamma interferon (IFN-γ) (40) (Table 2, orange).
In contrast, the most preserved host transcripts in infected cytoplasts were related to the phagolysosome, as evidenced by the enriched functional clusters related to the lysosome and vacuolar membrane (Fig. 4E). The subunit D of vacuolar ATPases, which is responsible for vesicular acidification, is more preserved in infected cytoplasts (Atp6v1d) and can act together with subunit d isoform 2 (Atp6v0d2) to promote L. amazonensis PV biogenesis (36). Another important preserved transcript is that encoding the Gabarap protein, which is involved in selective autophagic processes that could culminate in PV development by membrane fusion between PVs, autophagosomes, and lysosomes (41, 42) (Table 2, blue).
Interestingly, among the most conserved host cell transcripts in L. amazonensis-hosting cytoplasts (Table 2, blue), we found three class B serine-cysteine protease inhibitors (SerpinB)—SerpinB8, SerpinB9b, and SerpinB9e—that were specifically enriched in infected cytoplasts and formed a functional cluster of serine-type endopeptidase inhibitor activity in the GO analysis (Fig. 4E). We validated this transcriptomic evidence by performing quantitative RT-qPCR for SerpinB8, SerpinB9, SerpinB9b, and SerpinB9e transcripts present in fibroblasts cytoplasts that contained parasites and parasite-free fibroblasts and cytoplasts (see Fig. S2). SerpinB9b and SerpinB9e transcripts were confirmed more abundant in parasite-hosting cytoplasts compared with noninfected cytoplasts after 48 h of intracellular infection (see Fig. S2). The preservation of SerpinB transcripts in infected enucleated cells suggested a mechanism mediated by the parasite to shut down important pathways in the host immune response by inducing the stability of host mRNAs involved in the anti-inflammatory environment.
The host cell nucleus stimulates transcription of a specific set of L. amazonensis amastigote genes.
In addition to potentially revealing the minimal components of the host cell that support parasite multiplication, the transcriptome of infected cytoplasts also included the L. amazonensis intracellular parasite transcripts expressed in enucleated cells compared with intracellular parasites hosted in nucleated cells (see Tables S4 to S6).
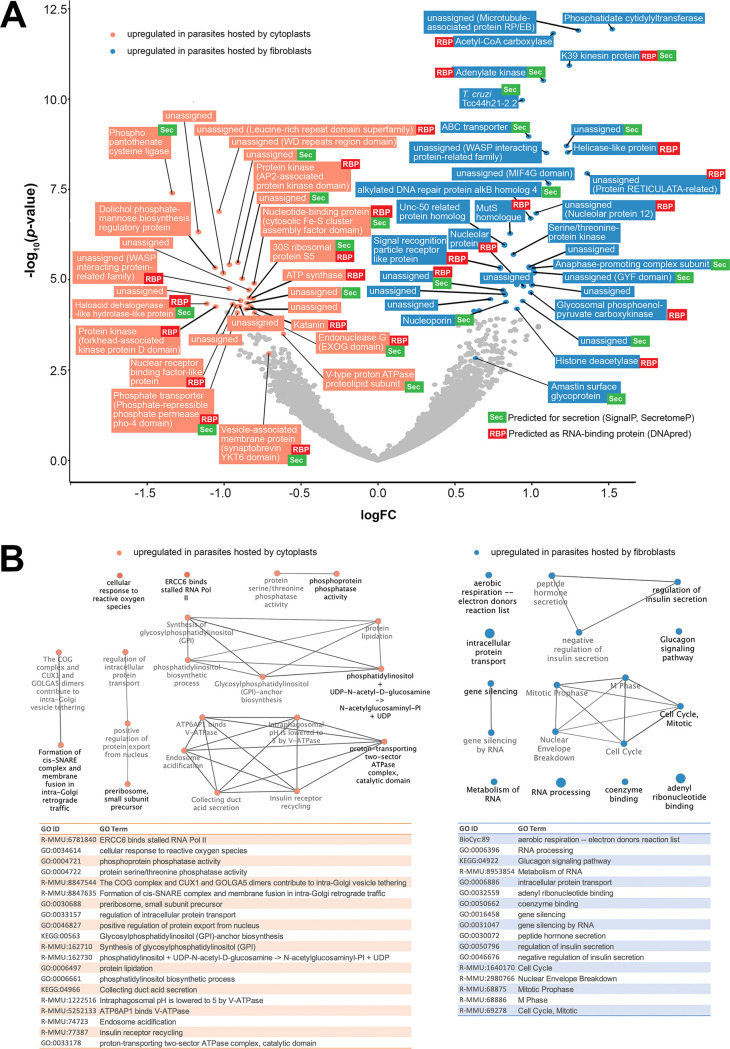
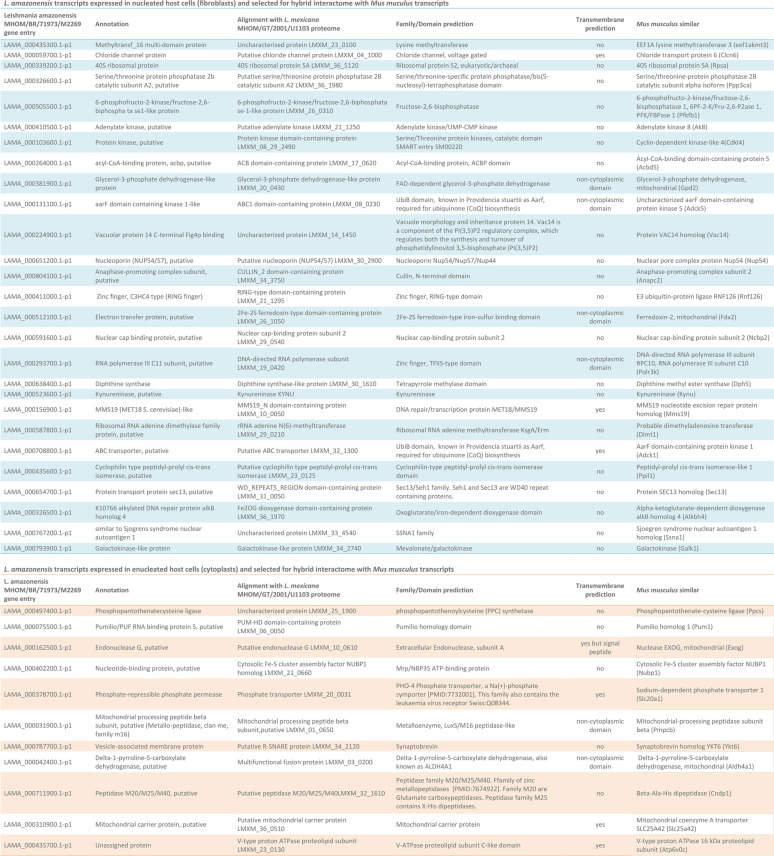
Intracellular parasites displayed upregulated expression of 135 genes when hosted by nucleated cells compared to their transcriptomic profile when hosted by cytoplasts. Among these upregulated genes, 60 (44%) encoded potential secreted proteins predicted for conventional, signal peptide-mediated or nonclassical, leaderless secretion pathways and 67 (49%) encoded RNA-binding proteins (RBP) (Table S5). Intracellular parasites upregulated the expression of 99 genes when hosted by cytoplasts compared to parasites hosted by nucleated cells, among them 46 messenger RNAs (46%) translated proteins predicted to be targeted to the secretory pathways and 47 (47%) translated potential RBPs (see Table S6). Around half of these differentially detected transcripts encoded unassigned proteins in databases (56 of 135 (41%) for parasites within fibroblasts and 53 of 99 (53%) for parasites within cytoplasts). Some of these unassigned proteins could be classified as belonging to protein families and superfamilies and were screened for conserved protein domains (see Tables S5 and S6; Fig. 5A).
FIG 5.
Transcriptomic profile and functional enrichment analysis of intracellular parasites hosted by cytoplasts and fibroblasts. (A) Volcano plot in a pairwise format showing –log10 (P value) and fold change (log2) of L. amazonensis transcripts detected in a pairwise comparison between parasites hosted by fibroblasts and cytoplasts. Transcripts with fold change negative signal correspond to parasite transcripts upregulated when parasites are hosted by cytoplasts (top lowest adjusted P value transcripts and other interesting transcripts with an adjusted P value of <0.1 identified in orange). Transcripts with positive fold change signal correspond to parasite transcripts upregulated when parasites are hosted by fibroblasts (top lowest adjusted P value transcripts and other interesting transcripts with adjusted P < 0.1 identified in blue). Parasite transcripts indicated by colors are also identified by gene annotations (orthology, protein family, and domain detection) and prediction for secretion (Sec, green) or RNA-binding properties (RBP, red). (B) Functional enrichment analysis performed by ClueGO and GeneMANIA software using parasite transcripts homologous to M. musculus and stratifying the analysis in parasite functional enrichment in cytoplasts (orange data) or parasite functional enrichment in fibroblasts (blue data). The upper panel shows the predicted connection between detected functional clusters formed with enriched GO terms that are described in the tables in the bottom panel.
Stratifying the analysis specifically to transcripts whose products were predicted to be involved in secretion, 43% of parasite factors potentially secreted inside cytoplasts were RBPs, compared to 35% of RBPs among parasite factors potentially secreted inside fibroblasts. Some of these RBPs were included in the top list of upregulated genes comparing cytoplasts and fibroblasts (Fig. 5A). In cytoplasts, for instance, intracellular parasites upregulated the expression of a secreted RBP with an endonuclease G (EXOG) domain involved in mitochondrial DNA repair (43) that could preserve host cytoplast mitochondrial DNA as well. These secreted transcripts included parasite-derived RBP that could be involved in preservation of host cell transcripts (44). The presence of a nucleus in the host cell and de novo synthesis of host transcripts in response to parasites specifically induced the expression of an amastin surface glycoprotein (LAMA_000534300). The role of this protein in the parasite-host relationship is poorly understood. It might participate in the intracellular establishment of parasites as a parasite counteraction to host cell responses (45).
From the list of parasite transcripts that were differentially expressed when parasites infected nucleated or enucleated cells, 70 of 135 (51%) differentially expressed genes (DEG) from parasites hosted by fibroblasts and 40 of 99 (40%) DEG from parasites hosted by cytoplasts were homologous to host Mus musculus genome (PSI-BLAST, E value cutoff = 10−5). This feature allowed for a GO analysis of the surrogate functional enrichment of parasite transcripts that were differentially expressed in nucleated versus enucleated hosts (Fig. 5B; see also Table S7). The data revealed that the absence of a host cell nucleus induced a transcriptional profile in infecting parasites that favored functions that included DNA repair, response to reactive oxygen species, endocytosis, and biosynthesis of glycosylphosphatidylinositol anchors (see Table S7; Fig. 5B, orange) compared to parasites in nucleated cells. The transcriptomic profile of the latter favored parasite multiplication, aerobic respiration, and control of parasite secretion (see Table S7; Fig. 5B, blue).
Hybrid interactome (Mus musculus-L. amazonensis protein-protein interactions) analyses between nucleated and enucleated fibroblasts in parasite infection.
The multiplication of L. amazonensis in cytoplasts suggested that intracellular parasite infection does not require the synthesis of new mRNAs by the host. Therefore, two facts could be deduced. First, the genes expressed by the host cell as a result of the recognition and internalization of L. amazonensis participate mainly in the cellular defense against the parasite and do not favor parasite’s intracellular maintenance. Second, L. amazonensis intracellular replication is supported by a native and restricted set of host cell genes, regardless of the genes expressed as a result of the recognition and internalization of these parasites.
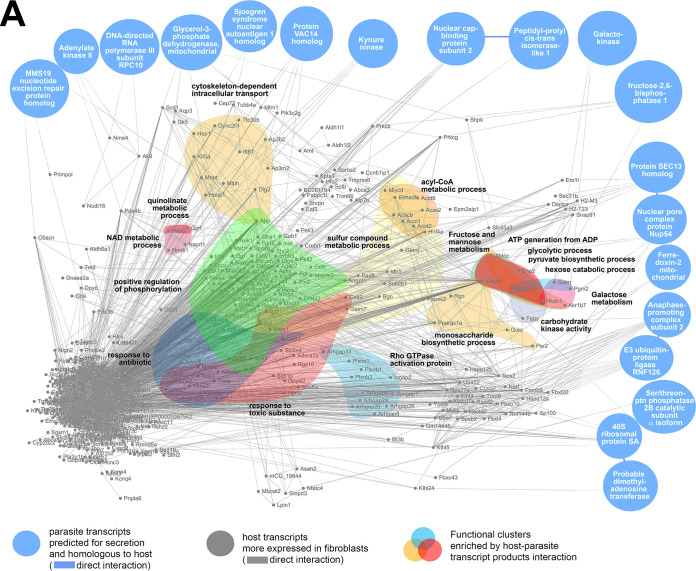
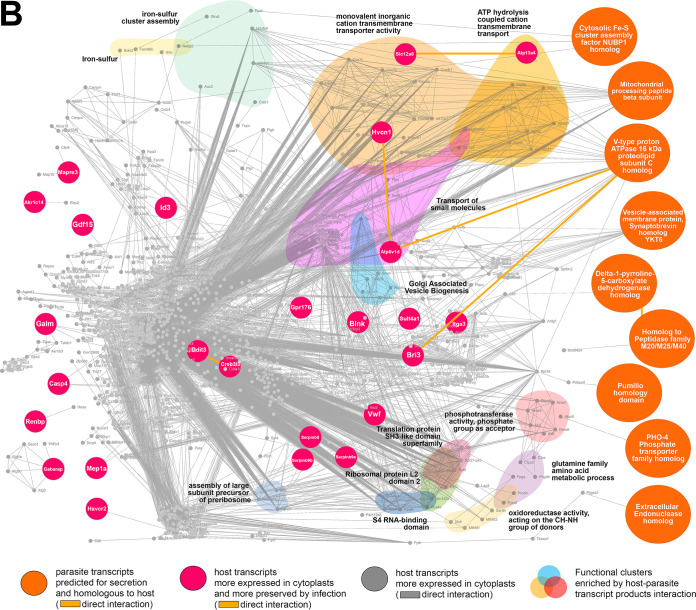
The biogenesis of PVs is an important indicator of the physiology of L. amazonensis amastigotes, which is possibly modulated by an emergent system composed of parasite and host cell factors (as a response to infection) that is not yet sufficiently clarified. To approach this complex system and evaluate the influence of host cell nucleus and de novo transcriptional response in host-parasite relationship, we constructed in silico networks of M. musculus-L. amazonensis protein-protein interactions (hybrid interactome) simulating the transcriptional landscape of infected nucleated cells (fibroblasts, Fig. 6A) and infected enucleated cells (cytoplasts, Fig. 6B) considering factors potentially secreted by the parasites.
FIG 6.
In silico hybrid M. musculus-L. amazonensis protein-protein interactomes constructed by host-parasite transcriptomic profiles of infected fibroblasts and cytoplasts. (A) Infected fibroblast hybrid interactome formed between the 1,950 most upregulated/preserved host transcripts detected in noninfected fibroblasts compared to noninfected cytoplasts (subset 1, gray nodes, protein-protein interactions indicated by gray lines) and the 27 most upregulated parasite transcripts expressed when hosted by fibroblasts compared to expression of parasites hosted by cytoplasts, whose products are at the same time predicted for secretion and homologous to M. musculus proteins (subset 2). Among these selected 27 parasite transcripts, 19 interacted directly or indirectly with host transcripts from subset 1 (blue nodes). A direct protein-protein interaction between parasite transcript products is indicated by blue lines. Protein-protein interactions between parasite and host transcript products are also indicated by gray lines. The generated hybrid network was submitted to functional enrichment analysis by STRING enrichment software allowing for localizing enriched functional clusters identified by colors and GO terms. (B) Infected cytoplast hybrid interactome formed between (i) the 1,950 most upregulated/preserved host transcripts detected in noninfected cytoplasts compared to noninfected fibroblasts (subset 3, gray nodes, protein-protein interactions indicated by gray lines); (ii) the 39 most preserved host transcripts in infected cytoplasts compared to noninfected cytoplasts (subset 4, magenta nodes); and (iii) the 11 most upregulated parasite transcripts expressed when hosted by cytoplasts compared to expression of parasites hosted by fibroblasts whose products are at the same time predicted for secretion and homologous to M. musculus proteins (subset 5). Among the 11 selected parasite transcripts, nine interacted directly or indirectly with host transcripts from subsets 3 and 4 (orange nodes). Protein-protein interactions between parasite and host transcript products are indicated by gray lines. A direct protein-protein interaction between parasite transcript products (orange nodes) and between these and parasite-mediated more preserved host transcript products (orange and magenta nodes interaction) is highlighted by the orange line. The generated hybrid network was submitted to functional enrichment analysis by STRING enrichment software allowing localization of enriched functional clusters identified by colors and GO terms.
For infected fibroblasts, the hybrid interactome comprised the two following data subsets. The first was the most upregulated/preserved host transcripts detected in noninfected fibroblasts compared to noninfected cytoplasts (subset 1, Table S1, fold change positive values, adjusted P < 0.1). The second was most upregulated parasite transcripts expressed in parasites hosted by fibroblasts compared to expression of parasites hosted by cytoplasts. This second subset included a subset of 27 transcripts whose products were simultaneously predicted for secretion and were homologous to M. musculus proteins (subset 2, Table 3, blue table). Among these 27 parasite transcripts, 19 translate products predicted to interact directly or indirectly with host transcript products from subset 1 (Fig. 6A, blue nodes). The networks generated using only subset 1 and the combined subsets 1 and 2 were compared in their functional enrichment analysis output. The interaction between the two subsets generated a network of protein interactions enriched in 72 GO terms that were not detected when network was built with subset 1 alone (see Table S8). These GO terms were detected only by the addition of parasite transcripts whose products were potentially secreted in the context of nucleated cell infection. Nonredundant GO terms that were significantly enriched and which could be translated as host cell functions following the interaction of parasites and nucleated host cells included NAD, quinolinate, sulfur compound and acyl coenzyme A metabolic processes (the latter were associated more with fatty acid oxidation as indicated by the nodes composing the cluster), response to antibiotics and toxic substances, positive regulation of phosphorylation, Rho GTPase activation protein, fructose/mannose and galactose metabolism, monosaccharide and pyruvate biosynthesis, carbohydrate kinase activity, and ATP generation from ADP as part of the functional clusters involving glycolytic and hexose catabolic processes (Fig. 6A, colored clusters).
TABLE 3.
List of L. amazonensis transcripts homologous to M. musculus and predicted for secretiona
The transcripts were used in the construction of hybrid interactomes simulating the host-parasite transcriptional landscape of infected fibroblasts (in the upper, blue portion of the table) or infected cytoplasts (in the lower, orange portion of the table). The table shows the gene entry according to the TriTryp database, the gene annotation, the aligned proteins on the L. mexicana proteome, the family/domain annotation, the transmembrane prediction, and the M. musculus homologous gene product.
For infected cytoplasts, the hybrid interactome comprised three data subsets. The first was the most upregulated/preserved host transcripts detected in noninfected cytoplasts compared to noninfected fibroblasts (subset 3, Table S1, fold change negative values, adjusted P < 0.1). The second was the most preserved host transcripts in infected cytoplasts compared to noninfected cytoplasts (subset 4, Table S3, fold change negative values, adjusted P < 0.1). The third was the most upregulated parasite transcripts expressed in parasites hosted by cytoplasts compared to expression of parasites hosted by fibroblasts. The latter included a subset of 11 transcripts that were simultaneously predicted for secretion and were homologous to M. musculus proteins (subset 5, Table 3, orange table). Among these selected 11 parasite transcripts, nine translate products predicted to interact directly or indirectly with host transcript products from subsets 3 and 4 according to the interactome predictions (Fig. 6B, orange nodes). Table S9 presents the 15 enriched GO terms detected specifically in the hybrid interactome that comprised subsets 3, 4, and 5 compared to interactomes generated from subset 3 alone or from subsets 3 and 4 combined. These GO terms could be translated in functional clusters arising from host-parasite protein-protein interaction in enucleated cells, highlighting the functional clusters of iron-sulfur, transfer of small molecules across membranes comprising monovalent inorganic cations and connected to ATP hydrolysis, Golgi associated vesicle biogenesis, translational regulation by proteins containing S4 RNA-binding, ribosomal protein L2 SH3 domains, phosphotransferase activity, glutamine family amino acid metabolic process, and oxidoreductase activity connected to proline metabolism (concluded from the protein nodes forming the cluster) (Fig. 6B, colored clusters).
DISCUSSION
The possibility of maintaining cytoplasts for several hours in vitro allowed intracellular infection assays to be performed in enucleated cells productively infected by different pathogens (20–27), including L. amazonensis in the present study. The use of cytoplasts as the host cell model is particularly useful in identifying which host cell transcripts are more or less preserved in response to the infection, thereby excluding de novo mRNA synthesis (since the host nucleus is removed prior to infection).
Macrophages are the main host cell for Leishmania parasites in the time course of leishmaniasis pathogenesis, a cell lineage extensively studied by us and others as host cell model for these parasites (35, 36, 46, 47). Unfortunately, enucleated macrophages do not survive enucleation long enough to support Leishmania replication, dying in culture in less than 12 h postenucleation (27). Interestingly, however, nonprofessional phagocytes, i.e., cells inefficient in performing phagocytosis, such as fibroblasts, also harbor Leishmania parasites both in vitro and in vivo (34, 47–51), indicating that these parasites are adapted to enter cells by processes other than classical phagocytosis. In fact, as recently demonstrated, L. amazonensis parasites are able to enter fibroblasts via host plasma membrane damage and lysosome-dependent host membrane repair in an actin cytoskeleton-independent way (34), thus bypassing classical phagocytosis which is an actin cytoskeleton-dependent process (52). Not all Leishmania species might be able to enter fibroblasts by such means as demonstrated by L. major assays (Fig. 3A).
The biogenesis of the large PVs in fibroblasts, where the parasites multiply, is the most remarkable similarity we have observed between macrophage and fibroblast intracellular infection (Fig. 3C). It is a definitive proof that fibroblasts fully support multiplication of the parasite species used in our study, making their cytoplasts a valid model for the investigation of host cell transcripts that are essential or minimally required for intracellular parasitism. Apart from their morphological similarity and their capacity to support parasite multiplication, PVs developed in cytoplasts and nucleated cells could differ in their membrane markers and in their content and pH, interfering in parasite gene expression and in the parasite-mediated preservation of host transcripts.
Hundreds of cellular genes are reprogrammed with time, being either induced or repressed, by the contact with microbial pathogens (53–60). The function and interaction of all host-cell genes modulated by intracellular parasites are poorly understood, and it is not known how many and what host transcripts protect the host cell (by innate immunity mechanisms, for example) or benefit the parasite (such as making nutrients available in the intracellular pathogen-containing compartment), or are neutral and do not participate in the parasite-host interaction (25, 27, 61). Transcriptional responses common to several pathogens have been described, while those that are pathogen specific are few (62). Most of the host cell genes whose expressions are stimulated by encounter with a pathogen relate to the immune response (e.g., genes associated with inflammation and the production of cytokines and chemokines). Thus, the demonstration that intracellular pathogens like L. amazonensis are able to invade and multiply into cells lacking nuclei suggests that host de novo transcription in response to the pathogen would participate more in counteracting parasite multiplication via host immune responses than in the promotion of intracellular parasitism.
We demonstrated that in the presence of host cell nucleus (hence, in the context of de novo mRNA synthesis in response to the parasite) there is discrete and statistically irrelevant alteration in the host transcript profile in response to infection. Concerning gene expression in response to the parasite, our study is in line with others showing that differentially expressed genes (DEG) upon intracellular infection are very few, if not statistically undetectable, at >4 h postinfection (29, 63): although DEG were not detected in our model of noninfected versus infected fibroblasts, transcripts are differentially conserved comparing cytoplasts hosting or not L. amazonensis. These parasite-mediated differentially conserved transcripts translate proteins implicated in functions required for the parasite intracellular establishment in macrophages (Fig. 4E), such as lysosome and phagolysosome biogenesis (36, 64–66), proton-transporting V-type ATPase complex (36, 67), and cellular lipid metabolism (57, 64, 68, 69).
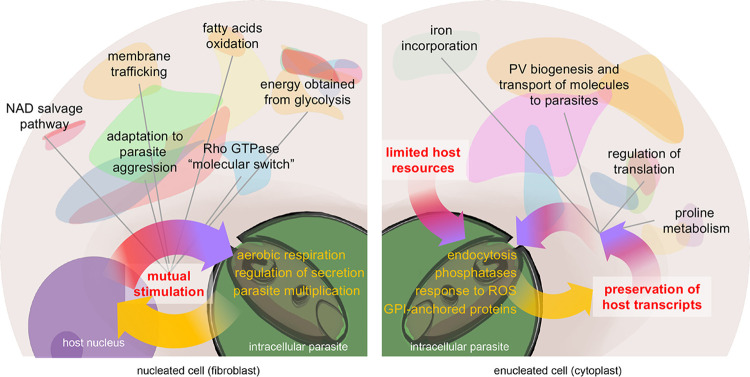
As summarized in Fig. 7, the cross talk between de novo gene expression in nucleated cells in response to intracellular parasite establishment and the parasite gene expression that occurs to counteract or benefit from these responses translates into a parasite transcriptional profile of increased parasite multiplication, aerobic respiration, and control of secreted factors. The potential secretion of parasite factors in response to host de novo transcription interferes in the process whereby host cells obtain energy for themselves and for the parasite, as highlighted by functional clusters, such as fatty acid oxidation and glycolysis, emerging from the host-parasite interaction. Another relevant interference in host cell functions emerging from host nucleus-parasite transcriptional cross talk is indicated by a large cluster formed by generic GO terms of response to antibiotics, response to toxic substances, which were connected to clusters referring to membrane trafficking, positive regulation of phosphorylation, and Rho GTPase activation. The latter clusters are known as molecular switches (70). The findings can be interpreted as an orchestrated adaptation of host cells to intracellular parasitism linked to signaling pathways and cytoskeleton-dependent membrane trafficking. The appearance of an NAD metabolism functional cluster in this scenario is also relevant and is implicated in energy production. NAD from the host cells is salvaged by Leishmania parasites, which are NAD auxotrophic organisms, which could potentially improve parasite fitness in nucleated host cells (71).
FIG 7.
Summary of the dual host-parasite transcriptomic profiling in the presence or absence of host cell nucleus. The left section shows the predicted landscape of host-parasite mutual stimulation generated by host de novo mRNA synthesis promoted by the nucleus in response to the parasite and the expression of parasite transcripts that are differentially regulated by the nuclear host response. The right section shows the predicted landscape of host-parasite cross talk when parasites established in cells without nucleus that display a self-limiting amount of resources for the parasite, promoting a parasite-mediated preservation of specific transcripts involved in maintaining host cell functions that are beneficial to the parasite. Functional clusters identified by in silico analysis of hybrid interactomes appear as colored regions.
Conversely, excluding de novo synthesis by cell enucleation (thus, the decay of transcripts regarding those that are more or less prevalent 48 h after infection of cytoplasts) revealed the remarkable role of intracellular infection in the stability or preservation of certain host cell transcripts. When this host nucleus-parasite transcriptional cross talk is blocked and parasites must establish within cytoplasts and exploit their self-limiting resources, the parasites are reprogrammed to be more endocytic (likely a response to nutrient deprivation) and resistant to host cell stress (by producing factors that will cope with reactive oxygen species and parasite DNA damage). Parasites also upregulate phosphatases and kinases when in the cytoplast host. These enzymes are important signal factors that have been implicated in different pathways, including the response to nutrient deprivation (72). In this scenario of intracellular infection in enucleated cells, the parasite will also secrete factors that control mRNA translation and possibly mRNA half-life, interfering in the pool of preserved cytoplast transcripts that will ultimately participate in the acquisition of energy by parasites (glutamine and proline metabolism) and construction of a safe intracellular niche (the PV) whose biogenesis is controlled by V-ATPase subunits (35, 36). The process is schematized in Fig. 7.
Belonging to a family of functionally diverse serine proteinase inhibitors, Serpins are divided in different groups or clades classified as A to P, and subgroups are classified by numbers (73). SerpinB proteins have no canonical signal peptide, are mainly found inside cells, and are responsible for the inhibition of nonspecific and deleterious intracellular proteolytic activity to prevent tissue damage and cell death (74, 75). Two homologs of SerpinB9 (SerpinB9b and SerpinB9e) were detected among the list of validated transcripts more preserved by L. amazonensis infection in cytoplasts, suggesting that preservation of SerpinB9 proteins in infected cytoplasts may preserve certain functions of host cytoplasts that are beneficial to the parasite.
SerpinB9 is a classic granzyme B inhibitor that prevents granzyme B induced cell death that is stimulated by the action of cytotoxic CD8+ T lymphocytes and natural killer cells (75–77). SerpinB9 also exerts effects independent of granzyme B, such as the inhibition of endogenous caspase-1 in human smooth muscle cells and cross-presentation of antigens in dendritic cells (78, 79). The function of SerpinB9 homologs SerpinB9b and SerpinB9e highlighted in our study is still elusive, although these homologs can share the same motifs of direct interaction with granzyme B, indicating different degrees of efficiency in inhibiting the latter and modulating cell-mediated immune responses (80). A parasite-mediated preservation of SerpinB9 host transcripts would benefit intracellular parasites in the context of this host cell-mediated cytotoxic response, which has been demonstrated to play a relevant role against Leishmania (81–83). A previous study also detected the upregulation of SerpinB9 mRNA in human macrophages infected by L. major (5-fold increase) or L. amazonensis (3-fold increase) after 4 h of infection (29). Considering the prosurvival factors that are relevant during cellular stress (84), the increased preservation of SerpinB9b and SerpinB9e in infected cytoplasts compared to noninfected cytoplasts may contribute to the inhibition of proteases that would ultimately also preserve proteins required for parasite survival or growth.
Although two SerpinB differentially expressed transcripts highlighted in our data were also validated by RT-qPCR in our model, this transcriptomic study has its inherent limitations to be taken into account and the other profiled transcripts here identified require experimental validation by other methods. Validation of protein synthesis from the detected host and parasite transcripts here presented is also required as Leishmania spp. modulate host cell mRNA translation, at least in macrophages (85), and as mRNA levels and protein abundance are not necessarily correlated. Furthermore, the transcript products here analyzed need to be approached functionally to assess their role in L. amazonensis establishment in macrophages, fibroblasts and other potential host cells.
In addition, a direct parasite-mediated influence on transcript half-life here presented must be further investigated as a particle uptake control could not be performed due to the use of nonprofessional phagocytes, L929 fibroblasts, as cytoplast model. These cells are inefficient in internalizing any control particle that we could have used as control for precisely assessing a parasite-mediated action on the preservation or degradation of host transcripts observed upon parasite internalization (29).
This direct action of intracellular parasites on host transcript half-life could be mediated by parasite-derived RNA-binding proteins (RBPs) expressed when these parasites are hosted by enucleated cells. RBPs in trypanosonomatids interfere with the regulation of gene expression mainly in posttranscriptional steps affecting mRNA transport, regulation of splicing, and modulation of mRNA translation. Presently, we observed the expression of 39 different proteins that may interact with mRNA. Of these, 12 were potentially secreted. In particular, Pumilio/PUF RNA-binding protein 5 (PUF5) is expressed in L. amazonensis, although its role in the parasite remains unclear. However, in the host cell, three pumilio homolog proteins were identified: PUM1, PUM2, and PUM3. PUM1 and PUM2 proteins were degraded largely in infected cytoplasts, whereas PUM3 (a nuclear protein) remained unaltered. These findings are of interest considering the prior description that related PUF6 protein can destabilize target L. infantum transcripts (86). The observations of degraded PUM1 and PUM2 in infected cytoplasts could indicate the contribution of Leishmania infection in the stabilization of host cell transcripts through the degradation of the Pumilio homolog proteins.
Some fundamental questions arise when we seek to understand parasite survival inside enucleated host cells. Although L. amazonensis intracellular parasitism apparently does not depend on the host response in terms of gene expression, would there be any posttranscriptional control mediated by the parasite on host’s transcripts? If so, what would they be? How would they be preserved in the infected cytoplasts to allow the parasite survival? These questions remain unaddressed.
Identifying the minimal factors for this interaction using cytoplasts as an experimental host cell model may serve as a tool to establish new conceptual bases of the parasite-host relationship, such as the influence of the parasite on the average life of host transcripts and the characterization of a minimal protein interaction network required for intracellular parasitism. In addition, this minimal interaction network might contribute to the development of new druggable targets and more specific therapeutic agents for leishmaniasis and other infectious diseases.
MATERIALS AND METHODS
Host cells.
Mouse tumor fibroblasts (L cells, clone 929; L929) were cultured in T25 culture flasks in high-glucose Dulbecco modified Eagle medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (Gibco) and antibiotics (complete medium). Cultures were maintained at 37°C in a 5% CO2 and 95% air humid atmosphere. Cells were collected from the culture bottles by treatment with a solution containing 2 mg/ml trypsin (Sigma-Aldrich) and 5 mM EDTA (Sigma-Aldrich). After being resuspended in complete medium for trypsin inactivation, the cells were centrifuged at 1,500 rpm at 4°C for 10 min, resuspended again in complete medium, and counted using a Neubauer chamber.
Enucleation of cell monolayers.
The method proposed by Prescott et al. was adapted to the laboratory conditions (2). For the enucleation assays, 3.5 × 104 L929 cells were seeded on a glass mini-slide (11 by 7 mm). Each mini-slide was cut out from a standard glass microscopy slide with the aid of a Diamond Bur glasscutter. The cells were then incubated in a 75-μl bubble of complete medium positioned on the slide for approximately 6 to 7 h at 37°C in the presence of 5% CO2 for adhesion. The unbound cells were washed out, and the attached cells were maintained in 0.5 ml of complete medium overnight. The mini-slides on which the L929 cells were attached were inserted into 2-ml tubes containing 1 ml of complete medium supplemented with cytochalasin B (20 μg/ml; Sigma-Aldrich). The tubes were centrifuged at 34°C for 60 min at 10,000 × g. After centrifugation, the mini-slides were removed from the tubes and placed in wells of 24-well plates for incubation in complete medium for at least 2 h at 34°C in a 5% CO2 atmosphere. The percentage of nucleated cells in the sample was assessed using fluorescence microscopy and Hoechst 33342 nuclear probe (Molecular Probes). Only samples containing >90% enucleated cells were retained for the subsequent step. Apoptosis and necrosis of the cytoplasts were assessed by using an Annexin V-Cy3 apoptosis detection kit (Sigma-Aldrich) according to the manufacturer’s instructions. Preservation of mitochondria in cytoplasts was assessed using MitoTracker Deep Red FM fluorescent reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Infection of host cells.
L. amazonensis (MHOM/BR/1973/M2269) was maintained in the laboratory by successive infections in BALB/c mice by inoculation of 2 × 106 amastigotes per footpad. Green fluorescent protein (GFP)-transfected L. amazonensis and L. major (MRHO/SU/1959/P) were generously donated by Silvia R. Uliana, ICB-USP, Brazil. Mice with visibly swollen but not ulcerated footpad lesions were used for parasite isolation (lesion-derived amastigotes). After euthanasia of the mice, infected footpads were removed and processed. Only the injured tissue was used, not bones and tendons. After being macerated with the aid of a homogenizer, each sample was centrifuged at low speed (500 rpm at 4°C for 5 min) to remove skin and bone remnants, and the pellet was resuspended in Hanks’ saline and then centrifuged and washed (1,000 rpm/4°C/8 min and 1,800 rpm/4°C/12 min, respectively). The pellet was resuspended in Hanks’ saline and amastigotes were counted using a Neubauer chamber (46). All animal procedures were carried out in accordance with guidelines of the Escola Paulista de Medicina Ethics Committee (CEUA 3398150715).
In the nucleated cell assays, 2 × 104 cells were plated per well in triplicate in six-well plates. L. amazonensis amastigotes were added to these cultures at a multiplicity of infection of 20 parasites per L929 cell (multiplicity of infection [MOI] of 20:1). In the enucleated cell assays, L. amazonensis amastigotes were added to wells of 24-well plates containing L929 enucleated cells attached to mini-slides, and the same MOI was used considering the L929 cell input before enucleation. After incubation for 6 h at 34°C in complete medium, L929 cell or cytoplast cultures were washed two to four times with Hanks’ saline to remove noninternalized parasites. Cultures were maintained at 34°C in complete medium for additional time periods depending on the experiments until paraformaldehyde fixation, live imaging, or RNA processing.
Microscopy.
Imaging of fluorescence-stained live or fixed cells was performed by using a model SP5 II tandem scanner confocal system (Leica Microsystems) or an IX70 inverted epifluorescence microscope (Olympus), equipped with 40× and 100× objective lenses. Live imaging of enucleated cells was performed using a Biostation IM Live cell recorder system (Nikon Corporation) at 34°C and an atmosphere of 5% CO2. The image acquisition of live cytoplasts attached to mini-slides deposited in round microscopy dishes (μ-Dish 35 mm, high wall; Ibidi, GmbH) was performed in 10 different microscopic fields with 40× objective (0.8 numerical aperture) at intervals of 5 min. The time of image acquisition started after 2 h of host cell-parasite interaction and is displayed as “day-hours:minutes” (dhh:mm). Acquired images were processed by Imaris v.7.4.2 software (Bitplane AG; Andor Technology).
RNA extraction and preparation of cDNA libraries.
The infected or noninfected control cells were washed twice in cold phosphate-buffered saline after 48 h of intracellular infection. For nucleated cells, 0.5 ml of TRIzol reagent (Life Technologies) was added in triplicate to wells of six-well plates containing the cells. For the enucleated cells, each mini-slide was repositioned in pairs in wells of a 12-well plate, and 0.5 ml of TRIzol reagent was added to each well. Approximately 40 to 70 mini-slides formed the pool of technical replicates per experiment. After 15 min at room temperature, the TRIzol-processed plates were stocked at –80°C until the total RNA extraction procedure.
For obtaining total RNA, RNeasy Protect minikit was used according to the manufacturer’s protocol (Qiagen). RNA concentration and purity were assessed using an ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies) by measuring the absorbance of samples under UV light. The RNA quality was evaluated by Bioanalyzer 2100 (Agilent) instrument, which provides an RIN index. The index varies from 1 to 10 (10 being the highest RNA integrity number). RNI indexes lower than 9 and 7, respectively, were discarded for nucleated and enucleated samples. Biological replicates used in transcriptomics represent experiments performed in different moments and selected based on percentage of infected cells/cytoplasts and RNA yield and purity (Table 1). RNA sequencing (RNA-seq) was performed after construction of cDNA libraries using an Illumina TruSeq stranded mRNA sample preparation kit on a HISEQ2500 Illumina platform (high output module, paired-end run).
Dual RNA-seq data generation and differential expression analysis.
RNA-seq data were trimmed and de novo reassembled into M. musculus and L. amazonensis genomes using HISAT2 software (https://ccb.jhu.edu/software/hisat2/; Center of Computational Biology, Johns Hopkins University) (87). M. musculus and L. amazonensis genome alignments were performed independently, and the transcripts that did not consistently align with the M. musculus genome database (version hg38/GRCh38) according to software standards were then aligned to the L. amazonensis genome (http://bioinfo08.ibi.unicamp.br/leishmania/) (88). Aligned transcripts were analyzed with HTSeq (https://htseq.readthedocs.io/en/) (89) and DESeq2 (90) for the analyses of DEG between two groups of samples. They were considered differentially expressed/present transcripts when the adjusted P value (Padj) < 0.1. The transcripts that displayed quantitative differences between the compared groups are presented as descriptive tables with their respective comparative fold change (log2 scale), adjusted P value, gene entries, and names according to databases, and functional annotation provided by transcriptomic databases or assessed by AUTOfact software v3.4 (91). Principal-component analysis (PCA) graphs were generated to evaluate biological replicates in these pairwise comparisons between groups of samples. Volcano plots were also generated using the Galaxy platform (92), comparing DEG in pairwise fashion, according to P values and fold change. Considering that standard nomenclature of gene expression, such as upregulated or downregulated genes, does not apply to cells without nucleus, transcripts detected in cytoplasts are considered more preserved/enriched or more absent/degraded in pairwise comparisons.
GO analysis and functional prediction of transcript products.
From a list of differentially expressed M. musculus genes, those displaying a nonadjusted P value < 0.05 were selected for GO analysis using the GOseq software package (93), which considers the transcript length bias of RNA-seq data in the GO analysis. The results are presented as plots showing in the y axis the top 20 overrepresented GO categories (KEGG, GO:CC, GO:BP, and GO:MF, Wallenius method) ranked by their adjusted P values (Benjamini-Hochberg procedure) that statistically indicates overrepresentation in a GO category and the percentage of DEG fitting in each category (x axis). Coordinates are dots whose size indicates the number of DEG detected in the GO category and whose color are determined by a colorimetric scale of P values.
To estimate the function of L. amazonensis DEG, parasite annotated proteins relative to the detected transcripts were first submitted to position-specific iterated (PSI)-BLAST (https://blast.ncbi.nlm.nih.gov/) to identify transcript products having sequence homology to M. musculus (cutoff E value < 10−5). The homologous transcript products were submitted to GO functional enrichment analyses using ClueGO software v2.5.5 (94) considering all evidence provided by the GO, KEGG, INTERPRO, and REACTOME databases. Only pathways with calculated adjusted P < 0.05 (two-sided hypergeometric test with Bonferroni step-down correction) were shown. Networks of related GO categories forming functional clusters from the provided list of L. amazonensis DEGs were generated using GeneMANIA software (95) in the Cytoscape suite v3.7.2 (The Cytoscape Consortium; http://www.cytoscape.org) (96).
In addition, L. amazonensis DEGs were submitted for functional prediction as RBPs using DNApred software (http://biomine.cs.vcu.edu/servers/DRNApred/) (97), which calculates propensities for binding RNA per amino acid residue of a transcript product (threshold for RNA-binding residue > 0.1493). A protein sequence with more than 10 RNA-binding residues was considered an RNA-binding transcript product. RNA-binding proteins were also identified by HMMER alignment with approximately 1,400 RBP proteins identified by proteomic analyses of macrophages infected with L. mexicana (98).
In silico prediction of host-parasite interactomes.
Before building a predictive network of interaction between parasites and host factors, we first detected, from the list of L. amazonensis DEG, which transcript products were potentially secreted by the parasite to allow for the interaction with host proteins. The task was performed using the SecretomeP 2.0 server (http://www.cbs.dtu.dk/services/SecretomeP/) (99) which uses the amino acid sequences of transcript products to predict secretion of proteins with (SignalP algorithm) or without (SecretomeP NN-score) an N-terminal signal peptide. Transcript products with a signal peptide predicted by SignalP algorithm and/or with an NN score > 0.6 were selected as predicted for secretion.
This new list of potentially secreted L. amazonensis factors was then submitted to PSI-BLAST/BLASTP (E value cutoff < 10−5) and InterPROscan (EMBL-EBI, Wellcome Genome Campus, Hinxton, UK) to identify which of these transcripts have sequence homology to the M. musculus host proteins, which of these transcripts belong to protein family or superfamily, and whether they present protein domains that could interact with host factors. InterPROscan was used to detect transmembrane sites. The SignalP algorithm also present in InterPROscan results confirmed or expanded the list of L. amazonensis secreted factors. To verify the actual expression of parasite transcripts into protein, the detected L. amazonensis transcript products were also aligned to the L. mexicana MHOM/GT/2001/U1103 proteome (UniProt Proteome ID UP000007259) and the annotated available proteome that most closely related to L. amazonensis was identified using HMMER software v3.2.1 (100).
From that analysis, a list of potentially secreted L. amazonensis parasite factors that also displayed homology to M. musculus host proteins was generated. This list was added to a list of M. musculus transcript products detected in the DEG analyses performed to generate a L. amazonensis-M. musculus hybrid interactome using STRING v1.5.0 software in the Cytoscape environment. This hybrid interactome was built by adding L. amazonensis-M. musculus transcript entries to a STRING protein query (confidence score cutoff = 0.4, 10 maximum additional interactors) and then creating two clustered networks using stringdb::experiments and stringdb::databases with clusterMaker2 software (101) (MCL cluster module; University of California, San Francisco) embedded in Cytoscape (inflation value = 4, edge cutoff adjusted to minimal value). After merging the two clustered networks based on stringdb::experiments and stringdb::databases, the merged network was set as a STRING network and expanded by 20 interactors (M. musculus) with a selectivity value of 0.5 (confidence cutoff the STRING network adjusted to 0.4). Disconnected nodes were excluded from the network and functional enrichment was retrieved by STRING enrichment software module (background: M. musculus genome).
Quantitative RT-qPCR.
RNA extraction was performed by using an RNeasy minikit (Qiagen) with in-column DNase I (Invitrogen) digestion according to the suppliers’ instructions. Up to 1 μg of RNA was reverse transcribed with a QuantiNova reverse transcription kit (Qiagen), and cDNA was subjected to real-time quantitative PCR with SYBR Select Master Mix (Applied Biosystems) on StepOne Plus equipment (Applied Biosystems). The primers used were as follows: hypoxanthine guanine phosphoribosyltransferase (HPRT) housekeeping gene, forward primer 5′-CCT AAG ATG AGC GCA AGT TGA A-3′ and reverse primer 5′-CCA CAG GAC TAG AAC ACC TGC TAA-3′; SerpinB9 gene, forward primer 5′-TAT CTC AGG CAC TTG GTT TGA ATA AA-3′ and reverse primer 5′-TTT CTG TCT GGC TTG TTC AGC TT-3′; SerpinB9b gene, forward primer 5′-CAA GCC CAC ATC CCT TTG AA-3′ and reverse primer 5′-TGT TGG CAG ATG ACT CAG TTA ATT TC-3′; SerpinB9e gene, forward primer 5′-ACA GCA TTT GGG AAT CTT GG-3′ and reverse primer 5′-ACA GCA TTT GGG AAT CTT GG-3′; and SerpinB8 gene, forward primer 5′-AAG TCA CGA AAC CTG TTC T-3′ and reverse primer 5′-GAC ATC TGG GTA GCA GTG TTT-3′. The primers were designed using PrimerQuest software (Integrated DNA Technologies, Inc.). All reactions were performed in duplicate. Melting curve analysis and agarose gel electrophoresis were performed to validate the specificity of amplicons. The results are expressed as the fold change expression that was first normalized to the HPRT housekeeping gene, and then expression after 48 h of culture was normalized by the expression at 6 h of culture to assess the preservation of transcripts. These normalizations were performed in each experimental group, namely, cytoplasts or fibroblasts, infected or not by L. amazonensis.
Data availability.
Raw RNA-seq sequence data are available in the NCBI Short Read Archive (SRA) database under BioProject accession number PRJNA552676.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the Life Sciences Core Facility (LaCTAD) from the State University of Campinas (UNICAMP), where all samples were sequenced. We gratefully acknowledge the support and advice of Eric Prina and Geneviève Milon, Institut Pasteur, France, and Morgane Bomsel, Institut Cochin, France.
This study was supported by funds from the Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (2014/25296-2 and 2016/15000-4), the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Graham DM, Andersen T, Sharek L, Uzer G, Rothenberg K, Hoffman BD, Rubin J, Balland M, Bear JE, Burridge K. 2018. Enucleated cells reveal differential roles of the nucleus in cell migration, polarity, and mechanotransduction. J Cell Biol 217:895–914. doi: 10.1083/jcb.201706097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescott DM, Myerson D, Wallace J. 1972. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res 71:480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- 3.Wright WE, Hayflick L. 1972. Formation of anucleate and multinucleate cells in normal and SV 40 transformed WI-38 by cytochalasin B. Exp Cell Res 74:187–194. doi: 10.1016/0014-4827(72)90496-x. [DOI] [PubMed] [Google Scholar]
- 4.Wright WE, Hayflick L. 1973. Enucleation of cultured human cells. Proc Soc Exp Biol Med 144:587–592. doi: 10.3181/00379727-144-37640. [DOI] [PubMed] [Google Scholar]
- 5.Goldman RD, Pollack R, Chang CM, Bushnell A. 1975. Properties of enucleated cells. III. Changes in cytoplasmic architecture of enucleated BHK21 cells following trypsinization and replating. Exp Cell Res 93:175–183. doi: 10.1016/0014-4827(75)90437-1. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RD, Pollack R, Hopkins NH. 1973. Preservation of normal behavior by enucleated cells in culture. Proc Natl Acad Sci U S A 70:750–754. doi: 10.1073/pnas.70.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise GE, Prescott DM. 1973. Ultrastructure of enucleated mammalian cells in culture. Exp Cell Res 81:63–72. doi: 10.1016/0014-4827(73)90111-0. [DOI] [PubMed] [Google Scholar]
- 8.Fleit HB, Lane BP. 1999. FC gamma receptor mediated phagocytosis by human neutrophil cytoplasts. Inflammation 23:253–262. doi: 10.1023/a:1020226020062. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson MD, Burne JF, Raff MC. 1994. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J 13:1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karsenti E, Kobayashi S, Mitchison T, Kirschner M. 1984. Role of the centrosome in organizing the interphase microtubule array: properties of cytoplasts containing or lacking centrosomes. J Cell Biol 98:1763–1776. doi: 10.1083/jcb.98.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RA, Ruddle FH. 1974. Enucleated neuroblastoma cells form neurites when treated with dibutyryl cyclic AMP. J Cell Biol 63:295–299. doi: 10.1083/jcb.63.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odell EW, Segal AW. 1988. The bactericidal effects of the respiratory burst and the myeloperoxidase system isolated in neutrophil cytoplasts. Biochim Biophys Acta 971:266–274. doi: 10.1016/0167-4889(88)90141-3. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Cleveland DW. 1985. Retention of autoregulatory control of tubulin synthesis in cytoplasts: demonstration of a cytoplasmic mechanism that regulates the level of tubulin expression. J Cell Biol 101:1941–1952. doi: 10.1083/jcb.101.5.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodionov V, Nadezhdina E, Borisy G. 1999. Centrosomal control of microtubule dynamics. Proc Natl Acad Sci U S A 96:115–120. doi: 10.1073/pnas.96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shay JW, Porter KR, Prescott DM. 1974. The surface morphology and fine structure of CHO (Chinese hamster ovary) cells following enucleation. Proc Natl Acad Sci U S A 71:3059–3063. doi: 10.1073/pnas.71.8.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf CM, Eastman A. 1999. Intracellular acidification during apoptosis can occur in the absence of a nucleus. Biochem Biophys Res Commun 254:821–827. doi: 10.1006/bbrc.1998.0132. [DOI] [PubMed] [Google Scholar]
- 17.Bruno J, Lucas JJ. 1983. Polypeptide synthesis in enucleated mouse fibroblasts. Cell Biol Int Rep 7:651–659. doi: 10.1016/0309-1651(83)90120-0. [DOI] [PubMed] [Google Scholar]
- 18.Follett EA. 1974. A convenient method for enucleating cells in quantity. Exp Cell Res 84:72–78. doi: 10.1016/0014-4827(74)90381-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldman RD, Pollack R. 1974. Uses of enucleated cells. Methods Cell Biol 8:123–143. doi: 10.1016/s0091-679x(08)60448-3. [DOI] [PubMed] [Google Scholar]
- 20.Romano JD, Bano N, Coppens I. 2008. New host nuclear functions are not required for the modifications of the parasitophorous vacuole of Toxoplasma. Cell Microbiol 10:465–476. [DOI] [PubMed] [Google Scholar]
- 21.Sethi KK, Pelster B, Piekarski G, Brandis H. 1973. Multiplication of Toxoplasma gondii in enucleated L cells. Nat New Biol 243:255–256. doi: 10.1038/newbio243255a0. [DOI] [PubMed] [Google Scholar]
- 22.Perara E, Yen TS, Ganem D. 1990. Growth of Chlamydia trachomatis in enucleated cells. Infect Immun 58:3816–3818. doi: 10.1128/IAI.58.11.3816-3818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatch TP. 1975. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun 12:211–220. doi: 10.1128/IAI.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stork E, Wisseman CL Jr. 1976. Growth of Rickettsia prowazekii in enucleated cells. Infect Immun 13:1743–1748. doi: 10.1128/IAI.13.6.1743-1748.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto D, Coimbra VC, Okuda K, Rabinovitch M. 2006. Enucleated L929 mouse fibroblasts support invasion and multiplication of Shigella flexneri 5a. Braz J Med Biol Res 39:749–758. doi: 10.1590/s0100-879x2006000600007. [DOI] [PubMed] [Google Scholar]
- 26.de Souza Carvalho C, Kasmapour B, Gronow A, Rohde M, Rabinovitch M, Gutierrez MG. 2011. Internalization, phagolysosomal biogenesis and killing of mycobacteria in enucleated epithelial cells. Cell Microbiol 13:1234–1249. doi: 10.1111/j.1462-5822.2011.01615.x. [DOI] [PubMed] [Google Scholar]
- 27.Coimbra VC, Yamamoto D, Khusal KG, Atayde VD, Fernandes MC, Mortara RA, Yoshida N, Alves MJ, Rabinovitch M. 2007. Enucleated L929 cells support invasion, differentiation, and multiplication of Trypanosoma cruzi parasites. Infect Immun 75:3700–3706. doi: 10.1128/IAI.00194-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combes C, de Buron I, Connors VA, Simberloff D. 2001. Parasitism: the ecology and evolution of intimate interactions. University of Chicago Press, Chicago, IL. [Google Scholar]
- 29.Fernandes MC, Dillon LA, Belew AT, Bravo HC, Mosser DM, El-Sayed NM. 2016. Dual transcriptome profiling of Leishmania-infected human macrophages reveals distinct reprogramming signatures. mBio 7:e00027-16. doi: 10.1128/mBio.00027-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maretti-Mira AC, Bittner J, Oliveira-Neto MP, Liu M, Kang D, Li H, Pirmez C, Craft N. 2012. Transcriptome patterns from primary cutaneous Leishmania braziliensis infections associate with eventual development of mucosal disease in humans. PLoS Negl Trop Dis 6:e1816. doi: 10.1371/journal.pntd.0001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westermann AJ, Gorski SA, Vogel J. 2012. Dual RNA-seq of pathogen and host. Nat Rev Microbiol 10:618–630. doi: 10.1038/nrmicro2852. [DOI] [PubMed] [Google Scholar]
- 32.Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. 2013. Case study for a vaccine against leishmaniasis. Vaccine 31(Suppl 2):B244–B249. doi: 10.1016/j.vaccine.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 33.Burza S, Croft SL, Boelaert M. 2018. Leishmaniasis. Lancet 392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 34.Cavalcante-Costa VS, Costa-Reginaldo M, Queiroz-Oliveira T, Oliveira ACS, Couto NF, Dos Anjos DO, Lima-Santos J, Andrade LO, Horta MF, Castro-Gomes T. 2019. Leishmania amazonensis hijacks host cell lysosomes involved in plasma membrane repair to induce invasion in fibroblasts. J Cell Sci 132:jcs226183. doi: 10.1242/jcs.226183. [DOI] [PubMed] [Google Scholar]
- 35.Real F, Mortara RA. 2012. The diverse and dynamic nature of Leishmania parasitophorous vacuoles studied by multidimensional imaging. PLoS Negl Trop Dis 6:e1518. doi: 10.1371/journal.pntd.0001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pessoa CC, Reis LC, Ramos-Sanchez EM, Orikaza CM, Cortez C, de Castro Levatti EV, Badaro ACB, Yamamoto J, D’Almeida V, Goto H, Mortara RA, Real F. 2019. ATP6V0d2 controls Leishmania parasitophorous vacuole biogenesis via cholesterol homeostasis. PLoS Pathog 15:e1007834. doi: 10.1371/journal.ppat.1007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogdan C, Donhauser N, Doring R, Rollinghoff M, Diefenbach A, Rittig MG. 2000. Fibroblasts as host cells in latent leishmaniasis. J Exp Med 191:2121–2130. doi: 10.1084/jem.191.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arend WP, Mehta G, Antonioli AH, Takahashi M, Takahashi K, Stahl GL, Holers VM, Banda NK. 2013. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J Immunol 190:6423–6433. doi: 10.4049/jimmunol.1300580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurung P, Kanneganti TD. 2015. Innate immunity against Leishmania infections. Cell Microbiol 17:1286–1294. doi: 10.1111/cmi.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. 2007. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol 8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M. 2016. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol 215:857–874. doi: 10.1083/jcb.201607039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veras PST, de Menezes JPB, Dias BRS. 2019. Deciphering the role played by autophagy in Leishmania infection. Front Immunol 10:2523. doi: 10.3389/fimmu.2019.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tann AW, Boldogh I, Meiss G, Qian W, Van Houten B, Mitra S, Szczesny B. 2011. Apoptosis induced by persistent single-strand breaks in mitochondrial genome: critical role of EXOG (5′-EXO/endonuclease) in their repair. J Biol Chem 286:31975–31983. doi: 10.1074/jbc.M110.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darnell JC, Richter JD. 2012. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb Perspect Biol 4:a012344. doi: 10.1101/cshperspect.a012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Paiva RM, Grazielle-Silva V, Cardoso MS, Nakagaki BN, Mendonca-Neto RP, Canavaci AM, Souza Melo N, Martinelli PM, Fernandes AP, daRocha WD, Teixeira SM. 2015. Amastin knockdown in Leishmania braziliensis affects parasite-macrophage interaction and results in impaired viability of intracellular amastigotes. PLoS Pathog 11:e1005296. doi: 10.1371/journal.ppat.1005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabinovitch M, Zilberfarb V, Ramazeilles C. 1986. Destruction of Leishmania mexicana amazonensis amastigotes within macrophages by lysosomotropic amino acid esters. J Exp Med 163:520–535. doi: 10.1084/jem.163.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rittig MG, Bogdan C. 2000. Leishmania-host cell interaction: complexities and alternative views. Parasitol Today 16:292–297. doi: 10.1016/s0169-4758(00)01692-6. [DOI] [PubMed] [Google Scholar]
- 48.Aebischer T. 1994. Recurrent cutaneous leishmaniasis: a role for persistent parasites? Parasitol Today 10:25–28. doi: 10.1016/0169-4758(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 49.Minero MA, Chinchilla M, Guerrero OM, Castro A. 2004. Infection of skin fibroblasts in animals with different levels of sensitivity to Leishmania infantum and Leishmania mexicana (Kinetoplastida: Trypanosomatidae). Rev Biol Trop 52:261–267. [PubMed] [Google Scholar]
- 50.Ramirez JL, Guevara P. 1997. Persistent infections by Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz 92:333–338. doi: 10.1590/s0074-02761997000300006. [DOI] [PubMed] [Google Scholar]
- 51.Schubach A, Haddad F, Oliveira-Neto MP, Degrave W, Pirmez C, Grimaldi G Jr, Fernandes O. 1998. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J Infect Dis 178:911–914. doi: 10.1086/515355. [DOI] [PubMed] [Google Scholar]
- 52.May RC, Machesky LM. 2001. Phagocytosis and the actin cytoskeleton. J Cell Sci 114:1061–1077. [DOI] [PubMed] [Google Scholar]
- 53.Coombes BK, Mahony JB. 2001. cDNA array analysis of altered gene expression in human endothelial cells in response to Chlamydia pneumoniae infection. Infect Immun 69:1420–1427. doi: 10.1128/IAI.69.3.1420-1427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eskra L, Mathison A, Splitter G. 2003. Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect Immun 71:1125–1133. doi: 10.1128/iai.71.3.1125-1133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldenberg RC, Iacobas DA, Iacobas S, Rocha LL, da Silva de Azevedo Fortes F, Vairo L, Nagajyothi F, Campos de Carvalho AC, Tanowitz HB, Spray DC. 2009. Transcriptomic alterations in Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect 11:1140–1149. doi: 10.1016/j.micinf.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, Lander ES, Hacohen N. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 57.Osorio y Fortéa J, de La Llave E, Regnault B, Coppée J-Y, Milon G, Lang T, Prina E. 2009. Transcriptional signatures of BALB/c mouse macrophages housing multiplying Leishmania amazonensis amastigotes. BMC Genomics 10:119. doi: 10.1186/1471-2164-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragno S, Estrada-Garcia I, Butler R, Colston MJ. 1998. Regulation of macrophage gene expression by Mycobacterium tuberculosis: downregulation of mitochondrial cytochrome c oxidase. Infect Immun 66:3952–3958. doi: 10.1128/IAI.66.8.3952-3958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauvonnet N, Pradet-Balade B, Garcia-Sanz JA, Cornelis GR. 2002. Regulation of mRNA expression in macrophages after Yersinia enterocolitica infection: role of different Yop effectors. J Biol Chem 277:25133–25142. doi: 10.1074/jbc.M203239200. [DOI] [PubMed] [Google Scholar]
- 60.Schwan WR, Kugler S, Schuller S, Kopecko DJ, Goebel W. 1996. Detection and characterization by differential PCR of host eukaryotic cell genes differentially transcribed following uptake of intracellular bacteria. Infect Immun 64:91–99. doi: 10.1128/IAI.64.1.91-99.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Modlin RL, Bloom BR. 2001. Chips, infections, and national security. Science 294:1279. doi: 10.1126/science.294.5545.1279b. [DOI] [PubMed] [Google Scholar]
- 62.Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, Belcher CE, Botstein D, Staudt LM, Brown PO, Relman DA. 2002. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci U S A 99:972–977. doi: 10.1073/pnas.231625398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aoki JI, Muxel SM, Zampieri RA, Muller KE, Nerland AH, Floeter-Winter LM. 2019. Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles. Sci Rep 9:19841. doi: 10.1038/s41598-019-56305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okuda K, Tong M, Dempsey B, Moore KJ, Gazzinelli RT, Silverman N. 2016. Leishmania amazonensis engages CD36 to drive parasitophorous vacuole maturation. PLoS Pathog 12:e1005669. doi: 10.1371/journal.ppat.1005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vieira OV, Botelho RJ, Grinstein S. 2002. Phagosome maturation: aging gracefully. Biochem J 366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson J, Huynh C, Kennedy KA, Ward DM, Kaplan J, Aderem A, Andrews NW. 2008. Control of parasitophorous vacuole expansion by LYST/Beige restricts the intracellular growth of Leishmania amazonensis. PLoS Pathog 4:e1000179. doi: 10.1371/journal.ppat.1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kissing S, Hermsen C, Repnik U, Nesset CK, von Bargen K, Griffiths G, Ichihara A, Lee BS, Schwake M, De Brabander J, Haas A, Saftig P. 2015. Vacuolar ATPase in phagosome-lysosome fusion. J Biol Chem 290:14166–14180. doi: 10.1074/jbc.M114.628891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semini G, Paape D, Paterou A, Schroeder J, Barrios-Llerena M, Aebischer T. 2017. Changes to cholesterol trafficking in macrophages by Leishmania parasite infection. Microbiologyopen 6:e00469. doi: 10.1002/mbo3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallochi AL, Teixeira L, Oliveira KDS, Maya-Monteiro CM, Bozza PT. 2018. Lipid droplet, a key player in host-parasite interactions. Front Immunol 9:1022. doi: 10.3389/fimmu.2018.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall A, Nobes CD. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci 355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gazanion E, Garcia D, Silvestre R, Gerard C, Guichou JF, Labesse G, Seveno M, Cordeiro-Da-Silva A, Ouaissi A, Sereno D, Vergnes B. 2011. The Leishmania nicotinamidase is essential for NAD+ production and parasite proliferation. Mol Microbiol 82:21–38. doi: 10.1111/j.1365-2958.2011.07799.x. [DOI] [PubMed] [Google Scholar]
- 72.Soulat D, Bogdan C. 2017. Function of macrophage and parasite phosphatases in leishmaniasis. Front Immunol 8:1838. doi: 10.3389/fimmu.2017.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, Silverman GA, Nebert DW, Vasiliou V. 2013. Update of the human and mouse SERPIN gene superfamily. Hum Genomics 7:22. doi: 10.1186/1479-7364-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mangan MS, Kaiserman D, Bird PI. 2008. The role of serpins in vertebrate immunity. Tissue Antigens 72:1–10. doi: 10.1111/j.1399-0039.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 75.Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S, Irving JA, Bird PI. 2004. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci 61:301–325. doi: 10.1007/s00018-003-3240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bots M, VAN Bostelen L, Rademaker MT, Offringa R, Medema JP. 2006. Serpins prevent granzyme-induced death in a species-specific manner. Immunol Cell Biol 84:79–86. doi: 10.1111/j.1440-1711.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 77.de Poot SA, Bovenschen N. 2014. Granzyme M: behind enemy lines. Cell Death Differ 21:359–368. doi: 10.1038/cdd.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mangan MS, Vega-Ramos J, Joeckel LT, Mitchell AJ, Rizzitelli A, Roediger B, Kaiserman D, Weninger WW, Villadangos JA, Bird PI. 2017. Serpinb9 is a marker of antigen cross-presenting dendritic cells. Mol Immunol 82:50–56. doi: 10.1016/j.molimm.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. 2000. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1β-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med 191:1535–1544. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaiserman D, Bird PI. 2010. Control of granzymes by serpins. Cell Death Differ 17:586–595. doi: 10.1038/cdd.2009.169. [DOI] [PubMed] [Google Scholar]
- 81.Pereira BA, Alves CR. 2008. Immunological characteristics of experimental murine infection with Leishmania (Leishmania) amazonensis. Vet Parasitol 158:239–255. doi: 10.1016/j.vetpar.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Colmenares M, Kima PE, Samoff E, Soong L, McMahon-Pratt D. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect Immun 71:3172–3182. doi: 10.1128/iai.71.6.3172-3182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alves CR, Benevolo-De-Andrade TC, Alves JL, Pirmez C. 2004. Th1 and Th2 immunological profile induced by cysteine proteinase in murine leishmaniasis. Parasite Immunol 26:127–135. doi: 10.1111/j.0141-9838.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- 84.Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird PI. 2010. Serpins flex their muscle. I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem 285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaramillo M, Gomez MA, Larsson O, Shio MT, Topisirovic I, Contreras I, Luxenburg R, Rosenfeld A, Colina R, McMaster RW, Olivier M, Costa-Mattioli M, Sonenberg N. 2011. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe 9:331–341. doi: 10.1016/j.chom.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Azizi H, Dumas C, Papadopoulou B. 2017. The Pumilio-domain protein PUF6 contributes to SIDER2 retroposon-mediated mRNA decay in Leishmania. RNA 23:1874–1885. doi: 10.1261/rna.062950.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Real F, Vidal RO, Carazzolle MF, Mondego JM, Costa GG, Herai RH, Wurtele M, de Carvalho LM, Carmona e Ferreira R, Mortara RA, Barbieri CL, Mieczkowski P, da Silveira JF, Briones MR, Pereira GA, Bahia D. 2013. The genome sequence of Leishmania (Leishmania) amazonensis: functional annotation and extended analysis of gene models. DNA Res 20:567–581. doi: 10.1093/dnares/dst031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anders S, Pyl PT, Huber W. 2015. HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koski LB, Gray MW, Lang BF, Burger G. 2005. AutoFACT: an automatic functional annotation and classification tool. BMC Bioinformatics 6:151. doi: 10.1186/1471-2105-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. 2018. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pages F, Trajanoski Z, Galon J. 2009. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. 2010. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu YH, Hu J, Song XN, Yu DJ. 2019. DNAPred: accurate identification of DNA-binding sites from protein sequence by ensembled hyperplane-distance-based support vector machines. J Chem Inf Model 59:3057–3071. doi: 10.1021/acs.jcim.8b00749. [DOI] [PubMed] [Google Scholar]
- 98.de Pablos LM, Ferreira TR, Dowle AA, Forrester S, Parry E, Newling K, Walrad PB. 2019. The mRNA-bound proteome of Leishmania mexicana: novel genetic insight into an ancient parasite. Mol Cell Proteomics 18:1271–1284. doi: 10.1074/mcp.RA118.001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. 2004. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel 17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 100.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morris JH, Apeltsin L, Newman AM, Baumbach J, Wittkop T, Su G, Bader GD, Ferrin TE. 2011. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinformatics 12:436. doi: 10.1186/1471-2105-12-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA-seq sequence data are available in the NCBI Short Read Archive (SRA) database under BioProject accession number PRJNA552676.