Abstract
Aim
The description of rare malignant ovarian tumours and the most suitable treatments. Alternative therapies different from intravenous chemotherapy are also explained.
Methods
Literature review and ongoing trial information have been used to elaborate this guide.
Results
Each ovarian cancer type must be identified and treated properly from diagnostic to surgery, adjuvant treatment and metastatic disease. Hormonotherapy can be useful as an alternative treatment, especially in low-grade ovarian cancer and endometrioid subtype. Tumour characterisation is appropriated for treatment selection when targeted therapy is indicated. MEK inhibitors, tyrosine-kinase inhibitors, EGFR inhibitors, therapies against integrins, antibody–drug conjugates and other strategies are described. Antiangiogenics, PARP inhibitors and immunotherapy are discussed in other parts of this publication.
Conclusion
Different ovarian cancer types must receive the appropriated treatment. Alternative therapies may be evaluated beyond the standard therapy, frequently in a clinical trial, and an individualised molecular study may help to find the best treatment.
Keywords: Rare malignant ovarian tumours, Hormonotherapy, Targeted therapy
Highlights
-
•
Rare Malignant Ovarian Tumors need a specific diagnosis and treatment.
-
•
Hormonotherapy is suitable for some ovarian cancers.
-
•
Molecular alterations analyses permits targeted therapy as a personalized medicine.
1. Introduction
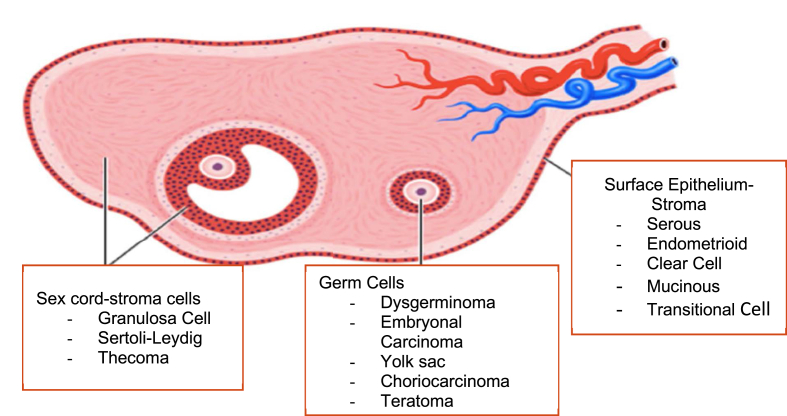
Low-frequency malignant tumours of the ovary are classified depending on their origin cells. Most of them are epithelial tumours. The last WHO classification in 2014 defined these main subtypes with less than 10% of frequency [See the complete classification in Ref. [1]]. (see Fig. 1)
-
−
Mucinous carcinoma (MC): 2–3%
-
−
Low-grade serous/endometrioid carcinoma (LGOC): <5%
-
−
Borderline tumours (BOT): 15%
-
−
Carcinosarcoma tumours (CS): 1–3%
-
−
Ovarian squamous cell carcinoma (OSCC): <1%
-
−
Ovarian carcinoid tumours (and struma ovarii) (OCT): 0.1%
-
−
Small cell ovarian tumours: <1%
-
−
Germ cell tumours (GCT): 3%
-
−
Sex cord–stroma cells tumours (SCST): <2%
-
−
Transitional and malignant Brenner tumours: <1%
-
−
Undifferentiated tumours: <5%
Fig. 1.
Ovarian cancer subtypes and its origin in the ovary.
Transitional, malignant Brenner and undifferentiated tumours are treated as high-grade, so they will not be discussed separately in this review.
1.1. Diagnosis and staging
Diagnosis is usually done as a result of the pathological report after the removal of a pelvic mass. These rare tumours must be referred to expert centres.
FIGO staging system is the same as for epithelial ovarian carcinomas. EOC.
Tumour markers can help in the initial diagnosis and can be used for response evaluation and follow-up if they are elevated in the diagnosis (see Table 1).
Table 1.
Tumour subtypes and associated tumoural markers.
| Tumour Subtype | Tumoural Markers |
|---|---|
| Endometrioid tumours | CA-125, CA-19.9 |
| Mucinous tumours | CA-125, CEA |
| Carcinoid tumours | CA-125, chromogranin A and B |
| Small cell tumours | CA-125, plasmatic calcium |
| Sex cord–stroma tumours | CA-125, Inhibin B, anti-Müllerian hormone |
| Germ cells tumours | CA-125, LDH, AFP, BHCG |
1.2. Surgery
Initial treatment is comprehensive surgery as in HGSC with the aim to achieve no residual tumour. Lymphadenectomy is not mandatory in borderline, mucinous tumours and stage IA GCC. Its role is not well stablished for OSCC. Fertility preservation can be considered in stage I–II MC and BOT; as well as stage I–II unilateral GCC, stage I SCST, stage I OCT and stage IA OSCC, although data are limited for the later. Nevertheless, surgery must be completed after childbearing [2]. This is not an option for carcinosarcoma and small cell carcinomas due to its bad prognosis [3].
1.3. Adjuvant treatment and treatment at recurrence
1.3.1. Mucinous carcinoma
MC of the ovary can be primary or metastatic in origin. Digestive endoscopies and appendectomy in surgery must be performed in order to rule out ovarian metastasis from digestive tumours. Most tumours are diagnosed at an early stage, and the prognosis after surgery is good. Yet, advanced or recurrent disease has poor response rate and prognosis [4]. Adjuvant treatment can be avoided in FIGO stage IA-B. In IC, it can be discussed. In II–IV stages, platinum-based doublet chemotherapy is indicated.
Advanced and recurrent diseases are commonly treated with chemotherapy. The classical regimen with carboplatin and paclitaxel (CP) can be substituted by other schemes more frequently used in gastrointestinal tumours such as 5-Fluorouracil and Leucovorin and Oxaliplatin, or Capecitabine and Oxaliplatin. Unfortunately, several trials that were studying these different regimens were prematurely closed due to slow accrual.
1.3.2. Low-grade serous and endometrioid carcinoma
Most of LGOC cases present with advanced-stage disease. Compared with HGSOC, LGOC is associated with younger age at diagnosis (median 43–55 years), similar progression-free survival (19.5 months), longer overall survival (median, 82–99 months), but higher rate of persistent disease after primary platinum-based chemotherapy [5].
The main treatment is cytoreductive surgery due to the relatively poor chemoresponsiveness of LGOC tumours [6]. Neoadjuvant chemotherapy is not recommended (only a 5% of response rate) [7,8].
No adjuvant treatment is recommended for early stages (IA–B). In IC–IV stages, the current recommendation is CP. Some groups avoid chemotherapy and use adjuvant hormonal therapy, despite only retrospective data being available (9% of RR and stable disease (SD) of 62%). A retrospective analysis of patients with LGSOC who received either maintenance hormonal treatment or observation, based on physician decision, following primary cytoreductive surgery and platinum-based chemotherapy, showed significantly longer median PFS (64.9 vs 26.4 months) for hormonotherapy compared to the observation group, without significant prolongation of median OS (OS, 115.7 vs 102.7 months) [9].
After recurrence, salvage surgery may be considered. Complete cytoreduction to no-gross residual disease has been found an independent prognostic factor for PFS, irrespective of platinum-free interval (PFI) [10]. In metastatic disease, chemotherapy regimens as for HGCS can be evaluated. But, due to relatively low responsiveness to chemotherapy, to choose a platinum-based regimen according to PFI may not be as prognostic as it is in HGOC [10]. Anti-angiogenic therapy with Bevacizumab, reported in retrospective reviews, has shown RR of 40%, alone or in combination with chemotherapy [11].
Targeted therapy has emerged after the molecular characterisation of these tumours, resulting in BRAF mutations in 6%, KRAS mutations in 20–41% [12,13] and NRAS mutations in 15% [13]. GOG-0239, a phase II trial, performed a mutational study and tested the MEK inhibitor, selumetinib, with an RR of 15.4%, SD of 65% and PFS of 11m, when the current PFS for other therapies is 7m [13]. Another randomised phase III trial, with MEK inhibitor (MEK162) versus investigator-chosen chemotherapy with paclitaxel, pegylated liposomal doxorubicin or topotecan was closed due to futility (NCT01849874). A randomised phase II/III trial with trametinib versus standard of care (SOC) treatment (GOG-281/NCT02101788) was recently reported. Median PFS for experimental arm was 13.0 versus 7.2 months in SOC [HR 0.48 (0.36–0.64), p < 0.0001], and RR of 26.2% versus 6.2% [OR 5.4 (2.4–12.2), 95% confidence interval (CI), p < 0.0001]. The differences are smaller if the comparison is with letrozole, but final data is pending in the publication. No genomic profile with KRAS mutations was presented [14].
1.3.3. Borderline tumours
They are classically divided into serous (67%) or mucinous (30%) subtypes. Molecular alterations are similar to LGOC. For staging, moreover for the FIGO stage, it is necessary to indicate the presence of invasive implants [2].
Adjuvant therapy is only considered if invasive implants are present. The treatment is similar to LGOC, as it is in case of recurrent or metastatic disease [15].
1.3.4. Carcinosarcomas
CSs have poor prognosis, with high recurrence rates. Molecular characterisation studies are limited, being TP53 the main mutation [16].
Adjuvant therapy is chemotherapy with CP, cisplatin and ifosfamide, or paclitaxel and ifosfamide. In recurrent or metastatic disease, the same chemotherapy schemes are useful. There are few prospective trials, most of the data is obtained from uterine CS studies [17].
1.3.5. Ovarian squamous cell carcinoma
Most of the OSCCs arise from a mature cystic teratoma malignisation.
Adjuvant therapy is evaluated in III and IV stages. In recurrent or metastatic disease, the prognosis is poor. The chemotherapy regimens are CP or gemcitabine. Clinical trials are very limited due to its low frequency [18].
1.3.6. Ovarian carcinoid tumours (and struma ovarii)
Carcinoid tumours are well-differentiated neuroendocrine tumours (NET). They are very rare and a metastatic origin must be ruled out; an octreoscan can be useful. The subtypes are insular (the most common), trabecular, stromal carcinoids, mucinous carcinoids (sometimes called goblet cells) and mixed endocrine/exocrine tumours.
Ovarian carcinoids may cause carcinoid heart disease without liver metastasis, or carcinoid syndrome. Somatostatin analogues are prescribed for these symptomatic patients.
It is important to exclude a primary NET cancer from another site causing metastasis to the ovary; these are usually bilateral. There is no evidence of benefit with adjuvant therapy (chemotherapy, radiotherapy or hormonal treatment).
Streptozocin-based regimens are the chemotherapy of choice. As in other NETs, mammalian target of rapamycin (mTOR) pathways inhibitors and peptide receptor radionuclide therapy are also of value [19].
Struma ovarii is a variant that commonly arises from a mature cystic teratoma, and it contains more than 50% of thyroid tissue. The treatment is similar to that of differentiated thyroid tumours, but it is currently under discussion, because it includes a total hysterectomy and bilateral salpingo-oophorectomy, thyroidectomy, radioiodine thyroid tissue ablation and thyroid hormone suppressive therapy [20]. The follow-up is with thyroglobulin levels and 131I scans.
1.3.7. Small cell ovarian tumours
They are neuroendocrine or carcinoid tumours. The three most common types are SCCOPT (pulmonary type); SCCOHT (hypercalcaemic type); and non-small-cell neuroendocrine carcinoma (large cell variant).
Hypercalcaemia is present in 70% of patients, and parathyroid hormone and parathyroid-hormone-related peptide may be measured. Inappropriate antidiuretic hormone secretion syndrome can be also present, especially in the pulmonary variant.
Only around 20–25% will have confined disease of the ovary at the diagnosis. The pattern of spread is very similar to HGSOC.
There is very little evidence regarding neoadjuvant chemotherapy.
The adjuvant therapy is indicated from stage I, and the combination of platinum (carboplatin or cisplatin) and etoposide is considered the most appropriate. Adjuvant radiotherapy seems to reduce the recurrence risk, but only stage I patients did not recur, so the evidence is very limited [21].
The prognosis is very poor after recurrence or in the metastatic setting. A second surgery can be evaluated. Moreover, platinum and etoposide scheme and test high-dose chemotherapy or CP, cyclophosphamide, doxorubicin, vincristine, topotecan.
1.3.8. Germ cell tumours
The main variants are dysgerminoma, yolk sac, embryonal carcinoma, choriocarcinoma and teratoma. 70% of cases are diagnosed in early stages.
The adjuvant treatment is not indicated in dysgerminoma or immature teratoma stage I. In stage IC–II, observation or chemotherapy can be discussed. Chemotherapy with BEP regimen (bleomycin, etoposide and cisplatin) is considered for stages III–IV. In some cases, embryonal yolk sac tumours and grade 2–3 immature teratomas, chemotherapy is considered in stages I–IV.
The recurrent or metastatic disease is treated with chemotherapy. The tested regimens are TIP (taxol, ifosfamide and cisplatin), VAC (vincristine, actinomycin D and cyclophosphamide), VeIP (vinblastine, ifosfamide and cisplatin), VIP (etoposide, ifosfamide and cisplatin) and other combinations with platinum (cisplatin–etoposide, docetaxel–carboplatin, CP), paclitaxel–gemcitabine, paclitaxel–ifosfamide, high-dose chemotherapy and RT [22].
1.3.9. Sex cord–stromal cell tumours
The most common variants are granulosa cells and Sertoli–Leydig cells tumours. Two mutations can be decisive in their identification: DICER-1 mutations in 60% of Sertoli–Leydig cells tumours, and FOXL2 mutations in most of the granulosa cells tumours.
A uterine curettage prior to fertility preservation surgery is necessary in order to rule out endometrial hyperplasia (55%) or endometrial adenocarcinoma (4–20%).
The adjuvant treatment is omitted in stages IA–B. In stage IC, chemotherapy is recommended if there are risk factors as poorly differentiated Sertoli–Leydig tumours, heterologous elements or size more than 10 cm. In stages II–IV, chemotherapy with CP or BEP regimen is indicated (a current trial is evaluating the best regimen between these two options SCST-01-NCT02429700-). RT with limited field can be also evaluated [23].
In the recurrent or metastatic setting, salvage surgery may be considered. Same chemotherapy combinations are used. Other alternative schemes are docetaxel, paclitaxel, paclitaxel and ifosfamide and VAC (vincristine, actinomycin D and cyclophosphamide). Hormonal therapy is an option, especially in limited recurrent disease, with aromatase inhibitors, diethylstilbestrol alternated with tamoxifen and GnRH agonists [24].
Bevacizumab was tested in a phase II trial with an RR of 16.7% and PFS of 9.3 months [25]. A trial is now active: Efficacy and safety of Bevacizumab (Avastin®) combined to weekly paclitaxel followed by Bevacizumab (Avastin®) alone in patients with relapsed ovarian sex cord–stromal tumours (ALIENOR-NCT01770301). Other targeted therapies are under evaluation (histone deacetylase inhibitors and anti-Müllerian hormone antibodies).
2. Hormonotherapy
Hormonal therapy (HT) is commonly used as a treatment option in patients with recurrent EOC who have exhausted or are not suitable for further standard lines of systemic chemotherapy.
The rate of ER positivity in EOC is reported to be 43–81%, depending on the definition and methodology used (the highest for endometrioid carcinoma and LGOC, intermediate for HGSC and the lowest for mucinous carcinoma and clear-cell carcinoma) [26]. Both ER and PgR expressions were associated with significantly improved survival in EOC.
Palieri et al. published a meta-analysis in 2017 with 53 trials and 2490 patients that shows a clinical benefit of 41% for aromatase inhibitors and 37% for progestins. A tendency in decreasing mortality was seen in the first line or in LGOC [27,28].
In LGOC, several retrospective trials have reported a clear benefit. Gersherson et al. found a median PFS of 26.4 months versus 64.9 months in HT arm that was dosed as a maintenance after primary surgery with and without residual disease. No statistically significant benefit was seen in OS [29].
Regarding those cases considered as platinum-resistant diseases, retrospective publications observe a PFS of 4 months, similar to that achieved with chemotherapy. The PARAGON (ANZGOG-0903), as a prospective phase II study, tested the activity of anastrozole in ER/PR-positive recurrent gynaecological tumours as a basket trial. It has shown a 6-month clinical benefit rate (CBR) of 61%, a 12-month CBR of 34% and median PFS of 11.1 months in LGOC and borderline tumours (36 patients) [30].
It is a well-tolerated therapy with low cost.
In Table 2 we collect the main trials and publications with HT in OC, and in Table 3 those specific for LGOC.
Table 2.
| Author | Year | Study | Disease stage | N | Treatment | ORR % |
CBR% | PFI m |
|---|---|---|---|---|---|---|---|---|
| Schwartz | 1982 | Phase II | Recurrent EOC | 13 | Tamoxifen 20 mg | 7.4 | 38.5 | |
| Weiner | 1987 | Phase II | Recurrent EOC | 31 | Tamoxifen 10 mg | 3.2 | 28.9 | |
| Hatch | 1991 | Phase II | Recurrent EOC | 105 | Tamoxifen 20 mg | 17.1 | 45.6 | |
| Bowman | 2002 | Phase II | Recurrent EOC | 50 | Letrozole 2.5 mg | 0 | 17 | |
| del Carmen | 2003 | Phase II | Recurrent EOC | 53 | Anastrozole 1 mg | 1.9 | 43.9 | |
| Papadimitriou | 2004 | Phase II | Recurrent EOC | 21 | Letrozole 2.5 mg | 15 | 29 | |
| Wagner | 2007 | Phase II | Platinum-resistant | 49 | Tamoxifen 40 mg + Gefinitib 500 mg | 0 | 32.7 | |
| Smyth | 2007 | Phase II | Recurrent, RE+ | 33 | Letrozole 2.5 mg | 9 | 51 | |
| Ramirez | 2008 | Phase II | Platinum-resistant | 31 | Letrozole 2.5 mg | 3 | 26 | |
| Argenta | 2009 | Phase II | Recurrent, RE+ | 26 | Fulvestrant | 0 | 50 | |
| Williams | 2010 | Cochrane | Recurrent EOC | 623 | Tamoxifen | 10 | 42 | |
| Stasenko | 2014 | Retrospective | Platinum-resistant | 99 | Any | – | – | 4.0 |
| Banerjee | 2016 | Phase II | Recurrent EOC | 42 | Abiraterone | 2 | 26 | |
| George | 2017 | Retrospective | Recurrent EOC | 97 | Tamoxifen 20–40 mg Letrozole 2.5 mg |
14 15 |
65 56 |
|
| Bonaventura | 2017 | Phase II | Platinum-resistant RE/RP+ |
49 | Anastrozole 1 mg | 0 | 27 | 2.7 |
Table 3.
| Author | Year | Study | Disease Setting | N | Treatment | RR % |
CBR % |
PFS mon |
|---|---|---|---|---|---|---|---|---|
| Gershenson | 2012 | Retrospective | Recurrent EOC | 64 | Tamoxifen 20 mg | 9 | 71 | 7.4 |
| Fader | 2017 | Retrospective | After primary surgery | 27 | Tam or Letrozole | – | – | 2-y: 82.8 |
| Gershenson | 2017 | Retrospective | After primary surgery | 203 | Tam or AI versus Observation | – | – | 64.9 vs 26.4 |
3. Targeted therapy
All the studies that have obtained molecular characterisation of EOC have identified a potentially targetable alterations. The most remarkable project is The Cancer Genome Atlas (TCGA) in HGSOC [31]. The main pathways are the homologous recombination deficiency, TP53 mutations and other cell-cycle alterations; in less percentage, Notch pathway (11%) and PI3K/RAS pathway alterations (45%) (see Table 4).
Table 4.
Main alterations described in different EOC subtypes [8].
| HGSOC | LGOC | Clear cell | Endometrioid | Mucinous | |
|---|---|---|---|---|---|
| Frequency | 70% | 3% | 12% | 11% | 3% |
| RE/RP | +/− | + | – | + | – |
| KRAS | – | +40% | + | +40% | +45% |
| BRAF | – | +5% | – | + | +5 |
| NRAS | – | + | |||
| p53 | +97% | – | – | – | |
| Via PI3K | +45% | +40% | + | +60% | + |
| Inactive PTEN | +3–8% | +33% | |||
| IGFR-1 | + | ||||
| Her2 | +15% | + | +18% | ||
| ARID1A | + | +19% | |||
| MSI | + | +19% |
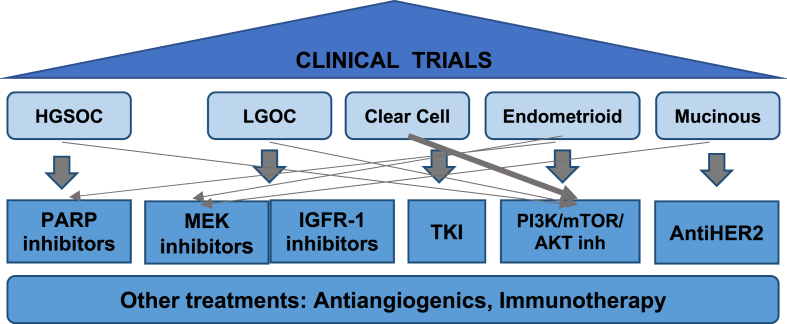
If we correlate the molecular profiling of each tumour with the most adequate treatment, several options can be discussed, and these therapies may be studied under clinical trials with new designs. In particular, in the most rare ovarian cancer tumours, the percentage of actionable somatic mutations reaches to 72% [32]. But, in all cases, an individual profile can be more accurate in selecting the optimal treatment. In this review, those therapies that are described in other parts of the supplement are excluded (see Fig. 2).
Fig. 2.
Ovarian cancer subtypes can be matched with different targeted therapies based on the molecular study findings, usually in clinical trials.
3.1. MEK inhibitors
These have been tested in several trials, mostly in combination with other targeted therapy. Some publications have suggested that MEK inhibitors may only be effective in patients with specific MAPK pathway alterations [33].
3.2. PI3K/mTOR/AKT inhibitors
All EOC subtypes present alterations in this pathway, especially clear-cell carcinoma [34]. A study with somatic PIK3CA mutations patients treated on various protocols involving mTOR pathway drugs showed two PR (22%) of nine EOC patients [35].
A recent review evaluated the activity of serine–threonine inhibitors against PI3K/mTOR/AKT pathways in EOC, reporting all drug regimens as a whole, an RR of 13%, and a CBR of 67%, a median PFS of 3.4 months and median OS of 13 months. The lowest RR was for AKT inhibitors [36].
The presence of RAS/BRAF mutations can be a biomarker for PI3K/mTOR/AKT inhibitors resistance. Metformin, an anti-diabetic agent typically used as an insulin sensitiser, is also being studied as an adjunct to cancer therapy due to its indirect inhibition of mTOR. These drugs can also be combined with anti-angiogenics and PARP inhibitors.
3.3. TYROSINE-KINASE inhibitors (TKI)
These are multi-targeted agents, with anti-angiogenic (see anti-angiogenics article), antiEGFR, antiSrc and other effects. These drugs are also being tested in clear-cell subtypes, due to their molecular profile [37].
Cabozantinib is a small-molecule TKI with potent activity towards MET and VEGFR2 and others including RET, KIT, AXL and FLT3. In a phase II trial designed for EOC (that included serous, endometrioid and clear-cell types), we observed an RR of 21% and median PFS of 5.5 months, for both patients with platinum-sensitive and platinum-resistant diseases [38].
3.4. Therapies against epidermal growth factor receptor
The PENELOPE trial tested Pertuzumab against placebo added to chemotherapy in platinum-resistant OC with low Her3 mRNA expression, resulting in lack of benefit [39].
Erlotinib, Gefitinib, Lapatinib, and Canertinib, have shown modest activity in several clinical trials. Another inhibitor, Neratinib, is ongoing in a trial targeting several tumors, including EOC, with EFR/HER2/HER3 mutations or EGFR amplification (NCT01953926).
3.5. Abagovomab
It is an anti-idiotypic antibody produced by a mouse hybridoma and generated against OC125, that causes a specific immune response (both tumoural and cellular). It was evaluated as a maintenance in advanced EOC in a phase III trial without benefit [40].
ADC are targeted against tumoural antigens that permit a direct delivery of the cytotoxic drug in cancer cells.
-
-
Alpha folate receptor (FOLR1) is expressed in ovarian (and endometrial) cancer cells, but rarely in normal cells. The phase III trials with FOLR1-targeting drugs, such as farletuzumab and vintafolide, have been disappointing. A new generation of drugs, such as Mirvetuximab Soravtansine (IMGN853), combines a folate receptor alpha-binding (FRα) antibody with maytansinoid, DM4. After phase I trial results, the FORWARD phase III study randomised patients who are not eligible for platinum-based regimens, to mirvetuximab soravtansine versus investigator's choice chemotherapy if FRα expression is positive. The results were presented recently without benefit in PFS for the experimental arm. MIRASOL trial is planned with a better selection of patients regarding FRα expression.
-
-
Mesothelin with ongoing trials.
-
-
Other: TROP-2; NOTCH3, MUC-16, Tissue factor: are in early stages of development.
3.6. Therapy against integrins
Several integrins have been identified as important mediators of EOC metastasis to the mesothelium, suggesting that use of integrin inhibitors could be a new therapeutic strategy to prevent the attachment of cancer cells to the peritoneal cavity [41]. Some examples are: Volociximab, against human α5β1-integrin, which showed insufficient activity; oncolytic adenovirus vector; Intetumumab, against αv-integrin family and anti-angiogenic, which also failed to phase II development; and conjugates of cytotoxic agents targeting several αvβ6-integrins.
4. Conclusions
The recognition of different ovarian cancer types as different diseases and their molecular features must lead to a personalised treatment. Alternative therapies may be evaluated beyond the standard therapy, frequently in clinical trials, and an individualised molecular study may help to design them. Due to its low incidence, international cooperation is crucial to achieve results.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of competing interest
Marta Gil-Martin has nothing to declare; Beatriz Pardo has nothing to declare; M Pilar Barretina reports personal fees from Roche, PharmaMar, Astra-Zeneca, Tesaro and Clovis, outside the submitted work.
Footnotes
This paper is part of a supplement supported by Pharma Mar S.A.
References
- 1.Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H. 4th ed. IARC WHO Classification of Tumours; 2014. WHO classification of tumours of female reproductive organs. 6. [Google Scholar]
- 2.du Bois A., Ewald-Riegler N., de Gregorio N., Reuss A., Mahner S., Fotopoulou C. Borderline tumours of the ovary: cohort study of the arbeitsgemeinschaft gynkologische onkologie AGO study group. Eur J Cancer. 2013;49:1905–1914. doi: 10.1016/j.ejca.2016.06.014. Eur J Cancer [Internet]. Elsevier; 2016 Sep 1;65:192–3. Available from: [DOI] [PubMed] [Google Scholar]
- 3.Reed N.S., Pautier P., Åvall-Lundqvist E., Choi C.H., Du Bois A., Friedlander M. Gynecologic cancer interGroup (GCIG) consensus review for ovarian small cell cancers. Int J Gynecol Cancer. 2014;24(9):S30–S34. doi: 10.1097/IGC.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 4.Ledermann J.A., Luvero D., Shafer A., O'Connor D., Mangili G., Friedlander M. Gynecologic cancer intergroup (GCIG) Consensus review for mucinous ovarian carcinoma. Int J Gynecol Cancer. 2014;24(9):S14–S19. doi: 10.1097/IGC.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 5.Gourley C., Farley J., Provencher D.M., Pignata S., Mileshkin L., Harter P. Gynecologic cancer intergroup (GCIG) consensus review for ovarian and primary peritoneal low-grade serous carcinomas. Int J Gynecol Cancer. 2014;24(9):S9–S13. doi: 10.1097/IGC.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 6.Grabowski Jacek P., Harter Philipp, Heitz Florian, du Bois A. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140(3):457–462. doi: 10.1016/j.ygyno.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Schmeler K.M., Sun C.C., Bodurka D.C., Deavers M T., Malpica A., Coleman R.L. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol [Internet] 2008 Mar 1;108(3):510–514. doi: 10.1016/j.ygyno.2007.11.013. Elsevier. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Groen R.S., Gershenson D.M., Fader A.N. Updates and emerging therapies for rare epithelial ovarian cancers: one size no longer fits all. Gynecol Oncol [Internet] 2015;136(2):373–383. doi: 10.1016/j.ygyno.2014.11.078. Elsevier Inc. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Gershenson D.M., Bodurka D.C., Coleman R.L., Lu K.H., Malpica A., Sun C.C. Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum. J Clin Oncol. 2017;35(10):1103–1111. doi: 10.1200/JCO.2016.71.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canaz E., Grabowski J.P., Richter R., Braicu E.I., Chekerov R., Sehouli J. Survival and prognostic factors in patients with recurrent low-grade epithelial ovarian cancer: an analysis of five prospective phase II/III trials of NOGGO metadata base. Gynecol Oncol. 2019;154:539–546. doi: 10.1016/j.ygyno.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Grisham R.N., Iyer G., Sala E., Zhou Q., Iasonos A., DeLair D. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer [Internet] 2014 Jul 1;24(6):1010. doi: 10.1097/IGC.0000000000000190. http://ijgc.bmj.com/content/24/6/1010.abstract LP-1014. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong K.-K., Tsang Y.T.M., Deavers M.T., Mok S.C., Zu Z., Sun C. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol [Internet] 2010 Oct 1;177(4):1611–1617. doi: 10.2353/ajpath.2010.100212. Elsevier. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farley J., Brady W., Vathipadiekal V., Lankes H., Coleman R., Morgan M. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14(2):134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershenson D.M., Miller A., Brady W., Paul J., Carty K., Rodgers W. ----; 2019. A randomized phase II/III study to assess the efficacy of trametinib in patients with recurrent or progressive low-grade serous ovarian or peritoneal cancer ESMO meeting; p. LBA61. [Google Scholar]
- 15.Harter P., Gershenson D., Lhomme C., Lecuru F., Ledermann J., Provencher D.M. Gynecologic cancer InterGroup (GCIG) consensus review for ovarian tumors of low malignant potential (Borderline Ovarian Tumors) Int J Gynecol Cancer. 2014;24(9):S5–S8. doi: 10.1097/IGC.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 16.Berton-Rigaud D., Devouassoux-Shisheboran M., Ledermann J.A., Leitao M.M., Powell M.A., Poveda A. Gynecologic cancer intergroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int J Gynecol Cancer. 2014;24(9):S55–S60. doi: 10.1097/IGC.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 17.Shylasree T.S., Bryant A., Athavale R. Chemotherapy and/or radiotherapy in combination with surgery for ovarian carcinosarcoma. Cochrane Database Syst Rev. 2013;2:CD006246. doi: 10.1002/14651858.CD006246.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasspool R.M., Martín A.G., Millan D., Lorusso D., Avall-Lundqvist E., Hurteau J.A. Gynecologic cancer intergroup (GCIG) consensus review for squamous cell carcinoma of the ovary. Int J Gynecol Cancer. 2014;24(9):S26–S29. doi: 10.1097/IGC.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 19.Reed N.S., Gomez-Garcia E., Gallardo-Rincon D., Barrette B., Baumann K., Friedlander M. Gynecologic cancer InterGroup (GCIG) consensus review for carcinoid tumors of the ovary. Int J Gynecol Cancer. 2014;24(9):S35–S41. doi: 10.1097/IGC.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 20.Shrimali R.K., Shaikh G., Reed N.S. Malignant struma ovarii: the west of Scotland experience and review of literature with focus on postoperative management. J Med Imaging Radiat Oncol. 2012;56(4):478–482. doi: 10.1111/j.1754-9485.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 21.Harrison M.L., Hoskins P., du Bois A., Quinn M., Rustin G.J.S., Ledermann J.A. Small cell of the ovary, hypercalcemic type; Analysis of combined experience and recommendation for management. A GCIG study. Gynecol Oncol [Internet] 2006 Feb 1;100(2):233–238. doi: 10.1016/j.ygyno.2005.10.024. Elsevier. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Brown J., Friedlander M., Backes F.J., Harter P., O'Connor D.M., De La Motte Rouge T. Gynecologic cancer intergroup (GCIG) consensus review for ovarian germ cell tumors. Int J Gynecol Cancer. 2014;24(9):S48–S54. doi: 10.1097/IGC.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 23.Ray-Coquard I., Brown J., Harter P., Provencher D.M., Fong P.C., Maenpaa J. Gynecologic cancer intergroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int J Gynecol Cancer. 2014;24(9):S42–S47. doi: 10.1097/IGC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 24.van Meurs H.S., van Lonkhuijzen L.R.C.W., Limpens J., van der Velden J., Buist M.R. Hormone therapy in ovarian granulosa cell tumors: a systematic review. Gynecol Oncol [Internet] 2014 Jul 1;134(1):196–205. doi: 10.1016/j.ygyno.2014.03.573. Elsevier. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Brown J., Brady W.E., Schink J., Van Le L., Leitao M., Yamada S.D. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120(3):344–351. doi: 10.1002/cncr.28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieh W., Köbel M., Longacre T.A., Bowtell D.D., deFazio A., Goodman M.T. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol [Internet] 2013 Aug 1;14(9):853–862. doi: 10.1016/S1470-2045(13)70253-5. Elsevier. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paleari L., Gandini S., Provinciali N., Puntoni M., Colombo N., DeCensi A. Clinical benefit and risk of death with endocrine therapy in ovarian cancer: a comprehensive review and meta-analysis. Gynecol Oncol [Internet] 2017;146(3):504–513. doi: 10.1016/j.ygyno.2017.06.036. The Authors. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Fader A.N., Bergstrom J., Jernigan A., Tanner E.J., Roche K.L., Stone R.L. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: reducing overtreatment without compromising survival? Gynecol Oncol [Internet] 2017;147(1):85–91. doi: 10.1016/j.ygyno.2017.07.127. Elsevier Inc. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Gershenson D.M., Sun C.C., Iyer R.B., Malpica A.L., Kavanagh J.J., Bodurka D.C. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2012;125:661–666. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang M., O'Connell R.L., Amant F., Beale P., McNally O., Sjoquist K.M. PARAGON: a Phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol. 2019;154:531–538. doi: 10.1016/j.ygyno.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Network T.C.G.A.R., Bell D., Berchuck A., Birrer M., Chien J., Cramer D.W. Integrated genomic analyses of ovarian carcinoma. Nature. 2011 Jun 29;474:609. doi: 10.1038/nature10166. [Internet]. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Freixinos V., Lheureux, Mandilaras V., Clarke B., Dhani N.C., Mackay H. Impact of somatic molecular profiling on clinical trial outcomes in rare epithelial gynecologic cancer patientsGynecol. Oncol Times. 2019;153:304–311. doi: 10.1016/j.ygyno.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Wainberg Z.A., Alsina M., Soares H.P., Braña I., Britten C.D., Del Conte G. A multi-arm phase I study of the PI3K/mTOR inhibitors PF-04691502 and gedatolisib (PF-05212384) plus irinotecan or the MEK inhibitor PD-0325901 in advanced cancer. Target Oncol [Internet] 2017;12(6):775–785. doi: 10.1007/s11523-017-0530-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto A., Glasspool R.M., Mabuchi S., Matsumura N., Nomura H., Itamochi H. Gynecologic cancer intergroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2014;24(9):S20–S25. doi: 10.1097/IGC.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 35.Janku F., Wheler J.J., Westin S.N., Moulder S.L., Naing A., Tsimberidou A.M. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol [Internet] 2012;30(8):777–782. doi: 10.1200/JCO.2011.36.1196. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciccone M.A., Maoz A., Casabar J.K., Machida H., Mabuchi S., Matsuo K. Clinical outcome of treatment with serine-threonine kinase inhibitors in recurrent epithelial ovarian cancer: a systematic review of literature. Expert Opin Investig Drugs [Internet] 2016;25(7):781–796. doi: 10.1080/13543784.2016.1181748. Taylor & Francis. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntanasis-Stathopoulos I., Fotopoulos G., Tzanninis I.G., Kotteas E.A. The emerging role of tyrosine kinase inhibitors in ovarian cancer treatment: a systematic review. Cancer Invest [Internet] 2016;34(7):313–339. doi: 10.1080/07357907.2016.1206117. Taylor & Francis. Available from: [DOI] [PubMed] [Google Scholar]
- 38.Vergote I.B., Smith D.C., Berger R., Kurzrock R., Vogelzang N.J., Sella A. A phase 2 randomised discontinuation trial of cabozantinib in patients with ovarian carcinoma. Eur J Cancer [Internet] 2017 Sep 1;83:229–236. doi: 10.1016/j.ejca.2017.06.018. Elsevier. Available from: [DOI] [PubMed] [Google Scholar]
- 39.Kurzeder C., Bover I., Marḿe F., Rau J., Pautier P., Colombo N. Double-blind, placebo-controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 mRNA-Expressing platinum-resistant ovarian Cancer (PENELOPE) J Clin Oncol. 2016;34(21):2516–2525. doi: 10.1200/JCO.2015.66.0787. [DOI] [PubMed] [Google Scholar]
- 40.Sabbatini P., Harter P., Scambia G., Sehouli J., Meier W., Wimberger P. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO-the MIMOSA study. J Clin Oncol. 2013;31(12):1554–1561. doi: 10.1200/JCO.2012.46.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi Masaki, Sawada Kenjiro, TTadashi K. Potential of integrin inhibitors for treating ovarian cancer: a literature review. Cancers (Basel) 2017;6(12):83. doi: 10.3390/cancers9070083. [DOI] [PMC free article] [PubMed] [Google Scholar]