Abstract
Ovarian epithelial cancer (OEC) is the most lethal gynecologic malignancy. Despite current chemotherapeutic and surgical options, this high lethality can be attributed to multiple factors, including late-stage presentation. In order to optimize OEC treatment, it is important to highlight that it is composed of five main subtypes: high-grade serous ovarian carcinoma (HGSOC), low-grade serous ovarian carcinoma (LGSOC), endometrioid ovarian carcinoma (EOC), ovarian clear cell carcinoma (CCOC), and mucinous ovarian carcinoma (MOC). These subtypes differ in their precursor lesions, as well as in epidemiological, morphological, molecular and clinical features. OEC is one of the tumours in which most pathogenic germline mutations have been identified. Accordingly, up to 20% OC show alterations in BRCA1/2 genes, and also, although with a lower frequency, in other low penetrance genes associated with homologous recombination deficiency (HRD), mismatch repair genes (Lynch syndrome) and TP53. The most important prognostic factor is the 2014 FIGO staging, while older age is also associated with worse survival. HGSOC in all stages and CCC and MOC in advanced stages have the worse prognosis among histological types. Molecular markers have emerged as prognostic factors, particularly mutations in BRCA1/2, which are associated with a better outcome. Regarding treatment, whereas a proportion of HGSOC is sensible to platinum-based treatment and PARP inhibitors due to HRD, the rest of the histological types are relatively chemoresistant. New treatments based in specific molecular alterations are being tested in different histological types. In addition, immunotherapy could be an option, especially for EOC carrying mismatch repair deficiency or POLE mutations.
Keywords: Ovarian cancer, Molecular markers, Histological groups, Hereditary cancer syndromes
Highlights
-
•
The five different histological types have different precursor lesions and epidemiological, morphological, genetic, epigenetic and clinical features.
-
•
Histological type is an important prognostic factor.
-
•
Drugs targeting homologous recombination deficiency have been approved for treatment.
-
•
The use of immunotherapy is limited due to lack of predictive biomarkers
1. Introduction
Ovarian epithelial cancer (OEC), as other malignant epithelial tumours in other organs, is a heterogeneous disease. Criteria for its classification has evolved during the last decades from a pure histogenetic approach to the current vision in which each major histological type is associated with different precursor lesions and specific epidemiological, morphological, genetic, epigenetic and clinical features [1,2]. Currently, five major histological types of EOC are recognized, including high-grade serous ovarian carcinoma (HGSOC), low-grade serous ovarian carcinoma (LGSOC), mucinous ovarian carcinoma (MOC), ovarian clear cell carcinoma (CCOC) and endometrioid ovarian carcinoma (EOC) [3]. Although HGSOC is the most common histological type, it is important to remember that the frequency of OEC histotypes depends on the stage at diagnosis. Thus, in early-stage OEC (FIGO stages I-IIC), the frequencies of HGSOC, EOC and CCOC are similar, according to both our own data in the RECLAMO cohort and previously reported series [4] (see Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
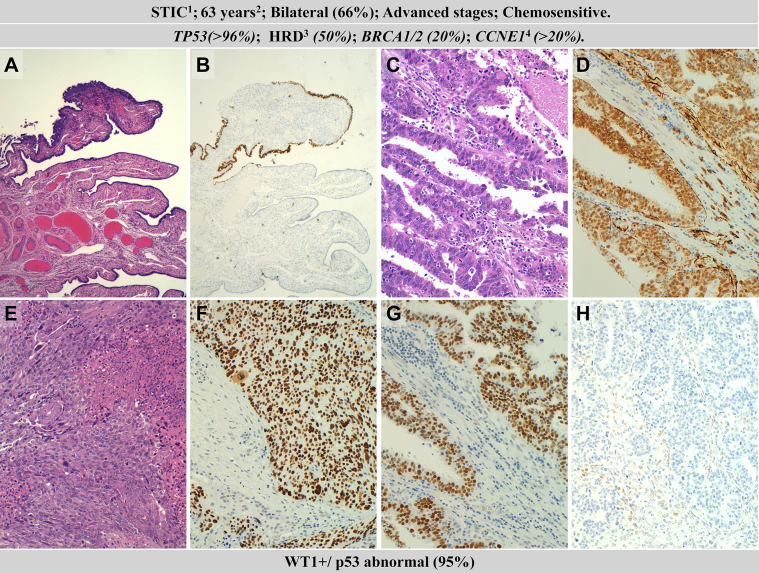
Fig. 1.
Clinicopathological and molecular features of high-grade serous ovarian carcinomas (HGSOC). Hematoxylin–eosin staining (A) and p53 staining of serous tubal intraepithelial carcinoma (STIC) of the Fallopian tube; HGSOC with a glandular pattern (C) and with WT1-positive expression (D); HGSOC with a solid pattern (E) and with WT1-positive expression (F); p53 mutated pattern abnormal (overexpression) (G), and p53 mutated pattern (null) (note focal positivity in some stromal cells) (H). 1precursor: serous tubal intraepithelial carcinoma, 2median age at diagnosis; 3homologous recombination deficiency; 3amplification.
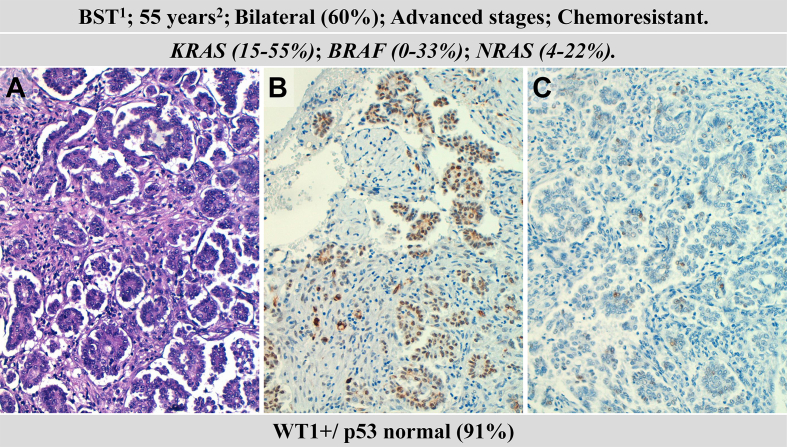
Fig. 2.
Clinicopathological and molecular features of a low-grade serous ovarian carcinoma (LGSOC). Hematoxylin–eosin staining (A); WT1-positive expression (B); wild-type pattern of p53 expression (C). 1precursor: borderline serous tumour, 2median age at diagnosis.
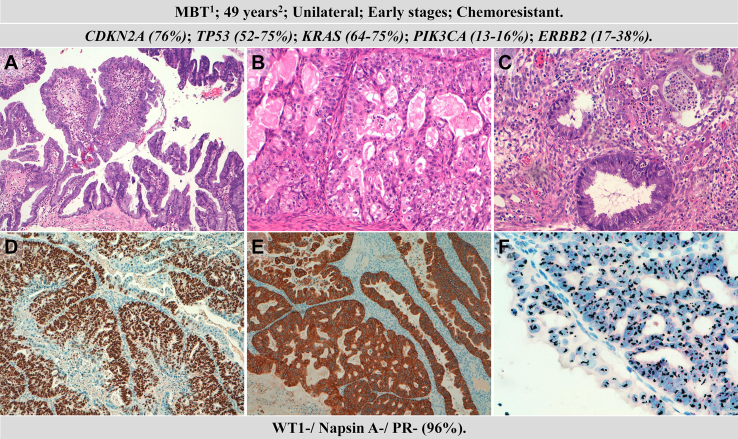
Fig. 3.
Clinicopathological and molecular features of mucinous ovarian carcinomas (MOC). Hematoxylin–eosin staining of a borderline tumour (A); MOC with expansive (B) and infiltrating pattern (C); mutated p53 expression (D), HER2 overexpression (E) and silver in situ hybridization (SISH) of ERBB2 amplification (F). 1precursor: mucinous borderline tumour, 2median age at diagnosis.
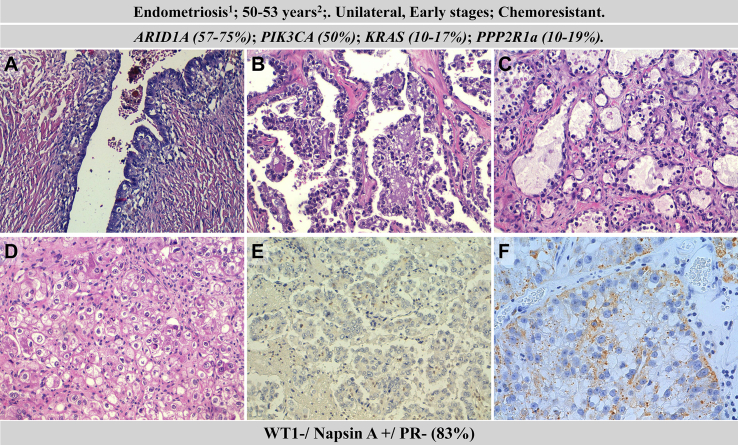
Fig. 4.
Clinicopathological and molecular features of clear ovarian clear cell carcinoma (CCOC). Hematoxylin–eosin staining of endometriosis (A), CCOCs with glandular (B), papilar (C) and solid (D) pattern; lack of Arid1A expression (note positive staining in inflammatory cells) (E); expression of Napsin A (F). 1precursor, 2median age at diagnosis.
Fig. 5.
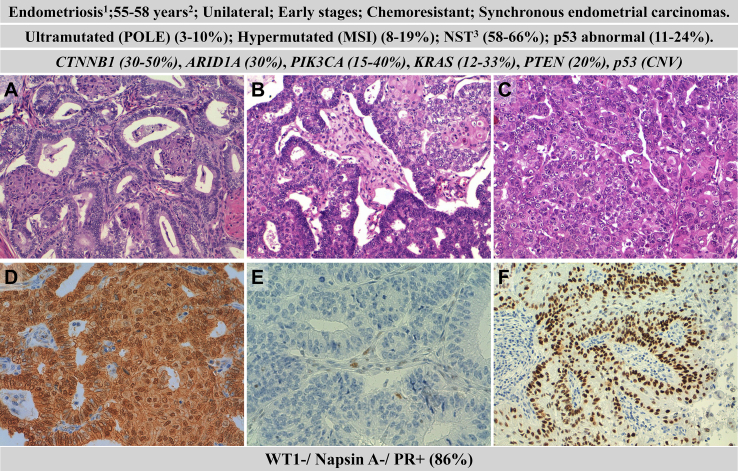
Clinicopathological and molecular features of endometrioid ovarian carcinomas (EOC). Hematoxylin–eosin staining of grade 1 (A), grade 2 (B) and grade 3 (C) EOCs; beta-catenin nuclear expression (D); loss of MSH6 (E); p53 wild-type expression (F). 1precursor, 2median age at diagnosis, 3no specific type.
Due to the high frequency of HGSOC, there is much research focused on this particular subtype. However, in order to optimize treatment, it is important to consider the characteristics of other histological types. The goal of this review is to highlight the most relevant histopathological and molecular features that define each major type of EOC and that could have an impact on the treatment of patients.
2. Epidemiology
Ovarian cancer is not the most frequent gynaecological cancer in women, but it is the most lethal. Thus, in countries with a high human development index (HDI) the most frequent gynaecological cancer is endometrial carcinoma, linked to obesity, while in countries with a low HDI, the most prevalent one is cervical cancer linked to HPV infection and limited screening strategies (GLOBOCAN) [5].
GLOBOCAN reported in 2018 an incidence of near 240.000 new OEC cases per year, representing 3.6% of all cancer cases worldwide. OEC was responsible of approximately 150.00 deaths yearly, representing 4.3% of all cancer deaths [5]. In Europe, the incidence was of 67,771 new cases per year and 44,576 deaths per year. These figures indicate that OEC is a highly lethal neoplasm, a fact partially due to the absence of specific early symptomatology and the lack of effective screening methods, which are responsible for a disproportionate number of OEC that are diagnosed in advanced stages.
The most important risk factor for developing OEC is inheritance (see below). Other risk factors include age beyond 63, infertility, increased body mass index, endometriosis [6] and tobacco [7]. Some studies have suggested an association with perineal talc application [8]. In addition, there are protective factors, such as gravidity, the use of oral contraceptives [9] and tubal ligation. The regular use of aspirin or nonsteroidal anti-inflammatory agents has also been associated with a reduced risk of OEC [10], which suggests their possible use in chemoprevention. It is important to note that most risk factors show substantial heterogeneity across the five histologic subtypes indicating different aetiologies for each subtype [8].
It is important to note that most risk factors show substantial heterogeneity across the five histologic subtypes indicating different etiologies for each subtype [8]. The most important risk factor for developing EOC is inheritance (see below). Nulliparity, early menarche or late menopause, which would all increase the total number of times od ovulation in a woman’s lifetime, increase the risk of ovarian cáncer. Other risk factors include age beyond 63, infertility, increased body mass index, endometriosis (associated with an increased risk of EOC and CCOC) [6] and tobacco (associated with an increased risk of MOC) [7]. Some studies have also suggested an association with perineal talc application [8]. In addition, there are protective factors, such as gravidity, the use of oral contraceptives [9], tubal ligation, and the regular use of aspirin or nonsteroidal anti-inflammatory agents [10]. A meta-analysis of 40 studies on the association between breastfeeding and ovarian cancer found a significant decrease in risk in women who did or did not breastfeed [11].
3. High grade serous ovarian carcinoma (HGSOC)
HGSOC is the most common histological type of OEC, it typically occurs in postmenopausal women with a mean age of 63 years, and most cases present in advanced stages. From a morphological point of view, HGSOC is composed of highly proliferative and markedly atypical cells. Although they typically adopt a papillary growth pattern, solid, glandular (pseudoendometrioid) and transitional patterns can also occur. These non-papillary patterns seem to be more frequent in BRCA1 mutation carriers [12].
Serous intraepithelial carcinoma (STIC) is a well-recognised precursor lesion of HGSOC. The identification of this lesion requires the systematic histological study of both Fallopian tubes by using the detailed Sectioning and Extensively Examining the FIMbriated End (SEE-FIM) protocol which should be performed in all cases of extrauterine HGSOC and risk-reducing prophylactic surgery [13]. STIC is present in 5%–10% of risk-reducing salpingo-oophorectomies from BRCA1 germline carriers [14], and according to a meta-analysis, in 11%–61% of HGSOC cases [15]. However, this figure is probably higher if we consider that even after using the SEE-FIM protocol a substantial portion of tissue remains to be histologically analysed. Recently, it has been proposed that earlier precursors, such as p53 signature (one small stretch of 12 or more cells with a TP53 mutation) and serous tubal intraepithelial lesions (early proliferative lesion without marked atypia characteristic of STIC) might “escape” progression into the tubal epithelium to develop STIC and, instead, develop transformation into extratubal locations, such as the ovary and peritoneum [16].
The concept of STIC as the precursor lesion of HGSOC, although not universally accepted for all cases, has important clinical implications, such as the use of opportunistic salpingectomy with ovarian preservation during routine hysterectomy for benign diseases, and the assignment of the primary site (Fallopian tube, ovary or peritoneum) of an extrauterine HGSOC [15].
Another important issue related to the histopathology of HGSOC is the evaluation of pathological response after neoadjuvant chemotherapy, a therapeutic modality that is being used with increasing frequency. The chemotherapy response score (CRS) is a reproducible, validated three-tiered morphological scoring system to assess the response of HGSOC to treatment. The interobserver agreement is shown to be substantial following online training, and women with CRS3 have significantly improved progression-free and overall survival [17].
The most frequent and early constant molecular alteration in HGSOC is TP53 mutation. This alteration is responsible for a high degree of chromosome instability that leads to frequent gains and losses of chromosomal regions. Accordingly, the amplification in oncogenes, such as CCNE1, MYC and MECOM is relatively common [18]. Approximately 50% of HGSOCs exhibit homologous recombination (HR) deficiency due to genetic or epigenetic alterations in the FANCONI–BRCA pathway. Germ-line BRCA1 and BRCA2 mutations are the most common alterations and are present in approximately 15–20% of HGSOCs, whereas somatic BRCA1 and BRCA2 mutations have been identified in 6%–7% of HGSOCs [[18], [19], [20], [21], [22]]. In addition, BRCA1 promoter hypermethylation has been reported in approximately 10%–20% of HGSOCs and is mutually exclusive of BRCA1/2 mutations, suggesting that there is strong selective pressure to inactivate BRCA via either mutation or epigenetic silencing in this disease [23]. Other HR pathway alterations include mutations in several Fanconi anemia genes, in core HR RAD genes, and in DNA damage response genes involved in HR, such as ATM, ATR, CHEK1, and CHEK2. Defective HR in OC may also occur via alterations in nonbona fide HR genes that are known to modulate the HR pathway, and thus, cause HR deficiency indirectly, such as PTEN deficiency, EMSY amplification and others [24]. According to the overall pathway analysis, more than half of HGSOCs show alterations of major genes involved in HR, which indicates that these tumours might be treatable with PARP inhibitors [25]. It is interesting to note that a resistance mechanism to PARP inhibitors that has been found in human tumours is genetic reversion that corrects or bypasses the original BRCA1-or BRCA2-inactivating mutation [26].
CCNE1-amplified cancers represent a large subgroup (20%) of poor prognosis HGSOC that lack BRCA mutations and HR deficiency. Thus, CCNE1 amplification is common in primary resistant and refractory HGSOC. Tumours without HR deficiency or CCNE1 amplification are commonly resistant to platinum-based therapy and have a poor prognosis [27].
Chen et al. analysed 45 HGSOC using high-resolution melting PCR (HRM-PCR) and NGS. They found mutations in MET (11%), PIK3CA (7%) and NRAS (4%), but none in KRAS or BRAF [28].
Several gene expression-based studies have identified four distinct HGSOC molecular subtypes C1/Mesenchymal, C2/Immunoreactive, C4/Differentiated, and the C5/Proliferative [[29], [30], [31]]. Whereas the mesenchymal and proliferative groups are associated with poor prognosis, the Immunoreactive and Differentiated groups have more favourable outcomes. At present, the clinical utility of this classification is limited due to the use of different non-standardized classification methods.
4. Low grade serous carcinoma
LGSOC presents typically in younger patients than high-grade serous ovarian cancer (HGSOC) with a median age at diagnosis of 43–55 years. In addition, LGSOC has a more indolent course than HGSOC, resulting in a longer overall survival (OS). However, LGSOC is more resistant to chemotherapy and has less than 5% response rate to first-line chemotherapy compared to the 80% response rate of HGSOC [32,33].
Cells of LGSOC resemble those of the fallopian tube, have mild to moderate nuclear atypia and a mitotic index of up to 12 mitoses per 10 high-powered fields. A borderline serous tumour (BST) is considered to be the precursor lesion of LGSOC and differs from it by the absence of stromal infiltration greater than 10 mm2.
LGSOC present few recurrent molecular alterations [34]. The most commonly mutated genes are KRAS and BRAF, with these mutations being mutually exclusive [25,34,35]. Singer et al., in 2003, reported the first analysis of KRAS and BRAF mutations in LGSOC [36], and since then, many studies have confirmed the presence of these mutations in LGSOC [33,[37], [38], [39]]. The frequency of mutations ranged from 0% to 32% (mean 7.8%) for BRAF and 15.4%–54.5% (mean 24.2%) for KRAS mutations. BRAF mutations are more frequent in SBT and early-stage LGSOC tumours and seem to be associated with a better prognosis [33]. Additional mutations in the MAPK pathway includes NRAS mutations, reported at a frequency of 3.6%–22% (mean 8.3%) [33,35]. Hunter et al. conducted whole-exome sequencing in 19 LGSOC cases and identified in addition to mutations in KRAS, BRAF, and NRAS, somatic mutations in USP9X and EIF1AX [35]. The latter two genes have both been linked to regulation of mTOR, suggesting that mTOR inhibitors may be useful in combination with MEK or RAF inhibitors in LGSOC.
The presence of CNV in LGSOC has been analysed and reviewed by Van Nieuwenhuysen [33]. The most frequently reported whole chromosome aberrations were loss of 1p (33%), 6q (24%), 9p (21%), 16p/q (21%), 17p (24%), 18p/q (21%), and 22q (40%) and gain in 1q (40%), 7p/q (26%), and 8q (29%). Loss of 1p36.33 was the most frequent focal alteration (54.1%). This region is frequently altered in a broad range of human cancers.
5. Mucinous ovarian carcinoma (MOC)
Malignant mucinous carcinoma in the ovary more frequently represent metastasis from gastrointestinal tumours. Primary MOCs tend to present as stage 1 large unilateral tumours.
The most common type of MOC is the intestinal variant, composed of cells resembling the gastrointestinal epithelium, which possess intracytoplasmic mucin and adopt a wide range of morphological patterns. There is little evidence for grading MOC; however, if grading is reported, it is recommended to use the same grading system that is used for endometrioid carcinomas [40]. It is also controversial whether the pattern of invasion in stage 1 MOC has prognostic significance. Some studies have shown that the expansive pattern is not associated with lymph node metastases and have a good prognosis, whereas the infiltrative pattern has a worse prognosis and is associated with lymph node metastases [41]. In spite of the conflicting results [42], the pattern of invasion in mucinous ovarian carcinomas should be recorded.
A recent study using both whole-genome sequencing for discovery and target sequencing for validation in a large cohort of MOC have demonstrated that the most frequent molecular alteration in this histological type affects CDKN2A gene (76% of cases) [43] Approximately 60% of MOC with CDKN2A alterations carried homozygous deletions, whereas the remaining 40% had mutations, more frequently of truncating type. In addition to CDKN2A alterations, mutations in KRAS and TP53 (both 64%), amplification of ERBB2 (26% of cases) and mutations in RNF43, BRAF, PIK3CA and ARID1A (8–12% of cases) were the next most frequent alteration in this series [43].
Previous studies have reported frequencies of TP53 mutations in 52%–75%, KRAS mutations in 64–75%, PIK3CA mutations in 13%–16%, and ARID1A mutations in 16%. Mueller et al. [44] reported that 25% and 8% of MOCs carried homozygous deletions and mutations in CDKN2A, respectively. Regarding HER2, the frequency of HER2 overexpression and gene amplification ranged from 17% to 38% in different series, being higher in the Asian population [[45], [46], [47]].
The comparison of the frequencies of these alterations between mucinous borderline tumours (MBTs) and MOCs, demonstrated that only TP53 mutations play a role in the progression from MBT to MOC since this alteration is only found in 10%–18% of MBTs [48,49]. In addition, Cheasley et al. (2019) reported that the fraction of the genome altered by copy number variation increases significantly from MBT to MOC and also with a grade in MOCs, and it was associated with patient outcome [43]. Specific copy number alterations associated with progression from MBT to grade 1 MOC were losses at 9pter-p21.2 and 17p. Increased 17p loss likely reflected the increased prevalence of TP53 mutations in MOC. Whereas loss of CDKN2A (9p21.3) was an early event in mucinous ovarian carcinogenesis, commonly seen in benign mucinous and MBT precursors, an expanded area of a deletion encompassing most of the chromosomal arm was enriched in MOC. This result suggests that other tumour suppressor genes may be located on chromosome 9 that is important for invasive progression. In addition, the copy number alterations most strongly enriched in grade 3 MOC were gains of 1p and 19p, affecting multiple oncogenes including JUN, JAK1, MYCL and BRD4 [43].
6. Ovarian clear cell carcinoma (CCOC)
Ovarian clear cell carcinoma (CCOC) shows a higher prevalence in women in Asia, and it is associated with a reduced response rate following platinum-based chemotherapy and a poorer prognosis, especially in an advanced stage, than other histological subtypes of OEC [50].
Histologically, it is composed of polygonal cells containing clear cytoplasm, in a ‘‘hobnail’’ arrangement, with an enlarged nucleus and prominent nucleolus. However, pleomorphism and mitotic index tend to be low. CCOC displays different architectural patterns, such as tubulocystic, papillary or solid. These conventional histopathological parameters have not been shown to be prognostically informative, and CCOC is considered to be a high-grade tumour [50].
Endometriosis has been associated with 33%–37% of OCCC, and the presence of endometriosis has a relative risk of 3.37 (1.24–9.14) for clear cell carcinoma [51]. Molecular studies have demonstrated the presence of somatic mutations in the epithelial cells of ovarian endometriosis, affecting more frequently the PIK3CA, KRAS and ARID1A genes [52].
The most frequently mutated gene in CCOC is ARID1A. Studies using Sanger sequencing reported mutations in 46% of the cases [53]. By using NGS techniques, slightly higher frequencies have been found (57–75%) [[54], [55], [56], [57]]. PIK3CA is the second most frequently mutated gene in CCOC: it is mutated in 50% of the tumours according to the latest studies by NGS [[56], [57], [58]]. According to in vivo studies, the inactivation of ARID1A is not effective for tumour initiation, and thus, additional alterations, such as mutations in PIK3CA, would be required for tumour evolution [59]. KRAS (10–17%) and PPP2R1a (10–19%) are other frequently mutated genes in CCOC [[56], [57], [58]]. Other less frequently mutated genes but detected in more than one study included PTEN (1–5%) and ARID1B (10–12.5%) [55,56,60]. The frequency of TP53 mutations is lower in CCOC than in the other histological types. Results from studies were Sanger sequencing was used show frequencies between 5 and 15% [[60], [61], [62]], while in NGS studies, mutations in TP53 have been reported in between 18 and 20% of the cases [58,63]. ERBB2 amplification has also been observed in 14% of CCOC [64]. Friedlander et al. highlight the frequent alterations in the PIK3CA/Akt/mTOR pathway in CCOCs and also the molecular heterogeneity of this group; the authors also pointed out the potential therapeutic targets to be tested in clinical trials in order to enable better selection of therapeutic options [58]. Although the frequency of MMRD in CCC differs among series, two recent studies based on a large number of cases have reported that 2.8% of CCOC lose at least one MMR protein [65,66].
7. Endometrioid ovarian carcinoma (EOC)
Endometriosis is a well-recognized precursor of EOC, and it has been reported that approximately 25% of patients with EOC have concomitant endometriosis [52]. Histologically, EOCs are typically formed by adjacent glands, similar to those observed in endometrioid endometrial carcinoma (EEC), with a confluent (cribriform) pattern. They sometimes exhibit squamous morules, mucinous differentiation and secretory, fusiform, ciliated, oxyphilic or clear cells. Similarly to its endometrial counterpart, three grades of differentiation have been established based on the percentage of a solid component (G1 <5%, G2 5%–50%, G3>50%). Although a binary classification has been proposed, including only low-grade carcinoma (G1 EOC) and high-grade carcinoma (G2 and G3 EC) [67], there is still controversy in considering G2 EOC as low or high-grade tumours [4,68,69].
Ten to thirty percent of OECs are associated with endometrioid endometrial cancer. Although there has been considerable controversy regarding the relationship/origin of such tumours, recent genomics studies have demonstrated that almost all such tumours are clonally related and likely the ovarian tumour developed from endometrial transformed cells reaching the ovary through transtubaric reflux [70,71]. It is important to note that outcomes of low-grade synchronous ovarian and endometrial endometrioid carcinomas, when confined to ovary and uterine corpus, are similar to that of solitary organ-confined endometrial or ovarian carcinoma, suggesting that the cells that spread to the ovary do not possess the ability to invade the circulation and thus colonise distant sites. The term ‘pseudometastasis’ has been proposed for this restricted dissemination and underlies the favourable prognosis seen in synchronous endometrial and ovarian carcinomas [72].
EOC shows the same spectrum of mutated genes than EEC but at different frequencies. For example, PTEN is more frequently mutated in EEC than EOC, but CTNNB1 is more frequently mutated in EOC than EEC [73,74]. EOC is frequently associated with mutations in CTNNB1 (30–50%), ARID1A (30%), PIK3CA (15–40%), KRAS (12–33%), PTEN (20%), and TP53 (6–24%) among others [25,50]. In addition, 10%–18% of EOC show MMRD [66]. The frequency of mutations in each gene varies depending on the histological grade. Thus, whereas in FIGO grade 1, EOC CTNNB1 is frequently mutated, but TP53 mutations are infrequent, the contrary pattern occurs in FIGO grade 3 EOC [75].
Recently, the new molecular classification of EEC has also been tested for EOC [[76], [77], [78]]. Based on a combination of mutations in POLE, expression of p53 and MMR protein expression, EOC could thus be classified into four groups: POLE mutated, MMR abnormal, p53 abnormal, and Nonspecial Type (NST, those without POLE mutations, MMRD or p53 abnormal expression). According to previous studies [79,80] and the results of the RECLAMO series (manuscript in preparation), the frequency of the four molecular groups was: 58%–61% NST, 11%–24% p53 abnormal, 8%–19% MMR abnormal, and 3%–10% POLE mutated. Interestingly, the frequency of POLE mutated and MMR abnormal tumours was lower in EOC than in EEC.
This classification could have important prognostic and therapeutic implications. Thus, no deaths were observed among POLE mutated tumour in these series [79,80], and the use of immunotherapy should be an option in POLE mutated and MMR abnormal tumours.
8. Refining EOC histotyping
Taking into account the differences among the major histological types of OEC, it is of great importance, both in the clinical and research settings, to achieve an appropriate histological typing of all tumours. By applying current morphological criteria, a high to medium reproducibility among Pathologists in assigning the correct histological type has been reported [81]. Assignation of the histological type can be difficult in some situations, such as to differentiate between LGSC and HGSOC in tumours with intermediate atypia, or HGSOC with a glandular or solid pattern from EOC, or EOC with extensive mucinous differentiation from MOC, or COCC from other histological types with clear cells. The most frequent discordance between the initial and final diagnosis is found in the differential diagnosis of endometrioid and serous carcinomas. In this sense, between 20% and 30% of the tumours initially diagnosed as EOC, are reclassified as HGSOC [82].
Histotype classification agreement notably improves by using a panel of immunohistochemical markers that includes at least WT1, p53, napsin A (NAPSA) and progesterone receptor (PR) [82]. WT1 expression is typical of serous carcinomas, although this marker does not have 100% sensitivity and specificity. The expression of p53 makes it possible to distinguish between HGSOC and LGSOC. Both overexpression (>70% of expressing tumour cells) and complete absence of expression in tumour cells (‘null pattern’) with focal expression in the stroma are regarded as abnormal patterns of expression indicative of TP53 mutation (‘mutated pattern’), characteristic of HGSOC. Infrequently (<5%) HGSOC have a cytoplasmic pattern of p53 expression or a wild non-mutated pattern of expression due to specific TP53 mutations [83]. In WTI-positive tumours, when p53 expression pattern is difficult to interpret, diffuse p16 staining supports a diagnosis of HGSOC. WT1-negative carcinomas include most CCC, EOC and MOC. NAPSA has been shown to be a highly sensitive and specific marker for CCC. However, NAPSA expression can be weak and focal in some tumours, and its expression may occasionally be detected in other histological types. In EOCs that express NAPSA, the possibility of a diagnosis of mixed EOC-CCC carcinoma should be considered, if supported by the morphology [4]. The use of vimentin may help in the differential diagnosis of PR-negative EOC with extensive mucinous differentiation, which is frequently vimentin-positive, from MOC, which are vimentin-negative [84]. Additional immunohistochemical findings that can help in refining EOC histotyping include: the absence of expression of ARID1A favour the diagnosis of CCC or EOC; nuclear beta-catenin expression favours the diagnosis of EOC; absence of expression of any MMR protein favours EOC or CCC, and HINF1B expression favour the diagnosis of CCC [4]. In order to exclude metastasis from the gastrointestinal tract when dealing with mucinous tumours, the use of SATB2 is recommended [85].
9. Hereditary cancer syndromes
Ovarian cancer is one of the tumours in which most pathogenic germline mutations have been identified. Thus, up to 20% of OEC harbour germline autosomal dominant alterations in BRCA1/2 genes, whereas other low penetrance genes associated with homologous recombination deficiency (HRD), mismatch repair genes (Lynch syndrome) and TP53 are less frequently present [22].
Song et al. analysed 2240 invasive OEC patients and found that the most common germline mutations were in BRCA1 (3.8%) and BRCA2 (4.2%), while mutations in mismatch repair genes were less common: MSH6 (0.45%) and MSH2 (0.18%) [86].
Other series have reported a higher incidence (up to 14%) of BRCA1/2 germline mutations in OEC [20,22]. When histological type is taken into account, BRCA1/2 mutations are not observed in MOC, but in 6% of CCC, 8,2% EOC and 17% of HGSOC [20,87]. Penetrance of these genes for the development of breast and/or ovarian cancer is high but incomplete. At 80 years, 65% and 37% of BRCA1 and BRCA2 carriers will ultimately develop OEC, respectively, [88]. To help to estimate ovarian cancer risk, we have to take into considerations that beyond carrying a germline pathogenic mutation, other factors, such as OEC in other family members, can increase the risk [89].
Regarding strategies to prevent OEC among BRCA1/2 carriers, prophylactic salpingo-oophorectomy (SOP) has shown to decrease overall mortality (10% vs. 3%; HR, 0.40 [95% CI, 0.26–0.61]), as well as breast cancer-specific mortality (6% vs. 2%; HR, 0.44 [95% CI, 0.26–0.76]) and ovarian cancer-specific mortality (3% vs. 0.4%; HR, 0.21 [95% CI, 0.06–0.80] [90]. SOP efficacy goes beyond and it also decreased breast (HR: 0.49; [95% IC: 0.37–0.65]) and ovarian cancer risk (HR: 0.21; [95% IC: 0.12–0.39]) [91]. This reduction was observed for both BRCA1 and BRCA2 mutation carriers [91]. Alternative options to SOP should be carefully discussed with the women since CA125 monitoring and periodical ultrasound evaluation have not shown any survival advantage [92]. The SOP is usually performed after childbearing desire has been fulfilled.
Currently, the use of gene panels that include, in addition to BRCA1/2, other genes with lower penetrance, is recommended for the screening of germline mutations in women with OEC. Thus, some studies have reported germline mutations in BRIP1 (1.71%), ATM (0.86%), and RAD51C (0.64%) in OEC patients [93]. In favour of their use is the possibility to identify further 25% [22] of non-BRCA mutations, while one of the disadvantages is the increased possibility of finding variants of uncertain significance (VUS) in up to 25% of the patients [94]. Besides the increased VUS, there is a risk of the psychological burden since the evidence supporting the management of low penetrance genes is scarce, as we will discuss later. Of note, not all mutations in other OEC risk genes are associated with increased breast cancer risk, as is BRCA1/2.
BRIP1 (BRCA1 interaction protein c-terminal helicase 1 gene) accounts for around 1.5% of OEC patients with a relative risk among carriers of 11.2 (95% IC, 3.22–34.10, p < 0.001) and of 14 for those with high grade serous histology (95% IC, 4.04–45.02, p < 0.001) [93,95]. RAD51C and RAD51D genes are mutated in less than 1% of OEC [96] non BRCA patients [19]. In case control studies, mutations in RAD51C were associated with increased OC risk (RR 5.88; 95% IC, 2,91-11.88, p < 0.001) [97], as well as mutations in RAD51D (RR 6.30; 95% IC, 2,86-13,85, p < 0.011) [98].
Lynch syndrome defined by mismatch repair genes mutations (MMR), such as MLH1, MSH2, MSH6, PMS2, accounts for 1–2% of hereditary OEC [93,99]. MMR protein loss and microsatellite instability for revised Bethesda criteria patients help to screen this population. The most frequent mutations are in MSH6, followed by mutations in MSH2 or MLH1, according to different series [93,99]. OEC penetrance is around 7%, making prophylactic measures less obvious to implement in these patients than in those carrying BRCA1/2 mutations. Most OEC in the setting or Lynch syndrome are endometrioid or clear cells. Other histological types are extremely infrequent when current classification criteria is applied [66,100].
Cowden syndrome is due to germline PTEN mutations, and it causes endometrial, breast and ovarian cancer [22,93]. Clinical criteria to identify patients at risk have been proposed and are available in the Cleveland Clinic PTEN risk calculator [101]. Li-Fraumeni syndrome due to TP53 germline mutations is responsible for 0.8% of OEC [22]. From a clinical standpoint, radiotherapy should be avoided, and there are limited strategic measures.
10. Prognostic factors
Several factors are associated with long-term survival, including younger age at diagnosis, nonserous histotypes, early-stage disease, no gross residual disease after cytoreductive surgery, absence of ascites and lower CA-125 levels.
The most important prognostic factor is the 2014 FIGO staging that divides early (I-IIB) versus advanced (III-IV) stage [102] (Table 1). If possible, staging should be done through a complete surgical diagnostic procedure.
Table 1.
FIGO staging system (Prat 2014).
| Stage I | Tumour confined to ovaries |
| IA | Tumour limited to one ovary, capsule intact, no tumour on surface, negative washing |
| IB | Tumour involves both ovaries, otherwise like IA |
| IC | Tumour limited to one or both ovaries |
| IC1 | Surgical spill |
| IC2 | Capsule rupture before surgery or tumour on ovarian surface |
| IC3 | Malignant cells in ascites or peritoneal washings. |
| Stage II | Tumour involves one or both ovaries with the pelvic extension (below the pelvic brim) or primary peritoneal cancer |
| IIA | Extension and/or implant on the uterus and/or Fallopian tubes |
| IIB | Extension to other intraperitoneal tissues |
| Stage III | Tumour involves one or both ovaries with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes |
| IIA | Positive retroperitoneal lymph nodes and/or microscopic metastasis beyond the pelvis. |
| IIA1 | Positive retroperitoneal lymph nodes only |
| IIIA1(i) Metastasis </ = 10 mm | |
| IIIA1 (5) Metastasis >10 mm | |
| IIIA2 | Microscopic, extrapelvic (above the brim) peritoneal involvement +/, positive retroperitoneal lymph nodes |
| IIIB | Macroscopic, extrapelvic, peritoneal metastases < or = 2 cm +/, positive retroperitoneal lymph nodes. Includes extension to the capsule of liver or spleen. |
| IIIC | Macroscopic, extrapelvic, peritoneal metastases >2 cm +/, positive retroperitoneal lymph nodes. Includes extension to the capsule of liver or spleen |
| Stage IV | Distant metastasis excluding peritoneal metastasis |
| IVA | Pleural effusion with positive cytology |
| IVB | Hepatic and/or spleen parenchymal metastasis to extraabdominal organs (including inguinal lymph nodes and lymph nodes outside the abdominal cavity) |
Histological type, according to current classification criteria [103], is an important prognostic factor. Considering all stages, HGSOC is the histological type with worse prognosis; however, it is important to remember that distant-stage MOC and CCC have similar or worse 10-year survival estimates than distant-stage HGSOC [103], whereas EOC is the histological type with a better prognosis, both in early and advanced stages [4,104].
Older age is associated with worse survival with a median overall survival of 18.7 months vs. 53.2 months comparing >70 years versus the range of 50–69 years [105]. Performance status of 2 versus 0–1 remains to be of prognostic importance in multivariate analysis [106]. Approximately 75% of the cases are of advanced stage and require expertise in gynaecological cancer treatment.
Another important prognostic factor is the expertise of the surgeon. Two quality surgery measures, the rate of lymphadenectomies in early stage (60%, 36% and 16%) and the rate of complete cytoreduction in advanced stages (58%, 51% and 40%), are higher among gynaecologic oncologists surgeons compared to general gynaecologist surgeons and general surgeons [107]. In a pooled analysis of 11,999 patients [108], when complete cytoreduction, 0.1–1 cm residual disease and >1 cm was achieved, the overall survival was 70, 40 and 30 months, respectively.
Molecular markers that are deeply reviewed in this article have emerged as prognostic factors, of which deficiency in DNA repair by homologous recombination has been clinically validated [22], particularly the status of BRCA1/2 mutation. As such, BRCA2 carriers have a better prognosis than BRCA1 carriers and BRCA1/2 carrier's better prognosis than BRCA wild type patients [109]. As previously stated, microarray-based transcriptome expression classification into molecular subgroups of HGSOC, although powerful, lack any clinical use at this moment [29].
Regarding other biological markers, the status of oestrogen and progesterone receptors are prognostic factors in OEC [110,111]. Moreover, several studies have demonstrated that the presence of CD8+ TILs within the epithelial component of OECs is associated with favourable prognosis in HGSOC [112,113]. However, little information is available regarding TILs in other histotypes. A recent study based on a large OEC cohort has confirmed the histotype-specific nature of immune infiltration, and it has also provided definitive evidence for a dose-response relationship between CD8+ TILs and HGSOC survival. Thus, also, the extent of infiltration is prognostic and not merely its presence or absence. In this study a survival benefit was additionally observed among women with endometrioid and mucinous carcinomas, but not the other histotypes [114].
Recently, the role of PD-L1 as a possible prognostic factor has been analysed in some studies, most frequently in HGSOC [115]. Results among series are discrepant due to differences in the samples analysed, antibodies used, and the evaluation criteria, including whether staining was evaluated in tumour or immune cells. For example, Darb-Esfahani [116] reported that 89% of HGSOC expressed PD-L1 in cancer cells and that positive expression was an independent favourable prognostic factor. In contrast, Wang et al. [117]and Schmoeckel et al. [118] observed that tumour PD-L1 expression, which was present in 24% of HGSOC, was a predictive factor for poorer overall survival. Regarding PD-L1 expression in inflammatory cells, Kim et al. have reported that PD-L1 expression in HGSOC is associated with a good prognosis. In histological types other than HGSOC, Willis et al. (2017) reported tumour cell PD-L1 staining in 43% of the CCOC cases [119], but Friedlander et al. reported only a 7% of positivity in this histological type [58].
11. General considerations for treatment
Although a multidisciplinary approach is mandatory in all tumours, this holds particularly true in OEC, since surgery and systemic treatment usually go together in a constant dialogue, which is usually preceded by imaging procedures and a precise pathologic report. Cytoreduction surgery is done at primary diagnosis (PDS) or after neoadjuvant chemotherapy (interval debulking surgery). Results of surgery (whether complete with no macroscopic residual tumour or not) are essential to treatment planning. The rate of complete cytoreduction is greater in centres with a high volume of patients [120] since the procedures are extensive and imply complete pelvic tumour resection, lymph node removal, stripping of diaphragm, and also selective bowel resections, in order to achieve quality indicators, such as those suggested by ESGO (www.esgo.org).
Medical treatment includes both cytostatic and targeted therapies. Although usually, the route of administration is endovenous, intraperitoneal route has some pharmacokinetic advantages [121], such as higher IP concentration of the drug, a longer half-life of the drug in the abdominal cavity and prolonged systemic exposure. Intraperitoneal chemotherapy after complete cytoreduction in stage III OEC has been associated with a decreased mortality rate (HR = 0.81; 95% confidence interval (CI): 0.72 to 0.90 for OS) [122]. However, despite its efficacy, it has not been widely adopted as a standard of care.
The first biological compound that was available to treat OEC was intravenous bevacizumab, targeting angiogenesis. Hypertension and proteinuria are the main side effects that should be closely monitored, and special attention should also be paid to bowel symptoms since patients are at increased risk of perforations. In Europe, this treatment is approved as first line for platinum-sensitive and resistant tumours.
Drugs targeting homologous recombination deficiency have been approved as maintenance or active drugs. These PARP inhibitors include, but are not limited to, olaparib, niraparib and rucaparib. Since these drugs are oral and are taken for long periods of time without interruption, some side effects are observed, including nausea, asthenia and neutropenia. Early management of these side effects is essential to achieve good adherence. Additionally, dose reduction should be considered when necessary.
Due to the relative chemoresistance of histological types other than HGSOC, new therapeutic approaches based on molecular alterations are being tested. Hormonal therapy (e.g. aromatase inhibitors) could be considered as maintenance therapy after the end of chemotherapy for LGSOC [123]. In addition, based on the known mutation in the KRAS/BRAF/MAPK and the PI3K/AKT/mTOR signalling pathway in LGSOC, several clinical trials have been designed, using targeted agents to target these pathways. Regarding CCC, direct and synthetic-lethal therapeutic strategies, in particular, focussing on ARID1A deficiency, are being evaluated in clinical trials [124]. For example, EZH2 facilitates epigenetic methylation to modulate gene expression, and both uterine and ovarian cancers show evidence of EZH2 overexpression. EZH2 inhibition in ARID1A mutated tumours acts in a synthetically lethal manner to suppress cell growth and promote apoptosis, revealing a unique new therapeutic opportunity.
Immunotherapy is becoming a standard of care in different human tumours. Despite the encouraging results in other malignant tumours, the use of single-agent antibodies inhibiting the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) or PD-1 or PD-L1 axis yielded only modest results in OEC with median response rates of 10–15% and control of disease was observed in less than half of the patients [125]. As a consequence, no immunotherapeutic agent has obtained regulatory approval for OEC so far. A limitation in the use of immunotherapy is the lack of predictive biomarkers. Recently, the role of P-L1 as a predictive marker of response for the immune checkpoint inhibitor Pembrolizumab has been evaluated in the Keynote-100 clinical trial. The authors used the combined positive score (CPS), defined as the number of PD-L1 staining cells (tumour cells, lymphocytes, macrophages) divided by the total number of viable tumour cells X100, and found that, although single-agent pembrolizumab showed only modest activity in advanced, recurrent ovarian cancer, a CPS ≥10 was correlated with higher response [126].
MMRD and POLE mutations are agnostic immunotherapy predictive markers. As previously discussed, pathogenic POLE mutations and MMRD occur in about 10% and 18% of EOC, respectively. In addition, less than 5% of CCCs are MMR-deficient. These molecular alterations are nearly absent in serous and mucinous carcinomas.
Tumour mutation burden (TMB) has been reported as a marker of response to immune checkpoint inhibitor in some tumours. There are few studies analysing TMB in OEC, and as for other biomarkers, most studies have focused on HGSOC. TMB in HGSOC tend to be lower than in other tumour types (around 1.5 non-synonymous mutations per megabase). The optimal cut-off for TMB as a possible predictor of response to immunotherapy has not been established in OEC [[127], [128], [129]].
12. Conclusions
In spite of advances in our understanding of its biology and the incorporation of new therapies during the last decades, the lethality of OEC remains high. The recognition of five major histological types of OEC is allowing the transition from a single therapeutic approach for all OECs, to a more precise one based on the specific characteristics of each subtype. However, as has been discussed, there are still problems in the correct classification of OEC, and also, substantial molecular heterogeneity occurs among tumours of each histological type. It is to be expected that the routine use of immunohistochemistry and molecular techniques, as next-generation sequencing, will improve OEC classification and will allow the identification of potential therapeutic targets. In addition to improving diagnosis, reduction of OEC incidence and mortality will need the implementation of primary prevention and early detection procedures, and the incorporation of new targeted therapies.
Funding
This article was funded by grants from the Instituto de Salud Carlos III (ISCIII), Spain, (PIE15/00050 and PI16/00887) and CIBERONC, Spain, (CB16/12/00316), co-financed by the European Development Regional Fund. ‘A way to achieve Europe’ (FEDER), by the Spanish Association Against Cancer Scientific Foundation (grants: AIO-aecc 2016 and Grupos Coordinados Traslacionales aecc 2018) and by the Spanish Group of Research in Ovarian Cancer (GEICO group).
Conflict of interest statement
Dr. Romero Noguera has nothing to disclose.
Dr. Leskela has nothing to disclose.
Dr. Belén Perez-Mies has nothing to disclose.
Dr. Poveda has nothing to disclose.
Dr. Palacios Calvo has nothing to disclose.
Footnotes
This paper is part of a supplement supported by Pharma Mar S.A.
References
- 1.Lheureux S., Braunstein M., Oza A.M. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA A Cancer J Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 2.Singh A., Gupta S., Sachan M. Epigenetic biomarkers in the management of ovarian cancer: current prospectives. Front Cell Dev Biol. 2019;7:182. doi: 10.3389/fcell.2019.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:12. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 4.Leskela S., Romero I., Cristobal E., Perez-Mies B., Rosa-Rosa J.M., Gutierrez-Pecharroman A. The frequency and prognostic significance of the histologic type in early-stage ovarian carcinoma: a reclassification study by the Spanish group for ovarian cancer research (GEICO) Am J Surg Pathol. 2019;44:149–161. doi: 10.1097/PAS.0000000000001365. [DOI] [PubMed] [Google Scholar]
- 5.WHO https://gco.iarc.fr/
- 6.Pearce C.L., Templeman C., Rossing M.A., Lee A., Near A.M., Webb P.M. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faber M.T., Kjaer S.K., Dehlendorff C., Chang-Claude J., Andersen K.K., Hogdall E. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24:989–1004. doi: 10.1007/s10552-013-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid B.M., Permuth J.B., Sellers T.A. Epidemiology of ovarian cancer: a review. Canc Biol Med. 2017;14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborative Group on Epidemiological Studies of Ovarian C, Beral V., Doll R., Hermon C., Peto R., Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 10.Trabert B., Poole E.M., White E., Visvanathan K., Adami H.O., Anderson G.L. Analgesic use and ovarian cancer risk: an analysis in the ovarian cancer cohort consortium. J Natl Cancer Inst. 2019;111:137–145. doi: 10.1093/jnci/djy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury R., Sinha B., Sankar M.J., Taneja S., Bhandari N., Rollins N. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96–113. doi: 10.1111/apa.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soslow R.A., Han G., Park K.J., Garg K., Olvera N., Spriggs D.R. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–636. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 13.Colombo N., Sessa C., Bois A.D., Ledermann J., McCluggage W.G., McNeish I. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Canc. 2019 doi: 10.1136/ijgc-2019-000308. [DOI] [PubMed] [Google Scholar]
- 14.Labidi-Galy S.I., Papp E., Hallberg D., Niknafs N., Adleff V., Noe M. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8:1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F., Gaitskell K., Garcia M.J., Albukhari A., Tsaltas J., Ahmed A.A. Serous tubal intraepithelial carcinomas associated with high-grade serous ovarian carcinomas: a systematic review. BJOG. 2017;124:872–878. doi: 10.1111/1471-0528.14543. [DOI] [PubMed] [Google Scholar]
- 16.Wu R.C., Wang P., Lin S.F., Zhang M., Song Q., Chu T. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. 2019;248:41–50. doi: 10.1002/path.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh P., Kaushal V., Rai B., Rajwanshi A., Gupta N., Dey P. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology. 2018;72:619–625. doi: 10.1111/his.13399. [DOI] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norquist B.M., Harrell M.I., Brady M.F., Walsh T., Lee M.K., Gulsuner S. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsop K., Fereday S., Meldrum C., deFazio A., Emmanuel C., George J. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harter P., Hauke J., Heitz F., Reuss A., Kommoss S., Marme F. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1) PloS One. 2017;12 doi: 10.1371/journal.pone.0186043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Canc Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruscito I., Dimitrova D., Vasconcelos I., Gellhaus K., Schwachula T., Bellati F. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients--a study of the tumour Bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD) Eur J Canc. 2014;50:2090–2098. doi: 10.1016/j.ejca.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Konstantinopoulos P.A., Ceccaldi R., Shapiro G.I., D'Andrea A.D. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Canc Discov. 2015;5:1137–1154. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmirotta R., Silvestris E., D'Oronzo S., Cardascia A., Silvestris F. Ovarian cancer: novel molecular aspects for clinical assessment. Crit Rev Oncol Hematol. 2017;117:12–29. doi: 10.1016/j.critrevonc.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Veneris J.T., Matulonis U.A., Liu J.F., Konstantinopoulos P.A. Choosing wisely: selecting PARP inhibitor combinations to promote anti-tumor immune responses beyond BRCA mutations. Gynecol Oncol. 2019;156:488–497. doi: 10.1016/j.ygyno.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Patch A.M., Christie E.L., Etemadmoghadam D., Garsed D.W., George J., Fereday S. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 28.Chen S., Cavazza E., Barlier C., Salleron J., Filhine-Tresarrieu P., Gavoilles C. Beside P53 and PTEN: identification of molecular alterations of the RAS/MAPK and PI3K/AKT signaling pathways in high-grade serous ovarian carcinomas to determine potential novel therapeutic targets. Oncol Lett. 2016;12:3264–3272. doi: 10.3892/ol.2016.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tothill R.W., Tinker A.V., George J., Brown R., Fox S.B., Lade S. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Canc Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 30.Konecny G.E., Wang C., Hamidi H., Winterhoff B., Kalli K.R., Dering J. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D. Ovarian cancer statistics, 2018. CA A Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaldawy A., Segev Y., Lavie O., Auslender R., Sopik V., Narod S.A. Low-grade serous ovarian cancer: a review. Gynecol Oncol. 2016;143:433–438. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 33.Van Nieuwenhuysen E., Busschaert P., Laenen A., Moerman P., Han S.N., Neven P. Loss of 1p36.33 frequent in low-grade serous ovarian cancer. Neoplasia. 2019;21:582–590. doi: 10.1016/j.neo.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones S., Wang T.L., Kurman R.J., Nakayama K., Velculescu V.E., Vogelstein B. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226:413–420. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter S.M., Anglesio M.S., Ryland G.L., Sharma R., Chiew Y.E., Rowley S.M. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget. 2015;6:37663–37677. doi: 10.18632/oncotarget.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer G., Oldt R., 3rd, Cohen Y., Wang B.G., Sidransky D., Kurman R.J. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 37.Wong K.K., Tsang Y.T., Deavers M.T., Mok S.C., Zu Z., Sun C. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsang Y.T., Deavers M.T., Sun C.C., Kwan S.Y., Kuo E., Malpica A. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol. 2013;231:449–456. doi: 10.1002/path.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grisham R.N., Sylvester B.E., Won H., McDermott G., DeLair D., Ramirez R. Extreme outlier analysis identifies occult mitogen-activated protein kinase pathway mutations in patients with low-grade serous ovarian cancer. J Clin Oncol. 2015;33:4099–4105. doi: 10.1200/JCO.2015.62.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.http://www.iccr-cancer.org/
- 41.Muyldermans K., Moerman P., Amant F., Leunen K., Neven P., Vergote I. Primary invasive mucinous ovarian carcinoma of the intestinal type: importance of the expansile versus infiltrative type in predicting recurrence and lymph node metastases. Eur J Canc. 2013;49:1600–1608. doi: 10.1016/j.ejca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Tabrizi A.D., Kalloger S.E., Kobel M., Cipollone J., Roskelley C.D., Mehl E. Primary ovarian mucinous carcinoma of intestinal type: significance of pattern of invasion and immunohistochemical expression profile in a series of 31 cases. Int J Gynecol Pathol. 2010;29:99–107. doi: 10.1097/PGP.0b013e3181bbbcc1. [DOI] [PubMed] [Google Scholar]
- 43.Cheasley D., Wakefield M.J., Ryland G.L., Allan P.E., Alsop K., Amarasinghe K.C. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun. 2019;10:3935. doi: 10.1038/s41467-019-11862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller J.J., Schlappe B.A., Kumar R., Olvera N., Dao F., Abu-Rustum N. Massively parallel sequencing analysis of mucinous ovarian carcinomas: genomic profiling and differential diagnoses. Gynecol Oncol. 2018;150:127–135. doi: 10.1016/j.ygyno.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao W.R., Lee M.Y., Ruan A., Sheng H.P., Hsu J.D., Han C.P. Assessment of HER2 status using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) techniques in mucinous epithelial ovarian cancer: a comprehensive comparison between ToGA biopsy method and ToGA surgical specimen method. PloS One. 2015;10 doi: 10.1371/journal.pone.0142135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chay W.Y., Chew S.H., Ong W.S., Busmanis I., Li X., Thung S. HER2 amplification and clinicopathological characteristics in a large Asian cohort of rare mucinous ovarian cancer. PloS One. 2013;8 doi: 10.1371/journal.pone.0061565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anglesio M.S., Kommoss S., Tolcher M.C., Clarke B., Galletta L., Porter H. Molecular characterization of mucinous ovarian tumours supports a stratified treatment approach with HER2 targeting in 19% of carcinomas. J Pathol. 2013;229:111–120. doi: 10.1002/path.4088. [DOI] [PubMed] [Google Scholar]
- 48.Ryland G.L., Hunter S.M., Doyle M.A., Caramia F., Li J., Rowley S.M. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7:87. doi: 10.1186/s13073-015-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackenzie R., Kommoss S., Winterhoff B.J., Kipp B.R., Garcia J.J., Voss J. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Canc. 2015;15:415. doi: 10.1186/s12885-015-1421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fadare O., Parkash V. Pathology of endometrioid and clear cell carcinoma of the ovary. Surg Pathol Clin. 2019;12:529–564. doi: 10.1016/j.path.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Khalique S., Lord C.J., Banerjee S., Natrajan R. Translational genomics of ovarian clear cell carcinoma. Semin Canc Biol. 2019 doi: 10.1016/j.semcancer.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Bulun S.E., Wan Y., Matei D. Epithelial mutations in endometriosis: link to ovarian cancer. Endocrinology. 2019;160:626–638. doi: 10.1210/en.2018-00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiegand K.C., Shah S.P., Al-Agha O.M., Zhao Y., Tse K., Zeng T. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones S., Wang T.L., Shih Ie M., Mao T.L., Nakayama K., Roden R. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abou-Taleb H., Yamaguchi K., Matsumura N., Murakami R., Nakai H., Higasa K. Comprehensive assessment of the expression of the SWI/SNF complex defines two distinct prognostic subtypes of ovarian clear cell carcinoma. Oncotarget. 2016;7:54758–54770. doi: 10.18632/oncotarget.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakami R., Matsumura N., Brown J.B., Higasa K., Tsutsumi T., Kamada M. Exome sequencing landscape analysis in ovarian clear cell carcinoma shed light on key chromosomal regions and mutation gene networks. Am J Pathol. 2017;187:2246–2258. doi: 10.1016/j.ajpath.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Shibuya Y., Tokunaga H., Saito S., Shimokawa K., Katsuoka F., Bin L. Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosomes Cancer. 2017;57:51–60. doi: 10.1002/gcc.22507. [DOI] [PubMed] [Google Scholar]
- 58.Friedlander M.L., Russell K., Millis S., Gatalica Z., Bender R., Voss A. Molecular profiling of clear cell ovarian cancers: identifying potential treatment targets for clinical trials. Int J Gynecol Canc. 2016;26:648–654. doi: 10.1097/IGC.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandler R.L., Damrauer J.S., Raab J.R., Schisler J.C., Wilkerson M.D., Didion J.P. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuo K.T., Mao T.L., Jones S., Veras E., Ayhan A., Wang T.L. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makii C., Oda K., Ikeda Y., Sone K., Hasegawa K., Uehara Y. MDM2 is a potential therapeutic target and prognostic factor for ovarian clear cell carcinomas with wild type TP53. Oncotarget. 2016;7:75328–75338. doi: 10.18632/oncotarget.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho E.S., Lai C.R., Hsieh Y.T., Chen J.T., Lin A.J., Hung M.H. p53 mutation is infrequent in clear cell carcinoma of the ovary. Gynecol Oncol. 2001;80:189–193. doi: 10.1006/gyno.2000.6025. [DOI] [PubMed] [Google Scholar]
- 63.Teer J.K., Yoder S., Gjyshi A., Nicosia S.V., Zhang C., Monteiro A.N.A. Mutational heterogeneity in non-serous ovarian cancers. Sci Rep. 2017;7:9728. doi: 10.1038/s41598-017-10432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan D.S., Iravani M., McCluggage W.G., Lambros M.B., Milanezi F., Mackay A. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin Canc Res. 2011;17:1521–1534. doi: 10.1158/1078-0432.CCR-10-1688. [DOI] [PubMed] [Google Scholar]
- 65.Rambau P.F., Duggan M.A., Ghatage P., Warfa K., Steed H., Perrier R. Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology. 2016;69:288–297. doi: 10.1111/his.12934. [DOI] [PubMed] [Google Scholar]
- 66.Leskela S., Romero I., Cristobal E., Pérez-Mies B., Rosa-Rosa J.M., Gutierrez-Pecharroman A. Mismatch repair deficiency in ovarian carcinoma: frequency, causes and consequences. Am J Clin Pathol. 2019 doi: 10.1097/PAS.0000000000001432. [accepted] [DOI] [PubMed] [Google Scholar]
- 67.McGee J., Bookman M., Harter P., Marth C., McNeish I., Moore K.N. Fifth ovarian cancer consensus conference: individualized therapy and patient factors. Ann Oncol. 2017;28:702–710. doi: 10.1093/annonc/mdx010. [DOI] [PubMed] [Google Scholar]
- 68.Parra-Herran C., Bassiouny D., Vicus D., Olkhov-Mitsel E., Cesari M., Ismiil N. FIGO versus silverberg grading systems in ovarian endometrioid carcinoma: a comparative prognostic analysis. Am J Surg Pathol. 2019;43:161–167. doi: 10.1097/PAS.0000000000001160. [DOI] [PubMed] [Google Scholar]
- 69.Assem H., Rambau P.F., Lee S., Ogilvie T., Sienko A., Kelemen L.E. High-grade endometrioid carcinoma of the ovary: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2018;42:534–544. doi: 10.1097/PAS.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 70.Schultheis A.M., Ng C.K., De Filippo M.R., Piscuoglio S., Macedo G.S., Gatius S. Massively parallel sequencing-based clonality analysis of synchronous endometrioid endometrial and ovarian carcinomas. J Natl Cancer Inst. 2016;108:djv427. doi: 10.1093/jnci/djv427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anglesio M.S., Wang Y.K., Maassen M., Horlings H.M., Bashashati A., Senz J. Synchronous endometrial and ovarian carcinomas: evidence of clonality. J Natl Cancer Inst. 2016;108:djv428. doi: 10.1093/jnci/djv428. [DOI] [PubMed] [Google Scholar]
- 72.Gilks C.B., Kommoss F. Synchronous tumours of the female reproductive tract. Pathology. 2018;50:214–221. doi: 10.1016/j.pathol.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Prat J., D'Angelo E., Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11–27. doi: 10.1016/j.humpath.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 74.McConechy M.K., Ding J., Senz J., Yang W., Melnyk N., Tone A.A. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 2014;27:128–134. doi: 10.1038/modpathol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geyer J.T., Lopez-Garcia M.A., Sanchez-Estevez C., Sarrio D., Moreno-Bueno G., Franceschetti I. Pathogenetic pathways in ovarian endometrioid adenocarcinoma: a molecular study of 29 cases. Am J Surg Pathol. 2009;33:1157–1163. doi: 10.1097/PAS.0b013e3181a902e1. [DOI] [PubMed] [Google Scholar]
- 76.Talhouk A., McConechy M.K., Leung S., Yang W., Lum A., Senz J. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123:802–813. doi: 10.1002/cncr.30496. [DOI] [PubMed] [Google Scholar]
- 77.Talhouk A., McConechy M.K., Leung S., Li-Chang H.H., Kwon J.S., Melnyk N. A clinically applicable molecular-based classification for endometrial cancers. Br J Canc. 2015;113:299–310. doi: 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosse T., Nout R.A., McAlpine J.N., McConechy M.K., Britton H., Hussein Y.R. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol. 2018;42:561–568. doi: 10.1097/PAS.0000000000001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parra-Herran C., Lerner-Ellis J., Xu B., Khalouei S., Bassiouny D., Cesari M. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod Pathol. 2017;30:1748–1759. doi: 10.1038/modpathol.2017.81. [DOI] [PubMed] [Google Scholar]
- 80.Cybulska P., Paula A.D.C., Tseng J., Leitao M.M., Jr., Bashashati A., Huntsman D.G. Molecular profiling and molecular classification of endometrioid ovarian carcinomas. Gynecol Oncol. 2019;154:516–523. doi: 10.1016/j.ygyno.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobel M., Bak J., Bertelsen B.I., Carpen O., Grove A., Hansen E.S. Ovarian carcinoma histotype determination is highly reproducible, and is improved through the use of immunohistochemistry. Histopathology. 2014;64:1004–1013. doi: 10.1111/his.12349. [DOI] [PubMed] [Google Scholar]
- 82.Kobel M., Rahimi K., Rambau P.F., Naugler C., Le Page C., Meunier L. An immunohistochemical algorithm for ovarian carcinoma typing. Int J Gynecol Pathol. 2016;35:430–441. doi: 10.1097/PGP.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casey L., Singh N. Ovarian high-grade serous carcinoma: assessing pathology for site of origin, staging and post-neoadjuvant chemotherapy changes. Surg Pathol Clin. 2019;12:515–528. doi: 10.1016/j.path.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 84.Woodbeck R., Kelemen L.E., Kobel M. Ovarian endometrioid carcinoma misdiagnosed as mucinous carcinoma: an underrecognized problem. Int J Gynecol Pathol. 2019;38:568–575. doi: 10.1097/PGP.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 85.Moh M., Krings G., Ates D., Aysal A., Kim G.E., Rabban J.T. SATB2 expression distinguishes ovarian metastases of colorectal and appendiceal origin from primary ovarian tumors of mucinous or endometrioid type. Am J Surg Pathol. 2016;40:419–432. doi: 10.1097/PAS.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 86.Song H., Cicek M.S., Dicks E., Harrington P., Ramus S.J., Cunningham J.M. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014;23:4703–4709. doi: 10.1093/hmg/ddu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Risch H.A., McLaughlin J.R., Cole D.E., Rosen B., Bradley L., Kwan E. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Evans D.G., Shenton A., Woodward E., Lalloo F., Howell A., Maher E.R. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Canc. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metcalfe K., Lubinski J., Lynch H.T., Ghadirian P., Foulkes W.D., Kim-Sing C. Family history of cancer and cancer risks in women with BRCA1 or BRCA2 mutations. J Natl Cancer Inst. 2010;102:1874–1878. doi: 10.1093/jnci/djq443. [DOI] [PubMed] [Google Scholar]
- 90.Domchek S.M., Friebel T.M., Singer C.F., Evans D.G., Lynch H.T., Isaacs C. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. J Am Med Assoc. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rebbeck T.R., Kauff N.D., Domchek S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stirling D., Evans D.G., Pichert G., Shenton A., Kirk E.N., Rimmer S. Screening for familial ovarian cancer: failure of current protocols to detect ovarian cancer at an early stage according to the international Federation of gynecology and obstetrics system. J Clin Oncol. 2005;23:5588–5596. doi: 10.1200/JCO.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 93.Minion L.E., Dolinsky J.S., Chase D.M., Dunlop C.L., Chao E.C., Monk B.J. Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecol Oncol. 2015;137:86–92. doi: 10.1016/j.ygyno.2015.01.537. [DOI] [PubMed] [Google Scholar]
- 94.LaDuca H., Stuenkel A.J., Dolinsky J.S., Keiles S., Tandy S., Pesaran T. Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genet Med. 2014;16:830–837. doi: 10.1038/gim.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramus S.J., Song H., Dicks E., Tyrer J.P., Rosenthal A.N., Intermaggio M.P. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song H., Dicks E., Ramus S.J., Tyrer J.P., Intermaggio M.P., Hayward J. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol. 2015;33:2901–2907. doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loveday C., Turnbull C., Ruark E., Xicola R.M., Ramsay E., Hughes D. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;44:475–476. doi: 10.1038/ng.2224. author reply 6. [DOI] [PubMed] [Google Scholar]
- 98.Loveday C., Turnbull C., Ramsay E., Hughes D., Ruark E., Frankum J.R. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879–882. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malander S., Rambech E., Kristoffersson U., Halvarsson B., Ridderheim M., Borg A. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol. 2006;101:238–243. doi: 10.1016/j.ygyno.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 100.Chui M.H., Ryan P., Radigan J., Ferguson S.E., Pollett A., Aronson M. The histomorphology of Lynch syndrome-associated ovarian carcinomas: toward a subtype-specific screening strategy. Am J Surg Pathol. 2014;38:1173–1181. doi: 10.1097/PAS.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 101.Mester J.L., Moore R.A., Eng C. PTEN germline mutations in patients initially tested for other hereditary cancer syndromes: would use of risk assessment tools reduce genetic testing? Oncol. 2013;18:1083–1090. doi: 10.1634/theoncologist.2013-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prat J. Oncology FCoG. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Peres L.C., Cushing-Haugen K.L., Anglesio M., Wicklund K., Bentley R., Berchuck A. Histotype classification of ovarian carcinoma: a comparison of approaches. Gynecol Oncol. 2018;151:53–60. doi: 10.1016/j.ygyno.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peres L.C., Cushing-Haugen K.L., Kobel M., Harris H.R., Berchuck A., Rossing M.A. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. 2019;111:60–68. doi: 10.1093/jnci/djy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kosary C., Ries L., Young J., Keel G., Eisner M., Lin Y. National Cancer Institute; Bethesda, MD: 2007. SEER survival monograph: cancer survival among adults: U.S. SEER program, 1988-2001, patient and tumor characteristics. Cancer of cervix uteri. SEER program. [Google Scholar]
- 106.du Bois A., Reuss A., Pujade-Lauraine E., Harter P., Ray-Coquard I., Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 107.Earle C.C., Schrag D., Neville B.A., Yabroff K.R., Topor M., Fahey A. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 108.Chiva L.M., Castellanos T., Alonso S., Gonzalez-Martin A. Minimal macroscopic residual disease (0.1-1 cm). Is it still a surgical goal in advanced ovarian cancer? Int J Gynecol Canc. 2016;26:906–911. doi: 10.1097/IGC.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 109.Bolton K.L., Chenevix-Trench G., Goh C., Sadetzki S., Ramus S.J., Karlan B.Y. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. J Am Med Assoc. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hogdall E.V., Christensen L., Hogdall C.K., Blaakaer J., Gayther S., Jacobs I.J. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the 'MALOVA' ovarian cancer study. Oncol Rep. 2007;18:1051–1059. [PubMed] [Google Scholar]
- 111.Sieh W., Kobel M., Longacre T.A., Bowtell D.D., deFazio A., Goodman M.T. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hwang W.T., Adams S.F., Tahirovic E., Hagemann I.S., Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J., Wang J., Chen R., Bai Y., Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8:15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ovarian Tumor Tissue Analysis C., Goode E.L., Block M.S., Kalli K.R., Vierkant R.A., Chen W. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang L.J., Deng X.F., Chang F., Wu X.L., Wu Y., Diao Q.Z. Prognostic significance of programmed cell death ligand 1 expression in patients with ovarian carcinoma: a systematic review and meta-analysis. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Darb-Esfahani S., Kunze C.A., Kulbe H., Sehouli J., Wienert S., Lindner J. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang L. Prognostic effect of programmed death-ligand 1 (PD-L1) in ovarian cancer: a systematic review, meta-analysis and bioinformatics study. J Ovarian Res. 2019;12:37. doi: 10.1186/s13048-019-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmoeckel E., Hofmann S., Fromberger D., Rottmann M., Luthardt B., Burges A. Comprehensive analysis of PD-L1 expression, HER2 amplification, ALK/EML4 fusion, and mismatch repair deficiency as putative predictive and prognostic factors in ovarian carcinoma. Virchows Arch. 2019;474:599–608. doi: 10.1007/s00428-019-02528-6. [DOI] [PubMed] [Google Scholar]
- 119.Willis B.C., Sloan E.A., Atkins K.A., Stoler M.H., Mills A.M. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol. 2017;30:1622–1632. doi: 10.1038/modpathol.2017.67. [DOI] [PubMed] [Google Scholar]
- 120.Vernooij F., Heintz P., Witteveen E., van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007;105:801–812. doi: 10.1016/j.ygyno.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 121.Dedrick R.L., Myers C.E., Bungay P.M., DeVita V.T., Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Canc Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 122.Jaaback K., Johnson N., Lawrie T.A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016:CD005340. doi: 10.1002/14651858.CD005340.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gourley C., Farley J., Provencher D.M., Pignata S., Mileshkin L., Harter P. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian and primary peritoneal low-grade serous carcinomas. Int J Gynecol Canc. 2014;24:S9–S13. doi: 10.1097/IGC.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 124.Khalique S., Naidoo K., Attygalle A.D., Kriplani D., Daley F., Lowe A. Optimised ARID1A immunohistochemistry is an accurate predictor of ARID1A mutational status in gynaecological cancers. J Pathol Clin Res. 2018;4:154–166. doi: 10.1002/cjp2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghisoni E., Imbimbo M., Zimmermann S., Valabrega G. Ovarian cancer immunotherapy: turning up the heat. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20122927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matulonis U.A., Shapira-Frommer R., Santin A.D., Lisyanskaya A.S., Pignata S., Vergote I. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 127.Yang S.Y.C., Lheureux S., Karakasis K., Burnier J.V., Bruce J.P., Clouthier D.L. Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Med. 2018;10:81. doi: 10.1186/s13073-018-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dai Y., Sun C., Feng Y., Jia Q., Zhu B. Potent immunogenicity in BRCA1-mutated patients with high-grade serous ovarian carcinoma. J Cell Mol Med. 2018 doi: 10.1111/jcmm.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang M., Fan W., Ye M., Tian C., Zhao L., Wang J. Molecular profiles and tumor mutational burden analysis in Chinese patients with gynecologic cancers. Sci Rep. 2018;8:8990. doi: 10.1038/s41598-018-25583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]