Abstract
Reading a book, understanding the news reports or any other behaviour involving the processing of meaningful stimuli requires the semantic system to have two main features: being active during an extended period of time and flexibly adapting the internal representation according to the changing environment. Despite being key features of many everyday tasks, formation and updating of the semantic “gestalt” are still poorly understood. In this fMRI study we used naturalistic stimuli and task manipulations to identify the neural network that forms and updates conceptual gestalts during time-extended integration of meaningful stimuli. Univariate and multivariate techniques allowed us to draw a distinction between networks that are crucial for the formation of a semantic gestalt (meaning integration) and those that instead are important for linking incoming cues about the current context (e.g., time and space cues) into a schema representation. Specifically, we revealed that time-extended formation of the conceptual gestalt was reflected in the neuro-computations of the anterior temporal lobe accompanied by multi-demand areas and hippocampus, with a key role of brain structures in the right hemisphere. This “semantic gestalt network” was strongly recruited when an update of the current semantic representation was required during narrative processing. A distinct fronto-parietal network, instead, was recruited for context integration, independently from the meaning associations between words (semantic coherence). Finally, in contrast with accounts positing that the default mode network (DMN) may have a crucial role in semantic cognition, our findings revealed that DMN activity was sensitive to task difficulty, but not to semantic integration. The implications of these findings for neurocognitive models of semantic cognition and the literature on narrative processing are discussed.
Keywords: Semantic, Context, Narrative, fMRI, ICA
Highlights
-
•
fMRI revealed areas and networks for semantic integration during narrative reading.
-
•
ATL has a key role in the formation of the conceptual gestalt.
-
•
IFG, pMTG and dAG support the update of the conceptual gestalt.
-
•
Left AG (mid-PGp) has a key role in context integration.
1. Introduction
Successful time-extended semantic cognition (e.g., understanding the news reports on the radio or sequential paragraphs while reading a book) relies on the ability of the semantic system to integrate information over time to build meaningful representations of the evolving world around us. Although semantic integration is often error-free and apparently effortless, the cognitive challenges are non-trivial. Thus, the semantic system is required over an extended-period of time to build a continuously evolving semantic representation from the torrent of words and non-verbal stimuli. This representation (that here we call “semantic gestalt”) is continuously evolving because, as each unit of information (words but also nonverbal stimuli) is integrated, the representation is revised. Importantly, sometimes the semantic context changes abruptly (which is often not overtly signalled but has to be inferred), and the semantic gestalt may require a major revision/update or resets to build a new representation afresh.
Despite being a core, everyday function of semantic cognition, the neural foundations of semantic gestalt formation and update are still uncharacterised. In fact, a handful of studies have utilised word pairs to explore the formation of meaning at the noun-phrase level (Price et al., 2015, 2016) without addressing the time-extended demands posed by everyday semantic cognition. Other studies, instead, have measured multi-item semantic combinations without task manipulations that distinguish between brain structures involved in semantic integration (or formation of the semantic gestalt) from those involved in extra-semantic neuro-computations (e.g., working memory, attentional control, “schema formation” etc.: e.g., Hasson et al., 2008; Lerner et al., 2011; Simony et al., 2016; Tylen et al., 2015). Note that these latter studies have largely investigated the construct of “schema” or “situation model”, a representation that summarises the interaction among entities and the environment in a scene or event (e.g., Ranganath and Ritchey, 2012). The formation of a schema representation relies on a set of mnemonic processes that match incoming cues about the current context (for example, information about agents, space, time and social interactions) to these situation models.
The neural basis of the schema formation has been widely investigated by comparing intact versions of stories against versions in which the order of the stories’ events is scrambled. Since the type of information integrated in these stories is semantic, it is likely that these scrambling operations also affect the formation of a semantic gestalt. However, in these studies it is not possible to differentiate brain responses reflecting formation of a semantic gestalt from those instead reflecting the formation of schema representations.
Accordingly, we designed a functional magnetic resonance imaging (fMRI) study to establish the brain regions and networks that support the formation and update of the semantic gestalt (i.e., regions that support time-extended semantic integration of the meaning of words and their associations, as well as major changes in semantic context, respectively). Our experiment also allowed us to differentiate these regions from brain structures that are important for linking incoming cues about the current context (e.g., time and space cues) into a schema representation. Importantly, in doing so we provide some timely evidence that bridges the existing work on narratives (e.g., Hasson et al., 2008; Lerner et al., 2011; Simony et al., 2016; Tylen et al., 2015) with the broader neurocognitive theories of semantic cognition (Lambon Ralph et al., 2017).
The neural foundations of conceptual gestalt formation and update were addressed through a straightforward fMRI experimental design. First, we used combinations of short paragraphs to reflect the time-extended demands posed by everyday semantic cognition and the challenges posed by the formation of larger conceptual gestalts. Second, we adopted a key manipulation to determine the neural networks involved in the formation of the conceptual gestalt and to differentiate them from brain structures supporting extra-semantic control mechanisms. Thus, participants were asked to read short narratives composed of two phases (context and target). For each narrative, the same second paragraph (target) was preceded by different types of context: (i) a highly congruent (HC) context which maximised the information contained in a single coherent semantic gestalt; (ii) a low-congruent (LC) paragraph with a divergent meaning, thus testing the semantic system’s ability to update the information presented context (see Table S1); and (iii) a no context control (NC) in which the same target was preceded by a number reading task (thus requiring a more substantial shift from a non-semantic to a semantic task and at the same time preventing context integration). The resultant fMRI data was analysed only for the identical target paragraph (thus ensuring that any observed differences must reflect the influence of the preceding contexts) that was compared across conditions to determine the neural foundations of formation (HC&LC > NC) and update (LC > HC) of the semantic gestalt. To establish the key brain areas and networks, we used both univariate and multivariate (independent component analysis; ICA) analyses. Particularly, we combined a whole-brain data-driven analytic approach, here particularly appropriate given the complexity of the investigated phenomena, with an independent region of interest (ROI) analysis approach, to link the present work to previous investigations on semantic cognition (Lambon Ralph et al., 2017).
According to previous studies, we expected to observe brain areas already implicated in semantic cognition as well as additional networks that might reflect the demands posed by formation and updating of conceptual contexts. A large body of cognitive and clinical neuroscience research, based primarily on the processing and representation of single concepts, has identified two interactive neural networks (Lambon Ralph et al., 2017). The first builds coherent, generalizable, multimodal conceptual representations through the interaction of an anterior temporal lobe (ATL) hub with a distributed set of secondary association cortices (Lambon Ralph et al., 2010; Patterson et al., 2007; Rogers et al., 2004). The computational models of this network (Chen et al., 2017b; Hoffman et al., 2018; Rogers et al., 2004) have always emphasised that the ATL hub allows information to be combined into coherent concepts from across verbal and nonverbal sources (words, sounds, vision, etc.) and also over time. Accordingly, it seems entirely possible that this network would be important for the formation of the conceptual gestalts conveyed in the narratives (i.e., HC&LC > NC). Indeed, a handful of previous studies have found evidence for a combinatorial semantic process in the superior ATL (Brennan and Pylkkanen, 2012; Humphries et al., 2006; Maguire et al., 1999; Noppeney and Price, 2004; Pallier et al., 2011; Vandenberghe et al., 2002).
The second established semantic network reflects the need to shape and mould semantic information to align with changing tasks and contexts demands (Jefferies and Lambon Ralph, 2006; Noonan et al., 2013). This executive semantic network is comprised of prefrontal, posterior middle temporal and intraparietal sulcus areas (Humphreys and Lambon Ralph, 2015; Noonan et al., 2013). It seems likely that this second network will be important in processing the meaning of the narratives, especially when there is a shift in the context (updating of the semantic gestalt, i.e., LC > HC).
Given the cognitive requirements posed by the formation of meaning across a complete narrative, additional brain regions and networks are likely to be engaged. ICA provides a data-driven approach for identifying independent spatiotemporal functional networks, which can then be tested for their sensitivity to the experimental conditions included in the fMRI task. Thus, ICA is particularly suitable for revealing which brain structures are recruited simultaneously for semantic integration. Previous studies have shown that task-active and resting-state fMRI reveal multiple spatiotemporal networks including a semantic-language network (SLN), an executive control network (ECN), a default mode network (DMN), etc. (Beckmann et al., 2005; Geranmayeh et al., 2014; Seeley et al., 2007; Wirth et al., 2011). Some of these networks overlap with the hypothesised neural networks for semantic representation and control (see above). Thus, consistent with the literature on narratives (Hasson et al., 2008; Lerner et al., 2011; Price, 2012; Simony et al., 2016; Vigneau et al., 2006; Xu et al., 2005) and the hub and spokes model (Lambon Ralph et al., 2017), we expected some of these to be engaged by the task and be sensitive to the task manipulations. In detail, the networks associated with dorsal executive and semantic control should be critically important for update of the semantic gestalt (i.e., LC > HC), as they have been shown to engage whenever semantic processing is demanding (Hoffman et al., 2015; Noonan et al., 2013; Tylen et al., 2015) or after changes of non-semantic rules (Vatansever et al., 2017b). Furthermore, the DMN – a network comprising midline brain regions and the angular gyrus (AG) – which is often anticorrelated with the dorsal executive network (Fox et al., 2005; Humphreys and Lambon Ralph, 2017; Spreng et al., 2010; Vincent et al., 2008, but see Dixon et al., 2017) – might also be a crucial network for the formation of conceptual gestalts. Its exact role, though, is hard to predict from the current literature on the DMN. On the one hand, some researchers have suggested that the DMN may support processes for semantic integration (Hasson et al., 2008; Lerner et al., 2011; Simony et al., 2016; Tylen et al., 2015; Wirth et al., 2011), which would align with the seminal work of Binder suggesting that ‘rest’ involves considerable spontaneous semantic-language activities (Binder et al., 2009). More recent evidence indicates that this hypothesis holds for nodes within the semantic network, such as the ATL, but the AG and other aspects of the DMN are deactivated by semantic and non-semantic tasks alike (Humphreys et al., 2015; Humphreys and Lambon Ralph, 2017). According to the proposal that DMN and AG are actively involved in the formation of semantic gestalt, this network should be positively engaged during semantic processing (i.e., in all conditions), in a way proportional to the amount (HC&LC > NC) and congruency (HC > LC) of the semantic information to be integrated. On the other hand, explorations of episodic memory have implicated the AG and DMN in vivid episodic retrieval and buffering (Rugg and Vilberg, 2013; van der Linden et al., 2017; Vatansever et al., 2017a; Vilberg and Rugg, 2008). Accordingly, it is possible that this buffering mechanism might be engaged by the time-extended narratives whilst participants build up a mental model for the story based on the pre-existing context/schema representation (i.e., positively engaged during HC and LC conditions only), predicting greatest activation for the consistent context condition (i.e., HC > LC) (van der Linden et al., 2017; Vatansever et al., 2017b). Interestingly, this hypothesis would be also in accord with evidence showing that the DMN is modulated by time-extended semantic integration (Hasson et al., 2008; Simony et al., 2016). Finally, an alternative hypothesis with an opposing prediction arises from recent studies that suggest that the DMN is engaged when switching between activities (Crittenden et al., 2015; Smith et al., 2018). If correct, then the DMN should be positively engaged during switches of task or semantic contexts (i.e., during NC and LC conditions, respectively) and particularly when switching from a number to language activity (i.e., NC > LC), but disengaged when no switch is perceived (i.e., HC conditions).
2. Materials and methods
2.1. Participants
Twenty-four volunteers took part in the study (average age = 23 years, standard deviation (SD) = 3; N female = 19). All participants were native English speakers with no history of neurological or psychiatric disorders and normal or corrected-to-normal vision. As a result of technical issues during the scanning session, only data from 22 participants (average age = 23 years, SD = 3; N female = 17) were useable for fMRI data analyses. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Furthermore, all participants gave written informed consent and the study was approved by the local ethics board.
2.2. Stimuli
A total of 40 narrative pairs, each one composed by two paragraphs, were created for the experimental study. For each narrative pair, the same second paragraph (target) was preceded by different first paragraphs (contexts) that could be either high-congruent (i.e., HC) or low-congruent (i.e., LC) with the target in terms of meaning. Both HC and LC context paragraphs could be integrated with the targets, though a major reworking of the evolving semantic representation was required after LC contexts only, because of a shift in the semantic context (see Table S1 for the complete list of the stimuli). Homonym words (e.g., bank) were employed in order to determine the exact point in the paragraph in which the shift in the semantic context should have been experienced.
To ensure that HC and LC conditions differed in respect to semantic associative strength between contexts and targets, we quantified in different ways semantic relatedness between the contexts and targets for both HC and LC conditions. First, we employed Latent Semantic Analysis (LSA) (Hoffman, 2019; Hoffman et al., 2013; Landauer and Dumais, 1997), a method to measure the semantic relationship between words based on the degree to which they are used in similar linguistic contexts. Thus, for each narrative pair, context and target paragraphs were converted in vectors of words that were successively compared (using a cosine similarity metric). From this comparison, an LSA value reflecting the associative strength between the context and target was obtained for both conditions. Results from LSA confirmed that semantic associative strength between the (same) target and the context was higher for HC (average score = 0.41, SD = 0.16) than LC conditions (average score = 0.23, SD = 0.1) [t(78) = 5.996, p < 0.001].
Second, we asked to a group of independent participants to indicate how much contexts and targets were perceived as being semantically related (0–5 scale). The results of this pre-experimental rating confirmed that HC (average score = 4.4, SD = 0.4) and LC (average score = 2.3, SD = 0.4) conditions were different [t(9) = 10.626, p < 0.001]. Moreover, to ensure that participants could perceive the shift of semantic context during the study, at end of each narrative the question “Was there any change of semantic context between part1 and part2?” was posed. Only pairs of narratives on which at least the 90% of participants responded correctly to the questions were employed in the study.
Finally, another condition was included in the design in order to measure the semantic integration processes in general. Precisely, in the NC condition the target (the same as in HC and LC conditions) was preceded by a string of numbers that could include from one to four-digit numbers.
2.3. Task procedures
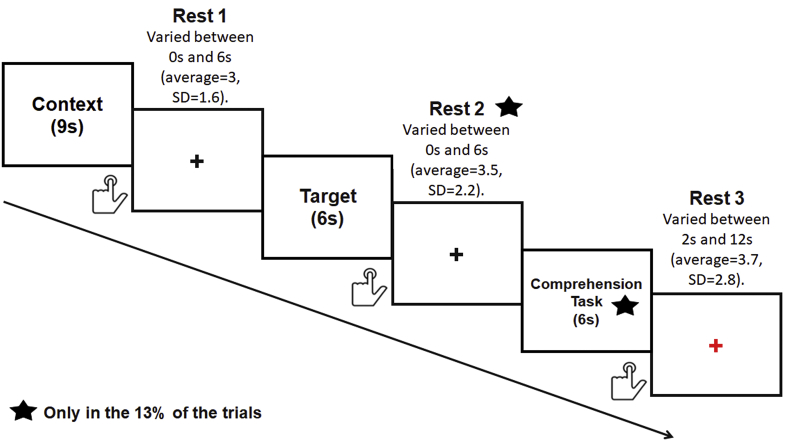
There were 40 items per condition presented using an event-related design with the most efficient ordering of events determined using Optseq (http://www.freesurfer.net/optseq). The details of trial presentation are reported in Fig. 1. Rest time was intermixed between trials and varied between 2 and 12s (average = 3.7, SD = 2.8). During this time a red fixation cross was presented to mark the end of each trial (each narrative composed by a context and a target paragraph). Each context paragraph was presented at once for 9s followed by the target paragraph, which was also presented at once for 6s. A black fixation cross was presented between contexts and targets and its duration varied between 0 and 6s (average = 3, SD = 1.6). During this time, similarly as during the presentation of the red fixation cross (see above), participants were required to rest.
Fig. 1.
Schematic depiction of trial structure and timing.
Participants were asked to read silently both contexts (verbal material and numbers) and targets (only verbal material). Our volunteers were instructed to press a button when arriving to the end of each paragraph (for both contexts and targets). The instruction emphasised speed, but also the need to understand the meaning of verbal contexts and targets, since at the end of some of the trials participants would have been asked to answer to some questions on the content of the narratives. We specified that in order to perform this task it would have been necessary to integrate the meaning between contexts and targets. Hence, following 13% of the trials a comprehension task was presented to ensure that participants were engaged in the task. When this happened, the target item was followed by a statement displayed on the screen for 6s at which participants were required to provide a response (true/false) via button press (see Table S2). A black fixation cross between the target and the comprehension task was presented during a time that varied between 0 and 6s (average = 3.5, SD = 2.2). Before starting the experimental study, all participants were given written instructions. Then they underwent to a practice session with few trials in order to allow them to familiarise with the task. The stimuli used in the practice session were different from those used in the experimental study.
2.4. Task acquisition parameters
Images were acquired using a 3T Philips Achieva scanner using a dual gradient-echo sequence, which is known to have improved signal relative to conventional techniques, especially in areas associated with signal loss (Halai et al., 2014). Thus, 31 axial slices were collected using a TR = 2s, TE = 12 and 35ms, flip angle = 95°, 80 × 79 matrix, with resolution 3 × 3mm, slice thickness 4mm. For each participant, 1492 volumes were acquired in total, collected in four runs of 746s each.
2.5. Data analysis
2.5.1. Behavioural data analyses
Behavioural analyses were performed on reading times (RTs) and the percentage of given responses. Two separate repeated-measures analyses of variance (ANOVAs), one for RTs and the other for percentage of given responses, with “Condition” as within-subjects factor with three levels (NC, LC and HC target conditions) were conducted. Bonferroni correction for multiple comparisons was applied to assess statistically significant effects.
2.5.2. fMRI data analyses
2.5.2.1. Preprocessing
The dual-echo images were averaged. Data was analysed using SPM8. After motion-correction images were co-registered to the participant’s T1 image. Spatial normalisation into MNI space was computed using DARTEL (Ashburner, 2007), and the functional images were resampled to a 3 × 3 × 3mm voxel size and smoothed with an 8mm FWHM Gaussian kernel.
2.5.2.2. General linear modelling (GLM)
The data was filtered using a high-pass filter with a cut-off of 128s and then analysed using a GLM. We ran a single GLM model in which at the individual subject level each condition of interest was modelled with a separate regressor (target NC, target LC and target HC) with time derivatives added. Events were convolved with the canonical hemodynamic response function, starting from the onset of the target paragraph. The number reading paragraph condition (context NC) was also modelled as a regressor of interest to have an active baseline against which to compare the semantic tasks (target NC, target LC and target HC). Also the other context paragraphs (LC and HC contexts) and the comprehension task were modelled as regressors. However, these data were not further analysed because not relevant for the scope of the present study. Each condition was modelled as a single event with a duration corresponding to 6s (target conditions and comprehension task trials) or 9s (context conditions). Motion parameters were entered into the model as covariates of no interest.
2.5.2.3. The semantic network
To identify the brain areas involved in semantic processing during the narrative reading task, we assessed the whole-brain contrast of semantic target conditions (NC, LC, HC collapsed) against rest and against the number reading task (context NC condition). All the contrasts were corrected for multiple comparisons with a voxel-wise false discovery rate (FDR) correction set at q < 0.05 (Benjamini and Hochberg, 1995) and a contiguity threshold ≥ 30 voxels. Note the contiguity threshold ≥ 30 voxels (see also below) was always applied after voxel-wise corrections for multiple comparisons to ensure that the size of the clusters was appropriate to run ROI analysis with spheres ≥ 8mm radius.
Having identified the semantic network, we conducted ROI analyses to assess the functional contribution of key semantic areas in respect to our task manipulations. All the ROI coordinates were independently derived from the literature (Table S3). Regarding the parietal ROIs, we investigated the functional role of three different portions of the AG (Humphreys et al., 2019). To select portions of frontal, parietal and temporal regions without including bordering areas of no interest or extending the selection outside the brain, we employed spheres of 10mm radius. Repeated-measures ANOVAs with “Condition” as within-subjects factor with three levels (NC, LC and HC targets) were conducted for temporal, semantic control and AG ROIs. Bonferroni correction for multiple comparisons was applied to assess statistically significant effects.
2.5.2.4. Whole-brain univariate analyses of the differences between experimental conditions
Semantic integration effect (or formation of the conceptual gestalt). To investigate the semantic integration effect the contrasts LC > NC and HC > NC were computed via whole-brain analysis. All the contrasts were corrected for multiple comparisons with a voxel-wise FDR correction set at q < 0.05 and a contiguity threshold ≥ 30 voxels.
Shift of semantic context (or update of the conceptual gestalt) and shift of task context. The shift of semantic context effect was established by running whole-brain analysis for the contrast LC > HC. To reveal brain regions that are important for task switches into language from a non-language task (i.e., shift of task context), we also conducted a NC > HC whole-brain contrast. Also these contrasts were corrected for multiple comparisons with a voxel-wise FDR correction set at q < 0.05 and a contiguity threshold ≥ 30 voxels.
Importantly, whole-brain contrast analyses alone do not inform on whether the observed differential activation is originated by task-positive or task-negative activation disparities. Hence, the precise contribution of each area was established by conducting ROI analysis (via repeated-measures ANOVA) on a set of key regions revealed by the whole-brain univariate analyses above. In this analysis we opted for spheres of 8mm radius to restrict our selection only to voxels found to be significantly activated in the univariate contrasts.
2.5.2.5. Task group spatial ICA
Spatial ICA applied to fMRI data identifies temporally coherent networks by estimating maximally independent spatial sources, referred to as spatial maps, from their linearly mixed fMRI signals, referred to as time courses. The pre-processed fMRI data was analysed in a group spatial ICA using the GIFT toolbox (http://mialab.mrn.org/software/gift) (Calhoun et al., 2001) to decompose the data into its components. GIFT was used to concatenate the subjects’ data and reduce the aggregated data set to the estimated number of dimensions using principal component analysis, followed by ICA using the Infomax algorithm (Bell and Sejnowski, 1995). Subject-specific spatial maps and time courses were estimated using GICA back-reconstruction method based on principal component analysis compression and projection (Calhoun et al., 2001).
The number of independent components estimated within the data was 38. The estimation was achieved by using the Minimum Description Length criteria, first per each individual data-set and then computing the group mean. The obtained 38 independent components were inspected to exclude from the analysis artefactual and noise-related components. Similar to previous studies (Geranmayeh et al., 2014; Griffanti et al., 2017), the criterion for assigning components as artefact was based on the spatial maps attained as a result of the one sample t-tests (threshold for voxel-wise significance was set at p < 0.05, corrected for family-wise error (FWE) and a contiguity threshold ≥ 30 voxels). The spatial maps were visually compared with the SPM grey matter template. Only components that had the majority of activity within the grey matter were selected (N = 22).
Establishing task-related functional networks. The 22 independent components were labelled according to the resting-state networks template provided in the GIFT toolbox. Then, a multiple regression analysis (implemented as “temporal sorting” function in GIFT) between independent component’s and task model’s time courses for each participant was conducted and allowed to identify the independent components related to target conditions (task-related functional networks). For that, for each participant the design matrix used for the GLM analysis, where rest periods were modelled implicitly as task baseline, was employed. For each independent component, the multiple regression analysis generated 3 beta weight values (one for each target condition, i.e., NC, LC, and HC) that were averaged across runs and participants. Beta weight values represent the correlations between time courses of the independent components and the canonical hemodynamic response model for each task condition. These values are thought to reflect engagement of the functional networks during specific task conditions (Xu et al., 2013).
Once extracted the beta weights for each independent component associated with each condition, task-relatedness for each component was assessed by testing group means of averaged beta weights for each task-condition employing one-sample t-tests (p < 0.05). Hence, a positive/negative beta weight value significantly different from zero indicates increase/decrease in activity of the independent component during a specific task condition relative to the baseline condition (i.e., rest). Once established the task-related functional networks, a repeated-measures ANOVA was used to assess the main differences between beta weights across different task conditions. Bonferroni correction for multiple comparisons was applied to assess statistically significant effects.
Functional network connectivity (FNC) analysis. To explore the relationship between task-related functional networks we conducted a FNC analysis using the Mancovan toolbox in GIFT. Hence, FNC was estimated as the Pearson’s correlation coefficient between pairs of time courses (Jafri et al., 2008). Subject specific time courses were detrended and despiked based on the median absolute deviation as implemented in 3dDespike (http://afni.nimh.nih.gov/), then filtered using a fifth-order Butterworth low-pass filter with a high frequency cutoff of 0.15 Hz. Pairwise correlations were computed between time courses, resulting in a symmetric c1 × c1 correlation matrix for each subject. For all FNC analyses, correlations were transformed to z-scores using Fisher’s transformation, z = atanh(k), where k is the correlation between two component time courses. One sample t-tests (corrected for multiple comparisons at α = 0.01 significance level using FDR) were conducted on task-related functional networks to reveal the significance of pairwise correlations.
Data availability statement. The data will be made available at http://www.mrc-cbu.cam.ac.uk/publications/opendata/.
3. Results
3.1. Behavioural results
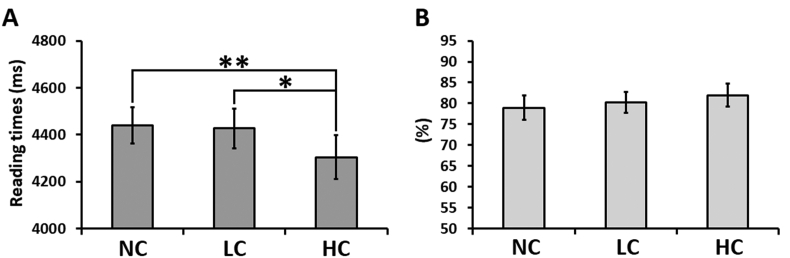
The RTs showed the expected behavioural effect of semantic coherence. Thus performance differed across experimental conditions [F(2,46) = 7.109, p = 0.002, ηp2 = 0.236], with reading speed in the NC and LC conditions being slower than in the HC condition (p = 0.008 and p = 0.019, respectively). There was no significant difference for reading the target paragraph after the NC or LC contexts (p > 0.999). The percentage of given responses was very high and similar across all experimental conditions [F(2,46) = 2.521, p = 0.091, ηp2 = 0.099], with a trend towards significance for percentage of given responses in the HC condition being higher than in the NC condition (p = 0.098) (Fig. 2).
Fig. 2.
Behavioural results for (A) reading times and (B) percentage of given responses for NC, LC and HC target conditions. Pairwise comparisons are Bonferroni-corrected. Error bars correspond to Standard Error (SE).
As mentioned in the “Materials and methods” section, two participants were excluded from the fMRI analyses. Hence, we also ran behavioural analyses for these 22 participants. The results remained unchanged from those reported above for both RTs [F(2,42) = 5.491, p = 0.008, ηp2 = 0.207; NC > HC (p = 0.02); LC > HC (p = 0.05); NC vs. LC (p > 0.999)] and percentage of given responses [F(2,42) = 2.841, p = 0.07, ηp2 = 0.119; NC < HC (p = 0.077); LC vs. HC (p > 0.999); NC vs. LC (p = 0.524)].
3.2. fMRI results
3.2.1. GLM results
3.2.1.1. The semantic network
Whole-brain univariate analysis revealed that semantic reading tasks (target conditions) against rest or non-semantic reading tasks (number context or context NC) recruit a similar network of brain areas (Fig. 3 and Table S4). This network includes frontal, temporal, and parietal brain areas, previously identified as key regions supporting semantic cognition (Lambon Ralph et al., 2017). Furthermore, time-extended semantic reading tasks recruit extensively also the right hemisphere and other areas, normally deactivated during semantic tasks (e.g., hippocampus, precuneus, and the mid-PGp portion of the left AG) (Humphreys et al., 2015; Humphreys and Lambon Ralph, 2015, 2017).
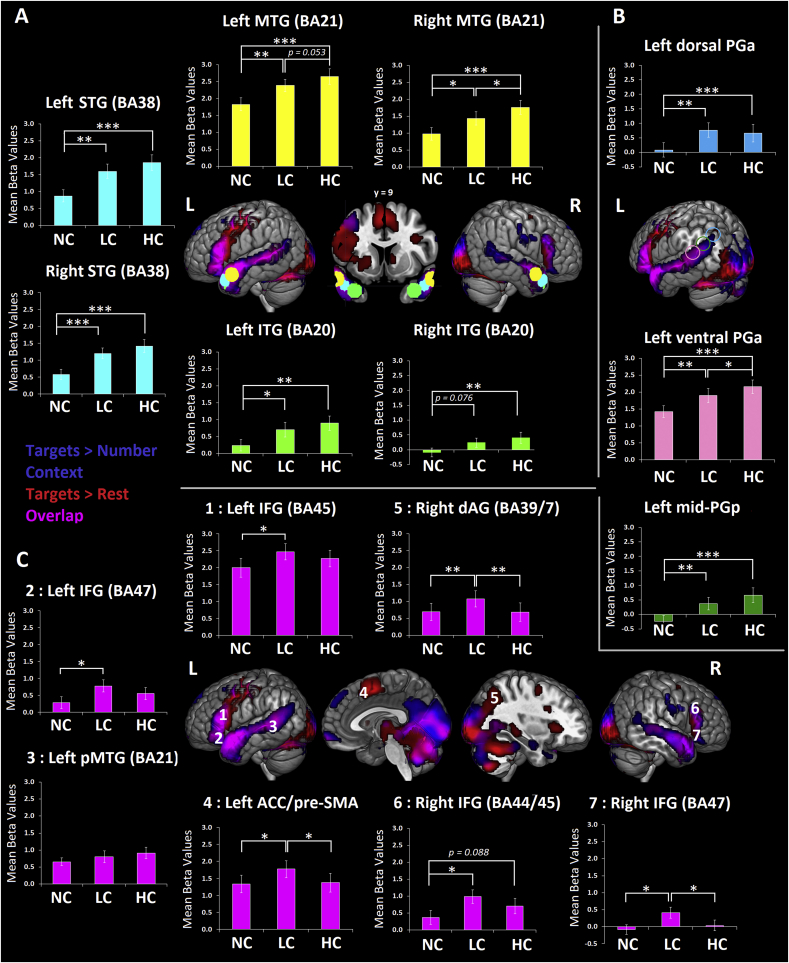
Fig. 3.
GLM results for target conditions against passive (rest) and active (number context or context NC) baselines and for different ROIs (derived from the literature) including (A) the ATL (B) the left AG and (C) the semantic control network. Note that in panel (A) and (B) ROI codes correspond to different colours. Instead, in panel (C) ROI codes correspond to different numbers. ROI mean beta values for each task condition were compared against rest. Pairwise comparisons are Bonferroni-corrected. Error bars correspond to SE.
To determine which parts of the semantic network were sensitive to task manipulations, a set of independently-derived ROIs were employed (see Table S3). These ROIs included ventral and dorsal portions of the ATL, left AG, posterior middle temporal gyrus (pMTG), inferior frontal gyrus (IFG), and anterior cingulate cortex/pre-supplementary motor area (ACC/pre-SMA) (Fig. 3).
Temporal lobe ROIs. We tested whether neural responses for each task-condition (NC, LC and HC) were statistically different across the three ATL regions, i.e., middle temporal gyrus (MTG), superior temporal gyrus (STG) and inferior temporal gyrus (ITG), and hemispheres (left and right), by conducting a repeated-measures ANOVA with “Region” (MTG, STG and ITG), “Hemisphere” (left and right) and “Condition” (NC, LC and HC) as within-subjects factors. A significant interaction between “Region” and “Condition” suggested that neural responses in the temporal ROIs were differently modulated by task conditions [F(4,84) = 7.472, p < 0.001, ηp2 = 0.262]. Bonferroni-corrected post-hoc t-tests revealed the following key finding: The MTG (~BA21) showed significantly increased responses for conditions with integration (LC and HC conditions) as compared to NC condition (p = 0.005 and p < 0.001, respectively), and particularly for coherent semantic endings (HC > LC, p = 0.016). Second, the STG (~BA38) showed increased neural responses for paragraphs preceded by a contextual support (LC and HC conditions) as compared with paragraphs without contextual integration (NC condition) (p = 0.001 and p < 0.001, respectively). However, no significant difference was observed between LC and HC conditions (p = 0.1). Third, the ITG (~BA20) showed sensitivity to integration of the contextual support similarly as the STG. In fact, neural activity in this region was increased for LC and HC conditions as compared with the NC condition (p = 0.043 and p = 0.001, respectively), but no significant difference was observed between LC and HC conditions (p = 0.184). Importantly, ITG did not show positive activation (compared to rest) across all experimental conditions. Precisely, negligible responses were observed for NC condition [left ITG: t(21) = 1.36, p = 0.188; right ITG: t(21) = -0.605, p = 0.552] and for LC condition in the right hemisphere [t(21) = 1.537, p = 0.139], suggesting that this area was mostly engaged during integration of coherent semantic information (HC condition). Finally, the “Region”, “Condition” and “Hemisphere” triple interaction was not significant, suggesting that the condition-specific modulations observed in MTG, STG and ITG were similar in the right and left hemispheres [F(4,84) = 0.466, p = 0.760, ηp2 = 0.022].
Left AG ROIs. We tested whether neural responses for each task-condition (NC, LC and HC) were statistically different across the three different left AG spheres. Hence, we conducted a repeated-measures ANOVA with “Region” (mid-PGp, ventral PGa and dorsal PGa) and “Condition” (NC, LC and HC) as within-subjects factors. A significant interaction between “Region” and “Condition” confirmed a different profile of engagement of the three different AG regions during the different task conditions [F (4,84) = 5.668, p < 0.001, ηp2 = 0.213]. Bonferroni-corrected post-hoc t-tests revealed that the mid-PGp showed increased neural responses for paragraphs preceded by a contextual support (LC and HC conditions) as compared with paragraphs without contextual integration (NC condition) (p = 0.009 and p < 0.001, respectively). However, no significant difference was observed between LC and HC conditions (p = 0.16). Similarly as the mid-PGp, the ventral PGa (vPGa) showed increased neural responses for LC and HC conditions as compared with paragraphs without contextual integration (NC condition) (p = 0.006 and p < 0.001, respectively). However, and differently from mid-PGp, this ventral portion of the AG showed also enhanced responses for coherent vs. less coherent semantic endings (HC > LC, p = 0.015), i.e., an effect of semantic coherence. Finally, the dorsal PGa (dPGa) showed a similar pattern of responses to the mid-PGp, i.e., increased neural responses for LC and HC conditions as compared with NC condition (p = 0.002 and p = 0.001, respectively) and no significant difference between LC and HC conditions (p > 0.999). Interestingly, all the different AG regions showed some sensitivity to the presence of contextual information. However, whilst the vPGa was positively activated (compared to rest) for NC conditions, the mid-PGp and dPGa exhibited negligible activation for the NC conditions (one sample t-tests revealed ps > 0.05 for NC conditions).
Semantic control network ROIs. We tested whether neural responses for each task condition (NC, LC and HC) were statistically different across the different ROIs of the semantic control network. Hence, we conducted a repeated-measures ANOVA with “Region” (see below) and “Condition” (NC, LC and HC) as within-subjects factors. A significant interaction between “Region” and “Condition” revealed a different profile of engagement of the semantic control regions during the different task conditions [F(12,252) = 2.48, p = 0.004, ηp2 = 0.106]. Of particular interest is the finding revealing that the effect of shift of semantic context was predominantly observed in the right hemisphere. In fact, the right IFG (~BA47) [LC > NC (p = 0.004), HC vs. NC (p > 0.999), and LC > HC (p = 0.017)] and the right dorsal AG (dAG) [LC > NC (p = 0.004), HC vs. NC (p > 0.999), and LC > HC (p = 0.006)] showed increased responses for LC > HC conditions. By contrast, with the exception of the left ACC/pre-SMA (~BA32/24/8/6) [LC > NC (p = 0.016), HC vs. NC (p > 0.999), and LC > HC (p = 0.015)], the effect of semantic update was not observed in the left hemisphere [left IFG (~BA45/44): LC > NC (p = 0.047), HC vs. NC (p = 0.145), and LC vs. HC (p = 0.324); left pMTG (~BA21/37/20): LC vs. NC (p = 0.912), HC vs. NC (p = 0.229), and LC vs. HC (p = 0.99); left IFG (~BA47): LC > NC (p = 0.043), HC vs. NC (p = 0.292), and LC vs. HC (p = 0.567)]. Finally, the right IFG (~BA44/45) showed a context integration effect [LC > NC (p = 0.01), and HC > NC (p = 0.088), and LC vs. HC (p = 0.102)].
To summarise, the independent ROI analyses revealed three key findings: First, the gyral distribution of semantic task activation in the temporal lobe supported previous research and revealed novel insights on the functional specialisation of the ATL for time-extended combinatorial processes. As expected, we observed a bilateral involvement of the ATL in semantic processing (Hoffman et al., 2015; Humphreys et al., 2015; Rice et al., 2015; Visser and Lambon Ralph, 2011). Interestingly, the effect of semantic coherence (HC > LC) was observed in the MTG. The STG and ITG showed a general effect of semantic integration (HC&LC > NC), as they were engaged more strongly – or uniquely in the case of ITG – for those condition preceded by a context paragraph (i.e., LC and HC conditions). Secondly, as found in many previous studies (Humphreys et al., 2019; Humphreys and Lambon Ralph, 2015, 2017; Seghier et al., 2010), the response profile in AG was found to shift rapidly and quickly across the AG region. The anterior ventral portion (vPGa) was sensitive to the semantic coherence of the information to be integrated, whereas instead the dorsal portion (dPGa) and mid-PGp were not. Instead, these two AG sub-regions showed a semantic integration effect as they were engaged only for those condition preceded by a semantic contextual support (i.e., LC and HC conditions). Finally, the update of the semantic gestalt (LC > HC) engages brain structures generally recruited when semantic processing requires increased executive control demands (Noonan et al., 2013). However, differently from previous studies, these effects are mainly observed in the right hemisphere.
3.2.1.2. Whole-brain univariate analyses of the differences between experimental conditions
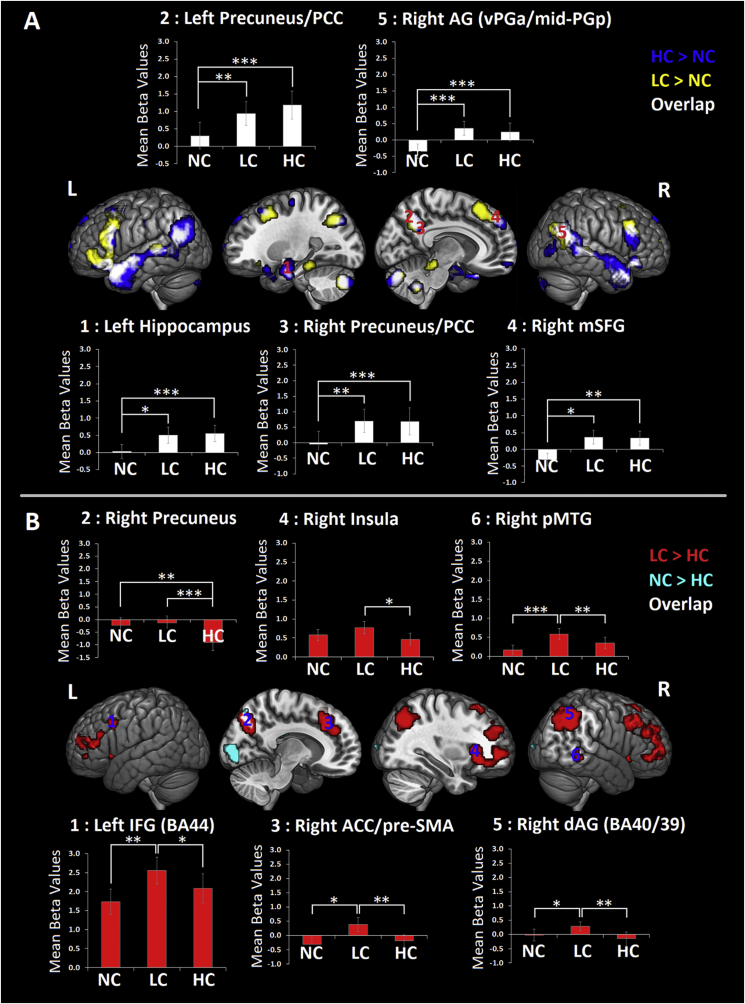
Semantic Integration effect (or formation of the conceptual gestalt). We compared HC > NC and LC > NC. Both contrasts generated very similar, overlapping neural semantic networks including different portions of the dorsal ATL, extending to posterior portions of the superior temporal sulcus (pSTS), ventral portions of the AG (vPGa) and bilateral IFG (Fig. 4A and Table S4). A crucial observation in accord with our hypothesis is that the overlap was also observed in correspondence of “extra-semantic” areas, i.e., areas that are not referred in the literature to semantic cognition in particular, such as the left hippocampus, the right AG, medial superior frontal gyrus (mSFG) and the precuneus/posterior cingulate cortex (PCC).
Fig. 4.
GLM results for (A) the semantic integration effect (HC&LC > NC) and five ROIs derived from the peaks of activation in areas of overlap. GLM results for (B) the shift of semantic and task context effects (LC > HC and NC > HC, respectively) and six ROIs derived from the peaks of activation. ROI codes correspond to different numbers. Pairwise comparisons are Bonferroni-corrected. Error bars correspond to SE.
Given neural responses within the semantic network were extensively investigated (see Fig. 3), the following ROI analysis focussed on overlapping extra-semantic regions in order to reveal their role in semantic integration. Therefore, we conducted a repeated-measures ANOVA with “Region” [left hippocampus (coordinate: x = −24, y = −9, z = −24), the right AG (~vPGa/mid-PGp; coordinate: x = 54, y = −60, z = 27), the right mSFG (~BA9/32; coordinate: x = 6, y = 48, z = 39), and the precuneus/PCC (coordinate: x = ±9, y = −54, z = 36)] and “Condition” (NC, LC and HC) as within-subjects factors. The “Region” and “Condition” interaction was not significant, suggesting a similar profile of engagement of these regions during the different task conditions [F(8,168) = 1.337, p = 0.229, ηp2 = 0.06]. A significant effect of “Condition” [F(2,42) = 22.003, p < 0.001, ηp2 = 0.512] revealed an important finding: Increased neural responses in these areas for LC and HC conditions as compared with NC condition (ps < 0.001) and no significant difference between HC and LC conditions (p > 0.999). Interestingly, as for two portions of the AG (Fig. 3B), these brain areas were all positively engaged (compared to rest) for LC and HC conditions (see Fig. 4A), but not for NC condition (one sample t-tests revealed all ps > 0.05 for NC condition).
To summarise these results, integration of meaning across a narrative engages areas of the semantic network (Lambon Ralph et al., 2017) as well as other brain regions. Unlike the semantic network regions, these extra-semantic areas are recruited only when contextual information can be integrated.
Shift of semantic context (or update of the conceptual gestalt) and shift of task context. We compared LC > HC conditions. The ACC/pre-SMA and the precuneus were activated more strongly for the LC than the HC condition. These regions were joined by other frontoparietal multi-demand (MD) regions (Duncan, 2010) (e.g., lateral IFG, superior parietal areas and insula) and the right pMTG (Fig. 4B and Table S4). We conducted a repeated-measures ANOVA with “Region” [dorsal right precuneus (~BA7; coordinate: x = 12, y = −72, z = 42), right dAG (~BA40/39; coordinate: x = 42, y = −51, z = 45), right ACC/pre-SMA (~BA32/8; coordinate: x = 3, y = 30, z = 42), left IFG (~BA44; x = −45, y = 12, z = 33), right insula (~BA47; coordinate: x = 30, y = 24, z = 6), and right pMTG (~BA21/20; coordinate: x = 60, y = −42, z = −6)] and “Condition” (NC, LC and HC) as within-subjects factors. A significant interaction between “Region” and “Condition” revealed a different profile of engagement of the selected ROIs during the different task conditions [F(10,210) = 8.504, p < 0.001, ηp2 = 0.288]. Bonferroni-corrected post-hoc comparisons revealed the following key results: Neural responses in the left IFG (~BA44) were increased for LC conditions as compared with HC and NC conditions (p = 0.036 and p = 0.003, respectively), but no significant difference was observed between the HC and NC conditions (p = 0.164). A similar profile of differential engagement was observed in right pMTG, right dAG and right ACC/pre-SMA [right pMTG: LC > NC (p < 0.001), HC vs. NC (p = 0.125), and LC > HC (p = 0.003); right dAG: LC > NC (p = 0.011), HC vs. NC (p = 0.652), and LC > HC (p = 0.006); right ACC/pre-SMA: LC > NC (p = 0.016), HC vs. NC (p > 0.999), and LC > HC (p = 0.007)]. Finally, neural activity in the right insula (~BA47) was increased for LC conditions as compared with HC conditions (p = 0.049), whilst no significant differences were observed between NC conditions as compared to LC and HC (p = 0.243 and p = 0.366, respectively).
The post-hoc comparison results also revealed that activity in a dorsal portions of the right precuneus (~BA7) was increased for LC and NC conditions as compared to HC conditions (p < 0.001 and p = 0.002, respectively). However, there was no significant difference between LC and NC conditions (p > 0.999).
Interestingly, in contrast with what we observed for the left IFG, right insula and right pMTG, the LC > HC differential activations measured in the dorsal right precuneus, right dAG and ACC/preSMA were all due to differential negligible or task-negative activation patterns (one sample t-tests revealed all ps-> 0.05 for LC condition). The HC > LC contrast did not reveal significant results.
Finally, we directly compared the NC > HC condition to establish which brain regions are important for task switches into language from a non-language task (number reading) and therefore to identify possible similarities between neuro-computations supporting shifts of semantic and task contexts. This contrast activated a right lateralised set of higher-order visual regions, including also a portion of the right precuneus activated by the LC > HC contrast (see Fig. 4B and Table S4).
To summarise, resetting the conceptual gestalt elicits a robust activation of a bilateral set of frontal regions and the right pMTG. The right precuneus was less deactivated for LC and NC conditions as compared with HC condition, a pattern that mirrored that of RTs (see Fig. 2).
3.2.2. Task group spatial ICA results
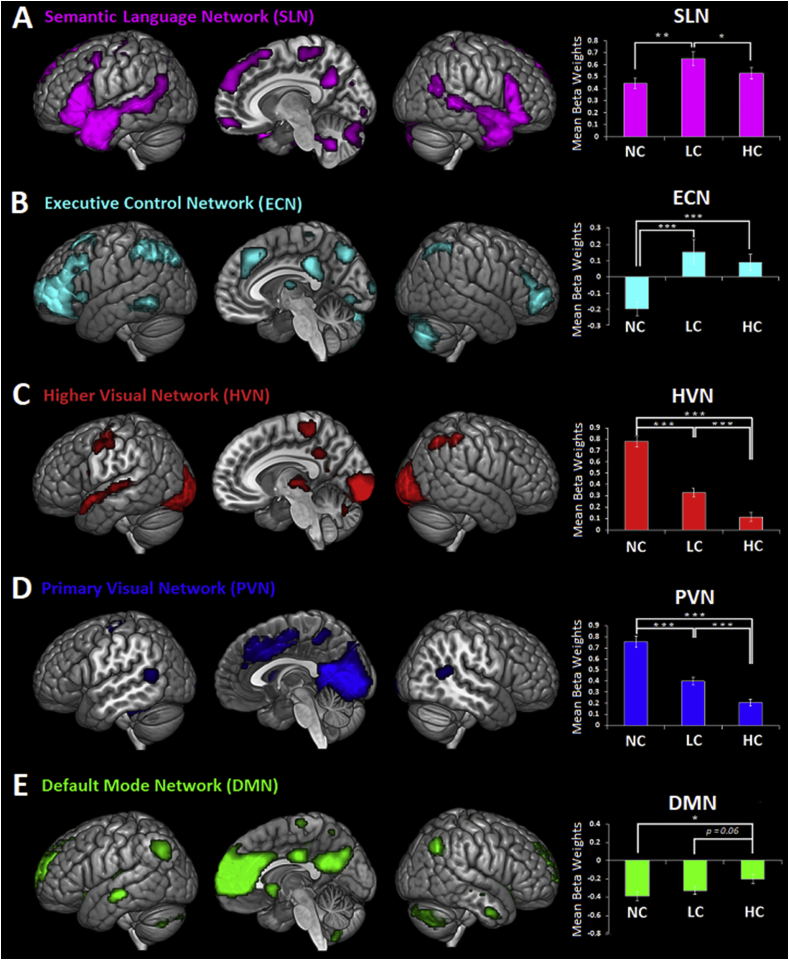
Task-related functional networks. ICA was used to explore which semantic and extra-semantic areas, revealed by univariate analyses, exhibited yoked activations – i.e., constituted functional networks rather than independent areas. ICA identified 22 independent components, of which 5 exhibited significant sensitivity to our task conditions: These were a semantic/language network (SLN), an executive control network (ECN) including fronto-parietal regions, a higher visual network (HVN), a primary visual network (PVN), and a DMN (Fig. 5 and Table S5).
Fig. 5.
Task-related functional networks and beta weights’ results. (A) Semantic language network (SLN); (B) Executive control network (ECN); (C) Higher visual network (HVN); (D) Primary visual network (PVN); (E) Default mode network (DMN). Comparisons relative to betaweights are Bonferroni-corrected. Error bars correspond to SE.
As well as a bilateral set of semantic brain areas, the SLN included extra-semantic regions (e.g., hippocampus), suggesting that these regions are recruited together with semantic areas to support time-extended semantic cognition. We conducted a repeated-measures ANOVA on the beta weight values. The results revealed a significant interaction between “Network” (SLN, ECN, HVN, PVN and DMN) and “Condition” (NC, LC and HC) within-subjects factors [F(8,168) = 42.698, p < 0.001, ηp2 = 0.670], suggesting different engagement of the networks in the different task conditions. In fact, the SLN was positively engaged by all three conditions (in comparison to rest), but that it was most active when the semantic context shifted (i.e., LC condition) [LC > NC (p = 0.007) and LC > HC (p = 0.032)]. A related pattern was observed in the ECN which was positively engaged by both the HC and LC conditions, i.e., when a previous semantic context was available for integration [LC > NC (p < 0.001) and HC > NC (p < 0.001)], though this was independent of whether the context was semantically congruent with the target or not [HC vs. LC (p = 0.774)]. The two visual networks (HVN and PVN), containing occipital but also attentional control regions, were recruited most heavily for the NC condition, in which there was a change in the cognitive task [HVN: NC > LC (p < 0.001), NC > HC (p < 0.001) and LC > HC (p < 0.001); PVN: NC > LC (p < 0.001), NC > HC (p < 0.001) and LC > HC (p = 0.001)].
Finally, unlike the four other components, the DMN was deactivated with respect to rest. It exhibited sensitivity to the task conditions, in that it was least deactivated in the HC condition [HC > NC (p = 0.025), HC > LC (p = 0.063) and NC vs. LC (p = 0.88)]. This pattern of deactivation mirrors the task performance (see Fig. 2), in which RTs for the target narrative were slowest for the LC and NC condition. Thus this result might reflect the common pattern that the degree of deactivation in the DMN is often correlated with task difficulty and other measures of behavioural stability (Esterman et al., 2013; Humphreys et al., 2015; Kucyi et al., 2016; Vatansever et al., 2017a). A negative correlation between averaged DMN time courses and averaged RTs was observed in our study, without however reaching statistical significance (r = −0.099, p = 0.662).
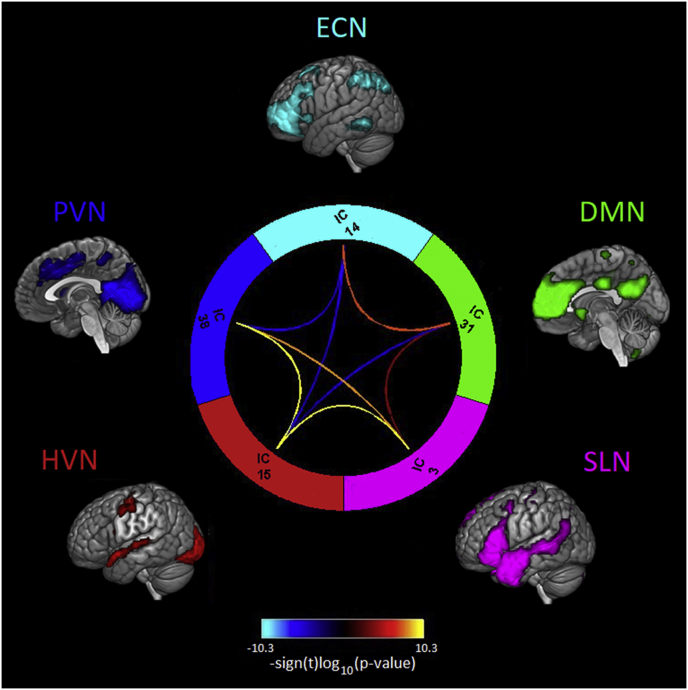
FNC analysis. Given our interest in exploring the interaction between the SLN and other networks involved in time-extended semantic cognition, we computed a FNC analysis (see Fig. 6). Significant positive correlations were observed between the time courses of DMN and SLN (r = 0.13) and between DMN and ECN (r = 0.23). Instead, the DMN was negatively correlated with HVN (r = −0.22). The ECN, the network not engaged during changes of task context, but only during semantic integration, showed a significant negative correlation with both HVN and PVN (r = −0.17 and r = −0.18, respectively), i.e., the networks maximally engaged during changes of task context (NC condition). In contrast with ECN, the SLN was positively correlated with both the HVN and PVN (r = 0.28 and r = 0.21, respectively). Finally, the time courses of the two visual networks were positively correlated (r = 0.44).
Fig. 6.
FNC results. Connectogram of the FNC results: Significant pairwise correlations (p < 0.05) were corrected for multiple comparisons (FDR, α = 0.01). Significance and direction of each pairwise correlation is displayed as the −sign(t)log10(p).
To summarise, these results suggest that time-extended semantic integration is supported by both the SLN and ECN. The SLN seems to reflect semantic update processes and the ECN context integration processes. These two networks seem to interact with the visual networks in different ways. Unlike the SLN, ECN recruitment is anticorrelated with the visual networks, being strongly recruited when switching from number to text reading (NC conditions, i.e., shift of task context). Thus, it might be that switching efficiently from a non-semantic to a semantic task requires the activation of semantic and language areas (SLN) and, at the same time, the disengagement of brain regions involved in integration of contextual information (ECN). The DMN seems to be sensitive to task difficulty only.
4. Discussion
Using both univariate and multivariate data-driven (ICA) approaches we revealed the brain regions and networks supporting time-extended formation and updating of conceptual representations. Unlike prior investigations (e.g., Hasson et al., 2008; Lerner et al., 2011; Simony et al., 2016; Tylen et al., 2015), in this fMRI study we established which neural networks are primarily evoked by the formation and updating of semantic representations, and we distinguished them from those that support other non-semantic processes required during narrative reading. The main findings on the functional specialisation of brain areas within and outside the classical semantic network are discussed below, followed by a discussion on the functional networks, i.e., how semantic and extra-semantic regions (i.e., regions outside the classical semantic network) are recruited together during meaning formation and update.
4.1. The semantic network
We hypothesised that the building of the conceptual gestalt would be supported by two interactive neural systems, reflecting representational and control aspects of semantic cognition, respectively. In accord with our hypothesis and previous findings (Hasson et al., 2008; Lerner et al., 2011; Silbert et al., 2014), our results revealed that integrating semantic information during narrative processing engages a bilateral set of frontal, parietal and temporal brain structures, known as the “semantic network” (Lambon Ralph et al., 2017). Within this network we identified hubs for formation of coherent concepts and control regions for context-sensitive regulation of semantic information.
4.1.1. Hubs for the formation of time-extended conceptual gestalts: The role of ATL
A first important result obtained from the univariate analysis (whole-brain and independent ROI analyses) is that the ATL supports time-extended combinatorial processes in addition to basic semantic combinations shown in previous studies (Brennan and Pylkkanen, 2012; Visser and Lambon Ralph, 2011), corroborating our hypothesis that this region is a key hub for the formation of conceptual representations (Noppeney and Price, 2004; Patterson et al., 2007; Lambon Ralph et al., 2017; Vandenberghe et al., 2002). In particular, the effect of semantic coherence (HC > LC) was observed in the MTG, suggesting that this subregion of the ATL may have a crucial role in the formation of time-extended conceptual gestalt in verbal tasks. Future studies will have to assess whether this effect is observed in other subregions of the ATL when meaningful stimuli are presented in non-verbal modalities. In contrast, the STG and the ITG showed a general effect of semantic integration (HC&LC > NC), as they were engaged more strongly – or uniquely in the case of ITG – for those conditions preceded by a context paragraph (i.e., LC and HC conditions).
The graded differential distribution of activation patterns observed within the ATL may reflect differences of structural connectivity between ATL subregions and primary input/output areas (Binney et al., 2012). The lack of sensitivity for NC conditions in the ITG indicates that neural activity in this ATL subregion is modulated by the presence of contextual information. This effect may be determined because of direct connections (via entorhinal cortex) with regions supporting the formation of contextual memories, such as in the parahippocampal gyrus and the hippocampus (Bar et al., 2008; Davachi, 2006; Dundon et al., 2018). A recent study has demonstrated the interplay between semantic processing in ATL and information encoding/retrieval in the hippocampus during the formation/retrieval of contextual memories (Griffiths et al., 2019). Accordingly, it is possible that these anatomical connections allow these regions to cooperate for the formation and retrieval of semantic and contextual information conveyed in the narrative (e.g., episodic details) (Ranganath and Ritchey, 2012), facilitating the flow of neocortical information into the hippocampus during encoding and the propagation of hippocampal signals into the ATL during retrieval. Future experimental studies combining high-temporal and high-spatial resolution techniques will be needed to test this possibility.
4.1.2. Brain regions for the update of time-extended conceptual gestalts: The contribution of the right hemisphere
In accordance with our hypothesis, a second important result is that increased semantic integration demands, induced by the need to update the semantic gestalt, elicit bilateral activation of ventral and dorsal portions of the frontal lobe (Fig. 4B). This result accords with previous studies that have employed tasks requiring multi-item combinations (Silbert et al., 2014; Tylen et al., 2015; Anderson et al., 2017; Wehbe et al., 2014). Conversely, it differs from others that have utilised semantic association tasks where the involvement of the IFG was mostly left lateralised (Humphreys et al., 2015; Noonan et al., 2013).
The involvement of the right hemisphere during natural-like language processing has been attributed to the complexity of the input. In other words, as language input gets increasingly complex, there is increasing involvement of right hemisphere homologues to classic left hemisphere language areas (Jung-Beeman, 2005). Particularly, the right hemisphere activation may become prominent and sustained when words and sentences are presented in a narrative context, and may here reflect coherence and inference at the propositional level and beyond, when readers make connections between sentences and paragraphs to form a coherent conceptual gestalt. This interpretation is consistent with the view that the right hemisphere, as compared to the left, would be involved in processing global aspects of linguistic contents (Hickok and Poeppel, 2007; Poeppel, 2003; St George et al., 1999).
Nevertheless, this proposal and our initial hypothesis do not entirely fit with our data. In fact, the univariate results revealed that some key nodes within the semantic control network (i.e., dAG, pMTG and ventral IFG) showed enhanced responses in the right but not in the left hemisphere when integration required major revision of the semantic context (LC > HC contrast). Hence, although the modulation of control regions in the right hemisphere aligns with the findings in the literature (Jung-Beeman, 2005; Xu et al., 2005), it is not clear what might have determined a shift in the lateralisation of the effect related to the update of the semantic gestalt. One possibility is that this effect is not observed in the left hemisphere because neural responses in these regions may have been too transient to be captured by fMRI.
Consistent with previous findings, the involvement of the right parietal and frontal regions may reflect the recruitment of domain-general working memory (Gajardo-Vidal et al., 2018; Vigneau et al., 2011) and inhibitory control mechanisms (Aron et al., 2004) applied when incongruences are detected across paragraphs of text. Particularly, the right ventral IFG (BA47) and the right insula might enact the sustained suppression of the incorrect interpretation of the ambiguous word and all the words semantically related to it, after a change of semantic context is detected (Mason and Just, 2007).
The role of the left pMTG in semantic tasks has been related to the modulation of semantic activation to focus on aspects of meaning that are appropriate to the task or context (Noonan et al., 2013). Accordingly, we were expecting that this region would also respond to shifts of semantic context (i.e., during the update the semantic gestalt). However, this effect was observed in the right pMTG that has different structural and functional connectivity properties as compared to the left pMTG (Vassal et al., 2016; Xu et al., 2015). For instance, Neurosynth (http://old.neurosynth.org/; Yarkoni et al., 2011) shows that resting-state co-activation maps from the left and right pMTG regions have different patterns of functional connectivity with the ATL. That is, the left but not the right pMTG shows intrinsic connectivity with the ATL semantic hub. Instead, the right pMTG shows connectivity with regions that, except for the left pMTG, constitute a right lateralised fronto-parietal network. This observation, along with evidence that this area is not involved in semantic tasks (Noonan et al., 2013) unless they require formation of time-extended contextual associations (Xu et al., 2005), may lead to the hypothesis that the right pMTG plays a critical role in capturing changes of context-sensitive meaning over longer periods of time, possibly by integrating information across working memory (frontal and parietal corticies) and semantic networks (left pMTG). Assessing this hypothesis will require the combination of high-temporal and high-spatial resolution techniques in an experimental design where semantic control demands can be manipulated at different levels of granularity (word, sentence and paragraph level).
4.2. The role of the left AG: Semantic hub or buffering system?
Our results reveal that a portion of the left AG (vPGa) supports meaning formation during time-extended semantic cognition similar to the MTG. In fact, an effect of semantic coherence was observed in the anterior ventral portion of the left AG (vPGa). This result aligns with the proposal that the left AG has a crucial role for the formation and retrieval of semantic representations (Binder and Desai, 2011; Binder et al., 2009; Geschwind, 1972). However, this general semantic role for the AG does not fit with a series of findings from neurological patients that have reported semantic impairments after ATL but not parietal damage (Lambon Ralph et al., 2017), and the demonstrations of equal (de) activation for non-semantic and semantic tasks in this region (Humphreys et al., 2015; Humphreys et al., 2019; Humphreys and Lambon Ralph, 2015, 2017).
Recent compelling evidence has led to an alternative proposal on the role of the left AG that would reconcile neuroimaging and neuropsychological findings. Rather than a hub for semantic integration, the left AG might support a domain-general mechanism that buffers time-, context- and space-varying inputs (Humphreys et al., 2019; Humphreys and Lambon Ralph, 2017). Since buffering becomes relevant only when the task requires combinations across multiple (internal or external) items (e.g., during encoding, integration, recollection, etc.), it is not surprising that left ventral AG is positively engaged in our study and in other semantic tasks that involve integration of information across multiple items (Price and Mechelli, 2005; van der Linden et al., 2017).
4.3. Beyond the semantic network
Besides brain areas implicated in semantic cognition, we also expected to observe additional brain regions and networks reflecting the demands posed by forming and updating conceptual contexts. Consistent with this hypothesis and previous findings (Hasson et al., 2008; Lerner et al., 2011; Price, 2012; Silbert et al., 2014; Simony et al., 2016; Vigneau et al., 2006; Xu et al., 2005), a set of brain regions comprising the hippocampus, the precuneus/PCC and the DMN AG region (i.e., mid-PGp) responded to semantic integration (LC&HC > NC contrast). Similar to the mid-PGp, these other regions have been identified as nodes of both task-positive (e.g., episodic memory, mind-wandering, etc.) and task-negative (DMN) networks, and have been related to multiple cognitive functions, including semantic cognition (Krieger-Redwood et al., 2016). In accord with what previous studies have found when contrasting intact (similar to our HC condition) versus scrambled (similar to NC condition) narratives (Hasson et al., 2008; Lerner et al., 2011; Simony et al., 2016), we revealed that activity in the hippocampus, the precuneus/PCC and the mid-PGp was modulated by the presence of contextual support. However, contrary to what it has been suggested, activity in these areas was not modulated by the semantic coherence per se. Without saying that these are fully independent neural processes, the intact versus scrambled comparison used in previous investigations does not necessarily distinguish between brain areas crucial for the formation of a semantic gestalt (meaning integration) and those important for scene construction, i.e., those processes that allow linking incoming cues about the current context (e.g., time and space cues) into a schema representation or situation model (for a discussion on these two different systems see Ranganath and Ritchey, 2012). Our study allowed us to draw out this distinction for the first time. Specifically, we compared brain activity elicited by coherent narratives (HC condition) not only against conditions without a contextual support (i.e., without a situation model, see NC condition), but also against conditions that, despite having a contextual support, nevertheless required an update of the ongoing semantic representation (i.e., the meaning associated to words and their combinations, see LC condition).
Therefore, we propose that whilst activity in the ATL is likely to reflect semantic integration per se (see above), activity in DMN regions (i.e., the hippocampus, the precuneus/PCC and the mid-PGp) may support processes needed to construct associations between the information conveyed in the target and context paragraphs (Davachi, 2006; Maguire et al., 1999; Ranganath and Ritchey, 2012; Spaniol et al., 2009; Staudigl and Hanslmayr, 2013).
Finally, contrary to what has been found in previous investigations (Crittenden et al., 2015; Smith et al., 2018), DMN regions (e.g., precuneus/PCC and mSFG) were not increasingly engaged by shifts of semantic or task contexts. These inconsistencies might be due to substantial differences in the tasks employed in our and other studies. Being a naturalistic-like task, the reading task was quite “passive” and resetting the task context did not require the cognitive manipulation (retrieval, inhibition, etc.) of instructions/rules associated to the task to be performed. Instead, switching between the highly novel tasks employed in previous studies (Crittenden et al., 2015; Smith et al., 2018) necessarily required this sort of process, given that each stimulus domain (i.e., stimuli depicting people, buildings and words) was associated to two possible classification rules (male/female and old/young for face stimuli; skyscraper/cottage and inside/outside view for building stimuli; first letter and last letter for word stimuli). Hence, the task-switch activity observed in DMN regions in previous studies may reflect the retrieval of task rules, rather than reinstatement and assessment of contextual representations. A related possibility is that, given the novelty of these tasks (learned prior to scanning), the DMN region activations reflect episodic retrieval of the task instructions.
4.4. The functional networks
ICA was employed in addition to univariate analysis to reveal how different brain regions were recruited for semantic integration. In the present study, ICA revealed a network sensitive to semantic control demands (SLN), a network sensitive to context integration (ECN) and two networks sensitive to domain-general attentional control demands (HVN and PVN). Finally, ICA revealed also a DMN modulated by task-condition difficulty.
4.4.1. SLN and ECN: Semantic update and context integration
In accord to previous research work (Geranmayeh et al., 2014; Price, 2012; Silbert et al., 2014; Simony et al., 2016; Vigneau et al., 2006) and an influential model of semantic cognition (Lambon Ralph et al., 2017), we expected two task-positive networks to support semantic processing and executive control. We were expecting these two networks to be modulated by shifts of semantic context (LC > HC). Accordingly, ICA revealed a SLN and a ECN, both positively engaged during the semantic task conditions.
In accordance with our predictions, the SLN, including semantic but also MD and other extra-semantic areas (e.g., hippocampus), was maximally recruited during shifts of semantic context (update of the semantic gestalt). This finding suggests that updating the information in the semantic system may require the orchestration of different neuro-computations, possibly including working memory, semantic processing and domain-general executive control.
The ECN, including a set of fronto-parietal and medial regions, was also positively engaged during the reading task. However, contrary to our predictions, rather than being sensitive to variations of semantic context (LC > HC), the ECN was sensitive to context integration in general (LC&HC > NC). That is, this network was positively recruited when the target conditions could be integrated with a previous context, independently of whether the context was highly congruent with the target. Instead, this network was disengaged when information could not be integrated with a previous context. The spatial distribution of this network and its sensitivity to contextual integration is consistent with different cognitive control processes, including also working memory (e.g., Vatansever et al., 2017a).
4.4.2. The visual networks support domain-general attentional control mechanisms
ICA revealed two additional visual networks, the PVN and HVN that were positively engaged during the reading task. Previous evidence suggests that the primary and higher visual systems dissociate not only in resting-state but also during functional tasks (Shen et al., 2019). Indeed, the PVN is specialised for processing information about static and moving objects, whereas the HVN is mostly associated with spatial awareness and guidance of actions (e.g., Nassi and Callaway, 2009). These networks, that in our study also included other cortical and subcortical control regions (e.g., IFG, pMTG, precentral gyrus, putamen, etc.), were strongly engaged during changes of task and semantic context. Based on the literature, we could not make strong hypotheses about the possibility of observing these two networks dissociating from the SLN or ECN. However, the observed sensitivity to changes of semantic context is particularly interesting. In fact, it shows that sensory-dorsal and posterior attentional networks are involved in narrative reading and support control processes that are important but not specific to semantic integration. Consistent with the idea that the visual networks might aid semantic integration, our exploratory FNC analyses revealed that HVN and PVN were both positively connected to the SLN. Future studies will have to establish whether the visual networks play a critical role for time-extended semantic processing in reading tasks.
4.4.3. The role of DMN during narrative processing
We expected the recruitment of a DMN including hippocampus, AG (mid-PGp), precuneus/PCC, and other medial prefrontal structures (Andrews-Hanna et al., 2010). According to the hypothesis that DMN supports semantic integration (Binder et al., 2009), we should have observed task-positive responses or some sensitiveness to semantic manipulations in the task. However, like previous findings, the DMN showed the typical task-negative response (Geranmayeh et al., 2014; Humphreys et al., 2015; Wirth et al., 2011). Whilst we acknowledge that the DMN involvement in semantic processing cannot be decided on the basis of this evidence alone, the observation that DMN activity was not modulated by semantic integration (LC vs. NC) is hard to reconcile with the semantic hypothesis (Binder et al., 2009). Furthermore, replicating previous studies (Humphreys et al., 2015), we observed similar DMN task-negative responses during semantic and non-semantic processing alike (see Figure S1C). This finding is clearly at odds with the hypothesis that the primary function of the DMN is representing and integrating semantic information.
A second hypothesis is that the DMN supports episodic retrieval and buffering (Rugg and Vilberg, 2013; van der Linden et al., 2017; Vatansever et al., 2017a; Vilberg and Rugg, 2008). If so, then one would expect to observe an activation profile similar to the ECN: positively engaged only for conditions allowing integration (LC and HC), and particularly for the most the consistent context condition (HC > LC). Conversely, not only was the DMN negatively engaged during meaning integration (HC and LC), but most importantly, it did not show differential responses between conditions preceded (LC) and not preceded by a contextual support (NC). Thus, this result is inconsistent with the proposal that DMN supports episodic retrieval and buffering during narrative reading.
A final and third hypothesis is that the DMN is involved in the reinstatement of context-relevant information (Crittenden et al., 2015; Smith et al., 2018). As such one would expect the DMN to be sensitive to a major switch to a new task (NC condition), when a completely different context representation is reawakened/reinstated. In contrast to this prediction, we found larger task-negative activations for NC as compared with HC.
It is worth noting that the evidence that we provide here might seem contradictory with previous reports suggesting a key role for the DMN in narrative processing (Ames et al., 2015; Baldassano et al., 2017, 2018; Chen et al., 2017a; Lerner et al., 2011; Simony et al., 2016; Yeshurun et al., 2017). One possibility is that we might have failed to observe DMN responses reflecting semantic integration in the current study because the stimuli employed were shorter than the much longer narratives used in previous studies (Baldassano et al., 2017, 2018; Chen et al., 2017a; Lerner et al., 2011; Yeshurun et al., 2017). There is, however, evidence to suggest that the involvement of DMN areas may not relate to the temporal duration of the stimuli, but rather to the presence of informational context. In fact, stimuli with the same informational content but different durations recruit the DMN to the same extent (Baldassano et al., 2017). Furthermore, short narratives with an interleaved design (similar to the one used here) recruit DMN regions, such as PCC and medial prefrontal cortex, responding to context integration (Ames et al., 2015).
Our GLM results revealed a positive engagement of DMN regions during context integration (i.e., HC&LC > NC). This set of regions included the same regions that have been found in other studies (Hasson et al., 2008; Simony et al., 2016). In apparent contrast with the GLM results, our ICA results revealed that the engagement of the DMN, including the regions noted above, reflected a task-negative engagement sensitive to task difficulty. An explanation for these apparently contrasting results might reside in common processes inherent to “cognitive ease” and “narrative processing”. For instance, one could hypothesise that during easier semantic integration (HC condition) as compared to harder semantic integration (LC) the DMN might support the formation of a schema representation and a conceptual gestalt. This type of cognitive processes, typically used for task-negative internal mentation (e.g., autobiographical memory and future planning), would facilitate the building of an internal model of the narrative and therefore facilitate its comprehension (i.e., the formation of a semantic gestalt). Therefore, despite the mean activity in DMN regions co-varies with an overall attentional state (and/or internal vs. external mentation) - aligning with the pattern of RT responses - yet at the same time the DMN neural circuits may be processing information related to the stimuli (formation of schema representation and conceptual gestalt). This hypothesis, however, contrasts with the observation that no significant difference between conditions with (LC condition) and without context-related schema representations (NC condition) was observed.
An alternative explanation of why some nodes of the DMN (e.g., ATL and hippocampus) were positively engaged (compared to rest) during our task conditions (GLM results), whilst the DMN overall activity is task-negative and co-varies with task difficulty, is that the DMN is not homogeneous and fractionates depending on the task contrasts (Axelrod et al., 2017; Buckner et al., 2008; Buckner and DiNicola, 2019; Humphreys et al., 2015). In parallel with previous investigations, we found that the same regions (ATL, mid-PGp and hippocampus) were aligned with the SLN during narrative reading but became a part of the extended DMN when semantic integration was not required (e.g., during number reading see Figure S1A-B). Finally, the observation that DMN responses mirror RTs (Fig. 2) (see also Humphreys and Lambon Ralph, 2017; Vatansever et al., 2017a) accords with the proposal that, when not critical for the task at hand, some brain regions may be deactivated, proportional to task difficulty, to save metabolic energy whilst preserving performance (Attwell and Laughlin, 2001).
CRediT authorship contribution statement
Francesca M. Branzi: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Project administration, Funding acquisition. Gina F. Humphreys: Methodology, Writing - review & editing. Paul Hoffman: Formal analysis, Writing - review & editing. Matthew A. Lambon Ralph: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
None.
Acknowledgements
This research was supported by a Medical Research Council Programme Grant (MR/R023883/1), an European Research Council Advanced Grant (GAP: 670428 - BRAIN2MIND_NEUROCOMP), a Postdoctoral Fellowship from the European Union’s Horizon 2020 research and innovation programme, under the Marie Sklodowska-Curie grant agreement No 658341, and Medical Research intramural funding (MC_UU_00005/18).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.116802.
Contributor Information
Francesca M. Branzi, Email: Francesca.Branzi@mrc-cbu.cam.ac.uk.
Matthew A. Lambon Ralph, Email: Matt.Lambon-Ralph@mrc-cbu.cam.ac.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ames D.L., Honey C.J., Chow M.A., Todorov A., Hasson U. Contextual alignment of cognitive and neural dynamics. J. Cognit. Neurosci. 2015;27:655–664. doi: 10.1162/jocn_a_00728. [DOI] [PubMed] [Google Scholar]
- Anderson A.J., Binder J.R., Fernandino L., Humphries C.J., Conant L.L., Aguilar M., Wang X., Doko D., Raizada R.D.S. Predicting neural activity patterns associated with sentences using a neurobiologically motivated model of semantic representation. Cerebr. Cortex. 2017;27:4379–4395. doi: 10.1093/cercor/bhw240. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cognit. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cerebr. Blood Flow Metabol. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Axelrod V., Rees G., Bar M. The default network and the combination of cognitive processes that mediate self-generated thought. Nat. Hum. Behav. 2017;1:896–910. doi: 10.1038/s41562-017-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C., Chen J., Zadbood A., Pillow J.W., Hasson U., Norman K.A. Discovering event structure in continuous narrative perception and memory. Neuron. 2017;95:709–721. doi: 10.1016/j.neuron.2017.06.041. e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C., Hasson U., Norman K.A. Representation of real-world event schemas during narrative perception. J. Neurosci. 2018;38:9689–9699. doi: 10.1523/JNEUROSCI.0251-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Aminoff E., Schacter D.L. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J. Neurosci. 2008;28:8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A.J., Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Binder J.R., Desai R.H. The neurobiology of semantic memory. Trends Cognit. Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebr. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney R.J., Parker G.J.M., Ralph M.A.L. Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion-weighted imaging probabilistic tractography. J. Cognit. Neurosci. 2012;24:1998–2014. doi: 10.1162/jocn_a_00263. [DOI] [PubMed] [Google Scholar]
- Brennan J., Pylkkanen L. The time-course and spatial distribution of brain activity associated with sentence processing. Neuroimage. 2012;60:1139–1148. doi: 10.1016/j.neuroimage.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., DiNicola L.M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019;20:593–608. doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Leong Y.C., Honey C.J., Yong C.H., Norman K.A., Hasson U. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 2017;20:115–125. doi: 10.1038/nn.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lambon Ralph M.A., Rogers T.T. A unified model of human semantic knowledge and its disorders. Nat Hum Behav. 2017;1 doi: 10.1038/s41562-016-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden B.M., Mitchell D.J., Duncan J. Recruitment of the default mode network during a demanding act of executive control. Elife. 2015;4 doi: 10.7554/eLife.06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dixon M.L., Andrews-Hanna J.R., Spreng R.N., Irving Z.C., Mills C., Girn M., Christoff K. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage. 2017;147:632–649. doi: 10.1016/j.neuroimage.2016.12.073. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cognit. Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Dundon N.M., Katshu M.Z.U., Harry B., Roberts D., Leek E.C., Downing P., Sapir A., Roberts C., d’Avossa G. Human parahippocampal cortex supports spatial binding in visual working memory. Cerebr. Cortex. 2018;28:3589–3599. doi: 10.1093/cercor/bhx231. [DOI] [PubMed] [Google Scholar]
- Esterman M., Noonan S.K., Rosenberg M., DeGutis J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cerebr. Cortex. 2013;23:2712–2723. doi: 10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajardo-Vidal A., Lorca-Puls D.L., Hope T.M.H., Jones O.P., Seghier M.L., Prejawa S., Crinion J.T., Leff A.P., Green D.W., Price C.J. How right hemisphere damage after stroke can impair speech comprehension. Brain. 2018;141:3389–3404. doi: 10.1093/brain/awy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F., Wise R.J., Mehta A., Leech R. Overlapping networks engaged during spoken language production and its cognitive control. J. Neurosci. 2014;34:8728–8740. doi: 10.1523/JNEUROSCI.0428-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Language and brain. Sci. Am. 1972;226:76–+. doi: 10.1038/scientificamerican0472-76. [DOI] [PubMed] [Google Scholar]
- Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-Almagro F., Glasser M.F., Duff E.P., Fitzgibbon S., Westphal R., Carone D., Beckmann C.F., Smith S.M. Hand classification of fMRI ICA noise components. Neuroimage. 2017;154:188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths B.J., Parish G., Roux F., Michelmann S., van der Plas M., Kolibius L.D., Chelvarajah R., Rollings D.T., Sawlani V., Hamer H., Gollwitzer S., Kreiselmeyer G., Staresina B., Wimber M., Hanslmayr S. Directional coupling of slow and fast hippocampal gamma with neocortical alpha/beta oscillations in human episodic memory. Proc. Natl. Acad. Sci. U. S. A. 2019;116:21834–21842. doi: 10.1073/pnas.1914180116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai A.D., Welbourne S.R., Embleton K., Parkes L.M. A comparison of dual gradient-echo and spin-echo fMRI of the inferior temporal lobe. Hum. Brain Mapp. 2014;35:4118–4128. doi: 10.1002/hbm.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Yang E., Vallines I., Heeger D.J., Rubin N. A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 2008;28:2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Opinion - the cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoffman P. Reductions in prefrontal activation predict off-topic utterances during speech production. Nat. Commun. 2019;10:515. doi: 10.1038/s41467-019-08519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P., Lambon Ralph M.A., Rogers T.T. Semantic diversity: a measure of semantic ambiguity based on variability in the contextual usage of words. Behav. Res. Methods. 2013;45:718–730. doi: 10.3758/s13428-012-0278-x. [DOI] [PubMed] [Google Scholar]
- Hoffman P., Binney R.J., Lambon Ralph M.A. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex. 2015;63:250–266. doi: 10.1016/j.cortex.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P., McClelland J.L., Lambon Ralph M.A. Concepts, control, and context: a connectionist account of normal and disordered semantic cognition. Psychol. Rev. 2018;125:293–328. doi: 10.1037/rev0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.F., Lambon Ralph M.A. Fusion and fission of cognitive functions in the human parietal cortex. Cerebr. Cortex. 2015;25:3547–3560. doi: 10.1093/cercor/bhu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.F., Lambon Ralph M.A. Mapping domain-selective and counterpointed domain-general higher cognitive functions in the lateral parietal cortex: evidence from fMRI comparisons of difficulty-varying semantic versus visuo-spatial tasks, and functional connectivity analyses. Cerebr. Cortex. 2017;27:4199–4212. doi: 10.1093/cercor/bhx107. [DOI] [PubMed] [Google Scholar]
- Humphreys G.F., Hoffman P., Visser M., Binney R.J., Lambon Ralph M.A. Establishing task- and modality-dependent dissociations between the semantic and default mode networks. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7857–7862. doi: 10.1073/pnas.1422760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.F., Jackson R.L., Lambon Ralph M.A. bioRxiv; 2019. Overarching Principles and Dimensions of the Functional Organisation in the Inferior Parietal Cortex; p. 654178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C., Binder J.R., Medler D.A., Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J. Cognit. Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E., Lambon Ralph M.A. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cognit. Sci. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Krieger-Redwood K., Jefferies E., Karapanagiotidis T., Seymour R., Nunes A., Ang J.W.A., Majernikova V., Mollo G., Smallwood J. Down but not out in posterior cingulate cortex: deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. Neuroimage. 2016;141:366–377. doi: 10.1016/j.neuroimage.2016.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A., Esterman M., Riley C.S., Valera E.M. Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proc. Natl. Acad. Sci. U. S. A. 2016;113:13899–13904. doi: 10.1073/pnas.1611743113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Sage K., Jones R.W., Mayberry E.J. Coherent concepts are computed in the anterior temporal lobes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Jefferies E., Patterson K., Rogers T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017;18:42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Landauer T.K., Dumais S.T. A solution to Plato’s problem: the latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychol. Rev. 1997;104:211–240. [Google Scholar]
- Lerner Y., Honey C.J., Silbert L.J., Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 2011;31:2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Frith C.D., Morris R.G.M. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122:1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Mason R.A., Just M.A. Lexical ambiguity in sentence comprehension. Brain Res. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi J.J., Callaway E.M. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K.A., Jefferies E., Visser M., Lambon Ralph M.A. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J. Cognit. Neurosci. 2013;25:1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Noppeney U., Price C.J. An FMRI study of syntactic adaptation. J. Cognit. Neurosci. 2004;16:702–713. doi: 10.1162/089892904323057399. [DOI] [PubMed] [Google Scholar]
- Pallier C., Devauchelle A.D., Dehaene S. Cortical representation of the constituent structure of sentences. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2522–2527. doi: 10.1073/pnas.1018711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K., Nestor P.J., Rogers T.T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as ’asymmetric sampling in time. Speech Commun. 2003;41:245–255. [Google Scholar]
- Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Mechelli A. Reading and reading disturbance. Curr. Opin. Neurobiol. 2005;15:231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Price A.R., Bonner M.F., Peelle J.E., Grossman M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. J. Neurosci. 2015;35:3276–3284. doi: 10.1523/JNEUROSCI.3446-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A.R., Peelle J.E., Bonner M.F., Grossman M., Hamilton R.H. Causal evidence for a mechanism of semantic integration in the angular gyrus as revealed by high-definition transcranial direct current stimulation. J. Neurosci. 2016;36:3829–3838. doi: 10.1523/JNEUROSCI.3120-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Rice G.E., Lambon Ralph M.A., Hoffman P. The roles of left versus right anterior temporal lobes in conceptual knowledge: an ALE meta-analysis of 97 functional neuroimaging studies. Cerebr. Cortex. 2015;25:4374–4391. doi: 10.1093/cercor/bhv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.T., Lambon Ralph M.A., Garrard P., Bozeat S., McClelland J.L., Hodges J.R., Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol. Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Vilberg K.L. Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 2013;23:255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Fagan E., Price C.J. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J. Neurosci. 2010;30:16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Tu Y., Gollub R.L., Ortiz A., Napadow V., Yu S., Wilson G., Park J., Lang C., Jung M., Gerber J., Mawla I., Chan S.T., Wasan A.D., Edwards R.R., Kaptchuk T., Li S., Rosen B., Kong J. Visual network alterations in brain functional connectivity in chronic low back pain: a resting state functional connectivity and machine learning study. Neuroimage Clin. 2019;22:101775. doi: 10.1016/j.nicl.2019.101775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert L.J., Honey C.J., Simony E., Poeppel D., Hasson U. Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4687–E4696. doi: 10.1073/pnas.1323812111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E., Honey C.J., Chen J., Lositsky O., Yeshurun Y., Wiesel A., Hasson U. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun. 2016;7:12141. doi: 10.1038/ncomms12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V., Mitchell D.J., Duncan J. Role of the default mode network in cognitive transitions. Cerebr. Cortex. 2018;28:3685–3696. doi: 10.1093/cercor/bhy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S., Kim A.S., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George M., Kutas M., Martinez A., Sereno M.I. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain. 1999;122:1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Staudigl T., Hanslmayr S. Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr. Biol. 2013;23:1101–1106. doi: 10.1016/j.cub.2013.04.074. [DOI] [PubMed] [Google Scholar]
- Tylen K., Christensen P., Roepstorff A., Lund T., Ostergaard S., Donald M. Brains striving for coherence: long-term cumulative plot formation in the default mode network. Neuroimage. 2015;121:106–114. doi: 10.1016/j.neuroimage.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Van der Linden M., Berkers R., Morris R.G.M., Fernandez G. Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. J. Neurosci. 2017;37:9474–9485. doi: 10.1523/JNEUROSCI.3603-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R., Nobre A.C., Price C.J. The response of left temporal cortex to sentences. J. Cognit. Neurosci. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Vassal F., Schneider F., Boutet C., Jean B., Sontheimer A., Lemaire J.J. Combined DTI tractography and functional MRI study of the language connectome in healthy volunteers: extensive mapping of white matter fascicles and cortical activations. PloS One. 2016;11 doi: 10.1371/journal.pone.0152614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D., Manktelow A.E., Sahakian B.J., Menon D.K., Stamatakis E.A. Angular default mode network connectivity across working memory load. Hum. Brain Mapp. 2017;38:41–52. doi: 10.1002/hbm.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D., Menon D.K., Stamatakis E.A. Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. U. S. A. 2017;114:12821–12826. doi: 10.1073/pnas.1710521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Herve P.Y., Duffau H., Crivello F., Houde O., Mazoyer B., Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Herve P.Y., Jobard G., Petit L., Crivello F., Mellet E., Zago L., Mazoyer B., Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage. 2011;54:577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Vilberg K.L., Rugg M.D. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Lambon Ralph M.A. Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. J. Cognit. Neurosci. 2011;23:3121–3131. doi: 10.1162/jocn_a_00007. [DOI] [PubMed] [Google Scholar]
- Wehbe L., Murphy B., Talukdar P., Fyshe A., Ramdas A., Mitchell T. Simultaneously uncovering the patterns of brain regions involved in different story reading subprocesses. PloS One. 2014;9 doi: 10.1371/journal.pone.0112575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M., Jann K., Dierks T., Federspiel A., Wiest R., Horn H. Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. Neuroimage. 2011;54:3057–3066. doi: 10.1016/j.neuroimage.2010.10.039. [DOI] [PubMed] [Google Scholar]
- Xu J., Kemeny S., Park G., Frattali C., Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhang S., Calhoun V.D., Monterosso J., Li C.S., Worhunsky P.D., Stevens M., Pearlson G.D., Potenza M.N. Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. Neuroimage. 2013;79:62–71. doi: 10.1016/j.neuroimage.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang J., Fan L., Li H., Zhang W., Hu Q., Jiang T. Tractography-based parcellation of the human middle temporal gyrus. Sci. Rep. 2015;5:18883. doi: 10.1038/srep18883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Swanson S., Simony E., Chen J., Lazaridi C., Honey C.J., Hasson U. Same story, different story: the neural representation of interpretive frameworks. Psychol. Sci. 2017;28:307–319. doi: 10.1177/0956797616682029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.