Abstract
Urinary tract infections (UTIs) are the third most common types of infection in human medicine worldwide. There is increasing appreciation for the pathogenic role of Gram-positive cocci (GPC) in UTIs, as they have a plethora of virulence factors, maintaining their pathogenicity and high affinity for the epithelial cells of the urinary tract. The study was carried out using microbiological data collected corresponding to the period between 2008 and 2017. Antimicrobial susceptibility testing was performed using the disk diffusion method and E-tests. The age range of patients affected from the outpatient and inpatient groups differed significantly (43 [range 0.7–99] vs. 68 [range 0.4–99] years; p = 0.008). 3962 GPCs were obtained from inpatient and 4358 from outpatient samples, corresponding to 20.5 ± 2.8% (range 17.5–26.8%) and 20.6 ± 2.6% (range 17.8–26.0%) of all positive urine samples (p > 0.05); in both groups, Enterococcus spp. were the most prevalent (outpatients: 79.6%; inpatients: 88.5%). High-level aminoglycoside resistance in enterococci was noted in 31.0–46.6% of cases. A pronounced increase in the number of MRSA was seen in the second half of the study period (0.6–1.9% vs. 9.8–11.6%; p = 0.038). The ratio of VRE isolates was 0.16%, no VISA/VRSA isolates were detected.
Subject terms: Antimicrobials, Bacteriology, Clinical microbiology, Urogenital diseases, Urological manifestations, Epidemiology
Introduction
Urinary tract infections (UTIs; ranging from uncomplicated cystitis to severe pyelonephritis and nephrolithiasis) are the third most common types of infection in human medicine worldwide (after respiratory tract infections and infections of the alimentary tract), and the second most commonly occurring infections in developed countries, with 100–180 million cases/year1–3. These infections affect outpatients and hospitalized patients to a significant extent (accounting for 25–50% of hospital-acquired infections overall), representing an important factor of morbidity, especially due to their recurring nature1,4. UTIs more commonly affect females, patients with immunosuppression or underlying diseases/developmental abnormalities of the urinary system and they are associated with some lifestyle choices (sexual promiscuity, public baths)1,5. If left untreated, these infections may lead to complications, debilitating sequelae and a decreased quality of life (QoL)6. UTIs should also be considered an important economic undertaking, as the medical care, pharmacotherapy and lost working days corresponding to these pathologies are estimated to be around 5–7 billion US$1,2,7. The etiology of UTIs are thought to be predictable, due to the relatively constant spectrum of pathogens implicated, however, due to the advancements of medical interventions, pharmacotherapy and the increasing number of patients affected by immunosuppression (disease-associated or iatrogenic), other less common pathogens are now emerging as prominent factors of disease1–6. The most common pathogens in UTIs are the members of the Enterobacterales order (Gram-negative bacteria found in the gut, namely Escherichia coli, Klebsiella spp., pathogens of the CES group [Citrobacter-Enterobacter-Serratia], members of the Proteae tribe [Proteus-Providencia-Morganella]), other causative agents include Gram-positive cocci (Enterococcus spp., Streptococcus spp., Staphylococcus saprophyticus and S. aureus), non-fermenting Gram-negative bacteria (Pseudomonas spp. and Acinetobacter spp.), atypical microorganisms (Mycoplasma, Ureaplasma species) and yeasts (Candida spp.)1–4,8–11.
Gram-positive facultative anaerobic cocci include several phenotypically heterogenous genera from the Firmicutes phylum: Staphycoccus spp. are members of the Bacillales order, while Streptococcus (Group A, B, C and G streptococci, based on Lancefield classification) and Enterococcus spp. (Group D streptococci) are members of the Lactobacillales order12. Staphylococcus spp. are ubiquitously found on the skin of humans, additionally, S. aureus is one of the most common pathogens of severe suppurative skin and soft tissue infections, abscesses, pneumonia, endocarditis and bacteremia; methicillin-resistant S. aureus (MRSA) is also one of the most commonly encountered nosocomial pathogens in the US and Europe13,14. S. aureus and coagulase-negative staphylococci (CoNS) were previously considered to be uncommon etiological agents in ascending UTIs in outpatients, however, they may have a more pronounced role in hospitalized, immunocompromised patients. The isolation of S. aureus from urine may also be an indicator of a more severe condition (e.g., bacteremia or endocarditis), where the microorganisms reach the kidneys through hematogenous dissemination15. The isolation frequency of S. aureus from UTIs is around 0.5–13% in the literature13,16. In contrast, S. saprophyticus is a well-characterized pathogen in both uncomplicated cystitis and catheter-associated UTIs. The pathogenic role of S. saprophyticus in UTIs (sometimes referred to as “honeymoon cystitis”) was described in the 1960s and since then, more and more evidence was found regarding the pathogenesis of this disease17. Most of epidemiological studies estimate S. saprophyticus as causative agents in 5–20% of UTIs, however, a study from Sweden found that this pathogen was the etiological agent in > 40% of uncomplicated UTIs in females18. Species of the Enterococcus genus are abundantly found in the gut microbiota of animals and humans, being one of the few Gram-positive bacteria that are resistant to bile19. Enterococci are also highly prevalent in aquatic environments and should be considered as an indicator of fecal contamination in urban areas20. E. faecalis and E. faecium are the most common species in bacteremia, endocarditis, central nervous system infections and UTIs, however, the emergence of non-faecalis enterococci should be taken into consideration21,22. Similarly to S. aureus, these pathogens are relevant in nosocomial infections worldwide23. Temporal changes in the occurrence of Gram-positive cocci in UTIs have been described, two peaks (one in early summer, the other in the winter months) were observed in multiple studies17,18. The role of companion animals as reservoirs of S. aureus and Enterococcus spp. and the consideration of these pathogens as zoonotic has been published by several reports24,25.
The therapy of UTIs in both inpatient and outpatient settings is becoming increasingly difficult, due to the emergence of drug resistance in these pathogens, leaving clinicians with few therapeutic options available26,27. Gram-positive cocci are no exception to this trend: the clinical significance of methicillin-resistant S. aureus [MRSA] is well known, in addition, vancomycin-resistant Enterococci [VRE] are a significant and a sharply increasing resistance problem worldwide. One must also mention the slow, but visible emerging threat of vancomycin-intermediate S. aureus [VISA] species, which will be daunting challenge for therapy25,28. Several factors contribute to the global emergence of antimicrobial resistance (AMR), however, the overuse and misuse of antimicrobials in human and animal healthcare, in addition to globalization (allowing for fast travel to geographically distant regions of the globe, leading to the spread of multidrug resistant pathogens) may be considered as some of the most important29. Multiple reports have demonstrated that resistance plasmids can continuously accumulate new resistance determinants for affected bacteria without losing previous ones, therefore bacteria carrying these plasmids end up with resistance against an extensive list of available antimicrobials30. Nevertheless, the resulting selection pressure will fuel the “antibiotic resistance spiral”, i.e., the more pronounced use of last-resort antibiotics against drug resistant pathogens, which unavoidably leads to the emergence of resistant strains against the last resort agents (e.g., in the case of the increasing prevalence of MRSA, which lead to the use of vancomycin, corresponding to the emergence of vancomycin-intermediate S. aureus [VISA], VRSA and VRE isolates, leading to the use of linezolid/daptomycin)31–33. The increasing use of oral vancomycin in the therapy of Clostridioides difficile infections may put further pressure on the selection of these MDR pathogens34. Nevertheless, gaining more and more resistance determinants also burdens the pathogenic bacteria (i.e. they might therefore lose from their original viability and their competitiveness, see the principle of cost–benefit), thus, they may lose “ground” in their fight for the respective niche against bacteria with less resistance-determinants35.
The epidemiology and antibiotic-susceptibility trends of urinary tract pathogens show pronounced variation, both temporally and regionally, therefore, the assessment of these data using analytical epidemiology is essential to reflect on the national situation, compared to international data36. The knowledge of these resistance trends may also aid treating physicians in the optimal choice for antibiotic therapy37. The aim of this study was to evaluate the resistance trends and epidemiology of Gram-positive cocci in the UTIs of inpatients and outpatients at the Albert Szent-Györgyi Clinical Center (Szeged, Hungary) retrospectively, during a 10-year study period.
Methods
Study location and design, data collection
The present microbiological study was carried out using data collected retrospectively, regarding the time period between January 1st, 2008 and December 31st 2017, at the Institute of Clinical Microbiology, University of Szeged. The Institute is the affiliated clinical microbiology laboratory of the Albert Szent-Györgyi Clinical Center, which is a 1,820-bed primary-and tertiary-care teaching hospital in the Southern Great Plain of Hungary (population: ~ 402,000 people; 2017)38. Data collection was performed electronically in the records of the laboratory information system by the authors, corresponding to urine samples positive for relevant Gram-positive bacteria.
Samples with clinically significant colony counts for uropathogenic Gram-positive cocci (> 105 CFU/mL; however, this was subject to interpretation by the senior clinical microbiologist, and on the basis of information provided on the clinical request forms for microbiological analysis and international guidelines) that were positive for nitrite and leukocyte-esterase tests were included in the data analysis. Only the first isolate per patient was included in the study; however, isolates with different antibiotic-susceptibility patterns (i.e. if the isolate showed different susceptibility to at least one tested antibiotic) from the same patient were considered as different individual isolates.
To evaluate the demographic characteristics of these infections, limited amount of patient data was also collected (sex, age at sample submission, date of samples submission, inpatient/outpatient status). The study was deemed exempt from ethics review by the Institutional Review Board of the University of Szeged (Szeged, Hungary), and informed consent was not required as data anonymity was maintained.
Identification of isolates
Ten microliters of each uncentrifuged urine sample was cultured on UriSelect chromogenic agar (Bio-Rad, Berkeley, CA, USA) and blood agar (bioMérieux, Marcy-l’Étoile, France) plates with a calibrated loop, according to the manufacturer’s instructions, and incubated at 37 °C for 24–48 h, aerobically. In the period between 2008 and 2012, presumptive, biochemical reaction-based methods and VITEK 2 Compact ID/AST (bioMérieux, Marcy-l’Étoile, France) were used for bacterial identification; from 2013 onward, MALDI-TOF MS (Bruker Daltonics, Germany) was introduced to the workflow of the Department of Bacteriology. Mass spectrometry was performed by the Microflex MALDI Biotyper (Bruker Daltonics, Germany) instrument, using the MALDI Biotyper RTC 3.1 software (Bruker Daltonics, Germany) and the MALDI Biotyper Library 3.1 for spectrum analysis. Sample preparation methodology and the technical details of MALDI-TOF MS measurements were described elsewhere39.
Susceptibility testing of relevant isolates
Antimicrobial susceptibility testing for the relevant Gram-positive species was performed using disk diffusion method (Liofilchem, Abruzzo, Italy) and E-tests (Liofilchem, Abruzzo, Italy) on Mueller–Hinton agar (MHA) plates, incubated at 35 ± 1 °C for 18–24 h before plate reading. The following antibiotic disks were used: penicillin (1 IU), ampicillin (2 μg for S. saprophyticus and 10 μg for Enterorococcus spp.), cefoxitin (30 μg), ceftraroline (5 μg), erythromycin (15 μg), clindamycin (2 μg), ciprofloxacin (5 μg), amikacin (30 μg), gentamicin (10 μg), nitrofurantoin (100 μg), rifampicin (5 μg), quinupristin–dalfopristin (15 μg), fusidic acid (10 μg), linezolid (10 μg), doxycycline (30 μg), tigecycline (15 μg), trimethoprim-sulfomethoxazole (23.75/1.25 μg) taking into account the intrinsic resistance mechanisms of the isolates and the clinical relevance of the listed antibiotics in the therapy of said infections40. The interpretation of the results was based on EUCAST breakpoints (https://www.eucast.org) valid at the time of interpretation, the re-analysis of susceptibilities based on revised breakpoints was not performed. Inducible clindamycin resistance was detected using the erythromycin-clindamycin D test, these strains were also reported as resistant to clindamycin. During data analysis, intermediately-susceptible results were grouped with and reported as resistant.
Methicillin-resistant S. aureus (MRSA) was detected using mannitol-salt agar (MSA) using cefoxitin disks (< 22 mm zone diameter) and PBP2′ Latex Agglutination Test Kit (Thermo Fisher Scientific Hungary Gmbh., Budapest, Hungary)28. After 2013, a combined MALDI-TOF MS and PBP2′ latex agglutination protocol was introduced in our laboratory41. MRSA-positive isolates were reported as resistant to all β-lactam antibiotics (except for 5th generation cephalosporins). Screening for high-level aminoglycoside resistance (HLAR) was done using gentamicin (30 μg) disks, while verification of positive results was performed using broth microdilution method (with a gentamicin concentration of 500 μg/ml)42.
S. aureus ATCC 29,213, S. aureus ATCC 43,300, E. faecalis ATCC 29,212, Proteus mirabilis ATCC 35,659, Escherichia coli ATCC 25,922 and Pseudomonas aeruginosa ATCC 27,853 were used as quality control strains43.
Statistical analyses
Statistical analyses, including descriptive analysis (means or medians with ranges and percentages to characterize data) and statistical tests (χ2-test, Student’s t-test and Mann–Whitney U test) were performed with SPSS software version 24 (IBM SPSS Statistics for Windows 24.0, Armonk, NY, USA, IBM Corp.). The normality of variables was tested using Shapiro–Wilk tests. p values < 0.05 were considered statistically significant43.
Results
Demographic characteristics of affected patients, sample types
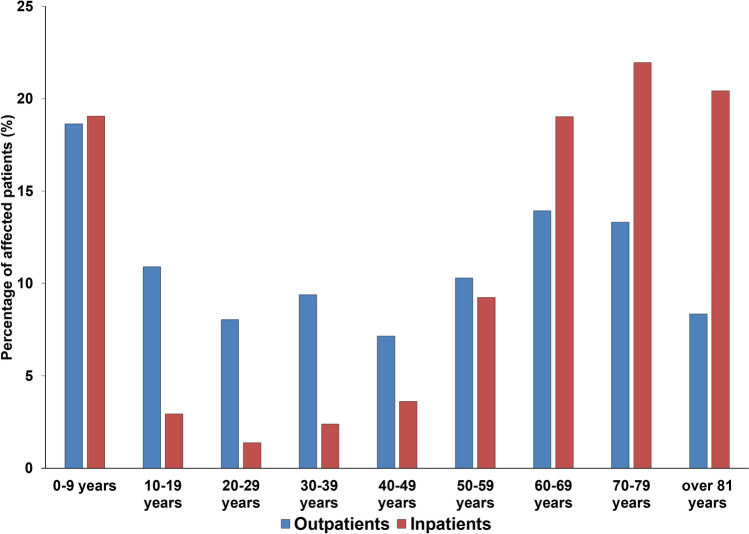
During the study period, the median age of outpatients affected by UTIs caused by Gram-positive cocci was 43 years, which showed the following variability during the two parts of the study period: age range for outpatients was 0.7–99 years, whereas the median age for the first half of the study period was 35, while for the second half was 54 years (p = 0.038) (see Fig. 1. for detailed age distribution). In contrast, for the inpatients, the median age overall was 68 years; the age rage range was 0.4–99 years, with a median age for the first half of the study period was 64, while for the second half was 70 years (p < 0.05). The female-to-male ratio of the outpatient group was 2.03 (that is 67.1% female), and 1.15 (that is 53.6% female) in the inpatient group, respectively. The observed difference in age distribution of the two patient groups (inpatients and outpatients) was statistically significant (43 vs. 68 years; p = 0.008). Patients under 10 (outpatients: 18.7%, inpatients: 19.1%) and over 60 years of age (outpatients: 35.6%, inpatients: 61.4%) were predominantly affected. The sample distribution among relevant urine samples was the following: among samples from outpatient clinics, the overwhelming majority was midstream urine (98.8%), with a minority of first-stream urine (1.2%); on the other hand, the distribution from inpatient departments was more variable: midstream urine (29.2%), first-stream urine (6.3%), catheter-specimen urine (63.6%) and suprapubic bladder taps (0.9%).
Figure 1.
Age distribution of the affected patients in the outpatient and inpatient group.
Distribution of Gram-positive cocci in urine samples
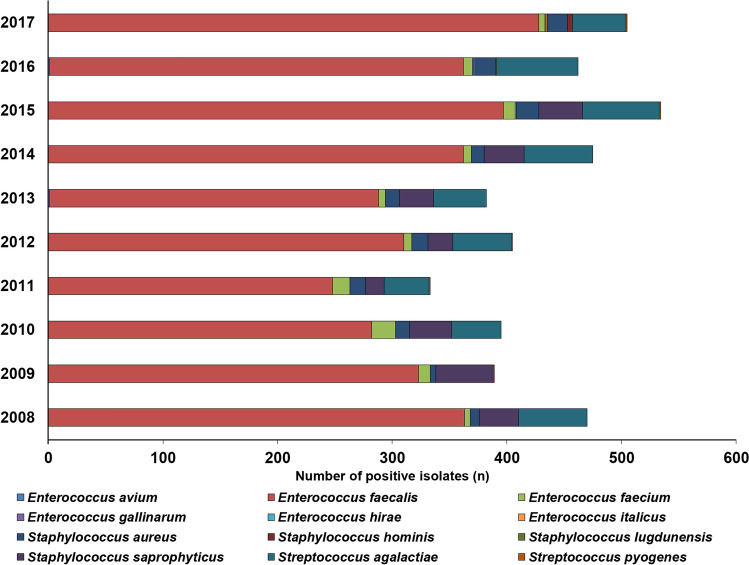
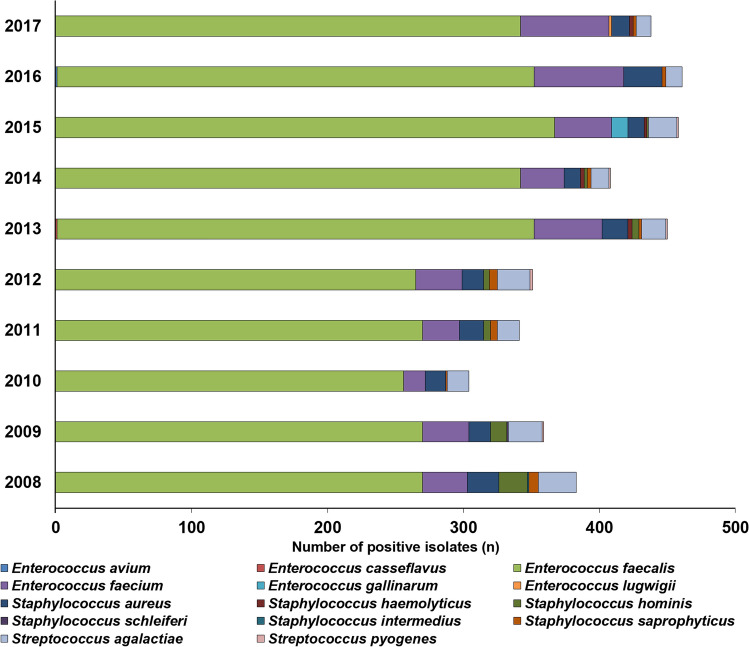
Between 2008 and 2017, the Institute of Clinical Microbiology received 21,150 urine samples from outpatient clinics and 19,325 samples from inpatient departments, from which a significant urinary pathogen was detected. 3962 Gram-positive coccus isolates were obtained from inpatients (396.2 ± 54.2/year) and 4358 from outpatients (435.8 ± 64.6/year). This corresponds to 20.5 ± 2.6% (range 17.8–26.0%) for outpatients, while 20.6 ± 2.8% (range 17.5–26.8%) of all positive urine samples for inpatients; (p > 0.05). In both groups, Enterococcus spp. (predominantly E. faecalis; outpatients: 79.6%; inpatients: 88.5%) were the most prevalent, while Staphylococcus spp. (outpatients: 9.2%, mainly S. saprophyticus; inpatients: 6.7%, mainly S. aureus) and Streptococcus spp. (predominantly S. agalactiae; outpatients: 11.2%; inpatients: 4.8%) were in a minority. The epidemiology and detailed species distribution of Gram-positive cocci in both patient groups are presented in Fig. 2 (outpatients) and Fig. 3 (inpatients). There was an obvious seasonal trend in the isolation of Gram-positive cocci in the outpatient group (24.7% was isolated in the June–July periods, 22.9% in the December-January periods), while no such tendency was noted in the inpatient group. In the inpatient group, 14 different species of Gram-positive urinary pathogens were isolated (median 7; range 5–9), while in the outpatient group, the species distribution was less diverse, with 12 different species (median 6; range 5–8) detected.
Figure 2.
Frequency and species distribution of Gram-positive bacterial isolates in outpatient samples (2008–2017).
Figure 3.
Frequency and species distribution of Gram-positive bacterial isolates in inpatient samples (2008–2017).
Antibiotic resistance trends among Gram-positive cocci isolated from UTIs
Antibiotic resistance data of the isolated enterococci, staphylococci and streptococci in the 10-year study period is presented in Tables 1, 2 and 3, respectively. To identify temporal developments in resistance trends, the 10-year study period was divided into two 5-year periods (2008–2012 and 2013–2017). The level of resistance in Enterococcus species was significantly higher in isolates originating from inpatients in both periods regarding ciprofloxacin, but not other antibiotics. Apart from intrinsic resistance, resistance rates against ciprofloxacin and HLAR did not show relevant differences among E. faecalis and non-faecalis enterococci (p > 0.05). Likewise, there was no significant increase noted in the ratio of resistance strains in either patient groups between the two 5-year periods. HLAR was detected in 31.0–46.6% of isolates overall and there was a numerical, but not significant increase in the second half of the study period (p = 0.067). Very few VRE isolates were recovered (0.16%; n = 11 from inpatients and n = 4 from outpatients, exclusively from E. faecium), while no linezolid-resistant isolates were detected (Table 1.).
Table 1.
Percentage of resistant Enterococcus strains to indicator antibiotics from inpatient and outpatient departments (2008–2017).
| 2008–2012 | 2013–2017 | |||||
|---|---|---|---|---|---|---|
| Outpatients (%) | Inpatients (%) | Statisticsa | Outpatients (%) | Inpatients | Statisticsa | |
| Ampicillinb | 0.1 | 0.3 | n.s | 0.2 | 0.4% | n.s |
| Imipenemb | 0.2 | 0.2 | n.s | 0.2 | 0.2% | n.s |
| Ciprofloxacin | 31.6 | 45.2 | p = 0.026 | 16.1% | 33.0 | p = 0.019 |
| HLARc | 31.0 | 39.8 | n.s | 45.8% | 46.6 | n.s |
| Vancomycind | 0.0 | 0.1 | n.s | 0.1% | 0.3 | n.s |
| QP/DPe | 0.0 | 0.0 | n.s | 0.0% | 0.0 | n.s |
| Tigecycline | 0.0 | 0.0 | n.s | 0.0% | 0.0 | n.s |
| Linezolid | 0.0 | 0.0 | n.s | 0.0% | 0.0 | n.s |
aComparison of resistance levels among isolates originating from outpatients and inpatients.
bCalculated for E. faecalis isolates only.
cHigh-level aminoglycoside resistance.
dRepresents the ratio of VRE strains.
eQuinpristin/dalfopristin.
n.s. not significant (p > 0.05).
Table 2.
Percentage of resistant Staphylococcus strains to indicator antibiotics from inpatient and outpatient departments (2008–2017).
| 2008–2012 | 2013–2017 | |||||
|---|---|---|---|---|---|---|
| Outpatients (%) | Inpatients (%) | Statisticsa | Outpatients (%) | Inpatients (%) | Statisticsa | |
| Penicillin | 94.8 | 96.6 | n.s | 95.2 | 96.9 | n.s |
| Ampicillinb | 10.8 | 12.2 | n.s | 12.4 | 13.7 | n.s |
| Cefoxitinc | 0.6 | 1.9 | n.s | 9.8 | 11.6 | n.s |
| Amikacin | 1.5 | 23.2 | p < 0.001 | 1.1 | 14.2 | p < 0.001 |
| Gentamicin | 4.6 | 23.2 | p = 0.028 | 2.1 | 24.8 | p < 0.001 |
| Azithromycin | 19.5 | 25.8 | p = 0.049 | 32.3 | 33.2 | n.s |
| Clindamycin | 17.9 | 21.9 | n.s | 22.1 | 26.3 | n.s |
| Ciprofloxacin | 9.7 | 35.7 | p < 0.001 | 12.1 | 25.6 | p = 0.042 |
| Doxycycline | 13.3 | 29.8 | p = 0.04 | 4.2 | 30.8 | p < 0.001 |
| Nitrofurantoin | 2.6 | 3.3 | n.s | 0.5 | 3.8 | p = 0.046 |
| SMX/TMPd | 5.6 | 23.2 | p < 0.001 | 1.1 | 27.1 | p < 0.001 |
| Rifampicin | 1.0 | 2.3 | n.s | 3.3 | 4.8 | n.s |
| Vancomycine | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
| QP/DPf | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
| Tigecycline | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
| Linezolid | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
| Fusidic acid | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
aComparison of resistance levels among isolates originating from outpatients and inpatients.
bCalculated for S. saprophyticus isolates only.
cRepresent the ratio of MRSA isolates in S. aureus.
dSulfamethoxazole-trimethoprim.
eRepresents the ratio of VISA/VRSA strains.
fQuinpristin/dalfopristin.
n.s.: not significant (p > 0.05).
Table 3.
Percentage of resistant Streptococcus strains to indicator antibiotics from inpatient and outpatient departments (2008–2017).
| 2008–2012 | 2013–2017 | |||||
|---|---|---|---|---|---|---|
| Outpatients (%) | Inpatients (%) | Statisticsa | Outpatients (%) | Inpatients (%) | Statisticsa | |
| Ampicillin | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
| Azithromycin | 16.0 | 17.8 | n.s | 19.4 | 21.3 | n.s |
| Clindamycin | 14.2 | 18.5 | n.s | 19.1 | 20.4 | n.s |
| Ciprofloxacin | 7.1 | 25.4 | p < 0.001 | 8.3 | 29.9 | p = 0.038 |
| SMX/TMPb | 3.3 | 19.4 | p = 0.031 | 2.6 | 20.8 | p < 0.001 |
| Rifampicin | 0.8 | 1.3 | n.s | 0.6 | 0.9 | n.s |
| Vancomycin | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
| Linezolid | 0.0 | 0.0 | n.s | 0.0 | 0.0 | n.s |
aComparison of resistance levels among isolates originating from outpatients and inpatients.
bSulfamethoxazole-trimethoprim.
n.s. not significant (p > 0.05).
The resistance levels in inpatient Staphylococcus samples were significantly higher for amikacin, gentamicin, azithromycin (in the 2008–2012 period), ciprofloxacin, doxycycline, nitrofurantoin (in the 2013–2017 period) and trimethoprim-sulfamethoxazole (SMX/TMP). There was no significant increase in the resistance of any tested antibiotics between 2008–2012 and 2013–2017, however, a numerical tendency was found for azithromycin (p = 0.071). The difference in the number of MRSA isolates among inpatient and outpatients was not significant, however, a pronounced increase in the number of MRSA was seen in the second half of the study period (0.6–1.9% vs. 9.8–11.6%; p = 0.038). No VISA/VRSA strains were found, in addition, none of the Staphylococcus strains were resistant to the supplementary antibiotics (quinpristin/dalfopristin, tigecycline, linezolid, fusidic acid) (Table 2.). In the case of streptococci, significant differences were observed in the resistance levels of ciprofloxacin and SMX/TMP, but not in the case of other antibiotics. Additionally, no significant temporal changes were noted between the two study periods. No vancomycin or linezolid-resistant strains were detected.
Discussion
The study presents the epidemiological trends and resistance levels of Gram-positive cocci in urinary tract infections (UTIs) in the southern part of Hungary, over a long surveillance period (10 years). Previous local studies have highlighted the epidemiological situation of other pathogens locally: E. coli (56.7% in outpatients, 42.0% in inpatients) and Klebsiella spp43. (8.9% vs. 13.0%) were detected in highest numbers, while members of the CES group44 (2.6% vs. 3.0%), Proteae45 (5.0% vs. 7.2%), non-fermenting Gram-negatives46 (3.4% vs. 5.5%) and Candida spp47. (0.4% vs. 6.0%) were present in lesser numbers. In contrast, these pathogens represented ~ 20% of the etiological agents in UTIs, both for inpatients and outpatients, therefore, their epidemiological significance should not be disregarded. Among the group of Gram-positive cocci, E. faecalis was the predominant species (~ 80% of isolates), which is not surprising, in light of global epidemiological reports on the causative agents for UTIs36. To the best of our knowledge, this is the longest-spanning study reporting on the prevalence and susceptibility of this group of uropathogens in Hungary. In contrast to previous studies, dating back some 20–30 years (where the reported prevalence of Enterococcus spp., S. aureus and S. saprophyticus was 2–20%, 0.2–6% and 0.5–8%, respectively), based on current literature results, their prevalence is around 8–35%, 0.5–13% and 5–20%, respectively48–55. This increase is prevalence is especially notable in patients affected by recurrent UTIs (recurrence 3 or more times in 6 months)44. The increased prevalence of these pathogens may be attributed to the increase in patients with lifestyle diseases (kidney diseases, diabetes), immunosuppression, patients undergoing surgical interventions56. Additionally, some reports suggest that Enterococcus spp. may be considered an indicator of more severe pathologies (e.g., diabetes, abnormality of the genitourinary tract)18–22,49,56. An overview of the literature published in the last 20 or so years, regarding the prevalence of UTIs caused by Gram-positive cocci outside Hungary is presented in Table 4. In contrast to the present report, most of these studies found that the prevalence of Gram-positive cocci was higher in inpatients (hospital-acquired infections). Of note, the seasonal occurrence/accumulation of these bacteria (particularly S. saprophyticus) was also verified by our results in the Southern Great Plain of Hungary.
Table 4.
Literature summary on the prevalence of Gram-positive cocci in urinary tract infections outside Hungary (1997–2019).
| First author | Study year | Country | Prevalence of Gram-positive cocci (%), most common isolate | Comments |
|---|---|---|---|---|
| Barros et al.56 | 1997–2005 | Brazil | 6.2%; E. faecalis | |
| Kothari et al.57 | 2005 | India | 9.6%; S. saprophyticus | CA-UTIs only |
| Toner et al.58 | 2005–2014 | United Kingdom | 14.7%; E. faecalis | 9.8% of isolates were resistant to vancomycin |
| Behzadi et al.11 | 2007 | Iran |
January-March 2007: 9.1%; Enterococcus spp. October-December 2007: 10.6%; Enterococcus spp. |
|
| Parameswarappa et al.59 | 2007–2009 | India | 12.1%; Enterococcus spp. | |
| Sorlózano-Puerto et al.60 | 2011–2014 | Spain | 22.4%; E. faecalis | Children only |
| Zarb et al.49 | 2010 | European Union | 17.2%; E. faecalis | HA-UTIs only |
| Lewis et al.50 | 2013 | South Africa | 10.8%; E. faecalis | CA-UTIs only |
| Baral et al.51 | 2013 | Nepal | 21.7%; S. aurues | |
| Goel et al.61 | 2013–2014 | India | 0.5%; Enterococcus spp. | CA-UTIs only |
| Prashamsa et al.52 | 2015 | India | 12.5; E. faecalis | |
| Dougnon et al.54 | 2016 | West Africa | 21.0%; E. faecalis | |
| Bardoroi et al.55 | 2017 | India | 26.7%; S. aureus | HA-UTIs only |
| Zaha et al.62 | 2017–2018 | Romania |
7.3%; Enterococcus spp. 4.9%; Staphylococcus spp. |
All patients were affected by diabetes |
| Petca et al.63 | 2018 | Romania | 18.7%; Enterococcus spp. | |
| Urmi et al.64 | 2018 | Bangladesh | 8.2%; Gram-positive cocci | |
| Shrestha et al.4 | 2018 | Nepal | 21.3%; E. faecalis | |
| Petca et al.65 | 2018–2019 | Romania |
13.3%; Enterococcus spp. 2.1%; Staphylococcus spp. |
Three different centers |
CA-UTI community-acquired urinary tract infection, HA-UTI hospital-acquired/catheter-associated urinary tract infection.
Although the susceptibility-reporting for some of the antibiotics (e.g., fusidic acid, rifampicin, erythromycin, clindamycin, doxycycline and tigecycline) might seem frivolous in the context of the therapy of UTIs (as these drugs are not used in the therapy of these infections), the reporting of these results for epidemiological purposes is of interest, especially because not many studies are available regarding GPCs as uropathogens from Europe66. The prevalence of MRSA/VRSA and VRE isolates from urinary samples was advantageous in our study, and the levels of these isolates were similarly low in other literature reports as well, especially if we compare resistance levels of urinary isolates with invasive isolates (vancomycin-resistant E. faecalis: 0.0% [2008], 0.4% [2017]; vancomycin-resistant E. faecium: 2.8% [2008], 28.3% [2017]; MRSA: 22.5% [2008], 23.6% [2017], data from the European Antimicrobial Resistance Surveillance Network [EARS-Net])48–56,67,68. On the other hand, the resistance levels to auxillary antimicrobials (aminoglycosides, fluoroquinolones) was shown to be high, and presenting in an increasing tendency (HLAR in enterococci: 53.3% [2008], 62.0% [2017], data from EARS-Net)48–56,67,68. S. saprophyticus is a common agent in UTIs, however, regarding its resistance patterns, it has proven so far to be mostly sensitive to the relevant antibiotics. Among the tested antibiotics, the highest levels of resistance were detected for ciprofloxacin and SMX/TMP, which could a consequence of their prevalent use, due to their broad-spectrum activity against both Gram-positive and Gram-negative bacteria. Out of the agents effective against Gram-positive bacteria, azithromycin and clindamycin had the highest resistance levels. Enterococci are a therapeutic challenge in general, because of their intrinsic resistance mechanisms against many antibiotics (aminoglycosides, cephalosporins), and due to their genetic plasticity, they can easily acquire additional resistance determinants against other antimicrobial drugs. This is especially concerning, as these bacteria normally live in the gastrointestinal tract, where they can pick up resistance plasmids from other members of the commensal flora69–72. Vancomycin resistance in Enterococcus species is therefore a severe therapeutic issue19–23,25. High-level aminoglycoside-resistance (HLAR) in Enterococcus spp. was detected in > 40% in the second part of the study period. This resistance is usually mediated by aminoglycoside-modifying enzymes (e.g., acetyltransferases, phospho-transferases and nucleotidyl-transferases). The detection of HLAR is relevant in antimicrobial therapy, for the combined use of a cell wall-acting agent (ampicillin, imipenem) and the aminoglycoside for their pharmacological synergy71,72.
Gram-positive cocci have a plethora of virulence determinants, maintaining their high affinity for the epithelial cells of the urinary tract, allowing for their survival. These virulence factors include fibrillar proteins (Ssp) mediating cell–cell interactions, fibronectin-binding proteins, elastin-binding protein, adhesins, hemagglutinin, elastase and lipase. In addition, most of S. saprophyticus and > 90% of S. aureus strains produce urease, breaking down carbamide (urea) in the urine11–23. Staphylococci may colonize the rectum, while Enterococcus spp. are present in fecal matter, therefore their anatomical proximity to the urinary tract may additionally enhance their UTI-causing capabilities20,28. Biofilm-production in these species is an another important factor for the emergence and the persistence of UTIs, with some reports suggesting that some 80% of uropathogenic Gram-positive cocci are biofilm-producers48. The presence of biofilm in urethral stents and catheters may lead to obstruction; furthermore, microorganisms embedded in biofilm may survive 1000-times higher concentrations of antibiotics, compared to non-embedded (i.e. planktonic) cells48,73,74. The diversification and time-dependent use of these virulence determinants allows for the infectivity and survival of these bacteria. At the onset of infection (i.e. low population density), the expression levels of adhesins is more significant, while if high population densities are achieved, genes corresponding for toxin secretion are activated74.
Several limitations of this present study need to be acknowledged. In addition to the retrospective study design, the authors were unable to access the charts of the individual patients affected, therefore the correlation between the existence of clinically relevant risk factors (apart from age, inpatient/outpatient status, and catheterization) could not be assessed. The clear differentiation between Gram-positive asymptomatic bacteriuria and clinically significant (symptomatic) urinary tract infection in the elderly is very difficult. Furthermore, the molecular characterization and genotyping of the isolates species (which could have provided us with important data, especially in case of MRSA or S. saprophyticus isolates) was not performed, due to financial constraints. Also, the selection bias of publication should also be noted, as most studies describing the prevalence of infectious diseases are tertiary-care centers or specialized centers, corresponds to patients with more severe conditions or underlying illnesses75. Nevertheless, the information presented in this report should be useful in both national and international comparisons for epidemiological purposes; additionally, the resistance trends presented here may aid clinicians in the selection of appropriate antimicrobial therapy76.
Conclusions
Although urinary tract infections are principally caused by Gram-negative bacteria, Gram-positives have emerged as important causative agents of UTIs, particularly among elderly patients, often associated with co-morbidities, pregnant women and catheterized patients, both in low- and high-income countries. In our study, corresponding to the southern region of Hungary, their prevalence was found to be around 20%, with Enterococcus spp. in highest numbers. While there was no relevant difference is their prevalence among inpatients and outpatients, the emergence of drug resistance in these pathogens to commonly used antibiotics is a worrisome finding, compromising therapeutic options in the clinical practice and leading to the use of agents with less advantageous side effect profiles, further contributing to the selection pressure on these microorganisms. In our local settings, the resistance rates for fluoroquinolones are particularly concerning (these agents are not recommended to be used empirically), in addition, the same goes for the use of most aminoglycosides for hospitalized patients. In contrast, the use of nitrofurantoin for staphylococci may still be regarded as safe in our settings, and the tested isolates are almost uniformly susceptible to the available last-resort antibiotics.
Acknowledgements
M.G. was supported by the János Bolyai Research Scholarship (BO/00144/20/5) of the Hungarian Academy of Sciences and the New National Excellence Programme (ÚNKP) of the Hungarian Ministry for Innovation and Technology (ÚNKP-20-5-SZTE-330). M.G. would also like to acknowledge the support of the ESCMID’s “30 under 30” Award. Support from Ministry of Human Capacities, Hungary grant 20391-3/2018/FEKUSTRAT is acknowledged.
Author contributions
M.G. conceived and designed the study. M.Á. and A.L. were the senior microbiologists and performed the identification and of the bacterial isolates and interpreted susceptibility-testing results during the study period. M.G. Á.M. and A.L. performed data collection and analysis, wrote and revised the full paper. K.B. wrote and revised the full paper.
Funding
This research received no external funding. The APC was funded by the University of Szeged Open Access Fund (Grant No. 4565).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiedemann B, Heisig A, Heisig P. Uncomplicated urinary tract infections and antibiotic resistance-epidemiological and mechanistic aspects. Antibiotics. 2014;3:341–352. doi: 10.3390/antibiotics3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobel JD, Kaye D. Chapter 74—Urinary tract infections. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8. Philadelphia: Elsevier; 2015. pp. 886–913.e3. [Google Scholar]
- 4.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeer AJ, Tambayh PA, Tenke P, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 5.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeer AJ, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 6.Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174–192. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callan A, O'Shea E, Galvin S, Duane S, Corry P, Vellinga A. The economic cost of urinary tract infections in the community: results from Ireland. Value Health. 2014;17:A468. doi: 10.1016/j.jval.2014.08.1319. [DOI] [PubMed] [Google Scholar]
- 8.Calzi A, Grignolo S, Caviglia I, Calevo MG, Losurdo G, Piaggio G, Bandettini R, Castagnola E. Resistance to oral antibiotics in 4569 Gram-negative rods isolated from urinary tract infection in children. Eur. J. Pediatr. 2016;175:1219–1225. doi: 10.1007/s00431-016-2763-1. [DOI] [PubMed] [Google Scholar]
- 9.Stefaniuk E, Suchocka U, Bosacka K, Hryniewicz W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1–7. doi: 10.1007/s10096-016-2673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behzadi P, Behzadi E, Ranjbar R. Urinary tract infections and Candida albicans. Cent. Eur. J. Urol. 2015;68:96–101. doi: 10.5173/ceju.2015.01.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behzadi P, Behzadi E, Yazdanbod H, Aghapour R, Cheshmeh A, Omran D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar) 2010;5:111–115. [PMC free article] [PubMed] [Google Scholar]
- 12.Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology. 9. Washington, DC: American Society for Microbiology; 2007. [Google Scholar]
- 13.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang CI, Song JH, Ko KS, Chung DR, Peck KR. Clinical features and outcome of Staphylococcus aureus infection in elderly versus younger adult patients. Int. J. Infect. Dis. 2011;15:e58–e62. doi: 10.1016/j.ijid.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Baraboutis IG, Tsagalou EP, Lepinski JL, Papakonstantinou I, Papastamopoulos V, Skoutelis AT, Johnson S. Primary Staphylococcus aureus urinary tract infection: the role of undetected hematogenous seeding of the urinary tract. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:1095–1010. doi: 10.1007/s10096-010-0967-2. [DOI] [PubMed] [Google Scholar]
- 16.Gajdács M. [Epidemiology and susceptibility patters of Staphylococcus aureus isolates from STI samples of male patients (2008–2017)] (article in Hungarian) Magyar Urol. 2019;31:66–68. [Google Scholar]
- 17.Adeghate J, Juhász E, Pongrácz J, Rimanóczy É, Kristóf K. Does Staphylococcus saprophyticus cause acute cystitis only in young females, or is there more to the story? A one-year comprehensive study done in Budapest, Hungary. Acta Microbiol. Immunol. Hung. 2016;63:57–67. doi: 10.1556/030.63.2016.1.4. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson A, Giske C, Ternhag A. The relative importance of Staphylococcus saprophyticus as a urinary tract pathogen: distribution of bacteria among urinary samples analysed during 1 year at a major Swedish laboratory. APMIS. 2012;121:72–78. doi: 10.1111/j.1600-0463.2012.02937.x. [DOI] [PubMed] [Google Scholar]
- 19.Vu J, Carvalho J. Enterococcus: review of its physiology, pathogenesis, diseases and the challenges it poses for clinical microbiology. Front. Biol. 2011;6:357–366. doi: 10.1007/s11515-011-1167-x. [DOI] [Google Scholar]
- 20.García-Solache M, Rice LB. The Enterococcus: a model of adaptability to its environment. Clin. Microbiol. Rev. 2019;32:e00058-18. doi: 10.1128/CMR.00058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulricha N, Vonberg RP, Gastmeier P. Outbreaks caused by vancomycin-resistant Enterococcus faecium in hematology and oncology departments: a systematic review. Heliyon. 2017;3:e00473. doi: 10.1016/j.heliyon.2017.e00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155:1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 23.Guzman-Prieto AM, Schaik W, Rogers MRC, Coque TM, Baquero F, Corander J, Willems RJL. Global emergence and dissemination of enterococci as nosocomial pathogens: attack of the clones? Front. Microbiol. 2016;7:e788. doi: 10.3389/fmicb.2016.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bierowiec K, Płoneczka-Janeczko K, Rypuła K. Is the colonisation of Staphylococcus aureus in pets associated with their close contact with owners? PLoS ONE. 2016;11:e0156052. doi: 10.1371/journal.pone.0156052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillay S, Zishiri OT, Adeleke MA. Prevalence of virulence genes in Enterococcus species isolated from companion animals and livestock. Onderstepoort J. Vet. Res. 2018;85:e1–e8. doi: 10.4102/ojvr.v85i1.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issakhanian L, Behzadi P. Antimicrobial agents and urinary tract infections. Curr. Pharm. Des. 2019;25:1409–1423. doi: 10.2174/1381612825999190619130216. [DOI] [PubMed] [Google Scholar]
- 27.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gajdács M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8:e52. doi: 10.3390/antibiotics8020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassini A, Högberg DL, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GN, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Burden of the AMR Collaborative Group Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 2019;19:55–56. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sultan I, Rahman S, Jan AT, Siddiqui MT, Mondal AH, Haq QHR. Antibiotics, resistome and resistance mechanisms: a bacterial perspective. Front. Microbiol. 2018;9:e2066. doi: 10.3389/fmicb.2018.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainardi JL, Viller R, Bugg TD, Mayer C, Arthur M. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria. FEMS Microbiol. Rev. 2008;32:386–408. doi: 10.1111/j.1574-6976.2007.00097.x. [DOI] [PubMed] [Google Scholar]
- 32.Tóth H, Fésüs A, Kungler-Gorácz O, Balázs B, Majoros L, Szarka K, Kardos G. Utilization of vector autoregressive and linear transfer models to follow up the antibiotic resistance spiral in Gram-negative bacteria from cephalosporin consumption to colistin resistance. Clin. Infect. Dis. 2019;69:1410–1421. doi: 10.1093/cid/ciy1086. [DOI] [PubMed] [Google Scholar]
- 33.Gajdács M, Paulik E, Szabó A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: a cross-sectional survey in Hungary (KAPPhA-HU) Antibiotics. 2020;9:e41. doi: 10.3390/antibiotics9020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunha BA, Sessa J, Blum S. Enhanced efficacy of high dose oral vancomycin therapy in Clostridium difficile diarrhea for hospitalized adults not responsive to conventional oral vancomycin therapy: antibiotic stewardship implications. J. Clin. Med. 2018;7:75. doi: 10.3390/jcm7040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013;26:185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int. J. Antimicrob. Agents. 2014;43:328–334. doi: 10.1016/j.ijantimicag.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Gajdács M. Dying bacteria allow others to survive and grow by absorbing antimicrobial compounds (article in Hungarian) Hung. Health Promot. J. 2019;60:29–35. [Google Scholar]
- 38.National Health Insurance Fund of Hungary. Hospital Bed Count and Patient Turnover Report 2017; National Health Insurance Fund of Hungary: Budapest, Hungary, 2017. https://www.neak.gov.hu/felso_menu/szakmai_oldalak/publikus_forgalmi_adatok/gyogyito_megelozo_forgalmi_adat/fekvobeteg_szakellatas/korhazi_agyszam.html. Accessed 26th August 2019.
- 39.Gajdács M, Spengler G, Urbán E. Identification and antimicrobial susceptibility testing of anaerobic bacteria: Rubik’s cube of clinical microbiology? Antibiotics. 2017;6:25. doi: 10.3390/antibiotics6040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leclercq R, Cantón R, Brown DFJ, Giske CG, Heisig P, MacGowan AP, Mouton JW, Nordmann P, Rodloff AC, Rossolini GM, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013;19:141–160. doi: 10.1111/j.1469-0691.2011.03703.x. [DOI] [PubMed] [Google Scholar]
- 41.Ábrók M, Lázár A, Szécsényi M, Deák J, Urbán E. Combination of MALDI-TOF MS and PBP2’ latex agglutination assay for rapid MRSA detection. J. Microbiol. Methods. 2018;144:122–124. doi: 10.1016/j.mimet.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Bertelloni F, Salvadori C, Lialotti G, Cocerri D, Ebani VV. Antimicrobial resistance in Enterococcus strains isolated from healthy domestic dogs. Acta Microbiol. Immunol. Hung. 2017;64:301–312. doi: 10.1556/030.63.2016.021. [DOI] [PubMed] [Google Scholar]
- 43.Gajdács M, Bátori Z, Ábrók M, Lázár A, Burián K. Characterization of resistance in Gram-negative urinary isolates using existing and novel indicators of clinical relevance: a 10-year data analysis. Life. 2020;10:16. doi: 10.3390/life10020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gajdács M, Urbán E. Resistance trends and epidemiology of Citrobacter-Enterobacter-Serratia in urinary tract infections of inpatients and outpatients (RECESUTI): a 10-year survey. Medicina (Kaunas) 2019;55:e285. doi: 10.3390/medicina55060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gajdács M, Urbán E. Comparative epidemiology and resistance trends of Proteae in urinary tract infections of inpatients and outpatients: a 10-year retrospective study. Antibiotics. 2019;8:91. doi: 10.3390/antibiotics8030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gajdács M, Burián K, Terhes G. Resistance levels and epidemiology of non-fermenting Gram-negative bacteria in urinary tract infections of inpatients and outpatients (RENFUTI): a 10-year epidemiological snapshot. Antibiotics. 2019;8:e143. doi: 10.3390/antibiotics8030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gajdács M, Dóczi I, Ábrók M, Lázár A, Burián K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: results from a 10-year retrospective survey. Cent. Eur. J. Urol. 2019;72:209–214. doi: 10.5173/ceju.2019.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrestha LB, Baral R, Khanal B. Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community acquired urinary tract infections and catheter-associated urinary tract infections. Infect. Drug. Res. 2019;12:957–963. doi: 10.2147/IDR.S200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarb P, Coignard B, Griskeviciene J, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, Goossens M, Vaerengerg S, Hopkins S, Catry B, Monnet DI, Goossens H, Suetens C, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012;17:e20316. doi: 10.2807/ese.17.46.20316-en. [DOI] [PubMed] [Google Scholar]
- 50.Lewis DA, Gumede LY, van der Hoven LA, de Gita GN, de Kock EJE, de Lange T, Maseko V, Kekana V, Smuts FP, Perovic O. Antimicrobial susceptibility of organisms causing community-acquired urinary tract infections in Gauteng Province, South Africa. S. Afr. Med. J. 2013;103:377–381. doi: 10.7196/SAMJ.6722. [DOI] [PubMed] [Google Scholar]
- 51.Baral R, Timilsina S, Jha P, Bhattarai NR, Poudyal N, Gurung R, Khanal B, Bhattacharya SK. Study of antimicrobial susceptibility pattern of Gram positive organisms causing UTI in a tertiary care hospital in eastern region of Nepal. Health Renaiss. 2013;11:119–124. doi: 10.3126/hren.v11i2.8218. [DOI] [Google Scholar]
- 52.Prashamsa K, Devi D, Madhup SK, Shrechand JB. Catheter associated urinary tract infection: prevalence, microbiological profile and antibiogram at a tertiary care hospital. Ann. Clin. Chem. Lab. Med. 2017;3:3–10. [Google Scholar]
- 53.Dougnon TV, Bankole HS, Johnson RC, Gildas H, Moussa TI, Christelle H, Michel B, Lamine BM. Catheter-associated urinary tract infections at a hospital in Zinvie, Benin (West Africa) Int. J. Infect. 2016;3:e34141. doi: 10.17795/iji-34141. [DOI] [Google Scholar]
- 54.Bardoloi V, Yogeesha BKV. Comparative study of isolates from community acquired and catheter-associated urinary tract infections with reference to biofilm-producing property, antibiotic sensitivity and multi-drug resistance. J. Med. Microbiol. 2017;66:927–936. doi: 10.1099/jmm.0.000525. [DOI] [PubMed] [Google Scholar]
- 55.Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab. Syndr. Obes. 2015;26:129–136. doi: 10.2147/DMSO.S51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barros M, Martinelli R, Rocha H. Enterococcal urinary tract infections in a university hospital: clinical studies. Braz. J. Infect. Dis. 2009;13:294–296. doi: 10.1590/S1413-86702009000400011. [DOI] [PubMed] [Google Scholar]
- 57.Kothari A, Sagar V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: a multicenter study. J. Infect. Dev. Ctries. 2008;2:354–358. doi: 10.3855/jidc.196. [DOI] [PubMed] [Google Scholar]
- 58.Toner L, Papa N, Aliyu SH, Dev H, Lawrentscuk N, Al-Hayek S. Vancomycin resistant enterococci in urine cultures: Antibiotic susceptibility trends over a decade at a tertiary hospital in the United Kingdom. ICUrology. 2016;57:29–134. doi: 10.4111/icu.2016.57.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parameswarappa J, Basavaraj VP, Basvaraj CM. Isolation, identification, and antibiogram of enterococci isolated from patients with urinary tract infection. Ann. Afr.Med. 2013;12:176–181. doi: 10.4103/1596-3519.117629. [DOI] [PubMed] [Google Scholar]
- 60.Sorlózano-Puerto A, Gómez-Luque JM, Luna-del-Castillo JD, Navarro-Marí JM, Gutiérrez-Fernández J. Etiological and resistance profile of bacteria involved in urinary tract infections in young children. BioMed Res. Int. 2017;2017:e4909452. doi: 10.1155/2017/4909452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goel V, Kumar D, Kumar R, Mathur P, Singh S. Community acquired enterococcal urinary tract infections and antibiotic resistance profile in North India. J. Lab. Phys. 2016;8:50–54. doi: 10.4103/0974-2727.176237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaha DC, Jurca CM, Daina LG, Vesa CM, Popa AR, Jurca AD, Muresan M, Micle M. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients. Farmacia. 2020;68:250–255. doi: 10.31925/farmacia.2020.2.9. [DOI] [Google Scholar]
- 63.Petca RC, Popescu RI, Mares C, Petca A, Mehedintu C, Sandu I, Maru N. Antibiotic resistance profile of common uropathogens implicated in urinary tract infection in Romania. Farmacia. 2019;67:994–1004. doi: 10.31925/farmacia.2019.6.9. [DOI] [Google Scholar]
- 64.Urmi UL, Jahan N, Nahar S, Rana M, Sultana F, Hossain B, Iqbal S, Hossain M, Mosaddek ASM, Islam S. Gram-positive uropathogens: empirical treatment and emerging antimicrobial resistance. Biomed. Res. Clin. Pract. 2019;4:1–4. [Google Scholar]
- 65.Petca RC, Mares C, Petca A, Negoita S, Popescu RI, Bot M, Barabás E, Chibelean CB. Spectrum and antibiotic resistance of uropathogens in Romanian females. Antibiotics. 2020;9:e472. doi: 10.3390/antibiotics9080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to 2011. Pharmaceuticals. 2013;6:1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.European Antimicrobial Resistance Surveillance Network (EARS-Net). https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-andlaboratory-networks/ears-net. Accessed 26th August 2019.
- 68.Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjor A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010;16:541–554. doi: 10.1111/j.1469-0691.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 69.Palmer KL, Kos VN, Gilmore MS. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010;13:632–639. doi: 10.1016/j.mib.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24:892. doi: 10.3390/molecules24050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khodabandeh M, Mohammadi M, Abdolsalehi MR, Hasannejad-Bibalan M, Gholami M, Alvandimanesh A, Pournajaf A, Rajabnia R. High-level aminoglycoside resistance in Enterococcus faecalis and Enterococcus faecium; as a serious threat in hospitals. Infect. Disord. Drug Targets. 2020;20:223–228. doi: 10.2174/1871526519666181130095954. [DOI] [PubMed] [Google Scholar]
- 72.Beganovic M, Luther MK, Rice LB, Arias CA, Rybak MJ, La Plante KL. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018;67:303–309. doi: 10.1093/cid/ciy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soto SM. Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv. Biol. 2014;2014:13. doi: 10.1155/2014/543974. [DOI] [Google Scholar]
- 74.Gomes-Fernandes M, Laabei M, Pagan N, Hidalgo J, Molinos S, Villar-Hernandez R, Domínguez-Villanueva D, Jenkins ATA, Lacoma A, Prat C. Accessory gene regulator (Agr) functionality in Staphylococcus aureus derived from lower respiratory tract infections. PLoS ONE. 2017;12:e0175552. doi: 10.1371/journal.pone.0175552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Influence of referral bias on the clinical characteristics 485 of patients with Gram-negative bloodstream infection. Epidemiol. Infect. 2011;139:1750–1756. doi: 10.1017/S095026881100001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Behzadi P, Urbán E, Matuz M, Benkő R, Gajdács M. The role of Gram-negative bacteria in urinary tract infections: current concepts and therapeutic options. Adv. Exp. Med. Biol. 2020;4:5. doi: 10.1007/5584_2020_566. [DOI] [PubMed] [Google Scholar]