Abstract
Three hundred and sixty 1-day-old male broiler chicks were randomly allocated to 4 treatments of 6 replicates to evaluate the effects of cLFchimera, a recombinant antimicrobial peptide (AMP), on gut health attributes of broiler chickens under necrotic enteritis (NE) challenge. Treatments were as follows: (T1) unchallenged group fed with corn-soybean meal (CSM) without NE challenge and additives (NC); (T2) group fed with CSM and challenged with NE without any additives (PC); (T3) PC group supplemented with 20 mg cLFchimera/kg diet (AMP); (T4) PC group supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/kg diet (antibiotic). Birds were sampled for villi morphology, ileal microbiota, and jejunal gene expression of cytokines, tight junctions proteins, and mucin. Results showed that AMP ameliorated NE-related intestinal lesions, reduced mortality, and rehabilitated jejunal villi morphology in NE challenged birds. While the antibiotic non-selectively reduced the count of bacteria, AMP restored microflora balance in the ileum of challenged birds. cLFchimera regulated the expression of cytokines, junctional proteins, and mucin transcripts in the jejunum of NE challenged birds. In conclusion, cLFchimera can be a reliable candidate to substitute growth promoter antibiotics, while more research is required to unveil the exact mode of action of this synthetic peptide.
Subject terms: Infection, Bacterial infection, Parasitic infection
Introduction
Necrotic enteritis (NE) is well-known as a detrimental disease in the poultry industry which results in production losses, increased mortality, reduced welfare of birds, and also increased risk of contamination of poultry products for human consumption1. The etiologic cause of NE is Clostridium perfringens (C. perfringens), a spore-forming Gram-positive bacterium that naturally inhabits the gastrointestinal tract of farm animals2.
Antibiotics have been widely used to control NE in poultry farms, while the administration of growth-promoting antibiotics is extensively forbidden due to the rapid spread of the antibiotic resistance in human cases3. This prohibition of antibiotic use in the livestock industry has inspired researcher into safe substitutions for antibiotics. Consequently, several additives have been introduced to the market, such as pro and prebiotics, essential oils, acidifiers, and antimicrobial peptides4.
Antimicrobial peptides (AMPs) are endo-exogenous polypeptides comprised of less than 50 amino acids, characterized by cationic amphipathic properties, and produced by host defense systems or synthetically supplied to the diet in order to protect a host from pathogenic microbes5,6. These peptides show broad-spectrum antimicrobial activities against various microorganisms, including Gram-positive and Gram-negative bacteria, fungi, and viruses7; they are well-known for their roles as competent alternatives for antibiotics in farm animal production8–11. Previous studies have demonstrated that AMPs could improve growth performance, nutrient digestibility and gut health, positively alter intestinal microbiota, and enhance immune function in pigs and broilers.
The AMP, cLFchimera, is a heterodimeric peptide designed to mimic two antimicrobial domains, Lactoferricin (LFcin) and Lactoferrampin (LFampin), which are present in the N1-domain of camel lactoferrin (cLF)12. More recently, the recombinant form of cLFchimera has been cloned and expressed in E. coli12 and L. lactic13. The results of in vitro studies showed that cLF36 has antibacterial12,13 antiviral14, and anticancer15 properties. Further, a previous in vivo study asserted that supplementing E. coli challenged broilers with cLFchimera improved jejunal villi morphology, restored microbial balance in the ileum, and improved gene expression of cytokines and tight junctions in the jejunum of challenged broiler chickens16. Therefore, the objective of this study was to evaluate the effectiveness of cLFchimera as an alternative to growth enhancer antibiotics on intestinal morphology, microflora, and gene expression of immune cells and junctional proteins in broiler chickens challenged with NE, as an animal model for infectious disease.
Materials and methods
All experimental protocols involving animals in the present study were approved by Institutional Animal Care and Use Committee of Ferdowsi University of Mashhad (Protocol number 3/42449) and performed in accordance with relevant guidelines and regulations to minimize animal pain, suffering, and distress.
Birds, treatments, and experimental design
A total of 360 1-day-old male chicks (Cobb 500) were purchased from a local hatchery, weighed and randomly assigned to 4 treatments with six replicates containing 15 birds in each replicate. Treatments were as follow: 1) negative control birds received a corn-soybean meal basal diet without AMPs, antibiotic, and NE challenge (NC); 2) positive control birds challenged with NE (PC); 3) PC birds challenged with NE and supplemented with 20 mg peptide/kg diet (AMP); 4) PC birds challenged with NE and supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/kg diet (antibiotic). All diets were in mash form and formulated to meet or exceed the minimum requirements of Cobb 500 (Table 1). Feed and water and were provided ad libitum. Chicks were reared in floor pens (1.1 m × 1.3 m) covered with wood shavings. Temperature and lighting programs were adjusted based on the guidelines of the Cobb 500 strain.
Table 1.
Composition of experimental diets.
| Ingredient (%)1 | Starter (0–10 days) | Grower (11–22 days) |
|---|---|---|
| Corn | 56.81 | 58.16 |
| Soybean meal (44.0%) | 36.01 | 34.80 |
| Soybean oil | 3.18 | 3.40 |
| Dicalcium phosphate | 1.79 | 1.65 |
| Limestone | 0.97 | 0.93 |
| Salt | 0.35 | 0.30 |
| Mineral-vitamin premix2 | 0.50 | 0.50 |
| DL-Methionine | 0.17 | 0.15 |
| L-Lysine HCl | 0.22 | 0.12 |
| Calculated nutrients | ||
| AME (kcal/kg) | 3000.00 | 3080.00 |
| Crude protein (%) | 21.00 | 19.00 |
| Calcium (%) | 0.90 | 0.84 |
| Available phosphorus (%) | 0.45 | 0.42 |
| Sodium (%) | 0.16 | 0.16 |
| Methionine (%) | 0.50 | 0.47 |
| Methionine + cystene (%) | 0.98 | 0.86 |
| Lysine (%) | 1.32 | 1.18 |
1Antibiotic (45 mg bacitracin methylene disalicylate/kg diet) and peptide (20 mg/kg diet) were added on top and thoroughly mixed.
2Added per kg of feed: vitamin A, 7,500 UI; vitamin D3 2100 UI; vitamin E, 280 UI; vitamin K3, 2 mg; thiamine, 2 mg; riboflavin, 6 mg; pyridoxine, 2.5 mg; cyanocobalamin, 0.012 mg, pantothenic acid, 15 mg; niacin, 35 mg; folic acid, 1 mg; biotin, 0.08 mg; iron, 40 mg; zinc, 80 mg; manganese, 80 mg; copper, 10 mg; iodine, 0.7 mg; selenium, 0.3 mg.
AMP production
cLF chimera was derived from camel lactoferrin (cLF) consisting of 42 amino acids and has primary sequence of DLIWKLLVKAQEKFGRGKPSKRVKKMRRQWQACKSSHHHHHH. In addition, the results of our previous study showed that cLFchimera had antioxidant activity (IC50: 310 μ/ml) and its activity was not affected after 40 min of boiling17 (for more details regarding production process, please review previous papers12,13,17). Briefly, preparation of recombinant plasmid vector was conducted through transforming recombinant expression vector harboring synthetic cLFchimera into DH5α bacterium12,13. Next, the latter bacterial colonies were cultured to harvest plasmid extraction. The recombinant vector was then transferred into E. coli BL21 (DE3) as an expression host and cultured in 2 ml Luria–Bertani broth (LB) medium for overnight according to standard protocol18. In the next step, cultured materials were inoculated in 50 ml LB and incubated at 37 °C with shaking at 200 rpm. Then, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM and incubated at 37 °C for 6 h after IPTG induction. Periplasmic protein was collected at different times after IPTG induction (2, 4, and 6 h) according to the method described by de Souza Cândido et al.19 and analyzed on 12% SDS-PAGE. To purify expressed peptide, Ni–NTA agarose column was used based on manufacturer's instruction (Thermo, USA). The quality of purified recombinant peptide was assessed on a 12% SDS-PAGE gel electrophoresis, while the Bradford method was used to analyze the quantity of recombinant peptide. More recently, an E. coli expression system was developed in our laboratory that is able to produce 0.42 g/L of recombinant peptide. Finally, four grams of peptide previously obtained from the recombinant E. coli were purified, lyophilized, and thoroughly mixed with 1 kg soybean meal and then supplemented to the relevant experimental diets.
NE challenge
A previously described method of inducing NE was used with some modifications20. Briefly, on day 16, chicks in NC group were administered a single 1 ml oral dose of sterile phosphate-buffered saline (PBS) (uninfected) as a sham control, while PC, peptide and the antibiotic groups were orally inoculated with 5,000 attenuated vaccine strain sporulated oocysts each of E. maxima, E. acervulina, and E. tenella (Livacox T, Biopharm Co., Prague, Czech Republic) in 1 mL of 1% (w/v) sterile saline. On days 20 and 21, birds in the NE groups were orally inoculated with 1 ml of broth containing C. perfringens isolated from broilers meat21, CIP (60.61) containing 107 cfu/ml in thioglycollate (Thermo-Fisher Scientific Oxoid Ltd, Basingstoke, UK) supplemented with peptone and starch. Inoculated C. perfringens was analysed using PCR to confirm the presence of netB gene required for inducing NE in broilers according to Razmyar et al.22. The unchallenged group received the same dose of sterilized broth.
Growth performance
On days 10 and 22, average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) were calculated using body weight (BW) and the weight of feed which remained in each pen. The ADG, ADFI and FCR were calculated over the specific and comprehensive periods of the study (0–10, 11–22, and 0–22 days). The feed conversion ratio for each period was readjusted based on the mortality data per pen per day, if any.
Sample collection and lesion score
On days 10 and 22, two birds from each pen (12 birds/treatment) were randomly selected for euthanasia by cervical dislocation. The viscera was excised from the specimens, the intestine discretely separated and the adherent materials were precisely removed. The ileum was gently pressed to aseptically collect ileal content into sterile tubes for microbiological analysis. A section of approximately 5 cm from mid-jejunal tissues was meticulously separated for morphological analysis. A 2 cm section from the mid-jejunum was detached, rinsed in cold phosphate-buffered saline (PBS), immediately immersed in RNAlater (Qiagen, Germantown, MD), and stored at − 20 °C for subsequent gene expression determination. On day 22, NE lesions of duodenum, jejunum, and ileum from 2 birds per pen were scored on a scale of 0 (none) to 6 (high) as described previously23.
Intestinal morphology
The method used to prepare samples for morphometry analysis was previously described by Daneshmand et al.24. Briefly, jejunal samples were stored in a 10% formaldehyde phosphate buffer for 48 h. The samples were then processed on a tissue processor (Excelsior AS, Thermo Fisher Scientific, Loughborough, UK), fixed in paraffin using an embedder (Thermo Fisher Histo Star Embedder, Loughborough, UK), and cut with a microtome (Leica HI1210, Leica Microsystems Ltd., Wetzlar, Germany) to a length of 3 cm per slice. The slices were placed on a slide and dehydrated on a hotplate (Leica ASP300S, Leica Microsystems Ltd., Wetzlar, Germany) and dyed with hematoxylin and eosin. Finally, the dyed slices of jejunal were examined under a microscope (Olympus BX41, Olympus Corporation, Tokyo, Japan). A total of 8 slides were prepared from the jejunal segment per bird, and ten individual well-oriented villi were measured per slide (80 villi/bird). The average of each measurement per sample was reported as the respective a mean for each bird. Villus width (VW) was measured at the base of each villus; villus height (VH) from the top of the villus to the villus-crypt junction, crypt depth (CD) from the base of the adjacent villus to the sub-mucosa, the ratio of VH/CD and villus surface area were calculated.
Microbial count
The methods used to count the populations of E. coli, Clostridium spp., Lactobacillus spp., and Bifidobacterium spp. in the ileal content were described elsewhere25. In summary, the ileal contents of a sample were thoroughly mixed, serially diluted tenfold from 10−1 to 10−7 with sterile PBS, and homogenized for 3 min. Next, dilutions were plated on different agar mediums. Regarding the enumeration of bacteria, Lactobacillus spp. and Clostridium spp. dilutions were plated on MRS agar (Difco, Laboratories, Detroit, MI) and SPS agar (Sigma-Aldrich, Germany) and anaerobically cultured at 37 °C for 48 h and 24 h, respectively. Black colonies of Clostridium spp. on SPS agar were counted. MacConkey agar (Difco Laboratories, Detroit, MI) and BSM agar (Sigma-Aldrich, Germany) were used to cultivate E. coli and Bifidobacterium spp. respectively, and incubated at 37 °C for 24 h. All microbiological analyses were performed in triplicate, and average values were used for statistical analyses and results were expressed in colony-forming units (Log10 cfu/g of ileal content).
RNA extraction and gene expression
The procedure of RNA extraction and gene expression was previously explained26. In summary, total RNA was extracted from chicken jejunum sampled on day 22 using the total RNA extraction kit (Pars Tous, Iran) following the manufacturer’s instructions. The purity and quality of extracted RNA were evaluated using an Epoch microplate spectrophotometer (BioTek, USA) based on 260/230 and 260/280 wavelength ratios, respectively. Genomic DNA was removed using DNase I (Thermo Fisher Scientific, Austin, TX, USA). The complementary DNA (cDNA) was synthesized from 1 µg of total RNA using the Easy cDNA synthesis kit (Pars Tous, Iran) following the manufacturer’s protocol.
Gene expression of two references (GAPDH and β-actin) and five targets (Interleukin-1 [IL-1], IL-6, mucin2 [MUC2], Claudin-1 [CLDN1], and Occludin [OCLN]) genes were determined by quantitative real-time PCR (qPCR) based on MIQE guidelines27. Each reaction was performed in a total volume of 20 μl in duplicate using an ABI 7300 system (Applied Biosystems, Foster City, CA) and 2 × SYBR Green Real Time-PCR master mix (Pars Tous, Iran). Primer details are shown in Table 2. All primers were designed according to MIQE criteria27 regarding amplification length and intron spanning. All efficiencies were between 90 and 110%, and calculated R2 was 0.99 for all reactions. The method 2−ΔΔCt Ct was used to calculate relative gene expression in relation to the reference genes28 (GAPDH and β-actin).
Table 2.
Sequences of primer pairs used for amplification of target and reference genes.1
| Gene2 | Strand | Sequence (5´ → 3´) | Ta | Product size (bp) | GenBank Accession No |
|---|---|---|---|---|---|
| ANXA1 | Forward | CTGCCTGACTGCCCTTGTGA | 63 | 98 | NM_206906.1 |
| Reverse | GTTTGTGTCGTGTTCCACTCCC | ||||
| TRAF3 | Forward | CTGAGAAAAGATTTGCCAGACCA | 63 | 101 | XM_421378 |
| Reverse | CATGAAACCATGACACACGGG | ||||
| MUC2 | Forward | ATGCGATGTTAACACAGGACTC | 60 | 110 | BX930545 |
| Reverse | GTGGAGCACAGCAGACTTTG | ||||
| CLDN1 | Forward | CATACTCCTGGGTCTGGTTGGT | 60 | 100 | NM_001013611.2 |
| Reverse | GACAGCCATCCGCATCTTCT | ||||
| OCLDN | Forward | CGCAGTCCAGCGGTTACTA | 58 | 178 | NM_205128.1 |
| Reverse | AGGATGACGATGAGGAACCCA | ||||
| GAPDH | Forward | TTGTCTCCTGTGACTTCAATGGTG | 63 | 128 | NM_204305 |
| Reverse | ACGGTTGCTGTATCCAAACTCAT | ||||
| β-Actin | Forward | CCTGGCACCTAGCACAATGAA | 63 | 175 | NM_205518.1 |
| Reverse | GGTTTAGAAGCATTTGCGGTG |
1For each gene the primer sequence for forward and reverse (5´ → 3´), the product size (bp), and the annealing temperature (Ta) in °C are shown.
2ANXA1, annexin A1; TRAF3, tumor necrosis factor receptor associated factor 3; MUC2, mucin2; CLDN1, claudin1; OCLDN, occludin; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Data were statistically analyzed in a completely randomized design by ANOVA using the General Linear Model (GLM) procedure of SAS (SAS Inst., Inc., Cary, NC). Tukey’s test was used to compare differences among means of treatments, and P values < 0.05 were considered to be significant.
Results
Lesion score and mortality
Table 3 shows the effects of experimental treatments on NE-inducing lesion scores in different segments of the intestine and mortality rate of broiler chickens. The results showed that none of the additives could rehabilitate the NE-inducing lesions in the intestine compared to NC. While the highest lesion scores in the intestine of PC group showed that NE was efficiently induced in broilers, AMP decreased (P < 0.05) lesions in duodenum, jejunum, and ileum of broilers compared to the challenged group.
Table 3.
Effects of treatments on necrotic enteritis lesion scores (d 22) and mortality (d 16–22) in broiler chickens.
| Treatments | Lesion score | Mortality2 (%) | ||
|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | ||
| NC1 | 0.000c | 0.000c | 0.000c | 0.000c |
| PC | 0.187a | 1.131a | 0.944a | 8.17a |
| AMP | 0.093b | 0.769b | 0.621b | 2.53bc |
| Antibiotic | 0.148ab | 0.955ab | 0.783ab | 3.44b |
| SEM3 | 0.119 | 0.285 | 0.373 | 0.819 |
| P-value | 0.034 | 0.001 | 0.013 | 0.001 |
a–cValues within a column with different letters differ significantly (P < 0.05).
1NC: negative control group received corn-soybean meal diet without challenge and additives; PC: positive control group received NC diet experimentally challenged with necrotic enteritis; AMP: PC received group supplemented with 20 mg antimicrobial peptide/ kg diet; Antibiotic: PC received group supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/kg diet.
2Only mortalities shown necrotic enteritis signs.
3SEM: standard error of means (results are given as means (n = 12) for each treatment).
Inducing NE in broilers increased (P < 0.05) mortality, while birds fed with peptide showed lower (P < 0.05) mortality rate compared to PC group and interestingly had similar results to NC group.
Broiler performance
Table 4 represents the effects of experimental diets on growth performance of broilers. While the antibiotic improved (P < 0.05) birds’ ADG at first 10 days of age, peptide showed the best FCR at the end of starter period when no challenge was induced yet. At d 22, NE challenge decreased (P < 0.05) ADG and increased (P < 0.05) feed intake, which devastated (P < 0.05) FCR in broilers. In the latter phase, peptide showed similar effects to NC group, while improved (P < 0.05) performance indices compared to PC group.
Table 4.
Effects of treatments on growth performance of broiler chickens from 0 to 22 days of age.
| Treatments | ADG2 (g) | ADFI (g) | FCR (g/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 11–22 | 0–22 | 0–10 | 11–22 | 0–22 | 0–10 | 11–22 | 0–22 | |
| NC1 | 26.25b | 58.95b | 85.20b | 24.93a | 74.79b | 99.37b | 0.950a | 1.269b | 1.170b |
| PC | 26.15b | 55.91c | 82.06c | 23.77ab | 88.18a | 111.95a | 0.909a | 1.578a | 1.364a |
| AMP | 27.05ab | 60.29ab | 87.34ab | 22.61b | 72.23b | 94.85b | 0.836b | 1.199b | 1.086c |
| Antibiotic | 27.78a | 61.94a | 89.72a | 24.64a | 75.26b | 99.90b | 0.888ab | 1.215b | 1.113bc |
| SEM3 | 0.318 | 0.446 | 0.644 | 0.422 | 1.401 | 1.577 | 0.0164 | 0.0257 | 0.0181 |
| P value | 0.007 | 0.001 | 0.001 | 0.005 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
a–cValues within a column with different letters differ significantly (P < 0.05).
1NC: negative control group received corn-soybean meal diet without challenge and additives; PC: positive control group received NC diet experimentally challenged with necrotic enteritis; AMP: PC received group supplemented with 20 mg antimicrobial peptide/ kg diet; Antibiotic: PC received group supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/kg diet.
2ADG: average daily gain; ADFI: average daily feed intake; FCR: feed conversion ratio.
3SEM: standard error of means (results are given as means of 6 pens of 15 birds/treatment).
Jejunal villi morphology
The effects of treatments on jejunal morphology are shown in Table 5. On day 10, experimental diets had no significant effects on the morphometry of the intestine. NE challenge significantly demolished villi structure and morphology, while AMP enhanced (P < 0.05) villus height, width, and surface area (VSA) compared to PC group and had a similar effect to NC group at 22 days of age. Experimental diets had no significant effects on CD and VH/CD at d 22. Although the antibiotic improved (P < 0.05) villi morphology compared to NE-challenged birds, it could not restore villi characteristics to those of NC.
Table 5.
Effects of treatments on villi morphology (µm) in the jejunum of broiler chickens at 10 and 22 days of age.
| Treatment | Day 10 | Day 22 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VH2 | VW | CD | VH/CD | VSA (µm2) | VH | VW | CD | VH/CD | VSA (µm2) | |
| NC1 | 621 | 188 | 144 | 3.29 | 367.7 | 1175a | 186a | 187 | 5.69 | 688.8a |
| PC | 592 | 192 | 125 | 3.09 | 356.6 | 827c | 153b | 201 | 5.04 | 396.9c |
| AMP | 681 | 194 | 138 | 3.52 | 414.2 | 1140ab | 187a | 171 | 6.06 | 671.5a |
| Antibiotic | 641 | 197 | 121 | 3.26 | 396.9 | 1017b | 174a | 180 | 6.50 | 557.0b |
| SEM3 | 26.4 | 3.5 | 13.7 | 0.164 | 16.42 | 35.4 | 5.1 | 22.8 | 0.632 | 25.78 |
| P value | 0.167 | 0.401 | 0.610 | 0.348 | 0.102 | 0.001 | 0.001 | 0.816 | 0.447 | 0.001 |
a–cValues within a column with different letters differ significantly (P < 0.05).
1NC: negative control group received corn-soybean meal diet without challenge and additives; PC: positive control group received NC diet experimentally challenged with necrotic enteritis; AMP: PC received group supplemented with 20 mg antimicrobial peptide/ kg diet; Antibiotic: PC received group supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/kg diet.
2VH: villus height; VW: villus width; CD: crypt depth; VH/CD: the ratio of VH to CD; VSA: villus surface area.
3SEM: standard error of means [results are given as means (n = 12) for each treatment].
Bacterial colonization
Table 6 summarizes the effects of experimental diets on ileal bacterial populations. At d 10, the antibiotic decreased (P < 0.05) the population of all bacteria compared to other groups. NE challenge increased (P < 0.05) the count of E. coli and expectedly Clostridium spp. and also decreased (P < 0.05) the number of Lactobacillus spp. and Bifidobacterium spp. in the ileum of birds. In the same period, the antibiotic had the lowest (P < 0.05) population of all cultured ileal bacteria compared to both PC and NC groups. Interestingly, AMP had similar effects to NC group, while it increased (P < 0.05) the population of Lactobacillus spp. and Bifidobacterium spp. and also decreased (P < 0.05) the colonization of E. coli and Clostridium spp. in the ileum of chickens compared to the challenged birds at 22 days of age.
Table 6.
Effects of treatments on ileal microflora (log10 CFU g-1) in broilers at 10 and 22 days of age.
| Treatments | Day 10 | Day 22 | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | Lactobacillus spp. | Bifidobacterium spp. | Clostridium spp. | E. coli | Lactobacillus spp. | Bifidobacterium spp. | Clostridium spp. | |
| NC1 | 3.03a | 5.69a | 6.17a | 1.62a | 4.09b | 7.36a | 6.41a | 2.74c |
| PC | 3.35a | 5.39a | 6.49a | 1.66a | 5.11a | 5.21b | 4.32b | 5.45a |
| AMP | 2.31ab | 5.31a | 6.47a | 1.48ab | 3.72bc | 6.69a | 5.86a | 4.68b |
| Antibiotic | 1.87b | 3.83b | 4.73b | 1.31b | 2.80c | 5.37b | 4.54b | 4.38b |
| SEM2 | 0.263 | 0.267 | 0.328 | 0.062 | 0.233 | 0.311 | 0.241 | 0.074 |
| P value | 0.007 | 0.002 | 0.007 | 0.008 | 0.001 | 0.009 | 0.001 | 0.001 |
a–cValues within a column with different letters differ significantly (P < 0.05).
1NC: negative control group received corn-soybean meal diet without challenge and additives; PC: positive control group received NC diet experimentally challenged with necrotic enteritis; AMP: PC received group supplemented with 20 mg antimicrobial peptide/ kg diet; Antibiotic: PC received group supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/ kg diet.
2SEM: standard error of means (results are given as means (n = 12) for each treatment).
Gene expression of immune cells and tight junctional proteins
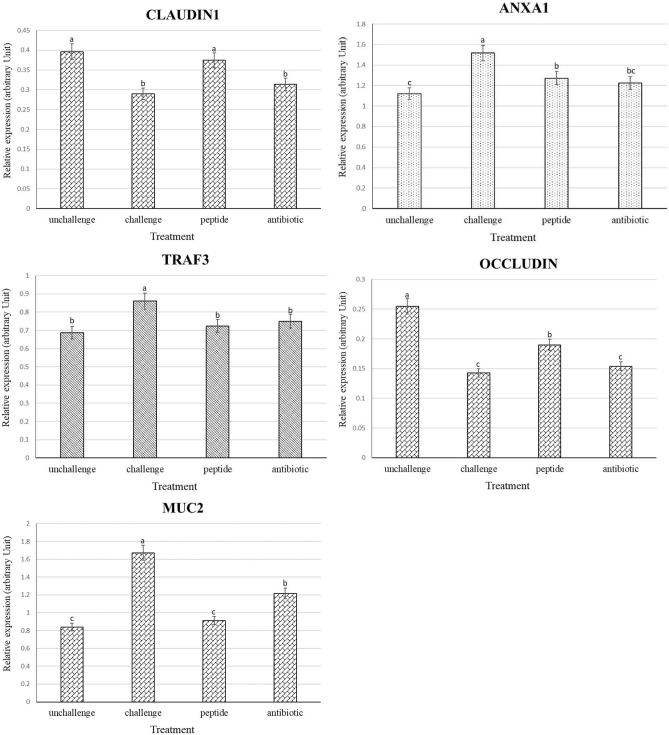
The effects of treatments on the expression level of immune and tight junction genes are presented in Fig. 1. While NE challenge increased (P < 0.05) TRAF3 and ANXA1 transcripts, adding the antibiotic and AMP to the diet reduced (P < 0.05) the expression of immune genes compared to PC group and the antibiotic had similar effects to those of non-challenged birds.
Figure 1.
Effects of treatments on the expression of different genes in the jejunum of broiler chickens on day 22. Samples were analyzed using qPCR, and GAPDH and β-actin were used as the reference genes. Abbreviations as follows: ANXA1, annexin A1; TRAF3, tumor necrosis factor receptor associated factor 3; MUC2, mucin2; unchallenge, control birds received a corn-soybean meal basal diet without AMPs, antibiotic and necrotic enteritis (NE) challenge; challenge, control birds challenged with NE; peptide, birds challenged with NE and supplemented with 20 mg cLFchimera/kg diet; Antibiotic, birds challenged with NE and supplemented with 45 mg antibiotic (bacitracin methylene disalicylate)/kg diet; The letters on the bar mean show significant difference (P < 0.05).
While NE challenge increased the expression of MUC2 in the jejunum of birds, AMP decreased (P < 0.05) the level of MUC2 trasncripts compared to PC group and had similar levels to NC group. Birds challenged with NE had the lowest expression levels of CLDN1 and OCLN genes in their jejunum. AMP increased (P < 0.05) expression patterns of CLDN1 in the jejunum of birds compared to PC group and had similar effects to those of non-challenged birds. Although birds fed with AMP had higher (P < 0.05) OCLN mRNA in the jejunum compared to PC group, this group had lower (P < 0.05) expression of this transcript compared to NC group. The antibiotic did not affect the level of tight junctions transcripts in the jejunum of NE challenged birds compared to both NC and PC groups.
Discussion
Necrotic enteritis is still a global concern with drastic losses in poultry farms, mainly due to retarded growth performance, increased mortality, and veterinary costs23. The outbreak of disease and consequently economic losses have been more prominent in the post-antibiotic era23. Recently, research focus has shifted to AMPs due to their beneficial roles on health attributes and their prophylactic effects against pathogenic invasion12,13,17. Therefore, the principal objective of the current study was to investigate the effects of antimicrobial peptide, cLFchimera, on various productive and health parameters in chickens challenged with NE.
Results of the current study showed that AMP decreased gut lesions and mortality induced by NE. Additional benefits include also improved growth attributes in challenged chickens similar to birds fed antibiotic, confirming the results of previous studies9,10. While most of the previous researches have studied the effects of AMPs in chickens in normal conditions, Hu et al.29 demonstrated that supplementing broilers diet with AMP improved their weight gain and FCR under heat stress. In another challenge study, Wu et al.30 inoculated weanling pigs with E. coli and supplemented the diet with AMP. The autors reported that AMP reduced the incidence of diarrhea and improved weight gain and FCR compared to the challenged group, which is similar to the present findings regarding the reduction in gut lesion and improvement of performance. Previous studies attributed the beneficial effects of AMPs on growth performance of chickens to their fundamental roles in maintaining microbial balance in the gut and consequently improvement in the intestinal morphometry9,10.
It has been well-documented that the villi play the critical roles in absorbing nutrients from the intestinal tract; consequently the morphometry of these villi can drastically affect the host’s performance and health31. The present study confirmed, AMP significantly improved the morphometry of villi in the jejunum of challenged chickens, similar to that of NC group9,10. It has been reported that AMPs extracted from pig intestine32 and rabbit sacculus rotundus33 enhanced jejunal villi characteristics in broiler chickens, which is in line with the present results. Generally, the critical strategy in maintaining villi structure in an infectious disease like NE is the elimination or minimization of the pathogens through provision of antimicrobial additives and manipulation of the intestinal microbiome34,35. Previous studies showed that the antibiotic and AMPs could improve villi morphology and nutrient absorption and consequently increase growth performance in chickens under disease conditions by manipulating the intestinal microflora9,10,17.
The intestinal commensal microbiome interacts with the host through different processes, including nutrients absorption, villi morphology, intestinal pH, and mucosal immunity36,37. In the current study, the supplementation of the antibiotic reduced the colonization of all bacteria, while AMP significantly enhanced the microflora balance in the ileum. In agreement with the present study, Tang et al.7 and Ohh et al.38 reported that AMPs significantly enhanced the microflora balance in the ileum of piglets and broilers, respectively. The antimicrobial action of Bacitracin Methylene Disalicylate (BMD) involves blocking the bacterial ribosome subunits and subsequently impeding protein synthesis, which finally reduces the colonization of the microbial community in the intestine39. Unfortunately, this antibiotic does not differentiate between commensal vs. pathogenic bacteria and may perturb microbial balance in the intestine and deprive the host of benefits of microbes’ roles and products39,40. There is no consensus on the mechanism by which AMPs influence bacterial colonization in the intestine, while two direct and indirect mechanisms have been proposed based on the physiological properties of peptides. The direct antimicrobial effect of AMPs has been attributed to different surface charges of peptides and pathogens41. In other words, AMPs possess positive charges contributing to electrostatically adhere to negatively charged bacterial membranes41,42. This attachment can either destroy the bacterial membranes through physical disruption or penetrate the bacterial cytoplasm without exerting any damage to the lipid layer41,43. Imported AMPs may interfere with intracellular signaling pathways like nucleic acids synthesis, enzyme activity, and protein biosynthesis42,43. In the indirect mode, AMPs might manipulate the microbial community of the intestine in favor of the colonization of beneficial bacteria (e.g. Lactobacillus spp. and Bifidobacterium spp.) and enhance the host health through various physiological mechanisms (e.g. competitive exclusion, secretion of short-chain fatty acids, activation of intestinal immune system, etc.)42. Previous findings suggested that cLF36 could attach to the bacterial membrane through electrostatic interactions and physically disrupt bacterial bilayer membranes12,13,16. In line with the previous reports44, the current results demonstrate that AMP can selectively prevent the bacterial growth in the intestine of NE challenged chickens, which may prove the competitive advantage of cLFchimera compared to antibiotics. Furthermore, previous research reported that the antimicrobial activities of AMPs against pathogens in the intestine might alert the host immune system to fight against invading agents45,46.
Mucosal immunity plays an important role in host defense against pathogens47. In broiler chickens, when Toll-like receptor (TLR)4 engages to microbe-associated molecular patterns (MAMP), it transmits the information to the cytoplasm of the phagocytes, which in turn leads to the expression of cytokines48,49. Different research groups have reported the controversial results regarding the effects of C. perfringens challenge on gene expression of TLR4 in the intestine of broiler chickens. For instance, while some researchers reported that C. perfringens upregulated the TLR4 gene expression in the intestine of chickens50,51, other investigators reported no apparent alteration of the TLR4 gene expression in C. perfringens challenged chickens52. Therefore, in the current study, we decided to analyze the gene expression of TRAF3, which is one step ahead of TLR4 activation to overcome the possible interference of other immune cells53. TRAF3 is a cytoplasmic protein that controls signal transduction from different receptor families, especially TLRs53. Following the activation of TLR4 with pathogen attachment, TRAF3 is recruited into signaling complexes, and its activation increases vital pro-inflammatory cytokines production54,55. Results of the present study showed that NE upregulated the expression of TRAF3 transcript in the jejunum of chickens, while the antibiotic and AMP significantly decreased the gene expression of this cytokine in the challenged birds, which is consistent with the results of previous studies54,56.
On the other hand, excessive and long-term production of pro-inflammatory cytokines might result in the gut damage and high energy demand57. To prevent the adverse effects of extra pro-inflammatory cells, pro-resolving mediators such as ANXA1 are released into the epithelial environment to orchestrate clearance of inflammation and restoration of mucosal homeostasis58,59. ANXA1 is a 37 kDa protein expressed in the apical and lateral plasma membrane in the intestinal enterocytes that facilitates the resolution of inflammation and repair60. In the current study, C. perfringens upregulated the expression of ANXA1 mRNA in the jejunum of PC group, which is in agreement with previous report54,61, while the antibiotic and AMP significantly decreased the expression of this cytokine, which is firstly reported herein. While there is no well-documented evidence to explain the results of cytokines expression, it could be inferred that antimicrobial activities of the antibiotic and AMP in the current study resulted in the reduction of invading pathogens (based on abovementioned microbial results) in the intestine of PC birds and possibly downregulating the expression of both pro- and anti-inflammatory (i.e. TRAF3 and ANXA1, respectively) cytokine-producing cells.
The epithelial barrier consists of tight junction proteins forming the primary lines of defence against a wide range of stimuli from feed allergens to commensal and pathogenic bacteria62,63. The disruption of these proteins may result in increasing the intestinal permeability to luminal pathogens62,63. Previous studies showed that C. perfringens might attach to the junctional proteins to form gaps between the epithelial cells and disrupt the intestinal integrity63,64. In the present study, NE challenge reduced the jejunal expression of OCLN and CLDN1 transcripts, which is in agreement with previous studies64,65. Previous studies showed that tight junction proteins, especially CLDN1 and OCLN, have a specific region (i.e. ECS2) containing a toxin-binding motif, NP (V/L)(V/L)(P/A), that is responsible for binding to C. perfringens63,66. Following attachment to junctional proteins, C. perfringens could digest these proteins67 and open the intracellular connection between adjacent epithelial cells resulting in more penetration of pathogens to deeper layers of lamina propria and transmitting to other organs63,68. AMP significantly upregulated the expression of these genes in PC group and the antibiotic had no significant effect on the gene expression of junctional proteins. In agreement with the current findings, previous reports demonstrated that AMPs could increase the expression of junctional proteins in different challenge conditions17,69. While no exact mechanism has been recognized, there are two suggested theories for the inhibitory effects of AMPs on C. perfringens regarding junctional proteins. In the first theory, it has been suggested that AMPs could directly switch on the expression of regulatory proteins (i.e. Rho family) in the intestine of challenged mice, upregulating the expression of tight junction proteins and ameliorating leaky gut69,70. The second theory attributed the beneficial effects of AMPs on tight junctions to their indirect roles in manipulating microflora populations in the intestine. In detail, previous studies showed that the intestinal commensal bacteria like Bifidobacteria and Lactobacilli secrete butyric acid that regulates epithelial O2 consumption and stabilization of hypoxia-inducible factor. This transcription factor protecting the epithelial barrier against pathogens, resulting in higher expression of junctional proteins71,72. Therefore, it can be hypothesized that AMP in the current study upregulated the expression of junctional proteins by both reducing the number of C. perfringens and inhibiting protein disruption by bacterial toxins. Surprisingly, the antibiotic did not change the expression of CLDN1 and OCLN transcripts in the jejunum of chickens, while it could be expected that the antibiotic upregulated the junctional proteins due to the antibacterial nature of antibiotics. In line with the current results, Yi et al.69 reported that antibiotics might not affect the gene expression of junctional proteins of the epithelial cells after pathogen removal, maybe because of controlling the microbial balance in the intestine.
Along with junctional proteins, the luminal mucus layer comprising of mucins plays a defensive role against invasive pathogens73. MUC2 widely expresses in goblet cells and secretes into the intestinal lumen to stabilize mucosal layer73,74. Any damage to the mucosal layer stimulates the expression of MUC2 to secrete more mucin and prevent further destruction74,75. In the current study, NE significantly increased the gene expression of MUC2 in the jejunum, which is in agreement with the results of previous studies76,77. On the other hand, the antibiotic and AMP significantly downregulated the level of this transcript, while the results for AMP was similar to those of NC group. According to the bacterial results in the present study, it could be inferred that the inhibitory effects of AMP on the population of C. perfringens and E. coli might reduce the colonization of these bacteria in the intestine, decrease the destruction of the mucosal layer, and subsequently lessen the expression of MUC2 transcript. The exact mechanism of these effects has not yet been revealed.
In conclusion, results of the current study propose that cLFchimera, an antimicrobial peptide originating from camel milk, could reduce mortality and attenuate NE-induced lesions in broilers. Beneficial consequences of AMP use include better growth performance and recovery of villi morphology in the jejunum of NE-imposed chickens. Further, supplementing NE challenged birds with cLFchimera restored the ileal microflora and consequently regulated the expression of cytokines, MUC2, and tight junctional proteins. Therefore, according to the desired results obtained in the present study, cLFchimera can be nominated as a candidate for replacing growth-promoting antibiotics against NE in chickens, while further studies may find other favourable effects of this AMP.
Acknowledgements
The authors would also like to express their gratitude to the Ferdowsi University of Mashhad for their support.
Author contributions
A.D. performed the experiment and wrote the main manuscript text. H.K. contributed to the experimental design. M.H.S. contributed to the writing of the manuscript. A.J. contributed to the experimental design. Mo.An. performed the experiment. Ma.Ah. performed the experiment. A.A. performed the experiment. All authors reviewed the manuscript.
Data availability
All generated and analysed data in the current study are included in this article, and also cited data were included in the reference list.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hassan Kermanshahi, Email: kermansh@um.ac.ir.
Mohammad Hadi Sekhavati, Email: sekhavati@um.ac.ir.
References
- 1.Truong AD, et al. Analysis of differentially expressed genes in necrotic enteritis-infected fayoumi chickens using RNA sequencing. Poult. Sci. J. 2017;54:121–133. doi: 10.2141/jpsa.0160053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper KK, Songer JG. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2019;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Aarestrup FM, Wegener HC, Collignon P. Resistance in bacteria of the food chain: epidemiology and control strategies. Expert Rev. Anti Infec. Ther. 2008;6:733–750. doi: 10.1586/14787210.6.5.733. [DOI] [PubMed] [Google Scholar]
- 4.Hong YH, Song W, Lee SH, Lillehoj HS. Differential gene expression profiles of β-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012;91:1081–1088. doi: 10.3382/ps.2011-01948. [DOI] [PubMed] [Google Scholar]
- 5.Seo MD, Won HS, Kim JH, Mishig-Ochir T, Lee BJ. Antimicrobial peptides for therapeutic applications: a review. Molecules. 2012;17:12276–12286. doi: 10.3390/molecules171012276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirkhezranian, Z., Tanhaeian, A., & Sekhavati, M. H. Expression of enterocin-P in HEK platform: evaluation of its cytotoxic effects on cancer cell lines and its potency to interact with cell-surface glycosaminoglycan by molecular modeling. Int J Pept Res Ther. 12 (2019).
- 7.Tang Z, et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin–lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2008;101:998–1005. doi: 10.1017/S0007114508055633. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Gutierrez E, Mayer MJ, Cotter PD, Narbad A. Gut microbiota as a source of novel antimicrobials. Gut Microbes. 2019;10:1–21. doi: 10.1080/19490976.2018.1455790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SC, et al. Effects of dietary supplementation with an antimicrobial peptide-P5 on growth performance, nutrient retention, excreta and intestinal microflora and intestinal morphology of broilers. Anim. Feed Sci. Technol. 2013;185:78–84. doi: 10.1016/j.anifeedsci.2013.07.005. [DOI] [Google Scholar]
- 10.Choi SC, et al. An antimicrobial peptide-A3: effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Brit Poult. Sci. 2013;54:738–746. doi: 10.1080/00071668.2013.838746. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, et al. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. J. Anim. Sci. 2015;93:4750–4760. doi: 10.2527/jas.2015-9284. [DOI] [PubMed] [Google Scholar]
- 12.Tanhaiean A, Azghandi M, Razmyar J, Mohammadi E, Sekhavati MH. Recombinant production of a chimeric antimicrobial peptide in E. coli and assessment of its activity against some avian clinically isolated pathogens. Microb. Pathog. 2018;122:73–78. doi: 10.1016/j.micpath.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Tanhaieian A, Sekhavati MH, Ahmadi FS, Mamarabadi M. Heterologous expression of a broad-spectrum chimeric antimicrobial peptide in Lactococcus lactis: its safety and molecular modeling evaluation. Microb. Pathog. 2018;125:51–59. doi: 10.1016/j.micpath.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Tahmoorespur M, Azghandi M, Javadmanesh A, Meshkat Z, Sekhavati MH. A novel chimeric anti-HCV peptide derived from camel lactoferrin and molecular level insight on its interaction with E2. Int. J. Pept. Res. Ther. 2019;19:1–13. [Google Scholar]
- 15.Tanhaeian A, Jaafari MR, Ahmadi FS, Vakili-Ghartavol R, Sekhavati MH. Secretory expression of a chimeric peptide in Lactococcus lactis: assessment of its cytotoxic activity and a deep view on its interaction with cell-surface glycosaminoglycans by molecular modeling. Probiot. Antimicrob. Proteins. 2019;11:1034–1041. doi: 10.1007/s12602-018-9496-6. [DOI] [PubMed] [Google Scholar]
- 16.Daneshmand A, Kermanshahi H, Sekhavati MH, Javadmanesh A, Ahmadian M. Antimicrobial peptide, cLF36, affects performance and intestinal morphology, microflora, junctional proteins, and immune cells in broilers challenged with E. coli. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-50511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanhaeian A, Mirzaii M, Pirkhezranian Z, Sekhavati MH. Generation of an engineered food-grade Lactococcus lactis strain for production of an antimicrobial peptide: in vitro and in silico evaluation. BMC Biotechnol. 2020;20:1–13. doi: 10.1186/s12896-020-00612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.de Souza Cândido E, et al. The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides. 2014;55:65–78. doi: 10.1016/j.peptides.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Wu SB, Stanley D, Rodgers N, Swick RA, Moore RJ. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Afshari A, Jamshidi A, Razmyar J, Rad M. Genotyping of Clostridium perfringens isolated from broiler meat in northeastern of Iran. Vet. Res. Forum. 2015;6:279. [PMC free article] [PubMed] [Google Scholar]
- 22.Razmyar J, Peighambari SM, Zamani AH. Detection of a newly described bacteriocin, perfrin, among Clostridium perfringens isolates from healthy and diseased ostriches and broiler chickens in Iran. Avian Dis. 2017;61:387–390. doi: 10.1637/11580-010517-ResNoteR. [DOI] [PubMed] [Google Scholar]
- 23.Keyburn AL, et al. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daneshmand A, Kermanshahi H, Danesh Mesgaran M, King AJ, Ibrahim SA. Effect of purine nucleosides on growth performance, gut morphology, digestive enzymes, serum profile and immune response in broiler chickens. Br. Poult. Sci. 2017;58:536–543. doi: 10.1080/00071668.2017.1335859. [DOI] [PubMed] [Google Scholar]
- 25.Kermanshahi H, et al. Effects of acidified yeast and whey powder on performance, organ weights, intestinal microflora, and gut morphology of male broilers. Braz. J. Poult. Sci. 2017;19:309–316. doi: 10.1590/1806-9061-2016-0351. [DOI] [Google Scholar]
- 26.Kermanshahi H, Ghofrani Tabari D, Khodambashi Emami N, Daneshmand A, Ibrahim SA. Effect of in ovo injection of threonine on immunoglobulin A gene expression in the intestine of Japanese quail at hatch. J. Anim. Physiol. Anim. Nutr. 2017;101:10–14. doi: 10.1111/jpn.12543. [DOI] [PubMed] [Google Scholar]
- 27.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu F, et al. Effects of antimicrobial peptides on growth performance and small intestinal function in broilers under chronic heat stress. Poult. Sci. 2017;96:798–806. doi: 10.3382/ps/pew379. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides. 2012;35:225–230. doi: 10.1016/j.peptides.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Hampson DJ. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986;40:32–40. doi: 10.1016/S0034-5288(18)30482-X. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, et al. Effects of rabbit sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult. Sci. 2008;87:250–254. doi: 10.3382/ps.2007-00353. [DOI] [PubMed] [Google Scholar]
- 33.Bao H, et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult Sci. 2009;88:291–297. doi: 10.3382/ps.2008-00330. [DOI] [PubMed] [Google Scholar]
- 34.Yegani M, Korver DR. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahiya JP, Wilkie DC, Van Kessel AG, Drew MD. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. doi: 10.1016/j.anifeedsci.2005.12.003. [DOI] [Google Scholar]
- 36.Apajalahti J, Kettunen A, Graham H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World Poult. Sci. J. 2004;60:223–232. doi: 10.1079/WPS20040017. [DOI] [Google Scholar]
- 37.Castanys-Muñoz E, Martin MJ, Vazquez E. Building a beneficial microbiome from birth. Adv. Nutrition. 2016;7:323–330. doi: 10.3945/an.115.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohh SH, et al. Effects of potato (Solanum tuberosum I. cv. golden valley) protein on performance, nutrient metabolizability, and cecal microflora in broilers. Arch Für Geflügelk. 2010;74:30–35. [Google Scholar]
- 39.Proctor A, Phillips GJ. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. Vet. Sci. 2019;6:114. doi: 10.3389/fvets.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koltes DA, et al. Effects of bacitracin methylene disalicylate and diet change on gastrointestinal integrity and endotoxin permeability in the duodenum of broiler chicken. BMC Res. Notes. 2017;10:470. doi: 10.1186/s13104-017-2781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang LJ, Gallo RL. Antimicrobial peptides. Curr. Biol. 2016;26:14–19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front. Immunol. 2012;3:310. doi: 10.3389/fimmu.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz J, Ortiz C, Guzman F, Fernandez-Lafuente R, Torres R. Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr. Med. Chem. 2014;21:2299–2321. doi: 10.2174/0929867321666140217110155. [DOI] [PubMed] [Google Scholar]
- 44.Alakomi HL, et al. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000;66:2001–2005. doi: 10.1128/AEM.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sørensen, O. E., Borregaard, N., & Cole, A. M. Antimicrobial peptides in innate immune responses. InTrends in innate immunity (Vol. 15, pp. 61–77). Karger Publishers (2008). [DOI] [PubMed]
- 46.Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Curr. Pharma Des. 2009;15:2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacDonald TT. The mucosal immune system. Parasite Immunol. 2003;25:235–246. doi: 10.1046/j.1365-3024.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- 48.Kannaki TR, Reddy MR, Shanmugam M, Verma PC, Sharma RP. Chicken toll-like receptors and their role in immunity. World Poult. Sci. J. 2010;66:727–738. doi: 10.1017/S0043933910000693. [DOI] [Google Scholar]
- 49.Adhikari P, Lee CH, Cosby DE, Cox NA, Kim WK. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2018;98:1235–1242. doi: 10.3382/ps/pey443. [DOI] [PubMed] [Google Scholar]
- 50.Cao L, et al. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poult. Sci. 2012;91:3065–3071. doi: 10.3382/ps.2012-02548. [DOI] [PubMed] [Google Scholar]
- 51.Du E, et al. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo S, Li C, Liu D, Guo Y. Inflammatory responses to a Clostridium perfringens type A strain and α-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 2015;44:81–91. doi: 10.1080/03079457.2015.1005573. [DOI] [PubMed] [Google Scholar]
- 53.Häcker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 2011;11:457. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 54.Kim DK, et al. Transcriptional profiles of host-pathogen responses to necrotic enteritis and differential regulation of immune genes in two inbreed chicken lines showing disparate disease susceptibility. PLoS ONE. 2014;9:e114960. doi: 10.1371/journal.pone.0114960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang HL, et al. Molecular cloning and expression analysis of TRAF3 in chicken. Genet. Mol. Res. 2015;14:4408–4419. doi: 10.4238/2015.April.30.14. [DOI] [PubMed] [Google Scholar]
- 56.Broom LJ, Kogut MH. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2018;98:1634–1642. doi: 10.3382/ps/pey535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SH, et al. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nut. 2013;110:840–847. doi: 10.1017/S0007114512006083. [DOI] [PubMed] [Google Scholar]
- 58.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune–epithelial interactions. Mucosal Immunol. 2015;8:959. doi: 10.1038/mi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leoni G, Nusrat A. Annexin A1: shifting the balance towards resolution and repair. Bio Chem. 2016;397:971–979. doi: 10.1515/hsz-2016-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fasina YO, Lillehoj HS. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult Sci. 2018;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulluwishewa D, et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 63.Saitoh Y, et al. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015;347:775–778. doi: 10.1126/science.1261833. [DOI] [PubMed] [Google Scholar]
- 64.Hashimoto Y, Yagi K, Kondoh M. Roles of the first-generation claudin binder, Clostridium perfringens enterotoxin, in the diagnosis and claudin-targeted treatment of epithelium-derived cancers. Pflügers Arch. 2017;469:45–53. doi: 10.1007/s00424-016-1878-6. [DOI] [PubMed] [Google Scholar]
- 65.Khodambashi Emami N, Calik A, White MB, Young M, Dalloul RA. Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:231. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell LA, Koval M. Specificity of interaction between Clostridium perfringens enterotoxin and claudin-family tight junction proteins. Toxins. 2010;2:1595–1611. doi: 10.3390/toxins2071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pruteanu M, Shanahan F. Digestion of epithelial tight junction proteins by the commensal Clostridium perfringens. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G740–G748. doi: 10.1152/ajpgi.00316.2012. [DOI] [PubMed] [Google Scholar]
- 68.McClane BA. The complex interactions between Clostridium perfringens enterotoxin and epithelial tight junctions. Toxicon. 2001;39:1781–1791. doi: 10.1016/S0041-0101(01)00164-7. [DOI] [PubMed] [Google Scholar]
- 69.Yi H, et al. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 2016;6:25679. doi: 10.1038/srep25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi H, Hu W, Chen S, Lu Z, Wang Y. Cathelicidin-WA improves intestinal epithelial barrier function and enhances host defense against enterohemorrhagic escherichia coli O157: H7 infection. J. Immunol. 2017;198:1696–1705. doi: 10.4049/jimmunol.1601221. [DOI] [PubMed] [Google Scholar]
- 71.Kelly CJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diao H, Jiao AR, Yu B, Mao XB, Chen DW. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019;14:4. doi: 10.1186/s12263-019-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forstner G, Forstner JF. Gastrointestinal mucus. In: Johnson R, Leonard P, editors. Physiology of the Gastrointestinal Tract. 3. New York: Raven Press; 1994. pp. 1255–1283. [Google Scholar]
- 74.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. PNAS. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kabat AM, Srinivasan N, Maloy KJ. Modulation of immune development and function by intestinal microbiota. Trends Immunol. 2014;35:507–517. doi: 10.1016/j.it.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collier CT, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Iimmunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 77.Forder RE, Nattrass GS, Geier MS, Hughes RJ, Hynd PI. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All generated and analysed data in the current study are included in this article, and also cited data were included in the reference list.